Abstract
Background
Outcome for critically ill patients with COVID-19 treated with continuous renal replacement therapy (CRRT) is largely unknown. We describe mortality and renal outcome in this group.
Methods
This observational study was conducted at a university hospital in Sweden. We studied critically ill adult COVID-19 patients with Acute Kidney injury (AKI) who received CRRT.
Results
In 451 patients, AKI incidence was 43.7%. 18.2% received CRRT. Median age of CRRT patients was 60 years (IQR 54–65), 90% were male, median BMI was 29 (IQR 25–32), 23.2% had Diabetes, 37.8% hypertension and 6.1% chronic kidney disease prior to admission. 100% required mechanical ventilation. 8.5% received Extra Corporeal Membrane Oxygenation. Median length of stay was 23 days (IQR 15–26). ICU mortality was 39% and 90-day mortality was 45.1%. Age, baseline creatinine values and body weight change were associated with 60 days mortality. Of the survivors, no patients required dialysis at hospital discharge, 73.8% recovered renal function and a median 10.5% of body weight was lost during admission.
Conclusions
Critically ill COVID-19 patients with AKI who received CRRT had a 90-day mortality of 45.1%. At follow-up, three quarters of survivors had recovered renal function. This information is important in the clinical management of COVID-19.
Keywords: Acute kidney injury, COVID-19, Renal replacement therapy, Outcomes, Death, Dialysis, Renal recovery
1. Background
In critically ill patients with Severe Acute Respiratory Syndrome (SARS) Coronavirus 2 (COVID-19), Acute kidney injury (AKI) is a common complication [[1], [2], [3]]. AKI in intensive care patients is generally associated with an increased risk of death and of developing chronic kidney disease (CKD) and End Stage Renal Disease (ESRD) [4]. Initial reports suggested that patients with COVID-19 and AKI may have up to a 13-fold increased risk of death compared to those who did not develop AKI [4,7,8]. Outcome data for this group remains limited, but in some health care settings renal replacement therapy requirement (RRT) has been suggested as a criterion for palliation [2]. It is therefore of paramount importance that outcome is elucidated.
Sweden has a population of 10.4 million people and has under normal circumstances 522 ICU beds in 55 hospitals [5,6]. Thus, there are 5.0 ICU beds per 100,000 people, this is among the lowest ICU accessibility per capita in Europe, where Germany leads with 35 ICU beds per 100,000 of the population [6]. ICU bed capacity increased to a peak of 753 at the height of the pandemic, on the 24th of April 2020, according to the Swedish Intensive Care register [7].The present study aimed to investigate the short-term risk of death, dialysis requirement at hospital discharge and renal function at follow-up, in patients who received CRRT at our university hospital in Stockholm. This information may help to guide resource allocation and treatment during ongoing COVID outbreaks.
2. Method
2.1. Study design
This is an observational study describing the characteristics of and outcome for patients with COVID-19 who received CRRT at a tertiary referral centre in Sweden.
2.2. Study population
The study population consisted of all adult patients registered as having SARS COV2 infection (detected by a positive reverse transcriptase-polymerase chain reaction test from samples obtained from the upper or lower airway), admitted between March 15th and July 16th, 2020 at intensive care units at the Karolinska university hospital. Patients aged less than 18 years were excluded. We studied patients treated with CRRT in detail.
2.3. Outcomes
The primary outcome was 60-day mortality. Secondary outcomes in CRRT patients were body weight change during ICU stay, IHD dependence at hospital discharge, 90-day mortality, renal recovery and estimated Glomerular Filtratin Rate (eGFR) at follow-up. We aimed to identify factors associated with risk of death at 60 days. The latest follow-up available for all data was the sixth of November 2020.
2.4. Definitions
AKI was defined according to KDIGO AKI criteria using serum creatinine values [8]. CRRT is defined as continuous renal replacement using continuous veno-venous hemodiafiltration or diafiltration, either with Baxter's Prismaflex or Fresenius Multifiltrate platforms. Karolinska University Hospital expanded its ICU capacity four-fold and treated patients at nine established or temporary ICUs at two sites. CRRT was initiated at each physician's discretion guided by KDIGO AKI guidelines for CRRT.
2.5. Data collection
Patients were identified in the hospital intensive care patient data management system Clinisoft (GE medical) by searching for the ICD-10 COVID-19 diagnosis code (U07.1). We identified patients who received CRRT using interventions codes and by review of medical notes. Patients who were transferred between ICUs within the hospital were treated as having had one ICU admission. In patients transferred from ICUs at other hospitals, only interventions occurring at our hospital were considered.
