Abstract
Background:
Transport of iron across the placenta is critical for appropriate development of the fetus. Iron deficiency during pregnancy remains a major public health concern, particularly in low and middle-income countries, often exacerbated by infectious diseases leading to altered iron trafficking via inflammatory responses. Herein, we investigate the role of hepcidin, a master regulator of iron homeostasis, on regulation of iron transport across trophoblast cells.
Methods:
We utilized the Jeg-3 choriocarcinoma cell line, for analysis of expression of transferrin receptor, ferritin and ferroportin as well as the export of 59Fe in the presence of hepcidin. Placental tissue from human term pregnancies was utilized for immunohistochemistry.
Results:
Hepcidin treatment of Jeg-3 cells decreased expression of ferroportin and transferrin receptor (TfR) and reduced the cellular export of iron. Lower expression of TfR on the syncytiotrophoblast was associated with the highest levels of hepcidin in maternal circulation, and ferroportin expression was positively associated with placental TfR. Placentas from small-for-gestational-age newborns had significantly lower levels of ferroportin and ferritin gene expression at delivery.
Conclusion:
Our data suggest hepcidin plays an important role in the regulation of iron transport across the placenta, making it a critical link in movement of iron into fetal circulation.
INTRODUCTION
Iron deficiency is the most common micronutrient deficiency and cause of anemia among infants worldwide (1, 2). In low and middle income countries (LMICs), over 60% of children less than five years of age are anemic, with the primary etiology being iron deficiency (3). During human pregnancy, maternal iron deficiency poses a significant threat to fetal iron endowment, which may decrease the iron available for the rapidly growing fetal and neonatal brain (4). In rodent models, this culminates in iron-dependent changes in neurometabolism and neuroanatomy during late gestation and the neonatal period, many of which are not reversible (5-7). Iron is also required for several important brain functions including myelination of nerve fibers, energy metabolism, and as a cofactor for a number of enzymes involved in neurotransmitter synthesis (8, 9). Therefore, understanding the dynamics of iron transfer in utero and factors that may compromise this is crucial.
The role of inflammatory responses in the fetal compartment in the pathogenesis of newborn iron insufficiency has not been well studied. Hepcidin has been identified as the putative link between inflammation and consequent anemia (10). Produced in the liver, hepcidin was first identified for its antimicrobial properties, and then found to be the dominant regulator of release of iron from macrophages and absorption from the gut (11-15). Hepcidin binds to the iron egress membrane protein ferroportin, culminating in its internalization and degradation (16, 17). This process leads to iron trapping within cells culminating in decreased iron bioavailability. Ferroportin is highly expressed in enterocytes of the duodenum as well as macrophages, consistent with its role in iron absorption from the gut and release from tissue macrophages, respectively (18-20). Although the process of hepcidin binding to ferroportin and resultant internalization has been extensively studied in macrophages, much less is known about the responsiveness of placental ferroportin to hepcidin, and the impact of either maternal or fetal inflammation and/or hepcidin on transplacental iron flux in utero. This is of paramount importance as ferroportin has been identified in placental tissue and specifically localized to the basal surface of the syncytiotrophoblast (20, 21). Given its localization, it is thought that signals in fetal blood, particularly hepcidin, contribute to the regulation of fetal iron acquisition through modulation of ferroportin expression on the syncytiotrophoblast. This hypothesis is supported by rodent models of embryonic overproduction of hepcidin, culminating in offspring with features of extreme iron deficiency anemia and intra-uterine growth restriction (IUGR) (12). Despite these data, a recent study examining fetal iron regulation suggests the fetal compartment has little control over iron flux across the placenta (22).
Understanding the dynamics of fetal iron acquisition and risk factors for newborn iron insufficiency are of great public health importance as newborn iron status is related to iron status throughout infancy (9, 23). The objectives of this study were to 1) evaluate whether hepcidin directly impacts the transport and release of iron from human trophoblasts in vitro, 2) understand the association between fetal production of hepcidin as assessed in cord blood and placental iron transport, and 3) evaluate the regulation of, and relationships among, key iron transport proteins using samples from a cohort in the Philippines.
METHODS
Cell culture
We used the choriocarcinoma cell line Jeg3 (ATCC, Manassas) for all in vitro studies. Cells were maintained at 37 °C with 5% CO2 in DMEM high glucose (Invitrogen, Grand Island, NY) supplemented with 1% L-glutamine (Invitrogen), 1% penicillin/streptomycin (Invitrogen) and 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA). Cells were treated with ferrous sulfate (20 μM) for 24 h prior to the addition of hepcidin (500 nM) in complete media or media only control.
