Abstract
Pediatric cases of coronavirus disease 2019 (COVID-19) have been reported. This meta-analysis was aimed at describing the clinical, laboratory, and imaging characteristics of children with COVID-19 based on published data of pediatric COVID-19 cases.
Search of PubMed, Embase, Web of Sciences, Science Direct, and Google Scholar for articles published until December 14, 2020, that described the clinical, laboratory, and imaging features of children with COVID-19. Data were extracted independently by 2 authors. Random-effects meta-analysis models were used to report pooled results.
Clinical data from 2874 children with COVID-19 from 37 articles were finally included for quantitative analyses. Fever (48.5%, 95% CI: 41.4%–55.6%) and cough (40.6%, 95% CI: 33.9%–47.5%) were the most common symptoms; asymptomatic infection and severe cases, respectively, accounted for 27.7% (95% CI: 19.7%–36.4%) patients and 1.1% of the 1933 patients included. Laboratory tests showed 5.5% (95% CI: 2.8%–8.9%) of the patients had lymphopenia. The pooled prevalence of leukopenia was 7.3% (95% CI: 3.4%–12.2%), and the C-reactive protein level was high in 14.0% (95% CI: 6.8%–22.8%). Chest computed tomography showed unilateral and bilateral lesions, and ground-glass opacity in 29.4% (95% CI: 24.8%–34.3%) and 24.7% (95% CI: 18.2%–31.6%), and 32.9% (95% CI: 25.3%–40.9%), respectively, and normal in approximately 36.0% (95% CI: 27.7%–44.7%).
We found that children with COVID-19 had relatively mild disease, with quite a lot of asymptomatic infections and low rate of severe illness. Data from more regions are needed to determine the prevention and treatment strategies for children with COVID-19.
Keywords: children, clinical features, coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2
1. Introduction
Coronavirus disease 2019 (COVID-19) is a type of atypical pneumonia that broke out in December 2019, the causative pathogen of which was isolated and named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Due to the rapid spread of COVID-19, the World Health Organization (WHO) has declared the disease a public health emergency of international concern.[1] Although the WHO and countries across the world have issued relevant prevention guidelines,[2] 199 million people in more than 200 countries have been infected, and it seems that the trend of an increase in infections will continue for a long time.
The SARS-CoV-2 belongs to betacoronavirus genus of viruses, to which the severe acute respiratory syndrome and Middle East respiratory syndrome viruses belong as well.[3] Previous studies have shown that COVID-19 can be transmitted from human to human through droplets and contact transmission,[4] the main susceptible population are individuals aged higher than 50 years,[5] the main symptoms are fever and cough, followed by myalgia, headache, and fatigue, and laboratory tests usually show lymphopenia and leukocytosis. Most of the infected people are mildly ill, but severely ill patients can deteriorate and develop a variety of serious complications and might even death.[6,7]
Some cases of infection in children have also been reported,[8] which overturned the previous conjecture that children were not susceptible to COVID-19. These sporadic reports indicated that the characteristics of COVID-19 in children differed from those of the disease in adults. For example, the symptoms of pediatric COVID-19 are mild, the decrease in lymphocytes is not obvious and severe cases are rare. However, due to the low incidence of pediatric COVID-19 and the scattered locations of the cases, the present research on pediatric COVID-19 is insufficient. Moreover, many clinical characteristics of pediatric COVID-19 were not fully understood for the limited sample sizes of these studies, and a consensus was not reached,[9] for example, previous studies have shown that the incidence of severe COVID-19 in children is lower than that in adults, however, it was difficult to obtain reliable quantitative data on the incidence of severe COVID-19.
Meta-analyses are usually performed to combine data from randomized clinical trials. However, in many instances, randomized clinical trials are not feasible and only observational studies can be performed (e.g., in the case of Zika virus infection).[10] Therefore, meta-analyses combining data from observational studies are widely conducted to provide more precise estimates of the effects of treatments or risk factors for diseases.[11] We conducted a systematic review and meta-analysis to describe the clinical, laboratory, and imaging characteristics of children with COVID-19.
2. Methods
2.1. Protocol and registration
This review protocol followed the recommendations established in the MOOSE statement and has been registered in the PROSPERO database (registration number CRD42020180827).[12]
2.2. Data sources and search strategy
PubMed, Embase, Web of Sciences, Science Direct, and Google Scholar were searched for articles published until December 14, 2020. The following search terms were used: “COVID-19,” “2019 novel coronavirus disease,” “COVID-19 pandemic,” “SARS-CoV-2 infection,” “COVID-19 virus disease,” “2019 novel coronavirus infection,” “2019-nCoV infection,” “coronavirus disease 2019,” “coronavirus disease-19,”“2019-nCoV disease,” “COVID-19 virus infection” and “pediatric,” “child,’ and “children.” No language restrictions were set.
2.3. Inclusion and exclusion criteria
Studies were considered eligible if the subjects included pediatric COVID-19 patients (age range: 0–18 years) diagnosed by real-time RT-PCR, and reported at least 1 of the clinical features, laboratory text, imaging characteristics, were included in the meta-analysis. Case reports, review articles, opinion articles, and letters lacking available data were excluded.
