Abstract
Background:
Thymic carcinoma represents a rare type of malignant mediastinal tumor and has been the subject of controversy. Although independent prognostic factors related to thymic carcinoma have been investigated previously, few studies have focused specifically on the survival outcomes associated with thymic squamous cell carcinoma (TSCC). This study aims at presenting a survival analysis in this rare malignant disease at population level.
Methods:
We extracted the data of 216 patients with TSCC recorded from 1973 to 2015 from the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute. The patients’ demographic features, clinical traits, and treatment factors were analyzed in order to identify prognostic factors, which correlate overall survival using the Kaplan–Meier method as well as a multivariate Cox regression model, for TSCC.
Results:
The majority of patients were male, Caucasian, married, and insured. Furthermore, 58.3%, 54.6%, and 59.7% of patients TSCC underwent surgery, radiotherapy, and chemotherapy respectively. In a multivariate analysis, age of diagnosis (hazard ratio [HR]: 1.022, 95% confidence interval [CI]: 1.003–1.040, P = .020), surgical treatment (HR: 0.282, 95% CI: 0.164–0.484, P = .000), and stage (regional vs distant HR: 0.532, 95% CI: 0.324–0.872, P = .013; localized vs distant HR: 0.297, 95% CI: 0.133–0.664, P = .003) correlated with increased overall survival, whereas adjuvant therapy, including chemotherapy and radiotherapy, did not correlate with survival. Among surgically treated patients, age of diagnosis and stage were associated with better overall survival, while chemotherapy and radiotherapy did not contribute significantly to overall survival.
Conclusion:
Surgery, age of diagnosis, and stage were associated with better overall survival among TSCC.
Keywords: population; prognosis; Surveillance, Epidemiology, and End Results database; survival; thymic squamous cell carcinoma
1. Introduction
Thymic carcinoma is a rare and highly malignant type of solid tumor that originates from the thymic epithelium and shows malignant cytological features with aggressive tumor invasiveness and high potential for metastasis. The mortality rate of thymic cancer is still in the top 10 among the chest tumors. Thymic squamous cell carcinoma (TSCC) is the main pathological type of thymic cancer, accounting for approximately 80% of all thymic carcinomas.[1,2] The consensus has not been reached due to the limited number of patients.
Although the latest National Comprehensive Cancer Network (NCCN) has general guidelines for thymic carcinom, but it is not specific for TSCC. However, in a single-center study by Zhao et al[4] enrolled 105 consecutive patients with TSCC who underwent surgery. In the comparison of the patients’ clinical and pathological data, only surgical completeness was found to have a strong effect on the overall survival and to extend disease-free survival[4] along with chemotherapy. Yet still, we need more research to have a consensus specifically on TSCC.
The Surveillance, Epidemiology, and End Results Program (SEER) database collects carcinoma incidence data from population-based cancer registries covering approximately 34.6% of the United State population. In this study, we used data from the SEER database mentioned above to identify the prognostic factors for overall survival among patients with TSCC.
2. Materials and methods
The SEER database is sponsored by the National Cancer Institute and has been used to track cancer incidence and survival since 1973. This database covers more than a third of the US population, that is, approximately 86.4 million people (2010 census from SEER official website).[5] We extracted information concerning cases of TSCC recorded during 1973 to 2015 from the SEER database, using SEER∗Stat Software, version 1 (National Cancer Institute, Bethesda, MD). The extracted information included patients’ time of diagnosis, demographics, stage of diagnosis, treatment, and follow-up to determine vital status. Cases of TSCC were identified using codes from the International Classification of Diseases for Oncology, Third Edition (ICD-O-3). First, the tumor primary site was identified as the Mediastinum (C339, C381–C383, C388, C390, C398, C399) or other endocrine, including thymic (C379, C740–C749, C750–C759). The histology was identified as squamous cell carcinoma (8070/3, 8070/6, 8071/3, 8072/3, 8073/3, 8074/3, 8075/3, 8076/2, 8076/3, 8078/3). The selected cases of thymic carcinoma were then restricted to only TSCC. Patients without a documented vital status at the end of follow-up (2015/12) were excluded.