Quantitative RT-PCR
Total RNA from trophoblasts in culture or whole villous tissue stored in RNAlater was isolated using Trizol (Invitrogen) according to manufacturer’s instructions. RNA was reverse transcribed using Superscript III reverse transcriptase (Invitrogen) and random hexamers. qPCR was performed using SYBRgreen technology (Applied Biosystems). All gene expression data was normalized to 18S levels within the respective sample, analyzed using the ΔΔCt method and are reported as relative quantity over control sample within each experiment. Primers for genes of interest are as follows – transferrin receptor F: CATTCTTTGGACATGCTCATCTG, R: TGTGATTGAAGGAAGGGAATCC; ferritin F: GTCAACAGCCTGGTCAATTTGTAC, R: GGTCGAAATAGAAGCCCAGAGA; ferroportin F: CCGACTACCTGACCTCTGCAA, R: ACATCCGATCTCCCCAAGTAGA.
Immunoblots
Whole cell protein lysates from trophoblasts in culture were prepared in radioimmunoprecipitation (RIPA) buffer with protease inhibitors (Thermo Scientific, Rockford, IL). Protein content was measured using a bicinchoninic acid (BCA) assay kit (Thermo Scientific), per manufacturer’s instructions. Proteins (10 μg) were fractionated by 4-15% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were then subjected to Western blot analysis for ferroportin, using an antibody generated against the C-terminus of human ferroportin (Genemed Synthesis, San Antonio, TX) or transferrin receptor (Proteintech, Rosemont, IL). Levels of GAPDH (Sigma Aldrich, St. Louis, MO) were monitored to assess protein loading. Primary antibodies were detected using secondary antibodies conjugated to alkaline phosphatase (Sigma) and detected with BCIP/NBT (Sigma).
Iron transport
Twelve-well plates of Jeg-3 cells, grown in DMEM with 10% FBS and 1% penicillin-streptomycin to 50-75% confluence, were washed twice with PBS, then 0.5 mL of pulse media (Optimem (ThermoFisher) + 59FeSO4 (Perkin Elmer) at 1 μCi/mL) was added per well. All plates were incubated overnight, with hepcidin (500 nM final concentration, dissolved in water) added either overnight or four hours prior to washing. Plates were then washed twice with PBS. 0.5 mL chase media (DMEM/10% FBS/1% penicillin-streptomycin with 10 μM ferrous ammonium sulfate) was added, and plates were incubated for 30 minutes or four hours. To harvest cells, plates were placed on ice. Conditioned media was removed and centrifuged at 4,000 g for 5 minutes at 4 °C, then the supernatants were counted by liquid scintillation using a Perkin Elmer Tri-Carb 2810 TR. 0.5 mL cell lysis buffer (1% Triton X-100, 150 mM NaCl, 50 mM Tris pH 7.4) was added to each well. Plates were incubated on ice for 10 minutes, then lysates were centrifuged at 14,000 g for 10 minutes at 4 °C. Lysate supernatants were counted by liquid scintillation. 59Fe cpm in media was expressed as a percent of all counts (media and lysate). Four technical replicates were included for each treatment, with the experiment performed three times.
Immunohistochemistry
A formalin-fixed paraffin embedded (FFPE) tissue microarray was prepared using the 4mm arraymold kit (Arraymold) with a 3x5 matrix for n=180 randomly selected samples. The block was mounted and sectioned with a Leica RM2255 microtome with a thickness of five microns. Sections were stained for transferrin receptor (Proteintech, Rosemont, IL). Protein expression was visualized with secondary antibody (DAKO Dual Link HRP; Agilent, Santa Clara, CA) and DAB substrate using the ImmPACT system (Vector Laboratories, Burlingame, CA).
Image analysis
Transferrin receptor (TfR) immunostained microarray slides were scanned with an Aperio scanner. Aperio ImageScope software, (v 12.3.05056) was used to acquire one 10x snapshot of TfR labeled samples. Image processing and analysis was performed using iVision image analysis software (BioVision Technologies, version 4.5.4 r14, Exton, PA.). Positive staining was defined through intensity thresholding for mean intensity measurements.
Primary human sample experiments – study site and population
We utilized blood and placenta villous samples from a completed study designed to evaluate the safety and efficacy of treatment of schistosomiasis with praziquantel during pregnancy. Characteristics and enrollment strategies of this study population have been described elsewhere (24). All women were positive for schistosomiasis and were randomized to praziquantel treatment or placebo at 12-16 weeks gestation. All women were provided with a daily multi-vitamin with iron (29 mg elemental iron) dispensed at the enrollment and 22 and 32 week visits. Of note, praziquantel did not impact birth weight, risk for low birth weight (LBW), or small for gestational age (SGA) (24).
