Abstract
Purpose
To investigate the adherence rate of neovascular age-related macular degeneration (nAMD) patients in treat-and-extend (TAE) protocol to their anti-vascular endothelial growth factor (anti-VEGF) intravitreal injection (IVI) appointments and to evaluate the functional and anatomical outcomes of the patients who attended and did not attend their IVI appointments during the coronavirus disease 2019 (COVID-19) restriction period (RP).
Methods
The patients with nAMD having IVI appointments between March 16 and June 1, 2020 (RP in Turkey) were included in this retrospective study. For adherence analysis, the patients who attended (Group 1, n = 44) and who did not attend (Group 2, n = 60) their IVI appointment visits during the RP (VRP) were evaluated according to their last visit before the RP (V0). For outcome analysis, the patients who attend VRP and have follow-up (Group 1a, 46 eyes) and who did not attend VRP but later attended for follow-up (Group 2a, 33 eyes) were evaluated for functional (best-corrected visual acuity, BCVA [logMAR]) and anatomical (optical coherence tomography [OCT] disease activity) outcomes at the first visit after RP (V1) and last visit within six months after RP (V2). Patients received a complete ophthalmologic evaluation with anti-VEGF (Aflibercept) IVI administration at all visits.
Results
The adherence rate of the patients to VRP was 42.3% (44/104). The patients in Group 1 were significantly younger (mean ± SD years, 71.0 ± 8.1 vs. 74.7 ± 8.0, p = 0.024), had better median [IQR] BCVA at their first presentation (0.30 [0.54] vs. 0.61 [1.08], p = 0.023) and V0 (0.40 [0.48] vs. 0.52 [0.70], p = 0.031), and had less hypertension (36.4% vs. 58.3%, p = 0.044) than Group 2. The mean ± SD delay of planned IVI at VRP in Group 2a was 13.9 ± 6.2 weeks. Disease activity in OCT was significantly higher in Group 2a than Group 1a at V1 (60.6% vs. 32.6%, p = 0.025). In Group 2a, the median (IQR) BCVA was significantly worse at V1 (0.70 [0.58]) and V2 (0.70 [0.59]) than V0 (0.52 [0.40], p = 0.047 and p = 0.035, respectively).
Conclusions
More than half of the scheduled nAMD patients in TAE protocol missed their IVI visits during the RP, which resulted in a delay of their treatments. The delay of IVI treatment in those patients resulted in an increase in OCT disease activity and a decrease in BCVA.
Keywords: Coronavirus disease-2019, COVID-19, Neovascular age-related macular degeneration, nAMD, Treat-and-extend protocol
Introduction
Macular neovascularization (MNV) secondary to neovascular age-related macular degeneration (nAMD) is the leading cause of progressive central vision loss among elderly patients [1, 2]. Although the treatment of nAMD with intravitreal injections (IVIs) of anti-vascular endothelial growth factor (anti-VEGF) proved to be effective in decreasing progression and improving vision, nAMD remains the third leading cause of severe irreversible vision loss worldwide [3, 4].
There are different anti-VEGF IVI protocols in the management of nAMD, including application in regular (monthly and bi-monthly) intervals or irregular (“pro-re-nata” [PRN, as needed] and “treat-and-extend” [TAE]) intervals after three consecutive monthly loading doses [5–8]. While the regular fixed-interval protocols adopt a strategy of applying IVI regardless of whether any sign of disease activity present, in the PRN treatment protocol, the IVI decision is made according to any sign of disease activity in monthly visits [5–7]. However, TAE is an individualized, proactive dosing regimen, in which, after three consecutive loading IVI doses, in-person visits and at-the-same-day IVI treatment intervals are extended (1 week to 4 weeks, to a maximum of 12 weeks) or shortened (1 week to 4 weeks, to a minimum of 4 weeks) according to predefined disease activity criteria [8, 9].
After being detected in Wuhan, China, in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes Coronavirus Disease 2019 (COVID-19), spread rapidly and was defined as a pandemic by the World Health Organization on March 11, 2020 [10, 11]. In healthcare practices, priority has focused on patients affected by the virus, and there have been substantial changes in specialties that are not directly related to COVID-19 [12, 13]. The American Academy of Ophthalmology (AAO) recommended that ophthalmologists should stop providing any treatment to patients other than emergency cases, and various organizations such as AAO, The Royal College of Ophthalmologists, and the Turkish Ophthalmological Association have tried to identify procedures that can be considered urgent and necessary during the COVID-19 pandemic period [14–16]. According to the American Society of Retina Specialists COVID-19 assessment dated March 20, 2020, a significant percentage of retina patients are at risk of permanent vision loss and should receive regular IVI treatment. Therefore, during the pandemic period, they are in a unique situation [17]. Even a group of experts in retinal diseases developed collective recommendations for managing patients who are receiving IVI during the pandemic [18].