The acquired data were input and organized into spreadsheets using Excel 2016 (Microsoft Corp., Redmond, WA). The information obtained included the time of diagnosis, sex, age of diagnosis, ethnicity, marital status, surgery data, chemotherapy data, radiotherapy data, SEER stage, cause of death, insurance, and survival duration. Given the small sample size, we established groups, each containing few samples for every factor. Patients were stratified by race/ethnicity as Caucasian, Asian or Pacific Islander, and others (unknown and African). The proportions of sexes and races/ethnicities were calculated and compared with the 2000 US standard population in the SEER database. Marital status was categorized into married or unmarried (single, divorced, widowed, or separated during the time of diagnosis). SEER stage was classified as localized disease (confined in the original organ without extension beyond the primary organ), regional disease (direct extension to adjacent organs or structures or by spreading to regional lymph nodes), or distant disease (spreading to parts of the body remote from the primary tumor). The primary end points of the study were overall survival (OS) and cancer-specific survival (CSS). Overall survival was calculated from the time of diagnosis to the time of death of any cause, while cancer-specific survival was calculated from the time of diagnosis to the time of death only of TSCC, and both durations were estimated by the Kaplan–Meier method. A Cox proportional hazard model of survival-associated risk factors was applied to conducting univariable and multivariable analyses and generating hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). Two-sided P-values <.05 were taken in to significant consideration statistically, and the survival curves were estimated also by the Kaplan–Meier method. A nomogram based on possible prognostic factors associated with OS was established using R software, on the basis of the Cox regression model, and its performance was measured by concordance index (c-index) and assessed by calibration curves. The concordance index (c-index), which can reflect the probability of the concordance between observed and predicted outcome, was used to evaluate the discrimination of nomograms.[6] The calibration curve was used to compare the predicted and observed probabilities of survival in the cohort study. Nomograms and calibration curves were developed along with bootstraps of 200 resamples to reduce the overfit bias. All statistical analyses will employ SPSS Statistics 20.0 (IBM, Inc., Armonk, NY).
Hubei Cancer Hospital Ethics Committee has approved the study. The patients in SEER database were informed of information collection and were consent to participate in the medical research.
3. Results
3.1. Demographics, clinical traits, and treatments
In our analysis of the SEER database, we identified 216 patients who were diagnosed with TSCC from 1973 to 2015. The patients had a median age of 54 years, and a bit more men were observed than women (54.6% vs 50.9% in the general population) (US Census, year 2010).[5] Furthermore, the cohort exhibited a significant predilection for Asian and Caucasian patients, who comprised 23.0% (compared with 8.5% in the US Census for 2000) and 83.6% (compared with 65.3% in the US Census for 2000) respectively. Regarding tumor characteristics, 46.3% of cases were classified as regional stage, while 31.6% were distant stage. Further details are listed in Table 1. According to the SEER database, the most common treatment option was surgery, which was chosen in 58.3% of cases. In addition to surgery, 54.6% and 59.7% of the cases received radiotherapy and chemotherapy respectively.
Table 1.
Characteristics of thymic squamous cell cancer in SEER database.
