Abstract
COVID-19, a highly transmissible pandemic disease, is affecting millions of lives around the world. Severely infected patients show acute respiratory distress symptoms. Sustainable management strategies are required to save lives of the infected people and further preventing spread of the virus. Diagnosis, treatment, and vaccination development initiatives are already exhibited from the scientific community to fight against this virus. In this review, we primarily discuss the management strategies including prevention of spread, prophylaxis, vaccinations, and treatment for COVID-19. Further, analysis of vaccine development status and performance are also briefly discussed. Global socioeconomic impact of COVID-19 is also analyzed as part of this review.
Keywords: SARS-CoV-2, COVID-19, Vaccinations, Treatments, Management, Social impact
A respiratory disease caused by the novel SARS-CoV-2 virus, Coronavirus disease 2019 (COVID-19), had outbroken from Wuhan seafood wholesale market in China in December 2019, and incontinently spread by the first quarter of 2020 [1]. The clinical manifestations related to the COVID-19 infected patients are usually cough, fever, respiratory distress, breathing difficulty, and sometimes asymptomatic [2]. The virus usually transmits via respiratory droplets (saliva, coughing, or sneezing) even via fecal transmission is also possible [[2], [3], [4]]. It has become a major global health concern, and the World Health Organization (WHO) declared this a ‘Pandemic’ in March 2020 [5]. More than 156 million individuals has been infected and is responsible for at least 3,262,000 deaths globally by May 06, 2021 and the surge continues [6,7].
Within three months of the reported outbreak, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) and Moderna, Inc have begun the first human trial of a SARS-CoV-2 vaccine, mRNA-1273 [8]. Recently, three vaccines have received Emergency Usage Authorization (EUA) by the Food and Drug Administration (FDA) in United States. Moreover, several other vaccine candidates have been approved and distributed globally. Besides, at present, several vaccine candidates, including genetic and inactivated whole virus-based vaccines, and treatments including monoclonal antibodies and small molecule antivirals are in the different phases of clinical trials [9,10]. Despite many of these attempts are ongoing under “warp speed”, it may take years to vaccinate entire population around the world. In the meantime, social distancing and lockdown measures have been taken intensively, including closing borders and halting international travels to mitigate viral transmission [11]. All these mitigation measures have severally impacted social encounters and financial dealings. In addition, the development of new mutant strains with various infection rates and their relationship with the uncertainty in responding to the current vaccines are other major problem in the management of this disease.
In this manuscript, we review primarily therapeutic and vaccinations for COVID-19. We discuss therapeutic development strategies, the efficiency of developed drugs, vaccine development technologies with comparative performance analysis, passive immunization, and the worldwide socioeconomic impact by the disease.
1. Genomic structure of SARS-CoV-2
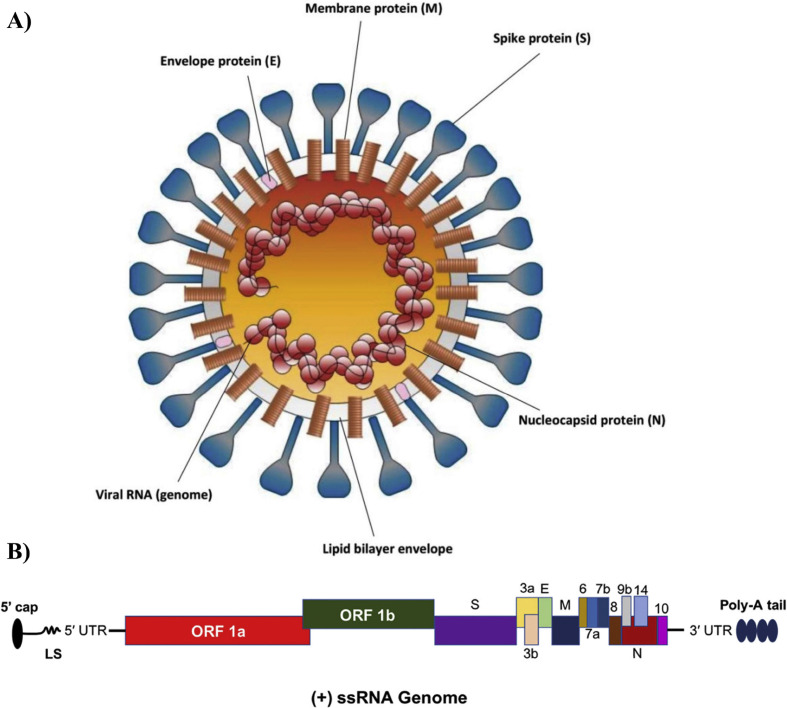
Seven coronaviruses infects humans in which four of them (HKU1, NL63, OC43, and 229E) usually shows very minor symptoms whereas rest of the three (SARS-CoV, MERS-CoV, and SARS-CoV-2) causes acute respiratory problems and causes high rate of fatality [14]. SARS-CoV-2 is a + sense ssRNA virus with a size of 100–160 nm [15]. The genomic analysis of this virus presented that the genomic length is approximately 30 kb and encodes two types of proteins (structural and non-structural proteins) [13]. The entire genome encodes 13–15 open reading frames (ORFs), and 12 are functional. The two encoded polyproteins (ORF1a and ORF1ab) creates 16 non-structural (NS) proteins (NSP1–NSP16) and structural proteins are known as spike (S), envelope (E), membrane (M) and nucleocapsid (N) (Fig. 1 A, B) [16]. Besides, it also contains two untranslated regions (UTR) including a 5′ cap terminal and 3′-poly-A tail. The ORFs are mainly replicase and protease however the S protein consists of two subunit S1 and S2. S protein plays a significant contribution in viral entry (S1) and fusion (S2) as a result they are considered a key target for designing/developing drugs/vaccine candidates against COVID-19 [13].
Fig. 1.
A) Structure of SARS-CoV-2 consisting four structural proteins including ES, M and N (Reprinted from Ref. [12]). B) Genomic organization of 30 kb long, positive-sense ssRNA having encoded both structural and nonstructural type proteins (Reprinted from Ref. [13]).
