Abstract
Since 27th December 2020, a mRNA vaccine from BioNTech / Pfizer (Comirnaty®) has been used across Germany. As of 12th March 2021, 286 fatalities of vaccinated German individuals were registered at the Paul-Ehrlich-Institute with time intervals after vaccination between one hour to 40 days. From our catchment area in northern Germany, we have so far become aware of 22 deaths in connection with vaccination in a 5 week period (range: 0–28 days after vaccination). Three death cases after vaccination with Comirnaty®, which were autopsied at the Institute of Legal Medicine Hamburg, are presented in more detail. All three deceased had severe cardiovascular diseases, among other comorbidities, and died in the context of these pre-existing conditions, while one case developed a COVID-19 pneumonia as cause of death. Taking into account the results of the postmortem examination a causal relation between the vaccination and the death was not established in any case. If there are indications of an allergic reaction, histological and postmortem laboratory examinations should be performed subsequent to the autopsy (tryptase, total IgE, CRP, interleukin-6, complement activity C3/C5).
Keywords: Vaccination, Comirnaty®, SARS-CoV-2, COVID-19 vaccine, Autopsy
1. Introduction
On 21st December 2020, the mRNA vaccine from BioNTech / Pfizer (Comirnaty®) was approved in the European Union (EU) as the first vaccine against the SARS-CoV-2 virus. Since 27th December 2020 this vaccine has been used nationwide in Germany. As in many other EU countries, highest vaccination priority in Germany is given to nursing home residents, persons older than 80, as well as medical and nursing staff [1]. In Germany, the Paul-Ehrlich-Institute (PEI) is responsible for the approval of vaccines, i.e., the evaluation of quality, efficacy, and drug safety after approval. After a vaccine has been approved, all reports of suspected side effects or vaccine complications, including deaths, are continuously recorded and evaluated. The PEI currently publishes weekly safety reports on suspected complications after vaccination including the number of fatalities registered.
There have been 7,093,082 vaccinations with Comirnaty® in Germany up to 12th March 2021 according to the Robert Koch-Institute (RKI) [2]. As shown in the safety report of the PEI [3] of 23rd March 2021, mainly transient local reactions and general reactions have been observed in adverse reactions reported after vaccination with Comirnaty®, without these being specified in more detail. As of 12th March 2021, 286 vaccinated individuals died in Germany from one hour to 40 days after vaccination. The average age of the deceased was 74 years. In the vast majority of individuals who did not die from COVID-19 infection, there were multiple pre-existing conditions, such as carcinoma, renal failure, and cardiovascular disease, which were assumed to be the cause of death [3]. The report does not provide more detailed information on the causes of death, However, it is not stated how many of these deceased had been autopsied.
According to press reports, Norway had already changed its vaccination indications about 3 weeks after the start of the vaccination period following 23 deaths in temporal connection with a COVID-19 vaccination (BioNTech / Pfizer and Moderna). The deaths had all occurred after initial vaccination of elderly (>75 years) and multimorbid patients [4]. Again, until now no information is available about the autopsy rate of these 23 cases. The Norwegian Office of Public Health (Folkehelseinstituttet) points out that even minor side effects could have serious consequences for seriously ill patients and that the positive effect of vaccination is thus lost. Therefore, a thorough consideration should be made in each individual case by the medical staff [5]. Meanwhile, Norway and Denmark, and temporarily Germany and other countries, have paused administration of AstraZeneca's vaccine because of the apparent clustering of thrombotic complications.
After the start of vaccination in Germany and abroad, anaphylactic reactions up to anaphylactic shock were reported [6]. Until 12th March 2021, 84 cases of anaphylactic reaction associated with a COVID-19 vaccination from BioNTech / Pfizer have been reported to PEI [3]; none of these cases ended up fatal. The anaphylactic symptoms occurred within the first 30 min after vaccination, so a medical monitoring of at least 15 min after vaccination is recommended by the RKI [1]. Currently, the anti-polyethylene glycol (PEG) antibody, which is an additive in cosmetics, pharmaceuticals and food and a component of the vaccine, is assumed to be the main allergen [3], [7]. PEG antibody 9996 was shown to account for hypersensitivity reactions entailing severe allergic symptoms or fatal anaphylaxis [8]. Therefore, in cases of death in close temporal relation to the application of the vaccine and corresponding symptoms, the presence of an anaphylactic reaction must be considered.
