Abstract
Background
National guidelines recommend that sexually active people with human immunodeficiency virus (PWH) who are men who have sex with men (MSM) be tested for hepatitis C virus (HCV) infection at least annually. Hepatitis C virus testing rates vary by race/ethnicity in the general population, but limited data are available for PWH.
Methods
We analyzed medical records data from MSM in the HIV Outpatient Study at 9 human immunodeficiency virus (HIV) clinics from January 1, 2011 through December 31, 2019. We excluded observation time after documented past or current HCV infection. We evaluated HCV antibody testing in each calendar year among HCV-seronegative MSM, and we assessed testing correlates by generalized estimating equation analyses.
Results
Of 1829 eligible MSM who were PWH, 1174 (64.2%) were non-Hispanic/Latino white (NHW), 402 (22.0%) non-Hispanic black (NHB), 187 (10.2%) Hispanic/Latino, and 66 (3.6%) of other race/ethnicity. Most were ≥40 years old (68.9%), privately insured (64.5%), with CD4 cell count/mm3 (CD4) ≥350 (77.0%), and with HIV viral load <200 copies/mL (76.9%). During 2011–2019, 1205 (65.9%) had ≥1 HCV antibody test and average annual HCV percentage tested was 30.3% (from 33.8% for NHB to 28.5% for NHW; P < .001). Multivariable factors positively associated (P < .05) with HCV testing included more recent HIV diagnosis, public insurance, lower CD4, prior chlamydia, gonorrhea, syphilis, or hepatitis B virus diagnoses, and elevated liver enzyme levels, but not race/ethnicity.
Conclusions
Although we found no disparities by race/ethnicity in HCV testing, low overall HCV testing rates indicate suboptimal uptake of recommended HCV testing among MSM in HIV care.
Keywords: epidemiology, HCV testing, HIV/AIDS, hepatitis, treatment-naive
There were no discernable racial/ethnic disparities in HCV testing among MSM within the HOPS during 2011-2019. However, low annual HCV testing rates across all racial and ethnic groups indicated suboptimal uptake of recommended HCV screening.
The prevention of hepatitis C virus (HCV) infection and associated health conditions (eg, cirrhosis and hepatocellular carcinoma) is a public health priority [1]. On the basis of a 2013–2016 survey study, approximately 2.4 million people in the United States were living with HCV [2]. However, national survey results indicate that approximately 50% of persons with HCV are unaware of their infection [3]. In addition, the awareness of HCV infection and the clinical cascade of HCV care varies substantially by race/ethnicity [3–6].
An estimated 20%–30% of the 1.1 million people with human immunodeficiency virus (PWH) in the United States are coinfected with HCV [7]. Liver-related mortality, primarily due to HCV, is a leading non-acquired immune deficiency syndrome (AIDS)-related cause of death among PWH [8–10]. In 2009, routine HCV testing was recommended for all PWH by the Centers for Disease Control and Prevention ([CDC] Atlanta, GA), National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America (IDSA), because sexual HCV transmission was increasingly reported in sexual networks of men living with human immunodeficiency virus (HIV), and heterosexual transmission of HCV might occur in persons with partners who were coinfected with HIV and HCV [11]. In 2011, the first orally available directly acting antiviral (DAA) for HCV treatment was approved by the US Food and Drug Administration, which expanded therapeutic options beyond lengthy and less effective interferon-based treatments [12]. Accumulated evidence has further shown DAA regimens to be safe, well tolerated, and result in high HCV cure rates (>95%) [13].
Diagnosis and awareness of HCV infection is the first essential step towards achieving cure of the infection and preventing onward transmission. Given the availability of the highly effective DAA treatments and growing recognition of sexually transmitted HCV infection among PWH, especially gay, bisexual, and other men who have sex with men (MSM), the CDC and IDSA recommended persons who inject drugs (PWID) and PWH who are MSM be tested for HCV at least annually, and more often if engaging in high-risk sexual or drug use practices [14–18]. However, results from pre-DAA era indicated HCV screening deficiencies. For example, in a large multisite cohort of PWH in primary care, although approximately 85% of PWH were screened for prevalent HCV infection at enrollment in care, only approximately half with nonreactive HCV antibody test at baseline ever had subsequent HCV testing [19]. An earlier study among the HIV Outpatient Study (HOPS) participants who were eligible for HCV testing (ie, had previously tested negative or had no tests) found that approximately 70% of MSM were ever tested for HCV in 2007 [20]. Men who have sex with men (compared with PWID and people with heterosexual risk for HIV) and white participants were overall less likely to be tested for HCV during 1996–2007[20]. A further HOPS analysis revealed that the incidence rate of HCV infection among MSM was 1.04 per 100 person-years during 2000–2013, and MSM comprised 59% of incident cases in the cohort [21].
To our knowledge, the rates of and the demographic disparities in HCV testing among MSM who are PWH have not been well described in the period covering more recent HIV and HCV management guidelines [22]. We sought to examine the rates of HCV testing among MSM in HIV care in diverse HOPS clinics and to explore disparities in HCV testing by race/ethnicity and other sociodemographic factors, including health insurance, during 2011 to 2019.
