Abstract
This study aims to establish an effective prognostic nomogram for small cell carcinoma of the esophagus (SCCE).
A total of 552 patients with SCCE from 1975 to 2016 were extracted from the surveillance, epidemiology, and end results (SEER) database. A Cox proportional hazard regression model was used to analyze the prognostic factors of patients, and a nomogram was constructed. The nomogram was then validated internally by using a consistency index (C-index) and a correction curve to evaluate its predictive value.
The Cox proportional hazard regression model showed that age, stage, surgery, primary site, radiotherapy, and chemotherapy were the prognostic factors of SCCE (P < .1), and they were used to construct the nomogram. The C-index of the nomogram for predicting survival was 0.749 (95% confidence interval [CI] = 0.722–0.776). The data were randomly divided into a modeling group and a validation group based on 7:3 for internal validation. The C-indices of the modeling and validation groups were 0.753 and 0.725, respectively, and they were close to 0.749. The calibration curves exhibited good consistency between the predicted and actual survival rates.
The nomogram of the survival and prognosis of patients with SCCE in this study had a good predictive value and could provide clinicians with accurate and practical predictive tools. It could also be used to facilitate a rapid and accurate assessment of patients’ survival and prognosis on an individual basis.
Keywords: database of the surveillance; epidemiology, and end results program; nomogram; small cell carcinoma of the esophagus; survival analysis
1. Introduction
According to the 2017 cancer statistics, 16,940 patients were diagnosed with esophageal cancer, and 15,690 patients died from this disease. Although the incidence of esophageal cancer is not included in the top 10 malignant tumors, the mortality of esophageal cancer in men ranks the 7th, indicating that esophageal cancer is one of the malignant tumors with poor prognosis.[1]
The main pathological types of esophageal cancer are squamous carcinoma and adenocarcinoma. The primary small cell carcinoma of the esophagus is an esophageal neuroendocrine tumor, which is a rare and highly malignant tumor affecting the esophagus. The most common extra pulmonary small cell carcinoma is the small cell carcinoma of the esophagus (SCCE), which accounts for about 53.0% to 71.0% of cases and has a high rate of early metastasis and poor prognosis.[2] The incidence of SCCE is observed in patients aged 60 to 70 years, and the incidence rate of men is 2 times higher than that of women.[3] SCCE has unique clinical, pathological, and prognostic characteristics. The biological behavior of SCCE is obviously different from that of other types of esophageal carcinoma. SCCE is highly malignant, so distant and lymphatic metastases occur in early stages. Most patients with SCCE die within 2 years after diagnosis, and their median survival time is only 8 to 13 months.
2. Material and methods
All analyses were based on SEER published database, thus no ethical approval and patient consent are required.
2.1. Database source
The data of patients diagnosed with primary esophageal cancer in 1973 to 2016 were extracted from the surveillance, epidemiology, and end results (SEER) database by using SEER∗Stat version 8.3.5 (https://seer.cancer.gov/seerstat/). The SEER database, established by the NCI in 1973, is one of the major sources of cancer data in the United States, and it has a large sample size, a wide population coverage, and a high data accuracy.
2.2. Patient selections
The clinicopathological characteristics of SCCE were obtained from the database of Incidence-SEER 18 Custom Data (with additional treatment fields), November 2017 Sub, 1973 to 2015 varying. SCCE was defined using the ICD for oncology version 3 (ICD-O-3). The primary site (Site recode: ICD-O-3/2008) was the esophagus, and 4 histopathological types, namely, small cell cancer (8041/2, 8041/3, 8042/3, 8043/3, 8044/3, 8045/3), were included. SEER historical stage A (classified as localized, regional, and distant) was used for tumor staging. Other variables included sex, age, race (white, black, and others [American Indian/AK Native and Asian/Pacific Islander]), and tumor site (upper third, middle third, lower third, or overlapping part of the esophagus). The patients were also categorized into the following age groups: 31 to 69, 70 to 79, and 80 to 97 years.
2.3. Statistical analysis
SPSS 21.0 was used for statistical analysis. Categorical variables were compared by using Chi-square or Fisher test. Survival curves were plotted with Kaplan–Meier (KM) method and compared via a log-rank test. Cox regression was conducted for multivariate analysis. Forest plots were drawn using the R software version 3.5.2. The optimal cutoff for age was determined with X-tile v3.6.1.
A nomogram graph was constructed using the rms package, and a consistency index (C-index) was calculated. Calibration curves were also drawn to evaluate the predicted value. Bootstrap method (self-sampling number B = 1000) was adopted for internal validation in the modeling and validation groups, respectively. In the ideal validation curve, the predicted value was equal to the actual observed value, and the curve was infinitely close to the ideal 45° oblique line. The C-index was similar to the area under the ROC curve and used to evaluate the predictive value of the nomogram, with a minimum value of 0.5 and a maximum value of 1.0. The R software (v3.5.2) was also used to make and check the nomogram, and rms and Hmisc[4,5] were the main software packages used. Data with P < .05 were considered statistically significant.
3. Results
3.1. Characteristics of patients
According to the data recorded in 1975 to 2016 in the SEER database, 90,864 patients were diagnosed with esophageal cancer. Of these patients, 552 were pathologically diagnosed with small cell carcinoma (Table 1). They were classified into 3 groups in terms of age by using the X-tile software (Fig. 1). The median age of the 552 patients upon diagnosis was 69 years.
