Abstract
Transcranial Doppler ultrasonography (TCD) is being used in many pediatric intensive care units (PICUs) to aid in the diagnosis and monitoring of children with known or suspected pathophysiological changes to cerebral hemodynamics. Standardized approaches to scanning protocols, interpretation, and documentation of TCD examinations in this setting are lacking. A panel of multidisciplinary clinicians with expertise in the use of TCD in the PICU undertook a three-round modified Delphi process to reach unanimous agreement on 34 statements and then create practice recommendations for TCD use in the PICU. Use of these recommendations will help to ensure that high quality TCD images are captured, interpreted, and reported using standard nomenclature. Furthermore, use will aid in ensuring reproducible and meaningful study results between TCD practitioners and across PICUs.
Keywords: transcranial doppler ultrasound, pediatric critical care, practice recommendations, protocol, pediatric intensive care unit
Introduction
Transcranial Doppler ultrasonography (TCD) allows for the noninvasive bedside evaluation and monitoring of cerebral blood flow velocities (CBFVs) in the major cerebral vessels. 1 2 3 4 5 6 TCD has had a long-standing role in pediatrics as a tool to identify abnormal CBFVs and then guide therapy to reduce stroke risk in children with sickle cell anemia. 7 8 The use of TCD in critically ill children in the pediatric intensive care unit (PICU) has increasingly being reported. 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 A recent survey of 27 centers that provide pediatric neurocritical care services revealed that 93% use TCD. 9 Most commonly TCD was used in the evaluation and management of patients with intracranial/subarachnoid hemorrhage (SAH) (20 hospitals), arterial ischemic stroke (14 hospitals), and traumatic brain injury (10 hospitals). Despite 74% (20 of 27) of the respondents reporting that TCD results were used to guide aspects of clinical care such as the performance of neuroimaging, titration of drugs to augment cerebral perfusion pressure, and/or the performance of a surgical intervention, only 30% (8/27) reported having a standardized, written protocol for the performance and interpretation of TCD examinations.
Standard protocols for TCD examinations when used as a diagnostic or monitoring tool in adult neurocritical care practice exist for several clinical scenarios including: the detection and monitoring of vasospasm in patients with spontaneous or traumatic SAH, in the evaluation of collateral pathways of intracranial blood flow, in the detection of cerebral microemboli or high intensity transient signals, in the detection of right-to-left cardiac shunts, in the assessment of cerebral vasomotor reactivity, as an adjunct to the clinical diagnosis of brain death, in the pre and postoperative assessment of arteriovenous malformations, and in the assessment of intracranial pressure, hydrocephalus, hypoxic-ischemic encephalopathy, or dural venous sinus patency. 55 56 57 58 59 60 61 62 63 64 65 Given the developmental differences of the pediatric brain, the normal age-related changes of cerebral blood flow, and the differences in the common pathologies cared for, simple adoption of these adult protocols for use in the pediatric neurocritical care setting is not possible.
The expanding use of TCD in the practice of pediatric neurocritical care highlights the urgent need to establish a consensus on the methods used for study acquisition, on the data collected, and on the interpretation and reporting of the results. We therefore performed this Delphi study to develop expert consensus guidelines focused on these topics. Findings from this study are intended to lead to standardization of TCD practices for both clinical care and research in critically ill children. This standardization will allow for improved generation of meaningful, reproducible results within and between centers.
Materials and Methods
The Institutional Review Board (IRB) at Nationwide Children's Hospital deemed this web-based survey exempt from IRB review.
Evidence Review
A steering committee (N.F.O., K.R.R., K.L.L.) performed a literature search using MeSH (medical subject heading) terms for “ultrasonography, Doppler, transcranial” and “intensive care unit, pediatric” and study quality was then assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach. 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 The steering group also reviewed studies describing normative values for TCD flow velocities in both healthy and critically ill individuals. 1 2 3 4 5 6 Lastly, the steering committee reviewed published recommendations for standardized TCD performance and interpretation in adults and children outside the ICU setting. 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 Practice recommendations for TCD use in these other reports fall into four general domains (1) request for examination standards, (2) technical performance standards, (3) data interpretation standards, and (4) data reporting standards. These general domains were thus used as a framework for the organization of statements throughout the Delphi process. Adapted statements from previously published practice recommendations were used to develop the open-ended survey questions circulated in the first round of the Delphi process.
Panel Member Selection
A multidisciplinary panel of members with special expertise in the use of TCD in the PICU was established for participation in the Delphi process. Panelists were identified through their participation in the TCD subgroup of the Pediatric Neurocritical Care Research Group (PNCRG) or as radiology collaborators of PNCRG members. PNCRG membership represents experts with an interest in and commitment to advancing the clinical care and research of children in the PICU with neurological and neurosurgical disorders to improve outcomes. At the time of this study, the PNCRG TCD focus group consisted of 22 members.
The Modified Delphi Process
The Delphi method is recommended for use in the health care setting as an effective and reliable means of determining expert group consensus around a clinical question where there is little or no definitive evidence and where opinion is important. 74 75 76 77 78 79 80 81 The method is an iterative process that uses a systematic progression of repeated rounds of voting on predetermined statements. The “modified” Delphi approach also includes a face-to-face meeting at the final stage of the process where panel members have an opportunity to provide further clarification and present arguments to justify viewpoints. Studies have demonstrated that this modified process can be superior to the original method as it is highly cooperative. 81 82
Surveys for each of the three rounds of the Delphi process were sent electronically (SurveyMonkey Inc, San Mateo, California, United States) from August 2019 to October 2019. Panelists were provided with a summary of the aims of the consensus project before each round. An in-person meeting was held in Vancouver, British Columbia during the annual Neurocritical Care Society Meeting in October 2019.
Round 1
Panelists received open-ended survey questions and were given an opportunity to suggest alternatives or possible considerations as it was related to the topics under discussion. The open-ended survey responses from round 1 were reviewed and categorized by the steering group to create a list of structured, Likert-type closed-ended questionnaire items for the second round of the survey administration.