Baseline creatinine level was chosen as the lowest creatinine value recorded in the year before ICU admission, or hospital admission value if this was missing (in cases where patients were transferred from other hospitals). Where no previous values where recorded, baseline creatinine was imputed by back calculating creatinine using the Modified Diet Renal Disease (MDRD) formula assuming a Glomerular filtration rate (GFR) of 75 ml/min/1.73m2, as recommended by KDIGO [9]. Renal recovery was defined as return to within 1.5 time baseline creatinine, using Acute Dialysis Quality Initiative (ADQI) definition of recovery and Acute Kidney Disease (AKD) [10].
Medical notes of all included patients were reviewed by medical staff from the electronic patient record system. Data obtained included demographics: age, sex, weight and height, baseline, admission and maximum ICU creatinine values. Occurrence and date of death were collated in all patients. In patients who received CRRT, daily laboratory data for renal function markers, serum creatinine, urea and Cystatin C, as well as myoglobin and weights were recorded. We collated intervention data regarding mechanical ventilation, CRRT treatment and duration as well as treatment with extra corporeal membrane oxygenation (ECMO). Outcome data including ICU discharge, hospital discharge, date of death and date of IHD treatments were recorded. We collated data on last available inpatient weight from hospital records. Follow-up data included serum creatinine, cystatin C, and urea and bodyweight. Data cleaning was performed to remove inaccurate data before analysis.
2.6. Ethical considerations
The study complied with the Declaration of Helsinki and was approved by the Swedish Ethical Review Authority (Diary number 2020–02859). Requirement for signed informed consent was waived.
2.7. Statistical analysis
Patient characteristics and variables are presented using frequencies and percentages for categorial data, and medians with interquartile range (IQR) are used for continuous variables. Differences between the groups were tested using t-test for normally distributed continuous variables, Wilcoxon rank sum for non-normally distributed continuous variables and chi-square test for categorical variables.
Survival analysis was performed using the Kaplan Meier method. Mortality data was extracted up until the end of follow-up, on the 6th of November 2020. We considered time from ICU admission to death or to end of follow-up, which ever occurred first. Cox regression was used to obtain mortality estimates, confidence intervals (CI) and Hazard ratios (HR) are given.
In patients who received CRRT, baseline data and clinical characteristics of patients who survived to 60 days were compared with those who died at or before 60 days. Variables of interest in these two groups included age, sex, body mass index (BMI), comorbidities, pre-ICU and ICU lab data, saps-III score, ECMO treatment and ICU body weight change. A univariate cox regression was performed using the above variables. We did not calculate HRs for the comorbidities affecting a small number of patients (less than 5 patients) due to lack of power. Variables with a p value <0.05 were introduced in a multivariate cox regression and stepwise regression was used. The least significant variables were removed, and the regression was stopped when all remaining variables had a p values smaller than 0.05. We tested for collinearity.
Statistical analyses were performed using Stata version 12 (StataCorp, College Station, US).
3. Results
Between March 15th and July 16th, 2020, 451 adult COVID-19 patients were admitted to ICUs at the Karolinska University Hospital. At least one admission creatinine value was available in all patients. Incidence of AKI overall was 43.7%, 9.5% had AKI grade I, 8.9% AKI grade II and 25.3% experienced AKI grade III (supplementary table I). Outcome data was available for all patients regarding survival. Compared to non-AKI patients, AKI patients were significantly older, 60 years (IQR 53–67), versus 58 (IQR 50–65), P = 0.010, and had a longer ICU length of stay, 17 days (IQR10–29) versus 8 days (IQR 3–16) P ≤0.001. AKI patients had higher SAPS III scores, with a median of 56 (IQR 49–63) compared to non-AKI patients with 51 (45–58) P ≤0.001), see Supplementary table 2.
3.1. Patients who received CRRT
82 patients (18.2%) received CRRT whilst on ICU and all these patients were included in the analysis. Data regarding receipt of CRRT at hospital discharge was available in all patients. Forty percent of patients (32) received both CRRT and IHD during their admission. Data on body weight change was available in 60 patients (73%). Follow-up renal function data was available in 93.3% of survivors (2 patients were transferred back to hospitals outside the Stockholm region and their follow-up data was not obtainable, 1 further patient has not been followed-up). Demographic and clinical characteristics of CRRT patients are presented in Table 1 . CRRT was initiated at a median of 5 days after ICU admission (IQR 2–10 days). At CRRT initiation, 77 of the 82 patients fulfilled KDIGO criteria for AKI grade III and four patients had AKI grade II. One patient had AKI grade 0, the CRRT indication was fluid overload.
