Abstract
The ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic is having a disastrous impact on global health. Recently, several studies examined the potential of vitamin D to reduce the effects of SARS-CoV-2 infection by modulating the immune system. Indeed, vitamin D has been found to boost the innate immune system and stimulate the adaptive immune response against SARS-CoV-2 infection. In this review, we provide a comprehensive update of the immunological mechanisms underlying the positive effects of vitamin D in reducing SARS-CoV-2 infection as well as a thorough survey of the recent epidemiological studies and clinical trials that tested vitamin D as a potential therapeutic agent against COVID-19 infection. We believe that a better understanding of the histopathology and immunopathology of the disease as well as the mechanism of vitamin D effects on COVID-19 severity will ultimately pave the way for a more effective prevention and control of this global pandemic.
Keywords: COVID-19, SARS-CoV-2, Vitamin D, Immune response, Immunopathogenesis
1. Introduction
The ongoing pandemic of the coronavirus disease-2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is having a devastating impact worldwide. The disease has been reported in 235 countries, with over 138 million infections and over 2.9 million deaths as of April 14, 2021. Although infections have been reported across essentially all demographics, the highest mortality rate is found in patients with comorbidities who are over 60 years old [1], [2], [3], [4], [5], [6]. Patients with COVID-19 present with pneumonia, severe acute respiratory distress syndrome (ARDS), septic shock, microvascular thrombosis, cytokine storms and cardiac failure [1], [7], [8], [9]. Comorbidities such as hypertension, chronic obstructive pulmonary disease, chronic cardiovascular diseases, chronic viral hepatitis, chronic renal disease, obesity and diabetes mellitus and other endocrine disorders are estimated to play a role in between 23% and 48% of the cases and are associated with the worst prognosis [3], [5], [10], [11], [12]. Existing data suggest that the severity of the disease caused by SARS-CoV-2 largely depends on an individual’s immune response, which can be significantly impaired by the comorbidities [13]. A weak innate immune response can lead to an increased SARS-CoV-2 viral load, resulting in an over-activation of the adaptive immune system and a massive production of pro-inflammatory cytokines such as IL (interleukin)-2, IL-6, IL-17, IL-21, IL-23 and IL-10 as well as an increase C-reactive protein (CRP) and d-dimer levels [14]. Recent data have indeed shown a robust correlation between massive cytokine production and the severity of COVID-19 [7]. This harmful overreaction of the immune system is often referred to “cytokine storm” [15], [16], [17], [18], [19] and is a main target of current treatment.
Recently, several experimental and meta-analysis studies have reported an association between serum vitamin D levels and the severity of COVID-19 [20], [21], [22], [23], [24], [25]. Indeed, many randomized control trials (RCTs) and clinical trials have shown that vitamin D supplementation can potentially reduce the severity of COVID-19 [26], [27], [28], [29], [30], [31], [32]. Still, several clinical trials are underway to investigate the impact of vitamin D in COVID-19 patients [29]. As this is a quickly evolving field, with reviews already discussing some of these findings [33], [34], [35], we will focus on providing the latest information regarding the immunological mechanisms of vitamin D in reducing SARS-CoV-2 infection as well as the most up-to-date information of the epidemiological evidence and clinical trials of vitamin D as a therapeutic agent in COVID-19 disease. We will also discuss recent advances of COVID-19 histopathology, immunopathology and the crucial mechanisms underlying disease severity, which are expected to pave the way for the prevention and control of this pandemic disease.
2. Information sources and review methodology
At the beginning of the SARS-CoV-2 outbreak, the initial focus of much of the research was the epidemiology, clinical signs, symptoms, and molecular diagnosis of SARS-CoV-2. Later on, there was greater emphasis on the genome sequence, transmission pattern, histopathology, immunopathology as well as vaccine and therapeutic options against COVID-19. As source material for the comprehensive review, we searched for the most up-to-date literature during the last 8 to 9 months in PubMed, PubMed Central, Google Scholar, ResearchGate, Science Direct, Bio Medical, Scopus and the World Health Organization COVID-19. Search terms used were ‘COVID-19 pandemic’, ‘SARS-CoV-2 infection’, ‘Seasonal variation in COVID-19′, ‘Histopathology in COVID-19′, ‘Immunopathology in COVID-19′, ‘Roles of vitamin D in respiratory infection’, ‘Roles of vitamin D in COVID-19′, ‘Vitamin D and immunity in COVID-19′, ‘Vitamin D deficiency and severity of COVID-19′, and ‘Vitamin D toxicity’.
3. COVID-19 infections are likely to be more prevalent in the winter season
Several studies have found that the prevalence of respiratory infectious diseases are seasonal, usually peaking in the winter season [36], [37], [38], [39], [40], [41], [42]. For instance, the respiratory diseases caused by a wide range of viruses including influenza virus, parainfluenza virus, human syncytial virus, rhinovirus and human coronavirus are more frequently found in the winter months [42]. Hirve et al. [43] performed a meta-analysis of 70 tropical and subtropical countries' influenza outbreak and suggested that the transmission of influenza and other coronavirus peaked during the winter season. Moreover, experimental studies with guinea pigs have shown that a low relative humidity of 20% to 35% is most favourable for coronavirus infections, with complete blockage of transmission at 80% humidity [44]. They also found that when guinea pigs were maintained at 5 °C, transmission occurred with higher frequency than at 20 °C, while at 30 to 35 °C, no transmission was detected. In addition, a recent systemic review on seasonal coronaviruses (sCoVs) from 21 countries has found that sCoVs are more prevalent in winter months in most temperate countries, whereas they tended to be less seasonal in tropical countries [45]. A lower temperature and higher relative humidity were also found to be associated with greater infectivity. It has also been reported that the immune system becomes weaker when the weather is cold and dry, increasing vulnerability to infection [46]. Notably, the initial outbreak of COVID-19 began in late December 2019 during the winter season in China, and the early epidemics in Iran, Italy, and much of Europe and the United States took place during the coldest months of the year [47], [48]. Considering the cycle of the present global pandemic, the extent of the epidemic gradually increased from January to May, then decreased from June to September, and then increased again starting in October at the beginning of winter [49]. Thus, similar to other respiratory disease viruses, SARS-CoV-2 appears to also have a higher epidemic prevalence in the winter season.
Low vitamin D levels during the winter have been suggested to be a “seasonal stimulus” of respiratory infectious diseases [50], [51]. A decrease in ultraviolet-B (UVB) exposure and/or insufficient sunlight exposure causes vitamin D deficiency or insufficiency, which increases during the winter season [51]. In fact, more than 1 billion people globally are believed to have vitamin D deficiency or insufficiency, with higher rates in the winter [52]. Experimental studies in vivo and in vitro have shown that low temperature and low UV indexes correlate with higher influenza infection [53]. Thus, these factors, which are maximal in winter, likely negatively influence the ability of the immune system to stave off respiratory infections, perhaps contributing to a greater prevalence of COVID-19 in the winter months.
4. Histopathology and immunopathology of COVID-19
After infection with SARS-CoV-2, COVID-19 patients may exhibit mild-to-no symptoms or can present with severe disease in need of immediate hospitalization. Severe patients often exhibit ARDS, reflecting septic shock and severe respiratory damage. Generally, in respiratory viral infections, the pathology may be dictated by the virus directly or by an exacerbated immune response, or both [54], [55], [56]. To date, the pathology of COVID-19 is not yet well defined, although several common morpho-functional characteristics have been reported between SARS-CoV, middle east respiratory syndrome coronavirus (MERS-CoV) and the SARS-CoV-2, including the interaction of the viral spike (S) glycoprotein with the human angiotensin-converting enzyme 2 (ACE2) [57], [58], [59], [60]. These similarities may help provide insight into the immuno-pathological mechanisms underlying COVID-19 and it may also pave the way for useful therapeutic approaches.
The respiratory system is the primary site of SARS-CoV-2 infection likely owing to the high concentration of ACE2 receptors in the epithelium lining [61], [62]. Nonetheless, SARS-CoV-2 can also infect the gastrointestinal, renal and nervous system due to the widespread distribution of the ACE2 receptors [62], [63]. Most COVID-19 patients indeed have mild symptoms, but approximately 15 to 20% of patients exhibit moderate symptoms of pneumonia characterized by fever, cough, and acute lung injury. Further, about 5% of patients eventually develop severe illness characterized by acute pneumonia, septic shock and multi-organ failure, a hallmark of ARDS [61], [64], [65], [66] and some cases exhibit diarrhoea, vomiting, hematuria, headache and paresthesia [63], [67]. As mentioned above, the severity of the COVID-19 disease increases in patients with comorbidities [65], [68].
SARS–CoV–2 binds to the human ACE2 receptor via the spike (S) protein to gain entry into cells [69] but requires proteolytic cleavage to complete entry through membrane fusion. The S protein can be cleaved either at the cell surface by a transmembrane serine protease (TMPRSS2), or by cathepsins following endocytic uptake [70], [71]. SARS-CoV-2 first invades the respiratory tract such as the nasal cavity, trachea, and the bronchial tree of the lung. The thin branches of the bronchial tree end in delicate air sacs, called alveoli, which are lined by a single layer of epithelial cells, pneumocytes (type I and type II) rich in ACE2 receptors [62], [63]. Usually, these alveoli permit exchange of oxygen into the capillaries that lie beside them to oxygenate the blood [72], [73], [74]. On binding to epithelial cells in the respiratory tract, SARS-CoV-2 begins to replicate and then migrates down to the airways and enters the alveolar epithelial cells in the lungs. The rapid replication of SARS-CoV-2 in the lungs triggers the inflammatory response. In patients with severe disease, congestion with patches of hemorrhagic necrosis appear in the lungs [65]. Microscopically, edema, proteinaceous exudates, congested capillaries, dilated alveoli and alveolar ducts, hyaline membrane formation and desquamation of pneumocytes have been observed [75]. At the ultrastructural level, SARS-CoV-2 viral particles have been seen in type I and type II pneumocytes and multinucleated giant cells [75], [76]. In other infected organs, renal microscopic lesions range from diffuse tubular injury with loss of brush border and capillary degeneration to glomerular endothelial cell necrosis [77]. Cardiovascular histological changes include hypertrophied cardiomyocytes along with inflammatory exudates, focal edema, cardiomyocytes hyperplasia, fibrosis, degeneration and necrosis [78]. Gastrointestinal tract histological changes include degeneration, necrosis and shedding of gastrointestinal mucosa [79].
