Abstract
Our understanding of the molecular events underpinning the development of mammalian organ systems has been increasing rapidly in recent years. With the advent of new and improved next-generation sequencing methods, we are now able to dig deeper than ever before into the genomic and epigenomic events that play critical roles in determining the fates of stem and progenitor cells during the development of an embryo into an adult. In this review, we detail and discuss the genes and pathways that are involved in mammary gland development, from embryogenesis, through maturation into an adult gland, to the role of pregnancy signals in directing the terminal maturation of the mammary gland into a milk producing organ that can nurture the offspring. We also provide an overview of the latest research in the single-cell genomics of mammary gland development, which may help us to understand the lineage commitment of mammary stem cells (MaSCs) into luminal or basal epithelial cells that constitute the mammary gland. Finally, we summarize the use of 3D organoid cultures as a model system to study the molecular events during mammary gland development. Our increased investigation of the molecular requirements for normal mammary gland development will advance the discovery of targets to predict breast cancer risk and the development of new breast cancer therapies.
Keywords: mammary development, gene expression, transcription regulation, cell heterogeneity
1. Introduction
Mammals are a diverse class of warm-blooded vertebrates with class-specific features that include the presence of hair and the nourishment of young offspring through the secretion of milk by the mammary glands of females. The structure and development of the mammary gland, as well as the nutritional constituents of milk (fat globules, casein micelles, whey proteins, and sugars) are highly conserved across mammals. The evolutionary origin of the mammary gland dates to 310 million years ago (mya), during the Carboniferous period [1,2], long before the appearance of mammals (190 mya). In the Carboniferous period, synapsids (mammalian ancestors evolved from basal amniotes) developed a glandular integument. During the various radiations of synapsids (mammals and mammaliaforms, therapsids), the ancestral integument became highly specialized to produce an abundant and nutritive secretion (milk) during lactation, leading to what is currently defined as the mammary gland. The primitive apocrine glands from which mammary glands originated played an initial role in keeping terrestrially-laid parchment-shelled eggs moist, and in protecting the skin of early synapsids from infection and injury [3].
More recently in evolution, emergence of the placenta diversified mammary gland structures in eutherians in terms of the number of glands and lobuloalveolar structures per nipple [4]. For instance, unlike nipples in mice and humans, cattle or ruminants have a teat formed by epithelial proliferation and gland cisterns that accumulate milk in between each milk harvest, offering a substantial yield of milk for the offspring and an economically advantageous milk supply [5].
During the first days or months of life, milk contributes significantly to nourishment, as well as to the regulation of basal metabolism and temperature of mammalian offspring. As lactation proceeds, caseins are synthetized, phosphorylated, and aggregated into large micelles that are insoluble in the milk and that function to carry calcium phosphate nanoclusters directly to the offspring’s body [6]. The presence of casein micelles correlates with an improvement in offspring nutrition, reducing the demand for egg yolk, and potentially leading to the inactivation of genes associated with yolk formation during evolution [7]. The three primary caseins, α-, β-, or κ-casein, present in the milk of monotremes, marsupials, and eutherians, respectively, diverged before these three taxa originated [8,9].
All the above processes arose more than 150 million years ago and shaped not only a new evolutionary feature to nourish offspring but also the development and anatomy of mammals. As the mammary gland specialized over time, molecular mechanisms have also evolved to control cellular differentiation of mammary epithelial cells and to support their production of milk during lactation. In this review, we will further discuss several molecular switches that control the identity of mammary epithelial cells (MECs) and the development of a fully-functional, milk-producing mammary gland.
2. Understanding fetal mammary gland development
Embryonic development of the mammary gland (Figure 1) is initiated during mid-gestation in many mammalian species. Several mammary gland features associated with sexual dimorphism differ among mammals: the number of primary and secondary sprouts, formation of teats and cisterns in cattle and ovines, and the timing of each developmental event [5].
Figure 1. The blooming of mammary gland development.
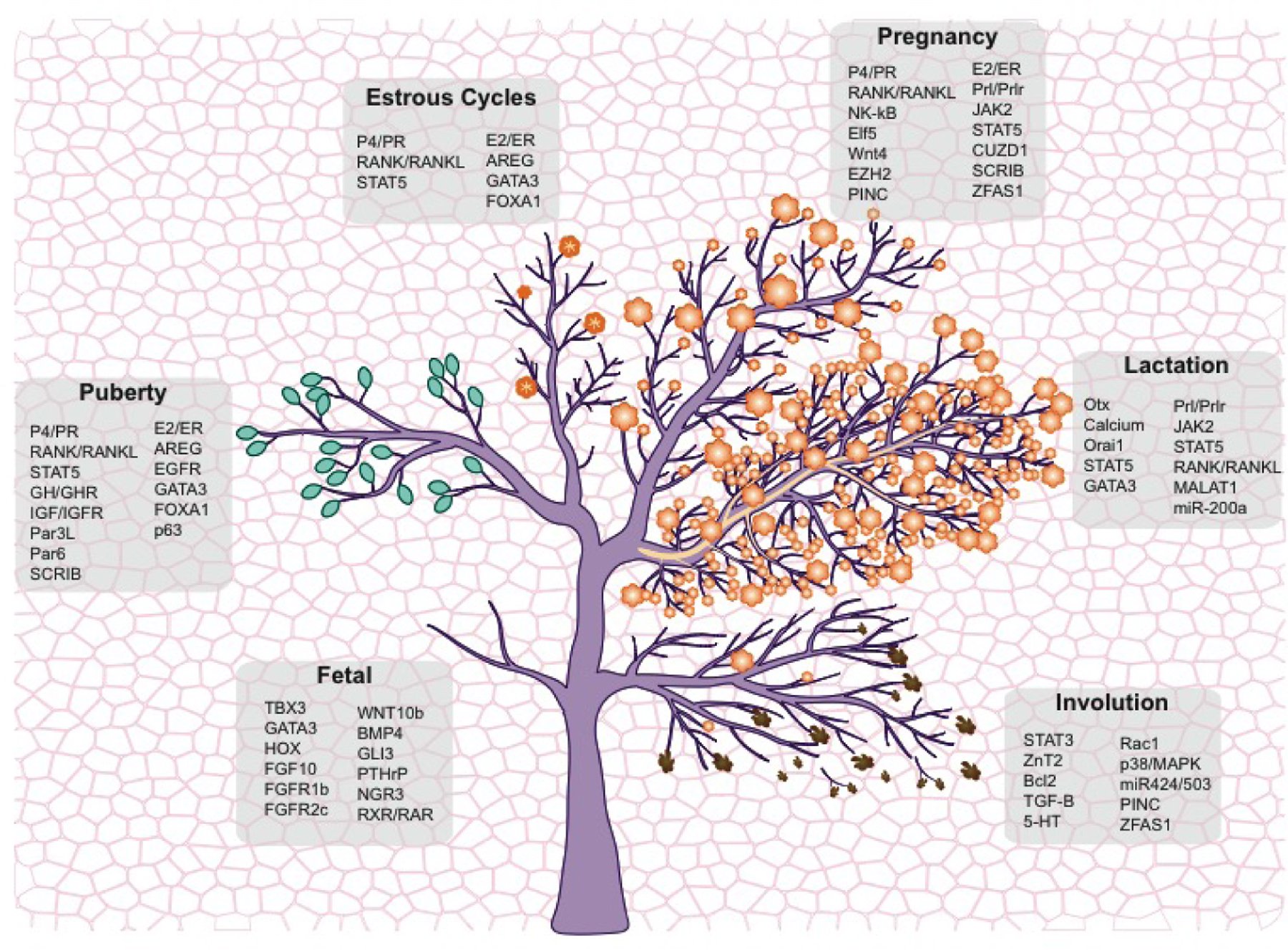
Schematic illustration of mammary gland developmental stages, showing fetal, puberty, estrous cycles, pregnancy, lactation and involution (from left to right). In puberty, green buds represent TEBs. Mammary alveoli are shown as orange flowers in estrous cycles, pregnancy and lactation. In lactation, the milk is represented as yellow sap flowing from the alveoli (flowers) to the ducts (branches). During involution, the regression of the mammary tissue is depicted with falling dead flowers and branches into the background, which portrays the fat pad. The basal compartment and luminal compartment are delineated with darker and lighter colors in the tree, respectively. The main molecular regulators of each developmental stage are highlighted in the grey squares.
In rodents, thick bands of ectodermal cells form bilateral and vertical mammary lines at embryonic day (E) 11.25 whereupon clumps of ectoderm (placodes) bloom along the mammary line at day E11.75, and these ultimately determine the number of breasts in each mammalian species. At day E12.5 the placodes intumesce into the mesoderm, forming an early mammary bud surrounded by a basement membrane (BM) and the first traces of a mammary mesenchyme. Between E13 and E14, the bud will give rise to mammary bulbs with an ectodermal stalk that will elongate into a sprout surrounded by the mesenchyme (fat pad) at E15.5. Lumen formation commences at day E17–18, involving the programmed death of ectodermal cells localized at the center of the mammary branches.
2.1. Signaling networks in the placode and mammary tissue formation
The first stage of mammary development occurs at the same time as the specialization and maturation of the embryonic mesoderm and ectoderm. The ectoderm shapes the structural organization of the mammary gland whereas mesenchymal signaling networks guide ectodermal modifications and expansion during mammary line positioning, placode assembly, and mammary bud formation and elongation. Members of the fibroblast growth factor (FGF) and the wingless-related integration site (WNT) protein families govern signaling in mammary embryonic tissues, and they regulate transcription factors (TFs) from the Homeobox gene family (HOX), GATA3 (GATA binding protein 3), and the T-box family (TBX), which are intermittently expressed either in the endoderm or mesoderm [10–12].
Hoxc8 is expressed until E12.5 and defines the location of mammary placodes, whereas Hox9 is expressed until shortly after birth, and their depletion results in mammary ductal hypoplasia [10,13]. Hox genes have been suggested to be the founders of the enhancer landscape in mammary epithelial cells, acting directly via mammary bud regulatory elements (MBRE) [14]. Such elements, including those associated with the Hoxd9 gene, have been identified in eutherians but not in monotremes or marsupials, suggesting that mammary gland development and a specific pool of regulatory factors perhaps guided evolution in eutherians.
Across all mammals, the TF TBX3 plays a role during fetal mammary gland development. The expression of fibroblast growth factor receptor 1b (FGFR1b), fibroblast growth factor receptor 2c (FGFR2c), and fibroblast growth factor 10 (FGF10) initially promote Tbx3 gene expression in mammary line mesenchyme and subsequently in the placodes [15]. Conversely, bone morphogenic protein 4 (BMP4) negatively regulates Tbx3, which influences the orientation of ectodermal cells, mammary line specification, and placode formation [16]. Furthermore, loss of Tbx3 expression in mice results in the absence of mammary placodes and abnormal gland development, due to loss of expression of Wnt10b and Lef1 (lymphoid enhancer-binding factor 1), key factors in mammary embryogenesis [17–20]. A similar phenotype is observed in FGF10+/− mice which have lost one of the master regulators of TBX3 [21,22].
Ectopic expression of the Wnt inhibitor Dickkopf (Dkk1) in the ectoderm compromises the formation of mammary placodes and impairs the localization of Wnt10b [23]. As abrogation of placode development is more severe in embryos ectopically expressing Dkk1 compared to FGF10- or FGFR-null mice, Wnt networks may be critical for placode initiation [23]. Later in mammary development, mutations in Wnt10b have been correlated with aggressive clinical outcomes for breast cancer, and rapid development of mammary tumors [24].
Other regulators of mammary embryogenesis include TFs that are part of the Hedgehog (Hh) pathway. For instance, mutations in the Gli3 TF lead to the loss of specific placodes, which can cause ductal defects in later stages of mammary development [22]. Through a signaling cascade with members of the Hh network, Gli3 activates gene-specific transcription that controls bud formation [25–27]. Gli2 functions in ductal branching through its localization in the tissue surrounding mammary branches (stroma) from embryogenesis to adulthood, but it becomes stromal and epithelial during pregnancy and lactation [28].
2.2. The nest of embryonic mammary tissue
The microenvironment surrounding mammary tissue plays a pivotal role in the gland development, predominantly via regulation of epithelial-to-mesenchymal transition (EMT), during which epithelial cells lose cell polarity and cell adhesion to become mesenchymal cells with migration and invasion properties. Both EMT and mesenchymal-epithelial transition (MET), the reverse of EMT, are associated with normal mammary development, such as the placodes during embryogenesis, and with cancer, as mammary tumor-initiating cells acquire stem-cell properties through the EMT [29,30]. EMT-inducing TFs (i.e. Zeb1, Slug, Twist) have been detected in cells at terminal end buds (TEBs) during puberty, and Wnt and transforming growth factor beta (TGF-β) signaling pathways in TEBs have also been reported as regulators of EMT [31]. More specifically, EMT-driven signals can determine the expression of extracellular matrix (ECM) components and epithelial cell adhesion receptors through Neuregulin-3 (NRG3), a member of the EGF family, which also localizes in the mesenchyme underlying the mammary line [32]. In Nrg3 mutant mice, FGF10 and mesenchymal Tbx3 expression levels were normal, however, Wnt10b and Lef1 levels were reduced or undetectable in specific placodes [33,34]. Nrg3 signaling through its ERBB4 receptor has been suggested to modulate cell adhesion, thus promoting transduction of somatic FGF10 signaling to the developing placodes.
From E15.5-E16.5 to the end of embryonic development, mammary buds continue to elongate and form rudimentary ductal sprouts that embed into the mesenchymal layer. Throughout this developmental time, transcriptional regulation and cellular signaling mediated by Tbx2–3, Wnt genes, parathyroid related hormone (PTHrP), MSX2 (a homeobox-containing transcription factor), and nuclear factor kappa B (NF-κB) function in concert to promote branching morphogenesis and expansion [27].
Specific immune cell populations are involved in mammary gland development, with recent studies demonstrating the presence of macrophages in the embryonic mammary gland. During embryonic development, macrophages invade and partially remove the mammary epithelium of males, implicating the microenvironment in the sexual dimorphism of the gland [35]. Macrophage-derived progenitor cells from the fetal liver or yolk sac persist in the gland from embryogenesis to adulthood, thus composing the majority of the macrophage population in the postnatal mammary fat pad [36]. Conversely, during puberty, mammary macrophages mostly originate from precursor cells located in the bone marrow, an observation that may suggest constant remodeling of the gland and the requirement for cellular renewal [37].
Macrophages have been observed in the mesenchyme surrounding the mammary buds, but not in close proximity to the epithelial cells during embryogenesis [35]. This is in marked contrast to postnatal stages of mammary development, in which macrophages are found in periductal locations, indicating their role as scavengers as part of the immune surveillance in the mammary microenvironment [35–38]. During postnatal stages, macrophages reach the intraductal niche through dendritic cell movements whereupon they have direct contact with both luminal and basal ductal compartments [37]. Thus, it is possible that fetal macrophages play a role that is distinct from their “sentinel” function during embryonic mammary development, which still remains to be elucidated. Accordingly, we still lack a clear picture of whether macrophages that populate fetal mammary glands remain dormant during post-birth mammary gland development, and whether they contribute to tissue surveillance after birth.
2.3. Not everything is about symmetry
Mammary gland development is bilateral and asymmetrical, like other paired organs [19]. Although the rate of asymmetry is relatively low for the mammary gland, molecular factors can contribute to left-right (L-R) asymmetry in somites during embryogenesis, including the retinoic acid receptors, RARs and RXRs. Lack of RXRα induces defects in the ductal networks, with thoracic mammary glands (TMGs) showing asymmetry marked by decreased ductal branching in the left gland, whereas inguinal mammary glands (IMGs) remain symmetric, with no alterations in the ductal profile [39].
Retinoic acid (RA) regulates the expression of Tbx3, Fgf8, sonic hedgehog protein (Shh), and human epidermal growth factor receptor 2 (ErbB2), all genes associated with MEC differentiation and proliferation [40,41]. In addition, forkhead box protein M1 (FoxM1) and Gata binding factor 3 (Gata3) are highly expressed in the left mammary gland, in contrast to retinoic acid-inducible G-protein coupled receptor 5D (Gprc5d) and neurogenic locus notch homolog protein 1 (Notch1), which are more abundant in the right gland [39]. Both pairs of genes play a role in luminal progenitor cell fate commitment, and in chemoresistance to cancer treatment, suggesting that asymmetrical development of the gland may engage programs that could alter the commitment and malignance of MECs. In fact, women whose breasts significantly vary in size have been reported to have an increased risk of developing breast cancer, with the left breast often being the most affected tissue [42,43], thus suggesting that misregulation of genes associated with left-sided breast development could play an important role as a prognostic marker of aggressive cancer development.
3. The “teen” years
During embryogenesis, the maternal hormones provide the initial stimuli to the rudimentary mammary gland for ductal development. However, after birth, cessation of maternal signaling reduces ductal and branching genesis in the postnatal mammary gland. This activity resumes with the start of puberty, a stage marked by the production of female sexual hormones, which will complete mammary morphogenesis and prepare the gland for milk production in the event of pregnancy.
