Abstract
Objective.
Trauma exposure is associated with a more severe, persistent course of affective and anxiety symptoms. Markers of reward neural circuitry function, specifically activation to reward prediction error[RPE], are impacted by trauma and predict future course of affective symptoms. This study’s purpose was to determine how lifetime trauma exposure influences relationships between reward neural circuitry function and the course of future affective and anxiety symptoms in a naturalistic, transdiagnostic observational context
Methods.
59 young adults ages 18 to 25[48F/11M, 21.5±2.0yrs] experiencing psychological distress completed the study. Participants were evaluated at baseline, six, and twelve months. At baseline, participants reported lifetime trauma events and completed a monetary reward fMRI task. Affective and anxiety symptoms were reported at each visit and trajectories were calculated using MPlus. Neural activation during RPE and other phases of reward processing were determined using SPM8. Trauma and reward neural activation were entered as predictors of symptom trajectories.
Results.
Trauma exposure moderated prospective relationships between left ventral striatum(β= −1.29,p=0.02) and right amygdala(β=0.58,p=0.04) activation to RPE and future hypo/mania severity trajectory: the interaction between greater trauma and greater left VS activation to RPE was associated with a shallower increase in hypo/mania severity whereas the interaction between greater trauma and greater right amygdala activation to RPE was associated with increasing hypo/mania severity.
Discussion.
Trauma exposure impacts prospective relationships between markers of reward circuitry function and affective symptom trajectories. Evaluating trauma exposure is thus critically important in naturalistic and treatment studies aiming to identify neural predictors of future affective symptom course.
Keywords: trauma, reward, striatum, amygdala, mania, fmri
Introduction
Trauma exposure during childhood and adolescence is common, and is associated with a more severe and persistent course of affective and anxiety symptoms(1–3). Individuals exposed to trauma have 2.0 greater odds of developing depressive symptoms, 2.7 greater odds of developing anxiety symptoms(4), and are at higher risk for bipolar disorder (BD)(5,6). Identifying objective markers of future affective symptom course in naturalistic and intervention studies can help elucidate neural mechanisms underlying progression or recovery from affective disorders(7,8). However, the neural systems underlying whether, and which, affective and anxiety symptoms develop after trauma are poorly understood.
Survival requires learning to make predictions about future events based on expectations. Accurately predicting future rewards increases motivation and behaviors to obtain such rewards. Deficits in reward prediction change motivation to obtain rewards, as potential rewards may not be perceived as rewarding or the experience of reward can be altered if a mismatch exists between expected versus received reward(9). The difference between expected and received rewards is the reward prediction error(RPE)(10). Affective and anxiety disorders are associated with altered RPE, where negative expectations in depression and anxious worry impairs reward prediction(11). In contrast, heightened sensitivity to rewards is associated with hypo/mania and predisposition to BD(12). Recent research suggests trauma influences RPE(13). Impaired RPE following trauma may be a mechanism linking trauma exposure and development of affective and anxiety disorders.
Neural regions underlying RPE include the ventral striatum(VS) and amygdala. The VS encodes RPE(14), the amygdala encodes valence and salience, and VS and amygdala interact during encoding of reward and punishment(15,16). Other reward regions, including anterior cingulate cortex(ACC), ventrolateral prefrontal cortex(vlPFC), and orbitofrontal cortex(OFC) support other phases of reward processing. Rostral-dorsal ACC regulates behavior in response to the incentive salience of stimuli to obtain rewards(17–19). vlPFC computes the behavioral significance of environmental cues by linking stimuli to future rewards(reward expectancy[RE])(20,21), while OFC encodes the incentive salience of expected rewards(16–18,22–27). Altered activation in neural regions supporting RPE is implicated in affective(28,29) and anxiety(30) disorders. Specifically, lower VS activation to RPE is observed in depressive and anxiety disorders(31–33) and is associated with depression severity(34) in many, but not all(see(35)), studies. Blunted VS response to RPE is associated with the development of future depression(36) and anhedonia(37). Reward processing is also altered in BD. BD is characterized by heightened VS activation to reward processing, heightened VS and amygdala activation to monetary and social reward(38–41), and heightened VS-amygdala connectivity during reward receipt(42). The pattern of elevated VS and amygdala activation, and VS-connectivity, to reward may underlie the arousal and reward sensitivity characteristic of hypo/mania(12,43), the pathognomonic symptom dimension of BD. Given the relationship between VS activation/connectivity to RPE and affective symptoms, neural activation and connectivity to RPE may be an important biomarker in the prediction of affective and anxiety symptoms.
Research suggests trauma exposure also impacts reward prediction and reward neural circuitry function(13), making RPE a plausible target linking trauma to affective and anxiety symptoms. Trauma exposure is associated with lower VS activation to reward, higher amygdala activation and ineffective ACC regulation of VS and amygdala activation(44,45). Greater life stress between ages 5-18 years is associated with lower VS activation to receipt of monetary reward(46) and lower VS reward activation relates to worse depression severity in those with life stress(47,48). Greater amygdala activation to valenced/salient stimuli in trauma-exposed individuals is also associated with reduced connectivity between amygdala and VS, particularly when trauma occurs earlier in life when such connections are developing(44). Lower VS-amygdala connectivity may blunt VS activation to reward and produce depressive symptoms in contrast from increased connectivity, which may produce hypo/mania(44,49, 50). In studies including positive and negative emotional stimuli, trauma-exposed individuals exhibited heightened amygdala activation to viewing emotional content(51). Amygdala activation correlated with hyperarousal, a feature of both hypo/mania and post-traumatic stress disorder (PTSD)(49). ACC regulation of VS, amygdala and other regions supporting reward processing, while adaptive, might, if excessive, contribute to depressive symptoms(52,53). In young adults, we demonstrated that lifetime trauma was associated with greater ventral ACC(vACC) activation but with lower vACC connectivity with frontoparietal regions supporting executive control to RPE; and that lower connectivity vACC-frontoparietal connectivity was associated with heightened affective and anxiety symptoms(45). Yet, the extent to which trauma influences the prospective relationships between reward neural circuitry function and future affective and anxiety symptoms remains unknown.
