Abstract
Rationale
The association between smoking status and severe Coronavirus Disease 2019 (COVID-19) remains controversial.
Objective
To assess the risk of hospitalization (as a marker of severe COVID-19) in patients by smoking status: former, current and never smokers, who tested positive for the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV2) at an academic medical center in the United States.
Methods
We conducted a retrospective cohort study in patients with SARS-COV2 between March-1-2020 and January-31-2021 to identify the risk of hospitalization due to COVID-19 by smoking status.
Results
We identified 10216 SARS-COV2-positive patients with complete documentation of smoking habits. Within 14 days of a SARS-COV2 positive test, 1150 (11.2%) patients were admitted and 188 (1.8%) died. Significantly more former smokers were hospitalized from COVID-19 than current or never smokers (21.2% former smokers; 7.3% current smokers; 10.4% never smokers, p<0.0001). In univariable analysis, former smokers had higher odds of hospitalization from COVID-19 than never smokers (OR 2.31; 95% CI 1.94-2.74). This association remained significant when analysis was adjusted for age, race and gender (OR 1.28; 95% CI 1.06-1.55), but became non-significant when analysis included Body Mass Index, previous hospitalization and number of comorbidities (OR 1.05; 95% CI 0.86-1.29). In contrast, current smokers were less likely than never smokers to be hospitalized due to COVID-19.
Conclusions
Significantly more former smokers were hospitalized and died from COVID-19 than current or never smokers. This effect is mediated via age and comorbidities in former smokers.
Keywords: COVID-19, SARS-COV2, Smoking, Hospitalization
1. Introduction
Since first being recognized in December 2019, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV2) has caused to date 130 million cases and 2.8 million deaths worldwide [1]. Coronavirus Disease 2019 (COVID-19), the infectious disease caused by SARS-COV2, can range in presentation from asymptomatic to severe disease. When symptomatic, the time from start of illness to when patients experience dyspnea ranges from 5 to 8 days. About 3–15% of individuals infected require hospitalization [[2], [3], [4], [5]]. In patients who develop severe disease the time from the beginning of illness to when patients develop acute respiratory distress syndrome (ARDS) ranges from 8 to 12 days. Also, the time from onset of illness to Intensive Care Unit (ICU) admission ranges from 9.5 to 12 days [6]. Risk factors for severe COVID-19 and greater risk of mortality have been identified [5,[7], [8], [9]]; specifically, these are: age greater than 65 years, coronary artery disease (CAD), congestive heart failure (CHF), cardiac arrhythmia, chronic obstructive pulmonary disease (COPD), diabetes mellitus, cancer, and chronic kidney disease (CKD), obesity, black race and Hispanic ethnicity [5,[8], [9], [10], [11], [12], [13]]. Despite agreement about the association between severe COVID-19 and the comorbidities mentioned above, the relationship between tobacco use and the severity of COVID-19 infection remains controversial [8,10,12,[14], [15], [16], [17], [18], [19], [20]].
Smoking has been associated with increased risk of infection and worse outcomes for a multitude of bacterial and viral pathogens [21]. For instance, smokers with or without chronic lung disease have higher risk of invasive pneumococcal disease and community acquired pneumonia [22,23]. Also, smoking is a risk factor for the development of and death from active tuberculosis infection [[24], [25], [26]]. Similarly, smokers are more likely to be infected with and develop clinical symptoms when exposed to rhinovirus, respiratory syncytial virus or coronavirus type 229 [27]. Influenza infection and severity are worse in smokers compared to non-smokers [[28], [29], [30]]. In contrast, studies throughout the world have reported a low prevalence of current smokers among people that tested positive for SARS-COV2 and/or were hospitalized due to COVID-19 [10,12,[18], [19], [20]]. The association between smoking and severe COVID-19 is unknown.
To evaluate the relationship between former, current, and never smoker status and severe COVID-19, we conducted a retrospective cohort study to identify the risk of hospitalization from COVID-19 (as a marker of severe infection) within 14 days of a positive SARS-COV2 test in former and current smokers compared to never smokers. Based on the known adverse effects of smoking, we hypothesize that smokers (former and/or current) may have higher risk of hospitalization from COVID-19 compared to never smokers.