Table 1.
Baseline characteristics, ICU parameters and outcome in patients admitted to ICU with COVID-19 who received CRRT.
| Baseline characteristics of CRRT cohort | ||
| Age(years) Median, (IQR) | 60 | (54–65) |
| Women N, % | 8 | 9.7 |
| Length of ICU stay (days) | 23 | (15–26) |
| SAPS III N = 75 Median Score, (IQR) | 61 | (53–71) |
| BMI (kg/m2) N = 72 Median, (IQR) | 29 | (25–32) |
| Over 30 kg/m2N, (% of N) | 28 | 38.9 |
| Over 40 kg/m2N, (% of N) | 4 | 5.5 |
| Comorbidity | N | % |
| Hypertension | 31 | 37.8 |
| Diabetes type I or II | 19 | 23.2 |
| Chronic renal failure (grade IV or V) | 5 | 6.1 |
| Any malignancy | 2 | 2.4 |
| Chronic pulmonary disease | 7 | 8.5 |
| Rheumatic disease | 2 | 2.4 |
| Cerebrovascular disease | 4 | 4.8 |
| Myocardial infarction | 1 | 1.2 |
| Peripheral vascular disease | 1 | 1.2 |
| Congestive cardiac failure | 4 | 4.0 |
| Lab values | Median | IQR |
| Baseline Creatinine(μmol/l)a | 83 | 72–94 |
| Admission Creatinine (μmol/l) | 83 | (72–110) |
| Highest ICU Creatinine (μmol/l) | 437 | (353–542) |
| Highest ICU Urea (mmol/l) | 36 | (25–42) |
| Highest ICU Cystatin C (mg/l) | 5.3 | (4.5–5.9) |
| Highest ICU myoglobin (ug/L) N = 80 | 4130 | (1488–6842) |
| Weight at ICU admission (N = 75) | 89 | (75–100) |
| Median discharge weight (N = 60) | 81 | (70–92) |
| Weight loss during ICU stay (kg) (N = 60) | −6.0 | (−11.8–1.6) |
| Percentage weight loss during ICU stay (%) (N = 60) | −7.2 | (−12.8–2.1) |
| Intervention | ||
| Mechanical ventilation N, (%) | 82 | 100 |
| ECMO N, (%) | 7 | 8.5 |
| CRRT only N, (%) | 62 | 76 |
| CRRT and IHD N, (%) on ICU | 32 | 40 |
| Number of days of CRRT Median (IQR) | 12 | [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]] |
| Number of days of IHD Median (IQR) | 3 | [[2], [3], [4], [5], [6]] |
| Mortality | N | % |
| ICU mortality | 32 | 39.0% |
| 60-day mortality | 37 | 45.1 |
| 90-day mortality | 37 | 45.1 |
| Hospital mortality | 37 | 45.1 |
| Follow-up data (N = 42) | Median | IQR |
| Creatinine umol/l, N = 42 | 97 | (73–116) |
| Last registered Cystatin C, (mg/l) N = 26 | 1.52 | (1.4–1.9) |
| eGFR creatinine-based (ml/min/1.73m2) N = 42 | 66 | (56–78) |
| eGFR cystatin C based (ml/min/1.73m2) N = 26 | 47 | (37–53) |
| Weight at follow-up (kg) N = 39 | 85 | (77–98) |
| Follow up weight as percent of baseline (%) N = 39 | −3 | (−17 − +17) |
| AKD n (% of survivors with data (N = 42)) | 11 | 26.2 |
IQR Interquartile range.
SAPS Simplified physiology score III.
BMI Body mass index.
ECMO Extra corporeal membrane oxygenation.
CRRT Continuous renal replacement therapy.
IHD Intermittent Haemodialysis.
AKD Acute Kidney Disease.
eGFR estimated glomerular filtration rate.
Estimated in 12 patients.
