Abstract
Recent discoveries have revealed that during viral infection the presence of the RNA modification N6-methyladenosine (m6A) on viral and cellular RNAs has profound impacts on infection outcome. While m6A directly regulates many viral RNA processes, its effects on cellular RNAs and pathways during infection have only recently begun to be elucidated. Disentangling the effects of m6A on viral and host RNAs remains a challenge for the field. m6A has been found to regulate host responses such as viral RNA sensing, cytokine responses, and immune cell functions. We highlight recent findings describing how m6A modulates host responses to viral infection and discuss future directions that will lead to a synergistic understanding of the processes by which m6A regulates viral infection.
Keywords: N6-methyladenosine, m6A, RNA sensing, Interferon (IFN), Cytokines
m6A modulates host responses to viral infection
During viral infection, the molecular processes of host cells are altered as viruses co-opt, usurp, or inhibit cellular machinery to facilitate their replication. Recent studies have revealed that chemical modification of RNA is an example of a category of host processes that viruses can exploit. In particular, the role of the RNA modification m6A (see Glossary) during viral infection has generated a great deal of interest. While m6A was first discovered on viral RNA in the 1970s [1–4], its functional roles in viral processes were only revealed more recently. m6A can regulate many aspects of RNA biology [5], including RNA structure, splicing, stability, localization, and translation [6–10], thus its effects on viral RNAs are diverse (reviewed in [11]). In addition to these effects, m6A has been shown to regulate cellular responses to viral infection, which is the focus of this review. m6A is deposited on mRNA primarily by a cellular complex of ‘writer’ enzymes, composed of methyltransferase like 3 (METTL3) and METTL14 (METTL3/14) [12] and other accessory proteins, such as WTAP, VIRMA, ZC3H13, and RBM15/RBM15B [13–17]. Additional enzymes such as METTL16, ZCCHC4, and METTL5 can catalyze the addition of m6A, mainly on non-coding RNA species [18–22]. m6A is the most abundant chemical modification to eukaryotic mRNA, and its effects on RNA metabolism are mediated by m6A ‘reader’ proteins, such as the YTH domain proteins (YTHDF1, YTHDF2, YTHDF3, YTHDC1, YTHDC2), and others [23]. Additionally, m6A can be removed from RNA by demethylase ‘eraser’ proteins including FTO and ALKBH5 [9, 24]. Because m6A can affect both viral and host processes, its regulatory effects on viral infection are complex. A more complete understanding of the effects of m6A at the virus-host interface will require additional understanding of its effects on the cellular response to infection.
Viruses are equipped with strategies to manipulate host gene expression and cellular processes in ways that promote their replication. Meanwhile, host cells modulate their own processes in response to infection to limit viral replication. First, host cells utilize pattern recognition receptors (PRRs) to detect pathogen associated molecular patterns (PAMPs) and initiate innate immune responses [25]. Signaling molecules called cytokines can then be produced to transmit innate immune signals for the expression of antiviral genes that directly limit viral infection and to orchestrate functional adaptive immune responses [26]. Many viruses have also developed mechanisms to inhibit host innate immune responses [27]. Therefore, the interplay between viruses and their host cells is intricate and complex, resulting in cells undergoing many dynamic changes during infection. m6A regulates many biological processes, including stress responses such as the integrated stress response, heat shock, and UV damage [28–30], and therefore likely regulates these or other stress responses important for viral infection. Recent research has shed light on the regulatory roles of m6A on host responses to viral infection, including detection of viral RNA, innate immune pathways, stress response pathways, and metabolism; however, many intriguing questions in this field have yet to be explored. This review highlights recent discoveries describing the mechanisms by which m6A regulates host processes during viral infection and some of the most pressing questions for future research.
Sensing of m6A-modified RNAs
Recognition of viral nucleic acids is an important cellular surveillance strategy, and the ability to distinguish foreign RNAs from host, or ‘self’, RNAs, is crucial for viral detection. Additionally, protection of self RNAs from detection by PRRs is essential to avoid autoimmune disease [31]. RNA modifications are known to serve as molecular signatures that can distinguish self and non-self RNAs, and roles for m6A in regulating PRR sensing of RNAs are now beginning to emerge. Other RNA modifications with well-established roles in shielding self RNAs from PRRs include the 7-methylguanosine cap (also known as cap0) and 2’-O-methylation of the first (cap1) and second (cap2). The cytosolic PRR RIG-I detects uncapped RNAs with 5’- tri- or diphosphate moieties that are generally found on viral RNA genomes or as viral replication intermediates [32–34]. 2’-O-methylation of the first transcribed nucleotide is also crucial for avoiding recognition by RIG-I [35, 36]. After binding non-self RNA, RIG-I induces signaling through the MAVS pathway that results in the production of type I and III interferons (IFNs) to activate antiviral responses [37]. The RNA binding protein Interferon Induced Protein With Tetratricopeptide Repeats 1 (IFIT1) can also detect RNAs that lack cap1 2’-O-methylation and can sequester these RNAs to inhibit their translation [38–40]. Thus, these RNA modifications play essential roles in distinguishing self from non-self RNAs within the cytosol of cells. Mimicry or co-option of capping and 2’-O-methylation processes by viruses to avoid detection by PRRs also demonstrates the importance of these modifications as key determinants of self [38, 41].
The role of m6A in modulating recognition of RNA substrates by PRRs is now beginning to be uncovered (Figure 1). For example, certain Toll-like receptors (TLRs) have been found to respond differently to RNAs derived from different organisms [42]. Human total RNA only weakly stimulates the Tumor Necrosis Factor alpha (TNF-α) response downstream of TLRs, while bacterial total RNA is a potent stimulator. Interestingly, tRNA from humans or bacteria does not strongly activate TNF-α. Given that tRNA species and human mRNAs are highly post-transcriptionally modified, these data suggested that these modifications may suppress TLR recognition of RNA. Indeed, early studies revealed that in vitro-transcribed RNAs containing modified nucleosides are less potent activators of TLRs than their unmodified counterparts, and m6A is particularly effective at inhibiting TLR activation [42]. Similarly, in vitro-transcribed polyU/UC RNA from hepatitis C virus (HCV), which is a strong RIG-I ligand, binds poorly to RIG-I when modified by m6A [43]. These studies suggest a role for m6A in shielding RNA species from detection by PRRs. An additional study found that m6A modification of human circular RNAs is necessary to inhibit their recognition by RIG-I, and this study proposes a model in which the m6A reader protein YTHDF2 sequesters circular RNAs away from RIG-I [44]. While YTHDF2 is generally known for its role in facilitating the degradation of its m6A-modified target RNAs, it has also been linked to mRNA localization and phase separation [8, 45], which could explain its role in circular RNA sequestration. However, the immunogenicity of unmodified circular RNA is controversial, and one study suggests that contaminating linear RNA may instead cause activation of RNA sensors [46]. Nevertheless, these studies suggest multiple roles for m6A in prevention of aberrant innate immune activation in response to endogenous RNAs. Additionally, the m6A writer Mettl3 was found to be essential for suppression of endogenous long double stranded RNA levels in murine hematopoietic stem cells. These RNA species activate cellular sensors such as MDA5, which specifically detects long double stranded RNAs and, like RIG-I, also signals through MAVS, as well as the Oligoadenylate Synthetase-Ribonuclease L (OAS-RNase L) and Protein Kinase-R-Eukaryotic Initiation Factor 2 alpha (PKR-eIF2α) pathways [37, 47, 48]. Thus, Mettl3 deletion resulted in aberrant upregulation of interferon-stimulated genes (ISGs) and failure of hematopoietic stem cells to differentiate [49]. The mechanisms by which METTL3 and m6A suppress endogenous double stranded RNA levels are not yet clear, although m6A could enable recognition by a reader protein such as YTHDF2 and subsequent degradation of these RNAs, or m6A could directly modulate the RNA structures to prevent recognition by the described cellular sensors. m6A-mediated effects on RNA structures have been previously described [6, 50], and these effects may be sufficient to interrupt long dsRNA structures and inhibit MDA5 recognition. Together these studies implicate m6A as an important molecular signature that can contribute to protecting self RNAs from innate immune sensing through sequestration by m6A reader proteins, preventing RNA binding protein interactions, direct structural changes, or other mechanisms that have not yet been identified. Interestingly, while m6A is a very prevalent modification on mRNA [51], not all mRNAs are m6A-modified, demonstrating that m6A modification must not be fully required for inhibition of host mRNA recognition by PRRs. Perhaps, in these contexts, other RNA modifications or structures serve redundant roles in inhibiting PRR sensing. Indeed, in vitro transcribed RNAs, for example, likely lack other internal modifications in addition to m6A and may not be bound by the same repertoire of RNA binding proteins as endogenous RNAs after transfection into cells. Thus, determining the specific contexts in which m6A is important for inhibiting PRR sensing will be crucial for understanding its importance as a signature of self RNA.
