Abstract
Background
The 2019 Coronavirus (COVID-19) pandemic poses a huge threat internationally; however, the role of the host immune system in the pathogenesis of COVID-19 is not well understood.
Methods
Cytokine and chemokine levels and characterisation of immune cell subsets from 20 COVID-19 cases after hospital admission (17 critically ill and 3 severe patients) and 16 convalescent patients were determined using a multiplex immunoassay and flow cytometry, respectively.
Results
IP-10, MCP-1, MIG, IL-6, and IL-10 levels were significantly higher in acute severe/critically ill patients with COVID-19, whereas were normal in patients who had reached convalescence. CD8 T cells in severe and critically ill COVID-19 patients expressed high levels of cytotoxic granules (granzyme B and perforin)and was hyperactivated as evidenced by the high proportions of CD38. Furthermore, the cytotoxic potential of natural killer (NK) cells, and the frequencies of myeloid dendritic cells and plasmacytoid dendritic cells was reduced in patients with severe and critical COVID-19; however, these dysregulations were found to be restored in convalescent phases.
Conclusion
Thus, elicitation of the hyperactive cytokine-mediated inflammatory response, dysregulation of CD8 T and NK cells, and deficiency of host myeloid and plasmacytoid DCs, may contribute to COVID-19 pathogenesis and provide insights into potential therapeutic targets and strategies.
Keywords: COVID-19, Cytokines /chemokines, Immune cell, Severe/critical infection
1. Background
In December 2019, an outbreak of pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was linked to a local seafood wholesale market in Wuhan, China [1]. After epidemiological and genome-wide analyses, it has been determined that this coronavirus is different from the known SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) [2], [3]. A wide spectrum of clinical manifestations develops from asymptomatic to critically ill disease, including acute respiratory distress syndrome (ARDS), multiple organ dysfunction, and death [4], [5]. Severe lung disease with extensive alveolar damage and progressive respiratory failure leads to fatal outcomes [6], [7]. A recent study reported an autopsy case with presence of remarkable interstitial lymphocyte infiltrates in the lung tissue, lymphopenia, and overactivation of T cells in the peripheral blood [8].
Innate and adaptive immune responses play a critical role in defending against viral infection and disease progression. Natural killer (NK) cells exert primary control during acute viral infection, but cytotoxic CD8 T lymphocytes (CTLs) are critical for long-term surveillance. However, increased elicitation of immune responses following infection has been associated with the production of excessive levels of proinflammatory cytokines and widespread tissue damage [9]. Preliminary studies have shown that SARS-CoV-2 infection triggers a cytokine storm and results in an increase in various cytokines levels [10], [11]. However, the cytokine levels in COVID-19 convalescent patients remain unclear. Previous studies have shown that the decreased frequency of circulating lymphocytes, including NK cells, CD4 and CD8 T cells, is closely related to COVID-19 severity [12], [13]. These results support the hypothesis that dysregulation of immune responses may exert detrimental effects on patients with COVID-19. There is an urgent need for an improved understanding of COVID-19 immunopathology in severe and critically ill patients, because COVID-19 is currently responsible for high morbidity and mortality in many countries. In this study, we investigated the immunological characteristics and inflammatory profiles in both acute severe/critically ill and convalescent patients with COVID-19.
2. Material and methods
2.1. Patients characteristics
This study enrolled 20 hospitalised patients with COVID-19 in the acute patient group, including 17 critically ill and 3 severe patients, based on the Guidelines for Diagnosis and Treatment of Corona Virus Disease 2019 issued by the National Health Commission of China(7th edition) (http://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_11815/202003/t20200305_214142.html). Severe patients who met one of the following criteria: (1) respiratory distress, respiratory rate ≥ 30 breaths min−1; (2) pulse oxygen saturation (SpO2) ≤ 93% without inhalation of oxygen support at quiet resting state;(3) arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤ 300 mm Hg; (4) computed tomography (CT) image shows there is more than 50% increase of lung infiltrating change within 24 to 48 h. Critically ill patients in this study generally required mechanical ventilation and exhibited respiratory failure, septic shock, and/or multiple organ dysfunction/failure that required monitoring and treatment in the intensive care unit[14]. COVID-19 was confirmed by the detection of SARS-CoV-2 by reverse-transcription polymerase chain reaction as previously described[15]. Blood samples were collected at a median of 3.75 days after hospital admission (range; 1–9 days). Sixteen convalescent patients recovered from the severe and critically ill COVID-19 patients, who received treatment and were subsequently discharged from the hospital. Blood samples were then collected at a median of 73.62 days after hospital admission (range; 23–166 days). Healthy individuals (n = 10) were enrolled as controls. Since archived specimens were used, written informed consent was waived. The study protocol was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University.