According to the available literature, it generally takes between 5 and 7 days following SARS-CoV-2 infection for patients to manifest clinical symptoms and activation of the immune system [80]. Since the majority of cases have mild symptoms, the immune system can usually successfully contain the SARS-CoV-2 infection. However, approximately 15 to 20% of cases exhibit overaggressive or dysregulated immune responses, leading to immunopathology [67]. This overaggressive/dysregulated immune response is characterized by substantial systemic and locally increased levels of pro-inflammatory cytokines, such as IL-1β, IL-2, IL-6, IL-8, IL-17, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), and chemokine (C-C motif) ligand 3 (CCL3) [61], [64], [81], [82]. Moreover, elevated levels of CRP and d-dimer have been found, with levels that positively correlate with the severity of COVID-19 disease [64]. The increased levels of the pro-inflammatory cytokines activate the immune system, resulting in substantially lower numbers of lymphocytes, including CD4+ T cells, CD8+ T cells, B cells and natural killer (NK) cells [7], [8], [64], [81], [82], as well as lower numbers of monocytes, eosinophils, and basophils [67], [81], altogether resulting in leukopenia. This significant overreaction of the immune system is often termed “cytokine storm” [64], [81] and the clinical condition is referred to as “cytokine release syndrome” [83]. As a result, there can be severe damage to many vital organs including the lungs, heart, liver, and kidney tissues, finally producing multiple organ failures and even death [16]. Interestingly, a recently cohort study of ~ 125 patients identified three immuno-types in hospitalized COVID-19 patients: one group of patients had robust activation and proliferation of CD4+ T cells and exhaustion of CD8+ T cells (likely a sign that B cell immunity was activated); a second group had robust activation and proliferation of CD8+ T cell responses and less robust CD4+ T cell responses (probably indicating T cell immunity was activated); and a third group exhibited a lack of significant lymphocyte response, suggesting a failure of immune activation [84]. This significance of the immune-response to infection may also underlie the better outcome of children to infection than adults. In particular, it is believed that children have less ACE2 receptor, especially in the upper respiratory tract, and have more NK cells than adults. Further, their NK cells respond to the virus much more quickly and more effectively [85].
5. Vitamin D transport and metabolism
Vitamin D is not only a fat-soluble vitamin but also a steroid hormone, playing a vital role in modulating the immune system together with maintaining serum calcium homeostasis [86], [87]. Most often, humans obtain vitamin D from sunlight exposure, diet and supplementation. The two major forms of vitamin D are ergocalciferol (vitamin D2), which is synthesized from yeast, sunlight exposed-mushrooms, cod liver oil, oily fish and plants, and cholecalciferol (vitamin D3, the major form of vitamin D), which is synthesized endogenously from 7-dehydrocholesterol in the skin dermis on exposure to sunlight (UVB radiation at 290 – 315 nm) [88], [89]. The circulating level of 25-hydroxyvitamin D [25(OH)D] is the clinically-accepted biomarker for vitamin D status. The conventional sites of vitamin D metabolism are the liver and proximal convoluted tubules of the kidney, where hydroxylases convert it to its active form, 1,25-dihydroxyvitamin D [1,25(OH)2D]. Hydroxylase activity is also found in immune cells, such as macrophages, dendritic cells and monocytes [90], [91], [92].
Because of its lipophilic nature, vitamin D must be carried in the bloodstream by a soluble protein, namely, vitamin D-binding protein (DBP). DBP is a liver-derived glycoprotein (serum α2-globulin) and a member of the albumin superfamily that includes albumin, α-albumin/afamin, and α-fetoprotein [93], [94]. It has been found that binding to DBP preserves 25(OH)D and prolongs the half-life of 1, 25(OH)2D [95], and also enables activation of 25(OH)D to 1,25(OH)2 D [96]. Interestingly, the total circulating 25(OH)D levels have been found to be lower in blacks than white Americans due to poor socio-economic status [97] while both groups have similar concentrations of the bioavailable 25(OH)D [98]. SARS-CoV-2 infection positivity is inversely associated with circulating 25(OH)D levels [99]. This could be of increased importance for the black Americans that who are disproportionately affected by both COVID-19 and vitamin D deficiency. A decrease in DBP levels has been found in diabetic [100], chronic liver disease [101], and chronic renal disease and cancer patients [102] and in acute inflammation [103], all of which are also associated with higher COVID-19 morbidity and mortality [1], [2], [3], [4], [5], [6]. In contrast, there is a significantly increased concentration of DBP during pregnancy and during estrogen therapy [104], [105]. Overall, DBP levels are higher in females than in males, which could be due to the stimulation of DBP synthesis by estrogen [104]. Interestingly, this mirrors a three times lower COVID-19 morbidity and mortality in females compared to males [106]. A correlation between geographic variations of COVID-19 prevalence and mortality and DBP gene polymorphism and vitamin D metabolism has also been noted [94] and polymorphism of the DBP increases the risk of COVID-19 infection and mortality [94].
Both 25(OH)D and 1,25(OD)2D are delivered to cells by DBP [107] where entry is mediated by the vitamin D receptor (VDR), a nuclear receptor. In particular, these receptors function as ligand-activated, transcriptional regulatory proteins, and so binding by 1,25(OH)2D leads to altered expression of selected genes in target cells [108], [109], [110].
6. Potential roles of vitamin D on respiratory infection
A growing body of evidence indicates that vitamin D plays an important role in regulating the immune system [86], [111], [112] and might thereby affect the response to respiratory infections including COVID-19. Indeed, VDR is present in many human cells including various types of immune cells (such as lymphocytes, monocytes, macrophages, and dendritic cells) and it regulates the expression of a large number of target genes (~1000) in these cells [108], [109], [110]. A meta-analysis of data from 10,933 participants in 25 RCTs has shown that vitamin D supplementation indeed reduces the risk of acute respiratory tract infections (RTI) [113], [114]. A prospective cohort study from 198 healthy adults over the fall and winter in 2009 to 2010 also showed a two-fold reduction in the risk of developing acute RTI with serum vitamin D levels of 38 ng/mL [115]. In another study, a randomized, double-blind, placebo-controlled trial of 344 schoolchildren showed that a vitamin D supplementation of 1200 IU/day reduced the incidence of influenza A infection [116]. Furthermore, a case-control study in New Zealand children reported that vitamin D deficiency increased the odds of hospitalization for acute RTI by 1.7-fold, as compared to those with outpatient acute RTI [117]. Moreover, Zhou and colleagues [118] performed a meta-analysis of eight vitamin D observational studies and noted an association of vitamin D levels with respiratory tract infection, suggesting a strong correlation between vitamin D deficiency and community-acquired pneumonia. Therefore, there is presently considerable evidence linking vitamin D deficiency and an increased risk of acute RTI.
7. Potential roles of vitamin D in COVID-19 infection
As mentioned above, there is generally a lower amount of vitamin D produced during the winter season, which could affect COVID-19 infections. In addition, there is also some dependence on latitude and location [119]. Countries proximal to the equator have been found to exhibit lower levels of COVID-19 fatalities than those further from the equator [120], [121], consistent with a potential role of vitamin D in these infections. A retrospective study of 216 COVID-19 patients from Spain showed that more than 80% of patients have vitamin D deficiency [21]. Recently, a series of studies have examined the impact of vitamin D on the ongoing COVID-19 pandemic and concluded that it is likely an important contributing factor for the higher prevalence of the pandemic in the winter months [20], [21]. For instance, in an observational study, Boston University researchers found that COVID-19 patients with 'sufficient' levels (30 ng/mL) of vitamin D were about 52% less likely to die after hospitalization, while rates of severe illness were about 13% lower in vitamin D-sufficient patients. They also noted that an estimated 42% of people suffer from vitamin D deficiency (<20 ng/mL), with a higher rate among the elderly [20]. In addition, Ilie and colleagues [122] performed a meta-analysis study of the association of vitamin D and COVID-19 in 20 European countries and found a negative correlation between the levels of vitamin D and morbidity/mortality associated with this infection. Moreover, a significantly lower level of vitamin D (p = 0.004) was found in PCR-positive COVID-19 patients compared with negative COVID-19 patients [99], [123]. Systemic inflammatory responses caused by respiratory infectious diseases, including COVID-19 infection, have been, in fact, found to lower circulating vitamin D levels [124], which might also contribute to pathology.
Several recent studies showed a strong link between vitamin D deficiency and COVID-19 mortality risk and hospitalization [30], [31], [32], [125], [126], [127], [128], [129]. With one, a retrospective cohort study revealed an inverse relation with serum vitamin D levels and severity of COVID-19 [126]. Similar results were also found in a prospective cohort study conducted between March 1 to April 30, 2020, which revealed that older adults with vitamin D deficiency are associated with worse outcomes of COVID-19 infection likely owing to higher peak levels of d-dimer [130]. Furthermore, Annweiler and colleagues [30] performed a quasi-experimental analysis to study the effects of vitamin D supplementation during or just before COVID-19 infection and found that supplementation was associated with less severe illness and better survival in the frail elderly. In addition, a recent clinical trial found that administration of a high dose of calcifediol (0.532 mg or 21,280 IU) significantly reduced the need for ICU treatment or other clinical outcomes for COVID-19 [129]. Another clinical trial (the SHADE study) in which SARS-CoV-2 RNA positive vitamin D deficient patients received 60,000 IU of vitamin D3 or placebo daily for 7 days showed that high-dose supplementation indeed led to a significantly greater number of SARS-CoV-2 RNA negative patients by day 21, with fibrinogen, a surrogate for inflammation, also significantly decreased as well [128]. Furthermore, Jain et al. [131] recently investigated vitamin D levels in critically ill COVID-19 patients and found a correlation with inflammatory markers: patients who had low vitamin D levels also had significantly higher serum IL-6, TNF-α and ferritin levels. Vitamin D levels were markedly lower in severe COVID-19 patients and the fatality rate was very high in vitamin D deficient patients. Still, a recent clinical trial study showed that supplementation with a single very large dose of 200,000 IU of cholecalciferol that increased serum vitamin D levels (21–44 ng/mL) was nonetheless ineffective in decreasing the length of hospital stay or any other clinical outcomes among hospitalized patients with severe COVID-19 [132]. Thus, while not necessarily causative, these results show that low levels of vitamin D are often associated with worse severity of COVID-19 infection [125], [126], [127], [128], [129], [132], with moderate-to-high dose vitamin D supplementation effective in reducing COVID-19 severity and mortality (Table 1 ).
Table 1.
Recent randomized clinical trials and retrospective cohort studies evaluating a relation between vitamin D supplementation and COVID-19 outcomes.
| Study type | Location | Size | Vitamin D dose | Outcomes | Ref. |
|---|---|---|---|---|---|
| Cross-sectional analysis | Iran | 235 | Potentially reduce the severity of morbidities and mortality of COVID-19 | [20] | |
| Retrospective Cohort | UK | 444 | Booster (high-dose) vitamin D therapy | Associated with a reduced risk of COVID-19 mortality | [32] |
| Retrospective Cohort | US | 489 | 1000–3000 IU/daily for 14 were administered before COVID-19. | Deficient vitamin D status was associated with increased COVID-19 risk | [126] |
| Quasi-experimental | France | 66 | 80,000 IU bolus in week following or previous month | Less severe COVID-19 and better survival in frail elderly | [30] |
| Quasi-experimental | France | 77 | 50,000 IU/month or 80,000–100,000 IU every 2–3 months before COVID-19) vs. 80,000 IU within “few hours” of COVID-19 | Regular bolus vitamin D3 supplementation was associated with less severe COVID-19 and better survival rate. | [31] |
| Randomized clinical trials | Spain | 76 | 0.532 mg (21,280 IU) on the day of admission and 0.266 mg on day 3, 7 and weekly | Significantly reduce the need for ICU of COVID-19 cases (e.g. reduced the severity of COVID) | [129] |
| Randomized clinical trials | Brazil | 240 | Single oral dose of 200,000 IU of vitamin D3 | Increase serum vitamin D levels (21–44 ng/mL) but did not significantly reduce hospital length of stay | [132] |
| Randomised clinical trials (SHADE study) | India | 40 | 60,000 IU of cholecalciferol daily for 7 days | Greater proportion of vitamin D-deficient individuals with SARS-CoV-2 infection turned SARS-CoV-2 RNA negative with a significant decrease in fibrinogen | [128] |
Hence, based on these results, it is likely beneficial to take vitamin D supplements, especially in the winter months, to better control the seasonal flu, common cold, influenza, and the ongoing COVID 19. It may be advisable for doctors to measure circulating vitamin D levels, and if the level is below normal, recommend vitamin D supplementation and/or enough sunlight exposure. Based on available guidelines, the threshold for healthy serum 25(OH)D is ~30 ng/ mL to maintain optimal serum calcium levels [133], [134], [135], [136]. Below 20 ng/mL (50 nmol/L) is considered vitamin D deficient, while 21 – 29 ng/mL (52.5 – 72.5 nmol/L) is vitamin D insufficient [133]. For an immunomodulatory effect, it has been suggested that a serum level of 30 ng/mL of 25(OH)D is essential [137], [138]. However, it remains under debate as to what is the optimum serum level of 25(OH)D to maximize its effect on the immune system against SARS-CoV-2 infection. A cross-sectional study with 235 COVID-19 patients showed that serum 25(OH)D levels of at least 30 ng/mL were associated with a significant reduction of clinical outcomes and mortality of COVID-19 [20]. Therefore, overall, a desirable serum level of 25(OH)D of at least 30/mL appears to be most useful for COVID-19 patients [20], [133]. The recommended supplementation and therapeutic dosage of vitamin D for vitamin D deficiency are compiled in Table 2 according to the Endocrine Society Guidelines. These dosages might be useful for the prevention and treatment of SARS-CoV-2 infection as well.
