Abstract
Since the discovery of insulin in 1921, the role of insulin in the brain has been investigated. The ability of insulin to act within the brain to regulate peripheral glucose levels helped evolve the research surrounding the ability of insulin to be transported into the brain. Investigations to determine the transport of insulin into the brain from the circulation soon followed. Once it was established insulin could enter the brain, the ability of insulin to bind brain microvessels and regulators of this process were determined. As technology advanced, quantitative measurements to specify the transport rate of insulin across the blood-brain barrier (BBB) and the impact of physiological conditions and diseases were the logical next steps. Lastly, with the advent of genetic mouse models and high-specificity antagonists, the specific role of the insulin receptor in mediating insulin transport could begin to be explored. In this review, we summarize the main findings throughout the decades regarding the interactions of insulin at the BBB.
Keywords: insulin, blood-brain barrier, insulin receptor, pharmacokinetics
Introduction
Insulin was first discovered in 1921 as the critical hormone in regulating blood glucose levels. Now a century later, we have discovered so much more about what insulin does and how it gets there. The purpose of this historical review is to present the primary advancements on the interactions of insulin at the blood-brain barrier (BBB) (Figure 1). Evidence for a barrier between the blood and the brain was provided by Paul Ehrlich (1) and others (2) well before the discovery of insulin and confirmed later by Goldmann (3). However, not until the early 1920s, was the phrase ‘blood-brain barrier’ used (4, 5), which actually referred to the blood-cerebrospinal fluid (CSF) barrier (BCSFB) as we now know (2). Therefore, the actual concept of the BBB and the discovery of insulin are nearly contemporaneous. Within the first few years of the discovery of insulin, the questions of whether insulin crossed the BBB, whether insulin affected the brain, and whether CNS insulin had peripheral effects were all addressed. However, no follow-up on BBB-insulin issues seems to have been conducted until the 1950’s. It was known by that time that insulin binding sites were widely prevalent throughout the brain. In addition, it had become well-accepted that insulin could act within the central nervous system (CNS) to affect peripheral metabolism. In this review, we will present critical advancements throughout the decades following the discovery of insulin regarding insulin interactions at the BBB. These advancements at the BBB go hand-in-hand with transport of insulin across the BCSFB located at the choroid plexus, which involves transport from the blood, across ependymal epithelium, and directly into the CSF. We have presented a historical perspective about what technologies were available and widely used at the time and how advancements in methods led to improved interpretations of many of the discoveries. Due to our focus specifically on insulin interactions at the BBB and length restrictions, we were unable to discuss the literature regarding the development of antibodies towards the insulin receptor for drug delivery which has been reviewed elsewhere (6).
Figure 1. Historical timeline on the evolution of interpreting insulin interactions at the BBB.
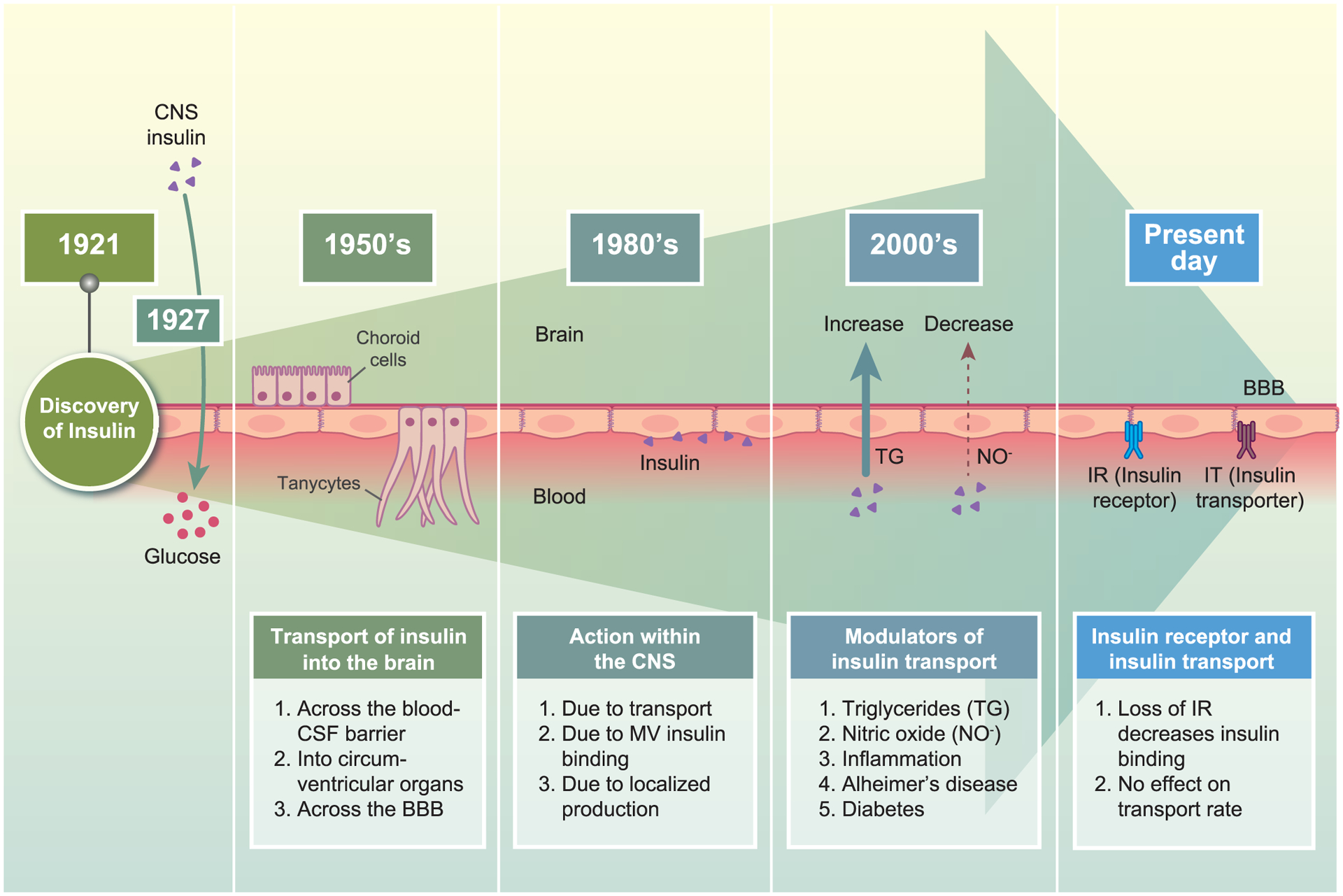
Since the discovery of insulin in 1921, the role of it in the brain, and thus the ability of the brain to access insulin, has been increasingly investigated over the decades. Since the early years after the discovery of insulin, the question of whether or not insulin was transported into the brain has been investigated. There was still uncertainty as to whether insulin crossed the BBB, the blood-CSF barrier, or entered through the circumventricular organs. In 1927, it was found that intracisternal injection of insulin led to a sharp increase in blood glucose levels, suggesting a regulatory role of CNS insulin in mediating peripheral metabolism. Follow-up studies investigated insulin-CNS connections and transport. The 1980s focused heavily on the ability of insulin to interact at the BBB and binding levels. Techniques to measure insulin transport into the brain became better. Once it was well established that insulin crossed the BBB, the focus in the 2000s shifted to modulators of this transport process. Most recently, the role of the insulin receptor in mediating transport and the impact of disease risk factors on insulin transport has been examined.