Round 2
Before round 2, panelists were provided with an anonymous summary of the responses from round 1. During round 2, the panel of experts were provided with the closed-ended survey questions in which they were asked to rate their agreement with each statement on a 1 to 10 scale (1 = absolutely do not agree to 10 = absolutely agree). The survey responses from round 2 were reviewed and analyzed. Questions that scored ≥ 9 by ≥ 80% of respondents were considered to have met our a priori definition of “near perfect or near unanimous agreement” and were deemed to have met consensus. Items meeting this a priori definition were not included in the next round of the Delphi process. Statements not achieving this level of agreement were revised for the next round.
Round 3
Before round 3, panelists were again provided with an anonymous summary of the responses from round 2. For round 3, a revised closed-ended Likert questionnaire was distributed to panelists. Results of round 3 were similarly analyzed according to the same a priori definition of agreement. Items meeting this a priori definition were not included in the next round of the Delphi process. Statements not meeting the a priori definition were sent to the group prior to the in-person meeting.
In-person Meeting
Panel experts attended an in-person meeting where participants worked together to attempt to reach a consensus agreement on the statements that did not reach near perfect agreement in the online survey process. All panel members had an equal opportunity to provide an opinion on each question. Panel members not able to attend the meeting had the opportunity to send their opinions electronically to the steering committee. These opinions were read and included in the discussion. Panel members not in attendance were also provided with a summary of the results of the discussion at the meeting. Abbreviations for terms used throughout the process are shown in Table 1 .
Table 1. Abbreviations used throughout the Delphi process and in the consensus document.
| Name | Abbreviations |
|---|---|
| Transcranial Doppler ultrasonography | TCD |
| Middle cerebral artery | MCA |
| Anterior cerebral artery | ACA |
| Posterior cerebral artery | PCA |
| Internal carotid artery | ICA |
| Extracranial internal carotid artery | Ex-ICA |
| Ophthalmic artery | OA |
| Vertebral artery | VA |
| Basilar artery | BA |
| Systolic flow velocity | Vs |
| Diastolic flow velocity | Vd |
| Mean flow velocity | Vm |
| Pulsatility index | PI |
| Lindegaard ratio | LR |
| Sviri ratio | SR |
| Soustiel ratio | SoR |
Results
Evidence Review
The steering committee reviewed 45 manuscripts that reported the use of TCD in the PICU. 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Overall, the quality of supporting evidence in the field related specifically to use of TCD in the PICU is weak with all reviewed evidence coming from surveys and observational cohort, case–control, or descriptive studies. Additionally, six manuscripts were reviewed that reported normative flow velocity values. 1 2 3 4 5 6 Seventeen publications that outline recommendations for standardized TCD performance and interpretation in populations outside the PICU were also evaluated. 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73
Panel Members
Round 1 of the Delphi survey was sent to 22 potential panelists with a 73% (16/22) response rate. All 16 (100%) respondents participated in Delphi rounds 2 and 3. A total of ten panelists participated in the in-person meeting with the other six submitting comments and opinions electronically. The specialties, years of clinical practice, and years of experience performing and/or interpreting TCD in clinical care or research are shown in Table 2 .
Table 2. Information on participating panel members.
| Description | Value |
|---|---|
| Medicine (number) | |
| Pediatric critical care | 5 |
| Pediatric neurology | 5 |
| Both pediatric critical care and neurology | 2 |
| Pediatric radiology | 2 |
| Nursing (number) | 1 |
| Ultrasound technician (number) | 1 |
| Years in clinical practice (mean, SD) | 12 (± 2) |
| Years using TCD in the PICU (mean, SD) | 7.5 (± 3) |
Abbreviations: PICU, pediatric intensive care unit; SD, standard deviation; TCD, transcranial Doppler ultrasound.
The Modified Delphi Process
A summary of the number of questions that achieved or did not achieve near unanimous consensus in each of the rounds is shown in Fig. 1 .
Fig. 1.
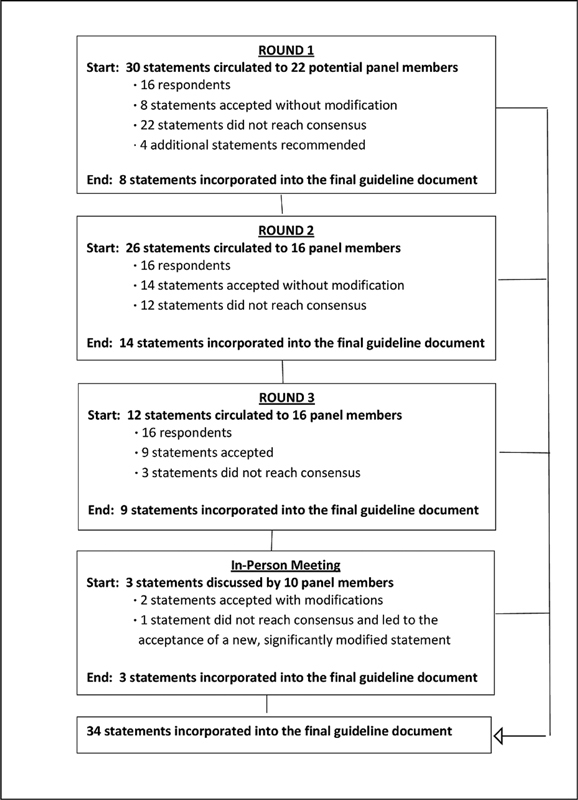
Modified Delphi methodology and results.
Summary of Recommendations
The panelists reached a consensus on 34 statements in the four domains. The full summary of statements achieving consensus is in Table 3 . In the Delphi process when an item does not reach consensus, an alternative agreement can be developed by the panelists. In this Delphi survey, one statement that read “It is necessary that the individual performing and interpreting the TCD in the PICU for clinical examinations should have received formal training and passed a written exam by a governing body” could not be agreed upon. Based on the review of the available evidence and following extensive discussion at the in-person meeting, panelists were able to reach agreement on an alternative statement “Individuals performing and interpreting TCD in the PICU should be accredited by their institution to do so.”