Table 2.
The supplementation and treatment dosage of vitamin D for vitamin D deficiency and prevention and treatment of SARS-CoV-2 infection. Compiled from the Endocrine Society Guidelines [133].
| Ages | Supplementation doses | Upper tolerable dose | Treatment and preventive doses |
|---|---|---|---|
| 0–1 year | 400–1000 IU/d. To achieve a serum level of 25(OH)D above 30 ng/mL need at least 1000 IU/d. | 2000 IU/d | 2000 IU/d or 50,000 IU/wkly for 6 wk to achieve a serum level of 25(OH)D above 30 ng/mL, followed by 400–1000 IU/d. |
| 1–18 years | 600–1000 IU/d. To achieve a serum level of 25(OH)D above 30 ng/mL need at least 1000 IU/d. | 4000 IU/d | 2000 IU/d or 50,000 IU/weekly for 6 wk to achieve a serum level of 25(OH)D above 30 ng/mL, followed by 600–1000 IU/d. |
| 19 –50 years | 600–1500 IU/d. To achieve a serum level of 25(OH)D above 30 ng/mL need at least 1500 –2000 IU/d. | 4000 IU/d | 6000 IU/d or 50,000 IU/weekly for 6 wk to achieve a serum level of 25(OH)D above 30 ng/mL, followed by 1500–2000 IU/d. |
| 50 –70 years | 800–2000 IU/d. To achieve a serum level of 25(OH)D above 30 ng/mL need at least 1500 –2000 IU/d. | 10,000 IU/d | 6000 IU/d or 50,000 IU/weekly for 8 wk to achieve a serum level of 25(OH)D above 30 ng/mL, followed by 1500–2000 IU/d. |
| 70 + years | 1000–2000 IU/d. To achieve a serum level of 25(OH)D above 30 ng/mL need at least 1500 –2000 IU/d. | 10,000 IU/d | 6000 IU/d or 50,000 IU/weekly for 8 wk to achieve a serum level of 25(OH)D above 30 ng/mL, followed by 1500–2000 IU/d. |
| Pregnant and lactating women | 1500–2500 IU/d. To achieve a serum level of 25(OH)D above 30 ng/mL need at least 1500 –2000 IU/d. | 10,000 IU/d | 6000 IU/d or 50,000 IU/weekly for 8 wk to achieve a serum level of 25(OH)D above 30 ng/mL, followed by 1500–2000 IU/d. |
| Obese and anticonvulsant medications | To achieve a serum level of 25(OH)D above 30 ng/mL need at least two to three times more vitamin D for their age group. | – | 2 to 3 times more for their age group to achieve a serum level of 25(OH)D above 30 ng/mL, followed by maintenance therapy of 3000–6000 IU/d. |
However, we note that a statistical error in the estimation of the Endocrine Society Guideline recommended dietary allowance (RDA) for vitamin D was recently discovered: in a correct reanalysis of the data used by the Institute of Medicine, it was found that 8895 IU/d was needed for 97.5% of individuals to achieve values of ≥ 50 nmol/L (20 ng/mL) [139]. Another study confirmed that 6201 IU/d was needed to achieve the Endocrine Society’s recommendation of 75 nmol/L (30 ng/mL) and 9122 IU/d was needed to reach 100 nmol/L (40 ng/mL) [140]. Based on this reanalysis, the recommended dose should be at least three-fourths of the upper tolerable dose originally proposed by the Endocrine Society, taken all year long, to achieve targeted levels of serum 25(OH)D. That is, for zero-old children, a dose of 1000 IU/d is recommended, while 1500 IU/d for breastfed children older than 6 months, 3000 IU/d for greater than 1-year age-old children and around 8000 IU/d for young adults and thereafter would be needed [141].
8. Potential mechanism of action of vitamin D in COVID-19 infection
The course of SARS-CoV-2 infection from infection to symptoms is about five days, and then after symptoms develop, there are about seven days where these symptoms become more and more progressive. About 20 percent of symptomatic patients need hospitalization, while the majority will actually get better, owing especially to a robust response of the innate immune system during the early phase of the disease [7]. Overall, when pathogens enter the body, the very first responder is the innate immunity followed by adaptive immunity [142].
Vitamin D supplementation can enhance innate immunity [112], [143], [144] as well as adaptive immunity [145], [146]. Because antigen-presenting cells (macrophages and dendritic cells) can synthesize 1,25(OH)2D from 25(OH)D, it has been postulated that vitamin D supplementation could improve the function of antigen-presenting cells, thereby ameliorating the overall immune response [147]. Thus, in what follows, we will focus on the two main mechanisms by which vitamin D enhances the immune system: (i) enhancing the innate immunity (including physical barriers), and (ii) enhancing the adaptive immunity [148].
8.1. Vitamin D and innate immunity
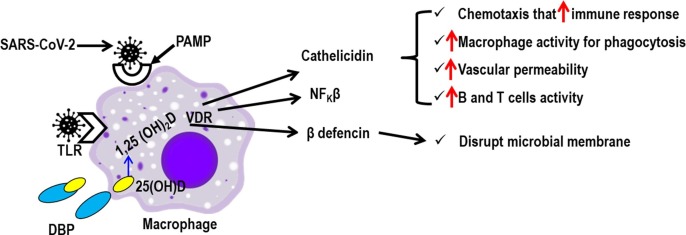
The innate immune system comprises the physical barriers, including the skin, mucosal membranes, and vascular endothelium, as well as specific immune cells, such as macrophages, monocyte, dendritic cells, neutrophils, and mast cells. Vitamin D has been found to stabilize the protective physical barriers, strengthening gap junctions, tight junctions and adherens junctions mediated by E-cadherin [149]. Vitamin D also has been found to enhance the innate immune response through the production of several antimicrobial peptides (such as defensins and cathelicidins) that have anti-bacterial, anti-viral, and anti-fungal properties as well as a modulation of cellular immunity [112]. Some of the mechanisms of the vitamin D-modulated innate immune response against microbial infection including SARS-CoV-2, particularly in macrophages, are compiled in Fig. 1 .
Fig. 1.
Possible pathway of vitamin D-modulated innate immunity in patients with SARS-CoV-2 infection. The SARS-CoV-2 infection results in the activation of antigen-presenting cells (APCs) (macrophages, dendritic cells, B cells) through toll-like receptors (TLR) and/or pathogen-associated molecular pattern (PAMP). Serum 25-hydroxyvitamin D [25-(OH)D] bound to vitamin D binding protein (DBP) allows intracellular access of free 25-(OH)D into the APCs . This triggers both the endogenous production (shown with long arrow) and action of 1,25(OH)2 D through the VDR, leading to induction of antimicrobial proteins, such as cathelicidin, nuclear factor kappa β (NFKβ) and β-defensins which overall destroy the SARS-CoV-2. Since the macrophage is a very professional immune cell that plays a vital role in innate immunity, the cell is chosen as a representative of innate immune cells to describe the mechanism.
SARS-CoV-2 infection results in the activation of antigen-presenting cells (macrophage, monocyte and dendritic cells) through toll-like receptor 3 (TLR3) and/or pathogen-associated molecular pattern (PAMP) [112]. This triggers both the endogenous production and action of 1,25(OH)2D through the VDR [90], [150], leading to induction of antimicrobial proteins, such as cathelicidins, nuclear factor kappa β (NFKβ) and β-defensins [112], [151], [152], [153]. Defensins disrupt microbial membranes including the SARS-CoV-2 virus envelope [154]. The NFKβ helps to produce defensins, while cathelicidins are involved in (i) chemo-attraction of various immune cells to increase immune response (chemotaxis), (ii) enhancing macrophage phagocytosis, (iii) increasing vascular permeability, and finally (iv) activation of B cells and T cells for proliferation [155], [156], [157]. Additionally, vitamin D is believed to stabilize the epithelial cell membrane which can reduce the ability of pathogens, including the SARS-COV-2, to infect cells. In in vitro and in vivo experiments in mice, the human cathelicidins, LL-37, was found to reduce influenza virus replication [156]. In another experimental study, rotavirus replication was found to be reduced in vivo and in vitro as a consequence of vitamin D treatment [158]. Finally, we note that a recent clinical trial found that a higher dose of vitamin D supplementation decreased dengue virus infection by improving the innate immune response [159].
8.2. Vitamin D and adaptive immunity
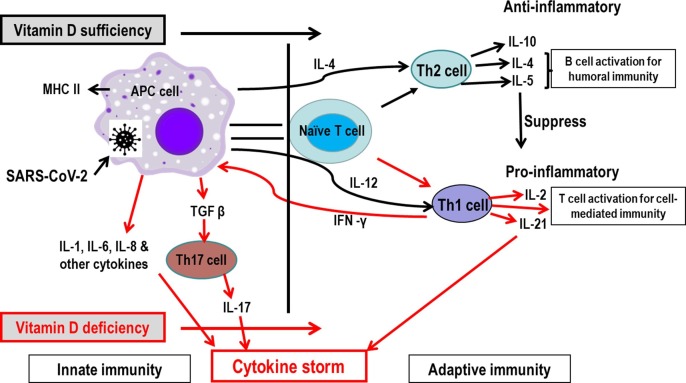
Vitamin D may be beneficial against COVID-19 infection by reducing both systemic and local inflammatory responses as well as cytokine responses. Indeed, vitamin D deficiency is directly associated with ARDS as shown by in vitro studies with primary cells and mouse models, as well as clinical studies [160]. Furthermore, vitamin D is also a modulator of adaptive immunity [148], [161], in part by reducing the cytokine storm which is a typical pathology of severe COVID-19 infections [7], [8], [81], [82]. Both the innate and adaptive immune systems produce pro-inflammatory and anti-inflammatory cytokines in response to viral and bacterial infections, as observed in COVID-19 patients [7]. Typically, the innate immune system fights against infection first until the adaptive immune system (T and B cells) becomes sufficiently activated, usually 7 to 10 days after primary infection. A schematic representation of the potential vitamin D mode of action on reducing the COVID-19 cytokine storm is presented in Fig. 2 .
