Abstract
Infectious diseases are a major threat to global human health, yet prophylactic treatment options can be limited, as safe and efficacious vaccines exist only for a fraction of all diseases. Notably, devastating diseases such as acquired immunodeficiency syndrome (AIDS) and coronavirus disease of 2019 (COVID-19) currently do not have vaccine therapies. Conventional vaccine platforms, such as live attenuated vaccines and whole inactivated vaccines, can be difficult to manufacture, may cause severe side effects, and can potentially induce severe infection. Subunit vaccines carry far fewer safety concerns due to their inability to cause vaccine-based infections. The applicability of protein nanoparticles (NPs) as vaccine scaffolds is promising to prevent infectious diseases, and they have been explored for a number of viral, bacterial, fungal, and parasitic diseases. Many types of protein NPs exist, including self-assembling NPs, bacteriophage-derived NPs, plant-derived NPs, and human virus-based vectors, and these particular categories will be covered in this review. These vaccines can elicit strong humoral and cellular immune responses against specific pathogens, as well as provide protection against infection in a number of animal models. Furthermore, published clinical trials demonstrate the promise of applying these NP vaccine platforms, which include bacteriophage-derived NPs, in addition to multiple viral vectors that are currently used in the clinic. The continued investigations of protein NP vaccine platforms are critical to generate safer alternatives to current vaccines, advance vaccines for diseases that currently lack effective prophylactic therapies, and prepare for the rapid development of new vaccines against emerging infectious diseases.
Keywords: protein nanoparticles, antigen delivery, infectious disease vaccines, immune response, virus-like particles
Graphical Abstract
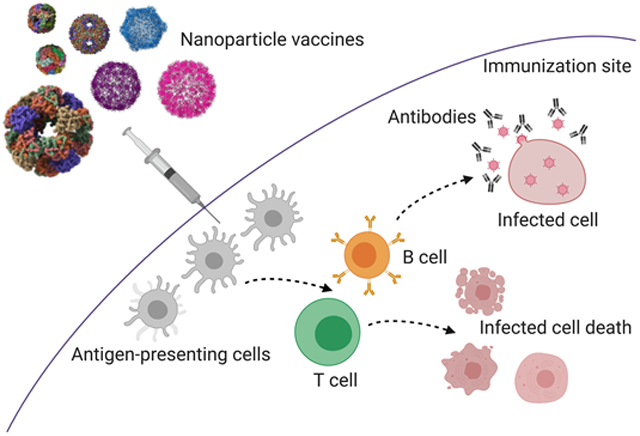
Various protein nanoparticles (NPs) have been utilized as scaffolds to display antigens for vaccination against infectious agents. NPs can stimulate humoral immune responses, cellular immune responses, or a combination of both.
1. Introduction
Bacteria, viruses, parasites, and fungi are the causative agents of infectious diseases [Lindahl & Grace, 2015], which vary in severity from the seasonal flu to fatal outbreaks of Ebola and endemic malaria [Fedson, 2016]. The most effective approach to prevent pathologies caused by these infectious organisms is vaccination [Omer et al., 2009][Delany et al., 2014]. Although drugs have been developed and approved to inhibit the growth or replication of these infectious organisms, these are only taken after an individual has contracted the organism. The power of vaccines lies in the fact that they can be administered long before patients come in contact with the organism, and they have the power to prevent disease pathology and systemic infection [Omer et al., 2009][Delany et al., 2014][Mbow et al., 2010]. Conventional vaccine approaches include live attenuated vaccines, inactivated whole organism vaccines, subunit vaccines, and toxoid vaccines [Kallerup & Foged, 2015]. The field has made significant progress in developing and bringing to market vaccines which protect against several diseases that pose serious threats to human health, including measles, mumps, rubella, smallpox, polio, hepatitis B (HBV), human papillomavirus (HPV), diphtheria, and influenza [Mailand & Frederiksen, 2017].
Despite the great benefit of vaccines, several have had documented issues with safety, which can have a serious impact on community trust, vaccination rates, and subsequently the loss of control of an infectious agent in a population. For example, live attenuated vaccines, although highly effective and protective, can be lethal. Dengvaxia®, the vaccine designed to prevent dengue disease, is a recent example of the dangers associated live attenuated vaccines; despite rigorous pre-clinical testing and clinical trials, the dangers of this vaccine were not realized until after it was deployed in a mass vaccination campaign of children in the Philippines [Cohen, 2019]. Historically and more recently, live attenuated oral polio vaccines have caused paralytic poliomyelitis [Desai et al., 2014][Alexander et al., 2004][Mbaeyi et al., 2018]. Early rotavirus vaccines resulted in intussusception in children and negatively impacted the public’s opinion and adoption of other vaccine candidates that were subsequently developed to protect against this highly contagious and transmissible diarrheal disease but which did not have these same safety concerns [Koch et al., 2017][Foster, 2007][Soares-Weiser et al., 2012]. Incidents of post-immunization autoimmunity have also been linked to adverse responses to vaccination [Agmon-Levin et al., 2009][Cerpa-Cruz et al., 2013][Vadala et al., 2017]. These examples demonstrate the need to research and implement alternative vaccine approaches that are safer and elicit better antigen-specific immunogenicity.
Subunit vaccines are considered the safest design approach, but often struggle to induce robust immune responses to immunization [Vartak & Sucheck, 2016]. This can be overcome with the use of adjuvant platforms, as evidenced by the many subunit vaccines that have garnered FDA approval, including Shingrix, DPT (diphtheria, pertussis, tetanus), Prevnar 13®, and Gardasil®9 [Shah, 2019][FDA, 2020b][Gupta et al., 1995][Sucher et al., 2011][Manini & Montomoli, 2018]. Many adjuvants have achieved FDA approval and others are actively being studied for their ability to increase immunogenicity [Lee & Nguyen, 2015][Sun et al., 2018][Jin et al., 2018]. Based on our experience, we believe nanoparticles (NPs) are an exciting adjuvant strategy that should be considered when novel subunit vaccines are being developed, especially for infectious diseases that still lack effective vaccines, including HIV-1, tuberculosis, and SARS-CoV2 (COVID-19) [Pati et al., 2018].
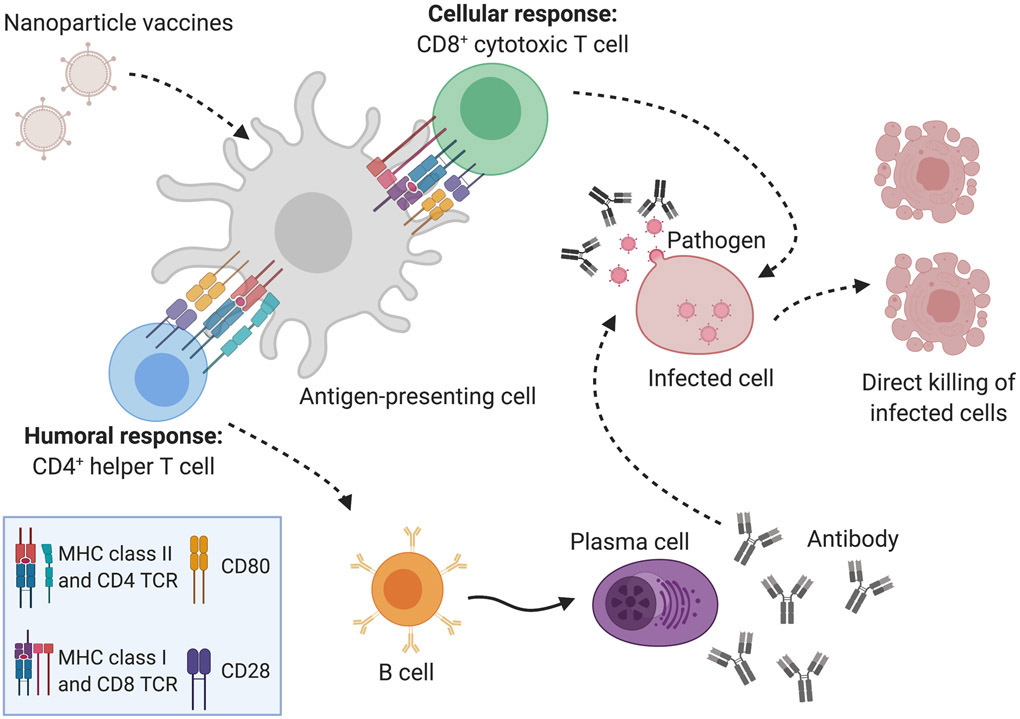
Nanoparticle (NP) scaffold platforms include inorganic NPs, polymeric NPs, liposomes, virus-like particles (VLPs), self-assembling NPs, and dendrimers [Pati et al., 2018][Al-Halifa et al., 2019]. NPs have already proven advantageous for the development of vaccines against cancer [Neek et al., 2019]. VLPs have virus-like nanostructures, but lack infectious genetic materials, providing safety while inducing humoral or cellular immune responses through the recognition of repetitive subunits on the surface of the particle by the immune system [Fuenmayor et al., 2017]. NPs with a size ranging from 20- to 200-nm can drain freely into lymph nodes and are presented to resident dendritic cells (DCs), making them ideal scaffolds for delivering antigens and immunostimulants [Manolova et al., 2008][Bachmann et al., 2010]. The goal of vaccination is to elicit antigen-specific, high affinity antibody responses, especially IgG and IgA [den Hartog et al., 2020], against the immunizing antigens. As summarized in Figure 1, antigen-presenting cells (APCs) including DCs, macrophages, and B cells, take up extracellular proteins and degrade them into peptides for presentation to CD4+ helper T cells, which subsequently generate humoral immune responses. The CD4+ T cell and B cell interaction is crucial for the production of long-lived plasma cells which generate antigen-specific antibodies [Bachmann et al., 2010][Look et al., 2010]. These antibodies can neutralize the infectious agent, thus preventing infection, or mark it for phagocytosis by APCs where it is destroyed in lysosomes. In addition to interacting with CD4+ helper T cells, DCs can also trigger cytotoxic CD8+ T cell (CTL) cellular responses, resulting in the direct killing of infected cells [Look et al., 2010].
Figure 1. The interaction between the nanoparticle (NP) vaccines and the immune system results in immunological memory and protection against future infections.
NP platforms are capable of stimulating the host immune response to generate both cellular (CD8+ T cell) and humoral (antibody) responses to the pathogen antigen(s) included in the vaccine formulation. As detailed in this review, each unique NP platform can generate antigen-specific CD8+, CD4+ and/or antibody responses to immunization following uptake of the NP vaccine by local antigen-presenting cells at the immunization site. These antigen-presenting cells travel back to draining lymph tissues, where they process the nanoparticle vaccine platforms into protein peptides that are presented in MHC molecules to both CD8+ and CD4+ T cells. CD8+ T cells (green) are capable of directly killing infected cells (pink). CD4+ T cells (blue) interact with B cells (orange) to stimulate the maturation of the B cells into antibody-secreting plasma cells (purple). The antibodies are specific to the pathogen and can neutralize their ability to infect and proliferate. The antigen-specific cellular and/or humoral responses generated by the NP platforms will be maintained as memory cells against the pathogen, which can be quickly activated when the immunized individual encounters that specific pathogen in the future. Future infection with that specific pathogen results in a rapid and robust immune response that controls the proliferation and dissemination of the pathogen and significantly reduces disease pathology in the immunized individual.
In this comprehensive review, we showcase the protein NPs that are being investigated for development into vaccine platforms for numerous infectious diseases, specifically focusing on self-assembling protein NPs, different types of VLPs (Fig. 2), and how they stimulate immune responses to vaccination. Furthermore, we discuss human-infecting viral vectors and their approved use as vaccine platforms for a number of infectious diseases [Sanchez et al., 2020][Ollmann, 2020]. Although others have also used these protein NP platforms as frameworks for drug and gene delivery systems [Lee et al., 2017][Ren et al., 2011][Molino et al., 2014], our main focus in this review is to highlight the investigations of protein NPs as structural scaffolds upon which to develop new vaccines. Examples of these vaccine NP scaffolds are shown in Figure 2. Furthermore, the humoral and cellular immune responses elicited from these NP vaccines, the adjuvants used, the route of administration, and other information will be covered.
Figure 2. Crystal structures of several protein NP scaffolds investigated for vaccine development.
Self-assembling, phage-derived, and plant-derived NPs are labeled in black, red, and blue, respectively. The inset shows TMV from the cross-sectional view (left) and side view (right). Scale bars denote 20-nm. NP structures were generated in ChimeraX using Protein Data Bank ID codes 1shs (sHsp), 1mfr (ferritin), 1b5s (E2), 5fmo (CPMV), 2ms2 (MS2), 1F15 (CMV), 3iyh (P22), and 6R7M (TMV).
2. Self-assembling Nanoparticles
In this section, studies on the development of infectious disease vaccines using non-viral self-assembling NPs as scaffolds will be reviewed. The NPs included in this section are small heat-shock protein, ferritin, and E2. We will discuss the structure of these NPs and the immune responses elicited after immunization with antigens presented on these NPs. A summary of this information is presented in Table 1.
Table 1. Immune responses elicited by self-assembling protein nanoparticle vaccines.
Abbreviations: Ab (antibodies), CD4+ T cell (T helper cell), CD8+ T cell (cytotoxic T cell), CTB (bacterial cholera toxin B subunit), DC (dendritic cell), FCA (Freud’s complete adjuvant), FIA (Freud’s incomplete adjuvant), HFMD (Hand, Foot, and Mouth Disease), ID (intradermal injection), IFN-γ (interferon gamma), IgA (immunoglobulin A), IgG (immunoglobulin G), IM (intramuscular injection), IP (intraperitoneal injection), MΦ (macrophage), SC (subcutaneous injection), sHSP (small heat-shock protein), TFH (T follicular helper), TNF-α (tumor necrosis factor alpha).
| Scaffold Platform |
Type of infectious disease |
Disease | In vitro, In vivo |
Route of Administration |
Adjuvant co- administered |
Elicited immune responses |
Specific immune responses |
References |
|---|---|---|---|---|---|---|---|---|
| sHSP | Viral | Influenza | In vivo | IN | sHsp, P22 | Humoral | Antigen-specific Abs (IgG and IgA), protection against challenge | (Richert et al., 2012), (Richert et al., 2013) |
| Cellular | TFH and CD4+ T cell expansion, local DC and aveolar MΦ accumulation | |||||||
| Ferritin | Viral | HIV-1 | In vitro, In vivo | IM | Adjuplex, MPLA, ISCOMATRIX | Humoral | Neutralizing Abs | (Georgiev et al., 2018), (Sliepen et al., 2015), (He et al., 2016) |
| Cellular | B cell activation | |||||||
| Influenza | In vitro, In vivo* | IM | Adjuplex | Humoral | Neutralizing Abs | (Georgiev et al., 2018) | ||
| Chronic hepatitis B | In vivo | SC | None | Humoral | IgG Abs, antigen-specific Abs, protection against challenge | (Wang et al., 2020) | ||
| Hepatitis C | In vitro, In vivo | IP | Inject Alum | Humoral | Antigen-specific Abs, neutralizing Abs | (Yan et al., 2020) | ||
| Rotavirus | In vivo | Oral | CTB | Humoral | IgA and IgG Abs, protection against challenge | (Li et al., 2019) | ||
| HFMV | In vitro, In vivo | SC | FCA or FIA | Humoral | IgG Abs, neutralization titers, protection against challenge | (Wang et al., 2019c) | ||
| Epstein-Barr virus infection | In vitro, In vivo | IM | SAS | Humoral | Neutralizing Abs, protection against challenge | (Kanekiyo et al., 2015) | ||
| Respiratory syncytial virus infection | In vivo | IM | AF03 | Humoral | Antigen-specific Abs, neutralizing Abs | (Swanson et al., 2020) | ||
| MERS | In vivo | IM | MF59 | Humoral | IgG Abs | (Kim et al., 2018) | ||
| Cellular | Expansion of CD4+ T cells that secreted IFN-γ and TNF-α | |||||||
| E2 | Viral | HIV-1 | In vitro, In vivo | SC, IM, ID | IFA, FIA | Humoral | IgG Abs, neutralizing Abs, | (Caivano et al., 2010), (Krebs et al., 2014), (He et al., 2016) |
| Cellular | CD8+ but not CD4+ T cells secreted IFN-γ, B cell activation | |||||||
| Dengue | In vitro, In vivo | IM, ID | Adjuplex | Humoral | Neutralizing Abs, protection against challenge | (McBurney et al., 2016) | ||
| Parasitic | Malaria | In vivo | Not specified | FCA or FIA | Humoral | Antigen-specific Abs | (Domingo et al., 2001) |
Asterisk indicates Phase I clinical trial.