2.4. Study selection
Two reviewers (ZL and SC) browsed the titles and abstracts to filter the search results, followed by the reading of the full text of the articles to determine whether they met the inclusion and exclusion criteria (Fig. 1), different opinions were resolved through consulted and discussed. When the same patient was reported at the same time in 2 articles, we performed statistical analyses with more complete information. References of included articles were also reviewed.
Figure 1.
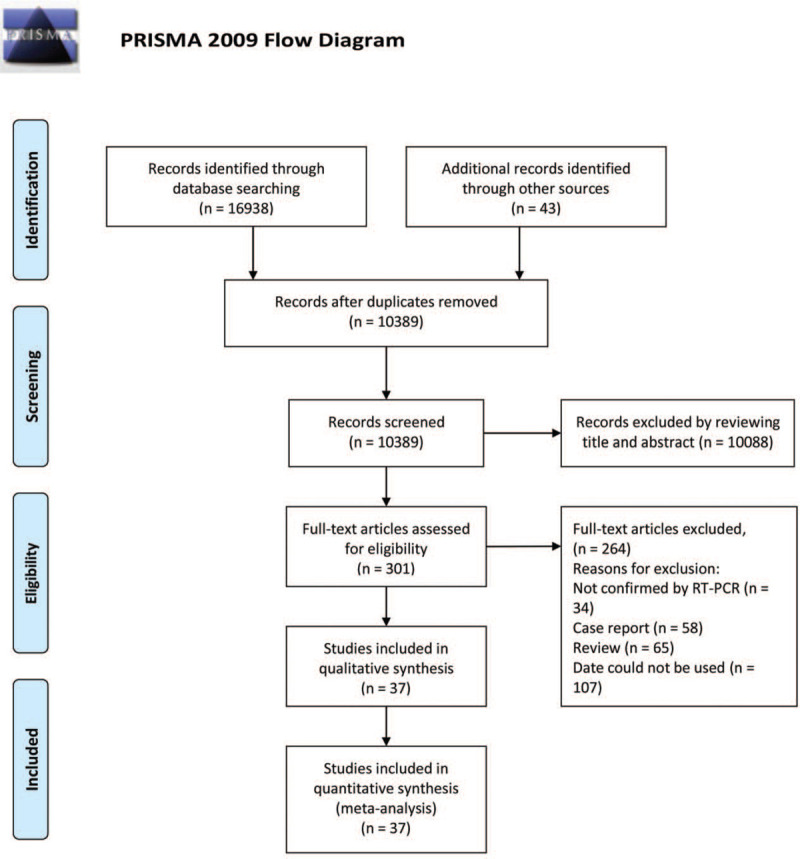
Flow diagram of papers screened and included.
2.5. Data collection process and data items
The following features were extracted by 2 investigators using a standardized data extraction form (ZL and SC): country/region, number of reported cases, number of severe cases, patient age, number of asymptomatic cases, history of exposure, clinical characteristics (e.g., fever and cough), laboratory test results (e.g., lymphocytopenia and biochemistry results), and chest computed tomography (CT) findings. All data were checked by a third researcher (XJJ).
2.6. Study quality evaluation
Two authors (Zwb and QK) screened and evaluated the literature independently. The quality of the case series was graded using The Joanna Briggs Institute (JBI) critical appraisal checklist.[13] The quality assessment method for descriptive/case series studies recommended by the JBI Reviewer's Manual includes nine quality items, which are judged as “yes,” “no,” “unclear,” or “not applied” for each question. Cross-sectional studies and cohort studies were graded using the Newcastle–Ottawa Scale (NOS).[14]
2.7. Statistical analysis
The meta-analyses were performed using Stata 15.0, metaprop command. Pooled prevalence and their 95% CIs were used to summarize the weighted effect sizes for each grouping variable in the study by using the binary random-effects model (the weighting took into consideration the sample sizes of the individual studies). Heterogeneity was evaluated using Cochran's Q statistic, the I2 index, and the tau-squared test. Sensitivity analysis was performed on the basis of outlier data. Funnel plot and Egger regression test were used to evaluate publication biases.
3. Results
3.1. Study selection and characteristics
A total of 16,938 articles were retrieved by the search strategy, of which 10,389 articles remained after deleting duplicate studies. After screening the abstracts and titles, 301 articles were selected for full-text assessment. Among them, 58 articles were case report, 65 were reviews and 107 were excluded for not showing confirmation analyses by RT-PCR or because of lack of available data. A total of 37 articles, with a total of 2874 patients, were finally included in the quantitative meta-analysis, consisting of 18 cross-sectional studies, 4 cohort studies, and 15 case series.[8,15–50] Twenty seven of the selected studies were from China and 10 were from abroad. Table 1 shows the main characteristics of these included studies. A total of 30 variables were quantitatively synthesized in this meta-analysis (Shown in Table 2).
Table 1.