2.2. Cytokines and chemokines assay
Cytokine and chemokine levels (interferon gamma-induced protein 10 [IP-10], macrophage inflammatory protein [MIP]-1β, interferon [IFN]-α, IFN-γ, monokine induced by IFN-γ [MIG], MIP-1α, monocyte chemoattractant protein-1 [MCP-1], interleukin [IL]-6, IL-10, and tumour necrosis factor [TNF]-α) in the serum were determined using bead-based multiplex LEGENDplex assay kits (BioLegend, San Diego, CA, USA), according to the manufacturer’s protocol. Data were analysed using the LEGEND ™ plex analysis software, version 8.0.
2.3. Detection of the changes in immune cell subsets using flow cytometry
Detection of changes in immune cell subsets was performed using flow cytometry. All antibodies for flow cytometry were purchased from BD Biosciences (San Jose, CA, USA). To determine CD4 T cells, CD8 T cells and NK cells, fluorescein isothiocyanate (FITC)-conjugated anti-CD8, phycoerythrin (PE)-conjugated perforin, PerCP-Cy5.5-conjugated anti-CD3, PE-Cy7-conjugated anti-CD56, allophycocyanin (APC)-conjugated granzyme B, and APC-Cy7-conjugated anti-CD45 were used. To determine plasmacytoid dendritic cells (pDCs) and myeloid DCs (mDCs), FITC-conjugated Lineage Cocktail, PE-conjugated anti-CD123, APC-Cy7-conjugated anti-HLA-DR, and APC-conjugated anti-CD11c were used. Activated T cells were identified using a combination of CD8-PE-CY7, CD3-Percp-CY5.5, CD4-FITC, and CD38-PE antibodies. Cells were stained according to the manufacturer’s instructions. All flow cytometry data were recorded and analysed using the BD FACSDiva software.
2.4. Statistical analysis
Statistical analysis was performed using the GraphPad Prism 6. Significant differences between the means of three groups were tested using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. To determine the difference between two groups, an independent t test was performed. Differences were considered significant at P < 0.05.
3. Results
Detailed clinical features, comorbidities, and treatments of severe and critically ill COVID-19 patients are shown in Table 1 . The mean age was 57.8 years, and most of the patients were men (14/20, 70%). Some patients had chronic diseases, including diabetes (6/20, 30%), hypertension (9/20, 45%), cardiovascular disease (5/20, 25%), and chronic obstructive pulmonary disease (3/20, 15%). The most common symptoms were fever (19/20, 95%), dyspnea (17/20, 85%), and cough (13/20, 65%). Most patients had one or more manifestations of organ dysfunction or failure. Patients with common complications included 16 (80%) with ARDS, 7 (35%) with acute kidney injury, 11 (55%) with cardiac injury, 7 (35%) with liver dysfunction, 6 (30%) with abnormal coagulant function, 7 (35%) with shock and 5 (25%)with multiorgan failure syndrome.
Table 1.
Clinical manifestations, comorbidities, and treatments of severe and critically ill COVID-19 patients.
| Characteristic | Severe and critically ill n = 20 |
|---|---|
| Age, years | 57.80 ± 14.63 |
| Sex (male,%) | 14(70%) |
| Chronic disease | |
| Diabetes | 6(30%) |
| Hypertension | 9(45%) |
| Cardiovascular disease | 5(25%) |
| Chronic obstructive | |
| Pulmonary disease | 3(15%) |
| Signs and symptoms | |
| Fever | 19(95%) |
| Cough | 13(65%) |
| Rhinorrhea | 0 |
| Expectoration | 5(25%) |
| Nasal congestion | 1(5%) |
| Dyspnea | 17(85%) |
| Pharyngalgia | 2(10%) |
| Myalgia | 6(30%) |
| Complications | |
| Acute respiratory distress | |
| Syndrome | 16(80%) |
| Acute kidney injury | 7(35%) |
| Cardiac injury | 11(55%) |
| Liver dysfunction | 7(35%) |
| Coagulant function abnormality | 6(30%) |
| Shock | 7(35%) |
| MODS | 5(25%) |
Abbreviations: MODS, multiple organ dysfunction syndrome.
