Abstract
By setting out from increased neutrophil count, decreased lymphocyte count, and increased mean platelet volume (MPV), which is a result of the effect of inflammation on blood cells, we aimed to investigate whether neutrophil to lymphocyte ratio (NLP) and MPV can be used as an auxiliary parameter for the diagnosis of early-onset neonatal sepsis (EOS). This study was conducted by analyzing term neonates with EOS and physiological jaundice who were admitted to the neonatal intensive care unit of Izmir Katip Celebi University Ataturk Training and Research Hospital. A total of 63 neonate files were examined to include 30 term neonates with EOS, and 77 neonate files were examined to include 30 term neonates with physiological jaundice as a control group. NLR had an area under the curve (AUC) of 0.891 for prediction of EOS. At a cut-off level of 1.42, NLR had a likelihood ratio (LR) of 5.5, sensitivity of 88%, a specificity of 84%, a positive predictive value (PPV) of 84.6%, and a negative predictive value (NPV) of 87.5%. MPV had an AUC of 0.666 for the prediction of EOS and at a cut-off level of 9.3 fL, MPV had an LR of 1.23, sensitivity of 84%, a specificity of 32%, a PPV of 55.2%, and an NPV of 66.6%. In conclusion, this study provides evidence that NLR and MPV can be used in addition to conventional parameters in the diagnosis of EOS.
Keywords: neutrophil, lymphocyte, mean platelet volume, sepsis
Introduction
Early-onset neonatal sepsis (EOS) is a common neonatal disease that occurs in the first 3 days of life and is a major cause of morbidity and mortality. 1 EOS develops with the transfer of microorganisms from mother to fetus in the intrapartum period. The passage of microorganisms into the bloodstream may result in inflammation, initially leading to systemic inflammatory response syndrome (SIRS) with potential progression to multiple organ failure (MOF) and death. Therefore, early diagnosis of sepsis may inhibit the progression of SIRS to MOF and therefore reduce the risk of sepsis-related morbidity and mortality in neonates with EOS. 2 The recognition of maternal risk factors and use of clinical and laboratory findings are used for the diagnosis of EOS. Maternal risk factors for EOS include maternal urinary tract infection, vaginitis, early membrane rupture, and chorioamnionitis. 3 Nonspecific and subtle clinical findings in the absence of sensitive and specific biomarkers used for the diagnosis of EOS cause a delay in diagnosis leading to unnecessary hospitalizations, overuse of antibiotic therapy, and subsequent antibiotic resistance. 4 Complete blood count and acute phase reactants, such as C-reactive protein (CRP) and procalcitonin (PCT), are used in combination to offset the known inefficiencies of blood cultures which is the gold standard for diagnosis of sepsis. These inefficiencies include a high false-negative rate, the inability to obtain results before 48 to 72 hours, false-positive results (contamination), and a low sensitivity of 19.2%. 5 6 In comparison, biomarkers such as CRP and PCT offer more rapid results but yet are limited as increases in CRP require up to 12 hours following onset of inflammation, and immediate rises in PCT within 3 to 4 hours subsequently decline to normal levels within 24 hours. As a result, the search for complementary biomarkers for the diagnosis of EOS 7 8 would be of value to assist the clinician with rapid identification and management of EOS. In this study, we aimed to investigate whether the use of neutrophil to lymphocyte ratio (NLR), and mean platelet volume (MPV) can be used as complimentary biomarkers for the diagnosis of EOS.