2. Methods
2.1. Data and cohort
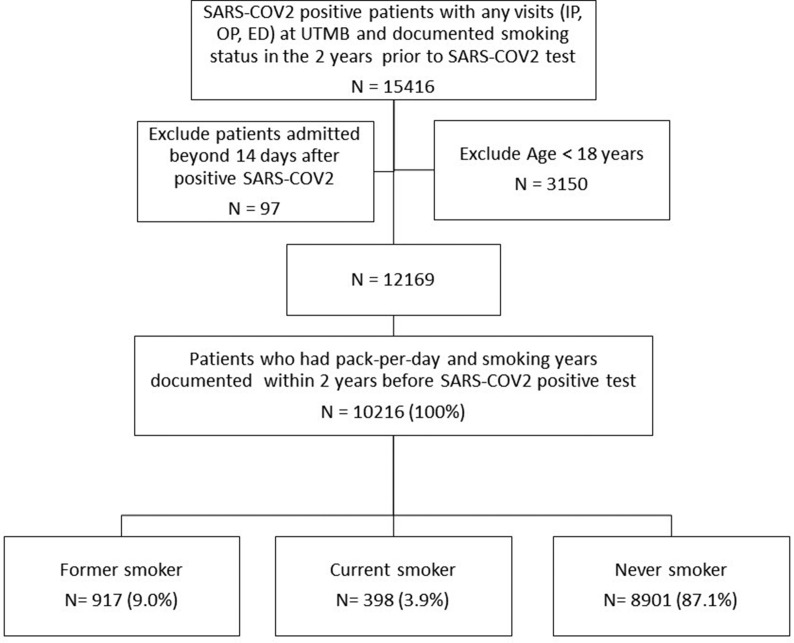
In this retrospective cohort study patients at the University of Texas Medical Branch (UTMB) Health System with any SARS-COV2 positive laboratory test result from March 1, 2020 to January 31, 2021 were included. The UTMB institutional review board approved the study (IRB# 20–0249). Data was collected from Epic, the institution's electronic health records system (EHR). Patients who were prisoners, younger than 18 years, admitted after 14 days of an initial positive SARS-COV2 result, had no clinical encounter in the prior 2 years in our health system or had incomplete smoking status records in the 2 years before SARS-COV2 test were excluded (Fig. 1 ). We divided the study cohort into former, current and never smokers based on each patient's lifetime count of cigarettes smoked and most recent self-reported smoking status. Patients who self-reported as a never smoker or who had smoked less than 100 cigarettes in their lifetime were considered never smokers; patients who smoked more than 100 cigarettes in their lifetime were considered smokers (former or current) [31]. Former smokers' status was assigned based on the patient's self-report of “former smoker” in the EHR.
Fig. 1.
Flow Diagram of our cohort selection process. Smoking status was obtained within 2 years before SARS-COV2 positive test result. For patients with multiple records, we took the latest. A never smoker was defined as a patient who: 1) self-reported as never having been a smoker or 2) had <100 cigarettes in their lifetime. A current or former smoker was defined as a patient who self-reported as a current or former smoker and had ≥100 cigarettes in their lifetime [31]. Current or former was based on latest self-report. Of patients who visited UTMB and had a positive test for SARS-COV2, 81.4% had smoking records. Of these patients who had a smoking record and fulfilled other eligibility criteria, 83.9% had complete records, including pack-per-day and smoking years and were included in the analysis. Admission was COVID-19 related inpatients (those admitted only to the Emergency Department were not considered). Definition of abbreviations: SARS-COV2= Severe Acute Respiratory Syndrome Coronavirus-2; COVID-19 = Coronavirus Disease 2019; IP= Inpatient; OP= Outpatient; ED = Emergency Department; UTMB= University of Texas Medical Branch.
2.2. Outcome and characteristics
Our main outcome was any COVID-19 related hospitalization within 14 days of the initial SARS-COV2-positive result. COVID-19-related hospitalization was defined as COVID-19 inpatient visits with any diagnosis of respiratory failure, cough, dyspnea, shortness of breath, respiratory distress, dyspnea on exertion, abnormal breathing, hypoxia, COVID-19, on supplemental oxygen, etc. (e-Table 1 in the online data supplement). For patients with multiple hospitalizations within 14 days of a positive SARS-COV2 result, we considered the first COVID-19-related hospitalization. We collected patient demographics, death date, Body Mass Index (BMI), and insurance status within a year of the initial SARS-COV2 test. Comorbidities, including diabetes, hypertension, COPD, asthma, CKD, end-stage renal disease (ESRD), stroke, CHF, cancer, CAD and liver disease, were collected from our EHR with the use of administrative diagnostic codes (International Classifications of Diseases version 10, [ICD-10]). Also collected were prednisone prescription within a year of the SARS-COV2 test and any history of inhaler prescriptions at the time of the test visit. We also collected each patient's prior year inpatient and emergency department visit records, home oxygen orders and smokeless cigarette usage.
2.3. Statistical analysis
Patient characteristics were summarized with mean, standard deviation (SD), frequency and percentage. In univariable analysis, we compared former, current and never smokers. For categorical characteristics, we used Chi-square test and Fisher's exact test; for continuous characteristics, we used analysis of variance. Multivariable analyses exploring the relationship of smoking status with the outcome were performed by fitting multivariable logistic regression models, adjusted for age, gender, race, BMI, number of comorbidities and number of inpatient stays within the previous year. The interaction of smoking status with other characteristics was evaluated. As sensitivity analysis, we split our cohort in three groups based on age (18–40, 41–64 and 65 years or older). Then, we fitted multivariable logistic regression models separately for each age group to explore any effect of smoking status on the risk of COVID-19 hospitalization. We also conducted sensitivity analyses with different BMI cutoffs, added Hispanic ethnicity and analyzed age as a continuous variable. Data management was performed with Python 3.7 and analysis was performed with SAS version 9.4 (SAS Inc., Cary, NC). All reported p-values were two-sided with p < 0.05.
3. Results
3.1. Demographics
Detailed characteristics of the cohort are presented in Table 1. During our study period, we found 10216 patients who had a positive test for SARS-COV2 and complete documentation of smoking habits in our EHR. Out of this group of patients, we identified 917 (9.0%) former smokers, 398 (3.9%) current smokers, and 8901 (87.1%) never smokers. In all, 35% were male, 43.0% were White, 39.1% were Hispanic, 15.3% were Black, 43.9% had BMI ≥30 kg/m2 and 3.6% had a smokeless tobacco use history. The mean age was significantly different for former (58.6 ± 15.96 years), current (48.8 ± 14.75 years) and never smokers (44.36 ± 17.93 years).
