Abstract
Genetically-selected Marchigian Sardinian alcohol-preferring (msP) rats display co-morbid symptoms of increased alcohol preference and elevated anxiety-like behavior. Heightened stress sensitivity in msPs is influenced by genetic polymorphisms of the corticotropin-releasing factor receptor in the central nucleus of the amygdala (CeA), as well as reduced influence of anti-stress mechanisms that normally constrain the stress response. Given this propensity for stress dysregulation, in this study we expand on the possibility that msPs may display differences in neuroendocrine processes that normally terminate the stress response. We utilized behavioral, biochemical and molecular assays to compare basal and restraint stress-induced changes in the hypothalamic-pituitary-adrenal (HPA) axis of male and female msPs relative to their non-selected Wistar counterparts. The results showed that msPs display deficits in marble-burying behavior influenced by environmental factors and procedures that modulate arousal states in a sex-dependent manner. While male msPs display evidence of dysregulated neuroendocrine function (higher adrenocorticotropic hormone levels and subthreshold reductions in corticosterone), females display restraint-induced elevations in corticosterone levels that were persistently higher in msPs. A dexamethasone challenge reduced the circulation of these stress hormones, although the reduction in corticosterone was generally attenuated in msP versus Wistar rats. Finally, we found evidence of diminished stress-induced glucocorticoid receptor (GR) phosphorylation in the hypothalamic paraventricular nucleus of msPs, as well as innate increases in phosphorylated GR levels in the CeA of male msPs. Collectively, these findings suggest that negative feedback processes regulating HPA responsiveness are diminished in msP rats, possibly underlying differences in the expression of anxiety-like behaviors.
Keywords: corticosterone, HPA axis, phosphorylation
INTRODUCTION
Genetically-selected Marchigian Sardinian alcohol-preferring (msP) rats have long been proposed as an innate phenocopy of alcohol dependence, characterized by high alcohol preference and excessive drinking, as well as innate negative affective symptoms that are commonly observed in individuals with alcohol use disorder (AUD)1. Genetic sequencing has revealed a series of single nucleotide polymorphisms of the corticotropin-releasing factor (CRF) receptor 1 gene that are associated with anxiety and alcohol-drinking behavior in msPs2,3. From a translational perspective, these gene polymorphisms are also conserved in the human CRF system and correlate with the diagnosis of AUD4–6, as well as co-occurring pathologies (e.g., anxiety) that conflate the issue of excessive drinking7. Systematic investigations in msPs have explored the association between dysregulated CRF signaling and the disruption of integral stress processes that regulate anxiety-like behavior, indicating that increased activation of the central nucleus of the amygdala (CeA) contributes to their anxious phenotype8–10.
Stress and anxiety are natural processes that impel an organism to react to potentially harmful stimuli. This can elicit a broad range of behavioral responses, including passive stress-coping behavior that emphasizes energy conservation and withdrawal and active stress-coping behavior that is observed with increased locomotion. Flexibility between these responses is mediated in part by neuroendocrine and autonomic nervous systems, although it is unclear whether deficits in activity may influence the development of pathological behavior. In this regard, msPs display a hyper-anxious phenotype related in part to a deficit in active stress-coping performance11. Currently, it is unclear whether innate deficiencies in the neurophysiological responses to stress bias msP behavior towards a more passive or withdrawn coping style. Investigations into the factors that contribute to stress regulation and their role in shaping behavioral performance will improve the distinction between natural responses to stress and those driven by pathological disorders.
The stress response can be conceptualized as a period when behavioral and physiological processes are activated for the purpose of attending to stressful stimuli. This is followed by a period of recovery in which adaptive mechanisms are engaged to restore these processes back to normal function, or homeostasis. Adaptation has been discussed in terms of the multiple factors that inhibit the stress response at various stages12. We and others have studied these concepts in terms of endogenous mechanisms that enable fast-acting inhibition of synaptic transmission in limbic areas of the brain, representing feedback during the initial stages of the stress response. Indeed, our studies support a growing consensus for the interaction of stress primers such as CRF and putative mechanisms that buffer stress signaling in the amygdala13,14. Interestingly, the dissociation between CRF signaling in limbic regions relative to those mediated at the hypothalamic level15 suggests that other inhibitory feedback systems may influence the persistence of anxiety-like effects. A further understanding of these processes will shed light on aspects of the msP rat line that are less plastic or less amenable to modification, such as innate hyperarousal, passive avoidance behavior and resistance to fear extinction11,16.
The adaptive stress response is widely-regarded to involve activation of the hypothalamic-pituitary-adrenal (HPA) axis beginning at the paraventricular nucleus of the hypothalamus (PVN). Stimulation of neuroendocrine/peptidergic neurons facilitates the release of CRF into the hypophyseal portal circulation that in turn, stimulates the anterior pituitary to release corticotropes such as adrenocorticotropic hormone (ACTH). ACTH is then transported through the bloodstream to the zona fasciculata of the adrenal gland where it contributes to the production and release of the glucocorticoid corticosterone/cortisol (CORT)17. CORT is initially important for sustaining the autonomic drive elicited by “fight or flight” responses, although prolonged exposure can present a metabolic challenge in its own right, and as such is tightly regulated by negative feedback systems18. In this regard, CORT binds to cytosolic glucocorticoid receptors (GR) in the pituitary and PVN regions that translocate to the nucleus and inhibit transcription machinery related to the synthesis of ACTH and CRF19.
The HPA axis is highly plastic and demonstrates a range of sensitivity based on the nature, frequency and duration of the stressor. Although repeated stress normally leads to habituation of the HPA axis20, exposure to novel stressors can re-potentiate HPA function in a manner that is not always consistent with the mechanisms of hypothalamic feedback21. In this regard, the CeA reciprocates connections with autonomic brainstem nuclei and PVN-innervating regions that are important for mediating arousal states, as well as the expression of anxiety-like behaviors22–26. Currently, it is unclear whether msPs would display deficiencies in HPA feedback processes that normally protect against excessive CORT exposure. To gain further insight on this possibility, we first examined the potential role of arousal-inducing stimuli in influencing anxiety-like performance in msP versus non-selected Wistar rats. The dexamethasone suppression test was then used to examine the integrity of HPA axis function on stress hormone circulation. When administered systemically, the synthetic glucocorticoid binds to corticosteroid receptors and engages negative feedback processes that lead to the suppression of ACTH and CORT27. Deficiencies in this response can provide insight into glucocorticoid-GR mediated responsivity. Finally, we employed pharmacological and phosphoprotein molecular assays to examine the integrity of HPA-driven feedback under basal and stress-activated states. We hypothesized that deficits in active stress-coping performance in msPs may depend on altered regulation of CORT-GR signaling that normally serves to constrain the stress response. Given the strong evidence suggesting underlying sex differences in the HPA axis28, we included behavioral and biological assessments of CORT-GR signaling in male and female msP versus Wistar rats.