METHODS
The HIV Outpatient Study
The HOPS is an ongoing prospective observational cohort study of adult PWH receiving care at 9 HIV clinics (university based, public, and private) in 6 US cities (Chicago, IL; Denver, CO; Stony Brook, NY; Philadelphia, PA; Tampa, FL; and Washington, DC) since 1993 [23]. Patient data, including sociodemographic characteristics, symptoms, diagnoses, treatments, and laboratory values, are abstracted from medical charts and entered into an electronic database by trained staff. These data are reviewed for quality and analyzed centrally. The present analysis is based on the HOPS dataset available as of September 30, 2020. Since 2007, the HOPS has used an optional supplemental survey to gather patient self-reported information on sociodemographic and behavioral measures, including sexual behaviors, substance use, and adherence to antiretroviral (ARV) therapy. The survey is conducted via telephone or web-based audio-computer assisted self-interview (ACASI). All data are reviewed for quality and analyzed centrally.
Ethics
Since its inception, the HOPS protocol has been reviewed and approved annually by the institutional review boards of the CDC, Cerner Corporation (Kansas City, MO), and each local site. The study protocol conforms to the guidelines of the US Department of Health and Human Services for the protection of human subjects in research.
Patient Consent Statement
The protocol of the HOPS was approved by the institutional review boards of the CDC, Cerner Corporation, and each local site, and all participants gave written informed consent.
Study Population
We analyzed medical records data from MSM receiving HIV care at 9 HOPS clinics, including 3 Ryan White Program-funded sites, during January 1, 2011 to December 31, 2019. We included participants with at least 2 HOPS encounters, at least 2 years of observation, and at least 1 CD4+ T-lymphocyte count (CD4) and plasma HIV ribonucleic acid ([RNA] viral load [VL]) test result performed during observation. We excluded participants with injection drug use HIV risk, or those who had a positive HCV antibody or HCV RNA (VL) test before or at the start of observation (baseline date, see definition below). In addition, we censored observation during follow-up for participants after their first positive HCV antibody or HCV VL test. We then evaluated HCV antibody testing during follow-up among MSM without evidence of current or prior HCV infection. We performed a subanalysis for those participants who completed at least 1 ACASI survey during 2011–2019.
Measurements and Definitions
Baseline was defined as the first instance when a patient was under active observation in the HOPS on or after January 1, 2011. Baseline demographic variables included age (<30, 30–39, 40–49, and ≥50 years); sex; race/ethnicity (non-Hispanic/Latino black [NHB], non-Hispanic/Latino white [NHW], Hispanic/Latino or other/unknown race/ethnicity); year of HIV diagnosis (≤2000, 2001–2010, 2011–2019); insurance (private, public, or none); HOPS clinic type (private and public); ARV history (experienced, naive, and other/unknown); CD4 (<200, 200–349, 350–499, and ≥500 cells/mm3); HIV VL (<200, 200–999, 1000–99 999, and ≥100 000 copies/mL); prior chlamydia, gonorrhea, syphilis, or hepatitis B virus (HBV) diagnoses; and aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels >2.5 times the upper limit of normal (ULN). Baseline CD4 and VL tests were those performed closest to baseline, whereas baseline AST and ALT tests were those done at or before the baseline date. Time-updated variables included age, insurance type, CD4, HIV VL, chlamydia or gonorrhea, syphilis, HBV diagnoses, and AST or ALT >2.5 times the ULN. The outcome variable was the receipt of an HCV antibody test, in each calendar year, during observation. Among the participant subset who completed at least 1 ACASI, factors of interest were engaging in substance use (any reported use of cocaine, “poppers,” heroin, methamphetamine, “club drugs,” or erectile dysfunction drugs) or condomless sex (insertive or receptive anal intercourse) during the 6 months before completing the ACASI.
Statistical Analyses
We plotted the percentage of persons tested for HCV by calendar year for each racial/ethnic group. Regression lines were fitted to plots of HCV testing percentages by calendar year, and corresponding slopes were estimated. Ninety-five percent confidence intervals (CIs) were calculated for testing percentages for each calendar year using the Mid-P exact test within OpenEpi (Open Source Epidemiologic Statistics for Public Health, Version 3.01), and the Cochran-Armitage trend test was used to obtain slope P values by race/ethnicity. For descriptive summaries of patient characteristics by race/ethnicity, we used Likelihood ratio χ 2 or Fisher exact tests for binary or class variables and Kruskal-Wallis or Wilcoxon rank-sum tests for comparing continuous variables. Univariate and multivariable generalized estimating equations (GEEs) analyses were performed to assess factors associated with undergoing HCV antibody testing in any calendar year of observation, and odds ratios (ORs) with associated 95% CIs were reported. Results with P < .05 were considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Among 11 331 HOPS participants who were enrolled in the HOPS as of September 30, 2020, we restricted analyses to those who (1) had at least 2 HOPS clinical encounters during 2011–2019 (excluded n = 6841), (2) had at least 2 years of follow-up (excluded n = 1081), (3) had at least 1 CD4 and VL test performed during the observation (excluded n = 37), (4) had MSM risk without injection drug use HIV risk (excluded n = 1422), and (5) had a prior positive HCV antibody or positive HCV VL test result (excluded n = 121) (Figure 1). Of the 1829 participants thus selected, 1174 (64.2%) were NHW, 402 (22.0%) were NHB, 187 (10.2%) were Hispanic/Latino, and 66 (3.6%) were of other race/ethnicity.
Figure 1.
Participant selection flow chart. Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; HOPS, HIV Outpatient Study; MSM, who are men who have sex with men.
Table 1 describes the characteristics of the study population, overall, and by race/ethnicity. Among the 1829 eligible MSM, most participants were ≥40 years of age (68.9%), diagnosed with HIV during 2000 or earlier (51.2%), and privately insured (64.5%), with CD4 ≥350 cells/mm3 (77.0%), HIV VL <200 copies/mL (76.9%), and prior ARV experience (83.2%). Approximately one quarter of the of participants had AST or ALT levels >2.5 times the ULN.
