Abstract
Background
Coronavirus disease 2019 (COVID-19) convalescent plasma (CCP) is being extensively investigated as a treatment, with mixed results to date. Overall, there has been a generalized lack of appropriateness in prescriptions, which, in the field of transfusion medicine, is termed patient-blood management.
Objectives
We aimed to separate study design variables that could affect clinical outcome after CCP therapy. We focus here on variables such as pretransfusion antibody testing in recipients, dose adjustments and antibody affinity measurements.
Sources
We searched PubMed and preprint servers for relevant preclinical and clinical studies discussing each of these variables in the field of CCP therapy.
Content
We show evidence that neglecting those variables has affected the outcomes of the vast majority of CCP clinical trials to date.
Implications
A better understanding of such variables will improve the design of the next generation of CCP clinical trials. This will likely lead to better clinical outcomes and will minimize risks of immune evasion from subneutralizing doses of neutralizing antibodies.
Keywords: Convalescent plasma, Coronavirus disease 2019, Neutralizing antibodies, Pharmacodynamics, Stoichiometry
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic had caused more than 150 million cases and 23.2 million deaths as of April 29, 2021. Although immunity largely depends on specific T lymphocytes [1], several therapeutics based on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibodies (nAb) are under investigation. COVID-19 convalescent plasma (CCP) is currently under study as a treatment for SARS-CoV-2 infection [2]. To date, clinical benefits have only been formally proved in early disease stages [[3], [4], [5]] (WHO scores 3 to 4 [6]). However, CCP has been extensively used in advanced disease stages (WHO scores 5 to 7), often as a last resort (compassionate) therapy in patients who have been hospitalized for weeks after onset of symptoms and have received multiple lines of treatment. Such practice has been generally approved by local ethics committees at the beginning of the pandemic, but we feel that it is time to test the scientific basis of this practice using the tools of evidence-based medicine. The field of transfusion medicine has pioneered patient-blood management (PBM), and we feel that CCP should also respond with prescription appropriateness. We recently solicited an urgent definition of CCP specifications by regulatory authorities (including, for example, the minimum antibody content, the methods that should be used to quantify such antibody content [7], and the shelf life of the unit) [8]. More points need to be addressed to elevate CCP from alchemy to a modern drug.
How can we define an appropriate dose adjustment?
To date, only a few trials have attempted to adjust the CCP dose, but this has invariably been accomplished by calculating the volume of CCP (mL) per recipient body weight (kg) [5]. Such an approach is legitimate when plasma is transfused to correct deficiencies of clotting factors, but it is completely lacking fundamental pharmacodynamic reasoning if the active ingredient is instead an nAb. It should be kept in mind that viral neutralization follows precise stoichiometry, and that a 200-mL CCP dose is finally diluted about ten-fold in about 2.5 L of plasma within the bloodstream. This means that in order to induce a measurable (two-fold) increase in nAb titres, the reinfused dose of nAb should be at least ten-fold higher than the one measured in the candidate recipient. Although plaque reduction neutralization test (PRNT) remains the reference standard for measuring nAb and can be safely used retrospectively, it takes days to report a measurement: hence, novel PRNT variants or surrogate high-throughput serology with high correlations [7] should be used prospectively when taking decisions on new patients.
How much neutralizing antibody is needed to exert a clinically significant impact on an ongoing SARS-CoV-2 infection?
