Abstract
There is no doubt that the development of transplantable synthetic kidneys could improve the outcome for the many millions of people worldwide suffering from chronic kidney disease. Substantial progress has been made in the last 6 years in the generation of kidney tissue from stem cells. However, the limited scale, incomplete cellular complexity and functional immaturity of such structures suggests we are some way from this goal. While developmental biology has successfully guided advances to date, these human kidney models are limited in their capacity for ongoing nephrogenesis and lack corticomedullary definition, a unified vasculature and a coordinated exit path for urinary filtrate. This review will reassess our developmental understanding of how the mammalian embryo manages to create kidneys, how this has informed our progress to date and how both engineering and developmental biology can continue to guide us towards a synthetic kidney.
Keywords: kidney development, organogenesis, synthetic embryology, organoid, pluripotent stem cell, directed differentiation
Introduction
The last decade has seen an explosion in the development of human tissue models, either via factor-based directed differentiation of pluripotent stem cells or enforced transdifferentiation of somatic cells using key transcription factors. The genes overexpressed and/or the growth factors used in such methods have been inferred from our understanding of mammalian embryology. The spontaneous capacity for pluripotent stem cells to recapitulate early embryogenic and organogenic events has been evident since the application of embryoid bodies as an assay of tri-germ layer potential [1–3]. However, the accuracy with which such spontaneous morphogenetic events were observed to occur when clumps of pluripotent cells are differentiated in 3D has been quite a revelation, leading to the ability to recreate models of regions of the brain, gastrointestinal tract and even the early embryo [4–10]. These observations led to the coining of the term ‘synthetic embryology’.
Synthetic embryology attempts to recapitulate developmental morphogenesis and has grown out of the observations of self-organisation of pluripotent stem cells when differentiated in vitro. By contrast, synthetic biology encompasses the molecular engineering of synthetic switches, devices or systems to modulate or redesign cellular identity and patterning. These two fields are clearly overlapping. Synthetic biology can extend to synthetic morphogenesis, such as the manipulation of cell-cell adhesion [11, 12]. While pluripotent stem cell cultures can also be manipulated at the molecular level to change or understand mechanism, the larger challenge is how to harness these emergent properties for the purposes of recreating functional tissue, which is more in the realm of synthetic embryology. The embryo gets this right every time. So how much can we deviate from what occurs in nature and still achieve a functional outcome? And how can we learn from nature to get around some of the challenges of in vitro organogenesis? My perspectives here come from the challenge of rebuilding a kidney, perhaps one of the most complicated organs one could attempt.
Synthetic embryology and how this applies to models of human kidney.
For the kidney, which is derived from the intermediate mesoderm, the step wise differentiation protocols for generating human kidney tissue from pluripotent stem cells involve an initial patterning to posterior primitive streak, intermediate mesoderm and then metanephric mesenchyme (reviewed in Little and Combes, 2019) [13]. Hence, in the early phases most kidney organoid protocols are similar to other mesodermal differentiation protocols, such as those developed for heart and blood [14, 15]. The resulting kidney organoid models represent remarkably complex multicellular tissues containing morphologically recognisable nephrons surrounded by a renal stroma and primitive vasculature [13] (Figure 1B). This is a testament to the capacity of cells to self-organise. Such kidney organoids have provided an unprecedented opportunity to better understand kidney morphogenesis [16–20] and model particularly genetic forms of human kidney disease [17–23]. The robustness and reproducibility of such protocols is evidenced in the adoption of such methods by groups around the globe, but most clearly from the increasing amount of single cell transcriptional profiling performed on kidney organoid models [24–29]. Comparison with the human fetal kidney would suggest strong but not perfect congruence with the target organ for many cell types [24, 29]. However, some components remain missing and the lack of maturity of individual nephron segments continues to limit their applicability as disease models [13]. More critically, kidney organoids are not kidneys as they lack of scale, are anatomically inaccurate and do not have an integrated vascular supply. The degree to which these deficits represent a challenge of ‘time in culture’ versus a mispatterning of cellular identity remains unclear, although the relevance of these limitations depend upon the application of the organoids. We can envisage perhaps two broad applications of pluripotent stem cell-derived kidney tissues in which synthetic embryology can be discussed. The first of these is the use of kidney organoids to dissect the kidney patterning and morphogenesis in vitro (Figure 1). This will not only provide opportunities to improve our understanding of human kidney development but facilitate improved modelling of particular disease states. The second is the generation of functional kidney tissue for use in vivo (Figure 1). This can be informed by the former, but also by our re-evaluation of how biology has achieved similar outcomes in different organisms or even in different tissues. For the purposes of clinical utility, a synthetic tissue may not have to perfectly replicate the original as long as it can deliver the required function. Perhaps this is where our reliance on a step-by-step replication of development has proven a hindrance rather than an asset. Perhaps our understanding of normal development remains insufficient to show us the way. And perhaps there are lessons yet to be learned from development that we have overlooked.
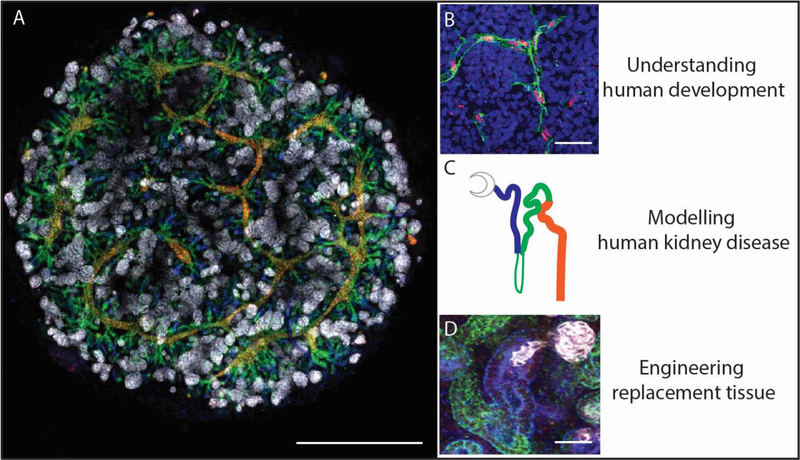
Figure 1.
Stem cell-derived kidney organoids contain patterning and segmenting nephrons with a surrounding stroma and vasculature. Such synthetic kidney tissue can be used to dissect the molecular basis of human development, model human kidney disease and potentially generate replacement functional renal tissue. A. Induced pluripotent stem cell-derived kidney organoid generated as a micromass culture on a Transwell filter [28]. White: NPHS1; Blue: lotus tetraglonolobus lectin; Green: E-cadherin; Red: GATA3. Scale bar = 1mm; B. Endothelial plexus formation within a kidney organoid. Green: CD31; Red; SOX17; Blue: DAPI. Scale bar = 50uM. C. Diagram of a forming nephrons colour coded to show the patterning and segmentation within A and D. D. Early nephron showing forming podocytes (white; NPHS1), proximal (blue; LTL) and distal (green; E-cadherin). Scale bar = 50uM.
This review will consider where the field of synthetic kidneys has reached, how confident we can be with the tissues generated, what alternatives could be deployed at the level of cell, tissue and organ, and whether we should reconsider our knowledge of urogenital development to change our engineering approach.
The mammalian kidney and how it develops
Let’s return to the embryo. Our kidneys are vital organs not simply for removing waste products but for fluid homeostasis, blood volume and pressure, red cell count and bone density. The postnatal human kidney is capable of filtering the entire blood volume 60 times a day to remove waste products whilst reclaiming 99% of the fluid filtered as well as selectively reabsorbing or excreting metabolites and ions. This is accomplished in mammals by the metanephroi, which are the paired retroperitoneal organs that represent the third pair of excretory organs formed during embryonic development. While all derived from the intermediate mesoderm, formation of the pronephroi, mesonephroi and then metanephroi occurs in that order as the body axis of the mammalian embryo extends (Figure 2A). All three of these paired organs forms ‘nephrons’ with a vascularised glomerulus at one end from which extends unbranching but regionally patterned tubules (Figure 2B). The blood is filtered within the glomerulus with the urinary filtrate passing down the nephron and into the collecting ducts before exiting the kidney via the ureter. Along the way, specialised segment-specific cell types reclaim water, amino acids and other metabolites whilst regulating pH and actively excreting ions. It is the arrangement and number of tubules that varies between pro-, meso- and metanephroi. In addition, the metanephroi are the only excretory organs that do not degenerate during mammalian embryogenesis. While in lower vertebrates such as fish the mesonephros represents the permanent kidney (a metanephros does not form), only remnant tubules remain of the human embryonic mesonephros. Despite this fact, similarities exist at the molecular and cellular level in the formation, patterning and segmentation of nephrons even between the pronephric tubules of a zebrafish and a mammalian metanephric tubule [30, 31] (Figure 2C). There is also molecular congruence between the nephrons in the murine meso- and metanephros [32].
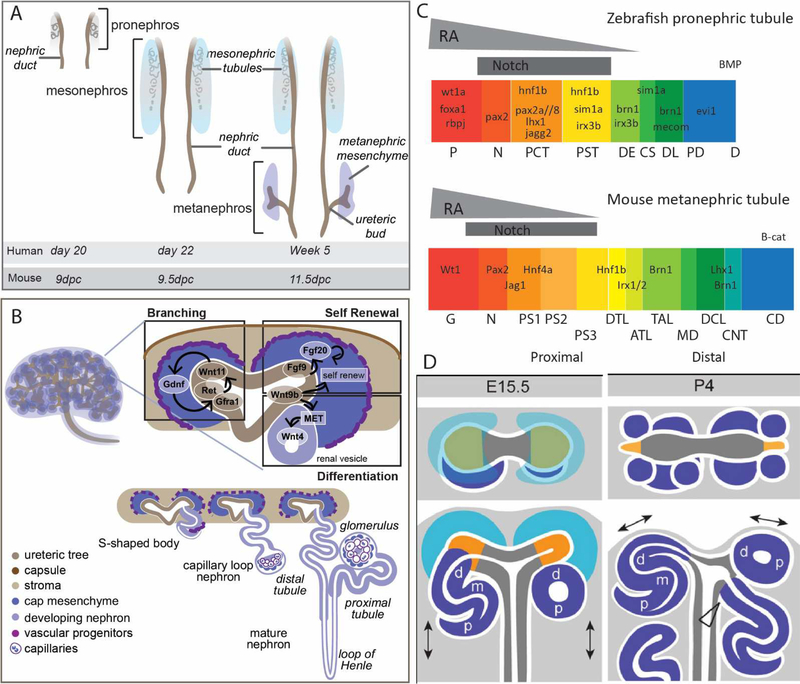
Figure 2. Vertebrate variations on a theme in building a kidney.
A. Three pairs of excretory organs arise from the intermediate mesoderm during mammalian development. From anterior to posterior, these are the pronephros, mesonephros and metanephros. The metanephros is the permanent postnatal kidney in mammals with both the pronephros and mesonephros degenerating. The appearance of each pair of organs during human and mouse gestation. dpc, days post coitum B. Diagram of the 15.5dpc mouse kidney showing the branched ureteric tree surrounded by the metanephric mesenchyme (top left). The nephrogenic zone of the forming metanephros (top right) contains branching ureteric tips each surrounded by cap mesenchyme. Signals from the cap mesenchyme promote tip branching (driven by Gdnf/Ret/Gfra1/Wnt11 signalling), while signals from the tip promote self-renewal (driven by FGF and Wnt signalling) and differentiation (driven by Wnt signalling). The cap mesenchyme cells undergo a mesenchyme the epithelial transition (MET) to initiate for formation of a renal vesicle. The patterning and segmentation of the nephron involves elongation from renal vesicle to S-shaped body, capillary loop and ultimately mature nephron (bottom right). C. Proximodistal nephron patterning and segmentation is conserved from zebrafish pronephros to mammalian mesonephros. The proximal end requires notch signalling with maturation promoted by retinoic acid. P, podocyte; N, neck; PCT, proximal convoluted tubule; PST, proximal straight tubule; DE, distal early; CS, corpus of Stannius; DL, distal late; PD, pronephric duct; G, glomerulus; PS, proximal segment; DTL, descending thin limb of the Loop of Henle; ATL, ascending thin limb of the Loop of Henle; TAL, thick ascending limb / distal straight tubule; MD, macula densa; DCL, distal convoluted limb; CNT, connecting tubule; CD, collecting duct. Adapted from [30, 31]. D. Diagram showing the shift in the relationship between the ureteric epithelium and the forming nephrons around cessation of nephrogenesis in the mouse. At embryonic day 15 in the mouse, the ureteric tips are bifurcating in response to the cap mesenchyme which surrounded the tips within the nephrogenic niche. New nephrons form and connect to the underside of the adjacent tip via the distal end with a patent connection present from early S-shaped body stage. At postnatal day 4 (P4), there is no remaining cap mesenchyme as this has all committed forming nephrons that surround the tips of the collecting duct epithelium. d, distal, m, medial, p, proximal. Adapted from [38].
