Abstract
Background
The first case of SARS-CoV-2 in Mexico was reported in February 2020, since then, high rates of mortality due to COVID-19 have been found. Cytokine storm is linked to the severity and decreasing the survival among infected patients by COVID-19. The serum levels of Interleukin 6 (IL-6) have been correlated to mortality in COVID-19 cases and could be used as indicator of mortality in COVID-19 cases. The aim of this study was to determine levels of IL-6 and assess its usefulness as indicator of mortality among COVID-19 patients from Mexico.
Methods
A cohort study among 38 adults (28 men, 10 women) was carried out in the Regional High Specialty Hospital of the Yucatan Peninsula in Merida, Yucatan, Mexico. Demographic and clinical biochemistry data were collected. The serum levels of IL-6 were measured in each patient by specific immunoassays.
Results
High frequency of mortality (36.84%) was found in the sample. The average age of individuals that non-survive was significantly higher (59.71 ± 13.83 years) than the survival group (43.29 ± 11.80 years). Serum levels of IL-6 were significantly higher in patients that did not survive. A correlation between IL-6 levels with lymphocyte count, LDH, CRP and procaciltonin was found. The optimal cutoff value of IL-6 was 30.95 pg/mL with high sensitivity and specificity.
Conclusion
Our findings demonstrate that level of IL-6 is an indicator of mortality among hospitalized COVID-19 patients in Mexico.
Keywords: SARS-CoV-2, COVID-19, Cytokine storm, Inflammatory cytokines, Interleukin 6, Mortality, Mexico
1. Introduction
The dissemination of SARS-CoV-2 is still increasing across the world and Mexico is one of the most affected countries [1]. The first case of COVID-19 in Mexico was reported in February 2020 [2] since then, the number of deaths due to COVID-19 have been found high in Mexican patients [3]. Remarkably, the prevalence of comorbidities as obesity, diabetes and hypertension is observed in a large proportion of adults in Mexico [4]. According with scientific reports, pre-existing comorbidities in infected patients with SARS-CoV-2 might result in the clinical worsening of the disease’ outcome [5]. Available studies performed among Mexican individuals on the mortality risk factors are limited. However, evidence shows that neutrophil-to-lymphocyte ratio (NLR), lactate dehydrogenase (LDH), albumin and invasive mechanical ventilation (IMV) are some of the risk factors for mortality in Mexican patients with COVID-19 [3].
Cytokine storm has been described as an excessive response to an external stimuli [6] that leads to an elevation of the level of inflammatory mediators [7]. This condition is linked to the severity of COVID-19 and decreased survival in patients infected by SARS-CoV-2 [8]. Among several pro-inflammatory biomarkers, the serum levels of Interleukin 6 (IL-6) were correlated to fatality [9], [10]. Accumulating evidence indicates that targeting the IL-6 mechanism may result in the regulation of the inflammatory levels, therefore it could be used as a therapeutic alternative for those patients [11]. Most studies that identify levels of IL-6 in COVID-19 cases in severe and in non-survivors are carried out China [12], [13]. Evidence related to IL-6 levels in infected patients with SARS-CoV-2 is not available among Mexican population. Therefore, we hypothesize that the COVID-19 cases have a similar association between mortality and IL-6 serum levels.
Accurate data regarding clinical worsening that results in death is crucial for timely interventions aimed to reduce mortality. Against this background, the aim of the present study was to determine levels of IL-6 and assess its usefulness as indicator of mortality among COVID-19 patients from Mexico.
2. Methods
2.1. Study design
The present study was carried out between April and May 2020. A total of 126 individuals attended in the COVID specialty unit at the Regional High Specialty Hospital (HRAEPY in the Spanish acronym) in Merida, Yucatan, localized in the southeast of Mexico. A sample of 38 hospitalized patients (28 men and 10 women) admitted at the COVID unit with SARS-CoV-2 infection confirmed by PCR, aged between 21 and 73 years were selected for this study. Hospitalized patients with COVID-19 were divided into severe or critical (patients that required mechanical ventilation, sedation and prolonged bed rest) [14]. The study was approved by the Ethics Committee of the HRAEPY (No. CONBIOETICA-31-CEI-002-20170731) in connection with a research project (Identification code: 2020-010).
