Abstract
The emerging SARS-CoV-2 infection is the cause of the global COVID-19 pandemic. To date, there are limited therapeutic options available to fight this disease. Here we examined the inhibitory abilities of two broad-spectrum antiviral natural products chebulagic acid (CHLA) and punicalagin (PUG) against SARS-CoV-2 viral replication. Both CHLA and PUG reduced virus-induced plaque formation in Vero-E6 monolayer at noncytotoxic concentrations, by targeting the enzymatic activity of viral 3-chymotrypsin-like cysteine protease (3CLpro) as allosteric regulators. Our study demonstrates the potential use of CHLA and PUG as novel COVID-19 therapies.
Keywords: SARS-CoV-2, Chebulagic acid, Punicalagin, 3CLpro, Allosteric inhibitor
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is the virus that causes a pneumonia like illness pandemic called coronavirus disease 2019 (COVID-19). The disease has reached to almost every country in the world, posing a serious threat to global public health and the economy (Hamid et al., 2020). As of March 4, 2021, more than 114.43 million cases have been confirmed with 2.54 million deaths globally (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). Encouragingly, 12 COVID-19 vaccines have been approved and globally available, and 22 vaccine candidates are in phase III clinical trials (https://covid19.trackvaccines.org/vaccines/). However, evidence is increasing that SARS-CoV-2 variants could evade immune responses triggered by either vaccines or previous infections, which may compromise the vaccine effectiveness (Callaway, 2021; Tegally et al., 2020). On the other hand, there is no specific antiviral therapy available for clinical usage, except remdesivir, of which the therapeutic effect is still under debate (Beigel et al., 2020). There is a pressing need to discover additional effective antivirals for the treatment of SARS-CoV-2 infection for use alone or in combination with already approved antiviral therapies.
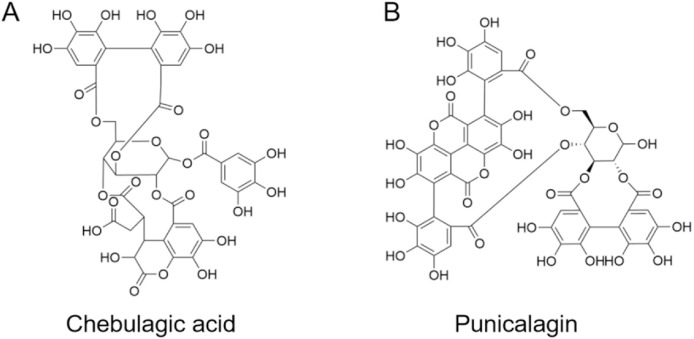
Broad spectrum antiviral agents are valuable candidates of inhibitors against emerging viruses (Zhang et al., 2020b). To our knowledge, two hydrolyzable polyphenolics, chebulagic acid (CHLA, Fig. 1 A) and punicalagin (PUG, Fig. 1B), have been well acknowledged as cost-effective and broad-spectrum antivirals. Previous studies have demonstrated CHLA and PUG can block interactions between cell surface glycosaminoglycan (GAG) and viral glycoproteins. Through this mechanism, they inhibit infections by diverse viruses that employ GAGs for host cell entry, such as herpes simplex virus type 1, human cytomegalovirus, and hepatitis C virus (Lin et al., 2011, 2013). Moreover, we recently identified that CHLA and PUG possess inhibitory effects against influenza viruses by targeting neuraminidase mediated virus release (Li et al., 2020b, 2020c).
Fig. 1.
The chemical structures of chebulagic acid (CHLA) and punicalagin (PUG).
In this study, we investigated the potent antiviral activity of CHLA and PUG against SARS-CoV-2.
2. Materials and methods
2.1. Virus and reagents
The clinical isolate of SARS-CoV-2 (SARS-CoV-2, Isolate USA-WA1/2020) was obtained from BEI Resources and manipulated in BSL3 containment at University of Illinois at Chicago (Chicago, IL). Compounds CHLA and PUG were purchased from MedChemExpress (MCE; Monmouth Junction, NJ, USA). The fluorescence resonance energy transfer (FRET)-based peptidic substrate (Dabcyl-KTSAVLQ/SGFRKME-Edans) was purchased from NJpeptide (Nanjing, China).