Our understanding of metanephric morphogenesis is drawn largely from studies in mouse. Here, as for the human, the metanephros forms as a result of reciprocal inductive events between two key cell populations, and epithelial ureteric bud (UB) and a mesenchymal self-renewing nephron progenitor population into which the UB grows (Figure 2B). The UB is a sidebranch of the nephric duct, itself the distal portion of the pronephros [33]. In a process that remains incompletely understood, once the UB reaches the mesenchyme, it undergoes a process of dichotomous branching events such that each tip first swells to form an ampulla before breaking symmetry to form two new branches. This process of dichotomous branching proceeds for 12 generations in the mouse showing a remarkably constrained branch angle and orientation [34]. The nephron progenitor population, also called the cap mesenchyme, remains positioned around each tip and moves with the new tips as they arise such that with each branching event the resulting tip and surrounding cap are smaller in cell number [34]. Secretion of GDNF from the cap mesenchyme signals via RET/GFRA1 on the tips to drive proliferation and branching. Conversely, FGF9/20 and WNT9B from the tip supports cap mesenchyme self-renewal and proliferation. This tip/cap niche is a defining feature of the metanephric kidney (Figure 2B). Positioned around the periphery of the growing organ, this represents a nephrogenic niche. The cap mesenchyme that surrounds each of the ureteric tips represents a motile swarming mesenchymal population driving ongoing ureteric tip branching and hence organ size and nephron number. The constant balance between attraction to and repulsion from the tip, together with variable adhesion between this population and the tip, drives continued tip branching like a carrot hung in front of a donkey [35].
Nephron formation requires a mesenchymal to epithelial transition (MET) of aggregates of cap mesenchyme cells. It is also a canonical Wnt signal from the ureteric tip that induces this commitment to nephron formation [36]. A single MET event forms a renal vesicle (RV), from which the nephron patterns. The cap mesenchyme expresses the transcription factor Six2 and lineage tracing studies confirmed that this Six2-expressing population represents a self-renewing nephron progenitor able to give rise to all cell types within the nephron [37]. Six2 expression in the mouse is downregulated as nephron formation is induced [38] and there is evidence that FGF8, Wnt4 and Notch signalling is required for commitment [39–42]. If the entire cap commits, branching ends, hence there is a delicate balance between self-renewal and nephron formation. To this end, commitment to nephron formation is a stochastic process in which the initiation of Wnt4 expression alone is not sufficient for epithelial transition [43].
As epithelialisation occurs to form a renal vesicle (RV) with a lumen, there are immediate transcriptional distinctions between the distal RV, which is closest to the adjacent ureteric tip, and the proximal RV, which will form the glomeruli [44] (Figure 2B). Indeed, careful analyses of this process in the human fetal kidney suggests the addition of cap mesenchyme-derived cells to the proximal end of the RV across time, rather than a process in which the entire structure forms simultaneously [45]. Early on in nephron patterning, the distal end of the nephron connects to the adjacent ureteric tip. This does not impact the subsequent ability of the UB to branch [46]. While there are limited studies available describing this process of connection in the mouse, it appears to involve invasion by the distal cells of the late renal vesicle / early S-shaped body such that these cells insert into the epithelium of the adjacent tip [47]. Enforced expression of the Notch pathway, which prevented distal identity, also prevented invasion, illustrating the essential requirement for distal patterning. This invasion event has been proposed to require the removal of the intervening basement membrane [38, 44]. Appropriate patterning of the proximal end of the nephron requires Notch signalling [48] with BMP, Pten, Wnt and notch signalling shown to influence segmentation along the nephron axis and Rho-kinase being required for proper axis formation and elongation [49, 50]. Differential cadherin expression is also seen along the forming nephron, with CDH4 and CDH6 on early, but CDH1 being restricted to the maturing distal end [44]. WT1 expression is maintained is the most proximal nephron, ultimately being expressed in the glomerular epithelial cells [44] whilst the clonal expansion of a medial Lgr5+ population has been shown to be involved in loop of Henle formation [51].
Gaps in our understanding
The relationship between the nephron progenitors and ureteric progenitors is the most completely studied during kidney development. However, the kidney is also comprised of stromal and endothelial populations also essential for organogenesis. Indeed, during kidney development, the stroma is a major component of the tissue. The cortical stroma of the developing kidney expresses the Foxd1 transcription factor with lineage tracing again suggesting that this is a self-renewing progenitor population able to give rise to much of the renal interstitium [52]. However, at the transcriptional level there is substantial heterogeneity within the Foxd1-derived stroma [53]. There is also a medullary stromal population within the kidney that arises in part from a distinct Tbx18-expressing population [54]. Functional evidence suggests that support of the nephron progenitors and patterning of both the nephron and collecting duct epithelium is influenced by the surrounding stroma [55, 56]. Indeed, it has recently been shown that stem cell-derived ureteric epithelium can adopt a ureter fate rather than a collecting duct fate in response to the type of surrounding stroma [57]. Finally, the endothelial progenitors of the murine kidney are also poorly defined and show considerable transcriptional variability [58], although the renal vasculature arises via both vasculogenesis and angiogenesis [59]. Equally, the anatomically constrained formation of peritubular capillaries is a mystery yet to be resolved.
While mesoscale quantitative imaging and global transcriptional profiling, including at the single cell level, have vastly increased the information available around the component cell types within the developing mammalian kidney, including recent single cell efforts around human fetal kidney, our template for the generation of a synthetic replica remains incomplete in time and space. The signalling pathways involved in early patterning events have in large part been defined, but the spatiotemporal regulation of nephron patterning and segmentation remains imperfectly understood. Hence, while we can mimic the initiation of a nephrogenic event in vitro, ensuring the contiguous appropriate maturation of all component cell types along the nephron is much harder to achieve. In vivo, the spatial relationship between the proximal tubules and the cortical stroma is likely to ensure convolution and retention in that region of the parenchyma, while the descent of the loops of Henle is encouraged by signals from the perfectly branched ureteric epithelium and the medullary stroma through which this epithelium grows [56, 60]. Before complete elongation and segmentation has occurred, each nephron commences filtration, providing the apical surface of the nephron epithelium with urinary filtrate which is likely to provide nutrients, sheer stress and a unique and constantly changing metabolic milieu as it transits along the tubule. How vital this is for nephron differentiation and maturation is not understood.
Kidney organoids as a model of the human fetal kidney
By comparison with what we know of mammalian kidney development, kidney organoids represent a remarkably accurate model of early organogenesis at the molecular, cellular and histological level. Patterned from human pluripotent stem cells via the stepwise induction of intermediate mesoderm patterning, the spontaneous formation of recognisable nephrons within 3D culture was very exciting when first described [61, 62]. While there are a variety of methods now described in the literature [13], these are quite similar in sequence, signalling pathways adopted and timing. Most protocols have shown the presence of a SIX2+ nephron progenitor population capable of responding to Wnt-induction to form renal vesicle-like structures (Figure 3A). These rapidly show distinct proximal to distal patterning with the formation of podocytes within a Bowman’s capsule at one end and distal tubule patterning at the other end. (Figure 3B). It has now definitively been demonstrated using CRISPR-edited human induced pluripotent stem cell lines that organoid nephrons arise from a SIX2-expressing progenitor population in response to a canonical Wnt signal and via a mesenchyme to epithelial transition [16] (Figure 3C). In addition, the relative proximodistal patterning of these structures can be altered by varying the exogenous signals applied. As originally described by [28], a decrease in GATA3 expression is seen with an increased duration of Wnt signalling during early patterning events. This was interpreted as shifting identity to a more anterior intermediate mesoderm state, as may be the case, but also results in proximalised nephrons. Low et al [63] also suggest the Wnt signalling modulates nephron patterning along this axis, which is not surprising given what is understood of proximodistal patterning in the mouse nephrons [50]. The glomeruli formed within organoids show patterning of parietal and visceral epithelial cells (podocytes) (Figure 3CD) with evidence of polarisation, slit diaphragm formation and maturing GBM components, including laminin β2 and early collagen IV switching [18]. Their diameter is in line with that of a human fetal glomerulus at 60–100um and the expression of known podocyte genes is much higher in 3D organoid glomeruli than 2D cultures of immortalised or primary podocytes [18].
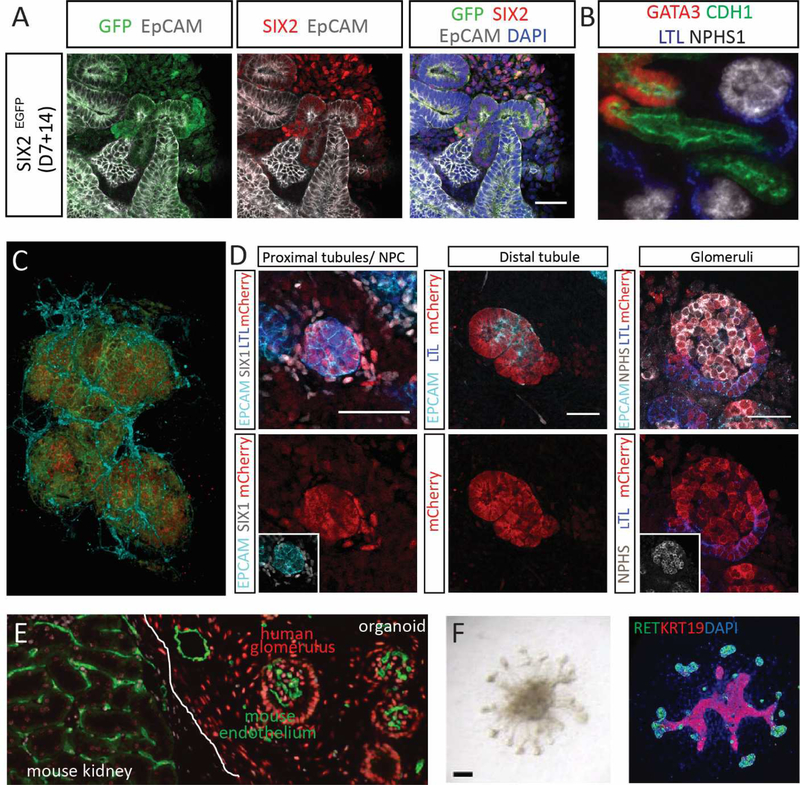
Figure 3. Cellular complexity, lineage relationships and maturation of kidney organoids.
