Highlights
-
•
Astrocytes and microglia key fulfil homeostatic and immune functions in the CNS.
-
•
Dysfunction of these cell types is implicated in neurodegenerative diseases.
-
•
Understanding cellular autonomy and early pathogenic changes is a key goal.
-
•
New human iPSC models will inform on disease mechanisms and therapy development.
Abbreviations: Aβ, Amyloid-β; AD, Alzheimer’s disease; APOE, Apolipoprotein E; APP, Amyloid precursor protein; BMPs, Bone morphogenetic proteins; ALS, Amyotrophic Lateral Sclerosis; C9ORF72, Chromosome 9 open reading frame 72; CCL2, Chemokine (C-C motif) ligand 2; CNS, central nervous system; CNTF, Ciliary neurotrophic factor; EGF, Epidermal growth factor; FACS, fluorescence activated cell sorting; FGF, Fibroblast growth factor; FTD, Frontotemporal dementia; GBA1, Glucocerebrosidase beta enzyme 1; GD, Gaucher disease; HD, Huntington’s disease; HTT, Huntingtin; IFN-γ, Interferon gamma; GM-CSF, Granulocyte-macrophage colony-stimulating factor; hiPSCs, human induced pluripotent stem cells; JAK-STAT, Janus kinase-signal transducer and activator of transcription; LIF, Leukemia inhibitory factor; LPS, lipopolysaccharide; LRRK2, Leucine-rich repeat kinase 2; M-CSF, Macrophage colony stimulating factor; NFIA, Nuclear factor 1 A-type; NGF-β, Nerve growth factor; NPCs, neural progenitor cells; PD, Parkinson’s disease; sALS, sporadic ALS; SOD1, Superoxide dismutase 1; SOX9, SRY-box 9; TDP-43, TAR DNA-binding protein 43; TGF-β, Transforming growth factor beta; TNF-α, Tumor necrosis factor alpha; TREM2, Triggering receptor expressed on myeloid cells 2; VCP, Valosin-containing protein
Keywords: Astrocyte, Microglia, Neurodegenerative disease, Human iPSC, In vitro
Abstract
Both astrocytes and microglia fulfil homeostatic and immune functions in the healthy CNS. Dysfunction of these cell types have been implicated in the pathomechanisms of several neurodegenerative diseases. Understanding the cellular autonomy and early pathological changes in these cell types may inform drug screening and therapy development. While animal models and post-mortem tissue have been invaluable in understanding disease processes, the advent of human in vitro models provides a unique insight into disease biology as a manipulable model system obtained directly from patients. Here, we discuss the different human in vitro models of astrocytes and microglia and outline the phenotypes that have been recapitulated in these systems.
1. Introduction
Astrocytes and microglia possess crucial homeostatic roles that are vital for neuronal function and survival, including metabolic support and synaptic regulation (Salter and Stevens, 2017; Vasile et al., 2017). Together, both cell types comprise the main immune component of the intact central nervous system (CNS) and can undergo dramatic reactive changes in response to their environment, with subsequent beneficial or detrimental effects on surrounding neurons (Anderson et al., 2016; Liddelow et al., 2017; Butovsky and Weiner, 2018). However, the spatiotemporal interplay between these cell types and their reactive states is only beginning to be resolved. Dysfunction of homeostatic processes and transformation to a reactive state in both astrocytes and microglia has been implicated in the pathomechanisms of several neurodegenerative diseases (Philips and Rothstein, 2014; Karran and De Strooper, 2016; Hickman et al., 2018; Clarke and Patani, 2020).
Although astrocytes and microglia share some common functions, their developmental origin is vastly different. Like neurons, astrocytes derive from the neuroectoderm and their precursors undergo a temporally regulated and likely epigenetically mediated gliogenic switch dependent on cardiotrophin and Notch signalling (Sloan and Barres, 2014; Tchieu et al., 2019). This process occurs through the activation of the Janus kinase-signal transducer and activator of transcription proteins (JAK-STAT) pathway and the critical transcription factors, SRY-box 9 (SOX9) and Nuclear factor 1 A-type (NFIA) (Kamakura et al., 2004; Kang et al., 2012). Astrocyte precursors migrate radially from the ventricular zone where they terminally differentiate and occupy mostly non-overlapping domains (Ge et al., 2012; Tien et al., 2012; Tsai et al., 2012). Both region specific encoding and local microenvironmental cues consolidate astrocytic identity likely depending on the specific requirements of interacting neurons within a particular neuroglial unit (Clarke et al., 2020a). Once mature, few astrocytes are proliferative even in response to either injury or disease (Colodner et al., 2005; Sofroniew and Vinters, 2010; Liddelow and Barres, 2017).
Meanwhile, microglia arise from mesodermal cells in the yolk sac which invade the CNS early during development, preceding astrogliogenesis (Ginhoux et al., 2010; Gomez Perdiguero et al., 2015). These yolk sac derived erythro-myeloid progenitors establish a distinct microglial identity different to macrophages through a combination of both ontological and environmental cues (Gosselin et al., 2017; Bennett et al., 2018). Microglial differentiation is independent of the transcription factor MYB but reliant on two other transcription factors, PU.1 and IRF8 (Schulz et al., 2012; Kierdorf et al., 2013). Consolidation of microglial identity is dependent on Macrophage colony-stimulating factor (M-CSF), Transforming growth factor beta (TGF-β) and IL-34 signaling (Butovsky et al., 2014; Muffat et al., 2016; Abud et al., 2017; Amos et al., 2017; Utz et al., 2020). Once mature, microglia establish non-overlapping domains in the adult CNS and, unlike astrocytes, remain highly mobile (Davalos et al., 2005; Nimmerjahn et al., 2005). The microglial pool is maintained locally without peripheral myeloid contribution and can proliferate upon stimulation with certain reactive stimuli (Bruttger et al., 2015; Tay et al., 2017; Rossi et al., 2018).
In neurodegeneration research, poor translation of findings from preclinical models has been in part attributed to the failure of animal models to faithfully recapitulate disease phenotypes. Human astrocytes and microglia display differences to their mouse counterparts in terms of their morphology, gene expression and function (Oberheim et al., 2012; Tarassishin et al., 2014; Zhang et al., 2016; Galatro et al., 2017; Gosselin et al., 2017). Access to post-mortem human astrocytes or microglia is limited and only provides information of the end stage of the disease. The seminal finding of the reprogramming of somatic nucleated mouse (Takahashi and Yamanaka, 2006) and later human cells (Takahashi et al., 2007) into induced pluripotent stem cells (hiPSCs) has greatly enhanced the ability of researchers to study different human CNS cell types in vitro, including both astrocytes and microglia. These advancements have enabled the investigation of the primacy of pathological events, cellular autonomy and cell type specific pathobiology in a human model while opening new possibilities for drug screening and development. hiPSC derived astrocytes and microglia allow for the study of highly pure populations of these cell types directly from patients, expressing mutations at physiological levels. Furthermore, the use of zinc finger nucleases or CRISPR technology has enabled the attainment of isogenic control lines, insertion of disease-causing mutations or knockout of disease-relevant genes, enabling the study of specific disease-causing genes in cell biology. However, it is important to consider the limitations of any model system, as each has advantages and disadvantages depending on the scientific question being addressed. Human in vitro models are more time-consuming and expensive to make than other in vitro models and cannot truly model the in vivo environment. It is also more difficult to model the complex multiple cell-cell interactions that exist in vivo. Furthermore, hiPSCs represent a fetal maturational status which do not capture some age-related phenotypes that are relevant to the study of neurodegenerative diseases (Patani et al., 2012; Ziff and Patani, 2019) and in particular to the study of astrocytes and microglia, which undergo major transcriptional changes during aging (Soreq et al., 2017). Human in vitro models are nevertheless emerging as an indispensable tool for investigating the primacy of pathological events and resolving cell autonomous pathological mechanisms. Here, we summarise the different human in vitro models of astrocytes and microglia and discuss recent findings of key disease phenotypes in these models in the context of neurodegenerative disorders.
1.1. Human in vitro astrocyte models
Protocols for obtaining human astrocytes or microglia in vitro attempt to recapitulate steps that occur during development (Table 1) (Tyzack et al., 2016; Haenseler and Rajendran, 2019). Human astrocytes have been derived from a variety of sources including hiPSCs (Krencik et al., 2011; Hall et al., 2017), embryonic stem cells (Gupta et al., 2012), immortalised cell lines (Price et al., 1999; Langley et al., 2009; Furihata et al., 2016), directly reprogrammed from fibroblasts (Meyer et al., 2014) or immunopanned from fetal or adult healthy donors (Zhang et al., 2016), each with their own advantages and disadvantages (Table 2). Human in vitro astrocyte models are able to recapitulate several physiological functions including glutamate uptake, inflammatory and calcium responses to stimuli and promoting neuronal synapse formation, neurite outgrowth and electrophysiological maturation (Hall et al., 2017; Bradley et al., 2019; Taga et al., 2019; Thelin et al., 2020). Furthermore, human astrocyte progenitors can be implanted into immunosuppressed rodents where they later express mature astrocyte markers and promote improved performance in cognitive tasks (Han et al., 2013; Haidet-Phillips et al., 2015).
Table 1.
Summary of different human in vitro astrocyte protocols.
| Citation Reference | Summary |
|---|---|
| Krencik et al. (2011) | Immature astrocytes specifiable to the forebrain or spinal cord with FGF8 or RA, respectively, from hiPSCs using a 180-day protocol. Progenitors expanded with EGF + FGF2 for >150 days and then terminally differentiated with CNTF for 7 days. Astrocytes elicited electrophysiological responses to glutamate, propagated calcium waves upon mechanical stimulation, performed glutamate uptake and promoted synapse formation of co-cultured neurons. |
| Gupta et al. (2012) | Human ES cell derived astrocytes obtained through a combination of BMP-mediated Smad and LIF-mediated JAK-STAT signalling. Neuroprotective properties of astrocyte conditioned media after exposure of human ES cell derived neurons to oxidative stress through glutathione-dependent and independent mechanisms. |
| Serio et al. (2013) | Astrocytes from TARDBP mutant hiPSCs. NPCs grown in suspension as neurospheres, enriched with LIF and EGF (4−6wks) followed by expansion with EGF and FGF2 and then CNTF for terminal differentiation. |
| Meyer et al. (2014) | Fibroblast derived astrocytes from SOD1A4V fALS, C9ORF72 fALS and sporadic ALS patients. Conversion to tripotent iNPCs through infection with Sox2, KLF4, Oct3/4, c-Myc, followed by switch to medium containing FGF2, EGF and heparin. Astrocytic differentiation initiated through seeding in NPC medium in fibronectin-coated dish, followed by 10% FBS and 0.3% N2. |
| Zhang et al. (2016) | Purification of astrocytes from fetal, juvenile and adult brains via immunopanning technique using anti-HepaCAM antibodies. |
| Hall et al. (2017) | iPSCs from VCP mutant fibroblasts. Astrocytes generated in monoculture throughout - FGF used to expand and BMP4 and LIF used to terminally differentiate. |
| Sloan et al. (2017) | Patterning 3D brain spheroids from hiPSCs to dorsal or ventral forebrain fate for up to 590 days. Astrocytes isolated by immunopanning with anti-HepaCAM antibodies perform phagocytic function, promote synapse formation and calcium signalling of co-cultured neurons. |
| Li et al. (2018) | Expression of NFIA and SOX9 speeds up iPSC derived astrocyte generation which display functional attributes including promoting neurite outgrowth, calcium waves after mechanical stimulation and glutamate uptake. |
Table 2.