Figure 1. m6A regulates innate immune signaling pathways.
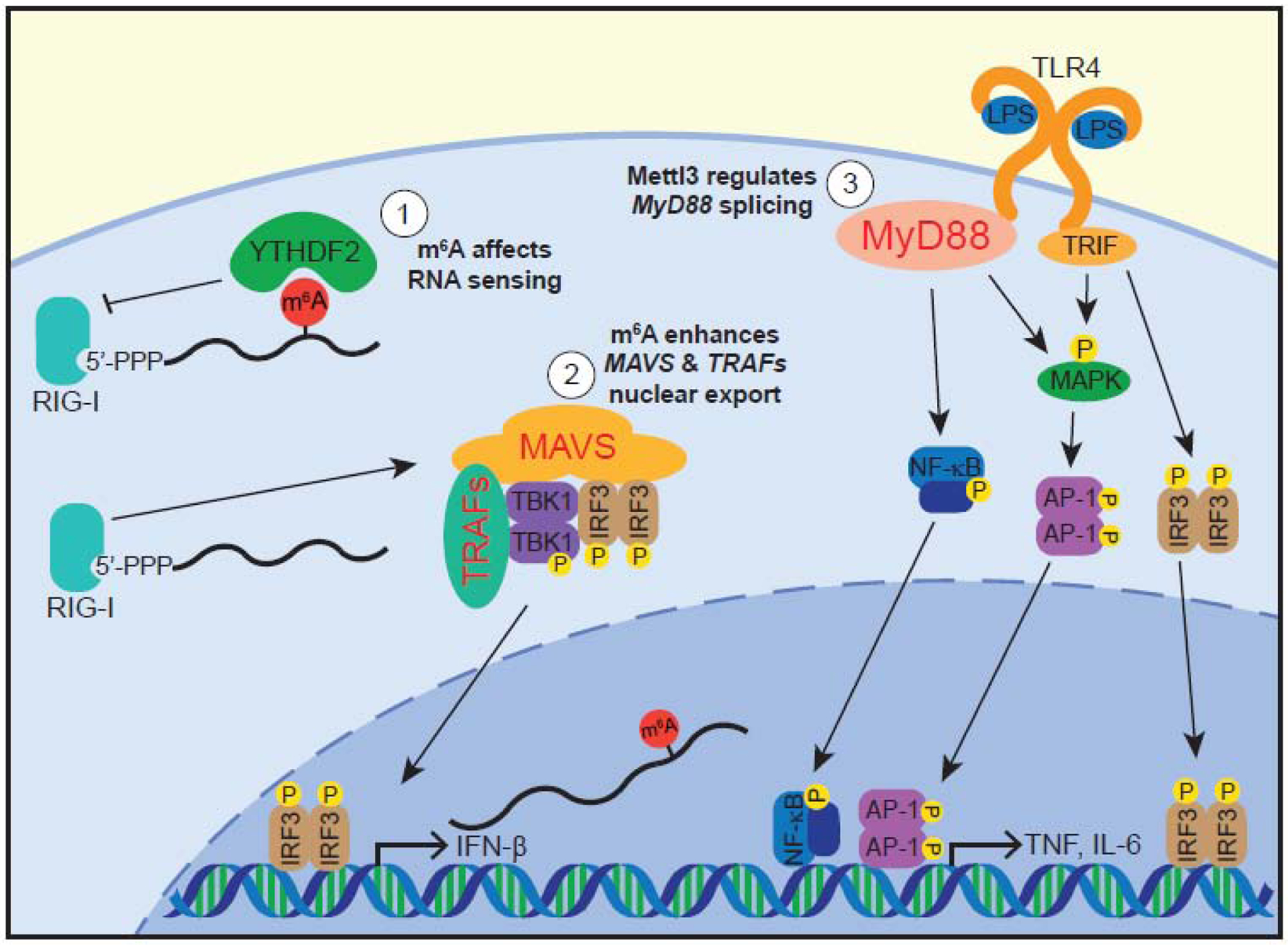
m6A regulates the induction of cytokine responses through multiple mechanisms. 1) m6A, perhaps via interactions with the YTHDF2 protein, inhibits detection of RNAs by RIG-I, whose role is to sense foreign RNAs and activate the MAVS signaling pathway [42–44, 49, 52, 53]. 2) m6A promotes the expression of MAVS and TRAFs by promoting the nuclear export of their mRNAs [64]. This induces signaling of the MAVS pathway, which activates the transcription factor IRF3 through the interaction of MAVS, TRAFs, and the kinase TBK1. Activated IRF3, along with NF-κB, induces transcription at the IFN-β promoter [37]. 3) METTL3 regulates MyD88 isoform usage in response to LPS stimulation of the TLR4 pathway [68]. TLR4 signals through proteins such as MyD88, MAPKs, and TRIF to activate the transcription factors NF-κB, AP-1, and IRF3. These transcription factors induce the production of proinflammatory cytokines like TNF, IL-6, and IFN-β [67]. YTHDF2 also regulates the TLR4 pathway [65] (not shown). Proteins whose expression are known to be regulated by m6A or m6A-related enzymes are shown in red text.