Methods
This retrospective cross-sectional study was conducted by analysis of term neonates admitted to the neonatal intensive care unit (NICU) of Izmir Katip Celebi University Ataturk Training and Research Hospital between May 2019 and February 2020. The records of 63 neonates with EOS and 77 neonates with unconjugated hyperbilirubinemia (UCH) were examined from the hospital database from which 30 term neonates with EOS were identified as the study group and 30 term neonates with UCH and without infection were identified as the control group in this study. The determination of this appropriate population base for this study was derived from the study by Can et al, 9 where using the NLR value (2.88 ± 0.16 and 0.21 ± 0.12) as a reference, ( α = 0.05 and p < 0.05), 30 term neonates with EOS and a control group of 30 term neonates with UCH the power was calculated to be 100%. 10
The study group included term neonates with EOS based on the criteria proposed by the European Medicines Agency (EMA) for the diagnosis of sepsis. 11 The presence of ≥ 2 clinical signs (body temperature, skin/subcutaneous lesions, cardiovascular instability, respiratory instability, gastrointestinal instability, and nonspecific findings) and ≥ 2 laboratory signs (leukopenia/leukocytosis, immature neutrophil count, thrombocytopenia, rise in CRP or PCT, hypoglycemia/hyperglycemia, and metabolic acidosis) constituted a diagnosis of EOS. All demographic data and laboratory findings were obtained upon review of patient records. The control group consisted of neonates admitted to the NICU with UCH and without signs, symptoms, or risk factors consistent with sepsis. Exclusion criteria included preterm neonates, term neonates with congenital pneumonia or other inflammatory diseases, metabolic disease, intrauterine growth restriction, perinatal asphyxia, congenital anomaly, congenital heart disease, chromosomal anomaly, and neonatal patients with insufficient data. Written informed consent was obtained from parents of all study and control group patients in compliance with ethical standards as outlined in the 2008 Declaration of Helsinki.
Statistical Analysis
Statistical analyses was performed utilizing SPSS 22.0 (SPSS Inc., Chicago, Illinois, United States). We assessed the normality of continuous data using Kolmogorov–Smirnov test. Statistical data of study and control groups were compared using Student's t -test or Mann–Whitney U-test.
A p -value less than 0.05 was considered statistically significant. The performance of laboratory biomarkers to accurately predict the diagnosis of EOS was analyzed using area under the curve-receiver operating curve (AUC-ROC).
Results
Comparison of the baseline demographic characteristics are presented in Table 1 indicating the presence of well-matched study and control groups with no significant differences. Biomarkers to facilitate recognition and subsequent management of EOS are presented in Table 2 . There were statistically significant increases in patients with EOS with regard to NLR ( p = 0.000), MPV ( p = 0.018), CRP ( p = 0.000), PCT ( p = 0.000), and neutrophil count ( p = 0.000). In contrast, lymphocyte count ( p = 0.012) and platelet count ( p = 0.000) were significantly lower in neonates with EOS. The WBC count was not statistically different between groups ( p = 0.108). The AUC-ROC analysis of laboratory findings to predict EOS in neonates detected statistically significant difference between the EOS group and the control group as summarized in Table 3 . NLR demonstrated the best AUC-ROC 0.891; at a cut-off level of 1.42, LR+ 5.5, sensitivity 88%, specificity 84%, PPV 84.6%, and NPV 87.5% ( Fig. 1 ). MPV had AUC-ROC 0.666; at a cut-off level of 9.3 fL, LR+ 1.23, sensitivity 84%, specificity 32%, PPV 55.2%, and NPV 66.6% ( Fig. 2 ). CRP revealed an AUC-ROC 0.826; at a cut-off level of 6.1 mg/L, LR+ 2.7, sensitivity 77.8%, specificity 68.3%, PPV 71%, and NPV 75.4%. PCT was associated with an AUC-ROC 0.865; at a cut of 1.35, LR+ 8.8, sensitivity of 69.1%, specificity of 97.6%, PPV of 96.6%, and NPV of 75.9%. In 5 of 30 (16.6%) neonates in the EOS group, blood cultures were positive for Escherichia coli in four, and Streptococcus agalactiae in one neonate. All CSF cultures were negative for these organisms.
