Abstract
Background:
To assess T-cell exhaustion mediated by programmed cell death 1 (PD-1) pathway in patients living with type 2 diabetes (T2D).
Methods:
MEDLINE and ProQuest electronic databases were searched for eligible studies from inception up to February 2020. The risk of bias and the quality of evidence were independently assessed by two reviewers using the modified Newcastle-Ottawa Scale adapted for cross-sectional studies and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool, respectively. The random effects model was used to calculate effect estimates.
Results:
We identified 5 studies involving 380 participants which met the inclusion criteria. The pooled estimates showed elevated T helper cell exhaustion in patients with T2D in comparison to controls (mean difference [MD]: 2.57% [95% confidence interval [CI]: –3.84, 8.97]; I2 = 100%, P < .00001). Likewise, T2D patients had increased levels of cytotoxic T-cells exhaustion (MD: 3.09% [95% CI: –12.96, 19.14]; I2 = 100%, P < .00001). Although the upregulation of PD-1 on T-cells did not affect glucose metabolism-related profiles, it was associated with inflammation and the development of cardiovascular disease.
Conclusion:
In patients living with T2D, immune dysfunction is at least in part due to T-cell exhaustion mediated by the upregulation of PD-1 expression. Therefore, the use of immune checkpoint inhibitors as a therapeutic strategy may be beneficial in restoring immune function in patients with T2D.
Keywords: chronic inflammation, immune activation, programmed cell death 1, T-cell exhaustion, type 2 diabetes mellitus
1. Introduction
Type 2 diabetes (T2D) is a low-grade inflammatory condition that is characterized by insulin resistance, hyperglycaemia, and immune dysregulation.[1,2] Notably, impaired glucose tolerance and obesity drive chronic inflammation in T2D.[3,4] This can lead to an exacerbated activation of both the innate and adaptive immune systems.[5] In fact, immune responses mediated by T-cells play a pivotal role in maintaining immune homeostasis.[6] In chronic inflammatory conditions, increased expression of negative co-stimulatory molecules is known to promote T-cell exhaustion.[7]
It is acknowledged that negative co-stimulatory molecules such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death-1 (PD-1) are essential in inducing immune tolerance during T-cell maturation, and regulating T-cell effector functions.[8] However, persistent immune activation in T2D leads to the aberrant expression of these markers and altered T-cell effector functions.[9] Of the various inhibitory molecules, PD-1 has been identified as one of the most potent negative regulators of T-cell function.[10] For instance, enhanced PD-1 signaling has been linked with cytotoxic (CD8+) T-cell exhaustion in viral infections.[11] Recent evidence also suggests the involvement of PD-1 in mediating T-helper (CD4+) cell exhaustion during chronic infections.[12,13] Notably, since T-cells are involved in the pathogenesis of T2D,[14] there has been a great interest in understanding the impact of PD-1 in T-cell mediated inflammation and dysfunction in conditions of metabolic syndrome. Interestingly, we have progressively explored the detrimental effects linking increased levels of T-cell activation and pro-inflammatory T-cell subsets with low-grade inflammation in T2D.[15–17] To date, available literature on the expression of PD-1 on T-cells of T2D patients is inconclusive, with dysregulated expression of PD-1 linked to the progression of low-grade inflammation in conditions of impaired glucose tolerance.[18,19] Therefore, in this systematic review and meta-analysis, we aimed to assess T-cell exhaustion mediated by PD-1 expression in patients living with T2D.
2. Methods
This systematic review and meta-analysis was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.[20] This study forms part of the registered protocol with the International prospective register of systematic reviews (PROSPERO), registration number: CRD42018099745. We conducted the qualitative and quantitative synthesis to answer the following questions;
Question 1: Are circulating T-cells exhausted in adult patients living with T2D?
Question 2: Does the PD-1 receptor mediate T-cell exhaustion in T2D?
2.1. Search strategy
A comprehensive search was conducted on the MEDLINE electronic database and ProQuest grey literature, from inception up until the February 20, 2020. The search was conducted by two independent reviewers (TMN and BBN), while a third reviewer (PVD) was consulted for arbitration in cases of disagreements. The search strategy was adapted to MEDLINE databases without any language restrictions using medical subjects heading (MeSH) terms and keywords such as “programmed cell death-1,” “T-cell exhaustion,” “type 2 diabetes mellitus,” and their respective synonyms and associated words or phrases. A detailed search strategy is provided as a supplementary file (see Table 1S, Supplemental Content, which illustrates the search strategy used). In addition to scanning reference lists of retrieved studies, the ProQuest grey literature database was also searched for relevant studies. The Mendeley reference manager software (Elsevier, Amsterdam, Netherlands) was used to manage the reference list and to remove study duplicates.