Table 3. Consensus statements for the performance of TCD in the pediatric intensive care unit (PICU).
| Domain | Consensus statement |
|---|---|
| Indication and request for examination standards |
• Any patient in the PICU with concern for pathophysiological changes to cerebral hemodynamics is a candidate to undergo TCD examination. • The written or electronic request must provide sufficient information to interpret the examination and should include relevant history (known or suspected acute or chronic diagnoses), signs or symptoms, and specific questions of the treating team. |
| Technical performance standards | • For positioning of a patient for a TCD in the PICU, 30–45 degrees head of bed positioning is ideal, but the exam can be performed in any position based on the patient's clinical requirements. • The vessel being insonated should always, at least in part, be identified by direction of flow, flow velocities, and depth of insonation. • A complete TCD examination includes evaluation of MCAs, ACAs, PCAs, VAs, BA, ICAs, and Ex-ICAs (bilaterally for all except BA). • A limited TCD examination can include any one or more of the vessels in the complete examination depending on the clinical indication for the examination. • The transtemporal window should be used to evaluate the MCA, ACA, PCA, and ICA. • The transorbital window should be used to evaluate the OA. Power should be reduced to as low as reasonably achievable (approximately 10%) for this portion of the examination. • For this portion of the examination. • The submandibular window should be used to evaluate the Ex-ICA and distal ICA. • The transforaminal window should be used to evaluate the bilateral VA and the BA. • Through the transtemporal window it is acceptable to locate the MCA/ACA bifurcation sonographically as an anatomical landmark to determine which vessels are being insonated when the probe angle is altered, or the depth is advanced or reduced. • The appropriate depth to insonate the MCA in adolescents and young adults is 65–35 mm. In pediatric patients, it may be less deep due to smaller head size. • The appropriate depth to insonate the ophthalmic artery in adolescents and young adults is 40–50 mm. In pediatric patients, it may be less deep due to smaller head size. The appropriate depth to insonate the ex-ICA and distal ICA in adolescents and young adults is 40–60 mm. In pediatric patients, it may be less deep. • The appropriate depth to insonate the BA in adolescents and young adults is 65–80 mm. In pediatric patients, it may be less deep due to smaller head size. • Measurements should be recorded every 2 mm. |
| Data interpretation standards |
• Temperature, mean arterial pressure, partial pressure of carbon dioxide, hemoglobin or hematocrit, the use of invasive or noninvasive mechanical ventilation, and the use and type of sedatives or anxiolytics at the time of TCD examination are necessary to record and consider when interpreting TCD examinations. • Intracranial pressure and cerebral perfusion pressure should also be included when available. • When interpreting a TCD examination of a nonintubated child <18 y of age in the PICU, normative values from Bode and Wais should be used. 2 • To diagnose abnormal flow, mean flow velocities ≤ or ≥ 2 SD from age and gender normal value can be used. • No Lindegaard ratio (LR) has been validated in children to differentiate between hyperemia and vasospasm in the MCA and thus using specific cut-offs for diagnosing, grading, or determining the clinical significance of vasospasm in the MCAs cannot be recommended. However, following LR values over time may have clinical utility to determine trends in cerebral blood flow. • No Sviri or Soustiel ratio has been validated in children to differentiate between hyperemia and vasospasm in the BA and thus using specific cut-offs for diagnosing, grading, or determining the clinical significance of vasospasm in the BA cannot be recommended. However, following Sviri/Soustiel values over time may have clinical utility to determine trends in cerebral blood flow. • Radiographic validation (with CT, MRI, etc.) of abnormal TCD findings should be strongly considered depending on the clinical indication for TCD examination. • Individuals performing and interpreting TCD in the PICU should be accredited by their institution to do so. |
| Data reporting standards |
• The initials of the operator should be included on the report. • The type of TCD machine (imaging vs non-imaging), window used for insonation, and side of examination (right vs. left vs. bilateral) are mandatory to report. • Sample volume size, gain, and power settings may be considered to include in the report. • The name, age, and gender of the child are mandatory to report. • It is necessary to report if the study was technically adequate to allow interpretation. • Depths of measurement (in mm) for Vs, Vd, Vm, and PI in each vessel are mandatory to report. • Abnormalities to the waveform characteristics such as delayed upstroke, reversal of flow, and embolic signals (if present) should be reported. • If serial examinations are performed, reporting trends in measured velocities and/or Lindegaard/Sviri/Soustiel ratio are encouraged. • Reporting the number of standard deviations from selected reference values for the measured flow velocities is encouraged. • The pediatric intensivist, neurosurgeon, and/or neurologist should be involved in the process of interpretation based on the patient's underlying pathophysiology. |
Abbreviations: ACA, anterior cerebral artery; BA, basilar artery; CT, computed tomography; ICA, internal carotid artery; MCA, middle cerebral artery; MRI, magnetic resonance imaging; OA, ophthalmic artery; PCA, posterior cerebral artery; SD, standard deviation; TCD, transcranial Doppler ultrasonography; VA, vertebral artery; Vd, diastolic flow velocity; Vm, mean flow velocity; Vs, systolic flow velocity.
Based on the statements that reached consensus as well as the panelists' collective experience in practice in the PICU, Supplementary Table S1 provides a recommended protocol for performance of TCD in the PICU ( Supplementary Material ). Fig. 2 provides a recommended reporting worksheet for TCD examinations done in the PICU. These two items are available for download at www.pncrg.org . Table 4 provides the normative values referenced in the recommended protocol. 2 10 All panel members supported the finalized consensus statements and protocols.
Fig. 2.