Primary human sample experiments - sample collection and clinical definitions
At enrollment, we collected health related epidemiologic and demographic data, described in our original publication (24). Maternal blood was collected at 32 weeks gestation and cord blood at delivery. Placental villous samples were collected at delivery and stored in RNAlater for a subset of participants (n=90). Newborn weight and length were measured within 24 hours of delivery. Size for gestational age was calculated based on the INTERGROWTH-21st healthy reference standard, which provides the percentile for birth weight by gestational age (25). Low birth weight was defined using the WHO standards of 2,499 g or less at delivery, regardless of gestational age. Anemia was defined according to the WHO definition of hemoglobin <11 g/dl (26).
Cytokine Assays
Multiplexed cytokine assays were performed on maternal and cord plasma as described previously (27). We utilized a multiplexed sandwich antibody-based assay already developed in our lab to measure IL-1, IL-6, IFNγ, TNFα, IL-4, IL-5, IL-10, IL-13, IL-12, IL-8, IL-2 and CXCL9 with a bead-based platform (BioPlex, Bio-Rad).
Statistical analysis
Statistical analyses were conducted using JMP Pro 12 (SAS Institute, Cary, NC). In vitro data was analyzed with ANOVA followed by Student’s T test, significance defined as P < 0.05. For experiments using primary human placental tissue, the relationship between gene expression and risk factor/outcomes was examined using multivariate logistic regression with maternal anemia status included in all regression models.
Ethical clearance and informed consent
For the immunohistochemistry and qPCR on whole villous tissue, written informed consent for use of banked specimens was obtained from each participant. The Institutional Review Boards of Rhode Island Hospital and the Research Institute for Tropical Medicine in the Philippines approved this study.
RESULTS
General characteristics of the subset of the study population from whom samples were collected and analyzed as part of this secondary analysis are outlined in Table 1. We did not observe an association with expression of TfR, ferritin or ferroportin in the placenta and maternal helminth infections (schistosomiasis, A. lumbricoides, T. trichuria, hookworm) or maternal treatment with praziquantel for the resolution of schistosomiasis early in gestation.
TABLE 1.
Characteristics of Study Population
| Maternal Characteristics | |
|---|---|
| Praziquantel administered; % (n) | 49% (106) |
| Ascaris lumbricoides infected (32±2wk); % (n) | 64% (138) |
| Trichuris trichiura infected (32±2wk); % (n) | 71% (152) |
| Hookworm infected (32±2wk); % (n) | 7% (16) |
| Maternal age; mean (SD) | 26 (6.3) |
| Socioeconomic status quartiles (Q1 = highest) | |
| Q1; % (n) | 23% (50) |
| Q2; % (n) | 27% (58) |
| Q3; % (n) | 37% (80) |
| Q4; % (n) | 13% (27) |
| Body Mass Index; median (IQR) | 21.6 (19.7-23.6) |
| Smoke during pregnancy; % (N) | 0% (0) |
| Alcohol consumption during pregnancy; % (N) | 72% (155) |
| Routine drinking during pregnancy; % (N) | 39% (84) |
| Parity; median (IQR) | 3 (2-5) |
| Infant Characteristics | |
| Sex, female; % (n) | 47% (102) |
| Gestational Age (weeks); median (IQR) | 38.7 (37.9-39.3) |
| Birth Weight (kg); mean (SD) | 2.86 (0.41) |
| Low Birth Weight; % (n) | 33% (71) |
| Small for Gestational Age; % (n) | 21% (46) |
| Premature; % (n) | 11% (23) |
Abbreviations: SD, standard deviation; IQR, interquartile range
Iron transport genes of the human placenta are responsive to hepcidin
To investigate the impact of hepcidin on iron transport and storage proteins specifically in human placental trophoblast cells, we used the choriocarcinoma cell line, Jeg-3, for in vitro studies. Jeg-3 cells that have been iron loaded prior to being treated with hepcidin show a modest but significant drop in total ferroportin protein expression by 4 h of treatment with hepcidin (500 nM; Figure 1A&B). This result was transient, with an initial increase in ferroportin levels in whole cell lysates, likely due to initial internalization of the protein immediately following primary hepcidin binding. Similarly, hepcidin treatment of iron loaded Jeg3 cells resulted in a modest but significant drop in transferrin receptor (TfR) protein expression (Figure 1C&D). We were unable to detect a difference in ferritin protein levels under these treatment conditions (data not shown).
Figure 1. Iron transporter expression and export is decreased in trophoblast cells after exposure to hepcidin.