Insulin and the brain: The Early Years
In less than five years after the discovery of insulin by Banting and Best (7), investigators were already investigating the role of insulin on brain function and the actions of CNS insulin. In 1924, Kasahara and Uetani showed that rabbits injected with subcutaneous insulin had 2–3 hours later a decrease in the level of reducing substance, which was by that time definitely known to be glucose, in the CSF (8). This was confirmed two years later in dogs (9). The resulting hypoglycorrhachia was eventually concluded to be the cause of insulin-induced seizures, thus providing a mechanistic explanation for an effect of peripheral insulin on brain function. In 1927, Supniewski et al showed that insulin administered into the brain paradoxically increased blood glucose, whereas subcutaneous insulin decreased glucose (Figure 2) (10). Thus, 5 years after the discovery of insulin, CNS insulin was associated with control of a peripheral end point. Later, intravenous insulin was shown to also produce a small drop in CSF potassium (11, 12) and the effect on glucose to be mediated by the vagus (13). These studies provided some of the first evidence for peripheral/central communication due to insulin.
Figure 2. Impact of insulin delivered to the brain (ICV) or periphery (SQ) on blood glucose.
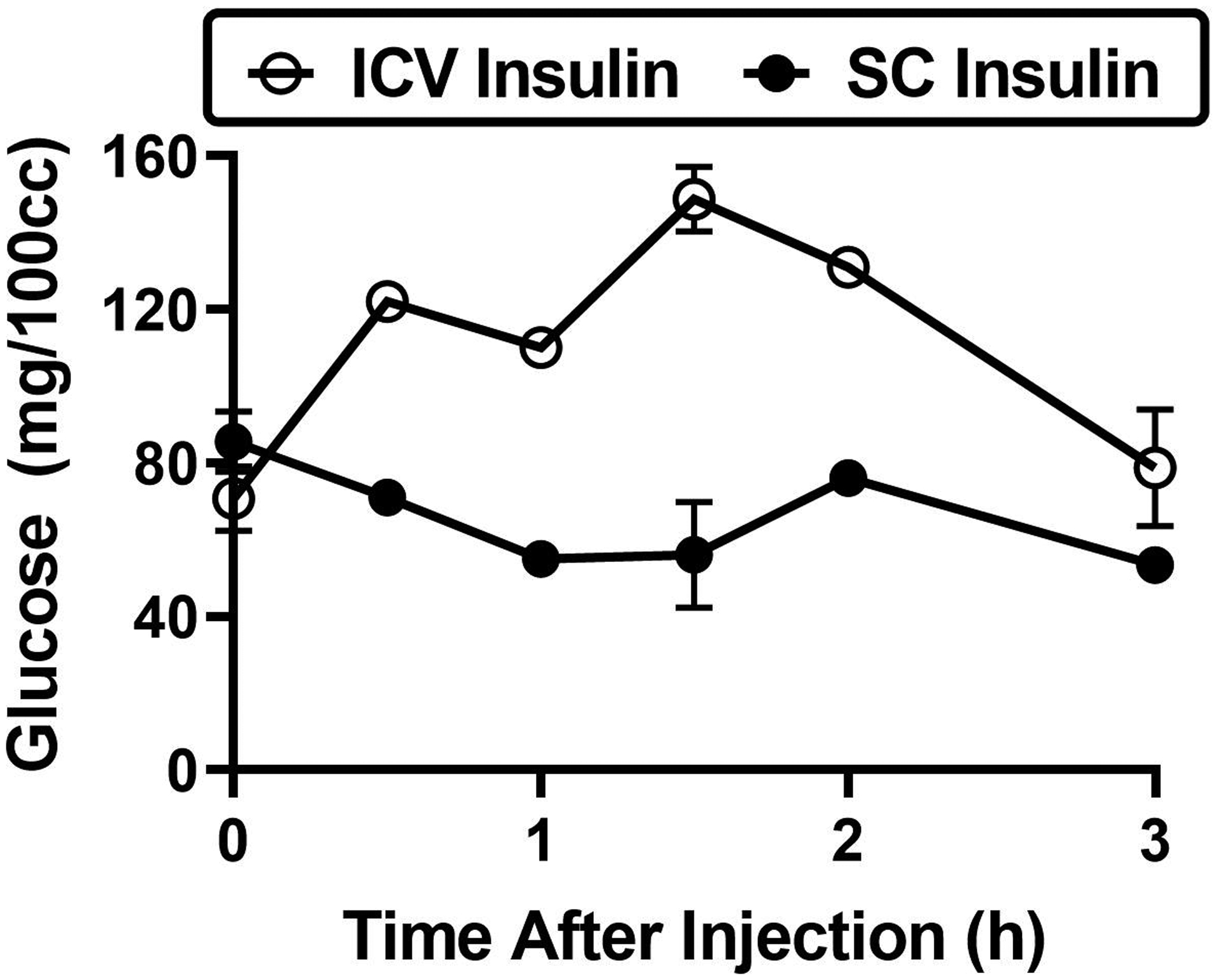
Dogs were injected with 3 U per kilogram body weight of insulin either by subcutaneous (SQ) injection or injection into the cerebellar cisterna magna (ICV). Blood and CSF samples were taken repeatedly up to 3 h later to determine the impact of insulin injection on glucose levels. Graph is drawn from Table III in Supniewski et al 1927.
Deciding whether insulin could cross the BBB was more controversial and the early history has been previously reviewed (14). In brief, two studies in the 1950’s injected radioactive insulin intravenously and sampled CSF or brain in lab animals and/or dying humans (15, 16). In 1962, Mahon et al performed a similar study with radioactivity in humans (17) and Schrader and Weinges administered insulin to humans, using an in vitro adipose fat assay for detection (18). All of these studies failed to find insulin in the CSF or brain tissue and concluded that insulin did not cross the BBB. One such possibility could have been too small level of insulin injected. Other reasons why these studies failed to detect insulin transport into the brain could have been due to technological limitations at the time in trying to detect a minimal amount of insulin transfer into the brain. Indeed, in 1967, two studies injected insulin peripherally and used the new radioimmunoassay of Berson and Yalow to measure insulin in the CSF. Mesdjian et al showed the appearance of peripherally administered beef insulin in the CSF of cats and that the radioimmunoassay did not detect endogenous feline insulin (19). Margolis and Altszuler showed that increasing doses of peripherally administered insulin resulted in increasingly higher levels of insulin in the CSF (20). However, the relation between blood levels of insulin and CSF levels of insulin was nonlinear, with the increase in CSF levels flattening at the highest blood levels. This led Margolis and Altszuler to postulate that insulin was transported into the brain by a saturable system, a conclusion endorsed by Greco et al in a human study (21). In 1977, Porte and Woods showed correlations between endogenous levels of insulin in the CSF and blood (22). They also showed that CSF levels of insulin slightly increased when blood levels were raised, but that the increase was low and slow. The culmination of these findings began to support that a transport system was in place for insulin at least across the BCSFB.