MATERIALS AND METHODS
Subjects.
A total of 217 rats were used in this study. Adult male (300–600g) and female (200–350g) msP rats were bred at The Scripps Research Institute (TSRI) from a colony obtained from the University of Camerino (Italy). Genotypic comparisons were made using age-matched Wistar rats (Charles River, Raleigh, NC) from which the msP rat line originally derived14. Rats were group-housed in a temperature- and humidity-controlled room on a 12h reverse light/dark cycle (lights off at 8:00 am) with food and water available ad libitum. We conducted all procedures in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the Institutional Animal Care and Use Committee policies of TSRI.
Marble-burying Task.
Rats (N=80) were divided evenly into 8 groups consisting of each genotype (Wistar and msP), sex (male and female) and experimental condition (low and high arousal). Rats from the low arousal group were habituated to the experimental procedures that included daily handling and acclimation to the testing environment for ~2–3h per day over a 7-day period. During this time, rats remained in their home cages and were acclimated to a procedural room under the presence of dim red lighting (~35 lux) and white noise (~50 decibels). Rats from the high arousal group were not handled and remained completely naïve to the testing environment. On the test day, rats were transported into the procedural room and allowed a 15 min acclimation period. Testing was staggered and counterbalanced across days such that each 35–40 min session consisted of a new group of rats (n= 4) of the same sex and arousal condition, but from each genotype. The low arousal group experienced testing under the same conditions that they were habituated to, while the high arousal group experienced testing under bright white lighting (~150 lux) and white noise (~50 decibels). Rats were individually transferred into standard-sized cages containing 5–6 cm height of saw-dust bedding. Cage tops were placed on top of the cages to prevent escape without hindering upward mobility or overhead camera view. Twenty marbles were aligned into 4 × 5 row grids spaced ~5 cm apart. The timer and overhead camera were activated, and the rats were left alone for a 20 min period. At the end of testing, rats were transferred back into their home cages and a new set of experimental cages were set in place. Data retrieved for subsequent analysis included the total count of marbles covered with at least 3/4 saw-dust bedding by the end of the test session and total immobility time scored independently by a neutral observer. Immobility time (in sec) was considered to be a distinct pause and absence in behavior for at least 3 sec and until overt movement was detected.
Dexamethasone Suppression Test.
Rats (N= 88) of each genotype and sex were subjected to dexamethasone suppression testing and restraint stress procedures to examine differences in stress hormonal release under basal and stress-induced conditions. Injections and testing occurred during the dark phase of the photo-schedule. Rats were injected intraperitoneally with a single dose of dexamethasone (0.3 mg/kg; 1 mL/kg) or vehicle (saline containing 5% dimethyl sulfoxide). Two hours later, rats were subjected to 30 min restraint stress, or left undisturbed in their home cages. This dose was chosen because it was previously shown to be the minimum dose tested that significantly suppressed CORT in acutely stressed rats versus controls29. For restraint stress, rats were placed in a cylindrical plexiglass tube inside of a cage with clean bedding with lights on. Females were placed in a tube of 15 cm long × 4.2 cm high with an inner diameter of 6 cm, while males were restrained in a tube of 23.6 cm long by × 6 cm high with an inner diameter of 9.2 cm to accommodate their larger size. Following the 30 min stressor, rats were removed from the restraint tube and maintained in the novel cage under dark conditions for an additional 30 min and then euthanized by rapid decapitation under isoflurane anesthesia. CORT levels in male and female rats have been previously demonstrated to remain significantly elevated above baseline at 30 min post-restraint.30 Trunk blood was collected in heparinized tubes and plasma was stored at −80°C until Luminex analysis.
Stress Hormonal Analysis.
ACTH and CORT were measured using Luminex 2-plex Rat Stress Hormone Panel (RSHMAG-69K). Plasma samples were diluted (1:4 ratio) in assay buffer and run in duplicate for simultaneous quantification of both hormones in each sample. Mean fluorescence intensities were analyzed on MAGPIX using xPONENT software (Luminex Corp, Austin TX). Data were fit using 5-parameter logistic regression for calculating analyte concentrations. Intra-assay and inter-assay % coefficient of variations is <10% for both stress hormones. One female Wistar rat was eliminated from the assessment for displaying aberrantly low CORT levels following dexamethasone treatment.
PVN and CeA Tissue Isolation.
Rats (N= 46–49) of each genotype and sex were either maintained naive to stress or experienced restraint stress for 30 min and then decapitated under isoflurane anesthesia to examine the effects of genotype, sex and stress on central GR phosphorylation. Brains were flash frozen in chilled isopentane and then stored at −80°C until microdissection. The frozen brains were mounted on a cryostat and sliced into 500 μm coronal sections. Three sections of the hypothalamus (approximately 0.84 to 2.34 mm caudal to bregma) were individually placed on glass slides. Bilateral tissue biopsies of the PVN region were centered at approximately medial-lateral ±1.0 and dorsal-ventral −7.8 from bregma in these sections. Similar strategies were applied to the collection of the CeA region from 4 sections of the amygdala (approximately 1.34 to 3.34 mm from bregma). Bilateral tissue biopsies were centered approximately at medial-lateral ±3.8 mm and dorsal-ventral −8.0 mm from bregma. The procured samples were stored at −80° C until western blot analysis.
Western Blot Analysis of Glucocorticoid Receptor Phosphorylation.
PVN and CeA tissue samples were homogenized by sonication in lysis buffer [320 mM sucrose, 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA), 1 mM ethylenediaminetetraacetic acid (EDTA), and 1% sodium dodecyl sulfate (SDS), with protease inhibitor cocktail and phosphatase inhibitor cocktails II and III, diluted 1:100; Sigma- Aldrich], followed by heating at 100°C for 5 minutes. Protein concentration was then determined by a colorimetric Lowry assay (DC protein assay, Bio-Rad, Hercules, CA). Samples were stored in 20μg protein aliquots at −80°C until use. Protein samples were subjected to SDS–polyacrylamide gel electrophoresis on 8% acrylamide gels using a Tris/ Glycine/SDS buffer system (Bio-Rad), followed by electrophoretic transfer to polyvinylidene difluoride (PVDF) membranes (GE Healthcare, Piscataway, NJ). Membranes were blocked for 1 hour in 5% non-fat milk at 4°C, then incubated in primary antibody overnight at 4°C. The primary antibodies utilized were phospho-GRS211 (1:1000; Cell Signaling, Danvers, MA; Cat. # 4161), total GR (1:1000; Invitrogen, Carlsbad, CA; Cat. #MA1–510), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1,000,000; Abcam, Cambridge, UK; Cat# AB9485). All antibodies have been validated by industry sources and have been published previously31. Note that the pGR antibody is specific for phosphorylation of serine 211 in humans and the corresponding serine 232 in rodents. Membranes were washed and labeled with species-specific peroxidase-conjugated secondary antibody (1:10,000; Bio-Rad) for 1 hour at room temperature. Following chemiluminescence detection (SuperSignal West Pico; Thermo Scientific, Rockford, IL), blots were stripped for 20 minutes at room temperature (Restore; Thermo Scientific) and were re-probed for total GR and GAPDH. Immunoreactivity was quantified by densitometry (ImageJ 1.45S; NIH, Bethesda, MD) under linear exposure conditions. To help ensure reliable quantification of changes, antibody concentrations and other conditions were initially optimized using 0.5x, 1x, and 2x of the amount of total protein loaded (e.g., 10, 20, 40 μg). Individual phosphoprotein levels were normalized to individual total GR protein levels and GAPDH to generate phosphorylation:total ratio values for statistical comparison. Densitized values were expressed as a percentage of the mean of control values for each gel to normalize data across blots.