Characteristics of the included studies on pediatric COVID-19.
| Author | Study type | Region | Year | N | Age | Sex (Male) | Quality score |
| CDC | Cross-sectional | The U.S.A | 2020 | 291 | Range: 0–17 yr | NA | 8∗ |
| Chen et al | Cross-sectional | China | 2020 | 12 | median: 14.5 yr | 6 | 8∗ |
| Dong et al | Cross-sectional | China | 2020 | 728 | Range: 2–13 yr | 418 | 8∗ |
| Du et al | Cross-sectional | China | 2020 | 14 | median: 6.2 yr | 6 | 7∗ |
| Han et al | Cross-sectional | China | 2020 | 7 | Range: 2–13 yr | 4 | 7∗ |
| Liang et al | Cross-sectional | China | 2020 | 9 | Range: 1–9 yr | 3 | 8∗ |
| Liu et al | Cross-sectional | China | 2020 | 4 | Range: 1–9 yr | 2 | 8∗ |
| Qiu et al | Cross-sectional | China | 2020 | 36 | mean: 8 3 yr | 23 | 9∗ |
| Wang et al | Cross-sectional | China | 2020 | 31 | Range: 1–17 yr | 15 | 8∗ |
| Xia et al | Cross-sectional | China | 2020 | 20 | median: 2 yr | 13 | 9∗ |
| Xie et al | Cross-sectional | China | 2020 | 13 | Range: 10–18 yr | 7 | 9∗ |
| Zheng et al | Cross-sectional | China | 2020 | 25 | Range: 1–14 yr | 14 | 9∗ |
| Zhu et al | Cross-sectional | China | 2020 | 10 | Range: 1–17 yr | 5 | 9∗ |
| Ma et al | Cross-sectional | China | 2020 | 50 | Range: 0–17 yr | 28 | 9∗ |
| Korkmaz et al | Cross-sectional | Turkey | 2020 | 81 | median: 9.5 yr | 48 | 8∗ |
| Wu et al | Cross-sectional | China | 2020 | 74 | median: 6 yr | 44 | 9∗ |
| Du et al | Cross-sectional | China | 2020 | 182 | median: 6 years | 120 | 9∗ |
| Parri et al | Cross-sectional | Italy | 2020 | 130 | median: 6 yr | 73 | 8∗ |
| Bo Li et al | Case series | China | 2020 | 22 | mean: 8 yr | 12 | 5/9† |
| Cai et al | Case series | China | 2020 | 10 | Range: 1–11 yr | 4 | 5/9† |
| Feng et al | Case series | China | 2020 | 15 | Range: 4–14 yr | 5 | 6/9† |
| Li et al | Case series | China | 2020 | 5 | Range: 1–6 yr | 4 | 7/9† |
| Lu et al | Case series | China | 2020 | 171 | mean: 6.7 yrs | 104 | 7/9† |
| Shen et al | Case series | China | 2020 | 9 | Range: 1–12 yr | 3 | 6/9† |
| Tan et al | Case series | China | 2020 | 10 | Range: 1–11 yr | 3 | 7/9† |
| Xu et al | Case series | China | 2020 | 10 | Range: 1–17 yr | 6 | 8/9† |
| Zhou et al | Case series | China | 2020 | 9 | Range: 1–3 yr | 4 | 7/9† |
| Steinberger et al | Case series | China | 2020 | 30 | median: 10 years | 15 | 7/9† |
| Wu et al | Case series | China | 2020 | 157 | median: 7 yr | 60 | 7/9† |
| Ma et al | Cohort study | China | 2020 | 216 | median: 7.25 yr | 134 | 9∗ |
| Han et al | Cohort study | Korea | 2020 | 91 | median: 11 yr | 53 | 9∗ |
| Kilani et al | Cohort study | Jordan | 2020 | 61 | median: 6 yr | 37 | 7∗ |
| Pablo et al | Case series | Worldwide | 2020 | 91 | median: 6.1 yr | 49 | 6/9† |
| Afshin et al | Case series | Iran | 2020 | 27 | mean: 4.7 ± 4.16 yr | 10 | 5/9† |
| Fakiri et al | Case series | Morocco | 2020 | 74 | median: 7 yr | 34 | 5/9† |
| Danah et al | Cohort study | Kuwait | 2020 | 134 | median: 8.8 yr | 74 | 7∗ |
| Mamishi et al | Case series | Iran | 2020 | 24 | median: 6 yr | 11 | 5/9† |
Table 2.
Meta-analysis outcomes of clinical manifestations, laboratory findings and CT imaging findings of pediatric COVID-19 patients∗.