2.2. Antiviral assay and cytotoxicity assay
To examine the anti-SARS-CoV-2 activity of CHLA or PUG, plaque reduction assay was conducted. Briefly, Vero-E6 monolayers grown in 12 well plates were pre-treated with increasing concentrations of test compound for 1 h, followed by infection with SARS-CoV-2 (MOI of 0.0001) in the presence of test compounds. DMSO and remdesivir (3 μM) were used as negative and positive controls respectively. After 1-h incubation, the medium was replaced with fresh MEM containing 1.25% Avicel and test compound, and the plates were incubated for another 48 h at 37 °C and 5% CO2. Then cells were fixed with 10% formalin and stained with 1% crystal violet to visualize plaques. The concentration required for the tested compound to reduce the plaque formation of the virus by 50% (the 50% effective concentration [EC50]) was determined. To estimate the cytotoxicity of CHLA or PUG on VERO-E6 cells, Cell-Titer Glo® luminescent cell viability assay (Promega) was performed according to the manufacturer's instruction. The half of the cytotoxic concentration (CC50) values were calculated from the percentages of cells whose viability was inhibited by CHLA or PUG at various concentrations.
2.3. Pseudotyped SARS-CoV-2 based entry inhibition assay
A lentiviral-based pseudovirus carrying the SARS-CoV-2 S protein (SARS-CoV-2pp) was prepared as previously described (Xia et al., 2020), while 293T cells transiently expressing human ACE2 and TMPRSS2 were adopted as target cells (Fig. S1A). The pseudovirus entry assay was conducted by inoculation of SARS-CoV-2pp to target cells in presence of increasing concentrations of test compound, with final concentrations ranged from 100 μM to 1.56 μM. After incubation for 48 h, luciferase activity was analyzed to monitor viral entry efficacy.
2.4. Enzymatic inhibition assay of 3-chymotrypsin-like cysteine protease (3CLpro)
The SARS-CoV-2 3CLpro was prokaryotic expressed and purified as previously described with slight modification (Ma et al., 2020b). For enzymatic inhibition assay, the recombinant 3CLpro (250 nM at a final concentration) was incubated with increasing concentrations of each compound in 90 μL reaction buffer (50 mM Tris–HCl, pH 7.3, 1 mM EDTA) and incubated for 30 min ( Dai et al., 2020 ). The reaction was initiated by adding 10 μL FRET-based peptidic substrate (Dabcyl-KTSAVLQ/SGFRKME-Edans) with a final concentration of 50 μM. The fluorescence signal was immediately measured every 20 s for 30 min with a Bio-Tek Synergy4 plate reader with filters for excitation at 336/20 nm and emission at 490/20 nm. The initial reaction velocities (V0) of reactions were calculated to indicate the enzymatic activities. Three independent experiments were performed and IC50 curves were analyzed using GraphPad Prism software.
2.5. In silico docking
For the molecular docking simulations, the 3CLpro structure from SARS-CoV-2 (PDB code: 6m2n) was used. The three-dimensional (3D) structure of CHLA and PUG was obtained from PubChem Compound database (NCBI). Before docking, polar hydrogen atoms were added to the 3CLpro protein using autodock tools. Docking was performed using a grid box covering the entire structure by AutoDock Vina software (Trott and Olson, 2010). After docking, the conformation of the compound was analyzed, and the 3D models were viewed with PyMOL.
