Abstract
Background
Candida empyema thoracis (pleural empyema) is an uncommon manifestation of invasive candidiasis, for which optimal treatment is unknown.
Methods
This is a retrospective study of patients with Candida empyema at 2 academic medical centers from September 2006 through December 2015.
Results
We identified 81 patients with Candida empyema (median age, 62 years; 68% men). Sixty-five percent of patients underwent surgery or an invasive intervention of the thorax or abdomen within the preceding 90 days. Candida empyema originated from intrathoracic (51%) or intra-abdominal sources (20%), spontaneous esophageal rupture (12%), pleural space manipulation (9%), and pneumonia (6%). Eighty-four percent and 41% of patients were intensive care unit residents and in septic shock, respectively, within 3 days of diagnosis. Causative species were Candida albicans (65%), Candida glabrata (26%), Candida parapsilosis (11%), Candida tropicalis (4%), Candida krusei (2%), and Candida dubliniensis (1%). Bacteria were recovered from empyemas in 51% of patients. Concurrent candidemia was diagnosed in only 2% of patients. Management included pleural drainage and antifungal treatment in 98% and 85% of patients, respectively. Mortality at 100 days was 27%, and it was highest for cases stemming from esophageal rupture (67%). Spontaneous esophageal rupture and echinocandin rather than fluconazole treatment were independent risk factors for death at 100 days (P = .003 and .04, respectively); receipt of antifungal therapy was an independent predictor of survival (P = .046).
Conclusions
Candida empyema mortality rates were lower than reported previously. Optimal management included pleural drainage and fluconazole treatment. Superiority of fluconazole over echinocandins against Candida empyema needs to be confirmed in future studies.
Keywords: azole antifungal, Candida, candidiasis, echinocandin, empyema
Candida empyema was most commonly caused by C albicans. Cornerstones of successful management were pleural drainage and antifungal treatment. Esophageal rupture, absence of antifungal treatment, and receipt of echinocandins rather than fluconazole were independent risk factors for death.
Candida empyema thoracis (pleural empyema) is an uncommon but severe manifestation of invasive candidiasis that is associated with high crude mortality. To our knowledge, there have been only 3 case series of fungal empyema in the peer-reviewed English language literature that have included 10 or more patients [1–3], only one of which focused solely on Candida empyema [1]. Infectious Diseases Society of America clinical practice guidelines for the management of candidiasis (2016) did not address Candida empyema, likely due to the scarcity of clinical data [4]. Indeed, the optimal treatment of Candida empyema is undefined. It is clear that some patients do well in the absence of antifungal therapy, particularly with drainage of infected pleural fluid [2]. No study has demonstrated that a particular antifungal agent is superior to others in treating Candida empyema. Fluconazole has been used most often among patients in case series. Echinocandins are endorsed as agents of choice for treatment of candidemia, the most common type of invasive candidiasis, and caspofungin is approved by the US Food and Drug Administration for treatment of Candida empyema. However, there are scant data on effectiveness of echinocandins against Candida empyema or echinocandin pharmacokinetics within empyemas or pleural fluid [5–7]. Breakthrough Candida empyema is well recognized among lung transplant recipients receiving echinocandin prophylaxis [8], calling into question their value in treating these infections.
We conducted a systematic review of Candida empyema at 2 US university hospitals. Our goal was to describe demographics, underlying disease mechanisms, management and patients’ outcomes, and identify risk factors for mortality.
METHODS
We performed a retrospective study of adults with pleural candidiasis at the University of Pittsburgh Medical Center-Presbyterian University Hospital and the University of California Davis Medical Center from September 2006 through December 31, 2015. Both hospitals are academic, acute care, adult, medical-surgical referral, organ transplant, and level 1 trauma centers.
Patients were identified by reviewing microbiologic culture results that were positive for Candida from pleural fluid. Empyema was defined as clinical evidence of infection and isolation of Candida from a sterile sample obtained from the pleural space. We excluded patients whose samples were collected from drains in place >24 hours and included only the initial episode of infection for each patient. Abstracted information included demographic data, underlying disease leading to empyema, immunocompromising conditions, clinical features, microbiology data, treatments, and outcomes.
Patient Consent
This study was reviewed by the Institutional Review Boards of both centers and qualified for “Exempt” status as defined by federal regulation.
Definitions
Sources of empyema are defined in Table 1. Contiguous spread of infection was defined in patients who had adjacent infection, such as esophageal rupture, pneumonia, mediastinitis, paraspinal abscess, or subdiaphragmatic liver abscess. Recurrent empyema was defined as culture-proven Candida infection occurring after apparent clinical resolution of an initial Candida empyema. Persistent empyema was defined as infection continuing for ≥5 days after appropriate source control, for which Candida was reisolated on culture from a pleural sample. Appropriate source control was defined as adequate drainage or surgical removal of infected material and correction of underlying pathology (eg, surgical repair of perforation or leak). Treatment was defined as receipt of a systemic antifungal agent for >48 hours. Combination therapy was defined as receipt of ≥2 classes of systemic antifungal agents (amphotericin B, azole, or echinocandin).