Laboratory findings of acute and convalescent patients are shown in Table 2 . The blood counts of severe and critically ill patients with COVID-19 on admission showed lymphopenia, whereas the lymphocyte levels returned to normal after recovery. The levels of prothrombin time, fibrinogen, D-dimer and serum glucose were increased in acute severe and critically ill COVID-19 patients, whereas in the convalescent phase, these levels were similar to normal levels. The levels of total protein and albumin were decreased in acute SARS-CoV-2-infected patients, whereas in the convalescent phase, these levels were returned to normal.
Table 2.
Laboratory findings of acute severe and critically ill COVID-19 patients and convalescent cases.
| Normal range | Severe and critically ill n = 20 |
Convalescent n = 16 |
P | |
|---|---|---|---|---|
| Blood routine tests | ||||
| WBC count (109 cells/L) | 4.0–10.0 | 7.76 ± 3.73 | 6.46 ± 1.79 | 0.180 |
| Neutrophil (109 cells/L) | 1.8–8.0 | 6.58 ± 3.73 | 3.96 ± 1.20 | 0.007 |
| Lymphocyte (109 cells/L) | 0.9–5.2 | 0.65 ± 0.32 | 1.77 ± 0.68 | <0.001 |
| Monocyte (109 cells/L) | 0.16–1.0 | 0.44 ± 0.27 | 0.54 ± 0.18 | 0.211 |
| Eosinophil (109 cells/L) | 0.05–0.3 | 0.07 ± 0.09 | 0.15 ± 0.11 | 0.020 |
| HGB (g/L) | 110–150 | 106.30 ± 19.96 | 120.31 ± 20.91 | 0.048 |
| PLT (109 /L) | 100–400 | 196.55 ± 90.19 | 224.16 ± 43.69 | 0.239 |
| Blood coagulation tests | ||||
| Prothrombin time, s | 11.0–14.5 | 14.52 ± 1.31 | 13.63 ± 0.79 | 0.016 |
| Activated partial thromboplastin time, s | 28–42.8 | 41.60 ± 6.61 | 38.23 ± 3.13 | 0.054 |
| D-dimer, ng/mL | 68–494 | 3533.20 ± 3386.12 | 508.06 ± 356.06 | 0.001 |
| Fibrinogen,g/L | 2.0–4.0 | 5.14 ± 1.35 | 3.43 ± 0.94 | <0.001 |
| Clinical immunology | ||||
| Complement 3 (g/L) | 0.9–1.5 | 0.89 ± 0.32 | 1.04 ± 0.16 | 0.184 |
| Complement 4 (g/L) | 0.2–0.4 | 0.21 ± 0.09 | 0.25 ± 0.08 | 0.267 |
| IgA (g/L) | 0.7–5.0 | 2.46 ± 1.21 | 2.28 ± 0.79 | 0.680 |
| IgG (g/L) | 6.0–16.0 | 13.25 ± 3.12 | 13.80 ± 2.67 | 0.638 |
| IgM (g/L) | 0.6–2.0 | 1.10 ± 0.61 | 0.81 ± 0.25 | 0.160 |
| Liver function | ||||
| Alanine aminotransferase (U/L) | 5–40 | 49.78 ± 46.43 | 45.24 ± 59.02 | 0.801 |