After the first case of a positive test for the virus in Turkey on March 11, 2020, the Ministry of Interior enacted many restrictions starting from March 16 to June 1, 2020, (namely, the restriction period) to reduce the risks posed by the rapid spread of the virus [19]. Our hospital was designated as a pandemic referral hospital as of March 16, 2020, our clinic’s appointments were reduced, routine examinations were postponed, and elective surgeries were ceased. However, our retina department decided to continue the IVIs on the condition that the recommendations of our hospital’s infection control committee (ICC) are followed. The patients whose IVI appointments have been scheduled were not postponed, and the IVIs of the patients who attended their visits were administered.
The purpose of this study is to determine the adherence rate of nAMD patients in the TAE protocol to their IVI appointments and evaluate the functional and anatomical results of the patients who both attended and did not attend their IVI appointments during the restriction period.
Methods
The study protocol was approved by the Institutional Review Board of Marmara University School of Medicine Hospital (No: 09.2020.1318). The study was performed in accordance with the Declaration of Helsinki principles, and written informed consent to use their medical information in the study analysis was routinely provided by all of the patients at their first presentation to our clinic.
Study participants
This retrospective study included nAMD patients in the TAE protocol scheduled for IVI (2 mg/0.05 mL aflibercept in all patients) during the restriction period (March 16 to June 1, 2020) at Marmara University Pendik Education and Research Hospital in Pendik, Istanbul. Patients in the loading phase of the TAE protocol, patients newly diagnosed with nAMD and scheduled for IVI during the restriction period, and patients with any maculopathy or MNV other than nAMD were excluded from the study.
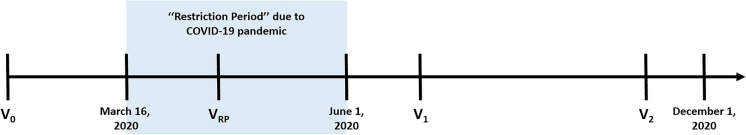
At all visits (Fig. 1), patients received a complete ophthalmologic examination, including the measurement of best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, dilated fundus examination, and optical coherence tomography (OCT) with the Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany). At all visits, 2 mg/0.05 mL aflibercept IVI was also administered in accordance with the TAE protocol. The patients’ next treatment interval was reduced (to a minimum of 4 weeks) or extended (to a maximum of 12 weeks) by 2 weeks according to the disease activity as a part of our TAE protocol. BCVA was assessed with an electronic Snellen chart, and the result was converted to the logarithm of the minimum angle of resolution (logMAR) [20]. The logMAR equivalent values for “counting fingers” and “hand motion” were assumed to be 1.85 and 2.30, respectively, based on the Freiburg Visual Acuity Test [21].
Fig. 1.
Graph showing the timeline and visits included in the study. V0 the last visit before the restriction period where the IVI at VRP was scheduled, VRP the visit of scheduled IVI in the restriction period, V1 the first visit after the restriction period ends, V2 the last follow-up visit within six months after the restriction period ends
Adherence and outcome analysis
The factors associated with the adherence of the patients to their visit appointments during the restriction period (VRP) were assessed by comparing the characteristics of the patients who have attended their appointments (Group 1) and those who did not attend their appointments (Group 2) (Fig. 2). Because the one who came to the VRP for one eye also came for the fellow eye (vice versa) in our study population, the appointments of the patients’ first eyes were considered as the study eyes for adherence analysis. The assessed characteristics are listed as follows: demographics (age and gender), disease-related features (follow-up time, previous IVI count, BCVA of the study eye and fellow eye at their first presentation and V0 [the last visit before the restriction period, where the IVI at VR was scheduled], and planned extension period at V0) and accompanying disorders (diabetes mellitus [DM], hypertension [HT], and coronary artery disease [CAD]).
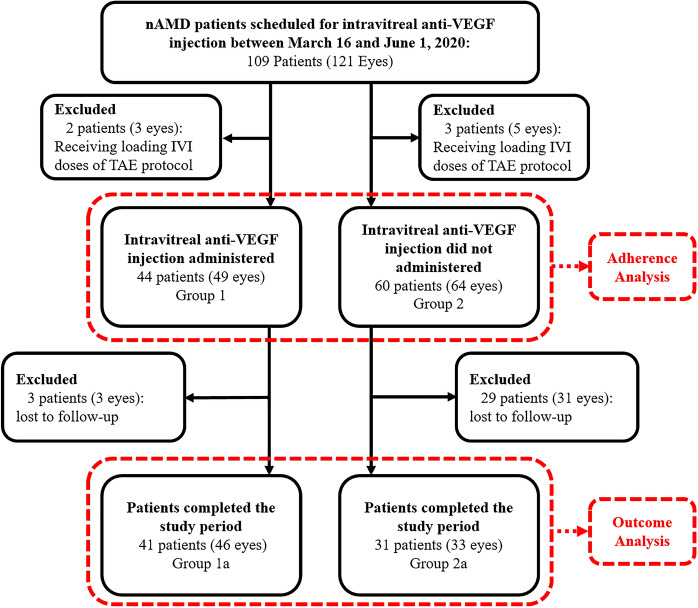
Fig. 2.