2.2. General data population, blood sample collection and cytokine assay
Demographic and clinical biochemistry data were collected following standard protocol during the 72 h after the hospital admission into the COVID unit. Biochemical parameters were determined in a pre-validated equipment (autoanalyzer COBAS® Integra 400 Plus, Roche Diagnostics). Briefly, blood samples were individually collected into pyrogen-free tubes (Vacutainer; BD Diagnostics) at room temperature. Serum was obtained by centrifugation of collection tubes at 3500 rpm for 10 min and stored at −20 °C until use. Serum levels of IL-6 were determined in duplicate by an enzyme-linked immunosorbent assay (ELISA) (Human IL-6 ELISA kit #BMS213-2, Bender MedSystems, Vienna, Austria), according to the instructions of the manufacturers. Raw data were analyzed with GraphPad Prism software.
2.3. Statistics
Statistical analyses were performed using SPSS statistical software (version 15.00) and the statistical package Jamovi (Version 1.2). Based on the status, the patients were assigned to either survival or non-survival group. Analysis were done taking the entire sample of men (n = 28) and women (n = 10). For categorical variables, absolute and relative frequencies by status were tabulated. The distribution of continuous variables was tested following the principal of the Shapiro-Wilk test (p < 0.05). Continuous variables between groups were compared using the student's t-test or the Mann-Whitney U test. Correlation analyses were performed by non-parametric Spearman test. The survival analysis was assessed using Kaplan-Meier survival function curve to test the statistical significance between the individuals who had a high level of cytokines and the subjects who had a low level of cytokines at 50% and 75% cutoff. A significance of 0.05 was considered with no correction for p-values.
3. Results
Mortality in the sample (n = 38) of patients infected by SARS-CoV-2 was remarkable (37%); three women (21%) and 11 (89%) men died due to COVID-19 complications. Seven (50%) patients died within 1–9 days of their first day of hospitalization; six (43%) within 10–19 days and one (7%) within 20–25 days. Fifteen individuals (39%) required mechanical ventilation and sedation, but only two patients from the survivor group were reported. Table 1 shows the baseline characteristics of COVID-19 patients by status. The average age of individuals that non-survive was significantly higher (59.71 ± 13.83 years) than the survival group (43.29 ± 11.80 years) (p = 0.001). Among the patients, the most common comorbidities were diabetes and hypertension (34%), and overweight or obesity (42.10%). Also, three individuals (8%) were living with human immunodeficiency virus (HIV) and under antiretroviral treatment. Shortness of breath was the most frequent symptom in the population (97%). Percentage values of COVID-19 patients that did not survive were higher (except having arthralgia and chest pain) in comparison with peers that survived.
Table 1.
Baseline characteristics of COVID-19 patients classified by status.
| Characteristics | Survivor (n = 24) n (%) |
Non-survivor (n = 14) n (%) |
p-value |
|---|---|---|---|
| Age (y), Mean ± SD | 43.29 ± 11.80 | 59.71 ± 13.83 | <0.001*** |
| Male, | 17 (70.83) | 11 (78.57) | 0.601 |
| Symptoms | |||
| Fever | 21 (87.50) | 14 (100) | 0.168 |
| Chills | 4 (16.66) | 4 (28.57) | 0.385 |
| Myalgia | 13 (54.16) | 8 (57.14) | 0.859 |
| Cough | 22 (91.66) | 13 (92.85) | 0.731 |
| Headache | 11 (45.83) | 7 (50.00) | 0.804 |
| Shortness of breath | 24 (100) | 13 (92.85) | 0.185 |
| Arthralgia | 11 (45.83) | 5 (35.71) | 0.542 |
| Chest pain | 15 (69.50) | 4 (28.57) | 0.044 |
| Comorbidities | |||
| Diabetes | 8 (33.33) | 5 (35.71) | 0.837 |
| Hypertension | 8 (33.33) | 7 (50.00) | 0.502 |
| Obesity | 10 (41.66) | 6 (42.85) | 0.788 |
| HIV | 2 (8.33) | 1 (7.14) | 0.622 |
Dependent variable: Non-survivor (yes = 1, no = 0); SD: standard deviation. HIV: Human Immunodeficiency Virus. p-Values *** < 0.001.