Table 1.
Baseline characteristics of the small cell carcinoma of esophagus patients.
| Total | Age 31–69 | Age 70–79 | Age 80–97 | P | ||||
| Sex | ||||||||
| Female | 214 | 106 | 37.1% | 62 | 40.3% | 46 | 41.1% | .689 |
| Male | 338 | 180 | 62.9% | 92 | 59.7% | 66 | 58.9% | |
| Race | ||||||||
| Black | 85 | 44 | 15.4% | 27 | 17.5% | 14 | 12.5% | .561 |
| Other | 32 | 20 | 7.0% | 6 | 3.9% | 6 | 5.4% | |
| White | 435 | 222 | 77.6% | 121 | 78.6% | 92 | 82.1% | |
| Primary site | ||||||||
| Lower third | 237 | 132 | 54.3% | 59 | 43.7% | 46 | 47.4% | .585 |
| Middle third | 162 | 74 | 30.5% | 52 | 38.5% | 36 | 37.1% | |
| Overlapping | 28 | 14 | 5.8% | 8 | 5.9% | 6 | 6.2% | |
| Upper third | 48 | 23 | 9.5% | 16 | 11.9% | 9 | 9.3% | |
| Stage | ||||||||
| Distant | 270 | 135 | 60.3% | 82 | 59.9% | 53 | 60.2% | .872 |
| Localized | 88 | 41 | 18.3% | 27 | 19.7% | 20 | 22.7% | |
| Regional | 91 | 48 | 21.4% | 28 | 20.4% | 15 | 17.0% | |
| Radiotherapy | ||||||||
| No | 295 | 146 | 51.6% | 79 | 51.3% | 70 | 63.6% | .073 |
| Yes | 252 | 137 | 48.4% | 75 | 48.7% | 40 | 36.4% | |
| Chemotherapy | ||||||||
| No | 410 | 209 | 73.1% | 118 | 76.6% | 83 | 74.3% | .719 |
| Yes | 142 | 77 | 26.9% | 36 | 23.4% | 29 | 25.7% | |
| Surgery | ||||||||
| No | 492 | 250 | 89.3% | 141 | 93.4% | 101 | 94.4% | .167 |
| Yes | 46 | 30 | 10.7% | 10 | 6.6% | 6 | 5.6% | |
Figure 1.
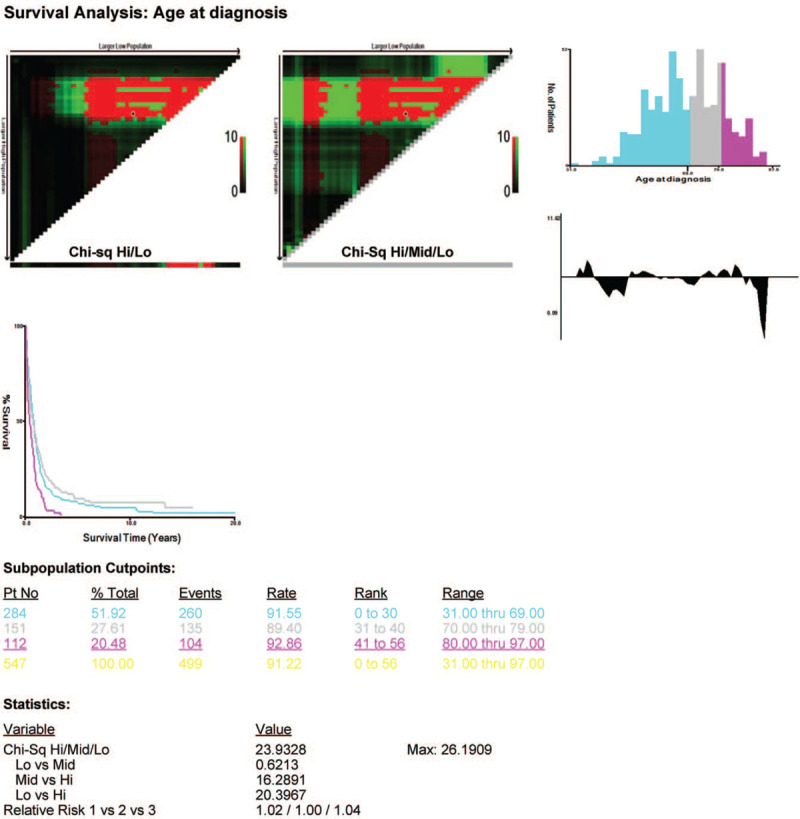
Estimation of the cut-off value for the age stratification as determined by the X-tile software.
No significant differences in sex (P = .689), primary site (P = .585), race (P = .585), surgery (P = .167), radiotherapy (P = .073), SEER historical stage (P = .872), and chemotherapy (P = .719) were observed in the 3 age groups. Therefore, these factors were comparable.
3.2. Survival
The median OS of the patients with SCCE was 7 (95% confidence interval [CI]: 6.025–7.975) months. They had 1-, 3-, and 5-year OS of 31.5%, 14.5%, and 6.4%, respectively. The KM curves of OS are shown in Fig. 2A. Of the 500 patients who died during follow-up, 378 died because of esophageal cancer.
Figure 2.