Flowchart of the study analysis anti-VEGF anti-vascular endothelial growth factor, IVI intravitreal injection, nAMD neovascular age-related macular degeneration, TAE treat-and-extend
The patients who have completed at least two follow-up visits within six months after the restriction period ended (December 1, 2020) were included in the outcome analysis according to whether they attended the VRP (Group 1a) or did not attend the VRP (Group 2a) (Fig. 2). The BCVA was compared between the groups at visits V0, V1 (first visit after the restriction period ends), and V2 (last follow-up visit within six months after the restriction period ends) as the functional outcome. The OCT images were qualitatively graded as active or inactive according to the findings suggestive of disease activity (subretinal fluid, intraretinal fluid, and subretinal hyperreflective material) at the same visits and compared between the groups as the anatomical outcome. Within-group comparisons were also made for functional and anatomical outcomes.
Statistical analysis
SPSS for Windows version 22.0 (IBM Corp., Armonk, NY, USA) was employed for statistical analysis of the data. The distribution of the data was determined by histogram graphs and the Shapiro–Wilk test. The data with normal distribution were presented as mean ± standard deviation (SD), and the data that did not have a normal distribution were given as the median (interquartile range [IQR]). Qualitative variables were assessed by the Pearson Chi-square test or Fisher’s exact test. Related samples were compared by the Wilcoxon signed-rank test or paired t test, and independent samples were compared by the Mann–Whitney U test or independent-samples t test, depending on the distribution of the data. The factors associated with patient adherence to their visits during the restriction period were evaluated with binary logistic regression analysis. A p value of less than 0.05 was considered statistically significant.
Results
During the restriction period, there were 109 nAMD patients (121 eyes) scheduled for IVI. Two patients (three eyes) who attended and three patients (five eyes) who did not attend the VRP were excluded from the study analysis due to receiving one of the three loading doses of IVI therapy in the TAE protocol.
Adherence rate of the patients to VRP
After excluding the patients receiving loading IVI doses, 104 patients were included in the adherence analysis (Fig. 2). Among them, 9 of the patients were scheduled for both eyes with different appointments during the restriction period. Considering that the patient who came to the VRP for one eye also came for the fellow eye (vice versa), the appointments of the patients’ first eyes were considered as study eyes.
Forty-four of the 104 patients (42.3%) attended the VRP (Group 1), while 60 of them (57.7%) did not (Group 2), resulting in an adherence rate of 42.3%. Regarding the factors that might affect the patients’ adherence to their appointment in VRP, the patients in Group 1 were significantly younger than those in Group 2 (mean ± SD [range] years, 71.0 ± 8.1 [50–85] vs. 74.7 ± 8.0 [58–93], p = 0.024). The BCVA of the patients’ study eye in Group 1 at their first presentation and V0 were also significantly better than that in Group 2 (median [IQR] logMAR, 0.30 [0.54] vs. 0.61 [1.08], p = 0.023, and 0.40 [0.48] vs. 0.52 [0.70], p = 0.031, respectively). The patients in Group 1 had significantly less hypertension than those in Group 2 (36.4% vs. 58.3%, p = 0.044). There was no significant difference among gender, follow-up time, BCVA of the fellow eye at first presentation and V0, IVI count before V0, planned extension period at V0, or accompanying disorders other than HT (any, DM, and CAD) (Table 1). A binomial logistic regression analysis was performed to ascertain the effects of age, BCVA of the study eye at presentation and V0, and accompanying HT on the likelihood to attend the VRP. The logistic regression model was statistically significant X2(4) = 15.955, p = 0.003 with a Nagelkerke R2 of 0.191. Of the four predictor variables, only the accompanying HT was statistically significant with an odds ratio of 2.50 (95% CI 1.06–5.84, p = 0.035).
Table 1.