Data Analysis.
Marble-burying counts were analyzed separately for each experimental condition. A Generalized Linear Model (GLIM) approach was used given that the frequency distribution of marble-burying is positively skewed. GLIM has been recently discussed as an alternative approach to Analysis of Variance (ANOVA) of count data that violate the test assumptions of general linear models, such as normality and homoscedasticity32. In general, GLIM allows for the assessment of dependent variables containing error residuals that are not normally distributed. GLIM also generalizes the linear regression approach by using a link function to estimate the predictive value of the dependent variable. Here, marbles buried were fitted into a negative binomial distribution with a log-linear link function and analyzed as a 2-way factorial model with sex (male versus female) and genotype (Wistar versus msP) as between-subject factors. Significant differences in the omnibus test and predictor estimates were probed further using the anti-log marginal means. Immobility time was assessed using a 2-way ANOVA with sex and genotype as between-subject factors. Stress hormone data (e.g., CORT and ACTH) were log-transformed to adjust for normal distribution and analyzed separately for each sex. We used a 3-way ANOVA with genotype, stress (restraint stress versus no stress) and drug treatment (vehicle versus dexamethasone) as between-subject factors. A ratio of the log-transformed values of CORT and ACTH was calculated for the vehicle-treated groups and analyzed separately as a 2-way ANOVA with genotype and stress as between-subject factors. Simple effects testing of the marginal means followed where significant interactions were detected. For the western blot data, we compared the normalized phosphoprotein levels using a 2-way ANOVA of genotype and stress with Tukey’s multiple comparisons tests as post-hoc analyses.
RESULTS
Arousal states modify anxiety-like performance in a genotype- and sex-dependent manner.
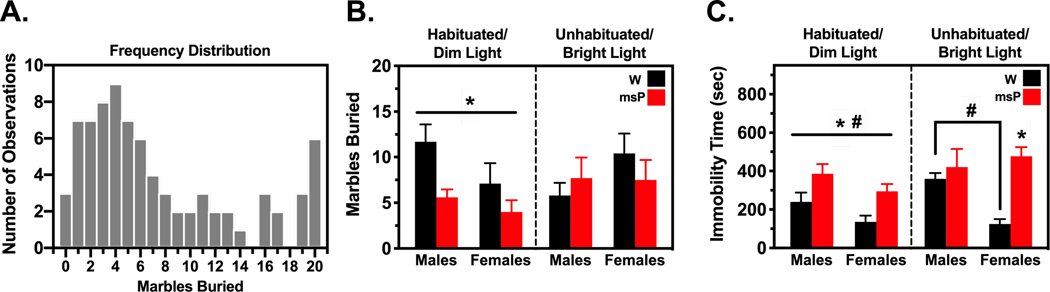
To determine whether arousal states influence anxiety-like performance, male and female Wistar and msP rats were either habituated to the experimental procedures and examined for marble-burying behavior under a dimly lit setting or were unhandled and tested under brightly lit conditions (Fig. 1). All statistical results for this study are reported in Tables S1–S2. To better discern group differences under conditions that were likely to result in baseline behavioral differences, we analyzed the data as a 2-way factorial model separately for each experimental condition. A GLIM approach was used given that the frequency distribution of marble-burying behavior was not normally distributed [Kolmogorov-Smirnov test (80)= 0.18, p<0.001)] and positively-skewed (M= 7.48±0.68, skewness= 0.88±0.27, kurtosis= −0.44±0.53) (Fig. 1A). Similar results were observed after parsing the analysis for the habituated/dim light (M= 7.10±0.92, skewness= 1.13±0.37, kurtosis= 0.37±0.73) and unhabituated/bright light (M= 7.85±1.01, skewness= 0.70±0.37, kurtosis= −0.92±0.73) conditions. In this regard, a negative binomial distribution provided a better goodness-of-fit for marble-burying counts relative to a Gaussian distribution32 (Akaike information criterion for habituated/dim light: 234.42 versus 251.93; for unhabituated/bright light: 254.09 versus 268.34, respectively). A 2-way factorial analysis of marble-burying (Fig. 1B) revealed a significant omnibus test [Likelihood χ2 Ratio (3)= 10.97, p<0.05] for the habituated condition. Genotype emerged as a significant predictor of the log marble-burying counts [Wald χ2(1)= 8.44, p<0.01], whereas sex was subthreshold for significance [Wald χ2(1)= 3.43, p= 0.064]. There was no interaction between these variables. Overall, msPs displayed a decrease in the number of marbles buried (M= 4.73) relative to their Wistar counterparts (M= 9.11) [Wald χ2(1)= 7.59, p<0.01]. A similar analysis of the unhabituated condition revealed no significant omnibus or predictor effects.
Fig. 1. Arousal states modify anxiety-like performance in a genotype- and sex-specific manner.
Male and female Wistar (W) and Marchigian Sardinian alcohol-preferring (msP) rats were either habituated to the testing conditions and examined for marble-burying behavior (B) and immobility (C) under a dimly-lit setting or were unhandled and tested under brightly-lit conditions (n= 10 per group). Frequency counts for marble-burying (A) revealed a positively-skewed distribution of the data and were thus analyzed using a generalized linear model approach. Genotypic differences emerged such that msPs displayed reduced marble-burying and increased immobility relative to their Wistar counterparts. The genotypic difference in immobility time persisted across testing conditions in females, while males more generally displayed increased immobility than females. Data reflect the number of observations, mean count or time in seconds (± SEM). Asterisks (*) indicate significant differences in genotype and pound signs (#) indicate significant differences in sex (p<0.05).