Advantages and disadvantages of human in vitro astrocyte models.
| Model | Strengths | Limitations |
|---|---|---|
| hiPSC | High purity | Expensive |
| Ability to self-renew | Time consuming | |
| Developmental model | ||
| ESCs | Same as hiPSCs | Same as hiPSCs plus ethical concerns |
| Transdifferentiated fibroblasts | Preserved age of donor | Limited supply |
| Faster than stem cell-based protocols | Reliant on expression of known factors | |
| Currently reliant on serum affecting reactivity | ||
| Immunopanned primary astrocytes | Ability to study cells exposed to in vivo cell-cell interactions | Limited supply |
| Can obtain from adult donors | ||
| Immortalised cell line | Fast | Karyotype abnormalities |
| Ability to self-renew | Abnormal proliferative state | |
| Currently reliant on serum affecting reactivity |
Directed differentiation of astrocytes from hiPSCs generally follows a method of converting hiPSCs to neural progenitors, patterning to a certain region of the neuraxis, propagation in vitro for a protracted period of time (e.g. 50–70 days) to permit the gliogenic switch followed by terminal differentiation to astrocytes (Tyzack et al., 2016). hiPSCs have the capacity to generate a high purity of terminally differentiated astrocytes from a potentially unlimited population of cells. They also have the advantage over embryonic stem cells of avoiding some ethical considerations. However, deriving astrocytes from this method is time-consuming and costly. Furthermore, hiPSC derived astrocytes are only partially mature compared to adult astrocytes found in vivo and are currently unable to capture age-related phenotypes that have been described in astrocytes transdifferentiated from patient fibroblasts (Meyer et al., 2014). Often a combination of 3D embryoid body and monolayer components are used to derive astrocytes, but protocols using exclusively monolayer cultures have also been developed (Hall et al., 2017), allowing for increased enrichment of cultures and therefore reduction of heterologous cell-cell interactions. However, a limitation of monoculture is that it does not mimic the 3D architecture of the developing nervous system. Indeed, the presence of other cell types can be beneficial as cell-cell interactions can be studied and purified populations can be dissociated and sorted (Krencik et al., 2017; Sloan et al., 2017). Many protocols have successfully generated highly pure cultures of mature astrocytes (>90 %) in the absence of fluorescence activated cell sorting (FACS), however FACS can be used as a tool to select for certain astrocyte-specific markers (Barbar et al., 2020).
hiPSCs are typically converted to neural progenitor cells via dual SMAD inhibition under feeder-free conditions. Dual SMAD inhibition results in the loss of pluripotency markers such as OCT4 and NANOG and the acquisition of neural progenitor markers such as NESTIN, MUSASHI, PAX6 and SOX1. Regional identity of neural progenitors can be achieved through the application of developmentally-inspired extrinsic cues including diffusible morphogens, such as Wnts, Bone morphogenetic proteins (BMPs), sonic hedgehog along the dorsoventral axis and retinoic acid, Wnts and Fibroblast growth factor (FGF) along the rostrocaudal axis (Krencik et al., 2011; Imaizumi et al., 2015; Hall et al., 2017; Bradley et al., 2019; Clarke et al., 2020b). Neural progenitor cells (NPCs) can be expanded using growth factors such as Epidermal growth factor (EGF) and/or FGF. Over time, neural progenitors spontaneously undergo a gliogenic switch due to the inhibition of neurogenesis and activation of gliogenic JAK-STAT pathways. This process can be accelerated using inducible expression of NFIA and/or SOX9 (Li et al., 2018; Tchieu et al., 2019). Terminal differentiation can be accelerated using BMPs in combination with IL-6 family cytokines, including Ciliary neurotrophic factor (CNTF) or Leukemia inhibitory factor (LIF), through neuregulin application or by exposure to serum. However, serum has been shown to induce a reactive state in astrocytes (Foo et al., 2011; Perriot et al., 2018) and so serum-free approaches to human astrocyte derivation are preferable. In addition to the use of serum, matrix topography has been implicated in driving astrocyte reactivity in vitro (Puschmann et al., 2013; Watson et al., 2017). For example, astrocytes cultured in 3D collagen hydrogels and nanofibres are less likely to exhibit reactive phenotypes than those grown in 2D matrices (East et al., 2009; Tiryaki et al., 2015).
As a model of ageing, transdifferentiation of human fibroblasts has the advantage over hiPSC cultures since it has been demonstrated that differentiated neurons retain age related transcripts and display impaired nucleocytoplasmic compartmentalisation due to loss of RANBP17 (Mertens et al., 2015). This model may be particularly useful in the study of neurodegenerative diseases where ageing is a key risk factor. Astrocytes can also be obtained faster than hiPSC-based protocols. However, this method generates a limited supply of cells and currently lacks the ability to pattern neural progenitors to different areas of the neuraxis. Furthermore, published protocols for transdifferentiated astrocytes have used serum containing media (Meyer et al., 2014; Varcianna et al., 2019) and so the feasibility of this approach in serum free conditions remains to be comprehensively demonstrated.
1.2. Human in vitro microglia models
Several human in vitro microglial models have been developed in recent years (Table 3) (Hasselmann and Blurton-Jones, 2020; Sabogal-Guáqueta et al., 2020). Cells obtained from these protocols display many microglial specific genes and carry out microglial functions such as phagocytosis and cytokine release in response to proinflammatory stimulation. However, several of these protocols produce macrophages which have not been validated as independent of the transcription factor MYB, a key determinant of the correct ontogeny to yolk sac-derived fetal macrophages (Schulz et al., 2012), and vary in the enrichment of microglia obtained (Buchrieser et al., 2017; Haenseler and Rajendran, 2019). Some protocols instead have distinguished microglia from macrophages by calcium responses to adenosine diphosphate (Douvaras et al., 2017), with respiratory bursts or displaying contact inhibition in vitro (Nimmerjahn et al., 2005; Pocock and Piers, 2018). Microglial specific markers P2RY12 or TMEM119 have also been used but this may not be reliable due to changes in different reactive states and the acquisition of certain macrophages expressing microglial specific markers upon their entry into the CNS during chronic inflammation (Grassivaro et al., 2020).
Table 3.
Summary of different human in vitro microglia protocols.
| Citation Reference | Summary |
|---|---|
| Etemad et al. (2012) | Primary monocytes cultured with M-CSF, GM-CSF, NGF and CCL2 acquire a ramified morphology and lower levels of CD45, CD14, HLA-DR, CD11b and CD11c. |
| Ohgidani et al. (2014) | Primary monocytes converted to microglia-like cells with incubation of GM-CSF and IL-34 to a more ramified morphology. |
| Muffat et al. (2016) | Microglia-like cells expressing TMEM119, from hESCs and hiPSCs. EBs cultured on murine embryonic fibroblast feeders before differentiation with M-CSF and IL-34. Can co-culture in 3D with neurons and astrocytes and they respond to LPS with cytokine secretion. |
| Abud et al. (2017) | Initially hiPSCs cultured in 5% oxygen conditions during haematopoiesis, then M-CSF, IL-34 and TGF-β followed by maturation with CD200 and CX3CL1 and then FACS for CD43. Microglia-like cells exhibit cytokine secretion, cell migration, responses of calcium and phagocytosis. |
| Douvaras et al. (2017) | Microglia-like cells isolated by FACS for CD14 and CX3CR1. Microglia-like cells express IBA1, CD11c, TMEM119, P2RY12, CD11b and CX3CR1. Release cytokines after LPS/IFNγ treatment, phagocytose and are calcium responsive after ADP treatment. |
| Haenseler et al. (2017a,b) | MYB-independent hiPSC derived microglia are motile and phagocytotic when co-cultured with iPSC derived cortical neurons and secrete cytokines upon treatment with LPS. |
| Pandya et al. (2017) | hiPSCs differentiated to microglia-like cells with GM-CSF, M-CSF, and IL-3 on astrocyte monolayers before being MACS sorted for CD11b/CD39. Express HLA-DR, CD45, TREM-2 and CX3CR1 but are negative for CD86, CD206, CD200R and CD80. Phagocytose and secrete TNF-α after LPS treatment. |
| McQuade et al. (2018) | Updated simplified Abud et al., protocol without the need for 5% oxygen or cell sorting. |
hiPSCs can be directed to mesodermal cell lineage through application of BMPs, VEGF and/or SCF. This is then followed by application of ligands such as M-CSF, IL-3, IL-34 and additional factors such as CD200 and CX3CL1 to promote microglial differentiation (Muffat et al., 2016; Abud et al., 2017; Douvaras et al., 2017; Haenseler et al., 2017a; Pandya et al., 2017; McQuade et al., 2018). Some protocols use cell sorting to obtain pure cultures of microglia, although this is not required for high purity (Abud et al., 2017; Douvaras et al., 2017; Pandya et al., 2017). Other protocols attempt to consolidate microglial identity through co-culture with neurons or astrocytes (Muffat et al., 2016; Haenseler et al., 2017a; Pandya et al., 2017). No direct comparison has been made between different protocols, but it is clear that extended incubation with microglial associated ligands,co-culture with CNS cell types, or application of neural precursor conditioned media (Banerjee et al., 2020) aids the consolidation of a microglial phenotype. Human microglia developed in vitro have been shown to successfully engraft into mice brains, retain their human microglial identity and perform key functions including synaptic pruning (Mancuso et al., 2019; Svoboda et al., 2019; Xu et al., 2020).
In addition to hiPSC based microglial protocols, human microglia have also been modelled using primary human monocytes (Etemad et al., 2012) and immortalised cell lines (Tsuchiya et al., 1980; Janabi et al., 1995). These cells can be obtained faster than hiPSC based protocols but are limited in terms of their supply or experience karyotype abnormalities, respectively. Furthermore, both of these methods lack the important distinct ontological background of microglia. Some studies have attempted to address this limitation by treating primary cells with factors used to promote microglial differentiation. Treatment of primary human peripheral blood cells with Granulocyte-macrophage colony-stimulating factor (GM-CSF), M-CSF, Chemokine (C-C motif) ligand 2 (CCL2), Nerve growth factor (NGF-β) and IL-34 induced a ramified morphology with increased expression of microglial specific genes such as TGFBR1, PROS1, P2RX7 and C1QB (Etemad et al., 2012; Ryan et al., 2017). Furthermore, treatment with only IL-34 and GM-CSF has been shown to be sufficient to recapitulate a more microglial identity with increased CX3CR1 and decreased CD45, CD14 and CCR2 (Ohgidani et al., 2014) (Table 4).
Table 4.
