Abstract
Tasked with identifying digital health solutions to support dynamic learning health systems and their response to COVID-19, the US Department of Health and Human Services Office of the Assistant Secretary for Preparedness and Response partnered with the University of New Mexico’s Project ECHO and more than 2 dozen other organizations and agencies to create a real-time virtual peer-to-peer clinical education opportunity: the COVID-19 Clinical Rounds Initiative. Focused on 3 “pressure points” in the COVID-19 continuum of care—(1) the out-of-hospital and/or emergency medical services setting, (2) emergency departments, and (3) inpatient critical care environments—the initiative has created a massive peer-to-peer learning network for real-time information sharing, engaging participants in all 50 US states and more than 100 countries. One hundred twenty-five learning sessions had been conducted between March 24, 2020 and February 25, 2021, delivering more than 58,000 total learner-hours of contact in the first 11 months of operation.
Introduction
Despite recent emergency use authorizations for both therapeutics and vaccines, COVID-19 continues to challenge the health care community.1, 2, 3, 4, 5, 6, 7 How can health care systems, clinicians, administrators, and policy makers quickly learn what is necessary to face these unprecedented and evolving challenges? Expanded use of digital health technologies has been suggested as one strategy for supporting learning health systems during a pandemic.8 This paper describes the realization of one COVID-19-specific digital health solution: COVID-19 Clinical Rounds.
Background: Project Echo
First developed in 2003, Project ECHO—Extension for Community Healthcare Outcomes—is a nonprofit telehealth initiative that uses a case-based collaborative learning approach to promote real-time, peer-to-peer, multidirectional learning among primary care teams, specialists, care managers, and public health practitioners.9, 10, 11 The idea is for clinicians to learn from each other, just as they do every day on the wards, but on a regional, national, or international scale. The Project ECHO model has been used to improve care and outcomes for both chronic and acute conditions throughout the United States and around the globe.12, 13, 14, 15, 16, 17
COVID-19 Clinical Rounds
In March 2020, the US Department of Health and Human Services (HHS) Office of the Assistant Secretary for Preparedness and Response (ASPR) engaged Project ECHO to create a new virtual education resource: the HHS ASPR Project ECHO COVID-19 Clinical Rounds Initiative, mentioned hereafter as “COVID-19 Clinical Rounds.” It uses a hybrid ECHO model to establish and support COVID-19-related learning networks through an interactive webinar platform. The platform includes virtual face-to-face engagement among expert panelists, guest presenters, and participants using multipoint videoconferencing as well as real-time polling, chat, and Q&A functions. The aim is to create peer-to-peer learning networks through which clinicians who have experience in treating patients with COVID-19 in the thick of the pandemic can share lessons learned, including successes and failures alike.
Dozens of professional societies and organizations, including the National Emerging Special Pathogen Education and Training Center (NETEC) network, collaborate in this public-private partnership (Figure 1 ). Three distinct COVID-19 Clinical Rounds learning networks focus on pressure points in the continuum of care: (1) the out-of-hospital and/or emergency medical services (EMS) setting, (2) emergency departments, and (3) inpatient critical care environments. Each learning network meets weekly for 1 hour. There are also additional ad hoc special topic sessions.
Figure 1.
COVID-19 Clinical Rounds collaborating professional societies and organizations.
| American Academy of Emergency Nurse Practitioners |
| American Ambulance Association |
| American Association of Nurse Anesthetists |
| American College of Chest Physicians |
| American College of Emergency Physicians |
| American College of Surgeons |
| American Geriatrics Society |
| American Society of Anesthesiologists |
| Association of American Medical Colleges |
| Commission on Accreditation of Medical Transport Systems |
| Dayton Metropolitan Medical Response System |
| US Department of Health & Human Services, Assistant Secretary for Preparedness and Response |
| Emergency Nurses Association |
| Emory University |
| Infectious Disease Society of America |
| International Association of Emergency Medical Services Chiefs |
| International Association of Fire Fighters |
| Massachusetts General Hospital |
| Mount Sinai Health System |
| National Association of Emergency Medical Technicians |
| National Association of EMS Physicians |
| National Association of State EMS Officials |
| National Emerging Special Pathogen Training and Education Center |
| US Department of Transportation, National Highway Transportation Safety Administration Office of Emergency Medical Services |
| New York City Health and Hospitals/Bellevue |
| Project ECHO |
| Society for Critical Care Medicine |
| Trauma Center Association of America |
| University of Nebraska Medical Center/Nebraska Medicine |
| University of New Mexico School of Medicine |
Presenters And Participants
COVID-19 Clinical Rounds presenters and panelists are recruited based on their lived experiences, not their reputation as experts or speakers. The majority of presenters and panelists are clinician leaders based at academic medical centers, state departments of health, and professional societies. The earliest presenters were from locations with the first or largest US outbreaks (eg, the University of Washington in Seattle, Bellevue Hospital in New York City) and from NETEC institutions with experience in managing special pathogen epidemics (ie, Emory University, the University of Nebraska, and Bellevue Hospital). Subsequent presenters have been nominated by the original speakers, panel members and participants, and by recruitment from COVID-19 “hot spots” (eg, Morristown, NJ; Detroit, MI; Singapore; Germany; United Kingdom).