A. The SIX2-expressing mesenchymal progenitor population within a kidney organoid generated from a SIX2-GFP fluorescent reporter iPSC line [132]. B. Nephrons within kidney organoids show patterning and segmentation into glomerulus (NPHS1, white), proximal (LTL, blue), distal (CHD1, green) and connecting (GATA3 (red) and CDH1 (green)) segments. C. Confocal imaging stack within a kidney organoid showing the presence of glomeruli comprised of podocytes (NPHS1, red) within a Bowman’s capsule of parietal epithelial cells (CLDN1, green) surrounded by an endothelial plexus (CD31; aqua). Image courtesy of Aude Dorison. D. SIX2 lineage tracing using an engineered induced pluripotent stem cell line reveals that the nephrons arise from a SIX2-expressing nephron progenitor. Initiation of SIX2 expression results in Cre-mediated excision of a GFP cassette, enabling the expression of mCherry fluorescent protein from the same locus. Lineage tracing showed that SIX2-expressing progenitors gave rise of proximal tubule, distal tubule and glomeruli within human kidney organoids [16]. E. Kidney organoid transplanted under the renal capsule of an immunocompromised mouse. After 14 days, there is clear evidence of angiogenic ingrowth of murine blood vessels (MECA32, green) forming glomerular capillaries within human organoid glomeruli. Cells of the organoid can be identified as human via staining with the human nuclear antibody (red). Image courtesy of Michelle Scurr. F. An example of a ureteric organoid generated via stepwise patterning to anterior intermediate mesoderm, subsequent isolation of nephric duct progenitors based upon surface expression of CXCR4/KIT and subsequent culture in Matrigel [76]. Ureteric organoids are shown here brightfield (left) and with immunofluorescence staining for RET and KRT19. Adapted from [76].
Surrounding the forming nephrons in kidney organoids is a renal stroma within which an endothelial plexus forms via vasculogenesis (Figure 1B, Figure 3C). Indeed, there is evidence for endothelial invasion of occasional Bowman’s spaces in vitro [18, 28] with this process improved in the presence of flow across organoid cultures [64]. It has been argued based on single cell profiling that these endothelial cells arise from nephron progenitors [63]. However, this was not seen in the organoid SIX2 lineage tracing studies [16] and there is no evidence for this in mouse. While the spontaneous vascularisation of organoid glomeruli is inefficient in vitro, despite evidence for the production of VEGF by the podocytes, this can be overcome via the transplantation of kidney organoids into recipient immunocompromised mice [65–67] When placed under the renal capsule, this results in a rapid (7–14 days) vascularisation of the transplanted tissue from the host such that mature glomerular capillary beds are formed with patent connections to the blood supply of the animal [65–67] (Figure 3E). Using kidney organoids generated from SOX17-reporter lines, we have shown evidence for a contribution of human endothelial cells to the resulting introrganoid plexi, however the capillary loops within the glomeruli appear to be mouse derived [65–67]. More remarkable is the fact that such host-vascularised organoid glomeruli display appropriate size-selective glomerular [68]. As such, a transplanted organoid can filter blood to produce urinary filtrate with this process resulting in substantial tubular maturation at the ultrastructural level. All is not perfect as yet. Off target differentiation, often to cartilage or stroma, has been observed in such transplants [65, 67, 69]. This varies with the starting organoid protocol and may represent non-renal off target populations in the initial differentiation or represent a response to the transplant by the host. While such challenges will need to be addressed to create functional tissue, progress to date is very promising.
Deficits of kidney organoids
Despite the remarkable self-organisation that occurs to form a kidney organoid, we are working with cells in vitro. As such, there are imperfections we can identify and others that are harder to pinpoint. There is also the challenge of technical variations between experiments and between starting stem cell line [26]. Hence it is worth considering some of the anomalies or curiosities of kidney organoid methods. The first is timing. While embryogenesis in mammals is grossly comparable, the timing of morphogenetic events clearly differs. Gestation is under 3 weeks in a mouse but 9 months in a human. It is now evident that pluripotent stem cells in vitro retain the developmental timing of their species of origin [70]. How then does the timing of kidney organoid patterning fit the picture? In the developing human, the ureteric bud reaches the metanephric mesenchyme at week 5. In the currently published kidney organoid protocols, starting with either mouse or human pluripotent stem cells, almost all protocols describe the initiation of nephron formation at around 15 days of culture [13], well before metanephrogenesis would occur in an intact human embryo. Given the lack of transcriptional difference between mesonephric and metanephric tubule patterning, it is challenging to definitively identify organoid nephrons as metanephric as opposed to mesonephric. Indeed, there is little analysis even within the mouse that would act as a suitable framework for comparison.
While there is substantial stroma within most kidney organoids, this does not express FOXD1. The ridge of intermediate mesoderm within which the kidney arises also forms the adrenogonadal primordium and the mesonephros, for which there is no available human transcriptional signatures. In mouse, the Foxd1+ mesenchyme is regarded as arising around the time of metanephric patterning and after the separation of the gonadal and adrenal primordia. The absence of this population may suggest a more anterior patterning (mesonephric) of current kidney organoids. Another cell type absent from current kidney organoid protocols is the resident macrophage, a cell type shown to be present throughout the metanephric kidney from approximately 12dpc in mouse [71]. These resident macrophages ultimately adopt a permanent position wrapped around the tubules of the nephron and, as the only cell type expressing CSF1R within the kidney, are likely to mediate the improved nephron formation observed in the presence of CSF1 [71]. Within the current protocols, there is clear evidence for a lack of epithelial maturation in most segments of the forming nephrons. For example, the lack of expression of OCT2 (SLC22A2), which is the most definitive for proximal tubule [72], remains a challenge to the application of kidney organoids for nephrotoxicity testing.
Perhaps most crucial deficit of kidney organoids is the lack of ongoing nephron formation within any currently described protocol. The lineage tracing studies in human kidney organoids, while confirming the contribution of SIX2-expressing progenitors to nephron formation, also showed that this process was lost across time [16]. Hence, there was a synchronous wave of nephron formation but not ongoing nephrogenesis. While not surprising given the absence of an anatomically defined nephrogenic niche, this has implications with respect to scaling up nephron number. It has long been proposed that as the ureteric bud arises from the nephric duct, which forms earlier and more anteriorly than the metanephros, that it was not possible to pattern both metanephric mesenchyme and ureteric epithelium simultaneously [61]. Despite this, the presence of a GATA3+PAX2+DBA+KRT8+ epithelium connecting the forming nephrons together was interpreted as evidence for collecting duct in some protocols [28, 62]. However, a branching ureteric epithelium was not formed and the expression of many of these markers is now know to occur in distal nephron and connecting segment in mouse and man [24, 73]. Several groups have now published distinct directed differentiation protocols with the specific objective of generating ureteric epithelium [74–77]. Again, by carefully mirroring mouse development, [76] created ureteric organoids (Figure 3F) via patterning through to a more anterior intermediate mesoderm and enriching by cell sorting for nephric duct progenitors expressing CXCR4 and KIT (Figure 3F). While feasible from human and mouse, it was shown that ureteric epithelium generated from mouse embryonic stem cells was able to connect to separately patterned nephrons when co-cultured with mouse embryonic stroma [76]. This ‘higher order’ kidney brought the prospect of recreating an organoid with a niche. However, while a similar population could be generated from human pluripotent stem cells, these did not successfully form a higher order kidney structure, potentially due to the lack of an appropriate stromal population. All more recent approaches to generating ureteric epithelium shorten the initial CHIR patterning, as was the approach taken by [28] to pattern more anteriorly. However, many involve multiple growth factor changes and surface marker-based cell sorting. Most of the growth factors used (retinoic acid, GDNF, Wnt, GDNF) are known to support the ureteric epithelium in mouse [78]. Most approaches also involve culture of the isolated epithelium in Matrigel, an approach not used for the nephron-forming organoids. Such ureteric organoids have successfully modelled autosomal dominant polycystic kidney disease in the relevant cell type [19] and can give rise to collecting duct and ureter [57]. However, as noted, patterning specifically to this epithelium has challenges for ultimate integration with the nephron forming differentiations.
In summary, current kidney organoids represent an astonishing advance with respect to how well they model the human kidney. However, the human kidney is such a large, multicellular and anatomically constrained organ that recreating a perfect replica seems unachievable. Setting aside our inability to provide an ex vivo environment within which such large tissue could be physiologically supported, there remain considerable challenges in simply getting the right components and appropriate functional configurations. The limitations to date continue to represent barriers to accurate modelling of many adult kidney diseases. If we envisage this as a synthetic embryology challenge, it is possible to consider alternative options from the level of recreating individual component cell types through to improved regulation of self-organising events or the reassembly of functional components to deliver a similar functional outcome.
Applying transdifferentiation to recreate the kidney one cell at a time.
While the power of embryogenic self-organisation to form kidney organoids is at one level remarkable, adopting a reductionist approach and simply patterning all required component cell types one by one could be considered. In fact, differentiation of human pluripotent stem cells to kidney endpoints began with differentiation to podocyte alone [79]. More recent podocyte protocols patterned first to early nephron and then inhibited Wnt signalling to provide a >90% pure podocyte population [80]. The challenge for kidney lies in the cellular complexity of the final organ. The adult kidney contains arguably more than 25 distinct cell types with specific functions. In principal, the recreation of nephron, ureteric epithelial, stromal and endothelial progenitors should allow the generation of all kidney cell types. As described above, there are now several protocols for the specific generation of ureteric epithelium, although this appears to remain plastic with an ability to move between ureteric tip, stalk and ureter. However, directed differentiation may be quite challenging for other progenitors.
An alternative would be transdifferentiation via the enforced overexpression of key pioneer transcription factors to redefine cellular identity. This is best exemplified in the reprogramming of somatic cells to an induced pluripotent state [81, 82]. By screening for minimal sets of transcription factors capable of driving a particular cellular endpoint, transdifferentiation has also proven to be effective for neural [83, 84] and even lung differentiation [85]. Indeed, there are now programs for predicting the required transcription factors for any given endpoint [86]. This approach has been tried for kidney. The conversion of fibroblasts to an induced renal epithelial cell (iREC) was achieved via the combined expression of Emx2, Hnf1b, Hnf4a and Pax8 [87]. A subsequent study directly introduced synthetic mRNAs encoding transcription factors into human pluripotent stem cells, describing the generation of nephron progenitors (FIGLA, PITX2, ASCL1 and TFAP2C) or pretubular aggregates (HNF1A, GATA3, GATA1 and EMX2) [88]. The latter were described as forming kidney organoid-like structures when cultured in 3D, suggesting that this represented patterning to a progenitor state.
Success in adopting this approach will rest with our understanding of the transcription factors required. In the case of the nephron progenitor, transcriptional profiling of this population allowed the identification of likely pioneer transcription factors. An initial pool of 6 transcription factors [89], that were subsequently reduced to a minimal pool of three (SIX1, OSR1, SNAI2), was shown to be sufficient for reprogramming to a nephron progenitor-like fate [90]. SIX2 may not have been required as, unlike the mouse, SIX1 is expressed along with SIX2 in the human nephron progenitor population. The challenge has been the difficulty of maintaining this population in isolation. The survival and self-renewal of the nephron progenitors is tightly dependent upon the surrounding stroma and the underlying ureteric epithelium. While a number of culture conditions have been defined to support isolated murine nephron progenitors [91–93], none of these have been shown to support human nephron progenitors within organoids. What these studies highlight is the interdependence of individual progenitor populations and the very early need for co-culture. However, there are a growing number of transcriptional datasets from fetal human kidney that may assist in the identification of distinguishing transcription factors for different progenitor cell types [68, 94, 95].