Most CRRT patients (90%) were male (Table 1). Median age was 60 years (IQR 54–65). SAPS III score was recorded in 75 patients (96%), median score was 61 (IQR 53–71). BMI was available in 72 patients; median BMI at ICU admission was 29 kg/m2 (IQR 25–32). BMI was greater than 30 kg/m2 in 38.9% and in 5.5% BMI was over 40 kg/m2. The most frequently reported comorbidities were hypertension (37.8%), diabetes mellitus type I or II (23.2%) and chronic pulmonary disease (8.5%). Five patients (6.1%) had chronic kidney disease prior to admission. Baseline serum creatinine was available in 70 patients (86%) and was estimated in 12; the median was 83 μmol/l IQR (72–94). The median ICU admission creatinine was 83 μmol/l (IQR 72–110). The highest values of renal function markers during ICU admission (group medians) were creatinine 437 μmol/l (IQR 353–542), urea 36 mmol/l (IQR (25–42) and Cystatin C 5.3 mg/l (IQR 4.5–5.9). Median Creatinine at discharge from the Karolinska hospital for all CRRT patients was 105 μmol/l (IQR 83–188), while it was 156 μmol/l for those who died (IQR 100–261) and 93 μmol/l (IQR 65–122) for survivors. 32 patients died on ICU, of these 23 (71.8%) received CRRT in the last 24 hours of their lives. The median last creatinine values were 129 μmol/l (IQR 99–162) in the non-survivors not receiving CRRT at the time of death and 102 (73–189) in those whodid. Maximum serum myoglobin during ICU admission was a median of 4130 μg/l (IQR 1488–6842). Body weight at admission was recorded in 75 patients and median was 89 kg (IQR 75–100). In those in whom baseline, and last ICU body weights were recorded (N = 60), median discharge weight was 81 kg (IQR =70–92). Median weight reduction was -6 kg (IQR -11-8–1.6 kg). This represents a median − 7.2% (IQR -12.8–2.1%) change in weight from baseline.
ICU stay for patients with COVID-19 and CRRT was a median of 23 days (IQR 15–26, range 1–85 days) (Table 1). All these patients were mechanically ventilated, and 7 patients (8.5%) received Veno-Venous ECMO; in 7 other cases, patients were referred, but not accepted for ECMO. Median treatment time with CRRT was 12 days (IQR 5–21) and the median number of IHD treatments per patient was 3 (IQR 2–6). IHD was given on ICU to 32 patients in the CRRT group (40%) and in the majority of cases, it was administered after a course of CRRT. 20 (40%) ICU survivors received at least one IHD treatment after ICU discharge. All survivors were discharged from hospital before end of follow-up, median time from ICU admission to hospital discharge was 53 days (IQR 38–70).
3.2. Primary outcome mortality
3.2.1. Overall mortality
Patients were followed for a median of 181 days (IQR 127–202). Overall mortality for the COVID-19 cohort (451 patients) was 19.1% at 30 days (with 86 patients dying) and 23.1% at 60 days, when 104 patients had died. Mortality was significantly higher in patients with AKI (42.6%) compared to patients with no-AKI (8.7%), crude hazard ratio was 5.9 P ≤0.001 (supplementary tables 1 and 3). Mortality increased with increased grade of AKI being 27.9% in grade 1, 40.0% in grade II and 49.1% in grade III (supplementary table 1).
3.2.2. Mortality in the CRRT group
In total 37 patients (45.1%) who received CRRT died up until the end of follow-up. Crude mortality estimates for CRRT are presented in Table 2 . 30-day mortality was 30.5% (CI 21.8–41.7), hospital, 60 day (primary outcome) and 90-day mortality were estimated as being 45.1% (CI 35.2–56.5). Patients died a median 21 days (IQR 16–35) after ICU admission. 32 patients (39.0%) died on ICU. A further 5 patients died in hospital a mean 7 days after ICU discharge. No patient in the CRRT group died after hospital discharge. Mortality in the CRRT group was significantly higher than in non-CRRT patients, HR 2.59 (CI 1.73–3.86) P ≤0.001. A Kaplan Meier survival curve comparing survival for CRRT patients with all other COVID-19 patients is displayed in Fig. 1 .
Table 2.
Crude estimated mortality predictions using Cox regression for COVID-19 ICU patients who received continuous renal replacement therapy.
| Time after admission (days) | Predicted mortality % | 95% Confidence interval |
|---|---|---|
| 30 | 30.5 | 21.8–41.7 |
| 60 | 45.1 | 36.1–56.5 |
| 90 | 45.1 | 35.2–56.5 |
Fig. 1.