Table 1. Comparison of the demographic characteristics of the groups.
| Characteristics | EOS group ( n = 30) |
Control group ( n = 30) | p -Value |
|---|---|---|---|
| GA, wk (mean ± SD) | 38.4 ± 1.2 | 39.1 ± 0.9 | 0.112 |
| BW, g (mean ± SD) | 3,140 ± 187 | 3,108 ± 223 | 0.256 |
| Male gender, n (%) | 18 (60) | 16 (53.3) | 0.098 |
| VD, n (%) | 20 (66.6) | 22 (73.3) | 0.103 |
| Apgar's score (minimum), median (minimum–maximum) 1 5 |
8 (7–9) 9 (7–10) |
8 (7–9) 9 (7–10) |
0.311 0.286 |
Abbreviations: BW, birth weight; EOS, early-onset sepsis; GA, gestational age; SD, standard deviation; VD, vaginal delivery.
Table 2. Comparison of the hematological parameters of the groups.
| Parameters (mean ± SD) |
EOS Group ( n = 30) |
Control Group ( n = 30) | p -Value |
|---|---|---|---|
| NLR | 3.16 ± 1.72 | 0.99 ± 0.75 | 0.000 |
| MPV (fL) | 10.2 ± 0.89 | 9.7 ± 0.60 | 0.018 |
| CRP (mg/L) | 17.3 ± 6.6 | 1.8 ± 0.4 | 0.000 |
| Procalcitonin (ng/mL) | 4.88 ± 2.21 | 0.34 ± 0.11 | 0.000 |
| WBC (×10 6 /L) | 18,452 ± 4,326 | 15,127 ± 3,899 | 0.108 |
| Neutrophil (×10 6 /L) | 13,256 ± 2,118 | 5,592 ± 977 | 0.000 |
| Lymphocyte (×/L) | 4,664 ± 1,036 | 5,234 ± 433 | 0.012 |
| Platelet (×10 9 /L) | 277 ± 86 | 342 ± 63 | 0.000 |
Abbreviations: CRP, C-reactive protein; EOS, early onset sepsis; MPV, mean platelet volume; NLR, neutrophil to lymphocyte ratio; SD, standard deviation; WBC, white blood cell.
Table 3. The performance of laboratory findings in diagnosis of early-onset sepsis.
| Parameters | AUC-ROC | Cut -off value | Sensitivity % | Specificity % | LR+ | PPV % |
NPV % |
|---|---|---|---|---|---|---|---|
| NLR | 0.891 | 1.42 | 88 | 84 | 5.5 | 84.6 | 87.5 |
| MPV (fL) | 0.666 | 9.3 | 84 | 32 | 1.23 | 55.2 | 66.6 |
| CRP (mg/L) | 0.826 | 6.1 | 77.8 | 68.3 | 2.7 | 71 | 75.4 |
| Procalcitonin (ng/mL) | 0.865 | 1.35 | 69.1 | 97.6 | 8.8 | 96.6 | 75.9 |
Abbreviations: AUC-ROC, area under-receiver operator curve; CRP, C-reactive protein; EOS, early-onset sepsis; LR + , likelihood ratio; MPV, mean platelet volume; NLR, neutrophil to lymphocyte ratio; NPV, negative predictive value; PPV, positive predictive value.
Fig. 1.