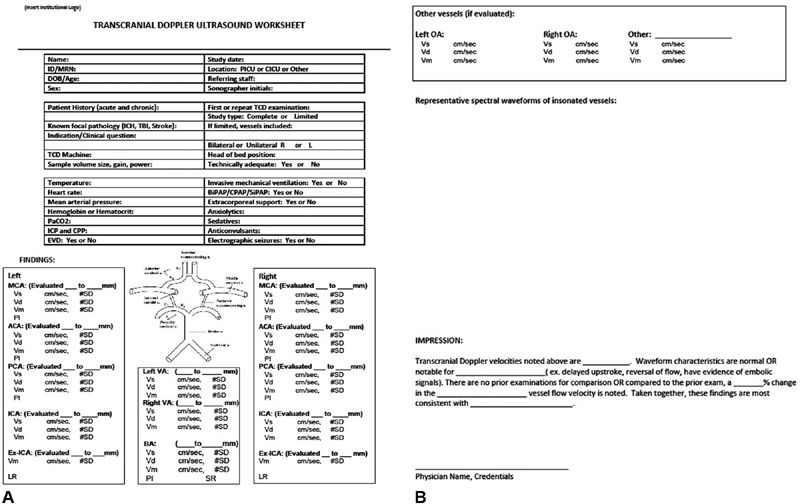
( A,B ) Example template for reporting of TCD results in the PICU. Printable and modifiable version available at: http://www.pncrg.org . PICU, pediatric intensive care units; TCD, transcranial Doppler ultrasonography.
Table 4. Recommended comparative values for the interpretation of TCD examinations in children (0–18 y) in the pediatric intensive care unit by age (mean [SD]) 2 10 .
| Age | MCA | ICA | ACA | PCA | BA |
|---|---|---|---|---|---|
| Systolic peak velocity: | |||||
| 0–10 d | 46 (10) | 47 (9) | 35 (8) | – | – |
| 11–90 d | 75 (15) | 77 (19) | 58 (15) | – | – |
| 3–11.9 mo | 114 (20) | 104 (12) | 77 (15) | – | – |
| 1–2.9 y | 124 (10) | 118 (24) | 81 (19) | 69 (9) | 71 (6) |
| 3–5.9 y | 147 (17) | 144 (19) | 104 (22) | 81 (16) | 88 (9) |
| 6–9.9 y | 143 (13) | 140 (14) | 100 (20) | 75 (10) | 85 (17) |
| 10–18 y | 129 (17) | 125 (18) | 92 (19) | 66 (10) | 68 (11) |
| Mean flow velocity a : | |||||
| 0–10 d | 24 (7) | 25 (6) | 19 (6) | – | – |
| 11–90 d | 42 (10) | 43 (12) | 33 (11) | – | – |
| 3–11.9 mo | 74 (14) | 67 (10) | 50 (11) | – | – |
| 1–2.9 y | 85 (10) | 81 (8) | 55 (13) | 50 (12) | 51 (6) |
| 3–5.9 y | 94 (10) | 93 (9) | 71 (15) | 48 (11) | 58 (6) |
| 6–9.9 y | 97 (9) | 93 (9) | 65 (13) | 51 (9) | 58 (9) |
| 10–18 y | 81 (11) | 79 (12) | 56 (14) | 45 (9) | 46 (8) |
| End-diastolic peak velocity: | |||||
| 0–10 d | 12 (7) | 12 (6) | 10 (6) | – | – |
| 11–90 d | 24 (8) | 24 (8) | 19 (9) | – | – |
| 3–11.9 mo | 46 (9) | 40 (8) | 33 (7) | – | – |
| 1–2.9 y | 65 (11) | 58 (5) | 40 (11) | 35 (7) | 35 (6) |
| 3–5.9 y | 65 (9) | 66 (8) | 48 (9) | 35 (9) | 41 (5) |
| 6–9.9 y | 72 (9) | 68 (10) | 51 (10) | 38 (7) | 44 (8) |
| 10–18 y | 60 (8) | 59 (9) | 46 (11) | 33 (7) | 36 (7) |
Abbreviations: ACA, anterior cerebral artery; BA, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; SD, standard deviation; TCD, transcranial Doppler ultrasonography.
Mean flow velocity = time mean of the maximal velocity envelope curve.
Discussion
Despite the lack of large studies and outcomes data, TCD findings appear to influence management in pediatric neurocritical care. 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Calcium channel blockers, phosphodiesterase inhibitors, and other interventions such as blood pressure augmentation or angioplasty have been administered based on TCD findings felt to be consistent with cerebral vasospasm. TCD results have been used to determine the timing of neuroimaging and drive intervention in cases of acute intracranial vascular occlusions. TCD results are also used to adjust hemodynamic targets and ventilation parameters in the management of intracranial hypertension. 9
Standards for the performance, interpretation, and reporting of TCD examinations in the PICU, however, are lacking. In clinical practice, inconsistency in these approaches may lead to inappropriate management or interventions. In clinical research, the lack of standardization limits the generalizability of results across research sites and between studies. Experts in the field thus participated in this modified Delphi process to develop consensus statements on approaches to scanning protocols, interpretation, and documentation when TCD is performed in the PICU.
Given the unique anatomy and physiology of children, several notable differences from published adult protocols are recommended in this consensus document. Head circumference progressively increases with age through late childhood. Thus recommending a standard depth that represents midline from which to begin the TCD evaluation in children is not possible. Furthermore, measuring the diameter of the head and then calculating the midline is often impractical in critically ill children given their positioning needs and the presence of life-support devices. The expert panelists thus agreed that it is recommended to identify the midline by sonographically locating the middle cerebral artery (MCA)/anterior cerebral artery (ACA) bifurcation as a landmark from which to trace the desired vessels (MCA, ACA, and PCA [posterior cerebral artery]). Additionally, given the relatively short length of the cerebral vessels in children, the consensus document recommends measurement at 2 mm increments along the entire length of each vessel, rather than every 5 mm as is recommended in adults. Lastly, expected CBFVs are age dependent in children, with values typically increasing through age 6 to 8 years before downtrending to adult levels by adolescence. Therefore, results of a TCD examination cannot be compared with a single normative value as in adults. The consensus document recommends CBFVs captured in children in the PICU be compared with normative values of healthy children, and that a measured flow velocity should be considered abnormal when it is ≥ or ≤ 2 standard deviations from the age-associated normal value. Multiple physiological factors that can impact measured CBFVs such as body temperature, mean blood pressure, partial pressure of carbon dioxide, and hematocrit are often deranged in this patient population. Additionally, treatments such as sedation and mechanical ventilation are known to impact measured CBFVs. 4 Thus, it is recommended that each of these aspects should be considered when interpreting the relevance of “abnormal values” when TCD is performed in a critically ill child in the PICU. A multidisciplinary approach to interpretation may therefore be beneficial.