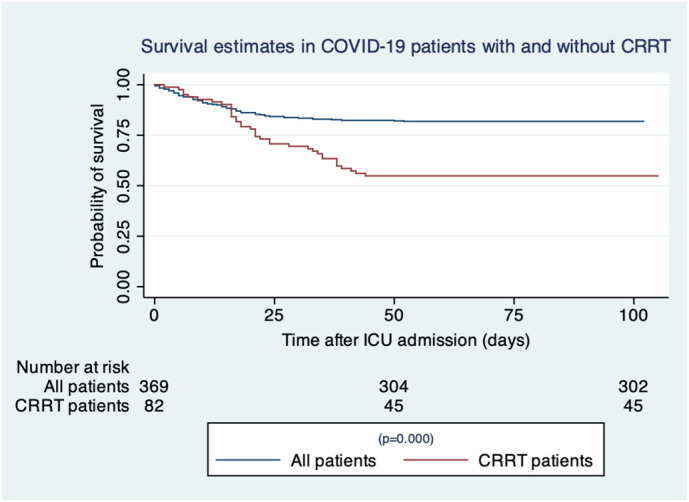
Survival estimates in COVID-19 patients with and without CRRT.
3.2.3. Predictors of survival to 60 days in CRRT patients
Characteristics of CRRT patients who survived 60 days after ICU admission are compared in Table 3 with those who died before 60 days. The characteristics of these groups were similar, but survivors differed in being younger than those who died (median 55 versus 63 years P ≤0.0001) and in having higher ICU maximum creatinine 463 μmol/l (IQR 397–560) versus 383umol/l(IQR 333–464) P = 0.032.
Table 3.
Comparison of baseline characteristics and ICU parameters in patients admitted to ICU with COVID-19 and who received RRT between those who survived up to 60-days and those who did not survive.
| Variable | Alive at 60 days (N = 45) | Dead at 60 days (N = 37) | P |
|---|---|---|---|
| Age. Median, (IQR) | 55 (52–64) | 63 (58–68) | <0.001 |
| Female. N % | 4 (8.9) | 4 (10.81) | 0.770 |
| SAPS III % (CI) | 64 (53–69) | 58 (54–72) | 0.70 |
| BMI % (CI) | 30 (27–32) | 28 (26–31) | 0.14 |
| Hypertension N % | 20 (44.0) | 11 (29.3) | 0.171 |
| Diabetes N % | 9 (20.0) | 10 (27.0) | 0.453 |
| Chronic renal disease N % | 1 (2.2) | 4 (10.8) | 0.106 |
| COPD N (%) | 6 (13.3) | 1 (2.7) | 0.086 |
| Congestive cardiac failure N % | 3 (6.7) | 1 (2.7) | 0.406 |
| Malignancy N % | 2 (4.4) | 0 (0) | 0.194 |
| ECMO N (%) | 5 (11.1) | 2 (5.4) | 0.358 |
| Median (IQR) | Median (IQR) | ||
| Admission Creatinine (μmol/l) | 84 (72–98) | 81(72–113) | 0.562 |
| Baseline Creatinine (μmol/l) | 83(70–93) | 80 (72–95) | 0.090 |
| Highest creatinine ICU (μmol/L) | 463 (397–560) | 383 (333–464) | 0.032 |
| Highest urea ICU (mmol/l) | 37 (28–41) | 34 (25–43) | 0.473 |
| Highest ICU cystatin (mg/l) | 5.5 (4.6–6.6) | 5.3 (4.4–5.7) | 0.135 |
| Highest ICU myoglobin (ug/L) | 4690 (2400–9030) | 3000 (1670–8440) | 0.205 |
| Weight change during admission (kg) | −9.0 (IQR −16–5) | −1 (−3.5 −+0.7) | <0.001 |
| Percentage weight change from baseline (%) | −10.5 (−15.3–6.7) | −1.2 (−4.1–+0.7) | <0.001 |
*Statistically significant difference between groups.
S.D. standard deviation.
CI Confidence interval.
SAPS Simplified physiology score III.
BMI Body mass index.
ECMO Extra corporeal membrane oxygenation.
Patients alive at 60 days had a median body weight change −9 kg (IQR −16–5) representing a median percentage change of −10.5% (IQR −15.3–6.7). Those who died differed in having a median weight change of −1 kg (IQR −3.5 − +0.7) with a percentage weight change of −1.2% (IQR −4.1- +0.7%) p ≤0.001.
In univariate cox regression, positive weight change during ICU admission, age and baseline creatinine values were associated with death at 60 days (supplementary table 4). These variables remained significant after stepwise multivariate regression and are included in the final model for prediction of survival to 60 days, which included 60 patients (Supplementary table 5). The Hazard ratio (HR) for every 1 kg increase in weight from admission was 1.13 (P < 0.001; CI 1.06–1.19). For every 1-year incremental increase in age, the HR for risk of death increased by 1.08 (p = 0.011; CI 1.01–1.14). The HR for a 1 unit-increase in baseline creatinine value was 1.01 (P = 0.012; CI 1.00–1.01).