| Number of patients | Percent of total number | |
| Total number | 216 | 100.0% |
| Mean age of diagnosis = 62 years | ||
| Time of diagnosis | ||
| 1997–2000 | 21 | 9.7% |
| 2000–2010 | 74 | 34.2% |
| 2010–2015 | 121 | 56.1% |
| Gender | ||
| Female | 98 | 45.4% |
| Male | 118 | 54.6% |
| Race | ||
| White | 141 | 65.3% |
| Asian or Pacific Islander | 51 | 23.6% |
| Others | 24 | 11.1% |
| Marital status | ||
| Married | 135 | 62.5% |
| Single | 70 | 32.4% |
| Unknown | 11 | 5.1% |
| SEER stage | ||
| Localized | 42 | 19.4% |
| Regional | 100 | 46.3% |
| Distant | 69 | 31.9% |
| Unknown | 5 | 2.4% |
| Surgery | ||
| Yes | 126 | 58.3% |
| None/Unknown | 90 | 41.7% |
| Radiotherapy | ||
| Yes | 118 | 54.6% |
| None/Unknown | 98 | 45.4% |
| Chemotherapy | ||
| Yes | 129 | 59.7% |
| None/Unknown | 87 | 40.3% |
| Insurance | ||
| Insured | 143 | 66.2% |
| Uninsured/unknown | 73 | 33.8% |
3.2. Survival
We used the Kaplan–Meier method and a stratified Cox proportional hazards model to evaluate the distributions of overall survival according to different variables. In the univariable analysis, we observed a significant difference in overall survival of non-surgery and surgery, with respective durations of 25 and 93 months. In the multivariable analysis, surgery was identified as a significant and independent prognostic factor for overall survival (HR: 0.282, 95% CI: 0.164–0.484, P = .000). In an age-stratified analysis, the risk of death increased by 2.2% every year and a multivariable Cox regression identified the age of diagnosis as an independent prognostic factor for survival (hazard ratio [HR]: 1.022, 95% CI: 1.003–1.040, P = .020). Moreover, patients with local-stage disease had a longer overall survival compared with those with regional- and distant-stage disease, and SEER stage was also found to have significant correlation with overall survival in a multivariable analysis (regional vs distant HR: 0.532, 95% CI: 0.324–0.872, P = .013; localized vs distant HR: 0.297, 95% CI: 0.133–0.664, P = .003). Kaplan–Meier analyses showed that surgical treatment and localized stage had better outcomes for OS (Fig. 1A and B). Furthermore, chemotherapy and radiotherapy did not show the ability to yield any significant benefits in terms of overall survival. Further details are listed in Table 2.
Figure 1.
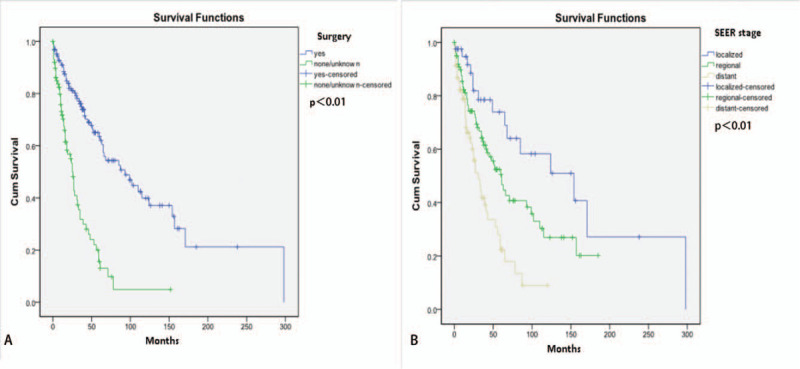
Overall survival curves of TSCC according to: patients in different stages (A); patients with and without surgery (B); all P < .05. TSCC = thymic squamous cell carcinoma.
Table 2.
Univariable and multivariable analysis of variables of overall survival in TSCC.
| Univariable analysis | Multivariable analysis | |||
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| Time of diagnosis | 1.004 (0.976–1.032) | .800 | 0.978 (0.935–1.023) | .331 |
| Age of diagnosis | 1.007 (0.992–1.023) | .346 | 1.022 (1.003–1.040) | .020 |
| Gender | ||||
| Male | ref | ref | ||
| Female | 1.372 (0.943–1.997) | .098 | 1.488 (0.943–2.350) | .088 |
| Race | ||||
| Asian or Pacific Islander | ref | ref | ||
| White | 0.903 (0.712–1.145) | .400 | 1.028 (0.615–1.718) | .915 |
| Marital status | ||||
| Unmarried | ref | ref | ||
| Married | 0.731 (0.492–1.085) | .120 | 1.022 (0.645–1.644) | .929 |
| SEER stage | ||||
| Distant | ref | ref | ||
| Regional | 0.511 (0.337–0.777) | .002 | 0.532 (0.324–0.872) | .013 |
| Localized | 0.266 (0.142–0.499) | .000 | 0.297 (0.133–0.664) | .003 |
| Surgery | ||||
| None/Unknown | ref | ref | ||
| Yes | 3.590 (2.411–5.345) | .000 | 0.282 (0.164–0.484) | .000 |
| Radiotherapy | ||||
| None/Unknown | ref | ref | ||
| Yes | 1.344 (0.927–1.949) | .116 | 1.188 (0.743–1.900) | .472 |
| Chemotherapy | ||||
| None/Unknown | ref | ref | ||
| Yes | 0.594 (0.400–0.882) | .010 | 1.166 (0.682–1.993) | .575 |
| Insurance | ||||
| Uninsured/unknown | ref | ref | ||
| Insured | 1.083 (0.726–1.616) | .696 | 0.812 (0.432–1.526) | .517 |
| Quamous cell cancer | ||||
3.3. The patients with TSCC who underwent surgical therapy
Surgical therapy was performed in 126 patients, among whom with localized stage and regional stage or of younger age were more likely to have better survival. However, the radiotherapy and chemotherapy may not contribute to longer survival for the patients with surgery (Table 3).