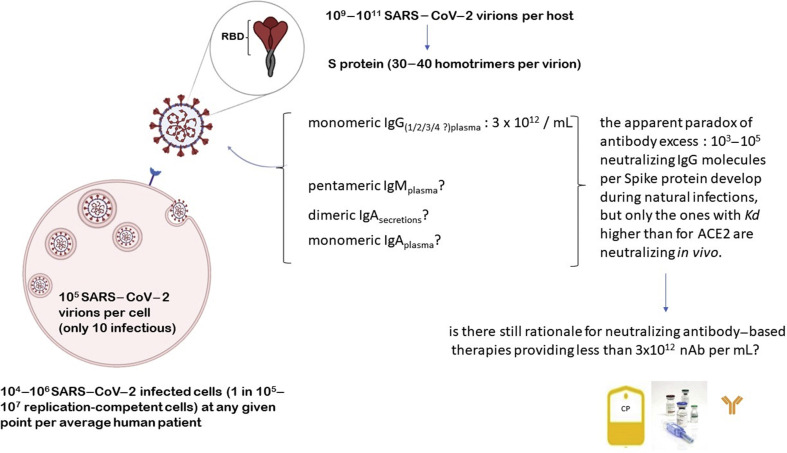
Several studies have measured the concentration of virions (in genome copies per gram of tissue) in respiratory, digestive and immune system tissues of rhesus macaques, after infection with SARS-CoV-2 [[9], [10], [11]]. An estimate for the total number of virions can be obtained from these measurements by multiplying the viral concentration of each tissue by the total tissue mass [12,13]. The lungs are the largest tissue in terms of mass (~1 kg) and have the highest viral concentration, so they contribute the most to the overall estimate. Other tissues, such as the nasal mucosa, larynx, bronchial tree and adjacent lymph nodes, contribute at most an additional 10% to the estimate based on the lungs, and viraemia appears negligible in most cases. Moving from macaque-to-human mass ratios and loads in human nasopharyngeal swabs [14], Sender et al. estimated that each infected person carries 109–1011 virions during peak infection, and transmission electron microscopy scans indicate that at a given moment there are ~105 viral particles within a single cell, although only about ten of them may be infectious [15]. A higher prevalence of detectable SARS-CoV-2 plasma viral load is associated with worse respiratory disease severity, lower absolute lymphocyte counts and increased markers of inflammation, including C-reactive protein and interleukin-6. SARS-CoV-2 viral loads, especially plasma viraemia, are associated with increased risk of mortality [16].
Total IgG antibody levels specific for SARS-CoV-2 Spike protein were measured 3 weeks following symptom onset showing a concentration in the serum in the order of ~10 μg/mL [17]. Only a fraction of ~5% of the total anti-Spike IgG antibodies has the capacity to neutralize the virus [18]. Combining the concentration of IgG nAb with a mean IgG molecular weight of 150 kDa, Sender et al. estimated that each millilitre of serum contains 3 × 1012 neutralizing IgG molecules [15]. Of course, these estimates neglect differences in the SARS-CoV-2 neutralizing ability of IgG subclasses [19], as well as the fundamental role in neutralization of IgM and monomeric IgA in plasma [20,21], and of dimeric IgA in secretions [22].
Combining this estimate with the measurement of viral concentration within the lung tissue [10] and accounting for 30–40 Spike homotrimers on each SARS-CoV-2 virion [23,24], Sender et al. estimated that there are 103–105 nAb per Spike protein [15]. Previous work on morphologically similar viruses such as influenza virus [25] and flavivirus [26] found that a ratio of one nAb to two to four receptor-binding proteins was sufficient to neutralize the binding of a virion to its cellular receptor. Hence, during the ongoing SARS-CoV-2 infection there is an apparent huge excess of nAb (Fig. 1 ), raising doubts about the rationale for nAb-based treatments.
Fig. 1.
The apparent neutralizing antibody excess paradox in severe acute respiratory syndrome coronavirus 2 infection.
Again, these estimates ignore other contributing factors, such as decoy receptors (e.g. angiotensin converting enzyme 2 (ACE2) -positive exosomes [27]) or antagonists (e.g. antagonistic exosomes [28], anti-OC43 antibodies [29], or ABO isoagglutinins [30]).