Advantages and disadvantages of human in vitro microglia models.
| Model | Strengths | Limitations |
|---|---|---|
| hiPSC | High purity | Expensive |
| Ability to self-renew | Time consuming | |
| Developmental model | ||
| Primary macrophages | Can obtain from adult donors | Limited supply |
| Different ontogeny | ||
| Immortalised cell line | Fast | Karyotype abnormalities |
| Ability to self-renew | Abnormal proliferative state | |
| Currently reliant on serum affecting reactivity |
2. Reactive transformation of hiPSC derived astrocytes and microglia
Astrocytes and microglia are able to undergo dramatic changes in gene expression in response to a wide array of stimuli in a process termed reactive gliosis. These states have been classified as a continuum between either M1/A1 or M2/A2 depending on their neurotoxic or neuroprotective attributes, respectively. However, this classification may be an oversimplification as a range of reactive states is likely to exist expressing both protective and detrimental markers in a non-mutually exclusive manner (Ransohoff, 2016; Liddelow and Barres, 2017). Reactive states may be transient and are likely to be temporally regulated depending on the chronicity of the stimulus and the age of the cell. Human in vitro models have further demonstrated the cellular autonomy of astrocyte and microglial reactive processes, as highly purified populations of these cell types can be obtained that are naive to interactions with other cell types.
hiPSC derived astrocytes respond differentially to different cytokines in terms of their transcriptional profiles and secretion of factors (Perriot et al., 2018; Thelin et al., 2020). As previously mentioned above, it is important to note that the presence of serum has been associated with the transformation to an irreversible reactive state and thus studies investigating reactivity should be performed under serum-free conditions (Foo et al., 2011; Perriot et al., 2018). The adoption of an A1 reactive state through stimulation with TNF-α, IL-1α and C1q has been recently recapitulated in an important comprehensive study using hiPSC derived astrocytes (Barbar et al., 2020). In this study, it was demonstrated that upon adoption of an A1 reactive state, astrocytes release proinflammatory factors whilst simultaneously losing homeostatic functions such as phagocytosis and glutamate uptake. It is currently unclear whether this increase in secretion of factors and the loss of homeostatic functions are mechanistically linked or merely correlated with one another.
hiPSC derived microglia have also been demonstrated to undergo reactive changes in response to inflammatory stimuli. Stimulation of microglia with lipopolysaccharide (LPS)/Interferon gamma (IFN-γ) resulted in morphological changes and cytokine release (Haenseler et al., 2017a; Garcia-Reitboeck et al., 2018). However, the impact of reactive stimulation on other functions of microglia is lacking. Furthermore, while both hiPSC derived astrocytes and microglia have been found to become reactive when stimulated with pro-inflammatory factors, the effects of anti-inflammatory stimuli on morphology, gene expression and function remain unexplored. It is important to note that both astrocyte and microglial hiPSC protocols may affect subsequent reactivity. Other than the effects of serum on reactive state, other factors including LIF, TGF-β and M-CSF used in published protocols have been associated with affecting reactive profiles of astrocytes and microglia (Gowing et al., 2009; Sofroniew and Vinters, 2010; Cekanaviciute et al., 2014).
3. Using human in vitro models to study astrocytes and microglia in neurodegeneration
While neurodegenerative diseases are characterized by the loss of specific neuronal populations, dysfunction of both astrocytes and microglia have been heavily implicated in contributing to neuronal death. Several genes which are highly expressed in astrocytes and/or microglia are linked to neurodegenerative diseases including APOE4, TREM2, CR1 and C9ORF72. Furthermore, prion-like spread of pathological proteins between neurons and glia has been reported including Amyloid-β (Aβ) in Alzheimer’s disease (AD) (Veeraraghavalu et al., 2014), α-synuclein in Parkinson’s disease (PD) (Lee et al., 2010; Loria et al., 2017), Huntingtin in Huntington’s disease (HD) (Pearce et al., 2015; Donnelly et al., 2020) and TAR DNA-binding protein 43 (TDP-43) in Amyotrophic Lateral Sclerosis (ALS) (Smethurst et al., 2020). Human in vitro models of astrocytes and microglia are able to capture some phenotypes of these neurodegenerative diseases and demonstrate that a particular phenotype is cell autonomous. Furthermore, findings from human in vitro models of neurodegenerative diseases have begun to shed light on the primacy of pathological events. With many genetic traits of neurodegenerative diseases occurring in glial cells, it is unsurprising that much focus has been on modelling astrocytes and microglia derived from patients with identified disease-linked mutations. iPSC-derived human in vitro models have provided valuable insight into the pathogenic mechanisms that underlie neurodegenerative disease, but remain limited in the study of sporadic cases.
3.1. Alzheimer’s disease
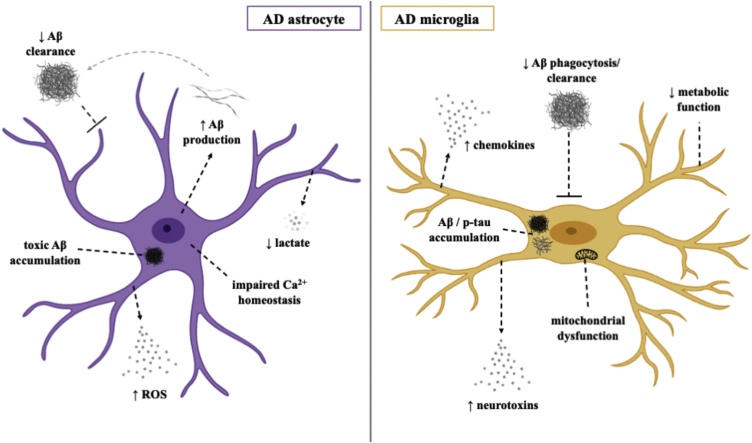
AD is the most common cause of dementia worldwide and is characterised by progressive decline in cognitive functions, particularly episodic memory. AD neuropathology includes the presence of extracellular Aβ plaques and intraneuronal neurofibrillary tangles made up of hyperphosphorylated Tau, both contributing to synaptic dysfunction and subsequent neurotoxicity, found throughout the cerebral cortex and sub-cortical regions in the post-mortem brain (Braak and Braak, 1991). Many of the GWAS-identified loci that confer genetic risk for late-onset AD are highly expressed in astrocytes and microglia, with the first identified and most studied being the APOE4 allele of the polymorphic APOE4 gene (Corder et al., 1993). Physiological Apolipoprotein E (APOE) serves as a lipid carrier and helps to support synaptic integrity and promote neuronal survival (Liu et al., 2013). With its primary source of expression being astrocytes, and to a lesser extent microglia and neurons, the APOE4 variant has been shown to disrupt physiological glial cell function, highlighting a potential mechanism by which astrocytes and microglia are involved in AD pathogenesis. Phenotypes associated with AD that have been identified in human in vitro models of astrocytes and microglia are summarised in Fig. 1.
Fig. 1.
Pathomechanisms of AD in human in vitro astrocytes and microglia.
3.1.1. Astrocytes
Astrocytes are crucial in the clearance of metabolites and toxins, including Aβ. In response to increasing levels of Aβ in the AD brain, astrocytes become reactive and undergo functional changes that impact clearance mechanisms, thus compromising synaptic and neuronal viability (Abramov et al., 2003; Rowland et al., 2018). In addition to the contribution of Aβ accumulation through defective clearance, reactive astrocytes have also been implicated in the production of Aβ possibly through an increase in β-Secretase 1 activity (Oksanen et al., 2017) a rate-limiting enzyme that catalyses the initial cleavage of Amyloid precursor protein (APP). While reactive astrogliosis represents an additional hallmark of AD, and one that precedes neuronal death, the direct contribution to disease progression remains unclear.
Such astrocyte pathology in AD has been recapitulated through various human in vitro iPSC models. iPSC-derived astrocytes from both familial and sporadic AD patients have been shown to exhibit disease phenotypes, including global morphological abnormalities and aberrant secretion of several factors (Jones et al., 2017). In an important study, hiPSC derived astrocytes generated from patients with PSEN1 exon 9 deletions exhibited increased Aβ production, impaired cytokine release, overproduction of reactive oxide species and disrupted calcium metabolism (Oksanen et al., 2017). Similar stress-induced changes were also reported in an hiPSC derived astrocyte model from AD patients with APP mutations, suggested to be attributed specifically to intracellular accumulation of Aβ oligomers (Kondo et al., 2013). Morphological abnormalities, namely generalized atrophy, and altered expression of astrocytic markers have also been described in hiPSC derived astrocytes from PSEN1M146L and APOE4+/+ patients (Jones et al., 2017; Lin et al., 2018). APOE4-hiPSC derived astrocytes were also shown to be less efficient in Aβ uptake and clearance than APOE3-astrocytes, an effect that correlated with changes in lipid metabolism (Lin et al., 2018). Importantly, conversion of APOE4 to APOE3 in a sporadic AD line enhanced the ability of both hiPSC derived astrocytes and microglia to perform Aβ uptake. Another recent study using hiPSC derived astrocytes to explore the role of APOE4 in AD pathogenesis demonstrated marked reduction in supportive neurotrophic and synaptogenic functions in APOE4+/+ astrocytes compared to those with an APOE3+/+ profile (Zhao et al., 2017).
3.1.2. Microglia
Aβ accumulation seems to instigate prolonged recruitment and clustering of microglia at the site of senile plaques in the AD brain. While microglia express receptors implicated in Aβ clearance and phagocytosis (Simard et al., 2006), such as CD36, and RAGE (Yan et al., 1996; El Khoury et al., 1998) why Aβ continues to accumulate and AD pathology progresses despite recruitment of supposedly protective cells remains unclear. However, evidence points to a combination of dysfunctional Aβ-clearing mechanisms (Hickman et al., 2008) and Aβ-induced activation of proinflammatory processes through upregulated secretion of cytokines and neurotoxins (Guillot-Sestier and Town, 2013). Microglial expression of known AD risk factors such as TREM2 and CD33 further emphasises a likely role microglia play in AD pathogenesis (Abud et al., 2017). A recent hiPSC derived microglial model from healthy patients demonstrated some physiological properties of microglia, including phagocytosis of Aβ, tau oligomers and human synaptosomes (Abud et al., 2017), providing a suitable platform to uncover pathogenic pathways.
A recent study comparing hiPSC derived microglia from AD patients and cognitively normal age-matched controls reported stronger phagocytic function in AD-microglia with or without a LPS induced inflammatory stimulus (Xu et al., 2019). High concentrations of LPS were significantly less toxic to AD-microglia and led to increased cytokine secretion, a phenotype that mimics pathological changes in AD microglia (Xu et al., 2019). Increased reactive oxide species has also been observed in hiPSC microglia from sporadic AD patients, as well as increased phagocytosis in response to H2O2 treatment (Zhang et al., 2020). When compared to those from PSEN1ΔE9 and APP mutation backgrounds, microglia from APOE4+/+ patients exhibit significantly impaired phagocytosis, migration and metabolic function as well as increased cytokine production (Konttinen et al., 2019). To ascertain the effect of APOE4 on AD pathology in a 3D system, hiPSC derived microglia from APOE3 and APOE4 backgrounds were co-cultured with APP duplication organoids (Lin et al., 2018). Identification of longer microglial processes, heightened levels of phosphorylated Tau and increased Aβ deposition in APOE4-organoids suggested that the APOE4+/+ genotype confers microglial dysfunction through reduced clearance of Aβ (Lin et al., 2018).
TREM2 represents another genetic risk factor associated with a greater risk of developing AD. TREM2 knockout hiPSC microglia have been shown to elicit increased apoptotic cell death and impaired phagocytic ability (Claes et al., 2019; Hall-Roberts et al., 2020; McQuade et al., 2020). In addition, TREM2 knockout microglia transplanted into a mouse model AD failed to effectively migrate and cluster around Aβ plaques. Significant metabolic deficits including mitochondrial dysfunction and an inability to carry out a glycolytic immunometabolic switch have been observed in TREM2R47H hiPSC derived microglia, due to dysregulation in PPARγ/p38MAPK signaling pathways (Piers et al., 2020). Activation of these pathways ameliorated these deficits and rescued microglial protective functions such as Aβ phagocytosis (Piers et al., 2020). However, other studies have found no dysfunction in phagocytic ability in TREM2R47H human in vitro models of microglia (Claes et al., 2019; Hall-Roberts et al., 2020).
