Abstract
A cell-surface heparan proteoglycan called Syndecan-1 (SDC-1) has multiple roles in healthy and pathogenic conditions, including respiratory viral infection. In this study, we explore the dynamic alternation in the levels of SDC-1 in cases with COVID-19. A total of 120 cases definitely diagnosed with COVID-19 were admitted to the Firoozgar Hospital, Tehran, Iran, from December 1, 2020, to January 29, 2021, and included in our study. Also, 58 healthy subjects (HS) were chosen as the control group. Patients were classified into two groups: 1) ICU patients and (63 cases) 2) non-ICU patients (57 cases). The dynamic changes of serum SCD-1, CRP, IL-6, IL-10, IL-18, and Vit D levels a well as the disease activity were investigated in three-time points (T1-T3). Our results indicated that the COVID-19 patients had significantly increased SCD-1, CRP, IL-6, IL-10, and IL-18 levels than in HS, while the Vit D levels in COVID-19 patients were significantly lower than HS. Further analysis demonstrated that the SCD-1, CRP, IL-6, IL-10, and IL-18 levels in ICU patients were significantly higher than in non-ICU patients. Tracking dynamic changes in the above markers indicated that on the day of admission, the SCD-1, CRP, IL-6, IL-10, and IL-18 levels were gradually increased on day 5 (T2) and then gradually decreased on day 10 (T3). ROC curve analysis suggests that markers mentioned above, SDC-1, IL-6, and IL-18 are valuable indicators in evaluating the activity of COVID-19. All in all, it seems that the serum SDC-1 levels alone or combined with other markers might be a good candidate for disease activity monitoring.
Keywords: COVID-19, Syndecan-1, ICU patients, SARS-CoV-2, Inflammatory markers, Disease activity
1. Introduction
Type I transmembrane heparan sulfate proteoglycans are called syndecans (SDCs) that can communicate with many ligands, such as chemokines, adhesion receptors, proteinases, and cytokines [1]. After the interaction between SDCs and their ligands, SDCs launch several biological signaling experiences connected to inflammation, angiogenesis cell adhesion, and tissue restoration [2], [3], [4], [5], [6]. The human genome encodes four syndecans, including SDC-1,-2, -3, and -4. SDCs in normal conditions support cell homeostasis and manage inflammatory reactions throughout trauma and infection [7]. The recent finding from studies performed on animal models of various diseases has rendered definite evidence that SDC-1 plays a crucial function in promoting inflammatory conditions, tumors, and infectious disease. These SDC-1 functions are critical in the pathophysiology of infectious diseases provided by studies using animal and cell culture-based infection models. It was observed that the loss or decline of SDC-1 enables mice or cells to resist infection by several viral and bacterial pathogens significantly [8], [9], [10], [11]. The underlying molecular mechanisms have yet to be accurately determined. However, numerous investigations have exhibited impressive features of how both cell surface and shed SDC-1 can increase pathogenesis by different molecular mechanisms [12]. Recently, humanity experience a new destructive viral pandemic after the pandemic flu (N5N1) in 1918; a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused horrifying disease accompanied by high mortality, economic, and health burden on the world community [13], [14]. The disease caused by the SARS-CoV-2 was called COVID-19, which originated from Wuhan, Hubei province, China, and then expanded worldwide [15], [16], [17], [18]. In addition to acute respiratory failure, the SARS-CoV-2 can damage other organs, which progresses rapidly and eventually drives to multiple organ collapse and death [19], [20]. The early diagnosis of COVID-19 is problematic, given that some patients do not have any specific signs/symptoms, or specific radiological abnormalities, in the early stages. Therefore, some investigation highlights that the early stages' immediate identification in the early stages can be necessary to halt the disease's spread and establish an effective treatment plan [21], [22]. In the previous study, several inflammatory factors, including white blood cells (WBC), platelet (PLT), CRP, lymphocyte (L), serum amyloid A (SAA), and procalcitonin (PCT) have been employed in the clinic as inflammation indicators [23]. Also, recently the Fraser et al. [24] and Suzuki et al. [25] investigate the possible role of SDC-1 in COVID-19 pathogenesis. Hence, we aimed to validate previous work [24], [25], [26] with a larger patient cohort and with graded severity in the present investigation. In summary, in the present work, we explore the dynamic changes in the levels of selected factors, including SDC1, CRP, IL-6, IL-18, IL-10, and vitamin D in the serum of patients with COVID-19, to help estimate the disease severity and management of COVID-19 diseases.
2. Material and methods
A comparative cross-sectional study was intended to understand the dynamic changes of the SDC-1 alone or combined with some factors include IL-6, IL-10, IL-18, CRP, and Vit D associated with the severity of COVID-19 patients admitted to the Firouzgar Hospital, Tehran, Iran. In order to catch legal and ethical authority for collecting the specimens, informed consent was obtained from all individuals who engaged in this study. Additionally, this research was authorized by the ethics committee of Iran University of Medical Sciences (IUMS) (ECIUMS; IR.IUMS.FMD.REC.1399.624). A total number of 120 patients with COVID-19 admitted to the Firouzgar Hospital, IUMS were recruited in this investigation and classified into two groups according to the Li et al. criteria as follows: the first group comprised 63 cases with COVID-19 (severe patients hospitalized in ICU), the second group consisted of 57 patients with COVID-19 (moderate patients). Also, in this study, 58 healthy subjects enrolled as the control group. We three times 5 ml peripheral blood take and collected from each patient, and quickly following sample gathering, the serum was isolated by centrifugation and put at −70 °C up to use.
2.1. Laboratory validation and treatment
The real-time polymerase chain reaction was used to detect and confirm SARS-CoV-2 infection within three hours after sample collection (sputum and throat swab specimens). Serum biochemistry and blood count were done on the day of admission. According to the COVID-19 Diagnosis and Treatment Plan declared by the National Health Committee of Iran, patients underwent supportive oxygen treatment, antiviral prescription, and other supportive therapies.
2.2. ELISA for SDC-1, IL-16, IL-10, IL-18, and CRP
For determining the serum levels of SDC-1, IL-6, IL-10, IL-18 (Abcam, Cambridge, MA, USA), and CRPBOSTER BIOLOGICAL TECHNOLOGY, EK7040), we used enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer's instructions. We used a quantitative chemiluminescent immunometric assay for assessing Vit D levels in serum (DiaSorin, spA, Via Crescentino, Vercelli, Italy). All specimens were tested in duplicate, and the mean values of individual sera were employed for the statistical interpretation. The intra-assay and interassay coefficients of variation (CV) were b12.0% for all calculated agents [27].
2.3. Statistical methods
Continuous and categorical variables were displayed as median (IQR) and n (%), sequentially. We applied the Wilcoxon rank-sum test, χ2 test, or Fisher's exact test where appropriate to analyze differences among different groups. The relationship between laboratory tests was analyzed using the Pearson correlation coefficient. The Receiver Operating Characteristic curve (ROC curve) was employed to determine the area under the curve (AUC) of SDC-1, IL-6, IL-18, IL-10, CRP, and Vit D meant to assess the sensitivity and specificity of these markers. A two-sided α of less than 0·05 was regarded statistically significant. Statistical analyses were done using R version 4.0.3 (2020-10-10).