Our expert panel was unable to achieve consensus regarding the recommended training and certification process for the performance and interpretation of TCD in the PICU. One significant challenge the panelists faced in reaching a consensus on this topic is that TCD has many potential roles in the PICU including: as a point of care ultrasound (POCUS) study; as a complete examination performed to gain extensive diagnostic information; as a continuous noninvasive monitor; and as one part of multimodal neuromonitoring. For TCD studies that are performed for clinical purposes that result in a report being placed in the medical record, the consensus agreed upon by the panelists is that the operator and practitioner interpreting the study must be approved to do so by their institution's credentialing body.
Due to the lack of clear supportive data, the recommendations outlined by the panel also do not include specific indications for when TCD would have a clear diagnostic or therapeutic benefit in the management of a critically ill child. In part, the lack of evidence for TCD use in pediatric neurocritical care is because of the limited generalizability of previous studies with variable procedural approaches and reporting. The recommendations made here may promote an improvement in future research that could assist in the creation of evidence-based guidelines outlining clear indications for TCD performance in routine clinical practice in pediatric neurocritical care.
Limitations
Our study has several limitations. There are no accepted requirements for what constitutes a TCD expert. Panel members differed in level of training, credentials, and experience with TCD. Additionally, the PNCRG, from which the expert panel was drawn, had limited number of participants from the field of radiology. Thus, the process may have excluded some individuals with relevant experience. However, the panel did have representatives from a variety of fields including pediatric critical care, pediatric neurology, pediatric radiology, pediatric nursing, and pediatric ultrasonography technicians that participated in the entire process. Additionally, the evidence available to support the recommendations developed here is weak. However, the majority of guidelines or recommendations in the field of pediatrics are developed similarly where limited high-quality evidence exists. This notable weakness should serve as a call to utilize the work presented here to guide standardized approaches in the performance of high-quality research that will enhance the available body of evidence.
There are also limitations to the Delphi method itself including unintentional bias from the steering committee, lack of anonymity, and incomplete participation during the in-person meeting. Furthermore, no formal process for conflict resolution such as facilitation or mediation was used when discussing the statements that did not achieve consensus throughout the Delphi process. However, three individuals with different backgrounds made up the steering committee, the group allowed for the inclusion of novel or alternative statements throughout the process, and the results from the in-person meeting were circulated for final remarks to address these limitations. Furthermore, the final documents reached unanimous agreement by the group.
Conclusion
The use of TCD to evaluate the cerebral hemodynamics during critical illness in children in the PICU is common despite not having standardized scanning protocols, methods for interpretation, or approaches to documentation. For safe and effective use of TCD in critically ill children, guidelines and standards on the performance and interpretation of TCD are needed. A multidisciplinary group of TCD experts undertook this modified Delphi study and developed 34 recommendations in four domains for these core aspects of TCD use. A standard basic protocol as well as reporting worksheet was created based on the consensus statements.
Footnotes
Conflict of Interest K.L.L. has a patent emboli detection method to identify mechanisms of brain injury in susceptible adults and children pending. Rest authors declare no conflict of interest.
Supplementary Material
References
- 1.Aaslid R, Markwalder T M, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57(06):769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 2.Bode H, Wais U. Age dependence of flow velocities in basal cerebral arteries. Arch Dis Child. 1988;63(06):606–611. doi: 10.1136/adc.63.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vavilala M S, Kincaid M S, Muangman S L, Suz P, Rozet I, Lam A M. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr Res. 2005;58(03):574–578. doi: 10.1203/01.PDR.0000179405.30737.0F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien N F. Reference values for cerebral blood flow velocities in critically ill, sedated children. Childs Nerv Syst. 2015;31(12):2269–2276. doi: 10.1007/s00381-015-2873-5. [DOI] [PubMed] [Google Scholar]
- 5.Lindegaard K F, Nornes H, Bakke S J, Sorteberg W, Nakstad P.Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements Acta Neurochir (Wien) 1989100(1-2):12–24. [DOI] [PubMed] [Google Scholar]
- 6.Sviri G E, Ghodke B, Britz G W.Transcranial Doppler grading criteria for basilar artery vasospasm Neurosurgery 20065902360–366., discussion 360–366 [DOI] [PubMed] [Google Scholar]
- 7.Adams R, McKie V, Nichols F. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326(09):605–610. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- 8.Adams R J, McKie V C, Hsu L. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(01):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 9.LaRovere K L, Tasker R C, Wainwright M. Transcranial Doppler ultrasound during critical illness in children: survey of practices in pediatric neurocritical care centers. Pediatr Crit Care Med. 2019;21(01):67–74. doi: 10.1097/PCC.0000000000002118. [DOI] [PubMed] [Google Scholar]