| Variable | Number of studies | Number of patients | Prevalence % | 95% CI | Q† | I2‡ | t2§ | P |
| Clinical manifestations | ||||||||
| Fever | 36 | 2146 | 48.5 | 41.4–55.6 | 293.28 | 88.07 | 0.13 | <.001 |
| Cough | 35 | 2116 | 40.6 | 33.9–47.5 | 260.34 | 86.94 | 0.11 | <.001 |
| Dyspnea | 21 | 1284 | 7.0 | 2.3–13.5 | 207.53 | 90.36 | 0.16 | <.001 |
| Myalgia | 13 | 985 | 7.1 | 2.0–14.2 | 107.13 | 88. 8 | 0.12 | <.001 |
| Runny nose | 16 | 1295 | 11.0 | 6.9–15.8 | 64.81 | 76.86 | 0.04 | <.001 |
| Sore throat | 23 | 1310 | 6.8 | 2.8–12.0 | 149.16 | 85.25 | 0.11 | <.001 |
| Headache | 13 | 947 | 9.2 | 4.1–15.7 | 83.75 | 85.67 | 0.09 | <.001 |
| Abdominal pain | 12 | 1047 | 3.6 | 1.7–6.0 | 21.44 | 48.7 | 0.01 | .029 |
| Nausea/Vomiting | 17 | 1388 | 5.7 | 3.6–8.1 | 32.38 | 50.59 | 0.01 | .009 |
| Diarrhea | 23 | 1571 | 7.2 | 5.0–9.5 | 40.25 | 45.34 | 0.01 | .010 |
| Fatigue | 12 | 814 | 5.7 | 2.7–9.4 | 23.95 | 54.08 | 0.02 | .013 |
| Asymptomatic | 28 | 2121 | 27.7 | 19.7–36.4 | 348.92 | 92.26 | 0.18 | <.001 |
| Severe cases | 20 | 1933 | 1.1 | 0–2.9 | 71.86 | 73.56 | 0.03 | <.001 |
| Laboratory findings | ||||||||
| Lymphocytosis | 15 | 542 | 8.5 | 3.1–15.4 | 52.01 | 73.08 | 0.08 | <.001 |
| Lymphopenia | 25 | 1303 | 5.5 | 2.8–8.9 | 80.43 | 70.16 | 0.05 | <.001 |
| Leukocytosis | 19 | 573 | 3.5 | 0.6–8.1 | 47.49 | 62.1 | 0.06 | <.001 |
| Leukopenia | 27 | 1320 | 7.3 | 3.4–12.2 | 146.21 | 82.22 | 0.10 | <.001 |
| High CRP | 24 | 1043 | 14.0 | 6.8–22.8 | 215.78 | 89.34 | 0.20 | <.001 |
| High LDH | 14 | 540 | 17.4 | 7.8–29.3 | 100.38 | 87.05 | 0.19 | <.001 |
| High ALT | 21 | 1035 | 6.2 | 2.7–10.6 | 81.36 | 75.42 | 0.07 | <.001 |
| High AST | 19 | 997 | 12.3 | 7.5–17.8 | 69.92 | 74.26 | 0.06 | <.001 |
| High CK-MB | 8 | 488 | 43 | 25.4–61.5 | 84.48 | 91.71 | 0.21 | <.001 |
| High ESR | 10 | 247 | 29.7 | 10.0–53.3 | 79.51 | 88.68 | 0.36 | <.001 |
| D-dimer increase | 11 | 508 | 9.3 | 5.1–14.3 | 16.38 | 38.94 | 0.02 | .089 |
| Procalcitonin increase | 15 | 850 | 22.2 | 9.6–37.7 | 277.41 | 94.95 | 0.35 | <.001 |
| CT imaging findings | ||||||||
| Normal | 28 | 1096 | 36.0 | 27.7–44.7 | 173.06 | 84.40 | 0.15 | <.001 |
| Unilateral lesion | 18 | 872 | 29.4 | 24.8–34.3 | 26.00 | 34.61 | 0.01 | 0.075 |
| Bilateral lesion | 18 | 872 | 24.7 | 18.2–31.6 | 56.56 | 69.94 | 0.05 | <.001 |
| Ground-glass opacity | 22 | 935 | 32.9 | 25.3–40.9 | 98.06 | 78.59 | 0.09 | <.001 |
| Contact with a confirmed case | 26 | 1354 | 93.6 | 88.9–97.3 | 146.42 | 82.93 | 0.10 | <.001 |
3.2. Quality assessment
According to the JBI critical appraisal checklist, the quality of the 15 case-series studies were evaluated. There were 5 scores of 5 points, 9 scores of 6 to 7 points, and 1 score of 8 points. Eighteen cross-sectional studies and 4 cohort studies conducted quality evaluation based on NOS scores, 18 of which were high-quality studies of 8 to 9 points, and 4 was medium-quality study of 7 points. (See Table S1 and S2, Supplemental Content, which shown the details of quality assessment of the articles).
3.3. Clinical characteristics
As shown in Table 2, fever (48.5%, 95% CI: 41.4–55.6%) and cough (40.6%, 95% CI: 33.9%–47.5%) were the most common symptoms, almost all studies have counted these 2 symptoms. Runny nose (11.0%, 95% CI: 6.9%–15.8%), headache (9.2%, 95% CI: 4.1%–15.7%), sore throat (6.8%, 95% CI: 2.8%–12.0%) were reported in 16, 13, and 23 studies, respectively. Other symptoms are relatively rare and few studies have been reported. Asymptomatic infection and severe cases, respectively, accounted for 27.7% (95% CI: 19.7%–36.4%) and 1.1% (95% CI: 0%–2.9%) of patients. (See Table S3 and Figure S2, Supplemental Content, which shown details of results of clinical characteristics of pediatric COVID-19 patients).
