Abstract
To explore the epidemiology of patients with spontaneous intracerebral hemorrhage (sICH) in Chengdu, China, we retrieved the data of patients with spontaneous cerebral hemorrhage admitted to the First Affiliated Hospital of Chengdu Medical College from January 2017 to December 2019. We performed a comprehensive analysis of the location of hemorrhage, demographics, factors of hemorrhage, condition of body, severity of disturbance of consciousness, treatment, length of stay (days), inpatient costs, prognosis, and mortality rate in patients with sICH. In total, data of 561 in patients with sICH were included. The hemorrhage site was primarily located in the basal ganglia and thalamus (64.71%). The mean patient age was 63.2 ± 12.4 years (64.17% men, 35.83% women). Male patients (mean age 62.3 ± 12.5 year) were younger than female patients (mean age 64.9 ± 12.1 year). The age of sICH onset in our sample was between 40 and 79 years; this occurred in 87.70% of the included cases. There were more males than females, which may be related to more daily smoking, longer drinking years, and overweight in males than in females. Cases occurred most frequently during the winter and spring months, and the relationship between sICH visits and hospitalizations appeared as a U-shape. The median time from illness onset to hospital admission was 3.0 hours. According to the Glasgow Coma Scale (GCS) score at admission, 20.50% of sICH cases were of mild intensity, 39.93% were moderate, and 39.57% were severe. Moderate disorder is the most common sICH severity. Factors influencing the disturbance of consciousness were blood glucose level at the time of admission as well as the number of years with hypertension. The lower the degree of disturbance of consciousness and the more they smoked per day indicated they had a higher likelihood of receiving surgical treatment while in hospital. The median hospital stay was 13.0 days, while the median inpatient cost was USD 3609. The 30-day mortality rate was 18.36%. sICH is an important public health problem in Chengdu, China. A governmental initiative is urgently needed to establish a sICH monitoring system that covers the Chengdu region to develop more effective and targeted measures for sICH prevention, treatment, and rehabilitation.
Keywords: clinical epidemiology, potential factors, prognosis, spontaneous intracerebral hemorrhage
1. Introduction
With the rapid economic development and corresponding improvements to people's living standards, the average life expectancy in China has increased significantly. However, this increasing number of older individuals has led to a steadily increasing number of stroke patients. There are approximately 2 million new stroke cases every year, with an incidence rate of 116 to 219 cases per 100,000 individuals.[1] Of these stroke cases, those caused by spontaneous cerebral hemorrhage (sICH) account for 10% to 20% of all cases. When compared with ischemic stroke, the morbidity, mortality, and disability rates resulting from sICH are notably higher.[2] Many factors affect the likelihood of an individual suffering from sICH, including heredity, regional differences, season, age, hypertension, diabetes, obesity, smoking, drinking, stroke history, oral anticoagulant and/or antiplatelet drug use, and race.[2,3] For instance, Wu et al[4] conducted an epidemiological study on 1298 sICH patients in Hefei and their results showed that developing sICH was related to a patient's history of hypertension, smoking, drinking, and stroke. O’Donnell et al[5] conducted a case-control study in 22 countries to analyze the risk factors of cerebral hemorrhage, and their work showed that hypertension, smoking, and drinking were all important risk factors. At present, there are few reports of major epidemiological cases of sICH in Chengdu. To address this need, we conducted a retrospective study and analysis of clinical data from 561 sICH patients treated at the First Affiliated Hospital of Chengdu Medical College from January 2017 to December 2019. Our results were then used to explore the epidemiological and clinical characteristics of this area of China.
2. Materials and methods
This study was approved by the First Affiliated Hospital of Chengdu Medical College. All sICH patient data were anonymized prior to data analysis.
2.1. Data sources
All cases for this study originated from the electronic medical record system and patient follow-up system of the First Affiliated Hospital of Chengdu Medical College. All patients were treated at the hospital between January 2017 and December 2019. We used the ICD-10 code I61 (non-traumatic intracerebral hemorrhage) to identify all sICH patients diagnosed and treated at the First Affiliated Hospital of Chengdu Medical College from January 2017 to December 2019. A total of 588 patients were retrieved using this approach. Of these, 27 patients with cerebral tumor stroke, hemorrhagic encephalomalacia, hemorrhage after cerebral venous occlusion, cerebrovascular inflammatory cerebral hemorrhage, multiple hospitalizations, and/or incomplete case data were excluded. This left 561 cases included 202 cases from 2017, 185 from 2018, and 174 from 2019. All cases were diagnosed in accordance with the Chinese guidelines for the diagnosis and treatment of cerebral hemorrhage (2014 edition)[6] and were confirmed by either craniocerebral CT or MRI.
2.2. Data collation
Indicators used in this study included sex, age, body mass index (BMI), seasonal variation, and time to hospitalization as well as history of hypertension, diabetes, smoking, drinking, stroke, and/or oral anticoagulant and/or antiplatelet drug use. Patients’ physical condition (e.g., liver, renal, and blood coagulation functioning) as well as side and location of bleeding were also included. Finally, inpatient days and inpatient costs were also included. Acute injury severity was functionally assessed using the GCS score[7] at the time of hospital admission. Patient outcomes were evaluated using the modified Rankin scale (mRS)[7] at 30 days after onset. Definitions for smoking and drinking history were as follows:
Smoking history[8]: Patients who smoked every day for more than 6 months were identified as smokers.
Drinking history[9]: patients who drank alcohol more than once a week in the past year were identified as alcohol drinkers.
According to the Chinese meteorological management system, the 4 seasons in Chengdu were classified as follows: winter (December to February), spring (March to May), summer (June to August), and autumn (September to November). Patients’ onset time was classified according to these 4 seasonal definitions.
The location of the cerebral hemorrhage was classified as:
-
1.
left, right, and bilateral according to lateral classification;
-
2.
according to location (divided into basal nucleus, thalamus, putamen, brain stem, cerebellum, caudate nucleus, primary ventricle, cerebral lobe (frontal, temporal, occipital, or parietal lobe), or mixed (2 or more cerebral lobes with evidence of hemorrhage).
If bleeding occurred in 2 or more sites, the hemorrhage was classified as ’multiple sites’.
Acute injury severity was functionally assessed by the GCS score at the time of hospital admission and was defined as follows: mild (GCS 13–15), moderate (GCS 9–12), or severe (GCS 3–8). During the 30-day follow-up, the modified Rankin scale (mRS) was used to evaluate patient prognosis as follows: 0 (no symptoms);
-
1.
(no significant disability; able to carry out all usual activities, despite some symptoms);
-
2.
(slight disability; able to look after one's affairs without assistance, but unable to carry out all previous activities);
-
3.
(moderate disability; requires some help, but able to walk unassisted);
-
4.
(moderately severe disability; unable to attend to one's own bodily needs without assistance and unable to walk unassisted);
-
5.
(severe disability; requires constant nursing care and attention, bedridden, incontinent);
-
6.
(deceased).
The patients’ neurological status (initial and 30th day after hemorrhage onset) was assessed using the Glasgow Coma Scale (GCS) score and modified Rankin Scale (mRS) score, respectively.
2.3. Treatment
All patients were managed in the Neurosurgery Intensive Care Unit with standard medical treatment and care. CT scans, routine blood tests, blood biochemistry (e.g., hepatic and renal function, electrolytes), and routine coagulation studies were performed immediately when the patients were admitted to the emergency department. The medical history and neurological physical examination were also recorded immediately after hospitalization in the Neurosurgery Intensive Care Unit. All patients had their vital signs monitored and were given supportive treatment at the same time, and all patients received standard medical treatments for sICH. The medical treatments included decreasing intracranial pressure, blood pressure control, prevention of complications, and other treatments individually. Mannitol was administered at an appropriate dose based on the clinical conditions of the patients. All medical management procedures followed the recommendations of the American Heart Association/American Stroke Association guidelines and clinical experience.[10,11]
2.4. Surgery
All surgeries were conducted by a special sICH treatment team, which consisted of well-trained neurosurgeons. The surgical method was decided by the surgeons preoperatively, which included craniotomy or neuroendoscopy. Under some conditions, extraventricular drainage was conducted before or after the operation, and decompressive craniotomy was conducted if necessary. All craniotomy hematoma evacuation was assisted by an operative microscope and followed the principle of minimally invasive procedures.