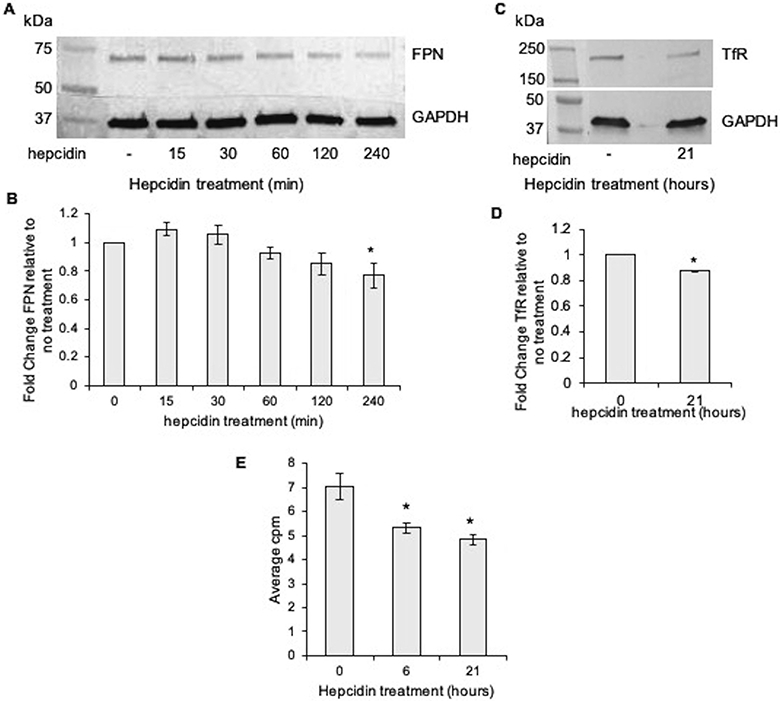
A) Jeg-3 cells were exposed to hepcidin for increasing times in culture, whole cell lysates collected, and ferroportin expression assayed via western blot. B) Ferroportin immunoreactivity quantitated by densitometry. Data presented as the fold change (mean±SEM) in ferroportin expression compared to the no treatment control within each experiment, n=4. * P = 0.01. C) Transferrin receptor (TfR) expression in Jeg3 cells exposed to hepcidin, assayed via western blot. D) TfR immunoreactivity quantitated by densitometry. Data presented as the fold change (mean±SEM) in TfR expression compared to the no treatment control within each experiment, n=3. * P < 0.01. E) Jeg3 cells treated with hepcidin display lower levels of 59Fe in the media, n=3. * P < 0.01 compared to no hepcidin control.
In order to evaluate the impact of hepcidin on iron efflux in trophoblast cells, Jeg3 cells were loaded with 59Fe prior to hepcidin exposure. As early as 6 h post initiation of hepcidin treatment, significantly less radiolabeled iron was detected in the media (Figure 1E). Together, these data suggest that hepcidin 1) alters the expression of iron transporters in the placenta, and 2) inhibits iron export from trophoblast cells.
Maternal hepcidin is inversely associated with placental TfR expression
In order to examine primary human tissues across a range of iron status during gestation and natural exposure to inflammatory insults, we utilized placental tissues collected from a randomized controlled trial conducted in the Philippines. This trial evaluated the safety and efficacy of praziquantel treatment for schistosomiasis during pregnancy (24) and thus also allowed for examination of the impact of potential modification of inflammation through treatment for schistosomiasis. Treatment had no effect on expression of TfR, ferritin or ferroportin at the maternal-fetal interface (IHC and qPCR). Similarly, pro-inflammatory cytokines measured in maternal serum or cord blood (i.e. IFNγ, IL-1, IL-6, IL-8, TNFα, CRP) were not associated with alterations in TfR, ferroportin or ferritin expression within the placenta. We did however, observe an inverse association between maternal hepcidin levels measured in peripheral blood collected at 32±2 weeks of gestation, and TfR staining in the syncytiotrophoblast (Figure 2). Conversely, cord blood hepcidin was not associated with TfR, ferritin, or ferroportin expression (data not shown). While no direct associations between ferritin or ferroportin (IHC and qPCR) were observed with hepcidin levels, there was a significant positive association between ferroportin mRNA levels and both ferritin and TfR mRNA in the placenta (Figure 3).
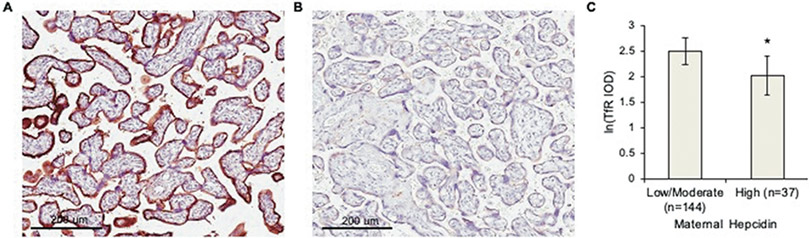
Figure 2. Women with the highest serum hepcidin levels late in gestation displayed lower levels of placental transferrin receptor (TfR) expression.