Table 2 describes the biochemical parameters of COVID-19 patients classified by status (24 survivors, 14 non-survivors). Interestingly, we observed significant differences in serum levels of leukocyte, neutrophil, lymphocyte, levels of LDH, procalcitonin and CRP between the groups; leukocyte and neutrophil counts were higher in non-survivors (p < 0.001). Lymphopenia (lymphocyte count < 0.8 × 109/L) was developed in 6 (43%) patients that died and only 4 (17%) that survived (p = 0.012). In addition, serum levels of LDH, procalcitonin and CRP were higher in cases that did not survive, suggesting an increased level of systemic inflammation among these patients (see Table 3 ).
Table 2.
Biochemical parameters of COVID-19 patients classified by status.
| Parameter | Survivor (n = 24) Mean (SD) |
Non-survivor (n = 14) Mean (SD) |
p-value |
|---|---|---|---|
| Leukocyte count (109 cells/L) | 9.52 (4.03) | 15.60 (5.26) | <0.001*** |
| Neutrophil count (109 cells/L) | 7.41 (3.93) | 14.18 (5.46) | <0.001*** |
| Lymphocyte count (109 cells/L) | 1.30 (0.67) | 0.80 (0.23) | 0.012** |
| Hemoglobin (gr/dL) | 12.64 (1.98) | 12.96 (1.83) | 0.627 |
| Platelet count (109 cells/L) | 376.08 (318.77) | 348.92 (115.06) | 0.586 |
| Fibrinogen (mg/dL) | 697.79 (235.22) | 763.00 (167.01) | 0.369 |
| D-dimer (ng/L) | 1.75 (4.90) | 3.19 (5.50) | 0.072 |
| Potassium (mmol/L) | 3.93 (0.48) | 4.18 (0.54) | 0.150 |
| ALT (U/L) | 49.73 (34.25) | 40.21 (20.22) | 0.661 |
| AST (U/L) | 51.36 (41.32) | 49.07 (29.37) | 0.783 |
| GGT (U/L) | 166.39 (161.57) | 152.35 (151.75) | 0.639 |
| LDH (U/L) | 716.66 (337.01) | 996.50 (278.75) | <0.001*** |
| Creatinine (mg/dL) | 0.76 (0.15) | 0.87 (0.27) | 0.120 |
| Urea (mg/dL) | 26.10 (12.01) | 45.12 (21.82) | 0.001*** |
| Uric acid (mg/dL) | 3.82 (1.30) | 4.38 (1.62) | 0.249 |
| Procalcitonin (ng/mL) | 0.81 (2.66) | 1.27 (2.32) | 0.016** |
| CRP (mg/L) | 155.57 (100.94) | 268.96 (118.89) | 0.008** |
| Ferritin (ug/L) | 1.53 (2.24) | 1.34 (0.66) | 0.767 |
| IL-6 (pg/mL) | 20.69 (18.73) | 71.69 (58.61) | <0.001*** |
Dependent variable: Non-survivor (yes = 1, no = 0); SD: standard deviation. ALT: Alanine aminotransferase; AST: Aspartate transaminase; GGT: Gamma-glutamil transferase; LDH: Lactate dehydrogenase; CRP: C-reactive protein, IL-6: Interleukin 6. p-Values ** < 0.01; *** < 0.001.
Table 3.