A 2-way ANOVA of immobility time (Fig. 1C) during the habituated condition revealed a significant main effect of genotype [F(1,36)= 12.48, p<0.001] and sex [F(1,36)= 5.11, p<0.05], but no interaction of these variables. Specifically, males spent a greater amount of time immobilized (M= 312.6 sec) than females (M= 215.3 sec). Immobility time was also nearly doubled in msPs displaying a higher propensity for freezing (M= 340.0 sec) than Wistars (M= 187.9 sec). A similar analysis of the unhabituated condition revealed an interaction of genotype and sex [F(1,36)= 6.63, p<0.05]. Specifically, female msPs demonstrated increased immobility (M= 476.4 sec) versus their Wistar counterparts (M= 124.8 sec) [F(1,36)= 19.41, p<0.001]. Wistar males also displayed increases in immobility (M= 359.4 sec) that were larger than females [F(1,36)= 8.64, p<0.05], whereas no sex differences were observed in msPs. Taken together, these data suggest that anxiety-like performance is modulated by experimental procedures that increase arousal levels in a genotype- and sex-dependent manner. Specifically, msPs displaying a hyper-anxious phenotype also show larger decrements in anxiety-like performance under conditions aimed to mitigate the influence of the testing environment. This effect appears to be stronger in males given the persistency of immobile states across testing conditions.
Negative feedback regulation of corticosterone is impaired in a genotype- and sex-dependent manner.
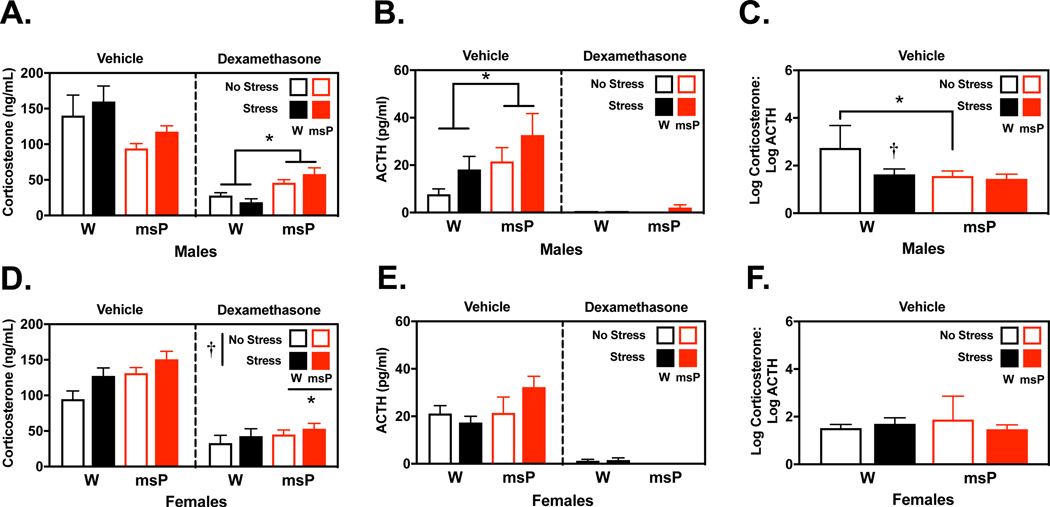
Circulating CORT and ACTH levels were examined in separate groups of male and female Wistar and msP rats that were pretreated with the synthetic glucocorticoid dexamethasone that normally engages hypothalamic feedback processes to inhibit CORT/ACTH release. Rats were then subjected to restraint stress procedures or left undisturbed (Fig. 2). All statistical results for this study are reported in Tables S3–S5. A 3-way ANOVA of log-transformed CORT values in males (Fig. 2A) revealed an interaction of genotype and drug [F(1,36)= 27.63, p<0.001]. First, basal CORT levels were marginally decreased in vehicle-treated msPs (M= 104.0 ng/mL) than Wistars (M= 141.3 ng/mL) [F(1,36)= 3.49, p=0.07]. Dexamethasone administration sharply decreased CORT levels, although probing the nature of the genotype by drug interaction revealed diminished influence of dexamethasone’s suppressing effects in msPs (M= 50.23 ng/mL) versus Wistars (M= 20.04 ng/mL) [F(1,36)= 30.84, p<0.001]. A 3-way ANOVA of log-transformed ACTH levels in males (Fig. 2B) revealed a similar genotype by drug interaction [F(1,36)= 5.45, p<0.05]. Here, basal ACTH levels were significantly higher in vehicle-treated msPs (M= 23.17 pg/mL) than Wistars (M= 8.51 pg/mL) [F(1,36)= 9.35, p<0.01], although dexamethasone substantially reduced ACTH release. Restraint stress marginally increased ACTH levels in males, although this effect did not reach statistical significance [F(1,36)= 3.84, p=0.058]. To further compare strain differences in HPA-feedback regulation, we analyzed the ratio of CORT to ACTH in vehicle-treated rats. A 2-way ANOVA of the log-transformed CORT:ACTH ratios (Fig. 2C) revealed an interaction of genotype and stress [F(1,17)= 5.15, p<0.05]. Non-stressed Wistars displayed larger ratios (M= 2.74) than msPs (M= 1.57) [F(1,17)= 15.62, p<0.001] suggesting that HPA axis feedback is differently regulated in Wistars versus msPs under basal conditions. This genotypic difference was no longer observable following restraint procedures which significantly reduced the ratio in Wistars (M= 1.64) [F(1,17)= 11.24, p<0.01], but not msPs.
Fig. 2. Negative feedback regulation of corticosterone is impaired in a genotype- and sex-dependent manner.
Male and female Wistar (W) and Marchigian Sardinian alcohol-preferring (msP) rats were examined for circulating corticosterone (A, D) and adrenocorticotropic hormone (ACTH) levels (B, E) 30 min following a restraint procedure or under no stress conditions. Rats also received pretreatment with either vehicle or the synthetic glucocorticoid dexamethasone (0.3 mg/kg) that suppresses hypothalamic-pituitary-adrenal axis signaling (n= 4–7 per group). A separate analysis examined the ratio of corticosterone and ACTH (C, F) in vehicle-treated groups only (n= 4–6 per group). Dexamethasone treatment significantly reduced corticosterone and ACTH levels in all groups, although genotypic differences emerged in a sex-dependent manner. In males, there was a non-significant trend for msPs to display reduced basal corticosterone levels, while dexamethasone resulted in diminished suppression of corticosterone release relative to Wistars. Alternatively, in females, restraint stress mobilized corticosterone release in both groups and remained persistently elevated in msPs than Wistars. Basal ACTH levels were generally elevated in msPs, although this effect was only significant in males. The analysis of corticosterone:ACTH ratios revealed significant differences in males but not females. Ratios were largest in non-stressed Wistar males but were reduced to comparable levels as their msP counterparts following restraint stress. Data reflect the mean concentration or log-transformed ratio of the circulating stress hormones (± SEM). Asterisks (*) indicate significant differences in genotype and daggers (†) indicate significant differences in stress conditions (p<0.05).