IHC for TfR in representative placentas from A) woman with low-moderate hepcidin or B) the highest tertile of hepcidin in this cohort in peripheral blood at 32±2 weeks gestation. C) Quantitation of all samples (n=179), * P < 0.02. IOD: Integrated Optical Density Data expressed as LS means +/− 95% confidence intervals and controlled for maternal anemia status with regression modeling.
Figure 3. Ferroportin mRNA expression at the maternal-fetal interface is correlated with ferritin and transferrin receptor expression.
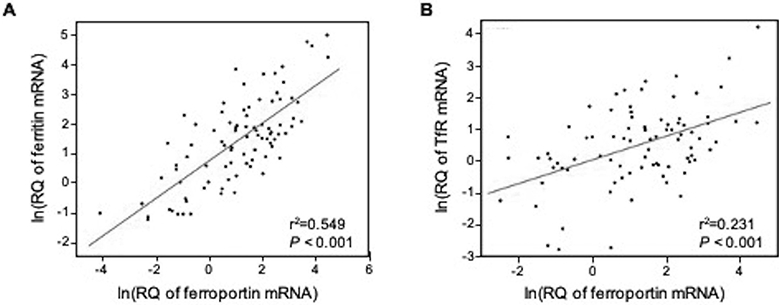
Both A) ferritin and B) TfR were highly correlated with ferroportin gene expression in RNAlater samples from human term placentas. Data displayed after log-transformation to adjust for non-normality. Pearson coefficients of 0.74 and 0.48 for ferritin and TfR, respectively. n=89
Placental iron transport proteins and adverse birth outcomes
We also examined the relationship between placental expression of iron transport genes and birth outcomes. While we were unable to discern a relationship between fetal iron stores (sTfR:ferritin in cord blood) and iron metabolism in trophoblasts, both ferritin and ferroportin gene expression was lower in placentas from pregnancies in which the infant was small-for-gestational age (SGA; Figure 4). Similar patterns were seen for newborns born with low birth weight (birthweight < 2.5 kg; data not shown).
Figure 4. Ferroportin and ferritin placental gene expression is lower in small-for-gestational age newborns.
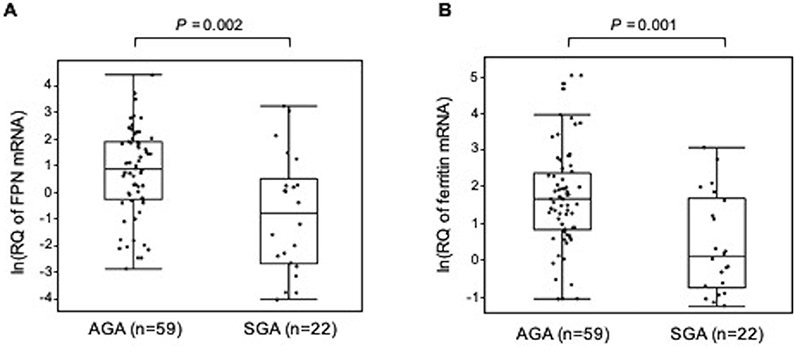
Those infants who were small for gestational age (SGA) based on the standards from the INTERGROWTH-21st study, had lower levels of both A) ferroportin and B) ferritin gene expression in the placenta at delivery, compared to AGA babies from the same cohort. Data is log transformed, presented as ln(relative quantity of gene expression) and adjusted for maternal anemia and cord blood hepcidin in regression analysis.
DISCUSSION
Transferrin bound iron is the primary source of iron uptake by the human placenta, with the majority of intracellular iron incorporated into ferritin (28, 29). Ferroportin has been localized to the basal surface of the syncytiotrophoblast in human placentas and the labyrinth zone of the murine placenta, suggesting that ferroportin is the primary means by which iron is exported from the trophoblast (20, 30, 31). Genetic manipulation of mouse models suggests that ferroportin is critical for iron uptake by the fetus, as embryos with reduced placental ferroportin expression are severely iron deficient (31, 32). Selective retention of ferroportin in placental tissues in a mouse model also rescues the iron deficiency and fetal demise phenotypes seen in global mutation models, highlighting the importance of ferroportin specifically at the maternal-fetal interface in mediating fetal iron availability (32, 33).