2. Management of COVID-19
The first step of management of a disease is to regulate the escalation of contagion. Orderly understanding the cause of the COVID-19 pandemic and adopting with different preventive measures plays vital role in controlling a pandemic like COVID-19. Besides, extensive researches on specific treatments, vaccine developments and administration have been ongoing with the advent of SARS-CoV-2. The currently available treatments include small molecule drugs that prevent viral entry into host cells or block viral replication and assembly, as well as biologics/antibodies that control COVID-19 associated pro-inflammatory immune responses to minimize the disease associated pathologies [17]. On the other hand, vaccines' goal is to produce an adaptive immunity counter to SARS-CoV-2 by generating required antibodies to neutralize the virus. Notable milestones in the developments of treatments and vaccines as well as protective actions are summarized below:
2.1. Prevention of spread as a strategy to reduce infections
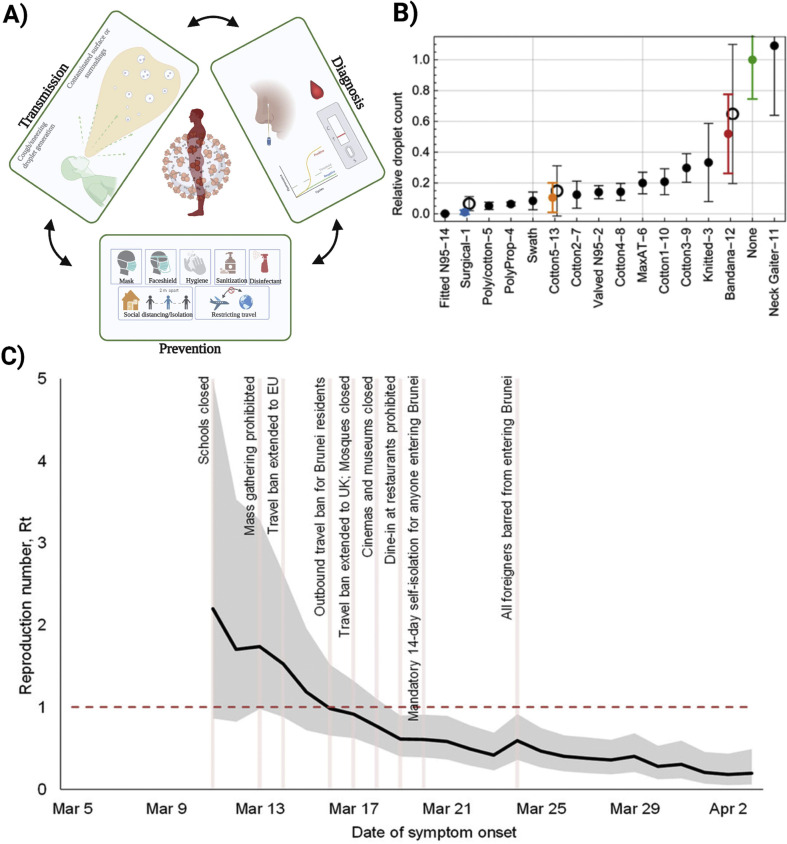
The primary transmission media is respiratory droplets and COVID-19 can be transmitted even before showing symptoms. To mitigate the virus's spread, two things need to be followed: first, an infected individual needs to be isolated and limited contact with non-infected patients and, secondly, decrease per contact transmission possibility [18]. Human behavior crucially affects the COVID-19 transmission pattern and adopting with the new behavioral changes are significant for further spreading [19]. Accurate and rapid diagnosis is the main key to fight against this kind of pandemic and proved to be fruitful in previous pandemics [[20], [21], [22], [23], [24]]. Utilization of ASSURED (Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free and Deliverable to end-users) criteria regulated by WHO to develop diagnosis devices should be considered [[25], [26], [27], [28]]. Isolation of the infected patients and maintaining social distancing actions, including mandating “shelter in place”, have been adopted to mitigate the spread of the virus globally, but at a huge cost to society and economy [19]. Proper practice of masks and gloves, hand sanitation, cleaning highly touchable areas with disinfectant have already proved to be beneficial (Fig. 2 A) [29]. A recent study about the effectiveness of mandating face masks in Germany outlined that the daily use of mask reduced the daily growth of COVID-19 infection by 47% [30]. Some studies also suggest that proper use of mask reduces the severity of the infection if someone gets infected by COVID-19 [31]. However, the effectiveness of masks also depends on the material or the fabrics it is made of. A study with 14 different types of masks represented that N-95/surgical masks are most effective against droplets (Fig. 2B) [32]. On the other hand, use of face shield also demonstrated to be fruitful from respiratory droplets but only for short time [33]. Gloves has been used since a long time to ensure the safety of healthcare workers in healthcare facilities. CDC has recommended all the healthcare professionals to use gloves who are engaged in providing care to infected people. While disinfecting the exposed/contaminated areas, always, CDC recommended guidelines should be followed [34]. However, maintaining social distancing, creating a barrier where frequent interaction occurs like in retail stores, avoid public gatherings, avoiding unwanted traveling also plays important role on mitigating the spread of the virus. Different countries, globally, have implemented travel restriction measures to downscale the intercontinental transmission [35]. A recent study regarding impact of imposing travel restrictions and banning public gatherings also demonstrated significantly reduced reproduction number of the virus infection (Fig. 2C) [36]. However, several safety measures should be considered if travel is necessary specially all passengers in an aircraft should be tested negative before boarding.
Fig. 2.
A) Prevention of spread by implementing behavioral changes. B) Comparative effectiveness analysis of masks made of different materials to provide protection against droplets (Reprinted from Ref. [32]). C) Estimation of reproduction no reduction after imposing travel restrictions, banning gatherings and closing schools in Brunei (Reprinted from Ref. [36]).
2.2. Developments and repurposing of small molecule drugs/biologics
With the outbreak of SARS-CoV-2, scientists/medical practitioners around the globe initially began testing the existing drugs as a repurposing strategy to treat affected patients without extensive clinical trials. Some of the most widely used/tested drugs include antivirals of other viral diseases, anti-inflammatory agents, immunomodulators, anticoagulants, and vasodilators [37,38]. While the antiviral drugs are mostly helpful in the early stage of infection, anti-inflammatory/immunomodulatory agents are almost exclusively effective in preventing disease progression in the late stage when there is a need to suppress hyperimmune responses; the anticoagulants can be useful to manage coagulation associated abnormalities caused by COVID-19 [37].
Most antiviral drugs (protease/polymerase. and reverse transcriptase inhibitors) that underwent clinical trials to treat COVID-19 were originally developed for similar infectious diseases including influenza, SARS-CoV, MERS, HIV, and Ebola with an understanding that known drugs that have worked against an infectious disease will also work against COVID-19 [39].
2.2.1. Protease inhibitors
Results from previous studies on diverse viruses reveal that, in many cases, while the viral proteases play essential roles in the intracellular processing of structural polyprotein into individual proteins for viral assembly, host cell proteases such as type 2 transmembrane serine protease (TMPRSS2) promote viral uptake by activating envelope glycoproteins [37]. Screening of viral protease inhibitors such as lopinavir-ritonavir combination (a.k.a. Kaletra™), which is an effectful therapy for HIV infections, did not show a clear benefit in the clinical trials of severely infected hospitalized patients [40,41] when equated to typical care alone [42]. This observation likely reflects that SARS-CoV-2 has viral proteases that are substantially different from other viral proteases and may not be efficiently inhibited by pan-protease inhibitors. In contrast, the inhibitors of host cell proteases (e.g. TMPRSS2) that process the S protein and help host cell entry appear to hold clear promises [37]. Notably, Nafamostat, a TMPRSS2 inhibitor, effectively inhibited MERS-CoV S protein-initiated membrane fusion [43], while Camostat mesylate was able to block entry of the virus into lung cells [44,45]. Both Camostat and Nafamostat are currently under extensive clinical investigations as potential antiviral treatments for COVID-19 [46]. However, another clinical protease inhibitor candidate developed by Pfizer (PF-07321332) has showed promising antiviral action in case of SARS-CoV-2 in lab studies [47]. Recently, they have started phase-1 clinical trial (NCT04756531) for this orally administrated therapeutic including single and multiple ascending doses in healthy adults [48]. Moreover, Pfizer is also studying the potent activity of an intravenously (IV) administered inhibitor (PF-07304814) for the hospitalized patients and currently conducting Phase 1b multi-dose clinical trial (NCT04535167) [47,49].
2.2.2. Polymerase inhibitors
Among the tested RNA-dependent RNA polymerase (RdRp) inhibitors, remdesivir, firstly formulated by Gilead Sciences (US) for Ebola/Marburg diseases, is probably the most promising agent despite mixed results from the clinical trials. Although remdesivir initially failed to demonstrate statistically significant clinical benefits on hospitalized patients with different levels of severity [50], however, more focused trials revealed promising data. The outcomes of a double-blinded, arbitrary, and placebo-controlled trial of 1063 individuals confirmed that patients with remdesivir had a faster recovery time compared to placebo-controlled group (11 vs. 15 days) [51,52]. Consequently, the FDA has permitted EUA of remdesivir to treat COVID-19 patients [52]. Among other RdRp inhibitors, EIDD-2801, an investigational drug, developed by Emory Institute for Drug Development (EIDD)/Ridgeback Therapeutics (US), underwent phase II/III clinical trials (NCT04405570 and NCT04405739) [53]. Both remdesivir and EIDD-2801 are ribonucleotide analogs that non-selectively block replication of multiple RNA viruses, although they appear to work in different ways, meaning they could be complementary [53]. Unlike remdesivir, which is administered intravenously, EIDD-2801 can be administered orally. However, Another study showed that MK-4482/EIDD-2801 successfully reduced the viral load in ferret models [54]. MK-4482/EIDD-2801 can be an auspicious antiviral therapeutic for suppressing the spread of COVID-19 in communities. Other polymerase inhibitors include favipiravir/avigan, which was initially developed for influenza in Japan by Fujifilm Toyama Chemical also tested against SARS-CoV-2. Although Favipiravir initially showed promising results in treating patients from Wuhan and Shenzhen with mild or moderate symptoms [55], more recent trials using favipiravir alone or in combination with another broad-spectrum antiviral drug, umifenovir/arbidol, suggested no significant improvement in clinical recovery; however, the combination product did expedite relief from fever and cough [56]. Moreover, Favipiravir has teratogenic effects on pregnancy; as a result, drug administration should be controlled [57].