3. Results
3.1. Demographic characteristics
As presented in Tables 1-1 and 1-2 , all individuals, including ICU patients, non-ICU patients, and HS, were equivalent in terms of sex and age as there were no meaningful differences between them (P < 0.05). Also, Table 1-1, Table 1-2 has illustrated that the laboratory findings and some risk factors such as smoking, kidney failure, lung disease, and others. As shown in Table 2 , ICU patients' death rate had significantly higher than non- ICU patients (P < 0.05). Also, the death event was positively associated with older age (P < 0.001). Besides, in Table 2, we illustrated the difference among laboratory findings and some mentioned risk factors between ICU and non-ICU patients. As presented in Table 1-1, Table 1-2, the lactate dehydrogenase (LDH), hemoglobin (Hb), glutamic-oxaloacetic transaminase (SGOT), death rate, Kidney failure in ICU patients were significantly higher than in non-ICU patients (P < 0.05). As demonstrated in Table 2, further analysis showed that some parameters such as age, lung disease, and heart disease were significantly higher in dead patients than in alive patients (P < 0.05).
Table 1-1.
Demographic and clinical parameters of patients with COVID-19.
| Group |
|||||
|---|---|---|---|---|---|
| Variable | N | Overalla, N = 120 | ICUa, N = 63 | Non ICUa, N = 57 | p-valueb |
| Sex | 120 | 0.092 | |||
| Female | 56 (47%) | 34 (54%) | 22 (39%) | ||
| Male | 64 (53%) | 29 (46%) | 35 (61%) | ||
| Age | 120 | 57 (38, 70) | 61 (47, 74) | 52 (36, 68) | 0.050 |
| ESR | 120 | 44 (28, 56) | 43 (26, 56) | 45 (30, 56) | 0.9 |
| Platelet | 120 | 191 (145, 236) | 183 (150, 233) | 192 (142, 257) | 0.9 |
| M.C.H | 120 | 28.5 (27.1, 30.3) | 29.2 (27.6, 31.2) | 28.1 (26.8, 29.5) | 0.049 |
| M.C.V | 120 | 88 (85, 92) | 88 (85, 91) | 88 (84, 93) | > 0.9 |
| Hct | 120 | 40 (34, 44) | 40 (35, 43) | 40 (32, 44) | 0.4 |
| Hb | 120 | 12.75 (10.85, 14.40) | 13.00 (11.80, 14.40) | 12.30 (9.70, 13.60) | 0.042 |
| R.B.C | 120 | 4.42 (3.90, 4.94) | 4.48 (3.92, 4.94) | 4.34 (3.67, 4.94) | 0.2 |
| W.B.C | 120 | 6.1 (5.0, 9.2) | 5.9 (5.0, 9.1) | 6.3 (5.0, 9.7) | 0.7 |
| LDH | 120 | 504 (418, 675) | 617 (481, 694) | 436 (356, 523) | <0.001 |
| SGPT | 120 | 21 (15, 34) | 24 (16, 36) | 18 (15, 31) | 0.075 |
| SGOT | 120 | 25 (19, 44) | 30 (22, 46) | 23 (16, 30) | 0.017 |
| Cigarette | 120 | 0.6 | |||
| Negative | 101 (84%) | 52 (83%) | 49 (86%) | ||
| Positive | 19 (16%) | 11 (17%) | 8 (14%) | ||
| Opium | 120 | 0.048 | |||
| Negative | 116 (97%) | 63 (100%) | 53 (93%) | ||
| Positive | 4 (3.3%) | 0 (0%) | 4 (7.0%) | ||
| Alcoholic_drinks | 120 | >0.9 | |||
| Lung_disease | 120 | 0.4 | |||
| Negative | 115 (96%) | 60 (95%) | 55 (96%) | ||
| Positive | 5 (4.2%) | 3 (4.8%) | 2 (3.5%) | ||
| Kidney_failure | 120 | 0.044 | |||
| Negative | 101 (84%) | 49 (78%) | 52 (91%) | ||
| Positive | 19 (16%) | 14 (22%) | 5 (8.8%) | ||
| Dialysis | 120 | >0.9 | |||
| Negative | 110 (92%) | 58 (92%) | 52 (91%) | ||
| Positive | 10 (8.3%) | 5 (7.9%) | 5 (8.8%) | ||
| Heart_disease | 120 | >0.9 | |||
| Negative | 97 (81%) | 51 (81%) | 46 (81%) | ||
| Positive | 23 (19%) | 12 (19%) | 11 (19%) | ||
| Death | 120 | 0.001 | |||
| Negative | 110 (92%) | 53 (84%) | 57 (100%) | ||
| Positive | 10 (8.3%) | 10 (16%) | 0 (0%) | ||
| CT.value | 120 | 25 (24, 33) | 25 (24, 33) | 27 (23, 33) | 0.7 |
n (%); Median (IQR).
Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test.
Table 1-2.
The age and sex characteristics of COVID-19 cases and healthy subjects.
| Variable | N | Overall, N = 176a | Group |
P valueb | ||
|---|---|---|---|---|---|---|
| Healthy, N = 56a | ICU, N = 63a | Non ICU, N = 57a | ||||
| Sex | 176 | 0.045 | ||||
| Female | 74 (42%) | 18 (32%) | 34 (54%) | 22 (39%) | ||
| Male | 102 (58%) | 38 (68%) | 29 (46%) | 35 (61%) | ||
| Age | 176 | 56 (39, 66) | 54 (44, 61) | 61 (47, 74) | 52 (36, 68) | 0.023 |
n (%); Median (IQR).
Pearson's Chi-squared test; Kruskal-Wallis rank sum test.
Table 2.
Difference between demographic characteristics and laboratory findings between ICU patients and non-ICU patients.
| Group |
|||||
|---|---|---|---|---|---|
| Variable | N | Overall, N = 120a | Negative, N = 110a | Positive, N = 10a | p-valueb |
| Group | 120 | Death | 0.001 | ||
| ICU | 63 (52%) | 53 (48%) | 10 (100%) | ||
| Non ICU | 57 (48%) | 57 (52%) | 0 (0%) | ||
| Sex | 120 | 0.7 | |||
| Female | 56 (47%) | 52 (47%) | 4 (40%) | ||
| Male | 64 (53%) | 58 (53%) | 6 (60%) | ||
| Age | 120 | 57 (38, 70) | 54 (37, 67) | 80 (76, 84) | <0.001 |
| ESR | 120 | 44 (28, 56) | 45 (27, 56) | 38 (34, 58) | 0.8 |
| Platelet | 120 | 191 (145, 236) | 198 (147, 237) | 160 (108, 174) | 0.075 |
| M.C.H | 120 | 28.5 (27.1, 30.3) | 28.5 (27.0, 30.3) | 28.6 (27.3, 30.1) | >0.9 |
| M.C.V | 120 | 88 (85, 92) | 88 (85, 92) | 87 (85, 89) | 0.6 |
| Hct | 120 | 40 (34, 44) | 40 (34, 44) | 41 (37, 48) | 0.2 |
| Hb | 120 | 12.75 (10.85, 14.40) | 12.70 (10.75, 14.25) | 13.75 (11.60, 15.45) | 0.2 |
| R.B.C | 120 | 4.42 (3.90, 4.94) | 4.42 (3.90, 4.88) | 4.61 (3.96, 5.26) | 0.4 |
| W.B.C | 120 | 6.1 (5.0, 9.2) | 6.0 (5.0, 9.1) | 7.4 (5.0, 9.2) | 0.7 |
| LDH | 120 | 504 (418, 675) | 492 (412, 675) | 624 (478, 670) | 0.3 |
| SGPT | 120 | 21 (15, 34) | 21 (15, 34) | 22 (15, 38) | 0.6 |
| SGOT | 120 | 25 (19, 44) | 25 (18, 43) | 24 (22, 71) | 0.6 |
| Cigarette | 120 | 0.7 | |||
| Negative | 101 (84%) | 93 (85%) | 8 (80%) | ||
| Positive | 19 (16%) | 17 (15%) | 2 (20%) | ||
| Opium | 120 | >0.9 | |||
| Negative | 116 (97%) | 106 (96%) | 10 (100%) | ||
| Positive | 4 (3.3%) | 4 (3.6%) | 0 (0%) | ||
| Alcoholic_drinks 120 | >0.9 | ||||
| Negative | 115 (96%) | 105 (95%) | 10 (100%) | ||
| Positive | 5 (4.2%) | 5 (4.5%) | 0 (0%) | ||
| Kidney_failure | 120 | 0.7 | |||
| Negative | 101 (84%) | 93 (85%) | 8 (80%) | ||
| Positive | 19 (16%) | 17 (15%) | 2 (20%) | ||
| Dialysis | 120 | 0.2 | |||
| Negative | 110 (92%) | 102 (93%) | 8 (80%) | ||
| Lung_disease | 120 | 0.042 | |||
| Negative | 102 (85%) | 96 (87%) | 6 (60%) | ||
| Positive | 18 (15%) | 14 (13%) | 4 (40%) | ||
| Heart_disease | 120 | 0.022 | |||
| Negative | 97 (81%) | 92 (84%) | 5 (50%) | ||
| Positive | 23 (19%) | 18 (16%) | 5 (50%) | ||
| CT.value | 120 | 25 (24, 33) | 26 (24, 33) | 24 (22, 34) | 0.7 |
n (%); Median (IQR).