Table 1.
Classification of Sources of Candida Empyema
| Classification | Description and Pathophysiology |
|---|---|
| Spontaneous esophageal rupture | Direct inoculation of Candida into the pleural space after spontaneous esophageal rupture without any antecedent cardiothoracic surgery or invasive procedure |
| Intrathoracic source | Candida inoculation into the pleural space via any of the following routes: (1) inoculation during intrathoracic (esophagus, lung, heart) surgery or invasive procedure; (2) inoculation via complications of these surgeries (eg, anastomotic leaks or perforation at the surgical site); (3) inoculation via trauma to the chest; (4) inoculation from oropharyngeal, retropharyngeal, or paraspinal infection to the pleural space; (4) esophageal cancer eroding into the lung with subsequent bronchopleural fistula formation; (5) bronchopleural fistula of unclear etiology |
| Diaphragmatic translocation from GI source | Candida inoculation from an intra-abdominal source, across the diaphragm. The infection can originate from the following: (1) rupture of an intestinal viscous; (2) primary or secondary Candida peritonitis; (3) intra-abdominal surgery, hepatobiliary surgery, or complications from these surgeries; (4) subdiaphragmatic liver abscess; (5) pancreatitis and/or pancreatic-pleural fistula |
| Recent pleural manipulation | Candida inoculation of the pleural space via repeated pleural effusion sampling or drainage, or indwelling chest tubes |
| Pneumonia | Candida empyema originating from an infection of the lung, as in cases of aspiration pneumonia |
| Unknown source | Most often involve chronic pleural effusion, for which the source of Candida is not apparent |
Abbreviations: GI, gastrointestinal.
Statistical Analyses
Data were analyzed using Stata, version 15 (StataCorp), and GraphPad Prism, version 8.0 (GraphPad Software). Descriptive statistics were used to summarize the data. The primary outcome was 100-day mortality, which was selected because complications of Candida empyema may occur several weeks after diagnosis. Associations between categorical variables were analyzed using Pearson’s χ 2 or 2-tailed Fisher’s exact tests. Risk factors for mortality were assessed using Mann-Whitney U and Fisher’s exact tests for continuous and categorical variables, respectively. Variables significant by univariate analysis (P ≤ .10) were entered into a multivariate logistic regression model to determine independent risk factors for death. Odds ratios with 95% confidence intervals were determined for each risk factor. Kaplan-Meier curves were used to estimate survival stratified by sources of infection, and log-rank test was used to compare curves between groups. Significance was defined as P ≤ .05 (2-tailed).
RESULTS
Patient Demographics
Eighty-one patients with clinical evidence of empyema and Candida species isolated from pleural fluid were identified. Patient demographics and clinical characteristics are shown in Table 2. Median age of patients was 62 years (range, 20–94). Sixty-eight percent (n = 55) of patients were men. Thirty-three percent (n = 27) of patients had a malignancy (all solid tumors). In 78% (21 of 27) of these patients, the site of malignancy was the source of Candida empyema. Esophageal malignancy was most common (n = 14), followed by pancreatic, hepatobiliary (n = 6 each), and gastrointestinal carcinoma (n = 1). Fourteen percent (n = 11) of patients were solid organ transplant recipients; the most common transplanted organs were lung and liver (n = 4 each). Sixty-five percent (n = 53) of patients underwent surgery or an invasive intervention of the thorax or abdomen within the preceding 90 days. Candida empyema was diagnosed at median 14 and 8 days postprocedure, respectively. Eighty-four percent (n = 68) of patients resided in intensive care units (ICUs) within 3 days of Candida empyema diagnosis; median simplified acute physiology II (SAPS II) score was 32 (12–72). Forty-one percent (n = 33) of patients were in septic shock at the time of Candida empyema diagnosis.
Table 2.
