Abstract
Bacterial microcompartments (MCPs) are extremely large (100–400 nm) and diverse proteinaceous organelles that compartmentalize multistep metabolic pathways, increasing their efficiency and sequestering toxic and/or volatile intermediates. This review highlights recent studies that have expanded our understanding of the diversity, structure, function, and potential biotechnological uses of MCPs. Several new types of MCPs have been identified and characterized revealing new functions and potential new associations with human disease. Recent structural studies of MCP proteins and recombinant MCP shells have provided new insights into MCP assembly and mechanisms and raised new questions about MCP structure. We also discuss recent work on biotechnology applications that use MCP principles to develop nanobioreactors, nanocontainers, and molecular scaffolds.
Keywords: microcompartment, carboxysome, glycyl radical, nanocompartment, enzyme scaffold
Proteinaceous Bacterial Organelles
Bacterial microcompartments (MCPs) are prokaryotic organelles which contain a set of metabolic enzymes encapsulated within a protein shell. MCPs are found in 23 bacterial phyla where they function in at least eight different catabolic processes and in autotrophic CO2 fixation [1–3]. Catabolic MCPs (metabolosomes; see Glossary) consist of degradative enzymes encased within a protein shell. Their function is to enhance the growth of bacteria on particular substrates by sequestering aldehyde intermediates that are toxic and/or volatile [4] (Figure 1). Metabolosomes are found in both commensal and pathogenic bacteria, and are prevalent in the human gut where they are linked to several diseases, including cancer and heart disease [4–6]. Carboxysomes are anabolic MCPs that enhance autotrophic CO2 fixation by the Calvin cycle. They are part of a CO2 concentrating mechanism that elevates CO2 levels in the immediate vicinity of RuBisCO increasing its reaction rate and suppressing photorespiration (Figure 1) [7, 8]. Carboxysomes are found in virtually all cyanobacteria and in many chemoautotrophs, where they play a major role in global carbon fixation [8]. Metabolosomes are typically encoded by a single continuous gene cluster that includes genes for the encapsulated pathway enzymes (including a signature enzyme, an aldehyde dehydrogenase, and an alcohol dehydrogenase) as well as canonical shell proteins and regulatory elements (Figure 2) [4, 6, 9]. Carboxysomes, which may be encoded by contiguous gene clusters or dispersed sets of genes [8], are built from the same types of shell proteins as metabolosomes, but encase RuBisCO and carbonic anhydrase [8, 10] rather than catabolic enzymes.
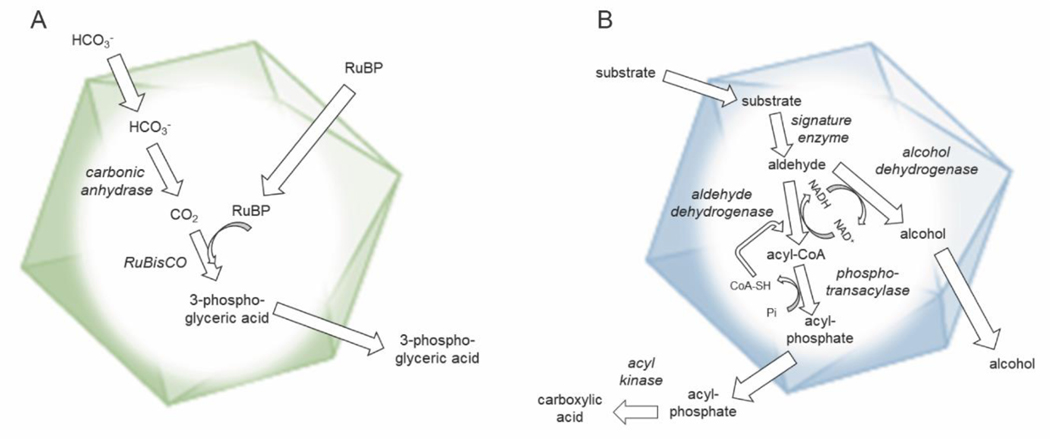
Figure 1: Mechanistic diagram of typical bacterial microcompartments (MCPs).
A) Schematic of a carboxysome. Relevant enzymes are italicized. The function of the carboxysome is to concentrate CO2 near RuBisCO to improve its catalytic efficiency and reduce photorespiration. B) Schematic of a generic metabolosome. Relevant enzymes are italicized. Most if not all metabolosomes are thought to sequester aldehyde intermediates which are toxic or volatile and subject to diffusive loss.
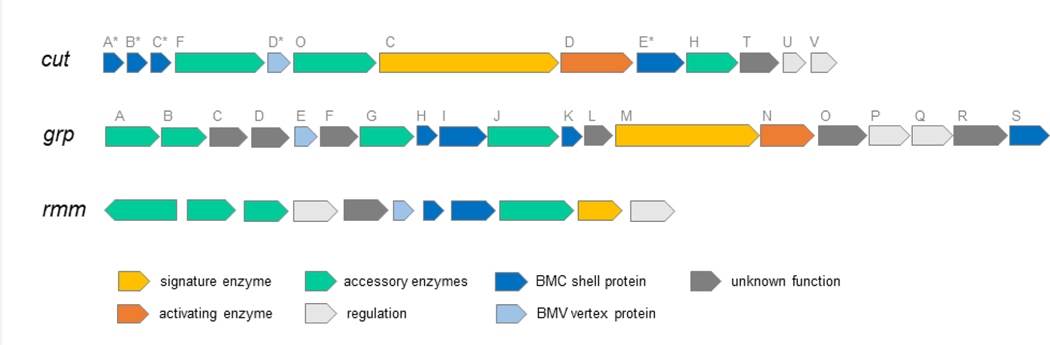
Figure 2: Operon layouts for several new classes of MCPs.
cut: choline utilization genes of E. coli 536 [5]. Asterisks on shell proteins designate different nomenclature; these genes are designated (choline microcompartment) cmcA-E rather than cut. grp: glycyl radical-propanediol genes from E. coli CFT073 [20]. rmm: Rhodococcus and Mycobacterium MCP from Mycobacteria smegmatis MC2 [25]. In general, MCPs are encoded by large operons that are sufficient for MCP formation. Signature enzymes are pathway specific enzymes characteristic of the metabolite processed by a particular MCP. Activating enzymes are Fe-S cluster dependent radical-SAM (S-adenosylmethionine) enzymes required to initiate glycyl radical formation in glycyl radical enzymes.