2.1. Small heat-shock protein
Heat shock proteins (HSPs) are ubiquitous chaperones that bind to non-native or misfolded proteins, preventing aggregation. They are an evolutionarily conserved protein family and have evolved to exhibit a diverse range of structures symmetries [Abisambra et al., 2011]. For example, small heat shock protein 16.5 (sHsp16.5), derived from the primitive thermophilic archaeon Methanocaldoccus jannaschii, assembles into a 24-subunit caged nanoparticle. Within the archaea, sHsp16.5 sequesters non-native or misfolded proteins, preventing their aggregation [Shi et al., 2013]. Heat shock proteins have been examined as adjuvants in cancer vaccines [Segal et al., 2006].
In the area of infectious disease, Harmsen and colleagues have utilized the sHsp16.5 scaffold to elicit enhanced immune response [Richert et al., 2012][Richert et al., 2013]. Ovalbumin (OVA) was conjugated to sHsp16.5 (OVA-sHsp) following expression and purification from E. coli [Richert et al., 2012]. Mice were vaccinated with OVA-sHsp and then challenged with influenza, resulting in enhanced OVA-specific IgG levels in mice compared to vaccination with OVA. Because the OVA-sHSP NP does not itself contain antigens specific for influenza, this shows that it acted as a non-specific adjuvant, making the lung environment more conductive to a local antibody response. Pre-treatment with unconjugated sHSP induced local IgA response and accelerated rates of influenza-specific IgG production [Richert et al., 2012]. Treatment with the caged NPs OVA-sHsp enhanced alveolar macrophage and dendritic cell (DC) numbers in inducible bronchus-associated lymphoid tissue and the tracheobronchial lymph node, which gave an accelerated response to the influenza challenge [Richert et al., 2013]. This migration pattern suggested augmented local immunity via increased phagocytic activity at the local site of infection. The vaccine promoted CD4+ T cell expansion and increased trafficking to the lungs, increasing accumulation in the tracheobronchial lymph node, inducible bronchus-associated lymphoid tissue, and spleen. Therefore, vaccination with sHSP increased immune activity both locally and systemically [Richert et al., 2013].
2.2. Ferritin
Ferritin is a spherical self-assembling protein NP that stores iron and is ubiquitous in living organisms. The main class of ferritin is 12-nm in diameter and contains 24 subunits, and antigens can be fused genetically to the N-terminus of a single ferritin subunit [Wang et al., 2019c] [Theil, 2013]. Georgiev et al. presented the possibility of placing two distinct antigens on one single ferritin while controlling the ratio and the geometric arrangement, and suggested that additional antigens can be attached, based on symmetry [Georgiev et al., 2018]. It was demonstrated that ferritin NPs were mostly recognized by DCs and macrophages expressing SIGNR1, and after subcutaneous injection in mice, fluorescently-labeled ferritin NPs were located abundantly in the medulla and interfollicular region [Wang et al., 2020].
Ferritin NP vaccines have been shown to induce humoral immune responses. For example, for vaccines in which Enterovirus 71 epitopes were displayed on the exterior of ferritin NPs, higher antibody titer responses were obtained [Wang et al., 2019c]. Serum from mice that were immunized with bacterioferritin fused to the receptor-binding domain (RBD) of Middle East Respiratory Syndrome (MERS-CoV) blocked the interaction between RBD and human receptor DPP4 (hDPP4) [Kim et al., 2018]. This is significant because the interaction between the RBD on MERS-CoV and the cellular receptor hDPP4 initiates MERS-CoV infection. The IgG1, IgG2a and IgG2b responses stimulated by RBD bacterioferritin were also stronger than RBD alone [Kim et al., 2018]. In another investigation, the display of multiple replicas of HIV-1 envelope glycoprotein trimers on ferritin resulted in greater neutralizing antibody titers and antibody responses against tier 1A viruses [Sliepen et al., 2015].
Ferritin has been examined in preclinical research as a vaccine scaffold for various viral infectious diseases; these include HIV-1; influenza (H1N1 strain); chronic hepatitis B (HBV); hepatitis C (HCV); Hand, Foot, and Mouth Disease (HFMD); rotavirus; Epstein-Barr; respiratory disease caused by respiratory syncytial virus, and Middle East Respiratory Syndrome (MERS-CoV) [He et al., 2016] [Swanson et al., 2020][Wang et al., 2019c][Georgiev et al., 2018][Wang et al., 2020][Li et al., 2019][Sliepen et al., 2015][Kanekiyo et al., 2013][Kanekiyo et al., 2015][Kim et al., 2018][Yan et al., 2020]. Ferritin vaccines have also been investigated for bacterial infections, including gonorrhea [Wang et al., 2017]. Phase 1 clinical trials were completed in 2019 to examine the safety of a ferritin-hemagglutinin (HA) vaccine for influenza, and another study has an estimated completion date in 2021 [ClinicalTrials.gov, 2020b][ClinicalTrials.gov, 2020c][Kanekiyo et al., 2013][Yassine et al., 2015].
2.3. E2
The pyruvate dehydrogenase complex of Geobacillus stearothermophilus includes a protein NP with icosahedral symmetry, formed from the E2 subunit of the complex. E2 is a 60-mer that assembles to form a hollow particle that is approximately 24-nm in diameter [Milne et al., 2002][Krebs et al., 2014]. It can be engineered with mutations to modify its properties while maintaining its dodecahedron structure and thermal stability; the internal, external, and inter-subunit interfaces can also be engineered to attach guest molecules [Dalmau et al., 2008][Neek et al., 2018] or modulate its pH-stability [Dalmau et al., 2009a][Dalmau et al., 2009b]. Foreign peptides and full-length proteins can be genetically attached to the N-terminus of each subunit, resulting in a display of up to 60 copies of the molecules [Domingo et al., 2001]. Multiple proteins can also be displayed on a single E2 NP, with the constraints being the protein expression of the protein-E2 fusion, its solubility, and steric hindrance [Caivano et al., 2010][Domingo et al., 2001]. This strategy has enabled the scaffold to be used as an antigen-delivery system for the development of cancer vaccines [Neek et al., 2018][Molino et al., 2016] and for infectious diseases that include HIV-1 [Krebs et al., 2014][Caivano et al., 2010][Jaworski et al., 2012][He et al., 2016], dengue fever [McBurney et al., 2016], and malaria [Domingo et al., 2001].
Domingo et al. showed that the surface of E2 can be simultaneously conjugated to green fluorescent protein and the MAL1 epitope from the circumsporozoite protein of Plasmodium falciparum, a parasite that can cause malaria [Domingo et al., 2001]. For HIV-1 vaccine studies, He et al. demonstrated that glycoprotein gp140 trimers can be stabilized on both E2 and ferritin scaffolds, and the resulting NPs are able to engage with B cells [He et al., 2016]. Caivano et al. demonstrated the possibility of genetically fusing the HIV-1 protein Gag(p17) on the N-terminus of the E2 while still resulting in the proper folding of the E2 scaffold [Caivano et al., 2010]. The IgG1 response was the dominant antibody isotype produced after the delivery of HIV-1 Gag(p17)-E2 NPs, indicating induction of Th2-type immune responses [Caivano et al., 2010]. In another study, by co-administrating E2 presenting the HIV-1 envelope (Env) third hypervariable region and Env(gp160) DNA, the antibody response and neutralizing antibody titer were increased and developed more rapidly [Jaworski et al., 2012]. In addition, the percentage of IFNγ-secreting CD8+ T cells increased when the antigen-E2 scaffold and antigen DNA were co-administered, compared to the groups that received DNA and E2 with antigen separately [Jaworski et al., 2012]. This co-administration strategy of antigen-E2 with DNA was applied to a dengue vaccine that was tested in macaques [McBurney et al., 2016].
3. Virus-like Particles (VLPs): Bacteriophage-derived
Bacteriophages are viruses that infect bacteria, but not human cells [Hess et al., 2020]. Several types of bacteriophage exist, each with unique-sized shape and dimensions. In particular, the P22, Qβ, λ, T4, MS2, and filamentous phage platforms have been developed for vaccination against numerous viral, bacterial, fungal, and parasitic infectious diseases (see Table 2). This section discusses recent advances in these platforms in infectious disease vaccines. While most vaccines are at the pre-clinical stage, an H1N1 Qβ vaccine showed promise in a Phase I clinical trial [Low et al., 2014].
Table 2. Immune responses elicited by bacteriophage-derived protein nanoparticle vaccines.
Abbreviations: Abs (antibodies), CT (cholera toxin), CTL (cytotoxic T cell lymphocyte), FIA (Freud’s incomplete adjuvant), FMDV (foot and mouth disease virus), IFN-γ (interferon gamma), IgG (immunoglobulin G), IL (interleukin), IM (intramuscular injection), IN (intranasal injection), IP (intraperitoneal injection), IT (intratracheal), IV (intravenous), MPLA (monophosphoryl lipid A), PO (per-orally), respiratory syncytial virus (RSV), SC (subcutaneous), SE (stable emulsion of GLA-SE TLR-4 agonist), TCI (transcutaneous immunization), Th1 (T helper 1), Th17 (T helper 17).
| Scaffold Platform |
Type of infectious disease |
Disease | In vitro, In vivo |
Route of Administration |
Adjuvant co- administered |
Elicited immune responses |
Specific immune responses |
References |
|---|---|---|---|---|---|---|---|---|
| P22 | Viral | Influenza | In vivo | IN, IT | None | Humoral | IgG Abs, protection against challenge | (Patterson et al., 2013), (Sharma et al., 2020) |
| Cellular | CTL response | |||||||
| Qβ | Viral | FMDV | In vitro, in vivo | Not specified (guinea pigs) | None | Humoral | Antigen-specific Abs | (Skamel et al., 2014) |
| HIV-1 | In vitro, in vivo | IM | Adjuplex | Humoral | Antigen-specific Abs, neutralization Abs | (Purwar et al., 2018) | ||
| Influenza | In vivo, in vitro | SC, IM | Alum | Humoral | IgG Abs, neutralization Abs, protection against challenge | (Jegerlehner et al., 2013), (Skibinski et al., 2013) | ||
| Cellular | Specific Th1 response, cytokine release (IFN-γ) | (Skibinski et al., 2013) | ||||||
| In vivo* | IM | Alum | Humoral | Antigen-specific antibodies, no serious adverse events | (Low et al., 2014) | |||
| Cellular | Cytokine release (IFN-γ, IL17A, IL-17F, IL-9, IL-5, IL-13, IL-21), specific CD4+ and CD8+ T cell proliferation | (Low et al., 2014), (Skibinski et al., 2018) | ||||||
| Parasitic | Malaria | In vivo | IP, SC | Alum, SE, Montanide | Humoral | IgG Abs, protection against challenge | (Khan et al., 2015) | |
| Leishmaniasis | In vitro, in vivo | SC | None | Humoral | IgG Abs, protection against challenge | (Moura et al., 2017) | ||
| λ | Viral | Hepatitis B | In vivo, in vitro | IM | None | Humoral | Antigen-specific Abs, protection against challenge | (March et al., 2004) and (Clark et al., 2011) |
| Cellular | Specific lymphocyte proliferation | (Clark et al., 2011) | ||||||
| T4 | Bacterial | Anthrax | In vivo | IM, TCI | Alhydrogel, heat-labile enterotoxin, MPLA | Humoral | IgG Abs, protection against challenge | (Peachmann et al., 2012) |
| Anthrax and plague | In vivo | IM | None | Humoral | IgG Abs, neutralization Abs, protection against challenge | (Tao et al., 2018) | ||
| MS2 | Viral | FMDV | In vitro, in vivo | IM | FIA | Humoral | Neutralizing Abs, protection against challenge | (Dong et al., 2015) |
| HPV | In vivo, in vitro | IM, PO | FIA, liquid CT, MPLA | Humoral | IgG Abs, neutralizing Abs, protection against challenge | (Tumban et al., 2012), (Tyler et al., 2014), and (Zhai et al., 2017) | ||
| RSV | In vivo | IN | None | Humoral | Protection against challenge | (Schwarz et al., 2016) | ||
| Cellular | Specific splenocyte proliferation | |||||||
| Filamentous (fd) | Fungal | Candida albicans (yeast infections) | In vivo, in vitro | Not specified | None | Humoral | IgG Abs | (Wang et al., 2014) |
| Cellular | Specific splenocyte proliferation, cytokine release (IFN-γ, IL-2, IL-12, and IL-17) | |||||||
| Sporotrichosis | In vivo, in vitro | IV | None | Humoral | IgG Abs, protection against challenge Th1 and Th17 proliferation | (Chen et al., 2017) |
Asterisk indicates Phase I clinical trial.
3.1. Salmonella virus P22 (P22)
The P22 phage is composed of a 60-nm icosahedral capsid that encapsulates double-stranded DNA (dsDNA), and a tail region which facilitates viral infiltration into Salmonella typhimurium bacteria [Wang et al., 2019b]. Researchers have recently utilized the P22 phage and its naturally-encapsulated DNA to develop influenza vaccines. In the study by Richert, et al., previously mentioned above, investigators evaluated the performance of sHSP conjugated to OVA antigen (OVA-sHsp) in an influenza murine model [Richert et al., 2012]. For some of the experiments, P22 phage was used as an adjuvant in combination with sHSP or OVA-sHsp. Pre-treatment of P22 prior to a single dose of OVA-sHsp enhanced OVA-specific serum IgG levels as early as 4 days after influenza A challenge. Since even non-modified P22 phages may be immunogenic, using P22 as a vaccine platform is promising to induce specific immune response. Others have also utilized a P22-based VLP by genetically fusing the conserved influenza A nucleoprotein antigen to the P22 capsid coat protein [Patterson et al., 2013]. Mice were vaccinated prior to lethal influenza challenge. The vaccine enhanced IgG antibody levels and antigen-specific CD8+ cytotoxic T cell activity, resulting in 80% survival compared to 0% of the unimmunized group. Another study developed a homologous influenza A virus (PR8)-specific P22 vaccine. Globular head domains of the hemagglutinin protein were fused onto the P22 platform through enzymatic conjugation. Mice were immunized then challenged with PR8 influenza A virus, leading to 100% survival compared to 50% for unvaccinated mice. Furthermore, the vaccine doubled antigen-specific IgG antibody levels compared to controls [Sharma et al., 2020].
The P22 platform has also been used in the development of a respiratory syncytial virus (RSV) vaccine. Two different RSV antigens, matrix (M) and matrix 2 (M2) proteins, were co-encapsulated through genetic fusion. Mice vaccinated with this formulation developed higher M- and M2-specific CD8+ and CD4+ T proliferation in the lungs, as well as more tissue-resident memory CD8+ T cells. Compared to PBS and un-conjugated P22 controls, treatment with P22 that co-encapsulated proteins M and M2 led to reduced viral titers in the lungs [Schwarz et al., 2016].
3.2. Escherichia virus Q-beta (Qβ)
Qβ is a bacteriophage that infects E. coli through the F-pilus protein, which is not present on mammalian cells, and therefore the virus is non-infectious to humans [Singleton et al., 2018]. Qβ is found in abundance in sewage and animal feces. Its icosahedral coat proteins encapsulate interior single-stranded RNA (ssRNA), forming a 25-nm capsid cage. Qβ has been used as a vaccine scaffold for both infectious disease as well as cancer vaccine applications [Hess et al., 2019][Speiser et al., 2010].
The use of Qβ-based vaccines has been most extensively applied towards preventing viral diseases. One study developed a vaccine for foot and mouth disease virus (FMDV) by expressing and purifying Qβ phages fused to FMDV viral antigen [Skamel et al., 2014]. Researchers performed multiple rounds of biopanning selection to allow for in vitro evolution of the phage vaccine, and the final selected FMDV antigen-displaying phages were used to vaccinate guinea pigs. Sera confirmed high antibody affinity for whole FMDV [Skamel et al., 2014]. These studies demonstrate that in vitro evolution is a feasible method to develop a Qβ-based vaccine with a higher diversity of presented antigen, recapitulating the plethora of viral antigen mutants that may be present in an animal infected with FMDV and thereby improving vaccine immune response. In another more recent study, several formulations of HIV-1 antigen fragment proteins displayed on the Qβ scaffold were developed through genetic engineering or chemical conjugation. Formulation performances were compared in a six-month long rabbit immunization study. Priming with Qβ VLPs followed by boost with full length HIV-1 antigen (subtype B JRFL gp120) resulted in superior neutralization antibody titers [Purwar et al., 2018]. Taken together, studies confirm that antigens can be displayed on Qβ-based vaccines through a variety of biochemical approaches.