The forensic or general pathologic examination of deaths after vaccination thus represents an important component in the processing and evaluation of such events and is still crucial for understanding complex or new entities and courses of diseases. Three deaths after vaccination with Comirnaty®, which were autopsied at the Institute of Legal Medicine Hamburg, Germany, are presented in more detail.
2. Case 1
An elderly female resident of a nursing home (age is not given for the small case group for anonymization) received her initial vaccination against SARS-CoV-2 with Comirnaty®. She was fine after the vaccination. Three days later, she developed a fever which was treated by the family doctor with an antipyretic drug as needed. Rapid antigen tests for SARS-CoV-2 virus infection on the 3rd and 4th day after vaccination were negative. The woman's general condition had deteriorated and she was exsiccated. On the 5th day after the vaccination, she was found lifeless in her room. Previous illnesses included coronary heart disease with a condition following bypass surgery, cardiac insufficiency, arterial hypertension, dementia and hyperthyroidism.
At the external postmortem examination, the doctor noted a duty to report the case given the possible causal association with a medical intervention and informed the responsible health and investigation authority.
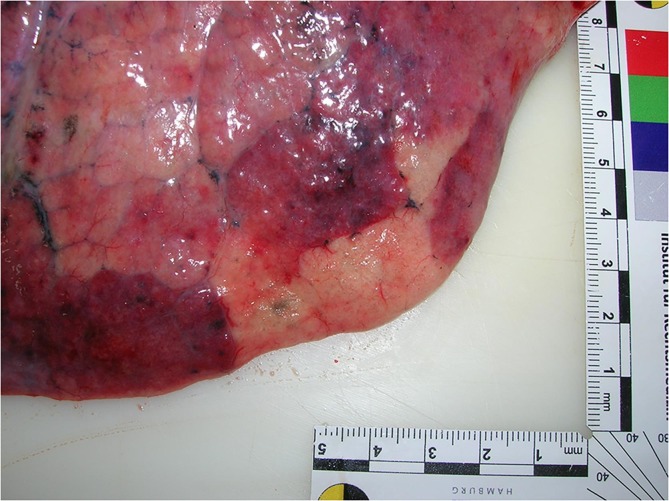
The forensic autopsy took place one day after death. In addition to the clinically indicated previous diseases, pulmonary emphysema, hiatus hernia, pseudomembranous colitis and dermatitis on the left lower leg were diagnosed. The cause of death was pulmonary artery embolism (Fig. 1 ) with infarction of the right lower lobe of the lung with deep leg vein thromboses on both sides. On the left upper arm, an injection site was found over the deltoid muscle. The axillary lymph nodes appeared inconspicuous macroscopically.
Fig. 1.
Central pulmonary artery embolism in the right lung.
As expected, with a time interval of five days between vaccination and death, no evidence of an allergic event was found morphologically and in the postmortem laboratory diagnostics (tryptase, total IgE). The pro-inflammatory parameters CRP and interleukin-6 were elevated, consistent with the colitis and dermatitis found. A postmortem nasopharyngeal swab for SARS-CoV-2 RNA was negative in real-time. quantitative Polymerase chain reaction (RT-qPCR).
A causal relation between the vaccination and the death did not emerge from the results of the autopsy. The death was due to an internal cause. The case and the autopsy evaluation were reported to the PEI.
3. Case 2
An elderly resident of a nursing home received his first COVID-19 vaccination (Comirnaty®). Seven days later, he developed the first symptoms of illness and was admitted to hospital for treatment 10 days after vaccination with respiratory insufficiency. A nasopharyngeal swab for SARS-CoV-2 RNA performed there 12 days after vaccination using RT-qPCR was positive. The man died clinically of COVID-19 pneumonia. Pre-existing conditions included chronic renal failure, anemia, atrial fibrillation, pulmonary artery embolism, arterial hypertension, peripheral artery disease, right thalamic infarction with left hemiparesis, recurrent tonic-clonic seizures, gait disorder with polyneuropathy, rheumatoid arthritis and prostate carcinoma with prostatectomy.