2.2. Inclusion criteria and study selection
This systematic review and meta-analysis included studies that reported on the expression of PD-1 on T-cells of individuals with T2D. Reviews, books, editorials, letters, and studies that reported PD-1 expression on other immune cells that are not T-cells were excluded. The studies identified by the search strategy were independently screened and selected by two reviewers (TMN and PVD) using the following pre-defined PECO: Participants: adults (≥18 years old); Exposure: T2D; Comparator: healthy controls (normoglycaemics); Outcome: T-cell exhaustion. In cases of disagreements, a third reviewer, BBN was consulted for arbitration.
2.3. Data extraction
Two independent investigators (TMN and VM) extracted the data items using a pre-defined data extraction sheet. A third reviewer (PVD) was consulted for arbitration in instances of disagreements. The extracted data items included the names of the authors, year of publication, study design, age of participants, T-cell subsets that PD-1 expression was reported on and the main findings.
2.4. Risk of bias and quality assessment
Two reviewers (TMN and VM) independently assessed risk of bias in the included studies using the modified Newcastle-Ottawa Scale adapted for cross-sectional studies.[21] Briefly, the tool uses 3 domains namely, selection of study groups, comparability of the groups, and outcome ascertainment to assess study quality. A study is considered unsatisfactory if the total score is ≤4, satisfactory (5–6), good (7–8), and very good (9–10). In cases of disagreements, a third reviewer (BBN) was consulted for arbitration. The same reviewers evaluated the quality of evidence using the Grading of Recommendations Assessment Development and Evaluation (GRADE) approach.[22]
2.5. Statistical analysis
Cohen kappa scores were used to measure interrater reliability.[23] The mean and standard deviation for each continuous effect measure was extracted or calculated using Hozo et al[24] method. In cases where standard deviations were not reported, the Cochrane guidelines were followed to estimate the values.[25] All statistical analysis was performed using REVMAN version 5.3 software (Cochrane Collaboration, Oxford, UK). Heterogeneity was quantitatively assessed using Higgin I2 index[26] and the random-effects model was used[27] to calculate the pooled estimates. The effect estimates were reported using mean difference (MD) and 95% confidence interval (CI). A p-value <.05 was considered statistically significant. A sensitivity analysis was conducted to investigate the sources of heterogeneity amongst the included studies and to assess the robustness of the reported estimates.
3. Results
3.1. Included studies
The search strategy identified a total of 12 citations and only 5 studies[9,18,19,28,29] met the inclusion criteria (overall agreement 95.45%, kappa = 0.88). A total of 5 studies were excluded at the abstract stage because 2 were reviews and 3 were not relevant to the topic of interest. Of the remaining 7 studies that were assessed for eligibility using full texts, 2 studies were excluded because there were not relevant to the topic of interest. As a result, a total of 5 studies were included in this systematic review and meta-analysis as indicated in Fig. 1.
Figure 1.
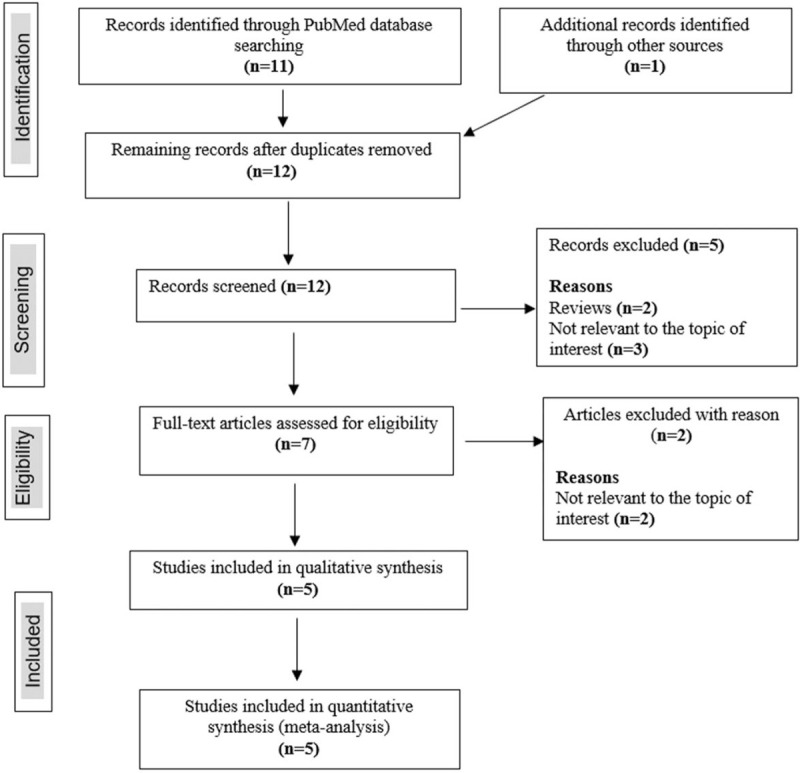
PRISMA diagram showing the study selection process. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
3.2. Study characteristics
The included studies were published between 2013 and 2019, and their characteristics are presented in Table 1. All citations were cross-sectional studies comprising a total of 380 participants. Of these, 198 were T2D patients and 182 were healthy controls. The study population had an average age of 55.64 ± 9.09 and a male to female ratio of 0.75. Overall, the included studies reported on participants from China (n = 3), Japan (n = 1), and South Africa (n = 1).