Table 1.
Characteristics of patients who tested positive for SARS-COV2 by smoking status at the University of Texas Medical Branch Health System between March 1. 2020 and January 31. 2021.
| Total n(%) | Former Smoker n(%) | Current Smoker n(%) | Never Smoker n(%) | p-value | |
|---|---|---|---|---|---|
| 10216(100) | 917(9.0) | 398(3.9) | 8901(87.1) | - | |
| Characteristics | - | - | - | - | - |
| Sex | - | - | - | - | <0.0001 |
| Female | 6632(64.9) | 479(52.4) | 240(60.3) | 5913(66.4) | - |
| Male | 3584(35.0) | 438(47.8) | 158(39.7) | 2988(33.6) | - |
| Age | - | - | - | - | <0.0001 |
| Mean (±SD) | 45.81(18.11) | 58.6(15.96) | 48.8(14.75) | 44.36(17.93) | - |
| N (%) | - | - | - | - | - |
| 0-40 | 4327(42.3) | 141(15.38) | 134(33.7) | 4052(45.52) | - |
| 40-49 | 1708(16.7) | 135(14.7) | 73(18.3) | 1500(16.9) | - |
| 50-64 | 2463(24.1) | 292(31.8) | 131(32.9) | 2040(22.9) | - |
| 65-74 | 1090(10.7) | 215(23.5) | 45(11.3) | 830(9.32) | - |
| 75-84 | 441(4.3) | 102(11.1) | 14(3.5) | 325(3.65) | - |
| >85 | 187(1.8) | 32(3.5) | 1(0.3) | 154(1.73) | - |
| Race | - | - | - | - | <0.0001 |
| White | 4402(43.1) | 529(57.7) | 237(59.55) | 3636(40.9) | - |
| Black | 1567(15.3) | 140(15.3) | 78(19.6) | 1349(15.2) | - |
| Other/Missing | 243(2.4) | 6(0.7) | 2(0.5) | 235(2.6) | - |
| Hispanic ethnicity | 4004(39.2) | 242(26.4) | 81(20.4) | 3681(41.4) | - |
| BMI Mean (±SD) | 31.41(7.63) | 31.76(7.80) | 30.89(7.79) | 31.39(7.60) | - |
| BMI | - | - | - | - | <0.0001 |
| Missing | 1541(15.1) | 62(6.8) | 52(13.1) | 1427(16.0) | - |
| Underweight | 98(1.0) | 16(1.7) | 8(2.0) | 74(0.8) | - |
| Normal | 1590(15.6) | 130(14.2) | 70(17.6) | 1390(15.6) | - |
| Overweight | 2500(24.5) | 251(27.4) | 101(25.4) | 2148(24.1) | - |
| Obese I(BMI 30–34) | 2178(21.3) | 221(24.1) | 76(19.1) | 1881(21.1) | - |
| Obese II(≥35) | 2309(22.6) | 237(25.9) | 91(22.9) | 1981(22.26) | - |
| Medicare | 1848(18.1) | 376(41) | 84(21.1) | 1388(15.59) | <0.0001 |
| Medicaid | 1196(11.7) | 98(10.7) | 78(19.6) | 1020(11.5) | <0.0001 |
| Dual eligibility | 87(0.9) | 26(2.8) | 13(3.3) | 48(0.5) | <0.0001 |
| Not insured | 1358(13.3) | 64(7.0) | 66(16.6) | 1228(13.8) | <0.0001 |
| Comorbidity | - | - | - | - | - |
| Diabetes | 1409(13.8) | 243(26.5) | 74(18.6) | 1092(12.3) | <0.0001 |
| Hypertension | 3045(29.8) | 536(58.5) | 166(41.7) | 2343(26.3) | <0.0001 |
| COPD | 1092(10.7) | 259(28.2) | 100(25.1) | 733(8.2) | <0.0001 |
| Asthma | 1108(10.8) | 146(15.9) | 59(14.8) | 903(10.1) | <0.0001 |
| CKD | 240(2.3) | 61(6.7) | 13(3.3) | 166(1.9) | <0.0001 |
| ESRD | 88(0.9) | 15(1.6) | 3(0.8) | 70(0.8) | <0.0001 |
| Stroke | 203(2.0) | 46(5.0) | 15(3.8) | 142(1.6) | <0.0001 |
| CHF | 356(3.5) | 124(13.5) | 22(5.5) | 210(2.4) | <0.0001 |
| Cancer | 127(1.2) | 35(3.8) | 8(2.0) | 84(0.9) | <0.0001 |
| CAD | 423(4.1) | 146(15.9) | 29(7.3) | 248(2.8) | <0.0001 |
| Liver disease | 530(5.2) | 124(13.5) | 35(8.8) | 371(4.2) | <0.0001 |
| Inhaler prescription | - | - | - | - | <0.0001 |
| No inhaler | 9885(96.8) | 829(90.4) | 362(91.0) | 8694(97.7) | - |
| LABA + LAMA + ICS | 13(0.1) | 7(0.8) | 3(0.8) | 3(0.03) | - |
| LABA + LAMA | 34(0.3) | 18(2.0) | 8(2.0) | 8(0.09) | - |
| LABA + ICS | 5(0.04) | 1(0.1) | 0(0) | 4(0.04) | - |
| LAMA + ICS | 17(0.2) | 3(0.3) | 1(0.3) | 13(0.2) | - |
| LABA | 167(1.6) | 44(4.8) | 12(3.0) | 111(1.3) | - |
| LAMA | 14(0.1) | 4(0.4) | 6(1.5) | 4(0.04) | - |
| ICS | 81(0.8) | 11(1.2) | 6(1.5) | 64(0.7) | - |
| Previous year admission | - | - | - | - | <0.0001 |
| 0 | 9293(91.0) | 785(85.6) | 350(87.9) | 8158(91.7) | - |
| 1 | 398(3.9) | 43(4.7) | 18(4.5) | 337(3.8) | - |
| 2 | 227(2.2) | 26(2.8) | 9(2.3) | 192(2.2) | - |
| ≥3 | 298(2.9) | 63(6.9) | 21(5.3) | 214(2.4) | - |
| Previous year ED visit | - | - | - | - | <0.0001 |
| 0 | 7867(77.0) | 626(68.3) | 257(64.6) | 6984(78.5) | - |
| 1 | 1493(14.6) | 155(16.9) | 77(19.4) | 1261(14.17) | - |