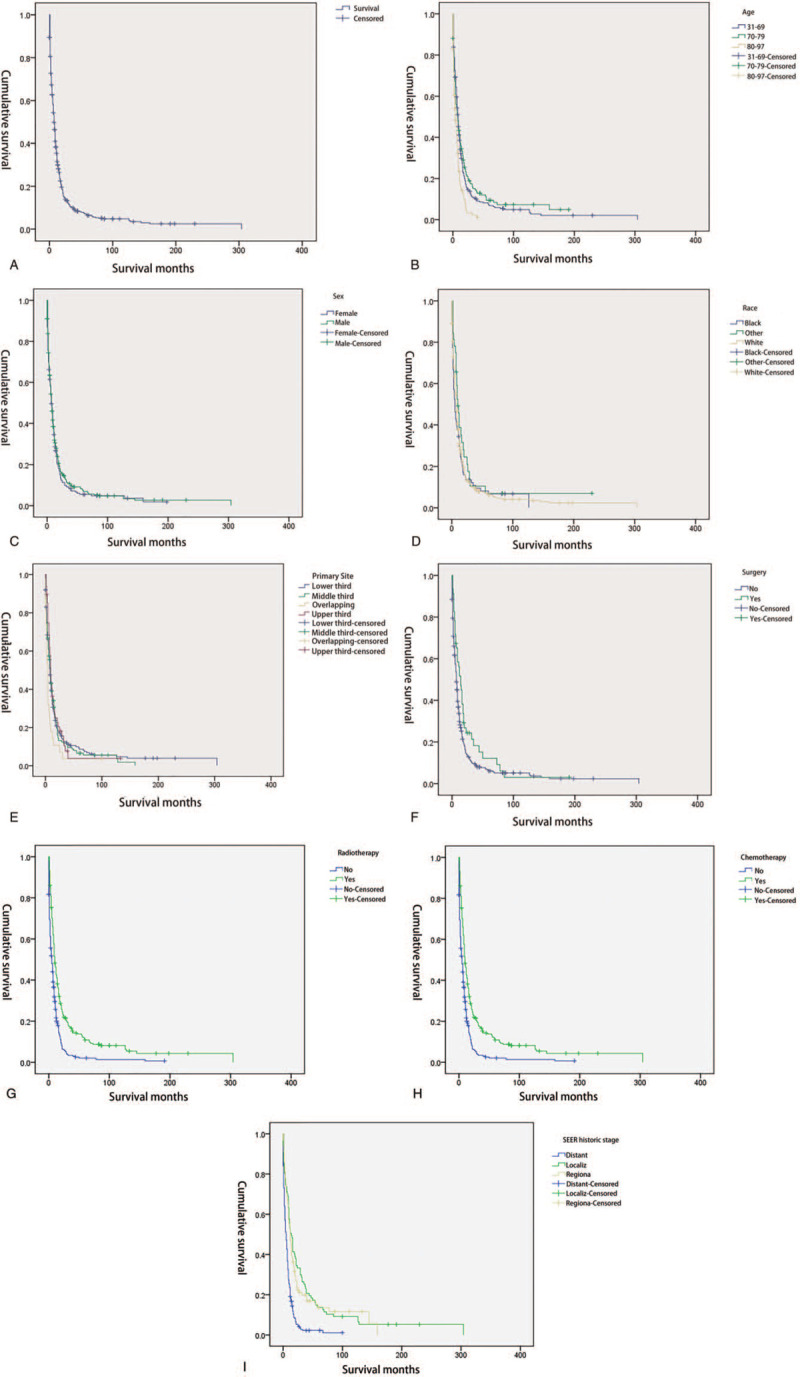
A. The overall survival curve of the elderly patients with esophageal squamous cell carcinoma in the whole group. B. Kaplan–Meier estimate of overall survival of patients by age group. C. Kaplan–Meier estimate of overall survival of patients by sex. D. Kaplan–Meier estimate of overall survival of patients by race. E. Kaplan–Meier estimate of overall survival of patients by primary site. F. Kaplan–Meier estimate of overall survival of patients by surgery. G. Kaplan–Meier estimate of overall survival of patients by radiotherapy. H. Kaplan–Meier estimate of overall survival of patients by chemotherapy. I. Kaplan–Meier estimate of overall survival of patients by SEER historic stage. SEER = surveillance, epidemiology, and end results.
3.3. Univariate survival analysis
A total of 552 patients with esophageal small cell carcinoma were subjected to univariate analysis via the KM method and compared with a log-rank test. The results showed that age, primary site, stage, surgery, radiotherapy, and chemotherapy were related to survival and prognosis, but sex and race were not associated with survival and prognosis (Table 2 and Fig. 2B–I).
Table 2.
