Abstract
Introduction
The antiviral drug favipiravir has been shown to have in vitro antiviral activity against severe-acute-respiratory-syndrome-coronavirus-2 (SARS-CoV-2). In this study, we investigated the clinical benefits and initiation of favipiravir treatment in patients with non-severe coronavirus-disease-2019 (COVID-19).
Methods
This study was a single-center retrospective cohort study. Receiver operating characteristic curves were drawn to calculate the area under the curve, and the optimal cut-off values for the time to initiate favipiravir treatment were calculated to predict defervescence within seven days. Univariate and multivariate Cox regression analyses were performed to identify potential influencing factors of defervescence. This was defined as a body temperature of less than 37 °C for at least 2 days.
Results
Data from 41 patients were used for the efficacy assessment. The days from the onset of fever to defervescence showed a positive correlation with the duration from the onset of fever to initiation of favipiravir treatment (r = 0.548, P < 0.001). The optimal cut-off value was the administration of favipiravir on day 4. Patients were assigned to two groups based on the optimal cut-off value from onset to initiation of favipiravir treatment: early treatment group (within 4-days) and late treatment group (more than 4-days). In the multivariate analysis, when adjusted for age, sex, and days from onset to initiation of favipiravir treatment, the significant factors were male sex and days of initiation of the favipiravir treatment.
Conclusions
We recommend that if favipiravir is to be used for treatment, it should be initiated as early as possible.
Keywords: Favipiravir, COVID-19, SARS-CoV-2, Early treatment, Defervescence
Abbreviations
- COVID-19
(coronavirus disease 2019)
- SARS-CoV-2
(severe acute respiratory syndrome coronavirus 2)
- ARDS
(acute respiratory distress syndrome)
- ROC
(receiver operating characteristics)
- AUC
(area under the curve)
- PPV
(positive predictive value)
- NPV
(negative predictive value)
1. Introduction
Coronavirus disease 2019 (COVID-19), first identified in Wuhan, China at the end of 2019, has become a global pandemic [1]. COVID-19 is a contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Many researchers are currently investigating treatments for COVID-19 [2]. The drugs under development for COVID-19 are divided into two categories: antiviral drugs that suppress the replication of the virus and drugs that prevent the “cytokine storm” or acute respiratory distress syndrome (ARDS) caused by severe forms of the illness. In particular, viral inhibition is expected to be most effective in the early stages of infection. The antiviral drug favipiravir, an oral RNA-dependent RNA polymerase inhibitor approved in Japan for the treatment of influenza, has a broad spectrum of activity against RNA viruses and exhibits good efficacy in animal models of human infections [3,4]. Favipiravir has shown promising results in clinical studies in China [5], Russia [6], India [7], and Japan [8], and more trials are underway in several countries [9]. In a non-randomized study from China with patients with non-severe forms of the disease, the use of favipiravir was associated with faster rates of viral clearance compared to that of lopinavir-ritonavir [5]. Doi et al. reported that favipiravir was associated with reductions in the time to defervescence [8]. Furthermore, Udwadia et al. showed that favipiravir treatment led to significant improvements in the time to clinical cure in mild-to-moderate COVID-19 cases [7]. Approximately 80% of infected COVID-19 patients are cured within a week or so after the onset of the disease. However, about 20% of patients become severely ill, with 5% requiring intensive care and admission to the ICU, and 2% of cases are potentially fatal [10]. The objective of this study was to evaluate the efficacy and safety of favipiravir treatment and to determine the optimal timing of initiation of favipiravir treatment for defervescence within 7 days in non-severe COVID-19 patients.
1.1. Patients and methods
This study was conducted in strict adherence to the principles of the Declaration of Helsinki and was approved by the Clinical Investigation Ethics Committee of Sapporo Medical University Hospital (Number 322–65).
1.2. Study subjects
This was a single-center, retrospective cohort study. We retrospectively enrolled consecutive patients who were admitted to the hospital with positive RT-PCR or antigen testing for SARS-CoV-2 from March 1, 2020, to November 12, 2020. Patients with fever, shortness of breath, decreased oxygen saturation, pneumonia on imaging, or worsening respiratory failure were eligible for treatment with favipiravir. The definition of onset of fever was a temperature of 37.5 °C or higher. The inclusion criteria were as follows: age of at least 18 years and duration from onset to initiation of favipiravir treatment of less than 8 days. Patients with severe clinical conditions on admission (meeting one of the following criteria: a resting respiratory rate > 30 per minute, oxygen saturation below 93%, oxygenation index (OI) < 300 mmHg (1 mmHg = 133.3 Pa), respiratory failure, shock, and/or combined failure of other organs that required ICU monitoring and treatment), patients with severe conditions within 3 days of admission and who were decided by doctors to be unsuitable for continued favipiravir treatment and patients with missing clinical data for analysis were excluded.