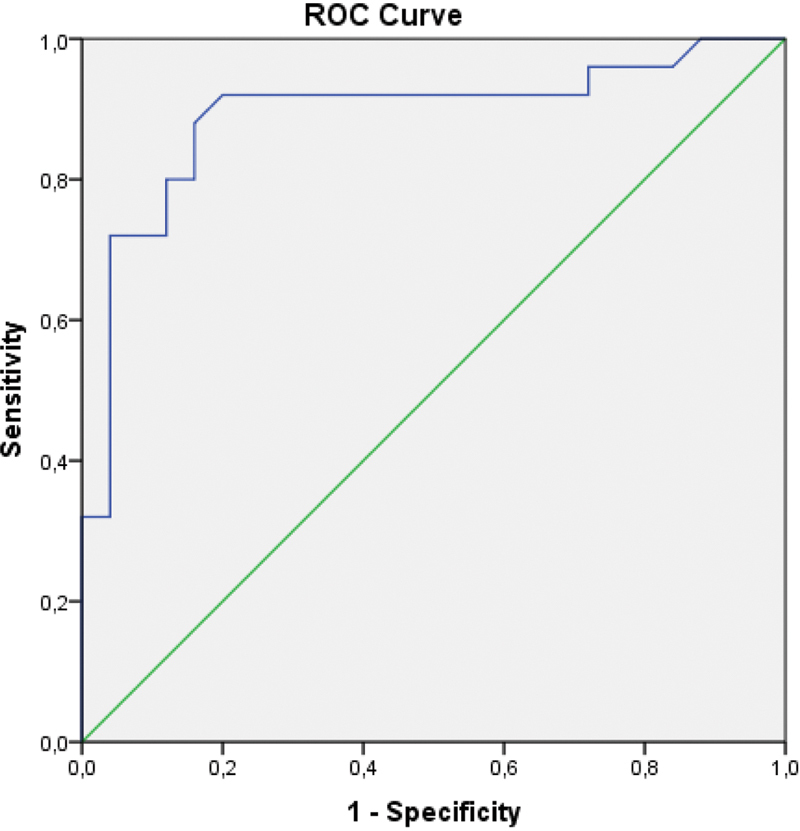
Area under the curve-receiver operating curve analysis of neutrophil to lymphocyte ratio. Diagonal segments are produced by ties.
Fig. 2.
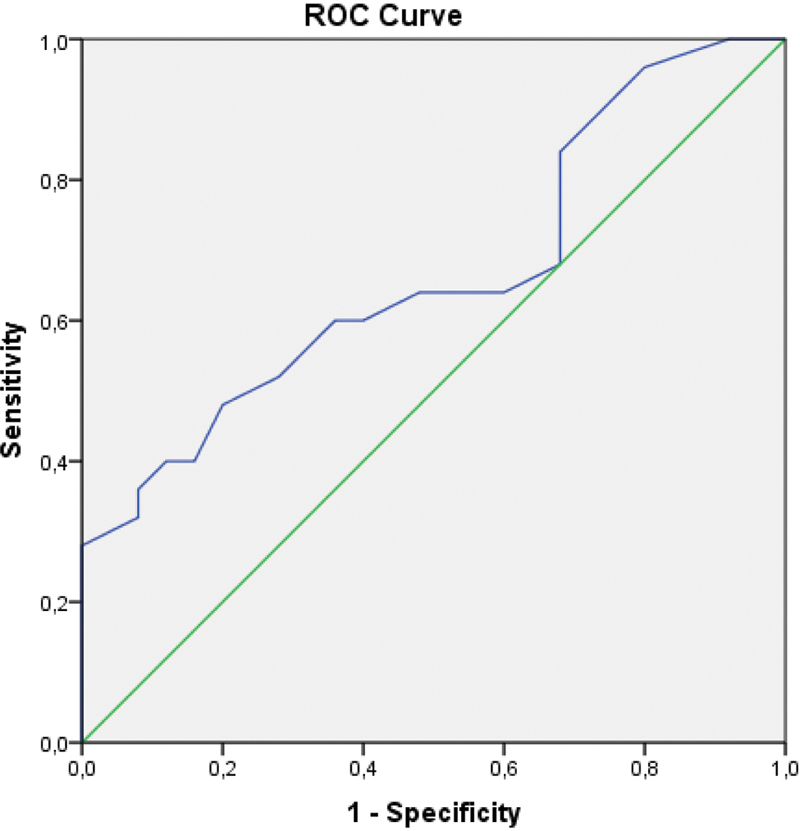
Area under the curve-receiver operating curve analysis of mean platelet volume. Diagonal segments are produced by ties.