Fig. 2.
Possible pathway of vitamin D-modulated immunity in reducing cytokine storm in patients with SARS-CoV-2 infection. SARS-CoV-2 infection results in antigen-presenting cells (APCs) activation for phagocytosis of SARS-CoV-2, then the cell type communicates with naïve T (Th-0) cells. In serum optimal level of vitamin D, the naïve T cell is shifted to T helper 2 (Th2) cell instead of Th1 phenotype and promotes anti-inflammatory cytokines such as IL-10, Il-5 and Il-4 production. The anti-inflammatory cytokines decrease the secretion of pro-inflammatory cytokines such as IFN-γ, IL-6, IL-2, and TNF-α mediated by down-regulating Th1 cells response. All of these result in an anti-inflammatory reaction and thus, the overreaction of the immune system is controlled. However, in deficiency of vitamin D, the adaptive immune response shifts towards the Th1 direction and may therefore cause hyper-inflammation/cytokine storm (in red colour). Furthermore, vitamin D also decreases Th17-cell responses and the differentiation of naive T cells into Th17 type cells via decreasing the synthesis of IL-12 which leads to a decrease in the production of pro-inflammatory cytokines, including IL-6, IL-17 and IL-23. Since the macrophage is a very professional immune cell that plays a vital role in immunity, the cell is chosen as a representative of innate immune cells to describe the mechanism.
Since the mechanisms of immunity against COVID-19 are presently incompletely understood, we will describe several processes by which vitamin D boosts immunity against viral infections more generally, which may also be applicable to SARS-CoV-2. In general, innate immune cells, macrophages, first phagocytize pathogens and then communicates with naïve T cells to induce activation. The activated naïve T cell converts into either T helper 1 (Th1) or Th2 cells. With vitamin D deficiency, the adaptive immune response shifts towards the Th1 direction, which may thereby cause the hyper-inflammation/ cytokine storm since these cells release the inflammatory cytokines, IL-2 and IL-21. Moreover, when Th1 cells are activated, they, in turn, activate macrophages by releasing IFN-γ, which leads to production of IL-6, IL-1, IL-8, and TGF-β, the latter of which activates Th17 cells to secrete IL-17. IL-1, IL-6, IL-8, IL-17 and IL-21 are all important pro-inflammatory cytokines (Fig. 2). Vitamin D has been found to suppress the adaptive immune response by decreasing T cell proliferation [162], [163]. In experimental mouse models, down-regulated adaptive immune responses mediated by Th1 cells were observed, as well as decreases in the levels of IFN-γ, IL-6, IL-2, and TNF-α [164], [165]. Moreover, down-regulation of Th1 cell-response cytokines, especially IFNγ, as well as stimulation of Th2-cell responses, were found [163], [166].
The Th2 cell type immune response is anti-inflammatory and thus works to control inflammation. This is effected by the secretion of IL-4, IL-5 and IL-10 by the Th2 cells. IL-4 and IL-5 activate B cells to proliferate and mature, which in turn release antibodies against pathogens. By contrast, IL-10 suppresses the activation of Th1 and the expression of major histocompatibility complex II (MHC-II) in macrophages, which reduces inflammation. IL-10 also results in a lower degree of communication between macrophages and naïve T cells. All of these effects prevent the over-activation of adaptive immunity that is hyper-inflammation. Generally, the COVID-19 patient’s immune system shifts to the Th2 phenotype in most cases [84] which helps with a quick control of the disease. Unfortunately, approximately 15–20% of COVID-19 cases undergo severe disease. Clinical data compiled by Daneshkhah and colleagues [167] indicate that vitamin D deficiency in severe COVID-19 patients is 34% (age ≥ 60 years), 22% (20 years ≤ age < 40 years), and 21% (40 years ≤ age < 60 years) more frequent than in patients with normal vitamin D levels. With the correct level of vitamin D, the immune system shifts to the Th2 phenotype direction that inhibits the production of pro-inflammatory cytokines [164], [165], resulting in an anti-inflammatory reaction and a control of the otherwise overreaction of the immune system in chronic-relapsing experimental allergic encephalomyelitis [168], [169]. Furthermore, a recent experimental study has shown that vitamin D can induce the production of more anti-inflammatory cytokines, such as IL-10, which in turn is expected to reduce the severity of the disease [170]. However, with vitamin D deficiency or insufficiency, the body’s immune system shifts towards the Th1 direction and hyper-inflammation/cytokine storm might occur [171]. Furthermore, vitamin D governs T-cell differentiation by regulating antigen-presenting dendritic cells, causing them to reduce the synthesis of IL-12 that promotes Th1-cell responses [172], [173]. Vitamin D also exerts an important effect on the activity of the immune system by decreasing Th17-cell responses and the differentiation of naive T cells into Th17 type cells which decreases secretion of several pro-inflammatory cytokines, including IL-6, IL-17 and IL-23 [174], [175]. In addition, vitamin D decreases the synthesis of IL-12 and simultaneously increases the production of IL-10 by dendritic cells (DCs) [170]. Overall, thus, these results indicate that vitamin D moves the adaptive immune response from a Th1- to a Th2-phenotype [168], [169], decreases the differentiation of naïve T cells into Th17 cells [174], [176] and enhances the T regulatory type response, leading to an inhibition of inflammatory processes [177], [178], [179], [180]. In this way, vitamin D might help in the treatment of COVID-19 by preventing the cytokine storm and subsequent ARDS which is a common cause of mortality [8].
9. Vitamin D deficiency and severity of COVID-19
The major cause of vitamin D deficiency is insufficient exposure to sunlight [181], with also some dependency of basal metabolic index (BMI) and aging [182], [183]. Patients with chronic kidney and liver diseases also have vitamin D deficiency [184]. For more than a century, vitamin D deficiency has been suggested to increase the susceptibility to RTI. The increased risk of RTIs in children with nutritional rickets [185] and vitamin D is considered of importance in the treatment of tuberculosis [186]. An observational study of 9548 adults aged 50 to 75 years in Germany has shown that vitamin D insufficiency and deficiency strongly increases respiratory disease mortality [187]. Consistent with this observational data, several randomized clinical trials show a strong link between vitamin D deficiency and COVID-19 severity and mortality, and that supplementation could reduce the severity [128], [129], [132]. Recent studies have provided further evidence that vitamin D is an important regulator of human immune function, perhaps, as described above, as a result of its stimulation of the innate immune response [112], [113], [188]. Since VDR is expressed in different cells of the myeloid and lymphoid lineage, vitamin D may increase the expression of antimicrobial peptides in human monocytes and neutrophils [189], [190]. The role of vitamin D in boosting the immune response in flu, common cold and other RTI have been shown [113], [153], [188], [191], [192]. Moreover, clinical data of vitamin D obtained during the 1918–1919 influenza pandemic found a potential role in reducing the cytokine storm [193]. Analysis of recent clinical data obtained from China showed a robust correlation between the cytokine storm and severity of COVID-19 [7]. Serum levels of inflammatory markers (IL-6, serum ferritin and TNF-α) were found to be higher in vitamin D deficient COVID-19 patients, which correlated with greater COVID-19 severity and increased mortality [131]. Overall, the correlations between COVID-19 and vitamin D deficiency from a biochemical standpoint are shown in Table 3 .
Table 3.
Biochemical correlations between COVID-19 and vitamin D deficiency. Data compoled from [130], [194], [195], [196], [197], [198], [199], [200], [201].
| Parameters | COVID-19 | Vitamin D deficiency |
|---|---|---|
| IL-6 | Increased | Increased |
| TNF-α | Increased | Increased |
| IFNγ | Increased (late in course) | Increased |
| C-reactive protein | Increased | Increased |
| D-dimer | Increased | Increased |
| Innate immune response | Decreased | Decreased |
| Th1 adaptive immune response | Increased (late in course) | Increased |
| Cytokine storm | Increased | Increased |
| ACE2 expression | Decreased | Decreased |
| Coagulability | Increased | Increased |
Recent work indeed has suggested that a weak innate immune response enables a higher SARS-CoV-2 load, which results in over-activation of the adaptive immune system and, consequently, increased cytokine release and severe COVID-19 [14]. Moreover, increased production of cytokines, including TNF-α and IL-1β, and elevated CRP were found in vitamin D deficient patients which may cause hyper-inflammation [171]. Thus, based on retrospective data and indirect evidence [7], [12], [14], [167], we suggest a possible relationship between vitamin D deficiency and COVID-19 severity (Fig. 3 ).
Fig. 3.
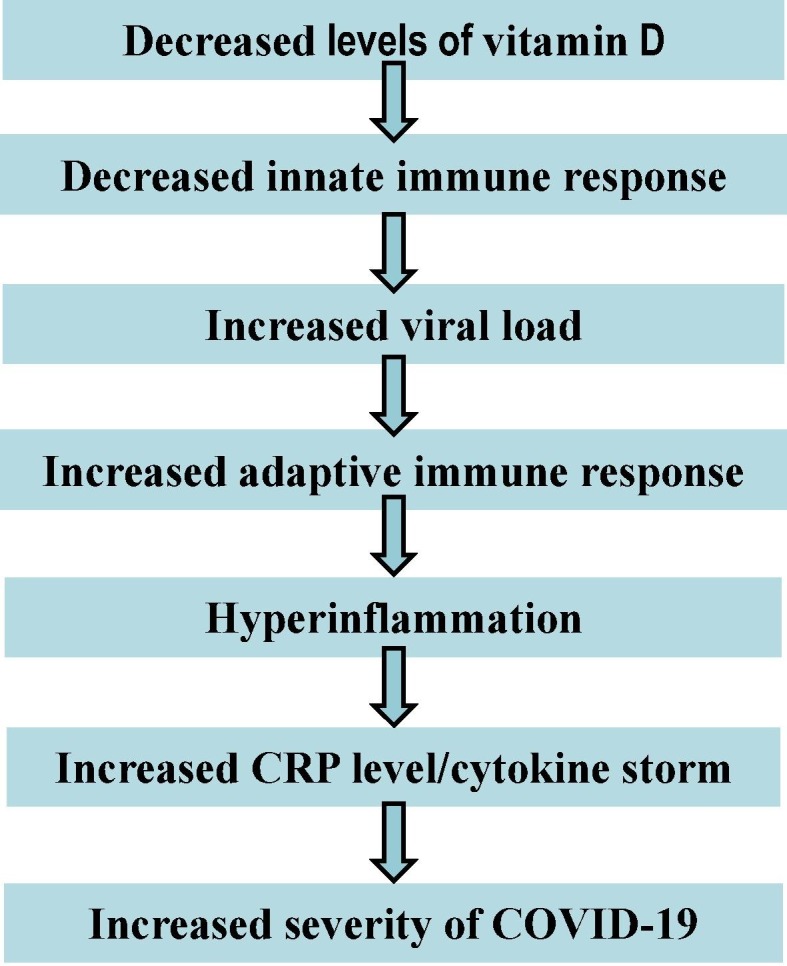
Schematic representation of a possible correlation between vitamin D deficiency and COVID-19 severity.