Engineering biophysical parameters and controlling cell placement
Having generated component cell types, there remains the question of assembly. Metzger [96] applied the term synthetic embryology to stem cell-derived models of gastrulation, neurulation and organogenesis, whilst noting the requirement to address reproducibility and control of tissue shape. In comparison to a developing embryo, which can reliably recreate an almost identical replica of all morphogenetic events in most individuals, there is substantial variability in the assembly arising using most pluripotent stem cell differentiation protocols in vitro. The application of engineering principles has improved the reproducibility of some models. For example, the control of size and shape in 2D and 3D has been critical for the successful modelling of gastrulation events [97]. Similarly, while pluripotent stem cells can form an epiblast-like cystic structure [10], it was alterations to the mechanical properties of the bed in which such cultures were grown that induced the formation of what resembled the pluripotent embryonic disc within an amniotic ectoderm of the early human embryo [6]. While a purely mechanical trigger facilitated the required symmetry breaking, once these structures were formed the streaming of cells from the embryonic disc occurred spontaneously, as anticipated during gastrulation [6]. This was not only observed in vitro, it could be inhibited in SNAI1 loss of function pluripotent stem cell lines, validating the role of epithelial to mesenchymal transition in this event. Hence, by controlling the biophysical parameters, such complex models can become reproducible enough to answer questions about human development in vitro.
While a number of variations on a theme have occurred around methods for generating kidney tissue from pluripotent stem cells, the application of biophysical or mechanical constraints, either as instructive signals, mechanical boundaries or to assist in quality control and reproducibility, is yet to be fully exploited. Most kidney organoid protocols initially pattern using monolayer culture prior to either aggregate culture within low attachment wells [98] or Transwell™ culture [28] as is used to grow the embryonic kidney ex vivo. Initiation of nephron formation is triggered using canonical Wnt agonists. Taguchi et al, (2014) pattern to metanephric mesenchyme prior to co-culture with an inducing tissue such as spinal cord. Somewhat by contrast, the method of [23] patterns within Matrigel to an epiblast-like structure before initiating mesodermal patterning. Several methods have more recently been described using suspension cultures, either commenced early [99] or after initial mesodermal patterning [100]. While this appears to facilitate substantial scale up, both studies reported challenges with longer term fibrotic changes. Both robotic liquid handling early in the differentiation process [101] or cellular extrusion bioprinting of intermediate mesoderm [102, 103] have been applied to provide improved throughout and quality control for screening. There have also been reports of shifts in maturation depending upon the culture conditions of the undifferentiated starting cells [104].
There remain many other engineering opportunities to address both scale and patterning. The use of photolithographic or hydrogel-based templates has been applied to the design of nephron elements, such as the engineering of individual tubules, to deliver flow [105, 106] and even provide an accompanying endothelial vessel [107]. These have largely not yet incorporated pluripotent stem cell-derived cell types, although [108] described the co-culture of pluripotent stem cell-derived podocytes with glomerular endothelial cells. While such modules may be very powerful for disease modelling and compound screening, they do not represent a scalable approach to forming a replacement organ. In contrast, we have recently demonstrated that altering the conformation of cell paste at bioprinting substantially increased the resulting nephron number and improved maturation at the single cell level [103] (Figure 4AB). Indeed, starting with the same number of cells, we saw a threefold increase in MAFB-positive glomeruli. The bioprinting of parallel lines of cell paste onto Transwell filters facilitated the manufacture of large sheets of renal tissue containing thousands of nephrons [103] (Figure 4A).
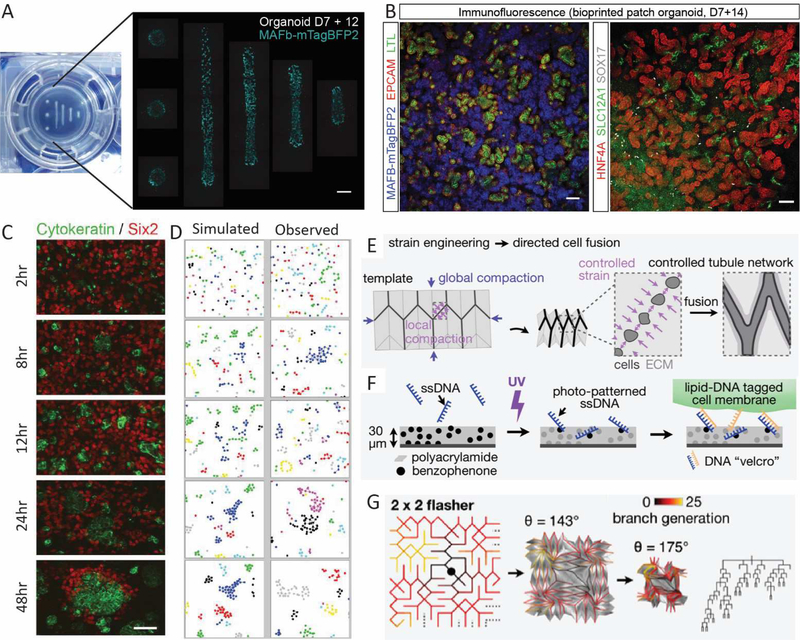
Figure 4. Key challenges to building a synthetic kidney.
A. Bioprinted cell paste showing that changing the conformation of printing results in an increase in glomerular number. Cell paste was bioprinted onto a Transwell™ filter on day 7 of differentiation in increasingly longer lines but with the same starting cell number (left). Imaging of the resulting structures 12 days after bioprinting cell paste generated using a MAFBmTAGBFP reporter line. This allows the visualisation of glomeruli (blue) as they form. Scale bar = 1mm. Adapted from [103] B. Immunofluorescence of sheets of uniformly patterned kidney tissue generated via bioprinting of cell paste in parallel lines onto a Transwell™ filter. Nephrons are comprised of glomeruli (MAFBmTAGBFP), proximal tubules (EPCAM, LTL, HNF4A) and distal tubules (EPCAM, SLC12A1). Vasculature is also present (SOX17). Scale bar = 100uM. Adapted from [103]. C. Quantitative imaging of dissociated 12dpc embryonic kidneys across the first 48 hours post recombination reveals rapid formation of ureteric epithelial cell clusters (cytokeratin, green) which arise prior to the reformation of a nephrogenic niche (Six2, red). [112] D. Simulated and observed clustering of ‘alike’ cells as a result of Brownian motion across the same time period. The congruence between observed and simulated cell clustering suggests that reformation of the ureteric epithelium arises due to selective cell-cell adhesion between ureteric epithelial cells [112]. E. Diagram illustrating an approach where strain engineering across a hydrogel layer onto which cells have been placed can result in directed cell fusion via apposition of cell clusters after compaction. This approach was applied to the generation of an epithelial network [113]. F. Diagram illustrating the approach of generating hydrogel sheets to which specific ssDNA sequences can be placed and photo-patterned to control the binding of cells with lipid-DNA tagged cell membranes. This approach can achieve a resolution of cell placement much finer than bioprinting [113]. G. The design of a folding pattern that will give rise to a branching tree similar to that characterised within the developing mouse kidney. Cell contraction-based compaction results in ‘origami’ folding of the template allowing the close apposition of MDCK cells for form and epithelial tree [113].
Guiding complex tissues to form – cell adhesion, kinomorphs and assembloids
Another approach to recreating shape and size is to understand and then manipulate cell-cell interactions. Even before cadherins were known, cell-cell adhesion had long been proposed to drive self-organisation [109, 110]. Self-organisation within kidney organoids is unsurprising given the knowledge that it is possible for a dissociated embryonic mouse kidney to recreate structure after reaggregation [111]. The morphogenetic role of cell-cell adhesion in this self-organisation was formally investigated using the dissociated mouse embryonic kidney [112]. Quantitative imaging of dissociated and then recombined 12dpc embryonic kidneys revealed rapid formation of ureteric epithelial cell clusters which preceded the reformation of a nephrogenic niche (Figure 4CD). The cellular behaviour of component cells observed across this first 12 hours was consistent with stochastic Brownian motion, with the emergence of self-associating structures therefore likely to reflect differential cell adhesion between ‘like’ cells. A tight congruence between the observed and mathematically predicted cell-cell association supported this concept. Indeed, the addition of a blocking antibody to P-cadherin, which was differentially expressed in ureteric epithelium, could delay the process [112]. It may be possible to harness such knowledge to guide cell placement in organogenesis. The nucleation of specific epithelial populations from within a complex mix may be possible at least in 2D. However, approaching organogenesis by recombining all required component cell types comes with the declining statistical likelihood of appropriate self-organisation as the number of different cell types increases. It also does not support global structural unity. For example, a recombined embryonic kidney forms many isolated ureteric epithelial structures, but not as a unified ureteric tree with a single existing ureter (Figure 4C). This may be where synthetic approaches can assist.
Approaches to artificial morphogenesis have included the use of DNA-based adhesion systems [12] where the placement of individual cells across a surface can be dictated via the printing of a particular DNA-sequence that will allow cells bearing the antisense sequence to adhere. This approach has also been applied to induce self-folding cellular constructs referred to as ‘origami’ where the placement of cells onto hydrogels in a particular 2D conformation results in a complex 3D conformation as a result of hydrogel folding in response to cellular constriction. The term ‘kinomorphs’ is used for tissue scaffolds that undergo a programmed shape change in response to the templated positioning of cells to reorganise the relationship of those cells to each other. Facilitated by photolithographic technology to dictate the placement of ssDNA strands, [113] have recently applied this approach to recreate an epithelial network designed to mimic the embryonic kidney ureteric tree. This starts by modelling the predicted shape changes that will occur with an extracellular matrix sheet in response to cellular contraction. Using photolithography to create this ssDNA pattern with a gel layer on a glass surface, cells can be spatially controlled to seed across a large field (Figure 4EFG). Using kinematic origami principles to design this placement enables this 2D structure to fold up in 3D to provide a very complex cellular structure with a final resolution with respect to cell positioning that is much finer than can be achieved through extrusion bioprinting (Figure 4EFG). While this approach is currently a proof of concept based upon our knowledge of the branching pattern of ureteric tree, such an approach may be central to recreating a uniform exit path.
The assembly of blastocyst-like structures with embryonic and extra-embryonic lineages can be achieved by combining separate differentiations of trophoblast stem cells and pluripotent stem cell-derived epiblasts [7]. The combining of organoids patterned separately to dorsal and ventral forebrain results in fusion to form an ‘assembloid’ that can recapitulate the interactions between glutamergic and GAGA-ergic neurons [114] (Figure 4). While neither example represent the way in which embryogenesis occurs, these have proven effective strategies to assemble larger or more complicated in vitro tissue models. We are yet to create an anatomically accurate cortex and medulla in a kidney organoid, but we do now have protocols for separate nephron and ureteric organoids which need to be united. However fusing one region to another will not be enough for renal function. Hence we have to again consider what function we want to achieve and then consider what options we have for form.
Nephrons joining to nephrons
Understanding the process by which nephrons form and how they connect to each other is likely to be critical in creating a synthetic kidney. Nephrogenesis in the mouse is regarded as a continuous process occurring throughout embryonic kidney development with new nephrons undergoing patterning and insertion into the adjacent ureteric tip in the same manner as each other. While in mouse all nephrons are regarded as forming in the same way, there is ultimately a distinction between those formed early and late with respect to the final length of their loops of Henle and the positioning of their glomeruli with respect to the corticomedullary axis. Recent studies in mouse highlight the transcriptional distinctions between these two types of nephrons [115] but all are regarded as connecting to ureteric tips. But is this process the same in mouse and man?