Table 1.
Patient Characteristics By Race/Ethnicity Among MSM in HIV Care: The HIV Outpatient Study, 2011–2019, N = 1829
| Patient Characteristics at Beginning of Observation, n (%) | Overall n = 1829 (100.0) |
NHW n = 1174 (64.2) |
NHB n = 402 (22.0) |
Hispanic/Latino n = 187 (10.2) |
Other/Unknown Race/ Ethnicity n = 66 (3.6) |
P Value |
|---|---|---|---|---|---|---|
| Age, Years | <.001 | |||||
| <30 | 230 (12.6) | 72 (6.1) | 111 (27.6) | 36 (19.3) | 11 (16.7) | |
| 30–39 | 339 (18.5) | 177 (15.1) | 96 (23.9) | 49 (26.2) | 17 (25.8) | |
| 40–49 | 628 (34.3) | 409 (34.8) | 122 (30.3) | 72 (38.5) | 25 (37.9) | |
| ≥50 | 632 (34.6) | 516 (44.0) | 73 (18.2) | 30 (16.0) | 13 (19.7) | |
| Year of HIV Diagnosis | <.001 | |||||
| ≤2000 | 936 (51.2) | 682 (58.1) | 166 (41.3) | 66 (35.3) | 22 (33.3) | |
| 2001–2010 | 627 (34.3) | 381 (32.5) | 143 (35.6) | 73 (39.0) | 30 (45.5) | |
| 2011–2019 | 266 (14.5) | 111 (9.5) | 93 (23.1) | 48 (25.7) | 14 (21.2) | |
| AIDS Diagnosis | 897 (49.0) | 570 (48.6) | 210 (52.2) | 92 (49.2) | 25 (37.9) | .17 |
| Insurance | <.001 | |||||
| Private | 1179 (64.5) | 864 (73.6) | 175 (43.5) | 109 (58.3) | 31 (47.0) | |
| Public | 386 (21.1) | 166 (14.1) | 156 (38.8) | 52 (27.8) | 12 (18.2) | |
| Other/unknown payer | 264 (14.4) | 144 (12.3) | 71 (17.7) | 26 (13.9) | 23 (34.8) | |
| HOPS Clinic Type | <.001 | |||||
| Private | 1387 (75.8) | 1052 (89.6) | 173 (43.0) | 111 (59.4) | 51 (77.3) | |
| Public | 442 (24.2) | 122 (10.4) | 229 (57.0) | 76 (40.6) | 15 (22.7) | |
| Current/prior tobacco smoker | 794 (43.4) | 504 (42.9) | 190 (47.3) | 80 (42.8) | 20 (30.3) | .06 |
| CD4+ Cell Count, Cells/mm3 | <.001 | |||||
| <200 | 104 (5.7) | 47 (4.0) | 38 (9.5) | 14 (7.5) | 5 (7.6) | |
| 200–349 | 203 (11.1) | 119 (10.1) | 54 (13.4) | 22 (11.8) | 8 (12.1) | |
| 350–499 | 351 (19.2) | 223 (19.0) | 81 (20.2) | 34 (18.2) | 13 (19.7) | |
| 500+ | 1057 (57.8) | 710 (60.5) | 197 (49.0) | 111 (59.4) | 39 (59.1) | |
| Median CD4+ (IQR) | 545 (373–734) | 574 (398–760) | 466 (283–656) | 534 (353–705) | 549 (374–699) | <.001 |
| Viral Load, Copies/mL | <.001 | |||||
| <200 | 1406 (76.9) | 995 (84.8) | 238 (59.2) | 126 (67.4) | 47 (71.2) | |
| 200–999 | 69 (3.8) | 34 (2.9) | 25 (6.2) | 9 (4.8) | 1 (1.5) | |
| 1000–99 999 | 256 (14.0) | 102 (8.7) | 107 (26.6) | 34 (18.2) | 13 (19.7) | |
| ≥100 000 | 98 (5.4) | 43 (3.7) | 32 (8.0) | 18 (9.6) | 5 (7.6) | |
| ARV History | <.001 | |||||
| Experienced | 1521 (83.2) | 1048 (89.3) | 283 (70.4) | 142 (75.9) | 48 (72.7) | |
| Naive | 277 (15.1) | 107 (9.1) | 114 (28.4) | 41 (21.9) | 15 (22.7) | |
| Other/Unknown | 31 (1.7) | 19 (1.6) | 5 (1.2) | 4 (2.1) | 3 (4.6) | |
| Prior Chlamydia Diagnosis | .17 | |||||
| Yes | 142 (7.8) | 84 (7.2) | 42 (10.4) | 12 (6.4) | 4 (6.1) | |
| No | 1687 (92.2) | 1090 (92.8) | 360 (89.6) | 175 (93.6) | 62 (93.9) | |
| Prior Gonorrhea Diagnosis | .97 | |||||
| Yes | 187 (10.2) | 117 (10.0) | 43 (10.7) | 20 (10.7) | 7 (10.6) | |
| No | 1642 (89.8) | 1057 (90.0) | 359 (89.3) | 167 (89.3) | 59 (89.4) | |
| Prior Syphilis Diagnosis | <.001 | |||||
| Yes | 340 (18.6) | 179 (15.2) | 118 (29.4) | 31 (16.6) | 12 (18.2) | |
| No | 1489 (81.4) | 995 (84.8) | 284 (70.6) | 156 (83.4) | 54 (81.8) | |
| Prior HBV Infection Diagnosis | .73 | |||||
| Yes | 175 (9.6) | 113 (9.6) | 41 (10.2) | 17 (9.1) | 4 (6.1) | |
| No | 1654 (90.4) | 1061 (90.4) | 361 (89.8) | 170 (90.9) | 62 (93.9) | |
| AST or ALT >2.5 × ULN | .25 | |||||
| Yes | 428 (23.4) | 292 (24.9) | 84 (20.9) | 39 (20.9) | 13 (19.7) | |
| No | 1401 (76.6) | 882 (75.1) | 318 (79.1) | 148 (79.1) | 53 (80.3) |
Abbreviations: AIDS, acquired immune deficiency syndrome; ALT, alanine aminotransferase; ARV, antiretroviral; AST, aspartate aminotransferase; HBV, hepatitis B virus; IQR, interquartile range; NHB, non-Hispanic/Latino black; NHW, non-Hispanic/Latino white; ULN, upper limit of normal.
