Abstract
Background and aims
Myocardial injury defined by elevation of cardiac troponins (cTn) in the course of coronavirus disease 2019 (COVID-19) pandemic has been reported, though not fully characterized yet. Using the Turkish nationwide centralized COVID-19 database, we sought to determine whether cTn measured within 24 h of admission may help identify 30-day all-cause mortality in hospitalized patients.
Methods
This retrospective cohort study was conducted at all hospitals in Turkey between March 11, 2020, and June 22, 2020. All hospitalized COVID-19 patients (≥18 years) who had cTn measurements within 24 h of admission were included. The primary outcome was 30-day all-cause mortality.
Results
A total of 14,855 COVID-19 patients (median age 49 years and 54% male) from 81 provinces of Turkey were included. Of these, 2020 patients (13.6%) were transferred to intensive care unit, 1165 patients (7.8%) needed mechanical ventilation, and 882 patients (5.9%) died during hospitalization. The prevalence of cTn positivity was 6.9% (n = 1027) in the hospitalized patients. cTn positivity was 5% in those patients alive at 30-day, and 44% in those who died. In multivariable Cox proportional hazard regression model, age, lactate dehydrogenase, and cTn were the strongest predictors of 30-day mortality, irrespective of cTn definition as a continuous, ordinal variable, or dichotomic variables.
Conclusions
A single measurement of cTn at admission in patients with COVID-19 is associated with 30-day all-cause mortality and may have an important prognostic role for optimizing risk stratification.
Keywords: COVID-19, Prognosis, Mortality, Troponin
Graphical abstract
1. Introduction
The novel severe acute respiratory syndrome coronavirus-2 disease (COVID-19) pandemic has brought an enormous burden to current healthcare systems worldwide. The assessment and determination of high-risk patients is the cornerstone of patient management in clinical practice. Therefore, reliable and practical prognostic predictors are needed at the time of admission or during hospitalization for COVID-19 patients [1].
Cardiac injury established by elevated troponin (cTnI or cTnT) levels is common in patients with COVID-19. Troponin positivity at admission or during hospitalization is reported from 10 to 50% of cases depending on variations in definitions, populations studied, timing of sampling and cTn assays [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10]]. Although certain risk factors have been associated with increased morbidity and mortality in patients admitted with COVID-19 [11], the impact of cardiac injury on the outcomes is not well described [12]. Early reports from China suggest that cTn values > 99th percentile of the upper normal limit (ULN) are powerful predictors of poor outcomes and mortality in COVID-19 patients [5,6,13]. However, a recent meta-analysis of studies comparing cTn levels in patients who were critically ill to those who were not demonstrated no significant differences between cTn levels [14]. Finally, the impact of elevated cTn on the outcomes in COVID-19 patients is not well studied and characterized [[15], [16], [17], [18], [19], [20]]. In the current nationwide study, we sought to determine whether cTn at admission may help identify 30-day all-cause mortality in hospitalized patients.
2. Patients and methods
2.1. Study cohort
We conducted an observational retrospective cohort study. All patients who were hospitalized in Turkey with at least one positive reverse transcriptase-polymerase chain reaction (PCR) test for COVID-19 between March 11, 2020 and June 22, 2020 were screened. Those patients in whom cTn was analyzed at admission to the emergency department or the same day of hospitalization were included in the study. We did not include patients with negative PCR results, age <18 years, or who were not hospitalized. The Turkish Ministry of Health approved the study with a waiver of informed consent for retrospective data analysis.
2.2. Outcomes
The primary outcome was 30-day all-cause mortality. We planned a retrospective cohort study design, and followed up patients until death, discharge or censoring on June 22, 2020.
2.3. National data collection
All data were obtained from two different national digital health record systems, including the “Public Health Management System module” (PHMS) to collect COVID-19 specific data (to obtain symptoms, biomarkers, medications, and clinical outcomes during index hospitalization) and the “e-Pulse” system to obtain ICD-10-CM codes in the last two years, as explained in detail in our previous reports [11,21]. On January 22, 2020, the COVID-19 Scientific Advisory Board for the Turkish Ministry of Health was set up, and COVID-19 dedicated PHMS was created as a real-time centralized national health registry system for all healthcare providers for outbreak surveillance during the COVID-19 era [21]. With the case tracking module designed under the PHMS, all COVID-19 possible cases (starting from their detection), people who come from abroad and needed isolation at home, and contacts of the COVID-19 cases are monitored, including their hospitalization process [21]. Since Turkey operates a mandatory universal system called General Healthcare Insurance, all Turkish residents with COVID-19 can receive medical services free of charge by the Social Security Institution, regardless of private or public hospital [21]. All study data were recalled from the above mentioned centralized national database, which was controlled by the Turkish Ministry of Health.