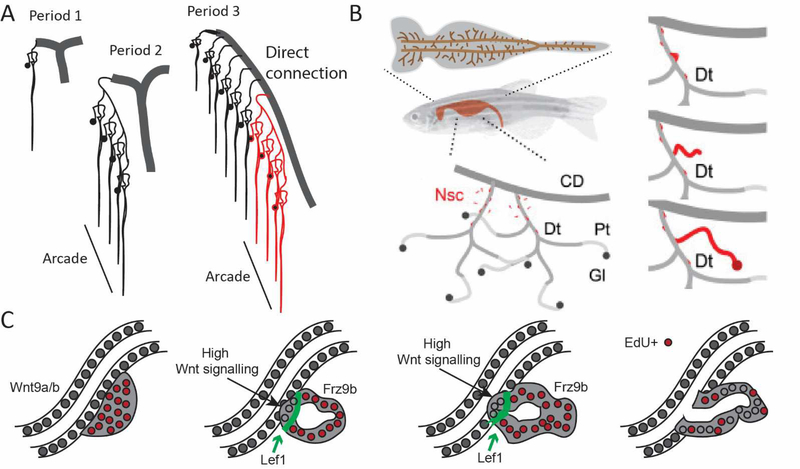
In a seminal series of anatomical descriptions of human kidney development, [116] described four distinct stages of collecting duct / nephron interactions in the human. In period 1 (gestational week 5 to 14), nephron induction does not commence until the ureteric bud has undergone up to 5 rounds of dichotomous branching. This is in contrast to mouse where nephron induction commences almost immediately. However, once nephrons begin to form, they connect to the ureteric tip as in the mouse during this period. Week 14 to 22 of human development (period 2) sees the end of ureteric tip branching but nephron formation continues with multiple nephrons now attaching to each other via the distal portion of the previous nephron (Figure 5A). This process has been called ‘arcading’, with a given arcade containing between 2 and 8 interconnected nephrons. This is not described in mouse, although it is likely to occur in the final wave of nephrogenesis around birth [38] where, during a very compressed time period, the final nephrons to form arise as a coincident commitment of all remaining cap mesenchyme around the final tips (Figure 2D). While we have no understanding of the molecular processes involved, the connection of one nephron to another via the connecting segment of a previous nephron is commonly seen in the mesonephros of the fish [117, 118]. Period 3 of human kidney development (week 22 to 36) sees a complete change in ureteric tip behaviour as there are no more branching events. The duct continues to elongate with new nephrons forming along the sides of the extending, non-branching collecting duct (Figure 5A). This is never seen in mouse. The final period of human kidney development reveals a single final arcade of nephrons attached to the terminal end of each collecting duct, but no new nephrons forming.
Figure 5. Nephron patterning and connection in human metanephros and zebrafish mesonephros.
A. Diagram showing the arrangement of nephrons with respect to the adjacent collecting duct across Periods 1 to 3 of human kidney development [116]. Collecting ducts are shown in grey. Period 1 represents the direct connection of new nephrons to the ureteric tip, as in mouse. Period 2 involves the formation of arcades in which multiple nephrons are attached to each other via their distal segments. During Period 3, the collecting duct extends without branching with new nephrons connecting into the stalk rather than the tip. Here a prior arcade formed during Period 2 is shown in red. Adapted from [116]. B. Neonephrogenesis occurs within the mesonephros (top) throughout adult zebrafish life with new nephrons connecting with the distal segments of previous nephrons (bottom left). Neonephrogenesis involves the formation of an aggregate from mesenchymal nephron stem cells (red) that then adhere to an existing distal epithelium and invade to form a connection. CD, collecting duct, Dt, distal tubule, PT, proximal tubule, Gl, glomerulus, Nsc, nephron stem cell. C. The process of invasion of a neo-nephron involves the attraction of the aggregate to the distal tubule of an existing nephron via the production of FGF4/10. The aggregates express Frz9b which responds to high Wnt9a/b secretion from the distal nephron. A front of invading cells forms beyond a region of high Lef1 expression with the invading cells showing loss of proliferation (become EdU negative). As a result, the neonephron creates a patent connection with the existing distal epithelium and process to elongate, pattern and segment. BC adapted from [117, 118].
At the time, these careful observations countered previously held views of how the human kidney patterned. While they describe distinct anatomical approaches to joining nephrons with a urinary exit path, the process of connection is likely to be very similar between periods at the molecular level. Our understanding of how arcading occurs will not come from mouse, but may come from fish. As noted above, fish continue to make new nephrons in response to injury or increased body mass with these new nephrons plumbing into existing mesonephric tubules (Figure 5B). Lhx1a-positive aggregates of cells form in response to both Wnt9a/b and FGF4/10 signalling from the distal pronephric tubule, or indeed the distal limb of another mesonephric tubule in the fish [117–119] (Figure 5B). These signals, sensed by Fgfr1 and Frz9b on the aggregates, lure them to the right place for fusion to occur. The invading aggregate strongly expresses Frz9b, Lef1 and Lhx1 with the invading portion experiencing high Wnt signalling (Figure 5C). The similarity between this and invasion by the distal end of the S-shaped body in mouse metanephros [44, 47] suggests a conservation of mechanism between species and between meso- and metanephros. Hence, a distal nephron can invade another distal nephron, but this requires signals from the target tubule to the new nephron. Could this be exploited to connect stem cell-derived nephrons to each other or to existing kidney tissue? This will depend upon whether the collecting duct / distal nephrons of the postnatal metanephric kidney are able to, or can be induced to, act as an attractive and receptive epithelium into which the new nephron can invade.
How development manages to scale nephron number
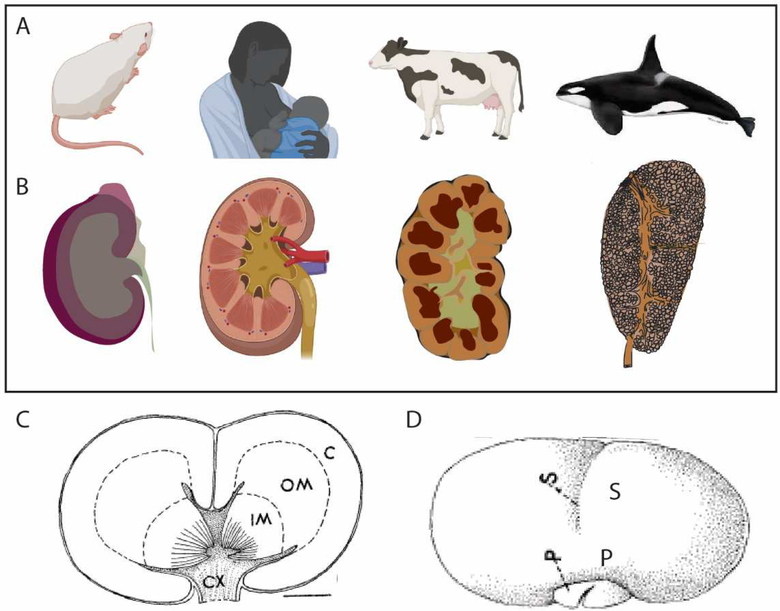
A major challenge to engineering a synthetic kidney is ‘scale’. Importantly for renal function, scale is not a function of size alone but the number of component nephrons and the connectivity of these structures. To recreate a synthetic kidney, it will be important to consider how many nephrons is enough? The average nephron number in the human is approximately 1 million, although this varies from 0.2 to 2 million per kidney [120]. All these nephrons arise during development as cessation of nephrogenesis in man appears to occur around 36 weeks of gestation [121, 122]. Even in the mouse, nephron number has been estimated at 16,000 per kidney [123] with the last nephrons forming in the first two days of life [38, 116, 124]. While it would appear logical that humans have more nephrons than mice, nature has varied the way in which nephrons can be arranged to provide adequate renal function for different animals (Figure 6). When you compare the kidney of a mouse (length 1cm, kidney weight 400mg,16,000 nephrons), with that of a killer whale (length >25cm, kidney weight 4.5kg, 10 × 106 nephrons), you may visualise a similar organ but just much bigger. However, the mouse kidney is a unipapillate structure comprised of one renal lobe or renicule, while Cetacea, such as the killer whale (Orcinus orca), gain renal capacity by having >1000 individual polyhedral unipapillate renicules, each surrounded by non-parenchymal capsules [125] (Figure 6AB). Not all lobes are visible at the surface of the organ with up to eight layers of renicules present with and packed together like a bunch of grapes (Figure 6C). Each individual renicule is approximately 430mg and 1cm in length, and hence not dissimilar to a mouse kidney [125]. Multilobar kidneys are common, but they are not usually comprised of individual separated renicules. The kidney of a sheep or a human has a smooth outer surface which hides the underlying lobular structure (Figure 6B). This fusiform cortical parenchyma may result from the shift in the relationship between nephron and collecting duct during periods of development, as described above. Individual lobes are more evident in bovine kidneys where they are arranged as a single layer and are visible from the outside of the organ (Figure 6B). The intrarenal separation of lobes seen in whales is more unusual.
Figure 6. Reniculism as an approach to creating more renal mass.
A. Mammal model. B. Structure of the adult kidney. The mouse kidney (length 1cm, mass 400mg) is a single lobe with a single papilla. While the human kidney (10–13cm, 110g) contains multiple lobes and papillae, the organ has a fusiform nature with a smooth outer capsule. The bovine kidney is multilobular with persistent separation between lobes with lobes arranged in a single layer. The kidney of the killer whale (>25cm, 4.5kg) is comprised of >1000 individual renicules each with one (82%) or two (18%) papillae that are arranged in up to 8 layers with individual renicules surrounded by separating connecting tissue. All renicules drain into a united ureteric tree for exit via a single ureter. C. Diagram of a longitudinal cross section of a single killer whale reniculate showing dual papillae. C, cortex; OM, outer medulla, IM, inner medulla, CX, calyx. Scale bar = 2.1 mm. D. External view of a single killer whale (Orcinus orca) renicule. Note that not all sulci represent separate lobes. P, papilla, S, sulcus. Killer whale data adapted from [125].
The proposed advantage of creating a larger organ by simply adding renicules is the provision of greater numbers of nephrons without having to increase the length of the component tubules [125]. This is not the approach always adopted. An alternative is to make each nephron larger. Indeed, an increase in the diameter of individual glomeruli with body mass is the approach of many large mammals. The diameter of glomeruli in both humans and killer whales is around 175uM, while in an elephant this can reach 350–400uM. As such, a killer whale has >8 x the number of nephrons compared to other species around the same body mass [125]. With larger glomeruli comes longer tubules. In non-mammalian species such as snakes, crocodiles and turtles, these longer tubules often require cilia to drive fluid down the tubules. This may in part be due to their lower arterial pressures. Birds and mammals with relatively high arterial pressures lack true cilia and their nephrons do not possess exceptionally long tubules.
Comparative biology suggests that the ability to make a synthetic renicule could be viewed as the primary engineering objective in a synthetic kidney, as long as each has a vascular supply and an exit path for urinary filtrate. Scale would then come with renicule number and/or glomerular size. The issue is how to generate individual renicules. Indeed, the formation of multiple lobes in the human kidney arises as a result of the very earliest branching of the invading ureteric bud [116]. Unlike the mouse, where nephron induction commences after the first branching event, in the human there are five rounds of UB branching that occur to establish the bases of each of the approximately 20 lobes within the final organ. At present, kidney organoids do not represent a renicule as they lack an architecturally defined cortex and medulla. While we see connections between nephrons (Figure 1A), these are not connected to a unified exit path. As described above, approaches for the generation of budding ureteric organoids have been developed [74–77]. If it were possible to generate a core branching tree and provide it with appropriate nephron and stromal progenitor populations, the engineering challenge will become how to vascularise such a structural unit, string together many units and still control the collection of urinary filtrate. Approaches such as kinomorphs, scaffolds and/or cellular bioprinting will be required.