1.3. Measurements
Patients were assessed for clinical characteristics, body temperature, blood biochemistry, C-reactive protein, and information from medical interviews. The axillary temperature of each patient was recorded daily. Adverse events and concomitant medications were also observed.
1.4. Treatment
The dose of favipiravir was 1800 mg twice daily on the first day, followed by 800 mg orally twice daily for up to 14 days. Standard care included oxygen inhalation, oral or intravenous rehydration, electrolyte correction, antipyretics, analgesics, and antiemetic drugs.
1.5. Efficacy and safety assessments
Efficacy was assessed by days from onset of fever to defervescence, which was defined as a body temperature of less than 37 °C for at least 2 days without antipyretic or systemic steroids. For safety, clinical symptoms and vital signs were assessed at least once daily. In addition, laboratory assessments (e.g., hematology) were performed throughout the study as warranted per standard management for COVID-19. The adverse events considered related to favipiravir treatment were recorded.
1.6. Statistical analysis
The optimal cut-off values for the time to initiate favipiravir treatment were calculated to predict defervescence within seven days by Receiver operating characteristic (ROC) curve analysis. The optimal cut-off was determined based on the Youden index. 4 days was found to be the optimal cutoff value. Clinical outcome was compared using this cutoff. Data are presented as mean ± standard deviation or median (interquartile range [IQR]: 25th-75th percentile) and expressed as frequencies and percentages. The Welch test or Mann-Whitney U test was used to compare continuous variables between the two groups. Differences in categorical variables between the two groups were examined using the χ2 test or Fisher’s exact test. Correlations were analyzed using Pearson’s correlation coefficient. We calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy to evaluate the diagnostic ability of defervescence within seven days. Kaplan–Meier curve analysis and log-rank test were used to compare the time to defervescence. Univariate and multivariate Cox regression analyses were performed to identify potential influencing factors of defervescence. This was defined as a body temperature of less than 37 °C for at least 2 days. Statistical significance was set at P < 0.05. EZR version 1.41 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) and JMP® 15 (SAS Institute Inc., Cary, NC, USA), were used for statistical analyses in this study.
2. Results
2.1. Patients
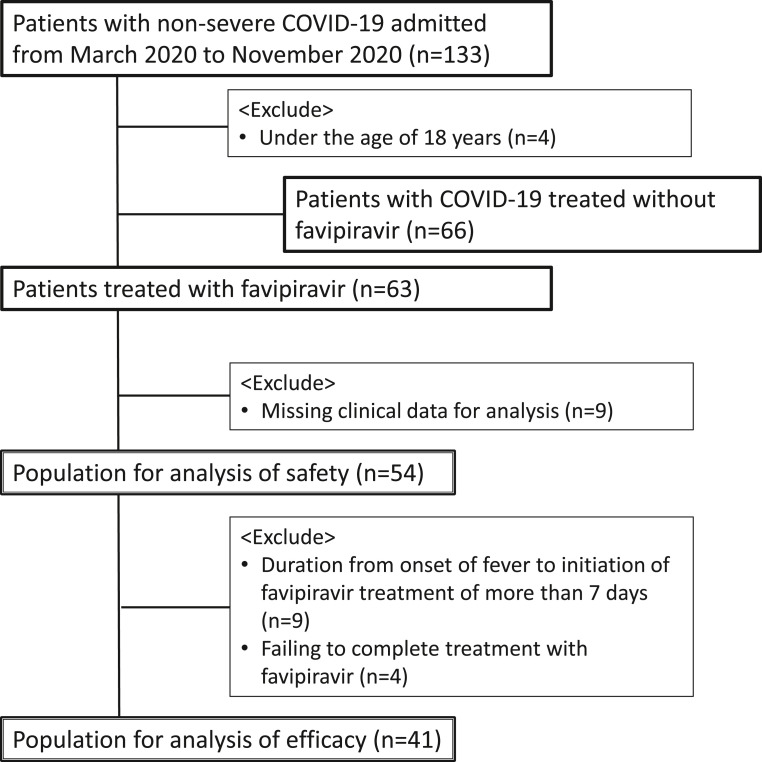
A total of 133 patients with non-severe COVID-19 with laboratory-confirmed SARS-CoV-2 infection were admitted to the Sapporo Medical University Hospital during the study period. Sixty-three patients were treated with favipiravir. Four patients who were below 18 years and nine patients with missing clinical data for analysis were excluded from the study population. Data from 54 patients were used for safety analyses. Nine patients who had a duration from onset of fever to initiation of favipiravir treatment for more than seven days and four patients who failed to complete the treatment with favipiravir were also excluded. Therefore, data from 41 patients were used for the efficacy assessment (Fig. 1 ).