Insulin binding at the BBB: The 1980’s
At this time, reagents and techniques were limited for studying insulin at the BBB and in the CNS. The insulin receptor was not cloned until the mid-1980’s (23) and therefore, antibodies were non-existent. A high-affinity antibody towards insulin, combined with a modified radioimmunoassay protocol that had enhanced sensitivity to detect very low levels of insulin, was developed by the Porte Lab at the Seattle VA Medical Center and University of Washington although antibody supply was limited. This allowed some groups to measure insulin levels in serum, CSF, and brain samples from rats. However, techniques used to collect or measure samples for insulin required special attention as insulin can bind non-specifically but with substantial avidity to glass and some types of plastics. Microdialysis to take repeated sampling of CSF had just come online (24) but again, due to the extensive tubing involved, insulin was often lost to the tubing. Luckily, at this time, highly purified insulin itself was readily available from Eli Lilly, although it was of porcine or bovine origin, which had different affinities for the receptor. Despite these differences in affinity, overall microvessel binding did not seem to vary much between species. Rat insulin was cloned in 1977 (25) and rapidly followed by the cloning of human insulin in 1979, with technical efforts to increase the yield in the years to follow (26). In 1983, recombinant human insulin became widely available (27). There was the ability to radiolabel insulin with 125I and this was used routinely to measure binding of insulin to tissues using Scatchard plots. However, these assays were not able to discriminate which proteins insulin was binding to. 125I-insulin was also being used to trace its entry into the CNS following cardiac infusion. The radioimmunoassay for insulin had already been developed in 1960 by Yalow and Berson and was well-accepted at this time (28).
There were three over-arching themes related to insulin action in the CNS entering this decade: 1) insulin transport from blood into the CNS, 2) insulin synthesis within the CNS, and 3) insulin action at the brain endothelial cell to elicit a metabolic response on the CNS side, acting in an endocrine fashion. The ability to measure mRNA was not readily available until the late 1980’s, therefore answering point 2 proved difficult at this time. Instead, the focus shifted to points 1 and 3, as reviewed below.
Researchers at the University of Washington, including Stephen Woods and Daniel Porte, during this decade helped to highlight the important role of CNS insulin on feeding behavior. Food deprivation in rats resulted in decreased levels of CSF insulin (29). Following re-feeding, CSF insulin levels took up to 85 min before increasing, compared to CSF glucose levels which only took half the time to increase. Due to this impact of insulin action within the CNS, it was concluded that insulin must be a signal within the brain. However, it was still unclear how insulin was able to do this and if there were conditions that altered the entry of insulin into the CNS.
In order to determine how insulin was able to act within CNS, much work during this decade was spent evaluating the interaction of insulin in brain microvessels, both ex vivo and in vivo. It is important to note that these studies simply represent the level of insulin binding to the endothelial cell surface, rather than binding specifically to the insulin receptor. Many articles continue to state that insulin receptors are widely expressed throughout the brain and cite key work by Havrankova et al (30). However, it is important to keep in mind that technology at the time only allowed one to measure insulin binding, rather than specificity to the insulin receptor as an antibody directed towards the insulin receptor had not yet been developed. Therefore, the conclusions at this time were that insulin binding sites were widely expressed throughout the brain.
Using isolated bovine brain microvessels, Pardridge et al found that iodinated porcine insulin bound microvessels, and this binding was specific for insulin as other hormones including thyroid stimulating hormone, growth hormone, and prolactin did not compete with binding but did compete with excess insulin, as unlabeled insulin decreased the amount of radiolabeled insulin binding (31). These studies showed that brain capillaries are capable of binding approximately 2–3 ng of insulin per gram of brain. At this time, in the early 1980’s, it was thought that insulin did not cross the BBB, though there was speculation of it crossing the BCSFB. Therefore, these observations that insulin bound brain microvessels were to support the idea that insulin could affect CNS function by binding the insulin receptor to alter brain endothelial cell signaling and transmit this metabolic signal to the brain cells. However, the specific protein insulin was binding to on the brain microvessels was not investigated. In vivo binding was determined using male rats and iodinated insulin (32). Radiolabeled insulin binding occurred on all brain blood vessels of all dimensions except the blood vessels along the perimeter of the brain. There were regional differences in binding intensity, with the neocortex, hippocampus, and hypothalamus having the highest binding. The corpus callosum had the least. This binding was dose-dependently reversible using unlabeled insulin from 1 μg to 830 μg. Electron micrographs were exposed for up to 4 months to identify insulin likely bound to the endothelial cell following intracardial injection. These studies were some of the first to describe insulin binding sites on blood vessels. One study around this time had shown insulin binding sites were present in a peripheral vascular bed (33)
Next steps were to determine what physiological states could alter insulin binding. To investigate insulin binding in developing microvessels, New Zealand rabbits of various developmental ages were used. Rabbits received a carotid bolus injection of radiolabeled insulin allowed to circulate for 15 sec, allowing for enough time of a single passage through the brain (34). Insulin was taken up 8 times greater than the inert vascular marker inulin (which coincidentally has a comparable molecular weight to insulin) in the newborn and 3 times more in the 11-wk old rabbits. While this partly represents differences in receptor binding on the endothelial surface, brain and CSF levels also proved to be significantly higher in the newborns, suggesting transport into the brain. Using similar methods, insulin binding to capillaries isolated from streptozotocin (STZ) diabetic rats was much lower than controls (35). It was also noted that brain insulin levels were decreased nearly 8-fold in the diabetic rats compared to controls, similar to a previous report in obese rats (36). Lastly, the degree of insulin binding to human brain capillaries was also investigated during this time (37). Fresh human autopsy brain was used to isolate brain capillaries and Scatchard plots were used to show insulin binding kinetics. These studies showed that insulin could bind rodent, rabbit, and human brain microvessels and this binding varied based on developmental or diabetic state.
Finally, towards the end of this decade, specific studies targeting the question of whether or not insulin crossed the BBB (and not the BCSFB) were executed. Again, using developing rabbits (<7 days old), transport of insulin across the BBB following cardiac perfusion in whole brain was 40% greater than that of 3H-albumin (38). This process was saturable, in that 100 μg/ml insulin co-perfusion blocked transport. The studies published during the 1980’s show great advancement in our current understanding of insulin interactions at the BBB. First, work further characterized the important role within the CNS in regulating peripheral metabolism. While we highlight here some of the important findings, more of this work will be discussed in other articles present in this special issue. Second, it was found that insulin could bind brain microvessels and that binding could change based on physiological state. Lastly, it seems as though the debate as to whether or not insulin crossed the BBB to enter the CNS was finally resolved.
Kinetic transport studies: The 1990’s
Now that it had been established insulin crossed both the BBB and the BCSFB, further modeling on this transport was performed. Woods and Porte found that mechanisms involving the transport across the BCSFB differed from other solutes known to cross at that time, with a delay in the ability of insulin to reach the CSF following a rise in blood levels (39). This proved to be due to a three-compartment uptake system during insulin infusions in which insulin traverses an intermediate compartment en route from the plasma to the CSF (40). This differs from the transport of inert molecules such as inulin that diffuse across the barrier in a two-compartment system. An intermediate compartment could be the choroid epithelial cells or could be interactions with the insulin receptor. It was also suggested that appearance of insulin in the CSF could be due to transport across the BBB, rather than the BCSFB (40). This data combined with BBB transport studies suggested the majority of insulin transport into the CNS occurred via the BBB.