Patient Demographics and Clinical Characteristics
| Demographics | Data, Percentage and/or Number | ||
|---|---|---|---|
| Age, median in years (range) | 62 (20–94) | ||
| Men (%, n) | 68%, 55 | ||
| Underlying Conditions | |||
| Malignancy (%, n) | 33%, 27 | ||
| Esophageal (n) | 14 | ||
| Pancreas (n) | 4 | ||
| Hepatobiliary (n) | 2 | ||
| GI (stomach, colon) (n) | 3 | ||
| Lung (n) | 1 | ||
| Others (prostate, skin glioblastoma) (n) | 3 | ||
| Solid Organ Transplantation (%, n) | 14%, 11 | ||
| Lung (n) | 4 | ||
| Liver (n) | 4 | ||
| Kidney (n) | 2 | ||
| Heart (n) | 1 | ||
| Diabetes Mellitus (%, n) | 22%, 18 | ||
| Cirrhosis (%, n) | 9%, 7 | ||
| Neutropenia (%, n) | 2%, 2 | ||
| Hemodialysis (%, n) | 2%, 2 | ||
| Surgery or Invasive Procedure Within 90 Days (%, n) | 65%, 53 | ||
| Thoracic surgery/procedure (n) | 31 | ||
| Abdominal surgery/procedure (n) | 13 | ||
| Both thoracic and abdominal surgery (n) | 6 | ||
| Other surgeries (orthopedics, brain) (n) | 3 | ||
| Severity of Illness at the Time of Empyema Diagnosis | |||
| Septic shock (%, n) | 41%, 33 | ||
| SAPS II, at time of diagnosis (median, range) | 32 (12–72) | ||
| ICU within 3 days of diagnosis (%, n) | 84% (68) | ||
| Hospital-acquired Candida empyema (%, n) | 65%, 53 |
Abbreviations: GI, gastrointestinal; ICU, intensive care unit; SAPS II, simplified acute physiology score II.
The expected in-hospital mortality rate for SAPS II of 32 is 12.8%.
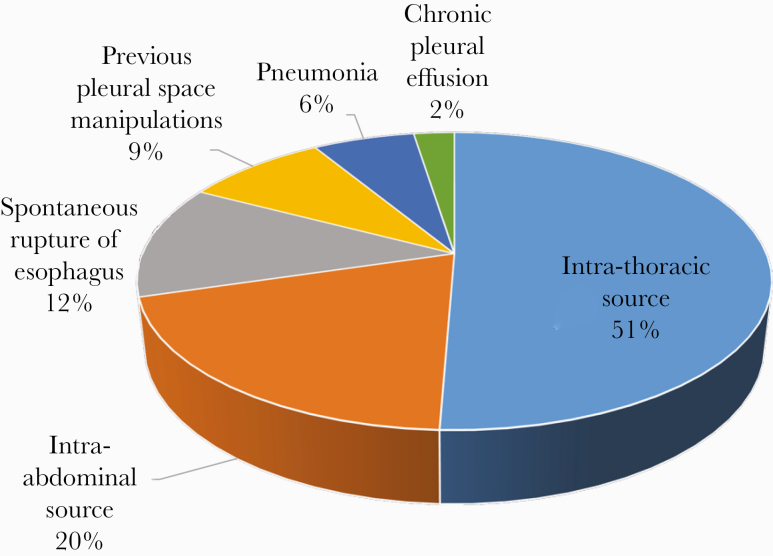
Candida empyema most commonly originated from an intrathoracic source (51%, n = 41), followed by intra-abdominal source (20%, n = 16), spontaneous esophageal rupture (12%, n = 10), previous pleural space manipulation (9%, n = 7), pneumonia (6%, n = 5), and chronic pleural effusion (2%, n = 2) (Figure 1, Table 3). Demographics and other data are presented by source of Candida empyema in Supplemental Table 1.
Figure 1.
Sources of Candida empyema. Most cases of Candida empyema stemmed from an intrathoracic source or diaphragmatic translocation from an intra-abdominal source. Details of intrathoracic and abdominal sources are summarized in the Table 3 below.
Table 3.
Details of Intrathoracic and Intra-Abdominal Sources of Candida Empyema
| Source of Empyema | Description | Number |
|---|---|---|
| Intrathoracic source | ||
| N = 41 | Esophageal surgery or procedure | 20 |
| (Esophageal surgery or procedure with leak) | (16) | |
| Lung surgery/VATS | 12 | |
| Extension from oro-/retropharyngeal infection | 4 | |
| Extension from paraspinal infection | 1 | |
| Esophageal cancer eroding into lungs with bronchopulmonary fistula | 2 | |
| Bronchopulmonary fistula of unclear cause | 2 | |
| Intra-Abdominal Source | Abdominal/pelvic surgery | 6 |
| N = 16 | Liver transplant | (1) |
| Surgery with complication (leaks or abscess) | (2) | |
| Hepatobiliary surgery | 5 | |
| Hepatibiliary surgery with complication (leak or abscess) | (4) | |
| Small bowel perforation | 4 | |
| Pancreatitis | 1 |
Abbreviations: VAPS, video-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery.
Microbiology
Eighty-nine Candida isolates were recovered from pleural fluid. Predominant Candida spp were Candida albicans (65%, n = 53) and Candida glabrata (26%, n = 21) (Table 4). More than 1 Candida species was isolated from 10% (n = 8) of patients. Concomitant bacteria were identified in 51% (n = 41) of patients, including more than 1 species in 31% (n = 25) (Table 4).