An understanding of MCP structure, function, and assembly has evolved considerably since the initial discovery of the carboxysome. Studies of B12-depedendent metabolosomes used for growth on ethanolamine or 1,2-propanediol and the carboxysome revealed that MCPs are used to sequester toxic or volatile metabolic intermediates and enhance autotrophic CO2 fixation by a carbon dioxide concentrating mechanism [4, 6, 8]. Bioinformatic studies illuminated the widespread and diverse nature of MCPs and identified many new MCP classes [1–3]. X-ray crystallography and cryo-electron microscopy (cryo-EM) uncovered many structural and mechanistic principles of individual MCP components and higher order assemblies [6, 10]. In addition, these fundamental advancements have paved the way for studies aimed at developing biotechnology applications based on MCP principles including their use as nanobioreactors for the enhanced production of renewable chemicals in varied hosts and the use of MCP proteins as molecular scaffolds for the spatial organization of enzymes [11–13].
This review focuses on recent work that has substantially advanced the MCP field and has raised important new questions. We discuss new types of MCPs that have recently been identified and characterized, emphasizing unique features, unanswered questions and relationships to human health. Also discussed are structural studies of MCP components and recombinant MCP shells that have provided a more detailed picture of MCP assembly and raised a number of structure-function questions. Lastly, we cover the major directions in MCP engineering, including MCPs in non-native hosts, MCPs with non-native cargo, and novel uses for MCP shell proteins.
New types of Bacterial Microcompartments
Recent bioinformatic and protein expression studies uncovered many new types of catabolic bacterial MCPs, including a widespread class whose signature enzymes use glycyl radical chemistry [2]. Later studies expanded this group identifying 5 subclasses that were termed glycyl radical MCPs 1–5 (GRMs 1–5) [1, 3, 14]. One GRM subclass metabolizes choline using a glycyl-radical choline-trimethylamine (TMA) lyase to catalyze the conversion of choline to acetaldehyde and TMA [14]. These choline utilizing (Cut) MCPs have implications for human health. Cut MCPs make a major contribution to the production of TMA in the gut which is thought to increase serum levels of trimethylamine N-oxide (TMAO) (a marker for heart and liver disease [15, 16]) and has been linked to cancer [17]. Other classes of GRMs have also recently been characterized [5, 14, 18, 19], including those processing 1,2-propanediol [20–22] or L-fuculose/rhamnose [23, 24]. Additionally, recent studies have begun to characterize another new class of MCPs referred to as Rhodococcus and Mycobacterium MCPs (RMM) which process aminoacetone using S-1-amino-2-propanol kinases (APK) [25, 26]. Below, we summarize recent work on these newly identified types of MCPs.
Cut MCPs were among the first reported B12-independent metabolosomes. Bioinformatic studies indicate that there are two types (type I and type II) which differ in regulation and shell protein composition, but share a related set of catabolic enzymes [27]. The signature enzyme for Cut MCPs is a glycyl radical choline TMA lyase (CutC) that catalyzes the conversion of choline to acetaldehyde and TMA, and was first characterized in Desulfovibrio desulfuricans [5, 14]. Similar to other glycyl radical enzymes, CutC is activated by a radical S-adenosylmethionine (SAM) activating enzyme which utilizes SAM and an Fe-S cluster to install the glycyl radical [14]. In early studies of choline degradation, the cutC gene was found in proximity to MCP shell genes, indicating that choline TMA lyase might be encapsulated within a MCP [14, 27]. Subsequent experiments in Escherichia coli 536 and Proteus mirabilis showed that CutC was associated with a MCP that is used for choline fermentation [5, 18]. Unlike other MCP gene clusters, type II cut loci lack genes for trimeric shell proteins, which are hypothesized to transport large metabolites such as enzymatic cofactors, suggesting there may be alternative mechanisms for transport of larger molecules in and out of MCPs. Type II cut gene clusters are also unique in containing a second operon that encodes three regulatory proteins (CutWXY). CutX and CutY are required for induction of the cut operon by choline, but the function of CutW is unknown [5]. In other work, a structure of a recombinant, miniature, empty type II Cut shell was determined by cryo-EM using a minimal set of proteins from Klebsiella pneumoniae [19]. This structure revealed important assembly interactions and identified a subset of the native shell proteins sufficient for shell formation. In another study, a type II Cut MCP was linked to swarming in P. mirabilis, revealing a competitive advantage for MCP-producing pathogenic bacteria [18].
Other GRMs have been identified that metabolize 1,2-propanediol, as does the well-studied propanediol utilization (Pdu) MCP. However, this type of MCP, referred to as a glycyl-radical propanediol (Grp) MCP, utilizes a glycyl radical enzyme for the conversion of 1,2-propanediol to propionaldehyde rather than a B12-depdendent enzyme [2, 20–22]. grp gene clusters have been explored in Rhodobacter capsulatus [22] and E. coli CFT073 [20]. Both encode canonical shell proteins, conserved pathway enzymes, and regulatory proteins (Figure 2). Growth studies with E. coli CFT073 showed that 1,2-propanediol fermentation by the Grp MCP stimulated anaerobic growth by providing cells with additional ATP [20]. Although B12-dependent 1,2-propanediol degradation is potentially linked to enteric pathogenesis [28], a similar association has not been reported for the Grp MCP. Interestingly, genetic studies in E. coli CFT073 showed that removal of the shell genes grpE, grpH, or grpI individually inhibits substrate fermentation, suggesting that the MCP shell plays an essential role in the enzymatic pathway, in contrast to other MCPs studied to date [20]. Another class of GRM, first identified in Clostridium phytofermentans, contains two additional encapsulated enzymes: a fuculose phosphate aldolase and lactaldehyde reductase, which perform the primary step of conversion from L-fuculose-phosphate to lactaldehyde to 1,2-propanediol [3, 23], with subsequent processing within the MCP similar to the above cases.
Elements of the less well-studied RMM MCPs found in Actinobacteria have also recently been characterized (Figure 2). Biochemical analyses of pathway enzymes identified an aminopropanol kinase (APK) that degrades aminoacetone, although the mechanism of action is still unclear [25]. Interestingly, this enzyme is found in both MCP-associated and MCP-independent contexts in Mycobacteria, implying multiple forms of the APK are optimized for different biological conditions [25]. Other MCPs studied to date contain oxygen-sensitive enzymes or volatile intermediates that require encapsulation, but enzymes and intermediates in the RMM pathway may function without the shell, raising the question of the purpose of the MCP in these instances [25]. In addition to GRM and RMM MCPs, a recent bioinformatics analysis has suggested additional MCP classes, including a novel type postulated to metabolize xanthine [29]. Such MCP types proposed from bioinformatics studies await experimental studies to confirm their functions.
Overall, newly characterized types of MCPs appear numerous, more widespread and functionally diverse than initially thought. Further study will be required to determine their specific roles in their bacterial hosts and in the human gut. Such studies should open new avenues regarding the importance of MCPs in pathogenesis and human health.