In an expanded transdiagnostic sample from Eckstrand et al.(37) we aimed to determine how lifetime trauma exposure influences relationships between reward neural circuitry function and the course of future affective and anxiety symptoms in a naturalistic, observational, transdiagnostic context. Based on findings described above, we determined whether greater amygdala and lower VS activation, and altered ACC connectivity with VS and amygdala, to RPE in the setting of greater trauma exposure predicted worsening affective and anxiety symptoms. We prospectively followed, over 1 year, this transdiagnostic sample of young adults aged 18-25 years to examine development of affective and anxiety symptoms/severity in a naturalistic setting, in accord with the NIMH Research Diagnostic Criteria approach(54). Lifetime trauma exposure was assessed, and reward neural circuitry function was examined using an established monetary reward paradigm at baseline, with affective and anxiety symptoms measured over 1 year. Existing findings and clinical neuroscience models of trauma allowed us to make the following hypotheses: 1.Greater VS and amygdala activation to RPE would predict increasing future hypo/mania severity, while lower VS activation to RPE would predict increasing future depression and anhedonia severity;2.Trauma would moderate these relationships, such that: a.greater trauma exposure and lower VS activation to RPE would predict increasing future depression and anhedonia severity, whereas greater trauma exposure and greater VS activation to RPE would predict decreasing future hypo/mania severity;b.greater trauma exposure and greater amygdala activation to RPE would predict increasing arousal, characterized by increasing hypo/mania and anxiety 3.Trauma would also impact the relationship between ACC-VS, ACC-amygdala, and VS-amygdala connectivity to RPE and future affective symptoms, such that greater trauma exposure, and greater ACC-VS connectivity and lower VS-amygdala connectivity, to RPE would predict increasing depression severity.
Methods
Participants/Study Design
This study was approved by the University of Pittsburgh Institutional Review Board. In an expanded sample from Eckstrand et al.(55)(61% overlap), 59 individuals between ages 18-25 years seeking mental healthcare for psychological distress were included in the final sample of this prospective, longitudinal study(Table1). The study goal was to recruit a young adult community sample, a period when psychiatric illnesses emerge, and observe the course of affective symptoms independent of providing specific treatment interventions, maximizing the likelihood of capturing significant changes in symptoms over time(56). Participants were recruited via local student counseling centers and advertisement. Individuals were right-handed and spoke fluent English(eMethods). 40 of the 59 participants met criteria for one or more diagnoses at initial visit(eTable 1), determined using the Structured Clinical Interview for DSM-5, Research Version(SCID-5-RV(57)). Participants completed three study visits(0 months[baseline visit], 6- and 12-months after baseline visit), as three visit is sufficient for longitudinal modeling in neuroimaging research(58). 6 months was selected for the duration between visits as this is the conventional timeframe for determining recovery from depression(59) and thus appropriate in a naturalistic study for evaluating clinical outcomes. At baseline, participants completed functional magnetic resonance imaging(fMRI);clinician-rated and self-report measures of depression, anxiety, and hypo/mania symptoms;and an assessment of lifetime trauma exposure. Symptom measures were completed at each follow-up. Details of medication usage are presented in the supplement.
Table 1.
Baseline Participant Demographics
| Mean ± SD or n | ||
|---|---|---|
| Age (yrs) | 21.47 ± 2.00 | |
| IQ | 107.76 ± 7.55 | |
| Gender | Female | 48 |
| Male | 11 | |
| Race | White | 33 |
| Black / African American | 10 | |
| Asian | 13 | |
| More than one race | 3 | |
| Parental Education | High school / GED | 11 |
| Some college | 34 | |
| Technical school | 1 | |
| College degree | 12 | |
| Graduate degree | 1 | |
| Psychotropic Load | 0.03 ± 0.18 | |
| Traumatic Events | 2.36 ± 1.61 | |
Trauma Exposure and Affective Measures
Lifetime trauma exposure was measured using the Trauma History Questionnaire(THQ) and quantified as the total number of events endorsed(eTable2)(60). Clinician-rated symptoms were measured with the Hamilton Rating Scale for Depression(HRSD)(61);Hamilton Anxiety Rating Scale(HAMA)(62);and Young Mania Rating Scale(YMRS)(63). Self-report measures included the Mood and Anxiety Symptom Questionnaire–Anhedonic Depression subscale(MASQ-AD)(64);MASQ–Anxious Arousal subscale(MASQ-AA);and the Snaith Hamilton Pleasure Scale(SHAPS)(65). See eMethods for measure scoring.
Monetary Reward fMRI Task
Reward neural circuitry was examined using an adapted event-related card-guessing task(45,66) that included win, loss, mixed, and neutral outcome trials and four expectancy contexts(eFigure 1) with a monetary value associated with trial outcome($1 per win;$0.75 deduction per loss;$0 for neutral). Reward prediction error(RPE), reward expectancy(RE), and outcome expectancy(OE) were derived from the monetary values associated with each trial type(eMethods). The outcome of interest, RPE, was computed as the difference between expected versus actual reward outcome based on the monetary value from each trial type:+$0.50 for a win;−$0.50 for no win in the possible win condition;+$0.375 for a no loss and −$0.375 for a loss in the possible loss condition;+$0.875 for a win and −$0.875 for a loss in the mixed condition and zero in the neutral condition. See eMethods for task description, RE/OE calculation, MRI acquisition parameters, and preprocessing.
Data Analyses
First level neuroimaging analysis used a fixed effect general linear model(GLM), implemented in Statistical Parametric Mapping software(SPM8), for each participant. The GLM included:the expectancy phase, including presentation of an upward or downward arrow, both or neither denoting one of the four possible expectancy contexts(above);the outcome phase, reflecting the result of the trial were entered;RPE, RE, and OE, each entered as parametric modulators, coupled to either the expectancy and outcome phases of each trial. RE was coupled to the expectancy phase, reflecting the expected value of the outcome;OE was coupled to the expectancy phase, reflecting the unsigned value of possible future outcomes;and RPE was coupled to the outcome, defined as the difference between the outcome and expected value. Another regressor modeled omission errors. Gram-Schmidt orthogonalization was applied to GLM regressors to eliminate collinearity between regressors. Physiological fluctuations, computed using CompCor(67,68) by calculating the mean signal within CSF, white matter, and high standard deviation voxels, and were included as covariates to reduce physiological and motion-related noise. Lastly, six motion parameters obtained during realignment were entered as covariates to control for head movement. A 60s high-pass filter and autoregressive modelling were implemented during fitting. The GLM was fit to the two task blocks separately and parameter estimates were combined across each.