In the mid 1990’s, Jaspan, Kastin, and Banks in collaboration with others performed a series of studies using multiple-time regression analysis (MTRA) and radioactive insulin. MTRA (41, 42) was developed by Patlak, Blasberg, and Fenstermacher to measure slow rates of transport across the BBB. Previous methods, such as the classic brain uptake index and indicator dilution method (43, 44), could only measure relative permeabilities of substances with rapid transport across the BBB, such as glucose, amino acids, and heroin, but not that of substances with slow transport, such as morphine or peptides. MTRA, in comparison, is able to measure exact influx constants of substances with entries as slow as that of albumin, a substance whose rate of transport across the BBB is so slow that it is classically used as a vascular marker or to measure BBB disruption. The great sensitivity of MTRA combined with that of the use of radioactivity allowed the rate of insulin transport to be quantified in vivo and within minutes in physiological and pathological conditions. Additionally, the use of radioactivity definitively demonstrated that insulin found in brain originated from the blood. This as well as studies using species specific immunoassays (19, 45), definitively showed that CNS insulin could be derived from the blood, as opposed to being synthesized by the CNS itself.
Additional findings from this decade have been previously summarized (14). In brief, using the MTRA method, it was found this transport process was saturable (46). This saturability is independent of species in that human insulin can reduce rat insulin transport and vice versa. In addition, these studies found that the insulin transporter was most efficient at euglycemic levels (46). In other words, insulin signaling to brain was occurring physiologically and was unrelated to the hypoglycemic response. Insulin transport is independent of leptin and amylin transport (47, 48). Insulin transport is highest in the olfactory bulb, pons-medulla, and hypothalamus with no or very low transport into the midbrain, thalamus, and occipital cortex (48, 49). It was also noted that once insulin reached the brain, it was sequestered there, meaning there is no efflux system in place (50). Starvation for 72 hours, but not fasting, increased the amount of insulin retained by the brain (50). This decade proved to be more quantitative in terms of insulin BBB transport, with studies beginning to investigate what could alter this transport rate.
Modulators of insulin BBB transport: The 2000’s
Now that insulin BBB transport had been widely accepted, there was an explosion of studies done to determine whether transport was altered in various disease states and what circulating factors were responsible for insulin BBB transport. Much of this work has already been nicely summarized (51), but we will briefly review it here. In addition, work during this time started to show an important role for insulin in improving memory and thus, interest in intranasal delivery of insulin into the brain became a big focus (52, 53). Intranasal delivery allows for direct delivery to the CNS, rather than entering the bloodstream, potentially affecting blood glucose levels, and having to navigate the BBB. As the focus of this review is on the interactions of insulin and the BBB, we direct readers to other reviews on this topic (54–56).
Initial obvious modulators of insulin BBB transport were related to obesity and diabetes. Obesity decreases insulin BBB transport whereas starvation increases transport (57). These changes in the insulin BBB transport rate were found to be due to serum factors rather than direct changes at the BBB. Since transport is saturable, an increase in serum insulin levels in the obese mice could be responsible for the decreased rate observed. In contrast, starvation increases circulating triglyceride levels which were shown to enhance insulin BBB transport. Diabetic mice (STZ-induced) have an increased rate of insulin transport that is not dependent on serum factors or vascular space, but rather changes at the BBB itself (58). STZ-diabetic rats also display a decreased function and expression of P-glycoprotein (Pgp), a critical efflux transporter, present in microvessels and insulin repletion was able to restore these deficiencies (59). These data suggest insulin can act on brain microvessels to regulate transporter expression and function. Inflammation, induced by LPS, is known to enhance insulin BBB transport through action on the neurovascular unit by activation of nitric oxide synthase (NOS) enzymes (60). Endothelial NOS (eNOS) and inducible NOS (iNOS) were primarily responsible for the LPS-induced increase in insulin BBB transport. These data suggest that regulators of NO production could have an indirect effect on insulin BBB transport.
To return to the studies done in the 80’s, the level of insulin binding the brain vasculature was renewed but used the MTRA technique. Since altered brain insulin signaling in Alzheimer’s disease had become an important topic, whether or not changes in the transport of insulin across the BBB occurred was investigated. Using a mouse model of Alzheimer’s disease, the SAMP8 mice, the level of insulin vascular binding in specific brain regions was higher in aged mice compared to young mice (61). What the consequences are of the increase in vascular binding remains to be determined.
Insulin receptor and BBB transport: The 2010’s
The role for the insulin receptor in mediating not only intracellular signaling but also transcytosis of insulin has been a question since transport of insulin across the BBB was first defined. Work by our group showed that insulin BBB transport could still occur either in the absence or inhibition of the insulin receptor in vivo (62). This work was further supported by an independent Denmark group in vitro (63). This data suggests that there is an insulin transporter that is not the insulin receptor, but this protein is currently unknown. The rate of insulin BBB transport, Ki, collected from Banks et al (48) was correlated with the level of insulin measured in the brain by Baskin et al (64) and the level of insulin receptor protein expression (65) (Figure 3). The correlation coefficient for rate and insulin content is much greater than the rate and insulin receptor expression, further suggesting the insulin receptor may not be the only protein involved in mediating insulin transcytosis across the brain endothelial cell. As the correlation in Figure 3B is trending towards significance, a greater number of data points may help in this interpretation. In addition, it makes sense that insulin transport into brain regions would correlate with receptor expression, based on action once present within the regions. A better correlation would be between the insulin receptor expressed on microvessels isolated from specific regions and the rate of BBB transport. However, these studies prove to be technologically difficult. The low density lipoprotein-related protein 2 (LRP2), also known as megalin, has been shown to bind and endocytose insulin (66). While the LRP2 inhibitor RAP (receptor-associated protein) was shown to not affect insulin BBB transport (62), further investigation on this system in mediating insulin BBB transport is warranted. In addition, LRP1 has been shown to be important for insulin receptor signaling at the BBB (67), and thus, could also act as a potential transporter for insulin. Studies using antibodies targeting the insulin receptor have suggested this transport system can be used to deliver substrates and therapeutics across the BBB using molecular Trojan horse technology (6, 68). However, it is possible these antibodies also recognize the protein responsible for insulin transport.
Figure 3. Correlations of the insulin BBB transport rate with insulin content and insulin receptor expression throughout the brain.