Table 3.
Multivariable analysis of variables of overall survival in thymic squamous cell cancer based on the surgery.
| Mean overall survival, mo | HR (95% CI) | P | |
| Age of diagnosis | – | 1.031 (1.001–1.062) | .044 |
| Time of diagnosis | |||
| 2010–2015 | 54 | ref | – |
| 2000–2010 | 103 | 2.346 (0681–8.074) | .176 |
| 1997–2000 | 102 | 5.807 (1.160–29.079) | .032 |
| Gender | |||
| Male | 115 | ref | – |
| Female | 120 | 0.515 (0.255–1.041) | .065 |
| Race | |||
| Asian or Pacific Islander | 146 | ref | – |
| White | 104 | 1.448 (0.676–3.100) | .340 |
| Marital status | |||
| Unmarried | 98 | ref | – |
| Married | 121 | 0.472 (0.205–1.084) | .077 |
| SEER stage | |||
| Distant | 54 | ref | – |
| Regional | 98 | 0.414 (0.192–0.892) | .024 |
| Localized | 159 | 0.169 (0.058–0.493) | .001 |
| Radiotherapy | |||
| None/Unknown | 116 | ref | – |
| Yes | 119 | 1.054 (0.515–2.158) | .885 |
| Chemotherapy | |||
| None/Unknown | 131 | ref | – |
| Yes | 89 | 1.743 (0.886–3.429) | .107 |
| Insurance | |||
| Uninsured/unknown | 119 | ref | – |
| Insured | 70 | 1.752 (0.568–5.406) | .329 |
3.4. Prognostic nomogram for OS
A prognostic nomogram that corporates all significant prognostic factors in multivariate analysis for OS is shown in Fig. 2. The c-index of prognostic nomogram for OS prediction was 0.566 (95% CI, 0.492–0.640) in the cohort. The calibration plot for the probability of OS at 3 or 5 years shows a positive correlation between the nomogram prediction and actual observation (Fig. 3).
Figure 2.
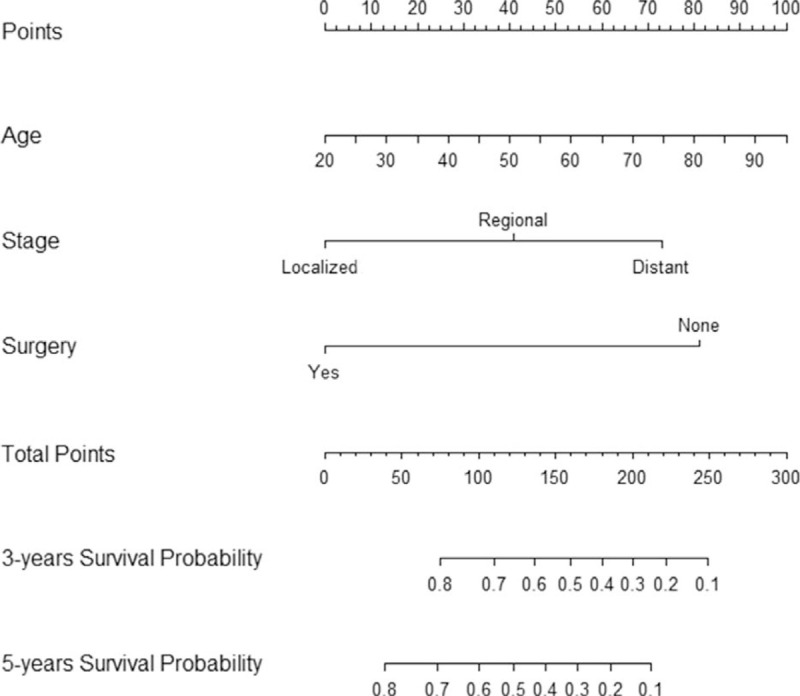
Nomogram to predict the probability of 3-year OS and 5-year OS. OS = overall survival.