First level contrast images were entered into second level SPM analyses. Participant age, gender, parental education, psychotropic load during the study period, and scanner type were entered as covariates in activation and connectivity models. Voxelwise analyses were constrained to a single mask comprising reward regions(eFigure 2). The mask included the main regions of interest, VS and amygdala, and other reward regions, comprising a mask of all key regions implicated in reward processing:bilateral amygdala, rostral-dorsal ACC(BA32), OFC(BA11), and vlPFC(BA47) defined in WFUPickAtlas;and VS as previously defined functionally in the present task(69,70). Functional connectivity maps(eMethods) were generated with ACC and VS as seed regions. Activation and connectivity maps within the mask were thresholded at the cluster level pFWE<0.05 with cluster extent threshold of kE>20 voxels. Contrast values representing BOLD activation and connectivity for regions with significant activation in second-level voxelwise analyses were extracted using Marsbar(http://marsbar.sourceforge.net/).
Intercepts and slopes of linear growth trajectories of individual symptoms over 1 year were calculated using baseline, 6-and 12-month timepoints with MPlus7(71). No variables had >5% missing data. MPlus uses full information maximum likelihood and all available data to estimate models, estimating each parameter directly without filling in missing values. Individual growth slopes over 1-year were used as dependent variables in subsequent analyses.
Data points for imaging markers, trauma exposure, and symptom trajectories were excluded from analyses if they were greater than 3SD from the mean to avoid the influence of extreme outliers(eResults). This included one data point from three different participants: right ACC RPE activation, right VS RPE activation, and left VS RPE activation.
Multivariate linear models implemented in SPSSv23 tested hypotheses of whether lifetime trauma exposure influenced relationships among markers of reward neural circuitry function and future affective and anxiety symptom trajectories over 6 months and 1year. Two separate multivariate linear models were run for each time span: one multivariate linear model examined clinician-rated symptom trajectories while the other multivariate linear model examined self-report symptom trajectories. Self-report and clinician-rated symptoms were examined in separate models as type of symptom rating scales contributes uniquely to symptom severity(72). Each multivariate linear model was structured similarly where models contained three nested univariate tests, one for each symptom trajectory. The clinician-rated multivariate linear models contained univariate tests predicting HAMA, HRSD, or YMRS symptom trajectories over 6 months or 1 year. The self-report multivariate linear models contained univariate tests predicting MASQ-AD, MASQ-AA, or SHAPS symptom trajectories over 6 months or 1 year. The same nine predictor variables were used in each nested univariate test: lifetime trauma exposure; the four neural regions with significant RPE activation(Results);and four interaction terms between lifetime trauma exposure and regions with significant RPE activation. Fixed intercepts were included in all models. The statistical threshold for each univariate model in each multivariate linear model was corrected for the number of univariate tests in each multivariate mode using sequential goodness of fit(SGoF+) metatests, as were the predictor-outcome relationships in each univariate model. SGoF+ is an effective tool for adjustment with high-dimensional biologic data to detect significant relationships without increasing the false discovery rate(73)(eMethods). Results are reported for each univariate test overall and the predictor-outcome relationships within the univariate tests. As reward neural activation had been corrected for demographic variables and psychotropic medication, these variables were excluded from growth trajectory and multivariate linear models. Post-hoc moderation analyses implemented using PROCESS in SPSSv23 examined effects of diagnosis, medication, and outpatient mental health treatment;Pearson correlations tested relationships between predictor and outcome variables(eResults).
Results
ROIActivation/Connectivity during RPE
In the fMRI main effects analysis examining neural activation to the contrast of interest, RPE, there was significant activation to RPE in four regions: the left and right VS, right amygdala, and right rostral-dorsal ACC(Table 2,Figure1A). Activation in these regions was used in subsequent models to examine the interactive effects with trauma and 1-year symptom trajectories. There were no areas of significant activation to RPE in OFC or vlPFC. There were no areas of significant ACC or VS connectivity to RPE. No ROIs were activated significantly, nor was significant ACC or VS connectivity observed, to RE and OE.
Table 2.
Neural activation during reward prediction error (RPE). Thresholded at pFWE<0.05, kE > 20 voxels
| Region | Hemisphere | Voxels | T-score | x | y | z |
|---|---|---|---|---|---|---|
| Ventral Striatum | R | 260 | 8.47 | 8 | 16 | −6 |
| L | 118 | 8.26 | −10 | 14 | −8 | |
| Amygdala | R | 20 | 6.29 | 18 | 4 | −16 |
| Anterior Cingulate Cortex | R | 43 | 4.75 | 4 | 44 | 4 |
Figure 1.
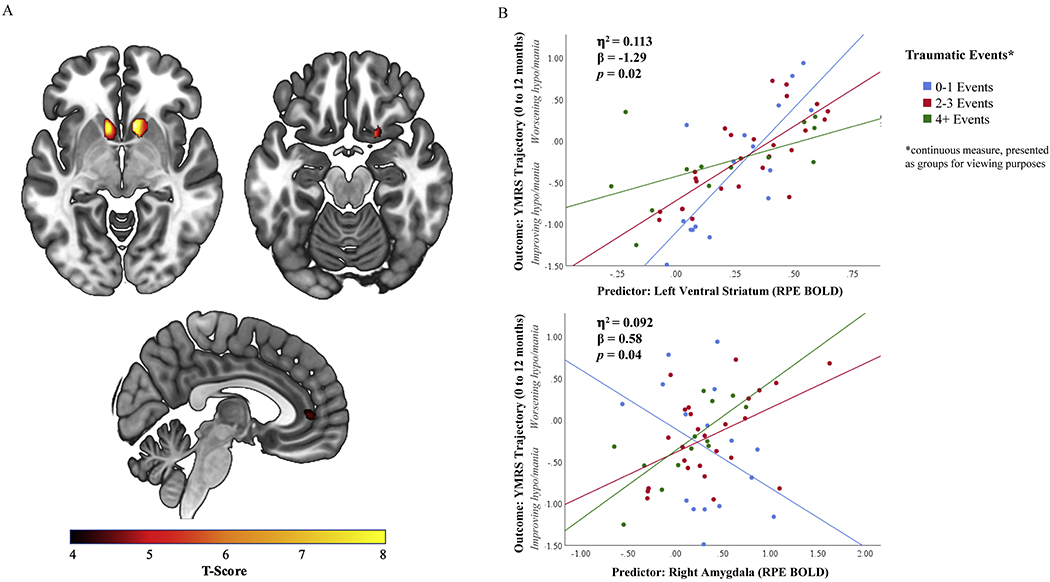
A. Activation to Reward Prediction Error (RPE) in the ventral striatum, amygdala, and anterior cingulate cortex. B. The interaction of trauma exposure and neural activation to RPE in the right amygdala and left ventral striatum predicts the development of hypo/mania symptoms.