Fisher's exact test; Wilcoxon rank sum test.
3.2. The test results of the assessment of CRP, IL-6, IL-10, 1L-18, SDC-1, and Vitamin D levels in patients with COVID-19
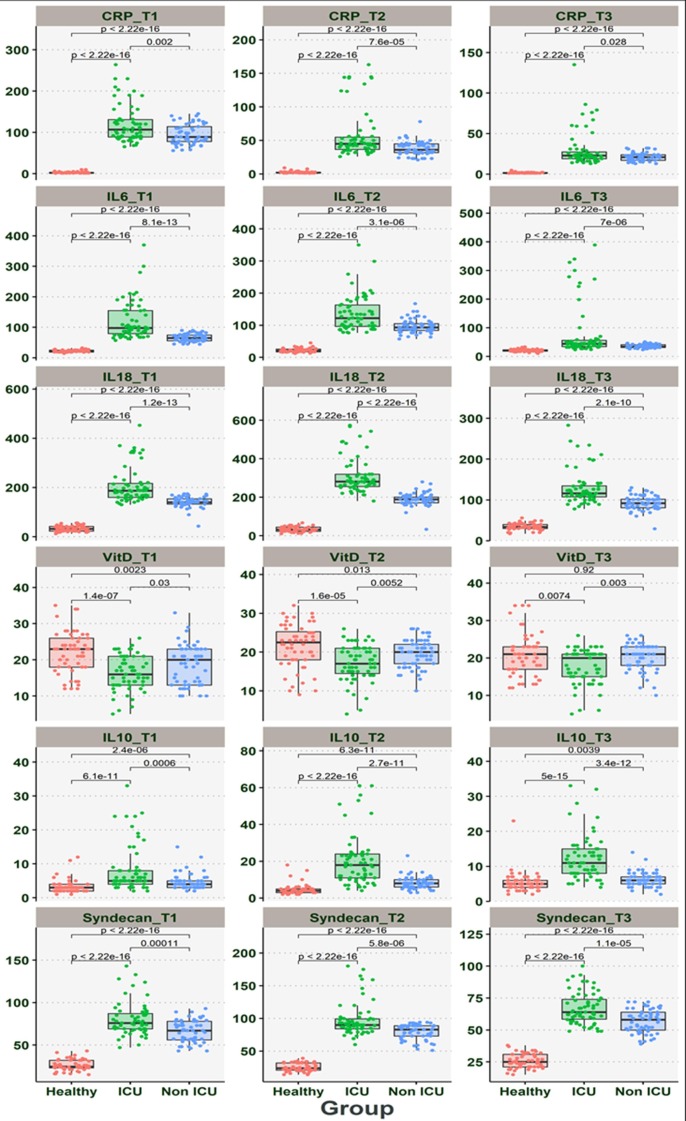
The relationship between CRP, IL-6, IL-10, 1L-18, SDC-1, and Vit D levels, were shown in Table 3 and Fig. 1 . According to Table 3 and Fig. 1, the levels of the above markers were significantly elevated in patients with COVID-19 than HS. Also, the CRP, IL-6, IL-10, 1L-18, SDC-1 levels in ICU patients were significantly higher than non-ICU patients (P < 0.001). Our result indicated that Vit D levels in COVID-19 significantly lower than in healthy subjects (P < 0.001). Further analysis shows that the Vit D levels had significantly lower in ICU patients when compared with non-ICU patients (P < 0.05). Also, our results indicated that with disease progression, the levels of CRP, IL-6, IL-10, 1L-18, SDC-1 gradually increase from the day of admission to day five and then decreased gradually on day 10 after hospitalization (Table 3 and Fig. 1). Concurrently, the Vit D levels from day of admission to day ten gradually decreased (P < 0.05).
Table 3.
The dynamic changes in the CRP, IL-6, IL-10, 1L-18, SDC-1, and Vitamin D levels among patients with COVID-19 and Healthy subjects.
| Group |
||||||
|---|---|---|---|---|---|---|
| Variable | N | Overall, N = 176a | Healthy, N = 56a | ICU, N = 63a | Non ICU, N = 57a | p-valueb |
| CRP_T1 | 176 | 84 (3, 110) | 2 (2, 3) | 107 (89, 131) | 89 (78, 114) | <0.001 |
| CRP_T2 | 176 | 34 (3, 45) | 2 (2, 3) | 45 (36, 55) | 36 (32, 45) | <0.001 |
| CRP_T3 | 176 | 18 (2, 23) | 2 (1, 2) | 23 (19, 28) | 21 (17, 24) | <0.001 |
| IL6_T1 | 176 | 65 (25, 86) | 21 (19, 25) | 98 (80, 155) | 65 (56, 75) | <0.001 |
| IL6_T2 | 176 | 88 (27, 115) | 21 (17, 26) | 122 (96, 164) | 93 (84, 105) | <0.001 |
| IL6_T3 | 176 | 32 (23, 43) | 21 (18, 23) | 44 (34, 56) | 34 (31, 41) | <0.001 |
| IL18_T1 | 176 | 140 (43, 168) | 32 (23, 42) | 187 (160, 217) | 140 (133, 154) | <0.001 |
| IL18_T2 | 176 | 190 (45, 267) | 32 (23, 45) | 281 (256, 320) | 189 (171, 201) | <0.001 |
| IL18_T3 | 176 | 90 (40, 113) | 34 (30, 40) | 116 (108, 134) | 92 (81, 102) | <0.001 |
| VitD_T1 | 176 | 19 (14, 23) | 23 (18, 26) | 16 (13, 21) | 20 (13, 23) | <0.001 |
| VitD_T2 | 176 | 20 (16, 23) | 22 (18, 25) | 17 (14, 21) | 20 (17, 22) | <0.001 |
| VitD_T3 | 176 | 21 (16, 23) | 21 (17, 23) | 20 (15, 21) | 21 (18, 23) | 0.004 |
| IL10_T1 | 176 | 4 (3, 6) | 3 (2, 4) | 5 (4, 8) | 4 (3, 5) | <0.001 |
| IL10_T2 | 176 | 8 (5, 13) | 4 (3, 5) | 18 (11, 24) | 8 (6, 10) | <0.001 |
| IL10_T3 | 176 | 6 (5, 9) | 5 (4, 6) | 11 (8, 15) | 6 (5, 7) | <0.001 |
| Syndecan_T1 176 | 64 (32, 78) | 24 (23, 32) | 76 (69, 87) | 67 (56, 78) | <0.001 | |
| Syndecan_T2 176 | 78 (32, 89) | 24 (21, 32) | 90 (84, 100) | 83 (73, 89) | <0.001 | |
| Syndecan_T3 176 | 54 (32, 64) | 25 (21, 31) | 64 (58, 74) | 58 (50, 64) | <0.001 | |
Median (IQR).