Characteristics of the patients in Group 1 and Group 2
| Group 1 (n = 44) |
Group 2 (n = 60) |
p | |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 71.0 ± 8.1 | 74.7 ± 8.0 | 0.024a |
| Gender, n (%) | 0.853b | ||
| Female | 20 (45.5) | 25 (41.7) | |
| Male | 24 (54.5) | 35 (58.3) | |
| Follow-up time, months | |||
| Median (IQR) | 30.50 (47.25) | 27.04 (41.00) | 0.927c |
| BCVA at presentation, logMAR | |||
| Median (IQR) | |||
| Study eye | 0.30 (0.54) | 0.61 (1.08) | 0.023c |
| Fellow-eye | 0.61 (1.75) | 0.40 (1.25) | 0.483c |
| BCVA at V0, logMAR | |||
| Median (IQR) | |||
| Study eye | 0.40 (0.48) | 0.52 (0.70) | 0.031c |
| Fellow-eye | 0.61 (1.36) | 0.52 (1.25) | 0.597c |
| IVI count before V0, n | |||
| Median (IQR) | 10.0 (8.75) | 10.0 (9.00) | 0.974c |
| Planned extension period at V0, weeks | |||
| Median (IQR) | 8.57 (5.96) | 10.0 (5.96) | 0.604c |
| Any accompanying disorders, n (%) | 0.095b | ||
| Present | 19 (43.2) | 37 (61.7) | |
| Absent | 25 (56.8) | 23 (38.3) | |
| Diabetes mellitus, n (%) | 0.971b | ||
| Present | 10 (22.7) | 15 (25.0) | |
| Absent | 34 (77.3) | 45 (75.0) | |
| Hypertension, n (%) | 0.044b | ||
| Present | 16 (36.4) | 35 (58.3) | |
| Absent | 28 (63.6) | 25 (41.7) | |
| Coronary artery disease, n (%) | 0.696d | ||
| Present | 3 (6.8) | 3 (5.0) | |
| Absent | 41 (93.2) | 57 (95.0) |
Statistical significance is highlighted in bold
BCVA best-corrected visual acuity, IVI intravitreal injection, IQR interquartile range, logMAR logarithm of the minimum angle of resolution, SD standard deviation, V0 the last visit before the restriction period where the IVI at restriction period scheduled
aIndependent-samples t test
bPearson Chi-square test with continuity correction
c Mann–Whitney U test
d Fisher’s exact test
Functional and anatomical outcomes of the patients
Within six months after the end of the restriction period, 31 patients (33 eyes) from Group 2 (51.6%) re-attended our clinic (Group 2a), whereas three patients (three eyes) from Group 1 lost to follow-up, leaving 41 patients (46 eyes) in Group 1a (95.3%) (Fig. 2). Thus, the functional and anatomical outcome analysis included a total of 72 patients with 79 eyes.
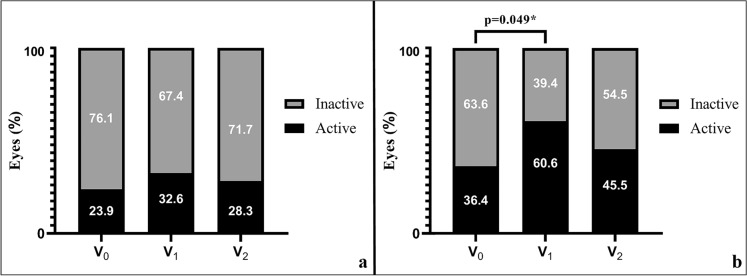
The age, gender, follow-up time, and planned extension period at V0, as well as the BCVA at presentation, V0, V1, and V2, were not significantly different between Group 1a and Group 2a (Table 2). The median (IQR) [range] IVI count administered within V1 and V2 was 2.00 (1.25) [1–5] and 2.00 (2.00) [1–5] in Group 1a and Group 2a, respectively (p = 0.856). The mean ± SD (min–max) delay of the planned IVI at VRP in Group 2a (time difference between V1 and VRP) was 97.9 ± 44.0 (28.0–197) days (13.9 ± 6.2 [4.0–28.1] weeks). Although the disease activity ratios in OCT were not significantly different between Group 1a and Group 2a at V0 and V2, the percentage of active disease was significantly higher in Group 2a than in Group 1a at V1 (60.6% vs. 32.6%, p = 0.025). The within-group comparisons of OCT disease activity yielded statistical significance only in Group 2a between V0 and V1 (Fig. 3).
Table 2.
Characteristics of the patients in Group 1a and Group 2a
| Group 1a 41 patients (46 eyes) |
Group 2a 31 patients (33 eyes) |
p | |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 70.7 ± 7.8 | 73.5 ± 7.7 | 0.127a |
| Gender, n (%) | 0.921b | ||
| Female | 19 (46.3) | 14 (45.2) | |
| Male | 22 (53.7) | 17 (54.8) | |
| Follow-up time, months | |||
| Median (IQR) | 32.00 (49.50) | 41.00 (53.00) | 0.223c |
| Planned extension period at V0, weeks | |||
| Median (IQR) | 8.64 (4.75) | 10.0 (6.00) | 0.940c |
| BCVA, logMAR | |||
| Median (IQR) | |||
| At presentation | 0.30 (0.56) | 0.30 (0.70) | 0.664c |
| At V0 | 0.45 (0.48) | 0.52 (0.40) | 0.719c |
| At V1 | 0.40 (0.58) | 0.70 (0.58) | 0.555c |
| At V2 | 0.40 (0.52) | 0.70 (0.59) | 0.216c |
| OCT, active/inactive | |||
| n (%) | |||
| At V0 | 11 (23.9)/35 (76.1) | 12 (36.4)/21 (63.6) | 0.342b |
| At V1 | 15 (32.6)/31 (67.4) | 20 (60.6)/13 (39.4) | 0.025b |
| At V2 | 13 (28.3)/33 (71.7) | 15 (45.5)/18 (54.5) | 0.181b |
| IVI count, n | |||
| Median (IQR) | |||
| Before V0 | 10.00 (9.50) | 13.00 (10.00) | 0.288c |
| Between V0 and V1 | 1.00 (0.25) | 0.00 (0.00) | < 0.001c |
| Between V1 and V2 | 2.00 (1.25) | 2.00 (2.00) | 0.856c |
| Between V0 and V2 | 3.00 (1.25) | 2.00 (2.00) | 0.001c |
Statistical significance is highlighted in bold
BCVA best-corrected visual acuity, IVI intravitreal injection, IQR interquartile range, logMAR logarithm of the minimum angle of resolution, OCT optical coherence tomography, SD standard deviation, V0 the last visit before the restriction period where the IVI at restriction period scheduled, V1 the first visit after the restriction period ends, V2 the last follow-up visit within six months after the restriction period ends
aIndependent-samples t test
bPearson Chi-square test with continuity correction
cMann–Whitney U test
Fig. 3.