Discussion
The basis for the pathogenesis of neonatal sepsis is inflammation. Common parameters used for the successful diagnosis and treatment of EOS, except for blood cultures, consist of inflammatory biomarkers. Accordingly, the study of inflammatory biomarkers is an important aspect of sepsis research. In particular, the effect of inflammation on blood cells such as neutrophils, lymphocytes, and platelets has become a focus of research on the diagnosis of sepsis. Neutrophils quickly react to infection and rapidly migrate toward the site of inflammation. As a result, compensatory increases in production of neutrophils in the bone marrow results in the release of more young neutrophils into the bloodstream. 12 In addition, neutrophil lifespan is prolonged due to delayed apoptosis, activation of nuclear factor kappa B, and reduction in caspase-3 levels related to sepsis induced stimulation of the inflammatory response. 13 The benefit of an increased number of circulating neutrophils with a prolonged half-life is enhancement of the host's immune response by directly killing microorganisms through phagocytosis, release of cytokines, and activation of T-cells. 14
Unfortunately, high levels of proinflammatory cytokines released by the host immune system in response to sepsis may also cause tissue injury and organ failure. As a result, compensatory release of anti-inflammatory cytokines induce lymphocyte apoptosis leading to lymphopenia and subsequent immunosuppression. 15 16 17 There is even evidence that detection of lymphopenia facilitates prediction of mortality in patients with sepsis. 18 19
In like fashion, platelets, an essential component of the coagulation system, inflammatory response, and host defense may undergo changes similar to that of neutrophils and lymphocytes. Platelet destruction and consumption lead to a reduction of platelets compensated through a marrow derived enhanced production and release of platelets with a larger volume than normal into the bloodstream. Subsequent sepsis research has been shown that the increase in MPV, which is the most sensitive parameter of platelet morphology, can be used for predicting inflammation. 20 21 22
Accordingly, increased neutrophil count and, decreased lymphocyte count leading to an increased N/L ratio, and an increased MPV may serve roles as complimentary biomarkers to identify the presence of and severity of neonatal EOS facilitating prompt and effective therapy leading to improved outcomes. In this study, we demonstrated that high NLR and MPV were more sensitive biomarkers than CRP and PCT, which are conventionally used parameters for the diagnosis of EOS.
Numerous studies on the diagnostic accuracy of blood culture and conventional parameters, such as CRP, used for the diagnosis of sepsis have reported a wide range of values. It has been reported that the sensitivity of blood cultures is 8 to 73%, and that of CRP (utilizing a cut-off value is 1.5–20 mg/L) is 74 to 98% with a related specificity of 71 to 94%. 23 24 We are of the opinion that the wide ranges of cut-off values, sensitivity, and specificity of conventional parameters commonly used for the diagnosis of neonatal sepsis may be due to the differences in gestational age, birth weight, and mode of delivery. The sensitivity of 83.1% and specificity of 85.9% at the most commonly accepted CRP-related cut-off value of 10 mg/L 24 is comparable with the sensitivity of 77.8% and a specificity of 68.3% at the CRP cut-off value of 6.1 mg/L noted in our study.
Studies evaluating the diagnostic value of NLR in sepsis have indicated variable results. Dursun et al reported a sensitivity of 75.6% and a specificity of 38.4%. 25 Can et al reported a sensitivity of 97.4% and a specificity of 100% at a cut-off value of 6.76, while Omran et al reported a sensitivity of 80% and a specificity of 57.1% at a cut-off value of 2.7. 9 26 In comparison, our findings of a sensitivity of 88% and a specificity of 84% at the NLR cut-off value of 1.42, and LR value of 5.5 contradicts the results of Can et al and Omran et al. This difference may be explained by the single-center design of the studies, and the inclusion of varying rates of proven versus suspected neonatal sepsis in the study groups. Although there is a wide range in cut-off values between these studies and our study, our findings support the use of NLR for the diagnosis of neonatal EOS due to its high AUC-ROC, sensitivity, specificity, LR + , PPV, NPV, and ease of use.
The diagnostic cut-off value of MPV in sepsis has been reported to be 10.4 to 11.4 fL with a sensitivity of 40.5 to 97.8% and a specificity of 78.7 to 100%. 27 28 29 30 31 In our study, MPV was found to have a sensitivity of 84% and a specificity of 32% at the cut-off value of 9.3 fL.