Structural and Organizational Principles
Structural studies have shed a tremendous amount of light on how MCPs function as metabolic organelles. An exterior protein shell, reminiscent of a roughly icosahedral viral capsid, is their defining feature. This outer protein layer sequesters and organizes interior enzymes and provides a mechanism for controlling metabolite diffusion in and out. Remarkably, diverse types of MCPs, with their distinct metabolic pathways, all have shells built from the same families of proteins. Based on their dispersed phylogeny, it appears that horizontal gene transfer played a major role in the diversification of this broad family of structures. Genetic, biochemical, and low-resolution electron microscopy investigations in thiobacillus spp., Nitrococcus mobilis, and Salmonella enterica LT2 laid out early concepts for metabolic organization and sequestration by MCPs [30, 31], but structural and mechanistic principles were lacking. Crystallographic studies on shell proteins from Synechocystis sp. PCC 6803 and Halothiobacillus neapolitanus, beginning in 2005 [32, 33], led to deeper understanding. To date nearly 100 crystal structures are known for the shell protein components of different MCPs. Two families of small proteins are now understood to be fundamental components of MCP shells. The first type comprises proteins with a roughly 100 amino acid BMC (bacterial microcompartment) domain (Pfam 00936). BMC proteins form symmetric (C6) hexameric units, which are the fundamental building blocks of the shell, tiling side-by-side [32, 34, 35] to form essentially flat facets of the polyhedral structure. A typical MCP operon contains between 3 to 6 paralogs of the BMC domain, which play specialized (or perhaps partially redundant) architectural and biochemical roles. Functional diversification of BMC proteins has occurred by various structural rearrangements, such as circular sequence permutations [36] and tandem domain duplications [37, 38]. In the latter case, homotrimers (known as BMC-T, as distinct from the designation BMC-H for canonical hexamers) comprise pseudohexameric units. Certain BMC subfamilies exhibit unusual flexibility [38, 39] and assembly polymorphism, including stacked double-disk forms [37], with potential implications for the somewhat irregular geometric nature of most native MCPs [40, 41]. Some BMC paralogs bind iron-sulfur clusters in their central pores [42 ], presumably to facilitate electron transfer processes that are not yet understood. A second protein family known as BMV (bacterial microcompartment vertex, Pfam PF03319), distinct and not homologous to the BMC family, was discovered to form pentamers, as required for closing up vertices in an icosahedral structure [43] (Figure 3). Owing to its much lower abundance, the BMV family was not detected as a component of purified MCPs in early studies and was revealed somewhat serendipitously by crystallographic studies.
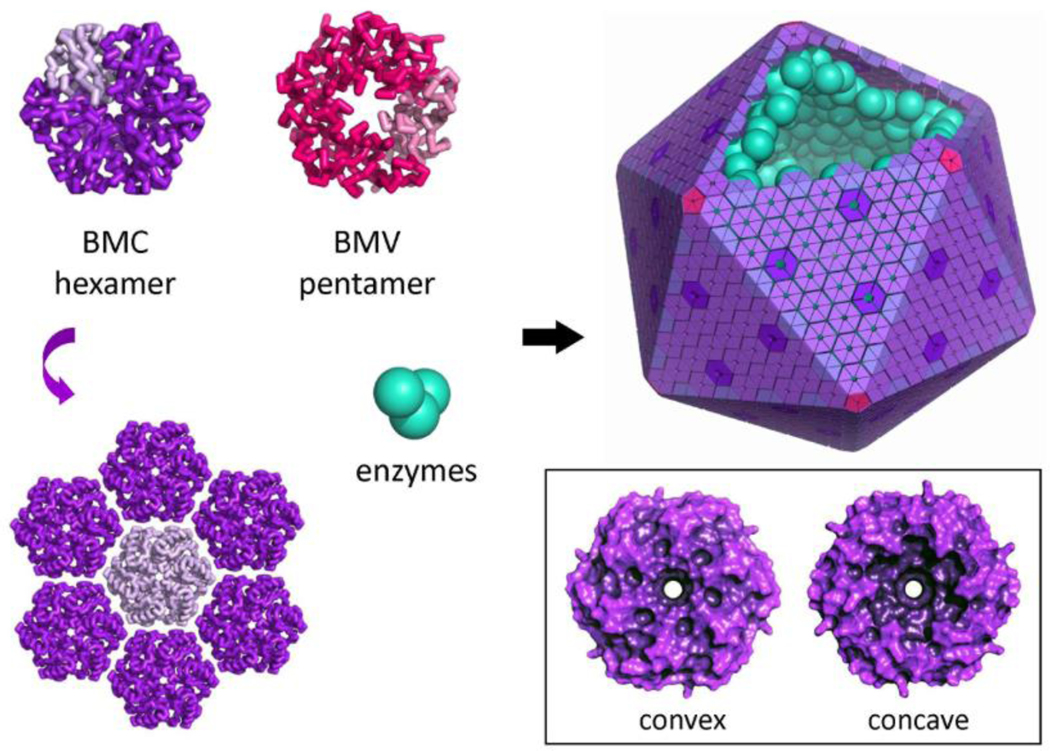
Figure 3: Structure of an MCP and canonical shell proteins.
An idealized scheme illustrating the structure and assembly of shell proteins and enzymes in an MCP. BMC hexamers (BMC-H) and trimeric pseudohexamers (BMC-T) tile into the protein sheets that form the bulk of the shell. Typical MCPs range in size from 100 – 400 nm. Pentamers belonging to the BMV family (alternatively designated BMC-P) form the vertexes. One subunit of the individual hexamer or pentamer units is shaded in a lighter color to show the subunit structure. Different paralogs of the BMC hexamer (or pseudohexamer) in an MCP shell are shaded differently. BMC shell proteins tend to be shaped like a disk with one relatively flat or convex side and one concave side with a central depression. Recent structural studies (refs: 48–52) argue that the concave sides of the BMC proteins face outward to the cytosol.
BMC hexamers possess central pores for molecular transport of substrates and products of the encapsulated reactions [32, 33]. Recent mutagenesis studies in S. enterica LT2 have emphasized the sensitivity of transport to pore structure [44–46]. Structure-guided mutagenesis of pore residues in the hexameric PduA shell protein showed that restriction of the pore compromises substrate influx into the Pdu MCP, while specific physiochemical alterations in the pore enable escape of the cytotoxic aldehyde intermediate [44]. Evidence so far suggests that transport is diffusive. Computational modeling and simulations have been informative. Molecular dynamics-based energetic calculations have examined whether BMC pores tend to favor inward diffusive movement of metabolic substrates compared to escape of volatile or toxic intermediates. Those calculations have suggested a preference for substrate influx versus intermediate escape by a factor between 3 and 10 in the case of 1,2-propanediol influx through the PduA pore [47]. For the carboxysome, similar calculations have led to a range of values for relative diffusion of HCO3− (influx) versus CO2 (escape), depending on the particular BMC shell protein examined; values have ranged from 1.4 to more than 1000 [45, 48]. Different computational approaches -- based on steady state solution of enzyme kinetic equations coupled to transport equations across the shell and inside the cell -- have been used to model whether selectivity of diffusion through pores is important for MCP function. Those studies have substantiated that facile substrate influx and limited intermediate efflux are important, though finding that favorable MCP function does not necessarily depend on an absolute selectivity preference for substrate influx [49], especially in the case of the carboxysome [50]. Beyond the canonical BMC-H shell proteins, trimeric BMC-T shell proteins present particularly interesting questions for molecular transport. In multiple cases, BMC-T shell proteins have been shown to undergo large pore-opening conformational changes, likely for the transit of larger metabolites or cofactors [37, 38, 51, 52]. Structural studies have provided evidence for allosteric gating of those conformations by key metabolites [51, 53].