Table 4.
Microbiology of Candida Empyema
| Microbiology | Percent (n/n) |
|---|---|
| Candida species | |
| Candida albicans | 65% (53/81) |
| Candida glabrata | 26% (21/81) |
| Candida parapsilosis | 11% (9/81) |
| Candida tropicalis | 4% (3/81) |
| Candida krusei | 2% (2/81) |
| Candida dubliniensis | 1% (1/81) |
| More than 1 Candida speciesa | 10% (8/81) |
| Concurrent bacteria in pleural spaceb | 51% (41/81) |
| Bloodstream Infectionsc | |
| Candidemia | 2% (1/52) |
| Bacteremia | 8% (4/52) |
aAn additional patient was coinfected with Saccharomyces cerevisiae.
bThirty-one percent of patients (25 of 81) had more than 1 bacterial species. Gram-positive organisms accounted for 71% (29 of 41) of bacterial isolates; 20% (8 of 41) and 17% (7 of 41) of bacteria were Lactobacillus and obligate anaerobes, respectively.
cFifty-two patients had blood cultures performed within 7 days of Candida empyema.
NOTE: Note that only 4 C albicans isolates from pleural fluid of 4 patients were tested for antifungal susceptibility in vitro. Each isolate was susceptible to fluconazole and echinocandins.
Patients with Candida parapsilosis empyema were more likely to have breakthrough infection while receiving azole prophylaxis than were those with empyema due to other Candida species (44% [4 of 9] vs 3% [2 of 72]; P = .001). Candida parapsilosis empyema developed later after a surgical procedure than empyema due to other spp (median 50 days [18–166] vs 11 days [1–216]; P = .01). Candida parapsilosis was less likely to occur among patients residing in the ICU (33% [3 of 9] vs 90% [65 of 72]; P < .0001). There were no differences in Candida spp with different sources of empyema, healthcare- versus community-acquired empyema, presence of bacterial coinfection, or bacteremia. Concurrent candidemia or bacteremia was diagnosed in 2% (1 of 52) and 8% (4 of 52) of patients in whom blood cultures were drawn within 7 days of Candida empyema.
Management and Outcome
Eight-five percent (69 of 81) of patients received antifungal therapy, which included fluconazole alone (58%, n = 47), echinocandin alone (22%, n = 18; micafungin, n = 10; caspofungin, n = 8), or an azole combined with echinocandin (5%, n = 4; caspofungin and fluconazole, n = 3; caspofungin and voriconazole, n = 1). Two percent (n = 2) of patients received agents sequentially. Antifungal therapy was started at median on the day of diagnostic intervention (interquartile range [IQR], −1 to 3 days); median duration of therapy was 21 days (IQR, 14–35 days).
Pleural drainage was performed in 98% (79 of 81) of patients. Types of drainage were percutaneous (96%, n = 78), surgical (32%, n = 26), and video-assisted thoracoscopic surgery ([VATS] 22%, n = 18). In 58% (47 of 81) and 40% (32 of 81) of patients, a single type of drainage or combined modality drainage was undertaken, respectively. Combined modalities included (1) percutaneous, surgical, and VATS (14%, n = 11), (2) percutaneous and surgical (19%, n = 15), and (3) percutaneous and VATS (7%, n = 6).
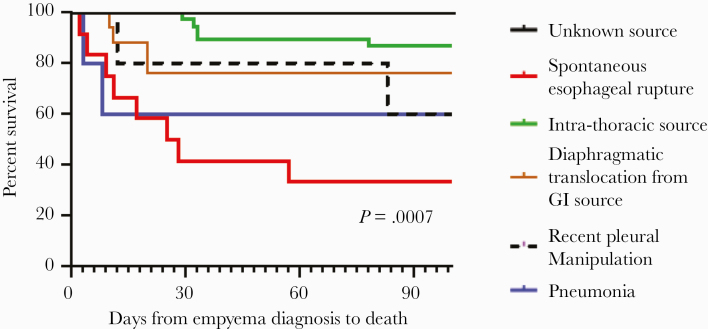
Mortality rates at 15, 30, and 100 days were 12% (n = 10), 20% (n = 16), and 27% (n = 22), respectively. The highest mortality was associated with esophageal rupture and direct introduction of Candida into the pleural cavity, followed by multiple pleural space interventions, pneumonia, diaphragmatic translocation from an intra-abdominal or hepatobiliary source, and intrathoracic source (Figure 2). Mortality was lowest for empyema of unclear source. There were no significant differences in 15-day, 30-day, and 100-day mortality between patients with and without concomitant bacterial empyema (12% vs 15% [P = .76], 17% vs 25% [P = .42], and 24% vs 32% [P = .47]). Likewise, there were no significant differences in mortality among patients with and without concomitant empyema with Pseudomonas aeruginosa, viridans Streptococcus or Lactobacillus, the most commonly recovered bacteria (data not shown). Mortality rates did not differ based on Candida species (Table 5), between contiguous and noncontiguous sources of empyema (30%, 18 of 61 vs 25%, 5 of 20; P = .78), or by type of pleural drainage (data not shown).