Figure 3.
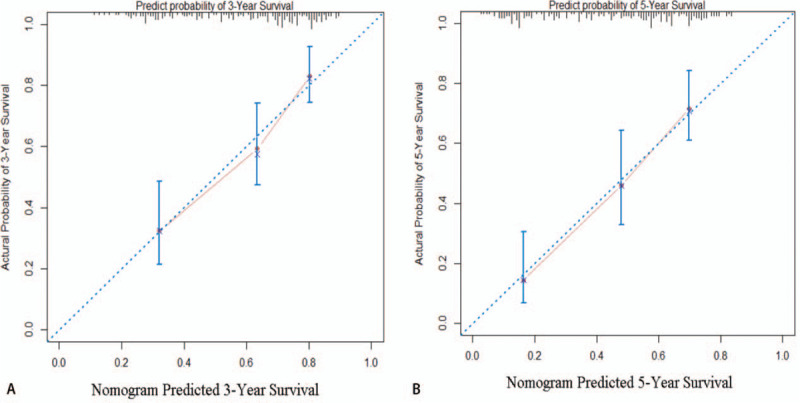
Calibration plot showing nomogram-predicted 3-year OS probabilities with the actual 3-year OS (A) and the nomogram-predicted 5-year OS with the actual 5-year OS (B). OS = overall survival.
4. Discussion
TSCC is the most common histological subtype of thymic carcinoma, accounting for approximately 1 in every 5 cases.[1,7] However, few studies have focused on specifically on survival among patients with TSCC. Notably, we found that age of diagnosis, surgical treatment, and SEER stage were associated with better prognosis. To our knowledge, this is the largest existing research study at population level to present clinical and pathology information regarding TSCC.
Currently, patients with TSCC have a relatively good overall prognosis, with median survival duration of 60 months and 1-, 3-, and 5-year survival rates of 83%, 55%, and 36% patients, respectively. SEER database with existing literature showed the median survival of the 290 included cases of thymic carcinoma was 48 months, and the 5-year survival rate was slightly better than that of TSCC.[8] This discrepancy has been attributed to differences in pathological types.
In all previous studies of thymic carcinoma, surgery has been the preferred curative treatment, with 5-year survival rates ranging between 58% and 80%.[8–12] Consistent with those findings and the NCCN guideline, our study observed the same survival benefits of surgery, with a 3-year overall survival rates of 73%. In a survival analysis of 105 surgically treated cases of TSCC, Zhao study reported median survival duration of 86.1 months after complete resection as well as 50.6 months after incomplete resection, and demonstrated that only the former had a significant impact on overall survival.[4] Suster et al[20] research also confirms the significance of the surgery. Undoubtedly, surgery remains the first choice for the treatment of TSCC. However, we must not overlook the fact that patients who choose to take surgery are more likely to be in a good condition and have localized and regional disease.