3. Results
3.1. CHLA and PUG exhibit antiviral activity in vitro
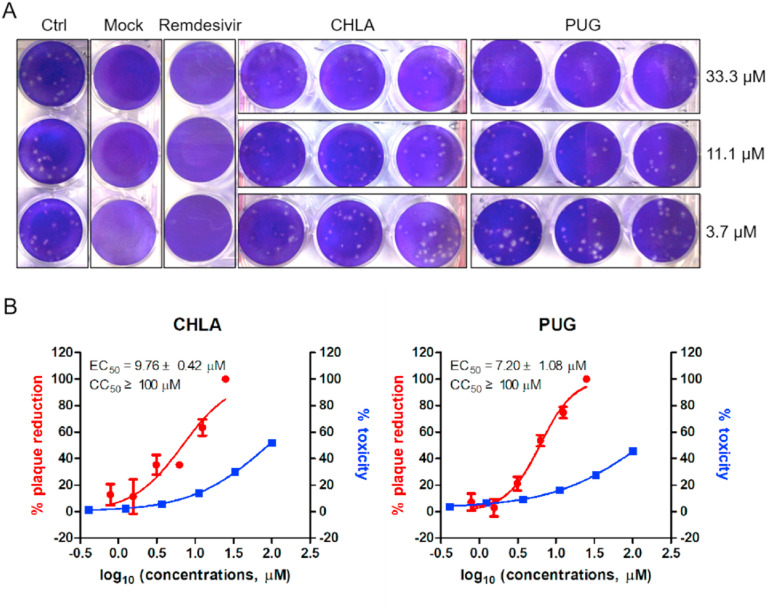
In order to determine whether CHLA and PUG can inhibit replication of SARS-CoV-2, a plaque reduction assay was performed with authentic SARS-CoV-2 using remdesivir as positive control. As a result, both CHLA and PUG inhibited the plaque formation of SARS-CoV-2 in a dose-dependent manner (Fig. 2 A), with EC50 values of 9.76 ± 0.42 μM and 7.20 ± 1.08 μM, respectively (Fig. 2B). To exclude the possibility that the inhibition of viral replication was due to compound-mediated cytotoxicity, a cell proliferation-based cytotoxicity assay was performed. As shown in Fig. 1B, the CC50 values of CHLA and PUG were both around 100 μM, conferring selectivity index (SI[CC50/EC50]) of above 10 and 13, respectively. These results suggest CHLA and PUG exhibit in-vitro anti-SARS-CoV-2 activity.
Fig. 2.
CHLA and PUG inhibit SARS-CoV-2 replication. A. Plaque reduction assay of CHLA and PUG as well as remdesivir (3 μM) against authentic SARS-CoV-2 at indicated concentrations. B. Dose-dependent inhibition of CHLA and PUG on SARS-CoV-2 replication. Antiviral activity and cytotoxicity are shown in red and blue, respectively. The EC50 and CC50 are displayed in the upper left corner for each compound. The data represent mean ± standard deviation (SD) of the triplicate measurements. IC50 values were determined by fitting the dose-response curves with four-parameter logistic regression in Prism GraphPad (version 8.1.2). All data was normalized to virus alone.
3.2. CHLA and PUG do not block SARS-CoV-2 entry
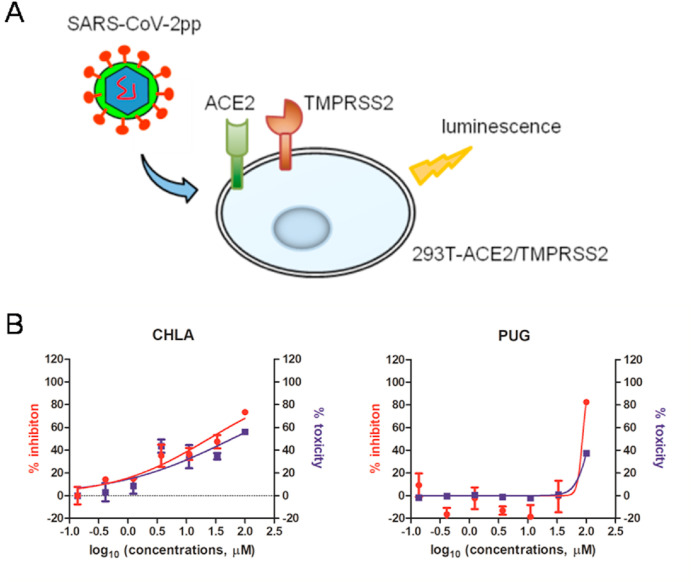
Recently, two independent studies have demonstrated that cell surface GAGs such as heparan sulfate are involved in SARS-CoV-2 entry, by interacting with motifs within viral spike (S) glycoprotein (Clausen et al., 2020; Kim et al., 2020). Considering the aforementioned fact that CHLA and PUG can prevent interactions between GAGs and viral glycoproteins, we therefore first tested whether CHLA and PUG act by blocking SARS-CoV-2 entry using a pseudotyped SARS-CoV-2 (SARS-CoV-2pp). However, upon adding CHLA or PUG during infection of SARS-CoV-2pp into target 293T cells transiently expressing ACE2 and TMPRSS2 (Fig. 3 A), neither CHLA nor PUG inhibited pseudovirus entry without interfering with target cell viability, indicating that CHLA and PUG inhibit SARS-CoV-2 replication by a mechanism other than preventing S-mediated viral entry (Fig. 3B).