The sponsoring agencies and organizations originally promoted participation through broadcast emails to their membership and contact lists; subsequent word-of-mouth promotion by participants has further publicized the sessions. Participation in COVID-19 Clinical Rounds is voluntary, and the rounds are not intended to be redundant or to replace other initiatives. They are also not intended as sources for policy recommendations or guidance. The shared experiences, perspectives, and opinions are those of the participants, and are not officially sanctioned or endorsed by the collaborating organizations.
Structure
Each COVID-19 Clinical Rounds session starts with brief presentations from 1 or 2 frontline clinicians describing the specific COVID-19 challenges they have faced and the response to those challenges (Figure E1, available at http://www.annemergmed.com/). Presentations are followed by a moderated discussion with a panel of experts consisting of the presenters, previous presenters, and professional organization leaders. Participants can submit questions in advance, and additional real-time interaction is achieved through the chat and Q&A functions. This allows participants to post questions for the panel as well as to share their individual questions, concerns, and experiences with all the other participants. The program organizers also routinely poll participants using the integrated polling function in order to gather information and insights to inform decisionmaking and policy at ASPR and other federal agencies. The sessions are recorded and made available through the Project ECHO website (https://echo.unm.edu/covid-19/sessions/hhs-aspr-clinical-rounds), which serves as a public repository for all COVID-19 Clinical Rounds presentations and session recordings.
Scope, Reach, And Initial Impact
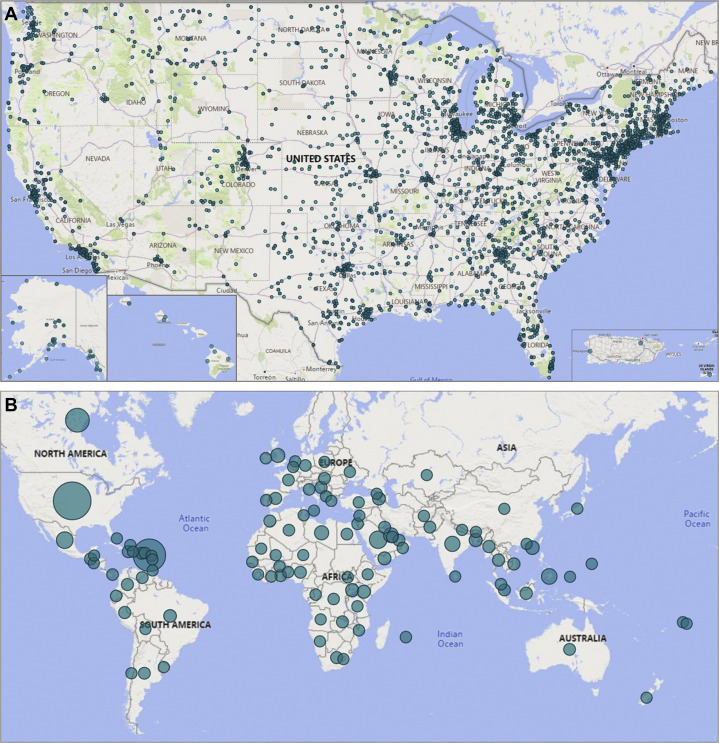
COVID-19 Clinical Rounds grew from idea to implementation within a week, debuting March 24, 2020. The initiative has since created a massive peer-to-peer network for information sharing among participants in all 50 states, the US territories, and more than 100 countries (Figure 2 ).
Figure 2.
Cumulative COVID-19 Clinical Rounds registered participants. A, United States. B, Worldwide.
One hundred twenty-five learning sessions had been conducted by February 2021, including 35 specific to EMS, 39 specific to ED care, 40 addressing critical care, and an additional 11 ad hoc “special sessions.” COVID-19 Clinical Rounds has delivered more than 58,000 total learner-hours of contact in the first 11 months. The EMS sessions have been the best attended (approximately 400/session), followed by critical care (approximately 360/session), and then ED care (approximately 300/session). The special sessions have averaged 450 participants. There have also been more than 12,600 views of the online COVID-19 Clinical Rounds recordings.