| 2 | 466(4.6) | 68(7.4) | 30(7.5) | 368(4.1) | - |
| ≥3 | 390(3.8) | 68(7.4) | 34(8.5) | 288(3.2) | - |
| Previous year Pulmonary clinic visit | 270(2.6) | 91(9.9) | 21(5.3) | 158(1.8) | <0.0001 |
| Previous year Prednisone prescription | 456(4.5) | 83(9.1) | 31(7.8) | 342(3.8) | <0.0001 |
| Home oxygen | 21(0.2) | 11(1.2) | 3(0.8) | 7(0.1) | <0.0001 |
| Smokeless cigarette (Vape) | - | - | - | - | <0.0001 |
| Current | 158(1.5) | 25(2.7) | 19 (4.8) | 114(1.3) | - |
| Former | 212(2.1) | 119(13.0) | 22(5.5) | 71(0.8) | - |
| Never | 8702(85.2) | 755(82.3) | 328(82.4) | 7619(85.6) | - |
| Unknown | 1144(11.1) | 18(2.0) | 29(7.3) | 1097(12.3) | - |
-Age calculated on SARS-COV2 testing date.
-BMI obtained within a year before COVID test. Excluded BMI outliers (0.1% extreme). For patients with multiple records, take the last before COVID test. BMI cut-offs reference CDC [50].
-Patients with missing BMI are not included in hypothesis testing and modeling.
-Persons self-identifying as Non-Hispanic ethnicity, where categorized based on race (White, Black, Other). Patients self-identifying as Hispanic ethnicity, were included in the ‘Hispanic’ group, regardless of race.
-Insurance obtained from bill payers within a year before SARS-COV2 testing date.
-Comorbidities collected from encounter diagnosis, problem list and medical history.
-Smoking status obtained from EHR (social history and flowsheet) within a year before SARS-COV2 testing date. For patients with multiple records, we use the last record before SARS-COV2 test.
-Inhaler medication prescription history obtained covers the SARS-COV2 testing date.
-Prednisone prescription obtained within a year of SARS-COV2 testing date.
-Home oxygen identified by ICD-10 diagnosis code J96.11 or Z99.81, and from the EHR flowsheet questionnaire.
-Vaping is self-reported and includes any smokeless tobacco product.
Definition of abbreviations: SARS-COV2= Severe Acute Respiratory Syndrome Coronavirus-2; COVID-19 = Coronavirus Disease 2019; BMI= Body Mass Index; EHR = Electronic Health Record; CAD= Coronary Artery Disease; CHF= Congestive Heart Failure; COPD= Chronic Obstructive Pulmonary Disease; CKD= Chronic kidney disease; ESRD = End Stage Renal Disease; ED = Emergency Department; LABA = Long Acting Beta Agonist; LAMA = Long Acting Muscarinic Agonist; ICS= Inhaled Corticosteroids; SD= Standard Deviation; ICD= International Classification of Diseases; CDC= Centers for Disease Control and Prevention.
3.2. Effect of smoking on hospitalization due to COVID-19
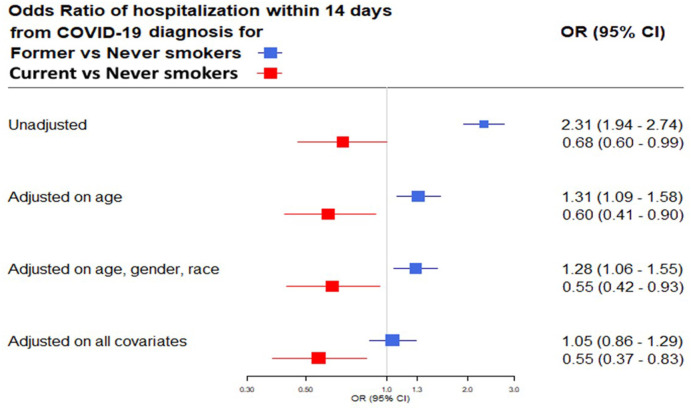
We found 1150 (11.2%) patients who were hospitalized due to COVID-19 within 14 days of a positive test for SARS-COV2. Significantly more former smokers were hospitalized from COVID-19 than current or never smokers (former smokers, 21.2%; current smokers, 7.3%; never smokers, 10.4%; p < 0.0001). (Table 2 ). Univariable analysis showed that former smokers had significantly higher odds of hospitalization from COVID-19 compared to never smokers (Odds Ratio [OR] 2.31; 95% Confidence Interval [CI] 1.94–2.74). In contrast, current smokers were less likely to be hospitalized from COVID-19 than never smokers (OR 0.68; 95% CI 0.60–0.99). When analysis was adjusted for age, race and gender, former smokers' odds of hospitalization due to COVID-19 remained higher than that of never smokers (OR 1.28; 95% CI 1.06–1.55); and current smokers' odds of hospitalization remained lower than that of never smokers. During further analysis (adding BMI, previous inpatient hospitalizations and number of comorbidities), there was a trend towards increase in odds of hospitalization for former smokers compared to never smokers, but the difference did not reach statistical significance (OR 1.05; 95% CI 0.86–1.29). Current smokers’ odds of hospitalization due to COVID-19 remained lower than that of never smokers (OR 0.55; 95% CI 0.37–0.83) (Fig. 2 ).