| Aspartate aminotransferase (U/L) | 5–40 | 52.16 ± 38.14 | 40.20 ± 54.29 | 0.449 |
| Total protein, g/L | 65–85 | 62.45 ± 5.98 | 69.56 ± 5.722 | 0.001 |
| Albumin, g/L | 35–55 | 35.51 ± 4.54 | 41.67 ± 4.15 | <0.001 |
| Total bilirubin, μmol/L | 1.7–22.2 | 15.98 ± 11.24 | 11.34 ± 3.83 | 0.125 |
| Direct bilirubin, μmol/L | 0.0–6.0 | 5.08 ± 5.86 | 2.11 ± 0.73 | 0.042 |
| Cardiac function | ||||
| Troponin I, μg/L | 0.0–0.04 | 0.14 ± 0.52 | 0.01 ± 0.01 | 0.330 |
| Creatine kinase-MB (U/L) | 3–25 | 10.35 ± 9.53 | 8.07 ± 3.80 | 0.388 |
| Renal function | ||||
| Serum glucose, mmol/L | 3.9–6.1 | 8.68 ± 2.24 | 5.67 ± 1.27 | <0.001 |
| BUN (mmol/L) | 2.9–7.2 | 8.48 ± 5.31 | 5.85 ± 3.19 | 0.075 |
| Creatinine (μmol/L) | 44.0–133.0 | 76.85 ± 30.26 | 75.19 ± 16.80 | 0.845 |
| Arterial blood gases | ||||
| pH | 7.35–7.45 | 7.41 ± 0.05 | 7.41 ± 0.02 | 0.376 |
| Paco2, mm Hg | 35.0–48.0 | 44.92 ± 6.19 | 41.86 ± 4.90 | 0.132 |
| Pao2, mm Hg | 83.0–108.0 | 110.43 ± 31.97 | 106.79 ± 32.99 | 0.691 |
| Oxygen saturation (%) | 95–99% | 96.05 ± 9.45 | 97.74 ± 1.28 | 0.512 |
3.1. Serum cytokine and chemokine levels in acute and convalescent patients with COVID-19
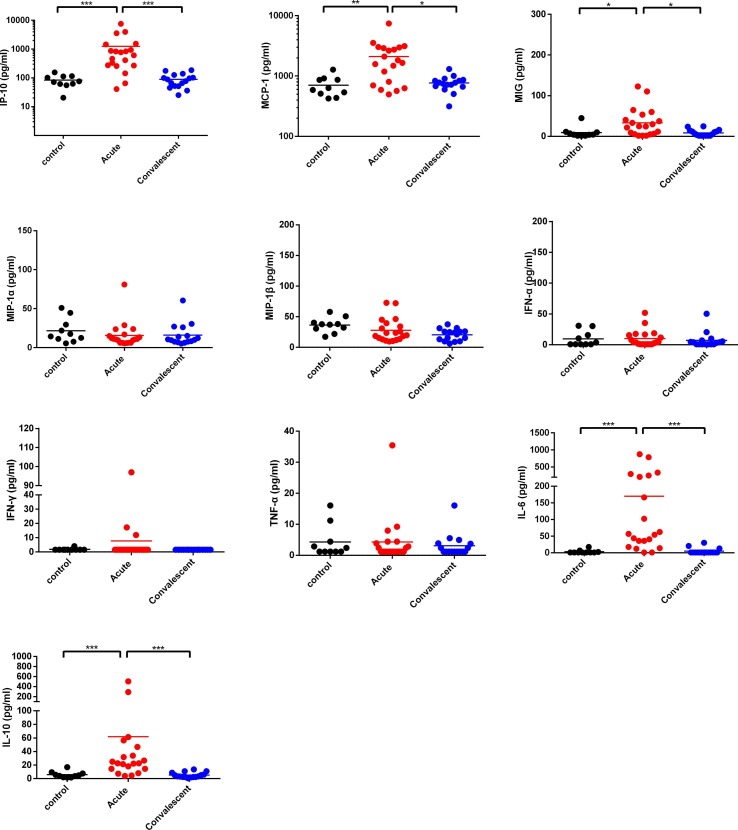
As shown in Fig. 1 , there were no statistically significant differences in the serum levels of IFN-α,IFN-γ,TNF-α,MIP-1α,and MIP-1β between acute severe and critically ill COVID-19 patients, convalescent cases and healthy controls. Compared to healthy controls, serum levels of IP-10, MCP-1 and MIG were significantly higher in acute severe and critically ill COVID-19 patients. The serum IL-6 and IL-10 levels in acute severe and critically ill COVID-19 patients were significantly higher than those in healthy controls. As expected, the serum levels of IP-10, MCP-1, MIG, IL-6, and IL-10 in convalescent cases returned to normal levels. Taken together, our results indicate that hypercytokinaemia in SARS-CoV-2-infected patients may be an important contributor to clinical severity.
Fig. 1.
Comparison of serum cytokine/chemokine levels (IFN-α, IFN-γ, TNF-α, IL-6, IL-10, IP-10, MCP-1, MIP-1α, MIP-1β, and MIG) between acute and convalescent patients and healthy controls. *P < 0.05, ** P < 0.01, and ***P < 0.001.