Table 1.
Characteristic features of included studies and the reported on the expression of programmed cell death 1 (PD-1) on T-cells in patients with type 2 diabetes (T2D).
| Study | Country | Study size | Male, n (%) | Age, y | T-cell subsets reported | Main findings |
| Shi et al, 2013[18] | China | 90 participants (42 T2D and 48 controls) | Not reported | 51.46 ± 10.68 | CD4+CD28– | The expression of PD-1 on CD4+CD28- T-cell subset was increased in T2D patients when compared to healthy controls. Moreover, the upregulation of PD-1 on these T-cells was associated with the development of atherosclerotic macrovascular diseases. |
| Fujisawa et al, 2015[28] | Japan | 48 participants (19 T2D and 29 controls) | 24 (50) | 49 ± 11.82 | CD4+ | The levels of PD-1 expression on T-helper cells were comparable between individuals with T2D versus healthy controls. |
| Jia et al, 2016[9] | China | 130 participants (80 T2D and 50 controls) | 70 (54) | 61 ± 4.10 | CD4+ and CD8+ | The expression of PD-1 on both CD4+ and CD8+ was increased in T2D when compared with healthy controls. In addition, the upregulation of PD-1 on T-cells positively correlated with the levels of C-reactive protein (CRP), an inflammation marker. |
| Nyambuya et al, 2018[29] | South Africa | 69 participants (34 T2D and 35 controls) | 10 (14) | 54.48 ± 4.45 | CD4+ | There was no difference in the expression of PD-1 on T-cells in T2D patients and healthy controls. Moreover, there was no correlation between the expression of PD-1 on T-cell and glucose metabolic profile. However, T2D patients had increased levels of inflammation. |
| Sun et al, 2019[19] | China | 43 participants (23 T2D and 20 controls) | 20 (47) | 57.47 ± 9.03 | CD4+ and CD8+ | CD4+ and CD8+ T-cells from individuals with T2D expressed lower levels of PD-1 when compared with healthy controls. However, no correlation was found between PD-1 expression and glucose metabolic profiles. |
3.3. Risk of bias assessment and publication bias
The overall median score range of included studies was 7 (4–8), one study was scored as unsatisfactory (4 points)[28] and the rest good (7–8 points)[9,18,19,29] (see Table 2S, Supplemental Content, which illustrates the modified Newcastle-Ottawa Scale used). Included studies had a selection median of 3 (2–4) out of possible 5 (overall agreement 100%, kappa = 1), comparability median of 2 (0–2) out of possible 2 (overall agreement 91.4%, kappa = 0.83) and outcome ascertainment median of 2 (2–3) out of 3 possible (overall agreement 93%, kappa = 0.87). We did not include funnel plots in this study since testing for funnel plot asymmetry is not recommended when included number of studies is less than 10.[25]
3.4. Glucose metabolic profiles
A total of 3 studies reported on glucose metabolism-related profiles of included participants. As expected, patients with T2D had significantly increased fasting blood glucose levels (MD: 2.81 mmol/L [95% CI: 0.28, 5.34]; I2 = 99%, P < .00001) and glycated hemoglobin levels (MD: 2.57% [95% CI: –0.08, 5.23]; I2 = 100%, P < .00001) (see Figure 1S, Supplemental Content, which demonstrates estimates of glucose metabolic profiles), thus indicating poor glucose control.