2.2.3. Immunosuppressants
Like other cytopathic viruses, SARS-CoV-2 infections trigger an innate immune response by recruiting macrophages, monocytes and release cytokines and chemokines, and prime T and B lymphocytes for adaptive immune response [37]. This procedure is successful in clearing the infection for more than 80% cases; nevertheless, in some patients, a dysfunctional immune response can lead to an overwhelming release of pro-inflammatory cytokines ((such as interferon gamma, interleukin (IL-1, IL-6), and complement factor 5a)) that mediates widespread lung inflammation, requiring intensive care in hospitals. Indeed, many people with COVID-19 do not die from the tissue damage caused by the virus but from an aggravated immune response leading to “cytokine release syndrome”, or “cytokine storm” [37]. In such cases, another treating technique consisting suppressing the inflammatory response by immunomodulatory monoclonal antibodies (mAbs) including the IL-6 inhibitors (tocilizumab and sarilumab), and IL-1β blockers (canakinumab) among others [58]. A clinical study for tocilizumab and sarilumab reported that they can reduce the death rate to critically ill patients by blocking the protein to detect IL-6 [59]. The use of corticosteroids, such as dexamethasone, was found to be beneficial in severe patients who required mechanical ventilation but not those in the early stages of infection [60]. In contrast, the antimalarial drugs, chloroquine/hydroxychloroquine, which also may have the positive immunomodulatory effects in vivo [61] in addition to their ability to prevent viral attack and endocytosis of the virus in vitro [62], have also been tested in several clinical trials with or without combining azithromycin, but did not show any clear benefit in hospitalized patients [63,64].
More recent approaches involve artificial intelligence (AI) to conduct exhaustive computational screening of large libraries of “redirected” or “repurposed” drugs against potential SARS-CoV-2 viral targets, followed by clinical investigations on selected candidates [65]. Such examples include losartan (antihypertensive) and ivermectin (anti-parasitic) [66] that were successful in several individual cases, but require larger clinical trials to demonstrate their potentials for COVID-19 management.
2.3. Passive immunization
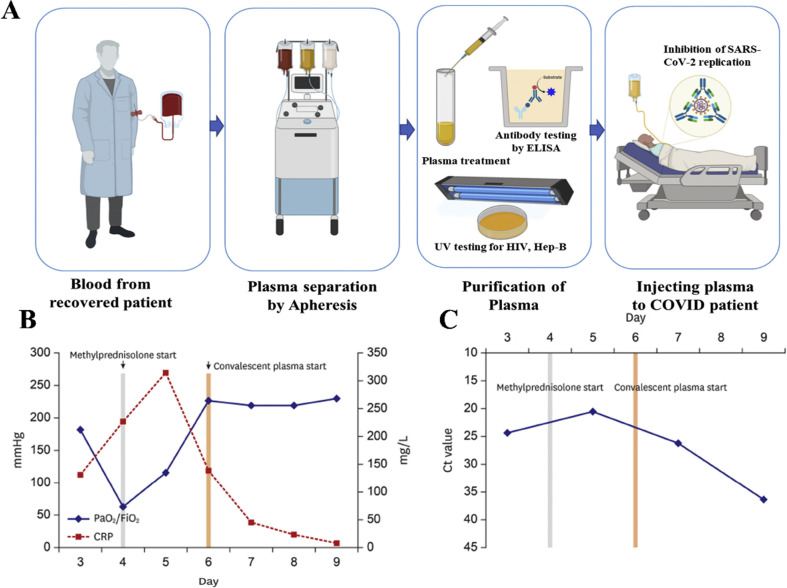
Passive immunization is one of the most effective means of treating viral infections by introducing virus-specific antibodies either from recovered patients or from immunized animals/cells to newly infected individuals (Fig. 3 A) [67,68], and was first demonstrated in 1890 for the treatment of tetanus and diphtheria [67]. Passive immunization involves the process of obtaining serum from immune individuals (convalescent plasma (CP), concentrating the immunoglobulin antibodies (hyperimmune immunoglobulins), or developing isolated antibodies (polyclonal or monoclonal) using an immunogen in animal models or cell lines [17,69]. This treatment is rapid in its action and can generate an immune response within hours or days, which is substantially faster than vaccines. Furthermore, antibody treatment has the potential to override the deficient immune system, especially in severe cases, when an infected individual is not in a condition to receive vaccination [70]. However, passive immunization lasts only for a few days or weeks because this treatment does not involve the generation of memory B cells [70] and can be uncertain for reasons that are context-dependent and not fully understood.
Fig. 3.
Passive immunization by convalescent plasma therapy. A) Step by step process plasma collection procedure from a recovered COVID-19 patient to infusing to a critically infected patient. B) Case study of a hospitalized patient showing the change in PaO2/FiO2 and CRP before and after the convalescent plasma therapy. C) Decrease of viral load shedding reflected in the detection threshold of ORF1b gene using RT-PCR of the same patient's sputum specimen before and after infusing the convalescent plasma (Reprinted from Ref. [73]).
Previous studies on SARS infection have shown that patients who received plasma therapy remained immune for shorter periods and had a reduced mortality rate than the high-dose steroids pulse therapy [71]. Using CP treatment on severely ill patients with COVID-19 reduced viral load measured by RT-PCR. It immediately increased correlation between partial pressure of arterial oxygen (PaO2) to the inspired oxygen (FiO2) (Fig. 3B, C) [72,73]. Therefore, considering the growing demand for COVID-19 treatment in a short time and due to the lack of specific treatments, many countries have adopted CP as their initial line of treatment. However, a clinical trial, including 228 COVID-19 infected patients who received CP and 105 patients who received placebo, didn't show a significant difference in the mortality and other clinical outcomes [74]. Nevertheless, the US-FDA has approved the use of passive immunization in treating COVID-19 for its immediate clinical benefits [75,76]. However, treatment with CP has several limitations, including difficulty in plasma collection, batch-to-batch variations in neutralizing antibody titers, potential contamination with blood-borne pathogens, and the requirement for matching blood type (Rh factor) [77].
Administration of isolated/refined antibodies may be a better alternative to CP because they are more antigen-selective and do not have many of the limitations associated with CP's. Several biotechnologies/pharmaceutical companies are currently developing hyperimmune immunoglobulins (H-IGs) as well as polyclonal antibodies (pAbs) from CPs. Notably, the “CoVIg-19 Plasma Alliance” is an alliance formed by a number of companies, that aims to develop plasma-derived H-IGs against COVID-19 [78]. However, the extraction of antibodies is not only technically challenging but also requires expensive laboratory procedures. Besides H-IGs and pAbs, there has been a major push many countries including USA to develop monoclonal antibodies (mAbs) to treat COVID-19. Since the identification of SARS-CoV-2, numerous research groups (both academic and industrial) have produced mAbs from the single B cells obtained from SARS-CoV-2 infected patients, or by immunization of humanized animals. Most neutralizing antibodies are aimed to prevent viral entry by masking the S protein from interacting with ACE2 or other host cell receptors, or by preventing the conformational changes of the S protein that enables membrane fusion [79].
Of particular notes, Elly Lilly developed two antibodies, LY-COV555 (in collaboration with AbCellera) and JS016 (with Shanghai Junshi Biosciences), both of which act by blocking the viral S protein [80]. However, ‘bamlanivimab’, which is clinically known as LY3819253 (LY-CoV555) was examined to have the better SARS-CoV-2 binding affinity than hundreds of other developed mAbs [81]. It has received the FDA-EUA clearance for treating the COVID-19 patients in the USA [82]. Besides, an antibody cocktail mixture of two human IgG1 antibodies named REGN- COV2 have recently been developed by Regeneron [83]. This cocktail prevents viral entry into human cells by targeting S protein's receptor-binding domain (RDB) [84]. The clinical trial (NCT04425629) data suggested a reduced amount of viral load, especially for patients with more significant viral load at the beginning, and this cocktail received FDA-EUA recently [82,85]. Sorrento Therapeutics is a US-based biotech company that currently tests antibody cocktails in patients with COVID-19. Sorrento's antibody cocktail (COVI-SHIELD) contains three mAbs attaching distinct epitopes on S protein. One of the COVI-SHIELD antibodies, STI-1499, binds specifically to the S1 subunit and can inhibit viral entry as observed in their preclinical studies [86]. Vir Biotechnology, another US company, developed VIR-7831 and VIR-7832 antibodies from recovered SARS patients and S-309 from the memory B cells of SARS-CoV-2 infected patients to target the RDB of SARS-CoV-2 S protein [87]. The company is currently conducting Phase 3 trials on COVID-19 patients with S-309 and has partnered with GSK for large scale production [79]. In addition to mAbs, Vir Biotechnology and Alnylam Pharmaceuticals are currently investigating the use of RNA interference to selectively block translation of proteins such as ACE2 and TMPRSS2 that are required for viral entry into host cells. Other companies, including ImmunoPrecise, Ligand Pharmaceuticals, are also in the process of testing their therapeutic antibodies in clinical trials.