2.4. Troponin measurements
Since cTnI (contemporary or high-sensitivity) have different values for the 99th percentile of the ULN according to different cTn assay manufacturers, we divided the measured cTn value by the ULN of each cTn assay and recorded the results as “x ULN” to standardize the cTn measurements. Then, we categorized these measurements as an ordinal variable as <½ of ULN,> 1/2 of ULN,> 1–2 x ULN,> 2–5 x ULN,> 5–10 x ULN,> 10–50 x ULN and> 50 x ULN. Some troponin assays were reported as “more than … " or “less than … "Measurements reported as “More than …. " were known as > 10,000 x ULN. In our study only 2 patients' value was recorded as “more than … " and we included them in the group> 50 x ULN. The measurements reported as “less than …. " were recorded as 0.001 x ULN arbitrarily. Also, we dichotomized each measured cTn value according to the ULN as “negative or positive”, as commonly used in clinical practice.
2.5. Statistics
Numerical variables were presented as median and interquartile ranges. Categorical data were shown as percentages and n.
2.6. Main candidate predictor and adjustment variables
Candidate predictors (main candidate predictor and adjustment variables) for the primary outcome should be clinically and biologically plausible, and their relationships with all-cause death should be demonstrated in previous studies [[15], [16], [17], [18], [19], [20]]. We considered all candidate predictors that we included in the model under these principles and finally draw a directed acyclic graph to inform regression models (Supplementary Fig. 1). The main candidate predictor, cTn measurements, was included in the model in three ways:
-
i)
we divided the measured cTn value by the ULN of each cTn assay and produced the result as “x ULN” (model-1)
-
ii)
we categorized cTn measurements ordinally as <1/2 x of ULN, >1/2 x of ULN, >1–2 x ULN, >2–5 x ULN, >5–10 x ULN, >10–50 x ULN and >50 x ULN (model-2)
-
iii)
we dichotomized each measured cTn value according to ULN as “negative or positive” (model-3).
Since the cTn measurements calculated as x ULN were significantly right-skewed, they were included in modeling as log (x ULN). Adjustment variables were determined as age, sex, neutrophil-lymphocyte ratio (NLR), D-Dimer, lactate dehydrogenase (LDH), C-reactive protein (CRP), hemoglobin, platelet count, coronary artery disease, heart failure (HF), chronic obstructive pulmonary disease (COPD), cerebrovascular disease, hypertension, diabetes mellitus, and chronic kidney disease (CKD).
2.7. Statistical modeling
Cumulative risk of all-cause death was displayed using corresponding Kaplan-Meier plots. To examine the relationship between all-cause death and cTn measurements, we used the Cox proportional hazard model. Continuous variables including log (x ULN), age, NLR, D-Dimer, LDH, CRP, hemoglobin, platelet count were included in the model as flexible smooth parameters using a restricted cubic spline.
We fitted different models according to the cTn measurement included in the model [cTn xULN were included as a continuous variable with and without spline function in model-1, ordinal variable in model-2, and dichotomic (positive/negative) variable in the model-3]. We also included the interaction term for cTn*age, cTn*HF, and cTn*CKD to test for the statistical significance of effect modification by age, HF, and CKD. We also tested the interaction between number of comorbities and cTn in a separate model. The associations between candidate predictors and all-cause death were quantified by the adjusted hazard ratio with a 95% confidence interval (CI). The hazard ratios (HR) for continuous variables with splines represents an increase from the 25th to the 75th percentile. We retained all candidate predictors in the model and did not remove any of these predictors based on statistical significance. The relative importance of each predictor in the models was estimated with partial X [2] value for each predictor divided by the model's total X [2], which estimated the independent contribution of each predictor to the variance of the outcome. The variables having missing value > 20% were not included in the model (except for D-Dimer and LDH, their missingness were about 40%), while for that < 20%, with the assumption of missing at random, multiple imputations were used either to minimize bias and to avoid exclusions of participants. Multiple imputations were applied for missing values using aregImpute function (rms). Five completed datasets were analyzed, and results were combined by Rubin's rule [22]. For all statistical analyses, we used R-software v. 3.5.1 (R statistical software, Institute for statistics and mathematics, Vienna, Austria).