NOTES: P value obtained using continuity adjusted, likelihood ratio, χ 2 tests, or Fisher exact test.
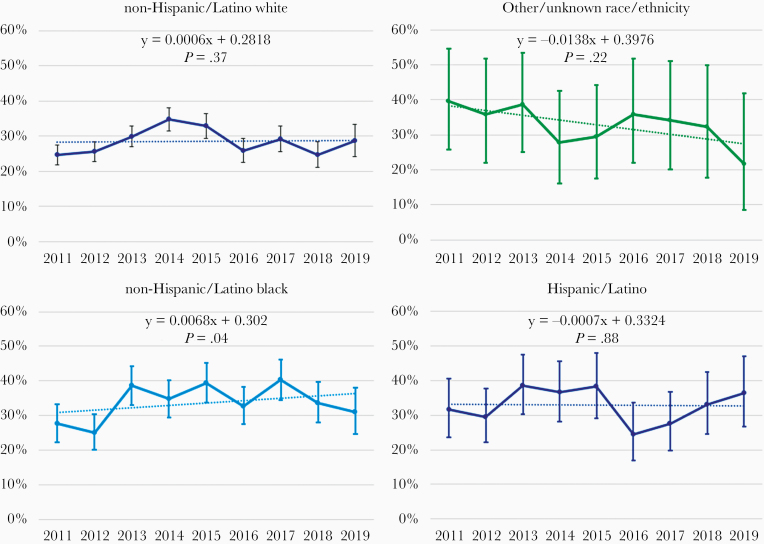
During 2011–2019, 1205 (65.9%) MSM underwent an HCV test, and the percentage with at least 1 HCV test during this period varied by race/ethnicity (72.9% for NHB, 72.7% for Hispanic/Latino, and 66.7% for other race/ethnicity, 62.4% for NHW; P < .001). The average annual HCV testing percentage during 2011–2019 was 30.3%, with the highest percentage for NHB men (33.8%), followed by men of other race/ethnicity (33.4%), Hispanic/Latino (32.9%), and NHW (28.5%) (P < .001; data not shown). The only statistically significant linear change in the percentage of participants tested annually for HCV among racial/ethnic groups occurred for NHB (P = .04) (Figure 2).
Figure 2.
Percentage of hepatitis C virus antibody testing among men with HIV who have sex with men by race/ethnicity and calendar year, the HIV Outpatient Study, 2011–2019, N = 1829. Note: Each graph has a fitted regression line, respective P value for difference between estimated slope and zero slope, and 95% confidence intervals for each data point.
In univariate GEE analyses, factors associated with receiving an HCV antibody test included age <30 years (compared with ≥50 years), NHB or Hispanic/Latino race/ethnicity (compared with white), having an HIV diagnosis in 2001 or later, having public or other/no insurance type, receiving care at a public clinic, being ARV-naive, as well as having a history of chlamydia or gonorrhea, syphilis, and HBV, and ALT or AST levels greater than 2.5 times the ULN (Table 2). In multivariable GEE analysis, compared with NHW, men who were NHB or of other race/ethnicity did not have significantly different odds of HCV testing. Multivariable factors positively associated with HCV testing included later year of HIV diagnosis versus year 2000 or before (OR = 1.18 and 95% CI = 1.02–1.37 for 2001–2010; and OR = 1.42 and 95% CI = 1.09–1.84 for 2011–2019), public versus private insurance (OR = 1.22, 95% CI = 1.07–1.39), and CD4 of 200–349 and 350–499 vs ≥500 cells/mm3 (OR = 1.28 and 95% CI = 1.08–1.52 and OR = 1.19 and 95% CI = 1.05–1.35, respectively), a history of chlamydia or gonorrhea (OR = 1.68, 95% CI = 1.42–2.00), syphilis (OR = 1.54, 95% CI = 1.29–1.84), hepatitis B virus (OR = 6.02, 95% CI = 2.59–13.98), and AST or ALT levels >2.5 times ULN (OR = 1.30, 95% CI = 1.13–1.49) (Table 2).
Table 2.