The biophysical parameters that govern the interaction between any antibody and its cognate antigen are its binding affinity and its concentration. Taken at face value, this estimate seems to suggest that CCP can lead to an excess of nAb molecules. This may be true even if the antibody concentrations in the lung tissue are lower than in the blood, and despite the extensive glycosylation patterns found on the Spike protein that shield many of its epitopes from nAb binding [24], and so decrease the efficiency of neutralization [31]. Hence variations in the dissociation constant K d (the equilibrium constant that measures the propensity of the antigen–antibody complex to dissociate reversibly) could contribute to explaining the imperfect correlation between high-throughput serology measuring anti-Spike receptor-binding domain IgG and viral neutralization test titres [7]: affinity and concentration can be directly measured in plasma samples of seropositive individuals using surface plasmon resonance [32] or microfluidic antibody affinity profiling [33]. In order to estimate the effectiveness of antibody neutralization, we need to estimate the fractional occupancy of the viral epitopes by antibodies. This fractional occupancy is determined by the strength of the binding of the nAb to the viral particles, given by K d [26]. The K d between human ACE2 and SARS-CoV-2 Spike protein are mostly in the range of 15 nM (using surface plasmon resonance [[34], [35], [36]]) to 30 nM (using microfluidics [37]), which is 10- to 20-fold higher than that between human ACE2 and the SARS-CoV-1 Spike protein [38]. This implies that COVID-19 plasma needs a K d ten-fold higher than in SARS-CoV-1, so that the 1:160 protective nAb titre threshold in SARS is hard to extrapolate for SARS-CoV-2. Hence the concentration of nAb in an average COVID-19 patient is 3 nM and the fractional occupancy needed is 25%–75%. The concentration of antibodies is needed to ensure that enough of the epitopes are bound, regardless of the high ratio between the number of nAb and viral particles [15]. Individual patients show K d of anti-receptor-binding domain antibodies spanning more than two orders of magnitude (from 80 pM to 25 nM) despite having similar antibody concentrations (8–69 nM). Moreover, these patients showed progressively higher antibody concentrations but constant K d values, suggesting that IgG affinities against most antigens did not mature with time [37,39]. Incomplete avidity maturation during acute infection might explain the observed decline of the humoral response during convalescence and follow up, potentially exposing individuals to SARS-CoV-2 reinfections. Peptides surrounding a key mutationally constrained B-cell epitope are predicted to bind poorly to common major histocompatibility complex class II alleles, suggesting a lack of major histocompatibility complex class II support in T-cell–B-cell cooperation, impacting the generation of high-potency nAb [40]. Higher levels of anti-Spike avidity were associated with older age, male sex and hospitalization, and also correlate with high nAb titres in viral neutralization tests [41].
The success of nAb-based treatment is dependent on the ratio between the concentration of antibodies and the dissociation constant of the neutralizing fraction of antibodies. During CCP treatment, the donor plasma (about 200 mL) is diluted by roughly a factor of 10 upon transfusion into the patient (2.5 L of plasma). For the donor antibodies to still bind viral proteins effectively following this dilution, in the case where the patient has produced no antibodies, the antibody binding site concentration should exceed the K d by at least a factor of 10. Therefore, only the patients harbouring anti-SARS-CoV-2 Spike antibodies with very strong K d values should be considered as CCP donors, a parameter that cannot be sufficiently estimated with conventional methods. Whereas Fc effector functions are not needed when nAb are administered as prophylaxis, they are necessary for optimal therapy [42]. That said, it should be remembered that if any of the systems downstream of neutralization (e.g. clearance of virus–nAb complexes by phagocytes and Fc receptors) becomes saturated, the virus–nAb complex is likely to dissociate.
Do neutralizing antibody levels correlate with disease severity?
It is now well established that individuals with mild/moderate disease display a slower rise and lower peak in anti-nucleocapsid (N) and anti-Spike S1 subunit IgG levels compared with individuals with severe/critical disease (although anti receptor-binding domain IgG and neutralization responses, but not anti-N, reached similar levels at 2–4 months [43]) [44]. Also, individuals with severe disease mount nAb with a higher frequency and a higher PRNT titre than those with moderate and mild disease [45]. According to the danger model of immunity [46], mild clinical presentations may be associated with a poor immune response. However, the fact that an individual with severe disease with high nAb is not able to neutralize the ongoing infection may sound counterintuitive. Although in these cases antibody-dependent enhancement of SARS-CoV-2 infection by pre-existing cross-reacting antibodies facilitating cell entry via Fc receptors cannot be formally excluded [47], antibodies with insufficient K d, as explained above, remain the most likely explanation.