3. Results
A total of 14,855 COVID-19 patients who had cTn measurement (all measurements were cTnI and 89.7% were high-sensitivity cTn) within 24 h of admission were included in the study [median age 49 years (36–62) and 54% male] between March 11 and June 22, 2020. Baseline clinical characteristics are summarized in Table 1 and Supplementary Table 1. Of these patients, 2020 patients (13.6%), were transferred to ICU, 1165 patients (7.8%) needed mechanical ventilation (MV), and 882 patients (5.9%) died during hospitalization.
Table 1.
Baseline characteristics of study participants according to troponin status.
| Variables | Overall n = 14,855 | cTn-negative |
cTn-positive |
p value |
|---|---|---|---|---|
| n = 13,828 | n = 1027 | |||
| Age, years | 49(36–62) | 48 (35–60) | 71 (61–80) | <0.001 |
| NLR | 2.33 (1.46–3.86) | 2.26 (1.44–3.69) | 4.03 (2.23–7.40) | <0.001 |
| D-Dimer,μg/ml | 0.36 (0.20–0.71) | 0.35 (0.19–0.65) | 0.93 (0.41–2.07) | <0.001 |
| LDH, U/L | 227(184–303) | 223 (183–294) | 331(242–480) | <0.001 |
| CRP, mg/dl | 5.40 (1.10–23.3) | 4.70 (1.00–19.9) | 41.3 (9.53–126) | <0.001 |
| Hemoglobin, mg/dl | 13.6 (12.3–14.8) | 13.7 (12.4–14.9) | 12.2 (10.7–13.6) | <0.001 |
| Platelet counts, x109/L | 208(169–256) | 209(170–256) | 197 (150–248) | <0.001 |
| Sex, male n (%) | 8272 (54.0) | 7705 (53.9) | 567 (54.8) | 0.598 |
| Dyspnea, n(%) | 5817 (38.0) | 5476 (38.3) | 341 (33.0) | 0.001 |
| Fever, n(%) | 5968 (39.0) | 5628 (39.4) | 340 (32.9) | <0.001 |
| Healthcare worker, n(%) | 793 (5.2) | 777 (5.4) | 16 (1.5) | <0.001 |
| Pregnancy, n(%) | 73 (0.5) | 72 (0.5) | 1 (0.1) | 0.109 |
| Valvular heart disease, n(%) | 175 (1.1) | 142 (1.0) | 33 (3.2) | <0.001 |
| Cardiac arrhythmias, n(%) | 1011 (6.6) | 796 (5.6) | 215 (20.8) | <0.001 |
| CAD, n(%) | 2341 (15.3) | 1864 (13.0) | 477 (46.1) | <0.001 |
| PAD, n(%) | 585 (3.8) | 474 (3.3) | 111 (10.7) | <0.001 |
| CTD, n(%) | 441 (2.9) | 395 (2.8) | 46 (4.4) | 0.002 |
| Malignancy, n(%) | 473 (3.1) | 389 (2.7) | 84 (8.1) | <0.001 |
| Lymphoma, n(%) | 51 (0.3) | 42 (0.3) | 9 (0.9) | 0.006 |
| Heart failure, n(%) | 776 (5.1) | 528 (3.7) | 248 (24.0) | <0.001 |
| Pneumonia on CT, n(%) | 10250 (66.9) | 9356 (65.5) | 894 (86.5) | <0.001 |
| COPD, n(%) | 3306 (21.6) | 2893 (20.3) | 413 (39.9) | <0.001 |
| DM, n(%) | 3056 (19.9) | 2671 (18.7) | 385 (37.2) | <0.001 |
| Cerebrovascular disease, n(%) | 976 (6.4) | 764 (5.3) | 212 (20.5) | <0.001 |
| Hypertension, n(%) | 5561 (36.3) | 4768 (33.4) | 793 (76.7) | <0.001 |
| Chronic liver disease, n(%) | 385 (2.6) | 349 (2.5) | 36 (3.5) | 0.071 |
| CKD, n(%) | 494 (3.2) | 304 (2.1) | 190 (18.4) | <0.001 |
| In-hospital treatments, n(%) | ||||
| Favipiravir | 3898 (25.4) | 3296 (23.1) | 602 (58.2) | <0.001 |
| HCQ | 13345 (87.1) | 12417 (86.9) | 928 (89.7) | 0.010 |
| High dose C-vitamin | 2374 (15.5) | 2044 (14.3) | 330 (31.9) | <0.001 |
| Oseltamivir | 7890 (51.5) | 7267 (50.9) | 623 (60.3) | <0.001 |
| Azithromycin | 9652 (63.0) | 8885 (62.2) | 767 (74.2) | <0.001 |
| Lopinavir/Ritonavir | 464 (3.0) | 366 (2.6) | 98 (9.5) | <0.001 |
| cTn, xULN | 0.08 (0.00–0.28) | – | – | – |
| cTn, positive, n(%) | 1027(6.9%) | – | – | – |
NLR, neutrophil-lymphocyte ratio; CRP, C-reactive protein; LDH, lactate dehydrogenase; CAD, coronary artery disease; PAD, peripheral artery disease; CTD, collagen tissue disorders; CT, computed tomography; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; CKD, chronic kidney disease; HCQ, hydroxy-chloroquine; cTn, cardiac troponin.