GEE Analyses of Factors Associated With Receiving an HCV Antibody Test: The HIV Outpatient Study, 2011–2019, N = 1829
| Participant Characteristics | Univariate OR (95% CI) | P Value | Multivariable OR (95% CI) | P Value |
|---|---|---|---|---|
| Age, Years* | ||||
| <30 | 1.35 (1.16–1.58) | <.001 | 1.11 (0.95–1.30) | .19 |
| 30–39 | 1.11 (0.98–1.26) | .11 | 0.99 (0.87–1.13) | .86 |
| 40–49 | 1.10 (0.97–1.25) | .12 | 1.08 (0.95–1.22) | .22 |
| ≥50 | Referent | |||
| Race/Ethnicity | ||||
| NHW | Referent | Referent | ||
| NHB | 1.25 (1.08–1.46) | .003 | 1.07 (0.90–1.26) | .44 |
| Hispanic/Latino | 1.29 (1.05–1.59) | .017 | 1.12 (0.90–1.40) | .30 |
| Other/Unknown | 1.32 (0.92–1.90) | .13 | 1.19 (0.82–1.71) | .36 |
| Year of HIV Diagnosis | ||||
| ≤2000 | Referent | Referent | ||
| 2001–2010 | 1.25 (1.08–1.44) | .002 | 1.18 (1.02–1.37) | .03 |
| 2011–2019 | 1.65 (1.38–1.98) | <.001 | 1.42 (1.09–1.84) | .009 |
| AIDS Diagnosis | ||||
| Yes | 0.88 (0.77–1.00) | .047 | 0.88 (0.76–1.01) | .06 |
| No | Referent | Referent | ||
| Insurance* | ||||
| Private | Referent | Referent | ||
| Public | 1.25 (1.11–1.41) | <.001 | 1.22 (1.07–1.39) | .003 |
| Other/unknown payer | 1.26 (1.08–1.46) | .003 | 1.22 (1.05–1.42) | .01 |
| HOPS Clinic Type | ||||
| Private | Referent | Referent | ||
| Public | 1.20 (1.04–1.38) | .010 | 0.90 (0.77–1.07) | .23 |
| Current/prior tobacco smoker | 1.09 (0.96–1.24) | .17 | 1.13 (0.99–1.28) | .08 |
| CD4+ Cell Count, Cells/mm3,* | ||||
| <200 | 1.14 (0.89–1.45) | .31 | 1.24 (0.96–1.60) | .09 |
| 200–349 | 1.16 (0.98–1.38) | .09 | 1.28 (1.08–1.52) | .005 |
| 350–499 | 1.03 (0.91–1.17) | .62 | 1.19 (1.05–1.35) | .007 |
| 500+ | Referent | Referent | ||
| Viral Load, Copies/mL* | ||||
| <200 | Referent | Referent | ||
| 200–999 | 1.53 (1.14–2.04) | .004 | 1.60 (1.20–2.14) | .001 |
| 1000–99 999 | 1.21 (0.97–1.50) | .09 | 1.12 (0.89–1.42) | .32 |
| ≥100 000 | 1.44 (0.87–2.38) | .15 | 1.33 (0.81–2.19) | .26 |
| ARV History | ||||
| Experienced | Referent | Referent | ||
| Naive | 1.39 (1.18–1.64) | <.001 | 0.95 (0.75–1.20) | .68 |
| Other/Unknown | 1.24 (0.76–2.02) | .40 | 1.14 (0.69–1.89) | .61 |
| Chlamydia or Gonorrhea Diagnosis* | ||||
| Yes | 1.81 (1.53–2.14) | <.001 | 1.68 (1.42–2.00) | <.001 |
| No | Referent | Referent | ||
| Syphilis Diagnosis* | ||||
| Yes | 1.71 (1.44–2.04) | <.001 | 1.54 (1.29–1.84) | <.001 |
| No | Referent | Referent | ||
| HBV Diagnosis* | ||||
| Yes | 6.57 (2.83–15.27) | <.001 | 6.02 (2.59–13.98) | <.001 |
| No | Referent | Referent | ||
| AST or ALT >2.5 × ULN* | ||||
| Yes | 1.32 (1.16–1.51) | <.001 | 1.30 (1.13–1.49) | <.001 |
| No | Referent | Referent |
Abbreviations: ALT, alanine aminotransferase; ARV, antiretroviral; AST, aspartate aminotransferase; CI, confidence interval; GEE, generalized estimating equation; HBV, hepatitis B virus; HCV, hepatitis C virus; NHB, non-Hispanic/Latino black; NHW, non-Hispanic/Latino white; OR, odds ratio, ULN, upper limit of normal.
*Time updated variables.
There were 1220 (66.7%) MSM in the HOPS who completed at least 1 ACASI during 2011–2019. Among them, 843 (69.1%) were NHW, 230 (18.9%) were NHB, 113 (9.3%) were Hispanic/Latino, 491 (40.2%) reported substance use, and 524 (43.0%) reported condomless anal sex, each in the time frame of the last 6 months (Supplementary Table 1). Among MSM who reported substance use, 157 (32.0%) had undergone at least 1 HCV test in the past 12 months including 16 MSM (3.3%) who received the test twice. Similarly, 180 (34.4%) of MSM who reported condomless sex had undergone at least 1 HCV test in the past 12 months before most recent ACASI completion including 12 MSM (2.3%) who received the test twice. In addition, no racial/ethnic difference in HCV testing was observed among these 2 high-risk subgroups (Supplementary Table 2a and b). In multivariable GEE analysis, neither substance use (OR = 0.94, 95% CI = 0.79–1.12) nor condomless sex (OR = 1.15, 95% CI = 0.96–1.37) was associated with receiving an HCV antibody test (Supplementary Table 3).