Stacked bar graph showing OCT disease activity in Group 1a (a) and Group 2a (b). V0 the last visit before the restriction period where the IVI at restriction period scheduled, V1 the first visit after the restriction period ends, V2 the last follow-up visit within six months after the restriction period ends. *Pearson Chi-square test
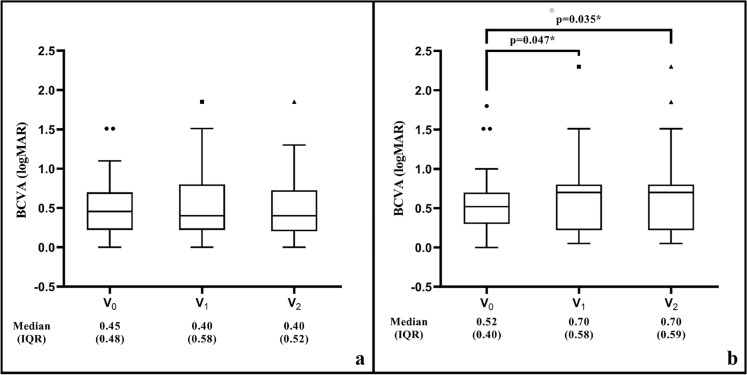
When we examine the changes in BCVA (logMAR) among the groups during the study period, in Group 1a, the median (IQR) BCVA was not significantly changed between V0 and V1 (0.45 [0.48] and 0.40 [0.58], respectively, p = 0.330); between V1 and V2 (0.40 [0.58] and 0.40 [0.52], respectively, p = 0.134), and between V0 and V2 (0.45 [0.45] and 0.40 [0.52], respectively, p = 0.762) (Fig. 4a). However, in Group 2a, the median (IQR) BCVA was significantly worse at V1 (0.70 [0.58]) and V2 (0.70 [0.59]) than at V0 (0.52 [0.40]) (p = 0.047 and p = 0.035, respectively); but was not significantly different between V1 and V2 (p = 0.310) (Fig. 4b).
Fig. 4.
Box-plot graphic showing the change in BCVA in the study period in Group 1a (a) and Group 2a (b). BCVA best-corrected visual acuity, IQR interquartile range, logMAR logarithm of the minimum angle of resolution, V0 the last visit before the restriction period where the IVI at restriction period scheduled, V1 the first visit after the restriction period ends, V2 the last follow-up visit within six months after the restriction period ends. *Wilcoxon signed-rank test
Discussion
This study demonstrated an attendance rate of 42.3% for nAMD patients in the TAE protocol during the COVID-19 restriction period. Among the patients who did not attend their IVI appointments, only 51.6% of them re-admitted to the clinic after the restriction period with a higher active disease ratio in OCT than the patients who attended their IVI appointments. The study also demonstrated a statistically significant decrease in the BCVA of the patients who missed their IVI appointment during the restriction period, which was not regained within six months after the restriction period. However, a stable BCVA was achieved in the patients who attended their visits. Although it seems that the number of IVIs before V0 is more in Group 2a than Group 1a (median [IQR], 13.00 [10.00] vs. 10.00 [9.50], respectively, p = 0.288), if we consider the clinical follow-up times of the groups before V0, it can be seen that Group 2a also has a longer follow-up time than Group 1a (median [IQR], 41.00 [53.00] vs. 32.00 [49.50], respectively). The difference in IVI counts before V0 might have been affected by the follow-up time of the patients, not necessarily by the severity of their diseases.
There was no guideline available to assist us in making decisions about our patients who were scheduled for IVI. We decided to continue IVI administration based on our hospital’s ICC recommendations, which includes reducing the number of patients and accompanying visitors in the waiting room; encouraging social distancing of 2 m; requiring the use of personal protective equipment (PPE), including N95 masks, ocular shields, and suits, by the staff; requiring the use of surgical masks by the patients; and establishing IVI intervals of 20 min with disinfecting the operating room between procedures. As time progressed, guidelines and algorithms were published for patients receiving IVI during the COVID-19 pandemic; even template letters were developed for the nAMD patients and their families [18, 22, 23]. New treatment protocol recommendations and telemedicine consultations were also included in the literature to ensure the continuity of treatment in nAMD patients during the pandemic period and to avoid in-person visits [24–26]. However, continuing with pre-adopted protocols was an option, which enabled us to compare the patients’ compliance with their IVI visits during the restriction period without any positive or negative interference.