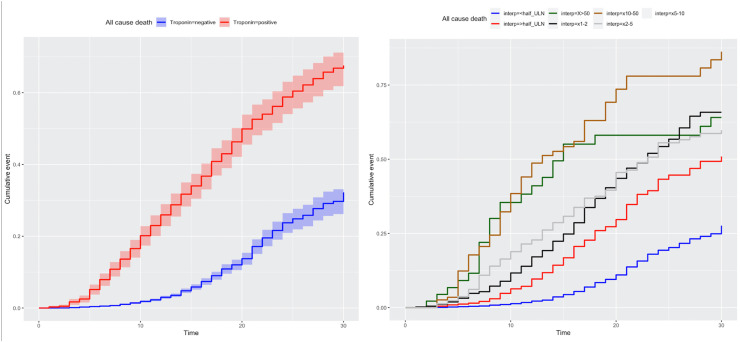
The distribution of cTn x ULN is showed in Supplementary Fig. 2. The median cTn x ULN was 0.08 (0.0001–0.28), cTn positivity rate was 6.9% (n = 1027) in the hospitalized population, 25.7% (n = 519) in those transferred to ICU, and 31.5% (n = 367) in those who needed MV. The frequency of 30-day death, transfer to ICU and need of MV is shown in Supplementary Fig. 3. There was a significant positive correlation between cTn x ULN and age regardless of sex (Supplementary Fig. 4). The median cTn x ULN was 0.071 (0.0001–0.23) and the frequency of cTn positivity (>xULN) was 5% in patients alive at 30-day, whereas the median cTn x ULN was 0.78 (0.26–2.68) and the frequency of cTn positivity (>xULN) was 44% in patients who died. 30-day cumulative death probabilities for cTn positive vs negative and ordinal cTn measurements are shown in Fig. 1.
Fig. 1.
Kaplan-Meier survival curves for troponin positivity (left panel) and ordinal xULN troponin measurements (right panel).
Patients with comorbidities (defined here as CAD, PAD, CVD, COPD, CKD, DM, HTN, malignancy, chronic liver disease, cardiac arrhytmias, valvular heart disease) had increased higher troponin xULN values than those without (Supplementary Table 2). Specifically, in those patients with HF, CAD, PAD, CKD, CVD, malignancy, valvular heart disease and cardiac arrhytmias, significant myocardial injury (troponin xULN >1) was more prevalent (the prevalence >15%) in comparison to those without. In addition to individual co-morbid condition, there was a positive corelation between cTn xULN and number of co-morbidities (Supplementary Fig. 5).
