Abstract
The presence of memory T cells in COVID-19 patients has been acknowledged, however the functional potency of memory responses is critical for protection. In this study, naïve, effector, effector memory, and central memory CD4+ and CD8+ T cells obtained from the COVID-19 survivors were re-exposed to autologous monocyte-derived DCs that were loaded with SARS-CoV-2 spike glycoprotein S1. Proliferation capacity, CD25, 4-1BB, and PD-1 expression, and IFN-γ, IL-6, granzyme, granulysin, and FasL secretion were enhanced in CD4+ and CD8+ effector memory and central memory T cells. Albeit being at heterogeneous levels, the memory T cells from the individuals with COVID-19 history possess functional capacities to reinvigorate anti-viral immunity against SARS-CoV-2.
Keywords: SARS-CoV-2; Central memory T cells, Effector memory T cells, Spike S1 glycoprotein; Dendritic cell; Helper T cells; Cytotoxic T lymphocytes
1. Introduction
Despite the emerging data on coronavirus disease 2019 (COVID-19), the immune response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains to be better defined. The strength and duration of the humoral and cellular responses against SARS-CoV-2 have been associated with neutralizing antibodies and memory T cells [1], [2]. Especially, the spike glycoprotein-S1 bears significant immunodominance since the neutralizing antibodies block its attachment to the angiotensin-converting enzyme 2 (ACE2) and hinder the viral entry [3]. Besides, approximately 50% of the patients displayed S1-specific T cell responses [4], [5].
Nevertheless, the heterogeneity observed amongst the COVID-19 cases has a confounding effect. While the majority of the patients develop anti-viral immunity, even the convalescent individuals may not be protected from the re-infection which is potentially due to the insufficient magnitude and/or stability of T cell and antibody production [6], [7], [8]. Intriguingly, in severe SARS-CoV-2 infections, the interaction between CD4+ helper T (Th) cells and B cells is blunted in the germinal center, which potentially dampens the longevity of antibody responses [9]. Together with the Th activities, the robustness of CD8+ cytotoxic T cells is also pivotal for a successful anti-viral immunity [10]. Previously, the presence of CD4+ or CD8+ memory T cells was reported in COVID-19, nevertheless the functional capacities of these cells need to be addressed thoroughly [11], [12], [13]. In this study, the functional responsiveness of naïve, effector, central memory, and effector memory CD4+ or CD8+ T cells, which were obtained from the patients with COVID-19 history, against monocyte-derived dendritic cells (DCs) bearing SARS-CoV-2 S1 antigen is confirmed.
2. Material and methods
2.1. Patients and sample collection
At two different time points, peripheral blood samples were freshly collected from the patients recovered from COVID-19 (Table 1 ) and peripheral blood mononuclear cells were separated by 1.077 g/mL Ficoll density gradient (Sigma-Aldrich). All protocols were approved by the local ethical committees and the Republic of Turkey Ministry of Health. İnformed consent was obtained from the patients. Patients with positive RT-PCR test and/or seropositivity were enrolled in the study. The clinical symptoms were categorized as mild (the non-hospitalized patients), moderate (the patients who had moderate pneumonia) and severe (the patients who had severe pneumonia and were hospitalized for more than 5 days). Blood samples from healthy donors [n = 10 (6 females, 4 males), median age 33 (min 28–max 55)] without SARS-CoV-2 history and seropositivity were used controls.
Table 1.
Patient data.
| COVID-19 patients | |
|---|---|
| Number (n = ) | 10 |
| Age median (range) | 37 (17–63) |
| Gender (female/male) | 5/5 |
| Clinical score (n = ) | |
| Mild | 6 |
| Moderate | 2 |
| Severe | 2 |
| Anti-S1 Ig titer median (range) | 8.4 RU/mL (1.9–9.5) |
| Timing of blood collectiona median (range) | 1 months (1–5) |
RU, relative units.
After the date of diagnosis.
2.2. Establishment of monocyte-derived DC and T cell co-cultures
DCs were generated from the monocytes (CD14 MACS, Miltenyi) according to a previously published protocol [14]. Antigen-loading with the recombinant S1 protein (S1; 10 µg/mL, Abcam) or HIV Gag antigen (10 µg/mL, TUBITAK Marmara Research Center) [15] or the tetanus toxoid (TT; 10 µg/mL, Turk Ilac) was simultaneously initiated with the maturation of monocyte-derived DCs with LPS (1 µg/mL, Sigma-Aldrich). Mature monocyte-derived DCs generated in the absence of a specific antigen were used as controls. At the end of 7-day-long incubation, the monocyte-derived DCs were characterized as a CD11bhiCD14loCD1a+CD83+ population.