2.5. Data analysis
Categorical variables were expressed as frequency and percentage, while quantitative variables were expressed as mean ± standard deviation; quantitative variables with non-normal distribution were described using median and quartiles. A Kolmogorov–Smirnov test was used to analyze the normal distribution of all quantitative variables. A Pearson Chi-Squared test was used for between-group comparisons of categorical variables. Either an independent two-sample t-test or the Mann–Whitney U test was performed for between-group comparisons of quantitative variables. Either a one-way analysis of variance or multivariate logistic regression was performed to compare data from more than 2 groups. The statistical package SPSS version 16.0. (IBM Corporation, Somers, NY) was used for all statistical analyses. P < .05 was considered statistically significant.
3. Results
3.1. Hemorrhage location
The majority of hemorrhages were found in deep (subcortical) sites, including the basal ganglia (49.38%), thalamus (15.33%), and brainstem (8.38%). In total, 52.58% of cases were unilateral left, 45.81% were unilateral right, and 1.60% were bilateral (Table 1).
Table 1.
Clinical data of 561 patients with sICH.
| Total | Male | Female | χ2/F/z/t | P | ||
| Total | n (%) | 561 | 360 (64.17) | 201 (35.83) | ||
| Age (yr) | 63.2 ± 12.4 | 62.3 ± 12.5 | 64.9 ± 12.1 | 2.439∗ | .015 | |
| Age group (yr) | 5.655† | .130 | ||||
24–39 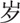
|
12 (2.14) | 8 (2.22) | 4 (1.99) | |||
40–59 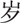
|
214 (38.15) | 150 (41.67) | 64 (31.84) | |||
60–79 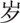
|
278 (49.55) | 169 (46.94) | 109 (54.23) | |||
80–96 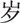
|
57 (10.16) | 33 (9.16) | 24 (11.94) | |||
| Onset season | 2.481† | .479 | ||||
| Spring | 154 (27.45) | 99 (27.50) | 55 (27.36) | |||
| Summer | 111 (19.79) | 77 (21.39) | 34 (16.92) | |||
| Autumn | 133 (23.71) | 86 (23.89) | 47 (23.38) | |||
| Winter | 163 (29.06) | 98 (27.22) | 65 (32.34) | |||
| Time to hospital encounter (h) | Median (P25, P75 ) | 3.00 (2.00, 5.00) | 3.00 (2.00, 5.00) | 3.00 (2.00, 4.00) | 0.527‡ | .598 |
| Hypertension | ||||||
| No | 297 (52.94) | 200 (55.56) | 97 (48.26) | 2.757† | .097 | |
| Yes | 264 (47.06) | 160 (44.44) | 104 (51.74) | |||
| Duration (yr) | Median (P25, P75 ) | 5.0 (3.0, 10.0) | 5.0 (3.0, 10.0) | 5.0 (3.0, 10.0) | 0.639‡ | .523 |
| Treatment | ||||||
| Regularly | 46 (17.42) | 26 (16.25) | 20 (19.23) | 0.496† | .780 | |
| Unregularly | 161 (60.98) | 100 (62.50) | 61 (58.65) | |||
| No | 57 (21.59) | 34 (21.25) | 23 (22.12) | |||
| Result | ||||||
| Reached | 10 (4.83) | 8 (6.35) | 2 (2.47) | 1.623† | .444 | |
| Unreached | 33 (15.94) | 20 (15.87) | 13 (16.05) | |||
| Unknown | 164 (79.23) | 98 (77.78) | 66 (81.48) | |||
| Admission systolic pressure (mm Hg) | Median (P25, P75 ) | 178.0 (155.0,198.0) | 177.0 (153.0,199.8) | 178.0 (157.5,197.0) | 0.438‡ | .661 |
| Admission diastolic pressure (mm Hg) | Median (P25, P75 ) | 98.0 (85.5,109.0) | 99.0 (85.3,110.0) | 96.0 (85.5,106.0) | 1.731‡ | .087 |
| Diabetes | ||||||
| No | 534 (95.19) | 343 (95.28) | 191 (95.02) | 0.018† | .893 | |
| Yes | 27 (4.81) | 17 (4.72) | 10 (4.98) | |||
| Duration (yr) | 7.7 ± 5.8 | 5.9 ± 3.9 | 10.8 ± 7.4 | 10.663∗ | .003 | |
| Treatment | ||||||
| Regularly | 8 (29.63) | 5 (29.41) | 3 (30.00) | 0.173† | .917 | |
| Unregularly | 7 (25.93) | 4 (23.53) | 3 (30.00) | |||
| No | 12 (44.44) | 8 (47.06) | 4 (40.00) | |||
| Result | ||||||
| Reached | 6 (40.00) | 3 (33.33) | 3 (50.00) | 0.417† | .519 | |
| Unreached | 9 (60.00) | 6 (66.67) | 3 (50.00) | |||
| Admission blood glucose (mmol/L) | Median (P25, P75 ) | 7.31 (6.14, 8.94) | 7.02 (6.09 8.62) | 7.54 (6.19, 9.26) | 1.555‡ | .120 |
| Smoking | ||||||
| No | 353 (62.92) | 162 (45.00) | 191 (95.02) | 138.355† | <.001 | |
| Yes | 208 (37.08) | 198 (55.00) | 10 (4.98) | |||
| Duration (yr) | Median (P25, P75 ) | 30.0 (20.0, 40.0) | 30.0 (20.0, 40.0) | 30.0 (20.0, 35.0) | 0.711‡ | .477 |
| Daily smoking volume (cigarettes / day) | Median (P25,P75) | 20.0 (10.0, 20.0) | 20.0 (10.0, 20.0) | 9.0 (5.0, 16.3) | 2.444‡ | .015 |
| Drinking | ||||||
| No | 359 (63.99) | 172 (47.78) | 187 (93.03) | 114.654† | <.001 | |
| Yes | 202 (36.01) | 188 (52.22) | 14 (6.97) | |||
| Duration (yr) | Median (P25, P75 ) | 30.0 (20.0, 40.0) | 30.0 (20.0, 40.0) | 20.0 (5.0, 30.0) | 2.802‡ | .005 |
| Daily drinking volume (g/d) | Median (P25, P75 ) | 100.0 (93.8, 200.0) | 100.0 (81.3, 200.0) | 100.0 (87.5, 100.0) | 1.330‡ | .183 |
| Stroke | ||||||
| No | 500 (89.13) | 327 (90.83) | 173 (86.07) | 3.020† | .082 | |
| Yes | 61 (10.87) | 33 (9.17) | 28 (13.93) | |||
| Cerebral hemorrhage | 41 (67.21) | 21 (63.64) | 20 (71.43) | 0.481† | .786 | |
| Cerebral infarction | 17 (27.87) | 10 (30.30) | 7 (25.00) | |||
| Both | 3 (4.92) | 2 (6.06) | 1 (3.57) | |||
| Oral anticoagulant and/or antiplatelet drugs | ||||||
| No | 552 (98.40) | 355 (98.61) | 197 (98.01) | 0.295† | .587 | |
| Yes | 9 (1.60) | 5 (1.39) | 4 (1.99) | |||
| Aspirin | 6 (66.67) | 4 (80.00) | 2 (50.00) | 1.575† | .455 | |
| Clopidogrel | 2 (22.22) | 1 (20.00) | 1 (25.00) | |||
| Warfarin | 1 (11.11) | 1 (25.00) | ||||
| BMI | Median (P25, P75 ) | 22.86 (20.81,24.66) | 23.03 (21.26,24.80) | 22.21 (20.03,24.03) | 2.912‡ | .004 |
| BMI group | 10.493† | .001 | ||||
| Normal | 370 (65.95) | 220 (61.11) | 150 (74.63) | |||
| Obese | 191 (34.05) | 140 (38.89) | 51 (25.37) | |||
| Liver function (ALT/AST) | ||||||
| Normal | 547 (97.50) | 352 (97.78) | 195 (97.01) | 0.308† | .579 | |
| Abnormal | 14 (2.50) | 8 (2.22) | 6 (2.99) | |||
| Renal function (CREA) | ||||||
| Normal | 538 (95.90) | 343 (95.28) | 195 (97.01) | 0.990† | .320 | |
| Abnormal | 23 (4.10) | 17 (4.72) | 6 (2.99) | |||
| Platelet (PLT) | ||||||
| Normal | 479 (85.38) | 308 (85.56) | 171 (85.07) | 0.024† | .877 | |
| Abnormal | 82 (14.62) | 52 (14.44) | 30 (14.93) | |||
| Blood coagulation function (PT/APTT/TT/FIB) | ||||||
| Normal | 556 (99.11) | 356 (98.89) | 200 (99.50) | 0.550† | .458 | |
| Abnormal | 5 (0.89) | 4 (1.11) | 1 (0.50) | |||
| Side of bleeding | ||||||
| Left | 295 (52.58) | 194 (53.89) | 101 (50.25) | 1.993† | 0.369 | |
| Right | 257 (45.81) | 162 (45.00) | 95 (47.26) | |||
| Bilateral | 9 (1.60) | 4 (1.11) | 5 (2.49) | |||
| Location of bleeding | ||||||
| Basal nucleus | 277 (49.38) | 186 (51.67) | 91 (45.27) | 21.171† | .048 | |