Supporting the role of m6A in preventing sensing of RNAs by PRRs, recent studies have found evidence that viruses use m6A to protect their RNA from recognition by PRRs. One study found that both the genome, antigenome, and mRNAs of the negative-sense, single-stranded RNA virus human metapneumovirus (HMPV) are m6A-modified [52]. By depleting m6A-related proteins, this study showed that m6A has a proviral effect for HMPV, likely through its effects on both host and viral RNAs. Abrogation of m6A sites in the HMPV genome resulted in viral mutants that induced more type I IFN and whose replication was attenuated. Interestingly, IFN induction by these mutants was dependent on RIG-I and appeared to be mediated by RIG-I specifically recognizing the m6A-deficient genome and anti-genome, rather than the viral mRNAs. While it is not yet clear how m6A inhibits RIG-I binding to HMPV RNA, it is possible that m6A also inhibits RIG-I oligomerization along the RNA, thus preventing downstream signaling [52]. This study provides evidence of a virus co-opting m6A modification to mask its RNA from cellular PRRs. An additional report suggests that both hepatitis B virus (HBV) and HCV, which have been shown to contain m6A, may utilize similar strategies to avoid innate immune detection [53]. m6A on HBV RNA was previously found to enhance reverse transcription and destabilize HBV transcripts [54], while we showed m6A on HCV inhibits packaging of its RNA genome [55]. A recent study tested the effect of m6A on the RNA of these viruses using in vitro transcribed viral RNA containing mutations at putative m6A sites and found that these m6A sites potentially inhibited RIG-I recognition of HBV and HCV RNA [53]. However, further work to ensure that the putative m6A sites mutated in these studies are indeed modified following transfection of the in vitro transcribed RNAs will be of importance. This may also help to reconcile other differences with published work that shows that m6A has antiviral roles during infection [54, 55].
The ability of m6A to serve as an additional feature beyond the m7G cap and 2’-O-methylation to mark cellular RNAs as self is an exciting function that the field is just beginning to understand. Future work detailing the mechanisms by which m6A on viral RNA inhibits activation of RIG-I and other RNA sensors will contribute to our understanding of the functions of m6A during viral infection, and also provide valuable information for designing attenuated vaccines, or for delivery of RNA therapeutics [56–58]. Additionally, whether m6A modification in certain structural or sequence contexts on viral RNA could actually serve as a molecular signature to recruit innate immune surveillance proteins will be interesting to explore further. Indeed, m6A-induced structural alterations in RNA have been shown to regulate RNA binding protein interactions [6, 59, 60]. Additionally, a recent report suggested that, in IFN-stimulated cells, the antiviral protein Interferon-Stimulated Exonuclease Gene 20 (ISG20) can specifically recognize an m6A-modified site in HBV RNA, perhaps through interaction with YTHDF2, and facilitate degradation of this RNA [61]. Other innate immune effector proteins are also known to recognize specific features of RNAs, such as IFIT1 which inhibits cap0 RNA translation [39, 40], or ZAP, which recognizes CG dinucleotides within viral RNA [62]. Therefore, there are many interesting possibilities to explore regarding the roles of m6A for RNA recognition by innate immune surveillance proteins.
Cytokine production and responses
After detection of foreign nucleic acids or other components of viruses, signaling pathways are activated by PRRs that detect specific PAMPs and drive the production of cytokines, such as IFNs, which initiate antiviral responses and orchestrate the adaptive immune response [25]. For example, as mentioned in the previous section, detection of viral RNA by RIG-I or MDA5 activates the MAVS pathway and a signaling cascade that activates proteins such as Tank Binding Kinase 1 (TBK1), Tumor Necrosis Factor Receptor-Associated Factor (TRAF) proteins (TRAF2, TRAF3, and TRAF6), and the transcription factors Interferon Regulatory Factors 3 and 7 (IRF3 and IRF7) [63]. Interestingly, m6A appears to play a role in the MAVS pathway by regulating the production of several of the signaling molecules in the pathway (Figure 1). In murine macrophages, it was found that Mavs, Traf3, and Traf6 transcripts are m6A-modified, and that DEAD-Box Helicase 46 (DDX46) can bind these mRNAs after viral infection to recruit the m6A eraser ALKBH5, which demethylates these transcripts. Following demethylation, Mavs, Traf3, and Traf6 mRNAs are increasingly retained in the nucleus, dampening signaling and production of IFNs [64]. These studies suggest that m6A can promote antiviral signaling pathways by regulating the expression of signaling molecules.
m6A may also be involved in regulation of other PAMP signaling pathways, such as the response to lipopolysaccharide (LPS) (Figure 1). A recent study found that the m6A reader protein YTHDF2 inhibits the inflammatory response to LPS, which signals through TLR4 to activate Nuclear Factor Kappa B (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) signaling [65–67]. However, it is unclear whether this YTHDF2-mediated regulation is dependent on m6A. Additional evidence of a role for m6A in LPS signaling was found in dental pulp cells, in which METTL3 depletion led to increased expression of a particular isoform of MyD88, a signaling molecule in the TLR4 pathway (Figure 1) [68]. This short isoform of MyD88 acts as a dominant-negative regulator of TLR4/MyD88 signaling, and therefore led to decreased activation of NF-κB and MAPK signaling [68]. Therefore, it appears METTL3 may modulate MyD88 splicing to promote the LPS-induced inflammatory response. While m6A can regulate the splicing of certain transcripts [69], it is not yet clear whether MyD88 mRNA is m6A-modified or whether METTL3 has other trans-regulatory effects on the transcript. Taken together, these studies do seem to suggest roles for m6A in the LPS response. In addition to its recently discovered roles in viral RNA and LPS-driven innate immune signaling pathways, m6A likely has additional roles in other signaling responses that stimulate induction of cytokines and inflammatory responses. Therefore, future explorations in these areas will be invaluable for understanding the role of m6A in inflammatory conditions and autoimmune disease.
In addition to regulating innate immune signaling pathways, m6A has recently been found to directly regulate the production of the important antiviral cytokine IFN-β [70, 71] (Figure 2). Following infection by human cytomegalovirus (HCMV), the expression of m6A writers, erasers, and reader proteins was found to increase substantially. Interestingly, m6A profiling revealed that after innate immune activation, the IFNB1 transcript was m6A-modified and that this methylation decreased its half-life [70]. These results suggest that m6A suppresses IFN-β production, which could be exploited by viruses by inducing m6A modification of IFNB1 mRNA. In further support of this hypothesis, an additional study found that HCMV replication was decreased in METTL3 depleted cells due to enhanced expression of IFN-β [71]. This study found that YTHDF2 binds to m6A-modified IFNB1 mRNA to facilitate its degradation [71]. Thus, these two studies converged on the idea that m6A dampens IFN-β production and contributes to turnover of this proinflammatory cytokine. This regulatory feature of m6A may be important for controlling inflammatory conditions and autoimmunity, which have been linked to excessive IFN production [72]. Additionally, the apparent ability of HCMV to exploit this control of IFN-β expression to facilitate its replication by increasing m6A modification of the IFNB1 transcript and thus decreasing its production is an exciting discovery. Additional m6A-mediated viral strategies for inhibiting IFN induction pathways also appear to exist. For example, HBV infection leads to increased m6A modification of the PTEN transcript, which encodes Phosphatase And Tensin Homolog, a positive regulator of IRF3 nuclear translocation [73]. PTEN mRNA is destabilized by m6A, thus increased m6A levels on PTEN mRNA during HBV infection lead to less PTEN expression and less IFN-β production [74]. It is likely that other viruses also influence the production of IFN-β or other cytokines by manipulating m6A modification on the transcripts of cytokines or molecules that regulate their production, and this will be an interesting avenue for future research.
Figure 2. m6A regulates IFN-β and the type I IFN response.