A 3D human tri-culture model of AD enabling the investigation of the important interactions between neurons, astrocytes and microglia has been recently developed (Park et al., 2018). This was achieved by overexpression of mutant APP in neural progenitor cells to produce neurons and astrocytes before immortalised adult microglia were added. Increased Aβ and phosphorylated Tau were observed in the 3D system compared to a 2D system of mutant APP neurons and astrocytes. Microglia cultured with APP mutant neurons and astrocytes became more motile in a CCL2 dependent process and released a number of pro-inflammatory cytokines and chemokines. Furthermore, microglia in this setting induced the death of neurons and astrocytes in a process partly dependent on TLR4 and IFN-γ. The development of this model has provided a unique opportunity to better understand cellular interplay in the CNS as well as the influence of cell specific effects of AD-associated mutations and the potential interactions between different mutations occurring in astrocytes, microglia and neurons.
3.2. Parkinson’s disease
PD is the second most prevalent neurodegenerative disease, affecting both motor and non-motor functions and is characterized by substantial loss of dopaminergic neurons in the substantia nigra pars compacta. While the distinguishing neuropathological hallmark of PD is the presence of Lewy bodies, made up of misfolded and abnormally aggregated α-synuclein, evidence of inflammatory activity in the CNS is another major pathological feature of PD including activation of both astrocytes and microglia (Imamura et al., 2003; Miklossy et al., 2006; Doorn et al., 2014).
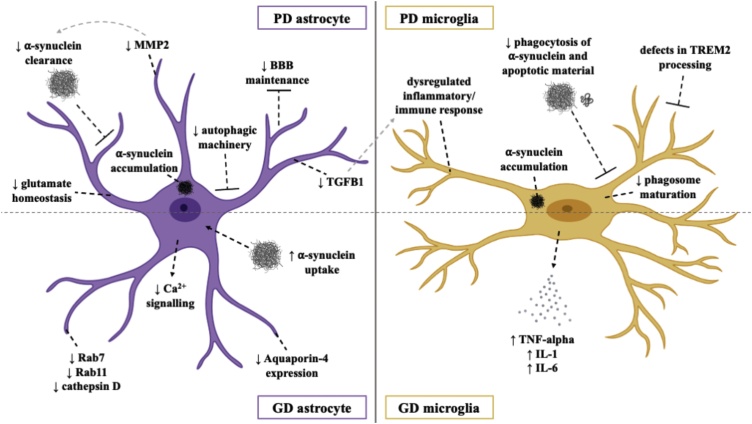
Mutations in Leucine-rich repeat kinase 2 (LRRK2) are known to cause autosomal dominant and sporadic PD, with the majority of mutations occurring within enzymatic domains. Such mutations have been found to upregulate inherent LRRK2 kinase activity and reduce GTPase activity (Ferreira and Massano, 2017). With LRRK2 variants also being associated with autoimmune and infectious diseases, it is unsurprising that LRRK2 is expressed in numerous immune cells such as microglia (Moehle et al., 2012; Lee et al., 2020). Studies using hiPSCs to investigate PD pathogenesis have focused predominantly on mechanisms underlying the degeneration and death of dopaminergic neurons. Studies using hiPSCs generated from PD patients with LRRK2 mutations have described the appearance of disease-specific phenotypes in hiPSC derived neurons, including impaired axonal outgrowth, increased susceptibility to oxidative stress (Nguyen et al., 2011), pathogenic activation of the unfolded protein response (Heman-Ackah et al., 2016) and deficient autophagic vacuole clearance (Sánchez-Danés et al., 2012). Phenotypes identified in human in vitro astrocyte and microglial models of PD are detailed below (Fig. 2).
Fig. 2.
Pathomechanisms of PD in human in vitro astrocytes and microglia.
3.2.1. Astrocytes
Post-mortem discovery of astrocytic accumulation of α-synuclein in the PD brain (Wakabayashi et al., 2000; Braak et al., 2007) prompted a shift in focus to the role of astrocyte dysfunction in PD pathogenesis. A myriad of recent studies have supported the emerging phenomena of pathogenic α-synuclein transfer and accumulation via aberrant neuron-astrocyte interactions, disruption to normal astrocytic function and subsequent loss of neuronal viability (Gu et al., 2010; Lee et al., 2010; Booth et al., 2017; Cavaliere et al., 2017). Such functional disruptions include impaired autophagy (di Domenico et al., 2019), glutamate homeostasis and blood-brain-barrier maintenance (Gu et al., 2010).
A recent notable study employed a co-culture system of hiPSC derived astrocytes from patients with PD-causing LRRK2 mutations and healthy hiPSC derived dopaminergic neurons to explore the pathogenic impact on neuron-astrocyte crosstalk. LRRK2 mutant astrocytes elicited a significant reduction in the survival rate of dopaminergic neurons, coupled with defective autophagic machinery and abnormal accumulation of endogenous α-synuclein (di Domenico et al., 2019). In the same study, treatment with an activator of chaperone-mediated autophagy ameliorated dysfunction of this process and prevented the manifestation of disease phenotypes in PD astrocytes. Another study, also using hiPSCs derived from PD LRRK2-positive patients, reported astrocyte-specific downregulation of genes associated with inhibition of microglial inflammatory responses and α-synuclein aggregate degradation (Booth et al., 2019), importantly implicating the loss of astrocyte neuroprotective capacity as a key player in the development of PD pathology.
Gaucher disease (GD) is a lysosomal storage disorder elicited by mutations in the gene that encodes the Glucocerebrosidase beta 1 (GBA1) enzyme, also representing a critical risk factor for PD and the related dementia with Lewy bodies (Sidransky et al., 2009; Nalls et al., 2013). Using hiPSC derived astrocytes from patients with GD type 1 (non-neuronopathic with and without PD) and type 2 (neuronopathic), a recent study reported astrogliosis in GD2-astrocytes as measured by increased GFAP and marked reduction in Aquaporin-4 expression (Aflaki et al., 2020) a channel implicated in blood-brain-barrier function, water transport and astrocyte migration (Nagelhus and Ottersen, 2013). GD type 1 and 2 hiPSC derived astrocytes also displayed dysfunctional calcium signalling and when incubated with α-synuclein monomers or fibrils showed increased uptake and reduced expression of endosomal markers RAB7 and RAB11 and lysosomal marker Cathepsin D, suggesting compromised α-synuclein clearance (Aflaki et al., 2020). PD and GD-associated astrocyte and microglial phenotypes identified through human in vitro models can be seen in Fig. 2.
3.2.2. Microglia
Microglial phenotypes have also been reported in hiPSC models of PD. An hiPSC derived microglia-like macrophage model from an early-onset PD patient with a SNCA triplication demonstrated a significant increase in both intracellular and extracellular α-synuclein when compared to controls (Haenseler et al., 2017b), mirroring effects seen in an hiPSC derived astrocyte model of PD. This study also reported altered expression of CXCL1, IL-18 and IL-22 and reduced phagocytic competence in PD microglial-like cells, which was replicated in control macrophages upon addition of monomeric α-synuclein to the culture medium (Haenseler et al., 2017b). Together, these findings suggest that a pathogenic build-up of α-synuclein in microglia-like cells results in a loss of function mechanism where the inherent clearance properties of microglia are jeopardised.
Recently, an elegant study uncovered a link between inflammation and neurodegeneration in PD using hiPSC-derived dopaminergic neurons and microglia carrying the LRRK2G2019S mutation (Panagiotakopoulou et al., 2020). It was demonstrated that LRRK2G2019S microglia display increased motility, phagocytic capacity and abnormal metabolic activity and immune responses to IFN-γ or LPS stimulation. Furthermore, LRRK2G2019S but not control microglial conditioned media treated with LPS reduced neurite length of hiPSC dopaminergic neurons, directly implicating altered microglial immune responses in neuronal dysfunction in PD.
Another study exploring LRRK2 function in hiPSC derived macrophages found genetic knockout or pharmacological inhibition of LRRK2 impeded phagosome maturation, contributing to mycobacterial replication and dampening innate immune responses (Härtlova et al., 2018). Using an hiPSC derived LRRK2 knockout microglial model and an isogenic control line, a more recent study highlighted a specific role of LRRK2 in the recruitment of RAB8a and RAB10 to phagosomes, identifying its function at the junction between phagosome maturation and recycling pathways (Lee et al., 2020). PD relevant microglial phenotypes have also been described in hiPSC microglia-like macrophages derived from GD patients with GBA1 mutations including elevated expression of TNF-α, IL-6 and IL-1, further exacerbated by LPS-stimulation (Panicker et al., 2012, 2014).
3.3. Huntington’s disease
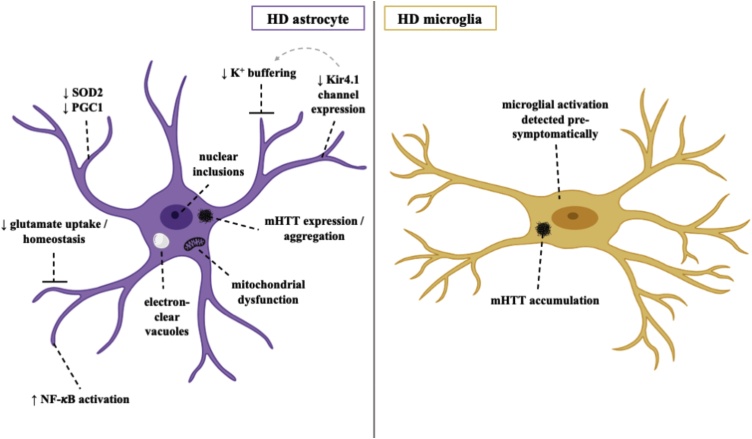
HD is a rare and rapidly progressive hereditary neurodegenerative disorder caused by CAG repeat expansions in the Huntingtin (HTT) gene, accumulation of its mutant protein (mHTT) (Bates et al., 2015) and consequent detriment to glial health and neuronal survival. Such neuropathology is most prevalent in the neostriatum of the HD brain, with marked loss of neostriatal medium spiny neurons (Benraiss et al., 2016). Several causative mechanisms have been proposed for this HD-specific degeneration profile, including non-cell-autonomous contribution from astrocytes (Juopperi et al., 2012) (Fig. 3).
Fig. 3.
Pathomechanisms of HD in human in vitro astrocytes and microglia.
3.3.1. Astrocytes
Mounting evidence implicating both reactive astrocytes and microglia in HD pathogenesis has led to the publication of many studies exploring their dysfunction, primarily in rodent models. Engraftment of mHTT-expressing human glial progenitor cells has been shown to inflict a disease phenotype on healthy mice, with healthy glia alleviating such effects in transgenic HD mice (Benraiss et al., 2016). Expression of mHTT in mouse astrocytes specifically has been shown to induce functional atrophic changes that may drive excitotoxicity, such as impaired glutamate homeostasis (Liévens et al., 2001; Bradford et al., 2009; Jiang et al., 2016), mitochondrial dysfunction (Schon and Przedborski, 2011) and diminished capacity to buffer extracellular potassium through downregulation of a striatum-specific Kir4.1 potassium channel (Tong et al., 2014). As with other neurodegenerative disorders, dysregulation in astrocytic neuroinflammatory signaling pathways is also implicated in HD pathogenesis such as chronic activation of NF-κB (Hsiao et al., 2013). While these findings are undeniably useful, few studies have investigated such effects in human astrocyte models of HD.
hiPSC derived HD astrocytes provide less functional support for neuronal maturation when co-cultured with HD and control neurons and are unable to protect neurons as effectively from glutamate-induced cytotoxicity (Garcia et al., 2019). Using hiPSC derived astrocytes from a father and daughter with adult onset and juvenile HD respectively revealed a unique vacuolation phenotype (Juopperi et al., 2012). This disease-specific astrocytic phenotype, marked by the presence of cytoplasmic electron-clear vacuoles, had previously only been observed in peripheral blood lymphocytes from HD patients, thus suggesting a potential role of vacuolation in HD pathogenesis (Juopperi et al., 2012). In a more recent study, an astrocyte differentiation protocol from hiPSCs of human oligomeric mHTT-injected HD monkeys was developed (Cho et al., 2019). Consistent with previous findings, astrocytic expression of mHTT induced a plethora of downstream pathogenic effects, including cytosolic mHTT aggregation and nuclear inclusion formation, impaired glutamate uptake capacity and down-regulated expression of SOD2 and PGC1 (Cho et al., 2019). Importantly, successful amelioration and reversal of such HD astrocyte-specific phenotypes was observed upon expression of anti-HTT small-hairpin RNA molecules. While there is undeniable demand for further study, these hiPSC-derived HD models collectively highlight the critical influence of mHTT on human astrocytes, thus challenging our view on the exclusivity of neuron-focused disease mechanisms and bringing alternative drug targets to light.