Though a few studies have examined the influence of maternal hepcidin levels on transplacental iron transfer (34, 35), much less is known with respect to the dynamics of fetal hepcidin regulation of iron transfer in utero. Hepcidin has been reported on the microvillus membrane of syncytiotrophoblast during early human pregnancy (36), however circulating hepcidin is suggested to elicit a greater impact on iron transport across the placenta late in gestation, when iron transport is the highest. Further, though murine models of fetal hepcidin overproduction culminate in phenotypes of IUGR and severe iron deficiency (14), models examining the activity of hepcidin specifically on the placenta have been lacking. Herein, we show that hepcidin can regulate iron efflux from trophoblast cells, as well as the expression of iron transporters such as ferroportin and transferrin receptor. Using an in vitro trophoblast cell model, exogenous hepcidin treatment resulted in reduced ferroportin and transferrin receptor protein expression on Jeg-3 trophoblast cells. In keeping with our data regarding transporter protein expression, iron efflux was also lower in hepcidin-exposed Jeg-3 cells. Given the different timeline in which we observe the effects of hepcidin on ferroportin and TfR (6 h versus 21 h, respectively), it is likely that exogenous hepcidin, causing ferroportin degradation with consequent iron retention in Jeg-3 cells, culminates in subsequent decreased transferrin receptor expression in response to the increase in intracellular iron stores. Together, these data provide evidence that hepcidin can directly impact the movement of iron across the maternal-fetal interface.
Concordant with these results, hepcidin overexpression in a transgenic mouse model is associated with lower placental transferrin receptor (TfR) gene expression, though no change in ferroportin expression was observed (37). Similarly, rat pups born to iron-deficient dams demonstrate a negative correlation between placental TfR expression and fetal hepcidin (38). However, a recent study using heterozygotic hepcidin deficient dams suggests that loss of fetal hepcidin does not alter the degree of iron transport across the placenta, and suggests maternal hepcidin may be more directly involved in mediating placental iron transport (22). Studies in humans have also demonstrated increased levels of placental TfR in the context of maternal iron deficiency, although the formal relationship between hepcidin, either of maternal or fetal origin, and placental TfR expression was not reported (39, 40). We have also previously reported that maternal iron deficiency anemia, but not anemia of inflammation, impact fetal iron stores (41). Our data support and extend these studies by suggesting that in the case of an iron replete mother (high circulating hepcidin levels) transferrin receptor expression on the syncytiotrophoblast is lower, likely because the fetus is also iron replete. Similarly, placentas displaying the highest gene expression of ferroportin in our study also displayed the highest levels of both ferritin and transferrin receptor gene expression. This suggests that pregnancies experiencing relative iron deficiency have transferrin receptor gene upregulation to maximize placental uptake of iron, necessitating increased ferritin and ferroportin for storage and efflux, respectively.
Infants born to anemic mothers often display normal hemoglobin levels at birth, and it is generally accepted that the fetus is protected from iron deficiency except in the most extreme cases, with preferential iron transport across the placenta at the expense of maternal stores (36, 38, 42). For this reason, it has long been presumed that the fetus regulates iron efflux. However, while some studies have linked placental expression of TfR with fetal iron status, others suggest both TfR and ferroportin placental expression is independent of fetal iron status (21, 22, 43). Mouse models have also shown that maternal genotype for Hfe, an upstream regulator of hepcidin, impacts fetal iron stores, but only in cases of maternal dietary iron restriction (44). Further, animal studies have demonstrated low concentrations of fetal hepcidin during normal gestation (12), (45) suggesting it may not play an important role in regulating the quantity of transmembrane ferroportin in syncytiotrophoblast cells. In humans, cord blood hepcidin has been positively associated with maternal hepcidin (46, 47) while maternal hepcidin (but not hepcidin of fetal origin) has also been correlated with fetal iron endowment (48, 49). We also were unable to find an association between fetal hepcidin levels and expression of iron transport genes within the trophoblast of the term placenta.
Infants of anemic mothers often are born preterm and/or low birth weight (50). Placental expression of TfR has also been demonstrated to be lower in intrauterine growth restriction (IUGR) pregnancies (51). Although we did not observe an association between placental TfR and growth outcomes, we did observe a significant reduction in placental gene expression of both ferritin and ferroportin in small-for-gestational-age (SGA) newborns, likely because less iron is available to be transferred to the developing fetus. This in turn causes decreased intracellular iron, which stabilizes the iron regulatory protein for ferritin mRNA such that it is blocked from translation and not produced (52, 53).
This study has limitations, including our inability to quantify ferroportin protein via IHC on placental sections. Despite the use of several antibodies previously validated in human placenta, ferroportin reactivity was diffusely distributed throughout the syncytium on all sections and could not be objectively quantified in these samples. This could be an artifact of the sample material and processing in the field, or high turnover rate for ferroportin in these samples. In addition, all women included in this study were positive for schistosomiasis at enrollment and only half were treated. Of note, we did not observe an association between praziquantel and mediators of iron transport, mitigating concerns that this factor has influenced our results. Finally, cord blood hepcidin was also measured following labor, a highly inflammatory event, and may not reflect concentrations throughout gestation.