3.4. Laboratory findings
As shown in Table 2, only 5.5% (95% CI: 2.8%–8.9%) of children with COVID-19 showed lymphopenia in laboratory tests, which is very common in adult COVID-19. The pooled prevalence of leukopenia in pediatric COVID-19 patients was estimated to be 7.3% (95% CI: 3.4–12.2%), while the corresponding values for high C-reactive protein level, high LDH level, high creatine kinase MB level, high AST level, and high erythrocyte sedimentation rate were 14.0% (95% CI: 6.8%–22.8%), 17.4% (95% CI: 7.8%–29.3%), 43% (95% CI: 25.4%–61.5%), 12.3% (95% CI: 7.5%–17.8%), and 29.7% (95% CI: 10.0%–53.3%), respectively. Abnormal results in laboratory tests for COVID-19 in children were not as common as those in adults. (See Table S4 and Figure S2, Supplemental Content, which shown details of results of laboratory characteristics of pediatric COVID-19 patients).
3.5. Imaging and demographic characteristics
In the chest CT imaging data summarized in Table 2, about 36.0% (95% CI: 27.7–44.7%) of the pediatric COVID-19 patients did not show abnormal imaging findings. Among patients with abnormal imaging findings, unilateral lesions, bilateral lesions, and ground-glass opacity accounted for 29.4% (95% CI: 24.8%–34.3%), 24.7% (95% CI: 18.2%–31.6%) and 32.9% (95% CI: 25.3%–40.9%), of the cases. In assessments of demographic characteristics, the results showed that 93.6% (95% CI: 88.9%–97.3%) of the pediatric COVID-19 patients had contact with a confirmed case. (See Table S5 and Figure S2, Supplemental Content, which shown details of results of Imaging characteristics, contact history, and severe cases of pediatric COVID-19 patients).
3.6. Publication bias
The funnel plot (standard error of fever) showed no obvious publication bias (see Figure S1, Content, Funnel-plot for publication bias of fever of pediatric COVID-19 patients), and the findings of Egger test also proved this conclusion (P = .428).
4. Discussion
As of September 21, 2020, there were 1 998 897confirmed cases of COVID-19 and 954 417 deaths attributable to the disease worldwide. Children were once considered not susceptible to COVID-19, but according to survey data released by the centers for disease control (CDC) on April 2, the United States recorded 2572 cases (1.7%) of children with COVID-19 under the age of 18 years.[15] A survey of 72,314 patients in China showed that children accounted for about 2% of the total number of cases.[51] In addition, children have atypical symptoms of infection and are unable to clearly describe their health status or exposure history, which poses serious challenges to the protection, diagnosis, and treatment of this population.[52] The increase in the number of children's infections and the particularity of clinical manifestations in pediatric COVID-19 infections necessitated a more comprehensive understanding of the clinical, laboratory, and imaging features of pediatric COVID-19.
In this systematic review and random-effects meta-analysis, we tried to conduct a comprehensive quantitative analysis of the published clinical data for COVID-19 in children to obtain clinical features with a larger sample size and more reliable results. Our results are robust due to the pooling of results after combining all the studies, which showed acceptable heterogeneity in forest plots for each of the variables (Table 2). Due to the small sample size of most included studies, the data do not conform to the normal distribution. Fixed-effect meta-analyses are inappropriate in such cases, so we used random-effects analyses.[53]
Most children with COVID-19 have mild clinical symptoms, and some infected patients show no obvious clinical manifestations. Lu et al reported the clinical information of 171 children with COVID-19 admitted to Wuhan Children's Hospital. Most of the patients had mild manifestations, with 15.8% (27/171) showing asymptomatic infection and only 3 showing severe disease.[28] Our results also show these characteristics in children with COVID-19. Fever is the most common symptom in children with COVID-19, but its incidence (50%, 95% CI: 43%–56%) was significantly lower than that in adults (about 70%).[54,55] The second most common infection symptoms were cough (40.6%, 95% CI: 33.9%–47.5%), myalgia (7.1%, 95% CI: 2.0%–14.2%), and sore throat (6.8%, 95% CI: 2.8%–12.0%). Notably, the probability of gastrointestinal symptoms in children was more than 15%, which suggested that gastrointestinal symptoms should receive special attention when diagnosing children with COVID-19.[56] Our results showed that children with severe illness account for only 1.1% of all hospitalized children, and all of these patients had severe underlying diseases. In contrast, the reported critical illness rate for adults is about 3% to 15%.[57] Corresponding to the lower rate of severe illness in children with COVID-19, about 27.7% of children COVID-19 were asymptomatic, while a study of 44672 diagnosed patients of all ages from Chinese Novel Coronavirus Pneumonia Emergency Response Epidemiology Team showed that asymptomatic infections account for only 1.2%.[58] Only 48.5% of children with COVID-19 showed symptoms of fever and 27.7% of the infections were asymptomatic, indicating the possibility of numerous omissions when screening pediatric COVID-19 patients by monitoring body temperature.