Whereas most structural studies on MCP shells have been on individual hexameric or pentameric components, a series of recent X-ray crystal and cryo-EM studies from Haliangium ochraceum, Halothece sp. PCC 7418, and S. enterica have investigated synthetic miniaturized versions of closed MCP shells [19, 54–57]. These are closed, icosahedral structures, smaller than native MCPs and lacking natively encapsulated enzymes, yet still capturing important shell assembly principles. The findings of those studies have solidified several fundamental ideas while also highlighting open questions. The side-by-side packing of hexamers and the occurrence of pentamers at icosahedral vertices have confirmed models proposed based on early crystallographic studies. However, the closed-shell structures obtained recently all show an inward versus outward orientation of the hexameric BMC units that is reversed compared to models favored by earlier sequence and biochemical studies. Typical BMC hexamers are distinctly sided (Fig. 3 inset). One side is relatively flat (referred to as the convex side). The other side features a bowl-shaped depression (referred to as the concave side) along with protruding C-terminal protein tails (in certain canonical BMC domains), which are often flexible. Mutagenesis studies of enzyme binding, structural inferences about cofactor binding, and other observations about sequence and structural properties, together suggested that the concave sides of the BMC proteins likely faced inward to the MCP lumen [58–60]. Yet the recent studies of synthetic miniaturized versions of MCP shells have all appeared with the concave sides of the BMC hexamers facing outward into the cytosol [19, 53–56]. This observation provides a compelling argument for inverting previous models for MCP shell architecture, while also emphasizing the need to obtain high resolution structures for bona-fide MCPs in situ, and in the presence of encapsulated enzymes. Shell interactions with core enzymes may be important for native shell assembly, which could drive topological differences between native and empty shells. Cryo-electron tomographic studies on the carboxysome have illuminated their overall polyhedral shape and the organization of interior RuBisCO enzymes [20, 61, 62], but the resolution of those studies have so far been insufficient to clarify structural details of the shell proteins. Ultimately, higher resolution studies on diverse systems could be critical for a deeper understanding of MCP architecture.
Important questions also remain about the higher-level organization of MCPs and their dynamic behavior. In metabolosomes, certain key interior enzymes bear N-terminal sequence extensions that have been shown to bind to BMC shell proteins, providing a key recognition element for encapsulation [60, 63, 64] as well as a route for novel engineering studies as described below. Recent studies further emphasize possible roles for interior enzymes in hierarchical organization of metabolosome MCPs [19]. Compared to metabolosome MCPs, organizational strategies appear to be somewhat different in carboxysomes, with distinctions even between the two (α and β) carboxysome lineages. In the β-carboxysome, a C-terminal encapsulating peptide has been identified in the CcmN protein, which binds to a BMC shell protein [65]. Dedicated carboxysomal proteins have been identified that provide critical interactions with oligomeric RuBisCO enzymes, thereby organizing and condensing the main MCP cargo. Biochemical and structural studies have illuminated how that function is provided by the CsoS2 protein in α-carboxysomes [66, 67] and by CcmM in β-carboxysomes [68, 69], with polyvalent interactions being critical; CcmM further binds to the CcmN protein noted above [65]. At least in β-carboxysomes, if not in other MCPs, the cargo enzymes (i.e. RuBisCO and carbonic anhydrase) become preorganized prior to enclosure by shell proteins [70, 71]. Carboxysome-specific chaperones and assembly factors are furthermore required for RuBisCO maturation [72–74]. Dynamic issues of how bacterial microcompartments assemble, propagate during cell division, age, and are degraded, are emerging from live-cell fluorescence imaging studies [70, 75–77]. A recent study highlights that individual β-carboxysomes in a cell show a wide range of activities and survival times, some potentially spanning as many as seven cell divisions [76]. In rod-shaped cyanobacteria, proper inheritance of MCPs is coupled to their spatial organization in the cell. A unique partitioning system, McdAB, with both similarities and notable differences to the well-studied ParAB system, is emerging as a key player in β-type cyanobacteria [77, 78], with polyvalent condensation also playing an important role in this system [79].
Biotechnology Based on Microcompartment Principles
Due to the modularity, multi-functionality, and high catalytic efficiency of MCPs, it has been possible to apply MCP principles for a variety of novel bioengineering applications (Figure 4). Recent highlights from this growing field are provided below.
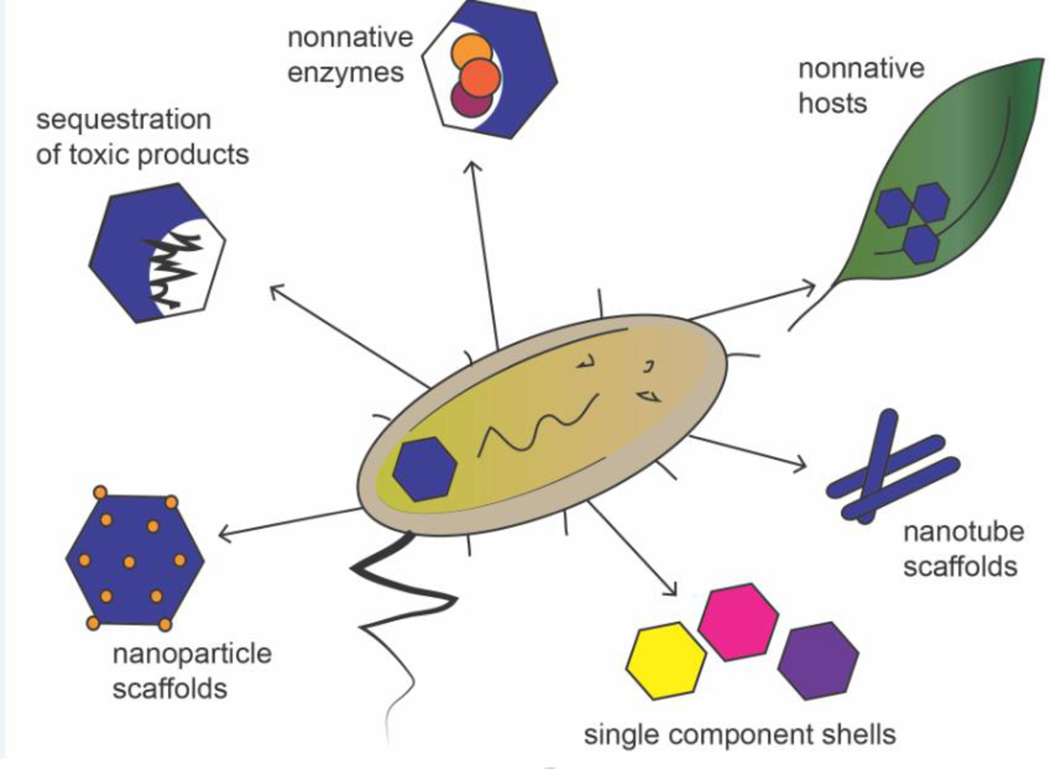
Figure 4: Biosynthetic applications of BMC shell proteins.