3.3.2. Microglia
The identification of reactive microglia in the neostriatum, cortex and globus pallidus of human HD brain tissue sparked interest in the role of microglia and neuroinflammation in HD pathogenesis, with the number of reactive microglia directly correlating with degree of neuronal cell death (Sapp et al., 2001). A correlation between microglial activation and disease severity has been described in both HD mice and patients (Pavese et al., 2006; Simmons et al., 2007), which when considered together with microglial suppression correlating with the prolonged lifespan of HD mice (Zwilling et al., 2011) reinforces microglial involvement in HD. The aberrant accumulation of mHTT in microglia has also been considered an integral component of progressive neurodegeneration in HD, with their activation detected pre-symptomatically in HD carriers as well as in the post-mortem HD brain (Yang et al., 2017). To the authors’ knowledge, however, a viable hiPSC derived microglia model for HD has yet to be reported.
3.4. Amyotrophic lateral sclerosis and frontotemporal dementia
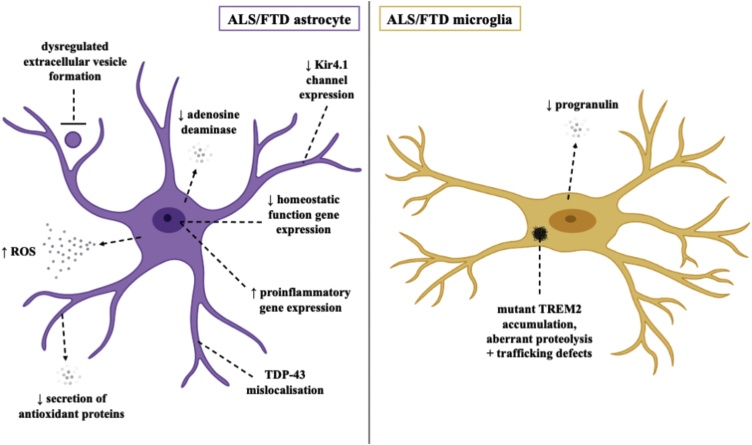
ALS is a rapidly progressing disease characterized by the degeneration of upper and lower motor neurons (Hardiman et al., 2017). Overlap of clinical, cellular and genetic aspects of disease with Frontotemporal dementia (FTD), a disease characterised by progressive cognitive decline and behavioural abnormalities, has reframed ALS and FTD as part of a disease spectrum. Several genes encoding proteins with diverse cellular functions have been implicated as causative for ALS including C9ORF72, SOD1, TARDBP, FUS and VCP. Both astrocytes and microglia have been implicated as important modulators of the pathological process of motor neuron death in ALS in animal models of the disease (Boillée et al., 2006; Yamanaka et al., 2008). Human in vitro models of astrocytes have recapitulated some of the phenotypes observed in human post-mortem tissue and animal models of ALS, while investigation of ALS human in vitro microglia models is currently lacking (Fig. 4).
Fig. 4.
Pathomechanisms of ALS/FTD in human in vitro astrocytes and microglia.
3.4.1. Astrocytes
The neuroprotective capacity of hiPSC derived astrocytes to motor neurons has been recently demonstrated using sporadic ALS (sALS) spinal cord extracts to seed aggregation of TDP-43 into hiPSC derived motor neurons (Smethurst et al., 2020). In this study, astrocyte conditioned media reduced mislocalization and aggregation of cytoplasmic TDP-43 and improved motor neuron survival.
hiPSC derived astrocytes harbouring ALS causing mutations have been shown to recapitulate some ALS phenotypes and be detrimental to co-cultured neurons. TDP-43 mutant astrocytes have been shown to display TDP-43 mislocalisation (Serio et al., 2013). While these mutant astrocytes did not affect the survival of co-cultured neurons, an important observation was that mutant astrocytes undergo cell death themselves. While TDP-43 mutant astrocytes did not cause the death of co-cultured neurons, ALS causing Valosin-containing protein (VCP) mutant astrocytes exhibited disrupted support of motor neuron survival in co-culture in addition to a cell autonomous survival phenotype (Hall et al., 2017). Furthermore, in an important study utilising astrocytes derived from transdifferentiated human fibroblasts from both sporadic and familial ALS cases including mutant Superoxide dismutase 1 (SOD1) and Chromosome 9 open reading frame 72 (C9ORF72), also were toxic to co-cultured motor neurons (Meyer et al., 2014). sALS astrocytes transplanted into the spinal cords of immunodeficient mice induced motor neuron death and motor deficits (Qian et al., 2017).
Astrocytes are thought to contribute to ALS pathology through both a transformation to a toxic reactive state and through the loss of homeostatic functions. In SOD1 mutant astrocytes derived from hiPSCs, many proinflammatory genes were increased while several genes associated with homeostatic functions were decreased (Tyzack et al., 2017). SOD1 mutant astrocytes also express decreased levels of Kir4.1, which is critical in regulating fast firing motor neuron function and may partly account for the specific vulnerability of fast firing motor neurons in ALS (Kelley et al., 2018). Downregulation of the adenosine deaminase was found in astrocytes transdifferentiated from C9ORF72 ALS human fibroblasts, correlating with increased toxicity of co-cultured motor neurons likely due to an increase in the substrate, adenosine, and decrease in product, inosine (Allen et al., 2019). These astrocytes have also been reported to display dysregulated extracellular vesicle formation (Varcianna et al., 2019). In this study, C9ORF72 astrocyte extracellular vesicles recapitulated toxicity of conditioned media to motor neurons, which may be due to the dysregulation of miRNAs such as miR-494-3p.
There has been some controversy over the toxicity of C9ORF72 ALS hiPSC derived astrocytes, with one group reporting loss of co-cultured hiPSC derived motor neuron electrophysiological function but no effect on viability (Zhao et al., 2020). However, other groups found that hiPSC derived C9ORF72 astrocyte conditioned media was toxic to both hiPSC derived motor neurons and mouse cortical neurons, possibly due to the acquisition of a senescent phenotype, decreased secretion of antioxidant proteins and increased reactive oxide species production (Madill et al., 2017; Birger et al., 2019). Further studies using the same lines with isogenic controls and standardized differentiation paradigms will help to reconcile these seemingly divergent results.
3.4.2. Microglia
Very few studies have investigated phenotypes of ALS/FTD in human in vitro models of microglia. hiPSC derived microglia have been developed from patients with FTD causing progranulin mutations (Almeida et al., 2012), however, other than reduced progranulin levels no phenotypes have been reported. hiPSC derived microglia have been generated from lines of the FTD-like syndrome, Nasu Hakola disease, which is due to missense mutations in TREM2 (Brownjohn et al., 2018). However, although deficits in TREM2 were observed in microglia, no functional defects in phagocytosis or secretion of inflammatory cytokines IL-6, TNF-α and IL-1β in response to LPS treatment were observed.
4. Conclusions
The advent of human in vitro models of astrocytes and microglia has opened exciting new possibilities for disease modelling and drug screening. Both astrocyte and microglia human in vitro models partially recapitulate the transcriptomes of their in vivo counterparts and are able to capture authentic disease phenotypes. Further investigation of monocultures to resolve cellular autonomy in disease, as well as co-culture paradigms between astrocytes and microglia and between neurons and glia will provide a deeper insight into the cellular interplay of different diseases and may lead to the development of cell type specific therapies.
Funding information
This work was supported by the Francis Crick Institute which receives its core funding from Cancer Research UK (FC010110), the UK Medical Research Council (FC010110), and the Wellcome Trust (FC010110). R.P. holds an MRC Senior Clinical Fellowship [MR/S006591/1].
Acknowledgements
This work was supported by the Francis Crick Institute which receives its core funding from Cancer Research UK (FC010110), the UK Medical Research Council (FC010110), and the Wellcome Trust (FC010110). R.P. holds an MRC Senior Clinical Fellowship [MR/S006591/1]. Figures were created with BioRender.com.