Fig. 1.
Flowchart of patient selection.
2.2. Baseline clinical characteristics
As shown in Table 1 , the median age of the patients was 62 years (interquartile range [IQR], 51–73 years) and 54% of the patients were male. The median body mass index (BMI) and body temperatures were 25.2 kg/m2 and 38.0 °C, respectively (Table 1). As for the patients, 66% (27/41) had at least one coexisting illness; 19 patients with hypertension, 10 patients with diabetes mellitus, 10 patients with CKD, and 3 patients with respiratory disease (Table 1).
Table 1.
Baseline clinical characteristics of the population for efficacy analysis.
| All (n = 41) | ||
|---|---|---|
| Age, years | 62 | (51, 73) |
| Male sex, n (%) | 22 | (54) |
| BMI, kg/m2 | 25.2 | (20.8, 29.7) |
| Body temperature, °C | 38.0 | (37.5, 38.5) |
| Complications | ||
| Hypertension, n (%) | 19 | (46) |
| Dyslipidemia, n (%) | 13 | (32) |
| Diabetes, n (%) | 10 | (24) |
| CKD, n (%) | 10 | (24) |
| Antipyretic drug, n (%) | 29 | (70) |
| Ciclesonide, n (%) | 14 | (34) |
| Oxygen administration, n (%) | 7 | (17) |
Data are presented as median (interquartile range [IQR]: 25th to 75th percentile) or numbers (with percentages). P < 0.05 was considered statistically significant. Abbreviations: BMI, body mass index; CKD, chronic kidney disease.
2.3. Antiviral-associated adverse events
During this trial, we detected 55 incidences of favipiravir-associated adverse events. The most common was hyperuricemia, which occurred in 30/54 (55.5%) patients. Other patients had liver function disorders (31.4%), drug eruption (7.4%), drug fever (5.5%), and increased eosinophil count (1.8%). Of the adverse events that appeared during this study period, cases with symptoms of adverse events were resolved by the time of hospital discharge. Some cases of abnormal laboratory values were discharged without retesting because they were minor abnormalities.
2.4. Correlations with time to initiate favipiravir treatment and time to defervescence
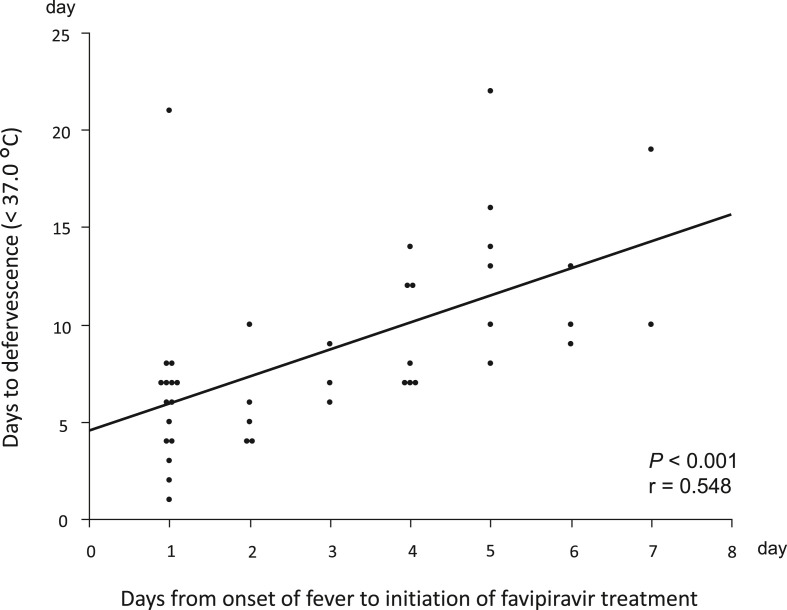
The correlations between the time to onset of fever to initiation of favipiravir treatment and time to defervescence are shown in Fig. 2 . The days from the onset of fever to defervescence showed a positive correlation with the duration from the onset of fever to initiation of favipiravir treatment (r = 0.548, P < 0.001).