A 3-way ANOVA of log-transformed CORT values in females (Fig. 2D) revealed a main effect of drug [F(1,35)= 101.24, p<0.001], stress [F(1,35)= 4.40, p<0.05] and genotype [F(1,35)= 7.83, p<0.01], but no interactions of these variables. Specifically, females displayed a modest rise in CORT levels following restraint stress (M= 77.44 ng/mL) versus non-stressed controls (M= 60.95 ng/mL). Moreover, msPs displayed persistently higher CORT levels (M= 80.54 ng/mL) than Wistars (M= 58.61 ng/mL) across all conditions. Alternatively, the analysis of ACTH levels (Fig. 2E) revealed only a main effect of drug [F(1,35)= 171.43, p<0.001] and there were no differences in the CORT:ACTH ratio analysis (Fig. 2F). Collectively, these studies suggest underlying sex differences in the activation of the HPA axis. Notably, in males there appear to be multiple levels of dysfunction in the mechanisms that mobilize and inhibit CORT release in response to stress.
GR signaling is dysregulated in msP rats.
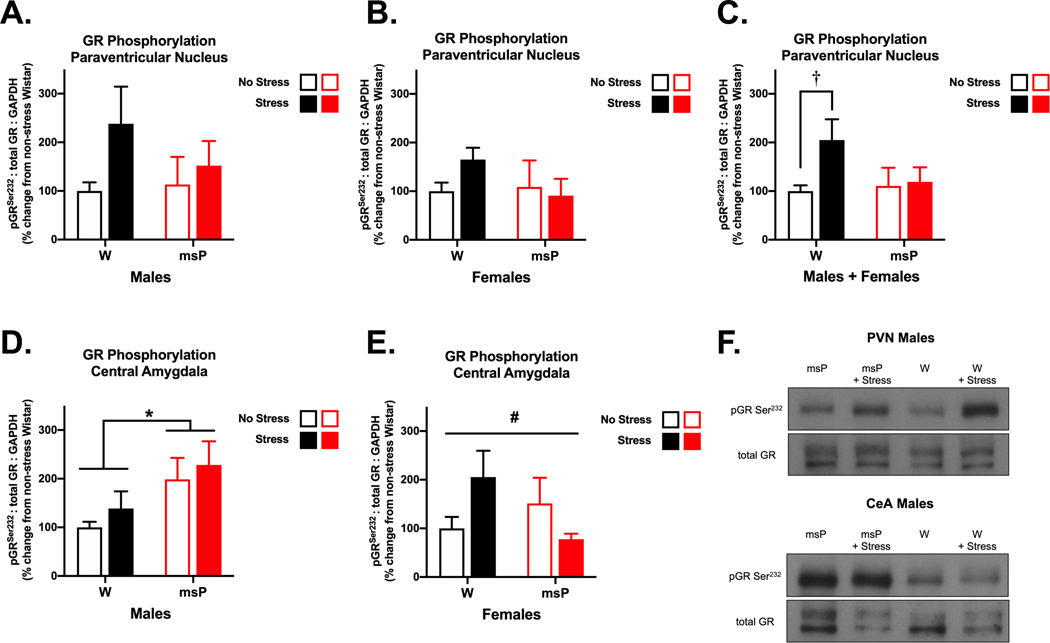
As msPs display tempered responses to dexamethasone treatment, we hypothesized that impaired GR-mediated feedback may underlie these genotypic differences. To examine this further, we measured the phosphorylation status of GR, which contributes to nuclear translocation and transcriptional regulation33. The hypothalamus (particularly the PVN) serves as a primary site for negative feedback regulation of the HPA axis. Here, we compared GR phosphorylation at the serine 232 site in male and female Wistar and msP rats subjected to restraint stress (or no stress) procedures (Fig. 3). All statistical results for this study are reported in Tables S6–S7. Initially, we did not observe any differences in the PVN data, although stress appeared to increase phosphorylated GR levels in Wistar rats (Fig. 3A and 3B). To better examine genotypic differences, we combined the PVN data for males and females together. Here, a 2-way ANOVA of phosphorylated GR levels (Fig. 3C) did not reveal any significant main effects or interactions of these variables, although there was a moderate trend for a stress main effect [F(1,42)= 3.03, p=0.089]. Acute stress is known to induce GR phosphorylation in rats34, and indeed planned comparisons revealed a 2-fold increase in this measure in restrained Wistars (M= 204.9%) versus their non-stressed counterparts [t(20)= 2.36, p<0.05]. The expected increase in GR phosphorylation appeared altogether absent in msPs.
Fig. 3. Glucocorticoid receptor signaling is dysregulated in msP rats.
Male and female Wistar (W) and Marchigian Sardinian alcohol-preferring (msP) rats were examined for phosphorylation of the serine 232 site on glucocorticoid receptors (GR) in the paraventricular nucleus of the hypothalamus (PVN) (A-C) and the central nucleus of the amygdala (CeA) (D, E) following a restraint procedure or under no stress conditions (n= 6–13 per group). A combined analysis of the PVN allowed us to better discern genotypic differences across sex. Here, restraint stress induced an increase in phosphorylated GR levels in Wistar, but not msP rats. In CeA tissue, male msPs displayed an innate increase in phosphorylated GR levels that remained persistently elevated across stress conditions. Comparatively, in females, we found a significant interaction of stress and genotype, although no specific group differences emerged. Data reflect mean changes in phosphorylation levels expressed as a percentage (± SEM) of non-stressed Wistar controls. The western blot images (F) depict representative data for significant differences in phosphorylation normalized for the expression of total GR. Asterisks (*) indicate significant differences in genotype, daggers (†) indicate significant differences in stress conditions and pound signs (#) indicate a significant interaction of genotype and stress (p<0.05).