Kruskal-Wallis rank sum test.
Fig. 1.
The dynamic changes in the concentration of CRP, IL-6, IL-10, 1L-18, SDC-1, and Vitamin D from day 0 or T1 (hospitalization), day five after hospitalization or T2, and day ten after hospitalization or T3 in ICU, non-ICU, and healthy subjects.
3.3. Comparison of dynamic changes in the levels of CRP, IL-6, IL-10, 1L-18, SDC-1, and Vitamin D between ICU and non-ICU patients
The CRP levels were significantly higher in ICU patients than those of non-ICU patients at the time of hospitalization (P < 0.001); however, the CRP levels gradually decreased from the day of admission to day ten. Other markers, including IL-6, IL-10, 1L-18, and SDC-1 levels, were significantly higher in ICU patients than those of non-ICU patients (P < 0.001). Also, the levels of these markers in both groups gradually increase from the day of hospitalization to day five and gradually decrease to day ten. Our result indicated that the levels of Vit D levels were significantly lower in ICU patients than non-ICU patients (P < 0.001). Concurrently, the Vit D levels gradually increased in both groups from the day of admission to day ten (Table 3) (Fig. 1).
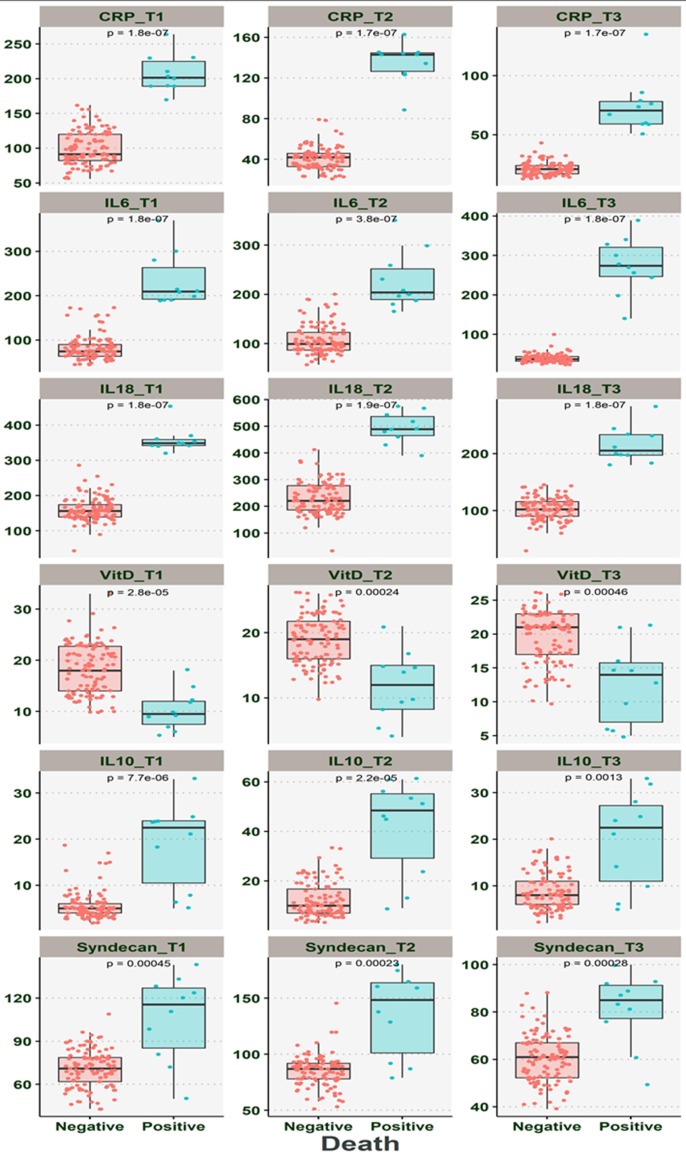
3.4. The comparison of the dynamic changes in the levels of CRP, IL-6, IL-10, 1L-18, SDC-1, and Vitamin D among death patients and alive patients
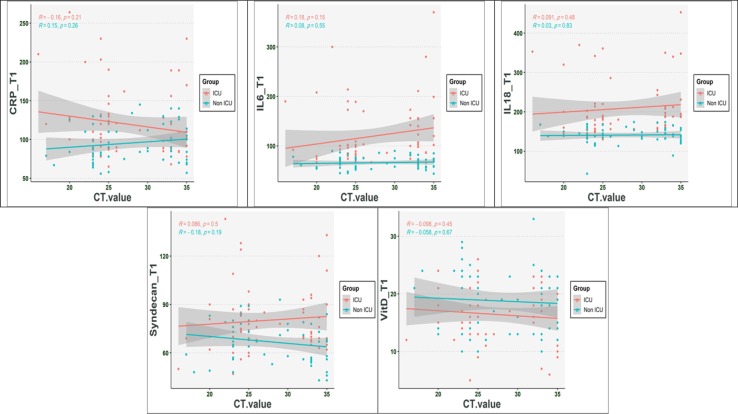
Our result indicated that the dead cases had significantly higher levels of CRP, IL-6, IL-10, 1L-18, and SDC-1 than in alive cases (P < 0.001) (Table 4 ) (Fig. 2 ). The Vit D levels in death cases were significantly lower than those of alive cases (P < 0.001). Further analysis showed that CRP, IL-6, IL-10, 1L-18, and SDC-1 levels at the three-time points in death cases were significantly higher than those alive (P < 0.001). While concurrently, our analysis demonstrated that the Vit D levels in three-time point significantly lower in death cases when compared to the alive cases (P < 0.001). Besides, more interpretation indicated that there was no relation between SDC-1, IL-6, IL-10, IL-18, CRP, and Vit D levels and cycle threshold (Fig. 3 ) (P > 0.05).
Table 4.
The association between dynamic changes in the serum levels of CRP, IL-6, IL-10, 1L-18, SDC-1, and Vitamin D with death in patients with COVID-19.