Table 2.
Hospitalization and mortality within 14 days after a positive test for SARS-COV2 by Smoking Status between March 1, 2020 and January 31, 2021.
| Total | Former Smoker | Current Smoker | Never Smoker | p-value | |
|---|---|---|---|---|---|
| N (%) | 10216(100) | 917(9.0) | 398(3.9) | 8901(87.1) | - |
| 14-day hospitalization | 1150(11.2) | 194(21.2) | 29(7.3) | 927(10.4) | <0.0001 |
| 14-day mortality | 188(1.8) | 47(5.1) | 3(0.8) | 138(1.6) | <0.0001 |
-Smoking status obtained from EHR (social history and flowsheet) within a year before SARS-COV2 testing date. For patients with multiple records, we use the last record before SARS-COV2.
-Former, current and never smokers categories were based on each patient's lifetime count of cigarettes smoked and most recent self-reported smoking status.
-Patients who self-reported as a never smoker or who had smoked less than 100 cigarettes in their lifetime were considered never smokers; patients who smoked more than 100 cigarettes in their lifetime were considered smokers (former or current) [31].
-Former smokers' status was assigned based on the patient's self-report of “former smoker” in the EHR.
Definition of Abbreviations: SARS-COV2= Severe Acute Respiratory Distress Coronavirus 2; EHR = Electronic Health Record.
Fig. 2.
Forrest plot of the multivariable analysis evaluating the effect of smoking status on the risk of 14-day hospitalization from COVID-19 unadjusted and adjusted for age, gender, race, BMI, number of comorbidities and inpatient hospital admission within the previous year. Definition of abbreviations: OR= Odds Ratio; CI: Confidence Interval; BMI: Body Mass Index; COVID-19: Coronavirus Disease 2019.
As sensitivity analysis, we stratified our cohort by age (18–40, 41–64, and 65 years or older) and adjusted for all other variables. Here we found an increasing trend for the association between being a former-smoker and hospitalization from COVID-19, but it did not reach statistical significance. Current smokers were less likely to be hospitalized due to COVID-19 compared to never smokers, but this was significant only in patients younger than 65 years (Table 4 ).
Table 4.
Stratified analysis by age group evaluating the effect of smoking status on risk for 14-Day Hospitalization from COVID-19.
| - | Age 18–40 OR (95% CI) | Age 41–64 OR (95% CI) | Age ≥ 65 OR (95% CI) |
|---|---|---|---|
| N(%) | 4327(42.4) | 4171(40.8) | 1718(16.8) |
| Characteristics | - | - | - |
| Smoking (ref = never smoker) | - | - | - |
| Former smoker | 1.01 (0.41–2.46) | 1.05 (0.77–1.42) | 1.08 (0.81–1.43) |
| Current smoker | 0.51 (0.18–1.44) | 0.50 (0.28–0.89) | 0.50 (0.24–1.03) |
Adjusted for Age, Gender, Race, BMI, Comorbidity Count, and Previous Year Inpatient Hospital Admission.
Definition of Abbreviations: OR= Odds Ratio; CI = confidence Interval; COVID-19 = Coronavirus Disease 2019; BMI= Body Mass Index; Ref = Reference; IP= Inpatient.
3.3. Effect of other independent variables on hospitalization due to COVID-19
Age: The odds of hospitalization from COVID-19 within 14 days following a positive test for SARS-COV2 increased gradually for each age group studied, ranging from 3.23 for those 40–49 years (OR 3.23; 95% CI 2.51–4.14) to 44.82 (OR 44.82; 95% CI 31.05–64.69) for those aged 85 years or more, compared to those aged 40 years or less (Table 3). As a sensitivity analysis, age was considered a continuous variable and results showed that the odds of hospitalization from COVID-19 increased by 6% for every year of age in the population studied after adjusting for other covariates (OR 1.06; 95% CI 1.06–1.07) (Data not shown).
Table 3.
Multivariable logistic regression model on the effect of smoking status and the risk for 14-day hospitalization among SARS-COV2 positive patients.
| Characteristics | OR (95% CI) |
|---|---|
| Smoking (ref = Never smoker) | - |
| Former smoker | 1.05 (0.86–1.28) |
| Current smoker | 0.55 (0.37–0.83) |
| Female Gender (ref = Male) | 0.57 (0.5–0.66) |
| Age (ref = 0–40) | - |
| 40–49 | 3.23 (2.51–4.14) |
| 50–64 | 5.51 (4.43–6.87) |
| 65–74 | 9.23 (7.24–11.76) |
| 75–84 | 18.78 (14.09–25.03) |
| >85 | 44.82 (31.05–64.69) |
| Race (ref = White) | 1.58 (1.37–1.81) |
| Obese, BMI≥ 30 kg/m2 (ref = non-obese) | 1.67 (1.45–1.92) |
| Comorbidity count ≥ 3 (ref= <3) | 1.45 (1.21–1.73) |
| Previous year IP admission(ref = No) | 3.64 (3.03–4.37) |
Adjusted for Age, Gender, Race, BMI, Comorbidity Count, And Previous Year Inpatient Hospital Admission.