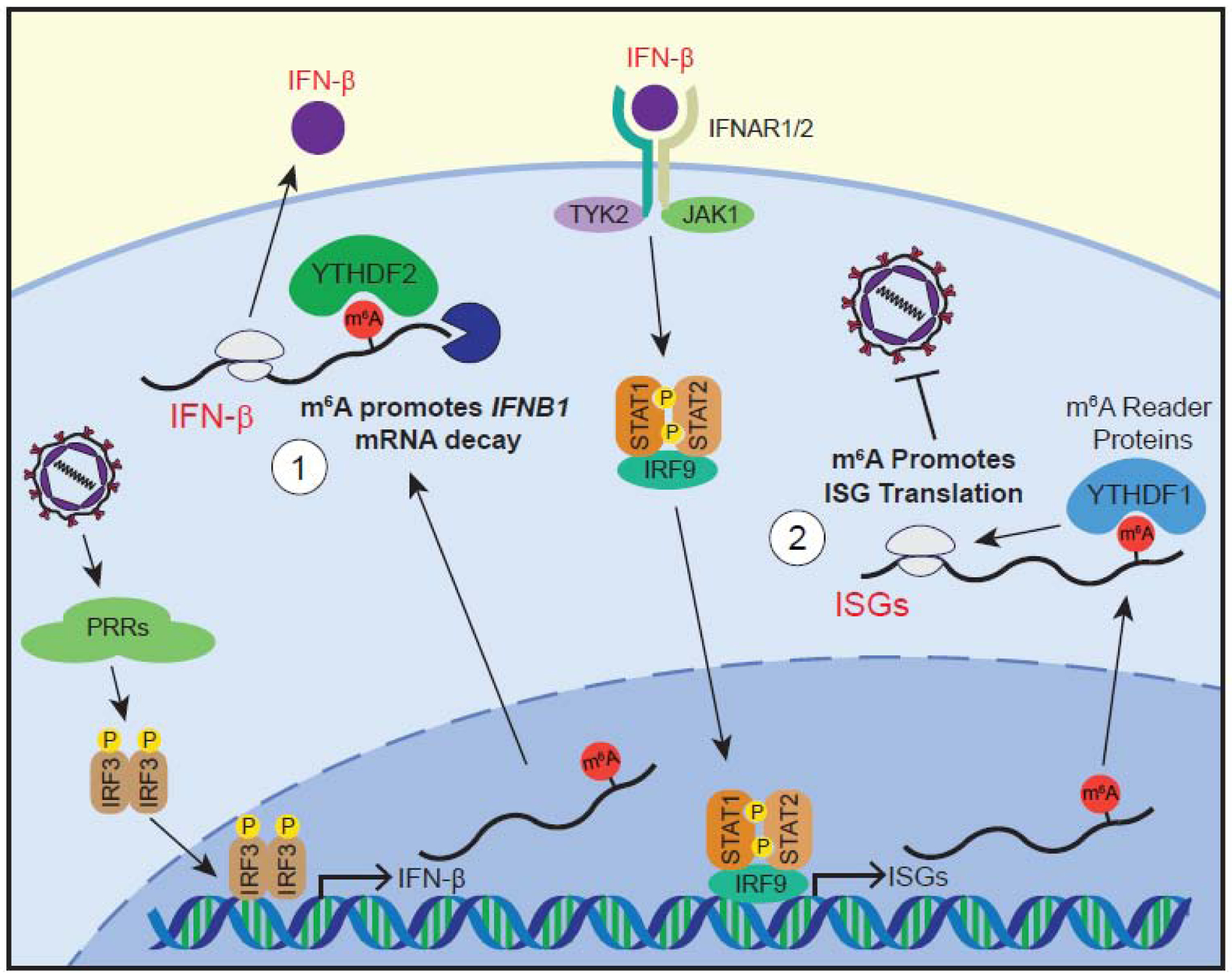
1) Following viral detection by PRRs and the activation of signaling pathways such as those described above, type I IFNs are produced [37]. The IFNB1 transcript is m6A modified, and m6A recruits the reader protein YTHDF2 to facilitate IFNB1 degradation, dampening the production of IFN-β [70, 71]. 2) IFN-β activates signaling through the JAK-STAT pathway, activating JAK1 and TYK2 kinases, which phosphorylate STAT1 and STAT2, which associate with IRF9, forming a transcription factor complex that drives the transcription of ISGs [72]. Many ISGs are m6A-modified, and m6A enhances the translation of a subset of these ISGs. This translation enhancement by m6A and reader proteins like YTHDF1 promotes the antiviral effects of the type I IFN response [75]. Proteins whose expression are known to be regulated by m6A or m6A-related enzymes are shown in red text.
As m6A has been found to regulate the pathways that lead to cytokine production and the transcripts of cytokines themselves, a role for m6A in cellular response pathways induced by cytokines is an interesting area to explore. We recently discovered a role for m6A in the response to type I IFN [75] (Figure 2). While the transcript levels of ISGs were not regulated by METTL3/14 after IFN stimulation, suggesting m6A does not regulate the JAK-STAT signaling pathway that leads to transcriptional activation of ISGs [76], we did find that METTL3/14 enhances the translation of a subset of m6A modified ISGs, including many with known antiviral functions. Importantly, depletion of METTL3/14, which decreased antiviral effector ISG expression, led to a higher percentage of IFN pretreated cells becoming infected with vesicular stomatitis virus (VSV) [75]. These results suggest that m6A enhances the antiviral effects of type I IFN, thus establishing a role for m6A in the type I IFN response. An additional study in mouse macrophages found that the m6A reader protein Ythdf3 indirectly regulates the transcription of ISGs by promoting the translation of Foxo3, which encodes Forkhead Box O3, which represses transcription of a subset of ISGs [77]. Surprisingly, Ythdf3 regulation of Foxo3 occurred independently of METTL3-mediated m6A modification. This study elucidated an interesting role for Ythdf3 in regulating the type I IFN response, although these studies have yet to be replicated in human cells. In addition to these findings, future research describing how m6A regulates responses to other cytokines will be an important avenue, especially as these results would help to inform how m6A regulates the cross-talk between the innate and adaptive immune responses.
Immune cell activation and function
Cytokines produced during viral infection recruit immune cells and influence their maturation and activation. As m6A can control cytokine production, as seen for IFN-β, it likely also regulates the communication between virus-infected cells and immune cells, although this specific type of regulation has yet to be described. However, roles have recently been described for m6A in regulating immune cell function (Figure 3). Dendritic cells (DCs) are a class of antigen presenting cells with important roles in linking innate and adaptive immune responses. m6A has now been shown to regulate DC maturation [78]. Using murine DCs, it was found that Mettl3 promoted DC maturation in a manner dependent on its m6A catalytic activity, likely through its promotion of the translation of the m6A-modified transcripts of Cluster of Differentiation 40 and 80 (CD40, CD80), and Tirap. TIR Domain Containing Adaptor Protein (Tirap) is a signaling protein in the TLR4/MyD88 pathway [67], and thus its expression is important for TLR4 signaling and downstream DC activation, whereas CD40 and CD80 are co-stimulatory molecules important for T cell activation [79]. Importantly, DCs lacking Mettl3 are deficient in their ability to promote T cell proliferation, demonstrating the importance of m6A in the maturation and function of DCs [78]. While the relevance of these findings have not been explored in the context of viral infection, many viruses also stimulate the MyD88 pathway through Tirap [80]. Additionally, it is known that DCs are potent producers of cytokines like type I IFNs in response to viral infection and are crucial for initiation of adaptive immune responses, as they activate naïve T cells [81]. Therefore, these findings provide some insight into potential roles of m6A in linking the innate and adaptive immune responses to control viral infection.
Figure 3. m6A regulates immune cell function.