The reduction in the average number of patients attending visits and IVI procedures during COVID-19 quarantine periods compared to the same periods from previous years has been demonstrated [27–30]. Considering the age and accompanying disorders of the nAMD patients, the fear of contracting COVID-19 from hospitals might have led to poor clinical attendance; therefore, informing the nAMD patients about precautions to minimize infection via an appointment letter or telephone call has been suggested to reduce non-attendance [31]. However, the decision to attend an IVI visit during the restriction period might have been affected by various other factors, such as traveling limitations, accompanied disorders, fear of contracting COVID-19 from sources such as public transportation, etc. [30, 32]. In their evaluation of 650 patients (with nAMD [76.6%], macular edema due to retinal vascular diseases [14.0%], and other causes of MNV [9.6%]), Viola et al. demonstrated an overall IVI attendance rate of 37% during the COVID-19 pandemic despite their stratification of patients as “emergent,” “urgent,” and “non-urgent” and after discussing their scheduled appointments via phone-calls [32]. Although we did not stratify the patients and did not interfere with their decision to attend, the adherence rate in our study was higher than that for nAMD patients in the study of Viola et al. (42.3% [44/104] vs. 35.7% [178/498]) [32]. In the same study, the patients who adhered to treatment were significantly younger and had a lower BCVA in the study eyes [32]. Patients who adhered to their appointments during the restriction period were also younger in our study; however, our patients had better BCVA in their study eyes at their first presentation and V0. This result might be explained by patients with better BCVA thought that they could maintain their visual acuity with IVI treatment, which may have motivated them to attend their appointments. Interestingly, we found that patients with hypertension were less likely to adhere to their IVI appointments during the restriction period than patients with diabetes or coronary artery disease. Although we did not evaluate the drugs used by the patients, we attribute this decision to arguments at that time that claimed that the use of antihypertensive medication (especially angiotensin-converting enzyme inhibitors and angiotensin receptor blockers) increases the likelihood of contracting COVID-19 and the severity of the disease [33, 34].
The number of anti-VEGF injections, clinical visits, and OCT was shown to be significant prognostic factors of BCVA maintenance or gain in nAMD patients [35]. Skipping even one anti-VEGF IVI has been associated with a decline in BCVA and increased OCT disease activity in nAMD treated with the PRN protocol [36]. Therefore, there was a justified concern about the impact of the COVID-19 pandemic on functional and anatomical results in nAMD patients [23, 37]. It was recently observed that PRN treatment intervals increased during the period of COVID-19 restrictions, with a mean ± SD difference of 29.9 ± 48.5 days between the preceding two visits before the pandemic [38]. This prolongation in the treatment interval also resulted in an increase in exudation in the structural OCT and a decrease in visual acuity, which was significantly associated with the extended interval time in multiple regression analysis [38]. To our knowledge, there is no study evaluating the TAE protocol during the COVID-19 pandemic in the literature. Recently, a study evaluating the unplanned extension of nAMD patients’ treatment intervals before the COVID-19 pandemic in the Fight Retinal Blindness! Registry showed that the 6-month vision outcomes in patients whose treatment intervals were extended up to 10–12 weeks were similar to those with ≤ 6-week intervals. However, there was a significant short-term risk to vision when the retreatment interval was extended beyond 12 weeks [39]. Similarly, in our study, a delay of mean ± SD of 13.9 ± 6.2 weeks in the patients’ treatment intervals resulted in a reduction in BCVA, which was not regained within 6 months.
The strengths of our study are its comparative and longitudinal design. However, the limitations of the study include its single-center retrospective design, relatively small sample size, heterogenicity of the cohort considering planned IVI intervals, IVI counts before V0, and the extensive range of BCVA. Moreover, the qualitative assessment of OCT parameters of exudation might have caused underestimation of progressive disease activity, especially in patients with exudative signs at V0.
Conclusion
In conclusion, although our study included a relatively small sample size, it is a real-life study showing that the missed appointment of nAMD patients in the TAE protocol with COVID-19 restrictions resulted in an increase in OCT disease activity and a decrease in BCVA. While the effectiveness and outcomes of previously adopted protocols in managing nAMD patients in the COVID-19 pandemic continue to be evaluated, perhaps more effective results will be obtained with the newly proposed protocols. We believe that our study results will be informative about the consequences of delays in the treatment of nAMD patients in the TAE protocol during this unprecedented period in which clinicians and patients have to make difficult decisions.
Acknowledgements
The authors would like to thank Zeynep Akyıldız for her significant contributions to the organization of the patients’ medical records.