Figure 2.
Mortality of Candida empyema stratified by source of infection. GI, gastrointestinal.
Table 5.
Risk Factors for 100-Day Mortality
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | ||||
| Factors | Nonsurvivors (N = 23) | Survivors (N = 58) | P Value | Model 1a | Model 2a | ||
| Demographics | |||||||
| Age (median, years) | 65 (24–83) | 60 (20–94) | .15 | ||||
| Men | 74% (17) | 66% (38) | .60 | ||||
| Whiteb | 65% (11/17) | 60% (41/68) | .79 | ||||
| Solid organ transplant | 13% (3) | 14% (8) | 1.0 | ||||
| Diabetes | 30% (7) | 19% (11) | .24 | ||||
| Malignancy | 35% (8) | 32% (19) | .79 | ||||
| Characteristic | |||||||
| Hospital-acquired infection | 65% (15) | 65% (38) | 1.0 | ||||
| SAPS II | 38.5 (12–72) | 31 (12–64) | .05 | .43 | |||
| Source of Infection | |||||||
| Spontaneous esophageal rupture | 35% (8) | 7% (4) | .003 | .003 | 10.5 (2.2–49.7) | .003 | 14 (2.4–81.3) |
| Contiguous source | 78% (18) | 74% (43) | .78 | ||||
| Microbiology | |||||||
| Candida albicans | 52% (12) | 69% (40) | .20 | ||||
| Candida glabrata | 30% (7) | 28% (16) | .79 | ||||
| Candida parapsilosis | 9% (2) | 12% (7) | 1.0 | ||||
| Candida tropicalis | 4% (1) | 3% (2) | 1.0 | ||||
| >1 Candida spp | 4% (1) | 14% (8) | |||||
| Polymicrobial | 43% (10) | 53% (31) | .47 | ||||
| Bacteremia | 11% (2/18) | 6% (2/34) | .60 | ||||
| Management | |||||||
| Source Control: | |||||||
| Percutaneous drainage | 100% (23) | 95% (55) | .55 | ||||
| VATSc | 7% (1/15) | 15% (6/40) | .66 | ||||
| Thoracotomyd | 33% (7/21) | 19% (8/42) | .23 | ||||
| Antifungal Therapy: | |||||||
| Receipt of antifungal | 74% (17) | 90% (52) | .09 | .046 | 0.24 (0.06–0.98) | ||
| Fluconazolee | 50% (8/16) | 80% (39/49) | .05 | .038 | 4.5 (1.1–18.8) | ||
| Caspofungine | 50% (8/15) | 20% (10/49) | |||||
Bold text denotes variables (found significant by univariate analyses at P < .10) that were entered into a multivariate logistic regression model.
Abbreviations: CI, confidence interval; SAPS II, simplified acute physiology score II; VAPS, video-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery.
aModel 1 included antifungal treatment for ≥48 hours as a variable. Model 2 included receipt of fluoconazole (as opposed to an echinocandin) as a variable. In Model 2, 4 patients who received combination antifungal therapy were excluded.
bRace was not recorded for 13 patients (7 in the nonsurvivor group, 6 in the survivor group).
cTwenty-six patients who received both VATS and thoracotomy were excluded from this analysis.
dEighteen patients who received both thoracotomy and VATS were excluded from this analysis.
eReceipt of antifungal agents for ≥48 hours. Four patients who received combination antifungal therapy were excluded from this analysis. P values are for comparisons of outcomes among patients receiving fluconazole vs those receiving an echinocandin.
Median length of hospital stay after diagnosis of Candida empyema was 32 days (IQR, 11 to 34 days). Persistent and recurrent Candida empyema occurred in 4% (3 of 81) and 7% (6 of 81) of patients, respectively. Two cases of recurrent empyema were in patients who did not receive antifungal treatment upon initial presentation.
Spontaneous esophageal rupture (P = .003), SAPS II (P = .05), and echinocandin (rather than fluconazole) therapy (P = .05) were significant risk factors for death by univariate analyses (Table 5); there was a trend toward an association between receipt of antifungal therapy and survival (P = .09). By multiple logistic regression analyses, SAPS II score was an independent risk factor for 15-day mortality (P = .046), and spontaneous esophageal rupture (P = .008 and 0.003) and antifungal therapy (P = .01 and P = .046) were independent risk factors for death at 30 and 100 days, respectively (Table 5, Supplemental Table 2). Receipt of an echinocandin (rather than an azole) was an independent predictor of mortality at days 15 and 100 (P = .03 and P = .04, respectively) (Table 5, Supplemental Table 2).