Recently, it has been suggested that the severity of COVID-19 infections are strongly associated with increased production of CRP [7], [167] and pro-inflammatory cytokines (IL-6, IL-17) [12], leading to increased risk of pneumonia [12], ARDS, and heart failure [202], and finally, multi-organ failure. In contrast, CRP and inflammatory cytokines (IL-6, IFN-γ) were attenuated in haemodialysis patients after vitamin D (calcitriol) treatment [203]. Although most of the studies are observational, it is thought that vitamin D can suppress cytokine and CRP production by simultaneous boosting the innate immune system, thus reducing the viral load and decreasing the over-activation of the adaptive immune system, thereby reducing COVID-19 mortality [167]. Clearly, randomized clinical trials are urgently needed to clarify the role of vitamin D in reducing COVID-19 infection.
10. Vitamin D toxicity
Recurrent vomiting, abdominal pain, polydipsia, polyuria, confusion, and apathy are the most often noted clinical symptoms of acute vitamin D toxicity (VDT) or vitamin D intoxication or hypervitaminosis D [204]. The Endocrine Society and the Institute of Medicine (IOM) have both stated that acute VDT is extremely rare [205], [206]. Although rare, if it is not identified quickly, the health effects can be serious. Serum 25(OH)D concentrations higher than 150 ng/mL (375 nmol/L) are the hallmark of VDT and the levels have been found to cause hypercalcemia [207]. Long-term consumption of vitamin D daily of more than 40,000 IU (1000 μg) has been found to cause hypercalcemia in healthy persons [208]. Hypercalcemia has a straightforward relation to serum 25(OH)D but not to 1,25(OH)2D levels [209]. The clinical findings associated with VDT are closely related to serum calcium concentration and duration of the hypercalcemic condition [207]. Long-term supplementation with vitamin D3 in doses ranging from 5000 to 50,000 IU/day is considered to be safe [210]. For instance, Pietras et al. [211] reported that healthy adults taking 50,000 IU (equivalent to approximately 3,300 IU/day) of vitamin D2 once every 2 weeks for 6 years maintained vitamin D levels of 40–60 ng/ml (100–150 nmol/l) without toxicity. Ekwaru et al. [212] noted that Canadian adults taking 20,000 IU of vitamin D3 per day had a significant increase of vitamin D levels, up to 60 ng/mL (150 nmol/L), with no evidence of toxicity. Dudenkov et al. [213] performed a retrospective population-based analysis from more than 20,000 measurements at the Mayo Clinic from 2002 to 2011 to determine the prevalence of VDT and demonstrated no acute clinical toxicity, with only one patient exhibiting a vitamin D level of 364 ng/mL (910 nmol/l) with hypercalcemia. In fact, Holick [214] showed that vitamin D is probably one of the least toxic fat-soluble vitamins.
11. Conclusions
Although the epidemic of SARS-CoV-2 started in late December 2019 in Wuhan, China, there is still great uncertainty as to how it will finally resolve. In the meantime, the COVID-19 vaccine has been discovered and is being administered globally. However, this does not mean that the crisis has been resolved or is going to resolve soon. The vaccine provides a specific immunity against disease, but no single vaccine can provide 100% protection to any diseases. Moreover, it is normal for many viruses to evolve through mutations and as a consequence, new variants of the virus emerge. It has been reported that the emergence of a different strain of SARS-CoV-2 may reduce the effectiveness of the vaccine [215]. In a true sense, vaccines alone, unless they achieve high population coverage, offer long-lasting protection, and are effective in preventing both SARS-CoV-2 transmission and COVID-19, but will not end the pandemic. Boosting immunity is a very important tactic to combat the ongoing and upcoming pandemic. The disease production by SARS-CoV-2 in an individual largely depends on host immunity, especially innate immunity, therefore, along with vaccination, there should be a priority to focus on enhancing host immunity. Vitamin D supplementation is one of the ways to boost host immunity. This review provides a rationale for the use of vitamin D against SARS-CoV-2 infection to improve the immune response based on recent observational studies and RCTs. Large-scale clinical trials are urgently needed to determine the definitive effects of vitamin D in reducing SARS-CoV-2 infection. Further studies are also necessary to improve our understanding of the immuno-pathogenesis of COVID-19 to control this public health emergency. Along with Vitamin D supplementation, we suggest that a poly-therapeutic intervention, such as sleeping at least eight hours a day, avoiding stress and fatigue, engaging in sufficient physical exercise, adequate sunlight exposure, as well as supplementation with other vitamins and macro/micro-nutrients (such as magnesium, zinc and selenium) will promote the maintenance of adequate homeostasis and proper activity of the immune system, ultimately aiding in the prevention of SARS-CoV-2 and other respiratory infectious diseases.
Authors contributions
MS Alam conceived the idea and wrote the original draft of the manuscript. MA Islam and MA Rahman helped MS Alam to write 'vitamin D transport and metabolism' section and 'EndNote references', respectively. Daniel and MS Alam reviewed and edited the final version of manuscript. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;105924 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.W. Ling, C-reactive protein levels in the early stage of COVID-19, Medecine et maladies infectieuses (2020). [DOI] [PMC free article] [PubMed]
- 3.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y. China; JAMA internal medicine: 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y., Zhang Z., Tian J., Xiong S. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Annals of palliative medicine. 2020;9(2):428–436. doi: 10.21037/apm.2020.03.26. [DOI] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China, The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet respiratory medicine. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.N. Zhu, D. Zhang, W. Wang, X. Li, B. Yang, J. Song, X. Zhao, B. Huang, W. Shi, R. Lu, China Novel Coronavirus, I., Research, T. A Novel Coronavirus from Patients with Pneumonia in China, (2019). [DOI] [PMC free article] [PubMed]
- 10.Saghazadeh A., Rezaei N. Immune-epidemiological parameters of the novel coronavirus–a perspective. Expert Review of Clinical Immunology. 2020:1–6. doi: 10.1080/1744666X.2020.1750954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D.C. Sanchez-Ramirez, D. Mackey, Underlying Respiratory Diseases, Specifically COPD, and Smoking Are Associated with Severe COVID-19 Outcomes: A Systematic Review and Meta-Analysis, Specifically COPD, and Smoking Are Associated with Severe COVID-19 Outcomes: A Systematic Review and Meta-Analysis (4/28/2020) (2020). [DOI] [PMC free article] [PubMed]
- 12.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. China, Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez C.R., Nomellini V., Faunce D.E., Kovacs E.J. Innate immunity and aging. Exp. Gerontol. 2008;43(8):718–728. doi: 10.1016/j.exger.2008.05.0168.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.P. Mehta, D.F. McAuley, M. Brown, E. Sanchez, R.S. Tattersall, J.J. Manson, H.A.S. Collaboration, COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet (London, England) 395(10229) (2020) 1033. [DOI] [PMC free article] [PubMed]
- 15.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 16.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dashti-Khavidaki S., Khalili H. Considerations for statin therapy in patients with COVID-19, Pharmacotherapy: The Journal of Human Pharmacology and Drug. Therapy. 2020;40(5):484–486. doi: 10.1002/phar.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun. Rev. 2020;102523 doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maghbooli Z., Sahraian M.A., Ebrahimi M., Pazoki M., Kafan S., Tabriz H.M., Hadadi A., Montazeri M., Nasiri M., Shirvani A. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE. 2020;15(9) doi: 10.1371/journal.pone.0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Hernández J.L., Nan D., Fernandez-Ayala M., García-Unzueta M., Hernández-Hernández M.A., López-Hoyos M., Muñoz-Cacho P., Olmos J.M., Gutiérrez-Cuadra M., Ruiz-Cubillán J.J. Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection. The Journal of Clinical Endocrinology & Metabolism. 2020 doi: 10.1210/clinem/dgaa733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.M. Pereira, A. Dantas Damascena, L.M. Galvão Azevedo, T. de Almeida Oliveira, J. da Mota Santana, Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis, Critical reviews in food science and nutrition (2020) 1-9. [DOI] [PubMed]
- 23.Liu N., Sun J., Wang X., Zhang T., Zhao M., Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: A systematic review and meta-analysis. International Journal of Infectious Diseases. 2021 doi: 10.1016/j.ijid.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah K., Saxena D., Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM: An International Journal of Medicine. 2021 doi: 10.1093/qjmed/hcab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A.A. Teshome A, Girma B and, M. ZA, The Impact of Vitamin D Level on COVID-19 Infection: Systematic Review and Meta-Analysis, Frontiers in Public Health 9 (2021). [DOI] [PMC free article] [PubMed]
- 26.McCartney D.M., Byrne D. Optimisation of vitamin D status for enhanced Immuno-protection against Covid-19. Ir Med J. 2020;113(4):58. [PubMed] [Google Scholar]
- 27.Panarese A., Shahini E. Covid-19, and vitamin D. Aliment. Pharmacol. Ther. 2020;51(10):993. doi: 10.1111/apt.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teymoori-Rad M., Marashi S.M. Vitamin D and Covid-19: From potential therapeutic effects to unanswered questions. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2159. [DOI] [PubMed] [Google Scholar]
- 30.C. Annweiler, B. Hanotte, C.G. de l’Eprevier, J.-M. Sabatier, L. Lafaie, T. Célarier, Vitamin D and survival in COVID-19 patients: A quasi-experimental study, The Journal of Steroid Biochemistry and Molecular Biology 204 (2020) 105771. [DOI] [PMC free article] [PubMed]
- 31.Annweiler G., Corvaisier M., Gautier J., Dubée V., Legrand E., Sacco G., Annweiler C. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12(11):3377. doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling S.F., Broad E., Murphy R., Pappachan J.M., Pardesi-Newton S., Kong M.-F., Jude E.B. High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study. Nutrients. 2020;12(12):3799. doi: 10.3390/nu12123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benskin L.L. A basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency. Front. Public Health. 2020;8:513. doi: 10.3389/fpubh.2020.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. Journal of infection and public health. 2020 doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bae M., Kim H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules. 2020;25(22):5346. doi: 10.3390/molecules25225346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lofgren E., Fefferman N.H., Naumov Y.N., Gorski J., Naumova E.N. Influenza seasonality: underlying causes and modeling theories. J Virol. 2007;81(11):5429–5436. doi: 10.1128/JVI.01680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.du Prel J.B., Puppe W., Grondahl B., Knuf M., Weigl J.A., Schaaff F., Schmitt H.J. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis. 2009;49(6):861–868. doi: 10.1086/605435. [DOI] [PubMed] [Google Scholar]
- 38.Shaw Stewart P.D. Seasonality and selective trends in viral acute respiratory tract infections. Med Hypotheses. 2016;86:104–119. doi: 10.1016/j.mehy.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drexler J.F., Corman V.M., Drosten C. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res. 2014;101:45–56. doi: 10.1016/j.antiviral.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Killerby M.E., Biggs H.M., Haynes A., Dahl R.M., Mustaquim D., Gerber S.I., Watson J.T. Human coronavirus circulation in the United States 2014–2017. J Clin Virol. 2018;101:52–56. doi: 10.1016/j.jcv.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price R.H.M., Graham C., Ramalingam S. Association between viral seasonality and meteorological factors. Sci Rep. 2019;9(1):929. doi: 10.1038/s41598-018-37481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of Respiratory Viral Infections. Annu Rev Virol. 2020;7(1):83–101. doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- 43.Hirve S., Newman L.P., Paget J., Azziz-Baumgartner E., Fitzner J., Bhat N., Vandemaele K., Zhang W. Influenza Seasonality in the Tropics and Subtropics - When to Vaccinate? PLoS ONE. 2016;11(4) doi: 10.1371/journal.pone.0153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowen A.C., Mubareka S., Steel J., Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3(10):1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y., Wang X., Nair H. Global seasonality of human seasonal coronaviruses: a clue for postpandemic circulating season of severe acute respiratory syndrome coronavirus 2? J. Infect. Dis. 2020;222(7):1090–1097. doi: 10.1093/infdis/jiaa436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kudo E., Song E., Yockey L.J., Rakib T., Wong P.W., Homer R.J., Iwasaki A. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc Natl Acad Sci U S A. 2019;116(22):10905–10910. doi: 10.1073/pnas.1902840116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamma Carleton, K.C. Meng, Causal empirical estimates suggest COVID-19 transmission rates are highly seasonal, Cold Spring Harbor Laboratory (CSHL), Yale University, United States, 2020.