- 10.LaRovere K L, O'Brien N F. Transcranial Doppler sonography in pediatric neurocritical care: a review of clinical applications and case illustrations in the pediatric intensive care unit. J Ultrasound Med. 2015;34(12):2121–2132. doi: 10.7863/ultra.15.02016. [DOI] [PubMed] [Google Scholar]
- 11.Abecasis F, Oliveira V, Robba C, Czosnyka M. Transcranial Doppler in pediatric emergency and intensive care unit: a case series and literature review. Childs Nerv Syst. 2018;34(08):1465–1470. doi: 10.1007/s00381-018-3877-8. [DOI] [PubMed] [Google Scholar]
- 12.LaRovere K L, O'Brien N F, Tasker R C. Current opinion and use of transcranial Doppler ultrasonography in traumatic brain injury in the pediatric intensive care unit. J Neurotrauma. 2016;33(23):2105–2114. doi: 10.1089/neu.2015.4344. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien N F, Maa T, Reuter-Rice K. Noninvasive screening for intracranial hypertension in children with acute, severe traumatic brain injury. J Neurosurg Pediatr. 2015;16(04):420–425. doi: 10.3171/2015.3.PEDS14521. [DOI] [PubMed] [Google Scholar]
- 14.Reuter-Rice K. Transcranial Doppler ultrasound use in pediatric traumatic brain injury. J Radiol Nurs. 2017;36(01):3–9. doi: 10.1016/j.jradnu.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuter-Rice K, Regier M, Bennett E, Laskowitz D. The effect of the relationship of APOE polymorphisms and cerebral vasospasm on functional outcomes in children with traumatic brain injury. Biol Res Nurs. 2018;20(05):566–576. doi: 10.1177/1099800418785982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovett M E, Maa T, Chung M G, O'Brien N F. Cerebral blood flow velocity and autoregulation in paediatric patients following a global hypoxic-ischaemic insult. Resuscitation. 2018;126:191–196. doi: 10.1016/j.resuscitation.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Lin J J, Hsia S H, Wang H S, Chiang M C, Lin K L. Transcranial Doppler ultrasound in therapeutic hypothermia for children after resuscitation. Resuscitation. 2015;89:182–187. doi: 10.1016/j.resuscitation.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 18.LaRovere K L. Transcranial Doppler ultrasound in children with stroke and cerebrovascular disorders. Curr Opin Pediatr. 2015;27(06):712–718. doi: 10.1097/MOP.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Roberts J S, Pihoker C. Transcranial Doppler-based assessment of cerebral autoregulation in critically ill children during diabetic ketoacidosis treatment. Pediatr Crit Care Med. 2014;15(08):742–749. doi: 10.1097/PCC.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 20.Ducharme-Crevier L, Mills M G, Mehta P M, Smith C M, Wainwright M S. Use of transcranial Doppler for management of central nervous system infections in critically ill children. Pediatr Neurol. 2016;65:52–5800. doi: 10.1016/j.pediatrneurol.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien N F, Mutatshi Taty T, Moore-Clingenpeel M. Transcranial Doppler ultrasonography provides insights into neurovascular changes in children with cerebral malaria. J Pediatr. 2018;203:116–124000. doi: 10.1016/j.jpeds.2018.07.075. [DOI] [PubMed] [Google Scholar]
- 22.Leliefeld P H, Gooskens R H, Peters R J. New transcranial Doppler index in infants with hydrocephalus: transsystolic time in clinical practice. Ultrasound Med Biol. 2009;35(10):1601–1606. doi: 10.1016/j.ultrasmedbio.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Cheatham S L, Chisolm J L, O'Brien N. Cerebral blood flow following hybrid stage I palliation in infants with hypoplastic left heart syndrome. Pediatr Cardiol. 2018;39(04):837–843. doi: 10.1007/s00246-018-1836-5. [DOI] [PubMed] [Google Scholar]
- 24.Lau V I, Arntfield R T. Point-of-care transcranial Doppler by intensivists. Crit Ultrasound J. 2017;9(01):21. doi: 10.1186/s13089-017-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien N, Hall M. Cerebral blood flow in children requiring extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2013;14:126–134. doi: 10.1097/PCC.0b013e3182712d62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rilinger J F, Smith C M, deRegnier R AO. Transcranial Doppler identification of neurologic injury during pediatric extracorporeal membrane oxygenation therapy. J Stroke Cerebrovasc Dis. 2017;26(10):2336–2345. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Pediatric Neurocritical Care Research Group (PNCRG) . O'Brien N F, Buttram S DW, Maa T, Lovett M E, Reuter-Rice K, LaRovere K L. Cerebrovascular physiology during pediatric extracorporeal membrane oxygenation: a multicenter study using transcranial doppler ultrasonography. Pediatr Crit Care Med. 2019;20(02):178–186. doi: 10.1097/PCC.0000000000001778. [DOI] [PubMed] [Google Scholar]
- 28.Itoh S, Suda K, Kishimoto S. Microembolic signals measured by transcranial Doppler during transcatheter closure of atrial septal defect using the Amplatzer septal occluder. Cardiol Young. 2011;21(02):182–186. doi: 10.1017/S1047951110001733. [DOI] [PubMed] [Google Scholar]
- 29.Wallace S, Døhlen G, Holmstrøm H, Lund C, Russell D. Cerebral microemboli detection and differentiation during transcatheter closure of atrial septal defect in a paediatric population. Cardiol Young. 2015;25(02):237–244. doi: 10.1017/S1047951113002072. [DOI] [PubMed] [Google Scholar]
- 30.Adelson P D, Clyde B, Kochanek P M, Wisniewski S R, Marion D W, Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr Neurosurg. 1997;26(04):200–207. doi: 10.1159/000121192. [DOI] [PubMed] [Google Scholar]
- 31.Adelson P D, Srinivas R, Chang Y, Bell M, Kochanek P M. Cerebrovascular response in children following severe traumatic brain injury. Childs Nerv Syst. 2011;27(09):1465–1476. doi: 10.1007/s00381-011-1476-z. [DOI] [PubMed] [Google Scholar]
- 32.Vavilala M S, Lee L A, Boddu K. Cerebral autoregulation in pediatric traumatic brain injury. Pediatr Crit Care Med. 2004;5(03):257–263. doi: 10.1097/01.pcc.0000123545.69133.c3. [DOI] [PubMed] [Google Scholar]
- 33.Vavilala M S, Muangman S, Tontisirin N.Impaired cerebral autoregulation and 6-month outcome in children with severe traumatic brain injury: preliminary findings Dev Neurosci 200628(4-5):348–353. [DOI] [PubMed] [Google Scholar]