Fig. 3.
Pseudoviral infection assay. A. representative diagram of pseudo-SARS-CoV-2 assay, where 293T cells transiently expressing ACE2 and TMPRSS2 were used as target cells. B. anti-pseudoviral activity and cytotoxicity are shown in red and blue, respectively. The data represent mean ± standard deviation (SD) of the triplicate measurements.
3.3. CHLA and PUG exhibited inhibitory effects against 3CLpro activity
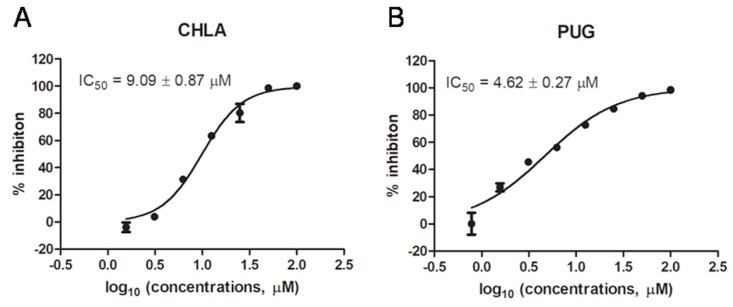
The 3-chymotrypsin-like cysteine protease (3CLpro) enzyme is one of the best characterized drug targets among coronaviruses (Zhang et al., 2020a). Along with the papain-like protease (PLpro), 3CLpro is an essential viral protease that processes the viral polyproteins, therefore, inhibiting the activity of this enzyme would block viral replication (Dai et al., 2020). Interestingly, an in silico study revealed that several hydrolyzable polyphenolics including CHLA are potential inhibitors of the SARS-CoV-2 3CLpro (Khalifa et al., 2020). To address this, CHLA and PUG were subjected to a SARS-CoV-2 3CLpro enzymatic inhibition assay. Upon mixture of 3CLpro with a FRET-based peptidic substrate in reaction buffer, the substrate gets hydrolyzed. After hydrolysis, the fluorophore Edans is no longer affected by the quencher molecule Dabcyl, resulting in an increase of fluorescence signal with proper filters (Fig. S1A). Both CHLA and PUG dose-dependently reduced fluorescence production (Fig. S1B), with IC50 values of 9.09 ± 0.87 μM and 4.62 ± 0.27 μM, respectively (Fig. 4 ). A fluorescent interference assay further illustrated that CHLA and PUG affected fluorescence only at higher concentrations (>50 μM, data not shown). Taken together, our study revealed that CHLA and PUG can block the enzymatic activity of 3CLpro. Of note, CHLA showed little effect on the enzymatic activity of SARS-CoV-2 PLpro, while PUG slightly inhibits the PLpro activity with IC50 of over 50 μM (Fig. S2). These data suggest that PUG is a potential dual-target SARS-CoV-2 inhibitor, although its inhibitory effect against the PLpro is weak.
Fig. 4.
Fluorescence resonance energy transfer (FRET)-based 3CLproenzymatic inhibition assay. The inhibitions of CHLA (A) and PUG (B) against 3CLpro were determined using a FRET-based cleavage assay. For each compound, the IC50 value is displayed in the upper left corner. The data represent mean ± standard deviation (SD) of the triplicate measurements.