DISCUSSION
To our knowledge, this is the largest published case series of Candida empyema. Our experience with 81 patients at 2 US teaching hospitals over a 10-year period was particularly notable for 3 findings. First, our mortality rate (20% and 27% at 30 and 100 days, respectively) was lower than the 31%–62% rates reported previously for patients with Candida empyema at centers in Taiwan and a cancer hospital in Houston [1–3]. Second, lack of antifungal therapy and use of an echinocandin rather than fluconazole were independent predictors of death. Antifungal therapy was an adjunct to pleural drainage, which was undertaken in 98% of patients. Therefore, our findings support combined treatment with pleural drainage and fluconazole for all patients with Candida empyema. Finally, spontaneous esophageal rupture as the source of empyema was also independently associated with mortality. The data advance our understanding of a distinct, but poorly characterized manifestation of invasive candidiasis, and provide important new insights for clinicians.
It is unclear why mortality rates here were relatively low. More of our patients underwent pleural drainage than in the previous studies (65%–76%), including by percutaneous procedures, VATS (with or without decortication), open thoracotomy, and combined modalities. Such interventions were associated with decreased mortality in earlier studies [1, 3]. Pleural drainage is a cornerstone of treating fungal and bacterial empyema because phagocytic activity within infected pleural fluid is diminished by low opsonin and complement concentrations, hypoxia, and acidity [9–12]. Disruption of loculations may enhance pleural drainage and allow better antifungal penetration [12]. Most of our patients developed Candida empyema within 90 days of undergoing thoracic or abdominal surgery (or other invasive procedures), which may have made cases more amenable to subsequent source control and drainage. Low mortality rates at our hospitals were especially notable given patients’ severity of illness. Eight-four percent of our patients were in the ICU, and 41% were in septic shock at time of Candida empyema diagnosis, figures that were consistent with those in prior reports [1, 3]. Severity of illness, measured by SAPS II, was an independent risk factor for death at 15 days. In keeping with the severity of illness, hospital stays were prolonged following the diagnosis of Candida empyema (median, 32 days).
Our observation that antifungal therapy was associated with significantly lower mortality was consistent with findings from a Taiwanese study of fungal empyema [3]. In the present study, antifungal treatment was initiated at median on the day of diagnostic intervention; prompt intervention may have contributed to therapeutic effectiveness. There is no consensus on the preferred antifungal regimen against Candida empyema. An echinocandin is endorsed in UpToDate (last accessed December 9, 2020) as the most appropriate initial therapy [13], although it is acknowledged that clinical or pharmacokinetic data supporting this recommendation are limited. Fluconazole and echinocandins were used as monotherapy in 58% and 22% of our patients, respectively. Fluconazole was the most commonly used agent against Candida empyema in earlier case series [1–3]; echinocandins were used in more than 1 patient in only 1 of these studies [2]. Some patients with Candida empyema can be managed successfully with source control and drainage in the absence of antifungal therapy [2]. However, as has been shown for candidemia and intra-abdominal candidiasis [14], clinicians cannot reliably identify patients who might do well without receiving antifungals. For this reason, positive Candida cultures from pleural samples collected under sterile conditions cannot be dismissed as colonizers or contaminants, and all patients merit early antifungal therapy.
This is the first study to establish the superiority of a particular antifungal agent in the treatment of Candida empyema. It is unclear why outcomes were superior in patients who received fluconazole compared with those who received an echinocandin. It is notable that breakthrough Candida empyema has been well described in lung transplant recipients receiving micafungin as prophylaxis [8]. There are limited data on antifungal pharmacokinetics within pleural fluid. In studies of patients with fungal infections, azoles such as fluconazole (200 mg daily) and voriconazole (300 mg twice a day) achieved pleural and pericardial fluid concentrations that were 20%–100% of those in paired plasma samples; levels increased over time [15–19]. Anidulafungin (100 mg daily) and micafungin (150 mg daily) concentrations within infected pleural fluid were 9%–15% and 57%–67% of those in plasma [5–7, 15]. It is difficult to interpret these data, given the heterogeneity of patients and study designs. Drug levels are lower in presence of chest tube drainage, larger effusion volumes, greater pleural inflammation, loculations, and thicker pleural walls [6, 20]. Moreover, antifungal activity may be impacted by factors such as pH, oxygen concentrations, purulence, or protein binding (which may be particularly relevant for echinocandins) within effusions. More pharmacokinetic-pharmacodynamic data are needed to define optimal antifungal treatment of Candida empyema. We cannot exclude that echinocandins were favored in patients who had unrecognized factors that accounted for worse outcomes. Further studies confirming the superiority of fluconazole are required.