3.2. SARS-CoV-2 infection results in T cell activation
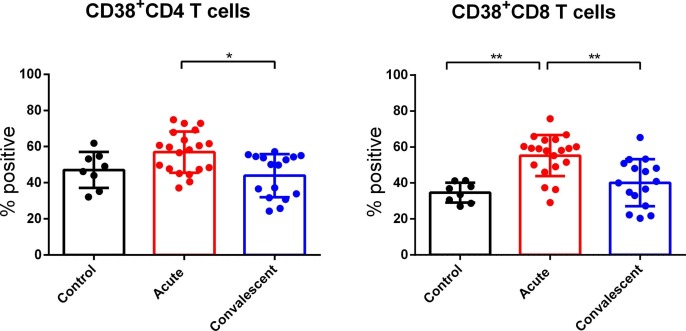
CD38 expression was detected to analyse the activation status of CD4 and CD8 T cells. As shown in Fig. 2 , we observed upregulated CD38 expression on CD8 T cells in acute severe and critically ill SARS-CoV-2-infected patients, compared to healthy controls. Moreover, we found that the expression of CD38 on CD4 and CD8 T cells in convalescent patients showed a downward trend compared to that in acute patients. Our results indicate that the CD8 T cell status was hyperactivated during acute SARS-CoV-2 infection.
Fig. 2.
Comparison of cells positive for CD38 among total CD4 and CD8 T cells from peripheral blood of acute SARS-CoV-2-infected patients, convalescent cases or healthy controls. *P < 0.05, ** P < 0.01.
3.3. The secretion of granzyme B and perforin by CD8 T cells and NK cells in acute and convalescent patients with COVID-19
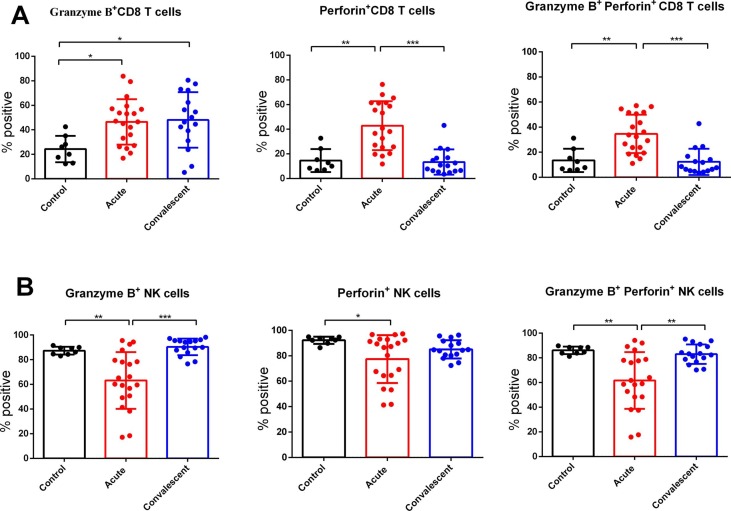
The expression of granzyme B and perforin in CD8 T and NK cells in acute severe and critically ill SARS-CoV-2-infected patients, convalescent patients, and healthy controls was detected to understand their cytotoxic potential. As shown in Fig. 3 , we found that CD8 T cells in the acute group had higher expression of these cytotoxic granules (granzyme B and perforin) compared to healthy controls. Moreover, the levels of perforin in CD8 T cells of convalescent patients were reduced and similar to those in healthy controls. However, the levels of granzyme B in CD8 T cells were still higher in convalescent patients than in healthy controls. In contrast, the expression of granzyme B and perforin in double positive NK cells was decreased in acute patients, compared to healthy controls, whereas in the convalescent phase, the cytotoxic potential in NK cells was restored.
Fig. 3.
Intracellular expression of Granzyme B and Perforin in CD8 T cells and NK cells in acute SARS-CoV-2-infected patients, convalescent cases or healthy controls. *P < 0.05, ** P < 0.01, and ***P < 0.001.
3.4. Myeloid and plasmacytoid DCs decreased in severe and critically ill COVID-19 patients
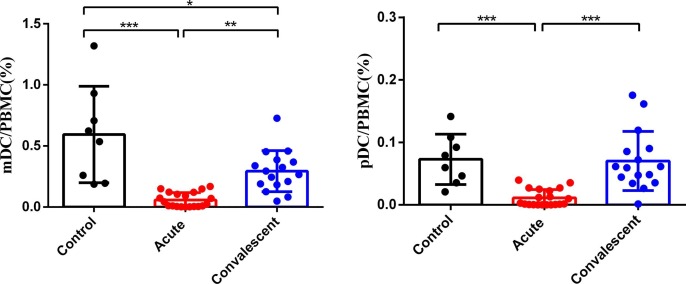
DCs play an important role in both innate and adaptive immune responses. Thus, we detected the proportion of mDCs and pDCs in peripheral blood mononuclear cells of acute severe and critically ill SARS-CoV-2-infected patients, convalescent cases and healthy controls. As shown in Fig. 4 , the frequencies of mDCs and pDCs in acute severe and critically ill SARS-CoV-2-infected patients were significantly lower than those in healthy controls. Of note, many convalescent patients had increased frequencies of mDCs and pDCs, and these frequencies were significantly higher than those in acute SARS-CoV-2-infected patients.