The Qβ platform has also been used to develop influenza vaccines. Researchers have created an influenza A H1N1 viral vaccine using the Qβ scaffold, a formulation showing high safety and immunogenicity in a Phase I clinical trial [Jegerlehner et al., 2013]. Initial studies used the Qβ platform to develop vaccines against influenza B and multiple influenza A strains, including H1N1, H5N1, and H3N2. For each, the ectodomain of HA (gH) was conjugated to Qβ, yielding strain-specific gH conjugation to Qβ. Mice were vaccinated and lethally challenged with the specific influenza strain. Each formulation enhanced survival rates and antibody titers at multiple long-term time points, including up to 48 days post-challenge [Jegerlehner et al., 2013]. These results showed that vaccine design using the Qβ scaffold provides strong protection in mice against H1N1 or other clinically relevant strains of influenza, depending on the antigen displayed.
Using the H1N1-specific vaccine (gH1-Qβ) formulation, further in vivo studies evaluated efficacy of the vaccine adjuvanted with alum (Alhydrogel®), an FDA-approved adjuvant widely used to enhance vaccine performance. In both mice and ferrets, gH1-Qβ with or without alum led to comparable levels of protection compared to the currently approved Panvax® H1N1 vaccine. However, the gH1-Qβ vaccine treatment in mice induced superior Th1-biased CD4 T cell response compared to Panvax® [Skibinski et al., 2013]. In a Phase I clinical trial, the gH1-Qβ vaccine resulted in 70.3% patient seroconversion by day 42 post-immunization, with no deaths or serious adverse events [Low et al., 2014]. The addition of a 2% Alhydrogel® adjuvant did not enhance seroconversion, perhaps because the natural ssRNA within the Qβ scaffold is sufficiently immunogenic [Low et al., 2014]. In another study, peripheral blood mononuclear cells of the patients treated with the gH1-Qβ vaccine were analyzed [Skibinski et al., 2018]. The gh1 peptide stimulation of samples collected post-vaccination had higher pro-inflammatory cytokine secretions levels including IFN-γ and IL17A than stimulated cells collected pre-treatment [Skibinski et al., 2018]. The Qβ platform in influenza vaccine development serves as a pioneer for protein VLP vaccination in clinical trials. Its results in these human trials continue to draw interest, encouraging further investigation of Qβ for other types of infectious diseases.
Qβ-based vaccines are also being developed to address parasitic infections. Malaria, caused by P. falciparum, is transmitted by mosquitos. The malaria antigen circumsporozoite protein (CSP) was conjugated onto the Qβ VLP [Khan et al., 2015]. Mice were vaccinated with the Qβ-CSP vaccine and challenged with sporozoites. Following a two-dose schedule, vaccinated mice exhibited higher antibody titer levels specific for both the full length CSP antigen as well as its central repeat epitope (termed NANP). NANP is similarly targeted by the currently approved malaria vaccine RTS,S, but in contrast the Qβ-CSP may further enhance protection by displaying the full-length CSP antigen [Khan et al., 2015]. Interestingly, the surface display of polysaccharides on Qβ can also serve as a vaccine. Moura et al. identified a trisaccharide epitope (α-Gal) found on the surface of leishmaniasis-causing parasites Leishmania infantum and Leishmania amazonensis [Moura et al., 2017]. These epitopes were displayed on the Qβ scaffold, and the resulting VLPs were used to immunize mice in a leishmaniasis model. Immunized mice had at least doubled IgG serum antibody levels at 3 weeks post-immunization versus naive mice. Following challenge with L. amazonensis or L. infantum, vaccinated mice had diminished parasite loads [Moura et al., 2017]. These results demonstrate the versatility of the Qβ phage platform in vaccine development, which is effective in both viral and parasitic models within preclinical research and preliminary clinical studies.
3.3. Escherichia virus lambda (λ)
λ phages can infect E. coli by entering the lytic or lysogenic replication pathways, which potentially allows for quiescent inheritance of phage by daughter cells. The dsDNA genetic material for phage replication is encapsulated in the icosahedron head, which is fused to a tail region that facilitates capsid ejection into E. coli cytoplasm [Fogg et al., 2010]. The λ phage platform has been investigated for development of a vaccine protective against hepatitis B virus (HBV). λ VLPs were engineered to also express hepatitis B antigen (HBsAg), and these were used to vaccinate mice and rabbits. HBsAg-specific antibody titers for both animal models over several weeks were enhanced compared to control treatment with HBsAg [March et al., 2004]. Given these promising results, further studies in rabbits compared the same formulation with the FDA-approved Engerix-B, a vaccine providing only limited protection to patients older than 60 years of age [Clark et al., 2011][Tohme et al., 2011]. Treatment with the λ phage vaccine led to consistently higher specific antibody titers compared to immunization with Engerix-B. These high antibody levels were maintained for more than 100 days in rabbits following multiple vaccinations with the λ vaccine [Clark et al., 2011]. It would be interesting to compare protection levels following challenge with HBV, especially since these results suggest potential advantages compared to an approved vaccine. However, we are not aware of more recent activity using the λ phage platform for infectious disease vaccines.
3.4. Escherichia virus T4 (T4)
The T4 phage consists of an icosahedron head with a tail region and is composed of 4 different proteins that form fibers, facilitating entry into E. coli [Bartual et al., 2010]. The outer capsid consists of a Hoc protein at the center of each major capsid and smaller Soc proteins [Shivachandra et al., 2006]. Compared to other phage-based vaccines, recombinant T4-based vaccines have been investigated mostly to protect against bacterial infection. Both of the following bacterial vaccines were developed by genetically fusing antigen to either the Hoc or Soc regions of the T4 phages.
Anthrax infection is caused by the Gram-positive bacterium Bacillus anthracis. Usually treated by antibiotics, inhalation of B. anthracis is more difficult to treat and can be fatal. One study investigated an anthrax vaccine by fusing protective antigen (PA) from B. anthracis to the N-terminus of the T4 Hoc regions [Peachmann et al., 2012]. The T4-PA vaccine was compared to alternative PA formulations, including a PA-based emulsion and PA-encapsulated liposomes with adjuvants. Rabbits were vaccinated with the different formulations and challenged with anthrax. The T4-PA vaccine enhanced IgG antibody levels and survival rates, although survival was 70%-80% that of the other formulations [Peachmann et al., 2012]. The T4-PA VLPs were immunogenic in themselves, likely due to the naturally-packaged dsDNA, and performance would likely be further improved by the inclusion of adjuvant (such as alum adjuvant used in the previously-mentioned Qβ studies).
More recently, a dual B. anthracis and plague (Yersinia pestis) vaccine was developed by genetically fusing antigens from both bacteria to the T4 Soc capsid proteins [Tao et al., 2018]. Following lethal doses of anthrax toxin and Y. pestis challenge in mice, rats, and rabbits, vaccination resulted in at least 80% survival, regardless of species. Neutralizing antibodies were also consistently higher in animals treated with recombinant T4 displaying antigen versus non-modified T4 [Tao et al., 2018]. As anthrax and plague are both potential biological weapons, the prospect of an effective dual vaccine has important clinical relevance.
3.5. Escherichia virus MS2 (MS2)
The MS2 phage consists of a 26-nm diameter icosahedron shell encapsulating ssRNA. Following infection of E. coli, virion assembly is instructed by multiple, specific RNA sequence sites [Stockley et al., 2016]. MS2-based vaccines are being developed for multiple anti-viral applications, including prevention of FMDV and human papillomavirus (HPV) infections.
To address FMDV, a coat protein epitope of a structural protein of FMDV (VP1) was genetically fused into the coat protein of MS2 [Dong et al., 2015]. Extensive in vivo studies were performed, including vaccination and FMDV challenge of mice, guinea pigs, and swine. Overall health, antibody levels, and endpoint survival were evaluated, revealing comparable performance to the standard inactivated vaccine that is commonly used in livestock husbandry [Dong et al., 2015]. FMDV is highly contagious, resulting in widespread livestock deaths and costing individual countries billions of dollars annually, yet no specific treatment currently exists [Knight-Jones & Rushton, 2013]. Therefore, it is especially promising that multiple phage platforms (including MS2 and Qβ) may offer potential prevention options.
Tumban and colleagues have recently developed an effective MS2-based HPV vaccine by genetically engineering MS2 VLPs to express a tandem HPV peptide (amino acids 17-31) with an HPV consensus peptide (amino acids 69-86). While the former peptide offers strong antibody response against HPV16 [Tumban et al., 2012], the latter offers a lower yet more widespread antibody response against several other HPV types [Tyler et al., 2014]. Vaccination of the mice with MS2 displaying tandem and consensus peptides not only protected mice from multiple HPV types, but antibody levels were comparable to those of mice immunized with the approved HPV vaccine, Gardasil®9 [Zhai et al., 2017]. This group further evaluated vaccine performance combined with 2 mucosal adjuvants in mice, including cholera toxin and the approved adjuvant monophosphoryl lipid A (MPLA). While the former study showed efficacy against only 6 types of HPV, these studies also showed efficacy against 8 additional HPV strains, including HPV16, which is associated with more than 70% of HPV-associated head and neck cancers. Notably, the MS2-based formulation provides protection against several additional HPV types associated with head and neck cancer versus the FDA-approved Gardasil®9 [Zhai et al., 2019]. These results suggest that a MS2-based vaccine may potentially protect against a more widespread range of HPV types than vaccines currently on the market.
3.6. Filamentous phage
While there have been a number of different filamentous phages used in antigen delivery (e.g., M13, fd, f1), all are rod-shaped with repetitive structure encapsulating ssDNA, which may be recognized by immune-activating toll-like receptor TLR9 [Henry et al., 2015]. Filamentous phage rod bodies consist of repeating units of pVIII coat protein, which are common sites engineered to express antigen peptides [Sartorius et al., 2019]. Filamentous vaccines, which others have previously reviewed [Henry et al., 2015], have been developed to vaccinate against bacterial, fungal, viral, and parasitic infections, and we highlight a few here.
Phage vaccine platforms have been examined for prevention of fungal infections. Wang et al. generated anti-fungal vaccines by genetically fusing 2 antigen epitopes from the fungus Candida albicans onto M13 bacteriophage [Wang et al., 2014]. Both epitopes were derived from the protein Sap2, a protease released by C. albicans known to degrade host proteins. Vaccination with the hybrid phage vaccine enhanced survival of mice challenged with C. albicans, in addition to higher levels of IgG antibody and pro-inflammatory cytokines, and reduced fungal levels in the spleen and kidney [Wang et al., 2014]. In another application, the prevention of fungal infection caused by Sporothrix globosa, a fungus present in soil and plant matter that causes sporotrichosis, has been investigated. Filamentous phages were genetically engineered with antigen epitopes of the fungus onto the major coat protein (pVIII). Mice vaccinated with the recombinant phage showed enhanced survival rates, even superior to those immunized with heat-killed S. globosa [Chen et al., 2017]. Because there are no currently approved anti-fungal vaccines [Nami et al., 2019], these examples of positive results are important for further evaluation.
Filamentous-based vaccines are also being developed to provide protection against parasitic infection. Researchers engineered fd filamentous phage to express highly immunodominant peptides trans-sialidase and amastigote surface protein-2, motifs accessible from the surface of the parasite Trypanisoma cruzi [Gomes-Neto et al., 2018]. Murine vaccination with either of the recombinant phage designs led to enhanced IgG antibody titer levels and superior survival rates following challenge with T. cruzi. Immunization was inhibited in TLR9 knockout mice, showing that immunogenicity of the filamentous phage-based vaccine requires receptor recognition of the naturally-encapsulated ssDNA adjuvant [Gomes-Neto et al., 2018]. Symptoms of Chagas disease, predominantly caused by transmission of T. cruzi from infected bugs, can be mediated by current anti-parasitic treatments. However, some individuals are chronically infected, and vertical transmission is of especially high concern in poorer regions of Latin America [Moncayo, 2017]. Therefore, the possibility of a vaccine would offer an encouraging option and merits further research.
4. Virus-like Particles (VLPs): Plant virus-derived
Plant viruses can infect specific plant types but lack the mammalian-replicable genetic material necessary to infect humans. In tandem with this characteristic and the utilization of genetic fusion to conjugate peptide antigens from a multitude of pathogens, these VLPs have been explored as vaccine platforms for numerous infectious diseases (see Table 3). This section will specifically focus on vaccine formulations and immunization studies that have used plant virus-derived VLPs as vaccine platforms.
Table 3. Immune responses elicited by plant virus-derived protein nanoparticle vaccines.
Abbreviations: Abs (antibodies), AIMV (alfalfa mosaic virus), BE (back of ear), CD4+ T cell (helper T cell), CD8+ T cell (cytotoxic T cell), CMV (cucumber mosaic virus), CpG 1826 (Oligodeoxynucleotide 1826), CPMV (cowpea mosaic virus), CT (cholera toxin), CTL (cytotoxic T cell lymphocyte), DC (dendritic cells), dIC (Polyinosinic-polycytidilic acid), di-GMP (Cyclic diguanylate monophosphate), DOPS (dioleoyl phosphatidylserine), FCA (Freud’s complete adjuvant), FIA (Freud’s incomplete adjuvant), FMDV (foot and mouth disease virus), HCV (Hepatitis C virus), HIV-1 (human immunodeficiency virus 1), IFN-γ (interferon-gamma), IL (interleukin), IM (intramuscular injection), IN (intranasal), IP (intraperitoneal injection), ISCOM (immune stimulating complex), IV (intravenous), LCMV (lymphocytic choriomeningitis virus), MCT (microcrystalline tyrosine), MΦ (macrophage), PO (per-orally), PapMV (papaya mosaic virus), PVX (potato virus X), RSV (respiratory syncytial virus), SC (subcutaneous), TBSV (tomato bushy stunt virus), TMV (tomato mosaic virus).
| Scaffold Platform |
Type of infectious disease |
Disease | In vitro, In vivo |
Route of Administration |
Adjuvant co- administered |
Elicited immune responses |
Specific immune response |
References |
|---|---|---|---|---|---|---|---|---|
| CPMV | Bacterial | Staph infection | In vivo | IN, SC | ISCOM Matrix, FCA, FIA, QS-21 | Humoral | Neutralizing Abs, opsonizing Abs | (Brannan et al., 1999a), (Brennan et al., 1999c), (Rennermalm et al., 2001) |
| Pseudomonas infection | In vivo | SC | FCA, FIA, Alum | Humoral | Opsonizing Abs, Protection upon challenge | (Brennan et al., 1999b) | ||
| Group B Streptococcus | In vivo | SC, IP | FCA, FIA | Humoral | Antigen-specific Abs | (Pomwised et. al, 2016) | ||
| Cellular | Cytokine release (IFN-γ) | (Pomwised et. al, 2016) | ||||||
| Viral | HIV-1 | In vivo | IN, PO, SC | Alum, FCA, Quil A, AdjuPrime, Ribi, CT | Humoral | Neutralizing Abs | (McInerney et al., 1999), (Durrani et al., 1998) | |
| Cellular | Antigen-specific T cell proliferation | (McInerney et al., 1999) | ||||||
| PVX | Viral | HIV-1 | In vivo | IN, IP | None | Humoral | Neutralizing Abs | (Marusic et al., 2001) |
| Influenza A (H1N1) | In vivo | SC | FIA | Cellular | Antigen-specific CD8+ T cell activation | (Lico et al., 2009) | ||
| HCV | In vitro, In vivo | SC | GERBU adjuvant MM | Humoral | Antigen-specific Abs | (Uhde-Holzem et al., 2010) | ||
| TMV | Bacterial | Pseudomonas infection | In vivo | SC, IM | FCA, FIA, Aluminum hydroxide | Humoral | Opsonizing Abs, protection against challenge | (Staczek et al., 2000) |
| Francisella tularensisis | In vivo, ex vivo | SC, IN | CpG 1826, Addavax, dIC, di-GMP | Humoral | Antigen-specific Abs, protection against challenge | (Banik et.al, 2015), (Mansour et.al, 2018), (McCormick et.al, 2018) | ||
| Anthrax | In vivo | IP | None | Humoral | Neutralizing Abs | (McComb et. al, 2015) | ||
| Yersinia pestis | In vivo | IN | None | Humoral | Antigen-specific Abs, protection against challenge | (Arnaboldi et. al, 2016) | ||
| Viral | Influenza (H1N1) | In vivo | SC | Alum, Addavax | Humoral | Antigen-specific Abs, protection against challenge | (Mallajosyula et al., 2014) | |
| FMDV | In vivo | IP, BE | Aluminum hydroxide | Humoral | Antigen-specific Abs, protection against challenge | (Jiang et al., 2006) | ||
| CMV | Viral | HCV | In vitro, In vivo | SC, IM | FIA | Humoral | Antigen-specific Abs | (Piazzola et al., 2005), (Nuzzaci et al., 2007) |
| Cellular | Cytokine release (IFN-γ, IL-12, and IL-15) | (Piazzola et al., 2005), (Nuzzaci et al., 2007) | ||||||
| Zika virus | In vitro, In vivo | IM | DOPS | Humoral | Neutralizing Abs | (Cabral-Miranda et. al, 2019) | ||
| Parasitic | Malaria | In vivo | IM | MCT, Alum | Humoral | Antigen-specific Abs, protection against challenge | (Cabral-Miranda et. al, 2017) | |
| Cellular | CD4+ and CD8+ T cell responses | (Cabral-Miranda et. al, 2017) | ||||||
| AIMV | Viral | RSV | In vivo | IM | None | Humoral | Antigen-specific Abs | (Yusibov et al., 2005) |
| Cellular | CD4+ and CD8+ T cell responses | (Yusibov et al., 2005) | ||||||
| Parasitic | Malaria | In vivo* | IM | Alhydrogel | Humoral | Neutralizing Abs | (Jones et al., 2013) (Chichester et. al, 2018) | |
| TBSV | Viral | HIV-1 | In vitro, In vivo | SC | None | Humoral | Antigen-specific Abs | (Joelson et al., 1997) |
| PapMV | Viral | Influenza | In vitro, In vivo | SC, IP, IM | Alum | Humoral | Antigen-specific Abs, B cell expansion, protection against challenge | (Denis et al., 2008), (Hanafi et al., 2010), (Bolduc et. al, 2018), (Carignan et. al, 2015), (Therien et al., 2017) |
| Cellular | Antigen-specific CD8+ T cell expansion and response | (Babin et al., 2013), (Leclerc et al., 2007), (Hanafi et al., 2010), (Laliberte-Gagne et al., 2019) | ||||||
| HCV | In vivo | SC | None | Humoral | Antigen-specific Abs | (Denis et al., 2007) | ||
| LCMV | In vivo | IV | None | Cellular | Antigen-specific CD8+ T cell activation, protection against challenge | (Lacasse et al., 2008) |
Asterisk indicates Phase I clinical trial.