The amygdala region and its subdivisions are an integral part of the extrahypothalamic system known to mediate the expression of fear and anxiety. Here, we focused on the CeA region that has long contributed to our understanding of genotypic differences in the msP rat line. A 2-way ANOVA of phosphorylated GR levels in males (Fig. 3D) revealed a main effect of genotype [F(1,20)= 6.24, p<0.05]. Specifically, msPs displayed an innate elevation in phosphorylated GR levels (M= 198.6%) and this genotypic difference was sustained across stress exposure. In addition, males generally displayed very mild stress-induced increases (30–40%) that were not significantly different from their respective (non-stressed) controls. In comparison, a 2-way ANOVA of phosphorylated GR levels in females (Fig. 3E) revealed a significant interaction of genotype and stress [F(1,21)= 5.25, p<0.05]. Here, stress induced a non-significant increase in Wistars (M= 205.5%) that was elevated relative to what appeared unchanged or even decreased in msPs (M= 77.9%); however, the post-hoc analysis revealed no significant group differences. Collectively, these data suggest that msPs exhibit dysregulated GR signaling in brain regions that are critically involved in stress regulation. Genotype-specific impairments in negative feedback may allow for the continued engagement of the HPA axis, thus promoting CORT-induced neuroadaptations in extrahypothalamic regions that modulate fear and anxiety. There are also likely sex-dependent differences in these changes in the CeA that may be tied to state-dependent effects in a more complex manner.
DISCUSSION
Previously, we found that msPs display an innate anxious phenotype characterized by increased sensitivity to stress-priming mechanisms in the CeA. More recently, we observed that endogenous anti-stress mechanisms are also deficient in this rodent line14. Here, we expand more on this conceptual framework to explore genotypic dysregulation in neuroendocrine processes that normally regulate the stress response. First, msPs displayed performance deficits in a classical model of behavioral anxiety (i.e., marble-burying), likely influenced by their hyper-anxious phenotype. Next, we probed basal and restraint-induced changes in neuroendocrine function to reveal genotypic dysregulation at multiple levels of the HPA axis. While male msPs demonstrated evidence of hypoactive CORT release, females were generally more responsive to restraint stress. Predictably, dexamethasone suppressed circulating levels of the stress hormones, but this inhibitory effect was generally diminished in msPs. We then examined changes in GR phosphorylation, representing the primary mechanism of CORT-mediated transcriptional activation, and found evidence of diminished stress-induced GR phosphorylation in the PVN of msPs. MsP males also demonstrated an innate upregulation of phosphorylated GR levels in the CeA, while females displayed an undefined genotype by stress interaction. Collectively, these findings suggest that hypothalamic processes regulating the neuroendocrine effects of stress are diminished in msPs. A reduced capacity to regulate the stress response is likely influencing sex-specific neuroadaptations to CORT-GR signaling in the CeA that may be important for the expression of anxiety-like behaviors.
It is well-established that the msP rat line exhibits co-morbid symptoms of excessive alcohol intake and innate negative affect. The latter symptom has been described using a variety of behavioral approaches supporting that msPs are highly anxious and sensitive to stress perturbations1. While the studies provide an important framework for the understanding of alcoholism and stress, there are several knowledge gaps. First, the majority of studies have almost exclusively used male msP rats, and there is a critical need to compare and contrast the biological and behavioral phenotypes in female msPs. Second, many of these studies utilized models that rely on exploratory behaviors for the assessment of anxiety-like performance. This is an important distinction given that increasing levels of arousal alter behavioral performance in an inverted U-shaped manner35. Indeed, our prior work demonstrates that minimal stress loads (e.g. bright lights and bedding-free cages) are sufficient to induce strong neophobic behavior in msPs14. In the current study, we examined the role of habituation and testing procedures as a means of manipulating arousal states36. We then utilized an anxiety-like model that would allow us to evaluate interactions with moderately stressful stimuli (i.e., marbles) over an extended period. We observed that genotypic differences were most evident following habituation to the testing environment performed in a dimly-lit setting. Under these conditions, msPs displayed reduced marble-burying behavior, which is interesting given what we know of the anxious phenotype. Indeed, reductions in marble-burying behavior are traditionally interpreted as evidence of diminished anxiety, although the extent to which task performance is modulated by arousal states has not, to our knowledge, been examined. The finding may suggest that msPs fall on an extreme end of the anxiogenic curve, placing them under heightened states of arousal that are likely to be performance-debilitating. Consistent with this, genotypic differences in marble-burying behavior were normalized under stressful testing conditions that likely increased basal arousal in Wistars. The findings are consistent with our previous work demonstrating that shock probe exposure facilitates a decrement in defensive burying behavior that is phenotypically similar in msPs as in pre-stressed Wistars11.
Complementing these findings, we also observed that msPs spent much of the test session immobilized. Generally, the shift from active to passive behavior is described as a parasympathetic “brake” on the motor system to gauge further action preparation37. As opposed to “freezing for action” however, msPs may instead be displaying evidence of behavioral inhibition. Similar phenotypes can be achieved in rodents that have been exposed to traumatic or unpredictable stress, and these characteristics generalize across multiple settings. Indeed, inescapable shock is known to sensitize rodent freezing behavior even under testing environments that are considered only mildly stressful, such as the elevated plus maze26. There are also sex differences in these responses given that immobility was detected at higher rates in males than females. The findings are consistent with extensive literature showing that female rodents are generally more active than males38, although the extent to which these findings portend differences in stress-coping behaviors remains to be fully explored. Recent studies have shown, for instance, that while female rats express predictable effects of fear conditioning, they are also more likely to express a combination of freezing and darting behaviors that facilitate better adaptive responses in later phases of testing39.
Acute stress leads to highly conserved physiological changes that characterize the stress response. This is achieved through the activation of the autonomic nervous system and the gradual ramping of stress hormonal circulation shortly thereafter. The processes regulating autonomic and neuroendocrine function are influenced by environmental factors and stressful life events that adjust the “gain” and sensitivity of stress activation24,40,41. This poses a unique challenge for the factors that regulate state-dependent effects versus those that drive pathological behavior. In this regard, msPs are well recognized for displaying innate elevations in CRF-CRF1 signaling, particularly in extrahypothalamic regions like the amygdala and hippocampus16. As the limbic system is heavily integrated with HPA and autonomic nuclei41, overactivity of these regions may present a competing pathway that occludes the stress-abating effects of the hypothalamus. Here, we capture such elements in msP males that display elevated levels of the HPA intermediate ACTH as compared to Wistars. Increased pituitary activation is consistent with evidence found in other selectively-bred anxiety lines, though it is not clear if this effect is directly attributable to CRF signaling in the PVN16,42. Interestingly, whereas elevations in ACTH might predict concomitant increases in CORT circulation, here we observed that msP males display marginally lower CORT levels. The innate reduction in CORT:ACTH ratios suggests that feedback modulation of glucocorticoids is inherently diminished in msPs and comparable to that of restrained Wistars. This is consistent with clinical reports showing an inverse relation between symptoms of hyperarousal in patients with post-traumatic stress disorder and CORT:ACTH ratios43. The altered physiology in msPs may arise from distinctions in selective-breeding procedures that also co-segregated heritable traits of alcohol dependence. Indeed, alcohol induces the release of ACTH and CORT, and over the course of repeated exposure and dependence, contributes to the development of neuroendocrine tolerance44. Similar parallels exist in the clinical literature among individuals with a family history of alcoholism who display blunted ACTH/CORT release45,46. A corollary for HPA hypo-reactivity is also observed in chronic stress models that are devoid of changes in PVN stress mechanisms and likely owing to the recruitment of upstream limbic processes47.