| Death |
|||||
|---|---|---|---|---|---|
| Variable | N | Overall, N = 120a | Negative, N = 110a | Positive, N = 10a | p-valueb |
| CRP_T1 | 120 | 98 (84, 124) | 92 (82, 120) | 202 (189, 225) | <0.001 |
| CRP_T2 | 120 | 42 (34, 48) | 42 (33, 46) | 143 (126, 145) | <0.001 |
| CRP_T3 | 120 | 21 (17, 26) | 21 (17, 24) | 70 (59, 78) | <0.001 |
| IL6_T1 | 120 | 76 (65, 100) | 74 (64, 90) | 210 (192, 264) | <0.001 |
| IL6_T2 | 120 | 102 (88, 134) | 100 (87, 123) | 204 (190, 252) | <0.001 |
| IL6_T3 | 120 | 38 (32, 46) | 37 (32, 44) | 274 (247, 321) | <0.001 |
| IL18_T1 | 120 | 158 (140, 187) | 156 (139, 174) | 349 (342, 359) | <0.001 |
| IL18_T2 | 120 | 230 (189, 284) | 221 (187, 278) | 489 (465, 536) | <0.001 |
| IL18_T3 | 120 | 108 (90, 122) | 102 (90, 116) | 206 (197, 234) | <0.001 |
| VitD_T1 | 120 | 18 (13, 21) | 18 (14, 23) | 10 (8, 12) | <0.001 |
| VitD_T2 | 120 | 19 (15, 21) | 19 (16, 22) | 12 (8, 15) | <0.001 |
| VitD_T3 | 120 | 21 (16, 22) | 21 (17, 23) | 14 (7, 16) | <0.001 |
| IL10_T1 | 120 | 5 (4, 6) | 5 (4, 6) | 22 (10, 24) | <0.001 |
| IL10_T2 | 120 | 10 (7, 19) | 10 (7, 17) | 48 (29, 55) | <0.001 |
| IL10_T3 | 120 | 8 (6, 12) | 8 (6, 11) | 22 (11, 27) | 0.001 |
| Syndecan_T1 120 | 73 (63, 81) | 71 (62, 79) | 116 (85, 127) | <0.001 | |
| Syndecan_T2 120 | 88 (78, 93) | 87 (78, 92) | 148 (101, 164) | <0.001 | |
| Syndecan_T3 120 | 61 (53, 69) | 61 (52, 67) | 85 (77, 91) | <0.001 | |
Median (IQR).
Wilcoxon rank sum test.
Fig. 2.
The comparison between dynamic changes in the CRP, IL-6, IL-10, 1L-18, SDC-1, and Vitamin D levels from three-time points (T1-T3) between alive (recovered) and dead patients.
Fig. 3.
The correlation between CRP, IL-6, IL-10, IL-18, SDC-1, and Vitamin D with the cycle threshold (Ct) value at day of hospitalization.
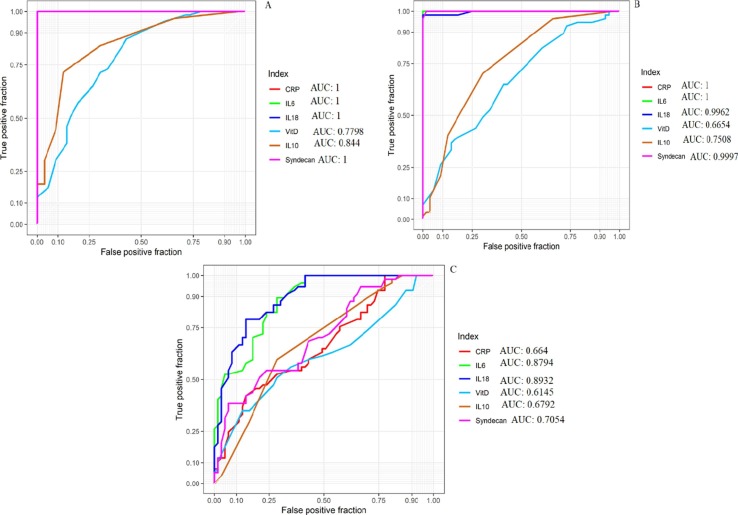
3.5. The value of SDC-1 on 1th day of admission compared with other indexes on the 1st for prognosis judgment of patients with COVID‑19
ROC curve analysis demonstrated that the SDC-1 and other indexes such IL-6, IL-10, IL-18, CRP, and Vit D were significantly different between COVID-19 cases and HS. Also, According to the ROC curve analysis results, the areas under the curve (AUC) of SDC-1 on the 1th and IL-6, IL-10, IL-18, CRP, and Vit D on the 1st day were 0.7054, 0.8794, 0.6792, 0.8932, 0.664, and 0.6145, respectively (Fig. 4 ). In sum, the ROC result indicated that the SDC-1 alone or coupled with IL-6 and IL-18 might be a good candidate for monitoring disease activity.
Fig. 4.
ROC curve analysis between, A: Healthy people vs. ICU patients, B: Healthy people vs. Non-ICU patients, and C: Non-ICU patients vs. ICU patients. AUC: Area under the curve.
4. Discussion
The novel pandemic infectious disease is called COVID-19 is a highly transmissible virus accompanied by high mortality and endangers human well-being and public safety [13], [15], [28], [29], [30]. Many investigations are launched for unraveling the pathogenesis mechanism of SARS-CoV-2. The previous studies demonstrated that RNA viruses such as hepatitis E virus [8], human papillomavirus [31], human immunodeficiency virus [32], [33], [34] for the attach to target cells use an SDC-1 on the cell surface and another viral pathogen for their attachment and establish infection have also been shown to bind to HSPGs on the cell surface [35], [36]. The recent investigation has provided further support for the performance of SDC-1 in the pathophysiology of viral infection; in this study, Bermejo-Jambrina et al. show that for SARS-CoV-2 infection in permissive cells, the cell surface HSPG, including SDC-1 and -4, are needed, and also in alveolar macrophages, the established infection by SARS-CoV-2 efficiently hindered via low molecular weight heparins (LMWH) [37]. Given the dilemma role of SDC-1 during the progression of inflammatory disease such as respiratory viral infection, in the current research, we explore and monitoring the dynamic alterations in the SDC-1 concentration along with some markers, including IL-6, IL-10, IL-18, CRP, and Vit D in cases with COVID-19.
Several inflammatory indexes are commonly employed to prognosticate, diagnose, and estimate many inflammatory conditions, including COVID-19, platelet count, procalcitonin, white blood count, CRP, SAA lymphocyte [23], [25]. The present result indicated that the levels of SDC-1 in cases with COVID-19 had significantly higher than healthy individuals. Also, we showed that the serum SDC-1 levels were significantly higher in ICU patients when compared with non-ICU patients. The levels of SDC gradually increased and then gradually decreased with disease duration. From day 0 (day of hospitalization) to day 5 (5 days after hospitalization), the levels of SDC-1 increased. However, from day 5 to day ten, the levels of SDC-1 gradually decreased in ICU and non-ICU patients. The complicated structure is called the glycocalyx, composed of proteoglycans (such as SDC-1), glycosaminoglycans, and numerous plasma proteins [38]. The glycocalyx is a crucial regulator of endothelial cell homeostasis, inflammatory processes, and tissue edema [39]. It consists of membrane-bound proteoglycans and glycoproteins and covers endothelial cells at the luminal vessel side [39], [40]. This weak boundary is interrupted in inflammatory conditions [41] and cardiovascular disorders [42], [43], is correlated with patient consequences [44], [45]. Recently, Fraser and colleagues highlight that the cases with COVID-19 had higher levels of SDC-1, P-selectin, and hyaluronic acid as glycocalyx-degradation products [24]. Also, Fraser and colleagues, according to the previous investigation, proposed that the glycocalyx-degradation may underlie platelet adhesion and thrombosis risk in COVID-19 patients [24]. In this way, according to Fraser et al. [25] and Suzuki et al. [37], our result highlighted that the levels of SDC-1 elevated in cases with COVID-19.
On the other hand, during infectious disease, SDC-1 obviously enhances the pathogenesis of some pathogens. It propitiates the binding and entrance of pathogens into host cells and represses host protection mechanisms. Besides, the lack of SDC-1 is a gain of function mutation that boosts the immunity of mice to various bacterial diseases. These findings recommend that one of the critical roles of mammalian SDC-1 in vivo is to guarantee the sufficient and accurate operative of inflammation [12]. Nevertheless, this beneficial role of SDC-1 arrives at a value as particular microbial pathogens, and tumor cells have both accommodated or emerged to catch the benefit of SDC-1 for their pathophysiology. Since various pathogens attach to HSPGs on the cell surface for their binding and entrance, it is unknown how? Soluble SDC-1 is not a host protection mechanism that immediately decreases microbial binding places. One possible explanation is that only highly efficient pathogens that hold excess mechanisms for binding, including some bacterial pathogen, including Staphylococcus aureus (S. aureus), can utilize soluble SDC-1 to support their pathophysiology [46]. Another possible reason is that some pathogens may employ both forms of SDC-1 (cell surfaces and shed SDC-1) to bind to host cells and hinder host immunity, whether SARS-CoV-2 like S. aureus hold the capability to provoke SDC-1 shedding to the increase of the production of SDC-1 to support their pathophysiology remains to be discovered.