Fig. 2.
Univariate correlations between the duration from onset of fever to initiation of favipiravir treatment and days from onset of fever to defervescence.
2.5. Cut-off value from the initiation point of the favipiravir treatment for defervescence in seven days
The ROC curve was analyzed with defervescence within seven days as the objective variable. The AUC of the time between fever onset and the initiation point of favipiravir treatment was 0.839 (95% confidence interval [CI], 0.711–0.968, P < 0.001). The diagnostic ability to administer favipiravir on day 1 for defervescence was 68%, 55%, and 84%, respectively (Table 2 ). On the other hand, the diagnostic ability to administer favipiravir on day 4 for defervescence had an accuracy of 81%, a sensitivity of 100%, and a specificity of 58% and this was the optimal cut-off value (Table 2).
Table 2.
Cut-off day from initiation of treatment with favipiravir to defervescence.
| Day | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| 1 | 0.545 | 0.842 | 0.800 | 0.615 | 0.683 |
| 2 | 0.727 | 0.789 | 0.800 | 0.714 | 0.756 |
| 3 | 0.818 | 0.737 | 0.783 | 0.778 | 0.780 |
| 4 | 1.000 | 0.579 | 0.733 | 1.000 | 0.805 |
| 5 | 1.000 | 0.263 | 0.611 | 1.000 | 0.659 |
Abbreviations: PPV, Positive Predictive Value; NPV, Negative Predictive Value.
2.6. Clinical characteristics of the early treatment group and late treatment group
The characteristics of the early treatment group (within four days) and late treatment group (more than four days) are shown in Table 3 . Although fewer patients in the early treatment group (5/30, 16%) had diabetes mellitus compared to those in the late treatment group (5/11, 45%), the difference was not statistically significant (P = 0.059). The two groups showed similar overall findings, including factors such as age, sex, complications, and laboratory data.
Table 3.
Clinical characteristics of the early treatment group and late treatment group.
| Early treatment group (n = 30) | Late treatment group (n = 11) | P value | |||
|---|---|---|---|---|---|
| Age, years | 64 | (33, 90) | 55 | (36, 73) | 0.195 |
| male, n (%) | 16 | (53) | 6 | (55) | 0.947 |
| BMI, kg/m2 | 24.5 | (17.6, 40.7) | 26.9 | (13.7, 45.9) | 0.149 |
| Body temperature, °C | 38.0 | (37.5, 38.4) | 38.6 | (37.9, 39.0) | 0.105 |
| Complications | |||||
| Hypertension, n (%) | 13 | (43) | 6 | (54) | 0.535 |
| Dyslipidemia, n (%) | 8 | (26) | 5 | (45) | 0.263 |
| Diabetes, n (%) | 5 | (16) | 5 | (45) | 0.059 |
| CKD, n (%) | 7 | (23) | 3 | (27) | 0.801 |
| Antipyretic drug, n (%) | 20 | (67) | 9 | (82) | 0.357 |
| Ciclesonide, n (%) | 12 | (40) | 2 | (18) | 0.271 |
| Oxygen administration, n (%) | 5 | (16) | 2 | (18) | 0.912 |
| Laboratory date | |||||
| WBC, × 1000/μL | 4.8 | (4.1, 6.2) | 4.3 | (3.4, 4.9) | 0.171 |
| Lymphocytes,/μL | 1023 | (812, 1207) | 958 | (878, 1100) | 0.837 |
| CRP, mg/dL | 1.80 | (0.45, 3.49) | 1.95 | (1.03, 4.17) | 0.769 |
| Platelet, × 1000/μL | 206 | (12.6, 458) | 166 | (90.0, 301) | 0.185 |
| Bilirubin, mg/dL | 0.6 | (0.4, 0.8) | 0.5 | (0.5, 0.7) | 0.976 |
| AST, U/L | 29 | (22, 39) | 30 | (24, 41) | 0.735 |
| ALT, U/L | 24 | (16, 40) | 27 | (22, 43) | 0.800 |
| ALP, U/L | 229 | (181, 287) | 219 | (171, 261) | 0.769 |
| Albumin, g/dL | 3.8 | (3.4, 3.9) | 3.8 | (3.7, 4.0) | 0.506 |
| Uric acid, mg/dL | 4.2 | (3.3, 5.3) | 5.0 | (4.5, 7.0) | 0.056 |
| Creatinine, mg/dL | 0.82 | (0.63, 1.00) | 0.79 | (0.56, 1,06) | 0.670 |
| eGFRcre, mL/min/1.73m2 | 69.8 | (61.7, 82.8) | 77.0 | (61.9, 81.8) | 0.735 |
Data are presented as medians (interquartile range [IQR]: 25th to 75th percentile) or numbers (with percentages).