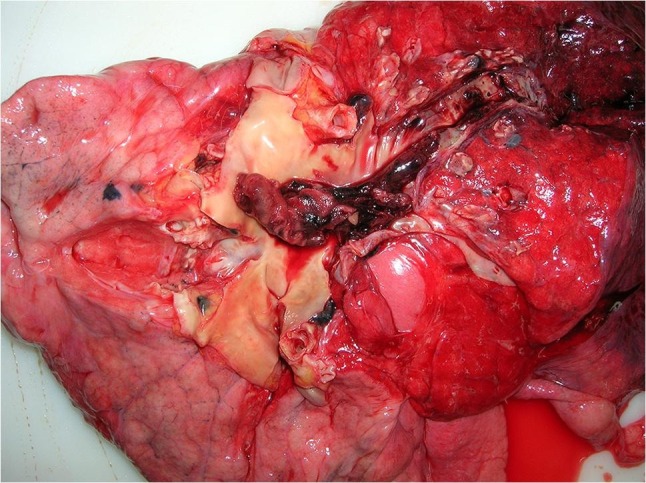
The forensic autopsy was performed three days post mortem. In addition to the clinically described pre-existing diseases, the autopsy revealed chronic and acute pancreatitis. Pneumonia was confirmed as the cause of death (Fig. 2, Fig. 3 ). Histologically, the markedly congested lungs showed alveoli filled with activated type II pneumocytes, fibroblasts, and partially lined with hyaline membranes. Giant cells and squamous metaplasia were present in some areas. The medium-sized arteries showed predominantly lymphocellular infiltrates in the outer wall layers. Microthromboses were found in small arterioles. The infection with SARS-CoV-2 had probably occurred shortly before or after vaccination, when vaccination protection was not yet available. According to the autopsy findings, this was a natural death in temporal coincidence but not causal relation to the SARS-CoV-2 vaccination. This conclusion was reported to the PEI afterwards.
Fig. 2.
Patchy pleural surface with segmental hyperemia.
Fig. 3.
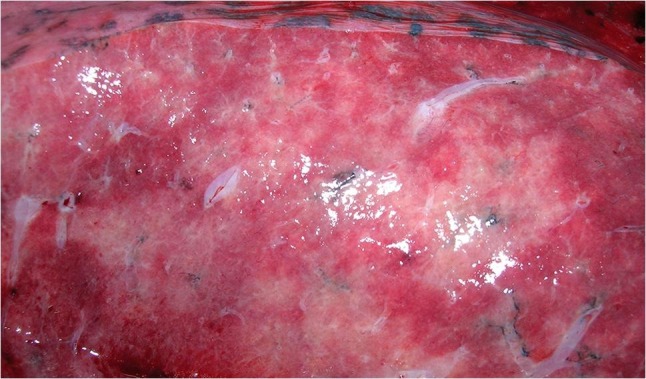
Cutting surface of the lung with alternating hyperemic and pale areas.
4. Case 3
On the occasion of a planned COVID-19 vaccination (Comirnaty®) of residents and staff of a nursing home, a senior resident suffering from dementia was presented to the responsible doctor of the vaccination team. Previous illnesses included apoplexy and myocardial infarction as well as arterial hypertension and type II diabetes mellitus. Otherwise, the resident was unremarkable (no fever, no allergies, vital signs without abnormal findings). After vaccination he had no acute pathological symptoms, his general condition was unremarkable. Two days later, he was suddenly found dead in front of his bed in the morning. According to the death certificate, the death was likely due to old age and multimorbidity and was declared to be of natural origin.
After the second external examination by a forensic pathologist prior to cremation three days after the death, the death was reported to the criminal investigation department as well as to the responsible public health department and the PEI under the suspicion of a shortening of life due to a fatal vaccination complication. The death certificate contained no reference to the previous vaccination. However, during the forensic medical examination, an injection-typical skin defect covered with plaster was found on the upper arm. The forensic autopsy was performed nine days after death. The known pre-existing conditions were confirmed, and further organ pathologies typical of old age were found in the form of signs of chronic obstructive pulmonary disease (COPD) and chronic renal dysfunction. The cause of death was a recurrent myocardial infarction (Fig. 4 ) with severe coronary heart disease and severe general arteriosclerosis. The lungs showed, besides advanced organised ones, a fresh, non-fulminant pulmonary artery thromboembolism in peripheral segments. Signs of an acute inflammatory event or a systemic abnormality (of the type of a vaccination complication) could not be verified; individual axillary lymph nodes were swollen near the injection site. From a forensic medical point of view, there was no causal relation between the vaccination and death that had taken place two days earlier.