It is also worth noting that we identified the age of diagnosis as an independent prognostic factor for overall survival among patients with TSCC. In this way, we analyze age as a continuous variable, which distinguishes our study from other researchers such as Zhao study, who selected a cut-off age value of 55 years in their analysis of surgical TSCC,[4] and Yasuko study, who selected 60 years as a cut-off age value in a study of resected thymic carcinoma.[12] However, neither of the 2 studies identified age as a significant prognostic factor for survival. In line with our study, in a propensity-matched analysis of thymic carcinoma,[13] significantly poorer survival was seen in patients with age ≥63 years (P = .008). We are currently not able to confirm those findings, but elderly people are suffering from a gradually increasing frailty and prone to accept palliative treatment to reduce their pain. Differences in therapeutic treatments may contribute to differences of survival time.
The most common stage of TSCC is Masaoka staging. Prognostic impact of Masaoka stage has been described in the previous studies.[7,8,12] As we know, Masaoka stage is still the most consistent prognostic factor so far. However, there is unique staging style provided by SEER database, which is localized, regional, distant stage. In our study, the SEER stage showed its benefits of overall survival. Zhai et al[14] also found the percentage of local-regional relapse free survivals were 81.4% and 54.4%, and that of distant metastasis-free survivals were 35.9% and 25.6% at 5 and 10 years. Localized stage is more likely to accept surgical treatment, just as the outcomes in our study, 78.6% of localized stage accept surgery which is far more than regional stage (63%) and distant stage (43%). This is probably the most important reason why stage was significantly related to the overall survival.
The ability of adjuvant therapy to improve overall survival remains controversial. According to our study, neither radiotherapy nor chemotherapy contributed to longer overall survival. Similarly, Zhao study reported that postoperative adjuvant therapy, which was comprised of radiotherapy or chemotherapy alone or in combination, did not yield overall survival benefits to patients with TSCC.[4] Other studies of thymic carcinoma, however, have reported different findings.[12,13,15,16] For example, a meta-analysis of 973 patients by Hamaji et al[17] demonstrated that postoperative radiotherapy (PORT) after thymic carcinoma resection could lead to improved overall and progression-free survival. Besides, Yen et al[18] reported that among 82 patients with recurrent thymic carcinoma after surgical intervention, postoperative chemotherapy yielded the best progression-free survival outcomes after recurrence. In a study of 86 cases of advanced thymic carcinoma by Song et al,[19] the objective response rate to first-line chemotherapy was 47.7%, and the disease control rate was 80.2%. Yabuki and Minowa[21] reported a case of long survival and recurrence of thymic carcinoma 10 years after resection followed by concurrent chemoradiotherapy. In other words, adjuvant therapy may still play a pivotal role in the treatment of thymic carcinoma. However, our study failed to demonstrate an overall survival benefit of adjuvant therapy for patients with TSCC. Still, for patients with advanced TSCC who do not meet the indications for surgery, adjuvant therapy should be considered for disease control or palliative use.
It is universally acknowledged that the death and incidence rate of some tumors are often related with race/ethinicity,[22–25] therefore, in our study, we also discuss whether race/ethnicity is associated with the survival of TSCC, and discovers that race/ethnicity does not have any correlation with the overall survival. The Gad MM's study has proposed that pancreatic adenocarcinoma is associated with race and confirmed the annual increasing mortality rate of Asians, which does not bear any correlation with age and stage.[25] It can be argued that the tumors related with race/ethnicity are such as lung cancer, colorectal cancer, cholangiocarcinoma, and high-grade glioma, which is relevant to their diet, lifestyle, and local social economy.[23] In the meantime, it is proved in the study of Elisabeth that these tumors mentioned before have significant differences and trends in molecular signatures of the 3 cancer types in African Americans and Caucasian cohorts.[24] As for TSCC, though its incidence rate might have little relation with the factors above, the study of its overall survival also corroborates that the incidence rate and mortality rate of TSCC has nothing to do with race.
Our study had some limitations of note. We were only able to describe the presence or absence of surgery, radiotherapy, and chemotherapy, as the SEER database does not provide information about the type of surgery (e.g., complete or incomplete resection or pathological biopsy). Furthermore, we lacked information about the types of radiotherapy and chemotherapy the patients received, including the details of the radiotherapy methods, total doses, daily fraction sizes, irradiation field volumes, and chemotherapy regimens. Moreover, the database lacked information about the pathologic resection margins, which inhibited the analysis of postoperative radiotherapy outcomes, as well as detailed Masaoka stage information, which denotes prognosis. Despite these limitations, the SEER database is an invaluable resource with which to study rare malignancies, especially TSCC. Our study is the first multicenter study of TSCC and thus provides insights into the epidemiology and survival prognostic factors of this type of malignancy. Hopefully, our findings will inform the clinical practice with our recommendations.