Hypothesis 1:RPE Activation predicting 1-year symptom trajectories
In the 1-year clinician-rated multivariate linear model including trauma exposure and clinician-rated outcome variables, there was a significant effect of predictor variables on 1-year clinician-rated hypo/mania trajectory(F[9,45]=2.302,η2=0.315,p=0.032), but not clinician-rated depression or anxiety trajectory. At the univariate level, after correction for multiple comparisons, the increase in 1-year hypo/mania severity was specifically predicted by greater left VS RPE activation(F[1,45]=8.543,β=4.133,η2=0.160,p=0.005;Table 3) and lower right amygdala RPE activation(F[1,45]=6.645,β=−0.174,η2=0.111,p=0.022;Table 3) and not by neural activation to RPE in the right VS or right rostral-dorsal ACC. No significant relationships with self-report symptoms were observed in the 1-year self-report multivariate linear model(eTable9).
Table 3.
1-year Clinician Rated Multivariate Linear Model showing association of neural activation during RPE with clinician rated affective symptoms between 0 and 12 months
|
Univariate Testsa |
Predictor-Outcome Relationships |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted | Partial | 95% CI | |||||||
| R2 | P-value | F | β | η2 | p-value | LL | UL | ||
| Anxiety (HAMA) | −0.074 | 0.800 | Intercept | 21.947 | −2.803 | 0.328 | <0.001 | −4.008 | −1.598 |
| Trauma Exposure | 0.928 | 0.177 | 0.020 | 0.341 | −0.193 | 0.546 | |||
| Left Ventral Striatum | 0.332 | 1.176 | 0.007 | 0.573 | −2.999 | 5.35 | |||
| Right Amygdala | 0.769 | −0.941 | 0.017 | 0.385 | −3.104 | 1.221 | |||
| Right ACC | 0.829 | 1.012 | 0.018 | 0.367 | −1.226 | 3.25 | |||
| Right Ventral Striatum | 0.606 | 1.916 | 0.013 | 0.440 | −3.04 | 6.873 | |||
| Left Ventral Striatum * Trauma | 0.348 | −0.465 | 0.008 | 0.558 | −2.051 | 1.122 | |||
| Right Amygdala * Trauma | 1.299 | 0.454 | 0.028 | 0.260 | −0.348 | 1.256 | |||
| Right ACC * Trauma | 0.354 | −0.255 | 0.008 | 0.555 | −1.118 | 0.609 | |||
| Right Ventral Striatum * Trauma | 0.579 | −0.747 | 0.013 | 0.451 | −2.725 | 1.231 | |||
| Depression (HDRS) | −0.094 | 0.487 | Intercept | 80.504 | −2.322 | 0.641 | <0.001 | −2.843 | −1.801 |
| Trauma Exposure | 0.965 | 0.078 | 0.021 | 0.331 | −0.082 | 0.237 | |||
| Left Ventral Striatum | 0.042 | 0.184 | 0.001 | 0.839 | −1.622 | 1.989 | |||
| Right Amygdala | 0.074 | −0.126 | 0.002 | 0.787 | −1.061 | 0.809 | |||
| Right ACC | 0.804 | 0.431 | 0.018 | 0.375 | −0.537 | 1.399 | |||
| Right Ventral Striatum | 0.776 | 0.938 | 0.017 | 0.383 | −1.206 | 3.082 | |||
| Left Ventral Striatum * Trauma | <0.001 | <−0.001 | <0.001 | 1.000 | −0.686 | 0.686 | |||
| Right Amygdala * Trauma | 0.120 | 0.060 | 0.003 | 0.731 | −0.287 | 0.407 | |||
| Right ACC * Trauma | 0.796 | −0.165 | 0.017 | 0.377 | −0.539 | 0.208 | |||
| Right Ventral Striatum * Trauma | 0.430 | −0.278 | 0.009 | 0.515 | −1.134 | 0.577 | |||
| Mania (YMRS) | 0.178 | 0.032† | Intercept | 6.267 | −1.022 | 0.122 | 0.016 | −1.844 | −0.200 |
| Trauma Exposure | 0.423 | 0.081 | 0.009 | 0.519 | −0.171 | 0.333 | |||
| Left Ventral Striatum | 8.543 | 4.133 | 0.160 | 0.005† | 1.285 | 6.982 | |||
| Right Amygdala | 5.645 | −1.740 | 0.111 | 0.022† | −3.215 | −0.265 | |||
| Right ACC | 0.873 | 0.708 | 0.019 | 0.355 | −0.819 | 2.235 | |||
| Right Ventral Striatum | 0.010 | −0.165 | <0.001 | 0.922 | −3.547 | 3.217 | |||
| Left Ventral Striatum * Trauma | 5.740 | −1.288 | 0.113 | 0.021† | −2.37 | −0.205 | |||
| Right Amygdala * Trauma | 4.533 | 0.579 | 0.092 | 0.039† | 0.031 | 1.126 | |||
| Right ACC * Trauma | 0.208 | −0.133 | 0.005 | 0.651 | −0.722 | 0.456 | |||
| Right Ventral Striatum * Trauma | 0.478 | 0.463 | 0.011 | 0.493 | −0.886 | 1.813 | |||
Significant after SGoF+ multiple comparisons correction
These statistics refer to the effect size and significance of the univariate tests nested in the multivariate linear model
HAMA, Hamilton Anxiety Rating Scale; HRSD, Hamilton Rating Scale for Depression; SGoF+, Sequential Goodness of Fit; YMRS, Young Mania Rating Scale
Hypotheses 2-3:Interaction between lifetime trauma exposure and RPE activation predicting 1-year symptom trajectories
In the 1-year clinician-rated multivariate linear model, at the univariate level, trauma exposure interacted with regions with significant RPE neural activation in the left VS(F[1,45]=5.740,β=−1.288,η2=0.113,p=0.021) and right amygdala(F[1,45]=4.533,β=0.579,η2=0.092,p=0.039) to predict 1-year change in clinician-rated hypo/mania severity(Figure1B). The interaction between greater trauma and greater left VS RPE activation was associated with a shallower increase in hypo/mania severity whereas the interaction between greater trauma and greater right amygdala RPE activation was associated with increasing hypo/mania severity(Table 3). There was no interaction between trauma exposure and neural RPE activation to predict 1-year clinician-rated depression or anxiety severity. These findings remained significant after accounting for diagnosis and psychotropic medication use during the study period(eResults). There was no interaction between trauma exposure and neural RPE activation predicting self-report symptom trajectories(eTable9).