Garcia-Beltran et al. reported that COVID-19 nAb predict disease severity and survival [48]. Accordingly, Chen et al. reported the highest nAb capacity in sera from patients with severe disease, whereas there was a lack of ability of asymptomatic patients to mount competent nAbs. Furthermore, the compositions of nAb subtypes were also different between recovered patients with severe symptoms and with mild-to-moderate symptoms [49]. Recently, Cervia et al. reported that systemic IgA and IgG production against SARS-CoV-2 develops mainly in severe COVID-19, with very high IgA levels seen in patients with severe acute respiratory distress syndrome, whereas mild disease may be associated with transient serum titres of SARS-CoV-2-specific antibodies but may stimulate mucosal SARS-CoV-2-specific IgA secretion [50].
As the baseline nAb titres are higher in these advanced COVID-19 stages, the nAb titre needed to achieve a clinically measurable benefit is likely to be much higher than the one required by individuals with mild-to-moderate disease.
Has neutralizing antibody-based therapy a chance to work in COVID-19?
So far, most randomized controlled trials have shown no statistically significant clinical benefit (defined as either requirement for mechanical ventilation or mortality) in patients treated after 72 hours from onset of symptoms [[3], [4], [5],51] and in the critically ill COVID-19 stages (WHO score 6–7). This has also proven true when high-titre CCP units have been used (defined as either PRNT >1:160 or with equivalent signal to cut-off ratio in high-throughput serology). This leaves room for exclusive usage of extremely high nAb titre CCP units in advanced stages. Of course, the landscape is different in patients with humoral immune deficiencies (e.g. B-cell alymphocytosis [52], common variable immune deficiency [53], or agammaglobulinaemia [[54], [55], [56]]), in whom lower nAb doses can be clinically useful. Unfortunately, this is exactly the converse of what happens at several transfusion centres, where CCP units not suitable for clinical trials (i.e. with nAb titres below the minimum threshold) are reserved for compassionate uses in advanced COVID-19 stages.
Few CCP trials correlated pretransfusion nAb titres in recipients with nAb titres in CCP units and clinical outcome: in one study, both nAb titres were associated with higher odds for this clinical improvement: pretransfusion nAb was associated with a significant reduction in length of intensive care unit stay and duration of mechanical ventilation [57]. Individuals with severe or life-threatening disease did not show benefit from high-nAb CCP units because they already had high pretransfusion nAb titres [58]. Accordingly, seronegative COVID-19 patients report greater drops in viral load when treated with the monoclonal antibody cocktail REGN-COV2 [59].
Another critical point is the rationale for multiple low-volume (200–300 mL) doses, which are the standard practice in most clinical trials, rather than a single loading dose. While division into sub-units would minimize the discard in case of in-transfusion adverse events, the vast majority of patients in the early stages of disease will be sufficiently haemodynamically stable to sustain transfusion of one or more plasmapheresis units.
As a further point, there is a rationale for transfusing CCP aliquots from different ABO-matched donors to maximize the polyclonality of the nAb. In countries where pathogen reduction technologies and nucleic acid testing on single CCP donations are mandatory, the additional risk for transfusion-transmitted infections associated with transfusion from different donors seems negligible.
Is SARS-CoV-2 replication still driving the pathology in advanced stages of COVID-19?