Some of the potential limitations of mAbs for COVID-19 treatment include unfamiliar bioavailability of passively infused IgG in tissues that are often largely affected by the disease and some inherently associated with undesirable effects of antibody treatments in general, including antibody-dependent enhancement (ADE) of infection [88]. Another important consideration is the effect of genetic diversity in the virus, which is mainly due to the error-prone nature of RdRps in RNA viruses such as CoVs [89]. With currently more than thousands of SARS-CoV-2 genome sequences has been deposited to date, around 200 recurrent mutations have been identified in the variable regions of the genome, including the S protein [89], which is the key target for antibody/vaccine design. Therefore, it is crucial to monitor the emergence of viral mutations that are either more virulent or that enable stronger adaptation to the human host due to antibody treatments or other factors.
2.4. Prophylaxis and vaccine development
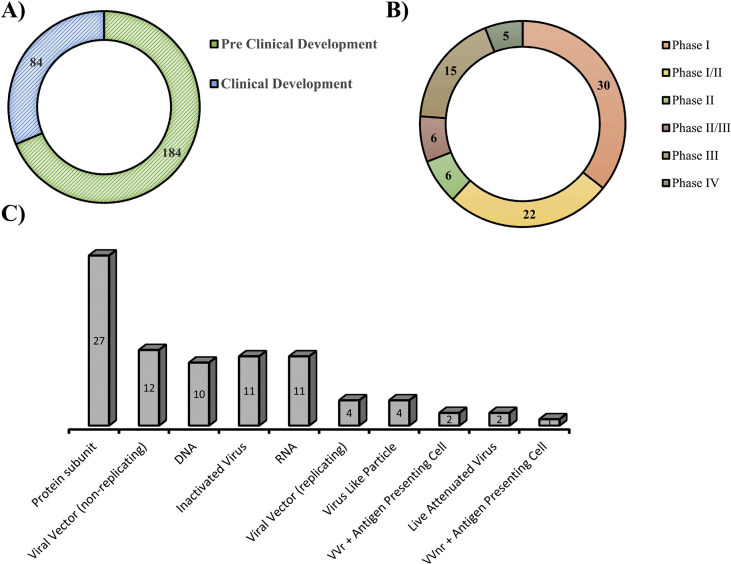
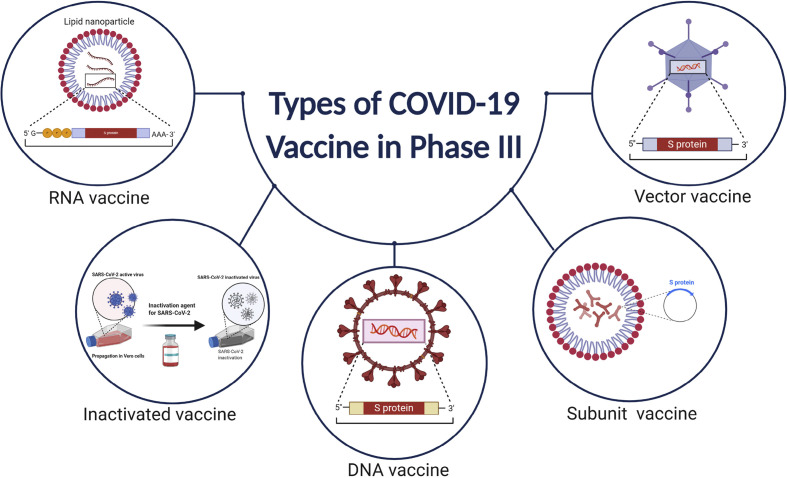
Vaccines are used as a preventive medicine that activates the adaptive host immune response to produce neutralizing antibodies against pathogens and induce memory B cells for long term immunity [90]. Because of the high degree of genomic sequence conservation of the CoVs, scientists could begin developing vaccines afterwards the genomic sequences were available, with currently more than hundreds of teams working on different vaccines [91]. Presently, three vaccines have received FDA EUA approval and several others vaccine candidate received approval in different countries around the world and are currently being given to people (Table 1 ). Currently, more than seven dozens of vaccines are in human trials in different phases (Fig. 4 (A,B)) [92]. Vaccine candidates were developed adopting various technologies, including genetic (DNA/RNA-based), recombinant protein-based, vector-based, and inactivated whole viral vaccines (Figs. 4C and 5 ) [91,93,94]. Many of these trials are sponsored by the Coalition for Epidemic Preparedness Innovations (CEPI), a charity foundation that was specially formed for developing vaccines to tackle epidemics. The most promising immunogen for developing vaccines for SARS-CoV-2 is the S protein, but whether the entire protein or only its RBD is appropriate to produce effective neutralizing antibodies remains to be investigated [93].
Table 1.
Vaccines candidates that are currently on/completed Phase III human trials. (Updated on April 02, 2021) [92,149,150].
| Type of Vaccine | Vaccine name | Developer | Trial No | Doses | Efficacy | Approved in countries |
|---|---|---|---|---|---|---|
| RNA vaccines | CVnCoV Vaccine | CureVac AG | NCT04674189 | 2 | – | – |
| Viral vector based vaccines | Ad5-nCoV/Convidecia | CanSino Biologics Inc., Academy of Military Medical Sciences, Hubei Provincial Center for Disease Control and Prevention | NCT04526990 | 1 | – | Chinese Military (Limited use), Mexico, Pakistan. |
| Ad26.COV2.S1 | Janssen Vaccines and Prevention, Beth Israel Deaconess Medical Center, Johnson & Johnson | NCT04505722 | 1 | USA-72% South Africa- 64%, Latin America-61% | WHO (EUA), EU, USA, Canada, Switzerland, Bahrain, Iceland, Liechtenstein, Norway, Thailand, | |
| Gam-COVID-Vac/Sputnik V | Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation, Acellena Contract Drug Research and Development |
NCT04530396 NCT04564716 |
2 | 91.4% | Russia and more than 50 other countries. | |
| Inactivated Vaccines | Inactivated SARS-CoV-2 vaccine | Sinopharm, China National Biotec Group Co, Beijing Institute of Biological Products | ChiCTR2000034780 NCT04560881 |
2 | Bahrain, China,Egypt, Hungary, Iraq, Jordan, Pakistan, Peru, Republic of Serbia, Seychelles, and United Arab Emirates | |
| Institute of Medical Biology, Chinese Academy of Medical Sciences | NCT04659239 | 2 | – | China. United Arab Emirates (Limited use). |
||
| QazCovid-in® | Research Institute for Biological Safety Problems, Rep of Kazakhstan | NCT04691908 | 2 | – | – | |
| Covaxin | Bharat Biotech International Limited, Iqvia Pty Ltd, Indian Council of Medical Research, | NCT04641481 | 2 | 80.6% | India, Iran, Mauritius, Nepal, Zimbabwe. | |
| Recombinant protein based vaccines | NVX-CoV2373 | Novavax | NCT04611802 | 2 | 96% against original coronavirus, 86% against B.1.1.7, 49% against B.1.351 | – |
| ZF2001 | Anhui Zhifei Longcom Biopharmaceutical, Chinese Academy of Sciences | ChiCTR2000040153, NCT04646590 | 3 | – | China, Uzbekistan. | |
| VAT00002 | Sanofi Pasteur + GSK | PACTR202011523101903 | 2 | – | – | |
| Soberana 1 | Instituto Finlay de Vacunas | RPCEC00000354 | 2 | – | – | |
| EpiVacCorona | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology "Vector" | NCT04780035 | 2 | – | Russia (Early use) | |
| CIGB-66 | Center for Genetic Engineering and Biotechnology (CIGB) | RPCEC00000359 | 3 | – | – | |
| DNA Based | nCov vaccine | Zydus Cadila | CTRI/2020/07/026352 | 3 | – | – |
Fig. 4.
Current stages of COVID-19 vaccine development. A, B) As of April 02, 2021, in total 268 vaccine candidates are in underdevelopment phases. Among them 84 candidates are in different phases of trials and 15 of them are in or completed phase 3 trials including 5 are continuing phase 4 trials. C) Different platforms have been adopted for developing COVID-19 vaccines including virus-like particles, DNA, viral vectors (replicating and non-replicating), inactivated vaccines, live-attenuated vaccines, subunit vaccines, and RNA vaccines. Source: World Health Organization (http://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/).
Fig. 5.
Types of technology used for developing COVID-19 vaccine for phase III candidate.