4.1. Cowpea Mosaic Virus (CPMV)
The 30-nm self-assembling icosahedral cowpea mosaic virus (CPMV) is one of the most studied plant-based platforms for protein nanoparticle vaccine development. It has been investigated as a vaccine platform against bacterial and viral pathogens, including Staphylococcus aureus, Pseudomonas aeruginosa, Group B Streptococcus, and HIV-1. In more recent years, CPMV has also been utilized in the development of cancer vaccines [Lizotte et al., 2016][Murray et al., 2018][Wang et al., 2019a]. Most formulations of CPMV utilize the technique of genetic fusion to load immunogenic epitope peptides from the proteins of pathogens.
CPMV fused to the D2 peptide of the fibronectin-binding protein B (FnBP) of S. aureus has elicited strong humoral immune responses. Immunizations of mice and rats with these CPMV-D2 formulations have yielded high titers of FnBP-specific antibodies, generated neutralizing antibodies capable of inhibiting the binding of fibronectin required for disease pathogenesis, produced antibodies that induced opsonization of S. aureus, protected animals from weight loss when intravenously administered with pre-opsonized S. aureus, and protected against endocarditis [Brennan et al., 1999a][Brennan et al., 1999b][Brennan et al., 1999c]. In a model of chronic pulmonary infection, a fusion protein of CPMV and peptide derived from the outer-membrane protein F of P. aeruginosa afforded protection upon challenge with two different immunotypes of the bacterium. Immunization generated high titers of antigen-specific opsonizing antibodies that marked the bacteria for phagocytosis by human neutrophils [Brennan et al., 1999b]. Against the pathogen group B Streptococcus, mice immunized with CPMV genetically fused to S9 peptide, a mimic of the group B streptococcal type III capsular polysaccharide, elicited a Th1 response. This response was characterized by the production of peptide-specific IgG2a antibodies, as well as the generation of IFN-γ by antigen-stimulated lymphocytes derived from treated mice [Pomwised et al., 2016].
In the application against a viral pathogen, formulations of CPMV fused with a gp41 peptide have been effective in inducing humoral and T cell-mediated immune responses against HIV-1. Immunization studies in mice have shown strong gp41-specific antibody responses, in vitro proliferation of T cells from splenocytes, and HIV-1 neutralization [McInerney et al., 1999]. Interestingly, intranasal immunization also elicited strong gp41 peptide-specific antibody responses, supporting the viability of CPMV as an intranasal vaccine delivery system that may improve patient compliance to vaccination [Durrani et al., 1998].
4.2. Potato Virus X (PVX)
Potato virus X (PVX) is a self-assembling rod-structured plant virus that naturally infects potatoes. Through genetic engineering, PVX can be modified for pathogen antigen conjugation. Specifically, fusing antigens onto the nanoparticle scaffold allows high loading capacity of repeating antigens, which is favored for eliciting an immune response.
Utilizing genetic fusions, the antigens gp41 peptide, nucleoprotein peptide, and R9 peptide have been conjugated to PVX to induce immune response against HIV-1, influenza (H1N1), and hepatitis C virus (HCV), respectively. Immunization studies in mice have elicited humoral response against both HIV-1 and HCV, including antigen-specific and neutralizing antibodies [Marusic et al., 2001][Uhde-Holzem et al., 2010]. In the case of HIV-1, the sera of immunized mice were also able to inhibit virus-induced syncytium, a phenomenon in which infected cells express glycoproteins from the virus, allowing infected cells to adhere to healthy cells, which in turn leads to accelerated disease progression. For infectious diseases that reside extensively within cells, a vaccine-elicited cellular response characterized by antigen-specific CD8+ T cell activation is desired for the eradication of infected cells. This activation of CD8+ T cells has been observed using this PVX-derived platform; when immunized with the PVX-nucleoprotein peptide formulation, mice produced antigen-specific CD8+ T cells [Lico et al., 2009].
4.3. Tobacco Mosaic Virus (TMV)
Tobacco mosaic virus (TMV) is a 300-nm x 20-nm rod-shaped plant virus [Butler, 1984]. As discussed, most plant virus-derived nanoparticle platforms utilize genetic engineering to load antigens, but studies with TMV have employed both genetic fusion and chemical conjugation to load peptide antigens. Due to the symmetry of the virus, the number of antigens per TMV particle can be high.
TMV has been studied as a vaccine platform for bacterial and viral pathogens. Mice immunized with a TMV-peptide antigen vaccine produced peptide-specific opsonic antibodies that reacted to all seven strains of P. aeruginosa, as well as conferred protection against challenge with wild-type P. aeruginosa in a mouse model of chronic pulmonary infection [Staczek et al., 2000]. The TMV platform has also been engineered against the bacterial pathogen Francisella tularensisis. Utilizing both genetic fusion and chemical conjugation, protein antigens including chaperone protein DnaK, OmpA-like protein (OmpA), SucB protein, and lipoprotein Tul4 have been conjugated onto TMV [Banik et al., 2015][Mansour et al., 2018][McCormick et al., 2018]. Immunization of mice with these formulations induced strong humoral responses predominated by IgG1 and IgG2 antibodies, as well as protection against lethal respiratory challenge of F. tularensis LVS (live vaccine strain) even after 163 days post-primary immunization [Banik et al., 2015][Mansour et al., 2018][McCormick et al., 2018]. In these studies, the magnitude and quality of immune responses were shown to vary based on immunization routes (subcutaneous or intranasal), adjuvant administration, and antigen selection. Against anthrax, immunization of mice with genetically-fused peptides from the protective antigen (PA) protein toxin of anthrax has shown to partially neutralize this toxin when challenged with anthrax spores [McComb et al., 2015]. In the case of Yersinia pestis, LcrV and F1 protein antigens that were chemically conjugated to TMV induced an immune response capable of complete protection against pneumonic infection after a lethal dose of Y. pestis in mice [Arnaboldi et al., 2016] .
For viral pathogens, TMV vaccines have generally elicited antigen-specific humoral responses. Interestingly, immunization of mice with a TMV-monomeric hemagglutinin protein (HA) vaccine not only elicited antibodies, but also better protected mice against H1N1 influenza virus challenge than the commonly-used trivalent inactivated H1N1 vaccine [Mallajosyula et al., 2014]. Against FMDV, a livestock disease, a TMV-based vaccine elicited peptide-specific antibodies, as well as protection against FMDV challenge in guinea pigs and swine [Jiang et al., 2006].
4.4. Cucumber Mosaic Virus (CMV)
Cucumber mosaic virus (CMV) is a plant virus with a 30-nm self-assembling icosahedral capsid. Immunization in rabbits with a fusion protein of CMV and peptide derived from the hepatitis C virus (HCV) envelope protein E2 (R9 mimotope) has led to increases in peptide-specific antibodies [Piazzolla et al., 2005]. In patients with chronic HCV infection, the CMV vaccine induced a significant release of IFN-γ, IL-12, and IL-15 by peripheral blood mononuclear cells, indicating a strong innate immune response to the peptide antigen [Piazzolla et al., 2005][Nuzzaci et al., 2007]. A CMV vaccine formulation using Zika virus envelope protein (EDIII) as its antigen induced high levels of antigen-specific antibodies after a single immunization; this was capable of neutralizing Zika virus without enhancing infection by dengue virus in vitro [Cabral-Miranda et al., 2019]. Unique from many plant-based VLPs, a CMV construct against the parasitic disease, malaria, has also been studied. Immunization with the chemically-conjugated malaria TRAP protein (from Plasmodium vivax) induced an increase in humoral responses and a strong T cell response, and it offered protection against challenge with recombinant Plasmodium berghei that expressed P. vivax TRAP antigen [Cabral-Miranda et al., 2019].
4.5. Alfalfa Mosaic Virus (AlMV)
Alfalfa mosaic virus (AlMV) particles have a bacilliform structure with a diameter of 19-nm and a length varying from 30- to 56-nm. As with other plant virus-derived VLP scaffolds, genetic fusion of pathogen antigens to AlMV has been utilized to construct infectious disease NP vaccines. AlMV has shown promise in inducing antigen-specific immune responses against both respiratory syncytial virus (RSV) and P. falciparum. Human DCs incubated with the AlMV-RSV formulation have been shown to stimulate vigorous CD4+ and CD8+ T cell responses [Yusibov et al., 2005]. Strong cellular and humoral RSV-specific immune responses have been generated in non-human primates [Yusibov et al, 2005]. In vaccine formulations against P. falciparum, AlMV has also shown promise in mice following immunization, where serum antibodies were observed with complete transmission blocking activity through a 6-month study period [Jones et al., 2013]. This formulation then entered a Phase 1 first-in-human study where a dose dependent antigen-specific antibody response was observed, as well as no evidence of dose-related toxicity. However, it was also noted that the transmission-reducing activity of the generated antigen-specific antibodies was weak, pointing to the need for further optimization [Chicester et al., 2018]. AlMV’s ability to elicit an immune response against a parasite using a peptide antigen gives this vaccine platform a unique position amongst the plant virus-based VLPs.
4.6. Papaya Mosaic Virus (PapMV)
This 500-nm x 13-nm rod-shaped virus was first reported in 1962 when studying diseased papaya plants in Florida [Conover, 1962]. In attempts to develop a universal influenza vaccine, PapMV fused with peptides from the influenza M2 ion channel protein have been shown to induce production of antigen-specific antibodies that can recognize infected cells and protect mice from influenza (H1N1) challenge [Therien et al., 2017][Carignan et al., 2015]. Combining this formulation with multimerized nucleoprotein nanoparticles protected mice from challenges by both H1N1 and H3N2 influenza strains [Bolduc et al., 2018]. More impressive was the ability of free (unconjugated) PapMV VLPs, used as adjuvants, to dramatically increase the immunogenicity of the PapMV-antigen vaccine. This increased immunogenicity led to 100% protection against a challenge with the WSN/33 influenza strain [Denis et al., 2008]. In vitro activation of B cells and expansion of antigen-specific T cells using PapMV-peptides derived from the nucleocapsid protein and M1 matrix protein of the influenza virus have been supported by immunizations of mice that exhibited antigen-specific CD8+ T cell responses [Babin et al., 2013][Leclerc et al., 2007][Hanafi et al., 2010][Laliberte-Gagne et al., 2019].
PapMV formulations using peptide antigen fusions have also been examined for vaccines against hepatitis C virus (HCV) and lymphocytic choriomeningitis virus (LCMV). Mice immunized with the PapMV-E2 glycoprotein epitope vaccine showed a long-lasting humoral response (more than 120 days) against the HCV peptide antigen [Denis et al., 2007]. Against LCMV, the PapMV-p33 epitope (derived from LCMV’s surface glycoprotein) vaccine activated large numbers of antigen-specific CTLs which rapidly expanded following LCMV challenge and protected vaccinated mice against LCMV infection in a dose-dependent manner [Lacasse et al., 2008].
5. Virus-like Particles (VLPs): Recombinant human-virus derived viral vectors (new section)
Viral vectors fall under a niche category of protein nanoparticle vaccine platforms. Unlike the other particles discussed in this review, these NPs have the ability to mimic infection in humans by entering host cells and inducing host-cell expression of foreign antigen using viral mechanisms; however, they lack viral extranuclear replication capabilities [Matthews et al., 2013][Ura & Shimada, 2014][Bull et al., 2019]. This in turn induces an immune response to those foreign, pathogenic antigens. The first published work in 1984 describing viral vectors as scaffolds used to deliver vaccine antigens was a recombinant vaccinia virus engineered to express hepatitis B antigen in host cells [Moss et al., 1984]. Since those initial studies, a plethora of viral vectors have been investigated, leading to several FDA-approved vaccines. While effective, safety concerns related to their innate pathogenic nature in humans have been raised [Rauch et al., 2018]. In this section, the use of human-tropism virus vectors as vaccine platforms for infectious disease will be summarized and discussed. Due to many extensive reviews already written in this area [Ho et al., 2020][Nieto & Salvetti, 2014][Ura & Shimada, 2014][Bull et al., 2019], we highlight only a few platforms that have been studied in the clinic, giving some examples which have been approved for use.
5.1. Hepatitis B surface antigen (HBsAg)
Globally, hepatitis B is a common viral infectious disease, and its surface envelope protein (HBsAg) has the ability to present foreign antigens for chimeric protein delivery and has been used in preclinical and clinical vaccine development [Ho et al., 2020][RTS,S Clinical Trials Partnership, 2015]. RTS,S/AS01 (RTS,S) (Mosquirix) is a malaria subunit vaccine developed by fusing a portion of Plasmodium falciparum-derived circumsporozoite (CS) onto HBsAg [Choi & Chang, 2013][Ho et al., 2020]. Naturally-acquired immunity against malaria is mediated by IgG levels and can be gained with age and exposure, decreasing risk of morbidity and mortality in adults; however, because children and infants may lack such protection, vaccination is important in this patient group [Dobano et al., 2019b][Doolan et al., 2009]. In recent years, clinical trials utilizing RTS,S/AS01 have examined safety, efficacies, immunogenicity, as well as a multitude of unique immune responses [Asante et al., 2020][Sanchez 2020]. Immune responses including unique antibody responses after variable doses and unique avidities of antibodies have been well documented [Sanchez et al., 2020][Dobano et al., 2019a] [Dobano et al., 2019b]. RTS,S vaccination of young children in Africa results in higher specific IgG antibody titers, correlating with increased protection against clinical malaria [Dobano et al., 2019b]. However, there are limitations; only about 56% of children receiving the RTS,S vaccine are protected from naturally-occurring malaria infection, with around 31% protection rates in infants [Dobano et al., 2019b][Vrieze, 2019]. The WHO has now set up a pilot vaccination program in Africa to monitor safety and efficacy concerns as the vaccine is deployed.