3.5. T-cell exhaustion mediated by increased PD-1 signaling pathway in T2D
Two studies[9,18] reported on increased expression of PD-1 on CD4+ and CD8+ T-cells in patients with T2D when compared with healthy controls. In contrast, 2 of the included studies[28,29] reported comparable levels, whereas 1 study[19] showed decreased levels of PD-1 expression on T-cells in patients with T2D and controls. Interestingly, pooled estimates showed increased PD-1 signaling on T helper cells (MD: 2.57% [95% CI: –3.84, 8.97]; I2 = 100%, P < .00001) and cytotoxic T-cells (MD: 3.09% [95% CI: –12.96, 19.14]; I2 = 100%, P < .00001) of T2D patients in comparison to controls (Fig. 2). Thus, suggesting PD-1 mediated T-cell exhaustion in a diabetic state.
Figure 2.
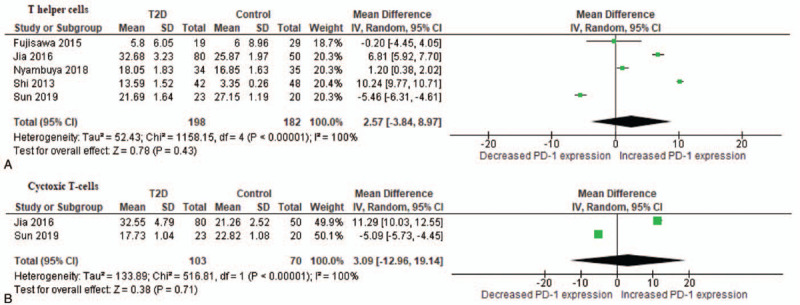
A comparison of mean difference of programmed cell death 1 (PD-1) expression on T helper cells (A) and cytotoxic T-cells (B) between patients with T2D and healthy controls. T2D = type 2 diabetes.
We performed a subgroup analysis based on extracted and computed values of PD-1 expression on T helper cells. Pooled estimates of studies where PD-1 expression values were extracted were lower (MD: 1.55% [–10.88, 13.99]; 100%, P < .00001) than studies where the values were computed (MD: 4.00% [–1.50, 9.50]; I2 = 99%, P < .00001) (see Table 3S, Supplemental Content, which demonstrates the subgroup analysis). To further investigate the sources of heterogeneity amongst the included studies and assess the robustness of the reported estimates, we conducted a sensitivity analysis and it revealed that sample type and risk of bias might be further sources of heterogeneity in the estimates of PD-1 mediated T-cell exhaustion. There was no difference on the levels of T-cell exhaustion between studies reporting on whole blood samples and those on peripheral blood mononuclear cells. Moreover, the level of statistical heterogeneity remained substantial (see Table 4S, Supplemental Content, which illustrates the sensitivity analysis). The sensitivity analysis did not change the direction of the polled effect estimate, thus suggesting our findings to be robust. A qualitative synthesis of included studies in this review revealed that the upregulation of PD-1 expression on T-cells had no association with glucose metabolism,[19,29] but was positively correlated with inflammation and the development of cardiovascular diseases (CVDs).[9,18] A summary of findings is provided in Table 2.
Table 2.
Summary of findings table.
| Type 2 diabetes compared with healthy controls | ||||||
| Patient or population: adults (≥18 y of age) | ||||||
| Exposure: type 2 diabetes mellitus (T2D) | ||||||
| Comparison: healthy controls (normoglycaemics) | ||||||
| Outcomes | Absolute effects∗ (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk in T2D patients | |||||
| T-cell exhaustionmeasured by PD-1 expression on CD4 T-cells | – | The mean level in the exposure group was 2.57 higher (–3.84–8.97) | – | 380 (5 observational studies) |
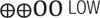 LOW LOW |
|
| T-cell exhaustion measured by PD-1 expression on CD8 T-cells | – | The mean level in the exposure group was 3.09 higher (–12.95 lower to 19.14 higher) | 242 (3 observational studies) |
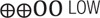 LOW LOW |
||
| ∗The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| CI: confidence interval; MD: mean difference; OR: odds ratio; NE: not estimable | ||||||
| GRADE Working Group grades of evidence | ||||||
| High certainty: We are very confident that the true effect lies close to that of the estimate of the effect | ||||||
| Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different | ||||||
| Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect | ||||||
| Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
4. Discussion
The aim of this study was to assess T-cell exhaustion mediated by PD-1 expression in patients living with T2D. Although T-cell exhaustion is associated with conditions of impaired glucose tolerance,[18,19] the regulation of PD-1 expression on T-cells of patients with T2D remains elusive. In this study, pooled estimates showed that in patients with T2D, PD-1 expression is upregulated when compared with healthy controls. Such findings are congruent with available evidence suggesting that PD-1 expression might promote inflammation and the development of CVDs.[9,18] Taken together, these findings support the notion that T-cell exhaustion may be mediated by PD-1 signaling pathway in patients living with T2D.