P < 0.05 was considered statistically significant. Abbreviations: BMI, body mass index; CKD, chronic kidney disease; WBC, white blood cell; CRP, C-reactive protein; eGFRcre, creatinine-based estimated glomerular filtration rate.
2.7. Days to defervescence (<37.0 °C) among the early treatment group and late treatment group
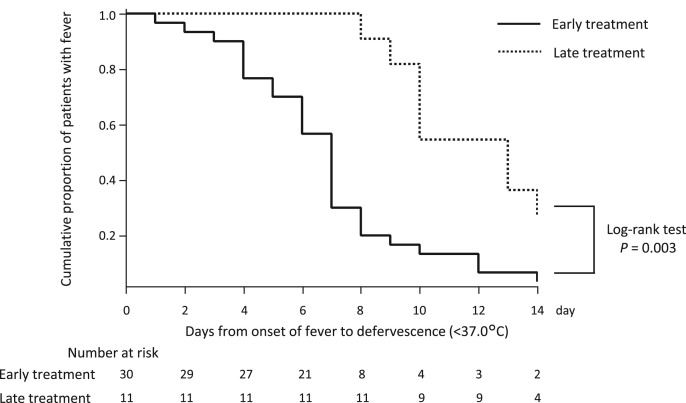
Kaplan–Meier curves and log-rank tests were performed to assess the cumulative defervescence between the early and late treatment groups. The median days to defervescence among patients who were symptomatic at baseline was significantly faster in the early treatment group than in the late treatment group (7 days [95%CI: 5 days, 8 days] vs. 13 days [95%CI: 10 days, 15 days], P = 0.001). The Kaplan–Meier curve of the early treatment group significantly differed from that of the late treatment group in the log-rank tests (P = 0.003). Thus, significantly earlier defervescence was achieved in the early treatment group (Fig. 3 ).
Fig. 3.
Days to deferves cencein the favipiravir-treated population.
2.8. Cox regression analysis of factors associated with defervescence time
Univariate and multivariate Cox regression analysis results for the risk factors related to time to defervescence in COVID-19 patients treated with favipiravir are shown in Table 4 . Univariate Cox regression was conducted before the multivariate analysis. The significant variables (P < 0.1) in the univariate analysis were the time between fever onset and initiation of favipiravir treatment. For the multivariate analysis, the HR of Model 1 was adjusted for age, sex, and time between fever onset and initiation of favipiravir treatment. The significant factors were male sex (hazard ratio [HR], 0.471; 95%CI, 0.233–0.952; P = 0.036) and time to initiate favipiravir treatment (HR, 0.679; 95%CI, 0.554–0.832; P < 0.001). Model 2 was adjusted for BMI, comorbidities (DM, HT), and medications (ciclesonide inhalation) in addition to Model 1. In model 2, as in Model 1, male sex (HR, 0.376; 95%CI, 0.172–0.824; P = 0.014) and time to initiate favipiravir treatment (HR: 0.618; 95%CI: 0.485–0.789; P < 0.001) were significantly associated with defervescence.
Table 4.
COX regression analysis of related factors to defervescence.