5.2. Adenovirus (Ad) vectors
One of the most studied viral vectors are adenovirus (Ad) vectors. Ad vectors have been examined in clinical trials for vaccines against HIV, influenza, malaria, and tuberculosis (TB), and more recently, SARS-CoV-2 [Mathews et al., 2013][Choi & Chang, 2013][Ura & Shimada, 2014][BioRender, 2020][Zhu et al., 2020][Ramezanpour et al., 2016][van Zyl-Smit et al., 2017][Liebowitz et al., 2020][van Zyl-Smit et al., 2017]. Ad-based vaccines for HIV-1 and malaria have been shown to induce antigen-specific CD4+ and CD8+ T cell responses and antibody responses in clinical trial volunteers [Mathews et al., 2013]. An Ad-based vaccine for malaria has been demonstrated to induce similar specific humoral and cellular immune responses as the RTS,S/AS01 vaccine described previously [Choi & Chang, 2013][Ramezanpour et al., 2016]. During the Ebola virus outbreak from 2014-2016, several Ad-based Ebola virus vaccines saw development and clinical trials through phase 1 and 2 [Lauer et al., 2017][Rauch et al., 2018]. More recently, Ad-based vaccines are being investigated to combat the outbreak of SARS-CoV-2, the causative infectious agent of COVID-19. Currently, three Ad-based SARS-CoV-2 vaccines are undergoing clinical trials with two candidates in phase 2 and one in phase 3 [BioRender, 2020][Zhu et al., 2020].
5.3. Vesicular stomatitis virus (VSV)
Vesicular stomatitis virus (VSV), a member of the Rhabdoviridae family, is an enveloped virus containing a single-stranded, negative-sense RNA [Rauch et al., 2018]. Due to the lack of a DNA intermediate during viral infection, VSV has become an attractive vaccine platform [Rauch et al., 2018]. In particular, a VSV-based vaccine has been developed for Ebola in which the VSV envelope glycoprotein was replaced by the corresponding protein from the Ebola virus [Marzi et al., 2011][Ollmann, 2020]. It progressed in clinical trials during the Ebola virus outbreak of 2014-2016, received conditional marketing authorization by the WHO, and was approved by the FDA in 2019. This vaccine (Ervebo) has now been used to vaccinate over 280,000 people (at the time of writing this article) during the ongoing 2018-2020 outbreak in Africa [Rauch et al., 2018][Ollmann, 2020].
5.4. Other viral vector platforms
The use of adeno-associated virus (AAV) vectors as vaccine platforms have also been studied for infectious diseases including HIV-1, HPV, influenza (H1N1), and hepatitis C [Nieto & Salvetti, 2014][Ura & Shimada, 2014][Ramezanpour et al., 2016][Demminger et al., 2020][Zhu et al., 2019][Naso et al., 2017][Vardas et al., 2010][ClinicalTrials.gov, 2020a]. The rarity of AAV-based vaccines in clinical trials is due to its relatively weak ability to induce humoral and cellular immune response when compared to other viral vectors [Ura & Shimada, 2014]. Alphaviruses replicate in the cytoplasm of infected cells, which eliminate the concern for potential integration of viral genes into the host genome, and a number of alphavirus-based vaccine formulations have been shown to induce both antigen-specific humoral and cellular responses [Choi & Chang, 2013] [Ramezanpour et al., 2016]. Chimeras of two alphaviruses, Venezuelan equine encephalitis virus (VEE)-Sindbis virus (SIN), have been pre-clinically shown to induce high neutralizing antibody titers and cell-mediated responses against HIV-1 [Choi & Chang, 2013]. Poxviruses have large genomes making them advantageous for larger antigen gene insertions and have been investigated as the viral vectors for malaria, tuberculosis, small pox, influenza, and HIV-1 vaccines in clinical trials [Choi & Chang, 2013][Ura & Shimada, 2014][Pitisuttithum et al., 2020][Laher et al., 2020][Levy et al., 2020][Pittman et al., 2019][Kreijtz et al., 2014][Minhinnick et al., 2016][Wilkie et al., 2020][de Barra et al., 2014] .
6. Future outlook
Since Edward Jenner’s first cowpox vaccine protected against smallpox, the field of vaccinology has made tremendous impacts in quality and length of life for untold millions of people around the world [Delany et al., 2014]. However, our work is not yet finished. Vaccines are still lacking for well-characterized infectious agents, including HIV-1 and tuberculosis, and new, emerging infectious agents, such as the 2009 H1N1 flu or SARS-COV2, will necessitate the rapid discovery and deployment of vaccine candidates to curb the threat of extended global pandemics [Pati et al., 2018][Lurie et al., 2020]. Although a live attenuated or inactivated whole organism approach may work for some of these organisms, subunit vaccines remain the safest design approach [Vartak & Sucheck, 2016]. These subunit vaccines will require adjuvants and alternative approaches to be efficacious; based on our experience and the data reviewed here, we believe a protein nanoparticle-based approach should be considered in the design of these vaccines.
As of the writing of this review, 174 vaccines against SARS-CoV2 (the causative agent of COVID-19) are in development, with 51 of these in clinical trials [BioRender, 2020]. Several approaches are being taken, including inactivated SARS-CoV2 particles, a genetically modified chimpanzee virus expressing SARS-CoV2 proteins, and nucleic acid based vaccines [Callaway, 2020]. Subunit vaccines with antigens attached to nanoparticles are also being developed, including a self-assembled VLP, NSP-10, which is expected to generate desired immune response after displaying SARS-CoV2 spike protein [Carter et al., 2020]. Several factors are important for the development of COVID-19 vaccines; it is crucial to display the correct antigens in the correct conformations to induce antigen-specific, high affinity neutralizing antibodies. It is also critical to avoid antibody-dependent enhancement of viral uptake and induction of Th2 immune responses which have the potential to cause allergic inflammation and vaccine-associated enhanced respiratory disease [Graham, 2020][Peeples, 2020][Vabret et al., 2020].
Great accomplishments have been achieved in developing nanoparticle vaccines for deployment in humans. However, these platforms still face a few challenges before they are ready for widespread inclusion in human vaccine formulations. The first limitation is the lack of in-depth understanding of how every permutation of each nanoparticle-based platform will perform in vivo. The high degree of customization inherent to NP design (factors including size, charge, and shape) is both a blessing and a curse; we have not yet established the ideal combination of each of these factors to achieve ideal targeting, biodistribution, and clearance for each pathogen of interest, but such information is important to advance the field. One can observe this challenge being surmounted as materials scientists, engineers, immunologists, and vaccinologists work together to generate vaccines using protein nanoparticle technologies.
The second challenge is maintaining reproducibility of synthesis as production scales up, which is a difficulty faced by every new therapeutic technology aiming to make the leap from pre-clinical development to clinical deployment. We know that this is not an insurmountable problem as multiple nanoparticle-based platforms have been reproducibly scaled for human clinical trials. Much like the first challenge, it is only a matter of time before the synthesis and large-scale production parameters are determined for each of these platforms.
Despite these challenges, the field is primed to see the results of clinical trials for vaccines using several of the platforms that are discussed in this review, and to envision where and how they will be applied next for the improvement of human health worldwide. As the collective field of vaccine research and design moves forward to meet the challenge of infectious and transmissible disease in the post-COVID-19 pandemic era, protein nanoparticle-based platforms have shown tremendous potential and should be considered as a possible strategy in vaccine development.
Table 4. Clinical trial progress of recombinant viral vector vaccine platforms.
Abbreviations: AAV (adeno-associated virus vector), Ad (adenovirus vector), FDA (US Food and Drug Administration), Hepatitis B surface antigen (HBsAg), HIV (human immunodeficiency virus), HPV (human papillomavirus), RTS,S (RTS,S/AS01 malaria vaccine (trade name Mosquirix™)), TB (tuberculosis), VSV (vesicular stomatitis virus).
| Scaffold Platform |
Type of infectious disease |
Disease | Clinical Status | References |
|---|---|---|---|---|
| HBsAg | Parasitic | Malaria | Pre-clinical, Phase 1, Phase 2, Phase 3, FDA approved | (RTS,S Clinical Trials Partnership, 2015) (Dobano et al., 2019a) (Doolan et al., 2009) (Vrieze, 2019) (Asante et al., 2020) (Dobano et al., 2019b) (Sanchez et al., 2020) |
| Ad | Bacterial | TB | Pre-clinical, Phase 1, Phase 2 | (Matthews et al., 2013) (Ura & Shimada, 2014) (van Zyl-Smit et al., 2017) |
| Viral | HIV | Pre-clinical, Phase 1, Phase 2 | (Choi & Chang, 2013) (Matthews et al., 2013) (Ura et al, 2014) | |
| Influenza | Pre-clinical, Phase 1, Phase 2 | (Ura & Shimada, 2014) (Lauer et al., 2017) (Liebowitz et al., 2020) | ||
| SARS-CoV-2 | Pre-clinical, Phase 1, Phase 2, Phase 3 | (BioRender, 2020) (Zhu et al., 2020) | ||
| Ebola virus | Pre-clinical, Phase 1, Phase 2 | (Lauer et al., 2017) (Rauch et al., 2018) | ||
| Parasitic | Malaria | Pre-clinical, Phase 1, Phase 2 | (Choi & Chang, 2013) (Matthews et al., 2013) (Ramezanpour et al., 2016) | |
| AAV | Viral | HIV | Pre-clinical, Phase 1, Phase 2 | (Ura & Shimada, 2014) (Nieto & Salvetti, 2014) (Ramezanpour et al., 2016) (Vardas et al., 2010) |
| HPV | Pre-clinical, Phase 1 | (Ura & Shimada, 2014) (Nieto & Salvetti, 2014) (ClinicalTrials.gov, 2020a) | ||
| Influenza (H1N1) | Pre-clinical | (Ura & Shimada, 2014) (Nieto & Salvetti, 2014) (Demminger et al., 2020) | ||
| Hepatitis C | Pre-clinical, Phase 1, Phase 2 | (Zhu et al., 2019) (Naso et al., 2017) | ||
| VSV | Viral | Ebola virus | Phase 1, Phase 2, Phase 3, FDA approved | (Rauch et al., 2018) (FDA, 2020a) (Ollmann, 2020) |
| Alphavirus | Viral | HIV | Pre-clinical, Phase 1, Phase 2 | (Choi & Chang, 2013) (Ramezanpour et al., 2016) |
| Influenza | Pre-clinical, Phase 1, Phase 2 | (Choi & Chang, 2013) (Ramezanpour et al., 2016) | ||
| Poxvirus | Viral | HIV | Pre-clinical, Phase 1, Phase 2, Phase 3 | (Choi & Chang, 2013) (Ura & Shimada, 2014) (Pitisuttithum et al., 2020) (Laher et al., 2020) (Levy et al., 2020) |
| Smallpox | Pre-clinical, Phase 1, Phase 2, Phase 3 | (Ura & Shimada, 2014) (Pittman et al., 2019) | ||
| Influenza | Pre-clinical, Phase 1, Phase 2 | (Lauer et al., 2017) (Kreijtz et al., 2014) | ||
| TB | Pre-clinical, Phase 1 | (Choi & Chang, 2013) (Minhinnick et al, 2016) (Wilkie et al., 2020) | ||
| Parasitic | Malaria | Pre-clinical, Phase1, Phase 2 | (Choi & Chang, 2013) (de Barra et al,. 2014) |
Acknowledgements
Figure 1 was created with software from BioRender. The authors have no conflicts of interest to declare.
Funding Information
This work was performed with support from the National Institutes of Health (R01EB027797), the Department of Defense (Defense Threat Reduction Agency, HDTRA11810036 and HDTRA11810035), the Department of Education (GAANN Fellowship for N.B.), and the National Science Foundation (Graduate Research Fellowship to A.R.).
Abbreviations
- AAV
adeno-associated virus
- Ab
antibody
- Ad
adenovirus
- AIDS
acquired immunodeficiency syndrome
- AIMV
alfalfa mosaic virus
- APC
antigen presenting cell
- CD28
cluster of differentiation 28, co-stimulatory molecule expressed on T cells
- CD80
cluster of differentiation 80, co-stimulatory molecule expressed on APCs
- CMV
cucumber mosaic virus
- COVID-19
coronavirus disease of 2019
- CPMV
cowpea mosaic virus
- CSP
circumsporozoite protein
- CT
cholera toxin
- CTL
cytotoxic T lymphocyte, CD8+ T cell
- DC
dendritic cell
- DNA
deoxyribonucleic acid
- dsDNA
double-stranded DNA
- ELISA
enzyme-linked immunosorbent assay
- FCA
Freund’s complete adjuvant
- FIA
Freund’s incomplete adjuvant
- FDA
U.S. Food and Drug Administration
- FMDV
foot and mouth disease virus
- HA
hemagglutinin
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HFMV
Hand, Foot, and Mouth Disease
- HIV-1
human immunodeficiency virus 1
- HPV
human papillomavirus
- IL
interleukin
- IM
intramuscular injection
- IN
intranasal administration
- IP
intraperitoneal injection
- ISCOM
immune stimulating complex
- IV
intravenous injection
- LCMV
lymphocytic choriomeningitis virus
- λ
Escherichia virus lambda
- MERS
Middle East Respiratory Syndrome
- MHC
major histocompatibility complex
- MPLA
monophosphoryl lipid A
- MS2
Escherichia virus MS2
- MVA
Modified vaccinia virus Ankara
- MΦ
macrophage
- NP
nanoparticle
- OVA
ovalbumin
- P22
salmonella virus P22
- PapMV
papaya mosaic virus
- PVX
potato virus X
- Qβ
Escherichia virus Q-beta
- RNA
ribonucleic acid
- RSV
respiratory syncytial virus
- RTS,S
RTS,S/AS01 malaria vaccine (trade name Mosquirix)
- SARS-CoV2
severe respiratory syndrome coronavirus 2
- SC
subcutaneous injection
- sHSP
small heat shock protein
- SIN
Sindbis virus
- ssRNA
single-stranded RNA
- T4
Escherichia virus T4
- TB
tuberculosis
- TCI
transcutaneous immunization
- TCR
T cell receptor
- TFH
T follicular helper cell, CD4+ T cell
- Th1
T helper 1 cell, CD4+ T cell
- Th17
T helper 17 cell, CD4+ T cell
- TMV
tobacco mosaic virus
- TNF-α
tumor necrosis factor alpha
- VEE
Venezuelan equine encephalitis virus
- VLP
virus-like particle
- VSV
vesicular stomatitis virus
- WHO
World Health Organization
References
- Agmon-Levin N, Paz Z, Israeli E, & Shoenfeld Y (2009). Vaccines and autoimmunity. Nat Rev Rheumatol, 5(11), 648–652. doi: 10.1038/nrrheum.2009.196 [DOI] [PubMed] [Google Scholar]
- Abisambra JF, Jinwal UK, Jones JR, Blair LJ, Koren J 3rd, & Dickey CA (2011). Exploiting the diversity of the heat-shock protein family for primary and secondary tauopathy therapeutics. Curr Neuropharmacol, 9(4), 623–631. doi: 10.2174/157015911798376226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander LN, Seward JF, Santibanez TA, Pallansch MA, Kew OM, Prevots DR, … Sutter RW (2004). Vaccine policy changes and epidemiology of poliomyelitis in the United States. JAMA, 292(14), 1696–1701. doi: 10.1001/jama.292.14.1696 [DOI] [PubMed] [Google Scholar]
- Al-Halifa S, Gauthier L, Arpin D, Bourgault S, & Archambault D (2019). Nanoparticle-Based Vaccines Against Respiratory Viruses. Front Immunol, 10, 22. doi: 10.3389/fimmu.2019.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaboldi PM, Sambir M, D'Arco C, Peters LA, Seegers JF, Mayer L, … Dattwyler RJ (2016). Intranasal delivery of a protein subunit vaccine using a Tobacco Mosaic Virus platform protects against pneumonic plague. Vaccine, 34(47), 5768–5776. doi: 10.1016/j.vaccine.2016.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante KP, Ansong D, Kaali S, Adjei S, Lievens M, Nana Badu L, … Ofori-Anyinam O (2020). Immunogenicity and safety of the RTS,S/AS01 malaria vaccine co-administered with measles, rubella and yellow fever vaccines in Ghanaian children: A phase IIIb, multi-center, non-inferiority, randomized, open, controlled trial. Vaccine, 38(18), 3411–3421. doi: 10.1016/j.vaccine.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babin C, Majeau N, & Leclerc D (2013). Engineering of papaya mosaic virus (PapMV) nanoparticles with a CTL epitope derived from influenza NP. J Nanobiotechnology, 11, 10. doi: 10.1186/1477-3155-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, & Jennings GT (2010). Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol, 10(11), 787–796. doi: 10.1038/nri2868 [DOI] [PubMed] [Google Scholar]