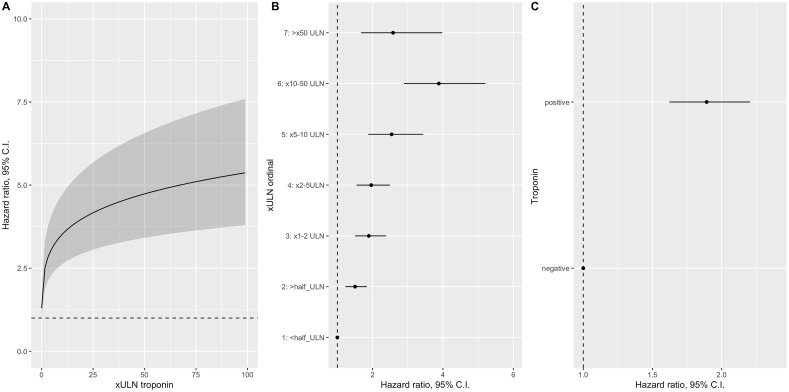
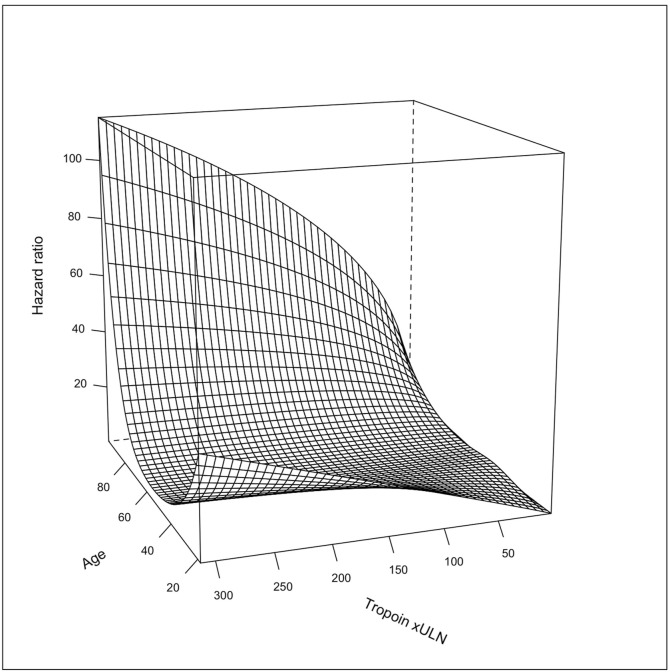
In all multivariable Cox proportional hazard models, the cTn measurements were the significantly associated with increased risk of death, after adjusting for age, sex, NLR, D-dimer, LDH, CRP, hemoglobin, platelet count, coronary artery disease, HF, COPD, cerebrovascular disease, hypertension, and diabetes mellitus (Table 2 , Supplementary Table 3 and Fig. 2 ). In the model, age, LDH, and cTn were the strongest predictors of death and explained 71% of the variability in the risk of death (Supplementary Fig. 6). There were also statistically significant interactions between cTn and age, cTn and HF, and cTn and CKD. The prognostic effect of cTn x ULN was more pronounced in advanced age as demonstrated in the three-dimensional plot (Fig. 3 ). Similarly, elevated cTn x ULN seemed to be more prognostic in patients with HF than without (Supplementary Fig. 7). Conversely, cTn x ULN seemed to have more prognostic value in those patients without CKD than those with CKD (Supplementary Fig. 8). There was also a significant interaction between cTnxULN and number of comorbidities. The effect of higher cTnxULN on the outcome was more pronounced in patients with lower number of comorbidities (Supplementary Fig. 9). We also presented DAG-informed model using adjustment variables without inflammation/thrombosis/coagulopathy variables (D-dimer, Hgb, platelet count, NLR, CRP, LDH) and multivariable cox proportional hazard model results in patients in the high sensitivity troponin subgroup. The results were similar to the main model (Supplementary Table 3 ).
Table 2.
Adjusted hazard ratios (95% CI) of various troponin xULN transformations for 30-day mortality.
| Troponin measurements | Hazard ratio, and 95% CI | Model AUC | Model R2 |
|---|---|---|---|
| Log (X ULN) – continuous | 2.00 (1.66–2.42) | 0.870 | 0.133 |
| Log (X ULN) – spline transformation | 0.876 | 0.138 | |
| 0.5 XULN | Ref | ||
| 1 XULN | 1.17 (1.13–1.21) | ||
| 2 XULN | 1.34 (1.26–1.43) | ||
| 5 XULN | 1.59 (1.42–1.78) | ||
| 50 XULN | 2.42 (1.90–3.09) | ||
| Ordinal X ULN | 0.876 | 0.137 | |
| <1/2x of ULN | Ref. | ||
| >1/2x of x ULN | 1.50 (1.23–1.84) | ||
| >1–2x ULN | 1.8 9(1.50–2.38) | ||
| >2–5x ULN | 1.96(1.54–2.49) | ||
| >5–10x ULN | 2.54 (1.87–3.44) | ||
| >10–50x ULN | 3.88 (2.89–5.21) | ||
| >50x ULN | 2.58 (1.68–3.98) | ||
| Positive (vs negative) | 1.89 (1.62–2.21) | 0.874 | 0.134 |
ULN, upper limit of normal; AUC, area under the curve.
Fig. 2.