Fig. 4.
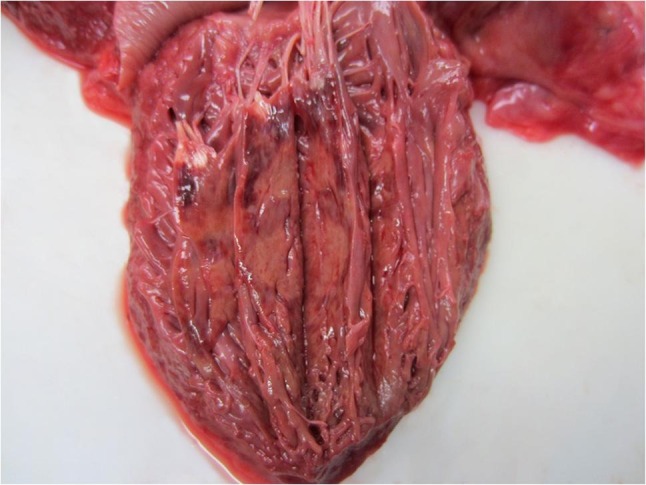
Acute myocardial infarction in the anterior papillary muscle.
5. Discussion
Since the start of SARS-CoV-2 vaccinations in Germany on 27th December 2020, we became aware of 22 deaths in relation to the vaccination (range: 0–28 days after vaccination) from our catchment area in northern Germany over the next 5 weeks (until the beginning of February). We received knowledge of these i) through the order of a forensic autopsy (four cases, one case was only discovered during the second external examination before cremation in another federal state), ii) through the second external postmortem examination before cremation (eleven cases, information in the death certificate or findings on the corpse – plaster at a location typical for vaccination with subsequent research in the nursing home), and iii) through external reports (seven cases, nursing home or local health authority). The deceased were on average 85.7 years old (range: 46–97 years, two were known to be younger than 80; 46 and 78 years).
Of these 22 cases, 7 cases were autopsied at the Institute of Legal Medicine in Hamburg. In the three reported cases, as well as three more autopsied a causal relation between the vaccination and death was not established in any case on the basis of the autopsy findings and the further postmortem examinations. All of the decedents had severe cardiovascular disease and other comorbidities and, with the exception of case 2 presented here, also died from these conditions.
It is not surprising that COVID-19 deaths also occur after vaccination, as was also reported in the PEI safety report. Polack et al. [9] report on the effectiveness of the vaccine from BioNTech and Pfizer that 9 cases of COVID-19 were observed among the participants at least 7 days after the second dose, which corresponds to a vaccine effectiveness of 94.6%. Thus, COVID-19 is possible even after receiving both doses. However, the disease course is usually less severe with only mild symptoms. Therefore, a post mortem nasopharyngeal swab to detect SARS-CoV-2 RNA should also be considered at the morgue or during autopsy if there is evidence of pneumonia.
All deaths that are causally and/or temporally related to a SARS-CoV-2 vaccination should be reported to the responsible investigating authority, the local health authority and to a higher-level reporting office, in Germany the PEI, if there are indications of a treatment complication.
Particular attention should also be paid at autopsy to signs of an allergic reaction and/or the onset of symptoms within the first 24 h after vaccination, also in very elderly and multimorbid persons. In the case of sudden deaths of a previously healthy or not significantly previously ill person, the time frame should be extended. The longer the time interval after vaccination, the less expectable is a causal relationship to death. A full postmortem examination to verify causality between vaccination and death should be sought in all these cases. In addition to the possibility of a public prosecutor's order or an autopsy with the consent of the family members in Germany, there is also the possibility of an order via the public health authorities according to the Infection Protection Act §25(4). The Infection Protection Act is a law for the prevention and control of infectious diseases, which, among other things, enables the public health officer to order an autopsy in the case of notifiable infectious diseases.
If there are signs, indications, patient histories or the suspicion of an allergic reaction, postmortem laboratory examinations should be performed in addition to the autopsy. Especially in these cases, a sample collection during the autopsy in the very early postmortem interval is necessary [10]. We recommend determination of tryptase, total immunoglobulin E (IgE), the inflammatory markers interleukin-6 and C-reactive protein (CRP) as well as complement activity C3/C5 (as an indicator of immune system activation) in femoral vein blood (serum), most probable sampled within 48 h after death to avoid significant postmortem alterations [11], [12]. Blood sampling should be done by groin puncture or from a clamped femoral/external iliac vein by aspiration instead of vessel scratching for comparable results [13].