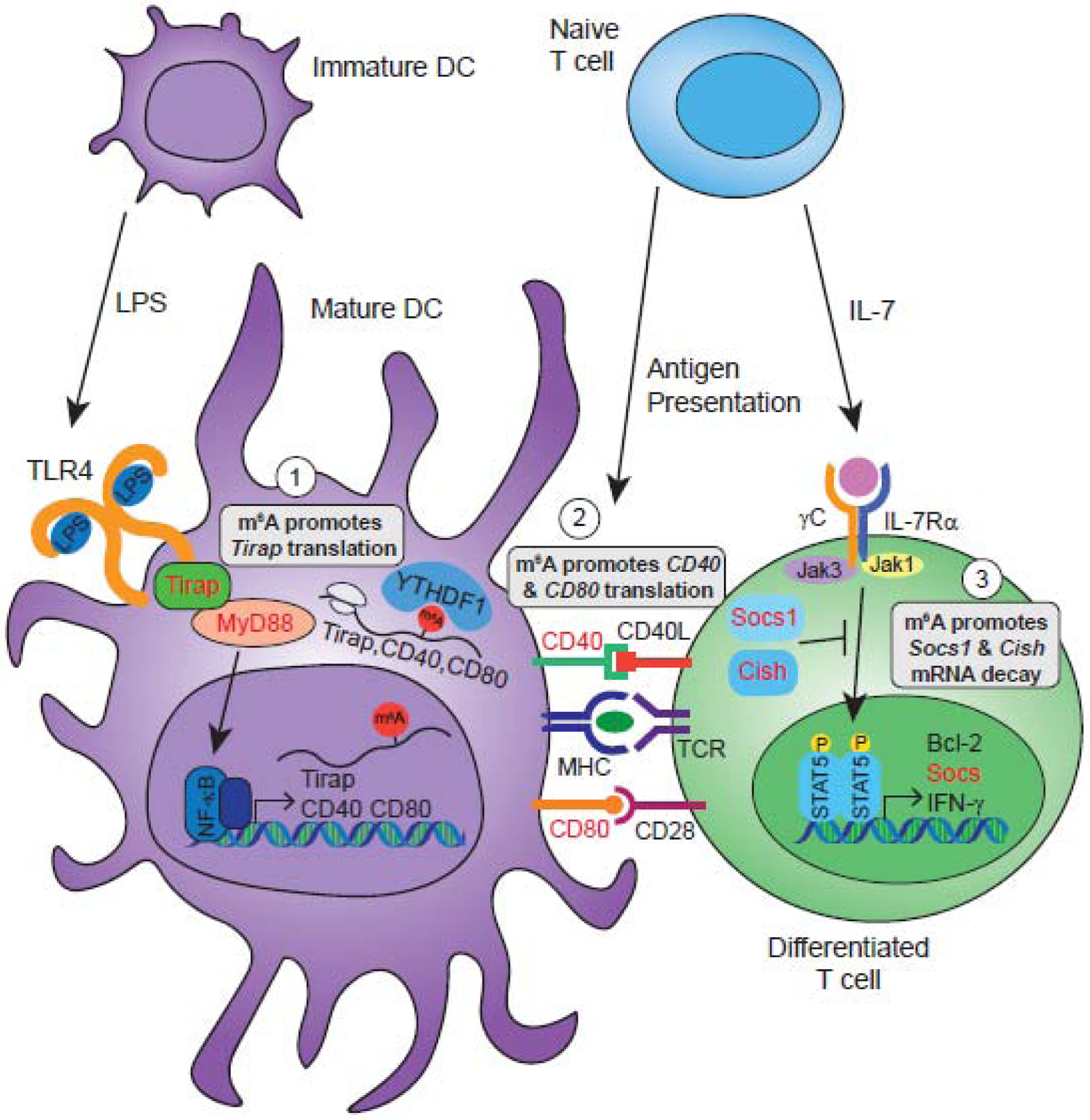
1) LPS treatment of murine DCs induces signaling through the TLR4/MyD88 pathway to activate the transcription factor NF-κB, which drives transcription of its target genes and DC maturation [67, 81]. m6A regulates DC maturation by promoting the translation of Tirap, which encodes an important adaptor protein for TLR4/MyD88 interaction [78]. m6A may also regulate MyD88 splicing [68]. 2) After activation, mature DCs can present antigens to T cells to influence their differentiation. This process involves MHC presentation of antigen peptides and interaction with T cell receptors (TCR), as well the interaction of co-stimulatory molecules such as CD40 and CD80 [81]. m6A contributes to this process by promoting the translation of CD40 and CD80 mRNAs [78]. 3) T cells can also be activated by cytokines like IL-7. IL-7 signals through its receptor composed of IL-7Rα and the common gamma chain (γC), activating JAK1 and JAK3, which induce the activation and homodimerization of STAT5, inducing cytokines like IFN-γ and genes involved in T cell survival and differentiation [83]. Additionally, this pathway induces suppressor of cytokine signaling (SOCS) proteins, such as SOCS1 and SOCS3, as well as Cish, all of which repress activation of the IL-7 pathway. The mRNA of Socs1, Socs3, and Cish are m6A-modified, and m6A destabilizes their transcripts, thus promoting the IL-7 pathway and T cell survival and differentiation [82]. Molecules whose expression are known to be regulated by m6A or m6A-related enzymes are shown in red text.
Interestingly, m6A also controls the homeostasis and differentiation of naïve T cells [82] (Figure 3). Recent work found that naïve T cells from conditional Mettl3 knockout mice are deficient in their ability to proliferate and differentiate into effector T cells. The model of T cell differentiation used in this study activates JAK1/STAT5 signaling [83], and in Mettl3 knockout T cells, this signaling was impaired, likely due to increased abundance of the transcripts and proteins of key suppressors of this pathway, Suppressor of Cytokine Signaling 1 and 3 (Socs1 and Socs3), and Cytokine-Inducible SH2-Containing Protein (Cish). These transcripts were all m6A-modified and lost m6A in Mettl3 knockout naïve T cells, which led to their stabilization [82]. Additionally, m6A was found to regulate CD4+ regulatory T cells, which are important for controlling inflammation, likely through similar mechanisms [84]. An additional role for METTL3/14 and m6A in the CD8+ T cell response to melanoma tumors has also recently been reported. In murine colorectal carcinoma models, Mettl3/14 depletion stabilizes the transcripts of Signal Transducer and Activator of Transcription 1 (Stat1) and Irf1 and increases the sensitivity of tumor cells to IFN-γ treatment, leading to growth inhibition of these cells [85]. These results suggest a role for m6A in STAT1-mediated signaling pathways through destabilization of the STAT1 transcript, although Mettl3 and m6A appear to stabilize the Stat1 transcript in mouse macrophages [86], thus more work will be required to determine the cell type-specific effects of m6A on STAT1 signaling pathways. Together, these results elucidate that m6A has a role in regulating T cell homeostasis and cytotoxic T cell functions.
While the functions of m6A in immune cells have not been well studied during viral infection, these studies clearly demonstrate its importance in the normal function and activation of immune cells, which are crucial for viral clearance. Additionally, these results demonstrate how understanding the transcript-specific roles of m6A in immune pathway regulation and control of immune cell functions will be useful for the development of future immunoregulatory therapies. As m6A is known to play important roles in stem cell fate decisions [87], cell development and maturation appears to be an important general biological function of m6A. Therefore, the discovery of additional roles of m6A in DCs and T cells, as well as other immune cell subsets, such as macrophages, natural killer cells, and B cells will be of great importance for our overall understanding of the roles of m6A in immunity.