| Thalamus | 86 (15.33) | 50 (13.89) | 36 (17.91) | |||
| Brain stem | 47 (8.38) | 33 (9.17) | 14 (6.97) | |||
| Others | 151 (26.92) | 91 (25.28) | 60 (29.85) | |||
| Admission GCS (score) | Median (P25, P75 ) | 9.0 (7.0, 11.0) | 9.0 (7.0, 11.0) | 10.0 (7.0, 11.0) | 2.088‡ | .037 |
| Degree of disturbance of consciousness on admission | ||||||
| Mild | 115 (20.50) | 75 (20.83) | 40 (19.90) | 3.327† | .189 | |
| Moderate | 224 (39.93) | 134 (37.22) | 90 (44.78) | |||
| Severe | 222 (39.57) | 151 (41.94) | 71 (35.32) | |||
| Surgical treatment | ||||||
| Yes | 173 (30.84) | 114 (31.67) | 59 (29.35) | 0.324† | .569 | |
| No | 388 (69.16) | 246 (68.33) | 142 (70.65) | |||
| Length of stay (days) | Median (P25, P75 ) | 13.0 (4.0, 22.0) | 12.0 (4.0, 23.0) | 14.0 (5.0, 20.0) | 0.730‡ | .466 |
| Inpatient costs (USD) | Median (P25, P75 ) | 3608 (1760, 8541) | 3500 (1742, 8774) | 3912 (1870, 8359) | 0.450‡ | .652 |
| Prognosis after 30 d of onset (mRS) | ||||||
| Slight disability | 22 (3.92) | 19 (5.28) | 3 (1.49) | 11.558† | .021 | |
| Moderate disability | 97 (17.29) | 58 (16.11) | 39 (19.40) | |||
| Moderately severe disability | 219 (39.04) | 128 (35.56) | 91 (45.27) | |||
| Severe disability | 120 (21.39) | 82 (22.78) | 38 (18.91) | |||
| Dead | 103 (18.36) | 73 (20.28) | 30 (14.93) | |||
3.2. Demographic characteristics
3.2.1. Distribution of ICH cases by sex and age groups
A total of 561 sICH cases were included in this study. There were 360 (64.17%) men and 201 (35.83%) women (male-to-female ratio: 1.79:1). Their mean age was 63.2 ± 12.4 years (range, 24–96 years). The average age of the male patients was significantly younger than that of the female patients (62.3 ± 12.5 years vs 64.9 ± 12.1 years; t = 2.439, P = .015). Inpatients aged 40 to 79 years accounted for 87.70% of the study population (Table 1).
3.2.2. Seasonal distribution and time to hospital encounter
Of all sICH occurrences included in this study, 27.45% occurred in the spring, 19.79% in the summer, 23.71% in the autumn, and 29.06% in the winter. The relationship between sICH visits and hospitalizations appeared as a U-shape. The median time from illness onset to hospital admission was 3.0 hours (interquartile range [IQR], 2.0–5.0 hours; range, 0.5–24.0 hours; Table 2).
Table 2.
Ordered multi-class logistic regression analysis of the factors affecting the degree of hospitalization in 561 patients with sICH.
| Factors | OR | OR 95% CI | P |
| Admission systolic pressure (mm Hg) | 1.001 | 0.99–1.01 | .841 |
| Admission diastolic pressure (mm Hg) | 1.009 | 0.99–1.03 | .283 |
| Admission blood glucose (mmol/L) | 1.160 | 1.06–1.27 | .001 |
| Duration of the hypertension (yr) | 1.053 | 1.01–1.10 | .028 |
3.3. Potential factors leading to ICH
3.3.1. Hypertension
The median blood pressure on admission was 178.0 ± 98.0 mm Hg (IQR, 155.0–198.0/85.5–109.0 mm Hg). A total of 264 patients (47.06%) had a history of hypertension, and the median duration of the disease was 5.0 years (IQR, 3.0–10.0 years). Of these patients with hypertension, 46 (17.42%) were treated regularly, 161 (60.98%) did not receive consistent treatment, and 57 (21.59%) were not treated. After treatment, 10 cases (4.83%) reached the recommended goal for blood pressure control,[12] 33 cases (15.94%) failed to achieve the recommended goal, and 164 cases (79.23%) had unknown control (Table 1).
3.3.2. Diabetes
The median blood glucose on admission was 7.3 mmol/L (IQR, 6.1–8.9 mmol/L). Of the included patients, 27 (4.81%) had a history of diabetes. The blood glucose of patients with a history of diabetes at the time of hospital admission was significantly higher than that of patients who did not have a history of diabetes (Z = 4.767, P < .001). Among the patients with a history of diabetes, the average number of years was 7.7 ± 5.8. Moreover, the number of years dealing with diabetes in females was significantly higher than that in males (t = 10.663, P = .003). Of those patients with diabetes, 8 were treated regularly (29.63%), 7 patients did not receive consistent treatment (25.93%), and 12 were untreated (44.44%). After treatment, 6 cases (40.00%) reached the recommended goal for diabetes control,[12] and 9 cases (60.00%) failed to achieve the recommended goal (Table 1).
3.3.3. Smoking history
A total of 208 patients (37.08%) had a history of smoking, including 198 (95.19%) men and 8 (4.81%) women. Significantly more males than females were smokers (χ2 = 138.355, P < .001), with the median years of smoking being 30.0 years (IQR, 20.0–40.0 years). The median daily smoking volume of smokers was 20 cigarettes per day (IQR, 10.0–20.0 cigarettes per day); notably, the median daily smoking amount of males was significantly higher than that of females (Z = 2.444, P = .015; Table 1).
3.3.4. Drinking history
A total of 202 patients (36.01%) had a history of drinking, including 188 (93.07%) men and 14 (6.93%) women. Significantly more males than females were drinkers (χ2 = 114.654, P < .001) and the median number of years of drinking was 30 years (IQR, 20.0–40.0 years), and the median daily drinking volume was 100.0 g/day (IQR, 93.8–200.0 g/day). The median number of years of drinking in males was significantly higher than that in females (Z = 2.802, P = .005; Table 1).
3.3.5. Stroke history
A total of 61 patients (10.87%) had a history of stroke, including 33 males (54.10%) and 28 females (45.90%) (Table 1). Of those with a history of stroke, 41 (67.21%) had cerebral hemorrhage, 17 (27.87%) had cerebral infarction, and 3 (4.92%) had both cerebral hemorrhage and cerebral infarction (Table 1).
3.3.6. Oral anticoagulant and/or antiplatelet drug history
A total of 9 patients (1.60%) had a history of oral anticoagulant and/or antiplatelet drug use. This included 6 (66.67%) patients who had used oral aspirin enteric-coated tablets, 2 (22.22%) who had used oral clopidogrel sulfate tablets, and 1 patient (11.11%) who had used oral warfarin tablets (Table 1).
3.3.7. Body mass index (BMI)
The median BMI of the 561 patients was 22.86 (IQR, 20.81–24.66). The median BMI of men was significantly higher than that of women (Z = 2.912, P = .004). According to the reference standard set forth by the Chinese BMI grouping,[13] patients were divided into 2 groups: normal and obese. There were 191 (34.05%) obese patients, including 140 (38.89%) men and 51 (25.37%) women. The number of obese men was significantly higher than that of women (χ2 = 10.493, P = .001; Table 1).