BMC shell proteins have been used for a variety of non-native purposes, and show promise for many biotechnological applications. (clockwise from the left) Functional MCPs have been used to catalyze the formation of regularly sized and spaced gold nano particles. MCPs have been used to sequester toxic products such as polyphosphate and compartmentalize non-native enzymes and catalytic cascades. MCPs have been introduced into non-native hosts to impart or enhance function (such as carbon fixation). MCP shell proteins have been developed to form novel scaffolding systems for spatial organization of biosynthetic systems in the cell. And single component shells are being/have been developed in order to reduce complexity and stress on the host cell when expressing foreign enzymes.
To pave the way for new applications, MCPs have been expressed in a number of industrially important hosts. Expressing MCPs in these hosts has the potential to allow improved function. For example, expressing carboxysomes in a crop plant could increase carbon fixation and overall plant growth. Recombinant expression of genes for Synechococcus elongatus β-carboxysomes produced functional, intact MCPs in an E. coli host [80]. Further, the β-carboxysomes shell proteins were successfully replaced with α-carboxysome or metabolosome homologs, highlighting the interchangeability of MCP components for the production of chimeric organelles [80]. Carboxysomes were also introduced into tobacco plants producing functional MCPs; however, plants displayed reduced photosynthetic performance relative to wild-type counterparts, suggesting additional modifications may be needed to optimize MCP function [81]. Introducing MCPs into heterologous hosts has been carried out in parallel with metabolosomes. In early studies, the Pdu MCP from Citrobacter freundii was expressed in E. coli [82]. More recently, the Pdu MCP from C. freundii, was successfully produced in the industrially relevant Corynebacterium glutamicum, where protein targeting systems based on synthetic scaffolding domains were also developed for encapsulation of non-native cargo [83]. In another study, the use of heat tolerant MCPs was explored by expressing thermophilic Pdu MCPs in Bacillus subtilis, expanding the range of industrial applications in which MCPs could be used [84]. In addition, a system based on the flp recombinase was used to rapidly transfer the Pdu MCP to a variety of heterologous hosts, increasing the ease and efficiency of creating recombinant MCP systems [85]. These examples highlight potential applications of MCPs and their emerging “plug-and-play” capabilities with respect to interchangeable parts, encapsulation of non-native cargo, and expression in diverse hosts.
MCP shells have also been employed to compartmentalize novel enzymes and reaction pathways to enhance pathway performance, inhibit counterproductive by-reactions, and isolate toxic molecules. The most commonly used approach has been to produce empty MCP shells together with desired cargo proteins in a suitable host [86]. Cargo proteins are targeted to the empty shell using encapsulation peptides (EPs), which are short, terminal peptides used for targeting native MCPs enzymes [63]. Using this approach, a proof-of-concept nanobioreactor, comprised of pyruvate decarboxylase and alcohol dehydrogenase from Zymomonas mobilis encapsulated within an MCP shell, was produced in E. coli and shown to increase ethanol production compared to expression of non-encapsulated enzymes [87]. In another recent example, esterase Est5 from soil metagenome, β-galactosidase, and glycerol dehydrogenase from E. coli were directed to an empty Pdu shell by attaching the 18-residue PduP EP [88]. Purified particles were functional, demonstrating the utility of EPs in trafficking diverse non-native cargo to MCPs. In other studies, toxic Lysis Protein E (from φ X174) was tagged with an EP and directed to a Pdu MCP shell, allowing enhanced toxic protein production [89]. MCP-encapsulated polyphosphate kinase was used to sequester inorganic phosphate (Pi), a common water pollutant. Uptake of Pi by the cell was increased, and cellular stability of polyphosphate, which is typically depolymerized in the cytosol, also increased indicating that compartmentalization within an MCP shell reduced undesired by-reactions [90]. In an effort to improve the efficiency of heterologous cargo encapsulation above that seen when native or synthetic EPs are used, Hagen et. al. utilized a SpyCatcher/SpyTag system [91]. The SpyCatcher element was inserted into a loop on the luminal side of a trimeric shell protein and the SpyTag was fused onto various fluorescent cargo proteins which were efficiently encapsulated. Similarly, Lee et al. developed a heterodimeric coiled-coil system (CC-Di-A/B), which was used to target fluorescent cargo proteins to the lumen of empty Pdu MCPs [92]. The development of these encapsulation systems shows promise for the efficient localization of varied enzymes and multi-enzyme pathways within MCPs, further enabling synthetic systems to take advantage of the natural ability of the MCP to enhance metabolic pathways by compartmentalization.
MCP components have also been repurposed as molecular scaffolds. Gold nanoparticles (NPs) were fabricated on the surface of purified Pdu MCPs where the hexameric BMC shell proteins promoted both regularity in the size of NPs as well as uniform spatial distribution [93]. These MCP-scaffolded NPs display unencumbered inorganic catalytic ability, and the activity of the MCPs enzymatic cargo was also maintained, resulting in a hybrid organic-inorganic catalyst. Additionally, single BMC hexameric and trimeric shell proteins from the Pdu MCP (PduA, PduB, and PduB’) have been purified and assembled at an oil/water interface into functional protein shells capable of encapsulating native and non-native enzymes as well as small molecules that can be used to assess the diffusive properties of these shells [94]. Single BMC proteins have also been employed to create scaffolds of novel structure. A version of PduA modified for increased solubility assembled into hollow, filamentous nanotubes [95]. Adding the heterodimeric coiled-coil system previously mentioned [96] allowed selective scaffolding of tagged proteins on these filaments. Similarly, a series of recent studies developed an immobilized catalytic cascade for cell-free biotransformation applications. The enzymes were attached to a EutM filament using the SpyCatcher system [13, 97, 98], resulting in a scaffolded multistep pathway for the production of chiral amines [99]. Expressing heterologous enzymes tagged with an EP, caused the formation of functional shell-free enzyme aggregates [95, 100], providing another flavor of molecular scaffold. Using nanotube forming BMC shell proteins from Haliangium ochraceum and the RMM MCP, Huang et al. attempted to functionalize BMC nanotubes with heme moieties using CxxCH motifs which are recognized by the cytochrome c maturation system to form redox chains. However, while the CxxCH insertions did not appear to affect nanotube formation, and heme was incorporated at the sites, the heme-BMC protein did not form nanotubes [101]. These applications highlight the use of MCP principles to engineer a variety of systems suited to perform diverse and important industrial roles, and also the need for a more thorough understanding of assembly mechanisms, and how the introduction of non-native proteins or functional groups interact with BMC proteins and affect their structure.