- 48.Sajadi M.M., Habibzadeh P., Vintzileos A., Shokouhi S., Miralles-Wilhelm F., Amoroso A. Temperature, Humidity, and Latitude Analysis to Estimate Potential Spread and Seasonality of Coronavirus Disease 2019 (COVID-19) JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO, COVID-19 CORONAVIRUS PANDEMIC, https://www.worldometers.info/coronavirus/, 2020.
- 50.Hope-Simpson R.E. The role of season in the epidemiology of influenza. J Hyg (Lond) 1981;86(1):35–47. doi: 10.1017/s0022172400068728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cannell J.J., Vieth R., Umhau J.C., Holick M.F., Grant W.B., Madronich S., Garland C.F., Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahota O. Understanding vitamin D deficiency. Age Ageing. 2014;43(5):589. doi: 10.1093/ageing/afu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ianevski A., Zusinaite E., Shtaida N., Kallio-Kokko H., Valkonen M., Kantele A., Telling K., Lutsar I., Letjuka P., Metelitsa N. Low temperature and low UV indexes correlated with peaks of influenza virus activity in Northern Europe during 2010–2018. Viruses. 2019;11(3):207. doi: 10.3390/v11030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 55.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.N. Vabret, G.J. Britton, C. Gruber, S. Hegde, J. Kim, M. Kuksin, R. Levantovsky, L. Malle, A. Moreira, M.D. Park, L. Pia, E. Risson, M. Saffern, B. Salome, M. Esai Selvan, M.P. Spindler, J. Tan, V. van der Heide, J.K. Gregory, K. Alexandropoulos, N. Bhardwaj, B.D. Brown, B. Greenbaum, Z.H. Gumus, D. Homann, A. Horowitz, A.O. Kamphorst, M.A. Curotto de Lafaille, S. Mehandru, M. Merad, R.M. Samstein, P. Sinai Immunology Review, Immunology of COVID-19: Current State of the Science, Immunity 52(6) (2020) 910-941. [DOI] [PMC free article] [PubMed]
- 57.Alam M.S., Alam M.Z., Nazir K., Bhuiyan M.A.B. The emergence of novel coronavirus disease (COVID-19) in Bangladesh: Present status, challenges, and future management. J Adv Vet Anim Res. 2020;7(2):198–208. doi: 10.5455/javar.2020.g410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu S., Ren S., Xu Y., Lai J., Hu J., Lu J., Huang M., Ma X., Chen J., Hu S. China legislates against violence to medical workers. Lancet Psychiatry. 2020;7(3) doi: 10.1016/S2215-0366(20)30005-5. [DOI] [PubMed] [Google Scholar]
- 60.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.I. Huang, R. Pranata, M.A. Lim, A. Oehadian, B. Alisjahbana, C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis, Ther Adv Respir Dis 14 (2020) 1753466620937175. [DOI] [PMC free article] [PubMed]
- 65.N. Zhu, D. Zhang, W. Wang, X. Li, B. Yang, J. Song, X. Zhao, B. Huang, W. Shi, R. Lu, P. Niu, F. Zhan, X. Ma, D. Wang, W. Xu, G. Wu, G.F. Gao, W. Tan, I. China Novel Coronavirus, T. Research, A Novel Coronavirus from Patients with Pneumonia in China, 2019, N Engl J Med 382(8) (2020) 727-733. [DOI] [PMC free article] [PubMed]
- 66.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang B., Zhou X., Qiu Y., Song Y., Feng F., Feng J., Song Q., Jia Q., Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE. 2020;15(7) doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M., Xiao S.Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2(1):33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gralinski L.E., Baric R.S. Molecular pathology of emerging coronavirus infections. J Pathol. 2015;235(2):185–195. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verma G. Fundamentals of histology, New Age. International. 2001 [Google Scholar]
- 75.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M., Galli M., Catena E., Tosoni A., Gianatti A., Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans, Lancet. Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y., Yi F., Yang H.C., Fogo A.B., Nie X., Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao X.H., He Z.C., Li T.Y., Zhang H.R., Wang Y., Mou H., Guo Q., Yu S.C., Ding Y., Liu X., Ping Y.F., Bian X.W. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020;30(6):541–543. doi: 10.1038/s41422-020-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.S. Council, The state council of the people’s republic of China. http:// english.www.gov.cn/ [Accessed 26 Jul 2020]. 2020.
- 80.Cheng Z.J., Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48(2):155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan. China, Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan M., Liu Y., Zhou R., Deng X., Li F., Liang K., Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou. China, Immunology. 2020;160(3):261–268. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., Alanio C., Kuri-Cervantes L., Pampena M.B., D’Andrea K. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508) doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee P.-I., Hu Y.-L., Chen P.-Y., Huang Y.-C., Hsueh P.-R. Are children less susceptible to COVID-19? J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sassi F., Tamone C., D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11):1656. doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holick M.F., Chen T.C. Vitamin D deficiency: a worldwide problem with health consequences. The American journal of clinical nutrition. 2008;87(4):1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 88.L.Y. Matsuoka, L. IDE, J. WORTSMAN, J.A. MACLAUGHLIN, M.F. HOLICK, Sunscreens suppress cutaneous vitamin D3 synthesis, The journal of clinical endocrinology & metabolism 64(6) (1987) 1165-1168. [DOI] [PubMed]
- 89.Matsuoka L.Y., Wortsman J., Dannenberg M.J., Hollis B.W., Lu Z., Holick M.F. Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. The Journal of Clinical Endocrinology & Metabolism. 1992;75(4):1099–1103. doi: 10.1210/jcem.75.4.1328275. [DOI] [PubMed] [Google Scholar]
- 90.Christakos S., Hewison M., Gardner D.G., Wagner C.L., Sergeev I.N., Rutten E., Pittas A.G., Boland R., Ferrucci L., Bikle D.D. Vitamin D: beyond bone. Ann. N. Y. Acad. Sci. 2013;1287(1):45. doi: 10.1111/nyas.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol. Cell. Endocrinol. 2010;321(2):103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haussler M.R., Whitfield G.K., Haussler C.A., Hsieh J.C., Thompson P.D., Selznick S.H., Dominguez C.E., Jurutka P.W. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998;13(3):325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 93.Constans J. Group-specific component is not only a vitamin-D-binding protein. Exp. Clin. Immunogenet. 1992;9(3):161–175. [PubMed] [Google Scholar]
- 94.Karcioglu Batur L., Hekim N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J. Med. Virol. 2021;93(3):1409–1413. doi: 10.1002/jmv.26409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bouillon R., Allewaert K., Xiang D.Z., Tan B.K., Van Baelen H. Vitamin D analogs with low affinity for the vitamin D binding protein: enhanced in vitro and decreased in vivo activity. J. Bone Miner. Res. 1991;6(10):1051–1057. doi: 10.1002/jbmr.5650061006. [DOI] [PubMed] [Google Scholar]
- 96.Berg J.P. Vitamin D-binding protein prevents vitamin D deficiency and presents vitamin D for its renal activation. Eur J Endocrinol. 1999;141(4):321–322. doi: 10.1530/eje.0.1410321. [DOI] [PubMed] [Google Scholar]
- 97.Liu X., Baylin A., Levy P.D. Vitamin D deficiency and insufficiency among US adults: prevalence, predictors and clinical implications. Br. J. Nutr. 2018;119(8):928–936. doi: 10.1017/S0007114518000491. [DOI] [PubMed] [Google Scholar]
- 98.Aloia J., Mikhail M., Dhaliwal R., Shieh A., Usera G., Stolberg A., Ragolia L., Islam S. Free 25 (OH) D and the vitamin D paradox in African Americans. The Journal of Clinical Endocrinology & Metabolism. 2015;100(9):3356–3363. doi: 10.1210/JC.2015-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaufman H.W., Niles J.K., Kroll M.H., Bi C., Holick M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE. 2020;15(9) doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blanton D., Han Z., Bierschenk L., Linga-Reddy M.P., Wang H., Clare-Salzler M., Haller M., Schatz D., Myhr C., She J.-X. Reduced serum vitamin D–binding protein levels are associated with Type 1 diabetes. Diabetes. 2011;60(10):2566–2570. doi: 10.2337/db11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stokes C.S., Volmer D.A., Grünhage F., Lammert F. Vitamin D in chronic liver disease. Liver International. 2013;33(3):338–352. doi: 10.1111/liv.12106. [DOI] [PubMed] [Google Scholar]
- 102.Denburg M.R., Kalkwarf H.J., de Boer I.H., Hewison M., Shults J., Zemel B.S., Stokes D., Foerster D., Laskin B., Ramirez A. Vitamin D bioavailability and catabolism in pediatric chronic kidney disease. Pediatric nephrology. 2013;28(9):1843–1853. doi: 10.1007/s00467-013-2493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waldron J.L., Ashby H.L., Cornes M.P., Bechervaise J., Razavi C., Thomas O.L., Chugh S., Deshpande S., Ford C., Gama R. Vitamin D: a negative acute phase reactant. J. Clin. Pathol. 2013;66(7):620–622. doi: 10.1136/jclinpath-2012-201301. [DOI] [PubMed] [Google Scholar]
- 104.Møller U., Streym S., Heickendorff L., Mosekilde L., Rejnmark L. Effects of 25OHD concentrations on chances of pregnancy and pregnancy outcomes: a cohort study in healthy Danish women. Eur. J. Clin. Nutr. 2012;66(7):862–868. doi: 10.1038/ejcn.2012.18. [DOI] [PubMed] [Google Scholar]
- 105.Møller U.K., Streym S.V., Jensen L.T., Mosekilde L., Schoenmakers I., Nigdikar S., Rejnmark L. Increased plasma concentrations of vitamin D metabolites and vitamin D binding protein in women using hormonal contraceptives: a cross-sectional study. Nutrients. 2013;5(9):3470–3480. doi: 10.3390/nu5093470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., Rosser E.C., Webb K., Deakin C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11(1):1–10. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haddad J.G. Plasma vitamin D-binding protein (Gc-globulin): multiple tasks. The Journal of steroid biochemistry and molecular biology. 1995;53(1–6):579–582. doi: 10.1016/0960-0760(95)00104-8. [DOI] [PubMed] [Google Scholar]
- 108.Maestro M.A., Molnár F., Mouriño A., Carlberg C. Vitamin D receptor 2016: novel ligands and structural insights. Expert Opin. Ther. Pat. 2016;26(11):1291–1306. doi: 10.1080/13543776.2016.1216547. [DOI] [PubMed] [Google Scholar]
- 109.Battault S., Whiting S., Peltier S., Sadrin S., Gerber G., Maixent J. Vitamin D metabolism, functions and needs: from science to health claims. Eur. J. Nutr. 2013;52(2):429–441. doi: 10.1007/s00394-012-0430-5. [DOI] [PubMed] [Google Scholar]
- 110.Adams J.S., Rafison B., Witzel S., Reyes R.E., Shieh A., Chun R., Zavala K., Hewison M., Liu P.T. Regulation of the extrarenal CYP27B1-hydroxylase. The Journal of steroid biochemistry and molecular biology. 2014;144:22–27. doi: 10.1016/j.jsbmb.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Charoenngam N., Holick M.F. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(7):2097. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., Ochoa M.T., Schauber J., Wu K., Meinken C. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 113.A.R. Martineau, D.A. Jolliffe, R.L. Hooper, L. Greenberg, J.F. Aloia, P. Bergman, G. Dubnov-Raz, S. Esposito, D. Ganmaa, A.A. Ginde, Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, bmj 356 (2017). [DOI] [PMC free article] [PubMed]