Lymphocytopenia, which is an important feature of adult COVID-19 and is considered to be one of the indicators to predict the severity of the disease was rare in children with COVID-19 (5.5%, 95% CI: 2.8%–8.9%).[59] Previous studies have shown that SARS-CoV2 will induce a series of immune responses after entering the body, which then induces inflammatory storms, leading to increases in inflammatory indicators and a decline in lymphocyte counts.[60,61] In our results, leukopenia, high C-reactive protein levels, high erythrocyte sedimentation rate, and high ALT levels were reported less frequently, which indicated that the immune responses of children with COVID-19 were weak. The imaging results showed that about 36% of children's chest CT scans had no imaging abnormalities, and many showed only slight local invasion, indicating that children with COVID-19 had only mild lung injury and a good prognosis.[62]
The data for clinical symptoms along with the findings of laboratory tests and imaging examinations of children with COVID-19 in this study indicate that the conditions of children with COVID-19 generally seem to be relatively mild. The possible reasons are as follows: first, children have healthier airways because they are not exposed to cigarette smoke and air pollution for long periods, both of which are thought to contribute to COVID-19.[62,63] Second, many other kinds of viruses are found in children's lungs and respiratory tract, which can restrict the growth of SARA-CoV2 through direct interaction and competition between viruses.[64] Third, the number of mature angiotensin-converting enzyme-2 (ACE2) receptors in children's lungs is lower than that in adults.[65,66] SARS-CoV2 uses the ACE2 receptors on the cell surface to enter human airway epithelial cells, and the limited number of ACE2 receptors enhance children's resistance to SARS-CoV2.[67] Fourth, the immune system of children is not yet mature. The SARS-CoV2 infection will not produce a large number of inflammatory factors, reducing the damage of autoimmunity to the lungs, heart, liver, and other organs, and the possibility of occurrence of an inflammatory storm, which is an important mechanism leading to the death of patients with severe disease.[68,69] Therefore, lymphocytes in children with COVID-19 rarely decline significantly, and the levels of inflammation indicators such as C-reactive protein are usually normal or transiently elevated. Fifth, since children indulge in relatively limited outdoor activities, they are usually infected by their families, and the virulence of these second- or third-generation infections may be lower in children.[49] The combined effect of these factors leads to a mild condition in children with COVID-19. However, these children are still contagious, and because of their concealed, mild, and asymptomatic disease, pediatric COVID-19 patients may be a key link in the community transmission of SARS-CoV2. Early detection and treatment of children with COVID-19 are of great significance to prevent the spread of SARS-CoV2.[8]
At present, Chang et al has made the 1 preliminary description of the clinical symptoms of children with COVID-19 through pool clinical data,[51] which was basically consistent with our conclusion. However, the number of articles included by Chang et al was limited, there were only 9 early studies with a small sample size, and their study did not collect data from laboratory tests, the description of the clinical characteristics of children with COVID-19 was not comprehensive enough. In addition, there are some studies had analyzed the clinical characteristics of COVID-19 in adults using si2milar methods.[70]
Our conclusions are robust, but there is still some heterogeneity, which can be attributed to the following reasons: First, all of the included studies were retrospective observational studies, and there were no randomized controlled studies. Second, because of the low incidence of COVID-19 in children, the sample size of many studies was small. Third, although all studies included were on children, the age ranges of children in each study were different. Fourth, the criteria for judging some indicators, such as headache and bilateral lesions, in each study may be different.
4.1. Limitations
Some limitations of this study must be pointed out. First, because of the low mortality rate of children with COVID-19, we did not collect enough data for synthesis analysis. Only a few large-scale statistical studies provided a small number of deaths. Of the 149,082 confirmed cases released by the CDC in the United States on April 2nd, only 3 deaths were recorded among children.[15] Data published on March 18 in Italy reported that of the 22,512 Italian cases of COVID-19, 1.2% involved children, with no mortalities.[71] Second, most of the studies included in the study are from China, with only 1 from the United States, 1 from Turkey and 1 from Italy, and more data from COVID-19 in children from other countries are needed to obtain more comprehensive conclusions. Third, to comprehensively describe the characteristics of COVID-19 in children, this study included a large number of articles, resulting in many sources of heterogeneity and the lack of effective means to reduce heterogeneity, but yielding a more comprehensive analysis. Therefore, our conclusions need to be verified by more rigorous large-sample studies.
5. Conclusions
This meta-analysis found that children with COVID-19 have relatively mild disease, with quite a lot of asymptomatic infections and a very low rate of severe illness. The results of laboratory tests were only slightly abnormal. Chest CT showed no obvious abnormality in quite a part of cases, and the scope of lung injury was limited. Data from studies on children with COVID-19 in more regions are needed to determine the best prevention and treatment strategies for pediatric COVID-19 cases.
Author contributions
XJJ and WYP designed the study; QK and ZWB interpreted data and wrote the manuscript; ZL and SC screened and extracted data; HS and DCH conducted statistical analyses; PJH and ZWX reviewed the results and made critical comments on the manuscript: All authors approved the final version of the manuscript.
Data curation: Chao Song.
Formal analysis: Li Zheng.
Investigation: Miao Ye, Sheng Hu.
Methodology: Chuanhui Duan.