DISCUSSION
In this large, ethnically diverse and prospectively followed group of MSM with HIV but without injection drug use, 65.9% received 1 or more tests for HCV infection over a 9-year period of observation. In unadjusted analyses, the overall annual HCV testing rate was 30.3%, and this differed significantly by race/ethnicity, ranging from 28.5% for NHW to 33.8% for NHB. We found that lower than recommended annual HCV testing levels held over time for all racial and ethnic groups. Having a more recent HIV diagnosis, public insurance, a history of chlamydia, gonorrhea, syphilis, or HBV diagnosis, and elevated ALT or AST levels were independently associated with HCV testing. After controlling for these factors, the odds of HCV testing among NHB and Hispanic/Latino MSM were not higher than the odds for NHW MSM.
The MSM who are PWH are at risk of HCV infection particularly when engaging in unprotected, mucosally traumatic sexual practices, such as anal receptive intercourse, and use of substances during sex [24, 25]. However, relatively low HCV testing levels for this population have been consistently reported in the literature, most of which comes from pre-DAA era [19, 26]. Garg et al [26] examined medical records data from Fenway Health in Boston and found that among 995 PWH (mostly MSM) whose initial HCV antibody results were negative, only 38% had more than 1 HCV test during 1997–2009. Freiman et al [19] found that among 9077 PWH enrolled in the Center for AIDS Research Network of Integrated Clinical Systems from 2000–2011 who had no evidence of current or prior HCV infection at enrollment, only 56% ever received additional HCV testing. Among 2715 MSM in that cohort whose first-time ALT results measured >40 IU/L, only 20% had a follow-up HCV test within 12 months [19]. In addition, Hoover et al [22] evaluated hepatitis prevention services among 1329 MSM PWH who had medical visits from 2004 to 2007 and found that only 54% were ever screened for HCV. Among 641 who had no suspected exposure to HCV infection, only 13% were retested over the years [22]. Absence of routine HCV testing as standard of care for PWH before 2009 may partially account for these observed low testing percentages. In this analysis, spanning time period while the national guidelines recommending at least annual HCV testing among MSM with HIV were in effect, the annual testing rate of 30.3% remains concerning. Suboptimal testing for HCV infection, as documented in the HOPS medical record, suggests missed opportunities for prompt HCV detection, treatment, and subsequent HCV prevention through behavioral risk reduction. The less frequent than recommended HCV testing for MSM might be, in part, attributed to the following factors: (1) underappreciation of risk of sexual transmission of HCV infection among healthcare providers and/or patients; (2) lack of awareness or noncompliance with updated HCV testing guidelines [11, 14] that recommend switching from symptom- and risk-based testing to routine annual testing for MSM with HIV; (3) low prioritization of such testing by providers due to outpatient visit time constraints; and (4) lack of awareness among PWH that HCV testing is readily available, minimally invasive, accurate, and routinely covered by most public and private insurance, as required by the Affordable Care Act. In this analysis, we were not able to identity the causes of suboptimal HCV testing rates in this population by using HOPS medical records data, and additional research including survey studies is warranted.
Our findings of no racial/ethnic disparities in HCV testing corroborate those from 3 previously published studies. Based on medical records data from 8 large US HIV clinics during 2004–2007, Hoover et al [22] found that HCV screening did not vary significantly by race or ethnicity. Similar findings were reported by Freiman [19], respectively. Previous HOPS data suggested that racial and ethnic differences in HIV testing existed among PWH, including PWID [20]. Possible explanations to account for differences in our current findings include the following: (1) our analysis focused on MSM with HIV but without injection drug use, instead of all PWH; and (2) use of data from more recent years, including the DAA era, in our current analysis and the availability of curative treatment may have contributed to more routine HCV screening among all groups.
Race/ethnicity is an important contributing factor to disparities in preventive services use and in health outcomes [5, 27–29]. Continued assessment of the potential roles of race and ethnicity in the uptake of HCV testing would help clarify reasons for low testing rates among PWH, especially MSM. It is noteworthy that, consistent with our findings, the analyses of data from HIV Research Network also found that PWH with public insurance were more likely to undergo HCV testing than those with private insurance [30]. The Ryan White Care Act-funded HIV/AIDS Programs strive to increase HCV testing and treatment among HIV/HCV-coinfected persons, which may, in part, explain HCV testing differences by insurance type. Last, our analysis corroborates other study findings that abnormal liver function tests and prior diagnoses of chlamydia, gonorrhea, or syphilis were positively associated with HCV testing [31, 32].
Hepatitis C virus acquisition is associated with condomless sex and injection and noninjection drug use, including the consumption of drugs to facilitate or enhance sexual performance and pleasure. In a recent study, the European AIDS Treatment Network (NEAT) acute hepatitis C consensus panel emphasized risk factors associated with sexual routes of mucosal HCV transmission in MSM, including fisting, receptive condomless anal intercourse, sharing sex toys, sharing anal douching equipment, sharing equipment during nasally administered drug use, and recommended that MSM engaging in the above-mentioned risk activities be tested for HCV infection every 3–6 months [33]. Our secondary analysis indicated that only one third of MSM with HIV in our study, who reported substance use or condomless sex on ACASI, had undergone an HCV test in the past 12 months before ACASI survey completion. The significant deficits in recommended HCV testing point to the need for additional support services for this high-risk population, such as improving the ascertainment of HCV risk in clinical settings through a comprehensive history taking, offering routine HCV testing for those at risk, and formulating other innovative strategies to detect, cure, and prevent HCV infection [34, 35]. A French modeling study has shown that combined test-and-treat and risk-reduction strategies could substantially reduce HCV acquisition and transmission, paving a way to HCV elimination among MSM with HIV [34].