Definition of Abbreviations: OR= Odds Ratio; CI = confidence Interval; COVID-19 = Coronavirus Disease 2019; BMI= Body Mass Index; Ref = Reference; IP= Inpatient.
Obesity: Obese (BMI ≥30 kg/m2) patients had 67% greater odds of hospitalization compared to non-obese individuals (OR 1.67; 95% CI 1.45–1.92). For sensitivity, we repeated the analysis with obesity at BMI 30–34 kg/m2 and at BMI ≥35 kg/m2 and compared each to the non-obese population (BMI<30 kg/m2). We found that the odds of hospitalization were similar to those in our previous model. More specifically, obese (BMI ≥35 kg/m2) patients had 82% greater odds of hospitalization compared to non-obese individuals (OR 1.8; 95% CI 1.52–2.14).
Race: Non-white patients had 58% higher odds of hospitalization compared to white patients (OR 1.58; 95% CI 1.37–1.81) (Table 3). For sensitivity, we repeated the analysis adding Hispanic ethnicity (Patients self-identifying as Hispanic were included in the Hispanic group, regardless of race) and separated groups by race based on self-identification as non-Hispanics (White, Black, Other). Hispanic patients had 62% (OR 1.62; 95% CI 1.39–1.9) and Black patients had 46% (OR 1.46; 95% CI 1.46–1.77) higher odds of hospitalization, respectively, compared to white patients (e-Table 2).
Comorbidities and prior inpatient admission: Patients with 3 or more comorbidities were more likely to be hospitalized than patients with fewer than 3 comorbidities (OR 1.45; 95% CI 1.21–1.73). The odds for hospitalization were more than 3 times higher in patients who had an inpatient admission in the prior year compared to those not admitted (OR 3.64; 95% CI 3.03–4.37).
Gender: In contrast to the above risk factors, women had 43% lower odds of admission compared to men (OR 0.57; 95% CI 0.5–0.66).
3.4. Effect of smoking on death after positive test for COVID-19
We found 188 patients (1.8%) who died within 14 days of a positive test for SARS-COV2. A significantly higher percentage of former smokers died within 14 days of positive testing for the virus than current or never smokers (former smokers 5.1%; current smokers 0.8%; never smokers 1.6%; p < 0.0001) (Table 2).
4. Discussion
In our study, patients who were former smokers had a higher percent of hospitalizations and death within 14 days of a positive test for SARS-COV2. After adjustment for other covariates, there was a trend for increase odds of hospitalization in former smokers compared to never smokers, but the trend did not reach statistical significance. Current smokers had a lower percentage of hospitalizations and death than did former or never smokers. They were also less likely than never smokers to be hospitalized due to COVID-19. Our results are in line with the observations of other authors about smoking and severe COVID-19 [14,[32], [33], [34]]. Our pragmatic use of “risk of 14-day hospitalization” as a marker for severe COVID-19 was based on the reported time from the onset of illness to the development of dyspnea and ARDS and the requirement for ICU admission [6].
Our findings of a higher percentage of hospitalizations and death for severe COVID-19 in former smokers compared to never smokers is intriguing. The risk of severe COVID-19 among former smokers is significantly driven by the effect of age and comorbidities. In our cohort, the mean age of former smokers was 10 years older than that of current smokers, and 12 years older than that of never smokers. Also, despite our initial analysis showing increased odds of hospitalization in former smokers compared to never smokers, when it was adjusted for age we found a modest reduction in odds of hospitalization due to COVID-19 in former smokers compared to never smokers. Furthermore, after the analysis was adjusted for comorbidities, the association was no longer significant. Smoking has been causally linked to stroke, coronary artery disease, chronic obstructive pulmonary disease, asthma, interstitial lung disease, diabetes, cancer, impaired immune function, overall diminished health and premature death [21,35,36]. The risks of smoking-related disease result largely from cumulative damage; hence, the consequences of smoking occur disproportionately among the elderly [21,35,37,38]. In our study, we estimated that the odds of hospitalization from COVID-19 increased by 6% for every year of age in the population studied. Older age and more comorbidities as shown in our and others’ analysis [5,[8], [9], [10],12] are significantly associated with severe COVID-19. Both variables may be significant confounders in the retrospective search for an association between smoking and severe COVID-19.
Several mechanisms have been proposed to describe the association between tobacco use and severe COVID-19. Tobacco smoke impairs mucociliary clearance, weakens innate and adaptive immune responses [39] and increases the risk of both viral and bacterial pulmonary infections [21,22,[24], [25], [26], [27], [28], [29], [30],40]. Tobacco use has been shown to increase levels of angiotensin-converting enzyme 2 (ACE2), the unique receptor used by SARS-COV2 to enter host cells [[41], [42], [43]].