5. Conclusion
TSCC is a rare type of carcinoma for which surgery should be considered as the best primary treatment option. Notably, the age of diagnosis and SEER stage were independent prognostic factors related to overall survival. Further studies are needed to confirm the effects of adjuvant therapy and better understand the effects of these treatments on overall survival when they are administered postoperatively to patients with TSCC. We hope that our findings will help to identify more appropriate and practical treatments for TSCC.
Acknowledgments
The authors would like to thank SEER for open access to their database.
Author contributions
Conceptualization: Jingyi Wu, Yanping Hu.
Data curation: Jingyi Wu, Yanping Hu.
Formal analysis: Jingyi Wu, Yanping Hu.
Funding acquisition: Jingyi Wu, Zhijun Wang, Caibao Jing, Yanping Hu.
Investigation: Jingyi Wu, Caibao Jing, Yanping Hu.
Methodology: Jingyi Wu, Zhijun Wang, Yanping Hu.
Project administration: Jingyi Wu, Zhijun Wang, Yang Hu, Yanping Hu.
Resources: Jingyi Wu, Yang Hu, Bing Yang, Yanping Hu.
Software: Jingyi Wu, Bing Yang, Yanping Hu.
Supervision: Jingyi Wu, Bing Yang, Yanping Hu.
Validation: Bing Yang, Yanping Hu.
Visualization: Bing Yang, Yanping Hu.
Writing – original draft: Yanping Hu.
Writing – review & editing: Yanping Hu.
Footnotes
Abbreviations: CIs = confidence intervals, CSS = cancer-specific survival, HRs = hazard ratios, NCCN = National Comprehensive Cancer Network, OS = overall survival, SEER = Surveillance, Epidemiology, and End Results Program, TSCC = thymic squamous cell carcinoma.
How to cite this article: Wu J, Wang Z, Jing C, Hu Y, Yang B, Hu Y. The incidence and prognosis of thymic squamous cell carcinoma: a SEER population-based study. Medicine. 2021;100:15(e25331).
Funding: Not applicable.
Availability of data and materials: Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Ethics approval and consent to participate: Not applicable.
Patient consent for publication: Not applicable.
The author declares that she has no competing interests
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
SEER = Surveillance, Epidemiology, and End Results Program.
SEER = Surveillance, Epidemiology, and End Results Program, TSCC = thymic squamous cell carcinoma.
SEER = Surveillance, Epidemiology, and End Results Program.
References
- [1].Fu H, Gu ZT, Fang WT, et al. Long-term survival after surgical treatment of thymic carcinoma: a retrospective analysis from the Chinese alliance for research of thymoma database. Ann Surg Oncol 2016;23:619–25. [DOI] [PubMed] [Google Scholar]
- [2].Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol 2015;10:1383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].NCCN. The NCCN Thymomas and Thymic Carcinomas clinical practice guidelines in oncology (version 2.2018) [EB/OL]. Fort Washington: NCCN; 2018. [Google Scholar]
- [4].Zhao Y, Zhao H, Hu D, et al. Surgical treatment and prognosis of thymic squamous cell carcinoma: a retrospective analysis of 105 cases. Ann Thorac Surg 2013;96:1019–24. [DOI] [PubMed] [Google Scholar]
- [5].“U.S. Census Bureau Announces 2010 Census Population Counts – Apportionment Counts Delivered to President” (Press release). United States Census Bureau; 2010. Archived from the original on December 24, 2010. Available at: January 9, 2011. [Google Scholar]
- [6].Wolbers M, Koller MT, Witteman JC, et al. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology 2009;20:555–61. [DOI] [PubMed] [Google Scholar]
- [7].Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95–100. 101.e1–101.e2. [DOI] [PubMed] [Google Scholar]