Maximum likelihood for mortality prediction.
| Parameter | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Age, years | 1.07 | [1.03, 1.12] | 0.001*** |
| Male gender | 0.85 | [0.235, 3.106] | 0.810 |
| IL-6 | 1.01 | [1.003, 1.020] | 0.011** |
| Diabetes | 1.11 | [0.364, 3.444] | 0.844 |
| Hypertension | 1.84 | [0.618, 5.514] | 0.272 |
IL-6: Interleukin 6. p-Values ** < 0.01; *** < 0.001.
Serum levels of IL-6 were significantly higher in the non-survivors group, with a median of 45.60 pg/mL compared to the median of 10 pg/mL from survivors (Supplementary Table 1). Using a cox model for overall survival indicated that the likelihood estimates of death were associated with age (HR, 1.07, 95% CI, 1.03–1.12, p = 0.001) and an increased levels of IL-6 (HR, 1.01, 95% CI, 1.00–1.02, p = 0.011). Suggesting that for every year that increased the probability of mortality increased 7%, and for each 100 units of pg/mL increased 10% the probability of dying.
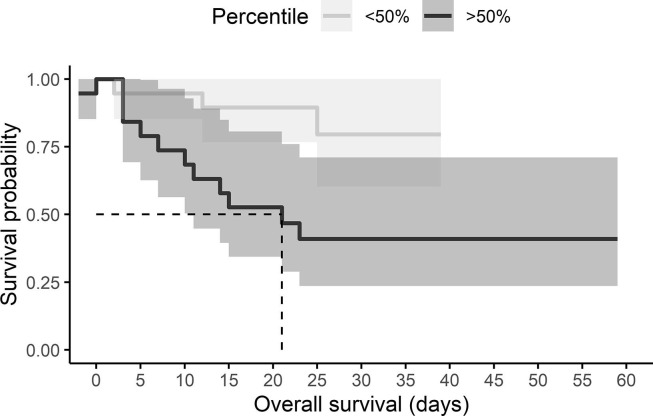
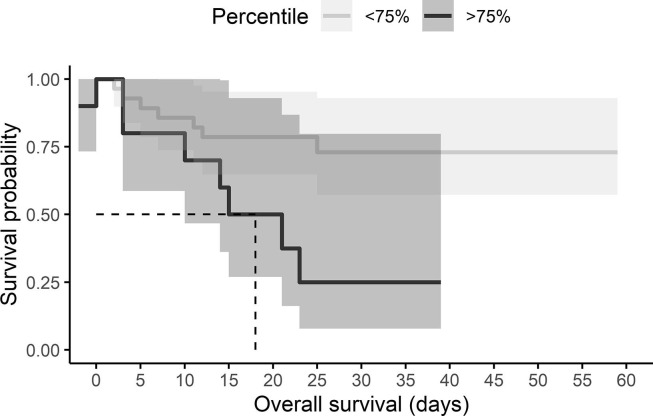
A cutoff value of 30.95 pg/mL with a sensitivity of 78.6% and specificity of 79.2% with a Youden Index of 0.57. The probability of death given an IL-6 level was calculated using survival analyses, using 50% and 75% cutoff. Half of the individuals who were above the 50% percentile in IL-6 levels died within 21 days (OR, 7.33, 95% CI, 1.58–33.96) (Fig. 1 ). Half of the individuals who were above the 75% percentile in IL-6 levels died within 18 days (OR, 7.00, 95% CI, 1.41–34.6) (Fig. 2 ) (see Fig. 3 ).
Fig. 1.
Kaplan–Meier curve according to IL-6 levels above the 50th percentile in COVID-19 patients. The cumulative mortality at a time to event (Non-survivor or survivor).
Fig. 2.
Kaplan–Meier curve according to IL-6 levels above the 75th percentile in COVID-19 patients. The cumulative mortality at a time to event (Non-survivor or survivor).