Authors’ contributions
MOS was involved in study supervision; concept and study design; data collection, interpretation, analysis, and statistics; and drafting, revision, and final approval of the manuscript. AA was involved in study supervision; concept and study design; data interpretation; and revision and final approval of the manuscript. GÖ was involved in concept and study design; data collection and interpretation; and revision and final approval of the manuscript. VD was involved in data interpretation, analysis, and statistics and revision and final approval of the manuscript. ÖŞ was involved in study supervision; concept and study design; data interpretation; and revision and final approval of the manuscript.
Funding
No funding was received for conducting this study.
Data availability
The data supporting the findings of the study are available from the corresponding author upon request.
Declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
The study protocol was approved by the Institutional Review Board of Marmara University School of Medicine Hospital (No: 09.2020.1318).
Informed consent
The study was performed in accordance with the Declaration of Helsinki principles, and written informed consent about having their medical information used in the study analysis was routinely provided from all of the patients at their first presentation to our clinic.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/s0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, Waheed NK, Chakravarthy U, Rosenfeld PJ, Holz FG, Souied EH, Cohen SY, Querques G, Ohno-Matsui K, Boyer D, Gaudric A, Blodi B, Baumal CR, Li X, Coscas GJ, Brucker A, Singerman L, Luthert P, Schmitz-Valckenberg S, Schmidt-Erfurth U, Grossniklaus HE, Wilson DJ, Guymer R, Yannuzzi LA, Chew EY, Csaky K, Monés JM, Pauleikhoff D, Tadayoni R, Fujimoto J. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–636. doi: 10.1016/j.ophtha.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3:005139. doi: 10.1002/14651858.CD005139.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Resnikoff S, Taylor HR. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1:e339–349. doi: 10.1016/s2214-109x(13)70113-x. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Wecker T, Grundel B, Reichl S, Stech M, Lange C, Agostini H, Böhringer D, Stahl A. Anti-VEGF injection frequency correlates with visual acuity outcomes in pro re nata neovascular AMD treatment. Sci Rep. 2019;9:3301. doi: 10.1038/s41598-019-38934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freund KB, Korobelnik JF, Devenyi R, Framme C, Galic J, Herbert E, Hoerauf H, Lanzetta P, Michels S, Mitchell P, Monés J, Regillo C, Tadayoni R, Talks J, Wolf S. Treat-and-extend regimens with anti-vegf agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015;35:1489–1506. doi: 10.1097/iae.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 9.Maruko I, Ogasawara M, Yamamoto A, Itagaki K, Hasegawa T, Arakawa H, Nakayama M, Koizumi H, Okada AA, Sekiryu T, Iida T. Two-year outcomes of treat-and-extend intravitreal aflibercept for exudative age-related macular degeneration: a prospective study. Ophthalmol Retina. 2020;4:767–776. doi: 10.1016/j.oret.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO 2020) WHO Director-General’s opening remarks at the media briefing on COVID-19-March 11, 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed 16 Mar 2020
- 12.Parravano M, Borrelli E, Costanzo E, Sacconi R, Varano M, Querques G. Protect healthcare workers and patients from COVID-19: the experience of two tertiary ophthalmology care referral centers in Italy. Ophthalmol Ther. 2020;9:231–234. doi: 10.1007/s40123-020-00251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iovino C, Caporossi T, Peiretti E. Vitreoretinal surgery tip and tricks in the era of COVID-19. Graefes Arch Clin Exp Ophthalmol. 2020;258:2869–2870. doi: 10.1007/s00417-020-04800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Türk Oftalmoloji Derneği (2020) Pandemi Nedeni ile Acil Kabul Edilen Göz Ameliyatları. https://koronavirus.todnet.org/pandemi-nedeni-ile-acil-kabul-edilen-gz-ameliyatlar. Accessed 20 Dec 2020
- 15.The Royal College of Ophthalmologists (2020) RCOphth: management of ophthalmology services during the covid pandemic. https://www.rcophth.ac.uk/wp-content/uploads/2020/10/Medical-Retinal-Management-Plan-During-COVID-19.pdf. Accessed 28 Mar 2020
- 16.American Academy of Ophthalmology (2020) New recommendations for urgent and nonurgent patient care. https://www.aao.org/headline/new-recommendations-urgent-nonurgent-patient-care. Accessed 27 Mar 2020
- 17.American Society of Retina Specialists (2020) COVID-19: updates and resources. American Society of Retina Specialists (ASRS) Member Alert Regarding the COVID-19 Pandemic. https://www.asrs.org/practice/asrs-member-alert-regarding-covid-19-pandemic. Accessed 20 Mar 2020
- 18.Korobelnik JF, Loewenstein A, Eldem B, Joussen AM, Koh A, Lambrou GN, Lanzetta P, Li X, Lövestam-Adrian M, Navarro R, Okada AA, Pearce I, Rodríguez FJ, Wong DT, Wu L. Guidance for anti-VEGF intravitreal injections during the COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258:1149–1156. doi: 10.1007/s00417-020-04703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.İçişleri Bakanlığı TC (2020) 81 İl Valiliğine Coronavirüs Tedbirleri Konulu Ek Bir Genelge Daha Gönderildi. https://www.icisleri.gov.tr/81-il-valiligine-koronavirus-tedbirleri-konulu-ek-genelge-gonderildi. Accessed 16 Mar 2020