In conclusion, this study provides the first evidence of direct regulation of iron efflux from human trophoblast cells by hepcidin. It also provides further support for the importance of maternal hepcidin, likely as a marker for both maternal and fetal iron status, as a key regulator of bioavailable iron across the placenta. Our failure to discern a clear association between cord blood hepcidin and placental iron transporters is in agreement with other studies and suggests that fetal hepcidin may remain low during gestation to maximize ferroportin integrity and iron transport. Given that we have previously shown an association between cord blood hepcidin and cord blood sTfR:ferritin (41) in this cohort, we do not expect that the lack of association between placental iron transport genes and cord hepcidin is due solely to increased hepcidin in the context of intrapartum inflammation.
IMPACT.
Hepcidin has a direct impact on iron transport across the human placenta
This study provides the first evidence of direct regulation of iron efflux from human trophoblast cells by hepcidin
These data extend our understanding of iron transport across the maternal-fetal interface, a process critical for fetal health and development
Acknowledgments
FUNDING: This work was supported by the National Institute of Allergy and Infectious Diseases [R21AI107520 to J.F.F. and K01AI13068 to E.A.M.] and by the National Institute of Diabetes and Digestive and Kidney Diseases [R01DK110049 to T.B.B.] at the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST: The authors report no conflict of interest.
CONSENT: Written informed consent was obtained by women early in gestation, which included agreement to inclusion of their tissue in data analysis and publication.
LITERATURE CITED
- 1.Fomon SJ, Nelson SE, Ziegler EE 2000. Retention of iron by infants. Annu Rev Nutr 20:273–290. [DOI] [PubMed] [Google Scholar]
- 2.Kretchmer N, Beard JL, Carlson S 1996. The role of nutrition in the development of normal cognition. Am J Clin Nutr 63:997S–1001S. [DOI] [PubMed] [Google Scholar]
- 3.McLean E, et al. 2008. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr 12:444–454. [DOI] [PubMed] [Google Scholar]
- 4.Lozoff B, Georgieff MK 2006. Iron deficiency and brain development. Semin Pediatr Neurol 13:158–165. [DOI] [PubMed] [Google Scholar]
- 5.Clardy SL, et al. 2006. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl:173–196. [DOI] [PubMed] [Google Scholar]
- 6.Felt BT, Lozoff B 1996. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr 126:693–701. [DOI] [PubMed] [Google Scholar]
- 7.de Deungria M, et al. 2000. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res 48:169–176. [DOI] [PubMed] [Google Scholar]
- 8.Beard JL 2001. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 131:568S–579S; discussion 580S. [DOI] [PubMed] [Google Scholar]
- 9.Rao R, Georgieff MK 2001. Neonatal iron nutrition. Semin Neonatol 6:425–435. [DOI] [PubMed] [Google Scholar]
- 10.Ganz T 2003. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102:783–788. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas G, et al. 2001. Lack of hepcidin gene expression and sever tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA 98:8780–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolas G, et al. 2002. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA 99:4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park C, Valore E, Waring A, Ganz T 2001. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276:7806–7810. [DOI] [PubMed] [Google Scholar]
- 14.Krause A, et al. 2000. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett 480:147–150. [DOI] [PubMed] [Google Scholar]
- 15.Pigeon C, et al. 2001. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276:7811–7819. [DOI] [PubMed] [Google Scholar]
- 16.Nemeth E, et al. 2004. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093. [DOI] [PubMed] [Google Scholar]
- 17.Delaby C, et al. 2005. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood 106:3979–3984. [DOI] [PubMed] [Google Scholar]
- 18.Abboud S, Haile DJ 2000. A Novel Mammalian Iron-regulated Protein Involved in Intracellular Iron Metabolism. J Biol Chem 275:19906–19912. [DOI] [PubMed] [Google Scholar]
- 19.McKie AT, et al. 2000. A Novel Duodenal Iron-Regulated Transporter, IREG1, Implicated in the Basolateral Transfer of Iron to the Circulation. Mol Cell 5:299–309. [DOI] [PubMed] [Google Scholar]
- 20.Donovan A, et al. 2000. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403:776–781. [DOI] [PubMed] [Google Scholar]
- 21.Bradley J, et al. 2004. Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta. Am J Physiol Regul Integr Comp Physiol 287:R894–901. [DOI] [PubMed] [Google Scholar]
- 22.Sangkhae V, et al. 2020. Effects of maternal iron status on placental and fetal iron homeostasis. J Clin Invest 130:625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgieff MK, Wewerka SW, Nelson CA, Deregnier RA 2002. Iron status at 9 months of infants with low iron stores at birth. J Pediatr 141:405–409. [DOI] [PubMed] [Google Scholar]