The increased mortality observed with spontaneous esophageal rupture (50% and 60% at 30 and 100 days, respectively) is in keeping with the severity of this event, which itself carries crude mortality as high as 46%, mostly commonly due to pleural empyema or pneumonia [21]. The esophagus is often colonized with high Candida burdens, and rupture can directly seed the pleural space. Recovery of Candida from pleural fluid or other intrathoracic sites after esophageal rupture is associated with increased risk of death [21]. Esophageal rupture was the third most common source of Candida empyema (15%), after thoracic procedures or trauma (51%) and diaphragmatic translocation from intra-abdominal or hepatobiliary sources (20%). Whereas bacterial empyema most often arises from pneumonia, Candida empyema was rarely associated with preceding lung infection. In a previous study, a noncontiguous source of Candida empyema, such as diaphragmatic translocation or hematogenous seeding, was an independent risk factor for death [1]. In our experience, outcomes were similar for patients with contiguous and noncontiguous sources (30% and 25% mortality, respectively). Relatively good outcomes among patients with empyema stemming from intra-abdominal and other noncontiguous sites likely accounted for our lower crude mortality compared with earlier reports [1, 3]. An unknown source of infection carried the lowest mortality rate here, which may reflect transient rather than ongoing seeding of the pleura, as may be seen after surgery or rupture of a gastrointestinal track viscus.
Candida albicans was the most common cause of Candida empyema (65%), followed by Candida glabrata (26%) and C parapsilosis (11%). Fifty-one percent of cases were complicated by bacterial coinfections; in 31% of cases, more than 1 bacterium was recovered. These microbiology data were broadly consistent with findings from previous case series of Candida empyema [1–3], as well as studies of intra-abdominal candidiasis [14]. Candida parapsilosis was more likely to cause azole breakthrough infections than were other spp. Of note, C parapsilosis was a common cause of echinocandin breakthrough empyema in lung transplant recipients [8]. In our experience, C parapsilosis also was diagnosed significantly longer after a surgical procedure, and it was more likely to occur among non-ICU patients. Candida parapsilosis is commonly associated with device and surgical infections, and it is less intrinsically virulent than other Candida spp [22, 23]. Mortality in this study did not differ by species, which likely reflects the relatively small size of the study and that non-microbiologic factors such as pleural drainage, source of infections, and host factors are major determinants of outcomes. Candidemia was diagnosed in only 2% of our patients with empyema, within the range of 2%–27% reported previously [1–3]. The prevalence of candidemia may have been understated by delayed collection of blood cultures in some patients and by the relative insensitivity of culture-based diagnosis [24]. It is also possible that our aggressive use of pleural drainage and early antifungal therapy may have limited dissemination from localized infections.
It is important to acknowledge that our study was limited by its retrospective design, and results may have been influenced by practices and patient populations at our hospitals. Experiences at other centers may differ. We used rigorous case definitions, which may have excluded cases for which positive cultures were collected from in-dwelling drains. In clinical practice, it can be difficult to distinguish Candida colonization from disease based on cultures from in-dwelling drains or catheters. In such cases, clinicians should not dismiss Candida-positive cultures out of hand, because we have shown that antifungal treatment improves outcomes if disease is present.