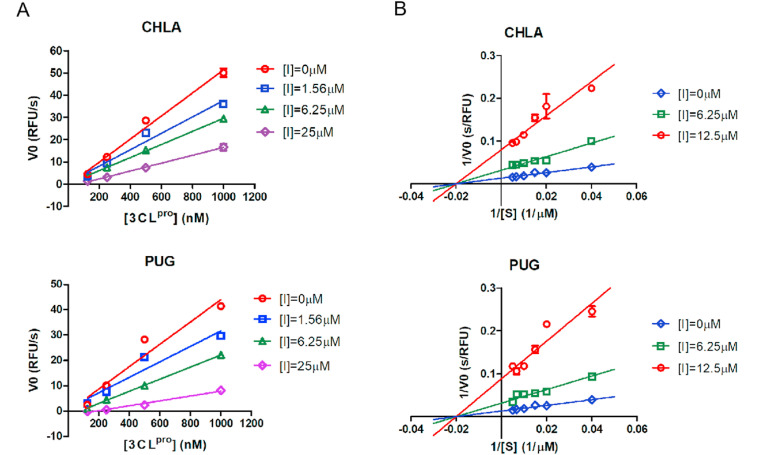
3.4. CHLA and PUG exhibited reversible, noncompetitive inhibition against 3CLpro
To better understand the kinetic modes involved in the interaction of CHLA and PUG with SARS-CoV-2 3CLpro, the proteolytic activity of the enzyme was measured using a series of enzyme concentrations and at various inhibitor concentrations. The substrate concentrations were held constant at sub-saturation levels. At each concentration of test inhibitor, the initial reaction velocities were plotted with the concentrations of 3CLpro, and fitted to a line using linear regression analysis. As indicated by Fig. 5 A, as the concentration of either CHLA or PUG increases, the slope of the line decreases, suggesting that both CHLA and PUG are reversible inhibitors. In order to deduce the mode of binding, Lineweaver-Burk plots were carried out at a constant concentration of 3CLpro (250 nM), increasing concentrations of substrate, and in absence or presence of various concentrations of CHLA or PUG. As a result, both CHLA and PUG exhibited noncompetitive modes of inhibition since all lines intercepting the x-axis (Fig. 5B).
Fig. 5.
Graphical determination of the type of enzymatic inhibition. A. Relationship of the enzymatic activity of SARS-CoV-2 3CLpro as function of enzyme concentrations at different concentration of CHLA or PUG. B. Lineweaver-Burk plot for inhibition of CHLA or PUG on SARS-CoV-2 3CLpro.
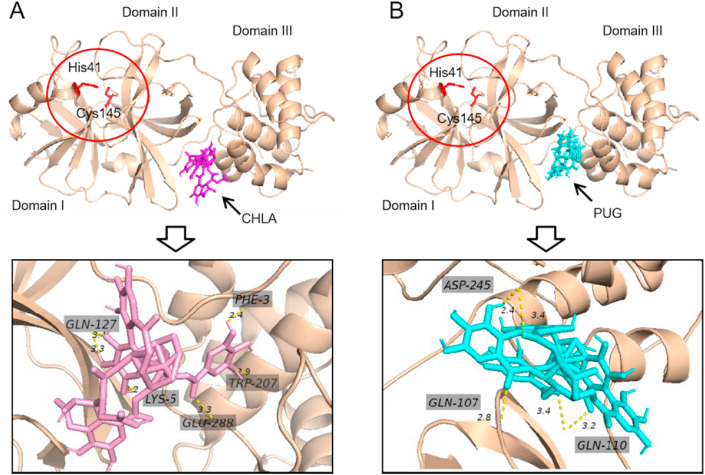
3.5. Molecular docking
According to above enzymatic assays, both CHLA and PUG are noncompetitive allosteric inhibitors. In order to search potential allosteric binding sites, in silico docking of CHLA and PUG to 3CLpro was thereafter performed using AutoDock Vina software. As depicted in Fig. 6 , both CHLA and PUG may interact with the cleft between domain II and domain III within 3CLpro with stable binding free energy. This binding site is located a little away from the substrate binding pocket, where His41 and Cys145 form the catalytic dyad (Dai et al., 2020).
Fig. 6.
Predicted binding modes of CHLA (A) and PUG (B) to 3CLpro(PDB ID:6m2n). The red circle indicates the catalytic site. The SARS-CoV-2 3CLpro is present in wheat cartoon, while CHLA and PUG are shown as sticks. The bonds are represented as the dashed lines, with the bond length measured between heavy atoms.
4. Discussion
In the urgent campaign to develop SARS-CoV-2 therapeutics, natural products from medicinal plants have been an important source of new lead compounds exhibiting different mechanisms of action, such as shikonin (Li et al., 2020a), salvianolic acid C (Yang et al., 2020), cepharanthine (Rogosnitzky et al., 2020), lycorine (Zhang et al., 2020b), ginkgolic acid (Chen et al., 2021) and so on (Zhang et al., 2020c). We herein report two additional natural products, CHLA and PUG as novel SARS-CoV-2 inhibitors. By binding to SARS-CoV-2 3CLpro at a pocket other that substrate binding site, CHLA and PUG act as allosteric inhibitors in reversible, noncompetitive manner.