Stress responses and metabolism
In addition to induction of immune responses, viral infection can induce cellular stress responses and can influence cellular metabolism [88]. As m6A can regulate many cellular pathways, including stress responses, its roles in infection-induced pathways will be important to understand. Indeed, many studies have found that diverse viral infections shape the m6A distribution within the host transcriptome [89–93]. However, in-depth functional validation of these m6A changes has been rare, and recent research suggests that some of these findings may be worth revisiting, as gene expression changes can influence m6A peak calling [51]. We recently profiled changes induced to the m6A epitranscriptome during Flaviviridae infection using rigorous analyses and investigated functional roles for some of these changes [93]. Of the viruses studied (dengue virus, Zika virus, West Nile virus, and HCV), each induced alterations to m6A modifications on certain transcripts, and some of these alterations were common across all viruses. Among the genes whose m6A status changed during infection by all of these viruses was RIOK3, a transcript that gained m6A during infection, which encodes RIO Kinase 3, a serine/threonine kinase that may regulate antiviral signaling. Interestingly, innate immune signaling driven by the transcription factor IRF3 was found to be important for the gain of m6A following infection, and m6A modification increased RIOK3 translation. The m6A status of CIRBP, which encodes Cold Inducible RNA Binding Protein, a stress-induced RNA binding protein, also changed in response to infection, although this transcript lost m6A and consequently was increasingly alternatively spliced to its short isoform. Importantly, ER stress-inducing treatment was sufficient to induce the loss of m6A on CIRBP, and Flaviviridae infection is known to induce ER stress responses [94]. While the precise mechanisms by which m6A status changes during infection are not clear, these data suggest that activation of host cell pathways during infection can influence the m6A status of individual transcripts. Additionally, many genes with m6A alterations were found to be capable of regulating Flaviviridae infection, including RIOK3 and CIRBP. These results point to functional roles for changes to the m6A landscape during viral infection, and set the stage for further investigation of the mechanisms responsible for m6A alterations [93].
Some possible mechanisms by which viral infection could induce changes to the m6A landscape include differential transcription rates of m6A-modified genes, changes in the expression, localization, or function of METTL3/14 or other RNA binding proteins involved in m6A targeting, or similar changes to m6A demethylase proteins like FTO or ALKBH5. Indeed, multiple viruses have been found to perturb the expression of the m6A machinery. These include HCMV, which increases the abundance of the m6A machinery [70, 71], or enterovirus 71, which increases the expression of METTL3 and METTL14 and changes the subcellular localization of reader, writer, and eraser proteins [95]. These alterations to the m6A machinery may benefit viruses by allowing modification of their RNAs or by influencing the m6A profile of the infected host cell. Interestingly, a recent study found that during VSV infection in mice, demethylation at a specific arginine residue in Alkbh5 impairs its m6A demethylase activity [96]. Alkbh5 deficiency in macrophages resulted in perturbations to cellular metabolism. In particular, Oxoglutarate Dehydrogenase (Ogdh), an enzyme involved in the citric acid cycle, was found to be strongly downregulated in these cells, as Alkbh5 normally demethylates the Ogdh transcript, which increases its stability and expression. Ogdh deficiency, in turn, was found to decrease the abundance of the metabolite itaconate, which was capable of promoting VSV replication [96]. Therefore, these results point to demethylation of Alkbh5 as a means of controlling Ogdh expression, which in turn regulates the production of itaconate. Importantly, this study identified a mechanism by which the m6A-modification of a cellular RNA can change in response to viral infection. Determining whether this interesting cellular response is specific to VSV infection, or relevant for other viruses, will be an important future step. Additionally, these results should set the stage for additional discoveries of mechanisms by which viral infection influences the cellular m6A landscape. Such discoveries will be of utmost importance for our understanding of how viruses manipulate m6A distribution for their benefit and how host cells utilize alteration of m6A to restrict viral replication.
Concluding Remarks
Our understanding of the functional roles of m6A in modulating host processes during viral infection is rapidly expanding, and these discoveries will also broaden our understanding of m6A biology. Because of the diverse, transcript-specific effects of m6A that can affect both viral and host RNAs, m6A regulates viral infection in complex ways (see Outstanding Questions). In order to achieve a more synergistic understanding of the mechanisms by which m6A and its related cellular machinery regulate viral infection, future research must continue to address the transcript-specific and position-specific roles of m6A in regulation of cellular pathways in response to individual, as well as pan-viral infection. Additional mechanistic understanding of how m6A regulates RNA sensing by PRRs, diverse cytokine production and responses, stress responses, immune cell biology, and cross-talk between the innate and adaptive immune system will be of great interest. Tissue- and cell type-specific m6A machinery knockout animal models will likely be very useful in gaining a better understanding of the roles of m6A in immune responses during viral infection. Finally, understanding whether and how viruses manipulate the m6A machinery and abundance or position of m6A in the host transcriptome will inform our understanding of the role of m6A at the virus-host interface and also elucidate potential m6A-based therapies for viral infection or immunopathies.
Outstanding Questions.
By what mechanisms does m6A modification of host and viral RNAs inhibit activation of RNA sensors like RIG-I? Could m6A modification of viral RNA recruit antiviral effector proteins?
What roles does m6A play in the production and response to cytokines beyond IFNs?
How does m6A regulate the cross-talk between the innate and adaptive immune responses?
In addition to dendritic cells and T cells, what other immune cells are regulated by m6A, and how does it modulate their development and effector processes?
What changes to the host RNA landscape are induced by diverse viral infections, and how do these changes modulate cellular responses to infection?
How do viruses manipulate m6A modification of host transcripts to benefit their replication?
Highlights.
The transcript-specific effects of N6-methyladenosine (m6A) exert control over host response pathways during viral infection.
m6A may serve as a molecular signature to regulate interactions between viral or host RNAs with antiviral RNA binding proteins.
Host responses such as cytokine production, cytokine signaling, and ER stress are regulated by m6A.
Immune cell activation pathways are modulated by m6A.
Acknowledgements
We would like to thank members of the Horner lab, especially Matthew Sacco and Matthew Thompson, for discussion of the manuscript. This work was supported by funds from Burroughs Wellcome Fund and National Institutes of Health (NIH) R01AI125416. MJM has received support from NIH T32CA009111.
Glossary
- ALKBH5
AlkB Homolog 5; a protein with m6A demethylase activity
- FTO
Fat Mass And Obesity-Associated Protein; a protein with m6A demethylase activity
- Interferon (IFN)
a family of proteins released from cells that induce interferon-stimulated genes to restrict viral replication
- Interferon-Stimulated Gene (ISG)
a class of genes whose transcription can be stimulated by interferons.