- 20.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–391. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- 21.Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47:1236–1240. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 22.Corazza P, D’Alterio FM, Younis S. Proposed algorithm during COVID-19 pandemic for patient management in medical retina clinic. Int J Retina Vitreous. 2020;6:20. doi: 10.1186/s40942-020-00226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korobelnik JF, Loewenstein A. Communicating with patients with nAMD and their families during the COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258:1335–1337. doi: 10.1007/s00417-020-04697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacconi R, Borrelli E, Vella G, Querques L, Prascina F, Zucchiatti I, Bandello F, Querques G. TriPla regimen: a new treatment approach for patients with neovascular age-related macular degeneration in the COVID-19 “era”. Eur J Ophthalmol. 2020 doi: 10.1177/1120672120963448. [DOI] [PubMed] [Google Scholar]
- 25.Antaki F, Dirani A. Treating neovascular age-related macular degeneration in the era of COVID-19. Graefes Arch Clin Exp Ophthalmol. 2020;258:1567–1569. doi: 10.1007/s00417-020-04693-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mintz J, Labiste C, DiCaro MV, McElroy E, Alizadeh R, Xu K. Teleophthalmology for age-related macular degeneration during the COVID-19 pandemic and beyond. J Telemed Telecare. 2020 doi: 10.1177/1357633x20960636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasser LM, Weill Y, Brosh K, Magal I, Potter M, Strassman I, Gelman E, Koslowsky M, Zadok D, Hanhart J. The Impact of COVID-19 on intravitreal injection compliance. SN Comp Clin Med. 2020 doi: 10.1007/s42399-020-00614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos A, Oliveira N, Martins J, Arruda H, Sousa J. The paradigm shift of ophthalmology in the COVID-19 era. Clin Ophthalmol. 2020;14:2625–2630. doi: 10.2147/opth.S267427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toro MD, Brézin AP, Burdon M, Cummings AB, Evren Kemer O, Malyugin BE, Prieto I, Teus MA, Tognetto D, Törnblom R, Posarelli C, Chorągiewicz T, Rejdak R. Early impact of COVID-19 outbreak on eye care: Insights from EUROCOVCAT group. Eur J Ophthalmol. 2020 doi: 10.1177/1120672120960339. [DOI] [PubMed] [Google Scholar]
- 30.Borrelli E, Grosso D, Vella G, Sacconi R, Querques L, Zucchiatti I, Prascina F, Bandello F, Querques G. Impact of COVID-19 on outpatient visits and intravitreal treatments in a referral retina unit: let’s be ready for a plausible “rebound effect”. Graefes Arch Clin Exp Ophthalmol. 2020;258:2655–2660. doi: 10.1007/s00417-020-04858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fung THM, Kuet ML, Patel MK, Puri P. Addressing COVID-19 fear to improve clinic attendance for patients with wet age-related macular degeneration. Acta Ophthalmol. 2020 doi: 10.1111/aos.14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viola F, Milella P, Giuffrida FP, Ganci S, Invernizzi A. The impact of coronavirus disease (COVID-19) pandemic on intravitreal injections treatment for macular diseases: report from a referral hospital in Milan. Retina. 2020 doi: 10.1097/iae.0000000000002941. [DOI] [PubMed] [Google Scholar]
- 33.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21–e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens. 2020;33:373–374. doi: 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Hykin P, Staurenghi G, Wittrup-Jensen K, Altemark A, Nilsson J, Kim K, Sivaprasad S. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016;100:1623–1628. doi: 10.1136/bjophthalmol-2015-308166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massamba N, Dirani A, Knoeri J, Pasquier B, Ingram A, Soubrane G. Evaluating the impact of summer vacation on the visual acuity of AMD patients treated with ranibizumab. Eye (Lond.) 2015;29:1453–1457. doi: 10.1038/eye.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corradetti G, Corvi F, Nguyen TV, Sadda SR. Management of neovascular age-related macular degeneration during the COVID-19 pandemic. Ophthalmol Retina. 2020;4:757–759. doi: 10.1016/j.oret.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borrelli E, Grosso D, Vella G, Sacconi R, Battista M, Querques L, Zucchiatti I, Prascina F, Bandello F, Querques G. Short-term outcomes of patients with neovascular exudative AMD: the effect of COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258:2621–2628. doi: 10.1007/s00417-020-04955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teo KYC, Nguyen V, Barthelmes D, Arnold JJ, Gillies MC, Cheung CMG. Extended intervals for wet AMD patients with high retreatment needs: informing the risk during COVID-19, data from real-world evidence. Eye (Lond.) 2020 doi: 10.1038/s41433-020-01315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the study are available from the corresponding author upon request.