- Banik S, Mansour AA, Suresh RV, Wykoff-Clary S, Malik M, McCormick AA, & Bakshi CS (2015). Development of a Multivalent Subunit Vaccine against Tularemia Using Tobacco Mosaic Virus (TMV) Based Delivery System. PLOS ONE, 10(6), e0130858. doi: 10.1371/journal.pone.0130858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartual SG, Otero JM, Garcia-Doval C, Llamas-Saiz AL, Kahn R, Fox GC, & van Raaij MJ (2010). Structure of the bacteriophage T4 long tail fiber receptor-binding tip. Proc Natl Acad Sci U S A, 107(47), 20287–20292. doi: 10.1073/pnas.1011218107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BioRender. COVID-19 Vaccine & Therapeutics Tracker (2020). Retrieved from https://biorender.com/covid-vaccine-tracker
- Bolduc M, Baz M, Laliberte-Gagne ME, Carignan D, Garneau C, Russel A, … Leclerc D (2018). The quest for a nanoparticle-based vaccine inducing broad protection to influenza viruses. Nanomedicine, 14(8), 2563–2574. doi: 10.1016/j.nano.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Brennan FR, Bellaby T, Helliwell SM, Jones TD, Kamstrup S, Dalsgaard K, … Hamilton WD (1999a). Chimeric plant virus particles administered nasally or orally induce systemic and mucosal immune responses in mice. J Virol, 73(2), 930–938. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9882293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FR, Gilleland LB, Staczek J, Bendig MM, Hamilton WDO, & Gilleland HE (1999b). A chimaeric plant virus vaccine protects mice against a bacterial infection. Microbiology, 145 (Pt 8), 2061–2067. doi: 10.1099/13500872-145-8-2061 [DOI] [PubMed] [Google Scholar]
- Brennan FR, Jones TD, Longstaff M, Chapman S, Bellaby T, Smith H, … Flock JI (1999c). Immunogenicity of peptides derived from a fibronectin-binding protein of S. aureus expressed on two different plant viruses. Vaccine, 17(15-16), 1846–1857. doi: 10.1016/s0264-410x(98)00485-x [DOI] [PubMed] [Google Scholar]
- Bull JJ, Nuismer SL, & Antia R (2019). Recombinant vector vaccine evolution. PLoS Comput Biol, 15(7), e1006857. doi: 10.1371/journal.pcbi.1006857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PJ (1984). The current picture of the structure and assembly of tobacco mosaic virus. J Gen Virol, 65 ( Pt 2), 253–279. doi: 10.1099/0022-1317-65-2-253 [DOI] [PubMed] [Google Scholar]
- Cabral-Miranda G, Heath MD, Mohsen MO, Gomes AC, Engeroff P, Flaxman A, … Bachmann MF (2017). Virus-Like Particle (VLP) Plus Microcrystalline Tyrosine (MCT) Adjuvants Enhance Vaccine Efficacy Improving T and B Cell Immunogenicity and Protection against Plasmodium berghei/vivax. Vaccines (Basel), 5(2). doi: 10.3390/vaccines5020010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caivano A, Doria-Rose NA, Buelow B, Sartorius R, Trovato M, D'Apice L, … De Berardinis P (2010). HIV-1 Gag p17 presented as virus-like particles on the E2 scaffold from Geobacillus stearothermophilus induces sustained humoral and cellular immune responses in the absence of IFN-gamma production by CD4+ T cells. Virology, 407(2), 296–305. doi: 10.1016/j.virol.2010.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E (2020). Coronavirus vaccine trials have delivered their first results - but their promise is still unclear. Nature, 581(7809), 363–364. doi: 10.1038/d41586-020-01092-3 [DOI] [PubMed] [Google Scholar]
- Carignan D, Therien A, Rioux G, Paquet G, Gagne ML, Bolduc M, … Leclerc D (2015). Engineering of the PapMV vaccine platform with a shortened M2e peptide leads to an effective one dose influenza vaccine. Vaccine, 33(51), 7245–7253. doi: 10.1016/j.vaccine.2015.10.123 [DOI] [PubMed] [Google Scholar]
- Carter DC, Wright B, Jerome WG, Rose JP, & Wilson E (2020). A Unique Protein Self-Assembling Nanoparticle with Significant Advantages in Vaccine Development and Production. Journal of Nanomaterials, 2020, 1–10. doi: 10.1155/2020/4297937 [DOI] [Google Scholar]
- Cerpa-Cruz S, Paredes-Casillas P, Landeros Navarro E, Bernard-Medina AG, Martinez-Bonilla G, & Gutierrez-Urena S (2013). Adverse events following immunization with vaccines containing adjuvants. Immunol Res, 56(2-3), 299–303. doi: 10.1007/s12026-013-8400-4 [DOI] [PubMed] [Google Scholar]
- Chen F, Jiang R, Wang Y, Zhu M, Zhang X, Dong S, … Wang L (2017). Recombinant Phage Elicits Protective Immune Response against Systemic S. globosa Infection in Mouse Model. Sci Rep, 7, 42024. doi: 10.1038/srep42024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichester JA, Green BJ, Jones RM, Shoji Y, Miura K, Long CA, … Yusibov V (2018). Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A Phase 1 dose-escalation study in healthy adults. Vaccine, 36(39), 5865–5871. doi: 10.1016/j.vaccine.2018.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, & Chang J (2013). Viral vectors for vaccine applications. Clin Exp Vaccine Res, 2(2), 97–105. doi: 10.7774/cevr.2013.2.2.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JR, Bartley K, Jepson CD, Craik V, & March JB (2011). Comparison of a bacteriophage-delivered DNA vaccine and a commercially available recombinant protein vaccine against hepatitis B. FEMS Immunol Med Microbiol, 61(2), 197–204. doi: 10.1111/j.1574-695X.2010.00763.x [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov. A study of safety, tolerability and immunogenicity of HPV-L2 vaccine in healthy adult male and female subjects (2020a). Retrieved from https://clinicaltrials.gov/ct2/show/study/NCT03929172
- ClinicalTrials.gov. Dose, Safety, Tolerability and Immunogenicity of an Influenza H1 Stabilized Stem Ferritin Vaccine, VRCFLUNPF099-00-VP, in Healthy Adults (2020b). Retrieved from https://www.clinicaltrials.gov/ct2/show/NCT03814720?term=ferritin&cond=influenza&draw=2&rank=2
- ClinicalTrials.gov. Influenza HA Ferritin Vaccine, Alone or in Prime-Boost Regimens With an Influenza DNA Vaccine in Healthy Adults (2020c). Retrieved from https://www.clinicaltrials.gov/ct2/show/NCT03186781?term=ferritin
- Cohen J (2019). Controversy over dengue vaccine risk. Science, 365(6457), 961–962. doi: 10.1126/science.365.6457.961 [DOI] [PubMed] [Google Scholar]
- Conover R (1962). Virus disease of papaya in Florida (abstract only). Phytopathology, 52(1), 6. [Google Scholar]
- Dalmau M, Lim S, Chen HC, Ruiz C, & Wang SW (2008). Thermostability and molecular encapsulation within an engineered caged protein scaffold. Biotechnol Bioeng, 101(4), 654–664. doi: 10.1002/bit.21988 [DOI] [PubMed] [Google Scholar]
- Dalmau M, Lim S, & Wang SW (2009a). Design of a pH-dependent molecular switch in a caged protein platform. Nano Lett, 9(1), 160–166. doi: 10.1021/nl8027069 [DOI] [PubMed] [Google Scholar]
- Dalmau M, Lim S, & Wang SW (2009b). pH-triggered disassembly in a caged protein complex. Biomacromolecules, 10(12), 3199–3206. doi: 10.1021/bm900674v [DOI] [PubMed] [Google Scholar]
- Delany I, Rappuoli R, & De Gregorio E (2014). Vaccines for the 21st century. EMBO Mol Med, 6(6), 708–720. doi: 10.1002/emmm.201403876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barra E, Hodgson SH, Ewer KJ, Bliss CM, Hennigan K, Collins A, … Hill AV (2014). A phase Ia study to assess the safety and immunogenicity of new malaria vaccine candidates ChAd63 CS administered alone and with MVA CS. PLOS ONE, 9(12), e115161. doi: 10.1371/journal.pone.0115161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog G, van Binnendijk R, Buisman AM, Berbers GAM, & van der Klis FRM (2020). Immune surveillance for vaccine-preventable diseases. Expert Rev Vaccines, 19(4), 327–339. doi: 10.1080/14760584.2020.1745071 [DOI] [PubMed] [Google Scholar]
- Demminger DE, Walz L, Dietert K, Hoffmann H, Planz O, Gruber AD, … Wolff T (2020). Adeno-associated virus-vectored influenza vaccine elicits neutralizing and Fcgamma receptor-activating antibodies. EMBO Mol Med, 12(5), e10938. doi: 10.15252/emmm.201910938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis J, Acosta-Ramirez E, Zhao Y, Hamelin ME, Koukavica I, Baz M, … Leclerc D (2008). Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform. Vaccine, 26(27-28), 3395–3403. doi: 10.1016/j.vaccine.2008.04.052 [DOI] [PubMed] [Google Scholar]
- Denis J, Majeau N, Acosta-Ramirez E, Savard C, Bedard MC, Simard S, … Leclerc D (2007). Immunogenicity of papaya mosaic virus-like particles fused to a hepatitis C virus epitope: evidence for the critical function of multimerization. Virology, 363(1), 59–68. doi: 10.1016/j.virol.2007.01.011 [DOI] [PubMed] [Google Scholar]
- Desai S, Diener T, Tan BK, Lowry N, Talukdar C, Chrusch W, & Wiebe S (2014). An unusual case of vaccine-associated paralytic poliomyelitis. Can J Infect Dis Med Microbiol, 25(4), 227–228. doi: 10.1155/2014/378320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobano C, Sanz H, Sorgho H, Dosoo D, Mpina M, Ubillos I, … Daubenberger C (2019a). Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat Commun, 10(1), 2174. doi: 10.1038/s41467-019-10195-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobano C, Ubillos I, Jairoce C, Gyan B, Vidal M, Jimenez A, … Moncunill G (2019b). RTS,S/AS01E immunization increases antibody responses to vaccine-unrelated Plasmodium falciparum antigens associated with protection against clinical malaria in African children: a case-control study. BMC Med, 17(1), 157. doi: 10.1186/s12916-019-1378-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo GJ, Orru S, & Perham RN (2001). Multiple display of peptides and proteins on a macromolecular scaffold derived from a multienzyme complex. J Mol Biol, 305(2), 259–267. doi: 10.1006/jmbi.2000.4311 [DOI] [PubMed] [Google Scholar]
- Dong YM, Zhang GG, Huang XJ, Chen L, & Chen HT (2015). Promising MS2 mediated virus-like particle vaccine against foot-and-mouth disease. Antiviral Res, 117, 39–43. doi: 10.1016/j.antiviral.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Doolan DL, Dobano C, & Baird JK (2009). Acquired immunity to malaria. Clin Microbiol Rev, 22(1), 13–36, Table of Contents. doi: 10.1128/CMR.00025-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrani Z, McInerney TL, McLain L, Jones T, Bellaby T, Brennan FR, & Dimmock NJ (1998). Intranasal immunization with a plant virus expressing a peptide from HIV-1 gp41 stimulates better mucosal and systemic HIV-1-specific IgA and IgG than oral immunization. J Immunol Methods, 220(1-2), 93–103. doi: 10.1016/s0022-1759(98)00145-8 [DOI] [PubMed] [Google Scholar]
- FDA. ERVEBO (2020a). Retrieved from https://www.fda.gov/vaccines-blood-biologics/ervebo
- FDA. Vaccines Licensed for Use in the United States (April. 24, 2020b). Retrieved from https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- Fedson DS (2016). Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and Ebola. Ann Transl Med, 4(21), 421. doi: 10.21037/atm.2016.11.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg PC, Allison HE, Saunders JR, & McCarthy AJ (2010). Bacteriophage lambda: a paradigm revisited. J Virol, 84(13), 6876–6879. doi: 10.1128/JVI.02177-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster S (2007). Rotavirus vaccine and intussusception. J Pediatr Pharmacol Ther, 12(1), 4–7. doi: 10.5863/1551-6776-12.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuenmayor J, Godia F, & Cervera L (2017). Production of virus-like particles for vaccines. N Biotechnol, 39(Pt B), 174–180. doi: 10.1016/j.nbt.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev IS, Joyce MG, Chen RE, Leung K, McKee K, Druz A, … Kwong PD (2018). Two-Component Ferritin Nanoparticles for Multimerization of Diverse Trimeric Antigens. ACS Infect Dis, 4(5), 788–796. doi: 10.1021/acsinfecdis.7b00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Neto JF, Sartorius R, Canto FB, Almeida TS, Dias AA, Barbosa CD, … Bellio M (2018). Vaccination With Recombinant Filamentous fd Phages Against Parasite Infection Requires TLR9 Expression. Front Immunol, 9, 1173. doi: 10.3389/fimmu.2018.01173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS (2020). Rapid COVID-19 vaccine development. Science, 368(6494), 945–946. doi: 10.1126/science.abb8923 [DOI] [PubMed] [Google Scholar]
- Gupta RK, Rost BE, Relyveld E, & Siber GR (1995). Adjuvant properties of aluminum and calcium compounds. Pharm Biotechnol, 6, 229–248. doi: 10.1007/978-1-4615-1823-5_8 [DOI] [PubMed] [Google Scholar]
- Hanafi LA, Bolduc M, Gagne ME, Dufour F, Langelier Y, Boulassel MR, … Lapointe R (2010). Two distinct chimeric potexviruses share antigenic cross-presentation properties of MHC class I epitopes. Vaccine, 28(34), 5617–5626. doi: 10.1016/j.vaccine.2010.06.024 [DOI] [PubMed] [Google Scholar]
- He L, de Val N, Morris CD, Vora N, Thinnes TC, Kong L, … Zhu J (2016). Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat Commun, 7, 12041. doi: 10.1038/ncomms12041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry K, Arabi-Ghahroudi M, & Scott J (2015). Beyond phage display: non-traditional applications of the filamentous bacteriophage as a vaccine carrier, therapeutic biologic, and bioconjugation scaffold. Front Microbiol, 6(755), 1–18. doi: 10.3389/fmicb.2015.00755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess KL, & Jewell CM (2020). Phage display as a tool for vaccine and immunotherapy development. Bioeng Transl Med, 5(1), e10142. doi: 10.1002/btm2.10142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JK, Jeevan-Raj B, & Netter HJ (2020). Hepatitis B Virus (HBV) Subviral Particles as Protective Vaccines and Vaccine Platforms. Viruses, 12(2). doi: 10.3390/v12020126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JP, Krebs SJ, Trovato M, Kovarik DN, Brower Z, Sutton WF, … Haigwood NL (2012). Co-immunization with multimeric scaffolds and DNA rapidly induces potent autologous HIV-1 neutralizing antibodies and CD8+ T cells. PLoS One, 7(2), e31464. doi: 10.1371/journal.pone.0031464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegerlehner A, Zabel F, Langer A, Dietmeier K, Jennings GT, Saudan P, & Bachmann MF (2013). Bacterially produced recombinant influenza vaccines based on virus-like particles. PLoS One, 8(11), e78947. doi: 10.1371/journal.pone.0078947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Li Q, Li M, Zhou Z, Wu L, Fan J, … Xu Z (2006). A modified TMV-based vector facilitates the expression of longer foreign epitopes in tobacco. Vaccine, 24(2), 109–115. doi: 10.1016/j.vaccine.2005.09.060 [DOI] [PubMed] [Google Scholar]
- Jin Y, Li P, & Wang F (2018). beta-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine, 36(35), 5235–5244. doi: 10.1016/j.vaccine.2018.07.038 [DOI] [PubMed] [Google Scholar]
- Joelson T, Akerblom L, Oxelfelt P, Strandberg B, Tomenius K, & Morris TJ (1997). Presentation of a foreign peptide on the surface of tomato bushy stunt virus. J Gen Virol, 78 ( Pt 6), 1213–1217. doi: 10.1099/0022-1317-78-6-1213 [DOI] [PubMed] [Google Scholar]
- Jones RM, Chichester JA, Mett V, Jaje J, Tottey S, Manceva S, … Yusibov V (2013). A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS One, 8(11), e79538. doi: 10.1371/journal.pone.0079538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallerup R, and Foged C (2015). Classification of Vaccines. In: Foged C, Rades T, Perrie Y, Hook S (eds) Subunit Vaccine Delivery. Advances in Delivery Science and Technology. Springer, New York, NY. [Google Scholar]