Alternatively, a potentially protective role of tobacco use in COVID-19 infection has been theorized. Nicotine, a highly addictive, psychoactive alkaloid contained in tobacco products, has shown immunomodulatory and anti-inflammatory effects [44,45]. Tobacco use has also been linked to increased nitric oxide production in the lungs, resulting in diminished viral replication and impaired viral entry into host cells [46]. Nicotine has also demonstrated a protective effect for ARDS animal models, and is thought to potentially inhibit Interleukin-6, a key player in the cytokine release syndrome induced by SARS-COV2 infection [38]. And while some studies report upregulation of ACE2 receptors for SARS-COV2 cell entry in tobacco users, others challenge this claim and report an interaction between nicotine and the renin-angiotensin system, resulting in reduced ACE2 levels [47]. Moreover, several series throughout the world have reported a low prevalence of current smokers among people that tested positive for SARS-COV2 and/or were hospitalized due to COVID-19 [10,12,[18], [19], [20]].
Our study has several limitations. We relied on patients' self-report of smoking habits and on identification of smokers (current and former) in our EHR; as shown in others’ analysis, smoking history can be accidently altered when providers update the fields for the “number of packs-smoked-per-day" and the “total-years-smoked" [48]. Also, we do not know exactly when patients stopped smoking, and for the definition of former smokers this may be significant. It is well known that a longer time of smoking cessation is associated with more health benefits to patients. For instance, patients who stop smoking before age 40 avoid more than 90% of the excess mortality caused by continuing smoking [49]. Despite these challenges, and to strengthen our analysis, we excluded patients with no complete documentation of smoking history. Finally, our retrospective analysis may only assume an association between smoking status (former and/or current) and risk of severe COVID 19, and not a cause and effect relation. Clinical trials regarding tobacco use among patients infected with COVID-19 are ongoing and will help clarify the effects of smoking in COVID-19. Future research directives should focus on systematic and standardized means of collecting data on the patterns of patient tobacco use in those with COVID-19.
5. Conclusions
In conclusion, the findings of our study showed that significantly more formers smokers were hospitalized and died from COVID-19 than current or never smokers. This effect is mediated through age and comorbidities in former smokers.
Acknowledgments
Daniel Puebla Neira, MD accepts full responsibility for the content of the manuscript including the data and analysis. Dr Kuo reports grants from the Agency of Healthcare Research and Quality (R01HS020642) during the conduct of this study.
The authors acknowledge and greatly appreciate the assistance in the preparation of this manuscript by:
-En Shuo Hsu.
Office of Biostatistics. UTMB
UTMB
-Sarah Toombs Smith, PhD, ELS.
Research Communications Manager and Fellow, Sealy Center on Aging.
Assistant Professor, Internal Medicine-Geriatrics.
Assistant Professor, Graduate School of Biomedical Sciences.
Faculty Associate, Hispanic Center of Excellence.
UTMB
-Tara N. Atkins, MLIS.
Reference Librarian.
Moody Medical Library/Academic Resources.
UTMB
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106414.
Credit author statement
All authors have contributed to the current manuscript in the following manner: Substantial contribution to the conception, design, data acquisition, analysis and interpretation of the manuscript. Drafted and/or revised the manuscript for important intellectual content. Approved the final version to be submitted for publication.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . 2021. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ [Google Scholar]
- 2.Salje H., Tran Kiem C., Lefrancq N., et al. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavezzo E., Franchin E., Ciavarella C., et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verity R., Okell L.C., Dorigatti I., et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . 2020. Ten Clinical Tips on COVID-19 for Healthcare Providers Involved in Patient Care.https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-tips-for-healthcare-providers.html [Google Scholar]
- 7.McElvaney O.J., McEvoy N.L., McElvaney O.F., et al. Characterization of the inflammatory response to severe COVID-19 illness. Am. J. Respir. Crit. Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Q., Meng M., Kumar R., et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J. Med. Virol. 2020;92:1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Y., Ling Y., Bai T., et al. COVID-19 with different severities: a multicenter study of clinical features. Am. J. Respir. Crit. Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend M.J., Kyle T.K., Stanford F.C. Outcomes of COVID-19: disparities in obesity and by ethnicity/race. Int. J. Obes. 2020;44:1807–1809. doi: 10.1038/s41366-020-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershengorn H.B., Patel S., Shukla B., et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021 doi: 10.1513/AnnalsATS.202011-1448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G., Henry B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur. J. Intern. Med. 2020;75:107–108. doi: 10.1016/j.ejim.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung J.M., Yang C.X., Sin D.D. Reply to: “Current smoking is not associated with COVID-19”. Eur. Respir. J. 2020;55:2001340. doi: 10.1183/13993003.01340-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkinson N.S., Rossi N., El-Sayed Moustafa J., et al. Thorax; 2021. Current Smoking and COVID-19 Risk: Results from a Population Symptom App in over 2.4 Million People. [DOI] [PubMed] [Google Scholar]