- JAK-STAT signaling pathway
Janus Kinase-Signal Transducer and Activator of Transcription; a pathway activated by various cytokines to induce transcriptional responses
- m6A
N6-methyladenosine; an adenosine residue containing a methyl group at its N6 position
- MAVS
Mitochondrial Antiviral Signaling; a mitochondria-localized adaptor protein that interacts with RIG-I or MDA5 to form a platform for the interaction of signaling proteins to stimulate IFN production
- MDA5
Melanoma Differentiation-Associated Protein 5; a cytosolic RNA helicase that recognizes long double stranded RNA
- Methyltransferase Like 3 (METTL3)
the catalytic enzyme subunit of the m6A methyltransferase complex
- METTL5
Methyltransferase Like 5; a protein with m6A methyltransferase ability that is responsible for m6A deposition on 18S rRNA
- METTL14
Methyltransferase Like 14; a protein that forms a heterodimer with METTL3 and is essential for m6A deposition
- METTL16
Methyltransferase Like 16; a protein with m6A methyltransferase ability that is responsible for U6 snRNA m6A deposition
- MyD88
Myeloid Differentiation Primary Response 88; a signaling protein involved in activation of TLR-driven pathways
- Pathogen-Associated Molecular Pattern (PAMP)
a molecular pattern that can be used to distinguish pathogens from their host cells
- Pattern Recognition Receptor (PRR)
a cellular protein that recognizes pattern-associated or damage-associated molecular patterns
- RBM15/RBM15B
RNA Binding Motif Protein 15 and RNA Binding Motif Protein 15B; accessory proteins in the m6A methyltransferase complex
- RIG-I
Retinoic Acid-Inducible Gene-I; a cytosolic RNA helicase that recognizes uncapped RNAs with 5’-tri or diphosphate moieties
- Toll-Like Receptors (TLR)
membrane-spanning receptors that recognize structurally conserved molecules derived from microbes
- VIRMA
Vir Like M6A Methyltransferase Associated; an accessory protein in the m6A methyltransferase complex
- WTAP
Wilms Tumor 1 Associated Protein; an accessory protein in the m6A methyltransferase complex
- YTHDC1
YTH Domain-Containing Protein 1; an m6A reader protein involved in regulation of splicing
- YTHDC2
YTH Domain-Containing Protein 2; an m6A reader protein that can promote both translation and decay of m6A-modified RNAs
- YTHDF1
YTH Domain Family 1. An m6A reader protein, primarily thought to promote translation of m6A-modified RNAs
- YTHDF2
YTH Domain Family 2. An m6A reader protein, primarily thought to promote decay of m6A-modified RNAs
- YTHDF3
YTH Domain Family 3. An m6A reader protein that can promote mRNA translation or decay through interactions with YTHDF1 and YTHDF2
- ZC3H13
Zinc Finger CCCH-Type Containing 13. An accessory protein in the m6A methyltransferase complex
- ZCCHC4
Zinc Finger CCHC-Type Containing 4. A protein with m6A methyltransferase ability that is responsible for m6A deposition on 28S rRNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lavi S and Shatkin AJ (1975) Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci U S A 72 (6), 2012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommer S et al. (1976) The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Res 3 (3), 749–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krug RM et al. (1976) Influenza viral mRNA contains internal N6-methyladenosine and 5’-terminal 7-methylguanosine in cap structures. J Virol 20 (1), 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss B et al. (1977) 5’-Terminal and internal methylated nucleosides in herpes simplex virus type 1 mRNA. J Virol 23 (2), 234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaccara S et al. (2019) Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol 20 (10), 608–624. [DOI] [PubMed] [Google Scholar]
- 6.Liu N et al. (2015) N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518 (7540), 560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X et al. (2014) FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res 24 (12), 1403–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X et al. (2014) N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505 (7481), 117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng G et al. (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49 (1), 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X et al. (2015) N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 161 (6), 1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams GD et al. (2019) Regulation of Viral Infection by the RNA Modification N6-Methyladenosine. Annu Rev Virol 6 (1), 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J et al. (2014) A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10 (2), 93–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz S et al. (2014) Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep 8 (1), 284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yue Y et al. (2018) VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knuckles P et al. (2018) Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev 32 (5–6), 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen J et al. (2018) Zc3h13 Regulates Nuclear RNA m(6)A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol Cell 69 (6), 1028–1038.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patil DP et al. (2016) m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537 (7620), 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warda AS et al. (2017) Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep 18 (11), 2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pendleton KE et al. (2017) The U6 snRNA m(6)A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell 169 (5), 824–835.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma H et al. (2019) N(6-)Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat Chem Biol 15 (1), 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Tran N et al. (2019) The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res 47 (15), 7719–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ignatova VV et al. (2020) The rRNA m(6)A methyltransferase METTL5 is involved in pluripotency and developmental programs. Genes Dev 34 (9–10), 715–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patil DP et al. (2018) Reading m(6)A in the Transcriptome: m(6)A-Binding Proteins. Trends Cell Biol 28 (2), 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia G et al. (2011) N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7 (12), 885–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi O and Akira S (2010) Pattern recognition receptors and inflammation. Cell 140 (6), 805–20. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki A and Medzhitov R (2015) Control of adaptive immunity by the innate immune system. Nat Immunol 16 (4), 343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beachboard DC and Horner SM (2016) Innate immune evasion strategies of DNA and RNA viruses. Curr Opin Microbiol 32, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J et al. (2018) N(6)-Methyladenosine Guides mRNA Alternative Translation during Integrated Stress Response. Mol Cell 69 (4), 636–647.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J et al. (2015) Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature 526 (7574), 591–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang Y et al. (2017) RNA m(6)A methylation regulates the ultraviolet-induced DNA damage response. Nature 543 (7646), 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlee M and Hartmann G (2016) Discriminating self from non-self in nucleic acid sensing. Nat Rev Immunol 16 (9), 566–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hornung V et al. (2006) 5’-Triphosphate RNA is the ligand for RIG-I. Science 314 (5801), 994–7. [DOI] [PubMed] [Google Scholar]
- 33.Goubau D et al. (2014) Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5’-diphosphates. Nature 514 (7522), 372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren X et al. (2019) RIG-I Selectively Discriminates against 5’-Monophosphate RNA. Cell Rep 26 (8), 2019–2027.e4. [DOI] [PubMed] [Google Scholar]
- 35.Schuberth-Wagner C et al. (2015) A Conserved Histidine in the RNA Sensor RIG-I Controls Immune Tolerance to N1–2’O-Methylated Self RNA. Immunity 43 (1), 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devarkar SC et al. (2016) Structural basis for m7G recognition and 2’-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc Natl Acad Sci U S A 113 (3), 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFadden MJ et al. (2017) Protect this house: cytosolic sensing of viruses. Curr Opin Virol 22, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daffis S et al. (2010) 2’-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature 468 (7322), 452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habjan M et al. (2013) Sequestration by IFIT1 impairs translation of 2’O-unmethylated capped RNA. PLoS Pathog 9 (10), e1003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar P et al. (2014) Inhibition of translation by IFIT family members is determined by their ability to interact selectively with the 5’-terminal regions of cap0-, cap1- and 5’ppp-mRNAs. Nucleic Acids Res 42 (5), 3228–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decroly E et al. (2011) Conventional and unconventional mechanisms for capping viral mRNA. Nat Rev Microbiol 10 (1), 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karikó K et al. (2005) Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23 (2), 165–75. [DOI] [PubMed] [Google Scholar]