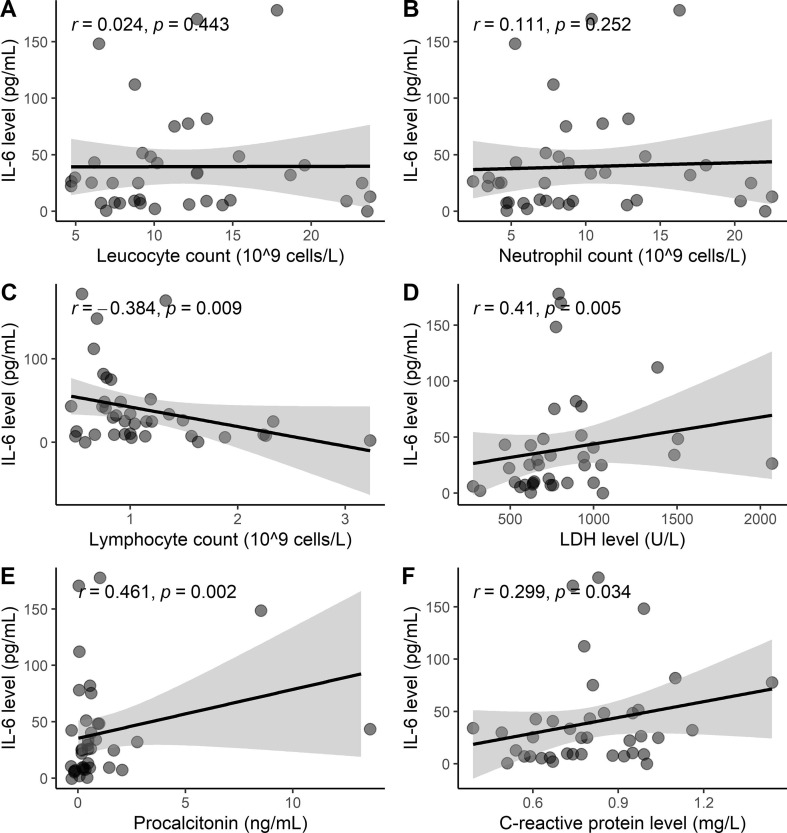
Fig. 3.
Statistical correlations of IL-6 with parameters related to (A) leucocyte count, (B) Neutrophil count, (C) Lymphocyte count; (D) Lactate dehydrogenase; (E) Procalcitonin; (F) C-reactive protein.
The IL-6 serum levels showed an increasing correlation with LDH (r = 0.409, p = 0.005), procalcitonin (r = 0.461, p = 0.002) and CRP (r = 0.299, p = 0.034). An inverse correlation with lymphocyte count (r = −0.384, p = 0.009), meaning levels of IL-6 significantly decreases as lymphocyte count increases.
4. Discussion
Results from this study showed that levels of IL-6 can predict survival among the Mexican population with COVID-19. The findings are in line with previous studies, that postulates the serum level of IL-6 as an indicator of severity and mortality due to SARS-CoV-2 infection [8]. Furthermore, using IL-6 levels in the first 72 h following hospital admission predicted mortality in patients with COVID-19. Although, mortality (37%) was found to be high in the population evaluated, the comorbidities as obesity, hypertension and diabetes were not significant between groups. However, the non-survivors group was significantly older than the survivor group.
Mortality rates vary among countries, this disparity may be due to different epidemiological features and/or accessibility to the health care services. According to data from Johns Hopkins Institute, Mexico is ranked on the top of the most affected countries by confirmed COVID-19 fatalities with 8.7% (observed case-fatality ratio) and 134,530 deaths per 100,000 population (as of November 30, 2020) [15]. Several factors contributed to the high rates of mortality in Mexico such as lowest testing rate for COVID-19 cases [16] delay in patients’ admission at health services for medical treatment [17] and the high prevalence of comorbidities namely obesity, hypertension and diabetes in Mexican adult population [5].