Supplementary Analyses:Interaction between lifetime trauma exposure and RPE activation predicting shorter-term, 6-month symptom trajectories
To examine the timing of this interaction we repeated analyses using baseline and 6-month data(eMethods). In the 6-month clinician-rated multivariate linear model, the interaction between left VS RPE activation in individuals with higher trauma exposure predicting a shallower increase in clinician-rated hypo/mania severity over 6 months missed significance and is reported here for transparency (F[1,31]=3.683,β=−3.301,η2=0.106,p=0.064;eTable10,eFigure2). Interestingly, the interaction between right amygdala RPE activation in individuals with more trauma exposure predicted increasing clinician-rated depression severity(F[1,31]=5.125,β=−10.211,η2=0.142,p=0.031) but did not predict increasing hypo/mania severity over 6 months. Consistent with our previously-reported findings(37), greater left VS RPE activation predicted improving anhedonia over 6 months (F[1,40]=4.428, β=−13.639,η2=0.099,p=0.042;eTable11).
Discussion
Functional abnormalities in neural regions underlying RPE are associated with affective and anxiety symptoms. Trauma exposure influences neural reward activation, including in VS and amygdala, and this may contribute to affective and anxiety symptoms. Altered VS and amygdala activation to RPE may facilitate development of future affective and anxiety symptoms following exposure to trauma. In this prospective, longitudinal study, we demonstrate that trauma exposure interacts with VS and amygdala RPE activation to predict affective symptom development. Specifically, the interaction between greater trauma and greater left VS RPE activation was associated with a shallower increase in hypo/mania severity whereas the interaction between greater trauma and greater right amygdala RPE activation was associated with increasing hypo/mania severity. Greater right amygdala RPE activation in individuals with greater trauma exposure predicted increasing clinician-rated depression severity over 6 months and increasing clinician-rated hypo/mania severity over 1 year.
The VS and amygdala are integral to the generation and learning of reward, and disruptions in reward neural circuitry function are associated with BD(38,39,74,75). The VS receives dopaminergic inputs from the ventral tegmental area which encode and transmit RPE signals to support goal-directed behavior(32). The VS also receives glutamatergic projections from the basolateral nucleus of the amygdala(BLA)(16), which translate emotional state and affective value of stimuli to enhance goal-directed(e.g.,reward-related), behavior(76,77). Heightened VS activation to RE is observed in BD(38), and elevated amygdala activation to RE and positive emotional stimuli, in hypo/mania(39,74,75). Amygdala activation can reduce reward-seeking and increase avoidance, however, via enhanced connectivity between BLA and the centromedial nucleus of the amygdala(CeM)(78), mediating fear conditioning and avoidance behaviors(79). Our present finding that greater VS activation to RPE predicted increasing 1-year hypo/mania severity, controlling for trauma exposure, parallels these findings and supports Hypothesis 1. Contrary to Hypothesis 1, yet consistent with previous research linking amygdala activation with reduced reward seeking behavior, we show that greater amygdala activation to RPE predicted lower hypo/mania severity over 1 year. The fact that left VS but right amygdala were predictors of future hypo/mania might reflect the left and right hemisphere roles in approach and avoidance behavior, respectively(80).
Trauma exposure blunts reward stimulus valuation(81) and VS activation(46), and increases amygdala activation(44,46,49). This parallels our findings, where the combination of greater VS RPE activation and greater trauma exposure resulted in a shallower increase in hypo/mania severity over 1 year, supporting Hypothesis 2a. While there were no direct effects of trauma on VS RPE activation(eTable3), greater trauma exposure likely blunts the magnitude of reward-related behavior associated with a given level of dopaminergically-modulated VS activation to RPE because of reduced BLA input to VS(50,78). Trauma exposure is also associated with heightened amygdala activation to viewing positive and negative emotional stimuli(51), and amygdala activation to valenced/salient stimuli is associated with arousal symptoms(e.g.,anxiety, affective lability) in trauma-associated disorders(44,49). Our finding that greater amygdala activation to RPE in those with greater trauma exposure predicted increasing hypo/mania over 1 year might reflect an association between trauma-related amygdala RPE activation and the arousal, but not reward-seeking, component of hypo/mania, in support of Hypothesis 2b. Together with evidence that greater RPE-related amygdala activation, controlling for trauma exposure, was associated with lower hypo/mania severity at 1 year, our findings highlight how trauma exposure can significantly alter the relationship between RPE-related amygdala activation and affective symptom development.
Six-month data regarding the RPE-related VS activation-hypo/mania relationship paralleled 1-year follow-up findings, with greater VS activation to RPE in individuals with greater trauma exposure predicting a shallower increase in hypo/mania over 6 months, although this missed significance. By contrast, greater amygdala RPE activation in individuals with more trauma predicted increasing depression, rather than hypo/mania, over 6 months. Taken together, there may be differential effects of trauma exposure on prospective relationships between RPE-related amygdala activation and future affective symptoms in the shorter and longer-term future. While we cannot make definitive statements about the timing of trauma exposure in the present study, one explanation for these differential effects is that greater trauma exposure might initially increase amygdala down-regulation of reward-related behavior and enhance avoidance behavior(78), due to greater BLA input to CeM at the expense of BLA input to VS(79), resulting in greater depression severity in the shorter term(6-months). Yet, greater trauma exposure might also enhance amygdala-modulated arousal in the longer term(1-year), potentially due to increased trauma-related BLA input to CeM increasing fear-conditioning-related arousal(44). While the mechanism for this latter relationship is unclear, it is unlikely to be the result of increased BLA input to the VS, as greater trauma exposure was associated with blunting of the VS activation-increasing hypo/mania relationship over 1 year. Future translational studies focused on the timing and recency of trauma exposure are necessary to explore these possibilities. Our findings might explain the relationship between trauma exposure and BD symptoms, as individuals with BD experience more traumatic events than those with unipolar depression or healthy controls(82,83), and trauma exposure is associated with earlier symptom onset, frequent mood episodes, and rapid cycling in BD(84).