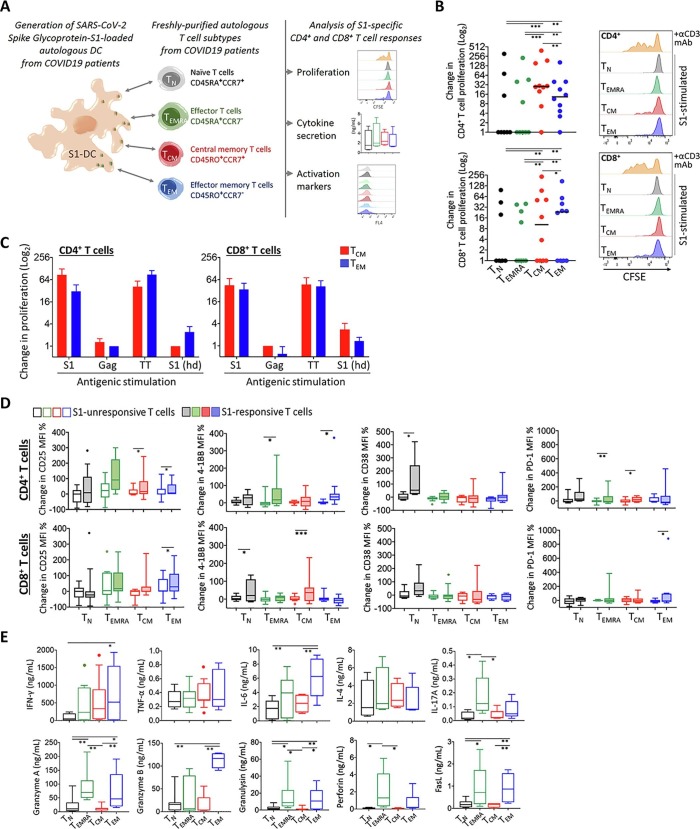
From the same COVID-19 patient, autologous naïve T (TN), terminally-differentiated effector T (TEMRA), central memory T (TCM), and effector memory T (TEM) cells were purified (≥96%) by FACS (FACSAria II; Becton Dickinson) as CD3-untouched, CD19- and CD56-negative lymphocytes according to the differential expression of CD45RA, CD45RO, and CCR7 markers (Fig. 1 A).
Fig. 1.
Assessment of functional responses in T cells from COVID-19 survivors. A) Graphical outline of the experimental setup is shown. Monocyte-derived dendritic cells were generated from the individuals with COVID-19 history and loaded with SARS-CoV-2 Spike Glycoprotein-S1 (S1-DC); then, autologous naïve T (TN), terminally-differentiated effector T (TEMRA), central memory T (TCM), and effector memory T (TEM) cells were purified and co-cultured with these DCs. T cell proliferation, expression of activation markers and cytokine secretion were measured after 96 h incubation. B) Change in CD4+ and CD8+ T cell proliferation was plotted for each patient in comparison to that obtained with control co-cultures with monocyte-derived DCs without specific antigen loading. Representative flow cytometry histograms are given on the right side. The co-cultures stimulated with an anti-CD3 monoclonal antibody served as a technical positive control for T cell proliferation. C) The patient-derived TCM and TEM cells’ proliferation response against the S1-DC was compared with those obtained with the HIV Gag or with the tetanus toxoid (TT) antigen-loaded DCs. T cells and S1-DCs obtained from healthy individuals (S1(hd)) were also used as controls. D) Changes in CD4+ and CD8+ T cell activation marker levels were shown in comparison to those obtained with control co-cultures with monocyte-derived DCs without specific antigen loading. E) Amount of T cell-associated cytokines secreted into the co-culture supernatants was assessed. (*p < 0.05, **p < 0.01).
The monocyte-derived DCs (5x104) were co-cultured with the purified subtypes of T cells (105) for 96 h in a round-bottom 96-well plate well containing 200 µL RPMI 1640 media completed with 10% FBS, 1% penicillin/streptomycin (Biological Industries), 5 ng/mL IL-2 (BioLegend). Prior to co-culturing, the T cell subtypes were labelled with 5 µM CFSE (BioLegend). As a positive control, anti-CD3 antibody (HIT3a, 25 ng/mL; BioLegend) was added into the co-cultures. The experimental setup is summarized in Fig. 1A.