| Univariate model |
Multivariate model 1 |
Multivariate model 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P value | HR | (95% CI) | P value | HR | (95% CI) | P value | |
| Age, per 1-year increase | 1.009 | 0.984–1.035 | 0.476 | 0.988 | 0.964–1.014 | 0.392 | 0.985 | 0.950–1.023 | 0.446 |
| Sex; male | 0.628 | 0.329–1.202 | 0.160 | 0.471 | 0.233–0.952 | 0.036 | 0.376 | 0.172–0.824 | 0.014 |
| Body mass index, per kg/m2 increase | 0.998 | 0.954–1.045 | 0.955 | 1.015 | 0.947–1.088 | 0.672 | |||
| Time to initiation of favipirevi treatment, per 1-day increase | 0.726 | 0.606–0.869 | <0.001 | 0.679 | 0.554–0.832 | <0.001 | 0.618 | 0.485–0.789 | <0.001 |
| Ciclesonide; yes | 0.933 | 0.458–1.900 | 0.849 | 0.757 | 0.347–1.654 | 0.486 | |||
| Hypertension; yes | 1.373 | 0.707–2.664 | 0.348 | 1.384 | 0.643–2.975 | 0.405 | |||
| Dyslipidemia; yes | 1.252 | 0.672–2.500 | 0.523 | ||||||
| Diabetes; yes | 0.779 | 0.355–1.707 | 0.533 | 1.886 | 0.696–5.110 | 0.212 | |||
| Chronic kidney disease; yes | 0.876 | 0.410–1.870 | 0.733 | ||||||
| Albumin, per 1.0 g/dL increase | 1.308 | 0.538–3.178 | 0.552 | ||||||
| Lymphocytes, per 1.0/μL increase | 1.028 | 0.987–1.070 | 0.177 | ||||||
| Log CRP | 0.695 | 0.387–1.248 | 0.223 | ||||||
P < 0.05 was considered statistically significant. Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; CKD, chronic kidney disease; CRP, C-reactive protein.
3. Discussion
Favipiravir is an antiviral agent that selectively and potently inhibits RNA-dependent RNA polymerase of RNA viruses [3,11]. Furthermore, it has been shown to have antiviral activity against SARS-CoV-2 [12,13]. However, the benefits of favipiravir treatment in patients with COVID-19 in clinical practice have not been fully investigated. In this study, we investigated the clinical benefits of favipiravir treatment and when to initiate favipiravir treatment in patients with non-severe COVID-19. The days from the onset of fever to defervescence showed a positive correlation with the duration from the onset of fever to initiation of favipiravir treatment. This result suggests that favipiravir treatment is associated with early defervescence in COVID-19. Early initiation of favipiravir treatment may shorten the duration of treatment for COVID-19. Doi and colleagues described that favipiravir is associated with a numerical reduction in time to defervescence [8]. In other words, it is important to initiate favipiravir treatment in COVID-19 patients as early as possible after the onset of infection. Similarly, in the treatment of acute viral diseases such as varicella, influenza, and shingles, it is recommended that antiviral drugs be initiated early after the onset of illness.
Furthermore, in non-severe COVID-19 patients, administration of favipiravir within four days of the onset of fever may reduce the duration of fever and lead to defervescence within seven days. The common initial symptoms of COVID-19 include fever or chills, cough, shortness of breath, difficulty breathing, fatigue, muscle or body aches, headache, loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, and diarrhea [2]. In one study on hospitalized patients, 89% of COVID-19 patients had a fever sometime during their hospitalization period [14]. Patients with severe COVID-19 may become critically ill with ARDS, which typically begins approximately one week after the onset of symptoms [15]. Dyspnea is the most common symptom of the severe form of the disease and is often accompanied by hypoxemia [16,17]. In patients who have a fever for >7 days, the condition may worsen suddenly [18]. Therefore, the extension of the fever period may be one of the factors affecting the severity of COVID-19. Early initiation of favipiravir therapy (within four days of onset) is expected to lead to fever resolution within seven days in non-severe COVID-19 patients and thus, may prevent the development of severe forms of disease.
Univariate analysis showed that the risk factor related to time to defervescence in COVID-19 patients treated with favipiravir was the time between fever onset and initiation of favipiravir treatment. Furthermore, in multivariate analysis, when adjusted for age, sex, and days from fever onset to initiation of favipiravir treatment, the significant factors were male sex and days to initiate favipiravir treatment. In other words, male sex and delayed initiation of favipiravir therapy may prolong the duration of fever in COVID-19. Peckham et al. in a meta-analysis, showed that the male sex is three times more likely than the female sex to have severe illness and 1.4 times more likely to die when infected with SARS-CoV-2 [19]. In the present study, we found that female COVID-19 patients treated with favipiravir may have significantly less time to defervescence than males. When treating non-severe COVID-19 patients with favipiravir, it is necessary to formulate a treatment strategy while also identifying differences in effects depending on sex.