Anti-PEG antibodies are no standard biomarker in biochemistry to our knowledge. However, specialized laboratories are able to establish a reference by coupling it to the antigen vaccine. For this purpose, aliquots should be stored at −80 °C for further analysis.
Histological examinations of the pharyngeal mucosa, the spleen, the injection site, regionally draining lymph nodes, especially axillary, and the deltoid muscle should be performed in addition. Histological evidence of mast cell degranulation can be obtained by Giemsa staining or CD117 immunohistochemistry. Former studies also evaluated anti-tryptase and anti-chymase immunostainings as further mast cell-typical antibodies [14]. Accumulations of eosinophilic granulocytes as well as shock signs of organs can be detected in the hematoxylin-eosin stain in case of fatal anaphylactic shock [15], [16]. Both formalin-fixed tissue samples and snap-frozen fresh tissue should be preserved for possible immunological investigations.
Due to the reported deaths, the authors believe that a general vaccination recommendation for all people over the age of 80 years should be reconsidered critically. In multimorbid patients in a very poor and/or deteriorating general condition in the days before vaccination, the general vaccination reactions and underlying immunological stimulation may be sufficient to lead to decompensation of their underlying diseases and even death. Likewise, the indication for palliative patients should be critically examined in individual cases, as is already recommended in Norway. Multimorbid patients with end-stage disease were not included in phase 3 of the vaccination studies, so that experience can only now be gained for this vulnerable group [9].
According to a recommendation of the PEI, COVID-19 vaccination can be carried out regularly in the case of existing allergies to food, insect venoms or inhalation allergies. However, a relative contraindication exists in patients with previous vaccination complications with ingredients of the vaccines against COVID-19. In the case of previous vaccination complications, the observation and monitoring time should be extended to 30 min after injection [17]. If an allergy to PEG is suspected, allergological clarification is recommended before vaccination [7]. In addition to this recommendation, the indication for vaccination in elderly and/or severely ill patients should, in the opinion of the authors, be decided on a case-by-case basis if there is a history of allergies with respiratory symptoms (e.g. dyspnea). Before vaccination, the availability of guideline-based therapy (epinephrine, antihistamines) should be checked.
The deaths reported in the PEI safety report and in this manuscript illustrate the importance of an autopsy/postmortem examination in each individual case and informing a higher-level reporting office in order to identify causal relationships between the occurrence of undesirable side effects, up to and including rare serious reactions such as death. The performance of detailed external postmortem examinations and reporting suspected cases through the coroner's inquest (information on the death certificate, vaccine patch on the outer side of the upper arm) play an important role in this context. This again highlights the desperate need of enough qualified forensic and/or clinical pathologists and high vulnerability of all authorities for current health care topics. With vaccine hesitancy still high among many people, it is especially important to carefully investigate deaths associated with vaccination and to objectively solve unclear circumstances. Otherwise, people's confidence in safe vaccination could easily be destroyed by individual cases sensationalized by the media.