Concluding remarks
In this review, we highlight our current understanding of MCPs along with some of the most recent advances in the MCP field. With the discovery of glycyl radical and other new types of MCPs, these organelles have shown themselves to be far more widespread and varied than initially thought, yet much of this diversity remains unexplored. Structural studies have revealed that MCP shells are built primarily from a family of small BMC domain proteins that have undergone remarkable divergence enabling wide variation in MCP function; despite exciting advances, many aspects underlying BMC diversification remain to be understood. As MCP principles have been uncovered, biotechnology applications have moved toward de novo construction of protein compartments using advanced engineering methods. Among the most challenging questions that surround MCPs are how thousands of individual diversified MCP components dynamically assemble into functional units and how this assembly influences function (see outstanding questions box). These queries mirror broad challenges in protein science generally. MCPs serve as ideal systems to explore how extremely large multiprotein complexes are assembled and functionally diversified. A more complete understanding of these principles will guide further biotechnology developments and may aid biomedical interventions that modulate MCP activities.
Outstanding Questions Box.
What are the salient similarities and differences between diverse MCP class types?
What protein-protein interactions drive assembly of functional MCPs, and is this pathway shared among varying MCP classes?
Are the molecular associations that organize the numerous enzymatic and structural components of metabolosomes entirely specific and deterministic, or might weaker and less-specific interactions operate more akin to the condensates formed by ‘membraneless organelles’ in eukaryotic cells?
How do the structures of native MCPs differ from cargoless and chimeric MCPs in size and composition?
How are enzyme components arranged within the MCP to enhance pathway efficiency?
How are large substrates and cofactors transported across MCP shells with and without the use of gated pores?
Highlights.
Toxic and volatile metabolites present a problem to biological systems which some bacteria overcome by producing bacterial microcompartments (MCPs) to sequester these molecules.
Originally thought to be broadly categorized by two types, carbon fixing or B12 dependent, recent studies have uncovered many new classes of MCPs a number of which are associated with human disease.
Recent structural studies of MCP proteins and higher order complexes have revealed many of the principles that underlie MCP assembly.
As the structural and biochemical knowledge of MCPs has grown, MCP principles have driven new bioengineering applications
Acknowledgements
This work was supported by grant AI081146 from the National Institutes of Health to T.A.B. and T.O.Y.
Glossary:
- Carboxysomes
A bacterial microcompartment which contains RuBisCO and carbonic anhydrase, and functions to increase the rate of carbon fixation.
- Chemoautotrophs
An organism which derives energy from the oxidation of inorganic compounds.
- Cryo-electron tomography
An electron microscopy technique for taking high-resolution (1 – 4 nm) three-dimensional images of (typically) biological complexes.
- Metabolosomes
A bacterial microcompartment that contains pathway enzymes for the catabolism of various metabolites including choline, ethanolamine, and 1,2-propanediol.
- Signature enzyme
The enzyme characteristic of a certain type of bacterial microcompartment.
- Trimeric shell proteins
Pseudohexameric BMC proteins built from three subunits that each contain 2 BMC domains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdul-Rahman F. et al. (2013) The distribution of polyhedral bacterial microcompartments suggests frequent horizontal transfer and operon reassembly. Mol. Phylogenetics Evol. 01 (4), 1–7. [Google Scholar]
- 2.Jorda J. et al. (2013) Using comparative genomics to uncover new kinds of protein-based metabolic organelles in bacteria. Protein Sci. 22 (2), 179–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarzycki J. et al. (2015) Bioinformatic characterization of glycyl radical enzyme-associated bacterial microcompartments. Appl. Environ. Microbiol. 81, 8315–8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhury C. et al. (2014) Diverse bacterial microcompartment organelles. Microbiol. Mol. Biol. Rev. 78 (3), 438–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herring TI et al. (2018) A bacterial microcompartment is used for choline fermentation by Escherichia coli 536. J. Bacteriol. 200, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerfeld CA et al. (2018) Bacterial microcompartments. Nat. Rev. Microbiol. 16, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long BM et al. (2016) Cyanobacterial CO2-concentrating mechanism components: function and prospects for plant metabolic engineering. Curr. Opin. Plant Biol. 31, 1–8. [DOI] [PubMed] [Google Scholar]
- 8.Rae BD et al. (2013) Functions, compositions, and evolution of the two types of carboxysomes: polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Microbiol. Mol. Biol. Rev. 77 (3), 357–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobik TA et al. (2015) Bacterial microcompartments: Widespread prokaryotic organelles for isolation and optimization of metabolic pathways. Mol. Microbiol. 98, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeates TO et al. (2011) The protein shells of bacterial microcompartment organelles. Curr. Opin. Struct. Biol. 21, 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MJ et al. (2019) Biotechnological advances in bacterial microcompartment technology. Trends Biotechnol. 37, 325–336. [DOI] [PubMed] [Google Scholar]
- 12.Plegaria JS and Kerfeld CA (2018) Engineering nanoreactors using bacterial microcompartment architectures. Curr. Opin. Biotechnol. 51, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt-Dannert S. et al. (2018) Building a toolbox of protein scaffolds for future immobilization of biocatalysts. Appl. Microbiol. Biotechnol. 102 (19), 8373–8388. [DOI] [PubMed] [Google Scholar]
- 14.Craciun S and Balskus EP (2012) Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. PNAS 109, 21307–21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YM et al. (2016) Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trøseid M. et al. (2015) Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 277, 717–726. [DOI] [PubMed] [Google Scholar]
- 17.Bae S. et al. (2014) Plasma choline metabolites and colorectal cancer risk in the women’s health initiative observational study. Cancer Res. 74, 7442–7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jameson E. et al. (2016) Anaerobic choline metabolism in microcompartments promotes growth and swarming of Proteus mirabilis. Environ. Microbiol. 18, 2886–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalnins G. et al. (2020) Encapsulation mechanisms and structural studies of GRM2 bacterial microcompartment particles. Nat. Commun. 11, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundin AP et al. (2020) Genetic characterization of a glycyl radical microcompartment used for 1,2-Propanediol fermentation by uropathogenic Escherichia coli CFT073 J. Bacteriol. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarzycki J. et al. (2017) In vitro characterization and concerted function of three core enzymes of a glycyl radical enzyme-associated bacterial microcompartment. Sci. Rep. 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindel HS et al. (2019) Characterization of a glycyl radical enzyme bacterial microcompartment pathway in Rhodobacter capsulatus. J. Bacteriol. 