Footnotes
The Peer Review Overview and Supplementary data associated with this article can be found in the online version, at doi:https://doi.org/10.1016/j.pneurobio.2020.101973.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abramov A.Y., Canevari L., Duchen M.R. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J. Neurosci. 2003;23(12):5088–5095. doi: 10.1523/JNEUROSCI.23-12-05088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abud E.M., Ramirez R.N., Martinez E.S., Healy L.M., Nguyen C.H.H., Newman S.A. iPSC-derived human microglia-like cells to study neurological diseases. Neuron. 2017;94(2):278–293. doi: 10.1016/j.neuron.2017.03.042. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflaki E., Stubblefield B.K., McGlinchey R.P., McMahon B., Ory D.S., Sidransky E. A characterization of Gaucher iPS-derived astrocytes: potential implications for Parkinson’s disease. Neurobiol. Dis. 2020;134 doi: 10.1016/j.nbd.2019.104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S.P., Hall B., Castelli L.M., Francis L., Woof R., Siskos A.P. Astrocyte adenosine deaminase loss increases motor neuron toxicity in amyotrophic lateral sclerosis. Brain. 2019;142(3):586–605. doi: 10.1093/brain/awy353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida S., Zhang Z., Coppola G., Mao W., Futai K., Karydas A. Induced pluripotent stem cell models of progranulin-deficient frontotemporal dementia uncover specific reversible neuronal defects. Cell Rep. 2012;2(4):789–798. doi: 10.1016/j.celrep.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos P.J., Fung S., Case A., Kifelew J., Osnis L., Smith C.L. Modulation of hematopoietic lineage specification impacts TREM2 expression in microglia-like cells derived from human stem cells. ASN Neuro. 2017;9(4) doi: 10.1177/1759091417716610. 1759091417716610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M.A., Burda J.E., Ren Y., Ao Y., O’Shea T.M., Kawaguchi R. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P., Paza E., Perkins E.M., James O.G., Kenkhuis B., Lloyd A.F., Burr K., Story D., Yusuf D., He X., Backofen R., Dando O., Chandran S., Priller J. Generation of pure monocultures of human microglia-like cells from induced pluripotent stem cells. Stem Cell Res. 2020;49 doi: 10.1016/j.scr.2020.102046. [DOI] [PubMed] [Google Scholar]
- Barbar L., Jain T., Zimmer M., Kruglikov I., Sadick J.S., Wang M. CD49f is a novel marker of functional and reactive human iPSC-derived astrocytes. Neuron. 2020;107(3):436–453. doi: 10.1016/j.neuron.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G.P., Dorsey R., Gusella J.F., Hayden M.R., Kay C., Leavitt B.R. Huntington disease. Nat. Rev. Dis. Primers. 2015;1:15005. doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- Bennett F.C., Bennett M.L., Yaqoob F., Mulinyawe S.B., Grant G.A., Hayden Gephart M. A combination of ontogeny and CNS environment establishes microglial identity. Neuron. 2018;98(6):1170–1183. doi: 10.1016/j.neuron.2018.05.014. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A., Wang S., Herrlinger S., Li X., Chandler-Militello D., Mauceri J. Human glia can both induce and rescue aspects of disease phenotype in Huntington disease. Nat. Commun. 2016;7:11758. doi: 10.1038/ncomms11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birger A., Ben-Dor I., Ottolenghi M., Turetsky T., Gil Y., Sweetat S. Human iPSC-derived astrocytes from ALS patients with mutated C9ORF72 show increased oxidative stress and neurotoxicity. EBioMedicine. 2019;50:274–289. doi: 10.1016/j.ebiom.2019.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillée S., Yamanaka K., Lobsiger C.S., Copeland N.G., Jenkins N.A., Kassiotis G. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Booth H.D.E., Hirst W.D., Wade-Martins R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017;40(6):358–370. doi: 10.1016/j.tins.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth H.D.E., Wessely F., Connor-Robson N., Rinaldi F., Vowles J., Browne C. RNA sequencing reveals MMP2 and TGFB1 downregulation in LRRK2 G2019S Parkinson’s iPSC-derived astrocytes. Neurobiol. Dis. 2019;129:56–66. doi: 10.1016/j.nbd.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H., Sastre M., Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114(3):231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Bradford J., Shin J.Y., Roberts M., Wang C.E., Li X.J., Li S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc. Natl. Acad. Sci. U. S. A. 2009;106(52):22480–22485. doi: 10.1073/pnas.0911503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.A., Shireman J., McFalls C., Choi J., Canfield S.G., Dong Y. Regionally specified human pluripotent stem cell-derived astrocytes exhibit different molecular signatures and functional properties. Development. 2019;146(13) doi: 10.1242/dev.170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownjohn P.W., Smith J., Solanki R., Lohmann E., Houlden H., Hardy J. Functional studies of missense TREM2 mutations in human stem cell-derived microglia. Stem Cell Rep. 2018;10(4):1294–1307. doi: 10.1016/j.stemcr.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruttger J., Karram K., Wörtge S., Regen T., Marini F., Hoppmann N. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity. 2015;43(1):92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Buchrieser J., James W., Moore M.D. Human induced pluripotent stem cell-derived macrophages share ontogeny with MYB-Independent tissue-resident macrophages. Stem Cell Rep. 2017;8(2):334–345. doi: 10.1016/j.stemcr.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O., Weiner H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018;19(10):622–635. doi: 10.1038/s41583-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O., Jedrychowski M.P., Moore C.S., Cialic R., Lanser A.J., Gabriely G. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere F., Cerf L., Dehay B., Ramos-Gonzalez P., De Giorgi F., Bourdenx M. In vitro α-synuclein neurotoxicity and spreading among neurons and astrocytes using Lewy body extracts from Parkinson disease brains. Neurobiol. Dis. 2017;103:101–112. doi: 10.1016/j.nbd.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Cekanaviciute E., Dietrich H.K., Axtell R.C., Williams A.M., Egusquiza R., Wai K.M. Astrocytic TGF-β signaling limits inflammation and reduces neuronal damage during central nervous system Toxoplasma infection. J. Immunol. 2014;193(1):139–149. doi: 10.4049/jimmunol.1303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I.K., Hunter C.E., Ye S., Pongos A.L., Chan A.W.S. Combination of stem cell and gene therapy ameliorates symptoms in Huntington’s disease mice. NPJ Regen. Med. 2019;4:7. doi: 10.1038/s41536-019-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes C., Van Den Daele J., Boon R., Schouteden S., Colombo A., Sebastian Monasor L., Fiers M., Ordovas L., Nami F., Bohrmann B., Tahirovic S., De Strooper B., Verfaille C.M. Human stem cell–derived monocytes and microglia‐like cells reveal impaired amyloid plaque clearance upon heterozygous or homozygous loss of TREM2. Alzheimers Dementia. 2019;15(3):453–464. doi: 10.1016/j.jalz.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Clarke B.E., Patani R. The microglial component of amyotrophic lateral sclerosis. Brain. 2020 doi: 10.1093/brain/awaa309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B.E., Taha D.M., Tyzack G.E., Patani R. Regionally encoded functional heterogeneity of astrocytes in health and disease: a perspective. Glia. 2020;69(1):20–27. doi: 10.1002/glia.23877. [DOI] [PubMed] [Google Scholar]
- Clarke B.E., Taha D.M., Ziff O.J., Alam A., Thelin E.P., Marco Garcia N., Helmy A., Patani R. Human stem cell-derived astrocytes exhibit region-specific heterogeneity in their secretory profiles. Brain. 2020;143(10):85. doi: 10.1093/brain/awaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colodner K.J., Montana R.A., Anthony D.C., Folkerth R.D., De Girolami U., Feany M.B. Proliferative potential of human astrocytes. J. Neuropathol. Exp. Neurol. 2005;64(2):163–169. doi: 10.1093/jnen/64.2.163. [DOI] [PubMed] [Google Scholar]
- Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Davalos D., Grutzendler J., Yang G., Kim J.V., Zuo Y., Jung S. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- di Domenico A., Carola G., Calatayud C., Pons-Espinal M., Muñoz J.P., Richaud-Patin Y. Patient-specific iPSC-derived astrocytes contribute to non-cell-autonomous neurodegeneration in Parkinson’s disease. Stem Cell Reports. 2019;12(2):213–229. doi: 10.1016/j.stemcr.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly K.M., DeLorenzo O.R., Zaya A.D., Pisano G.E., Thu W.M., Luo L. Phagocytic glia are obligatory intermediates in transmission of mutant huntingtin aggregates across neuronal synapses. Elife. 2020:9. doi: 10.7554/eLife.58499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorn K.J., Moors T., Drukarch B., van de Berg W.Dj, Lucassen P.J., van Dam A.M. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol. Commun. 2014;2:90. doi: 10.1186/s40478-014-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvaras P., Sun B., Wang M., Kruglikov I., Lallos G., Zimmer M. Directed differentiation of human pluripotent stem cells to microglia. Stem Cell Rep. 2017;8(6):1516–1524. doi: 10.1016/j.stemcr.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East E., Golding J.P., Phillips J.B. A versatile 3D culture model facilitates monitoring of astrocytes undergoing reactive gliosis. J. Tissue Eng. Regener. Med. 2009;3(8):634–646. doi: 10.1002/term.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J., Hickman S.E., Thomas C.A., Loike J.D., Silverstein S.C. Microglia, scavenger receptors, and the pathogenesis of Alzheimer’s disease. Neurobiol. Aging. 1998;19(Suppl. 1):S81–4. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- Etemad S., Zamin R.M., Ruitenberg M.J., Filgueira L. A novel in vitro human microglia model: characterization of human monocyte-derived microglia. J. Neurosci. Methods. 2012;209(1):79–89. doi: 10.1016/j.jneumeth.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Ferreira M., Massano J. An updated review of Parkinson’s disease genetics and clinicopathological correlations. Acta Neurol. Scand. 2017;135(3):273–284. doi: 10.1111/ane.12616. [DOI] [PubMed] [Google Scholar]
- Foo L.C., Allen N.J., Bushong E.A., Ventura P.B., Chung W.S., Zhou L. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71(5):799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furihata T., Ito R., Kamiichi A., Saito K., Chiba K. Establishment and characterization of a new conditionally immortalized human astrocyte cell line. J. Neurochem. 2016:92–105. doi: 10.1111/jnc.13358. [DOI] [PubMed] [Google Scholar]
- Galatro T.F., Holtman I.R., Lerario A.M., Vainchtein I.D., Brouwer N., Sola P.R. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 2017;20(8):1162–1171. doi: 10.1038/nn.4597. [DOI] [PubMed] [Google Scholar]
- Garcia V.J., Rushton D.J., Tom C.M., Allen N.D., Kemp P.J., Svendsen C.N. Huntington’s disease patient-derived astrocytes display electrophysiological impairments and reduced neuronal support. Front. Neurosci. 2019;13:669. doi: 10.3389/fnins.2019.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Reitboeck P., Phillips A., Piers T.M., Villegas-Llerena C., Butler M., Mallach A. Human induced pluripotent stem cell-derived microglia-like cells harboring TREM2 missense mutations show specific deficits in phagocytosis. Cell Rep. 2018;24(9):2300–2311. doi: 10.1016/j.celrep.2018.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W.P., Miyawaki A., Gage F.H., Jan Y.N., Jan L.Y. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012;484(7394):376–380. doi: 10.1038/nature10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D., Skola D., Coufal N.G., Holtman I.R., Schlachetzki J.C.M., Sajti E. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356(6344) doi: 10.1126/science.aal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing G., Lalancette-Hébert M., Audet J.N., Dequen F., Julien J.P. Macrophage colony stimulating factor (M-CSF) exacerbates ALS disease in a mouse model through altered responses of microglia expressing mutant superoxide dismutase. Exp. Neurol. 2009;220(2):267–275. doi: 10.1016/j.expneurol.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Grassivaro F., Menon R., Acquaviva M., Ottoboni L., Ruffini F., Bergamaschi A. Convergence between microglia and peripheral macrophages phenotype during development and neuroinflammation. J. Neurosci. 2020;40(4):784–795. doi: 10.1523/JNEUROSCI.1523-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X.L., Long C.X., Sun L., Xie C., Lin X., Cai H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol. Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot-Sestier M.V., Town T. Innate immunity in Alzheimer’s disease: a complex affair. CNS Neurol. Disord. Drug Targets. 2013;12(5):593–607. doi: 10.2174/1871527311312050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K., Patani R., Baxter P., Serio A., Story D., Tsujita T. Human embryonic stem cell derived astrocytes mediate non-cell-autonomous neuroprotection through endogenous and drug-induced mechanisms. Cell Death Differ. 