2.3. Immunophenotyping and function-related assays by flow cytometry
Immunophenotyping was performed with monoclonal antibodies anti-human-CD4 (OKT4), -CD8 (RPA-T8), -CD56 (MEM-188), -CD19 (SJ25C1), -CD45RA (HI100), -CD45RO (UCHL1), -CCR7 (G043H7), -CD25 (M-A251), -CD38 (HIT2), -4-1BB (4B4-1), -PD-1 (NAT105), -CD14 (M5E2), -CD11b (ICRF44) (BioLegend); -CD1a (REA736), -CD83 (REA714) (Miltenyi). Median fluorescence intensity (MFI) values were determined on CD4+ and CD8+ T cells, and according to CFSE dilution. The change in the MFI were calculated by comparing the data from the co-cultures with the antigen-loaded DCs and the control DCs.
The percentage of T cells with CFSE dilution was assessed for proliferation. The antigen-specific proliferation capacity of T cells was calculated as the change in proliferation wherein the data from the co-cultures with control monocyte-derived DCs were used as normalizer. The supernatants collected were used in a multiplex ELISA (LEGENDplex, BioLegend). All flow cytometric analyses were performed on a FACSAria II sorter.
2.4. Statistical analysis
The results are presented as median ± SEM. Kruskal-Wallis test and Bonferroni correction were used for the statistical analyses. A P value <0.05 was considered statistically significant.
3. Results
Following the incubation with the S1-DC, a small percentage of proliferating T cells was identified (range %, CD4+, TN 0.49–6.8, TEMRA 3.29–7.7, TCM 0.38–20.2, TEM 0.2–16.7; range %, CD8+, TN 0.64–5.6, TEMRA 2.16–10.3, TCM 0.8–15, TEM 1.6–19.4). Both CD4+ and CD8+ TCM and TEM cells exhibited a considerably increased frequency of proliferation than the naïve or effector T cell populations (Fig. 1B). CD4+ TCM cells and CD8+ TEM cells displayed the highest proliferative activity. The CD4+ memory T cell proliferation could be induced in ~90% of the COVID-19 patients, however the CD8+ memory T cell proliferation was only evidenced in ~60% of the patients (Fig. 1B). The monocyte-derived DCs were also loaded with an irrelevant viral antigen, HIV Gag; expectedly, no significant response was obtained in the T cells from COVID-19 survivors (Fig. 1C). Similarly, the T cells from the healthy individuals did not respond to S1-DC. On the other hand, the monocyte-derived DC presenting the TT antigen served as a positive control for T cell memory responses (Fig. 1C). In addition, the surface expression of certain activation-related markers, especially CD25, PD-1 and 4-1BB, was significantly upregulated on S1-responsive T cells (Fig. 1D). The S1-DC-stimulated TEM cells secreted the highest levels of IFN-γ and IL-6, which is largely produced by type 1 CD4+ T cells and CD8+ cytotoxic T cells. The markers of cytotoxic response, granzyme A, granulysin, Fas ligand (FasL), and especially granzyme B were also elevated in the co-cultures harboring TEM cells from COVID-19 patients (Fig. 1E). The co-culture supernatants from which the soluble factors were measured contained the mediators secreted by T cells and DCs; thus, as stated in the literature, moderate amounts of the cytokines such as TNF-α, IL-6, IL-4 and IFN-γ may also be produced by the DC [16], [17]. In some cases, the non-memory T cells responded to S1-DC, upregulated the CD38 and 4-1BB expression, and the secretion of IL-4 and TNF-α (Fig. 1). A heterogeneity was also noted between the T cell parameters studied and the time of blood sampling after recovery, the severity of clinical symptoms, or the level of anti-S1 antibodies (data not shown).