Fig. 4.
Comparison of the percentages of mDC and pDC in PBMC of acute SARS-CoV-2-infected patients, convalescent cases or healthy controls. *P < 0.05, ** P < 0.01, and *** P < 0.001.
4. Discussion
In this study, we systematically compared immunological responses and cytokine/chemokine profiles in acute and convalescent phases of COVID-19 infection. Previous studies have shown that high levels of cytokines and chemokines can induce cell damage and mediate destructive tissue inflammation during viral infections [16], [17], [18]. We found that serum levels of IP-10, MCP-1, MIG, IL-6, and IL-10, which are associated with the severity of COVID-19, were significantly higher in severe and critically ill COVID-19 patients. Our data are consistent with findings of previous studies [4], [12], [19]. Moreover, the results of the present study showed that the cytokine and chemokine levels in convalescent patients were restored to normal levels and similar to those in healthy controls. These observations suggest that IP-10, MCP-1, MIG, IL-6, and IL-10 could serve as potential biomarkers for monitoring COVID-19 disease recovery. Therefore, these findings indicate that cytokine and chemokine levels in the acute and convalescent stages of COVID-19 could contribute to our understanding of its pathogenesis and patient outcomes.
Lymphocytes, especially CD8 T cells and NK cells, are core components of the immune system that defend against viral infections. We recently reported that a SARS-CoV-2 infection resulted in broad immune cell reduction, including CD3 T cells, CD4 T cells, CD8 T cells, and NK cells, in critically ill COVID-19 patients [15].In order to further understand the immune status of CD4 T cells, CD8 T cells, and NK cells in severe and critically ill COVID-19 patients, we detected the activation status and cytotoxic granules of CD8 T cells and NK cells. Our results suggest that CD8 T cell activation, characterised by high expression of CD38, appears to correlate with disease severity in patients with COVID-19, which is consistent with other reports [20], [21]. Previous studies have shown that high expression of CD8 T cell cytotoxic molecules, such as granzyme B and perforin, are more prominent in severe cases compared to mild cases [21], [22]. In the present study, we found that CD8 T cells in severe and critically ill patients with COVID-19 had a high cytotoxic potential with high expression of granzyme B and perforin. Additionally, Liao et al. reported that CD8 T cells in the bronchoalveolar lavage fluid of severe COVID-19 cases expressed high levels of cytotoxic genes such as GZMB, GZMA, and GZMK [23]. Collectively, these data highlight the involvement of CD8 + T cells in the immunopathogenesis of a severe and critical ill COVID-19. Interestingly, when the cytotoxic potential of NK cells was assessed, we found lower expression levels of granzyme B and perforin in double positive NK cells in severe and critically ill patients with COVID-19, compared to healthy controls. These data demonstrated that SARS-CoV-2 infection led to decreased NK cell cytotoxic function in the acute group. Previous studies reported that the expression of granzyme A and B by NK cells in patients with COVID-19 was significantly lower than that in healthy controls [24], [25]. Thus, these data reveal that patients with COVID-19 have an NK cell functionally-impaired antiviral response. Importantly, in this study, convalescent patients with COVID-19 showed restoration of NK cell cytolytic functions along with enhanced levels of granzyme B and perforin.