Reinstating a nephrogenic niche within the postnatal kidney
A more tractable approach than making an entire synthetic kidney might be to supplement the existing organ with an exogenous nephrogenic niche. The postnatal human kidney has no capacity to grow new nephrons as nephrogenesis ceases before birth in the human [116, 121, 122] with the loss of nephron progenitors assumed to represent eventual commitment to epithelial transition. Even in the whale, all nephrons are formed by birth [125]. However, neonephrogenesis is not limited to organisms with mesonephroi and many reptiles show ongoing nephron formation within their adult metanephric kidney [126]. These animals retain a SIX2-expressing progenitor population [126] whereas this is lost in postnatal mammals. Would an artificially generated nephrogenic niche be supported if transplanted into an adult kidney? The missing piece here is what controls cessation of nephrogenesis during normal metanephric development. Studies looking into the behaviour of ‘young’ and ‘old’ nephron progenitors when transplanted into asynchronous nephron progenitor environments showed that an ‘old’ progenitor can be renewed if surrounded by ‘young’ progenitors, suggesting a non-cell autonomous signal. Can such a signal be provided to support self-renewal in a postnatal setting? The transcriptional distinction between ‘young’ and ‘old’ nephron progenitors from a number of studies points to a shift in cell adhesion and in metabolic state [127–129]. Critically, young nephron progenitors use glycolysis to generate energy, with this supporting self-renewal as opposed to nephron commitment by de-sensitizing the progenitors to Wnt signals from the ureteric tip [129]. With age, the nephron progenitors show reduced glycolysis and greater sensitivity to canonical Wnt signals which will favour commitment. They also show decreased FGF20 and increased mTOR pathway activity, which promotes mitochondrial biogenesis and reduces glycolysis. Such metabolic shifts with maturation occur in many tissues and have been employed in pluripotent stem cell protocols to enhance maturation, for example, of cardiomyocytes [130]. The addition of fatty acids to mature cardiomyocytes was described as mimicking the metabolic shift seen around birth. It is likely, therefore, that the environment of the postnatal kidney is optimised for the differentiated state rather than the metabolic demands of development. This may help us understand the environment required to maintain an exogenous nephrogenic niche in vitro or in vivo. What it does not answer is whether you can get a new nephron to connect to a postnatal kidney.
Synthesizing the road not taken
This review has focussed on what will be needed to synthesize a complete replacement organ. What should also be acknowledged is that imperfect but manipulable cellular models of development, such as kidney organoids, are not only useful for disease modelling but as a cellular testbed for exploring unnatural developmental outcomes and testing how patterning can come about. Just as the improved podocyte gene expression achieved within a 3D glomerulus within a kidney organoid is now clear [18], the ability to model a glomerular basement membrane disorder such as Alport syndrome, or a chronic systemic and vascular defect such as diabetic nephropathy, will need us to improve on what we have generated to date. This may not, however, require a full organ. The effective formation of a glomerular capillary bed within that 3D glomerulus, even if it is not within a complete organ, will further improve our ability to disease model.
Across developmental biology researchers have played with genetic and environmental perturbation or cellular rearrangements (microdissection, mosaicism, chimerism) to better understand cellular potential and fate [131]. Hence, even the existing imperfect organoid models provide the opportunity to perturb the system to better understand how it is controlled. Changing organoid conformation so as to improve our ability to image or readout phenotype, alongside the application of facile gene modification methods such as gene silencing, gene editing and base editing, provides us with a synthetic embryology sandpit within which to explore kidney morphogenesis. It is from these types of fundamental studies of form and function that we will in turn learn how we might engineer cellular replacements or improvements. Hence, we are at the beginning of the road with a long way to travel, but should remember that the ‘road not taken’ by evolution may not always be a dead end.
Highlights.
Summary of current approaches to generating human kidney tissue from human pluripotent stem cells
Comparison to normal human development, including an analysis of missing cell types
Discussion of challenges to recreating kidney tissue for renal replacement
Comparative biology of strategies for forming a kidney
Challenges of directed differentiation
Acknowledgements:
M.H.L. is a Senior Principal Research Fellow of the National Health and Medical Research Council of Australia (GNT1136085). Her research is supported by the National Institute of Health as part of ReBuilding a Kidney (DK107344), the NHMRC (GNT1156440), Australian Research Council (DP190101705) and the Medical Research Future Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Evans MJ and Kaufman MH, Establishment in culture of pluripotential cells from mouse embryos. Nature, 1981. 292(5819): p. 154–6. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR, Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A, 1981. 78(12): p. 7634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, and Jones JM, Embryonic stem cell lines derived from human blastocysts. Science, 1998. 282(5391): p. 1145–7. [DOI] [PubMed] [Google Scholar]
- 4.Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, and Sasai Y, Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature, 2011. 472(7341): p. 51–6. [DOI] [PubMed] [Google Scholar]
- 5.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, and Knoblich JA, Cerebral organoids model human brain development and microcephaly. Nature, 2013. 501(7467): p. 373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao Y, Taniguchi K, Townshend RF, Miki T, Gumucio DL, and Fu J, A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat Commun, 2017. 8(1): p. 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sozen B, Cox AL, De Jonghe J, Bao M, Hollfelder F, Glover DM, and Zernicka-Goetz M, Self-Organization of Mouse Stem Cells into an Extended Potential Blastoid. Dev Cell, 2019. 51(6): p. 698–712 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, and Wells JM, Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature, 2011. 470(7332): p. 105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, Yonemura S, Oiso Y, and Sasai Y, Self-formation of functional adenohypophysis in three-dimensional culture. Nature, 2011. 480(7375): p. 57–62. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi K, Shao Y, Townshend RF, Tsai YH, DeLong CJ, Lopez SA, Gayen S, Freddo AM, Chue DJ, Thomas DJ, Spence JR, Margolis B, Kalantry S, Fu J, O’Shea KS, and Gumucio DL, Lumen Formation Is an Intrinsic Property of Isolated Human Pluripotent Stem Cells. Stem Cell Reports, 2015. 5(6): p. 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gartner ZJ and Bertozzi CR, Programmed assembly of 3-dimensional microtissues with defined cellular connectivity. Proc Natl Acad Sci U S A, 2009. 106(12): p. 4606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todhunter ME, Jee NY, Hughes AJ, Coyle MC, Cerchiari A, Farlow J, Garbe JC, LaBarge MA, Desai TA, and Gartner ZJ, Programmed synthesis of three-dimensional tissues. Nat Methods, 2015. 12(10): p. 975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little MH and Combes AN, Kidney organoids: accurate models or fortunate accidents. Genes Dev, 2019. 33(19–20): p. 1319–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanovs A, Rybtsov S, Ng ES, Stanley EG, Elefanty AG, and Medvinsky A, Human haematopoietic stem cell development: from the embryo to the dish. Development, 2017. 144(13): p. 2323–2337. [DOI] [PubMed] [Google Scholar]
- 15.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, and Kamp TJ, Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res, 2012. 111(3): p. 344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howden SE, Vanslambrouck JM, Wilson SB, Tan KS, and Little MH, Reporter-based fate mapping in human kidney organoids confirms nephron lineage relationships and reveals synchronous nephron formation. EMBO Rep, 2019. 20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dvela-Levitt M, Kost-Alimova M, Emani M, Kohnert E, Thompson R, Sidhom EH, Rivadeneira A, Sahakian N, Roignot J, Papagregoriou G, Montesinos MS, Clark AR, McKinney D, Gutierrez J, Roth M, Ronco L, Elonga E, Carter TA, Gnirke A, Melanson M, Hartland K, Wieder N, Hsu JC, Deltas C, Hughey R, Bleyer AJ, Kmoch S, Zivna M, Baresova V, Kota S, Schlondorff J, Heiman M, Alper SL, Wagner F, Weins A, Golub TR, Lander ES, and Greka A, Small Molecule Targets TMED9 and Promotes Lysosomal Degradation to Reverse Proteinopathy. Cell, 2019. 178(3): p. 521–535 e23. [DOI] [PubMed] [Google Scholar]
- 18.Hale LJ, Howden SE, Phipson B, Lonsdale A, Er PX, Ghobrial I, Hosawi S, Wilson S, Lawlor KT, Khan S, Oshlack A, Quinlan C, Lennon R, and Little MH, 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun, 2018. 9(1): p. 5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuraoka S, Tanigawa S, Taguchi A, Hotta A, Nakazato H, Osafune K, Kobayashi A, and Nishinakamura R, PKD1-Dependent Renal Cystogenesis in Human Induced Pluripotent Stem Cell-Derived Ureteric Bud/Collecting Duct Organoids. J Am Soc Nephrol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanigawa S, Islam M, Sharmin S, Naganuma H, Yoshimura Y, Haque F, Era T, Nakazato H, Nakanishi K, Sakuma T, Yamamoto T, Kurihara H, Taguchi A, and Nishinakamura R, Organoids from Nephrotic Disease-Derived iPSCs Identify Impaired NEPHRIN Localization and Slit Diaphragm Formation in Kidney Podocytes. Stem Cell Reports, 2018. 11(3): p. 727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill AJ, Kim YK, Winston K, Tran LM, Diaz MA, Fu H, Finn LS, Pei Y, Himmelfarb J, and Freedman BS, Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater, 2017. 16(11): p. 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, Wilson S, Quinlan C, Ho G, Holman K, Bennetts B, Crawford J, Trnka P, Oshlack A, Patel C, Mallett A, Simons C, and Little MH, Patient-iPSC-Derived Kidney Organoids Show Functional Validation of a Ciliopathic Renal Phenotype and Reveal Underlying Pathogenetic Mechanisms. Am J Hum Genet, 2018. 102(5): p. 816–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, and Bonventre JV, Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun, 2015. 6: p. 8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Combes AN, Zappia L, Er PX, Oshlack A, and Little MH, Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med, 2019. 11(1): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harder JL, Menon R, Otto EA, Zhou J, Eddy S, Wys NL, O’Connor C, Luo J, Nair V, Cebrian C, Spence JR, Bitzer M, Troyanskaya OG, Hodgin JB, Wiggins RC, Freedman BS, and Kretzler M, Organoid single cell profiling identifies a transcriptional signature of glomerular disease. 2019. [DOI] [PMC free article] [PubMed]
- 26.Phipson B, Er PX, Combes AN, Forbes TA, Howden SE, Zappia L, Yen HJ, Lawlor KT, Hale LJ, Sun J, Wolvetang E, Takasato M, Oshlack A, and Little MH, Evaluation of variability in human kidney organoids. Nat Methods, 2019. 16(1): p. 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian A, Sidhom EH, Emani M, Vernon K, Sahakian N, Zhou Y, Kost-Alimova M, Slyper M, Waldman J, Dionne D, Nguyen LT, Weins A, Marshall JL, Rosenblatt-Rosen O, Regev A, and Greka A, Single cell census of human kidney organoids shows reproducibility and diminished off-target cells after transplantation. Nat Commun, 2019. 10(1): p. 5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva SM de Sousa Lopes, and M.H. Little, Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature, 2015. 526(7574): p. 564–8. [DOI] [PubMed] [Google Scholar]