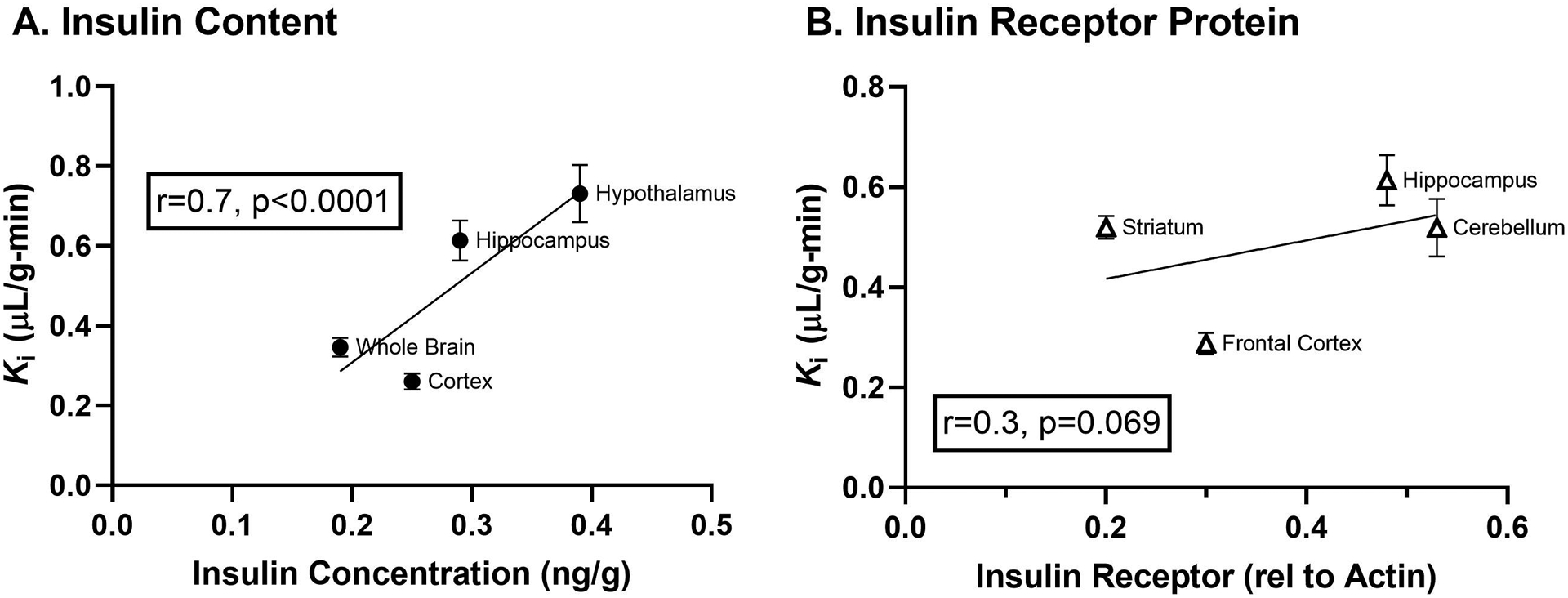
A) Insulin content present throughout the brain regions is significantly correlated with the transport rate across the BBB calculated for insulin in these areas. B) On the other hand, the protein expression of the insulin receptor does not correlate as strongly with the transport rate. Transport data has been graphed from Table 1 in (48), insulin content was taken from Figure 2 in (64), and insulin receptor protein expression is from Table 1 in (65).
It should also be noted research from another group suggested that inhibition of the insulin receptor led to a reduction in an insulin BBB transport in vitro (69). In vitro models are extremely sensitive to the culturing conditions and many important BBB characteristics can change due to these conditions (63). Most brain endothelial cells are cultured in high insulin conditions and therefore, could have a completely different protein expression. Indeed, expression of the insulin receptor from freshly isolated bovine brain capillaries increases with time in culture (63). Therefore, differences in in vitro culture conditions could explain the contradictions between these two transport studies. Both studies performed the transport experiments using co-cultures with astrocytes which are known to improve BBB integrity, and thus impart an important communication between these two cell types that is mostly through secreted factors.
The role of the insulin receptor on astrocytes in mediating insulin transport into the brain has partially been investigated recently. Genetic models lacking the insulin receptor in GFAP-expressing cells suggests this signaling pathway is implicated in glucose BBB transport and insulin BCSFB transport (70). However, the impact of the astrocytic insulin receptor, as well as other cell types within the neurovascular unit (71) on insulin BBB transport remains to be determined.
Recently, the impact of factors linked to insulin resistance on BBB insulin transport have been investigated. Apolipoprotein E isoforms (72, 73) and the insulin sensitizing drug, rosiglitazone (74), have been shown to alter insulin binding at the BBB. Cholecystokinin (CCK), another anorexigenic hormone, increases insulin transport at the BCSFB (75). When looking at transport of insulin into the brain, it is important to keep in mind whether the BBB is being investigated or the BCSFBas these two barriers are completely different.
Conclusions and future directions
The last 100 years of research has shown great advancement in our knowledge and understanding about the specifics on insulin interactions in the brain and more specifically at the BBB. We now know there are two barriers insulin uses to cross into the brain, the BBB and the BCSFB, though entry through the BBB seems to be the primary route. Insulin can bind brain microvessels and trigger intracellular signaling through the insulin receptor or be transcytosed across the brain endothelial cell by a protein other than the insulin receptor. This binding and transport varies based on physiological state and levels of known, and likely some unknown, serum factors, as extensively studied in the 1990’s and 2000’s. However, the magnitude of regulation the CNS imparts on BBB insulin transport is largely unknown. There are various cell types that make up the neurovascular unit (NVU) to regulate properties of the brain endothelial cells that encompass the BBB. These cells have been shown to communicate with one another to regulate critical properties of the BBB (Figure 4). While this topic could be reviewed entirely in a separate publication, we wanted to discuss some of the known signaling mechanisms between the cells and the possible relationship to the insulin receptor here.
Figure 4. Communication between cells of the neurovascular unit (NVU).

The dogma in the 1980’s was that insulin did not cross the BBB and instead, bound the brain endothelial cell to elicit an intracellular signaling response that released a signaling factor on the abluminal side to continue the endocrine response to act within the CNS. While this is still a possible signaling mechanism, we also now know and accept that insulin can cross the BBB to act directly within the CNS. Unfortunately, due to technical limitations, the fate of insulin once released on the abluminal side of the brain endothelial cell (BEC) is unclear. Does insulin interact with binding proteins during this transit route or is it released free into the interstitial fluid? Is insulin released into the interstitial space for diffusion throughout the CNS? Or is insulin taken up by tightly connected neighboring cells such as pericytes and astrocytes? While recent genetic models such as the endothelial cell, astrocyte, and neuron insulin receptor knock-out mice have allowed us to begin to explore some of these questions, most of the emphasis in these studies have been placed on insulin signaling and action, rather than feedback signals related to BBB transport. We know many serum factors that affect insulin entry into the CNS as highlighted in the 2000s, but CNS regulators that control insulin entry into the brain has been minimally investigated. It is known that astrocytes, neurons, pericytes, and BECs communicate with one another to regulate BBB properties. Some of these intracellular signaling events include cAMP, NF-kB, histamine, ATP, nitric oxide, calcium, and potassium signaling. The presence of aquaporin 4 (AQP4) on astrocytes also aids in communication with BECs, as do the gap junctions (green channels) present between pericytes and BECs. The communication between the cells of the NVU following insulin binding or internalization is a difficult question to answer but with advancing technologies such as single-cell RNA sequencing, genetic knock-out models, targeted regional delivery, and better in vitro co-culture BBB models, these intercellular connections can begin to be established in regards to insulin signaling.