- 24.Olveda R, et al. 2016. Efficacy and safety of praziquantel for the treatment of human schistosomiasis during pregnancy: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 16:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villar J, et al. 2014. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 384:857–868. [DOI] [PubMed] [Google Scholar]
- 26.WHO 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System:WHO/NMH/NHD/MNM/11.11. [Google Scholar]
- 27.Coutinho H, et al. 2005. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J Infect Dis 192:528–536. [DOI] [PubMed] [Google Scholar]
- 28.Cao C, Fleming M 2016. The placenta: the forgotten essential organ of iron transport. Nutr Rev 74:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas G, King B 1990. Uptake and processing of 125I-labelled transferring and 59Fe-labelled transferrin by isolated human trophoblast cells. Placenta 11:41–57. [DOI] [PubMed] [Google Scholar]
- 30.Bastin J, et al. 2006. Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol 134:532–543. [DOI] [PubMed] [Google Scholar]
- 31.Mok H, et al. 2004. Dysregulation of ferroportin 1 interferes with spleen organogenesis in polychthaemia mice. Development 131:4871–4881. [DOI] [PubMed] [Google Scholar]
- 32.Donovan A, et al. 2005. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1:191–200. [DOI] [PubMed] [Google Scholar]
- 33.Mao J, et al. 2010. The iron exporter ferroportin 1 is essential for development of the mouse embryo, forebrain patterning and neural tube closure. Development 137:3079–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young MF, et al. 2012. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr 142:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bah A, et al. 2017. Serum Hepcidin Concentrations Decline during Pregnancy and May Identify Iron Deficiency: Analysis of a Longitudinal Pregnancy Cohort in The Gambia. J Nutr 147:1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans P, et al. 2011. Hepcidin and iron species distribution inside the first-trimester human gestational sac. Mol Hum Reprod 17:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin M, et al. 2004. Transferrin receptor 1 mRNA is downregulated in placenta of hepcidin transgenic embryos. FEBS Lett 574:187–191. [DOI] [PubMed] [Google Scholar]
- 38.Gambling L, et al. 2009. Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol 296:R1063–1070. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Valdes L, et al. 2015. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes 39:571–578. [DOI] [PubMed] [Google Scholar]
- 40.Young M, et al. 2010. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta 31:1010–1014. [DOI] [PubMed] [Google Scholar]
- 41.Abioye A, et al. 2018. Anemia of inflammation during Human Pregnancy Does Not Affect Newborn Iron Endowment. J Nutr 148:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lubach G, Coe C 2006. Preconception maternal iron status is a risk factor for iron deficiency in infant rhesus monkeys (Macaca mulatta). J Nutr 136:2345–2349. [DOI] [PubMed] [Google Scholar]
- 43.Georgieff MK, Berry SA, Wobken JD, Leibold EA 1999. Increased Placental Iron Regulatory Protein-1 Expression in Diabetic Pregnancies Complicated by Fetal Iron Deficiency. Placenta 20:87–93. [DOI] [PubMed] [Google Scholar]
- 44.Balesaria S, et al. 2012. Fetal iron levels are regulated by maternal and fetal Hfe genotype and dietary iron. Haemtologica 97:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willemetz A, et al. 2014. Matriptase-2 is essential for hepcidin repression during fetal life and postnatal development in mice to maintain iron homeostasis. Blood 124:441–444. [DOI] [PubMed] [Google Scholar]
- 46.Lee S, et al. 2016. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res 79:42–48. [DOI] [PubMed] [Google Scholar]
- 47.Ervasti M, et al. 2009. Maternal pro-hepcidin at term correlates with cord blood pro-hepcidin at birth. Eur J Obstet Gynecol Reprod Biol 147:161–165. [DOI] [PubMed] [Google Scholar]
- 48.Young M, et al. 2012. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr 142:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koenig MD, et al. 2014. Hepcidin and Iron Homeostasis during Pregnancy. Nutrients 6:3062–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen L 2001. Biological mechanisms that might underlie iron's effects on fetal growth and preterm birth. J Nutr 131:581S–589S. [DOI] [PubMed] [Google Scholar]
- 51.Mando C, et al. 2011. Transferrin receptor gene and protein expression and localization in human IUGR and normal term placentas. Placenta 32:44–50. [DOI] [PubMed] [Google Scholar]
- 52.Gambling L, Lang C, McArdle HJ 2011. Fetal regulation of iron transport during pregnancy. Am J Clin Nutr 94:1903S–1907S. [DOI] [PubMed] [Google Scholar]
- 53.Rouault TA 2006. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol 2:406–414. [DOI] [PubMed] [Google Scholar]