BioNTech's lipid nanoparticle (LNP)-formulated, nucleoside-modified mRNA vaccine (BNT162b1) demonstrated adequate T-cell responses, and it is the first vaccine to receive FDA clearance in USA [[95], [96], [97]]. Another example of mRNA-based vaccine is Moderna's LNP-encapsulated mRNA-1273 that encodes for a whole, prefusion stabilized SARS-CoV-2's S protein and is the second vaccine that received FDA EUA approval [95]. Phase 3 data for both of the mRNA-based vaccines demonstrated about 95% efficacy [98], and they are currently being administrated in different countries globally. Currently, these two vaccines are continuing phase 4 clinical trials. Besides, CureVac, a German company, is also working on mRNA-based vaccines that recently underwent Phase 2 and initiated phase 3 trial [99,100].
Like mRNA vaccines, the viral vector-based vaccines are also in the advanced stages of clinical investigations. DNA/RNA of the spike protein is conveyed using another vector in this case to create the immunity required to fight against SARS-CoV-2. Johnson & Johnson has developed a vaccine named Ad26.COV2.S using human adenovirus to transport the mRNA for the S protein and completed the phase 3 trials with around 40,000 participants [101]. The phase 3 trial showed that the vaccine has 66.3% efficacy for preventing of symptomatic patients and the US-FDA has approved the administration of this vaccine on February 27, 2021 [102]. Besides, Gamaleya Research Institute of Epidemiology and Microbiology in Moscow developed a vaccine “Gam-COVID-Vac” which later named “Sputnik V” combining two Adenoviruses to express S protein inside the host cell and completed its phase 1 and 2 clinical trial (Trial no: NCT04436471) on August 10, 2020 [103]. The Russian authority has approved the vaccine's widespread use before completing the phase 3 clinical trial [104]. However, later, the phase 3 trial reported that it is 92% effective [105]. CanSino biologics (China) has started phase 3 clinical trials for its Ad5-nCoV vaccine with 20,000 participants (Trial no: NCT04526990), which utilizes adenovirus type-5 (Ad5) as a vaccine vector [106,107]. CanSino's Ad5-nCoV vaccine has been approved by the Chinese Military as a “specially needed drug” on June 25, 2020 and is currently one of the approved vaccines for COVID-19. The University of Oxford's Jenner Institute and the British-Swedish pharmaceutical company, AstraZeneca have already completed phase 3 trials on their ChAdOx1/AZD1222 vaccine and it has shown around 76% effective at preventing COVID-19 [[108], [109], [110], [111]]. The ChAdOx1/AZD1222 vaccine has received approval from UK's medicines regulator to be distributed in UK beginning of 2021 and currently being administrated in more than 100 countries globally [112]. Both Ad5-nCoV and ChAdOx1/AZD1222 use replication-deficient adenoviruses capable of encoding Sprotein of SARS-CoV-2, thus enabling formation of endogenous antibodies against SARS-CoV-2 [108,110]. However, recently more than 20 countries have halted the administration of this vaccine due to discrete reporting of rare blood-clotting problems in women (<55 years) [113]. WHO and the European Medicines Agency (EMA) stated that this vaccine is harmless and recommends it, though EMA couldn't rule out the possibilities. Another interesting example of an adenovirus-based vaccine formulation includes an oral pill, rather than injectables, made by Vaxart. This USA based company is currently conducting the phase 1 trial (NCT04563702). In addition, Sinovac Biotech and Wuhan Institute of Biological Products, and China National Pharmaceutical Group (Sinopharm) have started the clinical trials of their live attenuated whole virus vaccines on humans [106,114]. Inactivated vaccines utilize a whole, attenuated strain of the SARS-CoV-2 to initiate the immune response inside human body [115]. Even though the introduced viruses are attenuated, they are still capable of inducing both distinctive and adaptive immunity in humans [116]. Inovio has formulated a DNA plasmid vaccine, INO-4800, that showed excellent safety and tolerability with 100% immunogenicity [117]. No serious adverse effects in phase 1 were observed, and is currently continuing the phase 2/3 trials [118]. The Japanese biotechnology company AnGes in collaboration with Osaka University and Takara Bio, is also working on a DNA vaccine that is in the phase 2/3 stage.
A recombinant protein-based vaccine, RBD-S, consisting of a RDB of the SARS-CoV-2 S protein, is currently under in phase I/II trial by Kentucky BioProcessing [119,120]. Similarly, NVX-CoV2373, a USA-based company Novavax, has also developed a prefusion stabilized recombinant S protein-based vaccine that utilizes a proprietary nanoparticle technology for enhanced immune responses [121,122]. Other vaccines using the recombinant protein-based platform are made by ExpresS2ion, iBio, Baylor College of Medicine, University of Queensland, and Sichuan Clover Biopharmaceuticals [95].
All these vaccine platforms have their own advantages and disadvantages. For example, the genetic vaccines are relatively easy to handle, yet they can invoke aberrant immune responses and cause immunogenicity issues [95]. Similarly, the viral vector vaccines have proved to be excellent in preclinical and clinical phases for MERS and SARS-CoV; however, pre-existing vector immunity might reduce vaccine effectiveness [95]. Attenuated whole virus vaccines are a straightforward process, yet the relatively larger size of their genome can take a long time to create clones of the attenuated SARS-CoV-2 vaccine seeds [123]. Regardless of vaccine platforms, one key issue is the potential duration of immunity as the neutralizing antibody levels against SARS-CoV-2 seems to wear off relatively quickly, viz. within 2–3 months post-infection [124]. In contrast, recent reports demonstrated 70% and 100% of convalescent patients COVID-19 specific CD4+ and CD8+ T cell responses respectively, and it lasts for months if not years [125]. Collectively these studies indicate that the frequency of vaccine doses needed to confer immunity may be closely observed and/or adjusted consequently.
3. Social and economic impacts
3.1. Social impacts
The social effects created by the international transmission and pandemic due to COVID-19 is showing beyond imaginable consequences to the entire world. The total social impact is beyond comprehendible. After the health emergency declared by WHO, the world responded quickly to “flatten the curve” or limiting the virus's spread by prohibiting cross-border travels, closing non-essential businesses, closing educational institutions as well stopping all kind of large gatherings. Around 300 billion people are under full or partial lockdown currently even after more than a year since the first case was reported [126]. Since people are staying at home due to COVID-19, the first impact arises inside the home in the form of exacerbating or sparking domestic violence, including physical, phycological, and sexual violence, or some form of child abuse [[127], [128], [129]]. The longer time staying at home due to the lockdown means more prolonged exposure of abuser to the victim. A recent study summarizes the increment of domestic violence in different developed countries around the world, counting France by 32%–36%, USA by 21%–35%, 5% increase in domestic abuse calls to police in Australia, and 25% increase calls to the helpline to report abuses in UK [130].
On the other hand, more than 900 million learners are directly or indirectly affected by the closures of educational institutions from preschool to universities all over the world [131]. But, most of the developed countries avoided physical classroom activities and adopted online teaching with a campaign “School's Out, But Class's On” [132]. As a consequence of staying home for a more extended period, students are becoming more and more addicted to video games and pose the risk of setting unhealthy routine patterns [133]. Experts are concerned that this could increase childhood obesity as well as disparities in obesity risk [134]. The pandemic impacted religious activities in many ways, with canceling religious gatherings, worshiping of various faiths, religious festivities, and pilgrimages to religious sites [135,136]. The kingdom of Saudi Arabia has provisionally canceled the pilgrimage (Umrah as well as the yearly hajj) out of precaution for the first time after the Kingdom of Saudi Arabia was formed [137]. Apart from all this, the social impacts of the pandemic have an advert effect on the mental health of individuals staying under lockdown for more extended periods, which is intensified by anxiety, self-isolation, depression, and physical distancing [138]. Experts suggested that these factors could create perpetual fear and anxiety and also lead to suicidal tendencies [139].
3.2. Economic impacts
The extraordinary restrictions prompted due to the pandemic has halted multidimensional commercial/economic events throughout the year of 2020 globally. Gradually, the healthcare instruments demand is increasing parallelly with the rise of infection rate as well as fatality. Over 100 countries have shut their borders for non essential travels [140], and global air traffic plunged just after WHO's “Pandemic” announcement. This harshly obstructed the global supply chain, as well as intercontinental trade [140]. The economic experts agreed that COVID-19 will negatively impact the global economic growth, and this will possibly drive into a deep recession [141]. The Organization for Economic Cooperation and Development (OECD) has projected a monthly 2% decrease in global GDP, potentially leading us to an economic shrinkage similar to the great depression in the 1930s [140].