Partial effect plot for various troponin (xULN) measurements.
(A) Troponin (xULN) was modeled as restricted cubic spline transformation. (B) Troponin (xULN) was modeled as ordinal categories. (C) Troponin (xULN) was modeled as dichotomic (positive vs negative) variable. Y axis indicate hazard ratio and 95% CI.
Fig. 3.
Three-dimensional plot showed effect of age and cardiac troponin (xULN) interaction on 30-day mortality risk.
4. Discussion
We present the largest sample size study examining the association between cTn levels at hospital admission and 30-day mortality in patients with COVID-19. This finding is concordant with the report of Shi et al. [6] including 416 patients from Wuhan, China which demonstrated a hazard ratio of 3.41 [95% CI, 1.62–7.16] for death in patients with myocardial injury as compared to patients without. Measurement of cTn levels at the time of hospital admission for COVID-19 seems to be useful to identify patients at increased risk of worse outcome and those who may require more intensive treatment[2,3].
The prevalence of myocardial injury in our study (%6.8) was lower than reported in previous studies [1,[4], [5], [6], [7], [8], [9], [10]]. Our patient cohort had a younger age (median age: 49), and this likely explains the lower prevalence of cardiac injury in our cohort. The older COVID-19 patients were more prone to have elevated cTnI levels [1,[4], [5], [6],11]. In accordance with the previous studies [7], it was also more apparent in patients without CKD compared to those with CKD.
Considering the established risk of acute myocardial injury in acute infection settings regarding aggravated inflammatory and prothrombotic circumstances, the strong prognostic value of cTn in COVID-19 infection is not surprising. However, this issue with its underlying pathophysiology is not fully clarified[15]. Traditionally, in a reflective maneuver, elevated cTn concentrations have been considered equivalent to myocardial infarction. However, the noncardiac situations mainly caused by inflammatory storms such as critical illness, sepsis, or thromboembolic events seem to take more place in pathophysiology underlying elevated cTn levels in COVID-19 patients. Many critically ill COVID-19 patients demonstrate concomitant elevations in acute phase reactants such as procalcitonin (PCT), serum ferritin, erythrocyte sedimentation rate (ESR), CRP, interleukin (IL)-6 and the natriuretic peptides [23], supporting the main reason of a cardio-inflammatory response. It has been reported that elevated cTn served as a marker of risk of future adverse events based on the current severity of the infectious disease and inflammatory process, but not necessarily as a marker of hard cardiovascular outcomes [24]. In clinical practice, however, it is challenging to determine whether elevated cTnI levels are caused by direct COVID-19 infection or by the progress of these preexisting medical conditions [25].
The pattern of cTn release in the context of a clinical presentation of type 1 or 2 MI, myocarditis, or cytokine/stress-related cardiomyopathy is also not well defined in COVID-19 patients. In accordance with Zhou et al. cTnI did not increase at the beginning of the infection rather its rise throughout the illness was noted in patients with an increase in severity of COVID-19 [5,16]. While the Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment recommends evaluating myocardial enzymes in patients admitted with COVID-19 infection [26], the American College of Cardiology, based on the frequency and non-specific nature of abnormal cTn results in COVID-19 patients, advised clinicians to measure cTn only if the diagnosis of acute myocardial infarction is being considered on clinical grounds [27]. Similarly, the World Health Organization recommends laboratory testing for acute myocardial injury at admission and only when clinically indicated [28].
There are multiple strengths and limitations to our study. We used nationwide data and had a large sample size, which emphasizes the prevalence and prognostic role of cTn in COVID-19. We used the only cTn values at admission; however, the serial measurements of cTn during hospitalization might also be an important marker of severity and prognosis despite the normal initial level at admission [5,12,15,16,24,29]. The other limitation of our study was the analytic accuracy of the prognosticative value of cTn, given the different assays used at each hospital. Using standard reference laboratory cutoffs might underestimate the extent of cardiac injury [13]. Therefore, we opted to use the measured troponin value by the ULN of each cTn assay and recorded the results as “x ULN” to standardize the troponin measurements. We did not have available data regarding electrocardiograms and echocardiograms in all patients at the time of cTn measurement to provide a more comprehensive evaluation of myocardial injury. Finally, there might be a selection bias of the study subjects because myocardial markers might have been examined more frequently in patients with suspected myocardial injury or with signs of cardiovascular diseases than in the general patient population. Finally, the results do not represent the epidemiological data of COVID-19 and generalize our findings to all COVID-19 patients. Ongoing registries to collect cardiovascular data in COVID-19 patients, such as CAPACITY-COVID, will help improve our understanding of the disease's implications.