- Kanekiyo M, Bu W, Joyce MG, Meng G, Whittle JR, Baxa U, … Nabel GJ (2015). Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell, 162(5), 1090–1100. doi: 10.1016/j.cell.2015.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M, Wei CJ, Yassine HM, McTamney PM, Boyington JC, Whittle JR, … Nabel GJ (2013). Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature, 499(7456), 102–106. doi: 10.1038/nature12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F, Porter M, Schwenk R, DeBot M, Saudan P, & Dutta S (2015). Head-to-Head Comparison of Soluble vs. Qbeta VLP Circumsporozoite Protein Vaccines Reveals Selective Enhancement of NANP Repeat Responses. PLoS One, 10(11), e0142035. doi: 10.1371/journal.pone.0142035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Son A, Kim J, Kwon SB, Kim MH, Kim P, … Seong BL (2018). Chaperna-Mediated Assembly of Ferritin-Based Middle East Respiratory Syndrome-Coronavirus Nanoparticles. Front Immunol, 9, 1093. doi: 10.3389/fimmu.2018.01093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight-Jones TJ, & Rushton J (2013). The economic impacts of foot and mouth disease - what are they, how big are they and where do they occur? Prev Vet Med, 112(3-4), 161–173. doi: 10.1016/j.prevetmed.2013.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch J, Harder T, von Kries R, & Wichmann O (2017). Risk of Intussusception After Rotavirus Vaccination. Dtsch Arztebl Int, 114(15), 255–262. doi: 10.3238/arztebl.2017.0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs SJ, McBurney SP, Kovarik DN, Waddell CD, Jaworski JP, Sutton WF, … Haigwood NL (2014). Multimeric scaffolds displaying the HIV-1 envelope MPER induce MPER-specific antibodies and cross-neutralizing antibodies when co-immunized with gp160 DNA. PLoS One, 9(12), e113463. doi: 10.1371/journal.pone.0113463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreijtz JH, Goeijenbier M, Moesker FM, van den Dries L, Goeijenbier S, De Gruyter HL, … Osterhaus AD (2014). Safety and immunogenicity of a modified-vaccinia-virus-Ankara-based influenza A H5N1 vaccine: a randomised, double-blind phase 1/2a clinical trial. Lancet Infect Dis, 14(12), 1196–1207. doi: 10.1016/S1473-3099(14)70963-6 [DOI] [PubMed] [Google Scholar]
- Lacasse P, Denis J, Lapointe R, Leclerc D, & Lamarre A (2008). Novel plant virus-based vaccine induces protective cytotoxic T-lymphocyte-mediated antiviral immunity through dendritic cell maturation. J Virol, 82(2), 785–794. doi: 10.1128/JVI.01811-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laher F, Moodie Z, Cohen KW, Grunenberg N, Bekker LG, Allen M, … Tomaras GD (2020). Safety and immune responses after a 12-month booster in healthy HIV-uninfected adults in HVTN 100 in South Africa: A randomized double-blind placebo-controlled trial of ALVAC-HIV (vCP2438) and bivalent subtype C gp120/MF59 vaccines. PLoS Med, 17(2), e1003038. doi: 10.1371/journal.pmed.1003038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliberte-Gagne ME, Bolduc M, Therien A, Garneau C, Casault P, Savard P, … Leclerc D (2019). Increased Immunogenicity of Full-Length Protein Antigens through Sortase-Mediated Coupling on the PapMV Vaccine Platform. Vaccines (Basel), 7(2). doi: 10.3390/vaccines7020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer KB, Borrow R, & Blanchard TJ (2017). Multivalent and Multipathogen Viral Vector Vaccines. Clin Vaccine Immunol, 24(1). doi: 10.1128/CVI.00298-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel ME, Chartrand K, Leclerc D, & Lamarre A (2015). Plant Viruses as Nanoparticle-Based Vaccines and Adjuvants. Vaccines (Basel), 3(3), 620–637. doi: 10.3390/vaccines3030620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc D, Beauseigle D, Denis J, Morin H, Pare C, Lamarre A, & Lapointe R (2007). Proteasome-independent major histocompatibility complex class I cross-presentation mediated by papaya mosaic virus-like particles leads to expansion of specific human T cells. J Virol, 81(3), 1319–1326. doi: 10.1128/JVI.01720-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, … He TC (2017). Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis, 4(2), 43–63. doi: 10.1016/j.gendis.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, & Nguyen MT (2015). Recent advances of vaccine adjuvants for infectious diseases. Immune Netw, 15(2), 51–57. doi: 10.4110/in.2015.15.2.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y, Lacabaratz C, Ellefsen-Lavoie K, Stohr W, Lelievre JD, Bart PA, … Pantaleo G (2020). Optimal priming of poxvirus vector (NYVAC)-based HIV vaccine regimens for T cell responses requires three DNA injections. Results of the randomized multicentre EV03/ANRS VAC20 Phase I/II Trial. PLoS Pathog, 16(6), e1008522. doi: 10.1371/journal.ppat.1008522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Cui K, Wang H, Liu F, Huang K, Duan Z, … Liu Q (2019). A milk-based self-assemble rotavirus VP6-ferritin nanoparticle vaccine elicited protection against the viral infection. J Nanobiotechnology, 17(1), 13. doi: 10.1186/s12951-019-0446-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lico C, Mancini C, Italiani P, Betti C, Boraschi D, Benvenuto E, & Baschieri S (2009). Plant-produced potato virus X chimeric particles displaying an influenza virus-derived peptide activate specific CD8+ T cells in mice. Vaccine, 27(37), 5069–5076. doi: 10.1016/j.vaccine.2009.06.045 [DOI] [PubMed] [Google Scholar]
- Liebowitz D, Gottlieb K, Kolhatkar NS, Garg SJ, Asher JM, Nazareno J, … Tucker SN (2020). Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect Dis, 20(4), 435–444. doi: 10.1016/S1473-3099(19)30584-5 [DOI] [PubMed] [Google Scholar]
- Lindahl JF, & Grace D (2015). The consequences of human actions on risks for infectious diseases: a review. Infect Ecol Epidemiol, 5, 30048. doi: 10.3402/iee.v5.30048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, & Fiering S (2016). In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol, 11(3), 295–303. doi: 10.1038/nnano.2015.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look M, Bandyopadhyay A, Blum JS, & Fahmy TM (2010). Application of nanotechnologies for improved immune response against infectious diseases in the developing world. Adv Drug Deliv Rev, 62(4-5), 378–393. doi: 10.1016/j.addr.2009.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low JG, Lee LS, Ooi EE, Ethirajulu K, Yeo P, Matter A, … Novotny-Diermayr V (2014). Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine: results from a double-blinded, randomized Phase I clinical trial in healthy Asian volunteers. Vaccine, 32(39), 5041–5048. doi: 10.1016/j.vaccine.2014.07.011 [DOI] [PubMed] [Google Scholar]
- Lurie N, Saville M, Hatchett R, & Halton J (2020). Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med, 382(21), 1969–1973. doi: 10.1056/NEJMp2005630 [DOI] [PubMed] [Google Scholar]
- Mailand MT, & Frederiksen JL (2017). Vaccines and multiple sclerosis: a systematic review. J Neurol, 264(6), 1035–1050. doi: 10.1007/s00415-016-8263-4 [DOI] [PubMed] [Google Scholar]
- Mallajosyula JK, Hiatt E, Hume S, Johnson A, Jeevan T, Chikwamba R, … McCormick AA (2014). Single-dose monomeric HA subunit vaccine generates full protection from influenza challenge. Hum Vaccin Immunother, 10(3), 586–595. doi: 10.4161/hv.27567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini I, & Montomoli E (2018). Epidemiology and prevention of Human Papillomavirus. Ann Ig, 30(4 Supple 1), 28–32. doi: 10.7416/ai.2018.2231 [DOI] [PubMed] [Google Scholar]
- Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, & Bachmann MF (2008). Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol, 38(5), 1404–1413. doi: 10.1002/eji.200737984 [DOI] [PubMed] [Google Scholar]
- Mansour AA, Banik S, Suresh RV, Kaur H, Malik M, McCormick AA, & Bakshi CS (2018). An Improved Tobacco Mosaic Virus (TMV)-Conjugated Multiantigen Subunit Vaccine Against Respiratory Tularemia. Front Microbiol, 9, 1195. doi: 10.3389/fmicb.2018.01195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JB, Clark JR, & Jepson CD (2004). Genetic immunisation against hepatitis B using whole bacteriophage lambda particles. Vaccine, 22(13-14), 1666–1671. doi: 10.1016/j.vaccine.2003.10.047 [DOI] [PubMed] [Google Scholar]
- Marusic C, Rizza P, Lattanzi L, Mancini C, Spada M, Belardelli F, … Capone I (2001). Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J Virol, 75(18), 8434–8439. doi: 10.1128/jvi.75.18.8434-8439.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi A, Ebihara H, Callison J, Groseth A, Williams KJ, Geisbert TW, & Feldmann H (2011). Vesicular stomatitis virus-based Ebola vaccines with improved cross-protective efficacy. J Infect Dis, 204 Suppl 3, S1066–1074. doi: 10.1093/infdis/jir348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews Q, Gu L, Krendelchtchikov A, & C Z (2013). Viral Vectors for Vaccine Development. Novel Gene Therapy Approaches. Retrieved from https://www.intechopen.com/books/novel-gene-therapy-approaches/viral-vectors-for-vaccine-development
- Mbaeyi C, Wadood ZM, Moran T, Mjourn, Ather F, Stehling-Ariza T, … Sharaf M (2018). Strategic Response to an Outbreak of Circulating Vaccine-Derived Poliovirus Type 2 - Syria, 2017-2018. MMWR Morb Mortal Wkly Rep, 67(24), 690–694. doi: 10.15585/mmwr.mm6724a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbow ML, De Gregorio E, Valiante NM, & Rappuoli R (2010). New adjuvants for human vaccines. Curr Opin Immunol, 22(3), 411–416. doi: 10.1016/j.coi.2010.04.004 [DOI] [PubMed] [Google Scholar]
- McBurney SP, Sunshine JE, Gabriel S, Huynh JP, Sutton WF, Fuller DH, … Messer WB (2016). Evaluation of protection induced by a dengue virus serotype 2 envelope domain III protein scaffold/DNA vaccine in non-human primates. Vaccine, 34(30), 3500–3507. doi: 10.1016/j.vaccine.2016.03.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb RC, Ho CL, Bradley KA, Grill LK, & Martchenko M (2015). Presentation of peptides from Bacillus anthracis protective antigen on Tobacco Mosaic Virus as an epitope targeted anthrax vaccine. Vaccine, 33(48), 6745–6751. doi: 10.1016/j.vaccine.2015.10.075 [DOI] [PubMed] [Google Scholar]
- McCormick AA, Shakeel A, Yi C, Kaur H, Mansour AM, & Bakshi CS (2018). Intranasal administration of a two-dose adjuvanted multi-antigen TMV-subunit conjugate vaccine fully protects mice against Francisella tularensis LVS challenge. PLOS ONE, 13(4), e0194614. doi: 10.1371/journal.pone.0194614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney TL, Brennan FR, Jones TD, & Dimmock NJ (1999). Analysis of the ability of five adjuvants to enhance immune responses to a chimeric plant virus displaying an HIV-1 peptide. Vaccine, 17(11-12), 1359–1368. doi: 10.1016/s0264-410x(98)00388-0 [DOI] [PubMed] [Google Scholar]
- Milne JL, Wu X, Borgnia MJ, Lengyel JS, Brooks BR, Shi D, … Subramaniam S (2006). Molecular structure of a 9-MDa icosahedral pyruvate dehydrogenase subcomplex containing the E2 and E3 enzymes using cryoelectron microscopy. J Biol Chem, 281(7), 4364–4370. doi: 10.1074/jbc.M504363200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhinnick A, Satti I, Harris S, Wilkie M, Sheehan S, Stockdale L, … McShane H (2016). A first-in-human phase 1 trial to evaluate the safety and immunogenicity of the candidate tuberculosis vaccine MVA85A-IMX313, administered to BCG-vaccinated adults. Vaccine, 34(11), 1412–1421. doi: 10.1016/j.vaccine.2016.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molino NM, Neek M, Tucker JA, Nelson EL, & Wang SW (2016). Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials, 86, 83–91. doi: 10.1016/j.biomaterials.2016.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molino NM, & Wang SW (2014). Caged protein nanoparticles for drug delivery. Curr Opin Biotechnol, 28, 75–82. doi: 10.1016/j.copbio.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncayo A. a. S., AC. (2017). American Trypanosomiasis Chagas Disease (Second Edition), Chapter 4: Current epidemiological trends of Chagas disease in Latin America and future challenges: epidemiology, surveillance, and health policies. [DOI] [PubMed]
- Moss B, Smith GL, Gerin JL, & Purcell RH (1984). Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature, 311(5981), 67–69. doi: 10.1038/311067a0 [DOI] [PubMed] [Google Scholar]
- Moura APV, Santos LCB, Brito CRN, Valencia E, Junqueira C, Filho AAP, … Marques AF (2017). Virus-like Particle Display of the alpha-Gal Carbohydrate for Vaccination against Leishmania Infection. ACS Cent Sci, 3(9), 1026–1031. doi: 10.1021/acscentsci.7b00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AA, Wang C, Fiering S, & Steinmetz NF (2018). In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma. Mol Pharm, 15(9), 3700–3716. doi: 10.1021/acs.molpharmaceut.8b00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nami S, Mohammadi R, Vakili M, Khezripour K, Mirzaei H, & Morovati H (2019). Fungal vaccines, mechanism of actions and immunology: A comprehensive review. Biomed Pharmacother, 109, 333–344. doi: 10.1016/j.biopha.2018.10.075 [DOI] [PubMed] [Google Scholar]
- Naso MF, Tomkowicz B, Perry WL 3rd, & Strohl WR (2017). Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs, 31(4), 317–334. doi: 10.1007/s40259-017-0234-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neek M, Kim TI, & Wang SW (2019). Protein-based nanoparticles in cancer vaccine development. Nanomedicine, 15(1), 164–174. doi: 10.1016/j.nano.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neek M, Tucker JA, Kim TI, Molino NM, Nelson EL, & Wang SW (2018). Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials, 156, 194–203. doi: 10.1016/j.biomaterials.2017.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto K, & Salvetti A (2014). AAV Vectors Vaccines Against Infectious Diseases. Front Immunol, 5, 5. doi: 10.3389/fimmu.2014.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzaci M, Piazzolla G, Vitti A, Lapelosa M, Tortorella C, Stella I, … Piazzolla P (2007). Cucumber mosaic virus as a presentation system for a double hepatitis C virus-derived epitope. Arch Virol, 152(5), 915–928. doi: 10.1007/s00705-006-0916-7 [DOI] [PubMed] [Google Scholar]
- Ollmann Saphire E (2020). A Vaccine against Ebola Virus. Cell, 181(1), 6. doi: 10.1016/j.cell.2020.03.011 [DOI] [PubMed] [Google Scholar]
- Omer SB, Salmon DA, Orenstein WA, deHart MP, & Halsey N (2009). Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med, 360(19), 1981–1988. doi: 10.1056/NEJMsa0806477 [DOI] [PubMed] [Google Scholar]
- Pati R, Shevtsov M, & Sonawane A (2018). Nanoparticle Vaccines Against Infectious Diseases. Front Immunol, 9, 2224. doi: 10.3389/fimmu.2018.02224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DP, Rynda-Apple A, Harmsen AL, Harmsen AG, & Douglas T (2013). Biomimetic antigenic nanoparticles elicit controlled protective immune response to influenza. ACS Nano, 7(4), 3036–3044. doi: 10.1021/nn4006544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachman KK, Li Q, Matyas GR, Shivachandra SB, Lovchik J, Lyons RC, … Rao M (2012). Anthrax vaccine antigen-adjuvant formulations completely protect New Zealand white rabbits against challenge with Bacillus anthracis Ames strain spores. Clin Vaccine Immunol, 19(1), 11–16. doi: 10.1128/CVI.05376-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples L (2020). News Feature: Avoiding pitfalls in the pursuit of a COVID-19 vaccine. Proc Natl Acad Sci U S A, 117(15), 8218–8221. doi: 10.1073/pnas.2005456117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzolla G, Nuzzaci M, Tortorella C, Panella E, Natilla A, Boscia D, … Antonaci S (2005). Immunogenic properties of a chimeric plant virus expressing a hepatitis C virus (HCV)-derived epitope: new prospects for an HCV vaccine. J Clin Immunol, 25(2), 142–152. doi: 10.1007/s10875-005-2820-4 [DOI] [PubMed] [Google Scholar]
- Pitisuttithum P, Nitayaphan S, Chariyalertsak S, Kaewkungwal J, Dawson P, Dhitavat J, … group, R. V. s. (2020). Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: a double-blind, randomised controlled trial. Lancet HIV, 7(4), e238–e248. doi: 10.1016/S2352-3018(19)30406-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman PR, Hahn M, Lee HS, Koca C, Samy N, Schmidt D, … Chaplin P (2019). Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N Engl J Med, 381(20), 1897–1908. doi: 10.1056/NEJMoa1817307 [DOI] [PubMed] [Google Scholar]