3.2.4. Follow-up data CRRT patients
Follow-up time for survivors after CRRT was a median of 172 days (IQR 121–188), with range of 27–213 days. Of the 45 patients that survived, follow-up creatinine values were available in 42 cases (Table 1). Median Creatinine was 97 μmol/l (IQR 73–116). Follow-up creatinine was within 1.5 times the baseline value in 31 patients (73.8%), with 11 cases (26.2%) fulfilling criteria for non-recovery, according to AKD definition. Median Cystatin C, taken in 26 patients, was 1.52 mg/dl (IQR 1.4–1.9). Cystatin based eGFR was 47 ml/min/1.73m2 (37–53) and in these 26 patients, median creatinine eGFR was 66 ml/min/1.73m2 (IQR 50.3–71). Median weight at follow-up was 85 kg (IQR 77–97.7), with median percentage change from baseline at follow-up of −3% (IQR -17 − +17%).
3.2.5. Outcome for CRRT patients with chronic kidney disease
Five patients with CKD (grade IV or V) prior to admission received CRRT during ICU stay. Four patients (80%) died in hospital. The surviving patient did not require IHD after discharge.
3.2.6. AKI grade III patients who did not receive CRRT
There were 32 patients within the COVID-AKI cohort who fulfilled AKI grade III criteria, but who did not receive CRRT (Supplementary table 6). In 16 patients, treatment restriction decisions were made on the basis of futility, and mortality was 100%. Highest creatinine values in this group were most often recorded on the day of death. A further 15 patients were not treated with CRRT, because it was judged not to be clinically necessary and one further patient received intermittent haemodialysis, mortality in this group was 20%. Overall mortality in the AKI grade III group without CRRT was 60%.
4. Discussion
4.1. Key findings
In a cohort of COVID-19 Intensive care patients treated at the Karolinska University Hospital, AKI incidence was 43.7%. CRRT was given to 18.2% of patients and in this group, hospital, 60-day and 90-day mortality were 45.1%. Predictors of hospital mortality for CRRT patients were age at admission, weight change during hospital stay and baseline creatinine value. No patients required dialysis at hospital discharge or follow-up. CRRT COVID-19 survivors experienced a median 10.5% decrease in body weight during hospital stay. 73.8% of survivors had recovered renal function according to AKD criteria at follow-up.
4.2. Relationship with previous studies
Mortality in our AKI grade III CRRT population is low, compared to other published outcome data for RRT patients and even compared to mortality for COVID-AKI patients in general. In an Italian cohort of 99 COVID-19 patients treated at a tertiary referral centre, hospital mortality for AKI patients was 38.9% and for those who received CRRT, it was 52.9% [11]. In a second Italian cohort of 777 patients in Genoa, AKI incidence was 22.6% and 12% received RRT. AKI mortality at 60 days was 63%, hazard ratio for death in AKI versus non-AKI was 1.60 [95% CI 1.21–2.49] p = 0.002 [12]. Specific mortality for RRT was not reported.
Chans described a cohort of nearly 4000 hospitalised patients at Mount Sinai Health System in New York, 76% of the 976 ICU patients had AKI and 19% received RRT. In-hospital mortality in the AKI group was 50%, mortality for RRT patients was not separately reported. Odds ratio for mortality in AKI versus non-AKI populations was 9.2 (95%, CI 7.5–11.3) [13]. In a systematic review by Robbins-Juarez of 20 studies, which included over 13,000 patients hospitalised with COVID-19, prevalence of AKI was 17%, with 77% of these patients experiencing severe illness/ICU admission [14]. Mortality reported up until May 31st was reported as 52%. AKI increased the risk of death with an odds ratio of 15.3. Five percent of patients required RRT.
Only a few studies specifically report the outcome for CRRT patients. Results from a Dutch cohort of 37 COVID patients, of whom 13 received RRT, are in line with our results. Mortality for RRT group was 39% and for non-RRT AKI it was 44% [15]. Zahid's cohort from an inner-city hospital in New York, which is described as serving a severely economically deprived neighbourhood, had an in-hospital mortality in AKI patients of 71.1% and in those who received RRT, mortality was 90.7%. [2]. They concluded that the findings “should prompt the early involvement of a palliative care team to define goals of care in patients with COVID-19 disease and stage 3 AKI”. Ng's study of 9657 COVID-19 patients, reported mortality for the non-AKI group as 7.3%, for AKI patients without RRT it was 46.3% and for those receiving RRT it was 79.3% [16]. Additionally, of those who survived 30.6% were dialysis dependent at discharge. The authors concluded that the high mortality in the group is “underscoring the need for shared decision making in these critically ill patients”.