Project administration: Yiping Wei, Jianjun Xu.
Supervision: Jinhua Peng.
Visualization: Wenxiong Zhang.
Writing – original draft: Kai Qi, Weibiao Zeng.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ACE2 = angiotensin-converting enzyme-2, CDC = centers for disease control, COVID-19 = coronavirus disease 2019, CT = computed tomography, JBI = Joanna Briggs Institute, NOS = Newcastle–Ottawa Scale, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, WHO = World Health Organization.
How to cite this article: Qi K, Zeng W, Ye M, Zheng L, Song C, Hu S, Duan C, Wei Y, Peng J, Zhang W, Xu J. Clinical, laboratory, and imaging features of pediatric COVID-19: a systematic review and meta-analysis. Medicine. 2021;100:15(e25230).
KQ, WZ, and MY contributed equally to this work.
The study does not require ethical approval because the meta-analysis is based on published research and the original data are anonymous.
The authors have no funding and conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
Quality score based on the Newcastle-Ottawa quality assessment scale for cross-sectional studies (NOS).
Quality score based on the Joanna Briggs Institute quality assessment scale for case series studies (JBI).
CDC, CDC COVID-19 Response Team; NA = not available, not reported.
Using the binary random-effects model.
Cochran's Q statistic for heterogeneity.
I2 Index for the degree of heterogeneity.
Tau-Squared measure of heterogeneity.
95% CI = 95% confidence interval, CRP = C-reactive protein, CK-MB = creatine kinase-MB, CT = computed tomography, AST = aspartate transaminase, ALT = alanine transaminase, LDH = lactate dehydrogenase, ESR = erythrocyte sedimentation rate.
References
- [1].World Health Organization. Coronavirus disease (COVID-19) outbreak 2020. 2020; https://www.who.int. [access date 04 October, 2020]. [Google Scholar]
- [2].World Health Organization. Critical preparedness, readiness and response actions for COVID-19. 2020; https://www.who.int/publications-detail/critical-preparedness-readiness-and-response-actions-for-covid-19. [access date 02 October, 2020]. [Google Scholar]
- [3].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pongpirul WA, Pongpirul K, Ratnarathon AC, et al. Journey of a thai taxi driver and novel coronavirus. N Engl J Med 2020;382:1067–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci 2020;75:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect 2020;80:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alexpandi R, De Mesquita JF, Pandian SK, et al. Quinolines-Based SARS-CoV-2 3CLpro and RdRp Inhibitors and Spike-RBD-ACE2 Inhibitor for Drug-Repurposing Against COVID-19: An in silico Analysis. Front Microbiol 2020;11:1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020;145: [DOI] [PubMed] [Google Scholar]
- [9].She J, Liu L, Liu W. COVID-19 epidemic: disease characteristics in children. J Med Virol 2020;92:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cardona-Ospina JA, Henao-SanMartin V, Acevedo-Mendoza WF, et al. Fatal Zika virus infection in the Americas: a systematic review. Int J Infect Dis 2019;88:49–59. [DOI] [PubMed] [Google Scholar]
- [11].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [12].Moher D, Shamseer L, Clarke M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aromataris E FR, Godfrey C, Holly C, Kahlil H, P T. Methodology for JBI umbrella reviews, In Joanna Briggs Institute reviewer's manual 2014. 2014; http://joannabriggs.org/assets/docs/sumari/ReviewersManual-Methodology-JBI_Umbrella%20Reviews-2014.pdf. [access date Deceber 13, 2020]. [Google Scholar]
- [14].Wells G, Shea B, O Connell DL, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. 2014; https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-(NOS)-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf. [access date December 13, 2020]. [Google Scholar]
- [15].CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children - United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang D, Ju XL, Xie F, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi 2020;58:269–74. [DOI] [PubMed] [Google Scholar]
- [17].Jiehao C, Jin X, Daojiong L, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis 2020;71:1547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Du W, Yu J, Wang H, et al. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province. China Infection 2020;48:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Feng K, Yun YX, Wang XF, et al. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Zhonghua Er Ke Za Zhi 2020;58:E007. [DOI] [PubMed] [Google Scholar]
- [20].Li B, Shen J, Li L, et al. Radiographic and clinical features of children with coronavirus disease (COVID-19) pneumonia. Indian Pediatrics 2020;57:423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Qiu H, Wu J, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020;20:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xia W, Shao JB, Guo Y, et al. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol 2020;55:1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zheng F, Liao C, Fan QH, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci 2020;40:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhu L, Wang J, Huang R, et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol 2020;55:1430–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chandratre S. Promoting medical students’ interest in pediatric endocrinology during COVID-19. Clin Pediatr (Phila) 2020;59:1219. [DOI] [PubMed] [Google Scholar]
- [26].Li W, Cui H, Li K, et al. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol 2020;50:796–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu H, Liu F, Li J, et al. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect 2020;80:e7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lu XX, Zhang LQ, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med 2020;382:1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shen QX, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol 2020;55:1424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Su L, Ma X, Yu H, et al. The different clinical characteristics of corona virus disease cases between children and their families in China-the character of children with COVID-19. Emerging Microbes Infections 2020;9:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tan YP, Tan BY, Pan J, et al. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol 2020;127:104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xie M, Tian J, Hun M, et al. Analysis of Epidemiological and Clinical Characteristics of Children with Mild COVID-19 and Discussion on the Clinical Significance of SARS-CoV-2 Antibody. Available at SSRN: https://ssrn.com/abstract=3566189. [access date Deceber 13, 2020]. [Google Scholar]