4.1. Conclusion
Cardiac troponin measurements were significantly associated with 30-day mortality and might have a prognostic role for optimizing risk stratification.
Financial support
This study is supported by the Republic of Turkey Ministry of Health. The supporter had no role in design, analysis, and interpretation. The authors received no financial support for the research, authorship, and/or publication of this article.
Author contributions
Ibrahim Halil Tanboğa: conceived the study, cleaned data, did statistical analyses, drafted the manuscript, approved the final draft, Uğur Canpolat: collected the data, cleaned data, Data are verified, approved the final draft, Elif Hande Özcan Çetin: collected the data, cleaned data, drafted the manuscript, Sema Turan: collected the data, approved the final draft, Harun Kundi: collected the data, cleaned data, approved the final draft, Osman Çelik: collected the data, approved the final draft, Naim Ata: collected the data, approved the final draft, Serkan Çay: drafted the manuscript, conceived the study, collected the data, Özcan Özeke: conceived the study, collected the data, cleaned data, drafted the manuscript, Serkan Topaloğlu: drafted the manuscript, conceived the study, Cihangir Kaymaz: drafted the manuscript, approved the final draft, conceived the study, Murat Çağlayan: collected the data, approved the final draft,
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2021.04.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nie S.F., Yu M., Xie T., Yang F., Wang H.B., Wang Z.H., Li M., Gao X.L., Lv B.J., Wang S.J., Zhang X.B., He S.L., Qiu Z.H., Liao Y.H., Zhou Z.H., Cheng X. Cardiac troponin I is an independent predictor for mortality in hospitalized patients with COVID-19. Circulation. 2020;142:608–610. doi: 10.1161/CIRCULATIONAHA.120.048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lombardi C.M., Carubelli V., Iorio A., Inciardi R.M., Bellasi A., Canale C., Camporotondo R., Catagnano F., Dalla Vecchia L.A., Giovinazzo S., Maccagni G., Mapelli M., Margonato D., Monzo L., Nuzzi V., Oriecuia C., Peveri G., Pozzi A., Provenzale G., Sarullo F., Tomasoni D., Ameri P., Gnecchi M., Leonardi S., Merlo M., Agostoni P., Carugo S., Danzi G.B., Guazzi M., La Rovere M.T., Mortara A., Piepoli M., Porto I., Sinagra G., Volterrani M., Specchia C., Metra M., Senni M. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol. 2020;5(11):1274–1280. doi: 10.1001/jamacardio.2020.3538. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefanini G.G., Chiarito M., Ferrante G., Cannata F., Azzolini E., Viggiani G., De Marco A., Briani M., Bocciolone M., Bragato R., Corrada E., Gasparini G.L., Marconi M., Monti L., Pagnotta P.A., Panico C., Pini D., Regazzoli D., My I., Kallikourdis M., Ciccarelli M., Badalamenti S., Aghemo A., Reimers B., Condorelli G., Humanitas C.-T.F. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart. 2020;106:1512–1518. doi: 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- 4.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., Zhao S., Somani S., Van Vleck T., Vaid A., Chaudhry F., De Freitas J.K., Fayad Z.A., Pinney S.P., Levin M., Charney A., Bagiella E., Narula J., Glicksberg B.S., Nadkarni G., Mancini D.M., Fuster V., Mount Sinai C.I.C. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J. Am. Coll. Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao J., Zheng Y., Luo Z., Mei Z., Yao Y., Liu Z., Liang C., Yang H., Song Y., Yu K., Gao Y., Zhu C., Huang Z., Qian J., Ge J. Myocardial injury and COVID-19: serum hs-cTnI level in risk stratification and the prediction of 30-day fatality in COVID-19 patients with no prior cardiovascular disease. Theranostics. 2020;10:9663–9673. doi: 10.7150/thno.47980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bavishi C., Bonow R.O., Trivedi V., Abbott J.D., Messerli F.H., Bhatt D.L. Acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog. Cardiovasc. Dis. 2020;63(5):682–689. doi: 10.1016/j.pcad.2020.05.013. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonow R.O., Fonarow G.C., O'Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5:751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 10.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kundi H., Cetin E.H.O., Canpolat U., Aras S., Celik O., Ata N., Birinci S., Cay S., Ozeke O., Tanboga I.H., Topaloglu S. The role of frailty on adverse outcomes among older patients with COVID-19. J. Infect. 