Post COVID renal function is also reported by Chan and co-authors, who defined recovery as return to with-in 25% of baseline creatinine (or to a difference of less than 26.5umol/l from baseline). They found that 35% of patients had not recovered function at discharge [13]. We found renal recovery using creatinine-based eGFR estimates in our survivors to be good, with only 26.2% of patients fulfilling AKD criteria at follow-up and no patient requiring IHD post discharge. However estimated GFR was lower at follow-up, when assessed using cystatin C (median 46.5 versus 66 ml/min/1.73m2 for creatinine) and physicians should be aware that in the presence of muscle mass loss, creatinine may not accurately estimate renal function.
Much emphasis has been given to the potentially differing pathophysiological process occurring during COVID-19 AKI, which may include microthrombi formation, renal congestion secondary to right ventricular failure and direct viral injury. This study cannot elucidate on the mechanism of AKI, but does suggest a clear reversibility and recovery is comparable to that seen in AKI patients with different aetiologies, such as our previously reported mixed ICU cohort, where AKD incidence was 18.9% [17].
Factors that predicted 60-day survival were age, baseline creatinine and weight change on ICU. Patients who survived had a median weight change of -9 kg (−10.5%) during ICU admission. This may represent primarily sarcopenia occurring during a long duration of intensive care treatment. Those who died differed in having a median weight change of -1 kg (−1.2%) which may reflect a positive fluid balance, since they likely had a concomitant muscle mass loss. Maximum ICU creatinine values were significantly lower in RRT patients who died, and we could speculate that this may reflect a relative dilution of renal markers due to fluid overload. Fluid balance data was not available to us and is a limitation. More detailed data regarding hemodynamic status, urine production, urine analysis, diuretic use and hospital discharge cystatin Cvalues for calculation of the sarcopenia index would be necessary to elucidate the reason for weight and fluid balance differences between survivors and non survivors [18].
Outcome clearly depends on the setting, the demographics and the comorbid burden. The low mortality observed in our study may reflect that the patients were treated in a relatively wealthy public health care system, in which CRRT resources, although stretched, were always available and the normal standard of ICU care was largely maintained. Appropriate selection of patients judged most likely to benefit from CRRT may have contributed to the outcome observed. This may be reflected by low mortality in non-dialysed AKI grade III patients without treatment restrictions and 100% mortality in those in whom initiation of treatment was judged to be futile. The low mortality may also be due to the demographics of our patients, our cohort was relatively young (median age 60 years) and had a relatively low comorbid burden, seen from an international perspective. The population prevalence of CKD and diabetes in Sweden is of 4.3% and 6.8%, respectively, compared with USA, where CKD prevalence is 14.3% and diabetes 10.5% [[19], [20], [21], [22]]. Furthermore our cohort had access to a largely free health care system.
4.3. Implications of findings
Our study's findings show that COVID-19 respiratory failure combined with CRRT-requiring AKI is associated with a high, but not extreme mortality. The implications of the findings are that CRRT should be offered to patients (in the absence of other factors which would render treatment futile) and should not lead to automatic initiation of palliation in relatively wealthy health care settings similar to our own. ADQI has issued recommendation regarding AKI and CRRT initiation for COVID-19 AKI and they advise that initiation of acute CRRT “should be based on patient needs, local expertise and the availability of staff and equipment and that a coordinated response to an increase in RRT demand and/or supply chain failure at an organizational, regional and national level is needed” [3].
The weight loss experienced by survivors is an important finding because it has implications for patient's rehabilitation and the interpretation of their post COVID renal function test, where sarcopenia may result in low creatinine values and lead to falsely high GFR estimates, if based on serum creatinine measurements alone [17,23].
4.4. Strengths and limitations
This is one of only a very few studies that report outcome for COVID-19 CRRT patients. It is unique in presenting mortality, renal function and data on weight change up to 6 months after admission. The limitations of the study include its observational status, its size and its single centre nature (despite including 9 ICU's spread over 2 sites). Lack of detailed data regarding fluid balance is a limitation.
4.5. Generalisability
Our results are generalisable to COVID-19 patient populations in countries with similar resource abundant healthcare systems and similar comorbid burdens. The relatively low mortality reported here may not be achievable in other health care systems.