Variables associated with overall survival in univariate analyses for the entire study population.
| Independent variables | Median OS | P value |
| Age, yrs | <.001 | |
| 31–69 | 9.00 (7.74–10.26) | |
| 70–79 | 8.00 (5.34–10.66) | |
| 80–97 | 4.00 (2.09–5.91) | |
| Sex | .409 | |
| Female | 7.00 (5.56–8.44) | |
| Male | 7.00 (5.68–8.32) | |
| Race | .307 | |
| Black | 5.00 (2.76–7.24) | |
| Other | 10.00 (6.56–13.44) | |
| White | 8.00 (7.01–8.99) | |
| SEER historic stage | <.001 | |
| Distant | 5.00 (3.74–6.26) | |
| Localize | 14.00 (10.08–17.92) | |
| Regional | 12.00 (9.08–14.92) | |
| Primary site | .046 | |
| Lower t | 8.00 (6.57–9.43) | |
| Middle | 9.00 (7.68–10.32) | |
| Overlap | 4.00 (2.62–5.38) | |
| Upper t | 9.00 (7.09–10.91) | |
| Surgery | .022 | |
| No | 7.00 (6.00–8.00) | |
| Yes | 14.00 (8.81–19.19) | |
| Radiotherapy | <.001 | |
| No | 5.00 (3.51–6.49) | |
| Yes | 10.00 (8.34–11.66) | |
| Chemotherapy | <.001 | |
| No | 2.00 (1.45–2.55) | |
| Yes | 11.00 (9.86–12.14) |
3.4. Multivariate survival analysis
All the covariates with P < .1 in univariate survival analysis were also included in multivariate survival analysis. The results of Cox regression multivariate analysis are shown in Fig. 3. Multivariate survival analysis showed that age (P = .033), radiotherapy (P = .002), surgery (P = .008), stage (P < .001), and chemotherapy (P < .001) could be used to predict survival independently. The nomogram comprised all statistically significant prognostic factors, including age, surgery, radiotherapy, chemotherapy, and stage, in the multivariate risk ratio regression model. The prediction results of the nomograms of 1-, 3-, and 5-year survival rates are shown in Fig. 4. According to the different classifications of each feature, the score of each item could be obtained by projecting it upward to a small scale. The higher the score was, the worse the survival prognosis would be. The total score could be obtained by adding all the scores, and the survival rate of the patients could be obtained by projecting it downward from the total scale. This nomogram could be utilized to make an individual prediction of the survival rate based on various conditions of different patients and improve prediction efficiency and accuracy. The C-index of the nomogram was 0.749 (95% CI = 0.722–0.776), indicating its good predictive ability. The nomogram accuracy calibration chart (Fig. 5) was in good agreement with the actual and predicted 1-, 3-, and 5-year OS, and the slope was close to 45° (Table 3).
Figure 3.
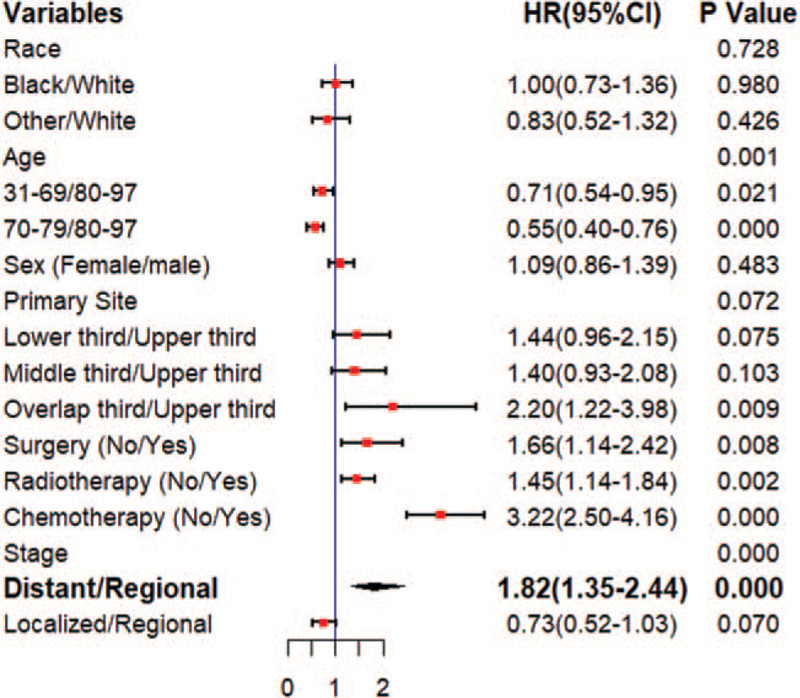
Forest plot of multivariate survival analysis.
Figure 4.
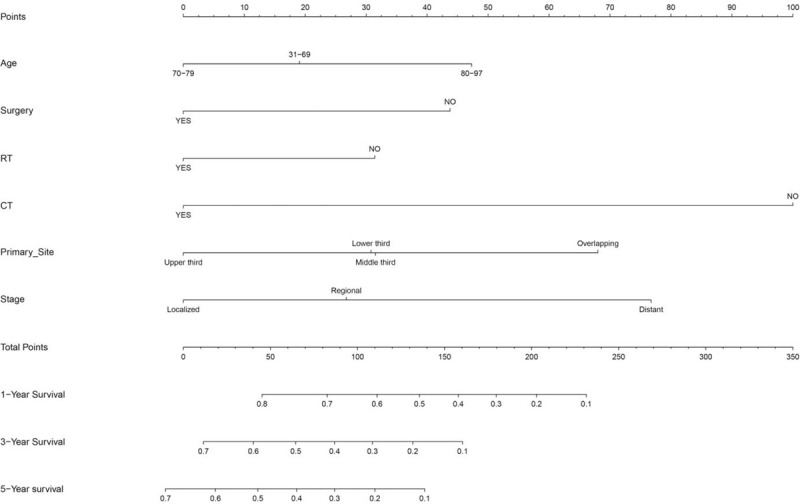
Nomogram for predicting the 1-, 3-, and 5-year.