There are two classes of inhibitors against a cysteine protease, covalent inhibitors and non-covalent ones. Development of covalent inhibitors is usually challenging due to their toxicity and lack of specificity resulting from covalent modification of untargeted cysteine residues, while encouraging strategies have been explored, for example, by rational design, to inhibit 3CLpro via its covalent inhibition (Dai et al., 2020; Jin et al., 2020; Qiao et al., 2021).
On the other hand, a non-covalent inhibitor may specifically bind to the target proteins reversibly. Compared to covalent inhibitors, non-covalent inhibitors can often be optimized to diffuse through membranes and bind to target proteins with high affinity (Zhang et al., 2015). Moreover, non-covalent inhibitors are often more chemical stable than their covalent counterparts since they don't have reactive warheads,and undesirable toxic effects can be reduced for their binding to host proteins and nucleic acids are reversible (El-Baba et al., 2020).
Virtual screens have placed hundreds chemically diverse molecules in the active site of 3CLpro, which is the main target for a non-covalent inhibitor (Douangamath et al., 2020; Gyebi et al., 2020; Khalifa et al., 2020). Although most of the predicted inhibitors either were not subjected to antiviral assays or failed to inhibit SARS-CoV-2 replication, compound ML188 has been validated as SARS-CoV-2 inhibitor by competitively binding to 3CLpro with natural substrate (Lockbaum et al., 2021). Besides, a non-covalent inhibitor may also act by binding to allosteric sites, which are away from the active site (El-Baba et al., 2020). Recently, a highly reactive pocket in the dimerization region at the domain III apex of SARS-CoV-2 3CLpro has been recognized as a possible allosteric site. Interestingly, our in silico docking data clearly reveal that both CHLA and PUG accommodates this pocket (Fig. 6).
Historically, in order to discover non-covalent cysteine protease inhibitors, reducing reagents were usually added in the enzymatic assay of cysteine protease to prevent nonspecific covalent modification of the catalytic cysteine (Ma et al., 2020a; Ratia et al., 2008). However, in the case of CHLA and PUG, addition of 4 mM DTT in the enzymatic assay of SARS-CoV-2 3CLpro shifted the IC50 curves to right by 7–10 folds, suggesting significant loss of inhibitory effects (Fig. S3). This is inconsistent with our finding that CHLA and PUG are reversible, noncompetitive inhibitors of 3CLpro. To address this discrepancy, we suspect that reducing reagents might induce slight conformational changes of 3CLpro, which would not affect the detectable enzymatic activity, while the cavities where an allosteric inhibitor binds might be impaired. Although more evidence is required to support this hypothesis, the scientific community should be cautious to add reducing reagents in the enzymatic assay of cysteine protease if allosteric inhibitors were anticipated.
In summary, our study demonstrates CHLA and PUG as a novel class of SARS-CoV-2 inhibitors, by allosterically regulating the 3CLpro activity.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We thank Dr. Shibo Jiang and Dr. Lu Lu from Fudan University for kindly providing the plasmid expressing SARS-CoV-2 Spike glycoprotein. We thank Ms. Yanyan Wang for the docking analysis. This work was supported by (1) the Key R & D Project in Shandong Province, China (Grant No. 2020CXGC010505); (2) Shandong Provincial Natural Science Foundation, China (Grant No. ZR2020MH383); (3) The Social Benefiting Technology Program of Qingdao (Grant No. 21-1-4-rkjk-15-nsh).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2021.105075.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. Fast-spreading COVID variant can elude immune responses. Nature. 2021 doi: 10.1038/d41586-021-00121-z. [DOI] [PubMed] [Google Scholar]