Puberty varies widely, from a few weeks to several years post-birth, in different mammalian species (5 weeks in mice and 9–18 years in humans). The onset of puberty is triggered by the increase in gonadotropin levels that lead to the secretion of ovarian hormones, mainly estrogen (E2) and progesterone (P4). Peak levels of E2 production are between the follicular phase and ovulation and, depending on the vertebrate, E2 synthesis occurs every 2–4 days in mice and once every month in humans [44].
In the pubertal and adult female, the mammary gland undergoes developmental modifications tightly correlated with ovarian/uterine reproductive cyclical repetitions (4–5 days in mice and 26–32 days in humans). The cycle (Figure 1) is divided between two major phases: Follicular (proestrus and estrus in mice) and luteal (metestrus and diestrus in mice) phases. In humans, the follicular phase begins on the first day of menstruation when P4 levels decrease, the previous corpus luteum degenerates, and a new preovulatory folliculum grows. During ovulation (also called estrus in mice), peak levels of E2 stimulate the high production of luteinizing hormone from the pituitary gland, causing the release of the ovum from the ovary whereupon the luteal phase begins. In mice, and in preparation for a potential pregnancy, the corpus luteum keeps up P4 production for a few days thus triggering mammary tissue expansion and lobuloalveologenesis. The percentage of dense tissue in women’s breasts (mammographic density), is amplified during this phase given the augmented mammary ductal branching and, in mice, high levels of P4 positively correlate with lobuloalveologenesis and tertiary branching [44,45]. The degradation of the corpus luteum and reduced levels of P4 mark the end of a cycle, which induces clearance of MECs through cell death and lobuloalveolar shedding.
The investigation of the molecular underpinnings of both mammary gland pubertal development and the fluctuations during the reproductive cycle will contribute to evaluate the effects of molecular and signaling perturbations in response to disease and cancer initiation, ductal alveologenesis, stromal composition, and in immune microenvironment studies.
3.1. Pubescent structure of the gland
The rapid increase in mammary morphogenesis through branch initiation, invasion of the fat pad, and ductal elongation, transforms a pre-formed, rudimentary mammary epithelium into an extensive ductal network. Hormonal signaling promotes differentiation and proliferation of MECs, culminating in an extensively branched mammary morphology, with pro-apoptotic factors, such as BH3-only BCL-2 protein (BIM), triggering apoptosis and cell clearance to allow lumen formation in the newly developed ducts [46]. The pubescent emergence of mammary ducts depends on TEBs, which emerge at the tip of the ducts and are responsible for promoting the invagination of ducts into the fat pad at a rate of ~0.5mm/day in mice [47]. TEBs (Figure 1) are elongated-shaped structures with an outer layer of cap cells and an inner multilayer of body cells.
The TEB cap cells have stem cell-like features such as self-renewal properties, being morphologically undifferentiated, and the ability to give rise to both luminal and myoepithelial cells in cleared fat pad transplants. In the “neck” region of TEBs, cap cells tend to differentiate into myoepithelial cells, while a fraction of the cap cells have high mobility, penetrate the lumen of the TEBs, and commit to a luminal cell fate [48,49]. Additional studies of cells expressing the p63 protein, a master regulator of MEC development, identified cap cells with a unipotent differentiation capacity towards a myoepithelial cell fate [50], thus suggesting a cellular hierarchy within mammary differentiation and cellular commitment. Conversely, and with the combination of biostatistical modeling and lineage tracing, recent studies dispute the contribution of cap-in-body cells to the luminal lineage. These migratory cap cells are more apoptotic and unlikely to contribute overall to either the luminal or the myoepithelial lineages [47,50]. Therefore, the role of TEB cap cells in ductal elongation remains controversial and warrants further studies.
As an additional cellular stage, the cap-in-body cells are mostly exclusive to the body of TEBs. These cells have a delayed cell cycle progression and increased apoptotic rate when compared to cap cells localized at the outer layer of the TEBs. Molecular analysis of cap-in-body cells identified the TF Forkhead box O protein 1 (FOXO1), and its downstream targets, as major regulators of apoptosis in cap-in-body cells, which contributed to the formation of the lumen during ductal expansion [50]. Collectively, these studies indicate that TEB cap and cap-in-body mammary cells may represent a pool of plastic, heterogeneous, undifferentiated cells that guide pubescent ductal expansion.
Moreover, signaling between MECs and the stroma plays a crucial role in ductal elongation during puberty. TEBs can secrete factors (i.e. eotaxin and interleukin 5) that recruit eosinophils to the tip of TEBs, thus orchestrating side branching [51,52]. Additionally, macrophages spread throughout the mammary ductal system encasing TEBs and perfusing the epithelial bilayer, where they perform a range of functions in guiding ductal outgrowth into the fat pad and phagocytizing body cells to form the lumen of the ducts [35,52,53].
3.2. Estrogen network
During embryogenesis, estrogen receptor (ER)α-depleted glands showed normal primitive mammary ducts, however, during puberty and the following stages of mammary development, lack of ER expression severely compromised ductal network development [54]. Transplantation of ERα−/− MECs, together with WT MECs, resulted in ductal elongation, suggesting that ER may also act in a paracrine manner, stimulating neighbor cells [55–57]. Local paracrine signals act downstream of ovarian hormones, as stroma-derived growth factors. Among these factors is amphiregulin (AREG), a ligand of the epidermal growth factor receptor (EGFR) in stromal cells, which functions as a membrane-anchored precursor and is expressed in luminal MECs and cells throughout the TEBs. AREG-depleted mice lack a mammary ductal network during puberty, similar to the phenotype observed in ERα−/− glands, and ectopic AREG overexpression rescues the ductal network phenotype in ERa knock out (KO) mice. [58,59]. AREG is cleaved by ADAM17 (disintegrin and metalloprotease family member) to promote signaling in stromal cells. The ADAM17 KO phenocopies the ablation of mammary ductal outgrowth seen in AREG- and EGFR-depleted mice during puberty, indicating that the E2-AREG-ADAM17 axis is a key network of paracrine signaling responsible for ductal outgrowth during mammary pubertal development [60]. Moreover, the epithelial expression of AREG is sufficient to induce mammary ductal formation, independently of the stromal signals. In contrast, stromal expression of EGFR is crucial for mammary tree formation compared to epithelial EGFR expression, demonstrating that depletion of EGFR family members also delayed ductal expansion with hyperplasia of cap-in-body cells and reduced body cell levels [60–62].
E2 binds to its receptor, ER, which translocates from the cytoplasm to the nucleus, where it activates the transcription of genes associated with expansion and growth of the mammary epithelium. ER executes its TF functions in both a ligand-independent (Activation function-1, AF-1) and ligand-dependent (Activation function-2, AF-2) manner, and both mechanisms support the transcriptional activation of paracrine factors which are crucial for ductal outgrowth and side branching [58]. Beyond its transcription activation role, ER can mediate a series of cellular signaling via its membrane localization. In fact, site-specific mutations in ERα protein that block its anchoring to cellular membrane resulted in delayed mammary development during puberty and an inability of MaSCs to repopulate mammary fat pads in transplantation assays. This phenotype was accompanied by alterations to the transcriptional state of MECs, thus bringing together a complex regulatory network of endogenous and secreted factors orchestrated by ERα [63].
Another TF, forkhead box A protein 1 (FOXA1), mediates E2 signaling by facilitating chromatin accessibility and, therefore, the interaction between ER and its gene targets. FOXA1 has been identified as a target of the GATA3 regulatory network in branching morphogenesis during puberty [64–67]. Loss of GATA3 has been shown to abrogate TEB formation and to reduce ductal outgrowth, and impair development of ER+ MECs, and perturb pathways regulated by P4, consistent with its role as a master regulator of hormone sensing during mammary gland development [11,67,68]. More recently, a reorganization of the chromatin landscape has been detected in cancer cells, leading to redistribution of ER- and FOXA1-binding sites and disruption of GATA3-ER-FOXA1 signaling network, implicating these TFs and their downstream targets in disease-related pathways [69].
As part of its function during mammary development, ER also recruits other coregulators. The ER co-factor abrogation of glutamic acid [E] and aspartic acid [D]-rich C-terminal domain 1 (CITED1) induced ductal hyperplasia, little to no lumen formation, and dilated ductal structures, thus delaying mammary maturation, although these effects were less pronounced in comparison to ERα−/− [70]. CITED1 also functions as a downstream target of the TGF-β family of TFs, suggesting that its role as a cofactor in these two major signaling pathways, ER and TGF-β, ensures a balance between proliferative and non-proliferative signals during puberty [71].
3.3. Progesterone signaling
In mammals there are two main nuclear progesterone receptor (PR) isoforms (PR-A and PR-B) and multiple variants that homodimerize or heterodimerize to perform distinct gene transcription functions. The ratio of PR-A:PR-B varies in humans (1:1) and in mice (2:1 – 3:1), and perturbation of the PR ratio has been associated with mammary oncogenesis in humans and atypical side-branching development and proliferation in mice [72,73]. Although PR-A can act as a dominant repressor of PR-B during murine puberty, ablation of PR-B results in the lack of conventional pubertal structures in the mammary gland [74,75]. Overexpression of PR-A in the pubertal gland induces mammary ductal hyperplasia and the development of abnormal TEBs [73]. In mice, during puberty, overall depletion of PR resulted in impaired mammary side branching and lobuloalveolar development [76,77]. Using mammary transplantation assays, injection of PR-depleted MECs resulted in impaired lobuloalveolar development in response to E2/P4 treatment, a phenotype mostly rescued with the co-transplantation of WT MECs and PR−/− MECs [77].
Increased P4 levels result in induction of side-branching morphogenesis, through the activation of a subset of quiescent ductal MECs and their reorganization into a multilayered epithelium that buds laterally [76,78]. Given the role of P4 signaling in side branching, we speculate that PR signaling is essential for MaSCs to promote tissue expansion and differentiation, although MaSCs have not been demonstrated to express PR [79–82]. PR is commonly expressed in luminal epithelial cells in mice and humans, and such cell types also engage in tissue expansion during puberty in response to elevated P4 levels, suggesting that a combination of paracrine and non-paracrine functions are regulated by the PR/P4 axis [79,80].
One of the P4-induced paracrine signaling mechanisms involves release of the receptor activator of nuclear factor kappa-Β-ligand (RANKL) and its interaction with receptor activator of nuclear factor kappa-Β (RANK) in PR-negative cells, which together control mammary alveologenesis during much of mammary gland development [83,84]. In addition to its paracrine function, P4 induces the proliferation of PR-positive MECs, potentially through the activation of its downstream target cyclin D1, a mitogenic regulator [83]. Cyclin D1-depleted mice show similar phenotypes as PR-null mice [85,86]. The abrogation of PR reduces cyclin D1 expression, resulting in cell cycle changes in highly proliferative cells. P4 also mediates Wnt4 signaling during puberty to promote ductal expansion, revealing that an additional signaling network is involved in mammary branching during major mammary development stages [87]. The balance between P4-induced cell proliferation and side branching may also rely on mediators and downstream targets of P4 signaling.
At puberty, P4 and insulin growth factor 1 (IGF-1) synergistically promote side branching, TEB expansion, and lobulo alveologenesis, with the combination of P4 and IGF-1 treatment having a 3-fold greater effect on ductal branching compared to IGF-1 treatment alone [76,88]. In pre-pubertal glands, IGF-1 and/or IGF1R have substantial, P4-independent effects on branching, as increased IGF-1 and/or IGF1R levels markedly enhanced ductal expansion, in some cases resulting in mammary tumors, whereas the transplantation of IGF-1R-depleted cells into mammary fat pads arrested MEC proliferation, causing defects in TEB formation [89–93].
The IGF-1 signaling network involves pituitary hormones required for TEB initiation. Growth hormone (GH) binds to its receptor (GHR) on stromal cells and activates IGF-1 transcription, which, in turn, is secreted and interacts with IGFR in epithelial cells. The depletion of IGF-1 leads to a delay in pubertal ductal outgrowth with few side branches, a similar phenotype as described in GHR KO glands [94]. As GH and E2 are upstream targets of IGF-1, the combination of GH and E2 treatment rescued the phenotype caused by IGF-1 depletion [94]. The expression of AREG, an E2-transcriptional target, is increased by P4 signaling and mediates TEB formation and expansion during puberty [95].
In summary, the P4 network involves a combination of paracrine signals and other hormonal pathways that coordinate mammary TEB development, ductal expansion into the fat pad, and side branching during puberty and adult reproductive cycles.
3.5. Regulation of cell polarity
The multicellular bi-layered organization of luminal and myoepithelial cells confers the mammary gland tissue with polarization, and polarity proteins regulate the differential apical-basal (A/B) asymmetry of MECs. Collectively, polarity proteins govern TEB expansion, likely through the regulation of MaSC homeostasis and mammary epithelial fate commitment. Golgi positioning and cytoskeleton organization ensures the orientation of vesicle trafficking, which also contributes to cell polarity. MEC cell polarity can also play a role in mammary lumen formation, in epithelial cell shape and, consequently, ductal branching [96,97]. Moreover, depletion of the TF p63 blocked mammary lumen establishment, and reduced levels of cell-cell adhesion proteins, a phenotype commonly found in mammary tissues with loss of cell polarity [98]. The loss of polarity proteins, i.e. partitioning-defective protein 3 (Par3), triggers atypical hyperplastic ductal morphology due to loss of A/B symmetry, deregulation of progenitor cell differentiation, and increases in cell proliferation and apoptosis [99]. This results in expansion of the diameter of primary ducts and limits the growth of secondary branches, thus arresting mammary branching.
The exogenous Par3-Like (Par3L) protein is another key polarity factor involved in mammary duct expansion. Par3L localizes in cap cells of TEBs and controls stem cell maintenance, as lack of Par3L significantly depleted a subset of MaSCs [100]. Mechanistically, Par3L has been suggested to interact with and inhibit Par4/LKB1 kinase activity, thus controlling stem cell maintenance and cell apoptosis [100]. In addition, the depletion of the focal adhesion protein Paxillin, induces loss of A/B cell polarity, misallocation of apical proteins, loss of microtubule acetylation, and disturbed acinar orientation [101].
Moreover, cell polarity determines the orientation of the mitotic spindle and, consequently, the plane of cell division. This also affects the differentiation and architecture of the expanding TEB during pubertal mammary development. In addition to the factors discussed above, a number of signaling pathways are involved in cell fate decisions. Notch/Numb/Musashi1 signaling, Wnt/β-catenin signaling, and p53 and its downstream effectors are all key pathways that regulate the symmetry of cell divisions based on microenvironmental cues at various developmental stages in the mammary gland (reviewed in detail by Santoro et al [102]).
The deregulation of proteins that control cell polarity is often associated with tumorigenesis. The ablation of Par3, besides impairing ductal growth, also induces STAT3-dependent cell invasion and migration, and it contributes to the invasiveness and metastasis of mammary tumors from ErbB2 mice [103]. Par6 is overexpressed in ER+ human breast tumors and in MCF10A human mammary cell lines, and the inhibition of ErbB2-Par6 signaling axis is sufficient to arrest cell invasion [104]. Although expression of Par6 does not alter A/B polarity, it does cause hyperplasia and induces EGF-independent cell proliferation [105]. Additionally, upregulation of scribble (SCRIB) as well as its mislocalization away from cell-cell junctions is correlated with poor breast cancer prognosis, and ectopic expression of SCRIB can activate oncogenic pathways (i.e. PTEN and mTOR) [106]. Pubertal rats subjected to 7,12-dimethylbenz(α)anthracene (DMBA) treatment developed more TEBs, which showed a higher proliferation rate compared to other mammary developmental stages, and these TEBs eventually underwent oncogenic transformation [107]. Therefore, TEB development and organization share some characteristics with oncogenic phenotypes (i.e. cell invasion, proliferation and an increase in vascular supply) and investigation of TEBs and pubertal development of the mammary gland may be relevant to breast cancer research.
Microtubule organization also plays a key role in determining the apicobasal polarity of MECs, orienting the mitotic spindle, and forming the mammary lumen structure during development and in response to pregnancy signals. Loss of stathmin (STM), a microtubule destabilizing protein, causes a significant delay in postnatal development and maturation of the mammary gland in mice, thus depriving them of the ability to nurse offspring. STM loss leads to decreased Prolactin receptor (PrlR) trafficking and STAT5 signaling, both known to be essential for normal functioning of the gland and to be involved in breast cancer [108]. Huntingtin (HTT) is another protein that regulates apicobasal polarity through microtubule-based dynamics. HTT has been shown to be required for the microtubule dependent apical localization of Par3 through vesicular transport and controls lumen formation in virgin, pregnant, and lactating mice [109]. Integrins also play a critical role in determining the apicobasal polarity of MECs through the integrin linked kinase (ILK)-microtubule network, by regulating tight junction proteins, basolateral surface, Golgi orientation, and consequently mammary acinar morphogenesis [110].
Additional components of the basement membrane, have also been reported in determining MEC cell polarity. For instance, loss of p120, a complex subunit essential for normal functioning of E-cadherin (epithelial cadherin), induced alterations to ductal architecture and TEB function, phenotypes that were likely resultant of an abnormal interaction of cadherin complexes with polarity proteins [111]. More recently, it was shown that depletion of the laminin-binding integrins α3α6 resulted in abnormal baso-apical polarization of luminal progenitor cells, and blocked alveologenesis during pregnancy, thus illustrating that maintenance of polarity via basement membrane function can influence the secretory potential of MECs [112].