3.4. Basic condition of hospitalized patients and severity of disturbance of consciousness (GCS score)
At the time of admission, most patients’ basic physical conditions (liver, renal, platelet, and blood coagulation functioning) were normal (97.50%, 95.90%, 85.38%, and 99.11%, respectively). There were no significant differences between men and women (P > .05; Table 1).
According to patients’ GCS scores, disturbance of consciousness was divided into mild (20.50%), moderate (39.93%), and severe (39.57%). These results showed that the higher the patient's blood glucose at admission, the longer their history of hypertension, and the more serious the disturbance of consciousness on admission (P < .05; Table 4, Table 2).
Table 4.
Clinical data related to the factors affecting the severity of hospitalization in 561 patients with sICH.
| Total | Mild | Moderate | Severe | F/χ2 | P | ||
| Total | n (%) | 561 | 115 (20.50) | 224 (39.93) | 222 (39.57) | ||
| Age (yr) | 63.2 ± 12.4 | 61.5 ± 12.8 | 63.8 ± 11.6 | 63.5 ± 12.9 | 1.439∗ | .238 | |
| Hypertension | 0.385† | .825 | |||||
| No | 297 (52.94) | 62 (20.88) | 115 (38.72) | 120 (40.40) | |||
| Yes | 264 (47.06) | 53 (20.08) | 109 (41.29) | 102 (38.64) | |||
| Duration (yr) | 6.5 ± 5.1 | 4.9 ± 3.3 | 6.8 ± 4.9 | 7.1 ± 5.9 | 3.593∗ | .029 | |
| Admission systolic pressure (mm Hg) | 178.0 ± 31.1 | 171.2 ± 28.2 | 174.9 ± 27.9 | 184.6 ± 34.2 | 9.065∗ | <.001 | |
| Admission diastolic pressure (mm Hg) | 98.1 ± 18.2 | 95.7 ± 16.1 | 96.2 ± 16.5 | 101.3 ± 20.4 | 5.773∗ | .003 | |
| Diabetes | 0.092† | .955 | |||||
| No | 534 (95.19) | 109 (20.41) | 213 (39.89) | 212 (39.70) | |||
| Yes | 27 (4.81) | 6 (22.22) | 11 (40.74) | 10 (37.04) | |||
| Duration (yr) | 7.7 ± 5.8 | 10.0 ± 8.1 | 7.9 ± 5.6 | 6.1 ± 4.5 | 0.846∗ | .441 | |
| Admission blood glucose (mmol/L) | 7.94 ± 2.81 | 7.05 ± 2.37 | 7.63 ± 2.72 | 8.70 ± 2.93 | 15.954∗ | <.001 | |
| Smoking | 2.557† | .278 | |||||
| No | 353 (62.92) | 65 (18.41) | 144 (40.79) | 144 (40.79) | |||
| Yes | 208 (37.08) | 50 (24.04) | 80 (38.46) | 78 (37.50) | |||
| Duration (yr) | 31.9 ± 12.9 | 31.3 ± 12.4 | 32.6 ± 13.7 | 31.4 ± 12.5 | 0.219∗ | .803 | |
| Daily smoking volume (cigarettes/d) | 15.7 ± 8.4 | 16.7 ± 6.8 | 14.8 ± 6.9 | 15.8 ± 10.4 | 0.768∗ | .465 | |
| Drinking | 0.149† | .928 | |||||
| No | 359 (63.99) | 72 (20.06) | 145 (40.39) | 142 (39.55) | |||
| Yes | 202 (36.01) | 43 (21.29) | 79 (39.11) | 80 (39.60) | |||
| Duration (yr) | 29.7 ± 12.5 | 30.8 ± 13.0 | 30.9 ± 12.5 | 27.9 ± 12.2 | 1.389∗ | .252 | |
| Daily drinking volume (g/day) | 139.5 ± 108.2 | 138.4 ± 68.9 | 128.7 ± 95.7 | 150.6 ± 134.2 | 0.814∗ | .444 | |
| Stroke | 0.816† | .665 | |||||
| Yes | 61 (10.87) | 15 (24.59) | 22 (36.07) | 24 (39.34) | |||
| No | 500 (89.13) | 100 (20.00) | 202 (40.40) | 198 (39.60) | |||
| Oral anticoagulant and/or antiplatelet drugs | 2.512† | .285 | |||||
| Yes | 9 (1.60) | 4 (44.44) | 5 (55.56) | ||||
| No | 552 (98.40) | 115 (20.83) | 220 (39.86) | 217 (39.31) | |||
| BMI | 22.83 ± 3.30 | 22.92 ± 3.29 | 22.74 ± 2.83 | 22.88 ± 3.72 | 0.161∗ | .851 | |
| BMI group | 0.449† | .799 | |||||
| Normal | 370 (65.95) | 73 (19.73) | 148 (40.00) | 149 (40.27) | |||
| Obese | 191 (34.05) | 42 (21.99) | 76 (39.79) | 73 (38.22) |
3.5. Surgical treatment
A total of 173 patients (30.84%) received surgical treatment during hospitalization. Patients with more daily smoking or less disturbance of consciousness on admission were more likely to receive surgical treatment (P < .05; Table 5, Table 3).
Table 5.
Clinical data related to operation in 561 patients with sICH.
| Total | Yes | No | χ2/Z/t | P | ||
| Total | n (%) | 561 | 173 (30.84) | 388 (69.16) | ||
| Gender | 0.324∗ | .569 | ||||
| Male | 360 (64.17) | 114 (65.90) | 246 (63.40) | |||
| Female | 201 (35.83) | 59 (34.10) | 142 (36.60) | |||
| Age (yr) | 63.2 ± 12.4 | 60.6 ± 10.8 | 64.4 ± 12.9 | 3.006† | .003 | |
| Hypertension | 3.761∗ | .052 | ||||
| No | 297 (52.94) | 81 (46.82) | 216 (55.67) | |||
| Yes | 264 (47.06) | 92 (53.18) | 172 (44.33) | |||
| Duration (yr) | 6.5 ± 5.1 | 6.4 ± 5.0 | 6.6 ± 5.2 | 0.086† | .770 | |
| Admission systolic pressure (mm Hg) | 178.0 ± 31.0 | 182.3 ± 30.0 | 176.0 ± 31.4 | 0.026† | .872 | |
| Admission diastolic pressure (mm Hg) | 98.1 ± 18.2 | 99.7 ± 19.2 | 97.4 ± 17.8 | 1.899† | .169 | |
| Diabetes | 0.083∗ | .774 | ||||
| No | 534 (95.19) | 164 (94.80) | 370 (95.36) | |||
| Yes | 27 (4.81) | 9 (5.20) | 18 (4.64) | |||
| Duration (yr) | Median (P25, P75 ) | 6.0 (3.0, 10.0) | 5.0 (3.5, 7.5) | 8.0 (1.5, 14.5) | 0.958‡ | .338 |
| Admission blood glucose (mmol/L) | 7.94 ± 2.81 | 8.47 ± 2.78 | 7.70 ± 2.79 | 1.529† | .217 | |
| Smoking | 0.047∗ | .829 | ||||
| No | 353 (62.92) | 110 (63.58) | 243 (62.63) | |||
| Yes | 208 (37.08) | 63 (36.42) | 145 (37.37) | |||
| Duration (yr) | Median (P25, P75 ) | 30.0 (20.0, 40.0) | 40.0 (20.0, 40.0) | 30.0 (20.0, 40.0) | 0.213∗ | .831 |
| Daily smoking volume (cigarettes / day) | Median (P25, P75 ) | 20.0 (10.0, 20.0) | 17.5 (10.0, 20.0) | 20.0 (10.0, 20.0) | 2.301‡ | .021 |
| Drinking | 0.106∗ | .745 | ||||
| No | 359 (63.99) | 109 (63.01) | 250 (64.43) | |||
| Yes | 202 (36.01) | 64 (36.99) | 138 (35.57) | |||
| Duration (yr) | Median (P25, P75 ) | 30.0 (20.0, 40.0) | 30.0 (20.0, 40.0) | 30.0 (20.0, 40.0) | 0.525‡ | .599 |
| Daily drinking volume (g/d) | 139.5 ± 108.2 | 118.5 ± 109.6 | 149.2 ± 106.6 | 2.609† | .108 | |
| Stroke | 0.003∗ | .956 | ||||
| Yes | 61 (10.87) | 19 (10.98) | 42 (10.82) | |||
| No | 500 (89.13) | 154 (89.02) | 346 (89.18) | |||
| Oral anticoagulant and/or antiplatelet drugs | 0.794∗ | .373 | ||||
| Yes | 9 (1.60) | 4 (2.31) | 5 (1.29) | |||
| No | 552 (98.40) | 169 (97.69) | 383 (98.71) | |||
| BMI | 22.83 ± 3.30 | 23.28 ± 3.38 | 22.63 ± 3.24 | 0.229† | .632 | |
| Side of bleeding | 1.669∗ | .434 | ||||
| Left | 295 (52.58) | 92 (53.18) | 203 (52.32) | |||
| Right | 257 (45.81) | 80 (46.24) | 177 (45.62) | |||
| Bilateral | 9 (1.60) | 1 (0.58) | 8 (2.06) | |||
| Location of bleeding | 21.201∗ | .048 | ||||