The vast majority of finding from the latest research has highlighted that inflammation represents an axial function in the pathogenesis of COVID-19 [47], [48], [49], [50]. Our work's data show that the levels of inflammatory indexes, such as IL-6, IL-18, and CRP, were significantly elevated in cases with COVID-19 than in HS. Further analysis revealed that the levels of these inflammatory markers in ICU patients were significantly higher than in non-ICU patients. We have also indicated that the IL-6 and IL-18 levels gradually increased from day 0 to day 5 in both ICU and non-ICU patients. However, the CRP levels gradually decreased from day 0 to day 10 in both groups of patients. Our result in agreement with the studies shows that the IL-6 [26], [51], [52], [53], [54], [55], [56], [57], IL-18 [26], [58], [59], [60], and CRP elevated in patients with COVID-19 [61], [62], [63]. Besides, our data demonstrated that the IL-10 levels were significantly higher in cases with COVID-19 than in HS.
Further analysis of our data indicated that the levels of IL10 were significantly higher in ICU patients than non-ICU patients. Our result agreed with several investigations that revealed the levels of IL-10 elevated in patients with COVID-19 [52], [64], [65], [66]. Also, dead patients significantly had higher IL-6, IL10, IL-18, and CRP levels than alive patients. The current evidence reflected that the inflammatory response might the tries of the immune system for the control of SARS-CoV-2 infection; however, the recent investigations showed that the SARS-CoV-2 could induce inflammation, necroptosis, and apoptosis in a mouse model infected with SARS-CoV-2 and lung sections of postmortem of fatal COVID-19 cases [67]. Also, the raised IL-10 levels in patients with COVID-19 may reflect the immune system's compensatory function to alleviate inflammation induced by SARS-CoV-2 [65]; nevertheless, a growing body of clinical data implies that dramatic early height of IL-10 levels may perform a pathological performance in the severity of COVID-19 pathogenesis [65]. As results showed, the levels of SDC-1 positively correlated with anti- and pro-inflammatory markers, including IL-6, IL-18, CRP, and IL-10, which one explanation is SDC-1 may act as a compensatory response for the alleviation of inflammatory response were provoked by SARS-CoV-2. In contrast, as mentioned before, another finding highlights that the soluble SDC-1 in the context of infectious disease has propathogenic functions to promote infection [12]. So, it is possible that the SARS-CoV-2 may subvert the production of SDC-1 for their advantage and promote the pathogenesis.
The vast majority of observational investigations have highlighted a low vitamin D status is correlated with a high experience of viral infection (respiratory viral infections), which globally display significant wellness and economic burdens [68], [69], [70], [71]. Our findings showed that the Vit D levels were significantly lower in COVID-19 cases than in HS. Also, our data revealed that the ICU patients significantly had lower levels of Vit D compared to non-ICU patients. Besides, the concentration of Vit D gradually decreased in both ICU and non-ICU patients from day 0 to day 10. Our result agreed with the studies that indicated that the COVID-19 patients had lower levels of Vit D [71], [72], [73], [74], [75]. As mentioned above, data from observational investigations have recommended that Vit D supplementation can lessen the odds of promoting respiratory diseases, especially in Vit D-deficient groups; however, randomized controlled trials (RCTs) have generated mixed outcomes [76]. Despite that, recent meta-analysis conclusions designate the inherent capacity of vitamin D in improving COVID-19 severity in hospitalized cases [77]; more robust data from RCTs are required to confirm its impacts on the severity and mortality rate of COVID-19.
Finally, the ROC curve report reveals that AUC that from high to low is: IL-18 > IL-6 > SDC-1 > IL-10 > CRP > Vit D, implying that SDC-1 along with IL-6 and IL-18 is a reliable indicator in differentiating severe SARS-CoV2 infection (ICU patients) cases from moderate ones (non-ICU patients).
Ultimately, the underlying molecular mechanisms in which SDC-1 governs in the physiological versus pathological situation remain opaque. Future investigations directed at determining the molecular aspects of how expression and shedding of SDC-1 are switched on or off and also how particular cellular and tissue parts throughout viral pathogenesis (such as SARS-CoV-2) are predicted to uncloak innovative therapeutic targets for a viral infection such as SARS-CoV-2.
In summary, Our present findings provide further support for using SDC-1 alone or combined with other markers such as IL-6, IL-10, IL-18, CRP, and Vit D might be applied as a significant indicator to designate and pursue inflammation states in cases with COVID-19. Also, SDC-1 and the indexes mentioned above are precious indicators in monitoring the disease activity of COVID-19. All in all, it seems that the dynamic monitoring alternation in the SDC-1 serum levels, coupled with some markers including IL-6 and IL-18, could be a valuable approach in diagnosing and establish an effective treatment plan in COVID-19 patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
On behalf of the authors, we sincerely thank Patients, healthy subjects, and clinicians (Firouzgar Hospital, Tehran, Iran) for their dedication.
Ethical approval
The Medical Ethics Review Board approved this study of Iran University of Medical Sciences (No. IR.IUMS.FMD.REC.1399.624(.