PD-1 is a receptor belonging to the CD28 family that delivers a negative signal upon interacting with its two ligands, programmed death ligand 1 or 2 (PD-L1 or PD-L2).[10,30] Successful T-cell activation requires two signals to induce their effector functions, whereby the first primary signal is via the T-cell receptor (TCR) and the second is through CD28 co-stimulation.[31] The upregulation of PD-1 blocks the co-stimulatory signaling, resulting in immune suppression.[32] Patients living with T2D patients are known to present with abnormally increased levels of circulating interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-17.[33,34] In fact, these pro-inflammatory cytokines have the ability to induce PD-1 signaling by upregulating the expression of its ligands in tumor environments, autoimmunity, or during chronic infections.[35] The activation of PD-1 signaling pathway dephosphorylates TCR signaling and zeta-chain-associated protein kinase (ZAP)70 by recruiting Src homology phosphatase (SHP)-1/2 which results in the dysregulation of a number of molecular mechanisms including; the insulin-dependent phosphoinositide 3-kinase/protein kinase B (PI3K/AKT); as well as the pro-inflammatory Janus kinase/signal transducers and activators of transcription 3 (JAK/STAT3) and nuclear factor kappa B (NF-κB) elements.[36] These pathways are essential for T-cell proliferation, activation, and survival.[37–39] In particular, JAK/STAT and NF-κB pathways are the principal signaling mechanisms for a variety of cytokines and growth factors implicated in cell death,[39,40] while the activation of PI3K/AKT is crucial for cell survival and proliferation, including improvements in glucose control.[37,41] Therefore, a pro-inflammatory state in T2D may induce the activation of PD-1 signaling leading to immune suppression and T-cell exhaustion.
The expression of PD-1 on T-cells and cytokine production is closely regulated in a physiological state. However, in a state of impaired glucose metabolism, it is apparent that PD-1 expression is dysregulated. For instance, exposure of murine T-cells to galactose or glucose substrates has been shown to enhance the expression of PD-1 in addition to increasing oxidative phosphorylation.[42] These findings suggest that PD-1 may play a significant role in regulating cellular metabolism, or could impact energy generating mechanisms such as the AMP-activated protein kinase (AMPK) pathway. To support this hypothesis, the anti-diabetic drug metformin, (is to activate AMPK activator), has already been shown to enhance T-cell function by altering the PD-L1/PD-1 axis.[43] In agreement with the findings reported in this study, it seems that the levels of PD-1 expression are elevated on T-cells of patients with T2D. This is especially true since the activation of PD-1 signaling in cultured T-cells from healthy donors altered energy seems metabolic pathways by inhibiting the uptake and utilization of glucose while promoting fatty acid β-oxidation as a source of ATP.[44] Thus, providing further evidence that tight regulation of T-cell function is necessary for optimal glucose metabolism. Therefore, in addition to the well-described chronic antigen stimulation and inflammation in T-cell exhaustion,[7] increased expression of PD-1 and loss of effector function is also influenced by cellular glucose metabolism. As a result, this evidence highlights the need to target PD-1 signaling pathway as a therapeutic mechanism to improve metabolic functions in conditions of metabolic stress.
Chronic inflammation is implicated in the development of CVD in patients living with T2D.[18] In that context, patients with T2D are at a 2- to 4-fold risk of developing CVD when compared with healthy controls.[45] Interestingly, increased levels of CRP, a sensitive systemic marker of inflammation has been associated with increased risk of developing CVDs in patients with T2D.[46] The evidence synthesized in this study demonstrated increased levels of CRP in patients with T2D[9,29] which was associated with increased expression of PD-1.[9] Moreover, the upregulation of PD-1 on T-cells was consistent with the development of atherosclerotic macrovascular diseases.[18] The use of immune checkpoint inhibitors which block co-stimulatory signaling pathway are known to be effective in rejuvenating T-cell effector function in chronic infections and tumor environments.[47] However, this consequence has been correlated with the new-onset of impaired glucose homeostasis.[48] Therefore, the use of immune checkpoint inhibitors in T2D as a treatment strategy may have a double-edged sword effect whereby it improves T-cell mediated immune responses but further impairing glucose control, as reported elsewhere.[49] This emphasizes the need to find a fine balance between improving T-cell function while enhancing glucose metabolism when targeting PD-1 signaling as a therapeutic strategy in T2D patients. The overall impact of PD-1 signaling on T-cell function is illustrated in Fig. 3.
Figure 3.