Our study is subject to several limitations. First, we did not have sexual behavior data systematically collected and available for all HOPS participants. Therefore, the study population in the main analysis did not exclude MSM with HIV for whom annual or more frequent HCV testing would not be recommended: those not sexually active and those with no history of condomless anal intercourse during the study period. However, in the subset analysis restricting to MSM who reported substance use or condomless sex, the HCV testing rate in the past 12 months before the most recent ACASI completion was still approximately 32%–34%, and no racial/ethnic difference was observed in these 2 subgroups reporting the risk indications for HIV testing. Second, we might have underestimated HCV testing because of incomplete capture of HCV tests conducted outside of HOPS clinics. Finally, our study findings, which were based on a convenience sample of patients from 9 HOPS clinics, may not be generalizable to MSM in other HIV clinic settings or those not in HIV care.
CONCLUSIONS
In conclusion, although we found no discernable racial/ethnic disparities in HCV testing among MSM within the HOPS, low HCV testing rates across all racial and ethnic groups indicate suboptimal uptake of recommended HCV screening, which is the first and indispensable step in the HCV care continuum. To end the epidemic of HCV in the United States and achieve the World Health Organization HCV elimination target—90% reduction of new infections by 2030—additional efforts to improve provider adherence to nationally recommended HCV testing guidelines are warranted [36, 37].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was funded by the Centers for Disease Control and Prevention (contract nos. 200-2001-00133, 200-2006-18797, and 200-2011-41872).
Potential conflicts of interest. F. J. P. has been a consultant and/or on the Speakers’ Bureau for Gilead Sciences, Janssen Pharmaceuticals, Merck and Co., and ViiV. R. M. N. is a consultant to ViiV and Gilead. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
The HOPS Investigators include the following persons and sites in 2019: Jun Li, Kate Buchacz, and Marcus D. Durham, Division of HIV/AIDS Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC) Atlanta, GA; Cheryl Akridge, Stacey Purinton, Nabil Rayeed, Selom Agbobil-Nuwoaty, Kalliope Chagaris, Kimberly Carlson, Carl Armon, Linda Battalora, and Jonathan Mahnken, Cerner Corporation, Kansas City, MO; Frank J. Palella, Saira Jahangir, Conor Daniel Flaherty, and Patricia Bustamante, Feinberg School of Medicine, Northwestern University, Chicago, IL; John Hammer, Kenneth S. Greenberg, Barbara Widick, and Rosa Franklin, Rocky Mountain Cares, Denver, CO; Douglas J. Ward, Troy Thomas, and Cheryl Stewart, Dupont Circle Physicians Group, Washington, DC; Jack Fuhrer, Linda Ording-Bauer, Rita Kelly, and Jane Esteves, State University of New York (SUNY), Stony Brook, NY; Ellen M. Tedaldi, Ramona A. Christian, Faye Ruley, Dania Beadle, and Princess Davenport, Lewis Katz School of Medicine at Temple University, Philadelphia, PA; Richard M. Novak, Andrea Wendrow, and Stockton Mayer, University of Illinois at Chicago, Chicago, IL; Mia Scott, Billie Thomas, and Loraine Van Slyke, APEX Family Medicine, Denver, CO; Cynthia Mayer, Terry Beitler, Karen Maroney, and Denise Franklin, SJH Comprehensive Research Institute, Tampa, FL.
Contributor Information
for the HIV Outpatient Study (HOPS) Investigators:
Jun Li, Kate Buchacz, Marcus D Durham, Cheryl Akridge, Stacey Purinton, Nabil Rayeed, Selom Agbobil-Nuwoaty, Kalliope Chagaris, Kimberly Carlson, Carl Armon, Linda Battalora, Jonathan Mahnken, Frank J Palella, Saira Jahangir, Conor Daniel Flaherty, Patricia Bustamante, John Hammer, Kenneth S Greenberg, Barbara Widick, Rosa Franklin, Douglas J Ward, Troy Thomas, Cheryl Stewart, Jack Fuhrer, Linda Ording-Bauer, Rita Kelly, Jane Esteves, Ellen M Tedaldi, Ramona A Christian, Faye Ruley, Dania Beadle, Princess Davenport, Richard M Novak, Andrea Wendrow, Stockton Mayer, Mia Scott, Billie Thomas, Loraine Van Slyke, Cynthia Mayer, Terry Beitler, Karen Maroney, and Denise Franklin
References
- 1. Ward JW, Valdiserri RO, Koh HK. Hepatitis C virus prevention, care, and treatment: from policy to practice. Clin Infect Dis 2012; 55(Suppl 1):S58–63. [DOI] [PubMed] [Google Scholar]
- 2. Hofmeister MG, Rosenthal EM, Barker LK, et al. . Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology 2019; 69:1020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim HS, Yang JD, El-Serag HB, Kanwal F. Awareness of chronic viral hepatitis in the United States: an update from the National Health and Nutrition Examination Survey. J Viral Hepat 2019; 26:596–602. [DOI] [PubMed] [Google Scholar]
- 4. Backus LI, Belperio PS, Loomis TP, Mole LA. Impact of race/ethnicity and gender on HCV screening and prevalence among U.S. veterans in Department of Veterans Affairs Care. Am J Public Health 2014; 104(Suppl 4):S555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim NJ, Locke CJ, Park H, et al. . Race and hepatitis C care continuum in an underserved birth cohort. J Gen Intern Med 2019; 34:2005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vutien P, Hoang J, Brooks L Jr, et al. . Racial disparities in treatment rates for chronic hepatitis C: analysis of a population-based cohort of 73 665 patients in the United States. Medicine (Baltimore) 2016; 95:e3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831–7. [DOI] [PubMed] [Google Scholar]
- 8. Ingle SM, May MT, Gill MJ, et al. ; Antiretroviral Therapy Cohort Collaboration. Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis 2014; 59:287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith CJ, Ryom L, Weber R, et al. . Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 10. Weber R, Sabin CA, Friis-Møller N, et al. . Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166:1632–41. [DOI] [PubMed] [Google Scholar]
- 11. Kaplan JE, Benson C, Holmes KK, et al. . Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009; 58(RR-4):1–207; quiz CE1-4. [PubMed] [Google Scholar]
- 12. Geddawy A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct acting anti-hepatitis C virus drugs: clinical pharmacology and future direction. J Transl Int Med 2017; 5:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, et al. . Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017; 166:637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Workowski KA, Bolan GA; Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 15. American Association for the Study of Liver Disease and Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org. Accessed 14 June 2016.