Another important interface for NK cells with the innate immune response is their interaction with DCs which play an important role in immune monitoring, initiation, and antigen presentation. This connects the innate and adaptive immunity. Moreover, pDCs can produce large amounts of type I IFN in response to viral infections [26]. DCs are sentinel cells involved in innate and adaptive immunity that affect the pathogenesis of MERS and SARS [27], [28]. To the best of our knowledge, the present study is the first to compare the frequencies of mDCs and pDCs in acute severe and critically ill COVID-19 patients and individuals in the convalescent phase. Our findings demonstrated that the frequencies of mDCs and pDCs in severe and critically ill COVID-19 patients upon symptom onset were significantly reduced. A recent study demonstrated that the proportion of DCs were significantly reduced with functional impairment in mild and severe COVID-19 cases compared to healthy controls [29]. In addition, the proportions of pDCs in the bronchoalveolar lavage fluid from severe COVID-19 patients were significantly lower than those in moderate cases as evidenced by single-cell RNA sequencing [23]. Yang et al. found that monocyte-derived DCs were permissive to SARS-CoV-2 infection, which might explain the deficiency of host myeloid DCs and plasmacytoid DCs that occurred in severe and critically ill patients with COVID-19 [30]. Therefore, the results of the present study imply that the significant loss of pDCs and mDCs, together with NK cell reduction and functional impairment among severe and critically ill patients could lead to abortive innate immune responses against the SARS-CoV-2 infection and delayed viral clearance.
In conclusion, our results suggest that a hyperactive cytokine-mediated inflammatory response, dysregulation of CD8 T cells and NK cells, and deficiency of host mDCs and pDCs occur in severe and critically ill patients with COVID-19. These immunological molecules play an important role in the pathogenesis of SARS-CoV-2 infection. Therefore, our findings may contribute to the current knowledge on the immunopathological mechanisms of SARS-CoV-2 infection and provide insights into potential therapeutic targets and strategies.
Notes
Author contributions. Q.C. and Y.L. played roles in the study design and writing of the manuscript. Q.C., B.Y., Y.Y., J.H., J.Z., L.L., Y.L., X.P., B.C. played roles in the experiments, data collection, data analysis, and data interpretation. All authors reviewed and approved the final version of the manuscript.
Financial support
This work was supported by Guangzhou Science and Technology Program key projects (grant number 201704020213)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.N. Zhu, D. Zhang, W. Wang, X. Li, B. Yang, J. Song, X. Zhao, B. Huang, W. Shi, R. Lu, P. Niu, F. Zhan, X. Ma, D. Wang, W. Xu, G. Wu, G.F. Gao, W. Tan, I. China Novel Coronavirus, T. Research, A Novel Coronavirus from Patients with Pneumonia in China, 2019, N Engl J Med 382(8) (2020) 727-733. [DOI] [PMC free article] [PubMed]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China, The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.X. Yang, Y. Yu, J. Xu, H. Shu, J.a. Xia, H. Liu, Y. Wu, L. Zhang, Z. Yu, M. Fang, T. Yu, Y. Wang, S. Pan, X. Zou, S. Yuan, Y. Shang, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, The Lancet Respiratory Medicine 8(5) (2020) 475-481. [DOI] [PMC free article] [PubMed]
- 7.N. Chen, M. Zhou, X. Dong, J. Qu, F. Gong, Y. Han, Y. Qiu, J. Wang, Y. Liu, Y. Wei, J.a. Xia, T. Yu, X. Zhang, L. Zhang, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, The Lancet 395(10223) (2020) 507-513. [DOI] [PMC free article] [PubMed]
- 8.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson B.T., Chambers R.C., Liu K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi Y., Ge Y., Wu B., Zhang W., Wu T., Wen T., Liu J., Guo X., Huang C., Jiao Y., Zhu F., Zhu B., Cui L. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J. Infect. Dis. 2020;222(5):746–754. doi: 10.1093/infdis/jiaa363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L., Gou J., Gao J., Huang L., Zhu Z., Ji S., Liu H., Xing L., Yao M., Zhang Y. Immune characteristics of severe and critical COVID-19 patients. Signal Transduct. Target Ther. 2020;5(1):179. doi: 10.1038/s41392-020-00296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J.Y., Wang X.M., Xing X., Xu Z., Zhang C., Song J.W., Fan X., Xia P., Fu J.L., Wang S.Y., Xu R.N., Dai X.P., Shi L., Huang L., Jiang T.J., Shi M., Zhang Y., Zumla A., Maeurer M., Bai F., Wang F.S. Single-cell landscape of immunological responses in patients with COVID-19. Nat. Immunol. 2020;21(9):1107–1118. doi: 10.1038/s41590-020-0762-x. [DOI] [PubMed] [Google Scholar]