References
- 1.Xian X., Gopal S., Couchman J.R. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2010;339(1):31–46. doi: 10.1007/s00441-009-0829-3. [DOI] [PubMed] [Google Scholar]
- 2.Gopal S., Bober A., Whiteford J.R., Multhaupt H.A., Yoneda A., Couchman J.R. Heparan sulfate chain valency controls syndecan-4 function in cell adhesion. J. Biol. Chem. 2010;285(19):14247–14258. doi: 10.1074/jbc.M109.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh E.-S., Woods A., Couchman J.R. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J. Biol. Chem. 1997;272(13):8133–8136. doi: 10.1074/jbc.272.13.8133. [DOI] [PubMed] [Google Scholar]
- 4.Oh E.-S., Woods A., Lim S.-T., Theibert A.W., Couchman J.R. Syndecan-4 proteoglycan cytoplasmic domain and phosphatidylinositol 4, 5-bisphosphate coordinately regulate protein kinase C activity. J. Biol. Chem. 1998;273(17):10624–10629. doi: 10.1074/jbc.273.17.10624. [DOI] [PubMed] [Google Scholar]
- 5.De Rossi G., Evans A.R., Kay E., Woodfin A., McKay T.R., Nourshargh S., Whiteford J.R. Shed syndecan-2 inhibits angiogenesis. J. Cell Sci. 2014;127(21):4788–4799. doi: 10.1242/jcs.153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Rossi G., Whiteford J.R. Syndecans in angiogenesis and endothelial cell biology. Biochem. Soc. Trans. 2014;42(6):1643–1646. doi: 10.1042/BST20140232. [DOI] [PubMed] [Google Scholar]
- 7.Gopal S. Syndecans in inflammation at a glance. Front. Immunol. 2020;11:227. doi: 10.3389/fimmu.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalia M., Chandra V., Rahman S.A., Sehgal D., Jameel S. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. J. Virol. 2009;83(24):12714–12724. doi: 10.1128/JVI.00717-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacsa S., Karasneh G., Dosa S., Liu J., Valyi-Nagy T., Shukla D. Syndecan-1 and syndecan-2 play key roles in herpes simplex virus type-1 infection. J. Gen. Virol. 2011;92(Pt 4):733. doi: 10.1099/vir.0.027052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashida A., Amano S., Park P.W. Syndecan-1 promotes Staphylococcus aureus corneal infection by counteracting neutrophil-mediated host defense. J. Biol. Chem. 2011;286(5):3288–3297. doi: 10.1074/jbc.M110.185165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.E. Freissler, A. Meyer auf der Heyde, G. David, T.F. Meyer, C. Dehio, Syndecan‐1 and syndecan‐4 can mediate the invasion of OpaHSPG‐expressing Neisseria gonorrhoeae into epithelial cells, Cellular Microbio. 2(1) (2000) 69–82. [DOI] [PubMed]
- 12.Teng Y.H.-F., Aquino R.S., Park P.W. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012;31(1):3–16. doi: 10.1016/j.matbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.P. Goodarzi, F. Mahdavi, R. Mirzaei, H. Hasanvand, M. Sholeh, F. Zamani, M. Sohrabi, A. Tabibzadeh, A.S. Jeda, M.H.K. Niya, Coronavirus disease 2019 (COVID-19): Immunological approaches and emerging pharmacologic treatments, Int. Immunopharmacol. (2020) 106885. [DOI] [PMC free article] [PubMed]
- 14.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 15.Mirzaei R., Mohammadzadeh R., Mahdavi F., Badrzadeh F., Kazemi S., Ebrahimi M., Soltani F., Kazemi S., Jeda A.S., Darvishmotevalli M. Overview of the current promising approaches for the development of an effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. Int. Immunopharmacol. 2020:106928. doi: 10.1016/j.intimp.2020.106928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirzaei R., Karampoor S., Sholeh M., Moradi P., Ranjbar R., Ghasemi F. A contemporary review on pathogenesis and immunity of COVID-19 infection. Mol. Biol. Rep. 2020;47(7):5365–5376. doi: 10.1007/s11033-020-05621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China, The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou T., Liu Q., Yang Z., Liao J., Yang K., Bai W., Lu X., Zhang W. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019-nCoV. J. Evidence-Based Med. 2020;13(1):3–7. doi: 10.1111/jebm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.N. Zhu, D. Zhang, W. Wang, X. Li, B. Yang, J. Song, X. Zhao, B. Huang, W. Shi, R. Lu, A novel coronavirus from patients with pneumonia in China, 2019, New Engl. J. Med. (2020). [DOI] [PMC free article] [PubMed]
- 21.Adhikari S.P., Meng S., Wu Y.-J., Mao Y.-P., Ye R.-X., Wang Q.-Z., Sun C., Sylvia S., Rozelle S., Raat H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infectious Dis. Poverty. 2020;9(1):1–12. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han W., Quan B., Guo Y., Zhang J., Lu Y., Feng G., Wu Q., Fang F., Cheng L., Jiao N. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J. Med. Virol. 2020;92(5):461–463. doi: 10.1002/jmv.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X., Long H., Wang Q., Wu Q. Serum Amyloid A is a biomarker of severe Coronavirus Disease and poor prognosis. J. Infect. 2020;80(6):646–655. doi: 10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D.D. Fraser, E.K. Patterson, M. Slessarev, S.E. Gill, C. Martin, M. Daley, M.R. Miller, M.A. Patel, C.C. Dos Santos, K.J. Bosma, Endothelial injury and glycocalyx degradation in critically ill coronavirus disease 2019 patients: Implications for microvascular platelet aggregation, Critical care Explorations 2(9) (2020). [DOI] [PMC free article] [PubMed]
- 25.Suzuki K., Okada H., Tomita H., Sumi K., Kakino Y., Yasuda R., Kitagawa Y., Fukuta T., Miyake T., Yoshida S. Possible involvement of Syndecan-1 in the state of COVID-19 related to endothelial injury. Thrombosis J. 2021;19(1):1–5. doi: 10.1186/s12959-021-00258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser D.D., Cepinskas G., Slessarev M., Martin C., Daley M., Miller M.R., O’Gorman D.B., Gill S.E., Patterson E.K., Dos Santos C.C. Inflammation profiling of critically ill coronavirus disease 2019 patients. Crit. Care Explor. 2020;2(6) doi: 10.1097/CCE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karampoor S., Zahednasab H., Ramagopalan S., Mehrpour M., Tameshkel F.S., Keyvani H. 25-hydroxyvitamin D levels are associated with multiple sclerosis in Iran: A cross-sectional study. J. Neuroimmunol. 2016;290:47–48. doi: 10.1016/j.jneuroim.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020:1–14. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirzaei R., Goodarzi P., Asadi M., Soltani A., Aljanabi H.A.A., Jeda A.S., Dashtbin S., Jalalifar S., Mohammadzadeh R., Teimoori A. Bacterial co-infections with SARS-CoV-2. IUBMB Life. 2020;72(10):2097–2111. doi: 10.1002/iub.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirzaei R., Mahdavi F., Badrzadeh F., Hosseini-Fard S.R., Heidary M., Jeda A.S., Mohammadi T., Roshani M., Yousefimashouf R., Keyvani H. The emerging role of microRNAs in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Int. Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafti-Keramat S., Handisurya A., Kriehuber E., Meneguzzi G., Slupetzky K., Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J. Virol. 2003;77(24):13125–13135. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A. Ceballos, F. Remes Lenicov, J. Sabatté, C. Rodríguez Rodrígues, M. Cabrini, C. Jancic, S. Raiden, M. Donaldson, R. Agustín Pasqualini Jr, C. Marin-Briggiler, Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells, J. Exp. Med. 206(12) (2009) 2717–2733. [DOI] [PMC free article] [PubMed]
- 33.Saphire A.C., Bobardt M.D., Zhang Z., David G., Gallay P.A. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 2001;75(19):9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.A.J. Smith, T.W. Schacker, C.S. Reilly, A.T. Haase, A role for syndecan-1 and claudin-2 in microbial translocation during HIV-1 infection, Journal of acquired immune deficiency syndromes (1999) 55(3) (2010) 306. [DOI] [PMC free article] [PubMed]
- 35.Spillmann D. Heparan sulfate: anchor for viral intruders? Biochimie. 2001;83(8):811–817. doi: 10.1016/s0300-9084(01)01290-1. [DOI] [PubMed] [Google Scholar]
- 36.Bartlett A.H., Park P.W. Proteoglycans in host–pathogen interactions: molecular mechanisms and therapeutic implications. Expert Rev. Mol. Med. 2010;12 doi: 10.1017/S1462399409001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bermejo-Jambrina M., Eder J., Kaptein T.M., Helgers L.C., Brouwer P.J., van Hamme J.L., Vlaar A.P., van Baarle F.E., de Bree G.J., Nijmeijer B.M. SARS-CoV-2 infection and transmission depends on heparan sulfates and is blocked by low molecular weight heparins. BioRxiv. 2020 [Google Scholar]