We did not find neural predictors of future anxiety symptoms with trauma exposure. This may be due to examining lifetime trauma, as anxiety symptoms undergo more rapid change over the 12 months following recent trauma before remitting or becoming stable/persistent(85,86). Contrary to Hypothesis 3, we did not find a relationship between RPE-related ACC activation, trauma exposure, and symptom trajectories, which may be due to the role of ACC in regulation of current affective symptoms rather than predicting future symptom severity. We also did not find relationships between neural RPE activation and self-report symptoms, likely due to self-report scales not assessing hypo/mania. We also did not observe significant OFC or vlPFC activation to RPE, likely due to the fact that these regions are not directly involved in RPE processing. The lack of significant activation to RE/OE might be because of greater inter-individual variation in RE/OE-related activation, due to individual variation in behavioral traits(66,87). Lastly, the lack of significant connectivity to RPE may be due to greater inter-individual variability, negating group-level effects. Future studies could examine inter-individual differences in activation and connectivity during RE, OE, and RPE that may be related to behavioral traits and future symptom trajectories.
While the severity of hypo/mania symptoms in the present sample was low, BD symptoms emerge during young adulthood(88) and subthreshold hypo/mania symptoms predict future conversion to BD(89). Thus, it is important to examine predictors of increasing hypo/mania severity in young adulthood as a first stage toward identifying predictors of future conversion to BD. The present findings help elucidate potential mechanisms for the development of hypo/mania symptoms, and future research could examine the present relationships over a multi-year period. While there was no relationship between RPE-related VS activation and depression severity over 1 year, there was a relationship between RPE-related amygdala activation and depression with greater trauma exposure at 6 months. Furthermore, in an expanded transdiagnostic sample from Eckstrand et al.(37) we again demonstrated the previously reported finding that greater RPE-related VS activation predicted improving anhedonia over 6 months. In a naturalistic, observational study over 1 year, where the expected course of remission is within 6 months(90,91), it is plausible that a linear trajectory does not adequately capture depressive symptom course over 1 year, whereas vulnerability to hypo/mania may emerge in this timeframe. Longitudinal studies have shown linear models to be superior to quadratic models in modeling hypo/mania symptoms over two years(92,93). While we performed preliminary analyses regarding recency of trauma exposure, these results must be interpreted with caution given that self-report measures are prone to biases(94). We also acknowledge that in the present design, RPE comprises unexpected positive and negative prediction errors and distinguishing these events may provide additional understanding to the observed relationships.
In conclusion, this longitudinal, observational study aimed to determine how trauma exposure impacts neural predictors of future affective symptoms. Our findings indicate that lifetime trauma exposure interacts with reward neural circuitry activation to impact future affective symptom trajectories in young adults. This suggests that evaluating trauma exposure is critically important for naturalistic and treatment studies aiming to identify neural predictors of future affective symptom course, and for targeting effective treatment interventions.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | Not applicable | Not applicable | Not applicable | Not applicable |
| Bacterial or Viral Strain | Not applicable | Not applicable | Not applicable | Not applicable |
| Biological Sample | Not applicable | Not applicable | Not applicable | Not applicable |
| Cell Line | Not applicable | Not applicable | Not applicable | Not applicable |
| Chemical Compound or Drug | Not applicable | Not applicable | Not applicable | Not applicable |
| Commercial Assay Or Kit | Not applicable | Not applicable | Not applicable | Not applicable |
| Deposited Data; Public Database | Not applicable | Not applicable | Not applicable | Not applicable |
| Genetic Reagent | Not applicable | Not applicable | Not applicable | Not applicable |
| Organism/Strain | Not applicable | Not applicable | Not applicable | Not applicable |
| Peptide, Recombinant Protein | Not applicable | Not applicable | Not applicable | Not applicable |
| Recombinant DNA | Not applicable | Not applicable | Not applicable | Not applicable |
| Sequence-Based Reagent | Not applicable | Not applicable | Not applicable | Not applicable |
| Software; Algorithm | Not applicable | Not applicable | Not applicable | Not applicable |
| Transfected Construct | Not applicable | Not applicable | Not applicable | Not applicable |
| Other | ||||
ACKNOWLEDGMENTS:
This work was supported by the National Institute of Mental Health (R01MH100041 and R37MH100041 to MLP) and the Pittsburgh Foundation (to MLP).
Role of Funder/Sponsor Statement: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PRESENTATION: This paper was accepted for presentation at the 2020 Annual Meeting of the Society of Biological Psychiatry.