Astrocytes can regulate many properties of the BBB including expression and polarized localization of transporters such as P-glycoprotein (Pgp) (76) and the glucose transporter 1 (GLUT1) (77). Endothelial cell expression of Pgp was later shown to be regulated by NF-kB nuclear translocation (78). This regulation was shown to occur with astrocyte conditioned medium, suggesting a secreted factor is responsible for the endothelial cell effect. A secreted factor was also shown to be responsible for the endothelial GLUT1 increase (77). Indeed, enhancement of many BBB properties often occurs through secreted factors as in vitro studies show co-cultures of astrocytes and brain endothelial cells do not require cell contact. Astrocytic aquaporin 4 is also known to regulate BBB properties and levels are altered in various diseases including traumatic brain injury, stroke, and Alzheimer’s disease. Therefore, changes in this protein expression or localization could affect endothelial cell signaling. Factors secreted by astrocytes include transforming growth factor-beta (TGFβ), glial-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), and angiopoetin 1 (ANG1) are all known to act on the BBB (79). Astrocytes can also signal to neurons through calcium and ATP (80). These signaling processes highlight the diverse ways astrocytes are able to communicate with brain endothelial cells.
Intracellular signaling processes that are known to regulate transporters expressed in brain endothelial cells include cyclic AMP, NF-kB, histamine, ATP, and calcium signaling (79). Exosomes and circular- or micro-RNAs (circRNAs/microRNAs) are also means of cell-cell communication. Specific circRNAs are known to be transmitted between pericytes and endothelial cells (81). Intracellular calcium spikes quickly and returns to baseline within 10 s after insulin stimulation of myotubes (82). While endothelial cells primarily express eNOS and iNOS and neurons express nNOS, astrocytes do have the ability to express iNOS (83). Insulin receptor signaling activation also leads to eNOS and nNOS phosphorylation and NO production (84, 85).
Insulin is more effective for enhancing pericyte proliferation than endothelial cells (86). Therefore, insulin could protect CNS damage by inducing pericyte survival, which is an important area of research in Alzheimer’s disease. Both potassium (Kir) and Ca2+-sensitive potassium channels are involved in insulin signaling in pericytes (87). Insulin increases pericyte VEGF expression (88), which as a secreted factor, could help promote endothelial survival. These processes directly link the beneficial effect and communication of insulin on both pericytes and brain endothelial cells.
In culmination, to further our understanding of insulin interactions at the BBB, it might be worth shifting the focus to CNS regulators. Utilizing genetic mouse models that lack the insulin receptor in individual cell types and in vitro culture systems provide a useful tool to study a lot of these cell-cell communications. However, validating the appropriate conditions for insulin transcytosis in vitro will be critical to correctly interpreting these data. Also, with advancements in proteomics, identifying other insulin binding proteins present on the brain endothelial cell surface could help in uncovering the molecular mechanisms for transcytosis and fate of insulin once release on the abluminal side.
Acknowledgements:
The authors would like to thank Dr. Dianne Figlewicz Lattemann at the University of Washington for providing a more in depth understanding about the historical perspective in the 1970s-1990s. We would like to thank Ms. Alison Schroeer for assistance in designing Figures 1 and 4.
Funding: This work was supported in part by the University of Washington’s Diabetes Research Center New Investigator Award (EMR), the National Institutes of Health: RF1AG059088, RO1AG046619 (WAB), and the Veterans Affairs Puget Sound Health Care System.
Footnotes
Declaration of interest: The authors declare no conflict of interest.
References
- 1.Ehrlich P Das Sauerstoffbedürfnis des Organismus. Eine Farbenanalytische Studie Berlin: Hirschwald. 1885. [Google Scholar]
- 2.Saunders NR, Dreifuss JJ, Dziegielewska KM, Johansson PA, Habgood MD, Mollgard K, Bauer HC. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front Neurosci. 2014; 8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldmann E Die äussere und innere Sekretion des gesunden und kranken Organismus im Lichte der ‘vitalen Färbung.’. Beiträg Klinische Chirurgie 1909; 64192–265. [Google Scholar]
- 4.Stern LG R Le passage dans le liquide céphalo-rachidien de substances introduites dans la circulation et leur action sur le système nerveux central chez les différentes espèces animales. R C R d Ia Soc de Phys et d’hist natur de Genève. 1918; 3591–4. [Google Scholar]
- 5.Stern LG R Passage simultané des substances dans le liquide céphalo-rachidien et dans les centres nerveux. R C R d Ia Soc de Phys et d’hist natur de Genève. 1918; 3558–60. [Google Scholar]
- 6.Pardridge WM. Delivery of Biologics Across the Blood-Brain Barrier with Molecular Trojan Horse Technology. BioDrugs. 2017; 31(6): 503–19. [DOI] [PubMed] [Google Scholar]
- 7.Banting FG, Best CH. The internal secretion of the pancreas. Journal of Laboratory and Clinical Medicine. 1922; 7251–66. [Google Scholar]
- 8.Kasahara M, Uetani E. The effect of insulin upon the reducing substance in the cerebropsinal fluid of normal rabbits. J Biol Chem. 1924; 59433–6. [Google Scholar]
- 9.Gutowski B, Wasilkowska H. The quantitative variation of the sugar in the blood and in cerebrospinal fluid under the effect of insulin. Comptes Rendus Des Seances De La Societe De Biologie Et De Ses Filiales. 1926; 94549–51. [Google Scholar]
- 10.Supniewski JV, Ishikawa Y, Geiling EMK. The effect of insulin injected into the cerebrospinal fluid. J Biol Chem. 1927; 74(2): 241–6. [Google Scholar]
- 11.Bekaert J, Demeester G. The influence of glucose and insulin upon the potassium concentration of serum and cerebrospinal fluid. Arch Int Physiol. 1951; 59(2): 262–4. [DOI] [PubMed] [Google Scholar]
- 12.Carey ME, Davson H, Bradbury MW. The effect of severe hypoglycemia upon cerebrospinal fluid formation, ventricular iodide clearance, and brain electrolytes in rabbits. J Neurosurg. 1981; 54(3): 370–9. [DOI] [PubMed] [Google Scholar]
- 13.Chowers I, Lavy S, Halpern L. Effect of insulin administered intracisternally on the glucose level of the blood and the cerebrospinal fluid in vagotomized dogs. Experimental neurology. 1966; 14(3): 383–9. [DOI] [PubMed] [Google Scholar]
- 14.Banks WA. The source of cerebral insulin. European Journal of Pharmacology. 2004; 4905–12. [DOI] [PubMed] [Google Scholar]
- 15.Elgee NJ, Williams RH, Lee ND. Distribution and degradation studies with insulin-I131. Journal of Clinical Investigation. 1954; 331252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haugaard N, Vaughan M, Haugaard ES, Stadie WC. Studies of radioactive injected labeled insulin. J Biol Chem. 1954; 208549–63. [PubMed] [Google Scholar]