High level of cytokines is reported in several diseases including viral infections, as dengue [18] HIV [15] and influenza [19] and also chronic diseases as aging [20] cancer [21] and others. Especially, high levels of IL-6 are correlated to mortality among older men [20]. Cytokine storm in COVID-19 cases has been reported at the beginning of the pandemic [22]. However, until now the results are not entirely clear. A retrospective study from Zhou F, et al. [22] showed that the IL-6 was clearly elevated in non-survivors. In addition, Han, et al. [12] concluded that values of IL-10 and IL-6 can be utilized to predict the severity of COVID-19 cases. There are no reports on the Mexican population regarding the link between the serum level of cytokines and mortality in infected patients by SARS-CoV-2. However, previous analysis obtained in our laboratory showed that the mean values of IL-6 serum levels were higher in COVID-19 patients (39.5 ± 45.2 pg/mL) in comparison with healthy individuals (1.78 ± 10.60 pg/mL) (unpublished data). A study by M. Mandel, et al. [10] reported that 15% and 25% of patients with high IL-6 levels (above the 50th and 75th percentiles), will die on day 5. Results from our study showed that half of the individuals who were above the 50% percentile in IL-6 levels would die within 21 days and half of the individuals who were above the 75% percentile would die within 18 days.
The analysis in this study showed that being older and having high levels IL-6 in serum were associated with non-survivals, but comorbidities as diabetes, obesity or hypertension did not contribute to mortality prediction. In contrast with Bello-Chavolla, et al. [5] that reported that diabetes and obesity are risk factors for lethality among Mexican patients with COVID-19. This could be due to our small sample size. However, regardless of the geographic region, higher mortality and morbidity have been associated with an increased age [23].
In the study sample, the cutoff value of IL-6 was 30.95 pg/mL with high sensitivity and specificity. It was similar to reported by Zhang J, et al. [13] that showed a cutoff value of 37.65 pg/mL for IL-6 level among 901 patients in China. Different cutoff values were obtained among different populations. In a cohort of 77 patients in Italy reported a cutoff value of 25 pg/mL of serum IL-6 and linked to a risk factor of progression for severe and mortality by COVID-19 [24]. In another cohort of 40 patients in Munich, showed higher cutoff values for IL-6 (>80 pg/mL) and associated with the need of mechanical ventilation [25]. Therefore, the increased serum levels of IL-6 during infection by SARS-CoV-2 may alert and help in the clinical decision support for treatment interventions [26]. Consequently, COVID-19 patients in Mexico could benefit of the use of IL-6 inhibitors in order to reduce the risk of mortality [27].
Clinical studies and case reports established differences in clinical, immunological, molecular and pathologic characteristics in COVID-19 cases. Several reports described some markers for predicting clinical worsening [28] and risk factors for mortality [29] in patients infected by SARS-CoV-2, as a lymphopenia and high levels of CRP [30] LDH [31] and procalcitonin [32]. Similar results were reported in our study, by comparing survivors and non-survivor groups, we found lower levels of lymphocyte count and higher levels of CRP, LDH and procalcitonin in non-survivors. Normally, these markers are found with normal levels on hospital admission or in other viral infections [33], [34], but in patients with COVID-19 abnormal levels are found (Supplementary Table 2).
Additionally, we found a correlation between serum level of IL-6 with CRP, LDH and procaciltonin, and inverse correlation with the lymphocyte count. This suggests that IL-6 levels may by influence by systemic values of these features. However, our work is only an associative study and additional research is needed to clarify the dynamics through which IL-6 would be able to inhibit the lymphocyte count and the increased levels of CRP, LDH and procaciltonin in individuals infected by SARS-CoV-2.
The present work has some limitations as the small number of patients compared to others studies performed among individuals in China and also lack of data on healthy patients as control group. However, the data presented in this work was collected during the beginning of the pandemic in Mexico, therefore number of patients seen at the hospital were very limited.