Millions of workers have lost/will lose their job, especially lower-income individuals or individuals working with daily wages [142]. Most of the workers related to retails and supply chain are exceptionally susceptible to COVID-19 in both health and monetarily as they are less likely to receive healthcare insurance provided by the employer [142]. In just five weeks after the pandemic, a record-breaking number of about 26 million Americans has filed for unemployment benefits, which clearly shows the impact of COVID-19 on US economy [143].
Fears of exponential widespread of the virus severely impacted the US financial market. Dow Jones, stock market measuring index for the most significant 30 companies' stock performance listed in the stock market, has started losing points just after the USA outbreak and became lowest in the recent history on March 23, 2020 [144]. Due to the lockdown and other travel restriction measures caused by the pandemic, the oil consumption demand has evaporated severely. For the first time from the US oil market's record-keeping history, the crude oil price became negative, which means the producers are paying the buyers to purchase the oil. The price of crude oil in the USA has experienced a record drop of around 300% on April 20, 2020, and oil was traded as low as -$40.32 a barrel [145].
The world tourism sector is experiencing an unexpected fall of between 20 and 30%, which is equivalent to $300–450 billion [123], and this could lead many airline companies to go bankrupt [146]. Many European countries like Portugal, Spain, and Greece, whose GDP vastly depends on tourism, will get the worst hit by COVID-19 [141]. Hotels and restaurants are mostly closed all around the world, where lockdown measures are imposed. As a result, the price for agricultural products used in restaurants dropped by almost 20% [131]. The housing and real estate sector is another sector that is walloped by the pandemic. An online real estate database company Zillow has conducted a survey regarding housing prices during past pandemics and concluded that housing prices didn't drop or decrease much in most cases. Still, there was a drop in home/real estate property sales [147]. Moreover, there are many other sectors directly linked with the economy that are severely impacted by COVID-19 lockdown and social distancing measures. Of note, the pandemic may create a financial collapse, and it can potentially create a recession. Although different countries have started immediate relief packages to save the economy, it is still very soon to judge what would be the total economic impact of COVID-19 to the entire world.
4. Future perspectives and conclusion
In addition to current approaches, more potential drugs will be investigated for their performance and effectiveness towards COVID-19 in coming years. Still, common protecting measures, especially social distancing, proper decontamination procedure and wearing masks, are proven to limit viral spread and should be executed widely. On the other hand, more than two hundred candidate vaccines are pacing the R&D to expedite the development and clinical trial process to fight against the pandemic. We also foresee availability of effective vaccines for all within the next few years as already some vaccine candidates are approved, and few are in the final stage of the trials and should be available commercially soon. Although it is very early to say as a conventional method of vaccine development to a human application takes years to decades with investigating long term effects, but this pandemic showed the progression of half a decade's work within just five months. The development of vaccines for a novel disease like this will always be a challenge as it has to gain the trust of the scientific community first. There are challenges needs to overcome after the vaccine development in the stages like delivery, distribution, and administration. For an idyllic vaccine the production cost and storage cost should be minimum. So, it is also important that after the successful and innocuous development of the vaccines, the mass level of production, as well as the distribution of vaccines globally, needs to coordinate effectively to save human life.
Moreover, one major concern for developing a vaccine is to produce effective and preventive immune response against mutated and genetically drifted variants of SARS-CoV-2 strains. Cases of reinfection of COVID-19 for some patients suggests that immune response due to previous infection may not be effective or the immune response is not sustained for longer period [148]. Considerations should be given in innovative vaccine developments and also potentiality od application of combinational vaccines (two different type vaccines) can also be explored.
Declaration of competing interest
U. Demirci is a founder of and has an equity interest in: (i) DxNow Inc., a company that is developing microfluidic IVF tools and imaging technologies for point-of-care diagnostic solutions, (ii) Koek Biotech, a company that is developing microfluidic technologies for clinical solutions, (iii) Levitas Inc., a company focusing on developing microfluidic products for sorting rare cells from liquid biopsy in cancer and other diseases, and (iv) Hillel Inc., a company bringing microfluidic cell phone tools to home settings, (v) Mercury Biosciences, a company focused on extracellular vesicles. UD's interests were viewed and managed in accordance with the conflict of interest policies. All other authors declare no financial conflict of interests. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgements
This manuscript has been supported by research support from the National Science Foundation (NSF) Rapid (2027890), and NSF CAREER (1942487).
References
- 1.Paz S., Mauer C., Ritchie A., Robishaw J.D., Caputi M. A simplified SARS-CoV-2 detection protocol for research laboratories. PloS One. 2020;15 doi: 10.1371/journal.pone.0244271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W., Du R.-H., Li B., Zheng X.-S., Yang X.-L., Hu B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microb Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen K., Yang Y., Wang T., Zhao D., Jiang Y., Jin R. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement. World J Pediatr. 2020:1–9. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L. The presence of SARS-CoV-2 RNA in feces of COVID-19 patients. J Med Virol. 2020;92(7):833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 5.Organization WH Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.2020 URL.
- 6.Organization WH . World Health Organization. Regional Office for the Eastern Mediterranean; 2020. Dengue and severe dengue. [Google Scholar]
- 7.Callaway E., Cyranoski D. China coronavirus: six questions scientists are asking. Nature. 2020;577:605. doi: 10.1038/d41586-020-00166-6. [DOI] [PubMed] [Google Scholar]
- 8.NIH . 2020. NIH clinical trial of investigational vaccine for COVID-19 begins. [Google Scholar]
- 9.Chen W.-H., Strych U., Hotez P.J., Bottazzi M.E. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. 2020:1–4. doi: 10.1007/s40475-020-00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu S. Timely development of vaccines against SARS-CoV-2. Emerg Microb Infect. 2020;9:542–544. doi: 10.1080/22221751.2020.1737580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadi J., Dunowska M., Wu S., Brightwell G. Control measures for SARS-CoV-2: a review on light-based inactivation of single-stranded RNA viruses. Pathogens. 2020;9:737. doi: 10.3390/pathogens9090737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2020;1866:165878. doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bar-On Y.M., Flamholz A., Phillips R., Milo R. Science forum: SARS-CoV-2 (COVID-19) by the numbers. Elife. 2020;9 doi: 10.7554/eLife.57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 18.Howard J., Huang A., Li Z., Tufekci Z., Zdimal V., van der Westhuizen H.-M. An evidence review of face masks against COVID-19. Proc Natl Acad Sci Unit States Am. 2021:118. doi: 10.1073/pnas.2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West R., Michie S., Rubin G.J., Amlôt R. Applying principles of behaviour change to reduce SARS-CoV-2 transmission. Nat Human Behav. 2020;4:451–459. doi: 10.1038/s41562-020-0887-9. [DOI] [PubMed] [Google Scholar]
- 20.Coleman B., Coarsey C., Kabir M.A., Asghar W. Point-of-care colorimetric analysis through smartphone video. Sensor Actuator B Chem. 2019;282:225–231. doi: 10.1016/j.snb.2018.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrada C.A., Kabir M., Altamirano R., Asghar W. Advances in diagnostic methods for zika virus infection. J Med Dev Trans ASME. 2018;12 doi: 10.1115/1.4041086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabir M.A., Zilouchian H., Caputi M., Asghar W. Advances in HIV diagnosis and monitoring. Crit Rev Biotechnol. 2020;40:623–638. doi: 10.1080/07388551.2020.1751058. [DOI] [PubMed] [Google Scholar]