Overall, several molecular pathways and factors act during puberty to promote mammary ductal maturation, and these pathways remain active throughout adulthood. As each reproductive cycle promotes lobulo alveologenesis and side branching, we speculate that the constant promotion of mammary cell differentiation and proliferation may induce tumorigenesis over time or otherwise elicit oncogenic pathways that are dormant in the first years of adulthood.
4. Parity signals and mammary development
A complete pregnancy cycle involves gestation, lactation, and involution and, collectively represents the second postnatal stage of mammary gland development, which prepares the gland to produce nourishment to support the offspring. Given the complexity and importance of each of these steps and their molecular programs in mammary gland development and maturation, we will discuss each of them separately.
As well as their role in sexual maturation and mammary development, increased E2 and P4 levels during early gestation are the main factors that induce and regulate MEC proliferation, differentiation, ductal branching, and alveolar development [113,114]. These effects on mammary gland development can be mimicked with subcutaneous implantation of slow-release 17-β-estradiol and progesterone pellets, which provide a temporally controlled approach for studies of the mammary gland in response to pregnancy hormones [115]. Similar to the pubertal mammary gland development, ER dimerizes and translocates to the nucleus in response to elevated E2 levels, thus acting as (a) a paracrine signal transducer that activates FGF and EGF (epidermal growth factor) pathways, and (b) a TF of that induces the expression of ER-responsive genes, during pregnancy [116,117].
During pregnancy (Figure 1), P4 and prolactin (Prl) orchestrate the differentiation of MECs into specialized alveolar structures, which are capable of synthetizing and secreting milk during lactation. Like its function during puberty, the main role of P4 during pregnancy is to promote extensive ductal side-branching but, in pregnancy, P4 signals substantially increase the number of alveolar structures to promote a lactation-competent gland. The absence of PRs in MECs impairs alveolar and side-branching formation, and its paracrine signaling is associated with Wnt4 and receptor activator of nuclear factor kappa-Β ligand (RANKL) [114]. The RANKL receptor (RANK) expressed in ER+ PR+ luminal cells increases WNT paracrine signaling in ER−PR− luminal progenitor cells, wherein P4 mediates Wnt4 and RANKL expression in luminal cells, promoting alveologenesis, expansion of mammary epithelium and milk-secreting acini during pregnancy [118–120]. In progenitor cells, P4-RANK/L signals upregulate R-spondin, a receptor agonist for Wnt. Thus, the Wnt and RANK pathways work in combination to control MEC differentiation [118]. As a consequence, Wnt4 KO does not completely abrogate alveologenesis, as other factors are able to compensate for the absence of Wnt4, promoting alveoli formation in late pregnancy [119]. However, RANKL and RANK KO pregnant mice have no lobuloalveolar milk-secreting structures and experience increased levels of apoptosis in alveolar MECs [121,122].
RANKL activates NF-kB in combination with IKK (IkB kinase), inducing the expression of cyclin D1 and promoting MEC proliferation during pregnancy [123]. The impairment of this pathway leads to underdeveloped ductal structures during pregnancy and a subsequent lactation deficiency due to the lack of MEC expansion [124]. RANKL expression in PR+ luminal cells induces mitogenic paracrine signaling to MaSCs and RANKL-expressing luminal progenitors. Elf5 is a downstream factor for the P4-RANK/L network that can induce progenitor specification towards luminal secretory cell fate [79,125]. The purification of subpopulations of MECs and organoid culture strategies will potentially help identify the specific cell lineages affected by RANK/L, its downstream targets, and the mechanisms and molecular switches triggered by P4.
The release of oxytocin (peptide and neuropeptide hormone, OXT) is one of the factors that control parturition (the act of giving birth) and lactation (Figure 1). OXT controls calcium uptake and contractibility of myoepithelial cells and induces mechanical constriction of luminal alveolar cells to eject milk droplets into the lumen of alveoli [126]. Abrogation of oxytocin production and release does not impact milk production, instead affecting myoepithelial cell contraction. This results in an accumulation of milk droplets in the luminal alveolar cells, nursing impairment, and the death of pups [127]. The inhibition of oxytocin master regulators, such as calcium release-activated calcium channel protein 1 (Orai1), delays alveolar contraction due to interference with calcium influx, and impairs lactation [128,129].
Lactation and milk production yield during late pregnancy have been associated with the presence of binucleated alveolar cells [130,131]. Aurora kinase A (AURKA) and polo-like kinase (PLK1), essential kinases that control cell cycle progression, were found to be upregulated during the onset of lactation, and binucleated cells were suggested to be a byproduct of cytokinesis failure during cell division [130]. These binucleated MECs not only have an altered ploidy index, but also display an enlarged cell volume, indicating the synthesis of high levels of milk protein. They are postulated to play a critical role during milk production, given that AURKA-depleted mammary glands lacked cells with increased DNA content, and were impaired for milk protein production, resulting in stunting of the pups. Similarly, small molecule inhibition of AURKA and PLK1 kinase activity reduced binucleation in luminal alveolar cells and impacted lactogenesis [130]. Such binucleated cells were also detected in the mammary tissue of humans, cows, seals, and wallabies, indicating their evolutionary conservation across mammalian species [146]. However, recent studies have yielded conflicting results, with some indicating that mitotic events are not involved in promoting alterations in DNA synthesis and milk protein production in murine MECs, while others have suggested that DNA synthesis and lactogenesis are directly associated. This highlights the complexity of mechanisms supporting polyploidy in mammary alveolar cells during pregnancy and lactation, which still remain to be elucidated [132–134].
4.1. Prolactin’s role in mammary gland development and lactation
Prolactin (Prl) was first described in 1929 when virgin rabbits injected with pituitary extracts from lactating mice showed pregnancy-like mammary architecture and lactating glands [135]. During the early stages of pregnancy, markedly increased Prl levels play a role in maintaining the corpus luteum, expression of E2 and P4, and in inducing mammary morphogenesis [136,137]. Prl KO mice suffer from impaired alveolar bud formation and reduced tertiary ductal branches, demonstrating the role of Prl during pregnancy-induced development of the mammary gland [138]. The defective mammary branching phenotype was restored by administration of P4 in Prl−/− ovariectomized mice, revealing that P4 and Prl potentially coordinate lobuloalveolar development [138]. Impaired alveologenesis, but not ductal branching, was rescued when the mammary fat pads of wild-type (WT) mice were transplanted with Prl−/− MECs, revealing that prolactin alone drives mammary alveologenesis. While the placental hormones regulate Prl function mid-pregnancy, Prl levels increase during lactation. Prl is mainly expressed by lactotrophic cells in the pituitary gland and released into the bloodstream, but it is also expressed locally in several tissues, including by MECs in the mammary glands. RANKL also acts downstream of PrlR signaling and its overexpression in virgin glands induces pregnancy-like mammary architecture. RANKL is also responsive to P4, and depletion of RANKL results in similar mammary phenotypes as in PR−/− and Prl−/− glands [48,84,139].
Prolactin binds to its receptor (PrlR), resulting in the activation of several signaling cascades, including the JAK2/STAT5 pathway in MECs [140,141]. Upon Prl binding, Janus kinase 1 and 2 (JAK1, JAK2) are recruited to PrlR, and their activation triggers the phosphorylation and nuclear localization of signal transduction and activator of transcription 5 (STAT5) [142]. STAT5(A/B) was first described as one of the progenitors and master regulators of stem cells in normal and leukemic hematopoiesis, and its activation allows for its binding to GAS motif responsive elements (TTCnnnGAA) at gene regulatory regions [143,144]. In MECs, STAT5 controls the expression of an array of genes, including whey acidic protein (Wap), β-casein, and others that together regulate differentiation and proliferation across multiple developmental stages [145,146]. STAT5 also binds to super-enhancers of mammary lineage-specific target genes, which then act collectively with additional TFs to control gene expression [147].
A downstream target of the PrlR pathway is the ETS transcription factor Elf5, a major regulator of alveolar cell fate and lobuloalveolar expansion, as mammary glands from Elf5+/− mice show arrested alveologenesis during pregnancy and lactation failure [148,149]. Elf5 expression increases during pregnancy and lactation, and falls immediately after lactation (involution), when alveolar structures are cleared [149,150]. Accordingly, induction of Elf5 expression in virgin mice leads to an increased expression of milk genes (β-casein and WAP), secretion of milk, inhibition of ductal expansion, and alveolar differentiation [148]. The depletion of Elf5 impacts other downstream targets of the prolactin pathway, as female mice and cultured cells that are haploinsufficient for Elf5 showed impairment of STAT5 activation during pregnancy. Elf5 responsive elements were found near the promoter region of STAT5, suggesting that Elf5 controls STAT5 expression [150]. Genome-wide analysis showed that ELF5 and STAT5 colocalize at mammary-specific STAT5-bound enhancers, including an intergenic enhancer that controls STAT5 activity, suggesting that they cooperate to induce gene expression [151–153].
Additional factors, such as zona pellucida-like domain-containing protein 1 (CUZD1) operate downstream of the JAK2/STAT5 pathway. Loss of CUZD1 induces abnormal mammary TEBs, and impairs the development of tertiary branches and alveologenesis, resulting in a critical reduction of milk proteins and milk production in pregnant and lactating mice [154]. CUZD1 deletion resulted in enhanced STAT5-mediated transcription activation of members of the EGF family, such as Areg (amphiregulin), Nrg1 (neuregulin-1), and Epgn (epigen), which interact with ErbB receptors and promote MEC proliferation [152,154–156].
During early (5–7 days) and mid (11–14 days) pregnancy in mice, the downregulation of SCRIB expression delayed alveologenesis though the reduction of Prl-induced activation of the JAK2/STAT5 pathway [157]. Loss of SCRIB induced PrlR accumulation in the Golgi complex and in recycling endosomes in both mouse and human cells. Given that lactation and milk flow were normal, it was hypothesized that SCRIB levels are restored in late pregnancy [157], however SCRIB depletion has not been fully investigated during involution, when A/B cell polarity is lost [158]. Late during gestation and early during lactation, formation of tight junctions during luminal cell specification controls cellular polarity, which is crucial for directional secretion of milk droplets into the lumen [159], which is principally coordinated by Prl/Jak2 modulation of Erk1/2 function [160].
4.2. The back-and-forth of mammary involution
Offspring weaning removes the suckling stimulus and causes milk stasis, which triggers a series of remodeling processes leading to regression of mammary tissue to a pre-pregnancy state, also known as involution (Figure 1). In humans involution lasts an average of 24 months, while in rodents it lasts for ~10–20 days and encompasses two main phases, the reversible phase (days 0–2 of involution) and the irreversible phase (days 8–18) [161,162].
The reversible phase is characterized by reduced milk production, milk absorption, epithelial cell shedding, alveolar cell death, phagocytosis of apoptotic cells by non-specialized epithelial cells, leukocyte infiltration, and breakdown of tight junctions. As the name implies, resumption of suckling and the suckling stimulus restores lactation through the release of accumulated milk. During lactation, the mammary gland may commence reversible involution after a few hours of milk accumulation, which restores milk-producing cells and avoids over production of milk.
Cell death in the reversible phase of involution occurs via non-apoptotic signals, where residual milk fat globules are taken up by the MECs through lysosomes, which induces lysosomal-mediated cell death (LCD) [163,164]. Of the factors that control LCD, the zinc transporter zinc transporter 2 (ZnT2) is involved in regulating mammary gland development during lactation and involution [165]. During lactation, ZnT2 regulates cell polarization, orientation of vesicles, lumen formation, and prolactin signaling, while induction of ZnT2 expression in MECs induced premature activation of involution and increased zinc concentration in mitochondria and lysosomes [166]. Mutations in ZnT2 have been found in women who produce milk deficient in zinc, which is an important nutritional constituent for newborns [167]. From day 0–6, ~80% of the mammary epithelium undergoes tightly regulated cell death [168]. Serotonin (5-HT) synthetized by MECs [169] regulates tight junction homeostasis through the activation of p38-MAPK signaling, with long-term exposure to 5-HT inducing tight junction breakdown and promoting cell death [170].
Such events occur concomitantly to the recruitment of STAT3-mediated signals, which is initially activated by LIF (leukemia inhibitory factor) in response to milk stasis during the first 3 days of involution [171,172]. Activation of LIF-STAT3 pathways induces the expression of oncostatin M (OSM), one of the main cytokines induced by STAT3-regulatory network, and essential factor for the control of the irreversible phase of involution [173]. In fact, mammary glands from either TGF-β−/−, interleukin 6−/− (IL6−/−), leukemia inhibitor factor−/− (LIF−/−), or STAT3fl/fl mice showed stalled involution and lower levels of cell death [171,172,174,175]. Similarly, STAT5 overexpression during early involution activates Akt1 transcription, a direct target of STAT5, and both proteins abrogate pro-apoptotic STAT3 signaling, leading to the survival of mammary cells and delayed involution, indicating that the Prl/Jak2/STAT5 pathway must be terminated during involution [176,177]. Understanding the mechanisms by which STAT3 functions during involution could lead to therapeutic strategies targeting STAT3 during breast cancer development and progression [178].
During the irreversible phase (days 2–6), the mammary extracellular matrix (ECM) undergoes substantial remodeling, with the activation of wound healing processes, via increased activity of matrix metalloproteases (MMPs), deposition of collagen and BM, in addition to changes in many signaling pathways [179]. For example, the metalloprotease disintegrin and metalloproteinase domain-containing protein 12 (ADAM12) directly activates the STAT3 pathway, one of the involution signals in MECs. In addition, MECs of both rodents and humans express the inflammatory activator gene cyclooxygenase-2 (COX-2) in response to collagen accumulation during involution, a signal that supports immune infiltration, but may also be associated with postpartum breast tumor development [180,181]. Finally, the drop of systemic Prl levels, and the increase of leptin hormone levels induces adipogenesis starting on involution day 2, allowing for the initial reestablishment of pre-pregnancy cellular density and architecture in the mammary gland [182,183].
In the irreversible phase of involution, macrophages and non-professional phagocytic MECs clear the remainder of the cellular debris, resulting in a second wave of inflammation and immune cell recruitment [184,185]. Activation of Ras-related C3 botulinum toxin substrate 1 (Rac1), a member of Rho-GTPase family, in MECs sustains their phagocytic activity, while chemotactic factors promote macrophage infiltration in order to eliminate dead cells [186,187]. Concordantly, Rac1−/− female mice showed accumulation of dead cells and milk protein in the mammary lumen due to loss of adhesion proteins in dying MECs, and inhibition of the phagocytic function of MECs.
The ECM also plays a role in immune cell recruitment and activation, as well as broader immune system functions, as collagen and laminin fragments may also induce an influx of macrophages and neutrophils to the involuting gland [188]. Accordingly, TGF-β regulates MEC cell death and phagocytosis, and helps in the maintenance of ECM integrity, thus also playing a role during the final stages of involution [189,190]. Signaling pathways and the high cell-turnover modulate mammary involution, and they also promote an increase in self-antigen reactions, creating an immune tolerant environment and a mucosal barrier. Increased numbers of RORγT+ FoxP3+ CD4+ T regulatory cells, dendritic cells, and memory Th17-Treg cells are observed during involution. The immune environment then reverts to its nulliparous state when involution comes to an end [191].
The immune tolerance observed during involution and lactation may be relevant for understanding postpartum breast cancer, which has a very poor clinical prognosis, as well as suggest potential routes to explore in cancer treatment [192]. For example, T-lymphocytes expressing the T-cell receptor γδ TCR, also called γδ T cells, may be potential targets for genetic engineering to treat a variety of cancers, including breast tumors [193]. γδ T-cells are involved in both innate and adaptive immune responses, and the mechanism of activation for γδ T-cells is major histocompatibility complex (MHC)-independent creating an opportunity for the development of pan-population immunotherapy.
Despite involution being both a complex and extensive developmental process, the post-involution gland is phenotypically indistinguishable from a mammary gland that has never been exposed to pregnancy hormones, and is competent to re-engage in the lactation process de novo, by repeating the process with each pregnancy. Part of this ability is based on stable molecular changes brought about by the first pregnancy cycle, which enhance milk production during a consecutive pregnancy, indicating that MECs undergo stable molecular changes that function as a memory of prior pregnancy [194,195]. These changes involve remodeling of the MEC epigenome, including alteration of the DNA methylation landscape and gain of the histone ‘active’ mark, H3K27ac, at genomic regions associated with early activation of milk-related genes during a subsequent exposure to pregnancy hormones [196–198]. These gene activation mechanisms are MEC-autonomous, given that a robust response to pregnancy hormones was also observed using fatpad transplantation and organoid cultures [198].
Involution shares many biological processes and gene networks with those implicated in breast cancer progression and metastasis [188,199]. Thus, research on involution-related processes will provide insights on the orchestration of mechanisms associated with ECM remodeling, regulation of gene transcription, maintenance of the epigenomic landscape, and immune surveillance in the normal environment of the gland, while also exploring their deregulation in contribution to cancer development.