DISCLOSURES/COIs: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li M, D’Arcy C, Meng X. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta-analysis, and proportional attributable fractions. Psychol Med. 2016;46(4):717–30. [DOI] [PubMed] [Google Scholar]
- 3.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry. 2012;169(2):141–51. [DOI] [PubMed] [Google Scholar]
- 4.Li M, D’arcy C, Meng X. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta-analysis, and proportional attributable fractions. Psychological medicine. 2016;46(4):717–30. [DOI] [PubMed] [Google Scholar]
- 5.Hanford LC, Eckstrand K, Manelis A, Hafeman DM, Merranko J, Ladouceur CD, et al. The impact of familial risk and early life adversity on emotion and reward processing networks in youth at-risk for bipolar disorder. PLoS One. 2019;14(12):e0226135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aas M, Henry C, Andreassen OA, Bellivier F, Melle I, Etain B. The role of childhood trauma in bipolar disorders. Int J Bipolar Disord. 2016;4(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85(3):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer A, Nelson B, Perlman G, Klein DN, Kotov R. A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. J Child Psychol Psychiatry. 2018;59(11):1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papalini S, Beckers T, Vervliet B. Dopamine: from prediction error to psychotherapy. Transl Psychiatry. 2020;10(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz W Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci. 2016;17(3):183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134(Pt 6):1751–64. [DOI] [PubMed] [Google Scholar]
- 12.Johnson SL, Edge MD, Holmes MK, Carver CS. The behavioral activation system and mania. Annu Rev Clin Psychol. 2012;8:243–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross MC, Lenow JK, Kilts CD, Cisler JM. Altered neural encoding of prediction errors in assault-related posttraumatic stress disorder. J Psychiatr Res. 2018;103:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131(Pt 8):2084–93. [DOI] [PubMed] [Google Scholar]
- 15.Baxter MG, Murray EA. The amygdala and reward. Nature reviews neuroscience. 2002;3(7):563. [DOI] [PubMed] [Google Scholar]
- 16.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1): 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15(2):56–67. [DOI] [PubMed] [Google Scholar]
- 18.Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70(6):1054–69. [DOI] [PubMed] [Google Scholar]
- 19.Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55(6):594–602. [DOI] [PubMed] [Google Scholar]
- 20.Lee SW, O’Doherty JP, Shimojo S. Neural computations mediating one-shot learning in the human brain. PLoS Biol. 2015;13(4):e1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boorman ED, Rajendran VG, O’Reilly JX, Behrens TE. Two Anatomically and Computationally Distinct Learning Signals Predict Changes to Stimulus-Outcome Associations in Hippocampus. Neuron. 2016;89(6):1343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55(4):359–66. [DOI] [PubMed] [Google Scholar]
- 23.Ramnani N, Elliott R, Athwal BS, Passingham RE. Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage. 2004;23(3):777–86. [DOI] [PubMed] [Google Scholar]
- 24.Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23(1):303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–7. [DOI] [PubMed] [Google Scholar]
- 26.Kahnt T, Park SQ, Haynes JD, Tobler PN. Disentangling neural representations of value and salience in the human brain. Proc Natl Acad Sci U S A. 2014;111(13):5000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard JD, Kahnt T. Identity-Specific Reward Representations in Orbitofrontal Cortex Are Modulated by Selective Devaluation. J Neurosci. 2017;37(10):2627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual review of clinical psychology. 2014;10:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, Labarbara EJ, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14(3):249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White SF, Geraci M, Lewis E, Leshin J, Teng C, Averbeck B, et al. Prediction error representation in individuals with generalized anxiety disorder during passive avoidance. American Journal of Psychiatry. 2016;174(2):110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macoveanu J, Meluken I, Chase HW, Phillips ML, Kessing LV, Siebner HR, et al. Reduced frontostriatal response to expected value and reward prediction error in remitted monozygotic twins with mood disorders and their unaffected high-risk co-twins. Psychol Med. 2020:1–10. [DOI] [PubMed] [Google Scholar]
- 32.Kumar P, Goer F, Murray L, Dillon DG, Beltzer ML, Cohen AL, et al. Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology. 2018;43(7):1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White SF, Geraci M, Lewis E, Leshin J, Teng C, Averbeck B, et al. Prediction Error Representation in Individuals With Generalized Anxiety Disorder During Passive Avoidance. Am J Psychiatry. 2017;174(2):110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberg T, Chase HW, Almeida JR, Stiffler R, Zevallos CR, Aslam HA, et al. Moderation of the Relationship Between Reward Expectancy and Prediction Error-Related Ventral Striatal Reactivity by Anhedonia in Unmedicated Major Depressive Disorder: Findings From the EMBARC Study. Am J Psychiatry. 2015;172(9):881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutledge RB, Moutoussis M, Smittenaar P, Zeidman P, Taylor T, Hrynkiewicz L, et al. Association of Neural and Emotional Impacts of Reward Prediction Errors With Major Depression. JAMA Psychiatry. 2017;74(8):790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. Am J Psychiatry. 2018:appiajp201817101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckstrand KL, Forbes EE, Bertocci MA, Chase HW, Greenberg T, Lockovich J, et al. Anhedonia reduction mediates relationship between left ventral striatal reward response and 6-month improvement in life satisfaction in young adults. JAMA Psychiatry. 2019;76(9):958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry. 2013;170(5):533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berghorst LH, Kumar P, Greve DN, Deckersbach T, Ongur D, Dutra SJ, et al. Stress and reward processing in bipolar disorder: a functional magnetic resonance imaging study. Bipolar Disord. 2016;18(7):602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160(7):1345–7. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55(6):578–87. [DOI] [PubMed] [Google Scholar]
- 42.Dutra SJ, Man V, Kober H, Cunningham WA, Gruber J. Disrupted cortico-limbic connectivity during reward processing in remitted bipolar I disorder. Bipolar Disord. 2017;19(8):661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, et al. High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: a prospective behavioral high-risk design. J Abnorm Psychol. 2012;121(2):339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fareri DS, Tottenham N. Effects of early life stress on amygdala and striatal development. Dev Cogn Neurosci. 2016;19:233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckstrand KL, Hanford LC, Bertocci MA, Chase HW, Greenberg T, Lockovich J, et al. Trauma-associated anterior cingulate connectivity during reward learning predicts affective and anxiety states in young adults. Psychol Med. 2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanson JL, Albert D, Iselin AM, Carre JM, Dodge KA, Hariri AR. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc Cogn Affect Neurosci. 2016;11(3):405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corral-Frias NS, Nikolova YS, Michalski LJ, Baranger DA, Hariri AR, Bogdan R. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol Med. 2015;45(12):2605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, et al. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Admon R, Lubin G, Rosenblatt JD, Stern O, Kahn I, Assaf M, et al. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cereb Cortex. 2013;23(1):28–35. [DOI] [PubMed] [Google Scholar]