COVID-19 Clinical Rounds’ impact is more than participant numbers. The multidirectional sharing informs practices among both the participants and the sponsoring organizations (Table E1, available at http://www.annemergmed.com/). In follow-up evaluations, participants self-rated their knowledge as higher after COVID-19 Clinical Rounds, with 73% reporting that they definitely or probably will incorporate learning from the sessions into their work (Table E2, available at http://www.annemergmed.com/). Further, questions and discussions raised during COVID-19 Clinical Rounds have directly informed the choice of topics for the NETEC COVID-19 webinar series. COVID-19 Clinical Rounds also provides federal departments and agencies with a finger on the pulse of a broad network of thousands of clinicians through polling responses and the questions and comments shared by the participants. This bottom-up sharing of experiences from the field with federal agencies has been as valuable as the more traditional “top-down” dissemination of information to health care professionals. The US Department of Health and Human Services Healthcare Resilience Working Group has described COVID-19 Clinical Rounds as “a critical source of clinical ground truth to guide the COVID response.”
Implications For The Continuing Response To Covid-19
Our understanding of COVID-19 remains incomplete, and the continuing knowledge gaps underscore several important concepts:
-
•
None of the information shared through COVID-19 Clinical Rounds should be adopted as dogma. This is shared information, not evidence.
-
•
It is important to regularly revisit issues and discussions. For example, in early April 2020, one ED rounds presenter described how his institution embedded mental health support for doctors, nurses, and other staff into their ED. Four weeks later, that presenter returned to report that the strategy had failed and the department was now trialing a “buddy system” for peer support.
-
•
COVID-19 Clinical Rounds must be able to evolve and adapt. Building on the success of COVID-19 Clinical Rounds, additional pandemic response learning networks have been developed to focus on (1) implementation of telemedicine in ambulatory care settings and (2) outpatient therapeutics, including monoclonal antibody therapy.
-
•
The COVID-19 experience is diverse; however, there is value in learning about experiences even in dissimilar settings, as one participant put it in a chat entry: “Great presentations today and very valuable to see the responses from the poll. … One observation I’d like to share. The Covid-19 experience is very different for each of us, based on geography and other factors. It is clear however, that all of us need to be vigilant … and have the right policies and procedures in place for the foreseeable future.”
-
•
Finally, COVID-19 is not constrained by national boundaries. With participation from more than 100 countries, COVID-19 Clinical Rounds has facilitated international knowledge sharing between and across nations, helping to support an effective global response to both the patient care and public health challenges of the pandemic.
Relationships Are Key
Relationships have been key to the success of COVID-19 Clinical Rounds. A preexisting relationship between federal agencies and Project ECHO set the foundation for the initiative. Relationships among the US Department of Homeland Security/Federal Emergency Management Agency, HHS/ASPR, the Department of Transportation's National Highway Traffic Safety Administration's Office of EMS, NETEC, and Project ECHO personnel were critical in rapidly assembling the consortium of collaborating organizations. Relationships among organizers, sponsors, and clinicians caring for patients were leveraged to recruit the earliest speakers. Relationships among the participants have been critical to building and expanding the 3 learning communities.
Limitations And Lessons Learned
There are challenges and limitations to the COVID-19 Clinical Rounds initiative. First, by design, the rounds do not necessarily present evidence-based medicine. Indeed, COVID-19 Clinical Rounds was conceived at a time when evidence was limited and for the express purpose of panelists sharing individual experiences. The real-time chat and Q&A functions enable participants to share similar or conflicting experiences, essentially crowd-sourcing knowledge in the absence of evidence. Nonetheless, developing evidence is included in the presentations. For example, early discussions around steroids were generally driven by anecdotes and opinions; however, once the RECOVERY trial was completed, an internist from the United Kingdom was invited to discuss those findings.18 Second, COVID-19 Clinical Rounds is a resource-intense undertaking, requiring time, technical expertise, and funding. Once the COVID-19 pandemic has passed, ongoing support will be required to ensure continued readiness. Third, virtual peer-to-peer learning was new to almost all of the presenters, panelists, and participants, and thus adaptation took some time. Future technological advances will likely necessitate additional adaptations. Finally, the dependence on existing relationships to identify speakers and panelists is both a strength and limitation of COVID-19 Clinical Rounds. Existing relationships allowed planners to quickly identify clinicians with experience in managing special pathogen outbreaks and provided access to ever-expanding networks of additional potential speakers and panelists. However, there is also some risk that clinicians with important experiences worthy of sharing are excluded simply because they are not known by or referred to the planners.
A Model For Future Public Health Emergencies Or Disasters
When the COVID-19 Clinical Rounds initiative has served its purpose and is no longer useful in its current form, the platform can be stood down as quickly as it was stood up. It can also be transitioned to focus on preparedness for the next emergency. Epidemics and pandemics, natural and manmade disasters, and the impacts of global warming and climate change are just a few examples of potential future crises. Whenever and whatever that next disaster is, COVID-19 Clinical Rounds provides a new model for multidirectional peer-to-peer learning at scale. When investing in and maintaining a platform, the expertise and—most importantly—the relationships necessary to quickly establish virtual networks that support dynamic learning health systems should be a priority for public health and disaster-oriented agencies and organizations.