CONCLUSIONS
Candida empyema thoracis is an uncommon but important disease that is associated with high mortality and prolonged hospitalizations. Keys to limiting mortality are recognition of the disease by clinicians and prompt pleural drainage, source control, and antifungal therapy. Our study lays the foundation for a prospective multicenter observational study of Candida empyema and other types of nonbloodstream invasive candidiasis that are less extensively investigated than candidemia. As we have shown, optimal treatment of nonbloodstream candidiasis may differ in important ways from that of candidemia. Any future study should verify that fluconazole is superior to an echinocandin in treating Candida empyema and assess antifungal pharmacokinetics within infected pleural fluid.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. C. J. C has been awarded investigator-initiated research grants from Astellas, Merck, Melinta, and Cidara for studies unrelated to this project, served on advisory boards or consulted for Astellas, Merck, the Medicines Company, Cidara, Scynexis, Shionogi, Qpex and Needham & Company, and has spoken at symposia sponsored by Merck and T2Biosystems. M. H. N. has been awarded investigator-initiated research grants from Astellas, Merck, Scynexis, Pulmocide, and Cidara for projects unrelated to this study and served on advisory boards for Astellas, Merck, the Medicines Company, Scynexis, and Shionogi. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lin KH, Liu YM, Lin PC, et al. Report of a 63-case series of Candida empyema thoracis: 9-year experience of two medical centers in central Taiwan. J Microbiol Immunol Infect 2014; 47:36–41. [DOI] [PubMed] [Google Scholar]
- 2. Nigo M, Vial MR, Munita JM, et al. Fungal empyema thoracis in cancer patients. J Infect 2016; 72:615–21. [DOI] [PubMed] [Google Scholar]
- 3. Ko SC, Chen KY, Hsueh PR, et al. Fungal empyema thoracis: an emerging clinical entity. Chest 2000; 117:1672–8. [DOI] [PubMed] [Google Scholar]
- 4. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moriyama B, Ditullio M, Wilson E, et al. Pharmacokinetics of anidulafungin in pleural fluid during the treatment of a patient with Candida empyema. Antimicrob Agents Chemother 2011; 55:2478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamada N, Kumada K, Kishino S, et al. Distribution of micafungin in the tissue fluids of patients with invasive fungal infections. J Infect Chemother 2011; 17:731–4. [DOI] [PubMed] [Google Scholar]
- 7. Welte R, Eller P, Lorenz I, Joannidis M, Bellmann R. Anidulafungin pharmacokinetics in ascites fluid and pleural effusion of critically ill patients. Antimicrob Agents Chemother 2018; 62:e02326–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baker AW, Maziarz EK, Arnold CJ, et al. Invasive fungal infection after lung transplantation: epidemiology in the setting of antifungal prophylaxis. Clin Infect Dis 2020; 70:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22:747–62; quiz 763–4. [DOI] [PubMed] [Google Scholar]
- 10. Lew DP, Despont JP, Perrin LH, et al. Demonstration of a local exhaustion of complement components and of an enzymatic degradation of immunoglobulins in pleural empyema: a possible factor favouring the persistence of local bacterial infections. Clin Exp Immunol 1980; 42:506–14. [PMC free article] [PubMed] [Google Scholar]
- 11. Sese E, Xiol X, Castellote J, et al. Low complement levels and opsonic activity in hepatic hydrothorax: its relationship with spontaneous bacterial empyema. J Clin Gastroenterol 2003; 36:75–7. [DOI] [PubMed] [Google Scholar]
- 12. Moores DW. Management of acute empyema. Chest 1992; 102:1316–7. [DOI] [PubMed] [Google Scholar]
- 13. Kauffman CA. Candida infections of the abdomen and thorax. In: Marr KA, ed. UpToDate, Waltham, MA. Available at: https://www.uptodate.com/contents/candida-infections-of-the-abdomen-and-thorax#H7. Accessed 9 December 2020.
- 14. Vergidis P, Clancy CJ, Shields RK, et al. Intra-abdominal candidiasis: the importance of early source control and antifungal treatment. PLoS One 2016; 11:e0153247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuda T, Koreeda Y, Mataki H, et al. A case of Aspergillus empyema successfully treated with combination therapy of voriconazole and micafungin: excellent penetration of voriconazole and micafungin into pleural fluid. Intern Med 2010; 49:1163–9. [DOI] [PubMed] [Google Scholar]
- 16. Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev 2014; 27:68–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rieder-Nelissen CM, Hasse J, Yeates RA, Sarnow E. Fluconazole concentrations in pulmonary tissue and pericardial fluid. Infection 1997; 25:192–4. [DOI] [PubMed] [Google Scholar]
- 18. Stern JB, Girard P, Caliandro R. Pleural diffusion of voriconazole in a patient with Aspergillus fumigatus empyema thoracis. Antimicrob Agents Chemother 2004; 48:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poupelin JC, Philit F, Richard JC, et al. Pericardial and pleural diffusion of voriconazole during disseminated invasive aspergillosis: report of a case with successful outcome. Intensive Care Med 2006; 32:939–40. [DOI] [PubMed] [Google Scholar]
- 20. Teixeira LR, Sasse SA, Villarino MA, et al. Antibiotic levels in empyemic pleural fluid. Chest 2000; 117:1734–9. [DOI] [PubMed] [Google Scholar]
- 21. Zimmermann M, Hoffmann M, Jungbluth T, et al. Predictors of morbidity and mortality in esophageal perforation: retrospective study of 80 patients. Scand J Surg 2017; 106:126–32. [DOI] [PubMed] [Google Scholar]
- 22. Nguyen MH, Peacock JE Jr, Morris AJ, et al. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med 1996; 100:617–23. [DOI] [PubMed] [Google Scholar]
- 23. Toth R, Nosek J, Mora-Montes HM, et al. Candida parapsilosis: from genes to the bedside. Clin Microbiol Rev 2019; 32:e00111–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clancy C, Nguyen M-H. Finding the “Missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis 2013; 56:1284–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.