- Chen Z., Cui Q., Cooper L., Zhang P., Lee H., Chen Z., Wang Y., Liu X., Rong L., Du R. Ginkgolic acid and anacardic acid are specific covalent inhibitors of SARS-CoV-2 cysteine proteases. Cell Biosci. 2021;11:45. doi: 10.1186/s13578-021-00564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., Thacker B.E., Glass C.A., Yang Z., Torres J.L., Golden G.J., Bartels P.L., Porell R.N., Garretson A.F., Laubach L., Feldman J., Yin X., Pu Y., Hauser B.M., Caradonna T.M., Kellman B.P., Martino C., Gordts P., Chanda S.K., Schmidt A.G., Godula K., Leibel S.L., Jose J., Corbett K.D., Ward A.B., Carlin A.F., Esko J.D. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043–1057 e1015. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., Li C., Li Y., Bai F., Wang H., Cheng X., Cen X., Hu S., Yang X., Wang J., Liu X., Xiao G., Jiang H., Rao Z., Zhang L.K., Xu Y., Yang H., Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douangamath A., Fearon D., Gehrtz P., Krojer T., Lukacik P., Owen C.D., Resnick E., Strain-Damerell C., Aimon A., Ábrányi-Balogh P., Brandão-Neto J., Carbery A., Davison G., Dias A., Downes T.D., Dunnett L., Fairhead M., Firth J.D., Jones S.P., Keeley A., Keserü G.M., Klein H.F., Martin M.P., Noble M.E.M., O'Brien P., Powell A., Reddi R.N., Skyner R., Snee M., Waring M.J., Wild C., London N., von Delft F., Walsh M.A. Crystallographic and electrophilic fragment screening of the SARS-CoV-2 main protease. Nat. Commun. 2020;11:5047. doi: 10.1038/s41467-020-18709-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Baba T.J., Lutomski C.A., Kantsadi A.L., Malla T.R., John T., Mikhailov V., Bolla J.R., Schofield C.J., Zitzmann N., Vakonakis I., Robinson C.V. Allosteric inhibition of the SARS-CoV-2 main protease: insights from mass spectrometry based assays*. Angew Chem. Int. Ed. Engl. 2020;59:23544–23548. doi: 10.1002/anie.202010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyebi G.A., Ogunro O.B., Adegunloye A.P., Ogunyemi O.M., Afolabi S.O. Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CL(pro)): an in silico screening of alkaloids and terpenoids from African medicinal plants. J. Biomol. Struct. Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1764868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid S., Mir M.Y., Rohela G.K. Novel coronavirus disease (COVID-19): a pandemic (epidemiology, pathogenesis and potential therapeutics) New microbes and new infections. 2020;35:100679. doi: 10.1016/j.nmni.2020.100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Khalifa I., Zhu W., Mohammed H.H.H., Dutta K., Li C. Tannins inhibit SARS-CoV-2 through binding with catalytic dyad residues of 3CL(pro) : an in silico approach with 19 structural different hydrolysable tannins. J. Food Biochem. 2020 doi: 10.1111/jfbc.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Jin W., Sood A., Montgomery D.W., Grant O.C., Fuster M.M., Fu L., Dordick J.S., Woods R.J., Zhang F., Linhardt R.J. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antivir. Res. 2020;181:104873. doi: 10.1016/j.antiviral.2020.104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhou X., Zhang Y., Zhong F., Lin C., McCormick P.J., Jiang F., Luo J., Zhou H., Wang Q., Fu Y., Duan J., Zhang J. Crystal structure of SARS-CoV-2 main protease in complex with the natural product inhibitor shikonin illuminates a unique binding mode. Sci. Bull. 2020 doi: 10.1016/j.scib.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Du R., Chen Z., Wang Y., Zhan P., Liu X., Kang D., Zhao X., Wang L., Rong L., Cui Q. Punicalagin is a neuraminidase inhibitor of influenza viruses. J. Med. Virol. 2020 doi: 10.1002/jmv.26449. [DOI] [PubMed] [Google Scholar]
- Li P., Du R., Wang Y., Hou X., Wang L., Zhao X., Zhan P., Liu X., Rong L., Cui Q. Identification of chebulinic acid and chebulagic acid as novel influenza viral neuraminidase inhibitors. Front. Microbiol. 2020;11:182. doi: 10.3389/fmicb.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.T., Chen T.Y., Chung C.Y., Noyce R.S., Grindley T.B., McCormick C., Lin T.C., Wang G.H., Lin C.C., Richardson C.D. Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J. Virol. 