- 16. Centers for Disease Control and Prevention. Recommendations for HIV prevention with adults and adolescents with HIV in the United States, 2014. Available at https://stacks.cdc.gov/view/cdc/26062. Accessed 14 June 2018.
- 17. Centers for Disease Control and Prevention. Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the U.S. Department of Health and Human Services. MMWR Recomm Rep 2012; 61:1–40. [PubMed] [Google Scholar]
- 18. Aberg JA, Gallant JE, Ghanem KG, et al. ; Infectious Diseases Society of America. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–34. [DOI] [PubMed] [Google Scholar]
- 19. Freiman JM, Huang W, White LF, et al. . Current practices of screening for incident hepatitis C virus (HCV) infection among HIV-infected, HCV-uninfected individuals in primary care. Clin Infect Dis 2014; 59:1686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spradling PR, Richardson JT, Buchacz K, et al. . Trends in hepatitis C virus infection among patients in the HIV Outpatient Study, 1996–2007. J Acquir Immune Defic Syndr 2010; 53:388–96. [DOI] [PubMed] [Google Scholar]
- 21. Samandari T, Tedaldi E, Armon C, et al. . Incidence of hepatitis C virus infection in the Human Immunodeficiency Virus Outpatient Study cohort, 2000–2013. Open Forum Infect Dis 2017; 4:ofx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoover KW, Butler M, Workowski KA, et al. ; Evaluation Group for Adherence to STD and Hepatitis Screening. Low rates of hepatitis screening and vaccination of HIV-infected MSM in HIV clinics. Sex Transm Dis 2012; 39:349–53. [DOI] [PubMed] [Google Scholar]
- 23. Buchacz K, Armon C, PalellaFJ, Jr., et al. . The HIV Outpatient Study-25 years of HIV patient care and epidemiologic research. Open Forum Infect Dis 2020; 7:ofaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaillon A, Sun X, Cachay ER, et al. . Primary incidence of hepatitis C virus infection among HIV-infected men who have sex with men in San Diego, 2000–2015. Open Forum Infect Dis 2019; 6:ofz160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS 2015; 29:2335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garg S, Taylor LE, Grasso C, Mayer KH. Prevalent and incident hepatitis C virus infection among HIV-infected men who have sex with men engaged in primary care in a Boston community health center. Clin Infect Dis 2013; 56: 1480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geter A, Sutton MY, Armon C, Buchacz K, Investigators HIVOS . Disparities in viral suppression and medication adherence among women in the USA, 2011–2016. AIDS Behav 2019; 23:3015–23. [DOI] [PubMed] [Google Scholar]
- 28. Sarkar S, Esserman DA, Skanderson M, Levin FL, Justice AC, Lim JK. Disparities in hepatitis C testing in U.S. veterans born 1945–1965. J Hepatol 2016; 65:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69:7–34. [DOI] [PubMed] [Google Scholar]
- 30. Radwan D, Cachay E, Falade-Nwulia O, et al. . HCV screening and treatment uptake among patients in HIV care during 2014–2015. J Acquir Immune Defic Syndr 2019; 80:559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coffin PO, Reynolds A. Ending hepatitis C in the United States: the role of screening. Hepat Med 2014; 6:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gamage DG, Read TR, Bradshaw CS, et al. . Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis 2011; 11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. European Treatment Network for HIV H, Global Infectious Diseases Consensus Panel. Recently acquired and early chronic hepatitis C in MSM: recommendations from the European treatment network for HIV, hepatitis and global infectious diseases consensus panel. AIDS 2020; 34:1699–711. [DOI] [PubMed] [Google Scholar]
- 34. Castry M, Cousien A, Supervie V, et al. . Impact of test-and-treat and risk reduction strategies on HCV transmission among MSM living with HIV in France: a modelling approach. BMJ 2020; 0:1–9. [DOI] [PubMed] [Google Scholar]
- 35. Künzler-Heule P, Engberg S, Battegay M, et al. ; Swiss HIV Cohort Study (SHCS). Screening HIV-positive men who have sex with men for hepatitis C re-infection risk: is a single question on condom-use enough? A sensitivity analysis. BMC Infect Dis 2019; 19:821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization. Guidelines for the screening care and treatment of persons with chronic hepatitis C infection. Updated version, April 2016. Guidelines. Available at: http://apps.who.int/iris/bitstream/10665/205035. Accessed 14 June 2018. [PubMed]
- 37. National Academies of Sciences, Engineering, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: National Academies Press; 2017: pp 1–202. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.