Relative to our findings in males, females display several differences in HPA function. First, restraint stress produced an overall increase in CORT levels versus their non-stressed counterparts. This is consistent with evidence showing that physical restraint induces the release of plasma ACTH and CORT within a 10–30 min timeframe and is restored to normal function several hours following stress cessation48,49. The acute effects of restraint stress are generally enhanced in female versus male rats50,51, resulting in larger overall increases in stress hormone circulation that appear to recover at slower rates52. The finding of increased CORT levels in female msPs also points to an opposing genotypic profile relative to our observations in males. Indeed, studies utilizing chronic restraint procedures have shown that male rats tend to habituate to the CORT-mobilizing effects of stress, while females display evidence of sensitization and/or a slower habituation to these procedures28,48. Collectively, this suggests that females may be especially sensitive to the CORT-mobilizing responses of restraint stress, thereby inducing longer periods of stress hormone circulation relative to their male counterparts. Interestingly, dexamethasone treatment strongly attenuated the release of stress hormones across all groups, but also exposed an underlying genotypic resistance to the CORT-suppressing effects of this drug. Thus, while we were able to capture subtle differences in sex-related responses, a more prevailing attribute in the msP phenotype appears to involve the dysregulation of hypothalamic processes that normally corral the stress response. While we highlight the interaction of CORT/ACTH as an important mediator of HPA dysfunction, it is also possible that marginal secretagogues of CORT (e.g., ghrelin)53, ACTH (e.g., vasopressin)42 or other sex-specific covariates (e.g., dehydroepiandrosterone)54 contribute to the effects reported here.
To explore molecular correlates of negative feedback dysregulation, we compared neuroadaptations in GR phosphorylation (pGR) in the hypothalamic PVN. While Wistars displayed an expected increase in pGR levels following restraint stress, this effect was altogether absent in msPs. The finding suggests that an important mechanism for regulating CORT levels in the brain is deficient in msP rats. In contrast to the regulation of the HPA axis, glucocorticoids exert a feed-forward response on CRF expression in the CeA55, and this mechanism is thought to contribute to stress sensitization, increased anxiety and escalation of alcohol drinking56. Alcohol-dependent male rats that display escalated alcohol intake also exhibit increased CeA pGR31, and these effects are blocked by local injections of a GR antagonist31,57. In the current study, elevated pGR appears to be constitutively present in male msPs, lending further support to the hypothesis that msPs reflect an innate phenocopy of alcohol dependence1. A more complex pattern of CeA GR regulation was evident in female msP rats, where restraint stress appeared to mobilize pGR in opposing manners for each genotype. Additional work will be necessary to uncover the functional significance of these region- and sex-specific changes. Notably, msP females demonstrate similar propensities for excessive alcohol intake and anxiety-like phenotypes as their male counterparts58, yet demonstrate a lower magnitude of pGR upregulation in the CeA. It is possible that other factors involved in GR receptor signaling overlap more discretely with these elements of co-morbidity. GR sensitivity to glucocorticoids, for instance, is moderated by co-chaperone proteins (peptidylprolyl isomerase D and FK506 binding protein) that alter the nuclear translocation profile of this receptor59. In this regard, emerging studies are elucidating the role of these regulator proteins in the expression of stress- and alcohol-induced phenotypes60,61.
Overall, our findings suggest that negative feedback processes regulating HPA function are blunted in msP versus Wistar rats, and this effect is likely contributing to the anxiogenic phenotype by altering CORT-GR responsivity in critical regions of the amygdala. While dysregulation in CORT-GR function may serve as a potential biomarker for co-morbid anxiety and alcoholism, it will be necessary to characterize the stress response in relation to stimulus-, time- and duration-dependent CORT release, as well as account for the potential interaction of these changes with circadian effects, sex differences and overlapping biomarkers62.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful for the support provided by the National Institute on Alcohol Abuse and Alcoholism via the following mechanisms: R37-AA017447, R01-AA015566, P60-AA006420, AA027700, AA021491, AA013498, K99/R00-AA025393, R01-AA025996, T32-AA007456, and the Pearson Center for Alcoholism and Addiction Research. This is Scripps manuscript number 30002. We sincerely appreciate the technical assistance from Caleigh Hasenfluck and Delaney Gaither for the behavioral analyses.
Footnotes
The authors state no competing financial interests, or interests otherwise that might be perceived to unduly influence the results and discussions generated in this report.
REFERENCES
- 1.Ciccocioppo R, Economidou D, Cippitelli A, et al. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11(3–4):339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12(1):30–34. [DOI] [PubMed] [Google Scholar]
- 3.Gehlert DR, Cippitelli A, Thorsell A, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27(10):2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63(2):146–151. [DOI] [PubMed] [Google Scholar]
- 5.Treutlein J, Kissling C, Frank J, et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11(6):594–602. [DOI] [PubMed] [Google Scholar]
- 6.Zorrilla EP, Heilig M, de Wit H, Shaham Y. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013;128(3):175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JP, Randall CL. Anxiety and alcohol use disorders: comorbidity and treatment considerations. Alcohol Res. 2012;34(4):414–431. [PMC free article] [PubMed] [Google Scholar]
- 8.Herman MA, Kallupi M, Luu G, et al. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology. 2013;67:337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman MA, Varodayan FP, Oleata CS, et al. Glutamatergic transmission in the central nucleus of the amygdala is selectively altered in Marchigian Sardinian alcohol-preferring rats: Alcohol and CRF effects. Neuropharmacology. 2016;102:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirson D, Oleata CS, Parsons LH, Ciccocioppo R, Roberto M. CB1 and ethanol effects on glutamatergic transmission in the central amygdala of male and female msP and Wistar rats. Addict Biol. 2018;23(2):676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cippitelli A, Ayanwuyi LO, Barbier E, et al. Polymorphism in the corticotropin-releasing factor receptor 1 (CRF1-R) gene plays a role in shaping the high anxious phenotype of Marchigian Sardinian alcohol-preferring (msP) rats. Psychopharmacology (Berl). 2015;232(6):1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol Learn Mem. 2009;92(2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray JM, Vecchiarelli HA, Morena M, et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 2015;35(9):3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natividad LA, Buczynski MW, Herman MA, et al. Constitutive Increases in Amygdalar Corticotropin-Releasing Factor and Fatty Acid Amide Hydrolase Drive an Anxious Phenotype. Biol Psychiatry. 2017;82(7):500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller MB, Zimmermann S, Sillaber I, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6(10):1100–1107. [DOI] [PubMed] [Google Scholar]