5. Conclusion
Critically ill COVID-19 patients with AKI who received RRT had 60 and 90-day mortality of 45.1%. Predictors of survival were age, baseline creatinine and body weight change during ICU stay. Three quarters of patients had recovered renal function at follow-up, suggesting COVID-19 related AKI is reversible. Survivors lost a median 10.5% of their body weight during admission. This data is important in the clinical management of COVID-19 patients and in the planning of acute RRT and outpatient nephrological services.
Financial disclosure
The project has not received funding.
Declaration of Competing Interest
The authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2021.04.002.
Appendix A. Supplementary data
Supplementary material
References
- 1.Rudnick M.R., Hilburg R. Acute kidney injury in COVID-19: another challenge for nephrology. Am J Nephrol. 2020;51:761–763. doi: 10.1159/000511161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahid U., Ramachandran P., Spitalewitz S., et al. Acute kidney injury in COVID-19 patients: an Inner City hospital experience and policy implications. Am J Nephrol. 2020;51:786–796. doi: 10.1159/000511160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadim M.K., Forni L.G., Mehta R.L., et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat Rev Nephrol. 2020;382:727. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stigare C., Frumento P., Bottai M., et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; A Swedish multi-centre cohort study. Critical Care (London, England) 2015;19:221. doi: 10.1186/s13054-015-0920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Statistics Sweden SCB Population Statistics. 2021. https://www.scb.seenfinding-statisticsstatistics-by-subject-areapopulationpopulation-compositionpopulation-statistics
- 6.Bauer J., Brüggmann D., Klingelhöfer D., et al. Access to intensive care in 14 European countries: a spatial analysis of intensive care need and capacity in the light of COVID-19. Intensive Care Med. 2020;46:2026–2034. doi: 10.1007/s00134-020-06229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swedish Intensive Care Register . Swedish Intensive Care register; 2021. Data portal. httpportalicuregsweorgutdataenhome. [Google Scholar]
- 8.Gibney N. 2012. KDIGO clinical guidelines for management of AKI; pp. 1–39. [Google Scholar]
- 9.Group KDIGOKCW KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 10.Chawla L.S., Bellomo R., Bihorac A., et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 11.Fominskiy E.V., Scandroglio A.M., Monti G., et al. Prevalence, characteristics, risk factors, and outcomes of invasively ventilated COVID-19 patients with acute kidney injury and renal replacement therapy. Blood Purif. 2020:1–8. doi: 10.1159/000508657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo E., Esposito P., Taramasso L., et al. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J Nephrol. 2020;55:105924. doi: 10.1007/s40620-020-00875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan L., Chaudhary K., Saha A., et al. Acute kidney injury in hospitalized patients with COVID-19. medRxiv. 2021;32(1):151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins-Juarez S.Y., Qian L., King K.L., et al. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep. 2020;5:1149–1160. doi: 10.1016/j.ekir.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilbers T.J., Koning M.V. Renal replacement therapy in critically ill patients with COVID-19: a retrospective study investigating mortality, renal recovery and filter lifetime. J Crit Care. 2020;60:103–105. doi: 10.1016/j.jcrc.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng J.H., Hirsch J.S., Hazzan A., et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77(2) doi: 10.1053/j.ajkd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stigare C., Ravn B., Awad A., et al. Creatinine- and cystatin C-based incidence of chronic kidney disease and acute kidney disease in AKI survivors. Crit Care Res Pract. 2018;2018:7698090. doi: 10.1155/2018/7698090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barreto E.F., Kanderi T., DiCecco S.R., et al. Sarcopenia index is a simple objective screening tool for malnutrition in the critically ill. JPEN J Parenter Enteral Nutr. 2019;43:780–788. doi: 10.1002/jpen.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holzmann M.J., Ivert T., Jungner I., et al. Renal function assessed by two different formulas and incidence of myocardial infarction and death in middle-aged men and women. J Intern Med. 2010;267:357–369. doi: 10.1111/j.1365-2796.2009.02171.x. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention National diabetes statistics report. 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 21.Centers for Disease Control, Prevention: Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance System. 2021. https://www.cdc.gov/kidneydisease/index.html Available from:
- 22.Andersson T., Ahlbom A., Carlsson S. Diabetes prevalence in Sweden at present and projections for year 2050. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prowle J.R., Kolic I., Purdell-Lewis J., et al. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol. 2014;9:1015–1023. doi: 10.2215/CJN.11141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