5. Beyond tissue development – the transcriptional regulation of MEC differentiation
Two main theories have been advanced concerning the cellular states between MaSCs and fully differentiated MECs [200]. The first hypothesis is based on a MaSC that differentiates directly into luminal or myoepithelial progenitors, thereby producing the respective differentiated MECs. The second theory postulates the existence of a bipotent stem cell generated by a multipotent stem cell that gives rise to basal and luminal progenitors. However, it is difficult to analyze lineage commitment and differentiation in a system where cell surface markers and signals are highly interchangeable. To address this point, several studies have focused on understanding determinants of cell identity and state, with the goal of defining gene expression dynamics that could identify bi- or unipotent cells and their ability to commit to a lineage during the process of mammary gland differentiation (summarized in Figure 2 and Table 1).
Figure 2. Molecular regulators of mammary hierarchy.
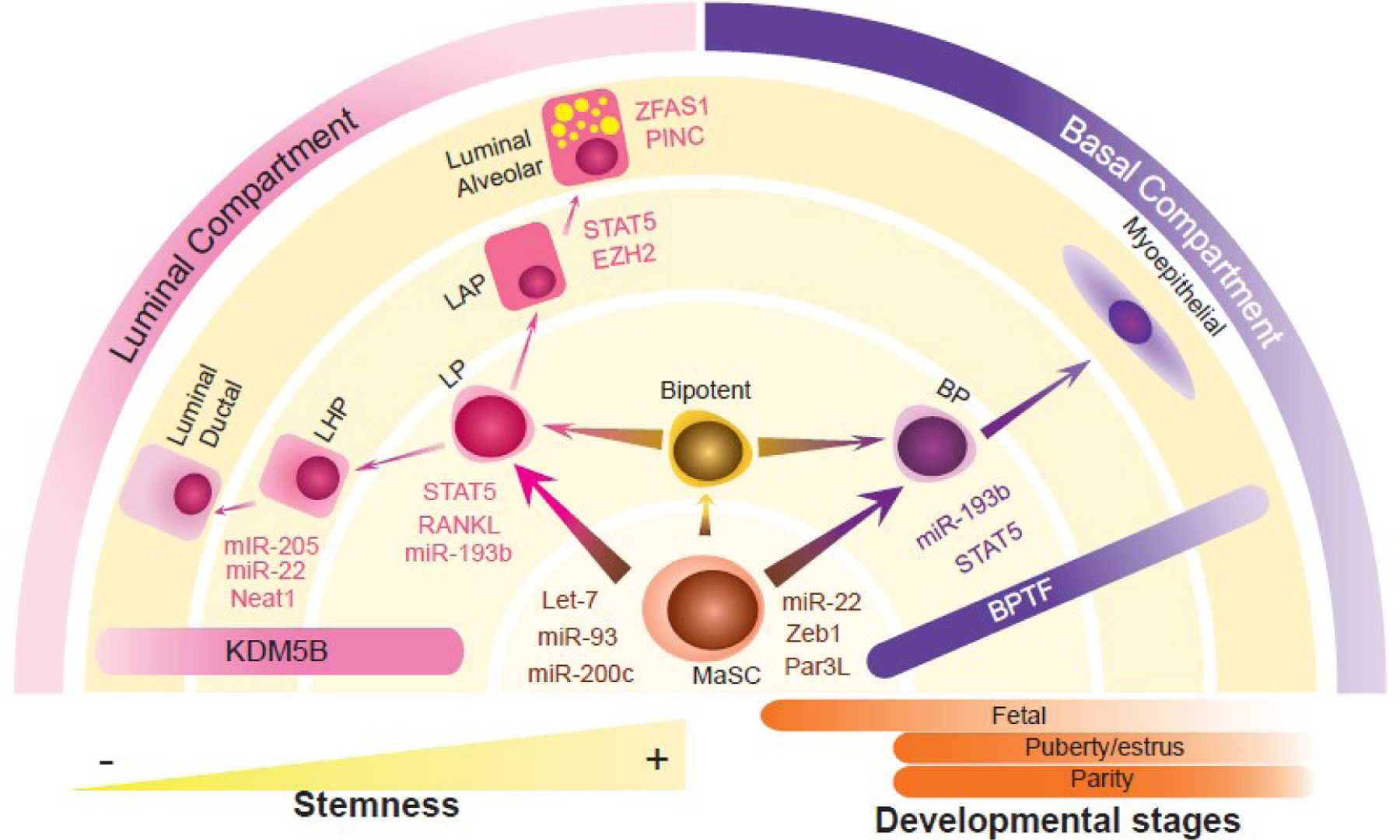
Schematic representation of molecular regulators of mammary cell lineage commitment. Two main differentiation pathways have been reported in literature. In one of them, mammary stem cell (MaSCs) can differentiate into luminal progenitors (LP) and basal progenitors (BP), which are committed to originate either luminal alveolar progenitor (LAP) and luminal hormone progenitors (LHP), or myoepithelial cells, respectively. Another possibility is that MaSCs differentiate into a bipotent MaSC that will give rise to LPs and BPs, subsequently driving luminal and basal differentiated cells. Signaling molecules regulating one MEC subpopulation are color coded according to the cell color and are adjacent to the cell type, whereas molecules regulating more than one cell fate are represented with a bar. Lighter yellow semi-circles indicate high cellular stemness whereas the darker yellow is representative of a differentiated state. The orange bars on the right highlight the most common MECs specification networks identified in each stage of the mammary gland. The gradient colors in the bars representing BPTF (Bromodomain Protein Transcription Factor) and KDM5B (Lysine-Specific Demethylase 5B) are directly related to the function of these proteins at the specified cell lineage.
Table 1. Transcriptional regulation of MEC differentiation –
Overall summary of bulk and scRNAseq expression profiles and their contribution to elucidating cellular state, master regulators, and lineage commitment across mammary epithelial tress.
| Gene name | Cell types | Function | Identification method | References |
|---|---|---|---|---|
| Axin2 | Multipotent cell fates | Lineage commitment and stemness | Lineage tracing, transplantation assays | [203,204] |
| BPTF | Basal epithelial cells, MaSC pool | MaSC self-renewal, ductal alveologenesis, regulation of chromatin accessibility | Gene knockdown, transgenic mice, RNA-seq, ATAC-seq | [231,333] |
| KDM5B | Luminal epithelial cells | Regulator of epigenetic and transcriptomic states | Single cell RNA-seq, mathematical and molecular modeling, gene knockdown, human tumor cell line cultures, inhibitor resistance | [206,207,211,213] |
| HOTAIR | Ductal carcinoma cells | Hormonal regulation of cell proliferation | Human tumor tissue analysis, microarray expression analysis, gene knockdown, PDX models | [263,264,266] |
| Let-7/miR-93/miR-200c | MaSCs | Maintenance of stemness and regulation of differentiation | 3D cell culture assays, miRNA expression sequencing | [248] |
| NEAT1 | Luminal ductal progenitors | Ductal morphogenesis throughout postnatal mammary development | Mouse tissue analysis, RNA in situ hybridization | [260,261] |
| PER2 | MaSC pool | MaSC lineage commitment during pubertal development, regulation of MEC identity, epithelial-mesenchymal transition (EMT) | Transgenic mice, transplantation assays, RNA-seq, | [238,239] |
| PRC2 complex – EZH2 | Luminal alveolar progenitors | Regulator of timing of differentiation and alveologenesis | Gene knockdown, transgenic mice, ES cell differentiation studies | [214,216,217] |
| PRC1 complex – Bmi1 | MaSC pool | Mammary ductal expansion, alveolar cell differentiation, self-renewal of MaSCs | Human MaSC cultures, PDX models, transgenic mice | [218,243] |
| PROM1 | ER+ luminal progenitors | Development and long-term homeostasis of ER+ luminal cells, alveologenesis | Transgenic mice, lineage tracing, transplantation assays | [334] |
| Pygo2 | Basal epithelial cells, MaSC pool | MaSC self-renewal, regulation of chromatin accessibility, lineage commitment and differentiation | Gene knockdown, transgenic mice, transplantation assays, ChIP microarrays | [232,233,335] |
| SOX9 | ER- luminal and basal progenitors | Development and long-term homeostasis of ER-luminal cells | Transgenic mice, lineage tracing, transplantation assays | [240,334] |
| STAT5 | Luminal and basal progenitors | Lineage commitment and differentiation during pregnancy | Inhibitor assays, transgenic mice, microarray analysis, transplantation assays, 3D cell culture systems | [139,147,153–156,236,237] |
| miR-193b | Luminal progenitors | Control of MaSC activity and alveolar differentiation | Transplantation assays. RNA-seq, gene knockdown | [254] |
| miR-205/miR-22 | MaSCs, MECs | Stemness, EMT, cell polarity, differentiation and specialization of MECs during late pregnancy through lactation, breast tumorigenesis | Transplantation assays, xenograft models, 3D cell culture, gene knockdown | [249–253] |
| miR-206/miR-150 | Luminal alveolar cells, MaSCs | Regulation of cell proliferation, differentiation, and stemness during pregnancy, mammary positioning during embryogenesis | 3D cell culture, microarray expression analysis, gene overexpression, transgenic mice | [255–257] |
| ZFAS1, PINC | Luminal alveolar cells | Terminal secretory differentiation during pregnancy and lactation, epigenetic control of mammary development | Gene knockdown, microarray expression analysis, RNA in situ hybridization | [259,261] |
5.1. Working forces of MEC differentiation
Axin2 is one such factor whose role in MEC differentiation has been investigated. Axin2 is a target of the Wnt/β-Catenin pathway and has been shown to mark stem cells localized at the bottom of intestinal crypts and to generate entire crypt/villus structures [201–203]. In the mammary gland, Axin2+ MECs exclusively give rise to MECs committed to the myoepithelial lineage in pre-pubescent mice. However, Axin2+ MECs demonstrated multipotent cell fates in mammary transplantation assays, suggesting that signals present during wound healing, or those coming from specific localization at TEBs during mammary branching, may dictate stemness (Table 1) [203,204].
In addition to signaling molecules, epigenetic factors have also been shown to play a role in lineage commitment and differentiation during mammary gland development. For instance, Lysine-specific demethylase 5B (KDM5B), a Jumonji protein, is a histone demethylase that removes H3K4me3 methyl marks and regulates ductal development during puberty and pregnancy through the control of the transcription of genes for luminal lineage maintenance [205]. Using a single-cell approach, KDM5B was identified as a regulator of epigenetic and transcriptomic states of differentiated luminal epithelial cells, as well as a regulator of transcriptomic heterogeneity in ER+ luminal breast cancer (Table 1) [206]. The inhibition of KDM5B was associated with a pattern of H3K4me3 marks that overall altered the transcriptomic profile in single cells. The KDM5 family of proteins has been implicated in the development of cancer, poor cancer survival, and cancer therapy resistance (e.g. lung, melanoma and breast) [207–210]. Recent studies have attempted to identify small molecules that target KDM5 [211–213].
Many components of the master epigenetic regulator Polycomb complex (PcG) have been implicated during mammary gland development. The H3K27me3 histone methyltransferase EHZ2, the catalytic subunit of the polycomb repressive complex 2 (PRC2), and a key factor in stem cell differentiation, regulates the timing of alveologenesis and luminal differentiation during mammary development [79,214–216]. Loss of EZH2 induced premature cell differentiation and luminal alveolar lineage commitment due to enhanced STAT5 occupancy at its genomic binding sites and increased expression of its downstream targets, suggesting that loss of a repressive marker catalyzed by EZH2 facilitated STAT5-dependent gene expression activation and luminal-biased differentiation (Table 1) [217]. During pregnancy, activation of genes associated with milk production and cellular differentiation was associated with loss of H3K27me3 signals, whereas genes that were repressed during pregnancy (and mammary morphogenesis) markedly gained H3K27me3, demonstrating a regulated gene expression switch that supports cellular commitment during pregnancy-development [79]. Conversely, the Polycomb repressive complex 1 (PRC1) ring finger protein 4, also known as Bmi-1, plays an essential role in controlling the stemness of MECs [214, 215]. Loss of Bmi-1 affected the pool of MaSCs and lineage committed progenitors, which resulted in defective mammary development in fat pad transplantation assays (Table 1), a phenotype that was rescued by further loss of Ink4a/Arf expression, one of the factors negatively regulated by PRC1/Bmi-1 [218].
Another key regulator of MaSC self-renewal and differentiation is the bromodomain protein transcription factor (BPTF). BPTF is the largest subunit of the Nucleosome remodeling factor (NURF) complex, classified as a histone acetylation reader that plays a role in the regulation of chromatin accessibility, modulating TF-DNA occupancy and gene expression levels. [219–223]. Conditional deletion of BPTF in KRT5+ MaSCs resulted in loss of accessibility at genomic regions occupied by many master regulators of mammary development, such as SOX2 [224], TFAP2 family of TFs [225], RUNX1 [226], SOX10 [227], TEAD1 [228], SOX6 [229] and ZEB1 [230], and impacted ductal alveologenesis during active stages of post-birth mammary gland development (Table 1) [231]. Pygopus 2 (Pygo2), is an additional epigenetic factor and histone methylation reader that in response to Wnt signaling controls gene expression, self-renewal and MEC differentiation. Conditional loss of Pygo2 in KRT14 expressing cells resulted in reduced mammary repopulation activity in fat pad transplantation assays (Table 1) due to a luminal-biased state of MaSCs, suggesting its role in sustaining the basal-like fate commitment of MECs [232,233]. Further gene-focused chromatin immunoprecipitation assays (ChIP-qPCR) suggested a mechanism through which Pygo2 chromatin association induces the active transcription of Notch3 mRNA, one of the drivers of luminal/alveolar cellular fate, indicating an epigenomic control of the timing of gene expression according to the cell differentiation state [234,235].
As well as epigenetic regulation of MEC differentiation, many TFs associated with DNA and gene expression regulation are required to determine lineage commitment and cellular differentiation. For example, STAT5 plays a role during pregnancy-development of the mammary gland, and has also been implicated in cellular differentiation [48,139,147,153–156]. Depletion of STAT5 expression in MaSCs resulted in loss of tissue engraftment in fat pad transplantation assays, abrogation of mammary branching, and reduced milk production, demonstrating a role for STAT5 in controlling lineage commitment (Table 1) [236]. STAT5 overexpression in MaSCs induced precocious alveologenesis, further indicating that STAT5 is involved in the differentiation of luminal alveolar progenitor cells [237].
Based on observations of genes that control MEC lineage commitment, a “developmental clock” has been proposed for MEC fate specification, which functions independently of normal circadian clocks. Period 2 (PER2) is one such gene that regulates MaSC lineage commitment and cell fate determination during the pubertal development of the mammary gland, given that PER2 KO mice exhibited underdeveloped mammary glands with reduced ductal branching, and MECs displayed a dual luminal/myoepithelial phenotype, demonstrating PER2’s role in cell fate determination (Table 1) [238]. Further studies showed that loss of PER2 resulted in alteration of MEC identity, with the deregulation of several factors that block or promote EMT progression and MaSC cell fate determination, suggesting that these MECs have increased cell fate plasticity and heterogeneity, which will need to be defined at the single cell level [239,240].
Additional TFs controlling MEC differentiation include the AP-1 complex, E2F, RUNX1, and BCL11B, which mainly control gene expression in response to changes in the levels of growth factors and hormones (such as FGF, EGF, and ER). For example, loss of function of the TFs AP-1and E2F suppressed mammary development at all postnatal stages, given their role in controlling genes such as cyclin D1, c-Myc, TIMP1, vimentin, and fibronectin, which together guide the proliferation, survival, and ECM remodeling of MECs [241,242].
Undoubtedly, this comprehensive overview of molecular regulators in murine MECs illustrates the intersection of complex events that guide murine mammary gland development. The need to understand how such processes take place in mammary tissue aims to answer outstanding questions regarding activation of programs associated with development and carcinogenesis in humans. For example, utilizing RNA interference targeting to induce Bmi-1 knockdown, severely impaired the mammary repopulating capacity of human MaSCs in humanized mammary fat pad transplantation assays, and reduced in vitro mammosphere formation, supporting an evolutionarily conserved role in controlling breast stem cell activity [243]. The notion of the evolutionarily conserved need for molecular regulators of breast development was further expanded in studies that defined cell specific cis-regulatory regions in isolated mammary cell populations from humans, implicating TP63, ELF5, and ESR1 as master regulators of human mammary epithelial lineage commitment [244]. In addition, the utilization of mouse and human comparative analyses revealed that pregnancy epigenetically modifies the p27 and TGFβ gene loci, an effect that influences the state of luminal progenitor cells in culturing systems [245]. Still, there remains an acknowledged gap in bridging breast cellular function, lifespan, and life events (pregnancy, environmental exposures, habits, etc) with epigenomic and molecular alterations that can influence MEC homeostasis and cancer development.
5.2. ncRNA regulation of MEC development
Non-coding RNAs (ncRNAs) are known to play key roles during tissue development and cellular lineage commitment. MicroRNAs (miRNAs) and long non-coding RNAs (lncRNAs) have been implicated in fate specification and specialization of MECs. Profiling of miRNA expression in comma-Dβ cells, a normal-like mammary cell line revealed a number of miRNAs potentially involved in MEC differentiation, including miR-205 and miR-22, which are highly expressed in Sca1high progenitor cells, and let-7 and miR-93, present in mammary undifferentiated cells (Table 1) [246–248].