5. Conclusion
Together, elevated levels of IL-6 may be an important indicator of mortality and deterioration of patients affected by COVID-19 in Mexico. A strong correlation was found between serum IL-6 levels and mortality in COVID-19 hospitalized patients. The mechanism in which IL-6 is released and how interacts with others inflammatory mediators in patients infected by SARS-CoV-2 needs further research. Up to the present, there is not a biomarker that is sensitive or specific enough to elucidate a diagnosis, stratification or mortality of COVID-19. Therefore, as more studies are available, biomarkers may emerge than can differentiated between patients at high risk of severe and mortality of COVID-19.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors are very grateful to the participants. Also, we would like to thank Mr. Julio Vega for helping in data analysis.
Ethics approval and consent to participate
This study was part of a project titled “Risk factors for the development of severe disease and its relationship with the levels of IL-6, IL-1, IL-8 and TNF-alpha in Yucatán” (No. 2020-010) that has been approved by Research Committee and the Ethics Committee from the Regional High Speciality Hospital of the Yucatan Peninsula (No. CONBIOETICA-31-CEI-002-20170731).
Consent of publication
Authors agree to allow the publication and declare that the submitted work is not presented and will not be published elsewhere in whatever language and published article will not be shared with anyone without earlier written permission of the publisher, except for academic purposes.
Availability of data and material
The authors agree to allow the publication and distribution of the materials submitted in all available forms, without limiting territory or language, provided that the material is accepted for publication. Authors confirm that all information is original and free from plagiarism.
Funding
This research received no external funding and the IL-6 ELISA kit was funded by Hospital Regional de Alta Especialidad de la Península de Yucatán.
Authors' contributions
AL Gutiérrez-Solis: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. A Ávila-Nava: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. A Cortes-Telles: Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. D Torres- Erazo: Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. S López-Romero: Data curation, Investigation, Resources, Validation, Visualization, Writing - original draft. R Chim Aké: Data curation, Investigation, Resources, Validation, Writing - original draft.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cyto.2021.155543.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Salud, Información Internacional y Nacional sobre nuevo Coronavirus (COVID-2019). https://www.gob.mx/salud/documentos/informacion-internacional-y-nacional-sobre-nuevo-coronavirus-2019-ncov.
- 2.Suárez V., Suarez Quezada M., Oros Ruiz S., Ronquillo De Jesús E. Epidemiology of COVID-19 in Mexico: from the 27th of February to the 30th of April 2020. Rev. Clin. Esp. (Barc) 2020;220(8):463–471. doi: 10.1016/j.rceng.2020.05.008. Epub 2020 Jul 9.doi: 10.1016/j.rce.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortés-Tellés A., López-Romero S., Mancilla-Ceballos R., Ortíz-Farías D.L., Núñez-Caamal N., Figueroa-Hurtado E. Risk factors for mortality among hospitalized patients with COVID-19. an overview in Mexican population. Tuberc. Respir. Dis. 2020 doi: 10.4046/trd.2020.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Secretaría de Salud, Instituto Nacional de Salud Pública, Instituto Nacional de Estadística y Geografía, Encuesta Nacional de Salud y Nutrición (ENSANUT) 2018. Presentación de resultados, 2018.
- 5.Bello-Chavolla O.Y., Bahena-López J.P., Antonio-Villa N.E., Vargas-Vázquez A., González-Díaz A., Márquez-Salinas A., Fermín-Martínez C.A., Naveja J.J., Aguilar-Salinas C.A. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J. Clin. Endocrinol. Metab. 2020;105(8) doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm' in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valle D., Kim-Schulze S., Huang H., Beckmann N., Nirenberg S., Wang B., Lavin Y., Swartz T., Madduri D., Stock A. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J., Li F. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa449. 2020.02.29.20029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandel M., Harari G., Gurevich M., Achiron A. Cytokine prediction of mortality in COVID19 patients. Cytokine. 2020;134 doi: 10.1016/j.cyto.2020.155190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F., Jiang Y., Cheng X., Zhu C., Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes. Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J., Hao Y., Ou W., Ming F., Liang G., Qian Y., Cai Q., Dong S., Hu S., Wang W., Wei S. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. J. Transl. Med. 2020;18(1):406. doi: 10.1186/s12967-020-02571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organization W.H. World Health Organization; 2020. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. [Google Scholar]
- 15.M. Analyses, Johns hopkins coronavirus resource center, 2020.