| Basal nucleus | 277 (49.38) | 95 (54.91) | 182 (46.91) | |||
| Thalamus | 86 (15.33) | 26 (15.03) | 60 (15.46) | |||
| Brain stem | 47 (8.38) | 9 (5.20) | 38 (9.79) | |||
| Cerebellum | 38 (6.77) | 7 (4.05) | 31 (7.99) | |||
| Mixed (2 or more than 2 cerebral lobes hemorrhage) | 32 (5.70) | 8 (4.62) | 24 (6.19) | |||
| Multiple sites (bleeding in 2 or more sites) | 30 (5.35) | 11 (6.36) | 19 (4.90) | |||
| Primary ventricle | 15 (2.67) | 5 (2.89) | 10 (2.58) | |||
| Temporal lobe | 10 (1.78) | 10 (2.58) | ||||
| Parietal lobe | 10 (1.78) | 6 (3.47) | 4 (1.03) | |||
| Frontal lobe | 6 (1.07) | 2 (1.16) | 4 (1.03) | |||
| Putamen | 5 (0.89) | 1 (0.58) | 4 (1.03) | |||
| Occipital lobe | 4 (0.71) | 3 (1.73) | 1 (0.26) | |||
| Caudate nucleus | 1 (0.18) | 1 (0.26) | ||||
| Admission GCS (score) | Median (P25, P75 ) | 9.06 ± 2.70 | 7.80 ± 1.66 | 9.62 ± 2.89 | 64.794† | <.001 |
| Degree of disturbance of consciousness on admission | 92.449∗ | <.001 | ||||
| Mild | 115 (20.50) | 1 (0.58) | 114 (29.38) | |||
| Moderate | 224 (39.93) | 59 (34.10) | 165 (42.53) | |||
| Severe | 222 (39.57) | 113 (65.32) | 109 (28.09) |
Table 3.
Ordered multi-classification logistic regression analysis of influencing factors of operation in 561 patients with sICH.
| Factors | OR | OR 95% CI | P |
| Age | 1.015 | 0.99–1.04 | .289 |
| Daily smoking volume | 1.049 | 1.01–1.09 | .021 |
| Degree of disturbance of consciousness on admission | 0.232 | 0.14–0.39 | <.00 |
3.6. Hospitalization days and inpatient expenses
When assessing the length of hospitalization (in days) and expenses (USD) of patients with sICH, the median days of hospitalization was 13.0 days (IQR, 4.0–22.0 days), and the median hospitalization cost was $3,609 (IQR, USD 1760–8543; Table 1).
3.7. Prognosis after 30 days of onset (mRS)
A total of 103 (18.36%) patients died within 30 days after sICH onset. The remaining 458 (81.64%) patients survived, including 22 (3.92%) who had slight disability, 97 (17.29%) had moderate disability, 219 (39.04%) had moderately severe disability, and 120 (21.39%) had severe disability. The prognosis of men was generally worse than that of women, and the 30-day mortality rate was significantly higher in men than in women (20.28% vs 14.93%, respectively; χ2 = 11.558, P < .021; Table 1). The older the patient, the higher their mortality (F = 2.440, P = .046; Schedule 3). Finally, the lower the admission GCS score, the worse the 30-day prognosis (P < .01; Table 6).
Table 6.
Clinical data related to 30-day prognosis of 561 patients with sICH.
| Total | Slight disability | Moderate disability | Moderately severe disability | Severe disability | Dead | χ2/F | P | ||
| Total | n (%) | 561 | 22 (3.92) | 97 (17.29) | 219 (39.04) | 120 (21.39) | 103 (18.36) | ||
| Gender | 11.558∗ | .021 | |||||||
| Male | 360 (64.17) | 19 (5.28) | 58 (16.11) | 128 (35.56) | 82 (22.78) | 73 (20.28) | |||
| Female | 201 (35.83) | 3 (1.49) | 39 (19.40) | 91 (45.27) | 38 (18.91) | 30 (14.93) | |||
| Age (yr) | 63.2 ± 12.4 | 61.8 ± 15.4 | 62.7 ± 12.8 | 62.4 ± 11.9 | 62.4 ± 11.3 | 66.6 ± 13.2 | 2.440† | .046 | |
| Onset season | 15.716∗ | .205 | |||||||
| Spring | 154 (27.45) | 6 (3.90) | 19 (12.34) | 66 (42.86) | 31 (20.13) | 32 (20.78) | |||
| Summer | 111 (19.79) | 4 (3.60) | 23 (20.72) | 38 (34.23) | 22 (19.82) | 24 (21.62) | |||
| Autumn | 133 (23.71) | 4 (3.01) | 31 (23.31) | 50 (37.59) | 34 (25.56) | 14 (10.53) | |||
| Winter | 163 (29.06) | 8 (4.91) | 24 (14.72) | 65 (39.88) | 33 (20.25) | 33 (20.25) | |||
| Time to hospital encounter (h) | 4.55 ± 5.25 | 4.75 ± 5.06 | 5.13 ± 6.17 | 4.57 ± 5.27 | 4.39 ± 4.87 | 4.09 ± 4.73 | 0.536† | .709 | |
| Hypertension | 8.690∗ | .069 | |||||||
| No | 297 (52.94) | 15 (5.05) | 52 (17.51) | 116 (39.06) | 52 (17.51) | 62 (20.88) | |||
| Yes | 264 (47.06) | 7 (2.65) | 45 (17.05) | 103 (39.02) | 68 (25.76) | 41 (15.53) | |||
| Duration (yr) | 6.5 ± 5.1 | 4.6 ± 2.2 | 5.9 ± 4.1 | 6.7 ± 5.2 | 6.3 ± 4.9 | 7.5 ± 6.5 | 0.871† | .482 | |
| Admission systolic pressure (mm Hg) | 178.0 ± 31.1 | 159.7 ± 30.1 | 174.4 ± 27.6 | 175.7 ± 27.8 | 178.9 ± 33.3 | 188.9 ± 35.1 | 5.947† | <.001 | |
| Admission diastolic pressure (mm Hg) | 98.1 ± 18.2 | 91.9 ± 15.6 | 96.9 ± 16.8 | 97.3 ± 17.2 | 98.5 ± 19.9 | 101.8 ± 19.8 | 1.885† | .112 | |
| Diabetes | 6.647∗ | .156 | |||||||
| No | 534 (95.19) | 20 (3.75) | 92 (17.23) | 211 (39.51) | 110 (20.60) | 101 (18.91) | |||
| Yes | 27 (4.81) | 2 (7.41) | 5 (18.52) | 8 (29.63) | 10 (37.04) | 2 (7.41) | |||
| Duration (yr) | 7.7 ± 5.8 | 12.0 ± 11.3 | 11.2 ± 8.5 | 7.5 ± 4.1 | 5.3 ± 4.4 | 7.5 ± 3.5 | 1.191† | .342 | |
| Admission blood glucose (mmol/L) | 7.94 ± 2.81 | 7.12 ± 2.14 | 7.13 ± 2.48 | 7.64 ± 2.59 | 8.23 ± 2.79 | 9.14 ± 3.26 | 8.545† | <.001 | |
| Smoking | 4.949∗ | .293 | |||||||
| No | 353 (62.92) | 10 (2.83) | 57 (16.15) | 146 (41.36) | 76 (21.53) | 64 (18.13) | |||
| Yes | 208 (37.08) | 12 (5.77) | 40 (19.23) | 73 (35.10) | 44 (21.15) | 39 (18.75) | |||
| Duration (yr) | 31.9 ± 12.9 | 35.4 ± 12.2 | 30.8 ± 11.8 | 31.3 ± 13.8 | 31.5 ± 12.9 | 33.4 ± 12.8 | 0.472† | .756 | |
| Daily smoking volume (cigarettes / day) | 15.7 ± 8.4 | 16.7 ± 4.9 | 16.6 ± 7.3 | 14.4 ± 7.3 | 15.3 ± 10.0 | 17.2 ± 10.0 | 0.965† | .428 | |
| Drinking | 1.403∗ | .844 | |||||||
| No | 359 (63.99) | 13 (3.62) | 63 (17.55) | 145 (40.39) | 76 (21.17) | 62 (17.27) | |||
| Yes | 202 (36.01) | 9 (4.46) | 34 (16.83) | 74 (36.63) | 44 (21.78) | 41 (20.30) | |||
| Duration (yr) | 29.7 ± 12.5 | 30.8 ± 11.8 | 31.2 ± 14.4 | 30.4 ± 11.9 | 28.6 ± 13.1 | 27.9 ± 11.8 | 0.488† | .745 | |
| Daily drinking volume (g/d) | 169.5 ± 149.1 | 172.2 ± 61.8 | 138.2 ± 70.8 | 127.0 ± 99.0 | 126.7 ± 106.7 | 169.5 ± 149.1 | 1.408† | .233 | |
| Stroke | 0.301∗ | .990 | |||||||
| Yes | 61 (10.87) | 3 (4.92) | 10 (16.39) | 23 (37.70) | 13 (21.31) | 12 (19.67) | |||
| No | 500 (89.13) | 19 (3.80) | 87 (17.40) | 196 (39.20) | 107 (21.40) | 91 (18.20) | |||
| Oral anticoagulant and/or antiplatelet drugs | 6.532∗ | .163 | |||||||
| Yes | 9 (1.60) | 5 (55.56) | 4 (44.44) | ||||||