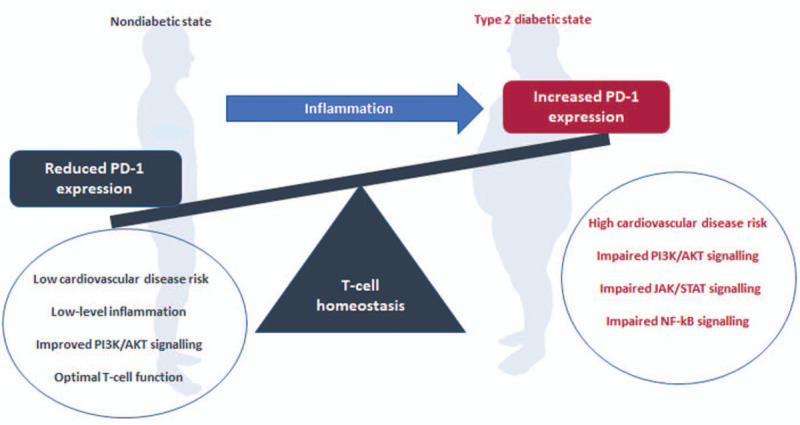
An overview of mechanisms that implicates programmed cell death-1 signaling and its modulatory effects on T-cell function. In brief, chronic immune activation in type 2 diabetes mellitus is known to increase cardiovascular risk and T-cell exhaustion, which is likely to be mediated by the upregulation of programmed cell death-1 (PD-1), a negative T-cell regulator. Thus, increased expression of PD-1 can alter glucose metabolism by inhibiting the actions of phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling. It further appears that PD-1 expression promotes T-cell proliferation and survival but inhibit their effector functions by upregulating the detrimental mechanisms such Janus kinase/signal transducers and activators of transcription (JAK/STAT) and nuclear factor kappa B (NF-κB) signaling pathways.
Overall, this systematic review and meta-analysis had a few limitations. Firstly, the number of included studies was low as well as the quality of evidence due to their cross-sectional nature. Secondly, there was substantial amount of unexplained statistical heterogeneity in this study. Lastly, the level of T-cell exhaustion is influenced by the duration of exposure to chronic inflammation, of which the majority of included studies did not report on disease duration. Nevertheless, our study has a unique strength in that to our knowledge, it is the first systematic review and meta-analysis to assess T-cell exhaustion in T2D. Moreover, the methodologies employed in this study were robust as indicated by high levels of inter-rater agreements. Results from sensitivity analysis indicated that the reported pooled effect sizes were not influenced by a single study, thus making the findings reported herein robust. Lastly, the current findings are important as they pave way for future therapeutic strategies to explore the use of immune checkpoint inhibitors in order to resuscitate immune responses mediated by T-cells. This will thus potentially correct the immune dysfunction observed in T2D patients.
5. Conclusion
Low-grade inflammation in conditions of impaired glucose tolerance is associated with chronic immune activation and dysfunction, which collectively increases the risk of developing diabetes-associated cardiovascular complications. The evidence synthesised here suggests that immune dysfunction observed in T2D is in part due to T-cell exhaustion mediated by increased expression of PD-1. Therefore, the use of immune checkpoint inhibitors in rejuvenating the immune response in these patients may be an effective therapeutic strategy.
Author contributions
Conceptualization: Tawanda Maurice Nyambuya, Phiwayinkosi Vusi Dludla, Bongani Brian Nkambule.
Data curation: Tawanda Maurice Nyambuya.
Formal analysis: Tawanda Maurice Nyambuya, Bongani Brian Nkambule.
Funding acquisition: Bongani Brian Nkambule.
Investigation: Tawanda Maurice Nyambuya, Vuyolwethu Mxinwa, Bongani Brian Nkambule.
Software: Tawanda Maurice Nyambuya.
Supervision: Phiwayinkosi Vusi Dludla, Bongani Brian Nkambule.
Validation: Tawanda Maurice Nyambuya, Bongani Brian Nkambule.
Writing – original draft: Tawanda Maurice Nyambuya.
Writing – review & editing: Tawanda Maurice Nyambuya, Phiwayinkosi Vusi Dludla, Vuyolwethu Mxinwa, Bongani Brian Nkambule.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AMPK = AMP-activated protein kinase, ATP = adenosine triphosphate, CD4+ = T-helper cells, CD8+ = Cytotoxic T-cells, CRP = C-reactive protein, CTLA-4 = cytotoxic T-lymphocyte-associated protein 4, CVD = cardiovascular disease, IFN-γ = interferon gamma, IL = interleukin, JAK/STAT3 = Janus kinase/signal transducers and activators of transcription 3, MD = mean difference, NF-κB = nuclear factor kappa B, PD-1 = programmed cell death-1, PD-L1/2 = programmed death ligand 1/2, PI3K/AKT = phosphoinositide 3-kinase/protein kinase B, T2D = type 2 diabetes, TCR = T-cell receptor, TNF-α = tumor necrosis factor alpha.