- 114.Martineau A.R., Jolliffe D.A., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol. Assess. 2019;23(2):1–44. doi: 10.3310/hta23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sabetta J.R., DePetrillo P., Cipriani R.J., Smardin J., Burns L.A., Landry M.L. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS ONE. 2010;5(6) doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Urashima M., Segawa T., Okazaki M., Kurihara M., Wada Y., Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. The American journal of clinical nutrition. 2010;91(5):1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 117.Ingham T.R., Jones B., Camargo C.A., Kirman J., Dowell A.C., Crane J., Stanley T.V., Grimwood K., W.T.R.S. Group Association of vitamin D deficiency with severity of acute respiratory infection: A case-control study in New Zealand children. Eur. Respir. J. 2014;44(Suppl 58) [Google Scholar]
- 118.Zhou Y.-F., Luo B.-A., Qin L.-L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine. 2019;98(38) doi: 10.1097/MD.0000000000017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Engelsen O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients. 2010;2(5):482–495. doi: 10.3390/nu2050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rhodes J.M., Subramanian S., Laird E., Kenny R.A. low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020;51(12):1434–1437. doi: 10.1111/apt.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Whittemore P.B. COVID-19 fatalities, latitude, sunlight, and vitamin D. Am. J. Infect. Control. 2020;48(9):1042–1044. doi: 10.1016/j.ajic.2020.06.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.P.C. Ilie, S. Stefanescu, L. Smith, The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality, Aging Clinical and Experimental Research (2020) 1-4. [DOI] [PMC free article] [PubMed]
- 123.D’Avolio A., Avataneo V., Manca A., Cusato J., De Nicolò A., Lucchini R., Keller F., Cantù M. 25-hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smolders J., van den Ouweland J., Geven C., Pickkers P., Kox M. Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metabolism-Clinical and Experimental. 2020 doi: 10.1016/j.metabol.2020.154434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I., Frenkel-Morgenstern M. Low plasma 25 (OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. The FEBS journal. 2020;287(17):3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.D.O. Meltzer, T.J. Best, H. Zhang, T. Vokes, V. Arora, J. Solway, Association of vitamin D status and other clinical characteristics with COVID-19 test results, JAMA network open 3(9) (2020) e2019722-e2019722. [DOI] [PMC free article] [PubMed]
- 127.Radujkovic A., Hippchen T., Tiwari-Heckler S., Dreher S., Boxberger M., Merle U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients. 2020;12(9):2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rastogi A., Bhansali A., Khare N., Suri V., Yaddanapudi N., Sachdeva N., Puri G., Malhotra P. SHADE study); Postgraduate medical journal: 2020. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study. [DOI] [PubMed] [Google Scholar]
- 129.Castillo M.E., Costa L.M.E., Barrios J.M.V., Díaz J.F.A., Miranda J.L., Bouillon R., Gomez J.M.Q. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. The Journal of steroid biochemistry and molecular biology. 2020;203 doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baktash V., Hosack T., Patel N., Shah S., Kandiah P., Van Den Abbeele K., Mandal A.K., Missouris C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2020 doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jain A., Chaurasia R., Sengar N.S., Singh M., Mahor S., Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S., Silva C.B., Franco A.S., Macedo M.B., Dalmolin H.H. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe covid-19: a randomized clinical trial. JAMA. 2021 doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 134.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. The Journal of Clinical Endocrinology & Metabolism. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bordelon P., Ghetu M.V., Langan R.C. Recognition and management of vitamin D deficiency. Am. Fam. Physician. 2009;80(8):841–846. [PubMed] [Google Scholar]
- 136.Dawson-Hughes B., Mithal A., Bonjour J.-P., Boonen S., Burckhardt P., Fuleihan G.-H., Josse R., Lips P., Morales-Torres J., Yoshimura N. IOF position statement: vitamin D recommendations for older adults. Osteoporos. Int. 2010;21(7):1151–1154. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 137.Shirvani A., Kalajian T.A., Song A., Holick M.F. Disassociation of Vitamin D’s calcemic Activity and non-calcemic Genomic Activity and individual Responsiveness: A Randomized controlled Double-Blind clinical trial. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-53864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Niculescu D.A., Deacu L.G., Caragheorgheopol A., Dusceac R., Procopiuc C., Petris R., Poiana C. Seasonal periodicity of serum parathyroid hormone and its relation with vitamin D in Romania. Archives of osteoporosis. 2020;15(1):1–8. doi: 10.1007/s11657-020-00744-1. [DOI] [PubMed] [Google Scholar]
- 139.Veugelers P.J., Ekwaru J.P. A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients. 2014;6(10):4472–4475. doi: 10.3390/nu6104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.R. Heaney, C. Garland, C. Baggerly, C. French, E. Gorham, Letter to Veugelers, PJ and Ekwaru, JP, A Statistical Error in the Estimation of the Recommended Dietary Allowance for Vitamin D. Nutrients 2014, 6, 4472–4475, Nutrients 7(3) (2015) 1688-1690. [DOI] [PMC free article] [PubMed]
- 141.Papadimitriou D.T. The big vitamin D mistake. Journal of Preventive Medicine and Public Health. 2017;50(4):278. doi: 10.3961/jpmph.16.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gruber-Bzura B.M. Vitamin D and influenza—prevention or therapy? Int. J. Mol. Sci. 2018;19(8):2419. doi: 10.3390/ijms19082419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aglipay M., Birken C.S., Parkin P.C., Loeb M.B., Thorpe K., Chen Y., Laupacis A., Mamdani M., Macarthur C., Hoch J.S. Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. 2017;318(3):245–254. doi: 10.1001/jama.2017.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Campbell G.R., Spector S.A. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012;8(10):1523–1525. doi: 10.4161/auto.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Laplana M., Royo J.L., Fibla J. Vitamin D Receptor polymorphisms and risk of enveloped virus infection: A meta-analysis. Gene. 2018;678:384–394. doi: 10.1016/j.gene.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 146.van Etten E., Mathieu C. Immunoregulation by 1, 25-dihydroxyvitamin D3: basic concepts. The Journal of steroid biochemistry and molecular biology. 2005;97(1–2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 147.Bilezikian J.P., Bikle D., Hewison M., Lazaretti-Castro M., Formenti A.M., Gupta A., Madhavan M.V., Nair N., Babalyan V., Hutchings N. Mechanisms in endocrinology: vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183(5):R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rondanelli M., Miccono A., Lamburghini S., Avanzato I., Riva A., Allegrini P., Faliva M.A., Peroni G., Nichetti M., Perna S. Self-care for common colds: the pivotal role of vitamin D, vitamin C, zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds—practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evidence-Based Complementary and Alternative Medicine. 2018;2018 doi: 10.1155/2018/5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schwalfenberg G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011;55(1):96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 150.M. Hewison, Vitamin D and the immune system: new perspectives on an old theme, Endocrinol Metab Clin North Am 39(2) (2010) 365-79, table of contents. [DOI] [PMC free article] [PubMed]
- 151.Prietl B., Treiber G., Pieber T.R., Amrein K. Vitamin D and immune function. Nutrients. 2013;5(7):2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 153.Aranow C. Vitamin D and the immune system. J. Invest. Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tripathi S., Tecle T., Verma A., Crouch E., White M., Hartshorn K.L. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. The Journal of general virology. 2013;94(Pt 1):40. doi: 10.1099/vir.0.045013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shahmiri M., Enciso M., Adda C.G., Smith B.J., Perugini M.A., Mechler A. Membrane core-specific antimicrobial action of cathelicidin LL-37 peptide switches between pore and nanofibre formation. Sci. Rep. 2016;6:38184. doi: 10.1038/srep38184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barlow P.G., Svoboda P., Mackellar A., Nash A.A., York I.A., Pohl J., Davidson D.J., Donis R.O. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sousa F.H., Casanova V., Findlay F., Stevens C., Svoboda P., Pohl J., Proudfoot L., Barlow P.G. Cathelicidins display conserved direct antiviral activity towards rhinovirus. Peptides. 2017;95:76–83. doi: 10.1016/j.peptides.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao Y., Ran Z., Jiang Q., Hu N., Yu B., Zhu L., Shen L., Zhang S., Chen L., Chen H. Vitamin D Alleviates Rotavirus Infection through a Microrna-155-5p Mediated Regulation of the TBK1/IRF3 Signaling Pathway In Vivo and In Vitro. Int. J. Mol. Sci. 2019;20(14):3562. doi: 10.3390/ijms20143562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martínez-Moreno J., Hernandez J.C., Urcuqui-Inchima S. Effect of high doses of vitamin D supplementation on dengue virus replication, Toll-like receptor expression, and cytokine profiles on dendritic cells. Mol. Cell. Biochem. 2020;464(1–2):169–180. doi: 10.1007/s11010-019-03658-w. [DOI] [PubMed] [Google Scholar]
- 160.Dancer R.C., Parekh D., Lax S., D'Souza V., Zheng S., Bassford C.R., Park D., Bartis D., Mahida R., Turner A.M. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70(7):617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cantorna M.T. Mechanisms underlying the effect of vitamin D on the immune system. Proc. Nutr. Soc. 2010;69(3):286–289. doi: 10.1017/S0029665110001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bhalla A.K., Amento E.P., Krane S.M. Differential effects of 1, 25-dihydroxyvitamin D3 on human lymphocytes and monocyte/macrophages: inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell. Immunol. 1986;98(2):311–322. doi: 10.1016/0008-8749(86)90291-1. [DOI] [PubMed] [Google Scholar]
- 163.Chun R.F., Liu P.T., Modlin R.L., Adams J.S., Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front. Physiol. 2014;5:151. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Carvalho J.T.G., Schneider M., Cuppari L., Grabulosa C.C., Aoike D.T., Redublo B.M.Q., Batista M.C., Cendoroglo M., Maria Moyses R., Dalboni M.A. Cholecalciferol decreases inflammation and improves vitamin D regulatory enzymes in lymphocytes in the uremic environment: A randomized controlled pilot trial. PLoS ONE. 2017;12(6) doi: 10.1371/journal.pone.0179540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xie Z., Chen J., Zheng C., Wu J., Cheng Y., Zhu S., Lin C., Cao Q., Zhu J., Jin T. 1, 25-dihydroxyvitamin D3-induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells. Immunology. 2017;152(3):414–424. doi: 10.1111/imm.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hewison M. Elsevier; Vitamins & hormones: 2011. Vitamin D and innate and adaptive immunity; pp. 23–62. [DOI] [PubMed] [Google Scholar]