| No | 552 (98.40) | 22 (3.99) | 97 (17.57) | 214 (38.77) | 116 (21.01) | 103 (18.66) | |||
| BMI | 22.83 ± 3.30 | 22.08 ± 2.57 | 23.04 ± 3.66 | 23.14 ± 3.16 | 22.85 ± 3.26 | 22.13 ± 3.34 | 2.014† | .091 | |
| Location of bleeding | 57.195∗ | .171 | |||||||
| Basal nucleus | 277 (49.38) | 9 (3.25) | 44 (15.88) | 113 (40.79) | 70 (25.27) | 41 (14.80) | |||
| Thalamus | 86 (15.33) | 1 (1.16) | 21 (24.42) | 32 (37.21) | 16 (18.60) | 16 (18.60) | |||
| Brain stem | 47 (8.38) | 2 (4.26) | 4 (8.51) | 14 (29.79) | 9 (19.15) | 18 (38.30) | |||
| Cerebellum | 38 (6.78) | 2 (5.26) | 10 (26.32) | 18 (47.37) | 3 (7.89) | 5 (13.16) | |||
| Mixed (2 or more than 2 cerebral lobes hemorrhage) | 32 (5.70) | 1 (3.13) | 7 (21.88) | 12 (37.50) | 5 (15.63) | 7 (21.88) | |||
| Multiple sites (bleeding in 2 or more sites) | 30 (5.35) | 2 (6.67) | 5 (16.67) | 8 (26.67) | 6 (20.00) | 9 (30.00) | |||
| Primary ventricle | 15 (2.67) | 3 (20.20) | 2 (13.33) | 4 (26.67) | 4 (26.67) | 2 (13.33) | |||
| Temporal lobe | 10 (1.78) | 1 (10.00) | 2 (20.00) | 4 (40.00) | 1 (10.00) | 2 (20.00) | |||
| Parietal lobe | 10 (1.78) | 1 (10.00) | 5 (50.00) | 3 (30.00) | 1 (10.00) | ||||
| Frontal lobe | 6 (1.07) | 1 (16.67) | 3 (50.00) | 1 (16.67) | 1 (16.67) | ||||
| Putamen | 5 (0.89) | 1 (20.00) | 2 (40.00) | 1 (20.00) | 1 (20.00) | ||||
| Occipital lobe | 4 (0.71) | 3 (75.00) | 1 (25.00) | ||||||
| Caudate nucleus | 1 (0.18) | 1 | |||||||
| Admission GCS (score) | 9.1 ± 2.7 | 12.7 ± 0.8 | 12.2 ± 0.9 | 9.7 ± 1.9 | 7.7 ± 1.3 | 5.7 ± 1.9 | 265.052† | <.001 | |
| Degree of disturbance of consciousness on admission | 570.254∗ | <.001 | |||||||
| Mild | 115 (20.50) | 22 (19.13) | 80 (69.57) | 13 (11.30) | |||||
| Moderate | 224 (39.93) | 17 (7.59) | 156 (69.64) | 42 (18.75) | 9 (4.02) | ||||
| Severe | 222 (39.57) | 50 (22.52) | 78 (35.14) | 94 (42.34) |
4. Discussion
We conducted an epidemiological investigation of 561 sICH patients in Chengdu, and our results revealed that the hemorrhage site was primarily located in the basal ganglia and thalamus (64.71%). The mean patient age was 63.2 ± 12.4 years (64.17% men, 35.83% women). Male patients (mean age 62.3 ± 12.5 year) were younger than female patients (mean age 64.9 ± 12.1 year). The age of sICH onset in our sample was between and 40 to 79 years; this occurred in 87.70% of the included cases. There were more males than females in our study. Notably, male patients had increased daily smoking relative to female patients as well as a longer time drinking alcohol. Male patients were also more likely to be overweight than women. Cases occurred most frequently during the winter and spring months, and the relationship between sICH visits and hospitalizations appeared as a U-shape. The median time from illness onset to hospital admission was 3.0 hours. According to the GCS score at admission, 20.50% of sICH cases were of mild intensity, 39.93% were moderate, and 39.57% were severe. The factors influencing the disturbance of consciousness were blood glucose level at the time of admission as well as the number of years with hypertension. In sICH patients, the lower the degree of disturbance of consciousness and the more they smoked per day indicated they had a higher likelihood of receiving surgical treatment while in hospital. The median hospital stay was 13.0 days, while the median inpatient cost was USD 3609. Finally, the 30-day mortality rate was 18.36%. Collectively, these patient characteristics provide necessary data for the prevention and treatment of sICH in Chengdu.
The preponderance of male patients with sICH (male-to-female ratio: 1.79:1) was consistent with the findings of previous Chinese studies on sICH. For instance, Zhang et al.[14] conducted an analysis of the clinical characteristics of 6374 sICH patients and showed that the male-to-female ratio was 1.34:1. Similarly, Wu et al[4] conducted a study on the epidemiological characteristics of 1298 sICH patients and showed that the male-to-female ratio was 1.73:1. Hou et al[15] analyzed the clinical characteristics of 515 sICH patients and found that the male-to-female ratio was 1.4:1. Foreign studies on sICH have reported similarly high male-to-female ratios, with a ratio of 1.29:1 in India,[16] 1.6:1 in the United States,[17] 1.11:1 in the United Kingdom,[18] and 1.28:1 in Norway.[19] The reason why there are consistently more male sICH patients remains unclear. However, results from this study indicate that it may be related to more daily smoking in males, longer drinking years in males, and/or greater obesity in males than in females. Further research is needed to definitively tie any one or more of these factors to the greater likelihood of sICH in males. Additionally, our results indicated that the hospitalization age of patients with sICH was mostly between 40 and 79 years, with most patients being over 60 years (43.5% vs 56.5%, respectively). This finding is consistent with the results of studies conducted both in China and in other countries, which have found that patients with sICH are most commonly elderly and usually over 50 years of age.[4,20]
Most sICH patients were hospitalized in the winter and spring, showing a “U-shaped” distribution. This finding was consistent with other published literature from Chinese populations.[15,21] This pattern of hospitalizations may be due to the greater temperature change in the winter and spring, which stimulates the sympathetic nerve to release a large amount of catecholamines. This causes vasoconstriction and increased blood pressure, thereby increasing the risk of sICH.[22] At the same time, high temperature and low atmospheric pressure can increase the risk of sICH.[23] Similar findings have been reported in other countries.[24] Chengdu is located in the Sichuan Basin, which has a unique climate of abundant heat, rich rainfall, and 4 distinct seasons. It also belongs to the subtropical monsoon climate, with characteristically high temperatures and low atmospheric pressure.