- 50.Damme KS, Young CB, Nusslock R. Elevated nucleus accumbens structural connectivity associated with proneness to hypomania: a reward hypersensitivity perspective. Soc Cogn Affect Neurosci. 2017;12(6):928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambuco N, Bradley M, Herring D, Hillbrandt K, Lang PJ. Transdiagnostic trauma severity in anxiety and mood disorders: Functional brain activity during emotional scene processing. Psychophysiology. 2020;57(l):el3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu DT, Langenecker SA, Kennedy SE, Zubieta JK, Heitzeg MM. fMRI BOLD responses to negative stimuli in the prefrontal cortex are dependent on levels of recent negative life stress in major depressive disorder. Psychiatry Res. 2010;183(3):202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010; 121(l):33–40. [DOI] [PubMed] [Google Scholar]
- 54.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010; 167(7):748–51. [DOI] [PubMed] [Google Scholar]
- 55.Eckstrand KL, Forbes EE, Bertocci MA, Chase HW, Greenberg T, Lockovich J, et al. Anhedonia Reduction and the Association Between Left Ventral Striatal Reward Response and 6-Month Improvement in Life Satisfaction Among Young Adults. JAMA Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kessler RC, Amminger GP, Aguilar - Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Current opinion in psychiatry. 2007;20(4):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.First M, Williams J, Karg R, Spitzer R. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association. 2015:1–94. [Google Scholar]
- 58.King KM, Littlefield AK, McCabe CJ, Mills KL, Flournoy J, Chassin L. Longitudinal modeling in developmental neuroimaging research: Common challenges, and solutions from developmental psychology. Dev Cogn Neurosci. 2018;33:54–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Health NIf, Excellence C. Depression in Adults: Recognition and Management. NICE Guideline (CG90). 2009. [PubMed] [Google Scholar]
- 60.Hooper LM, Stockton P, Krupnick JL, Green BL. Development, Use, and Psychometric Properties of the Trauma History Questionnaire. Journal of Loss and Trauma. 2011;16(3):258–83. [Google Scholar]
- 61.Hamilton M A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23(1): 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamilton M The assessment of anxiety states by rating. British journal of medical psychology. 1959;32(l):50–5. [DOI] [PubMed] [Google Scholar]
- 63.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. [DOI] [PubMed] [Google Scholar]
- 64.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991; 100(3):316–36. [DOI] [PubMed] [Google Scholar]
- 65.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995; 167(1):99–l03. [DOI] [PubMed] [Google Scholar]
- 66.Chase HW, Fournier JC, Bertocci MA, Greenberg T, Aslam H, Stiffler R, et al. A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Transl Psychiatry. 2017;7(4):e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fournier JC, Chase HW, Almeida J, Phillips ML. Model specification and the reliability of fMRI results: implications for longitudinal neuroimaging studies in psychiatry. PLoS One. 2014;9(8):e105169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15(8):839–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50(7):1252–66. [DOI] [PubMed] [Google Scholar]
- 71.Muthén L, Muthén B. Mplus User’s Guide Seventh Edition ed. Los Angeles: CA: Muthén & Muthén. 2015. [Google Scholar]
- 72.Uher R, Perlis RH, Placentino A, Dernovsek MZ, Henigsberg N, Mors O, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety. 2012;29(12):1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carvajal-Rodriguez A, de Una-Alvarez J, Rolan-Alvarez E. A new multitest correction (SGoF) that increases its statistical power when increasing the number of tests. BMC Bioinformatics. 2009;10:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bermpohl F, Dalanay U, Kahnt T, Sajonz B, Heimann H, Ricken R, et al. A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disord. 2009;11(1):70–5. [DOI] [PubMed] [Google Scholar]
- 75.Cattarinussi G, Di Giorgio A, Wolf RC, Balestrieri M, Sambataro F. Neural signatures of the risk for bipolar disorder: A meta-analysis of structural and functional neuroimaging studies. Bipolar Disord. 2019;21(3):215–27. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe N, Sakagami M, Haruno M. Reward prediction error signal enhanced by striatum-amygdala interaction explains the acceleration of probabilistic reward learning by emotion. J Neurosci. 2013;33(10):4487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59(4):648–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, et al. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520(7549):675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468(7321):277–82. [DOI] [PubMed] [Google Scholar]
- 80.Davidson RJ, Shackman AJ, Maxwell JS. Asymmetries in face and brain related to emotion. Trends Cogn Sci. 2004;8(9):389–91. [DOI] [PubMed] [Google Scholar]
- 81.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry. 2009;66(3):206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCraw S, Parker G. The prevalence and outcomes of exposure to potentially traumatic stressful life events compared across patients with bipolar disorder and unipolar depression. Psychiatry Res. 2017;255:399–404. [DOI] [PubMed] [Google Scholar]
- 83.Kefeli MC, Turow RG, Yildirim A, Boysan M. Childhood maltreatment is associated with attachment insecurities, dissociation and alexithymia in bipolar disorder. Psychiatry Res. 2018;260:391–9. [DOI] [PubMed] [Google Scholar]
- 84.Etain B, Aas M, Andreassen OA, Lorentzen S, Dieset I, Gard S, et al. Childhood trauma is associated with severe clinical characteristics of bipolar disorders. J Clin Psychiatry. 2013;74(10):991–8. [DOI] [PubMed] [Google Scholar]
- 85.Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M, et al. Early PTSD symptom trajectories: persistence, recovery, and response to treatment: results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS). PLoS One. 2013;8(8):e70084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Donnell ML, Alkemade N, Creamer M, McFarlane AC, Silove D, Bryant RA, et al. A Longitudinal Study of Adjustment Disorder After Trauma Exposure. Am J Psychiatry. 2016;173(12):1231–8. [DOI] [PubMed] [Google Scholar]
- 87.Edmiston EK, Fournier JC, Chase HW, Bertocci MA, Greenberg T, Aslam HA, et al. Assessing Relationships Among Impulsive Sensation Seeking, Reward Circuitry Activity, and Risk for Psychopathology: A Functional Magnetic Resonance Imaging Replication and Extension Study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kennedy N, Everitt B, Boydell J, Van Os J, Jones PB, Murray RM. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855–63. [DOI] [PubMed] [Google Scholar]
- 89.Hafeman DM, Merranko J, Goldstein TR, Axelson D, Goldstein BI, Monk K, et al. Assessment of a Person-Level Risk Calculator to Predict New-Onset Bipolar Spectrum Disorder in Youth at Familial Risk. JAMA Psychiatry. 2017;74(8):841–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171–80. [DOI] [PubMed] [Google Scholar]
- 91.Ramana R, Paykel ES, Cooper Z, Hayhurst H, Saxty M, Surtees PG. Remission and relapse in major depression: a two-year prospective follow-up study. Psychol Med. 1995;25(6):1161–70. [DOI] [PubMed] [Google Scholar]
- 92.Galynker II, Yaseen ZS, Koppolu SS, Vaughan B, Szklarska-Imiolek M, Cohen LJ, et al. Increased sleep duration precedes the improvement of other symptom domains during the treatment of acute mania: a retrospective chart review. BMC Psychiatry. 2016;16:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Findling RL, Jo B, Frazier TW, Youngstrom EA, Demeter CA, Fristad MA, et al. The 24-month course of manic symptoms in children. Bipolar Disord. 2013;15(6):669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Althubaiti A Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