- 29.Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, and Humphreys BD, Comparative Analysis and Refinement of Human PSC-Derived Kidney Organoid Differentiation with Single-Cell Transcriptomics. Cell Stem Cell, 2018. 23(6): p. 869–881.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desgrange A and Cereghini S, Nephron Patterning: Lessons from Xenopus, Zebrafish, and Mouse Studies. Cells, 2015. 4(3): p. 483–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wingert RA and Davidson AJ, The zebrafish pronephros: a model to study nephron segmentation. Kidney Int, 2008. 73(10): p. 1120–7. [DOI] [PubMed] [Google Scholar]
- 32.Georgas KM, Chiu HS, Lesieur E, Rumballe BA, and Little MH, Expression of metanephric nephron-patterning genes in differentiating mesonephric tubules. Dev Dyn, 2011. 240(6): p. 1600–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costantini F and Kopan R, Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell, 2010. 18(5): p. 698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Short KM, Combes AN, Lefevre J, Ju AL, Georgas KM, Lamberton T, Cairncross O, Rumballe BA, McMahon AP, Hamilton NA, Smyth IM, and Little MH, Global quantification of tissue dynamics in the developing mouse kidney. Dev Cell, 2014. 29(2): p. 188–202. [DOI] [PubMed] [Google Scholar]
- 35.Combes AN, Lefevre JG, Wilson S, Hamilton NA, and Little MH, Cap mesenchyme cell swarming during kidney development is influenced by attraction, repulsion, and adhesion to the ureteric tip. Dev Biol, 2016. 418(2): p. 297–306. [DOI] [PubMed] [Google Scholar]
- 36.Carroll TJ, Park JS, Hayashi S, Majumdar A, and McMahon AP, Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell, 2005. 9(2): p. 283–92. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, and McMahon AP, Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell, 2008. 3(2): p. 169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rumballe BA, Georgas KM, Combes AN, Ju AL, Gilbert T, and Little MH, Nephron formation adopts a novel spatial topology at cessation of nephrogenesis. Dev Biol, 2011. 360(1): p. 110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyle SC, Kim M, Valerius MT, McMahon AP, and Kopan R, Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development, 2011. 138(19): p. 4245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung E, Deacon P, and Park JS, Notch is required for the formation of all nephron segments and primes nephron progenitors for differentiation. Development, 2017. 144(24): p. 4530–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, and Lewandoski M, Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development, 2005. 132(17): p. 3859–71. [DOI] [PubMed] [Google Scholar]
- 42.Tanigawa S, Wang H, Yang Y, Sharma N, Tarasova N, Ajima R, Yamaguchi TP, Rodriguez LG, and Perantoni AO, Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev Biol, 2011. 352(1): p. 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawlor KT, Zappia L, Lefevre J, Park JS, Hamilton NA, Oshlack A, Little MH, and Combes AN, Nephron progenitor commitment is a stochastic process influenced by cell migration. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, Taylor D, Grimmond SM, Potter SS, McMahon AP, and Little MH, Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol, 2009. 332(2): p. 273–86. [DOI] [PubMed] [Google Scholar]
- 45.Lindstrom NO, De Sena Brandine G, Tran T, Ransick A, Suh G, Guo J, Kim AD, Parvez RK, Ruffins SW, Rutledge EA, Thornton ME, Grubbs B, McMahon JA, Smith AD, and McMahon AP, Progressive Recruitment of Mesenchymal Progenitors Reveals a Time-Dependent Process of Cell Fate Acquisition in Mouse and Human Nephrogenesis. Dev Cell, 2018. 45(5): p. 651–660.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Short KM, Combes AN, Lisnyak V, Lefevre JG, Jones LK, Little MH, Hamilton NA, and Smyth IM, Branching morphogenesis in the developing kidney is not impacted by nephron formation or integration. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kao RM, Vasilyev A, Miyawaki A, Drummond IA, and McMahon AP, Invasion of distal nephron precursors associates with tubular interconnection during nephrogenesis. J Am Soc Nephrol, 2012. 23(10): p. 1682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, and Kopan R, Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development, 2007. 134(4): p. 801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindström NO, Hohenstein P, and Davies JA, Nephrons require Rho-kinase for proximal-distal polarity development. 2013. [DOI] [PMC free article] [PubMed]
- 50.Lindstrom NO, Lawrence ML, Burn SF, Johansson JA, Bakker ER, Ridgway RA, Chang CH, Karolak MJ, Oxburgh L, Headon DJ, Sansom OJ, Smits R, Davies JA, and Hohenstein P, Integrated beta-catenin, BMP, PTEN, and Notch signalling patterns the nephron. Elife, 2015. 3: p. e04000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barker N, Rookmaaker MB, Kujala P, Ng A, Leushacke M, Snippert H, van de Wetering M, Tan S, Van Es JH, Huch M, Poulsom R, Verhaar MC, Peters PJ, and Clevers H, Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep, 2012. 2(3): p. 540–52. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, and McMahon AP, Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Reports, 2014. 3(4): p. 650–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.England AR, Chaney CP, Das A, Patel M, Malewska A, Armendariz D, Hon GC, Strand DW, Drake KA, and Carroll TJ, Identification and characterization of cellular heterogeneity within the developing renal interstitium. Development, 2020. 147(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohnenpoll T, Bettenhausen E, Weiss AC, Foik AB, Trowe MO, Blank P, Airik R, and Kispert A, Tbx18 expression demarcates multipotent precursor populations in the developing urogenital system but is exclusively required within the ureteric mesenchymal lineage to suppress a renal stromal fate. Dev Biol, 2013. 380(1): p. 25–36. [DOI] [PubMed] [Google Scholar]
- 55.Das A, Tanigawa S, Karner CM, Xin M, Lum L, Chen C, Olson EN, Perantoni AO, and Carroll TJ, Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol, 2013. 15(9): p. 1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, and McMahon AP, A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development, 2009. 136(1): p. 161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saleem M, Palakkan AA, Mills CG, Tarnick J, Elhendawi M, Marson L, and Davies JA, Differentiation of a contractile ureter-like tissue from exmbryonic stem cell-derived ureteric bud and ex fetu mesenchyme. J Am Soc Nephrol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daniel E, Azizoglu DB, Ryan AR, Walji TA, Chaney CP, Sutton GI, Carroll TJ, Marciano DK, and Cleaver O, Spatiotemporal heterogeneity and patterning of developing renal blood vessels. Angiogenesis, 2018. 21(3): p. 617–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohamed T and Sequeira-Lopez MLS, Development of the renal vasculature. Semin Cell Dev Biol, 2019. 91: p. 132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, and Carroll TJ, Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet, 2009. 41(7): p. 793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, and Nishinakamura R, Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell, 2014. 14(1): p. 53–67. [DOI] [PubMed] [Google Scholar]
- 62.Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, and Little MH, Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol, 2014. 16(1): p. 118–26. [DOI] [PubMed] [Google Scholar]
- 63.Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, Lian MM, Liu M, Aizawa E, Rodriguez Esteban C, Yong KSM, Chen Q, Campistol JM, Fang M, Khor CC, Foo JN, Izpisua Belmonte JC, and Xia Y, Generation of Human PSC-Derived Kidney Organoids with Patterned Nephron Segments and a De Novo Vascular Network. Cell Stem Cell, 2019. 25(3): p. 373–387 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, and Morizane R, Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods, 2019. 16(3): p. 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bantounas I, Ranjzad P, Tengku F, Silajdzic E, Forster D, Asselin MC, Lewis P, Lennon R, Plagge A, Wang Q, Woolf AS, and Kimber SJ, Generation of Functioning Nephrons by Implanting Human Pluripotent Stem Cell-Derived Kidney Progenitors. Stem Cell Reports, 2018. 10(3): p. 766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharmin S, Taguchi A, Kaku Y, Yoshimura Y, Ohmori T, Sakuma T, Mukoyama M, Yamamoto T, Kurihara H, and Nishinakamura R, Human Induced Pluripotent Stem Cell-Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation. J Am Soc Nephrol, 2016. 27(6): p. 1778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, Howden SE, Takasato M, Little MH, and Rabelink TJ, Renal Subcapsular Transplantation of PSC-Derived Kidney Organoids Induces Neo-vasculogenesis and Significant Glomerular and Tubular Maturation In Vivo. Stem Cell Reports, 2018. 10(3): p. 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hochane M, van den Berg PR, Fan X, Berenger-Currias N, Adegeest E, Bialecka M, Nieveen M, Menschaart M, Chuva de Sousa Lopes SM, and Semrau S, Single-cell transcriptomics reveals gene expression dynamics of human fetal kidney development. PLoS Biol, 2019. 17(2): p. e3000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nam SA, Seo E, Kim JW, Kim HW, Kim HL, Kim K, Kim TM, Ju JH, Gomez IG, Uchimura K, Humphreys BD, Yang CW, Lee JY, Kim J, Cho DW, Freedman BS, and Kim YK, Correction to: Graft immaturity and safety concerns in transplanted human kidney organoids. Exp Mol Med, 2020. 52(1): p. 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barry C, Schmitz MT, Jiang P, Schwartz MP, Duffin BM, Swanson S, Bacher R, Bolin JM, Elwell AL, McIntosh BE, Stewart R, and Thomson JA, Species-specific developmental timing is maintained by pluripotent stem cells ex utero. Dev Biol, 2017. 423(2): p. 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, Grimmond SM, Hume DA, Ricardo SD, and Little MH, Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol, 2007. 308(1): p. 232–46. [DOI] [PubMed] [Google Scholar]
- 72.Soo JY, Jansen J, Masereeuw R, and Little MH, Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat Rev Nephrol, 2018. 14(6): p. 378–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindstrom NO, Guo J, Kim AD, Tran T, Guo Q, De Sena Brandine G, Ransick A, Parvez RK, Thornton ME, Basking L, Grubbs B, McMahon JA, Smith AD, and McMahon AP, Conserved and Divergent Features of Mesenchymal Progenitor Cell Types within the Cortical Nephrogenic Niche of the Human and Mouse Kidney. J Am Soc Nephrol, 2018. 29(3): p. 806–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mae SI, Ryosaka M, Sakamoto S, Matsuse K, Nozaki A, Igami M, Kabai R, Watanabe A, and Osafune K, Expansion of Human iPSC-Derived Ureteric Bud Organoids with Repeated Branching Potential. Cell Rep, 2020. 32(4): p. 107963. [DOI] [PubMed] [Google Scholar]
- 75.Mae SI, Ryosaka M, Toyoda T, Matsuse K, Oshima Y, Tsujimoto H, Okumura S, Shibasaki A, and Osafune K, Generation of branching ureteric bud tissues from human pluripotent stem cells. Biochem Biophys Res Commun, 2018. 495(1): p. 954–961. [DOI] [PubMed] [Google Scholar]
- 76.Taguchi A and Nishinakamura R, Higher-Order Kidney Organogenesis from Pluripotent Stem Cells. Cell Stem Cell, 2017. 21(6): p. 730–746.e6. [DOI] [PubMed] [Google Scholar]
- 77.Zeng Z, Huang B, Parvez RK, Li Y, Chen J, Vonk A, Thornton ME, Patel T, Rutledge EA, Kim AD, Yu J, Pastor-Soler N, Hallows Kenneth R., Grubbs Brendan H., McMahon JA, McMahon AP, and Li Z, Generation of kidney ureteric bud and collecting duct organoids that recapitulate kidney branching morphogenesis BioRxiv, 2020. [Google Scholar]
- 78.Yuri S, Nishikawa M, Yanagawa N, Jo OD, and Yanagawa N, In Vitro Propagation and Branching Morphogenesis from Single Ureteric Bud Cells. Stem Cell Reports, 2017. 8(2): p. 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, and Ricardo SD, The directed differentiation of human iPS cells into kidney podocytes. PLoS One, 2012. 7(9): p. e46453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshimura Y, Taguchi A, Tanigawa S, Yatsuda J, Kamba T, Takahashi S, Kurihara H, Mukoyama M, and Nishinakamura R, Manipulation of Nephron-Patterning Signals Enables Selective Induction of Podocytes from Human Pluripotent Stem Cells. J Am Soc Nephrol, 2019. 30(2): p. 304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, and Yamanaka S, Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007. 131(5): p. 861–72. [DOI] [PubMed] [Google Scholar]
- 82.Takahashi K and Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006. 126(4): p. 663–76. [DOI] [PubMed] [Google Scholar]