- 16.Ibarra-Nava I., Cardenas-de la Garza J.A., Ruiz-Lozano R.E., Salazar-Montalvo R.G. Mexico and the COVID-19 Response. Disaster Med. Public Health Prep. 2020;14(4):e17–e18. doi: 10.1017/dmp.2020.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman J., Calderón-Villarreal A., Bojorquez L., Hernández C.V., Schriger D.L., Hirashima E.T. Excess out-of-hospital mortality and declining oxygen saturation: the sentinel role of EMS data in the COVID-19 crisis in Tijuana, Mexico. Ann. Emerg. Med. 2020 doi: 10.1101/2020.05.13.20098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredeking T.M., Zavala-Castro J.E., González-Martínez P., Moguel-Rodríguez W., Sanchez E.C., Foster M.J., Diaz-Quijano F.A. Dengue patients treated with doxycycline showed lower mortality associated to a reduction in IL-6 and TNF levels. Recent Pat. Antiinfect. Drug Discov. 2015;10(1):51–58. doi: 10.2174/1574891X10666150410153839. [DOI] [PubMed] [Google Scholar]
- 19.Liu S., Yan R., Chen B., Pan Q., Chen Y., Hong J., Zhang L., Liu W., Wang S., Chen J.-L. Influenza virus-induced robust expression of SOCS3 contributes to excessive production of IL-6. Front. Immunol. 2019;10:1843. doi: 10.3389/fimmu.2019.01843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baune B.T., Rothermundt M., Ladwig K.H., Meisinger C., Berger K. Systemic inflammation (Interleukin 6) predicts all-cause mortality in men: results from a 9-year follow-up of the MEMO Study. Age (Dordr) 2011;33(2):209–217. doi: 10.1007/s11357-010-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern L., Mittenbühler M.J., Vesting A.J., Ostermann A.L., Wunderlich C.M., Wunderlich F.T. Obesity-induced TNFα and IL-6 signaling: the missing link between obesity and inflammation—driven liver and colorectal cancers. Cancers. 2019;11(1):24. doi: 10.3390/cancers11010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang S.J., Jung S.I. Age-related morbidity and mortality among patients with COVID-19. Infect. Chemother. 2020;52(2):154–164. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grifoni E., Valoriani A., Cei F., Lamanna R., Gelli A.M.G., Ciambotti B., Vannucchi V., Moroni F., Pelagatti L., Tarquini R., Landini G., Vanni S., Masotti L. Interleukin-6 as prognosticator in patients with COVID-19. J. Infect. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.M. Tobias Herold, C. Arnreich, J.C. Hellmuth, M. Matthias Klein, M. Tobias Weinberger, Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients, medRxiv. doi: http://dx.doi.10.1101/2020.04.01.20047381.
- 26.M. Zhao, Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies, Int. J. Antimicrob. Agents. (2020).doi: http://dx.doi.10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed]
- 27.Kaye A.G., Siegel R. The efficacy of IL-6 inhibitor Tocilizumab in reducing severe COVID-19 mortality: a systematic review. PeerJ. 2020;8 doi: 10.7717/peerj.10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y., Li B., Song X., Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Z., Chen A., Hou W., Graham J.M., Li H., Richman P.S., Thode H.C., Singer A.J., Duong T.Q. Prediction model and risk scores of ICU admission and mortality in COVID-19. PloS one. 2020;15(7) doi: 10.1371/journal.pone.0236618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu R., Han C., Pei S., Yin M., Chen X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020;58(7):1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors agree to allow the publication and distribution of the materials submitted in all available forms, without limiting territory or language, provided that the material is accepted for publication. Authors confirm that all information is original and free from plagiarism.