2012;19(5):779–787. doi: 10.1038/cdd.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenseler W., Rajendran L. Concise Review: Modeling Neurodegenerative Diseases with Human Pluripotent Stem Cell-Derived Microglia. Stem Cells. 2019;37(6):724–730. doi: 10.1002/stem.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenseler W., Sansom S.N., Buchrieser J., Newey S.E., Moore C.S., Nicholls F.J. A highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Rep. 2017;8(6):1727–1742. doi: 10.1016/j.stemcr.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenseler W., Zambon F., Lee H., Vowles J., Rinaldi F., Duggal G. Excess α-synuclein compromises phagocytosis in iPSC-derived macrophages. Sci. Rep. 2017;7(1):9003. doi: 10.1038/s41598-017-09362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidet-Phillips A.M., Doreswamy A., Gross S.K., Tang X., Campanelli J.T., Maragakis N.J. Human glial progenitor engraftment and gene expression is independent of the ALS environment. Exp. Neurol. 2015;264:188–199. doi: 10.1016/j.expneurol.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.E., Yao Z., Choi M., Tyzack G.E., Serio A., Luisier R. Progressive motor neuron pathology and the role of astrocytes in a human stem cell model of VCP-related ALS. Cell Rep. 2017;19(9):1739–1749. doi: 10.1016/j.celrep.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Roberts H., Agarwal D., Obst J., Smith T.B., Monzón-Sandoval J., Di Daniel E., Webber C., James W.S., Mead E., Davis J.B., Cowley S.A. TREM2 Alzheimer’s variant R47H causes similar transcriptional dysregulation to knockout, yet only subtle functional phenotypes in human iPSC-derived macrophages. Alzheimers Res Ther. 2020;12 doi: 10.1186/s13195-020-00709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Chen M., Wang F., Windrem M., Wang S., Shanz S. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12(3):342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers. 2017;3:17071. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- Härtlova A., Herbst S., Peltier J., Rodgers A., Bilkei-Gorzo O., Fearns A. LRRK2 is a negative regulator of. EMBO J. 2018;37(12) doi: 10.15252/embj.201798694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmann J., Blurton-Jones M. Human iPSC-derived microglia: a growing toolset to study the brain’s innate immune cells. Glia. 2020;68(4):721–739. doi: 10.1002/glia.23781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heman-Ackah S.M., Bassett A.R., Wood M.J. Precision modulation of neurodegenerative disease-related gene expression in human iPSC-derived neurons. Sci. Rep. 2016;6:28420. doi: 10.1038/srep28420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S.E., Allison E.K., El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008;28(33):8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S., Izzy S., Sen P., Morsett L., El Khoury J. Microglia in neurodegeneration. Nat. Neurosci. 2018;21(10):1359–1369. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao H.Y., Chen Y.C., Chen H.M., Tu P.H., Chern Y. A critical role of astrocyte-mediated nuclear factor-κB-dependent inflammation in Huntington’s disease. Hum. Mol. Genet. 2013;22(9):1826–1842. doi: 10.1093/hmg/ddt036. [DOI] [PubMed] [Google Scholar]
- Imaizumi K., Sone T., Ibata K., Fujimori K., Yuzaki M., Akamatsu W. Controlling the regional identity of hPSC-derived neurons to uncover neuronal subtype specificity of neurological disease phenotypes. Stem Cell Rep. 2015;5(6):1010–1022. doi: 10.1016/j.stemcr.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K., Hishikawa N., Sawada M., Nagatsu T., Yoshida M., Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106(6):518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Janabi N., Peudenier S., Heron B., Ng K., Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci. Lett. 1995:105–108. doi: 10.1016/0304-3940(94)11792-h. [DOI] [PubMed] [Google Scholar]
- Jiang R., Diaz-Castro B., Looger L.L., Khakh B.S. Dysfunctional calcium and glutamate signaling in striatal astrocytes from Huntington’s disease model mice. J. Neurosci. 2016;36(12):3453–3470. doi: 10.1523/JNEUROSCI.3693-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juopperi Ta, Kim Wr, Chiang Ch, Yu H., Margolis Rl, Ross Ca. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Mol. Brain. 2012;5:17. doi: 10.1186/1756-6606-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura S., Oishi K., Yoshimatsu T., Nakafuku M., Masuyama N., Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat. Cell Biol. 2004;6(6):547–554. doi: 10.1038/ncb1138. [DOI] [PubMed] [Google Scholar]
- Kang P., Lee H.K., Glasgow S.M., Finley M., Donti T., Gaber Z.B. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron. 2012;74(1):79–94. doi: 10.1016/j.neuron.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E., De Strooper B. The amyloid cascade hypothesis: are we poised for success or failure? J. Neurochem. 2016;139(Suppl. 2):237–252. doi: 10.1111/jnc.13632. [DOI] [PubMed] [Google Scholar]
- Kelley K.W., Ben Haim L., Schirmer L., Tyzack G.E., Tolman M., Miller J.G. Kir4.1-dependent astrocyte-fast motor neuron interactions are required for peak strength. Neuron. 2018;98(2):306–319. doi: 10.1016/j.neuron.2018.03.010. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K., Erny D., Goldmann T., Sander V., Schulz C., Perdiguero E.G. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013;16(3):273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kondo T., Asai M., Tsukita K., Kutoku Y., Ohsawa Y., Sunada Y. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell. 2013;12(4):487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Konttinen H., Cabral-da-Silva M.E.C., Ohtonen S., Wojciechowski S., Shakirzyanova A., Caligola S. PSEN1ΔE9, APPswe, and APOE4 confer disparate phenotypes in human iPSC-derived microglia. Stem Cell Rep. 2019;13(4):669–683. doi: 10.1016/j.stemcr.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R., Weick J.P., Liu Y., Zhang Z.J., Zhang S.C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 2011;29(6):528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R., Seo K., van Asperen J.V., Basu N., Cvetkovic C., Barlas S. Systematic three-dimensional coculture rapidly recapitulates interactions between human neurons and astrocytes. Stem Cell Rep. 2017;9(6):1745–1753. doi: 10.1016/j.stemcr.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley R.R., Fan D., Guo L., Zhang C., Qingtang L., Brantley E.C. Generation of an immortalized astrocyte cell line from H-2Kb-tsA58 mice to study the role of astrocytes in brain metastasis. Int. J. Oncol. 2009:665–672. doi: 10.3892/ijo_00000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Suk J.E., Patrick C., Bae E.J., Cho J.H., Rho S. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010;285(12):9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Flynn R., Sharma I., Haberman E., Carling P.J., Nicholls F.J. LRRK2 is recruited to phagosomes and co-recruits RAB8 and RAB10 in human pluripotent stem cell-derived macrophages. Stem Cell Rep. 2020;14(5):940–955. doi: 10.1016/j.stemcr.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Tao Y., Bradley R., Du Z., Kong L., Dong Y. Fast generation of functional subtype astrocytes from human pluripotent stem cells. Stem Cell Rep. 2018;11(4):998–1008. doi: 10.1016/j.stemcr.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S.A., Barres B.A. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46(6):957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liévens J.C., Woodman B., Mahal A., Spasic-Boscovic O., Samuel D., Kerkerian-Le Goff L. Impaired glutamate uptake in the R6 Huntington’s disease transgenic mice. Neurobiol. Dis. 2001;8(5):807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- Lin Y.T., Seo J., Gao F., Feldman H.M., Wen H.L., Penney J. APOE4 causes widespread molecular and cellular alterations associated with alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98(6):1294. doi: 10.1016/j.neuron.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria F., Vargas J.Y., Bousset L., Syan S., Salles A., Melki R. α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol. 2017;134(5):789–808. doi: 10.1007/s00401-017-1746-2. [DOI] [PubMed] [Google Scholar]
- Madill M., McDonagh K., Ma J., Vajda A., McLoughlin P., O’Brien T. Amyotrophic lateral sclerosis patient iPSC-derived astrocytes impair autophagy via non-cell autonomous mechanisms. Mol. Brain. 2017;10(1):22. doi: 10.1186/s13041-017-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso R., Van Den Daele J., Fattorelli N., Leen W., Balusu S., Burton O., Liston A., Sierksma A., Fourne Y., Poovathingal S., Arranz-Mendiguren A., Sala Frigerio C., Claes C., Serneels L., Theys T., Perry H.V., Verfaillie C., Fiers M., De Strooper B. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat. Neurosci. 2019;22:2111–2116. doi: 10.1038/s41593-019-0525-x. [DOI] [PubMed] [Google Scholar]
- McQuade A., Coburn M., Tu C.H., Hasselmann J., Davtyan H., Blurton-Jones M. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol. Neurodegener. 2018;13(1):67. doi: 10.1186/s13024-018-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade A., Kang Y.J., Hasselmann J., Jairaman A., Sotelo A., Coburn M. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat. Commun. 2020;11(1):5370. doi: 10.1038/s41467-020-19227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J., Paquola A.C.M., Ku M., Hatch E., Böhnke L., Ladjevardi S. Directly reprogrammed human neurons retain aging-associated transcriptomic signatures and reveal age-related nucleocytoplasmic defects. Cell Stem Cell. 2015;17(6):705–718. doi: 10.1016/j.stem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K., Ferraiuolo L., Miranda Cj, Likhite S., McElroy S., Renusch S. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc. Natl. Acad. Sci. U. S. A. 2014;111(2):829–832. doi: 10.1073/pnas.1314085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J., Doudet D.D., Schwab C., Yu S., McGeer E.G., McGeer P.L. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp. Neurol. 2006;197(2):275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Moehle M.S., Webber P.J., Tse T., Sukar N., Standaert D.G., DeSilva T.M. LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 2012;32(5):1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffat J., Li Y., Yuan B., Mitalipova M., Omer A., Corcoran S. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 2016;22(11):1358–1367. doi: 10.1038/nm.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelhus E.A., Ottersen O.P. Physiological roles of aquaporin-4 in brain. Physiol. Rev. 2013;93(4):1543–1562. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls M.A., Duran R., Lopez G., Kurzawa-Akanbi M., McKeith I.G., Chinnery P.F. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 2013;70(6):727–735. doi: 10.1001/jamaneurol.2013.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.N., Byers B., Cord B., Shcheglovitov A., Byrne J., Gujar P. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8(3):267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Oberheim N.A., Goldman S.A., Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgidani M., Kato T.A., Setoyama D., Sagata N., Hashimoto R., Shigenobu K. Direct induction of ramified microglia-like cells from human monocytes: dynamic microglial dysfunction in Nasu-Hakola disease. Sci. Rep. 2014;4:4957. doi: 10.1038/srep04957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen M., Petersen A.J., Naumenko N., Puttonen K., Lehtonen Š, Gubert Olivé M. PSEN1 mutant iPSC-derived model reveals severe astrocyte pathology in Alzheimer’s disease. Stem Cell Rep. 2017;9(6):1885–1897. doi: 10.1016/j.stemcr.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakopoulou V., Ivanyuk D., De Cicco S. Interferon-γ signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat. Commun. 2020;11:5163. doi: 10.1038/s41467-020-18755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya H., Shen M.J., Ichikawa D.M., Sedlock A.B., Choi Y., Johnson K.R. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat. Neurosci. 2017;20(5):753–759. doi: 10.1038/nn.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker L.M., Miller D., Park T.S., Patel B., Azevedo J.L., Awad O. Induced pluripotent stem cell model recapitulates pathologic hallmarks of Gaucher disease. Proc. Natl. Acad. Sci. U. S. A. 2012;109(44):18054–18059. doi: 10.1073/pnas.1207889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker L.M., Miller D., Awad O., Bose V., Lun Y., Park T.S. Gaucher iPSC-derived macrophages produce elevated levels of inflammatory mediators and serve as a new platform for therapeutic development. Stem Cells. 2014;32(9):2338–2349. doi: 10.1002/stem.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Wetzel I., Marriott I., Dréau D., D’Avanzo C., Kim D.Y. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 2018;21(7):941–951. doi: 10.1038/s41593-018-0175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patani R., Lewis P.A., Trabzuni D., Puddifoot C.A., Wyllie D.J., Walker R. Investigating the utility of human embryonic stem cell-derived neurons to model ageing and neurodegenerative disease using whole-genome gene expression and splicing analysis. J. Neurochem. 2012;122(4):738–751. doi: 10.1111/j.1471-4159.2012.07825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N., Gerhard A., Tai Y.F., Ho A.K., Turkheimer F., Barker R.A. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology. 2006;66(11):1638–1643. doi: 10.1212/01.wnl.0000222734.56412.17. [DOI] [PubMed] [Google Scholar]