- 34.Vavilala M S, Muangman S, Waitayawinyu P. Neurointensive care; impaired cerebral autoregulation in infants and young children early after inflicted traumatic brain injury: a preliminary report. J Neurotrauma. 2007;24(01):87–96. doi: 10.1089/neu.2006.0058. [DOI] [PubMed] [Google Scholar]
- 35.Figaji A A, Zwane E, Fieggen A G. Pressure autoregulation, intracranial pressure, and brain tissue oxygenation in children with severe traumatic brain injury. J Neurosurg Pediatr. 2009;4(05):420–428. doi: 10.3171/2009.6.PEDS096. [DOI] [PubMed] [Google Scholar]
- 36.Trabold F, Meyer P G, Blanot S, Carli P A, Orliaguet G A. The prognostic value of transcranial Doppler studies in children with moderate and severe head injury. Intensive Care Med. 2004;30(01):108–112. doi: 10.1007/s00134-003-2057-8. [DOI] [PubMed] [Google Scholar]
- 37.Chaiwat O, Sharma D, Udomphorn Y, Armstead W M, Vavilala M S. Cerebral hemodynamic predictors of poor 6-month Glasgow outcome score in severe pediatric traumatic brain injury. J Neurotrauma. 2009;26(05):657–663. doi: 10.1089/neu.2008.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer P G, Ducrocq S, Rackelbom T, Orliaguet G, Renier D, Carli P. Surgical evacuation of acute subdural hematoma improves cerebral hemodynamics in children: a transcranial Doppler evaluation. Childs Nerv Syst. 2005;21(02):133–137. doi: 10.1007/s00381-004-1016-1. [DOI] [PubMed] [Google Scholar]
- 39.Figaji A A, Zwane E, Fieggen A G, Siesjo P, Peter J C. Transcranial Doppler pulsatility index is not a reliable indicator of intracranial pressure in children with severe traumatic brain injury. Surg Neurol. 2009;72(04):389–394. doi: 10.1016/j.surneu.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Melo J R, Di Rocco F, Blanot S. Transcranial Doppler can predict intracranial hypertension in children with severe traumatic brain injuries. Childs Nerv Syst. 2011;27(06):979–984. doi: 10.1007/s00381-010-1367-8. [DOI] [PubMed] [Google Scholar]
- 41.Benedik M P, Zaletel M, Meglic N P, Podnar T. A right-to-left shunt in children with arterial ischaemic stroke. Arch Dis Child. 2011;96(05):461–467. doi: 10.1136/adc.2010.203992. [DOI] [PubMed] [Google Scholar]
- 42.Perkovič-Benedik M, Zaletel M, Pečarič-Meglič N, Podnar T. A right-to-left shunt and prothrombotic disorders in pediatric patients presenting with transient ischemic attack. Eur J Pediatr. 2013;172(02):239–245. doi: 10.1007/s00431-012-1875-5. [DOI] [PubMed] [Google Scholar]
- 43.Goh D, Minns R A. Cerebral blood flow velocity monitoring in pyogenic meningitis. Arch Dis Child. 1993;68(01):111–119. doi: 10.1136/adc.68.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robba C, Cardim D, Czosnyka M. Ultrasound non-invasive intracranial pressure assessment in paediatric neurocritical care: a pilot study. Childs Nerv Syst. 2020;36(01):117–124. doi: 10.1007/s00381-019-04235-8. [DOI] [PubMed] [Google Scholar]
- 45.Fanelli A, Vonberg F W, LaRovere K L. Fully automated, real-time, calibration-free, continuous noninvasive estimation of intracranial pressure in children. J Neurosurg Pediatr. 2019;23:1–11. doi: 10.3171/2019.5.PEDS19178. [DOI] [PubMed] [Google Scholar]
- 46.O'Brien N F, Maa T, Moore-Clingenpeel M, Rosenberg N, Yeates K O. Relationships between cerebral flow velocities and neurodevelopmental outcomes in children with moderate to severe traumatic brain injury. Childs Nerv Syst. 2018;34(04):663–672. doi: 10.1007/s00381-017-3693-6. [DOI] [PubMed] [Google Scholar]
- 47.Philip S, Chaiwat O, Udomphorn Y. Variation in cerebral blood flow velocity with cerebral perfusion pressure >40 mm Hg in 42 children with severe traumatic brain injury. Crit Care Med. 2009;37(11):2973–2978. doi: 10.1097/CCM.0b013e3181a963f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowe L H, Morello F P, Jackson M A, Lasky A. Application of transcranial Doppler sonography in children with acute neurologic events due to primary cerebral and West Nile vasculitis. AJNR Am J Neuroradiol. 2005;26(07):1698–1701. [PMC free article] [PubMed] [Google Scholar]
- 49.Polito A, Ricci Z, Di Chiara L. Cerebral blood flow during cardiopulmonary bypass in pediatric cardiac surgery: the role of transcranial Doppler—a systematic review of the literature. Cardiovasc Ultrasound. 2006;4:47. doi: 10.1186/1476-7120-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kussman B D, Gauvreau K, DiNardo J A. Cerebral perfusion and oxygenation after the Norwood procedure: comparison of right ventricle-pulmonary artery conduit with modified Blalock-Taussig shunt. J Thorac Cardiovasc Surg. 2007;133(03):648–655. doi: 10.1016/j.jtcvs.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 51.Hillier S C, Burrows F A, Bissonnette B, Taylor R H. Cerebral hemodynamics in neonates and infants undergoing cardiopulmonary bypass and profound hypothermic circulatory arrest: assessment by transcranial Doppler sonography. Anesth Analg. 1991;72(06):723–728. doi: 10.1213/00000539-199106000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Cheng H H, Wypij D, Laussen P C. Cerebral blood flow velocity and neurodevelopmental outcome in infants undergoing surgery for congenital heart disease. Ann Thorac Surg. 2014;98(01):125–132. doi: 10.1016/j.athoracsur.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Misrahi S, Reuter-Rice K. Transcranial Doppler ultrasound use in pediatric patients with penetrating traumatic brain injuries. J Radiol Nurs. 2020;39(01):39–43. doi: 10.1016/j.jradnu.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lovett M E, Maa T, Moore-Clingenpeel M, O'Brien N F. Transcranial Doppler ultrasound findings in children with moderate-to-severe traumatic brain injury following abusive head trauma. Childs Nerv Syst. 2020;36(05):993–1000. doi: 10.1007/s00381-019-04431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guyatt G, Oxman A D, Akl E A. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(04):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 56.ACR–AIUM–SPR–SRU Practice parameter for the performance of transcranial Doppler ultrasound. Available at:https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-Transcranial.pdf?la=en [DOI] [PubMed]