- 23.Kabir M.A., Soto-Acosta R., Sharma S., Bradrick S.S., Garcia-Blanco M.A., Caputi M. An antibody panel for highly specific detection and differentiation of Zika virus. Sci Rep. 2020;10:1–8. doi: 10.1038/s41598-020-68635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabir M.D.A., Ahmed R., Iqbal S.M.A., Chowdhury R., Paulmurugan R., Demirci U. Diagnosis for COVID-19: current status and future prospects. Expert Rev Mol Diagn. 2021:1–20. doi: 10.1080/14737159.2021.1894930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coarsey C., Coleman B., Kabir M.A., Sher M., Asghar W. Development of a flow-free magnetic actuation platform for an automated microfluidic ELISA. RSC Adv. 2019;9:8159–8168. doi: 10.1039/c8ra07607c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabir M.A., Zilouchian H., Sher M., Asghar W. Development of a flow-free automated colorimetric detection assay integrated with smartphone for Zika NS1. Diagnostics. 2020;10:42. doi: 10.3390/diagnostics10010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S., Kabir M.A., Asghar W. Lab-on-a-Chip zika detection with reverse transcription loop-mediated isothermal amplification–based assay for point-of-care settings. Arch Pathol Lab Med. 2020;144:1335–1343. doi: 10.5858/arpa.2019-0667-OA. [DOI] [PubMed] [Google Scholar]
- 28.Sher M., Coleman B., Caputi M., Asghar W. Development of a point-of-care assay for HIV-1 viral load using higher refractive index antibody-coated microbeads. Sensors. 2021;21:1819. doi: 10.3390/s21051819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen M.S., Corey L. American Association for the Advancement of Science; 2020. Combination prevention for COVID-19. [DOI] [PubMed] [Google Scholar]
- 30.Mitze T., Kosfeld R., Rode J. 2020. Face masks considerably reduce Covid-19 cases in Germany-A synthetic control method approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peeples L. Face masks: what the data say. Nature. 2020;586:186–189. doi: 10.1038/d41586-020-02801-8. [DOI] [PubMed] [Google Scholar]
- 32.Fischer E.P., Fischer M.C., Grass D., Henrion I., Warren W.S., Westman E. Low-cost measurement of face mask efficacy for filtering expelled droplets during speech. Sci Adv. 2020;6 doi: 10.1126/sciadv.abd3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindsley W.G., Noti J.D., Blachere F.M., Szalajda J.V., Beezhold D.H. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg. 2014;11:509–518. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takagi G., Yagishita K. Principles of disinfectant use and safety operation in medical facilities during coronavirus disease 2019 (COVID-19) outbreak. SN Compr Clin Med. 2020;2:1041–1044. doi: 10.1007/s42399-020-00413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daon Y., Thompson R., Obolski U. 2020. Estimating COVID-19 outbreak risk through air travel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong J., Chaw L., Koh W.C., Alikhan M.F., Jamaludin S.A., Poh W.W.P. Epidemiological investigation of the first 135 COVID-19 cases in Brunei: implications for surveillance, control, and travel restrictions. Am J Trop Med Hyg. 2020;103:1608–1613. doi: 10.4269/ajtmh.20-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon D., Jang G., Bouhaddou M., Xu J., Obernier K., O'Meara M. A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug repurposing. bioRxiv. 2020 doi: 10.1101/2020.03.22.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livescienc.com . 2020. Treatments for COVID-19: drugs being tested against the coronavirus. [Google Scholar]
- 43.Yamamoto M., Matsuyama S., Li X., Takeda M., Kawaguchi Y., Inoue J-i. Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. 2016;60:6532–6539. doi: 10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu H., Du W., Song M., Liu Q., Herrmann A., Huang Q. Spontaneous binding of potential COVID-19 drugs (Camostat and Nafamostat) to human serine protease TMPRSS2. Comput Struct Biotechnol J. 2021;19:467–476. doi: 10.1016/j.csbj.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. 2020. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrobial Agents and Chemotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfizer.com . 2021. Pfizer initiates phase 1 study OF novel oral antiviral therapeutic agent against SARS-COV-2. [Google Scholar]
- 48.ClinicalTrials.gov ULoM . 2021. Study OF PF-07321332 IN healthy participants. [Google Scholar]
- 49.ClinicalTrials.gov ULoM . 2021. First-in-human study to evaluate safety, tolerability, and pharmacokinetics following single ascending and multiple ascending doses of PF-07304814 in hospitalized participants with COVID-19. [Google Scholar]
- 50.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ledford H. Hopes rise for coronavirus drug remdesivir. Nature. 2020 doi: 10.1038/d41586-020-01295-8. [DOI] [PubMed] [Google Scholar]
- 53.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020:12. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cox R.M., Wolf J.D., Plemper R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2020:1–8. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad B. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.03.17.20037432. [DOI] [Google Scholar]
- 57.Hashemian S.M., Farhadi T., Velayati A.A. 2020. A review on favipiravir: the properties, function, and usefulness to treat COVID-19. Expert review of anti-infective therapy; pp. 1–9. [DOI] [PubMed] [Google Scholar]
- 58.Thng Z.X., De Smet M.D., Lee C.S., Gupta V., Smith J.R., McCluskey P.J. COVID-19 and immunosuppression: a review of current clinical experiences and implications for ophthalmology patients taking immunosuppressive drugs. Br J Ophthalmol. 2020;105(3):306–310. doi: 10.1136/bjophthalmol-2020-316586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., Arabi Y.M. Interleukin-6 receptor antagonists in critically ill patients with covid-19–preliminary report. medRxiv. 2021 doi: 10.1101/2021.01.07.21249390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L. Dexamethasone in hospitalized patients with covid-19-preliminary report. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 62.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N Engl J Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaishya R., Javaid M., Khan I.H., Haleem A. Artificial Intelligence (AI) applications for COVID-19 pandemic. Diabetes Metab Syndrome: Clin Res Rev. 2020;14(4):337–339. doi: 10.1016/j.dsx.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heidary F., Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot. 2020:1–10. doi: 10.1038/s41429-020-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slifka M.K., Amanna I.J. Plotkin's Vaccines; 2018. Passive immunization; p. 84. [Google Scholar]
- 68.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baxter D. Active and passive immunity, vaccine types, excipients and licensing. Occup Med. 2007;57:552–556. doi: 10.1093/occmed/kqm110. [DOI] [PubMed] [Google Scholar]
- 70.Stiehm E.R., Keller M.A. 7 - passive immunization. In: Plotkin S.A., Orenstein W.A., Offit P.A., editors. Vaccines. 6th ed. W.B. Saunders; London: 2013. pp. 80–87. [Google Scholar]
- 71.Soo Y.O., Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect : Offl Publ Eur Soc Clin Microbiol Infect Dis. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890–1901. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Kor Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jaffe-Hoffman M. The Jerusalem Post; 2020. 29-year-old COVID-19 patient treated with Israel's new ‘passive vaccine’. [Google Scholar]
- 76.Tanne J.H. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;368:m1256. doi: 10.1136/bmj.m1256. [DOI] [PubMed] [Google Scholar]
- 77.Malani A.N., Sherbeck J.P., Malani P.N. Convalescent plasma and COVID-19. J Am Med Assoc. 2020 Aug 4;324(5):524. doi: 10.1001/jama.2020.10699. [DOI] [PubMed] [Google Scholar]
- 78.Hartmann J., Klein H.G. Supply and demand for plasma-derived medicinal products-A critical reassessment amid the COVID-19 pandemic. Transfusion. 2020;60:2748–2752. doi: 10.1111/trf.16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun Y., Ho M. Emerging antibody-based therapeutics against SARS-CoV-2 during the global pandemic. Antib Therapeut. 2020;3:246–256. doi: 10.1093/abt/tbaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.News CaE . 2020. Lilly begins first clinical trial of antibody that targets SARS-CoV-2. [Google Scholar]
- 81.Tuccori M., Ferraro S., Convertino I., Cappello E., Valdiserra G., Blandizzi C. Taylor & Francis; Mabs: 2020. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline; p. 1854149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang Y., Sun H., Yu H., Li S., Zheng Q., Xia N. Antibody Therapeutics; 2020. Neutralizing antibodies against SARS-CoV-2: current understanding, challenge and perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N Engl J Med. 2020;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Therapeutics S. Neutralizing Antibody Cocktail); 2020. COVID-19 treatment: COVI-SHIELD. [Google Scholar]
- 87.Pinto D., Park Y.-J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020:1–6. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 88.Marovich M., Mascola J.R., Cohen M.S. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA. 2020;324:131–132. doi: 10.1001/jama.2020.10245. [DOI] [PubMed] [Google Scholar]
- 89.van Dorp L., Acman M., Richard D., Shaw L.P., Ford C.E., Ormond L. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect Genet Evol. 2020:104351. doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy K., Weaver C. Garland science; 2016. Janeway's immunobiology. [Google Scholar]
- 91.Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580:576. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- 92.Organization W.H. World; 2020. DRAFT landscape of COVID-19 candidate vaccines. [Google Scholar]