4. Discussion
In the COVID-19 survivors, the circulating S1-specific TEM and TCM cells retained functional responsiveness and displayed augmented effector capacities such as activation, proliferation, and secretion of immune mediators. Even though the proliferative response in the memory CD8+T cells was not as potent as in the CD4+ memory T cells, our data suggest that these cells can quickly advance to an effector state when exposed to SARS-CoV-2 S1 antigen. The TEM cells are critical gatekeepers since they tend to locate into the tissues prone to invasion by the pathogenic microorganism, whereas the TCM cells are recruited into the secondary lymphoid organs for accelerating the immune reactions inaugurated by the antigen-presenting DCs [18]. Albeit covering a limited number of COVID-19 cases, in our study, the majority of the patients harbored TEM and TCM cells that functionally responded to S1 protein in terms of at least one parameter tested. These preliminary findings may indicate a probable disparity between functional competence of T cells and COVID-19 severity. A previous study reported a higher frequency of S1-specific T cells than the T cells specific for N and M proteins [8]. Correspondingly, the functional responsiveness of T cells to other SARS-CoV-2 antigens remains to be better elucidated. Recently published seminal work demonstrated the presence of long-term memory in the T cells from COVID-19 patients [19], [20]. By using a distinct experimental approach wherein the autologous monocyte-derived DCs were used as a feasible element for testing the T cell response, our study confirmed the function and character of T cells previously mentioned in the individuals with COVID-19 history. Accumulating evidence on the immunity established in the COVID-19 survivors would provide a better understanding of disease pathogenesis, therapeutic approaches, and vaccine development.
CRediT authorship contribution statement
Ece Tavukcuoglu: Investigation, Visualization, Formal analysis, Writing - review & editing. Utku Horzum: Investigation, Visualization, Formal analysis, Writing - review & editing. Ahmet Cagkan Inkaya: Conceptualization, Resources. Serhat Unal: Conceptualization, Resources. Gunes Esendagli: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mazzoni A., Maggi L., Capone M., Spinicci M., Salvati L., Colao M.G., Vanni A., Kiros S.T., Mencarini J., Zammarchi L., Mantengoli E., Menicacci L., Caldini E., Romagnani S., Liotta F., Morettini A., Rossolini G.M., Bartoloni A., Cosmi L., Annunziato F. Cell-mediated and humoral adaptive immune responses to SARS-CoV-2 are lower in asymptomatic than symptomatic COVID-19 patients. Eur. J. Immunol. 2020;50:2013–2024. doi: 10.1002/eji.202048915. [DOI] [PubMed] [Google Scholar]
- 2.Tan Y., Liu F., Xu X., Ling Y., Huang W., Zhu Z., Guo M., Lin Y., Fu Z., Liang D., Zhang T., Fan J., Xu M., Lu H., Chen S. Durability of neutralizing antibodies and T-cell response post SARS-CoV-2 infection. Front. Med. 2020;14:746–751. doi: 10.1007/s11684-020-0822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conte C., Sogni F., Affanni P., Veronesi L., Argentiero A., Esposito S. Vaccines against coronaviruses: The State of the Art. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C.K., Wu H., Yan H., Ma S., Wang L., Zhang M., Tang X., Temperton N.J., Weiss R.A., Brenchley J.M., Douek D.C., Mongkolsapaya J., Tran B.H., Lin C.L., Screaton G.R., Hou J.L., McMichael A.J., Xu X.N. T cell responses to whole SARS coronavirus in humans. J. Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koblischke M., Traugott M.T., Medits I., Spitzer F.S., Zoufaly A., Weseslindtner L., Simonitsch C., Seitz T., Hoepler W., Puchhammer-Stockl E., Aberle S.W., Fodinger M., Bergthaler A., Kundi M., Heinz F.X., Stiasny K., Aberle J.H. Dynamics of CD4 T cell and antibody responses in COVID-19 patients with different disease severity. Front. Med. (Lausanne) 2020;7 doi: 10.3389/fmed.2020.592629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poland G.A., Ovsyannikova I.G., Kennedy R.B. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou R., To K.K., Wong Y.C., Liu L., Zhou B., Li X., Huang H., Mo Y., Luk T.Y., Lau T.T., Yeung P., Chan W.M., Wu A.K., Lung K.C., Tsang O.T., Leung W.S., Hung I.F., Yuen K.Y., Chen Z. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53:864–877 e865. doi: 10.1016/j.immuni.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A. Grifoni, D. Weiskopf, S.I. Ramirez, J. Mateus, J.M. Dan, C.R. Moderbacher, S.A. Rawlings, A. Sutherland, L. Premkumar, R.S. Jadi, D. Marrama, A.M. de Silva, A. Frazier, A.F. Carlin, J.A. Greenbaum, B. Peters, F. Krammer, D.M. Smith, S. Crotty, A. Sette, Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals, Cell, 181 (2020) 1489-1501 e1415. [DOI] [PMC free article] [PubMed]