2020;81(6):944–951. doi: 10.1016/j.jinf.2020.09.029. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raad M., Dabbagh M., Gorgis S., Yan J., Chehab O., Dagher C., Jamoor K., Hussein I.H., Cook B., Van Harn M., Singh G., McCord J., Parikh S. Cardiac injury patterns and inpatient outcomes among patients admitted with COVID-19. Am. J. Cardiol. 2020;133:154–161. doi: 10.1016/j.amjcard.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin J.J., Cheng X., Zhou F., Lei F., Akolkar G., Cai J., Zhang X.J., Blet A., Xie J., Zhang P., Liu Y.M., Huang Z., Zhao L.P., Lin L., Xia M., Chen M.M., Song X., Bai L., Chen Z., Zhang X., Xiang D., Chen J., Xu Q., Ma X., Touyz R.M., Gao C., Wang H., Liu L., Mao W., Luo P., Yan Y., Ye P., Chen M., Chen G., Zhu L., She Z.G., Huang X., Yuan Y., Zhang B.H., Wang Y., Liu P.P., Li H. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID-19. Hypertension. 2020;76:1104–1112. doi: 10.1161/HYPERTENSIONAHA.120.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson D., Dominic P., Sheth A., Modi M. Prognostic value of cardiac biomarkers in COVID-19 infection: a meta-analysis. Res Sq. 2020 doi: 10.21203/rs.3.rs-34729/v1. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A.K., Jneid H., Addison D., Ardehali H., Boehme A.K., Borgaonkar S., Boulestreau R., Clerkin K., Delarche N., DeVon H.A., Grumbach I.M., Gutierrez J., Jones D.A., Kapil V., Maniero C., Mentias A., Miller P.S., Ng S.M., Parekh J.D., Sanchez R.H., Sawicki K.T., Te Riele A., Remme C.A., London B. Current perspectives on coronavirus disease 2019 and cardiovascular disease: a white paper by the JAHA editors. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aikawa T., Takagi H., Ishikawa K., Kuno T. Myocardial injury characterized by elevated cardiac troponin and in-hospital mortality of COVID-19: an insight from a meta-analysis. J. Med. Virol. 2020;93(1):51–55. doi: 10.1002/jmv.26108. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X.W., Lai J.S., Cheng J., Wang M.W., Liu Y.J., Xiao Z.C., Xu C., Li S.S., Zeng H.S. [Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients] Zhonghua Xinxueguanbing Zazhi. 2020;48:456–460. doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 19.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.04.052. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.B Ka, Chaudhuri D. A review of acute myocardial injury in coronavirus disease 2019. Cureus. 2020;12 doi: 10.7759/cureus.8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanboğa I.H., Canpolat U., Çetin E.H.Ö., Kundi H., Çelik O., Çağlayan M., Ata N., Özeke Ö., Çay S., Kaymaz C., Topaloğlu S. Development and validation of clinical prediction model to estimate the probability of death in hospitalized patients with COVID-19: insights from a nationwide database. J. Med. Virol. 2021;93(5):3015–3022. doi: 10.1002/jmv.26844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin D.B. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 23.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh N., Anchan R.K., Besser S.A., Belkin M.N., Dela Cruz M., Lee L., Yu D., Mehta N., Nguyen A.B., Alenghat F.J. High sensitivity troponin-T for prediction of adverse events in patients with COVID-19. Biomarkers. 2020:1–26. doi: 10.1080/1354750X.2020.1829056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franks C.E., Scott M.G., Farnsworth C.W. Elevated cardiac troponin I is associated with poor outcomes in COVID-19 patients at an academic medical center in midwestern USA. J Appl Lab Med. 2020;5:1137–1139. doi: 10.1093/jalm/jfaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition). http://kjfymeetingchinaorg/msite/news/show/cn/3337html 2020. Accessed November 2020.
- 27.American College of Cardiology. https://wwwaccorg/latest-in-cardiology/articles/2020/03/18/15/25/troponin-and-bnp-use-in-covid19 2020 Available at: Accessed November 2020.
- 28.Clinical management of COVID-19. https://wwwwhoint/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected 2020 Accessed November 2020.
- 29.Li C., Jiang J., Wang F., Zhou N., Veronese G., Moslehi J.J., Ammirati E., Wang D.W. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J. Mol. Cell. Cardiol. 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.