- 16.Hansson AC, Cippitelli A, Sommer WH, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103(41):15236–15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505–2511. [DOI] [PubMed] [Google Scholar]
- 18.Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gjerstad JK, Lightman SL, Spiga F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress. 2018;21(5):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav. 1988;43(1):47–55. [DOI] [PubMed] [Google Scholar]
- 21.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–1039. [DOI] [PubMed] [Google Scholar]
- 22.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gozzi A, Jain A, Giovannelli A, et al. A neural switch for active and passive fear. Neuron. 2010;67(4):656–666. [DOI] [PubMed] [Google Scholar]
- 24.Herman JP, Figueiredo H, Mueller NK, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. [DOI] [PubMed] [Google Scholar]
- 25.Roozendaal B, Koolhaas JM, Bohus B. Central amygdala lesions affect behavioral and autonomic balance during stress in rats. Physiol Behav. 1991;50(4):777–781. [DOI] [PubMed] [Google Scholar]
- 26.Pliota P, Bohm V, Grossl F, et al. Stress peptides sensitize fear circuitry to promote passive coping. Mol Psychiatry. 2020;25(2):428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole MA, Kim PJ, Kalman BA, Spencer RL. Dexamethasone suppression of corticosteroid secretion: evaluation of the site of action by receptor measures and functional studies. Psychoneuroendocrinology. 2000;25(2):151–167. [DOI] [PubMed] [Google Scholar]
- 28.Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44(1):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology. 2001;26(5):443–459. [DOI] [PubMed] [Google Scholar]
- 30.Gabriel KI, Ellis L, Yu W, Weinberg J. Variations in corticosterone feedback do not reveal differences in hpa activity after prenatal ethanol exposure. Alcohol Clin Exp Res. 2001;25(6):907–915. [PubMed] [Google Scholar]
- 31.Vendruscolo LF, Estey D, Goodell V, et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125(8):3193–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazic SE. Analytical strategies for the marble burying test: avoiding impossible predictions and invalid p-values. BMC Res Notes. 2015;8:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies L, Karthikeyan N, Lynch JT, et al. Cross talk of signaling pathways in the regulation of the glucocorticoid receptor function. Mol Endocrinol. 2008;22(6):1331–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adzic M, Djordjevic J, Djordjevic A, et al. Acute or chronic stress induce cell compartment-specific phosphorylation of glucocorticoid receptor and alter its transcriptional activity in Wistar rat brain. J Endocrinol. 2009;202(1):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldi E, Bucherelli C. The inverted “u-shaped” dose-effect relationships in learning and memory: modulation of arousal and consolidation. Nonlinearity Biol Toxicol Med. 2005;3(1):9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morena M, Leitl KD, Vecchiarelli HA, Gray JM, Campolongo P, Hill MN. Emotional arousal state influences the ability of amygdalar endocannabinoid signaling to modulate anxiety. Neuropharmacology. 2016;111:59–69. [DOI] [PubMed] [Google Scholar]
- 37.Roelofs K. Freeze for action: neurobiological mechanisms in animal and human freezing. Philos Trans R Soc Lond B Biol Sci. 2017;372(1718). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenfeld CS. Sex-dependent differences in voluntary physical activity. J Neurosci Res. 2017;95(1–2):279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM. Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol Psychiatry. 2015;78(3):186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583(2–3):194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wigger A, Sanchez MM, Mathys KC, et al. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology. 2004;29(1):1–14. [DOI] [PubMed] [Google Scholar]
- 43.Golier JA, Schmeidler J, Legge J, Yehuda R. Twenty-four hour plasma cortisol and adrenocorticotropic hormone in Gulf War veterans: relationships to posttraumatic stress disorder and health symptoms. Biol Psychiatry. 2007;62(10):1175–1178. [DOI] [PubMed] [Google Scholar]
- 44.Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28(8):1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59(3):203–209. [DOI] [PubMed] [Google Scholar]
- 46.Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59(3):210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147(4):2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chadda R, Devaud LL. Differential effects of mild repeated restraint stress on behaviors and GABA(A) receptors in male and female rats. Pharmacol Biochem Behav. 2005;81(4):854–863. [DOI] [PubMed] [Google Scholar]
- 49.McClennen SJ, Cortright DN, Seasholtz AF. Regulation of pituitary corticotropin-releasing hormone-binding protein messenger ribonucleic acid levels by restraint stress and adrenalectomy. Endocrinology. 1998;139(11):4435–4441. [DOI] [PubMed] [Google Scholar]
- 50.Babb JA, Masini CV, Day HE, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience. 2013;234:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livezey GT, Miller JM, Vogel WH. Plasma norepinephrine, epinephrine and corticosterone stress responses to restraint in individual male and female rats, and their correlations. Neurosci Lett. 1985;62(1):51–56. [DOI] [PubMed] [Google Scholar]
- 52.Goel N, Workman JL, Lee TT, Innala L, Viau V. Sex differences in the HPA axis. Compr Physiol. 2014;4(3):1121–1155. [DOI] [PubMed] [Google Scholar]
- 53.Stevanovic D, Milosevic V, Starcevic VP, Severs WB. The effect of centrally administered ghrelin on pituitary ACTH cells and circulating ACTH and corticosterone in rats. Life Sci. 2007;80(9):867–872. [DOI] [PubMed] [Google Scholar]
- 54.Kent M, Bardi M, Hazelgrove A, et al. Profiling coping strategies in male and female rats: Potential neurobehavioral markers of increased resilience to depressive symptoms. Horm Behav. 2017;95:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640(1–2):105–112. [DOI] [PubMed] [Google Scholar]
- 56.Edwards S, Little HJ, Richardson HN, Vendruscolo LF. Divergent regulation of distinct glucocorticoid systems in alcohol dependence. Alcohol. 2015;49(8):811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology. 2012;37(4):906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borruto AM, Fotio Y, Stopponi S, et al. NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area. Br J Pharmacol. 2020;177(7):1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourke CH, Raees MQ, Malviya S, Bradburn CA, Binder EB, Neigh GN. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38(1):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konig L, Kalinichenko LS, Huber SE, et al. The selective FKBP51 inhibitor SAFit2 reduces alcohol consumption and reinstatement of conditioned alcohol effects in mice. Addict Biol. 2020;25(3):e12758. [DOI] [PubMed] [Google Scholar]
- 61.Li H, Su P, Lai TK, et al. The glucocorticoid receptor-FKBP51 complex contributes to fear conditioning and posttraumatic stress disorder. J Clin Invest. 2020;130(2):877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dhama K, Latheef SK, Dadar M, et al. Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values. Front Mol Biosci. 2019;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.