- 17.Mahon WA, Mitchell ML, Steinke J, McKhann GM. MEASUREMENT OF I131-INSULIN AND OF INSULIN-LIKE ACTIVITY IN CEREBROSPINAL FLUID OF MAN. Metab-Clin Exp. 1962; 11(4): 416–&. [PubMed] [Google Scholar]
- 18.Schrader A, Weinges KF. [Comparative determinations of insulin-like activity in the blood and cerebrospinal fluid]. Klin Wochenschr. 1962; 40344–6. [DOI] [PubMed] [Google Scholar]
- 19.Mesdjian E, Waltregny A, Lyagoubi S, Boeuf G, Depieds R, Gastaut H. [Study of the passage of insulin (bovine insulin) from plasma to the cerebrospinal fluid in cats]. C R Seances Soc Biol Fil. 1967; 161(7): 1619–24. [PubMed] [Google Scholar]
- 20.Margolis RU, Altszuler N. Insulin in the cerebrospinal fluid. Nature. 1967; 2151375–6. [DOI] [PubMed] [Google Scholar]
- 21.Greco AV, Ghirlanda G, Fedeli G, Gambassi G. Insulin in the cerebro spinal fluid of man. Eur Neurol. 1970; 3303–7. [DOI] [PubMed] [Google Scholar]
- 22.Woods SC, Porte D Jr Relationship between plasma and cerebrospinal fluid insulin levels of dogs. American Journal of Physiology. 1977; 233E331–E4. [DOI] [PubMed] [Google Scholar]
- 23.Ebina Y, Ellis L, Jarnagin K, Edery M, Graf L, Clauser E, Ou JH, Masiarz F, Kan YW, Goldfine ID, et al. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985; 40(4): 747–58. [DOI] [PubMed] [Google Scholar]
- 24.Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of brain microdialysis. Curr Protoc Neurosci. 2009; Chapter 7Unit7 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ullrich A, Shine J, Chirgwin J, Pictet R, Tischer E, Rutter WJ, Goodman HM. Rat insulin genes: construction of plasmids containing the coding sequences. Science (New York, NY). 1977; 196(4296): 1313–9. [DOI] [PubMed] [Google Scholar]
- 26.Goeddel DV, Kleid DG, Bolivar F, Heyneker HL, Yansura DG, Crea R, Hirose T, Kraszewski A, Itakura K, Riggs AD. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979; 76(1): 106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson IS. Human insulin from recombinant DNA technology. Science (New York, NY). 1983; 219(4585): 632–7. [DOI] [PubMed] [Google Scholar]
- 28.Yalow RS. Radioimmunoassay: a probe for the fine structure of biologic systems. Science (New York, NY). 1978; 200(4347): 1236–45. [DOI] [PubMed] [Google Scholar]
- 29.Steffens AB, Scheurink AJ, Porte D Jr, Woods SC Penetration of peripheral glucose and insulin into cerebrospinal fluid in rats. The American journal of physiology. 1988; 255(2 Pt 2): R200–4. [DOI] [PubMed] [Google Scholar]
- 30.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978; 272(5656): 827–9. [DOI] [PubMed] [Google Scholar]
- 31.Frank HJ, Pardridge WM. A direct in vitro demonstration of insulin binding to isolated brain microvessels. Diabetes. 1981; 30(9): 757–61. [DOI] [PubMed] [Google Scholar]
- 32.van Houten M, Posner BI. Insulin binds to brain blood vessels in vivo. Nature. 1979; 282(5739): 623–5. [DOI] [PubMed] [Google Scholar]
- 33.Bar RS, Hoak JC, Peacock ML. Insulin receptors in human endothelial cells: identification and characterization. The Journal of clinical endocrinology and metabolism. 1978; 47(3): 699–702. [DOI] [PubMed] [Google Scholar]
- 34.Frank HJ, Jankovic-Vokes T, Pardridge WM, Morris WL. Enhanced insulin binding to blood-brain barrier in vivo and to brain microvessels in vitro in newborn rabbits. Diabetes. 1985; 34(8): 728–33. [DOI] [PubMed] [Google Scholar]
- 35.Frank HJ, Pardridge WM, Morris WL, Rosenfeld RG, Choi TB. Binding and internalization of insulin and insulin-like growth factors by isolated brain microvessels. Diabetes. 1986; 35(6): 654–61. [DOI] [PubMed] [Google Scholar]
- 36.Baskin DG, Stein LJ, Ikeda H, Woods SC, Figlewicz DP, Porte D, Jr., Greenwood MR, Dorsa DM. Genetically obese Zucker rats have abnormally low brain insulin content. Life sciences. 1985; 36(7): 627–33. [DOI] [PubMed] [Google Scholar]
- 37.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier insulin receptor. Journal of neurochemistry. 1985; 44(6): 1771–8. [DOI] [PubMed] [Google Scholar]
- 38.Duffy KR, Pardridge WM. Blood-Brain-Barrier Transcytosis of Insulin in Developing Rabbits. Brain Res. 1987; 420(1): 32–8. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz MW, Sipols A, Kahn SE, Lattemann DF, Taborsky GJ, Bergman RN Jr, Woods SC, Porte D Jr Kinetics and specificity of insulin uptake from plasma into cerebrospinal fluid. The American journal of physiology. 1990; 259(3 Pt 1): E378–83. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz MW, Bergman RN, Kahn SE, Taborsky GJ, Fisher LD Jr, Sipols AJ, Woods SC, Steil GM, Porte D Jr Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. The Journal of clinical investigation. 1991; 88(4): 1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blasberg RG, Fenstermacher JD, Patlak CS. Transport of alpha-aminoisobutyric acid across brain capillary and cellular membranes. Journal of Cerebral Blood Flow and Metabolism. 1983; 38–32. [DOI] [PubMed] [Google Scholar]
- 42.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Journal of Cerebral Blood Flow and Metabolism. 1983; 31–7. [DOI] [PubMed] [Google Scholar]
- 43.Oldendorf WH. Brain uptake of radio-labelled amino acids, amines and hexoses after arterial injection. American Journal of Physiology. 1971; 2211629–39. [DOI] [PubMed] [Google Scholar]
- 44.Barry DI, Paulson OB, Hertz MM. The blood-brain barrier: an overview with special reference to insulin effects on glucose transport. Acta Neurol Scand Suppl. 1980; 78147–56. [DOI] [PubMed] [Google Scholar]
- 45.Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: Novel demonstration by species-specific enzyme immunoassays. Peptides. 1997; 181257–62. [DOI] [PubMed] [Google Scholar]
- 46.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997; 18(9): 1423–9. [DOI] [PubMed] [Google Scholar]
- 47.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996; 17305–11. [DOI] [PubMed] [Google Scholar]
- 48.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998; 19(5): 883–9. [DOI] [PubMed] [Google Scholar]
- 49.Banks WA, Kastin AJ, Pan W. Uptake and degradation of blood-borne insulin by the olfactory bulb. Peptides. 1999; 20(3): 373–8. [DOI] [PubMed] [Google Scholar]
- 50.Cashion MF, Banks WA, Kastin AJ. Sequestration of centrally administered insulin by the brain: effects of starvation, aluminum, and TNF-alpha. Hormones and behavior. 1996; 30(3): 280–6. [DOI] [PubMed] [Google Scholar]
- 51.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacology & therapeutics. 2012; 136(1): 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benedict C, Hallschmid M, Schultes B, Born J, Kern W. Intranasal insulin to improve memory function in humans. Neuroendocrinology. 2007; 86(2): 136–42. [DOI] [PubMed] [Google Scholar]
- 53.Hanson LR, Frey WH. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008; 9 Suppl 3S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meredith ME, Salameh TS, Banks WA. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. The AAPS journal. 2015; 17(4): 780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cholerton B, Baker LD, Craft S. Insulin, cognition, and dementia. Eur J Pharmacol. 2013; 719(1–3): 170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhea EM, Salameh TS, Banks WA. Routes for the delivery of insulin to the central nervous system: A comparative review. Experimental neurology. 2019; 31310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008; 149(7): 3592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banks WA, Jaspan JB, Kastin AJ. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides. 1997; 18(10): 1577–84. [DOI] [PubMed] [Google Scholar]