- 83.Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Sudhof TC, and Wernig M, Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell, 2011. 9(4): p. 374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, and Wernig M, Direct conversion of fibroblasts to functional neurons by defined factors. Nature, 2010. 463(7284): p. 1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong AP, Shojaie S, Liang Q, Xia S, Di Paola M, Ahmadi S, Bilodeau C, Garner J, Post M, Duchesneau P, Waddell TK, Bear CE, Nagy A, and Rossant J, Conversion of human and mouse fibroblasts into lung-like epithelial cells. Sci Rep, 2019. 9(1): p. 9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rackham OJ, Firas J, Fang H, Oates ME, Holmes ML, Knaupp AS, Consortium F, Suzuki H, Nefzger CM, Daub CO, Shin JW, Petretto E, Forrest AR, Hayashizaki Y, Polo JM, and Gough J, A predictive computational framework for direct reprogramming between human cell types. Nat Genet, 2016. 48(3): p. 331–5. [DOI] [PubMed] [Google Scholar]
- 87.Kaminski MM, Tosic J, Kresbach C, Engel H, Klockenbusch J, Muller AL, Pichler R, Grahammer F, Kretz O, Huber TB, Walz G, Arnold SJ, and Lienkamp SS, Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors. Nat Cell Biol, 2016. 18(12): p. 1269–1280. [DOI] [PubMed] [Google Scholar]
- 88.Hiratsuka K, Monkawa T, Akiyama T, Nakatake Y, Oda M, Goparaju SK, Kimura H, Chikazawa-Nohtomi N, Sato S, Ishiguro K, Yamaguchi S, Suzuki S, Morizane R, Ko SBH, Itoh H, and Ko MSH, Induction of human pluripotent stem cells into kidney tissues by synthetic mRNAs encoding transcription factors. Sci Rep, 2019. 9(1): p. 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hendry CE, Vanslambrouck JM, Ineson J, Suhaimi N, Takasato M, Rae F, and Little MH, Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am Soc Nephrol, 2013. 24(9): p. 1424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vanslambrouck JM, Woodard LE, Suhaimi N, Williams FM, Howden SE, Wilson SB, Lonsdale A, Er PX, Li J, Maksimovic J, Oshlack A, Wilson MH, and Little MH, Direct reprogramming to human nephron progenitor-like cells using inducible piggyBac transposon expression of SNAI2-EYA1-SIX1. Kidney Int, 2019. 95(5): p. 1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown AC, Muthukrishnan SD, and Oxburgh L, A synthetic niche for nephron progenitor cells. Dev Cell, 2015. 34(2): p. 229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Z, Araoka T, Wu J, Liao HK, Li M, Lazo M, Zhou B, Sui Y, Wu MZ, Tamura I, Xia Y, Beyret E, Matsusaka T, Pastan I, Rodriguez Esteban C, Guillen I, Guillen P, Campistol JM, and Izpisua Belmonte JC, 3D Culture Supports Long-Term Expansion of Mouse and Human Nephrogenic Progenitors. Cell Stem Cell, 2016. 19(4): p. 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tanigawa S, Taguchi A, Sharma N, Perantoni AO, and Nishinakamura R, Selective In Vitro Propagation of Nephron Progenitors Derived from Embryos and Pluripotent Stem Cells. Cell Rep, 2016. 15(4): p. 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindstrom NO, McMahon JA, Guo J, Tran T, Guo Q, Rutledge E, Parvez RK, Saribekyan G, Schuler RE, Liao C, Kim AD, Abdelhalim A, Ruffins SW, Thornton ME, Basking L, Grubbs B, Kesselman C, and McMahon AP, Conserved and Divergent Features of Human and Mouse Kidney Organogenesis. J Am Soc Nephrol, 2018. 29(3): p. 785–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Menon R, Otto EA, Kokoruda A, Zhou J, Zhang Z, Yoon E, Chen YC, Troyanskaya O, Spence JR, Kretzler M, and Cebrian C, Single-cell analysis of progenitor cell dynamics and lineage specification in the human fetal kidney. Development, 2018. 145(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Metzger JJ, Simunovic M, and Brivanlou AH, Synthetic embryology: controlling geometry to model early mammalian development. Curr Opin Genet Dev, 2018. 52: p. 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warmflash A, Sorre B, Etoc F, Siggia ED, and Brivanlou AH, A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods, 2014. 11(8): p. 847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, and Bonventre JV, Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol, 2015. 33(11): p. 1193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Przepiorski A, Sander V, Tran T, Hollywood JA, Sorrenson B, Shih JH, Wolvetang EJ, McMahon AP, Holm TM, and Davidson AJ, A Simple Bioreactor-Based Method to Generate Kidney Organoids from Pluripotent Stem Cells. Stem Cell Reports, 2018. 11(2): p. 470–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar SV, Er PX, Lawlor KT, Motazedian A, Scurr M, Ghobrial I, Combes AN, Zappia L, Oshlack A, Stanley EG, and Little MH, Kidney micro-organoids in suspension culture as a scalable source of human pluripotent stem cell-derived kidney cells. Development, 2019. 146(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, Martins TJ, Pippin JW, Fu H, Kretzler M, Shankland SJ, Himmelfarb J, Moon RT, Paragas N, and Freedman BS, High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell Stem Cell, 2018. 22(6): p. 929–940 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Higgins JW, Chambon A, Bishard K, Hartung A, Arndt D, Brugnano J, Xuan Er P, Lawlor KT, Vanslambrouck JM, Wilson S, Combes AN, Howden SE, Tan KS, Kumar SV, Hale LJ, Shepherd B, Pentoney S, Presnell SC, Chen AE, and Little MH, Bioprinted pluripotent stem cell-derived kidney organoids provide opportunities for high content screening. bioRxiv, 2018: p. 505396. [Google Scholar]
- 103.Lawlor KT, Vanslambrouck JM, Higgins JW, Chambon A, Bishard K, Arndt D, Er PX, Wilson S, Howden SE, Tan KS, Li F, Hale LJ, Shepherd B, Pentoney S, Presnell SC, Chen AE, and Little MH, Cellular extrusion bioprinting improves kidney organoid throughput, scale reproducibility and conformation. Nature Materials, 2020(In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garreta E, Prado P, Tarantino C, Oria R, Fanlo L, Marti E, Zalvidea D, Trepat X, Roca-Cusachs P, Gavalda-Navarro A, Cozzuto L, Campistol JM, Izpisua Belmonte JC, Hurtado Del Pozo C, and Montserrat N, Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat Mater, 2019. 18(4): p. 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, and Lewis JA, Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci Rep, 2016. 6: p. 34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rein JL, Heja S, Flores D, Carrisoza-Gaytan R, Lin NYC, Homan KA, Lewis JA, and Satlin LM, Effect of luminal flow on doming of mpkCCD cells in a 3D perfusable kidney cortical collecting duct model. Am J Physiol Cell Physiol, 2020. 319(1): p. C136–C147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin NYC, Homan KA, Robinson SS, Kolesky DB, Duarte N, Moisan A, and Lewis JA, Renal reabsorption in 3D vascularized proximal tubule models. Proc Natl Acad Sci U S A, 2019. 116(12): p. 5399–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, and Ingber DE, Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng, 2017. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steinberg MS, Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool, 1970. 173(4): p. 395–433. [DOI] [PubMed] [Google Scholar]
- 110.Trinkaus JP and Groves PW, DIFFERENTIATION IN CULTURE OF MIXED AGGREGATES OF DISSOCIATED TISSUE CELLS. Proc Natl Acad Sci U S A, 1955. 41(10): p. 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weiss P and Taylor AC, Reconstitution of Complete Organs from Single-Cell Suspensions of Chick Embryos in Advanced Stages of Differentiation. Proc Natl Acad Sci U S A, 1960. 46(9): p. 1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lefevre JG, Chiu HS, Combes AN, Vanslambrouck JM, Ju A, Hamilton NA, and Little MH, Self-organisation after embryonic kidney dissociation is driven via selective adhesion of ureteric epithelial cells. Development, 2017. 144(6): p. 1087–1096. [DOI] [PubMed] [Google Scholar]
- 113.Viola JM, Porter CM, Gupta A, Alibekova M, Prahl LS, and Hughes AJ, Guiding Cell Network Assembly using Shape-Morphing Hydrogels. Adv Mater, 2020. 32(31): p. e2002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sloan SA, Andersen J, Pasca AM, Birey F, and Pasca SP, Generation and assembly of human brain region-specific three-dimensional cultures. Nat Protoc, 2018. 13(9): p. 2062–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ransick A, Lindstrom NO, Liu J, Zhu Q, Guo JJ, Alvarado GF, Kim AD, Black HG, Kim J, and McMahon AP, Single-Cell Profiling Reveals Sex, Lineage, and Regional Diversity in the Mouse Kidney. Dev Cell, 2019. 51(3): p. 399–413 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Osathanondh V and Potter EL, Development of Human Kidney as Shown by Microdissection. Iii. Formation and Interrelationship of Collecting Tubules and Nephrons. Arch Pathol, 1963. 76: p. 290–302. [PubMed] [Google Scholar]
- 117.Gallegos TF, Kamei CN, Rohly M, and Drummond IA, Fibroblast growth factor signaling mediates progenitor cell aggregation and nephron regeneration in the adult zebrafish kidney. Dev Biol, 2019. 454(1): p. 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kamei CN, Gallegos TF, Liu Y, Hukriede N, and Drummond IA, Wnt signaling mediates new nephron formation during zebrafish kidney regeneration. Development, 2019. 146(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, Zhu H, Ikenaga T, Ono F, Englert C, Cowan CA, Hukriede NA, Handin RI, and Davidson AJ, Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature, 2011. 470(7332): p. 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, and Bertram JF, Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int, 2003. 63(6): p. 2113–22. [DOI] [PubMed] [Google Scholar]
- 121.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, and van Velzen D, Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest, 1991. 64(6): p. 777–84. [PubMed] [Google Scholar]
- 122.Ryan D, Sutherland MR, Flores TJ, Kent AL, Dahlstrom JE, Puelles VG, Bertram JF, McMahon AP, Little MH, Moore L, and Black MJ, Development of the Human Fetal Kidney from Mid to Late Gestation in Male and Female Infants. EBioMedicine, 2018. 27: p. 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Merlet-Benichou C, Gilbert T, Vilar J, Moreau E, Freund N, and Lelievre-Pegorier M, Nephron number: variability is the rule. Causes and consequences. Lab Invest, 1999. 79(5): p. 515–27. [PubMed] [Google Scholar]
- 124.Hartman HA, Lai HL, and Patterson LT, Cessation of renal morphogenesis in mice. Dev Biol, 2007. 310(2): p. 379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maluf NS and Gassman JJ, Kidneys of the killerwhale and significance of reniculism. Anat Rec, 1998. 250(1): p. 34–44. [DOI] [PubMed] [Google Scholar]
- 126.Camarata T, Howard A, Elsey RM, Raza S, O’Connor A, Beatty B, Conrad J, Solounias N, Chow P, Mukta S, and Vasilyev A, Postembryonic Nephrogenesis and Persistence of Six2-Expressing Nephron Progenitor Cells in the Reptilian Kidney. PLoS One, 2016. 11(5): p. e0153422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brunskill EW, Georgas K, Rumballe B, Little MH, and Potter SS, Defining the molecular character of the developing and adult kidney podocyte. PLoS One, 2011. 6(9): p. e24640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen S, Brunskill EW, Potter SS, Dexheimer PJ, Salomonis N, Aronow BJ, Hong CI, Zhang T, and Kopan R, Intrinsic Age-Dependent Changes and Cell-Cell Contacts Regulate Nephron Progenitor Lifespan. Dev Cell, 2015. 35(1): p. 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu J, Edgington-Giordano F, Dugas C, Abrams A, Katakam P, Satou R, and Saifudeen Z, Regulation of Nephron Progenitor Cell Self-Renewal by Intermediary Metabolism. J Am Soc Nephrol, 2017. 28(11): p. 3323–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Batho CAP, Mills RJ, and Hudson JE, Metabolic Regulation of Human Pluripotent Stem Cell-Derived Cardiomyocyte Maturation. Curr Cardiol Rep, 2020. 22(8): p. 73. [DOI] [PubMed] [Google Scholar]
- 131.Davies J, Using synthetic biology to explore principles of development. Development, 2017. 144(7): p. 1146–1158. [DOI] [PubMed] [Google Scholar]
- 132.Vanslambrouck JM, Wilson SB, Tan K,S,, Soo YC, Scurr M, Spijker HS, Starks LT, Neilson A, Cui X, Jain S, Little MH, Howden SE A toolbox to characterize human induced pluripotent stem cell-derived kidney cell types and organoids. J Am Soc Nephrol, 2019. 30(10):1811–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]