Meanwhile, there have been reports of complications and deaths in comparatively young people after administration of AstraZeneca's vaccine. There have been suspicions that the combination of thrombocytopenia and cerebral venous sinus thrombosis (CVST) may have been clustered. On March 18, 2021, the European Medicines Agency (EMA) stated that there was no evidence for a general clustering of thromboembolic events, while a slightly increased risk for thrombocytopenia and CVST could at least not be excluded [18]. It is possible that selection effects play a role in the group of patients who received the AstraZeneca vaccine, feigning a higher incidence of CVST. Either way, Denmark and Norway are currently continuing to pause administration of the vaccine. We have not investigated any deaths after AstraZeneca vaccination until april in Hamburg. Data on suspected cases of severe vaccine complications are available to regulatory authorities such as the EMA or the PEI, but not yet to the wider scientific public. This makes it all the more important to quickly make such data available for scientific review in the form of single case reports and carefully analyzed case collections.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Robert Koch-Institut . 2021. COVID-19 und Impfen.https://www.rki.de/SharedDocs/FAQ/COVID-Impfen/gesamt.html (accessed 28 March 2021) [Google Scholar]
- 2.Robert-Koch-Institut . 2021. Impfquotenmonitoring.https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Impfquotenmonitoring.html;jsessionid=A045CDFF871ED37808105E1918F5C435.internet122?nn=13490888 (accessed 28 March 2021) [Google Scholar]
- 3.Paul-Ehrlich-Institut . 2021. Sicherheitsbericht.https://www.pei.de/SharedDocs/Downloads/DE/newsroom/dossiers/sicherheitsberichte/sicherheitsbericht-27-12-bis-12-03-21.pdf?__blob=publicationFile&v=4 (accessed 28 March 2021) [Google Scholar]
- 4.Frankfurter Rundschau . 2021. Todesfälle nach Corona-Impfung – Norwegen spricht eine krasse Warnung aus.https://www.fr.de/panorama/corona-impfung-todesfaelle-norwegen-nebenwirkungen-patienten-impfstoff-richtlinien-biontech-warnung-zr-90174341.html (accessed 28 March 2021) [Google Scholar]
- 5.Norwegian Institute of Public Health . 2021. Coronavirus Vaccine – Information for the Public.https://www.fhi.no/en/id/vaccines/coronavirus-immunisation-programme/coronavirus-vaccine/ (accessed 28 March 2021) [Google Scholar]
- 6.Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N. Engl. J. Med. 2020 doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zylka-Menhorn V. Anaphylaxie in der Anamnese. Dtsch. Arztebl. 2021;118:20–21. [Google Scholar]
- 8.Kozma G.T., Shimizu T., Ishida T., Szebeni J. Anti-PEG antibodies: properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020;154–155:163–175. doi: 10.1016/j.addr.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garland J., Ondruschka B., Da Broi U., Palmiere C., Tse R. Post mortem tryptase: a review of literature on its use, sampling and interpretation in the investigation of fatal anaphylaxis. Forensic Sci. Int. 2020;314 doi: 10.1016/j.forsciint.2020.110415. [DOI] [PubMed] [Google Scholar]
- 11.Ondruschka B., Woydt L., Bernhard M., Franke H., Kirsten H., Löffler S., Pohlers D., Hammer N., Dreßler J. Post-mortem in situ stability of serum markers of cerebral damage and acute phase response. Int. J. Legal Med. 2019;133:871–881. doi: 10.1007/s00414-018-1925-2. [DOI] [PubMed] [Google Scholar]
- 12.Woydt L., Bernhard M., Kirsten H., Burkhardt R., Hammer N., Gries A., Dreßler J., Ondruschka B. Intra-individual alterations of serum markers routinely used in forensic pathology depending on increasing post-mortem interval. Sci. Rep. 2018;8:12811. doi: 10.1038/s41598-018-31252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garland J., Philcox W., McCarthy S., Hensby-Bennet S., Ondruschka B., Woydt L., Da Broi U., Palmiere C., Lam L., Ahn Y., Kesha K., Stables S., Tse R. The effects of different sampling techniques on peripheral post mortem tryptase levels: a recommended sampling method. Int. J. Legal Med. 2019;133:1477–1483. doi: 10.1007/s00414-019-02038-9. [DOI] [PubMed] [Google Scholar]
- 14.Osawa M., Satoh F., Horiuchi H., Tian W., Kugota N., Hasegawa I. Postmortem diagnosis of fatal anaphylaxis during intravenous administration of therapeutic and diagnostic agents: evaluation of clinical laboratory parameters and immunohistochemistry in three cases. Leg. Med. 2008;10:143–147. doi: 10.1016/j.legalmed.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Byard R.W. Anaphylaxis at autopsy. Forensic Sci. Med. Pathol. 2017;13:269–271. doi: 10.1007/s12024-016-9799-4. [DOI] [PubMed] [Google Scholar]
- 16.Ondruschka B., Habeck J.O., Schwarz M., Dreßler J., Bayer R. Atypisches Ertrinken bei anaphylaktischem Schock. Rechtsmedizin. 2016;26:124–128. [Google Scholar]
- 17.Paul-Ehrlich-Institut . 2021. Empfehlungen zur Coronaimpfung für Allergikerinnen und Allergiker.https://www.pei.de/SharedDocs/Downloads/DE/newsroom/mitteilungen/201223-stellungnahme-empfehlung-allergiker.pdf?__blob=publicationFile&v=6 (accessed 28 March 2021) [Google Scholar]
- 18.European Medicines Agency. https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots, 2021 (accessed 28 March 2021).