- [8].Weksler B, Dhupar R, Parikh V, et al. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013;95:299–303. [DOI] [PubMed] [Google Scholar]
- [9].Weissferdt A, Moran CA. Thymic carcinoma, part 1: a clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol 2012;138:103–14. [DOI] [PubMed] [Google Scholar]
- [10].Filosso PL, Guerrera F, Rendina AE, et al. Outcome of surgically resected thymic carcinoma: a multicenter experience. Lung Cancer 2014;83:205–10. [DOI] [PubMed] [Google Scholar]
- [11].Shimosato Y, Kameya T, Nagai K, et al. Squamous cell carcinoma of the thymus. An analysis of eight cases. Am J Surg Pathol 1977;1:109–21. [DOI] [PubMed] [Google Scholar]
- [12].Hosaka Y, Tsuchida M, Toyabe S, et al. Masaoka stage and histologic grade predict prognosis in patients with thymic carcinoma. Ann Thorac Surg 2010;89:912–7. [DOI] [PubMed] [Google Scholar]
- [13].Lim YJ, Song C, Kim JS. Improved survival with postoperative radiotherapy in thymic carcinoma: a propensity-matched analysis of Surveillance, Epidemiology, and End Results (SEER) database. Lung Cancer 2017;108:161–7. [DOI] [PubMed] [Google Scholar]
- [14].Zhai Y, Hui Z, Ji W, et al. A single-center analysis of the treatment and prognosis of patients with thymic carcinoma. Ann Thorac Surg 2017;104:1718–24. [DOI] [PubMed] [Google Scholar]
- [15].Zhao Y, Gu H, Fan L, et al. Comparison of clinical features and survival between thymic carcinoma and thymic carcinoid patients. Eur J Cardiothorac Surg 2017;52:33–8. [DOI] [PubMed] [Google Scholar]
- [16].Fernandes AT, Shinohara ET, Guo M, et al. The role of radiation therapy in malignant thymoma: a Surveillance, Epidemiology, and End Results database analysis. J Thorac Oncol 2010;5:1454–60. [DOI] [PubMed] [Google Scholar]
- [17].Hamaji M, Shah RM, Ali SO, et al. A meta-analysis of postoperative radiotherapy for thymic carcinoma. Ann Thorac Surg 2017;103:1668–75. [DOI] [PubMed] [Google Scholar]
- [18].Yen YT, Lai WW, Chang KW, et al. Factors predicting recurrence and postrecurrence survival in completely resected thymic carcinoma. Ann Thorac Surg 2014;97:1169–75. [DOI] [PubMed] [Google Scholar]
- [19].Song Z, Yu X, Zhang Y. Chemotherapy and prognosis in advanced thymic carcinoma patients. Clinics (Sao Paulo) 2015;70:775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Suster D, Pihan G, Mackinnon AC, et al. Poorly differentiated nonkeratinizing squamous cell carcinoma of the thymus: clinicopathologic and molecular genetic study of 25 cases. Am J Surg Pathol 2018;42:1224–36. [DOI] [PubMed] [Google Scholar]
- [21].Yabuki H, Minowa M. Long survival and recurrence of thymic carcinoma 10 years after resection. Asian Cardiovasc Thorac Ann 2019;27:407–9. [DOI] [PubMed] [Google Scholar]
- [22].Holowatyj AN, Lewis MA, Pannier ST, et al. Clinicopathologic and racial/ethnic differences of colorectal cancer among adolescents and young adults. Clin Transl Gastroenterol 2019;10:e00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee RM, Liu Y, Gamboa AC, et al. Race, ethnicity, and socioeconomic factors in cholangiocarcinoma: what is driving disparities in receipt of treatment? J Surg Oncol 2019;120:611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heath EI, Lynce F, Xiu J, et al. Racial disparities in the molecular landscape of cancer. Anticancer Res 2018;38:2235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gad MM, Găman MA, Saad AM, et al. Temporal trends of incidence and mortality in Asian-Americans with pancreatic adenocarcinoma: an epidemiological study. Ann Gastroenterol 2020;33:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