Several of these miRNAs have subsequently been shown to either promote or block MEC stemness. For example, loss of miR-205 expression resulted in a stemness phenotype in MECs, promoting EMT, and altering cell polarity and symmetric division, through increased Zeb1/2 and Notch expression [249]. During late-pregnancy through lactation, miR-200a stabilizes the levels of E-cadherin and other cell polarity proteins, such as Par6b, by potentially downregulating Zeb1/2, thereby inducing MEC differentiation and specialization [250]. Conversely, miR-22 overexpression resulted in Zeb1 upregulation, leading to the amplification of the MaSC pool, and breast tumorigenesis [251]. Zeb1 and Bmi-1 are also target of miR-200c, a microRNA detected in CD44+CD24− mammary repopulating cells and downstream to the p53 oncogene [252,253]. miR-193b has been identified as a STAT5 target, and mice lacking miR-193b showed accelerated stem/progenitor cell activity and proliferation during puberty and pregnancy (Table 1) [254]. Given miR193b is downstream of prolactin signaling, other miRNAs may potentially be targets of hormonal pathways, and their signaling is yet to be elucidated.
Besides its expression in ER+ breast cancers, miR-206 regulates the transcription of genes that control cell proliferation, differentiation, and stemness in non-tumorigenic mammary cells during pregnancy (Table 1) [255]. Among the genes upregulated by mir-206 in mammary buds are Tbx3 and Lef1, TFs required for mammary positioning during embryogenesis (see above) [256]. The levels of miR-206 and miR-150 decrease between pregnancy and lactation [255,257]. Constitutive expression of miR-150 in MECs resulted in the failure of alveoli formation, and therefore, lactation, liked caused by reduced the expression and phosphorylation of STAT5 [257], however the exact mechanism is currently not known. miR424–503 has been shown to activate apoptosis during involution [258]. As the offspring are weaned, the TGF-β pathway is activated, upregulating miR-424–503 and resulting in the expression of apoptotic factors while reducing the activity of Akt and ERK1–2 pathways [258].
Amongst the lncRNAs identified in MECs, ZNFX1 antisense RNA 1 (ZFAS1) and pregnancy induced non-coding RNA (PINC), are highly expressed in alveolar cells during pregnancy, however, their expression is significantly decreased during lactation with a subsequent increase during involution (Table 1). The reduction of PINC levels during lactation is crucial for terminal secretory differentiation of the gland as knockdown of PINC induces differentiation whereas its overexpression interferes negatively with lactogenic differentiation [259]. PINC inhibits the differentiation of alveolar secretory cells through its interaction with retinoblastoma-associated protein 46 (RbAp46) and PRC2, which results in the deposition of H3K27me3 marks that repress the transcription of target genes [259]. PINC modulates the epigenetic control of mammary development to prevent overproduction of milk and uncontrolled differentiation of luminal progenitor cells into alveolar secretory cells.
Similarly, the lncRNA nuclear paraspeckle assembly transcript 1 (Neat1) is expressed in luminal cells and is required for ductal morphogenesis throughout postnatal mammary gland development, as KO mice for Neat1 show impaired lactation progression (Table 1) [260]. In parous glands, PINC and Zfas1 localize to the terminal ductal-lobular structures, where their function is associated with cell survival and cell division [261,262]. Taken together, ZFAS1, Neat1, and PINC expression is tightly regulated during mammary development, and collectively these ncRNAs control the differentiation of mammary alveolar structures.
Hox transcript antisense intergenic RNA (HOTAIR) is another lncRNA that recruits PRC2 to control and repress the transcription of target genes (Table 1) [263,264]. Given that E2 regulates HOTAIR, and that HOTAIR regulates cancer cell proliferation and cancer invasion in ER+ breast tumors, this suggests that a feedback loop exists between HOTAIR and mammary hormones [265,266]. MALAT1 is a lncRNA upregulated by oxytocin during lactation and in the breast of postmenopausal women [267]. MALAT1 has also been reported to induce invasion and metastasis and to regulate the oncogene p53 [268].
With their wide-ranging role in gene expression regulation, miRNAs and lncRNAs have been considered as suitable drug targets to abrogate the establishment and progression malignant mammary development. While a series of strategies, including the development of tiny-LNAS, have been shown to be efficient in silencing programs regulated by miRNAs [269], recent studies utilizing anti-sense oligo targeting approaches have successfully reduced tumor growth and metastasis by inhibiting MALAT1 expression, thus providing a potential therapeutic strategy to target breast tumors.
5.3 . New Insights into mammary hierarchy – at single cell resolution
The need for more refined strategies to isolate MaSCs and differentiated MECs has led to the development of flow cytometry-based methods for the prospective isolation of mammary cells, the identification of multiple cellular markers that, coupled with molecular profiling, are starting to reveal the mechanistic basis of mammary lineage commitment [200,204,270–277]. These approaches are also filling in the gaps in our understanding of cell specification and plasticity, while raising questions about our appreciation of cellular heterogeneity in MECs. Recent advances in next generation sequencing coupled with bioengineering approaches for single-cell isolation (e.g. microfluidics) offer versatile and agnostic tools able to address global molecular classification and cell identity at high-resolution.
Single cell RNA-seq (scRNAseq) studies are beginning to analyze mammary epithelial cell developmental trajectories in order to address, for example, the existence of bipotent MaSCs and/or lineage-committed progenitors during mammary development, and the temporal switches that govern lineage segregation [278]. To investigate the onset of the mammary epithelial differentiation during embryogenesis, the transcriptomes of a limited pool of FACS-isolated E14 cells have been analyzed, using scRNAseq [279]. Although a transcriptional signature for multipotency was still detected in E13 mammary tissue, unsupervised analysis revealed a composite of gene expression signatures from both luminal and basal cells at E14 [279]. This data provides insight into the molecular regulation that shifts multipotency to unipotency in a developmentally timed fashion, and also indicates the presence of a rare population of bipotent cells early during mammary fetal development. Consistent with these results, scRNAseq data from mouse mammary tissue at E16 and E18 revealed hybrid transcriptional signatures consistent with both basal and luminal lineage specifiers within a single fMaSC cluster, in addition to signatures associated with chromatin remodeling, indicating a role of the epigenetic reprogramming of stemness during mammary embryogenesis [280]. Single-nucleus ATAC-seq (snATACseq) data revealed an epigenetically poised state in fMaSCs from E18, an observation consistent with earlier lineage tracing studies [281,282]. This poised state may guide bidirectional cell commitment to either the basal or luminal lineage after birth [283].
MEC specification accompanies mammary tissue remodeling during subsequent postnatal developmental stages, involving multiple repeated lineage segregation events. Single-cell transcriptome analyses of prepubertal glands revealed signs of an intermediate cellular state between embryonic and adult cells, with MECs assuming more luminal/alveolar features, which potentially indicates the emergence of a luminal precursor or immature state [280,284]. Such an intermediate cellular state was also observed in studies that analyzed MaSCs microdissected from TEBs, which demonstrated a heterogenous transcription state for lineage committed MaSCs, thus introducing the concept that perhaps pools of MaSCs are more likely to drive mammary expansion and growth [285]. High-resolution transcriptomics of sorted cells also captured a largely heterogeneous population of MECs wherein myoepithelial cells showed gene profiles correlated to luminal cells, thus suggesting a common ancestry [286].
Single-cell transcriptomics has also revealed and confirmed epithelial cell types within the mammary gland. For instance, scRNA-seq captured Procr+ cells, Bcl11+ cells, and Cdh5+ cells within myoepithelial cell clusters, corroborating previous studies that characterized populations of MaSCs that reside within the basal compartment, consistent with their ability to fully reconstitute the mammary epithelium in transplantation assays [286–289]. A series of studies have described modifications in the transcriptional landscape of luminal MECs across mammary development. scRNA-seq studies revealed that parity stably affects the transcriptional program of luminal cells, and biases luminal differentiation towards an alveolar fate [288]. E2 treatment correlated with the enrichment of four populations within the luminal progenitor and mature luminal pools, consisting of: (a) ER1+PR+; (b) ER1+PR−; (c) ER1−PR+; and (d) ER1−PR− cells, revealing hormone receptor expression in cells of the luminal fate [290]. Single-cell transcriptomics captured a shift within the hormone-sensing alveolar secretory luminal cells, from high numbers in young murine glands to very low numbers in nulliparous perimenopausal murine glands, indicating a role for aging in luminal cellular fate [291]. Pseudotemporal reconstruction of scRNAseq data from adult breast epithelial cells have also identified a linear trajectory that may give rise to three distinct cellular populations, connecting one myoepithelial cell cluster with two luminal-committed cell clusters [292]. These further divide into subpopulations that were all proliferative, indicating a specific progenitor cell-type for each cell subcluster.
Although single-cell RNA-seq and ATAC-seq are groundbreaking technologies, standardization and reproducibility are critical issues for these methods. Some of the potential pitfalls in these systems arise from single-cell isolation techniques, variation in cell counts, and computational data analysis that considers both technical and biological effects [293]. Furthermore, we still lack a clear general signature of all cells within mammary tissue, including stromal and immune cells, data that would allow effective cellular identification across developmental stages and could consider cellular fluctuations that could influence tumorigenesis. Regardless, single-cell transcriptomics has been critical in the reconstruction of the cell populations that constitute the mammary gland and in disentangling the transcriptional and epigenomic programs critical for mammary lineage segregation and development.
6. Organoids and modeling normal mammary gland development
Over the last several decades, it has become clear that the functional differentiation and development of tissues is dependent on three-dimensional architecture. Consequently, there has been a surge in studies that use 3D cultures to model mammary gland development. Numerous protocols have been developed for the 3D culture of tissues and organs, and the resulting structures are collectively referred to as “organoids”. However, the definition of the organoids depends on the source tissue. In the case of mammary glands, organoids are cultures derived from of mammary tissue fragments in 3D gels, whose composition is similar to the in vivo mammary ECM [294,295].
Cultures of mammary epithelial cells in collagen I gels were first established in the late 1970s. Together with other contemporary studies, they demonstrated that the normal functional differentiation of MECs is dependent on their interaction with a flexible biological substrate [296–299]. MECs harvested from virgin female mice grown in collagen gels showed the ability to reorganize and form structures that can express milk proteins when stimulated with hormones [300–303]. There were also alterations in differentiation and proliferation of MECs depending on whether the collagen gel matrix was attached to a substrate or if it was suspended in dome-like structures [299,304–306].
Mammary organoids can also been grown in commercial 3D matrices such as Matrigel [307] or collagen I [308], which contain BM matrix proteins required for epithelial cell growth and differentiation. Culturing mammary organoids in Matrigel gives rise to organized clusters of bi-layered mammary epithelium, which can be stimulated into branching morphogenesis with growth factors, partially resembling normal in vivo mammary gland development [309,310]. Such organoid systems can also be used as models to study the modifications that pregnancy brings about to the mammary gland. By culturing organoids with pregnancy hormones, organoids can be stimulated to secrete milk proteins (lactation), and removal of such signals can mimic some of the stages seen during involution [198,311] (Figure 3). Additionally, to understand the role of various stromal components during normal mammary gland development, several co-culture assays for MECs or primary mammary organoids with fibroblasts have been developed [312,313]. These assays involve culturing MECs or primary organoids in Matrigel containing fibroblasts isolated by enzymatic digestion and differential centrifugation. There are also 3D-printing strategies for controlled placement of cells in the hydrogel matrix, which allows for reproducible, high-throughput experiments [314].
Figure 3. Mammary organoid cultures can replicate characteristics of normal development.
Representative images of mammary organoid culture derived from pre-pregnancy MECs (Balb/C mice), grown with either essential media or media with pregnancy hormones (containing estrogen (E2), progesterone (P4), and prolactin (Prl). (a) H&E stained organoids (b) light microscope image of organoids in culture (c) Immunofluorescence image of an organoid showing the branching morphogenesis phenotype (d) Immunofluorescence image of an organoid treated with pregnancy hormones expressing Csn2, a milk protein. Scale: 200 µm. Mammary organoid growth conditions and IF staining were performed as described on [198]. Images credits to Chen Chen and Michael F. Ciccone.
3D mammary organoids cultures has led to the elucidation of many factors that drive signaling transduction, gene expression regulation, cell-to-cell junction and tissue remodeling, and that together influence mammary development and MEC differentiation [189,315–320]. More recently, mammary organoids have been used to define a role for SUZ12 and EED, core components of the PRC2 complex, in sustaining progenitor activity of MECs via regulation of cell type specific gene silencing [321]. In addition, high levels of Rspo induced the expansion of undifferentiated myoepithelial cells, revealing that the combination of Nrg1 treatment with low concentrations of Rspo can help maintaining mammary organoids in culture for extended periods of time [322]. Moreover, mammary organoid cultures have also been utilized to analyze the signals controlling mTOR regulation of MaSCs, and the specific contribution of EMT-associated genes in controlling MEC migration and invasion, demonstrating the use of such a system to understand signals that control stemness and cellular plasticity [323–325].
Recently, protocols have also been developed for modeling mammary gland developmental and molecular processes engaged during pregnancy, lactation and involution, and as well for culturing irradiated organoids to simulate radiotherapy, a standard protocol for treating many types of breast cancer [311,326,327]. Furthermore, it has been shown by single-cell analysis that normal and pre-malignant organoid cultures can retain the complex system of multiple MEC states (stem/progenitor and differentiated) and protein expression patterns [328]. Although lacking the complex interactions with the microenvironment, human tissue organoids can be used as a model system to characterize cellular and molecular changes during development and to test the susceptibility of an individual to a variety of therapies.
7. Final remarks
In this review we discuss many aspects of the molecular basis of mammary epithelial differentiation, as well as the mechanisms that are engaged during the various mammary gland developmental stages. Our goal was to highlight many of the significant advances in mammary gland biology research from the last several decades, and to highlight important mechanisms that must be considered as we move toward addressing questions about lineage commitment, cellular differentiation, and mammary gland development. Many of the points discussed here have implications for either the initiation, maintenance or progression of mammary tumorigenesis, underlining the disease relevance in the understanding of normal breast development.
Technological developments - the exploration of 3D organoid cultures and single-cell strategies — are deepening our understanding of specific perturbations that influence the mammary tissue as a whole. Unquestionably, the combination of such strategies with novel mathematical models and lineage tracing has the means to provide quantifiable metrics of cell fate and expression heterogeneity and will yield valuable insights into the coordination of tissue development, as well as help in the prediction of novel factors that modulate risk and treatment response of breast tumors. For instance, quantification of MEC-specific apoptosis and proliferation rates, cell size, cell number, and duct morphology, offer parameters that sufficiently predicted the outcome of ductal elongation in TEBs during puberty [47]. In fact, previous studies have also successfully demonstrated the use of mathematical modeling and lineage tracing to correlate the number of cells and differentiation states with age, pregnancy, and the risk for breast cancer development [329–331]. Moreover, the integration of 3D imaging of breast tumor spheroids with the analysis of biophysical parameters using mathematical models has brought us a step closer toward assessing tumor aggressiveness and response to treatment [332], thus offering robustness and a method for the analysis of retrospective clinical data.
Altogether, these strategies hold the promise of answering long-standing questions about the connections between the regulation of gene expression and tissue maintenance, and microenvironment homeostasis and cellular diversity; evidence of which will hopefully elucidate, address, and perhaps even provide preventative strategies against malignant transformation.