The median time from sICH onset to treatment was 3.0 hours, which was shorter than the median time of 7.3 hours as reported in Taipei.[25] This median time to treatment was also shorter than the median visit time of 7.33 hours reported in Turkey.[26] At present, there are no reports from other parts of mainland China regarding the time from sICH onset to treatment.
The main risk factors for sICH included hypertension, smoking, drinking, diabetes, oral anticoagulant and/or antiplatelet drug use, heredity, and race.[2,3] Hypertension was the most important risk factor for the development of sICH, and the contribution of hypertension was greater for deep sICH than for lobar sICH.[27] Current smoking and heavy alcohol consumption were also associated with an increased risk of sICH.[28,29] A prospective study showed that diabetes was also a risk factor for sICH, with a relative risk of 1.6 (95% confidence interval 1.2 to 2.1) when compared with non-diabetic patients.[3,30] The use of anticoagulants and/or antiplatelets, especially warfarin, increased the risk of sICH by two- to five-fold, depending upon the intensity of anticoagulation.[31] However, several case-control studies did not show an increased sICH risk with antiplatelet use, so the relationship may be more complicated.[32]
SICH usually occurs in the basal ganglia and thalamus (49.38% and 15.33%, respectively), which is consistent with previous research conducted in China and abroad.[4,15,33] The main cause of hemorrhage in the basal ganglia and thalamus was hypertension, which may be related to the distribution and anatomical relationship of blood vessels in the basal ganglia area. More specifically, the thick artery is nearly perpendicular to its branches, resulting in greater blood pressure on the vessel wall. The common cause of cerebral lobe hemorrhage is amyloidosis.[34] The causes and treatment of different parts of cerebral hemorrhage will require further study.
According to the GCS scores obtained in this study, disturbance of consciousness was divided into mild (20.50%), moderate (39.93%), and severe (39.57%). An epidemiological study in Germany showed that mild sICH accounted for 38% of all sICH cases, moderate accounted for 38.6%, and severe accounted for 23.4%. Taken together, these results show that moderate disorder is the most common sICH severity.[35]
In this study, the surgical treatment rate of sICH inpatients was 30.84%, which was lower than that observed in northwest China (61.55%)[16] as well as in the United Kingdom (51.08%).[36] Our results indicated that the average hospital stay of sICH patients was 16 days, which was shorter than the 17.9 days observed in Taiwan[37] and longer than that observed in Canada (8 days).[38] In this study, the median cost of hospitalization was $3,608, which was lower than the $5,456 observed in Germany.[39] Finally, our results showed that the 30-day mortality rate of sICH patients was 18.36%, which was lower than that observed in western China (22.67%)[16] and that observed in Taiwan (19.8%).[40]
Poor prognostic factors for ICH include large hematoma volume, hematoma expansion, intraventricular hemorrhage, infra-tentorial location, old age, and the use of anticoagulation therapy.[2] In studies using Asian populations, fever, low initial Glasgow Coma Scale, large hematoma, intra-ventricular hemorrhage, and diabetes have all been shown to be independent predictors for poorer outcomes in patients with sICH.[41,42] Guo et al[43] conducted a meta-analysis on 6527 patients with sICH and showed that hyperglycemia significantly increased the risk of death in patients with sICH. Moreover, the risk of death relative risk was 1.14 for every 1 mmol increase in fasting blood glucose (95% CI: 1.06–1.22). Liu et al[44] examined the prognosis of 908 patients with sICH in Chengdu, China. Their work showed that hyperglycemia and a low GCS score at admission were both independent risk factors for poor prognosis after 3 months. Satu et al[45] investigated 967 patients with sICH in Finland and found that, compared to patients with non-intracerebral hemorrhage, patients with intraventricular hemorrhage had lower GCS at admission, larger cerebral hemorrhage, were more prone to hydrocephalus, and had higher mortality. It is also worth noting that this study showed that intraventricular hemorrhage was a powerful predictor of three-month mortality in patients with sICH.
This study has some limitations. Since the data originated from the first affiliated Hospital of Chengdu Medical College, there is a lack of multi-center data with which to draw a more robust conclusion across a wider sample size. The results of this study also do not reflect the epidemiology of sICH in the entire region of Chengdu, China. Moreover, this study did not include information about patients with sICH who visited private clinics, received emergency medical attention, and/or those who did not visit the hospital. Moreover, the lack of confirmation of sICH diagnosis was another limitation. It is possible that the ICD-10 coding used in this study to identify patients for inclusion missed other sICHs that may not have been identified under this ICD-10 code. We did not compare the effect of surgical and conservative complications on patients’ clinical outcomes, so we did not discuss infectious complications in the present study.
sICH is an important public health problem in Chengdu, China, as both the mortality and disability rates of sICH are high. For patients in particular, their resulting lower quality of life and higher psychological pressure can result in negative emotions and affect their recovery. For families and society at large, sICH increases the economic, human, and material resource burden, even affecting societal development as a whole. A governmental initiative is urgently needed to establish a sICH monitoring system that covers the Chengdu region. Moreover, this initiative should also be used to develop more effective and targeted measures for sICH prevention, treatment, and rehabilitation.
Author contributions
Conceptualization: Kai Yu.
Data curation: Kai Yu.
Formal analysis: Kai Yu, Shu Zhu, Lie Zhang.
Funding acquisition: Lie Zhang.
Investigation: Kai Yu, Zongxi Li.
Methodology: Kai Yu.
Project administration: Mingjie He, Lie Zhang, Zhao Sui.
Resources: Zhao Sui.
Software: Kai Yu, Shu Zhu.
Supervision: Kai Yu.
Writing – original draft: Kai Yu.
Writing – review & editing: Shu Zhu, Xun Xia, Yunming Li.
Footnotes
Abbreviations: BMI = body mass index, GCS = Glasgow Coma Scale, IQR = interquartile range, mRs = modified Rankin scale, sICH = spontaneous intracerebral hemorrhage.
How to cite this article: Yu K, Zhu S, He M, Li Z, Zhang L, Sui Z, Li Y, Xia X. Epidemiological characteristics of 561 cases of intracerebral hemorrhage in Chengdu, China. Medicine. 2021;100:15(e24952).
KY and SZ contributed equally to this work.
This work was supported by the Natural Science Foundation of Chengdu Medical College (project number: CYZ19-32); Recipient: Kai Yu.
All relevant data are within the paper and its Supporting Information files.
The authors have no conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The t value of the independent two-sample t test.
The χ2 value of Pearson Chi-Squared test.
The Z value of rank sum test. P < .05.
BMI = body mass index, GCS = Glasgow Coma Scale, mRs = modified Rankin scale, sICH = spontaneous intracerebral hemorrhage.
sICH = spontaneous intracerebral hemorrhage. P < .05.
sICH = spontaneous intracerebral hemorrhage. P < .05.
The F value of one-way analysis of variance.
The χ2 value of Pearson Chi-Squared test. P < .05.
BMI = body mass index, sICH = spontaneous intracerebral hemorrhage.
The χ2 value of Pearson Chi-Squared test.
The t value of the independent two-sample t test.
The Z value of rank sum test. P < .05.
BMI = body mass index, sICH = spontaneous intracerebral hemorrhage.
The χ2 value of Pearson Chi-Squared test.
The F value of one-way analysis of variance. P < .05.
BMI = body mass index, sICH = spontaneous intracerebral hemorrhage.