2011;85:4386–4398. doi: 10.1128/JVI.01492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.T., Chen T.Y., Lin S.C., Chung C.Y., Lin T.C., Wang G.H., Anderson R., Lin C.C., Richardson C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013;13:187. doi: 10.1186/1471-2180-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockbaum G.J., Reyes A.C., Lee J.M., Tilvawala R., Nalivaika E.A., Ali A., Kurt Yilmaz N., Thompson P.R., Schiffer C.A. Crystal structure of SARS-CoV-2 main protease in complex with the non-covalent inhibitor ML188. Viruses. 2021;13 doi: 10.3390/v13020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Hu Y., Townsend J.A., Lagarias P.I., Marty M.T., Kolocouris A., Wang J. Ebselen, disulfiram, carmofur, PX-12, tideglusib, and shikonin are nonspecific promiscuous SARS-CoV-2 main protease inhibitors. ACS Pharmacol Transl Sci. 2020;3:1265–1277. doi: 10.1021/acsptsci.0c00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Sacco M.D., Hurst B., Townsend J.A., Hu Y., Szeto T., Zhang X., Tarbet B., Marty M.T., Chen Y., Wang J. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–692. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., Li Y.S., Zeng R., Liu F.L., Luo R.H., Huang C., Wang Y.F., Zhang J., Quan B., Shen C., Mao X., Liu X., Sun W., Yang W., Ni X., Wang K., Xu L., Duan Z.L., Zou Q.C., Zhang H.L., Qu W., Long Y.H., Li M.H., Yang R.C., Liu X., You J., Zhou Y., Yao R., Li W.P., Liu J.M., Chen P., Liu Y., Lin G.F., Yang X., Zou J., Li L., Hu Y., Lu G.W., Li W.M., Wei Y.Q., Zheng Y.T., Lei J., Yang S. SARS-CoV-2 M(pro) inhibitors with antiviral activity in a transgenic mouse model. Science. 2021 doi: 10.1126/science.abf1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratia K., Pegan S., Takayama J., Sleeman K., Coughlin M., Baliji S., Chaudhuri R., Fu W., Prabhakar B.S., Johnson M.E., Baker S.C., Ghosh A.K., Mesecar A.D. A noncovalent class of papain-like protease/deubiquitinase inhibitors blocks SARS virus replication. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16119–16124. doi: 10.1073/pnas.0805240105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosnitzky M., Okediji P., Koman I. 2020. Cepharanthine: a Review of the Antiviral Potential of a Japanese-approved Alopecia Drug in COVID-19. Pharmacological Reports : PR; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., Mlisana K., von Gottberg A., Walaza S., Allam M., Ismail A., Mohale T., Glass A.J., Engelbrecht S., Van Zyl G., Preiser W., Petruccione F., Sigal A., Hardie D., Marais G., Hsiao M., Korsman S., Davies M.-A., Tyers L., Mudau I., York D., Maslo C., Goedhals D., Abrahams S., Laguda-Akingba O., Alisoltani-Dehkordi A., Godzik A., Wibmer C.K., Sewell B.T., Lourenço J., Alcantara L.C.J., Pond S.L.K., Weaver S., Martin D., Lessells R.J., Bhiman J.N., Williamson C., de Oliveira T. 2020. Emergence and Rapid Spread of a New Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Lineage with Multiple Spike Mutations in South Africa. medRxiv. [Google Scholar]
- Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Pan X., Xu X., Cheng C., Huang Y., Li L., Jiang S., Xu W., Xiao G., Liu S. Salvianolic acid C potently inhibits SARS-CoV-2 infection by blocking the formation of six-helix bundle core of spike protein. Signal transduction and targeted therapy. 2020;5:220. doi: 10.1038/s41392-020-00325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Xue X., Zhang L., Zhang L., Liu Z. Comparative analysis of pharmacophore features and quantitative structure-activity relationships for CD38 covalent and non-covalent inhibitors. Chem. Biol. Drug Des. 2015;86:1411–1424. doi: 10.1111/cbdd.12606. [DOI] [PubMed] [Google Scholar]
- Zhang Y.N., Zhang Q.Y., Li X.D., Xiong J., Xiao S.Q., Wang Z., Zhang Z.R., Deng C.L., Yang X.L., Wei H.P., Yuan Z.M., Ye H.Q., Zhang B. Gemcitabine, lycorine and oxysophoridine inhibit novel coronavirus (SARS-CoV-2) in cell culture. Emerg. Microb. Infect. 2020;9:1170–1173. doi: 10.1080/22221751.2020.1772676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.R., Zhang Y.N., Li X.D., Zhang H.Q., Xiao S.Q., Deng F., Yuan Z.M., Ye H.Q., Zhang B. A cell-based large-scale screening of natural compounds for inhibitors of SARS-CoV-2. Signal transduction and targeted therapy. 2020;5:218. doi: 10.1038/s41392-020-00343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.