Figure 5.
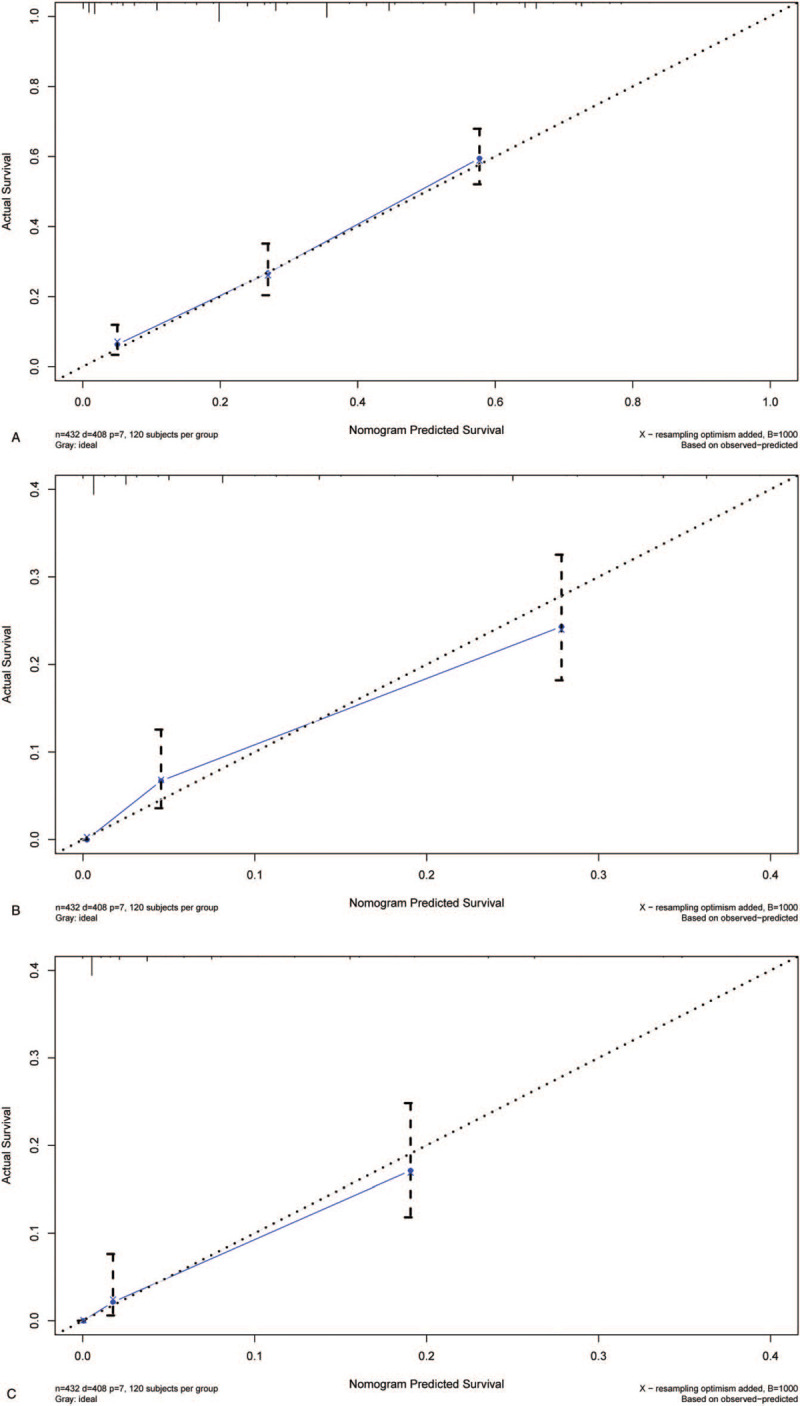
A. Calibration plots of the nomogram prediction of 1-year. B. Calibration plots of the nomogram prediction of 3-year. C. Calibration plots of the nomogram prediction of 5-year.
Table 3.
C-indices of nomogram and a single independent factor for OS prediction.
| SE | C-indices | 95% CI | |
| Stage | 0.014 | 0.609 | (0.582, 0.636) |
| Surgery | 0.007 | 0.523 | (0.509, 0.537) |
| Radiotherapy | 0.012 | 0.597 | (0.573, 0.621) |
| Chemotherapy | 0.011 | 0.650 | (0.628, 0.671) |
| Nomogram | 0.014 | 0.749 | (0.722, 0.776) |
3.5. Comparison of predictive accuracy between the nomogram and a single independent factor
In the Cox regression analysis of factors, the risk ratios of stage, surgery, radiotherapy, and chemotherapy were higher than those of other factors. The risk ratios of these factors were compared with those presented in the nomogram. The C-indices of stage, surgery, radiotherapy, and chemotherapy were 0.609, 0.523, 0.597, and 0.65, respectively, and they were significantly lower than that of the nomogram.
3.6. Nomogram validation
Data were segmented using the caret package in the R software and randomly divided into the modeling and validation groups at 7:3 for internal validation. The C-indices of the modeling and validation groups were 0.753 and 0.725, respectively, and they were close to 0.749. The calibration curve of the modeling group was accurately consistent between the predicted and observed 1-, 3-, and 5-year survival rates (Fig. 6A–C). All the C-indices were significantly lower than those of the nomogram (P < .001).