- 59.Liu H, Liu X, Jia L, Liu Y, Yang H, Wang G, Xie L. Insulin therapy restores impaired function and expression of P-glycoprotein in blood-brain barrier of experimental diabetes. Biochem Pharmacol. 2008; 75(8): 1649–58. [DOI] [PubMed] [Google Scholar]
- 60.Banks WA, Dohgu S, Lynch JL, Fleegal-DeMotta MA, Erickson MA, Nakaoke R, Vo TQ. Nitric oxide isoenzymes regulate lipopolysaccharide-enhanced insulin transport across the blood-brain barrier. Endocrinology. 2008; 149(4): 1514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banks WA, Farr SA, Morley JE. Permeability of the blood-brain barrier to albumin and insulin in the young and aged SAMP8 mouse. J Gerontol A Biol Sci Med Sci. 2000; 55(12): B601–6. [DOI] [PubMed] [Google Scholar]
- 62.Rhea EM, Rask-Madsen C, Banks WA. Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. J Physiol. 2018; 596(19): 4753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hersom M, Helms HC, Schmalz C, Pedersen TA, Buckley ST, Brodin B. The insulin receptor is expressed and functional in cultured blood-brain barrier endothelial cells but does not mediate insulin entry from blood to brain. Am J Physiol Endocrinol Metab. 2018; 315(4): E531–E42. [DOI] [PubMed] [Google Scholar]
- 64.Baskin DG, Porte D, Guest K Jr, Dorsa DM Regional concentrations of insulin in the rat brain. Endocrinology. 1983; 112(3): 898–903. [DOI] [PubMed] [Google Scholar]
- 65.Xu WH, Huber R, Riepe MW. Gender- and region-specific expression of insulin receptor protein in mouse brain: effect of mild inhibition of oxidative phosphorylation. J Neural Transm (Vienna). 2007; 114(3): 373–7. [DOI] [PubMed] [Google Scholar]
- 66.Orlando RA, Rader K, Authier F, Yamazaki H, Posner BI, Bergeron JJ, Farquhar MG. Megalin is an endocytic receptor for insulin. J Am Soc Nephrol. 1998; 9(10): 1759–66. [DOI] [PubMed] [Google Scholar]
- 67.Gali CC, Fanaee-Danesh E, Zandl-Lang M, Albrecher NM, Tam-Amersdorfer C, Stracke A, Sachdev V, Reichmann F, Sun Y, Avdili A, Reiter M, Kratky D, Holzer P, Lass A, Kandimalla KK, Panzenboeck U. Amyloid-beta impairs insulin signaling by accelerating autophagy-lysosomal degradation of LRP-1 and IR-beta in blood-brain barrier endothelial cells in vitro and in 3XTg-AD mice. Mol Cell Neurosci. 2019; 99103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood-brain barrier (BBB). J Drug Target. 2011; 19(2): 125–32. [DOI] [PubMed] [Google Scholar]
- 69.Gray SM, Aylor KW, Barrett EJ. Unravelling the regulation of insulin transport across the brain endothelial cell. Diabetologia. 2017; 60(8): 1512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Caceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi CX, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Aleman I, Kahn CR, Gotz M, Horvath TL, Tschop MH. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell. 2016; 166(4): 867–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhea EM, Banks WA. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Frontiers in Neuroscience. 2019; 13(521). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhea EM, Torres ERS, Raber J, Banks WA. Insulin BBB pharmacokinetics in young apoE male and female transgenic mice. PLoS One. 2020; 15(1): e0228455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rhea EM, Raber J, Banks WA. ApoE and cerebral insulin: Trafficking, receptors, and resistance. Neurobiology of disease. 2020; 137104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galindo DC, Banks WA, Rhea EM. The impact of acute rosiglitazone on insulin pharmacokinetics at the blood-brain barrier. Endocrinol Diabetes Metab. 2020; 3(3): e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.May AA, Liu M, Woods SC, Begg DP. CCK increases the transport of insulin into the brain. Physiology & behavior. 2016; 165392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaillard PJ, van der Sandt IC, Voorwinden LH, Vu D, Nielsen JL, de Boer AG, Breimer DD. Astrocytes increase the functional expression of P-glycoprotein in an in vitro model of the blood-brain barrier. Pharm Res. 2000; 17(10): 1198–205. [DOI] [PubMed] [Google Scholar]
- 77.Regina A, Morchoisne S, Borson ND, McCall AL, Drewes LR, Roux F. Factor(s) released by glucose-deprived astrocytes enhance glucose transporter expression and activity in rat brain endothelial cells. Biochimica et biophysica acta. 2001; 1540(3): 233–42. [DOI] [PubMed] [Google Scholar]
- 78.Qosa H, Lichter J, Sarlo M, Markandaiah SS, McAvoy K, Richard JP, Jablonski MR, Maragakis NJ, Pasinelli P, Trotti D. Astrocytes drive upregulation of the multidrug resistance transporter ABCB1 (P-Glycoprotein) in endothelial cells of the blood-brain barrier in mutant superoxide dismutase 1-linked amyotrophic lateral sclerosis. Glia. 2016; 64(8): 1298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews Neuroscience. 2006; 7(1): 41–53. [DOI] [PubMed] [Google Scholar]
- 80.Allen NJ, Eroglu C. Cell Biology of Astrocyte-Synapse Interactions. Neuron. 2017; 96(3): 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu C, Ge HM, Liu BH, Dong R, Shan K, Chen X, Yao MD, Li XM, Yao J, Zhou RM, Zhang SJ, Jiang Q, Zhao C, Yan B. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc Natl Acad Sci U S A. 2019; 116(15): 7455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Espinosa A, Estrada M, Jaimovich E. IGF-I and insulin induce different intracellular calcium signals in skeletal muscle cells. The Journal of endocrinology. 2004; 182(2): 339–52. [DOI] [PubMed] [Google Scholar]
- 83.Zhao ML, Liu JS, He D, Dickson DW, Lee SC. Inducible nitric oxide synthase expression is selectively induced in astrocytes isolated from adult human brain. Brain Res. 1998; 813(2): 402–5. [DOI] [PubMed] [Google Scholar]
- 84.Kellogg DL, McCammon KM 3rd, Hinchee-Rodriguez KS, Adamo ML, Roman LJ Neuronal nitric oxide synthase mediates insulin- and oxidative stress-induced glucose uptake in skeletal muscle myotubes. Free Radic Biol Med. 2017; 110261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009; 104(9): 1085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.King GL, Buzney SM, Kahn CR, Hetu N, Buchwald S, Macdonald SG, Rand LI. Differential responsiveness to insulin of endothelial and support cells from micro- and macrovessels. The Journal of clinical investigation. 1983; 71(4): 974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fadini GP, Mancuso P, Bertolini F, de Kreutzenberg S, Avogaro A. Amelioration of glucose control mobilizes circulating pericyte progenitor cells in type 2 diabetic patients with microangiopathy. Exp Diabetes Res. 2012; 2012274363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Escudero CA, Herlitz K, Troncoso F, Guevara K, Acurio J, Aguayo C, Godoy AS, Gonzalez M. Pro-angiogenic Role of Insulin: From Physiology to Pathology. Front Physiol. 2017; 8204. [DOI] [PMC free article] [PubMed] [Google Scholar]