Acknowledgments
This work was performed with the support by the CSHL and Northwell health affiliation, the Rita Allen Scholar Award, the AACR-Breast Cancer Research Foundation Award, the Pershing Square Sohn Prize for Cancer Research, the NIH/NCI grant R01CA248158-01 (C.O.D.S.), and Bristol-Myers Squibb Fellowship (A.V.H.S.). We deeply apologize to all investigators whose works were not cited owing to space limitations. This work was also supported with editing services provided by Dr. Riddihough from Life Science Editors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Oftedal OT (2002) The origin of lactation as a water source for parchment-shelled eggs. J. Mammary Gland Biol. Neoplasia 7, 253–66 [DOI] [PubMed] [Google Scholar]
- 2.Oftedal OT (2002) The mammary gland and its origin during synapsid evolution. J. Mammary Gland Biol. Neoplasia 7, 225–52 [DOI] [PubMed] [Google Scholar]
- 3.Vorbach C et al. (2006) Evolution of the mammary gland from the innate immune system? Bioessays 28, 606–16 [DOI] [PubMed] [Google Scholar]
- 4.Capuco AV and Ellis SE (2013) Comparative aspects of mammary gland development and homeostasis. Annu. Rev. Anim. Biosci 1, 179–202 [DOI] [PubMed] [Google Scholar]
- 5.Rowson AR et al. (2012) Growth and development of the mammary glands of livestock: a veritable barnyard of opportunities. Semin. Cell Dev. Biol 23, 557–66 [DOI] [PubMed] [Google Scholar]
- 6.Smyth E et al. (2004) A biological perspective on the structure and function of caseins and casein micelles. Int. J. Dairy Technol 57, 121–126 [Google Scholar]
- 7.Brawand D et al. (2008) Loss of egg yolk genes in mammals and the origin of lactation and placentation. PLoS Biol 6, e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefèvre CM et al. (2009) Characterisation of monotreme caseins reveals lineage-specific expansion of an ancestral casein locus in mammals. Reprod. Fertil. Dev 21, 1015–27 [DOI] [PubMed] [Google Scholar]
- 9.Rijnkels M (2002) Multispecies comparison of the casein gene loci and evolution of casein gene family. J. Mammary Gland Biol. Neoplasia 7, 327–45 [DOI] [PubMed] [Google Scholar]
- 10.Carroll LS and Capecchi MR (2015) Hoxc8 initiates an ectopic mammary program by regulating Fgf10 and Tbx3 expression and Wnt/β-catenin signaling. Development 142, 4056–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asselin-Labat M-L et al. (2007) Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol 9, 201–209 [DOI] [PubMed] [Google Scholar]
- 12.Davenport TG et al. (2003) Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development 130, 2263–73 [DOI] [PubMed] [Google Scholar]
- 13.Chen F and Capecchi MR (1999) Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc. Natl. Acad. Sci 96, 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schep R et al. (2016) Control of Hoxd gene transcription in the mammary bud by hijacking a preexisting regulatory landscape. Proc. Natl. Acad. Sci 113, E7720–E7729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eblaghie MC et al. (2004) Interactions between FGF and Wnt signals and Tbx3 gene expression in mammary gland initiation in mouse embryos. J. Anat 205, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho K-W et al. (2006) Molecular interactions between Tbx3 and Bmp4 and a model for dorsoventral positioning of mammary gland development. Proc. Natl. Acad. Sci. U. S. A 103, 16788–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Genderen C et al. (1994) Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 8, 2691–703 [DOI] [PubMed] [Google Scholar]
- 18.Boras-Granic K et al. (2006) Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev. Biol 295, 219–31 [DOI] [PubMed] [Google Scholar]
- 19.Veltmaat JM et al. (2013) Positional variations in mammary gland development and cancer. J. Mammary Gland Biol. Neoplasia 18, 179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerome-Majewska LA et al. (2005) Tbx3, the ulnar-mammary syndrome gene, and Tbx2 interact in mammary gland development through a p19Arf/p53-independent pathway. Dev. Dyn 234, 922–33 [DOI] [PubMed] [Google Scholar]
- 21.Veltmaat JM et al. (2006) Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development 133, 2325–35 [DOI] [PubMed] [Google Scholar]
- 22.Hatsell SJ and Cowin P (2006) Gli3-mediated repression of Hedgehog targets is required for normal mammary development. Development 133, 3661–70 [DOI] [PubMed] [Google Scholar]
- 23.Chu EY et al. (2004) Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development 131, 4819–29 [DOI] [PubMed] [Google Scholar]
- 24.Wend P et al. (2012) The role of WNT10B in physiology and disease. Acta Physiol. (Oxf) 204, 34–51 [DOI] [PubMed] [Google Scholar]
- 25.Lee MY et al. (2013) Hedgehog and Gli Signaling in Embryonic Mammary Gland Development. J. Mammary Gland Biol. Neoplasia 18, 133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tickle C and Jung H-S (2016) Embryonic Mammary Gland Development. In eLS pp. 1–10, John Wiley & Sons, Ltd [Google Scholar]
- 27.Robinson GW (2007) Cooperation of signalling pathways in embryonic mammary gland development. Nat. Rev. Genet 8, 963–972 [DOI] [PubMed] [Google Scholar]
- 28.Hatsell SJ and Cowin P (2006) Gli3-mediated repression of Hedgehog targets is required for normal mammary development. Development 133, 3661–3670 [DOI] [PubMed] [Google Scholar]
- 29.Creighton CJ et al. (2009) Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. U. S. A 106, 13820–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye X et al. (2015) Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nassour M et al. (2012) Slug controls stem/progenitor cell growth dynamics during mammary gland morphogenesis. PLoS One 7, e53498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panchal H et al. (2007) Neuregulin3 alters cell fate in the epidermis and mammary gland. BMC Dev. Biol 7, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard BA and Gusterson BA (2000) The characterization of a mouse mutant that displays abnormal mammary gland development. Mamm. Genome 11, 234–7 [DOI] [PubMed] [Google Scholar]
- 34.Howard B et al. (2005) Identification of the scaramanga gene implicates Neuregulin3 in mammary gland specification. Genes Dev 19, 2078–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart TA et al. (2019) Developmental Stage-Specific Distribution of Macrophages in Mouse Mammary Gland. Front. cell Dev. Biol 7, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jäppinen N et al. (2019) Fetal-derived macrophages dominate in adult mammary glands. Nat. Commun 10, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawson CA et al. (2020) Tissue-resident ductal macrophages survey the mammary epithelium and facilitate tissue remodelling. Nat. Cell Biol 22, 546–558 [DOI] [PubMed] [Google Scholar]
- 38.Gyorki DE et al. (2009) Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res 11, R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robichaux JP et al. (2015) Mammary glands exhibit molecular laterality and undergo left-right asymmetric ductal epithelial growth in MMTV-cNeu mice. Oncogene 34, 2003–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ginestier C et al. (2009) Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle 8, 3297–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhinn M and Dolle P (2012) Retinoic acid signalling during development. Development 139, 843–858 [DOI] [PubMed] [Google Scholar]
- 42.Cheng SA et al. (2018) Breast cancer laterality and molecular subtype likely share a common risk factor. Cancer Manag. Res DOI: 10.2147/CMAR.S182254 [DOI] [PMC free article] [PubMed]
- 43.Amer MH (2014) Genetic factors and breast cancer laterality. Cancer Manag. Res DOI: 10.2147/CMAR.S60006 [DOI] [PMC free article] [PubMed]
- 44.Fata JE et al. (2001) Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle. Biol. Reprod 65, 680–8 [DOI] [PubMed] [Google Scholar]
- 45.Robinson GW et al. (1995) Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development 121, 2079–90 [DOI] [PubMed] [Google Scholar]
- 46.Mailleux AA et al. (2007) BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev. Cell 12, 221–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paine I et al. (2016) A Geometrically-Constrained Mathematical Model of Mammary Gland Ductal Elongation Reveals Novel Cellular Dynamics within the Terminal End Bud. PLoS Comput. Biol 12, e1004839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srivastava S et al. (2003) Receptor activator of NF-kappaB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J. Biol. Chem 278, 46171–8 [DOI] [PubMed] [Google Scholar]
- 49.Williams JM and Daniel CW (1983) Mammary ductal elongation: Differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev. Biol 97, 274–290 [DOI] [PubMed] [Google Scholar]
- 50.Sreekumar A et al. (2017) WNT-Mediated Regulation of FOXO1 Constitutes a Critical Axis Maintaining Pubertal Mammary Stem Cell Homeostasis. Dev. Cell 43, 436–448.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sferruzzi-Perri AN et al. (2003) Interleukin-5 transgene expression and eosinophilia are associated with retarded mammary gland development in mice. Biol. Reprod 69, 224–33 [DOI] [PubMed] [Google Scholar]
- 52.Gouon-Evans V et al. (2000) Postnatal mammary gland development requires macrophages and eosinophils. Development 127, 2269–82 [DOI] [PubMed] [Google Scholar]
- 53.Ingman WV et al. (2006) Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev. Dyn 235, 3222–9 [DOI] [PubMed] [Google Scholar]
- 54.Feng Y et al. (2007) Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc. Natl. Acad. Sci. U. S. A 104, 14718–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russo J et al. (1999) Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res. Treat 53, 217–27 [DOI] [PubMed] [Google Scholar]
- 56.Zeps N et al. (1998) Estrogen receptor-negative epithelial cells in mouse mammary gland development and growth. Differentiation 62, 221–6 [DOI] [PubMed] [Google Scholar]
- 57.Clarke RB et al. (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57, 4987–91 [PubMed] [Google Scholar]
- 58.Ciarloni L et al. (2007) Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc. Natl. Acad. Sci. U. S. A 104, 5455–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kenney NJ et al. (2003) Effect of exogenous epidermal-like growth factors on mammary gland development and differentiation in the estrogen receptor-alpha knockout (ERKO) mouse. Breast Cancer Res. Treat 79, 161–73 [DOI] [PubMed] [Google Scholar]
- 60.Sternlicht MD et al. (2005) Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development 132, 3923–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiesen JF et al. (1999) Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development 126, 335–44 [DOI] [PubMed] [Google Scholar]
- 62.Jackson-Fisher AJ et al. (2004) ErbB2 is required for ductal morphogenesis of the mammary gland. Proc. Natl. Acad. Sci. U. S. A 101, 17138–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gagniac L et al. (2020) Membrane expression of the estrogen receptor ERα is required for intercellular communications in the mammary epithelium. Dev DOI: 10.1242/dev.182303 [DOI] [PMC free article] [PubMed]
- 64.Hurtado A et al. (2011) FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat. Genet 43, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laganière J et al. (2005) From the Cover: Location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc. Natl. Acad. Sci. U. S. A 102, 11651–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carroll JS et al. (2005) Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122, 33–43 [DOI] [PubMed] [Google Scholar]
- 67.Kouros-Mehr H et al. (2006) GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127, 1041–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takaku M et al. (2018) GATA3 zinc finger 2 mutations reprogram the breast cancer transcriptional network. Nat. Commun. 9, 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takaku M et al. (2020) Cancer-specific mutation of GATA3 disrupts the transcriptional regulatory network governed by Estrogen Receptor alpha, FOXA1 and GATA3. Nucleic Acids Res 48, 4756–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howlin J et al. (2006) CITED1 homozygous null mice display aberrant pubertal mammary ductal morphogenesis. Oncogene 25, 1532–42 [DOI] [PubMed] [Google Scholar]
- 71.Ewan KB et al. (2002) Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am. J. Pathol 160, 2081–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson E (2002) The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4, 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shyamala G et al. (1998) Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc. Natl. Acad. Sci. U. S. A 95, 696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conneely OM et al. (2001) Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol. Cell. Endocrinol 179, 97–103 [DOI] [PubMed] [Google Scholar]
- 75.Mulac-Jericevic B et al. (2000) Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289, 1751–4 [DOI] [PubMed] [Google Scholar]
- 76.Brisken C et al. (1998) A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. U. S. A 95, 5076–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Humphreys RC et al. (1997) Use of PRKO mice to study the role of progesterone in mammary gland development. J. Mammary Gland Biol. Neoplasia 2, 343–54 [DOI] [PubMed] [Google Scholar]
- 78.Lain AR et al. (2013) Research resource: progesterone receptor targetome underlying mammary gland branching morphogenesis. Mol. Endocrinol 27, 1743–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pal B et al. (2013) Global Changes in the Mammary Epigenome Are Induced by Hormonal Cues and Coordinated by Ezh2. Cell Rep 3, 411–426 [DOI] [PubMed] [Google Scholar]
- 80.Shehata M et al. (2012) Phenotypic and functional characterisation of the luminal cell hierarchy of the mammary gland. Breast Cancer Res 14, R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shyamala G et al. (2002) Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J. Steroid Biochem. Mol. Biol 80, 137–48 [DOI] [PubMed] [Google Scholar]
- 82.Schams D et al. (2003) Expression and localisation of oestrogen and progesterone receptors in the bovine mammary gland during development, function and involution. J. Endocrinol 177, 305–17 [DOI] [PubMed] [Google Scholar]
- 83.Beleut M et al. (2010) Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc. Natl. Acad. Sci. U. S. A 107, 2989–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fernandez-Valdivia R et al. (2009) The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev. Biol 328, 127–39 [DOI] [PubMed] [Google Scholar]
- 85.Sicinski P et al. (1995) Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82, 621–30 [DOI] [PubMed] [Google Scholar]
- 86.Fantl V et al. (1995) Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 9, 2364–72 [DOI] [PubMed] [Google Scholar]
- 87.Rajaram RD et al. (2015) Progesterone and Wnt4 control mammary stem cells via myoepithelial crosstalk. EMBO J 34, 641–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruan W et al. (2005) Progesterone stimulates mammary gland ductal morphogenesis by synergizing with and enhancing insulin-like growth factor-I action. Endocrinology 146, 1170–8 [DOI] [PubMed] [Google Scholar]
- 89.Jones RA et al. (2007) Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene 26, 1636–44 [DOI] [PubMed] [Google Scholar]
- 90.Carboni JM et al. (2005) Tumor development by transgenic expression of a constitutively active insulin-like growth factor I receptor. Cancer Res. 65, 3781–7 [DOI] [PubMed] [Google Scholar]
- 91.Richert MM and Wood TL (1999) The insulin-like growth factors (IGF) and IGF type I receptor during postnatal growth of the murine mammary gland: sites of messenger ribonucleic acid expression and potential functions. Endocrinology 140, 454–61 [DOI] [PubMed] [Google Scholar]
- 92.de Ostrovich KK et al. (2008) Paracrine overexpression of insulin-like growth factor-1 enhances mammary tumorigenesis in vivo. Am. J. Pathol 173, 824–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bonnette SG and Hadsell DL (2001) Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology 142, 4937–45 [DOI] [PubMed] [Google Scholar]
- 94.Ruan W and Kleinberg DL (1999) Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology 140, 5075–81 [DOI] [PubMed] [Google Scholar]
- 95.Aupperlee MD et al. (2013) Amphiregulin mediates progesterone-induced mammary ductal development during puberty. Breast Cancer Res. 15, R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCaffrey LM et al. (2012) Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell 22, 601–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guyer RA and Macara IG (2015) Loss of the polarity protein PAR3 activates STAT3 signaling via an atypical protein kinase C (aPKC)/NF-κB/interleukin-6 (IL-6) axis in mouse mammary cells. J. Biol. Chem 290, 8457–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar S et al. (2020) Inducible knockout of ∆Np63 alters cell polarity and metabolism during pubertal mammary gland development. FEBS Lett. 594, 973–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCaffrey LM and Macara IG (2009) The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev 23, 1450–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huo Y and Macara IG (2014) The Par3-like polarity protein Par3L is essential for mammary stem cell maintenance. Nat. Cell Biol 16, 529–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu W et al. (2019) Paxillin-dependent regulation of apical-basal polarity in mammary gland morphogenesis. Development 146, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Santoro A et al. (2016) Molecular mechanisms of asymmetric divisions in mammary stem cells. EMBO Rep DOI: 10.15252/embr.201643021 [DOI] [PMC free article] [PubMed]
- 103.Xue B et al. (2013) Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion. Nat. Cell Biol 15, 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chatterjee S et al. (2012) Dysregulation of cell polarity proteins synergize with oncogenes or the microenvironment to induce invasive behavior in epithelial cells. PLoS One 7, e34343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nolan ME et al. (2008) The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res 68, 8201–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feigin ME et al. (2014) Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with basal breast cancer. Cancer Res 74, 3180–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Russo IH and Russo J (1978) Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenz[a]anthracene. J. Natl. Cancer Inst 61, 1439–49 [PubMed] [Google Scholar]
- 108.Segatto I et al. (2019) Stathmin Is Required for Normal Mouse Mammary Gland Development and Δ16HER2-Driven Tumorigenesis. Cancer Res 79, 397–409 [DOI] [PubMed] [Google Scholar]
- 109.Elias S et al. (2015) Huntingtin Is Required for Epithelial Polarity through RAB11A-Mediated Apical Trafficking of PAR3-aPKC. PLoS Biol DOI: 10.1371/journal.pbio.1002142 [DOI] [PMC free article] [PubMed]
- 110.Akhtar N and Streuli CH (2013) An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat. Cell Biol DOI: 10.1038/ncb2646 [DOI] [PMC free article] [PubMed]
- 111.Kurley SJ et al. (2012) p120-catenin is essential for terminal end bud function and mammary morphogenesis. Development DOI: 10.1242/dev.072769 [DOI] [PMC free article] [PubMed]
- 112.Romagnoli M et al. (2020) Laminin-binding integrins are essential for the maintenance of functional mammary secretory epithelium in lactation. Dev DOI: 10.1242/dev.181552 [DOI] [PubMed]
- 113.Bocchinfuso WP et al. (2000) Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology 141, 2982–94 [DOI] [PubMed] [Google Scholar]
- 114.Lydon JP et al. (1995) Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9, 2266–78 [DOI] [PubMed] [Google Scholar]