- 15.Wang W., Liu X., Wu S., Chen S., Li Y., Nong L., Lie P., Huang L., Cheng L., Lin Y., He J. Definition and Risks of Cytokine Release Syndrome in 11 Critically Ill COVID-19 Patients With Pneumonia: Analysis of Disease Characteristics. J. Infect. Dis. 2020;222(9):1444–1451. doi: 10.1093/infdis/jiaa387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J., Luo W., Chen T., Qin Q., Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171(8):850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 17.Chi Y., Zhu Y., Wen T., Cui L., Ge Y., Jiao Y., Wu T., Ge A., Ji H., Xu K., Bao C., Zhu Z., Qi X., Wu B., Shi Z., Tang F., Xing Z., Zhou M. Cytokine and chemokine levels in patients infected with the novel avian influenza A (H7N9) virus in China. J. Infect. Dis. 2013;208(12):1962–1967. doi: 10.1093/infdis/jit440. [DOI] [PubMed] [Google Scholar]
- 18.M.D. de Jong, C.P. Simmons, T.T. Thanh, V.M. Hien, G.J. Smith, T.N. Chau, D.M. Hoang, N.V. Chau, T.H. Khanh, V.C. Dong, P.T. Qui, B.V. Cam, Q. Ha do, Y. Guan, J.S. Peiris, N.T. Chinh, T.T. Hien, J. Farrar, Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia, Nat Med 12(10) (2006) 1203-7. [DOI] [PMC free article] [PubMed]
- 19.Y. Yang, C. Shen, J. Li, J. Yuan, J. Wei, F. Huang, F. Wang, G. Li, Y. Li, L. Xing, L. Peng, M. Yang, M. Cao, H. Zheng, W. Wu, R. Zou, D. Li, Z. Xu, H. Wang, M. Zhang, Z. Zhang, G.F. Gao, C. Jiang, L. Liu, Y. Liu, Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19, J Allergy Clin Immunol 146(1) (2020) 119-127 e4. [DOI] [PMC free article] [PubMed]
- 20.Jiang Y., Wei X., Guan J., Qin S., Wang Z., Lu H., Qian J., Wu L., Chen Y., Chen Y., Lin X. COVID-19 pneumonia: CD8(+) T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin Immunol. 2020;218 doi: 10.1016/j.clim.2020.108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J.-W., Zhang C., Fan X., Meng F.-P., Xu Z., Xia P., Cao W.-J., Yang T., Dai X.-P., Wang S.-Y., Xu R.-N., Jiang T.-J., Li W.-G., Zhang D.-W., Zhao P., Shi M., Agrati C., Ippolito G., Maeurer M., Zumla A., Wang F.-S., Zhang J.-Y. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng H.-Y., Zhang M., Yang C.-X., Zhang N., Wang X.-C., Yang X.-P., Dong X.-Q., Zheng Y.-T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 24.Mazzoni A., Salvati L., Maggi L., Capone M., Vanni A., Spinicci M., Mencarini J., Caporale R., Peruzzi B., Antonelli A., Trotta M., Zammarchi L., Ciani L., Gori L., Lazzeri C., Matucci A., Vultaggio A., Rossi O., Almerigogna F., Parronchi P., Fontanari P., Lavorini F., Peris A., Rossolini G.M., Bartoloni A., Romagnani S., Liotta F., Annunziato F., Cosmi L. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Invest. 2020;130(9):4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald-Bocarsly P., Dai J., Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19(1):3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law H.K., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W., Nicholls J.M., Peiris J.S., Lau Y.L. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106(7):2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu H., Zhou J., Wong B.H., Li C., Cheng Z.S., Lin X., Poon V.K., Sun T., Lau C.C., Chan J.F., To K.K., Chan K.H., Lu L., Zheng B.J., Yuen K.Y. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. 2014;454–455:197–205. doi: 10.1016/j.virol.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou R., To K.K., Wong Y.C., Liu L., Zhou B., Li X., Huang H., Mo Y., Luk T.Y., Lau T.T., Yeung P., Chan W.M., Wu A.K., Lung K.C., Tsang O.T., Leung W.S., Hung I.F., Yuen K.Y., Chen Z. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity. 2020;53(4):864–877 e5. doi: 10.1016/j.immuni.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang D., Chu H., Hou Y., Chai Y., Shuai H., Lee A.C., Zhang X., Wang Y., Hu B., Huang X., Yuen T.T., Cai J.P., Zhou J., Yuan S., Zhang A.J., Chan J.F., Yuen K.Y. Attenuated Interferon and Proinflammatory Response in SARS-CoV-2-Infected Human Dendritic Cells Is Associated With Viral Antagonism of STAT1 Phosphorylation. J. Infect. Dis. 2020;222(5):734–745. doi: 10.1093/infdis/jiaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]