In the meantime, anyone interested in participating in upcoming COVID-19 Clinical Rounds sessions, or reviewing resources from prior sessions, can find more information at https://echo.unm.edu/covid-19/sessions/hhs-aspr-clinical-rounds.
Acknowledgments
The authors thank Dan Hanfling, MD, for helpful review of the manuscript.
Footnotes
Editor’s Note: This article is part of a series that describes the many ways that the Department of Health and Human Services interacts with the emergency care system. The Department of Health and Human Services includes many divisions that are well known to the health care world, including the Centers for Medicare & Medicaid Services, the Health Resources and Services Administration, the National Institutes of Health, and the Agency for Healthcare Research and Quality. The goal of the series is to increase the visibility of federal emergency care–related activities within the emergency care community.
Supervising editor: Brendan G. Carr, MD, MS. Specific detailed information about possible conflict of interest for individual editors is available at https://www.annemergmed.com/editors.
Authorship: All authors attest to meeting the 4 ICMJE.org authorship criteria: (1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Fundingandsupport: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (seewww.icmje.org). RCH, JTR, and JH are employed by HHS/ASPR which organized COVID-19 Clinical Rounds. BTJ is employed by Aveskha, Inc. and provides direct contract support to HHS/ASPR. BBS, SA, AJA, AMD, and CAB are affiliated with the ECHO Institute, which provides the platform for COVID-19 Clinical Rounds. JRK and LHB have stated that no such relationships exist. The ECHO Institute at the University of New Mexico Health Sciences Center is the recipient of funding from HHS/ASPR to provide administrative and technical support for COVID-19 Clinical Rounds.
Disclaimer: The views expressed are those of the authors and do not necessarily represent the official policies of the Office of the Assistant Secretary for Preparedness & Response, US Department of Health & Human Services or the Office of EMS, National Highway Traffic Safety Administration, US Department of Transportation.
Supplementary Data
References
- 1.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 Novel Coronavirus pneumonia in Wuhan, China: A Descriptive Study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization COVID-19 Weekly Epidemiological Update - 23 February 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update---23-february-2021 Accessed.
- 5.Anonymous. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment (press release). Washington, DC: U.S. Food and Drug Administration. Accessed December 20, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment
- 6.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen L.H., Drew D.A., Graham M.S., et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.In-Q-Tel . Q-Tel; Washington, DC: 2019. Roundtable Report – Leveraging digital health technologies during large-scale epidemics.https://www.bnext.org/wp-content/uploads/2019/12/Digital-Health-Roundtable-Report.pdf Accessed December 20, 2020. [Google Scholar]
- 9.Struminger B., Arora S., Zalud-Cerrato S., et al. Building virtual communities of practice for health. Lancet. 2017;390:632–634. doi: 10.1016/S0140-6736(17)31666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora S., Kalishman S., Thornton K., et al. Project ECHO (Project Extension for Community Healthcare Outcomes): A national and global model for continuing professional development. J Cont Educ Health Prof. 2016;36(Suppl 1):48–49. doi: 10.1097/CEH.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 11.Struminger B.B., Arora S. Leveraging telehealth to improve health care access in rural America: it takes more than bandwidth. Ann Intern Med. 2019;171:376–377. doi: 10.7326/M19-1200. [DOI] [PubMed] [Google Scholar]
- 12.Arora S., Thornton K., Murata G., et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–2207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams S.L., Kaigler A., Armistad A., et al. Creating a public health community of practice to support American Indian and Alaska Native communities in addressing chronic disease. Prev Chronic Dis. 2019;16:E109. doi: 10.5888/pcd16.190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heard-Garris N., Arora S., Lurie N. Building physician networks as part of the Zika response. Disaster Med Public Health Prep. 2017;11:259–261. doi: 10.1017/dmp.2017.24. [DOI] [PubMed] [Google Scholar]
- 15.Bikinesi L., O’Bryan G., Roscoe C., et al. Implementation and evaluation of a Project ECHO telementoring program for the Namibian HIV workforce. Hum Resour Health. 2020;18:1–10. doi: 10.1186/s12960-020-00503-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Africa Centers for Disease Control and Prevention . African Union; 2020. Extension for Community Healthcare Outcomes (ECHO). Addis Ababa, Ethiopia.https://africacdc.org/programme/public-health-information-systems/extension-for-community-healthcare-outcomes-echo/ Accessed December 20, 2020. [Google Scholar]
- 17.National Academies of Sciences, Engineering and Medicine . The National Academies Press; Washington, DC: 2016. A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths After Injury. Accessed December 20, 2020. [DOI] [PubMed] [Google Scholar]
- 18.The RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. New Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.