Figure 6.
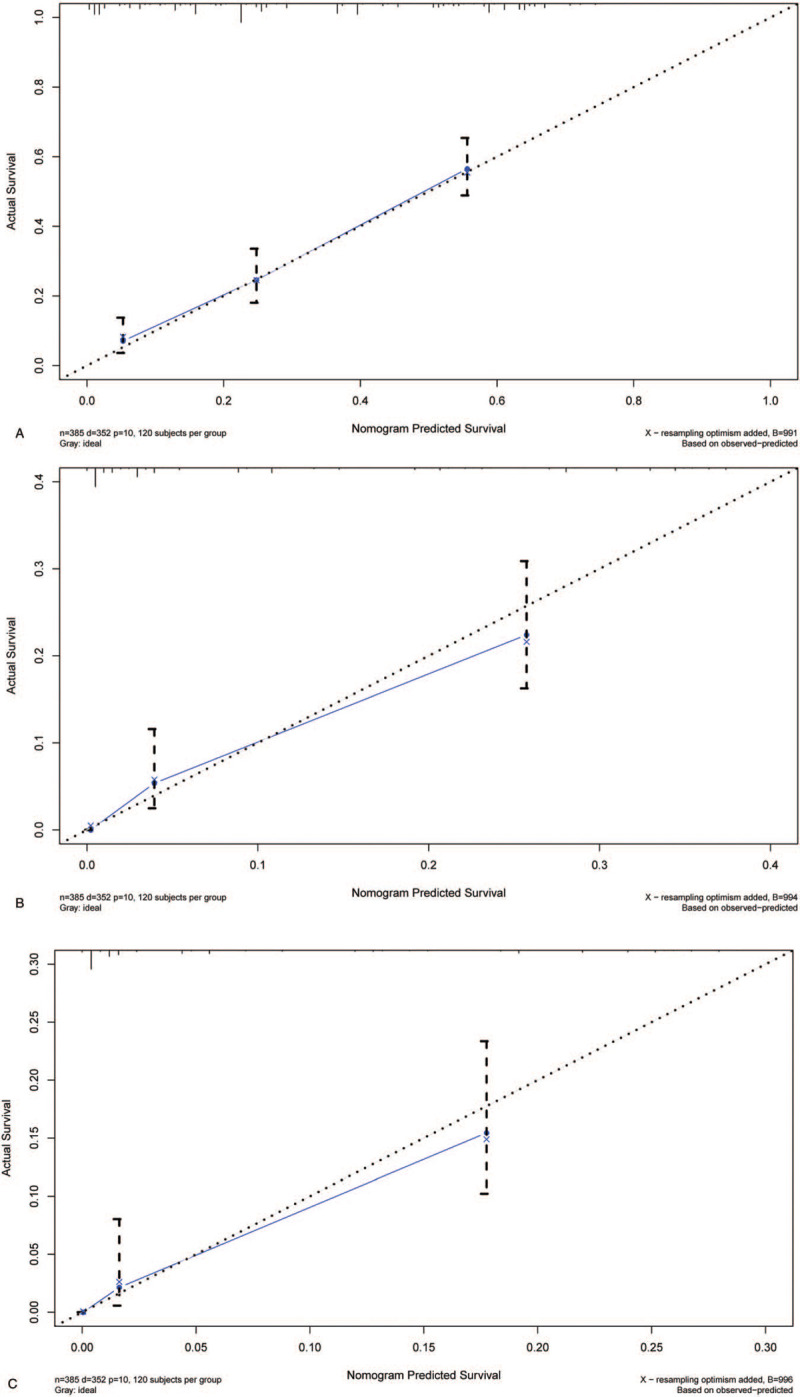
A. The calibration curves for predictions of overall survival in the validation cohort at 1-year. B. The calibration curves for predictions of overall survival in the validation cohort at 3-year. C. The calibration curves for predictions of overall survival in the validation cohort at 5-year.
4. Discussion
No consensus has been reached on the standard of SCCE treatment, which may be related to the small number of cases and the lack of large-scale clinical control studies. No standard treatment plan has also been established because of insufficient large sample randomized controlled studies and case reports. This study aimed to characterize the clinicopathologic features of SCCE. Our results revealed that age, stage, surgery, radiotherapy, and chemotherapy were independent prognostic factors of SCCE, and a nomogram was derived to accurately predict the prognosis of SCCE.
In this study, the number of cases of SCCE was 552, which accounted for 0.6% of the total number of cases of esophageal cancer, and this finding was consistent with previous results.[6,7] According to a multicenter study published by Vos et al,[8] in 2011, the median age is 59 to 69 years, and the median age in the SEER database is 69 years; this result was also consistent with a previous study. Among the 552 patients, 338 were men, accounting for 61.2%, but the distribution difference between men and women was not statistically significant. No difference was observed in the race or location distribution of the primary site, suggesting that no sex or race predisposition to SCCE is found.[9]
Some studies have shown that SCCE and small cell carcinoma of the lungs have similar biological behaviors with a high malignant degree and rapid progress. Chemotherapy is the main treatment method, but its efficacy is limited, especially for patients at the extensive stage of poor prognosis. The factors affecting prognosis remain controversial.[10,11] Surgery can significantly improve the survival rate of patients, and the efficacy of surgery plus chemotherapy for locally limited SCCE is significantly better than that of chemotherapy alone. Therefore, most studies have suggested a combination of surgery and chemoradiotherapy. The median OS of SCCE in our study was 7 months, which was shorter than the median survival reported by Bennouna et al[12] and Medgyesy et al[13]. This difference might be attributed to the large proportion of patients with distant metastasis in this study and the low proportion of surgery and radiotherapy.