- 17.Adams S.H., Park M.J., Schaub J.P., Brindis C.D., Irwin C.E., Jr. Medical vulnerability of young adults to severe COVID-19 illness-data from the National Health Interview Survey. J. Adolesc. Health. 2020;67:362–368. doi: 10.1016/j.jadohealth.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J.J., Dong X., Cao Y., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 19.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. J. Am. Med. Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds H.R., Adhikari S., Pulgarin C., et al. Renin–angiotensin–aldosterone system inhibitors and risk of covid-19. N. Engl. J. Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arcavi L., Benowitz N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 22.Nuorti J.P., Butler J.C., Farley M.M., et al. Cigarette smoking and invasive pneumococcal disease, active bacterial core surveillance team. N. Engl. J. Med. 2000;342:681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 23.Almirall J., González C.A., XBalanzó IBolíbar. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest. 1999;116:375–379. doi: 10.1378/chest.116.2.375. [DOI] [PubMed] [Google Scholar]
- 24.Yu G.P., Hsieh C.C., Peng J. Risk factors associated with the prevalence of pulmonary tuberculosis among sanitary workers in Shanghai. Tubercle. 1988;69:105–112. doi: 10.1016/0041-3879(88)90072-4. [DOI] [PubMed] [Google Scholar]
- 25.Levy M.H., Connolly M.A., O'Brien R.J. Cigarette smoking as a risk factor for tuberculosis in young adults: a case-control study. Tuber. Lung Dis. 1996;77:570. doi: 10.1016/s0962-8479(96)90060-x. [DOI] [PubMed] [Google Scholar]
- 26.Gajalakshmi V., Peto R., Kanaka T.S., Jha P. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43000 adult male deaths and 35000 controls. Lancet. 2003;362:507–515. doi: 10.1016/S0140-6736(03)14109-8. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S., Tyrrell D.A., Russell M.A., Jarvis M.J., Smith A.P. Smoking, alcohol consumption, and susceptibility to the common cold. Am. J. Publ. Health. 1993;83:1277–1283. doi: 10.2105/ajph.83.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finklea J.F., Sandifer S.H., Smith D.D. Cigarette smoking and epidemic influenza. Am. J. Epidemiol. 1969;90:390–399. doi: 10.1093/oxfordjournals.aje.a121084. [DOI] [PubMed] [Google Scholar]
- 29.Kark J.D., Lebiush M. Smoking and epidemic influenza-like illness in female military recruits: a brief survey. Am. J. Publ. Health. 1981;71:530–532. doi: 10.2105/ajph.71.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kark J.D., Lebiush M., Rannon L. Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N. Engl. J. Med. 1982;307:1042–1046. doi: 10.1056/NEJM198210213071702. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention Glossary. General concepts. 2017. https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm#:~:text=Current smoker%3A accessed.
- 32.Usman M.S., Siddiqi T.J., Khan M.S., et al. Is there a smoker's paradox in COVID-19? BMJ Evid Based Med. 2020 doi: 10.1136/bmjebm-2020-111492. [DOI] [PubMed] [Google Scholar]
- 33.Miyara M., Tubach F., Pourcher V., Morelot-Panzini C., et al. Qeios; 2020. Low Rate of Daily Active Tobacco Smoking in Patients with Symptomatic COVID-19. [Google Scholar]
- 34.Farsalinos K., Barbouni A., Niaura R. Qeios; 2020. Smoking, Vaping and Hospitalization for COVID-19. [Google Scholar]
- 35.National Center for Chronic Disease Prevention and Health Promotion (US) Centers for Disease Control and Prevention (US); Atlanta (GA): 2014. Office on Smoking and Health, the Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. [PubMed] [Google Scholar]
- 36.Bauer C.M.T., Morissette M.C., Stämpfli M.R. The influence of cigarette smoking on viral infections: translating bench science to impact COPD pathogenesis and acute exacerbations of COPD clinically. Chest. 2013;143:196–206. doi: 10.1378/chest.12-0930. [DOI] [PubMed] [Google Scholar]
- 37.Burns D.M. Cigarette smoking among the elderly: disease consequences and the benefits of cessation. Am. J. Health Promot. 2000;14:357–361. doi: 10.4278/0890-1171-14.6.357. [DOI] [PubMed] [Google Scholar]
- 38.Farsalinos K., Barbouni A., Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med. 2020;15:845–852. doi: 10.1007/s11739-020-02355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu F., Liang C.L., Liu H., et al. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget. 2017;8:268–284. doi: 10.18632/oncotarget.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buskin S.E., Gale J.L., Weiss N.S., Nolan C.M. Tuberculosis risk factors in adults in King County, Washington, 1988 through 1990. Am. J. Publ. Health. 1994;84:1750–1756. doi: 10.2105/ajph.84.11.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polverino F. Cigarette smoking and COVID-19: a complex interaction. Am. J. Respir. Crit. Care Med. 2020;202:471–472. doi: 10.1164/rccm.202005-1646LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai G., Bossé Y., Xiao F., et al. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;201:1557–1559. doi: 10.1164/rccm.202003-0693LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung J.M., Yang C.X., Tam A., et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur. Respir. J. 2020;55:20000688. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mabley J., Gordon S., Pacher P. Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury. Inflammation. 2011;34:231–237. doi: 10.1007/s10753-010-9228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polosa R., Caci G. COVID-19: counter-intuitive data on smoking prevalence and therapeutic implications for nicotine. Intern Emerg Med. 2020;15:853–856. doi: 10.1007/s11739-020-02361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akerström S., Mousavi-Jazi M., Klingström J., et al. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oakes J.M., Fuchs R.M., Gardner J.D., et al. Nicotine and the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R895–R906. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarabichi Y., Kats D.J., Kaelber D.C., et al. The impact of fluctuations in pack-year smoking history in the electronic health record on lung cancer screening practices. Chest. 2018;153:575–578. doi: 10.1016/j.chest.2017.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pirie K., Peto R., Reeves G.K., et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133–141. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention . 2020. Defining Adult Overweight and Obesity.https://www.cdc.gov/obesity/adult/defining.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.