201, e00343–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petit E. et al. (2013) Involvement of a bacterial microcompartment in the metabolism of fucose and rhamnose by Clostridium phytofermentans. PLoS ONE 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaMattina JW et al. (2016) 1,2-Propanediol dehydration in Roseburia inulinivorans: structural basis for substrate and enantiomer selectivity. J. Biol. Chem. 291 (30), 15515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallette E and Kimber MS (2018) Structural and kinetic characterization of (S)-1-amino-2-propanol kinase from the aminoacetone utilization microcompartment of Mycobacterium smegmatis. J. Biol. Chem. 293 (51), 19909–19918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallette E and Kimber MS (2018) Structure and Kinetics of the S-(+)-1-Amino-2-propanol Dehydrogenase from the RMM Microcompartment of Mycobacterium smegmatis. Biochemistry 57 (26), 3780–3789. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-del Campo A. et al. (2015) Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. mBio 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faber F. et al. (2017) Respiration of microbiota-derived 1,2-propanediol drives Salmonella expansion during colitis. PLoS Pathog. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravcheev DA et al. (2019) Comparative genomic analysis reveals novel microcompartment-associated metabolic pathways in the human gut microbiome. Front. Genet. 10, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shively JM (1974) Inclusion bodies of prokaryotes. Annu. Rev. Microbiol. 28 (0), 167–87. [DOI] [PubMed] [Google Scholar]
- 31.Bobik TA et al. (1999) The propanediol utilization (pdu) operon of Salmonella enterica serovar Typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B12-dependent 1,2-propanediol degradation. J. Bacteriol. 181, 5967–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerfeld CA et al. (2005) Microbiology: Protein structures forming the shell of primitive bacterial organelles. Science 309, 936–938. [DOI] [PubMed] [Google Scholar]
- 33.Tsai Y. et al. (2007) Structural analysis of CsoS1A and the protein shell of the Halothiobacillus neapolitanus carboxysome. PLoS Biol. 5 (6), e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dryden KA et al. (2009) Two-dimensional crystals of carboxysome shell proteins recapitulate the hexagonal packing of three-dimensional crystals. Protein Sci. 18 (12), 2629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutter M. et al. (2016) Visualization of bacterial microcompartment facet assembly using high-speed atomic force microscopy. Nano Lett. 16 (3), 1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowley CS et al. (2008) Structure of the PduU shell protein from the Pdu microcompartment of Salmonella. Structure 16, 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein MG et al. (2009) Identification and structural analysis of a novel carboxysome shell protein with implications for metabolite transport. J. Mol. Biol. 392, 319–333. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka S. et al. (2010) Structure and mechanisms of a protein-based organelle in Escherichia coli. Science 327, 81–84. [DOI] [PubMed] [Google Scholar]
- 39.Ochoa JM et al. (2020) Symmetry breaking and structural polymorphism in a bacterial microcompartment shell protein for choline utilization. Protein Sci. 29 (11), 2201–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy NW et al. (2020) Apparent size and morphology of bacterial microcompartments varies with technique. PLoS ONE. 15 (3), e0226395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bag S. et al. (2016) Classification of polyhedral shapes from individual anisotropically resolved cryo-electron tomography reconstructions. BMC Bioinformatics 17 (1), 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowley CS et al. (2010) Structural insight into the mechanisms of transport across the Salmonella enterica Pdu microcompartment shell. J. Biol. Chem. 285, 37838–37846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka S. et al. (2008) Atomic-level models of the bacterial carboxysome shell. Science 319, 1083–1086. [DOI] [PubMed] [Google Scholar]
- 44.Chowdhury C. et al. (2015) Selective molecular transport through the protein shell of a bacterial microcompartment organelle. PNAS 112, 2990–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faulkner M. et al. (2020) Molecular simulations unravel the molecular principles that mediate selective permeability of carboxysome shell protein. Sci. Rep. 10 (1), 17501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slininger Lee MF et al. (2017) Evidence for improved encapsulated pathway behavior in a bacterial microcompartment through shell protein engineering. ACS Synth. Biol. 6 (10), 1880–1891. [DOI] [PubMed] [Google Scholar]
- 47.Park J. et al. (2017) Molecular dynamics simulations of selective metabolite transport across the propanediol bacterial microcompartment shell. J. Phys. Chem. B 121, 8149–8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahinthichaichan P. et al. (2018) Selective permeability of carboxysome shell pores to anionic molecules. J. Phys. Chem. B 122 (39), 9110–9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jakobson CM et al. (2017) A systems-level model reveals that 1,2-Propanediol utilization microcompartments enhance pathway flux through intermediate sequestration. PLoS Comput. Biol. 13 (5), e1005525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mangan NM et al. (2016) pH determines the energetic efficiency of the cyanobacterial CO2 concentrating mechanism. PNAS 113 (36), E5354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsson AM et al. (2017) Crystal structures of beta-carboxysome shell protein CcmP: ligand binding correlates with the closed or open central pore. J. Exp. Bot. 68 (14), 3857–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mallette E and Kimber MS (2017) A complete structural inventory of the mycobacterial microcompartment shell proteins constrains models of global architecture and transport. J. Biol. Chem. 292 (4), 1197–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson MC et al. (2015) An allosteric model for control of pore opening by substrate binding in the EutL microcompartment shell protein. Protein Sci. 24, 956–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greber BJ et al. (2019) The plasticity of molecular interactions governs bacterial microcompartment shell assembly. Structure 27, 749–763.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jorda J. et al. (2016) Structure of a novel 13 nm dodecahedral nanocage assembled from a redesigned bacterial microcompartment shell protein. Chem. Comm. 52 (28), 5041–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sutter M. et al. (2017) Assembly principles and structure of a 6.5-MDa bacterial microcompartment shell. Science 356, 1293–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutter M. et al. (2019) Structure of a Synthetic beta-Carboxysome Shell. Plant Physiol. 181 (3), 10501058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Held M. et al. (2016) Engineering formation of multiple recombinant Eut protein nanocompartments in E. coli. Sci. Rep. 6, 24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson MC et al. (2014) Structure of a bacterial microcompartment shell protein bound to a cobalamin cofactor. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 70 (Pt 12), 1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan C. et al. (2012) Interactions between the termini of lumen enzymes and shell proteins mediate enzyme encapsulation into bacterial microcompartments. PNAS 109, 14995–15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai W. et al. (2018) Visualizing individual RuBisCO and its assembly into carboxysomes in marine cyanobacteria by cryo-electron tomography. J. Mol. Biol. 430, 4156–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iancu CV et al. (2010) Organization, structure, and assembly of alpha-carboxysomes determined by electron cryotomography of intact cells. J. Mol. Biol. 396 (1), 105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan C. et al. (2010) Short N-terminal sequences package proteins into bacterial microcompartments. PNAS 107, 7509–7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choudhary S. et al. (2012) Engineered protein nano-compartments for targeted enzyme localization. PLoS ONE. 7 (3), e33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kinney JN et al. (2012) Elucidating essential role of conserved carboxysomal protein CcmN reveals common feature of bacterial microcompartment assembly. J. Biol. Chem. 287, 17729–17736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oltrogge LM et al. (2020) Multivalent interactions between CsoS2 and RuBisCO mediate alpha-carboxysome formation. Nat. Struct. Mol. Biol. 27 (3), 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y. et al. (2018) Deciphering molecular details in the assembly of alpha-type carboxysome. Sci. Rep. 8 (1), 15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryan P. et al. (2019) The small RbcS-like domains of the beta-carboxysome structural protein CcmM bind RuBisCO at a site distinct from that binding the RbcS subunit. J. Biol. Chem. 294 (8), 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H. et al. (2019) RuBisCO condensate formation by CcmM in beta-carboxysome biogenesis. Nature 566 (7742), 131–135. [DOI] [PubMed] [Google Scholar]
- 70.Chen AH et al. (2013) The bacterial carbon-fixing organelle is formed by shell envelopment of preassembled cargo. PLoS ONE 8 (9), e76127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cameron JC et al. (2013) Biogenesis of a bacterial organelle: the carboxysome assembly pathway. Cell 155 (5), 1131–40. [DOI] [PubMed] [Google Scholar]
- 72.Wheatley NM et al. (2014) Structure and identification of a pterin dehydratase-like protein as a ribulose-bisphosphate carboxylase/oxygenase (RuBisCO) assembly factor in the alpha-carboxysome. J. Biol. Chem. 289 (11), 7973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang F. et al. (2019) Roles of RbcX in carboxysome biosynthesis in the Cyanobacterium Synechococcus elongatus pcc7942. Plant Physiol. 179 (1), 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang F. et al. (2020) Rubisco accumulation factor 1 (Raf1) plays essential roles in mediating Rubisco assembly and carboxysome biogenesis. PNAS 117 (29), 17418–17428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai F. et al. (2013) The structure of CcmP, a tandem bacterial microcompartment domain protein from the β-carboxysome, forms a subcompartment within a microcompartment. J. Biol. Chem. 288, 16055–16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill NC et al. (2020) Life cycle of a cyanobacterial carboxysome. Sci. Adv. 6 (19), eaba1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Savage DF et al. (2010) Spatially ordered dynamics of the bacterial carbon fixation machinery. Science 327 (5970), 1258–61. [DOI] [PubMed] [Google Scholar]
- 78.MacCready JS et al. (2018) Protein gradients on the nucleoid position the carbon-fixing organelles of cyanobacteria. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacCready JS et al. (2020) Origin and evolution of carboxysome positioning systems in cyanobacteria. Mol. Biol. Evol. 37 (5), 1434–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang Y. et al. (2018) Engineering and modulating functional cyanobacterial CO2-fixing organelles. Front. Plant Sci. 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Long BM et al. (2018) Carboxysome encapsulation of the CO2-fixing enzyme RuBisCO in tobacco chloroplasts. Nat. Commun. 9, 3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parsons JB et al. (2008) Biochemical and structural insights into bacterial organelle form and biogenesis. J. Biol. Chem. 283, 14366–14375. [DOI] [PubMed] [Google Scholar]
- 83.Huber I. et al. (2017) Construction of recombinant Pdu metabolosome shells for small molecule production in Corynebacterium glutamicum. ACS Synth. Biol. 6, 2145–2156. [DOI] [PubMed] [Google Scholar]
- 84.Wade Y. et al. (2019) Heterologous microcompartment assembly in bacillaceae: Establishing the components necessary for scaffold formation. ACS Synth. Biol. 8, 1642–1654. [DOI] [PubMed] [Google Scholar]
- 85.Graf L. et al. (2018) Transfer and analysis of Salmonella pdu genes in a range of gram-negative bacteria demonstrate exogenous microcompartment expression across a variety of species. Microb. Biotechnol. 11, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parsons JB et al. (2010) Synthesis of empty bacterial microcompartments, directed organelle protein incorporation, and evidence of filament-associated organelle movement. Mol. Cell 38, 305–315. [DOI] [PubMed] [Google Scholar]
- 87.Lawrence AD et al. (2014) Solution structure of a bacterial microcompartment targeting peptide and its application in the construction of an ethanol bioreactor. ACS Synth. Biol. 3 (7), 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wagner HJ et al. (2017) Engineering bacterial microcompartments with heterologous enzyme cargos. Eng. Life Sci. 17 (1), 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yung MC et al. (2017) Re-directing bacterial microcompartment systems to enhance recombinant expression of lysis Protein E from bacteriophage φX174 in Escherichia coli. Microb. Cell Fact. 16, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liang M. et al. (2017) Bacterial microcompartment-directed polyphosphate kinase promotes stable polyphosphate accumulation in E. coli. Biotechnol. J. 12. [DOI] [PubMed] [Google Scholar]
- 91.Hagen A. et al. (2018) Programmed loading and rapid purification of engineered bacterial microcompartment shells. Nat. Commun. 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee MJ et al. (2018) De novo targeting to the cytoplasmic and luminal side of bacterial microcompartments. Nat. Commun. 9 (1), 3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bari NK et al. (2018) Nanoparticle fabrication on bacterial microcompartment surface for the development of hybrid enzyme-inorganic catalyst. ACS Catal. 8, 7742–7748. [Google Scholar]
- 94.Bari NK et al. (2020) Functional protein shells fabricated from the self-assembling protein sheets of prokaryotic organelles. J. Mater. Chem. B 8, 523–533. [DOI] [PubMed] [Google Scholar]
- 95.Lee MJ et al. (2018) Engineered synthetic scaffolds for organizing proteins within the bacterial cytoplasm. Nat. Chem. Biol. 14 (2), 142–147. [DOI] [PubMed] [Google Scholar]
- 96.Fletcher JM et al. (2013) Self-assembling cages from coiled-coil peptide modules. Science 340 (6132), 595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang G. et al. (2019) Developing a protein scaffolding system for rapid enzyme immobilization and optimization of enzyme functions for biocatalysis. ACS Synth. Biol. 8 (8), 1867–1876. [DOI] [PubMed] [Google Scholar]
- 98.Zhang G. et al. (2019) Protein-based scaffolds for enzyme immobilization. Meth. Enzymol. 617, 323–362. [DOI] [PubMed] [Google Scholar]
- 99.Mutti FG et al. (2015) Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades. Science 349 (6255), 1525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee MJ et al. (2016) Employing bacterial microcompartment technology to engineer a shell-free enzyme-aggregate for enhanced 1,2-propanediol production in Escherichia coli. Metab. Eng. Commun. 36, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang J. et al. (2019) Functionalization of bacterial microcompartment shell proteins with covalently attached heme. Front. Bioeng. Biotechnol. 7, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]