- Pearce M.M.P., Spartz E.J., Hong W., Luo L., Kopito R.R. Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat. Commun. 2015;6:6768. doi: 10.1038/ncomms7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriot S., Mathias A., Perriard G., Canales M., Jonkmans N., Merienne N. Human induced pluripotent stem cell-derived astrocytes are differentially activated by multiple sclerosis-associated cytokines. Stem Cell Rep. 2018;11(5):1199–1210. doi: 10.1016/j.stemcr.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T., Rothstein J.D. Glial cells in amyotrophic lateral sclerosis. Exp. Neurol. 2014;262(Pt B):111–120. doi: 10.1016/j.expneurol.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piers T.M., Cosker K., Mallach A., Johnson G.T., Guerreiro R., Hardy J. A locked immunometabolic switch underlies TREM2 R47H loss of function in human iPSC-derived microglia. FASEB J. 2020;34(2):2436–2450. doi: 10.1096/fj.201902447R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock J.M., Piers T.M. Modelling microglial function with induced pluripotent stem cells: an update. Nat. Rev. Neurosci. 2018;19(8):445–452. doi: 10.1038/s41583-018-0030-3. [DOI] [PubMed] [Google Scholar]
- Price T.N.C., Burke J.F., L.V M . In Vitro Cellular & Developmental Biology – Animal. Springer; 1999. A novel human astrocyte cell line (A735) with astrocyte-specific neurotransmitter function; pp. 279–288. [DOI] [PubMed] [Google Scholar]
- Puschmann T.B., Zandén C., De Pablo Y., Kirchhoff F., Pekna M., Liu J., Pekny M. Bioactive 3D cell culture system minimizes cellular stress and maintains the in vivo-like morphological complexity of astroglial cells. Glia. 2013;61(3):432–440. doi: 10.1002/glia.22446. [DOI] [PubMed] [Google Scholar]
- Qian K., Huang H., Peterson A., Hu B., Maragakis N.J., Ming G.L. Sporadic ALS astrocytes induce neuronal degeneration in vivo. Stem Cell Rep. 2017;8(4):843–855. doi: 10.1016/j.stemcr.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R.M. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 2016;19(8):987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- Rossi C., Cusimano M., Zambito M., Finardi A., Capotondo A., Garcia-Manteiga J.M. Interleukin 4 modulates microglia homeostasis and attenuates the early slowly progressive phase of amyotrophic lateral sclerosis. Cell Death Dis. 2018;9(2):250. doi: 10.1038/s41419-018-0288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland H.A., Hooper N.M., Kellett K.A.B. Modelling sporadic Alzheimer’s disease using induced pluripotent stem cells. Neurochem. Res. 2018;43(12):2179–2198. doi: 10.1007/s11064-018-2663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K.J., White C.C., Patel K., Xu J., Olah M., Replogle J.M. A human microglia-like cellular model for assessing the effects of neurodegenerative disease gene variants. Sci. Transl. Med. 2017;9(421) doi: 10.1126/scitranslmed.aai7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabogal-Guáqueta A.M., Marmolejo-Garza A., de Pádua V.P., Eggen B., Boddeke E., Dolga A.M. Microglia alterations in neurodegenerative diseases and their modeling with human induced pluripotent stem cell and other platforms. Prog. Neurobiol. 2020;190 doi: 10.1016/j.pneurobio.2020.101805. [DOI] [PubMed] [Google Scholar]
- Salter M.W., Stevens B. Microglia emerge as central players in brain disease. Nat. Med. 2017;23(9):1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- Sánchez-Danés A., Richaud-Patin Y., Carballo-Carbajal I., Jiménez-Delgado S., Caig C., Mora S. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol. Med. 2012;4(5):380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp E., Kegel K.B., Aronin N., Hashikawa T., Uchiyama Y., Tohyama K. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J. Neuropathol. Exp. Neurol. 2001;60(2):161–172. doi: 10.1093/jnen/60.2.161. [DOI] [PubMed] [Google Scholar]
- Schon E.A., Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70(6):1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C., Gomez Perdiguero E., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Serio A., Bilican B., Barmada S.J., Ando D.M., Zhao C., Siller R. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc. Natl. Acad. Sci. U. S. A. 2013;110(12):4697–4702. doi: 10.1073/pnas.1300398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E., Nalls M.A., Aasly J.O., Aharon-Peretz J., Annesi G., Barbosa E.R. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009;361(17):1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard A.R., Soulet D., Gowing G., Julien J.P., Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49(4):489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Simmons D.A., Casale M., Alcon B., Pham N., Narayan N., Lynch G. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington’s disease. Glia. 2007;55(10):1074–1084. doi: 10.1002/glia.20526. [DOI] [PubMed] [Google Scholar]
- Sloan S.A., Barres B.A. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 2014;27:75–81. doi: 10.1016/j.conb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan S.A., Darmanis S., Huber N., Khan T.A., Birey F., Caneda C. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron. 2017;95(4):779–790. doi: 10.1016/j.neuron.2017.07.035. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smethurst P., Risse E., Tyzack G.E., Mitchell J.S., Taha D.M., Chen Y.R. Distinct responses of neurons and astrocytes to TDP-43 proteinopathy in amyotrophic lateral sclerosis. Brain. 2020;143(2):430–440. doi: 10.1093/brain/awz419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq L., Rose J., Soreq E., Hardy J., Trabzuni D., Cookson M.R. Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep. 2017;18(2):557–570. doi: 10.1016/j.celrep.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda D.S., Barrasa M.I., Shu J., Rietjens R., Zhang S., Mitalipova M. Human iPSC-derived microglia assume a primary microglia-like state after transplantation into the neonatal mouse brain. Proc. Natl. Acad. Sci. U. S. A. 2019;116(50):25293–25303. doi: 10.1073/pnas.1913541116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga A., Dastgheyb R., Habela C., Joseph J., Richard J.P., Gross S.K. Role of human-induced pluripotent stem cell-derived spinal cord astrocytes in the functional maturation of motor neurons in a multielectrode array system. Stem Cells Transl. Med. 2019;8(12):1272–1285. doi: 10.1002/sctm.19-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tarassishin L., Suh H.S., Lee S.C. LPS and IL-1 differentially activate mouse and human astrocytes: role of CD14. Glia. 2014;62(6):999–1013. doi: 10.1002/glia.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay T.L., Mai D., Dautzenberg J., Fernández-Klett F., Lin G., Sagar A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 2017;20(6):793–803. doi: 10.1038/nn.4547. [DOI] [PubMed] [Google Scholar]
- Tchieu J., Calder E.L., Guttikonda S.R., Gutzwiller E.M., Aromolaran K.A., Steinbeck J.A. NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nat. Biotechnol. 2019;37(3):267–275. doi: 10.1038/s41587-019-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin E.P., Hall C.E., Tyzack G.E., Frostell A., Giorgi-Coll S., Alam A. Delineating astrocytic cytokine responses in a human stem cell model of neural trauma. J. Neurotrauma. 2020;37(1):93–105. doi: 10.1089/neu.2019.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien A.C., Tsai H.H., Molofsky A.V., McMahon M., Foo L.C., Kaul A. Regulated temporal-spatial astrocyte precursor cell proliferation involves BRAF signalling in mammalian spinal cord. Development. 2012;139(14):2477–2487. doi: 10.1242/dev.077214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiryaki V.M., Ayres V.M., Ahmed I., Shreiber D.I. Differentiation of reactive-like astrocytes cultured on nanofibrillar and comparative culture surfaces. Nanomedicine. 2015;10(4):529–545. doi: 10.2217/nnm.14.33. [DOI] [PubMed] [Google Scholar]
- Tong X., Ao Y., Faas G.C., Nwaobi S.E., Xu J., Haustein M.D. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 2014;17(5):694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.H., Li H., Fuentealba L.C., Molofsky A.V., Taveira-Marques R., Zhuang H. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337(6092):358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int. J. Cancer. 1980:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Tyzack G., Lakatos A., Patani R. Human stem cell-derived astrocytes: specification and relevance for neurological disorders. Curr. Stem Cell Rep. 2016;2:236–247. doi: 10.1007/s40778-016-0049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzack G.E., Hall C.E., Sibley C.R., Cymes T., Forostyak S., Carlino G. A neuroprotective astrocyte state is induced by neuronal signal EphB1 but fails in ALS models. Nat. Commun. 2017;8(1):1164. doi: 10.1038/s41467-017-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz S.G., See P., Mildenberger W., Thion M.S., Silvin A., Lutz M. Early fate defines microglia and non-parenchymal brain macrophage development. Cell. 2020;181(3):557–573. doi: 10.1016/j.cell.2020.03.021. e18. [DOI] [PubMed] [Google Scholar]
- Varcianna A., Myszczynska M.A., Castelli L.M., O’Neill B., Kim Y., Talbot J. Micro-RNAs secreted through astrocyte-derived extracellular vesicles cause neuronal network degeneration in C9orf72 ALS. EBioMedicine. 2019;40:626–635. doi: 10.1016/j.ebiom.2018.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile F., Dossi E., Rouach N. Human astrocytes: structure and functions in the healthy brain. Brain Struct. Funct. 2017;222(5):2017–2029. doi: 10.1007/s00429-017-1383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavalu K., Zhang C., Zhang X., Tanzi R.E., Sisodia S.S. Age-dependent, non-cell-autonomous deposition of amyloid from synthesis of β-amyloid by cells other than excitatory neurons. J. Neurosci. 2014;34(10):3668–3673. doi: 10.1523/JNEUROSCI.5079-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Hayashi S., Yoshimoto M., Kudo H., Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol. 2000;99(1):14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- Xu M., Zhang L., Liu G., Jiang N., Zhou W., Zhang Y. Pathological changes in Alzheimer’s disease analyzed using induced pluripotent stem cell-derived human microglia-like cells. J. Alzheimers Dis. 2019;67(1):357–368. doi: 10.3233/JAD-180722. [DOI] [PubMed] [Google Scholar]
- Watson P.M.D., Kavanagh E., Allenby G., Vassey M. Bioengineered 3D Glial Cell Culture Systems and Applications for Neurodegeneration and Neuroinflammation. SLAS Discovery. 2017;22(5):583–601. doi: 10.1177/2472555217691450. [DOI] [PubMed] [Google Scholar]
- Xu R., Li X., Boreland A.J., Posyton A., Kwan K., Hart R.P. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat. Commun. 2020;11(1):1577. doi: 10.1038/s41467-020-15411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K., Chun S.J., Boillee S., Fujimori-Tonou N., Yamashita H., Gutmann D.H. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 2008;11(3):251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S.D., Chen X., Fu J., Chen M., Zhu H., Roher A. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yang H.M., Yang S., Huang S.S., Tang B.S., Guo J.F. Microglial activation in the pathogenesis of Huntington’s disease. Front. Aging Neurosci. 2017;9:193. doi: 10.3389/fnagi.2017.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sloan S.A., Clarke L.E., Caneda C., Plaza C.A., Blumenthal P.D. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89(1):37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Xu M., Ren Q., Liu G., Meng S., Xiahou K. Human-induced pluripotent stem cell-derived neural cells from Alzheimer’s disease patients exhibited different susceptibility to oxidative stress. Stem Cells Dev. 2020;29(22):1444–1456. doi: 10.1089/scd.2020.0103. [DOI] [PubMed] [Google Scholar]
- Zhao J., Davis M.D., Martens Y.A., Shinohara M., Graff-Radford N.R., Younkin S.G. APOE ε4/ε4 diminishes neurotrophic function of human iPSC-derived astrocytes. Hum. Mol. Genet. 2017;26(14):2690–2700. doi: 10.1093/hmg/ddx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Devlin A.C., Chouhan A.K., Selvaraj B.T., Stavrou M., Burr K. Mutant C9orf72 human iPSC-derived astrocytes cause non-cell autonomous motor neuron pathophysiology. Glia. 2020;68(5):1046–1064. doi: 10.1002/glia.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff O.J., Patani R. Harnessing cellular aging in human stem cell models of amyotrophic lateral sclerosis. Aging Cell. 2019;18(1) doi: 10.1111/acel.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwilling D., Huang S.Y., Sathyasaikumar K.V., Notarangelo F.M., Guidetti P., Wu H.Q. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145(6):863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.