When the value of NLR and MPV in the diagnosis of EOS was compared with conventional parameters such as CRP and PCT, it was found in our study that both NLR and MPV had higher sensitivity than CRP and PCT. Individually, NLR had higher specificity, LR + , and NPV than CRP.
The strengths of our study are that the power of our study was found to be 100% through the use of an appropriate sized patient population based on a validated reference study, and the diagnosis of EOS through use of accepted EMA criteria which are based on clinical and laboratory findings.
Our study has some limitations. The first is that the neonates with EOS included in the study were not separated as to suspected versus proven EOS. The second is the reliability upon results of a single-center study that may not be generalizable to neonates in other geographic and resource available locations.
In conclusion, in this study, we offer evidence that NLR and MPV may be used in addition to conventional parameters such as CRP and PCT in the diagnosis and subsequent management of EOS. In addition, NLR and MPV are inexpensive and readily available tools in comparison to other relatively more expensive tools such as PCT. However, we recommend that these results be verified through use of multicenter studies rigorously analyzing our findings through study of large number of neonates to accurately determine the suitability of NLR and MPV to aid in the diagnosis of EOS.
Footnotes
Conflict of Interest None declared.
References
- 1.Committee on Fetus and Newborn . Polin R A. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129(05):1006–1015. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Vincent J L, Adhikari N KJ. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15(05):581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 3.Gerdes J S.Diagnosis and management of bacterial infections in the neonate Pediatr Clin North Am 20045104939–959., viii–ix [DOI] [PubMed] [Google Scholar]
- 4.Mukhopadhyay S, Taylor J A, Von Kohorn I. Variation in sepsis evaluation across a national network of nurseries. Pediatrics. 2017;139(03):e20162845. doi: 10.1542/peds.2016-2845. [DOI] [PubMed] [Google Scholar]
- 5.Jyothi P, Basavaraj M C, Basavaraj P V. Bacteriological profile of neonatal septicemia and antibiotic susceptibility pattern of the isolates. J Nat Sci Biol Med. 2013;4(02):306–309. doi: 10.4103/0976-9668.116981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benitz W E. Adjunct laboratory tests in the diagnosis of early-onset neonatal sepsis. Clin Perinatol. 2010;37(02):421–438. doi: 10.1016/j.clp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Laborada G, Rego M, Jain A. Diagnostic value of cytokines and C-reactive protein in the first 24 hours of neonatal sepsis. Am J Perinatol. 2003;20(08):491–501. doi: 10.1055/s-2003-45382. [DOI] [PubMed] [Google Scholar]
- 8.Chiesa C, Pacifico L, Rossi N, Panero A, Matrunola M, Mancuso G. Procalcitonin as a marker of nosocomial infections in the neonatal intensive care unit. Intensive Care Med. 2000;26 02:S175–S177. doi: 10.1007/BF02900733. [DOI] [PubMed] [Google Scholar]
- 9.Can E, Hamilcikan Ş, Can C. The value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for detecting early-onset neonatal sepsis. J Pediatr Hematol Oncol. 2018;40(04):e229–e232. doi: 10.1097/MPH.0000000000001059. [DOI] [PubMed] [Google Scholar]
- 10.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(04):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency (EMA) Report on the expert meeting on neonatal and pediatrics sepsisAccessed June 8, 2010 at:https://www.ema.europa.eu/en/documents/report/report-expert-meeting-neonatal-paediatric-sepsis_en.pdf
- 12.Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(01):5–14. [PubMed] [Google Scholar]