- 43.Durbin AF et al. (2016) RNAs Containing Modified Nucleotides Fail To Trigger RIG-I Conformational Changes for Innate Immune Signaling. mBio 7 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen YG et al. (2019) N6-Methyladenosine Modification Controls Circular RNA Immunity. Mol Cell 76 (1), 96–109.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J et al. (2020) Binding to m(6)A RNA promotes YTHDF2-mediated phase separation. Protein Cell 11 (4), 304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wesselhoeft RA et al. (2019) RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol Cell 74 (3), 508–520.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peisley A et al. (2011) Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition. Proc Natl Acad Sci U S A 108 (52), 21010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peisley A et al. (2012) Kinetic mechanism for viral dsRNA length discrimination by MDA5 filaments. Proc Natl Acad Sci U S A 109 (49), E3340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y et al. (2020) m(6)A Modification Prevents Formation of Endogenous Double-Stranded RNAs and Deleterious Innate Immune Responses during Hematopoietic Development. Immunity 52 (6), 1007–1021.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu B et al. (2018) A potentially abundant junctional RNA motif stabilized by m(6)A and Mg(2). Nat Commun 9 (1), 2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McIntyre ABR et al. (2020) Limits in the detection of m(6)A changes using MeRIP/m(6)A-seq. Sci Rep 10 (1), 6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu M et al. (2020) N(6)-methyladenosine modification enables viral RNA to escape recognition by RNA sensor RIG-I. Nat Microbiol 5 (4), 584–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim GW et al. (2020) N (6)-Methyladenosine modification of hepatitis B and C viral RNAs attenuates host innate immunity via RIG-I signaling. J Biol Chem 295 (37), 13123–13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imam H et al. (2018) N6-methyladenosine modification of hepatitis B virus RNA differentially regulates the viral life cycle. Proc Natl Acad Sci U S A 115 (35), 8829–8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gokhale NS et al. (2016) N6-Methyladenosine in Flaviviridae Viral RNA Genomes Regulates Infection. Cell Host Microbe 20 (5), 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pardi N et al. (2017) Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543 (7644), 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pardi N et al. (2018) Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat Commun 9 (1), 3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lieberman J (2018) Tapping the RNA world for therapeutics. Nat Struct Mol Biol 25 (5), 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edupuganti RR et al. (2017) N(6)-methyladenosine (m(6)A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol 24 (10), 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arguello AE et al. (2017) RNA Chemical Proteomics Reveals the N(6)-Methyladenosine (m(6)A)-Regulated Protein-RNA Interactome. J Am Chem Soc 139 (48), 17249–17252. [DOI] [PubMed] [Google Scholar]
- 61.Imam H et al. (2020) Interferon-stimulated gene 20 (ISG20) selectively degrades N6-methyladenosine modified Hepatitis B Virus transcripts. PLoS Pathog 16 (2), e1008338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takata MA et al. (2017) CG dinucleotide suppression enables antiviral defence targeting non-self RNA. Nature 550 (7674), 124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Refolo G et al. (2020) Mitochondrial Interactome: A Focus on Antiviral Signaling Pathways. Front Cell Dev Biol 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng Q et al. (2017) The RNA helicase DDX46 inhibits innate immunity by entrapping m(6)A-demethylated antiviral transcripts in the nucleus. Nat Immunol 18 (10), 1094–1103. [DOI] [PubMed] [Google Scholar]
- 65.Yu R et al. (2019) m6A Reader YTHDF2 Regulates LPS-Induced Inflammatory Response. Int J Mol Sci 20 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medzhitov R et al. (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388 (6640), 394–7. [DOI] [PubMed] [Google Scholar]
- 67.Lester SN and Li K (2014) Toll-like receptors in antiviral innate immunity. J Mol Biol 426 (6), 1246–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng Z et al. (2018) METTL3 regulates alternative splicing of MyD88 upon the lipopolysaccharide-induced inflammatory response in human dental pulp cells. J Cell Mol Med 22 (5), 2558–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou KI et al. (2019) Regulation of Co-transcriptional Pre-mRNA Splicing by m(6)A through the Low-Complexity Protein hnRNPG. Mol Cell 76 (1), 70–81.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubio RM et al. (2018) RNA m(6) A modification enzymes shape innate responses to DNA by regulating interferon beta. Genes Dev 32 (23–24), 1472–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winkler R et al. (2019) m(6)A modification controls the innate immune response to infection by targeting type I interferons. Nat Immunol 20 (2), 173–182. [DOI] [PubMed] [Google Scholar]
- 72.Rodero MP and Crow YJ (2016) Type I interferon-mediated monogenic autoinflammation: The type I interferonopathies, a conceptual overview. J Exp Med 213 (12), 2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li S et al. (2016) The tumor suppressor PTEN has a critical role in antiviral innate immunity. Nat Immunol 17 (3), 241–9. [DOI] [PubMed] [Google Scholar]
- 74.Kim GW et al. (2020) HBV-Induced Increased N6 Methyladenosine Modification of PTEN RNA Affects Innate Immunity and Contributes to HCC. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McFadden MJ et al. (2020) Post-Transcriptional Regulation of Antiviral Gene Expression by N6-Methyladenosine. bioRxiv, 2020.08.05.238337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horvath CM (2004) The Jak-STAT pathway stimulated by interferon alpha or interferon beta. Sci STKE 2004 (260), tr10. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y et al. (2019) RNA-binding protein YTHDF3 suppresses interferon-dependent antiviral responses by promoting FOXO3 translation. Proc Natl Acad Sci U S A 116 (3), 976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H et al. (2019) Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat Commun 10 (1), 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Gool SW et al. (1996) CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev 153, 47–83. [DOI] [PubMed] [Google Scholar]
- 80.Bonham KS et al. (2014) A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell 156 (4), 705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Banchereau J et al. (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18, 767–811. [DOI] [PubMed] [Google Scholar]
- 82.Li HB et al. (2017) m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 548 (7667), 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong C et al. (2012) Intrathymic IL-7: the where, when, and why of IL-7 signaling during T cell development. Semin Immunol 24 (3), 151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tong J et al. (2018) m(6)A mRNA methylation sustains Treg suppressive functions. Cell Res 28 (2), 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L et al. (2020) m(6) A RNA methyltransferases METTL3/14 regulate immune responses to anti-PD-1 therapy. Embo j 39 (20), e104514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y et al. (2019) The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 facilitates M1 macrophage polarization through the methylation of STAT1 mRNA. Am J Physiol Cell Physiol 317 (4), C762–c775. [DOI] [PubMed] [Google Scholar]
- 87.Zhang M et al. (2020) Roles of N6-Methyladenosine (m(6)A) in Stem Cell Fate Decisions and Early Embryonic Development in Mammals. Front Cell Dev Biol 8, 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moreno-Altamirano MMB et al. (2019) Virus Control of Cell Metabolism for Replication and Evasion of Host Immune Responses. Front Cell Infect Microbiol 9, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lichinchi G et al. (2016) Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol 1, 16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tirumuru N et al. (2016) N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lichinchi G et al. (2016) Dynamics of Human and Viral RNA Methylation during Zika Virus Infection. Cell Host Microbe 20 (5), 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan B et al. (2018) Viral and cellular N(6)-methyladenosine and N(6),2’-O-dimethyladenosine epitranscriptomes in the KSHV life cycle. Nat Microbiol 3 (1), 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gokhale NS et al. (2020) Altered m(6)A Modification of Specific Cellular Transcripts Affects Flaviviridae Infection. Mol Cell 77 (3), 542–555.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blázquez AB et al. (2014) Stress responses in flavivirus-infected cells: activation of unfolded protein response and autophagy. Front Microbiol 5, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hao H et al. (2019) N6-methyladenosine modification and METTL3 modulate enterovirus 71 replication. Nucleic Acids Res 47 (1), 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y et al. (2019) N (6)-methyladenosine RNA modification-mediated cellular metabolism rewiring inhibits viral replication. Science 365 (6458), 1171–1176. [DOI] [PubMed] [Google Scholar]