References
- [1].Wang Y, Zhang S, Zhang L, et al. Chinese guidelines for Secondary Prevention of Ischemic Stroke and transient Ischemic attack 2010. Chin J Neurol 2010;43:154–9. [Google Scholar]
- [2].An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke 2017;19:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep 2012;14:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu Yan, Li Huaiyu, Li Hu. Analysis of epidemiology and clinical characteristics of 1298 cases of spontaneous Cerebral Hemorrhage. Chin J Evid based Med 2010;10:1256–8. [Google Scholar]
- [5].O”Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): a case-control study. XXX 2010;376:0–123. [DOI] [PubMed] [Google Scholar]
- [6].Zhang S, Xu Y, Zhu S, et al. Guidelines for diagnosis and treatment of Cerebral Hemorrhage in China(2014). Chin J Neurol 2015;48:435–44. [Google Scholar]
- [7].Maas MB, Francis BA, Sangha RS, et al. Refining Prognosis for Intracerebral Hemorrhage by Early Reassessment. Cerebrovasc Dis 2017;43:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang R, Cao Q, Lu Y. The analysis of cigarette smoking behaviors and its influencing factors among Chinese urban and rural residents. Acta Universitatis Medicinalis Nanjing(Natural Sciences) 2014;34:84–9. [Google Scholar]
- [9].Stockwell T, Zhao J, Panwar S, et al. Do “Moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. J Stud Alcohol Drugs 2016;77:185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American heart association/American stroke association stroke council, high blood pressure research council, and the quality of care and outcomes in research interdisciplinary working group. Circulation 2007;116:e391–413. [DOI] [PubMed] [Google Scholar]
- [11].Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–60. [DOI] [PubMed] [Google Scholar]
- [12].Ji L, Zhi X, Lu J, et al. Hyperglycemia and blood pressure treatment goal: a cross sectional survey of 18350 patients with type 2 diabetes in 77 tertiary hospitals in China. PLoS One 2014;9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang W, Sun Y, Sun J. Combined effects of FTO rs9939609 and MC4R rs17782313 on obesity and BMI in Chinese Han populations published correction appears in Endocrine. 2011 Feb; 39 (1):99. Endocrine 2011;39:69–74. [DOI] [PubMed] [Google Scholar]
- [14].Zhang R, Wang X, Tang Z, et al. Analysis of clinical characteristics and treatment of 6374 patients with hypertensive intracerebral hemorrhage. Chin J Neuromed 2013;12:57–61. [Google Scholar]
- [15].Hou Jiguang, Dawa, Li Yimin, et al. Clinical characteristics of 515 patients with spontaneous intracerebral hemorrhage in Xigaze area of Tibet Autonomous region. Chin J Neurosurg 2019;35:70–2. [Google Scholar]
- [16].Sandeep YS, Guru MR, Jena RK, et al. Clinical study to assess the outcome in surgically managed patients of spontaneous intracerebral hemorrhage. Int J Crit Illn Inj Sci 2017;7:218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mittal MK, Lele A. Predictors of poor outcome at hospital discharge following a spontaneous intracerebral hemorrhage. Int J Neurosci 2011;121:267–70. [DOI] [PubMed] [Google Scholar]
- [18].Parry-Jones AR, Abid KA, Di Napoli M. Accuracy and clinical usefulness of intracerebral hemorrhage grading scores: a direct comparison in a UK population. Stroke 2013; 44:1840–45. [DOI] [PubMed] [Google Scholar]
- [19].Hole BJ, Kloster R. Spontaneous intracerebral haemorrhages in Vestfold country. Spontane intracerebrale blødninger i Vestfold. Tidsskr Nor Laegeforen 2017;137:793–7. Published 2017 Jun 6. [DOI] [PubMed] [Google Scholar]
- [20].Nzwalo H, Nogueira J, Félix C, et al. Incidence and case-fatality from spontaneous intracerebral hemorrhage in a southern region of Portugal. J Neurol Sci 2017;380:74–8. [DOI] [PubMed] [Google Scholar]
- [21].Wang K, Li H, Liu W, et al. Temporal epidemiological characteristics of cerebral hemorrhage in Chengdu, Sichuan. Chin J Neurosurg 2011;27:759–63. [Google Scholar]
- [22].Chu SY, Cox M, Fonarow GC, et al. Temperature and precipitation associate with ischemic stroke outcomes in the United States. J Am Heart Assoc 2018;7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bao J, Guo Y, Wang Q, et al. Effects of heat on first-ever strokes and the effect modification of atmospheric pressure: A time-series study in Shenzhen, China. Sci Total Environ 2019;654:1372–8. [DOI] [PubMed] [Google Scholar]
- [24].Telman G, Sviri GE, Sprecher E, et al. Seasonal variation in spontaneous intracerebral hemorrhage in northern Israel. Chronobiol Int 2017;34:563–70. [DOI] [PubMed] [Google Scholar]
- [25].Tsou YJ, Lan KP, Fan JS. Relationship between changes in prehospital blood pressure and early neurological deterioration in spontaneous intracerebral hemorrhage. Adv Emerg Nurs J 2019;41:163–71. [DOI] [PubMed] [Google Scholar]
- [26].Akpinar E, Gürbüz MS, Berkman MZ. Factors affecting prognosis in patients with spontaneous supratentorial intracerebral hemorrhage under medical and surgical treatment. J Craniofac Surg 2019;30:e667–71. [DOI] [PubMed] [Google Scholar]
- [27].Martini SR, Flaherty ML, Brown WM, et al. Risk factors for intracerebral hemorrhage differ according to hemorrhage location. Neurology 2012;79:2275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang Y, Tuomilehto J, Jousilahti P, et al. Lifestyle factors on the risks of ischemic and hemorrhagic stroke. Arch Intern Med 2011;171:1811–8. [DOI] [PubMed] [Google Scholar]
- [29].O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): a case–control study. Lancet 2010;376:112–23. [DOI] [PubMed] [Google Scholar]
- [30].Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Flaherty ML, Tao H, Haverbusch M, et al. Warfarin use leads to larger intracerebral hematomas. Neurology 2008;71:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].García-Rodríguez LA, Gaist D, Morton J, et al. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology 2013;81:566–74. [DOI] [PubMed] [Google Scholar]
- [33].Zhang HL, Linn J, Bruckmann H, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhao FF, Gao HY, Gao Y, et al. A correlational study on cerebral microbleeds and carotid atherosclerosis in patients with ischemic stroke. J Stroke Cerebrovascular Dis 2018;27:2228–34. [DOI] [PubMed] [Google Scholar]
- [35].Safatli DA, Günther A, Schlattmann P, et al. Predictors of 30-day mortality in patients with spontaneous primary intracerebral hemorrhage. Surg Neurol Int 2016;7: Suppl 18: S510–7. Published 2016 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial published correction appears in Lancet. 2013 Aug 3; 382(9890):396. Lancet 2013;382:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ting HW, Chien TY, Lai KR, et al. Differences in spontaneous intracerebral hemorrhage cases between urban and rural regions of Taiwan: big data analytics of government open data. Int J Environ Res Public Health 2017;14:1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Specogna AV, Turin TC, Patten SB, et al. Hospital treatment costs and length of stay associated with hypertension and multimorbidity after hemorrhagic stroke. BMC Neurol 2017;17:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dodel RC, Haacke C, Zamzow K, et al. Resource utilization and costs of stroke unit care in Germany. Value Health 2004;7:144–52. [DOI] [PubMed] [Google Scholar]
- [40].Chan CL, Ting HW, Huang HT. The incidence, hospital expenditure, and, 30day and 1year mortality rates of spontaneous intracerebral hemorrhage in Taiwan. J Clin Neurosci 2014;21:91–4. [DOI] [PubMed] [Google Scholar]
- [41].Poungvarin N, Suwanwela NC, Venketasubramanian N, et al. Grave prognosis on spontaneous intracerebral haemorrhage: GP on STAGE score. J Med Assoc Thai 2006;89: Suppl 5: S84–93. [PubMed] [Google Scholar]
- [42].Chen HS, Hsieh CF, Chau TT, et al. Risk factors of in-hospital mortality of intracerebral hemorrhage and comparison of ICH scores in a Taiwanese population. Eur Neurol 2011;66:59–63. [DOI] [PubMed] [Google Scholar]
- [43].Guo X, Li H, Zhang Z, et al. Hyperglycemia and mortality risk in patients with primary intracerebral hemorrhage: a meta-analysis. Mol Neurobiol 2015;14:1–7. [DOI] [PubMed] [Google Scholar]
- [44].Liu J, Wang D, Yuan R, et al. Prognosis of 908 patients with intracerebral hemorrhage in Chengdu, Southwest of China. Int J Neurosci 2017;127:586–91. [DOI] [PubMed] [Google Scholar]
- [45].Mustanoja S, Satopää J, Meretoja A, et al. Extent of secondary intraventricular hemorrhage is an independent predictor of outcomes in intracerebral hemorrhage: data from the Helsinki ICH Study. Int J Stroke 2015;10:576–81. [DOI] [PubMed] [Google Scholar]