- 13.Taneja R, Parodo J, Jia S H, Kapus A, Rotstein O D, Marshall J C. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit Care Med. 2004;32(07):1460–1469. doi: 10.1097/01.ccm.0000129975.26905.77. [DOI] [PubMed] [Google Scholar]
- 14.Drewry A M, Samra N, Skrupky L P, Fuller B M, Compton S M, Hotchkiss R S. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(05):383–391. doi: 10.1097/SHK.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotchkiss R S, Dunne W M, Swanson P E.Role of apoptosis in Pseudomonas aeruginosa pneumonia Science 2001294(5548):1783. [DOI] [PubMed] [Google Scholar]
- 16.Rodrick M L, Wood J J, Grbic J T. Defective IL-2 production in patients with severe burns and sepsis. Lymphokine Res. 1986;5 01:S75–S80. [PubMed] [Google Scholar]
- 17.O'Sullivan S T, Lederer J A, Horgan A F, Chin D H, Mannick J A, Rodrick M L.Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection[See comments]Ann Surg 199522204482–490, discussion 490–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heffernan D S, Monaghan S F, Thakkar R K, Machan J T, Cioffi W G, Ayala A. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Crit Care. 2012;16(01):R12. doi: 10.1186/cc11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menges T, Engel J, Welters I. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med. 1999;27(04):733–740. doi: 10.1097/00003246-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Speth C, Hagleitner M, Ott H W, Würzner R, Lass-Flörl C, Rambach G. Aspergillus fumigatus activates thrombocytes by secretion of soluble compounds. J Infect Dis. 2013;207(05):823–833. doi: 10.1093/infdis/jis743. [DOI] [PubMed] [Google Scholar]
- 21.Speth C, Löffler J, Krappmann S, Lass-Flörl C, Rambach G. Platelets as immune cells in infectious diseases. Future Microbiol. 2013;8(11):1431–1451. doi: 10.2217/fmb.13.104. [DOI] [PubMed] [Google Scholar]
- 22.Becchi C, Al Malyan M, Fabbri L P, Marsili M, Boddi V, Boncinelli S. Mean platelet volume trend in sepsis: is it a useful parameter? Minerva Anestesiol. 2006;72(09):749–756. [PubMed] [Google Scholar]
- 23.Nachman S A. 2nd edition. Philadelphia, PA: Elsevier Mosby; 2005. Infection control and specific bacterial, viral, fungal and protozoan infections of the fetus and neonate; pp. 1083–1114. [Google Scholar]
- 24.Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(01):25–36. doi: 10.1159/000336629. [DOI] [PubMed] [Google Scholar]
- 25.Dursun A, Ozsoylu S, Akyildiz B N. Neutrophil-to-lymphocyte ratio and mean platelet volume can be useful markers to predict sepsis in children. Pak J Med Sci. 2018;34(04):918–922. doi: 10.12669/pjms.344.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omran A, Maaroof A, Saleh M H, Abdelwahab A. Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J Pediatr (Rio J) 2017 doi: 10.1016/j.jped.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Aydın B, Dilli D, Zenciroğlu A, Karadağ N, Beken S, Okumuş N. Mean platelet volume and uric acid levels in neonatal sepsis. Indian J Pediatr. 2014;81(12):1342–1346. doi: 10.1007/s12098-014-1417-4. [DOI] [PubMed] [Google Scholar]
- 28.Yao Y, Tu Y, Lu Q. [Values of C-reactive protein, percentage of neutrophils and mean platelet volume in early diagnosis of neonatal sepsis] Zhongguo Dang Dai Er Ke Za Zhi. 2015;17(05):425–429. [PubMed] [Google Scholar]
- 29.Shaaban H A, Safwat N. Mean platelet volume in preterm: a predictor of early onset neonatal sepsis. J Matern Fetal Neonatal Med. 2018;22:1–6. doi: 10.1080/14767058.2018.1488161. [DOI] [PubMed] [Google Scholar]
- 30.Catal F, Tayman C, Tonbul A. Mean platelet volume (MPV) may simply predict the severity of sepsis in preterm infants. Clin Lab. 2014;60(07):1193–1200. doi: 10.7754/clin.lab.2013.130501. [DOI] [PubMed] [Google Scholar]
- 31.Patrick C H, Lazarchick J. The effect of bacteremia on automated platelet measurements in neonates. Am J Clin Pathol. 1990;93(03):391–394. doi: 10.1093/ajcp/93.3.391. [DOI] [PubMed] [Google Scholar]