- 115.Silberstein GB et al. (1994) Essential role of endogenous estrogen in directly stimulating mammary growth demonstrated by implants containing pure antiestrogens. Endocrinology 134, 84–90 [DOI] [PubMed] [Google Scholar]
- 116.Kumar V and Chambon P (1988) The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell 55, 145–56 [DOI] [PubMed] [Google Scholar]
- 117.Skandalis SS et al. (2014) Cross-talk between estradiol receptor and EGFR/IGF-IR signaling pathways in estrogen-responsive breast cancers: Focus on the role and impact of proteoglycans. Matrix Biol 35, 182–193 [DOI] [PubMed] [Google Scholar]
- 118.Joshi PA et al. (2015) RANK Signaling Amplifies WNT-Responsive Mammary Progenitors through R-SPONDIN1. Stem Cell Reports 5, 31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brisken C et al. (2000) Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 14, 650–4 [PMC free article] [PubMed] [Google Scholar]
- 120.Mulac-Jericevic B et al. (2003) Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. U. S. A 100, 9744–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fata JE et al. (2000) The Osteoclast Differentiation Factor Osteoprotegerin-Ligand Is Essential for Mammary Gland Development. Cell 103, 41–50 [DOI] [PubMed] [Google Scholar]
- 122.Gonzalez-Suarez E et al. (2007) RANK Overexpression in Transgenic Mice with Mouse Mammary Tumor Virus Promoter-Controlled RANK Increases Proliferation and Impairs Alveolar Differentiation in the Mammary Epithelia and Disrupts Lumen Formation in Cultured Epithelial Acini. Mol. Cell. Biol 27, 1442–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cao Y et al. (2001) IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107, 763–75 [DOI] [PubMed] [Google Scholar]
- 124.Rao S et al. (2018) RANKL and RANK: From Mammalian Physiology to Cancer Treatment. Trends Cell Biol 28, 213–223 [DOI] [PubMed] [Google Scholar]
- 125.Lee HJ et al. (2013) Progesterone drives mammary secretory differentiation via RankL-mediated induction of Elf5 in luminal progenitor cells. Development 140, 1397–1401 [DOI] [PubMed] [Google Scholar]
- 126.Moore DM et al. (1987) Effect of calcium on oxytocin-induced contraction of mammary gland myoepithelium as visualized by NBD-phallacidin. J. Cell Sci 88 ( Pt 5), 563–9 [DOI] [PubMed] [Google Scholar]
- 127.Young WS III et al. (1996) Deficiency in Mouse Oxytocin Prevents Milk Ejection,but not Fertility or Parturition. J. Neuroendocrinol 8, 847–853 [DOI] [PubMed] [Google Scholar]
- 128.Davis FM et al. (2015) Essential role of Orai1 store-operated calcium channels in lactation. Proc. Natl. Acad. Sci 112, 5827–5832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stewart TA et al. (2019) Mammary mechanobiology: PIEZO1 mechanically-activated ion channels in lactation and involution. bioRxiv DOI: 10.1101/649038 [DOI] [PubMed]
- 130.Rios AC et al. (2016) Essential role for a novel population of binucleated mammary epithelial cells in lactation. Nat. Commun 7, 11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vonderhaar BK et al. (1978) Difference between mammary epithelial cells from mature virgin and primiparous mice. Cancer Res 38, 4059–65 [PubMed] [Google Scholar]
- 132.Vonderhaar BK and Smith GH (1982) Dissociation of cytological and functional differential in virgin mouse mammary gland during inhibition of DNA synthesis. J. Cell Sci 53, 97–114 [DOI] [PubMed] [Google Scholar]
- 133.Smith GH and Vonderhaar BK (1981) Functional differentiation in mouse mammary gland epithelium is attained through DNA synthesis, inconsequent of mitosis. Dev. Biol 88, 167–79 [DOI] [PubMed] [Google Scholar]
- 134.Smith GH and Medina D (1988) A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J. Cell Sci 90 ( Pt 1), 173–83 [DOI] [PubMed] [Google Scholar]
- 135.Hennighausen L and Robinson GW (2001) Signaling pathways in mammary gland development. Dev. Cell 1, 467–75 [DOI] [PubMed] [Google Scholar]
- 136.Ormandy CJ et al. (1997) Mammary gland development in prolactin receptor knockout mice. J. Mammary Gland Biol. Neoplasia 2, 355–64 [DOI] [PubMed] [Google Scholar]
- 137.Ormandy CJ et al. (1997) Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 11, 167–78 [DOI] [PubMed] [Google Scholar]
- 138.Vomachka AJ et al. (2000) Prolactin gene-disruption arrests mammary gland development and retards T-antigen-induced tumor growth. Oncogene 19, 1077–84 [DOI] [PubMed] [Google Scholar]
- 139.Cordero A et al. (2016) Rankl Impairs Lactogenic Differentiation Through Inhibition of the Prolactin/Stat5 Pathway at Midgestation. Stem Cells 34, 1027–1039 [DOI] [PubMed] [Google Scholar]
- 140.Rui H et al. (1994) Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J. Biol. Chem 269, 5364–8 [PubMed] [Google Scholar]
- 141.Wakao H et al. (1994) Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J 13, 2182–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ali S (1998) Prolactin receptor regulates Stat5 tyrosine phosphorylation and nuclear translocation by two separate pathways. J. Biol. Chem 273, 7709–16 [DOI] [PubMed] [Google Scholar]
- 143.Li G et al. (2007) STAT5 requires the N-domain to maintain hematopoietic stem cell repopulating function and appropriate lymphoid-myeloid lineage output. Exp. Hematol 35, 1684–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu F et al. (2008) Csf3r mutations in mice confer a strong clonal HSC advantage via activation of Stat5. J. Clin. Invest 118, 946–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li S and Rosen JM (1995) Nuclear factor I and mammary gland factor (STAT5) play a critical role in regulating rat whey acidic protein gene expression in transgenic mice. Mol. Cell. Biol 15, 2063–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schmitt-Ney M et al. (1991) Beta-casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol. Cell. Biol 11, 3745–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shin HY et al. (2016) Hierarchy within the mammary STAT5-driven Wap super-enhancer. Nat. Genet 48, 904–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oakes SR et al. (2008) The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes Dev 22, 581–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhou J et al. (2005) Elf5 is essential for early embryogenesis and mammary gland development during pregnancy and lactation. EMBO J 24, 635–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Choi YS et al. (2009) Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: Failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev. Biol 329, 227–241 [DOI] [PubMed] [Google Scholar]
- 151.Metser G et al. (2016) An autoregulatory enhancer controls mammary-specific STAT5 functions. Nucleic Acids Res 44, 1052–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Komurasaki T et al. (1997) Epiregulin binds to epidermal growth factor receptor and ErbB-4 and induces tyrosine phosphorylation of epidermal growth factor receptor, ErbB-2, ErbB-3 and ErbB-4. Oncogene 15, 2841–8 [DOI] [PubMed] [Google Scholar]
- 153.Zeng X et al. (2016) Lineage-Specific and Non-specific Cytokine-Sensing Genes Respond Differentially to the Master Regulator STAT5. Cell Rep 17, 3333–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mapes J et al. (2017) CUZD1 is a critical mediator of the JAK/STAT5 signaling pathway that controls mammary gland development during pregnancy. PLOS Genet 13, e1006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu B-M et al. (2012) Genome-wide analyses reveal the extent of opportunistic STAT5 binding that does not yield transcriptional activation of neighboring genes. Nucleic Acids Res 40, 4461–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elizalde PV et al. (2016) ErbB-2 nuclear function in breast cancer growth, metastasis and resistance to therapy. Endocr. Relat. Cancer 23, T243–T257 [DOI] [PubMed] [Google Scholar]
- 157.Baker L et al. (2016) Scribble is required for pregnancy-induced alveologenesis in the adult mammary gland. J. Cell Sci 129, 2307–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McDaniel SM et al. (2006) Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am. J. Pathol 168, 608–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rodriguez-Boulan E and Macara IG (2014) Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol 15, 225–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu F et al. (2015) Prolactin/Jak2 directs apical/basal polarization and luminal linage maturation of mammary epithelial cells through regulation of the Erk1/2 pathway. Stem Cell Res 15, 376–83 [DOI] [PubMed] [Google Scholar]
- 161.Jindal S et al. (2014) Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast Cancer Res 16, R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sharp JA et al. (2007) The fur seal-a model lactation phenotype to explore molecular factors involved in the initiation of apoptosis at involution. J. Mammary Gland Biol. Neoplasia 12, 47–58 [DOI] [PubMed] [Google Scholar]
- 163.Baxter FO et al. (2007) The beginning of the end: death signaling in early involution. J. Mammary Gland Biol. Neoplasia 12, 3–13 [DOI] [PubMed] [Google Scholar]
- 164.Sargeant TJ et al. (2014) Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nat. Cell Biol 16, 1057–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hennigar SR et al. (2015) ZnT2 is a critical mediator of lysosomal-mediated cell death during early mammary gland involution. Sci. Rep 5, 8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee S et al. (2017) Zinc transporter 2 interacts with vacuolar ATPase and is required for polarization, vesicle acidification, and secretion in mammary epithelial cells. J. Biol. Chem 292, 21598–21613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chowanadisai W et al. (2006) Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem 281, 39699–707 [DOI] [PubMed] [Google Scholar]
- 168.Walker NI et al. (1989) Cell death by apoptosis during involution of the lactating breast in mice and rats. Am. J. Anat 185, 19–32 [DOI] [PubMed] [Google Scholar]
- 169.Matsuda M et al. (2004) Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev. Cell 6, 193–203 [DOI] [PubMed] [Google Scholar]
- 170.Pai VP and Horseman ND (2011) Multiple cellular responses to serotonin contribute to epithelial homeostasis. PLoS One 6, e17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kritikou EA et al. (2003) A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development 130, 3459–68 [DOI] [PubMed] [Google Scholar]
- 172.Schere-Levy C et al. (2003) Leukemia inhibitory factor induces apoptosis of the mammary epithelial cells and participates in mouse mammary gland involution. Exp. Cell Res 282, 35–47 [DOI] [PubMed] [Google Scholar]
- 173.Tiffen PG et al. (2008) A dual role for oncostatin M signaling in the differentiation and death of mammary epithelial cells in vivo. Mol. Endocrinol DOI: 10.1210/me.2008-0097 [DOI] [PMC free article] [PubMed]
- 174.Nguyen AV and Pollard JW (2000) Transforming growth factor beta3 induces cell death during the first stage of mammary gland involution. Development 127, 3107–18 [DOI] [PubMed] [Google Scholar]
- 175.Humphreys RC et al. (2002) Deletion of Stat3 blocks mammary gland involution and extends functional competence of the secretory epithelium in the absence of lactogenic stimuli. Endocrinology 143, 3641–50 [DOI] [PubMed] [Google Scholar]
- 176.Creamer BA et al. (2010) Stat5 Promotes Survival of Mammary Epithelial Cells through Transcriptional Activation of a Distinct Promoter in Akt1. Mol. Cell. Biol 30, 2957–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schmidt JW et al. (2014) Stat5 Regulates the Phosphatidylinositol 3-Kinase/Akt1 Pathway during Mammary Gland Development and Tumorigenesis. Mol. Cell. Biol 34, 1363–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kordon EC and Coso OA (2017) Postlactational Involution: Molecular Mechanisms and Relevance for Breast Cancer Development. In Current Topics in Lactation InTech
- 179.Green KA and Lund LR (2005) ECM degrading proteases and tissue remodelling in the mammary gland. Bioessays 27, 894–903 [DOI] [PubMed] [Google Scholar]
- 180.Fornetti J et al. (2014) Physiological COX-2 expression in breast epithelium associates with COX-2 levels in ductal carcinoma in situ and invasive breast cancer in young women. Am. J. Pathol 184, 1219–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lyons TR et al. (2011) Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat. Med 17, 1109–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ben-Jonathan N and Hugo E (2015) Prolactin (PRL) in adipose tissue: regulation and functions. Adv. Exp. Med. Biol 846, 1–35 [DOI] [PubMed] [Google Scholar]
- 183.Lin Y and Li Q (2007) Expression and function of leptin and its receptor in mouse mammary gland. Sci. China. Ser. C, Life Sci 50, 669–75 [DOI] [PubMed] [Google Scholar]
- 184.Stein T et al. (2004) Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res 6, R75–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Monks J et al. (2005) Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ 12, 107–114 [DOI] [PubMed] [Google Scholar]
- 186.Akhtar N et al. (2016) Rac1 Controls Both the Secretory Function of the Mammary Gland and Its Remodeling for Successive Gestations. Dev. Cell 38, 522–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schuler F et al. (2016) The BH3-only protein BIM contributes to late-stage involution in the mouse mammary gland. Cell Death Differ 23, 41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jena MK et al. (2019) Molecular mechanism of mammary gland involution: An update. Dev. Biol 445, 145–155 [DOI] [PubMed] [Google Scholar]
- 189.Xu J et al. (2009) TGF-β-induced epithelial to mesenchymal transition. Cell Res 19, 156–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pang M-F et al. (2016) TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 35, 748–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Betts CB et al. (2018) Mucosal Immunity in the Female Murine Mammary Gland. J. Immunol 201, 734–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fornetti J et al. (2014) Mammary Gland Involution as an Immunotherapeutic Target for Postpartum Breast Cancer. J. Mammary Gland Biol. Neoplasia 19, 213–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Janssen A et al. (2020) γδ T-cell Receptors Derived from Breast Cancer-Infiltrating T Lymphocytes Mediate Antitumor Reactivity. Cancer Immunol. Res 8, 530–543 [DOI] [PubMed] [Google Scholar]
- 194.Zuppa AA et al. (1988) Relationship between maternal parity, basal prolactin levels and neonatal breast milk intake. Biol. Neonate 53, 144–7 [DOI] [PubMed] [Google Scholar]
- 195.Ingram J et al. (2001) Breastfeeding: it is worth trying with the second baby. Lancet (London, England) 358, 986–7 [DOI] [PubMed] [Google Scholar]
- 196.Huh SJ et al. (2015) Age- and Pregnancy-Associated DNA Methylation Changes in Mammary Epithelial Cells. Stem Cell Reports 4, 297–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.dos Santos CO et al. (2015) An Epigenetic Memory of Pregnancy in the Mouse Mammary Gland. Cell Rep 11, 1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Feigman MJ et al. (2020) Pregnancy reprograms the epigenome of mammary epithelial cells and blocks the development of premalignant lesions. Nat. Commun 11, 2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wallace TR et al. (2019) Studies of postpartum mammary gland involution reveal novel pro-metastatic mechanisms. J. Cancer Metastasis Treat 2019, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fu NY et al. (2020) Stem Cells and the Differentiation Hierarchy in Mammary Gland Development. Physiol. Rev 100, 489–523 [DOI] [PubMed] [Google Scholar]
- 201.Muncan V et al. (2006) Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol. Cell. Biol 26, 8418–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Korinek V et al. (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet 19, 379–83 [DOI] [PubMed] [Google Scholar]
- 203.van Amerongen R et al. (2012) Developmental Stage and Time Dictate the Fate of Wnt/β-Catenin-Responsive Stem Cells in the Mammary Gland. Cell Stem Cell 11, 387–400 [DOI] [PubMed] [Google Scholar]
- 204.Van Keymeulen A et al. (2011) Distinct stem cells contribute to mammary gland development and maintenance. Nature 479, 189–93 [DOI] [PubMed] [Google Scholar]
- 205.Zou MR et al. (2014) Histone Demethylase Jumonji AT-rich Interactive Domain 1B (JARID1B) Controls Mammary Gland Development by Regulating Key Developmental and Lineage Specification Genes. J. Biol. Chem 289, 17620–17633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hinohara K et al. (2018) KDM5 Histone Demethylase Activity Links Cellular Transcriptomic Heterogeneity to Therapeutic Resistance. Cancer Cell 34, 939–953.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yamamoto S et al. (2014) JARID1B is a luminal lineage-driving oncogene in breast cancer. Cancer Cell 25, 762–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Roesch A et al. (2013) Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell 23, 811–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Roesch A et al. (2010) A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 141, 583–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sharma SV et al. (2010) A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Horton JR et al. (2016) Characterization of a Linked Jumonji Domain of the KDM5/JARID1 Family of Histone H3 Lysine 4 Demethylases. J. Biol. Chem 291, 2631–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Johansson C et al. (2016) Structural analysis of human KDM5B guides histone demethylase inhibitor development. Nat. Chem. Biol 12, 539–45 [DOI] [PubMed] [Google Scholar]
- 213.Pippa S et al. (2019) Small Molecule Inhibitors of KDM5 Histone Demethylases Increase the Radiosensitivity of Breast Cancer Cells Overexpressing JARID1B. Molecules 24, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Michalak EM et al. (2013) Polycomb group gene Ezh2 regulates mammary gland morphogenesis and maintains the luminal progenitor pool. Stem Cells 31, 1910–1920 [DOI] [PubMed] [Google Scholar]
- 215.Margueron R et al. (2008) Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol. Cell 32, 503–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shen X et al. (2008) EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell 32, 491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yoo KH et al. (2015) Loss of EZH2 results in precocious mammary gland development and activation of STAT5-dependent genes. Nucleic Acids Res 43, 8774–8789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pietersen AM et al. (2008) Bmi1 Regulates Stem Cells and Proliferation and Differentiation of Committed Cells in Mammary Epithelium. Curr. Biol DOI: 10.1016/j.cub.2008.06.070 [DOI] [PubMed]
- 219.Goller T et al. (2008) Transcriptional Regulator BPTF/FAC1 Is Essential for Trophoblast Differentiation during Early Mouse Development. Mol. Cell. Biol 28, 6819–6827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Landry JW et al. (2011) Chromatin remodeling complex NURF regulates thymocyte maturation. Genes Dev 25, 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Koludrovic D et al. (2015) Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells. PLOS Genet 11, e1005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ruthenburg AJ et al. (2011) Recognition of a mononucleosomal histone modification pattern by BPTF via multivalent interactions. Cell 145, 692–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xu B et al. (2018) The Chromatin Remodeler BPTF Activates a Stemness Gene-Expression Program Essential for the Maintenance of Adult Hematopoietic Stem Cells. Stem cell reports 10, 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Domenici G et al. (2019) A Sox2-Sox9 signalling axis maintains human breast luminal progenitor and breast cancer stem cells. Oncogene 38, 3151–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jäger R et al. (2010) Loss of transcription factor AP-2gamma/TFAP2C impairs branching morphogenesis of the murine mammary gland. Dev. Dyn 239, 1027–33 [DOI] [PubMed] [Google Scholar]