- Pomwised R, Intamaso U, Teintze M, Young M, & Pincus SH (2016). Coupling Peptide Antigens to Virus-Like Particles or to Protein Carriers Influences the Th1/Th2 Polarity of the Resulting Immune Response. Vaccines (Basel), 4(2). doi: 10.3390/vaccines4020015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwar M, Pokorski JK, Singh P, Bhattacharyya S, Arendt H, DeStefano J, … Varadarajan R (2018). Design, display and immunogenicity of HIV1 gp120 fragment immunogens on virus-like particles. Vaccine, 36(42), 6345–6353. doi: 10.1016/j.vaccine.2018.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezanpour B, Haan I, Osterhaus A, & Claassen E (2016). Vector-based genetically modified vaccines: Exploiting Jenner's legacy. Vaccine, 34(50), 6436–6448. doi: 10.1016/j.vaccine.2016.06.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S, Jasny E, Schmidt KE, & Petsch B (2018). New Vaccine Technologies to Combat Outbreak Situations. Front Immunol, 9, 1963. doi: 10.3389/fimmu.2018.01963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Kratz F, & Wang SW (2011). Protein nanocapsules containing doxorubicin as a pH-responsive delivery system. Small, 7(8), 1051–1060. doi: 10.1002/smll.201002242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennermalm A, Li YH, Bohaufs L, Jarstrand C, Brauner A, Brennan FR, & Flock JI (2001). Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine, 19(25-26), 3376–3383. doi: 10.1016/s0264-410x(01)00080-9 [DOI] [PubMed] [Google Scholar]
- Richert LE, Harmsen AL, Rynda-Apple A, Wiley JA, Servid AE, Douglas T, & Harmsen AG (2013). Inducible bronchus-associated lymphoid tissue (iBALT) synergizes with local lymph nodes during antiviral CD4+ T cell responses. Lymphat Res Biol, 11(4), 196–202. doi: 10.1089/lrb.2013.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert LE, Servid AE, Harmsen AL, Rynda-Apple A, Han S, Wiley JA, … Harmsen AG (2012). A virus-like particle vaccine platform elicits heightened and hastened local lung mucosal antibody production after a single dose. Vaccine, 30(24), 3653–3665. doi: 10.1016/j.vaccine.2012.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RTS,S Clinical Trials Partnership (2015). Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet, 386(9988), 31–45. doi: 10.1016/S0140-6736(15)60721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez L, Vidal M, Jairoce C, Aguilar R, Ubillos I, Cuamba I, … Dobano C (2020). Antibody responses to the RTS,S/AS01E vaccine and Plasmodium falciparum antigens after a booster dose within the phase 3 trial in Mozambique. NPJ Vaccines, 5, 46. doi: 10.1038/s41541-020-0192-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Sampedro L, Perdiguero B, Mejias-Perez E, Garcia-Arriaza J, Di Pilato M, & Esteban M (2015). The evolution of poxvirus vaccines. Viruses, 7(4), 1726–1803. doi: 10.3390/v7041726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius R, D'Apice L, Prisco A, & De Berardinis P (2019). Arming Filamentous Bacteriophage, a Nature-Made Nanoparticle, for New Vaccine and Immunotherapeutic Strategies. Pharmaceutics, 11(9). doi: 10.3390/pharmaceutics11090437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz B, Morabito KM, Ruckwardt TJ, Patterson DP, Avera J, Miettinen HM, … Douglas T (2016). Viruslike Particles Encapsidating Respiratory Syncytial Virus M and M2 Proteins Induce Robust T Cell Responses. ACS Biomater Sci Eng, 2(12), 2324–2332. doi: 10.1021/acsbiomaterials.6b00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BH, Wang XY, Dennis CG, Youn R, Repasky EA, Manjili MH, & Subjeck JR (2006). Heat shock proteins as vaccine adjuvants in infections and cancer. Drug Discov Today, 11(11-12), 534–540. doi: 10.1016/j.drudis.2006.04.016 [DOI] [PubMed] [Google Scholar]
- Shah RA, Limmer AL, Nwannunu CE, Patel RR, Mui UN, & Tyring SK (2019). Shingrix for Herpes Zoster: A Review. Skin Therapy Lett, 24(4), 5–7. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31339679 [PubMed] [Google Scholar]
- Sharma J, Shepardson K, Johns LL, Wellham J, Avera J, Schwarz B, … Douglas T (2020). A Self-Adjuvanted, Modular, Antigenic VLP for Rapid Response to Influenza Virus Variability. ACS Appl Mater Interfaces, 12(16), 18211–18224. doi: 10.1021/acsami.9b21776 [DOI] [PubMed] [Google Scholar]
- Shi J, Koteiche HA, McDonald ET, Fox TL, Stewart PL, & McHaourab HS (2013). Cryoelectron microscopy analysis of small heat shock protein 16.5 (Hsp16.5) complexes with T4 lysozyme reveals the structural basis of multimode binding. J Biol Chem, 288(7), 4819–4830. doi: 10.1074/jbc.M112.388132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivachandra SB, Rao M, Janosi L, Sathaliyawala T, Matyas GR, Alving CR, … Rao VB (2006). In vitro binding of anthrax protective antigen on bacteriophage T4 capsid surface through Hoc-capsid interactions: a strategy for efficient display of large full-length proteins. Virology, 345(1), 190–198. doi: 10.1016/j.virol.2005.10.037 [DOI] [PubMed] [Google Scholar]
- Singleton RL, Sanders CA, Jones K, Thorington B, Egbo T, Coats MT, & Waffo AB (2018). Function of the RNA Coliphage Qbeta Proteins in Medical In Vitro Evolution. Methods Protoc, 1(2). doi: 10.3390/mps1020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamel C, Aller SG, & Bopda Waffo A (2014). In vitro evolution and affinity-maturation with Coliphage qbeta display. PLoS One, 9(11), e113069. doi: 10.1371/journal.pone.0113069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinski DA, Hanson BJ, Lin Y, von Messling V, Jegerlehner A, Tee JB, … Connolly JE (2013). Enhanced neutralizing antibody titers and Th1 polarization from a novel Escherichia coli derived pandemic influenza vaccine. PLoS One, 8(10), e76571. doi: 10.1371/journal.pone.0076571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinski DAG, Jones LA, Zhu YO, Xue LW, Au B, Lee B, … Connolly JE (2018). Induction of Human T-cell and Cytokine Responses Following Vaccination with a Novel Influenza Vaccine. Sci Rep, 8(1), 18007. doi: 10.1038/s41598-018-36703-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliepen K, Ozorowski G, Burger JA, van Montfort T, Stunnenberg M, LaBranche C, … Sanders RW (2015). Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology, 12, 82. doi: 10.1186/s12977-015-0210-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Weiser K, Maclehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E, … Cunliffe N (2012). Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev, 11, CD008521. doi: 10.1002/14651858.CD008521.pub3 [DOI] [PubMed] [Google Scholar]
- Speiser DE, Schwarz K, Baumgaertner P, Manolova V, Devevre E, Sterry W, … Bachmann MF (2010). Memory and effector CD8 T-cell responses after nanoparticle vaccination of melanoma patients. J Immunother, 33(8), 848–858. doi: 10.1097/CJI.0b013e3181f1d614 [DOI] [PubMed] [Google Scholar]
- Staczek J, Bendahmane M, Gilleland LB, Beachy RN, & Gilleland HE Jr. (2000). Immunization with a chimeric tobacco mosaic virus containing an epitope of outer membrane protein F of Pseudomonas aeruginosa provides protection against challenge with P. aeruginosa. Vaccine, 18(21), 2266–2274. doi: 10.1016/s0264-410x(99)00571-x [DOI] [PubMed] [Google Scholar]
- Stockley PG, White SJ, Dykeman E, Manfield I, Rolfsson O, Patel N, … Twarock R (2016). Bacteriophage MS2 genomic RNA encodes an assembly instruction manual for its capsid. Bacteriophage, 6(1), e1157666. doi: 10.1080/21597081.2016.1157666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher AJ, Chahine EB, Nelson M, & Sucher BJ (2011). Prevnar 13, the new 13-valent pneumococcal conjugate vaccine. Ann Pharmacother, 45(12), 1516–1524. doi: 10.1345/aph.1Q347 [DOI] [PubMed] [Google Scholar]
- Sun B, Yu S, Zhao D, Guo S, Wang X, & Zhao K (2018). Polysaccharides as vaccine adjuvants. Vaccine, 36(35), 5226–5234. doi: 10.1016/j.vaccine.2018.07.040 [DOI] [PubMed] [Google Scholar]
- Swanson KA, Rainho-Tomko JN, Williams ZP, Lanza L, Peredelchuk M, Kishko M, … Nabel GJ (2020). A respiratory syncytial virus (RSV) F protein nanoparticle vaccine focuses antibody responses to a conserved neutralization domain. Sci Immunol, 5(47). doi: 10.1126/sciimmunol.aba6466 [DOI] [PubMed] [Google Scholar]
- Tao P, Mahalingam M, Zhu J, Moayeri M, Sha J, Lawrence WS, … Rao VB (2018). A Bacteriophage T4 Nanoparticle-Based Dual Vaccine against Anthrax and Plague. mBio, 9(5). doi: 10.1128/mBio.01926-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil EC (2013). Ferritin: the protein nanocage and iron biomineral in health and in disease. Inorg Chem, 52(21), 12223–12233. doi: 10.1021/ic400484n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therien A, Bedard M, Carignan D, Rioux G, Gauthier-Landry L, Laliberte-Gagne ME, … Leclerc D (2017). A versatile papaya mosaic virus (PapMV) vaccine platform based on sortase-mediated antigen coupling. J Nanobiotechnology, 15(1), 54. doi: 10.1186/s12951-017-0289-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohme RA, Awosika-Olumo D, Nielsen C, Khuwaja S, Scott J, Xing J, … Spradling PR (2011). Evaluation of hepatitis B vaccine immunogenicity among older adults during an outbreak response in assisted living facilities. Vaccine, 29(50), 9316–9320. doi: 10.1016/j.vaccine.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumban E, Peabody J, Tyler M, Peabody DS, & Chackerian B (2012). VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS One, 7(11), e49751. doi: 10.1371/journal.pone.0049751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler M, Tumban E, Dziduszko A, Ozbun MA, Peabody DS, & Chackerian B (2014). Immunization with a consensus epitope from human papillomavirus L2 induces antibodies that are broadly neutralizing. Vaccine, 32(34), 4267–4274. doi: 10.1016/j.vaccine.2014.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde-Holzem K, Schlosser V, Viazov S, Fischer R, & Commandeur U (2010). Immunogenic properties of chimeric potato virus X particles displaying the hepatitis C virus hypervariable region I peptide R9. J Virol Methods, 166(1-2), 12–20. doi: 10.1016/j.jviromet.2010.01.017 [DOI] [PubMed] [Google Scholar]
- Ura T, Okuda K, & Shimada M (2014). Developments in Viral Vector-Based Vaccines. Vaccines (Basel), 2(3), 624–641. doi: 10.3390/vaccines2030624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zyl-Smit RN, Esmail A, Bateman ME, Dawson R, Goldin J, van Rikxoort E, … Bateman ED (2017). Safety and Immunogenicity of Adenovirus 35 Tuberculosis Vaccine Candidate in Adults with Active or Previous Tuberculosis. A Randomized Trial. Am J Respir Crit Care Med, 195(9), 1171–1180. doi: 10.1164/rccm.201603-0654OC [DOI] [PubMed] [Google Scholar]
- Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, … Sinai Immunology Review, P. (2020). Immunology of COVID-19: Current State of the Science. Immunity, 52(6), 910–941. doi: 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadala M, Poddighe D, Laurino C, & Palmieri B (2017). Vaccination and autoimmune diseases: is prevention of adverse health effects on the horizon? EPMA J, 8(3), 295–311. doi: 10.1007/s13167-017-0101-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardas E, Kaleebu P, Bekker LG, Hoosen A, Chomba E, Johnson PR, … Schmidt C (2010). A phase 2 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 vaccine based on adeno-associated virus. AIDS Res Hum Retroviruses, 26(8), 933–942. doi: 10.1089/aid.2009.0242 [DOI] [PubMed] [Google Scholar]
- Vartak A, & Sucheck SJ (2016). Recent Advances in Subunit Vaccine Carriers. Vaccines (Basel), 4(2). doi: 10.3390/vaccines4020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze J (2019). First malaria vaccine rolled out in Africa—despite limited efficacy and nagging safety concerns. Science. doi: 10.1126/science.aba3207 [DOI] [Google Scholar]
- Wang C, Beiss V, & Steinmetz NF (2019a). Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties. J Virol, 93(21). doi: 10.1128/JVI.00129-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Tu J, Liu J, & Molineux IJ (2019b). Structural dynamics of bacteriophage P22 infection initiation revealed by cryo-electron tomography. Nat Microbiol, 4(6), 1049–1056. doi: 10.1038/s41564-019-0403-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xing D, Le Van A, Jerse AE, & Wang S (2017). Structure-based design of ferritin nanoparticle immunogens displaying antigenic loops of Neisseria gonorrhoeae. FEBS Open Bio, 7(8), 1196–1207. doi: 10.1002/2211-5463.12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhou X, Bian Y, Wang S, Chai Q, Guo Z, … Zhu M (2020). Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat Nanotechnol, 15(5), 406–416. doi: 10.1038/s41565-020-0648-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Su Q, Dong S, Shi H, Gao X, & Wang L (2014). Hybrid phage displaying SLAQVKYTSASSI induces protection against Candida albicans challenge in BALB/c mice. Hum Vaccin Immunother, 10(4), 1057–1063. doi: 10.4161/hv.27714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xu L, Yu H, Lv P, Lei Z, Zeng Y, … Cheng T (2019c). Ferritin nanocage-based antigen delivery nanoplatforms: epitope engineering for peptide vaccine design. Biomater Sci, 7(5), 1794–1800. doi: 10.1039/c9bm00098d [DOI] [PubMed] [Google Scholar]
- Wilkie M, Satti I, Minhinnick A, Harris S, Riste M, Ramon RL, … McShane H (2020). A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime - MVA85A boost in healthy UK adults. Vaccine, 38(4), 779–789. doi: 10.1016/j.vaccine.2019.10.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Wang X, Lou P, Hu Z, Qu P, Li D, … Zhong H (2020). A Nanoparticle-Based Hepatitis C Virus Vaccine With Enhanced Potency. J Infect Dis, 221(8), 1304–1314. doi: 10.1093/infdis/jiz228 [DOI] [PubMed] [Google Scholar]
- Yassine HM, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, … Graham BS (2015). Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med, 21(9), 1065–1070. doi: 10.1038/nm.3927 [DOI] [PubMed] [Google Scholar]
- Yusibov V, Mett V, Mett V, Davidson C, Musiychuk K, Gilliam S, … Mann D (2005). Peptide-based candidate vaccine against respiratory syncytial virus. Vaccine, 23(17-18), 2261–2265. doi: 10.1016/j.vaccine.2005.01.039 [DOI] [PubMed] [Google Scholar]
- Yusuf Y, Yoshii T, Iyori M, Yoshida K, Mizukami H, Fukumoto S, … Yoshida S (2019). Adeno-Associated Virus as an Effective Malaria Booster Vaccine Following Adenovirus Priming. Front Immunol, 10, 730. doi: 10.3389/fimmu.2019.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Peabody J, Pang YS, Schiller J, Chackerian B, & Tumban E (2017). A novel candidate HPV vaccine: MS2 phage VLP displaying a tandem HPV L2 peptide offers similar protection in mice to Gardasil-9. Antiviral Res, 147, 116–123. doi: 10.1016/j.antiviral.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Yadav R, Kunda NK, Anderson D, Bruckner E, Miller EK, … Tumban E (2019). Oral immunization with bacteriophage MS2-L2 VLPs protects against oral and genital infection with multiple HPV types associated with head & neck cancers and cervical cancer. Antiviral Res, 166, 56–65. doi: 10.1016/j.antiviral.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Wang Y, Xu Z, Qu H, Zhang H, Niu L, … He H (2019). Novel adenoassociated virusbased genetic vaccines encoding hepatitis C virus E2 glycoprotein elicit humoral immune responses in mice. Mol Med Rep, 19(2), 1016–1023. doi: 10.3892/mmr.2018.9739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F-C, Li Y-H, Guan X-H, Hou L-H, Wang W-J, Li J-X, … Chen W (2020). Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet, 395(10240), 1845–1854. doi: 10.1016/s0140-6736(20)31208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]