- 167.Daneshkhah A., Agrawal V., Eshein A., Subramanian H., Roy H.K., Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clinical and Experimental Research. 2020;32(10):2141–2158. doi: 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mattner F., Smiroldo S., Galbiati F., Muller M., Di Lucia P., Poliani P.L., Martino G., Panina-Bordignon P., Adorini L. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1, 25-dihydroxyvitamin D3. Eur. J. Immunol. 2000;30(2):498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 169.Boonstra A., Barrat F.J., Crain C., Heath V.L., Savelkoul H.F., O’Garra A. 1α, 25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J. Immunol. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 170.McGregor R., Chauss D., Freiwald T., Yan B., Wang L., Nova-Lamperti E., Zhang Z., Teague H., West E.E., Bibby J. An autocrine Vitamin D-driven Th1 shutdown program can be exploited for COVID-19. BioRxiv. 2020 [Google Scholar]
- 171.Panichi V., De Pietro S., Andreini B., Bianchi A.M., Migliori M., Taccola D., Giovannini L., Tetta C., Palla R. Calcitriol modulates in vivo and in vitro cytokine production: a role for intracellular calcium. Kidney Int. 1998;54(5):1463–1469. doi: 10.1046/j.1523-1755.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 172.D'Ambrosio D., Cippitelli M., Cocciolo M.G., Mazzeo D., Di Lucia P., Lang R., Sinigaglia F., Panina-Bordignon P. Inhibition of IL-12 production by 1, 25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J. Clin. Investig. 1998;101(1):252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Penna G., Adorini L. 1α, 25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000;164(5):2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 174.Daniel C., Sartory N.A., Zahn N., Radeke H.H., Stein J.M. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 175.Penna G., Amuchastegui S., Cossetti C., Aquilano F., Mariani R., Sanvito F., Doglioni C., Adorini L. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J. Immunol. 2006;177(12):8504–8511. doi: 10.4049/jimmunol.177.12.8504. [DOI] [PubMed] [Google Scholar]
- 176.Tang J., Zhou R., Luger D., Zhu W., Silver P.B., Grajewski R.S., Su S.-B., Chan C.-C., Adorini L., Caspi R.R. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J. Immunol. 2009;182(8):4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Barrat F.J., Cua D.J., Boonstra A., Richards D.F., Crain C., Savelkoul H.F., de Waal-Malefyt R., Coffman R.L., Hawrylowicz C.M., O'Garra A. In vitro generation of interleukin 10–producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)–and Th2-inducing cytokines. J. Exp. Med. 2002;195(5):603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gorman S., Kuritzky L.A., Judge M.A., Dixon K.M., McGlade J.P., Mason R.S., Finlay-Jones J.J., Hart P.H. Topically applied 1, 25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+ CD25+ cells in the draining lymph nodes. J. Immunol. 2007;179(9):6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 179.Penna G., Roncari A., Amuchastegui S., Daniel K.C., Berti E., Colonna M., Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+ Foxp3+ regulatory T cells by 1, 25-dihydroxyvitamin D3. Blood. 2005;106(10):3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 180.Jeffery L.E., Burke F., Mura M., Zheng Y., Qureshi O.S., Hewison M., Walker L.S., Lammas D.A., Raza K., Sansom D.M. 1, 25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009;183(9):5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moan J., Porojnicu A.C., Dahlback A., Setlow R.B. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc. Natl. Acad. Sci. 2008;105(2):668–673. doi: 10.1073/pnas.0710615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wortsman J., Matsuoka L.Y., Chen T.C., Lu Z., Holick M.F. Decreased bioavailability of vitamin D in obesity. The American journal of clinical nutrition. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 183.MacLaughlin J., Holick M.F. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Investig. 1985;76(4):1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Williams S., Malatesta K., Norris K. Vitamin D and chronic kidney disease. Ethn. Dis. 2009;19(4 Suppl 5):S5. [PMC free article] [PubMed] [Google Scholar]
- 185.Khajavi A., Amirhakimi G. The rachoitic lung: pulmonary findings in 30 infants and children with malnutritional rickets. Clin. Pediatr. 1977;16(1):36–38. doi: 10.1177/000992287701600106. [DOI] [PubMed] [Google Scholar]
- 186.Kearns M.D., Tangpricha V. The role of vitamin D in tuberculosis. Journal of clinical & translational endocrinology. 2014;1(4):167. doi: 10.1016/j.jcte.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brenner H., Holleczek B., Schöttker B. Vitamin D insufficiency and deficiency and mortality from respiratory diseases in a cohort of older adults: potential for limiting the death toll during and beyond the COVID-19 pandemic? Nutrients. 2020;12(8):2488. doi: 10.3390/nu12082488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Goncalves-Mendes N., Talvas J., Dualé C., Guttmann A., Corbin V., Marceau G., Sapin V., Brachet P., Evrard B., Laurichesse H. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front. Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.White J.H. Vitamin D metabolism and signaling in the immune system. Reviews in endocrine and metabolic disorders. 2012;13(1):21–29. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 190.Wang T.-T., Nestel F.P., Bourdeau V., Nagai Y., Wang Q., Liao J., Tavera-Mendoza L., Lin R., Hanrahan J.W., Mader S. Cutting edge: 1, 25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 191.Charan J., Goyal J.P., Saxena D., Yadav P. Vitamin D for prevention of respiratory tract infections: A systematic review and meta-analysis. Journal of pharmacology & pharmacotherapeutics. 2012;3(4):300. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bergman P., Lindh Å.U., Björkhem-Bergman L., Lindh J.D. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grant W.B., Giovannucci E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918–1919 influenza pandemic in the United States. Dermato-endocrinology. 2009;1(4):215–219. doi: 10.4161/derm.1.4.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Laird E., McNulty H., Ward M., Hoey L., McSorley E., Wallace J., Carson E., Molloy A., Healy M., Casey M. Vitamin D deficiency is associated with inflammation in older Irish adults. The Journal of Clinical Endocrinology & Metabolism. 2014;99(5):1807–1815. doi: 10.1210/jc.2013-3507. [DOI] [PubMed] [Google Scholar]
- 195.Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7(6):4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zdrenghea M.T., Makrinioti H., Bagacean C., Bush A., Johnston S.L., Stanciu L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017;27(1) doi: 10.1002/rmv.1909. [DOI] [PubMed] [Google Scholar]
- 197.de Souza W.N., Norde M.M., Oki É., Rogero M.M., Marchioni D.M., Fisberg R.M., Martini L.A. Association between 25-hydroxyvitamin D and inflammatory biomarker levels in a cross-sectional population-based study. São Paulo, Brazil, Nutrition Research. 2016;36(1):1–8. doi: 10.1016/j.nutres.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 198.Ragab D., Soliman D., Samaha D., Yassin A. Vitamin D status and its modulatory effect on interferon gamma and interleukin-10 production by peripheral blood mononuclear cells in culture. Cytokine. 2016;85:5–10. doi: 10.1016/j.cyto.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 199.Rhodes J.M., Subramanian S., Laird E., Griffin G., Kenny R.A. Perspective: Vitamin D deficiency and COVID-19 severity–plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J. Intern. Med. 2021;289(1):97–115. doi: 10.1111/joim.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Malek Mahdavi A. A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: Implications for a potential treatment for COVID-19. Rev. Med. Virol. 2020;30(5) doi: 10.1002/rmv.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Blondon M., Biver E., Braillard O., Righini M., Fontana P., Casini A. Thrombin generation and fibrin clot structure after vitamin D supplementation. Endocrine Connections. 2019;8(11):1447–1454. doi: 10.1530/EC-19-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wu C.-C., Chang J.-H., Chen C.-C., Su S.-B., Yang L.-K., Ma W.-Y., Zheng C.-M., Diang L.-K., Lu K.-C. Calcitriol treatment attenuates inflammation and oxidative stress in hemodialysis patients with secondary hyperparathyroidism. The Tohoku journal of experimental medicine. 2011;223(3):153–159. doi: 10.1620/tjem.223.153. [DOI] [PubMed] [Google Scholar]
- 204.Marcinowska-Suchowierska E., Kupisz-Urbańska M., Łukaszkiewicz J., Płudowski P., Jones G. Vitamin D toxicity–a clinical perspective. Front. Endocrinol. 2018;9:550. doi: 10.3389/fendo.2018.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jacobus C.H., Holick M.F., Shao Q., Chen T.C., Holm I.A., Kolodny J.M., Fuleihan G.E.-H., Seely E.W. Hypervitaminosis D associated with drinking milk. N. Engl. J. Med. 1992;326(18):1173–1177. doi: 10.1056/NEJM199204303261801. [DOI] [PubMed] [Google Scholar]
- 206.Del Valle H.B., Yaktine A.L., Taylor C.L., Ross A.C. National Academies Press; 2011. Dietary reference intakes for calcium and vitamin D. [PubMed] [Google Scholar]
- 207.Özkan B., Hatun S., Bereket A. Vitamin D intoxication. Turk. J. Pediatr. 2012;54(2):93. [PubMed] [Google Scholar]
- 208.Klontz K.C., Acheson D.W. Dietary supplement–induced vitamin D intoxication. N. Engl. J. Med. 2007;357(3):308–309. doi: 10.1056/NEJMc063341. [DOI] [PubMed] [Google Scholar]
- 209.Araki T., Holick M.F., Alfonso B.D., Charlap E., Romero C.M., Rizk D., Newman L.G. Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. The Journal of Clinical Endocrinology & Metabolism. 2011;96(12):3603–3608. doi: 10.1210/jc.2011-1443. [DOI] [PubMed] [Google Scholar]
- 210.McCullough P.J., Lehrer D.S., Amend J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. The Journal of steroid biochemistry and molecular biology. 2019;189:228–239. doi: 10.1016/j.jsbmb.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 211.Pietras S.M., Obayan B.K., Cai M.H., Holick M.F. Vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch. Intern. Med. 2009;169(19):1806–1818. doi: 10.1001/archinternmed.2009.361. [DOI] [PubMed] [Google Scholar]
- 212.Ekwaru J.P., Zwicker J.D., Holick M.F., Giovannucci E., Veugelers P.J. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dudenkov D.V., Yawn B.P., Oberhelman S.S., Fischer P.R., Singh R.J., Cha S.S., Maxson J.A., Quigg S.M., Thacher T.D. Changing incidence of serum 25-hydroxyvitamin D values above 50 ng/mL: a 10-year population-based study. Mayo Clinic Proceedings, Elsevier. 2015:577–586. doi: 10.1016/j.mayocp.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Holick M.F. Vitamin D is not as toxic as was once thought: a historical and an up-to-date perspective. Mayo Clinic Proceedings, Elsevier. 2015:561–564. doi: 10.1016/j.mayocp.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 215.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infection, genetics and evolution. 2020;81 doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]