Good prognostic evaluation is important for treatment, but no relatively complete scoring system for SCCE is currently available. However, nomograms can be used to integrate the effects of various prognostic risk factors in clinical practice and visually present results. Predictions can be made more quickly, conveniently, and accurately by using nomograms than by utilizing traditional methods, and the prediction value of the former is also better than that of other evaluation systems. Some nomograms based on the SEER database have been widely used in studies on lymphoma, breast cancer, and colorectal cancer.[14–16] In this database, demographic, tumor, and survival data are collected from 17 regional populations distributed throughout the United States, covering approximately 28% of the United States population; its data accuracy is up to 95%, providing good data support for the establishment of line charts that may be applied to general single-center studies.
A variety of factors affecting the prognosis of SCCE were included in this study. A study has shown that the OS of young patients with SCCE is significantly better than that of elderly patients, with the median OS of 16 and 12.5 months and 5-year survival rates of 11.8% and 6.1%, respectively (P < .001). Sex slightly affected the survival and prognosis of patients with SCCE, and this finding was consistent with the results of a previous study.[17] However, studies on whether different race groups affect the survival of SCCE are limited. In the present study, univariate and multivariate analyses found no significant differences in survival among black, white, and other races, and this observation was consistent with previous findings.[18,19] In the previous prognostic analyses of SCCE based on the SEER database, no studies have shown that the primary site has a different prognosis. In the present study, KM survival analysis was carried out on the primary site, and the patients with an overlapping location had the shortest survival period (4 months, P = .046), with statistically significant differences. Stage is classified as distant, localized, and regional in the SEER database. In the present study, univariate analysis showed that different stages could affect prognosis. The median OS of patients with distant SCCE was 5 months, which was significantly shorter than that of regional and localized SCCE (P < .001). This finding was consistent with the conclusions of previously published articles.[20,21] Radical surgery is the only way to cure limited esophageal cancer, but for SCCE. Previous studies also revealed that chemotherapy should be the primary treatment.[22] As such, the optimal and standard treatment of SCCE has not been established,[22] even though surgery, radiotherapy, and chemotherapy are the main treatment methods for SCCE. In our study, these methods are also independent prognostic factors. Patients who underwent surgery, radiotherapy, and chemotherapy had better prognosis regardless of their univariate or multivariate characteristics. The OS of patients with SCCE and treated with the combination of surgery, radiotherapy, and chemotherapy was 25 months, and their 5-year survival rate was 38.9%, which was significantly better than that of other treatment modes.[23] Another study has reported that the OS of patients who undergo surgery combined with chemotherapy is 60.7 months, which is longer than that of patients who have surgery alone (23.2 months). Their difference is statistically significant.[24]
In this study, the above independent prognostic factors, including staging, surgery, radiotherapy, and chemotherapy, were considered, and the different classification conditions of each indicator were quantified to build a relatively systematic and complete evaluation system. The nomogram constructed in this study had C-indices of 0.749 and 0.753 in the modeling and validation cohorts, respectively. They also had a high predictive value, and the calibration curve showed good consistency.
This study was conducted at an appropriate time. To our knowledge, no relevant studies have provided the latest information in the field of SCCE nomograms. However, this study has some limitations. Radiotherapy and chemotherapy, as one of the main methods for SCCE treatment, have an important impact on prognosis, but specific information on radiotherapy and chemotherapy is not provided in the SEER database. In addition, the SEER database lacks surgical records and complete tumor recurrence records, so these factors were not evaluated. The SEER database, as the major clinical tumor database in the United States, is ethnically diverse, but patients are mainly white and black, and few clinical data about Asian populations are recorded. Therefore, the application of this study to Asian populations is limited. Nevertheless, this study included the most basic clinical features that affected the survival and prognosis of SCCE, and our nomogram could provide clinicians with more accurate and practical prediction tools. It could be used to quickly and accurately evaluate the survival prognosis of patients with SCCE after surgery, chemotherapy, and radiotherapy.
5. Conclusions
In conclusion, stage, surgery, radiotherapy, and chemotherapy are independent prognostic factors of SCCE. Based on these factors, the established nomogram can be utilized to predict the 1-, 3-, and 5-year OS of patients accurately. Further studies are required to confirm whether it is applicable to other races.
Acknowledgments
Thanks to Mr Xu Xiaolong for his contribution to the statistical, picture, and table processing of this study.
Author contributions
Methodology: Shuai Qie, Xuefeng Wang, Guimin Cui.
Software: Shuai Qie, Xuefeng Wang, Yuge Ran.
Supervision: Miaoling Liu, Hongyun Shi.
Validation: Shuai Qie.
Writing – original draft: Shuai Qie.
Footnotes
Abbreviations: KM = Kaplan–Meier, OS = overall survival, SCCE = small cell carcinoma of the esophagus, SEER = surveillance, epidemiology, and end results.