- [33].Zhou Y, Yang GD, Feng K, et al. Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children. Chin J Contemp Pediatr 2020;22:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wu Q, Xing Y, Shi L, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics 2020;146: [DOI] [PubMed] [Google Scholar]
- [35].Wu H, Zhu H, Yuan C, et al. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open 2020;3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Steinberger S, Lin B, Bernheim A, et al. CT features of coronavirus disease (COVID-19) in 30 pediatric patients. AJR Am J Roentgenol 2020;215:1303–11. [DOI] [PubMed] [Google Scholar]
- [37].Parri N, Magistà AM, Marchetti F, et al. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur J Pediatr 2020;179:1315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ma HJ, Hu JN, Tian J, et al. A single-center, retrospective study of COVID-19 features in children: a descriptive investigation. BMC Med 2020;18:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Korkmaz MF, Türe E, Dorum BA, et al. The epidemiological and clinical characteristics of 81 children with COVID-19 in a pandemic hospital in Turkey: an observational cohort study. J Korean Med Sci 2020;35: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Du H, Dong X, Zhang JJ, et al. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy 2021;76:510–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr 2021;175:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kilani MM, Odeh MM, Shalabi M, et al. Clinical and laboratory characteristics of SARS-CoV2-infected paediatric patients in Jordan: serial RT-PCR testing until discharge. Paediatr Int Child Health 2020;01–10. [DOI] [PubMed] [Google Scholar]
- [43].Mohammadi A, Mohebbi I, Khademvatani K, et al. Clinical and radiological characteristics of pediatric patients with COVID-19: focus on imaging findings. Jpn J Radiol 2020;38:987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Caro-Dominguez P, Shelmerdine SC, Toso S, et al. Thoracic imaging of coronavirus disease 2019 (COVID-19) in children: a series of 91 cases. Pediatr Radiol 2020;50:1354–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fakiri KE, Nassih H, Sab IA, et al. Epidemiology and clinical features of coronavirus disease 2019 in moroccan children. Indian Pediatr 2020;57:808–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Alsharrah D, Alhaddad F, Alyaseen M, et al. Clinical characteristics of pediatric SARS-CoV-2 infection and coronavirus disease 2019 (COVID-19) in Kuwait. J Med Virol 2020;10.1002/jmv.26684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mamishi S, Heydari H, Aziz-Ahari A, et al. Novel coronavirus disease 2019 (COVID-19) outbreak in children in Iran: Atypical CT manifestations and mortality risk of severe COVID-19 infection. J Microbiol Immunol Infect 2020;S1684-1182(20)30177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ma N, Li P, Wang X, et al. Ocular manifestations and clinical characteristics of children with laboratory-confirmed COVID-19 in Wuhan, China. JAMA Ophthalmol 2020;138:1079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen J, Zhang ZZ, Chen YK, et al. The clinical and immunological features of pediatric COVID-19 patients in China. Genes Dis 2020;7:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020;26:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- [52].Chen ZM, Fu JF, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr 2020;16:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: a comparison between DerSimonian-Laird and restricted maximum likelihood. Stat Methods Med Res 2012;21:657–9. [DOI] [PubMed] [Google Scholar]
- [54].Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hu Tuo FL, Junling Wang, Jingping Ye. Clinical characteristics of 2019 novel coronavirus (2019-nCoV) infection in children and family prevention and control (in Chinese). Med J Wuhan Univ 2020;41:357–61. [Google Scholar]
- [57].Xu T, Chen C, Zhu Z, et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis 2020;94:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:145–51. [DOI] [PubMed] [Google Scholar]
- [59].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Liu WJ, Zhao M, Liu K, et al. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res 2017;137:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cai JH, Wang XS, Ge YL, et al. First case of 2019 novel coronavirus infection in children in Shanghai. Zhonghua Er Ke Za Zhi 2020;58:86–7. [DOI] [PubMed] [Google Scholar]
- [63].Zhu Y, Xie J, Huang F, et al. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci Total Environ 2020;727:138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nickbakhsh S, Mair C, Matthews L, et al. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci U S A 2019;116:27142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fang F, Luo XP. Facing the pandemic of 2019 novel coronavirus infections: the pediatric perspectives. Zhonghua Er Ke Za Zhi 2020;58:E001. [DOI] [PubMed] [Google Scholar]
- [66].Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov 2020;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Brodin P. Why is COVID-19 so mild in children? Acta Paediatr 2020;109:1082–3. [DOI] [PubMed] [Google Scholar]
- [69].Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proceedings Biological Sci 2015;282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Emami A, Javanmardi F, Pirbonyeh N, et al. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- [71].Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA 2020;323:1335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