- 93.Le T.T., Andreadakis Z., Kumar A., Roman R.G., Tollefsen S., Saville M. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y., Wang K., Massoud T.F., Paulmurugan R. SARS-CoV-2 vaccine development: an overview and perspectives. ACS Pharmacol Transl Sci. 2020;3:844–858. doi: 10.1021/acsptsci.0c00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M. Concurrent human antibody and TH1 type T-cell responses elicited by a COVID-19 RNA vaccine. medRxiv. 2020 doi: 10.1101/2020.07.17.20140533. [DOI] [Google Scholar]
- 97.Oliver S.E., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M. The advisory committee on immunization practices' interim recommendation for use of pfizer-BioNTech COVID-19 vaccine—United States, december 2020. MMWR (Morb Mortal Wkly Rep) 2020;69:1922. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gostin L.O., Salmon D.A., Larson H.J. Mandating COVID-19 vaccines. J Am Med Assoc. 2020;325(6):532–533. doi: 10.1001/jama.2020.26553. [DOI] [PubMed] [Google Scholar]
- 99.Dolgin E. COVID-19 vaccines poised for launch, but impact on pandemic unclear. Nat Biotechnol. 2020 doi: 10.1038/d41587-020-00022-y. [DOI] [PubMed] [Google Scholar]
- 100.BioSpace . 2020. CureVac begins phase IIb/III trial of its mRNA COVID-19 vaccine. [Google Scholar]
- 101.Forni G., Mantovani A. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28:626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oliver S.E., Gargano J.W., Scobie H., Wallace M., Hadler S.C., Leung J. The advisory committee on immunization practices' interim recommendation for use of Janssen COVID-19 vaccine—United States. MMWR (Morb Mortal Wkly Rep) 2021;70:329. doi: 10.15585/mmwr.mm7009e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.ClinicalTrials.gov ULoM . 2020. An open study of the safety, tolerability and immunogenicity of the drug "Gam-COVID-Vac" vaccine against COVID-19. [Google Scholar]
- 104.news N. Russia's fast-track coronavirus vaccine draws outrage over safety. In: Callaway E., editor. 2020. [DOI] [PubMed] [Google Scholar]
- 105.Callaway E. Russia announces positive COVID-vaccine results from controversial trial. Nature. 2020 doi: 10.1038/d41586-020-03209-0. [DOI] [PubMed] [Google Scholar]
- 106.Akst J. COVID-19 vaccine frontrunners. Scientist. April 2020;7 [Google Scholar]
- 107.Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alharbi N.K., Padron-Regalado E., Thompson C.P., Kupke A., Wells D., Sloan M.A. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine. 2017;35:3780–3788. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;10249:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahase E. British Medical Journal Publishing Group; 2020. Covid-19: UK approves Oxford vaccine as cases of new variant surge. [DOI] [PubMed] [Google Scholar]
- 111.Callaway E., Mallapaty S. Latest results put Oxford-AstraZeneca COVID vaccine back on track. Nature. 2021 doi: 10.1038/d41586-021-00836-z. [DOI] [PubMed] [Google Scholar]
- 112.Rimmer A. British Medical Journal Publishing Group; 2020. Covid-19: doctors must be vaccinated without delay, says BMA. [DOI] [PubMed] [Google Scholar]
- 113.Mallapaty S., Callaway E. What scientists do and don't know about the Oxford-AstraZeneca COVID vaccine. Nature. 2021;592:15–17. doi: 10.1038/d41586-021-00785-7. [DOI] [PubMed] [Google Scholar]
- 114.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pearson H. Nature Publishing Group; 2003. SARS vaccines speed towards clinic. [Google Scholar]
- 116.PublicHealth.org . 2020. HOW vaccines work. [Google Scholar]
- 117.Tebas P., Yang S., Boyer J.D., Reuschel E.L., Patel A., Christensen-Quick A. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. E Clin Med. 2020:100689. doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith T.R., Patel A., Ramos S., Elwood D., Zhu X., Yan J. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11:1–13. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang S., Bottazzi M.E., Du L., Lustigman S., Tseng C.-T.K., Curti E. Roadmap to developing a recombinant coronavirus S protein receptor-binding domain vaccine for severe acute respiratory syndrome. Expet Rev Vaccine. 2012;11:1405–1413. doi: 10.1586/erv.12.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020:1–10. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 121.Negahdaripour M. The battle against COVID-19: where do we stand now? Iran J Med Sci. 2020;45:81. doi: 10.30476/ijms.2020.46357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tian J.-H., Patel N., Haupt R., Zhou H., Weston S., Hammond H. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 elicits immunogenicity in baboons and protection in mice. bioRxiv. 2020 doi: 10.1101/2020.06.29.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pololikashvili Z. International Trade Centre; 2020. Tourism and the sustainable development agenda: seizing opportunity in crisis. International trade forum; pp. 16–17. [Google Scholar]
- 124.Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020:1–5. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 125.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Insider B. 2020. A third of the global population is on coronavirus lockdown. [Google Scholar]
- 127.van Gelder N., Peterman A., Potts A., O'Donnell M., Thompson K., Shah N. COVID-19: reducing the risk of infection might increase the risk of intimate partner violence. E Clin Med. 2020;21 doi: 10.1016/j.eclinm.2020.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peterman A., Potts A., O’Donnell M., Thompson K., Shah N., Oertelt-Prigione S. Center for Global Development; Washington, DC: 2020 Apr 1. Pandemics and violence against women and children. [Google Scholar]
- 129.Saladino V., Algeri D., Auriemma V. The psychological and social impact of Covid-19: new perspectives of well-being. Front Psychol. 2020;11:2550. doi: 10.3389/fpsyg.2020.577684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Usher K., Bhullar N., Durkin J., Gyamfi N., Jackson D. Family violence and COVID-19: increased vulnerability and reduced options for support. Int J Ment Health Nurs. 2020:549–552. doi: 10.1111/inm.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C. The socio-economic implications of the coronavirus and COVID-19 pandemic: a review. Int J Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou L., Wu S., Zhou M., Li F. 2020. 'School's out, but Class' on', the largest online education in the world today: taking China's practical exploration during the COVID-19 epidemic prevention and control as an example. But Class' on', the largest online education in the world today: taking China's practical exploration during the COVID-19 epidemic prevention and control as an example. (March 15, 2020) [Google Scholar]
- 133.King D., Delfabbro P., Billieux J., Potenza M. Problematic online gaming and the COVID-19 pandemic. J Behav Addict. 2020;9(2):184–186. doi: 10.1556/2006.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rundle A.G., Park Y., Herbstman J.B., Kinsey E.W., Wang Y.C. COVID-19 related school closings and risk of weight gain among children. Obesity. 2020;28(6):1008–1009. doi: 10.1002/oby.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahmed Q.A., Memish Z.A. The cancellation of mass gatherings (MGs)? Decision making in the time of COVID-19. Trav Med Infect Dis. 2020:101631. doi: 10.1016/j.tmaid.2020.101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rodriguez-Morales A.J., Sah R., Paniz-Mondolfi A. 2020. Should the holy week 2020 be cancelled in Latin America due to the COVID-19 pandemic? Travel medicine and infectious disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ali I., Alharbi O.M. COVID-19: disease, management, treatment, and social impact. Sci Total Environ. 2020:138861. doi: 10.1016/j.scitotenv.2020.138861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yao H., Chen J.-H., Xu Y.-F. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatr. 2020;7:e21. doi: 10.1016/S2215-0366(20)30090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gunnell D., Appleby L., Arensman E., Hawton K., John A., Kapur N. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatr. 2020;7(6):468–471. doi: 10.1016/S2215-0366(20)30171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mishra M.K. 2020. The world after COVID-19 and its impact on global economy. [Google Scholar]
- 141.Fernandes N. 2020. Economic effects of coronavirus outbreak (COVID-19) on the world economy. Available at: SSRN 3557504. [Google Scholar]
- 142.brookings.com . 2020. COVID-19 puts America's low-wage workforce in an even worse position. [Google Scholar]
- 143.today U . 2020. Record 26 million Americans filed for jobless benefits over 5 weeks as layoffs wind on. [Google Scholar]
- 144.finance Y . 2020. Dow jones industrial average. [Google Scholar]
- 145.TImes F . 2020. US oil price below zero for first time in history. [Google Scholar]
- 146.Insider B . 2020. Many of the world's airlines could be bankrupt by May because of the COVID-19 crisis. [Google Scholar]
- 147.Research Z . 2020. Information from past pandemics, and what we can learn: a literature review. [Google Scholar]
- 148.Koyama T., Weeraratne D., Snowdon J.L., Parida L. Emergence of drift variants that may affect COVID-19 vaccine development and antibody treatment. Pathogens. 2020;9:324. doi: 10.3390/pathogens9050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Biorender.com . 2020. COVID-19 vaccine & therapeutics tracker. [Google Scholar]
- 150.NYTimes . JCaS-LW carl zimmer. NYTIMES; 2021. Coronavirus vaccine tracker. [Google Scholar]