How to cite this article: Qie S, Wang Xf, Ran Yg, Liu Ml, Cui Gm, Shi Hy. Nomogram for predicting the survival of patients with small cell carcinoma of the esophagus: a population study based on the SEER database. Medicine. 2021;100:15(e25427).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
OS = overall survival, SEER = surveillance, epidemiology, and end results.
CI = confidence interval; OS = overall survival.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Lee SS, Ha HK, Kim AY, et al. Primary extrapulmonary small cell carcinoma involving the stomach or duodenum or both: findings on CT and barium studies. AJR Am J Roentgenol 2003;180:1325–9. [DOI] [PubMed] [Google Scholar]
- [3].Yekeler E, Koca T, Vural S. A rare cause of the cough: primary small cell carcinoma of esophagus-case report. Case Rep Med 2012;2012:870783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang S, Ma K, Chen Z, et al. A Nomogram to predict prognosis in malignant pleural mesothelioma. World J Surg 2018;42:2134–42. [DOI] [PubMed] [Google Scholar]
- [5].Sun F, Ma K, Yang X, et al. A nomogram to predict prognosis after surgery in early stage non-small cell lung cancer in elderly patients. Int J Surg 2017;42:11–6. [DOI] [PubMed] [Google Scholar]
- [6].Kikuchi H, Takeuchi H. Surgery for limited-stage primary small cell carcinoma of the esophagus: is it feasible and for whom is it indicated? J Thorac Dis 2018;10: suppl: S1037–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saddoughi SA, Taswell J, Harmsen WS, et al. Surgical resection of rare esophageal cancers. Ann Thorac Surg 2016;101:311–5. [DOI] [PubMed] [Google Scholar]
- [8].Vos B, Rozema T, Miller RC, et al. Small cell carcinoma of the esophagus: a multicentre Rare Cancer Network study. Dis Esophagus 2011;24:258–64. [DOI] [PubMed] [Google Scholar]
- [9].Morita M, Saeki H, Nakaji YU, et al. Conversion to neuroendocrine carcinoma from squamous cell carcinoma of the esophagus after definitive chemoradiotherapy. Anticancer Res 2016;36:4045–9. [PubMed] [Google Scholar]
- [10].Yanagawa H, Shimada K, Tanaka H, et al. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Anticancer Res 1998;18:2877–80. [PubMed] [Google Scholar]
- [11].Klaase JM, Hulscher JB, Offerhaus GJ, et al. Small cell carcinoma of the esophagus: the Tata Memorial Hospital experience. Ann Surg Oncol 2003;10:261–7. [DOI] [PubMed] [Google Scholar]
- [12].Bennouna J, Bardet E, Deguiral P, et al. Small cell carcinoma of the esophagus: analysis of 10 cases and review of the published data. Am J Clin Oncol 2000;23:455–9. [DOI] [PubMed] [Google Scholar]
- [13].Medgyesy CD, Wolff RA, Putnam JB, Jr, et al. Small cell carcinoma of the esophagus: the University of Texas M. D. Anderson Cancer Center experience and literature review. Cancer 2000;88:262–7. [PubMed] [Google Scholar]
- [14].Zhang Y, Zhang J, Zeng H, et al. Nomograms for predicting the overall and cancer-specific survival of patients with classical Hodgkin lymphoma: a SEER-based study. Oncotarget 2017;8:92978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu J, Su M, Hong S, et al. Nomogram predicts survival benefit from preoperative radiotherapy for non-metastatic breast cancer: a SEER-based study. Oncotarget 2017;8:49861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang ZY, Luo QF, Yin XW, et al. Nomograms to predict survival after colorectal cancer resection without preoperative therapy. BMC Cancer 2016;16:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiao Q, Xiao H, Ouyang S, et al. Primary small cell carcinoma of the esophagus: comparison between a Chinese cohort and Surveillance, Epidemiology, and End Results (SEER) data. Cancer Med 2019;8:1074–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Song Y, Wang W, Tao G, et al. Survival benefit of radiotherapy to patients with small cell esophagus carcinoma: an analysis of Surveillance Epidemiology and End Results (SEER) data. Oncotarget 2016;7:15474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kukar M, Groman A, Malhotra U, et al. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol 2013;20:4239–44. [DOI] [PubMed] [Google Scholar]
- [20].Ishida H, Kasajima A, Onodera Y, et al. A comparative analysis of clinicopathological factors between esophageal small cell and basaloid squamous cell carcinoma. Medicine (Baltimore) 2019;98:e14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Deng HY, Ni PZ, Wang YC, et al. Neuroendocrine carcinoma of the esophagus: clinical characteristics and prognostic evaluation of 49 cases with surgical resection. J Thorac Dis 2016;8:1250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yamashita H, Nakagawa K, Asari T, et al. Concurrent chemoradiation alone with curative intent for limited-disease small-cell esophageal cancer in nine Japanese patients. Dis Esophagus 2009;22:113–8. [DOI] [PubMed] [Google Scholar]
- [23].Hou X, Wei JC, Wu JX, et al. Multidisciplinary modalities achieve encouraging long-term survival in resectable limited-disease esophageal small cell carcinoma. PLoS One 2013;8:e69259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li J, Li D, Xu M. Combined chemotherapy and surgery in primary small cell carcinoma of the esophagus. Minerva Chir 2015;70:69–75. [PubMed] [Google Scholar]