- 9.Canete P.F., Vinuesa C.G. COVID-19 makes B cells forget, but T cells remember. Cell. 2020;183:13–15. doi: 10.1016/j.cell.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulien I., Kemming J., Oberhardt V., Wild K., Seidel L.M., Killmer S., Sagar F., Daul M.S., Lago A., Decker H., Luxenburger B., Binder D., Bettinger O., Sogukpinar S., Rieg M., Panning D., Huzly M., Schwemmle G., Kochs C.F., Waller A., Nieters D., Duerschmied F., Emmerich H.E., Mei A.R., Schulz S., Llewellyn-Lacey D.A., Price T., Boettler B., Bengsch R., Thimme M., Hofmann C.-H. Characterization of pre-existing and induced SARS-CoV-2-specific CD8(+) T cells. Nat. Med. 2021;27:78–85. doi: 10.1038/s41591-020-01143-2. [DOI] [PubMed] [Google Scholar]
- 11.Odak I., Barros-Martins J., Bosnjak B., Stahl K., David S., Wiesner O., Busch M., Hoeper M.M., Pink I., Welte T., Cornberg M., Stoll M., Goudeva L., Blasczyk R., Ganser A., Prinz I., Forster R., Koenecke C., Schultze-Florey C.R. Reappearance of effector T cells is associated with recovery from COVID-19. EBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oja A.E., Saris A., Ghandour C.A., Kragten N.A.M., Hogema B.M., Nossent E.J., Heunks L.M.A., Cuvalay S., Slot E., Linty F., Swaneveld F.H., Vrielink H., Vidarsson G., Rispens T., van der Schoot E., van Lier R.A.W., Ten Brinke A., Hombrink P. Divergent SARS-CoV-2-specific T- and B-cell responses in severe but not mild COVID-19 patients. Eur. J. Immunol. 2020;50:1998–2012. doi: 10.1002/eji.202048908. [DOI] [PubMed] [Google Scholar]
- 13.Kratzer B., Trapin D., Ettel P., Kormoczi U., Rottal A., Tuppy F., Feichter M., Gattinger P., Borochova K., Dorofeeva Y., Tulaeva I., Weber M., Grabmeier-Pfistershammer K., Tauber P.A., Gerdov M., Muhl B., Perkmann T., Fae I., Wenda S., Fuhrer H., Henning R., Valenta R., Pickl W.F. Immunological imprint of COVID-19 on human peripheral blood leukocyte populations. Allergy. 2020 doi: 10.1111/all.14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruger A.M., Vanhaver C., Bruderek K., Amodio G., Tavukcuoglu E., Esendagli G., Gregori S., Brandau S., van der Bruggen P. Protocol to assess the suppression of T-cell proliferation by human MDSC. Methods Enzymol. 2020;632:155–192. doi: 10.1016/bs.mie.2019.05.046. [DOI] [PubMed] [Google Scholar]
- 15.Younes S.A., Yassine-Diab B., Dumont A.R., Boulassel M.R., Grossman Z., Routy J.P., Sekaly R.P. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frucht D.M., Fukao T., Bogdan C., Schindler H., O'Shea J.J., Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556–560. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- 17.Gubernatorova E.O., Gorshkova E.A., Namakanova O.A., Zvartsev R.V., Hidalgo J., Drutskaya M.S., Tumanov A.V., Nedospasov S.A. Non-redundant functions of IL-6 produced by macrophages and dendritic cells in allergic airway inflammation. Front. Immunol. 2018;9:2718. doi: 10.3389/fimmu.2018.02718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahnke Y.D., Brodie T.M., Sallusto F., Roederer M., Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Eur. J. Immunol. 2013;43:2797–2809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 19.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., Nakao C., Rayaprolu V., Rawlings S.A., Peters B., Krammer F., Simon V., Saphire E.O., Smith D.M., Weiskopf D., Sette A., Crotty S. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.L.B. Rodda, J. Netland, L. Shehata, K.B. Pruner, P.A. Morawski, C.D. Thouvenel, K.K. Takehara, J. Eggenberger, E.A. Hemann, H.R. Waterman, M.L. Fahning, Y. Chen, M. Hale, J. Rathe, C. Stokes, S. Wrenn, B. Fiala, L. Carter, J.A. Hamerman, N.P. King, M. Gale, Jr., D.J. Campbell, D.J. Rawlings, M. Pepper, Functional SARS-CoV-2-specific immune memory persists after mild COVID-19, Cell, 184 (2021) 169-183 e117. [DOI] [PMC free article] [PubMed]