As in other studies [6,7], the most commonly observed adverse events were increased serum uric acid and liver enzyme levels. These adverse events were transient and not serious. Previous reports suggest that favipiravir may be safe and tolerable for short-term use [20]. Most of the patients with elevated serum uric acid levels were asymptomatic. However, one patient who presented with hyperuricemia developed a gout attack. This suggests that caution should be exercised in patients with a history of gout or hyperuricemia. In addition, because of the teratogenic risk of favipiravir, its use in women with childbearing potential is contraindicated unless adequate contraceptive methods are employed [21].
The antiviral drug therapy is effective in the early course of acute viral infections. As with many acute viral infections, COVID-19 treatment with antiviral drugs might be effective if treatment is started early. The data presented in this study support the early use of medications to reduce viral replication in COVID-19 patients, as reported by Doi et al. Early defervescence in non-severe COVID-19 patients is expected to shorten the length of hospital stay due to early fulfillment of discharge requirements. Furthermore, this may prevent the development of severe COVID-19.
The present study had some limitations. First, since this was a retrospective observational study using a small number of patients in a single-center, selection bias might have occurred in the study participants. Second, viral clearance and length of hospital stay of COVID-19 patients treated with favipiravir were not assessed. This is because the discharge criteria changed from time to time. Finally, the favipiravir non-treatment group was not evaluated. To objectively evaluate the efficacy of favipiravir, it is necessary to compare the clinical course and outcome of patients with similar severity and risk that are treated and not treated with favipiravir. We made the following efforts to minimize selection bias; in the study of safety, we examined as many cases as possible except for the small number of cases that did not have all the data necessary for analysis. In a retrospective study of efficacy, there is a risk that selection bias will occur and the patient background may differ depending on if favipiravir is administered or not. Therefore, in this study, we compared cases of favipiravir treatment.
In conclusion, we recommend that if favipiravir is to be used for treatment, it should be initiated as early as possible (within four days of onset). We believe that this will not only prevent the severity of COVID-19 but also prevent hospitals from becoming overwhelmed.
Authorship statement
Contributors: SF was responsible for the organization and coordination of the trial. YI and TI were responsible for the data analysis. ST was the primary investigator. All authors contributed to the writing of the final manuscript and contributed to the management and administration of the trial.
Declaration of competing interest
There is no COI to be disclosed in this original research.
Acknowledgments
We thank FUJIFILM Toyama Chemical for providing the favipiravir.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus Disease 2019 (COVID-19): a review. J Am Med Assoc. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209:107512. doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tani H., Fukuma A., Fukushi S., Taniguchi S., Yoshikawa T., Iwata-Yoshikawa N., et al. Efficacy of T-705 (favipiravir) in the treatment of infections with lethal severe fever with thrombocytopenia syndrome virus. mSphere. 2016;1 doi: 10.1128/mSphere.00061-15. e00061-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020;6:1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivashchenko A.A., Dmitriev K.A., Vostokova N.V., Azarova V.N., Blinow A.A., Egorova A.N., et al. AVIFAVIR for treatment of patients with moderate COVID-19: Interim results of a Phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udwadia Z.F., Singh P., Barkate H., Patil S., Rangwala S., Pendse A., et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, Phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71. doi: 10.1016/j.ijid.2020.11.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi Y., Hibino M., Hase R., Yamamoto M., Kasamatsu Y., Hirose M., et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01897-20. e01897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi S., Parkar J., Ansari A., Vora A., Talwar D., Tiwaskar M., et al. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021;102:501–508. doi: 10.1016/j.ijid.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 11.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaptein S.J.F., Jacobs S., Langendries L., Seldeslachts L., Ter Horst S., Liesenborghs L., et al. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci U S A. 2020;117:26955–26965. doi: 10.1073/pnas.2014441117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berlin D.A., Gulick R.M., Martinez F.J. Severe covid-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura K., Ide S., Saito S., Kinoshita N., Kutsuna S., Moriyama Y., et al. COVID-19 can suddenly become severe: a case series from Tokyo, Japan. Glob Health Med. 2020;2:174–177. doi: 10.35772/ghm.2020.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilkington V., Pepperrell T., Hill A. A review of the safety of favipiravir - a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6:45–51. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pharmaceuticals and Medical Devices Agency . Evaluation and Licensing Division, Japan, Pharmaceutical and Food Safety Bureau; 2011. Report on the deliberation results - Avigan.www.pmda.go.jp/files/000210319.pdf [Google Scholar]