- 226.Hong D et al. (2017) Runx1 stabilizes the mammary epithelial cell phenotype and prevents epithelial to mesenchymal transition. Oncotarget 8, 17610–17627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Athwal HK et al. (2019) Sox10 Regulates Plasticity of Epithelial Progenitors toward Secretory Units of Exocrine Glands. Stem cell reports 12, 366–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Huh HD et al. (2019) Regulation of TEAD Transcription Factors in Cancer Biology. Cells 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kendrick H et al. (2008) Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics 9, 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sikandar SS et al. (2017) Role of epithelial to mesenchymal transition associated genes in mammary gland regeneration and breast tumorigenesis. Nat. Commun 8, 1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Frey WD et al. (2017) BPTF Maintains Chromatin Accessibility and the Self-Renewal Capacity of Mammary Gland Stem Cells. Stem Cell Reports 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gu B et al. (2009) Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation. J. Cell Biol DOI: 10.1083/jcb.200810133 [DOI] [PMC free article] [PubMed]
- 233.Gu B et al. (2013) Chromatin effector Pygo2 mediates wnt-notch crosstalk to suppress luminal/alveolar potential of mammary stem and basal cells. Cell Stem Cell DOI: 10.1016/j.stem.2013.04.012 [DOI] [PMC free article] [PubMed]
- 234.Bernstein BE et al. (2006) A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell DOI: 10.1016/j.cell.2006.02.041 [DOI] [PubMed]
- 235.Mikkelsen TS et al. (2007) Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature DOI: 10.1038/nature06008 [DOI] [PMC free article] [PubMed]
- 236.Yamaji D et al. (2009) Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev 23, 2382–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vafaizadeh V et al. (2010) Mammary epithelial reconstitution with gene-modified stem cells assigns roles to Stat5 in luminal alveolar cell fate decisions, differentiation, involution, and mammary tumor formation. Stem Cells 28, 928–38 [DOI] [PubMed] [Google Scholar]
- 238.McQueen CM et al. (2018) PER2 regulation of mammary gland development. Development 145, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hwang-Verslues WW et al. (2013) Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy. Proc. Natl. Acad. Sci. U. S. A 110, 12331–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Guo W et al. (2012) Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148, 1015–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shen Q et al. (2006) The AP-1 transcription factor regulates postnatal mammary gland development. Dev. Biol 295, 589–603 [DOI] [PubMed] [Google Scholar]
- 242.To B and Andrechek ER (2018) Transcription factor compensation during mammary gland development in E2F knockout mice. PLoS One 13, e0194937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liu S et al. (2006) Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res DOI: 10.1158/0008-5472.CAN-06-0054 [DOI] [PMC free article] [PubMed]
- 244.Pellacani D et al. (2016) Analysis of Normal Human Mammary Epigenomes Reveals Cell-Specific Active Enhancer States and Associated Transcription Factor Networks. Cell Rep DOI: 10.1016/j.celrep.2016.10.058 [DOI] [PubMed]
- 245.Huh SJ et al. (2015) Age- and pregnancy-associated DNA methylation changes in mammary epithelial cells. Stem cell reports 4, 297–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Danielson KG et al. (1984) Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc. Natl. Acad. Sci. U. S. A 81, 3756–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Johnson JR et al. (2019) Microarray and pathway analysis of two COMMA-Dβ derived clones reveal important differences relevant to their developmental capacity in-vivo. Oncotarget 10, 2118–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ibarra I et al. (2007) A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev 21, 3238–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chao C-H et al. (2014) MicroRNA-205 signaling regulates mammary stem cell fate and tumorigenesis. J. Clin. Invest 124, 3093–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Nagaoka K et al. (2013) Epithelial cell differentiation regulated by MicroRNA-200a in mammary glands. PLoS One 8, e65127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Song SJ et al. (2013) MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 154, 311–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chang C-J et al. (2011) p53 regulates epithelial–mesenchymal transition and stem cell properties through modulating miRNAs. Nat. Cell Biol 13, 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shimono Y et al. (2009) Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 138, 592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yoo KH et al. (2014) The STAT5-regulated miR-193b locus restrains mammary stem and progenitor cell activity and alveolar differentiation. Dev. Biol 395, 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang J et al. (2019) A miR-206 regulated gene landscape enhances mammary epithelial differentiation. J. Cell. Physiol 234, 22220–22233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lee M-J et al. (2013) Expression of miR-206 during the initiation of mammary gland development. Cell Tissue Res 353, 425–33 [DOI] [PubMed] [Google Scholar]
- 257.Heinz RE et al. (2016) Constitutive expression of microRNA-150 in mammary epithelium suppresses secretory activation and impairs de novo lipogenesis. Development 143, 4236–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Llobet-Navas D et al. (2014) The miR-424(322)/503 cluster orchestrates remodeling of the epithelium in the involuting mammary gland. Genes Dev 28, 765–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shore AN et al. (2012) Pregnancy-induced noncoding RNA (PINC) associates with polycomb repressive complex 2 and regulates mammary epithelial differentiation. PLoS Genet 8, e1002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Standaert L et al. (2014) The long noncoding RNA Neat1 is required for mammary gland development and lactation. RNA 20, 1844–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Askarian-Amiri ME et al. (2011) SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA 17, 878–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ginger MR et al. (2006) A noncoding RNA is a potential marker of cell fate during mammary gland development. Proc. Natl. Acad. Sci. U. S. A 103, 5781–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gupta RA et al. (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464, 1071–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bhan A and Mandal SS (2015) LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim. Biophys. Acta 1856, 151–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Amândio AR et al. (2016) Hotair Is Dispensible for Mouse Development. PLoS Genet 12, e1006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tao S et al. (2015) Estradiol induces HOTAIR levels via GPER-mediated miR-148a inhibition in breast cancer. J. Transl. Med 13, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Russo J et al. (2012) Pregnancy-induced chromatin remodeling in the breast of postmenopausal women. Int. J. cancer 131, 1059–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Richard JLC and Eichhorn PJA (2018) Deciphering the roles of lncRNAs in breast development and disease. Oncotarget 9, 20179–20212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Obad S et al. (2011) Silencing of microRNA families by seed-targeting tiny LNAs. Nat. Genet 43, 371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kordon EC and Smith GH (1998) An entire functional mammary gland may comprise the progeny from a single cell. Development 125, 1921–30 [DOI] [PubMed] [Google Scholar]
- 271.Stingl J et al. (2006) Purification and unique properties of mammary epithelial stem cells. Nature DOI: 10.1038/nature04496 [DOI] [PubMed]
- 272.Rohrschneider LR et al. (2005) The intron 5/6 promoter region of the ship1 gene regulates expression in stem/progenitor cells of the mouse embryo. Dev. Biol 283, 503–21 [DOI] [PubMed] [Google Scholar]
- 273.Rios AC et al. (2014) In situ identification of bipotent stem cells in the mammary gland. Nature 506, 322–327 [DOI] [PubMed] [Google Scholar]
- 274.Trejo CL et al. (2017) Lgr5 is a marker for fetal mammary stem cells, but is not essential for stem cell activity or tumorigenesis. npj Breast Cancer 3, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.dos Santos CO et al. (2013) Molecular hierarchy of mammary differentiation yields refined markers of mammary stem cells. Proc. Natl. Acad. Sci 110, 7123–7130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bai L and Rohrschneider LR (2010) s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev 24, 1882–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Centonze A et al. (2020) Heterotypic cell–cell communication regulates glandular stem cell multipotency. Nature DOI: 10.1038/s41586-020-2632-y [DOI] [PMC free article] [PubMed]
- 278.Cristea S and Polyak K (2018) Dissecting the mammary gland one cell at a time. Nat. Commun 9, 2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wuidart A et al. (2018) Early lineage segregation of multipotent embryonic mammary gland progenitors. Nat. Cell Biol 20, 666–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Giraddi RR et al. (2018) Single-Cell Transcriptomes Distinguish Stem Cell State Changes and Lineage Specification Programs in Early Mammary Gland Development. Cell Rep 24, 1653–1666.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Elias S et al. (2017) Long-lived unipotent Blimp1-positive luminal stem cells drive mammary gland organogenesis throughout adult life. Nat. Commun 8, 1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lilja AM et al. (2018) Clonal analysis of Notch1-expressing cells reveals the existence of unipotent stem cells that retain long-term plasticity in the embryonic mammary gland. Nat. Cell Biol 20, 677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chung C-Y et al. (2019) Single-Cell Chromatin Analysis of Mammary Gland Development Reveals Cell-State Transcriptional Regulators and Lineage Relationships. Cell Rep 29, 495–510.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Pal B et al. (2017) Construction of developmental lineage relationships in the mouse mammary gland by single-cell RNA profiling. Nat. Commun 8, 1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Scheele CLGJ et al. (2017) Identity and dynamics of mammary stem cells during branching morphogenesis. Nature 542, 313–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sun H et al. (2018) Single-cell RNA-Seq reveals cell heterogeneity and hierarchy within mouse mammary epithelia. J. Biol. Chem 293, 8315–8329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cai S et al. (2017) A Quiescent Bcl11b High Stem Cell Population Is Required for Maintenance of the Mammary Gland. Cell Stem Cell 20, 247–260.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bach K et al. (2017) Differentiation dynamics of mammary epithelial cells revealed by single-cell RNA sequencing. Nat. Commun 8, 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wang D et al. (2015) Identification of multipotent mammary stem cells by protein C receptor expression. Nature 517, 81–84 [DOI] [PubMed] [Google Scholar]
- 290.Kanaya N et al. (2019) Single-cell RNA-sequencing analysis of estrogen- and endocrine-disrupting chemical-induced reorganization of mouse mammary gland. Commun. Biol 2, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Li CM-C et al. (2019) Aging-associated alterations in the mammary gland revealed by single-cell RNA sequencing. bioRxiv DOI: 10.1101/773408 [DOI] [PMC free article] [PubMed]
- 292.Nguyen QH et al. (2018) Profiling human breast epithelial cells using single cell RNA sequencing identifies cell diversity. Nat. Commun 9, 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Luecken MD and Theis FJ (2019) Current best practices in single-cell RNA-seq analysis: a tutorial. Mol. Syst. Biol 15, e8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Simian M et al. (2001) The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 128, 3117–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shamir ER and Ewald AJ (2014) Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol 15, 647–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Emerman JT et al. (1979) Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell 11, 109–19 [DOI] [PubMed] [Google Scholar]
- 297.Shannon JM and Pitelka DR (1981) The influence of cell shape on the induction of functional differentiation in mouse mammary cells in vitro. In Vitro 17, 1016–28 [DOI] [PubMed] [Google Scholar]
- 298.Emerman JT and Pitelka DR (1977) Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro 13, 316–28 [DOI] [PubMed] [Google Scholar]
- 299.Emerman JT et al. (1977) Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc. Natl. Acad. Sci. U. S. A 74, 4466–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tonelli QJ and Sorof S (1982) Induction of biochemical differentiation in three-dimensional collagen cultures of mammary epithelial cells from virgin mice. Differentiation 22, 195–200 [DOI] [PubMed] [Google Scholar]
- 301.Haeuptle MT et al. (1983) Effect of cell shape change on the function and differentiation of rabbit mammary cells in culture. J. Cell Biol 96, 1425–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Durban EM et al. (1985) Comparative analysis of casein synthesis during mammary cell differentiation in collagen and mammary gland development in vivo. Dev. Biol 109, 288–98 [DOI] [PubMed] [Google Scholar]
- 303.Flynn D et al. (1982) Growth and differentiation of primary cultures of mouse mammary epithelium embedded in collagen gel. Differentiation 22, 191–4 [DOI] [PubMed] [Google Scholar]
- 304.Lee EY et al. (1984) Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J. Cell Biol 98, 146–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lee EY et al. (1985) Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc. Natl. Acad. Sci. U. S. A 82, 1419–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Aggeler J et al. (1991) Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J. Cell Sci 99 ( Pt 2), 407–17 [DOI] [PubMed] [Google Scholar]
- 307.Kleinman HK and Martin GR (2005) Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol 15, 378–86 [DOI] [PubMed] [Google Scholar]
- 308.Wolf K et al. (2009) Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol 20, 931–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Jamieson PR et al. (2017) Derivation of a robust mouse mammary organoid system for studying tissue dynamics. Development 144, 1065–1071 [DOI] [PubMed] [Google Scholar]
- 310.Florian S et al. (2019) A human organoid system that self-organizes to recapitulate growth and differentiation of a benign mammary tumor. Proc. Natl. Acad. Sci. U. S. A 116, 11444–11453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sumbal J et al. (2020) Primary Mammary Organoid Model of Lactation and Involution. Front. cell Dev. Biol 8, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Koledova Z and Lu P (2017) A 3D Fibroblast-Epithelium Co-culture Model for Understanding Microenvironmental Role in Branching Morphogenesis of the Mammary Gland. Methods Mol. Biol 1501, 217–231 [DOI] [PubMed] [Google Scholar]
- 313.Krause S et al. (2008) A novel 3D in vitro culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng. Part C. Methods 14, 261–71 [DOI] [PubMed] [Google Scholar]
- 314.Reid JA et al. (2018) Consistent and reproducible cultures of large-scale 3D mammary epithelial structures using an accessible bioprinting platform. Breast Cancer Res 20, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Barcellos-Hoff MH et al. (1989) Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105, 223–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chen LH and Bissell MJ (1989) A novel regulatory mechanism for whey acidic protein gene expression. Cell Regul 1, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mroue R and Bissell MJ (2013) Three-dimensional cultures of mouse mammary epithelial cells. Methods Mol. Biol 945, 221–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lin CQ et al. (1995) Extracellular matrix regulates whey acidic protein gene expression by suppression of TGF-alpha in mouse mammary epithelial cells: studies in culture and in transgenic mice. J. Cell Biol 129, 1115–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Streuli CH et al. (1991) Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J. Cell Biol 115, 1383–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Alcaraz J et al. (2008) Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J 27, 2829–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Michalak EM et al. (2018) Canonical PRC2 function is essential for mammary gland development and affects chromatin compaction in mammary organoids. PLoS Biol 16, e2004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Jardé T et al. (2016) Wnt and Neuregulin1/ErbB signalling extends 3D culture of hormone responsive mammary organoids. Nat. Commun 7, 13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Mohapatra B et al. (2017) An essential role of CBL and CBL-B ubiquitin ligases in mammary stem cell maintenance. Development 144, 1072–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Horibata S et al. (2017) Role of peptidylarginine deiminase 2 (PAD2) in mammary carcinoma cell migration. BMC Cancer 17, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lourenço AR et al. (2020) C/EBPɑ is crucial determinant of epithelial maintenance by preventing epithelial-to-mesenchymal transition. Nat. Commun 11, 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hacker BC et al. (2019) Growth and Characterization of Irradiated Organoids from Mammary Glands. J. Vis. Exp DOI: 10.3791/59293 [DOI] [PMC free article] [PubMed]
- 327.Ciccone MF, Trousdell MC, dos Santos CO (2020) Characterization of 3D organoid culture to study the effects of pregnancy hormones on the epigenome and transcriptional output of mammary epithelial cells. J. Mammary Gland Neoplasia [DOI] [PMC free article] [PubMed]
- 328.Rosenbluth JM et al. (2020) Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages. Nat. Commun 11, 1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Moolgavkar SH et al. (1980) Two-stage model for carcinogenesis: Epidemiology of breast cancer in females. J. Natl. Cancer Inst [PubMed]
- 330.Pike MC et al. (1983) “Hormonal” risk factors, “Breast tissue age” and the age-incidence of breast cancer. Nature DOI: 10.1038/303767a0 [DOI] [PubMed]
- 331.Rosner B et al. (1994) Reproductive risk factors in a prospective study of breast cancer: The nurses’ health study. Am. J. Epidemiol DOI: 10.1093/oxfordjournals.aje.a117079 [DOI] [PubMed]
- 332.Bowers HJ et al. (2020) Characterization of multicellular breast tumor spheroids using image data-driven biophysical mathematical modeling. Sci. Rep DOI: 10.1038/s41598-020-68324-4 [DOI] [PMC free article] [PubMed]
- 333.Koedoot E et al. (2019) Uncovering the signaling landscape controlling breast cancer cell migration identifies novel metastasis driver genes. Nat. Commun DOI: 10.1038/s41467-019-11020-3 [DOI] [PMC free article] [PubMed]
- 334.Wang C et al. (2017) Lineage-Biased Stem Cells Maintain Estrogen-Receptor-Positive and -Negative Mouse Mammary Luminal Lineages. Cell Rep 18, 2825–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Bouras T et al. (2008) Notch Signaling Regulates Mammary Stem Cell Function and Luminal Cell-Fate Commitment. Cell Stem Cell 3, 429–441 [DOI] [PubMed] [Google Scholar]