- 57.https://www.intersocietal.org/vascular/standards/html/2018/fs.htm https://www.intersocietal.org/vascular/standards/html/2018/fs.htm
- 58.Consensus Group on Transcranial Doppler in Diagnosis of Brain Death.Latin American consensus on the use of transcranial Doppler in the diagnosis of brain death Rev Bras Ter Intensiva 20142603240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ducrocq X, Hassler W, Moritake K. Consensus opinion on diagnosis of cerebral circulatory arrest using Doppler-sonography: task force group on cerebral death of the Neurosonology Research Group of the World Federation of Neurology. J Neurol Sci. 1998;159(02):145–150. doi: 10.1016/s0022-510x(98)00158-0. [DOI] [PubMed] [Google Scholar]
- 60.TCCS Consensus Group . Nedelmann M, Stolz E, Gerriets T. Consensus recommendations for transcranial color-coded duplex sonography for the assessment of intracranial arteries in clinical trials on acute stroke. Stroke. 2009;40(10):3238–3244. doi: 10.1161/STROKEAHA.109.555169. [DOI] [PubMed] [Google Scholar]
- 61.Sociedad Española de Neurosonología . Calleja S, Tembl J I, Segura T. Recommendations of the use of transcranial Doppler to determine the existence of cerebral circulatory arrest as diagnostic support of brain death [in Spanish] Neurologia. 2007;22(07):441–447. [PubMed] [Google Scholar]
- 62.Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology . Sloan M A, Alexandrov A V, Tegeler C H. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004;62(09):1468–1481. doi: 10.1212/wnl.62.9.1468. [DOI] [PubMed] [Google Scholar]
- 63.Blanco P, Abdo-Cuza A. Transcranial Doppler ultrasound in neurocritical care. J Ultrasound. 2018;21(01):1–16. doi: 10.1007/s40477-018-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalanuria A, Nyquist P A, Armonda R A, Razumovsky A. Use of transcranial Doppler (TCD) ultrasound in the neurocritical care unit. Neurosurg Clin N Am. 2013;24(03):441–456. doi: 10.1016/j.nec.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Rasulo F A, De Peri E, Lavinio A. Transcranial Doppler ultrasonography in intensive care. Eur J Anaesthesiol Suppl. 2008;42:167–173. doi: 10.1017/S0265021507003341. [DOI] [PubMed] [Google Scholar]
- 66.Saqqur M, Zygun D, Demchuk A. Role of transcranial Doppler in neurocritical care. Crit Care Med. 2007;35 05:S216–S223. doi: 10.1097/01.CCM.0000260633.66384.FB. [DOI] [PubMed] [Google Scholar]
- 67.Bulas D I. Transcranial Doppler applications in neonates and children. Ultrasound Clin. 2009;4:533–551. [Google Scholar]
- 68.Verlhac S. Transcranial Doppler in children. Pediatr Radiol. 2011;41 01:S153–S165. doi: 10.1007/s00247-011-2038-y. [DOI] [PubMed] [Google Scholar]
- 69.Bulas D I, Jones A, Seibert J J, Driscoll C, O'Donnell R, Adams R J. Transcranial Doppler (TCD) screening for stroke prevention in sickle cell anemia: pitfalls in technique variation. Pediatr Radiol. 2000;30(11):733–738. doi: 10.1007/s002470000317. [DOI] [PubMed] [Google Scholar]
- 70.Lowe L H, Bulas D I. Transcranial Doppler imaging in children: sickle cell screening and beyond. Pediatr Radiol. 2005;35(01):54–65. doi: 10.1007/s00247-004-1257-x. [DOI] [PubMed] [Google Scholar]
- 71.Bulas D. Screening children for sickle cell vasculopathy: guidelines for transcranial Doppler evaluation. Pediatr Radiol. 2005;35(03):235–241. doi: 10.1007/s00247-005-1417-7. [DOI] [PubMed] [Google Scholar]
- 72.Tsivgoulis G, Alexandrov A V, Sloan M A. Advances in transcranial Doppler ultrasonography. Curr Neurol Neurosci Rep. 2009;9(01):46–54. doi: 10.1007/s11910-009-0008-7. [DOI] [PubMed] [Google Scholar]
- 73.American Society of Neuroimaging Practice Guidelines Committee . Alexandrov A V, Sloan M A, Tegeler C H. Practice standards for transcranial Doppler (TCD) ultrasound. Part II. Clinical indications and expected outcomes. J Neuroimaging. 2012;22(03):215–224. doi: 10.1111/j.1552-6569.2010.00523.x. [DOI] [PubMed] [Google Scholar]
- 74.Holey E A, Feeley J L, Dixon J, Whittaker V J. An exploration of the use of simple statistics to measure consensus and stability in Delphi studies. BMC Med Res Methodol. 2007;7:52. doi: 10.1186/1471-2288-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalaian S A, Kasim R M. Terminating sequential Delphi survey data collection. Pract Assess, Res Eval. 2012;17:1–10. [Google Scholar]
- 76.Bennett C, Vakil N, Bergman J. Consensus statements for management of Barrett's dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology. 2012;143(02):336–346. doi: 10.1053/j.gastro.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meshkat B, Cowman S, Gethin G. Using an e-Delphi technique in achieving consensus across disciplines for developing best practice in day surgery in Ireland. J Hosp Adm. 2014;3:1–8. [Google Scholar]
- 78.Murphy M K, Black N A, Lamping D L.Consensus development methods, and their use in clinical guideline development Health Technol Assess 1998203i–iv., 1–88 [PubMed] [Google Scholar]
- 79.Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41(04):376–382. doi: 10.1046/j.1365-2648.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- 80.Global Consensus Group Vakil N, van Zanten S V, Kahrilas P, Dent J, Jones R.The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus Am J Gastroenterol 2006101081900–1920., quiz 1943 [DOI] [PubMed] [Google Scholar]
- 81.Wood L, Bjarnason G A, Black P C. Using the Delphi technique to improve clinical outcomes through the development of quality indicators in renal cell carcinoma. J Oncol Pract. 2013;9(05):e262–e267. doi: 10.1200/JOP.2012.000870. [DOI] [PubMed] [Google Scholar]
- 82.Graefe A, Armstrong J S. Comparing face-to-face meetings, nominal groups, Delphi and prediction markets on an estimation task. Int J Forecast. 2016;27:183–195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