How to cite this article: Nyambuya TM, Dludla PV, Mxinwa V, Nkambule BB. A systematic review and meta-analysis on the regulation of programmed cell death-1 on T-cells in type 2 diabetes. Medicine. 2021;100:15(e25488).
Ethics approval and consent to participate: Not applicable since data were generated from reported data.
Availability of data and materials: The authors confirm that the data supporting the findings of this study are available within the article and its supplementary files.
The current study is partially funded by the National Research Foundation (NRF) of South Africa (Grant Number: 107519 to BB Nkambule). BBN is also a University of KwaZulu-Natal (UKZN) Developing Research Innovation, Localisation and Leadership in South Africa (DRILL) fellow. DRILL, is a NIH D43 grant (D43TW010131) awarded to UKZN in 2015 to support a research training and induction programme for early career academics. PV Dludla was partially supported as a Post-Doctoral Fellow by funding from Research Capacity Division of the South African Medical Research Council (SAMRC) through its division of Research Capacity Development under the Intra-Mural Post Doctorial Fellowship Programme from funding received from the South African Treasury. The content hereof is the sole responsibility of the authors and do not necessary present the official views of SAMRC or the funders.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files];
Supplemental digital content is available for this article.
References
- [1].Viardot A, Heilbronn L, Samocha-Bonet D, et al. Obesity is associated with activated and insulin resistant immune cells. Diabetes Metab Res Rev 2012;28:01–18. [DOI] [PubMed] [Google Scholar]
- [2].Ip BC, Hogan AE, Nikolajczyk BS. Lymphocyte roles in metabolic dysfunction: of men and mice. Trends Endocrinol Metab 2015;26:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].van Greevenbroek MMJ, Schalkwijk CG, Stehouwer CDA. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: Causes and consequences. Neth J Med 2013;71:174–87. [PubMed] [Google Scholar]
- [4].Hameed I, Masoodi SR, Mir SA, et al. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J Diabetes 2015;6:598–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27:813–23. [DOI] [PubMed] [Google Scholar]
- [6].Felderhoff-Mueser U, Taylor DL, Greenwood K, et al. Fas/CD95/APO-1 can function as a death receptor for neuronal cells in vitro and in vivo and is upregulated following cerebral hypoxic-ischemic injury to the developing rat brain. Brain Pathol 2000;10:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wherry EJ. T cell exhaustion. Nat Immunol 2011;12:492–9. [DOI] [PubMed] [Google Scholar]
- [8].Okazaki T, Chikuma S, Iwai Y, et al. A rheostat for immune responses- the unique properties of PD-1 and their advantage for clinical application. Nat Immunol 2013;14:1212–8. [DOI] [PubMed] [Google Scholar]
- [9].Jia Y, Zhao Y, Li C, et al. The expression of programmed Death-1 on CD4+ and CD8+ T lymphocytes in patients with type 2 diabetes and severe sepsis. PLoS One 2016;11:01–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jin H-T, Ahmed R, Okazaki T. Role of PD-1 in regulating T-Cell immunity. Curr Top Microbiol Immunol 2011;358:17–37. [DOI] [PubMed] [Google Scholar]
- [11].Feuth T, Arends JE, Fransen JH, et al. Complementary role of HCV and HIV in T-cell activation and exhaustion in HIV/HCV coinfection. PLoS One 2013;8:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Saeidi A, Zandi K, Cheok YY, et al. T-cell exhaustion in chronic infections: Reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front Immunol 2018;9:01–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dong Y, Li X, Zhang L, et al. CD4+ T cell exhaustion revealed by high PD-1 and LAG-3 expression and the loss of helper T cell function in chronic hepatitis B. BMC Immunol 2019;20:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xia C, Rao X, Zhong J. Role of T lymphocytes in type 2 diabetes and diabetes-associated inflammation. J Diabetes Res 2017;2017:01–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nyambuya TM, Dludla PV, Mxinwa V, et al. Obesity-induced inflammation and insulin resistance: a mini-review on T-cells. Metab Open 2019;3:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mahlangu T, Dludla PV, Nyambuya TM, et al. A systematic review on the functional role of Th1/Th2 cytokines in type 2 diabetes and related metabolic complications. Cytokine 2020;126:01–9. [DOI] [PubMed] [Google Scholar]
- [17].Nyambuya TM, Dludla PV, Nkambule BB. T cell activation and cardiovascular risk in type 2 diabetes mellitus: a systematic review and meta-analysis. Clin Immunol 2019;210:01–12. [DOI] [PubMed] [Google Scholar]
- [18].Shi B, Du X, Wang Q, et al. Increased PD-1 on CD4+CD28- T cell and soluble PD-1 ligand-1 in patients with T2DM: association with atherosclerotic macrovascular diseases. Metabolism 2013;62:778–85. [DOI] [PubMed] [Google Scholar]