- 38.Ahrens T.D., Bang-Christensen S.R., Jørgensen A.M., Løppke C., Spliid C.B., Sand N.T., Clausen T.M., Salanti A., Agerbæk M.Ø. The role of proteoglycans in cancer metastasis and circulating tumor cell analysis. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.S. Reitsma, D.W. Slaaf, H. Vink, M.A. Van Zandvoort, M.G. oude Egbrink, The endothelial glycocalyx: composition, functions, and visualization, Pflügers Archiv-Eur. J. Physiol. 454(3) (2007) 345–359. [DOI] [PMC free article] [PubMed]
- 40.Pries A.R., Secomb T.W., Gaehtgens P. The endothelial surface layer. Pflügers Archiv. 2000;440(5):653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 41.Nelson A., Berkestedt I., Bodelsson M. Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol. Scand. 2014;58(1):36–43. doi: 10.1111/aas.12223. [DOI] [PubMed] [Google Scholar]
- 42.Wadowski P.P., Kautzky-Willer A., Gremmel T., Koppensteiner R., Wolf P., Ertl S., Weikert C., Schörgenhofer C., Jilma B. Sublingual microvasculature in diabetic patients. Microvasc. Res. 2020;129 doi: 10.1016/j.mvr.2019.103971. [DOI] [PubMed] [Google Scholar]
- 43.Salmon A.H., Satchell S.C. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J. Pathol. 2012;226(4):562–574. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt E.P., Overdier K.H., Sun X., Lin L., Liu X., Yang Y., Ammons L.A., Hiller T.D., Suflita M.A., Yu Y. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2016;194(4):439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beurskens D.M., Bol M.E., Delhaas T., van de Poll M.C., Reutelingsperger C.P., Nicolaes G.A., Sels J.-W.E. Decreased endothelial glycocalyx thickness is an early predictor of mortality in sepsis. Anaesth. Intensive Care. 2020;48(3):221–228. doi: 10.1177/0310057X20916471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henry-Stanley M.J., Hess D.J., Erlandsen S.L., Wells C.L. Ability of the heparan sulfate proteoglycan syndecan-1 to participate in bacterial translocation across the intestinal epithelial barrier. Shock. 2005;24(6):571–576. doi: 10.1097/01.shk.0000184286.95493.78. [DOI] [PubMed] [Google Scholar]
- 47.Cron R.Q. COVID-19 cytokine storm: targeting the appropriate cytokine, The Lancet. Rheumatology. 2021 doi: 10.1016/S2665-9913(21)00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front. Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fara A., Mitrev Z., Rosalia R.A., Assas B.M. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10(9) doi: 10.1098/rsob.200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhar S.K., Vishnupriyan K., Damodar S., Gujar S., Das M. IL-6 and IL-10 as predictors of disease severity in COVID 19 patients: Results from Meta-analysis and Regression. MedRxiv. 2020 doi: 10.1016/j.heliyon.2021.e06155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nasonov E., Samsonov M. The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomed. Pharmacother. 2020:110698. doi: 10.1016/j.biopha.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavalli G., Larcher A., Tomelleri A., Campochiaro C., Della-Torre E., De Luca G., Farina N., Boffini N., Ruggeri A., Poli A. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study, The Lancet. Rheumatology. 2021 doi: 10.1016/S2665-9913(21)00012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J., Hao Y., Ou W., Ming F., Liang G., Qian Y., Cai Q., Dong S., Hu S., Wang W. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. J. Translational Med. 2020;18(1):1–8. doi: 10.1186/s12967-020-02571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guirao J.J., Cabrera C.M., Jiménez N., Rincón L., Urra J.M. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. Mol. Immunol. 2020;128:64–68. doi: 10.1016/j.molimm.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun H., Guo P., Zhang L., Wang F. Serum Interleukin-6 Concentrations and the Severity of COVID-19 Pneumonia: A Retrospective Study at a Single Center in Bengbu City, Anhui Province, China, in January and February 2020. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020;26:e926941–1. doi: 10.12659/MSM.926941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Satış H., Özger H.S., Yıldız P.A., Hızel K., Gulbahar Ö., Erbaş G., Aygencel G., Tunccan O.G., Öztürk M.A., Dizbay M. Prognostic value of interleukin-18 and its association with other inflammatory markers and disease severity in COVID-19. Cytokine. 2020;137 doi: 10.1016/j.cyto.2020.155302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tao W., Zhang G., Wang X., Guo M., Zeng W., Xu Z., Cao D., Pan A., Wang Y., Zhang K. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Med. Microecol. 2020;5 doi: 10.1016/j.medmic.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gea-Mallorquí E. IL-18-dependent MAIT cell activation in COVID-19. Nat. Rev. Immunol. 2020;20(12) doi: 10.1038/s41577-020-00467-x. 719–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahnach M., Zbiri S., Nejjari S., Ousti F., Elkettani C. C-reactive protein as an early predictor of COVID-19 severity. J. Med. Biochem. 2020;39(4):500. doi: 10.5937/jomb0-27554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo X., Zhou W., Yan X., Guo T., Wang B., Xia H., Ye L., Xiong J., Jiang Z., Liu Y. Prognostic value of C-reactive protein in patients with coronavirus 2019. Clin. Infect. Dis. 2020;71(16):2174–2179. doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L. C-reactive protein levels in the early stage of COVID-19. Medecine et maladies infectieuses. 2020;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu L., Zhang H., Dauphars D.J., He Y.-W. A Potential Role of Interleukin-10 in COVID-19 Pathogenesis. Trends Immunol. 2020 doi: 10.1016/j.it.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.J. Li, L. Rong, R. Cui, J. Feng, Y. Jin, Y. Yu, X. Chen, R. Xu, Dynamic changes in serum IL-6, IL-8, and IL-10 are associated with the outcome of patients with severe COVID-19 in ICU, (2020). [DOI] [PubMed]
- 67.Li S., Zhang Y., Guan Z., Li H., Ye M., Chen X., Shen J., Zhou Y., Shi Z.-L., Zhou P. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduction Targeted Therapy. 2020;5(1):1–10. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bryson K., Nash A., Norval M. Does vitamin D protect against respiratory viral infections? Epidemiol. Infect. 2014;142(9):1789–1801. doi: 10.1017/S0950268814000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balla M., Merugu G.P., Konala V.M., Sangani V., Kondakindi H., Pokal M., Gayam V., Adapa S., Naramala S., Malayala S.V. Back to basics: review on vitamin D and respiratory viral infections including COVID-19. J. Commun. Hospit. Int. Med. Perspect. 2020;10(6):529–536. doi: 10.1080/20009666.2020.1811074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charan J., Goyal J.P., Saxena D., Yadav P. Vitamin D for prevention of respiratory tract infections: A systematic review and meta-analysis. J. Pharmacol. Pharmacotherapeutics. 2012;3(4):300. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jain A., Chaurasia R., Sengar N.S., Singh M., Mahor S., Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I., Frenkel-Morgenstern M. Low plasma 25 (OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carpagnano G.E., Di Lecce V., Quaranta V.N., Zito A., Buonamico E., Capozza E., Palumbo A., Di Gioia G., Valerio V.N., Resta O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Invest. 2020:1–7. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tee S., Panagiotou G., Ihsan Y., Athar W., Marchitelli G., Kelly D., Boot C.S., Stock N., Macfarlane J., Martineau A. Low serum 25-hydroxyvitamin D (25 [OH] D) levels in patients hospitalised with COVID-19 are associated with greater disease severity, 22nd European Congress of Endocrinology. BioScientifica. 2020 doi: 10.1111/cen.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kralj M., Jakovac H. Vitamin D and COVID-19 in an immunocompromised patient with multiple comorbidities—A Case Report. Clin. Case Rep. 2021 doi: 10.1002/ccr3.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.E. The Lancet Diabetes, Vitamin D and COVID-19: why the controversy?, lancet. Diabetes Endocrinol. 9(2) (2021) 53. [DOI] [PMC free article] [PubMed]
- 77.Shah K., Saxena D., Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM: Int. J. Med. 2021 doi: 10.1093/qjmed/hcab009. [DOI] [PMC free article] [PubMed] [Google Scholar]