- [19].Sun P, Jin Q, Nie S, et al. Unlike PD-L1, PD-1 is downregulated on partial immune cells in type 2 diabetes. J Diabetes Res 2019;2019:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shamseer L, Moher D, Ghersi D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Br Med J 2015;7647:01–25. [DOI] [PubMed] [Google Scholar]
- [21].Newcastle-Ottawa. Quality assessment scale adapted for cross-sectional studies. Available at: https://wellcomeopenresearch.s3.amazonaws.com/supplementary/13880/ea30a2fb-a15a-44a9-b35e-5f0914db80b3.docx. Accessed May 9, 2020. [Google Scholar]
- [22].Balshem H, Helfand M, Sch HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- [23].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [24].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:01–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].2011;Higgins J, Green S. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Version 5. doi:10.1109/ISIT.2017.8006970. [Google Scholar]
- [26].Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [27].Schroll JB, Moustgaard R, Gøtzsche PC. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med Res Methodol 2011;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fujisawa R, Haseda F, Tsutsumi C, et al. Low programmed cell death-1 (PD-1) expression in peripheral CD4+ T cells in Japanese patients with autoimmune type 1 diabetes. Clin Exp Immunol 2015;180:452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nyambuya T, Davison GM, Hon G, et al. T-cell activation and dysfunction in hyperglycaemia. Med Technol South Africa 2018;32:24–7. [Google Scholar]
- [30].Sharpe AH, Wherry EJ, Ahmed R, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007;8:239–45. [DOI] [PubMed] [Google Scholar]
- [31].Xia F, Qian CR, Xun Z, et al. TCR and CD28 Concomitant Stimulation Elicits a Distinctive Calcium Response in Naive T Cells. Front Immunol 2018;9:2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shi L, Chen S, Yang L, et al. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol 2013;6:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Qiao Y, Shen J, He L, et al. Changes of regulatory T cells and of proinflammatory and immunosuppressive cytokines in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Diabetes Res 2016;2016:01–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mahmoud F, Al-Ozairi E. Inflammatory cytokines and the risk of cardiovascular complications in type 2 diabetes. Dis Markers 2013;35:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Qin W, Hu L, Zhang X, et al. The diverse function of PD-1/PD-L pathway beyond cancer. Front Immunol 2019;10:01–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 2019;18:01–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Han JM, Patterson SJ, Levings MK. The role of the PI3K signaling pathway in CD4+ T cell differentiation and function. Front Immunol 2012;3:01–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].O'Shea JJ, Plenge R. JAKs and STATs in immunoregulation and immune-mediated disease. Immunity 2013;36:542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Oh H, Ghosh S. NF-κB: roles and regulation in different CD4+ T cell subsets. Immunol Rev 2013;252:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wunderlich CM, Hövelmeyer N, Wunderlich FT. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAKSTAT 2013;2:e238781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal 2012;5:01–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chang C, Curtis JD, Maggi LB, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013;153:1239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cha J, Yang W, Cha J, et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of article metformin promotes antitumor immunity degradation of PD-L1. Mol Cell 2018;71:606–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Patsoukis N, Bardhan K, Chatterjee P, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 2015;6:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schöndorf T, Lübben G, Karagiannis E, et al. Increased prevalence of cardiovascular disease and risk biomarkers in patients with unknown type 2 diabetes visiting cardiology specialists: results from the DIASPORA study. Diabetes Vasc Dis Res 2010;7:145–50. [DOI] [PubMed] [Google Scholar]
- [46].Kanmani S, Kwon M, Shin MK, et al. Association of C-reactive protein with risk of developing type 2 diabetes mellitus, and role of obesity and hypertension: a large population-based Korean cohort study. Sci Rep 2019;9:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lee J, Ahn E, Kissick HT, et al. Reinvigorating exhausted T cells by blockade of the PD-1 pathway. For Immunopathol Dis Therap 2015;6:07–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kotwal A, Haddox C, Block M, et al. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care 2019;7:e000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Reiche ME, Den TM, Willemsen L, et al. Deficiency of T cell CD40L has minor induced beneficial effects on obesity-induced- metabolic dysfunction. BMJ Open Diabetes Res Care 2019;7:01–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