This is probably the most difficult question to answer. Post-mortem virology studies detected the presence of SARS-CoV-2 genome [[60], [61], [62], [63]], and antigens [63,64]. Several risk factors suggest autoimmunity largely contributes to pathology in advanced stages. The leading cause of death seems angiocentric. Casciola-Rosen et al. showed IgM autoantibodies against ACE2 in 27% of 66 individuals with severe COVID-19 compared with 3.8% of 52 non-hospitalized patients [65]. Accordingly, Gupta et al. reported an increased prevalence of pre-existing anti-interferon-α autoantibodies in systemic lupus erythematosus patients with COVID-19 compared with the reported prevalence in individuals with systemic lupus erythematosus but without COVID-19 and the general population with severe COVID-19 [66]. Franke et al. identified autoantibodies against Yo or NMDA receptor, vessel endothelium, astrocytic proteins and neuropil of basal ganglia, hippocampus or olfactory bulb in 11 severely ill COVID-19 patients presenting with unexplained neurological symptoms [67]. Woodruf et al. identified anti-nuclear autontibodies (ANA) in 44% of 31 critically ill patients with COVID-19 and no known history of autoimmunity [68]: specifically, Gomes et al. showed that anti-DNA antibodies determined upon hospital admission were high in 16% of patients and correlated strongly with later development of severe disease, showing a positive predictive value of 89.5% and accounting for 22% of total severe cases [69]. The same predictive value was true for anti-annexin A2 antibodies in 86 hospitalized patients [70].
Similarly, Bastard et al. identified nAb against type I interferon-α2 and interferon-ω in about 10% of patients with severe COVID-19 pneumonia [71]. The positive rate of anti-MDA5 antibody in 274 patients with COVID-19 was 48.2% (132/274) and the anti-MDA5 antibody-positive patients tended to present with severe disease (88.6% versus 66.9%, p < 0.0001) [72]. Althaus et al. reported that severe COVID-19 is associated with antibody-mediated up-regulation of platelet apoptosis [73]. Severe illness significantly correlated with elevated anti-cardiolipin IgA, anti-cardiolipin IgM, and anti-β2 glycoprotein-1 IgA [74,75]. Nevertheless, Borghi et al. reported that anti-phospholipid antibodies show a low prevalence in COVID-19 patients and are not associated with major thrombotic events. Anti-phospholipid antibodies in COVID-19 patients are mainly directed against β2-glycoprotein-1 but display an epitope specificity different from antibodies in anti-phospholipid syndrome [76].
Wang et al., using a high-throughput autoantibody discovery technique called Rapid Extracellular Antigen Profiling to screen a cohort of 194 SARS-CoV-2-infected patients and health-care workers for autoantibodies against 2770 extracellular and secreted proteins (the ‘exoproteome’), found that COVID-19 patients exhibit dramatic increases in autoantibody reactivities compared with uninfected controls, with a high prevalence of autoantibodies against immunomodulatory proteins including cytokines, chemokines, complement components and cell surface proteins [77].
It is unlikely that nAb can benefit this kind of autoimmunity, which could instead be the target for non-convalescent polyclonal immunoglobulins, which are currently under study [78].
Are there risks from subneutralizing antibody doses?
It has been recently reported that treatment of immunocompromised patients with nAb-based therapeutics can develop accelerated SARS-CoV-2 genome evolution [[79], [80], [81]]: under these circumstances, emergence of viral strains with unprecedented numbers of mutations in the Spike protein is a risk [82] with obvious implications for public health (e.g. the emergence of new strains can impair the efficacy of vaccine campaigns). The phenomenon does not seem common or fast, because none of eight onco-haematological patients (recipients of haematopoietic stem cell transplants or chimeric antigen receptor T lymphocytes) treated with CCP who remained SARS-CoV-2-positive for 2 months showed significant mutations compared with wild-type strain [83]. Avanzato et al. reported within-host genomic evolution in a patient affected by chronic lymphocytic leukaemia and iatrogenic hypogammaglobulinaemia treated with CCP and shedding infectious SARS-CoV-2 for 70 days, and subgenomic RNA for 105 days [79]. Kemp et al. reported an immune suppressed individual who showed little evolutionary change in the first 65 days while on remdesivir, but who developed D796H and ΔH69/ΔV70 mutations twice after two unsuccessful courses of CCP. In vitro, the mutant showed similar infectivity to wild-type strain but resistance to many CCP donors [81]. Hence it seems advisable to avoid treatment with subneutralizing CCP doses that could exert selective pressures, and to monitor patients showing a lack of response for Spike variants.
Conclusion
Several SARS-CoV-2 variants are emerging [82,84,85], and the efficacy of anti-Spike monoclonal antibodies against them seems lower than CCP [86]. Even the currently marketed anti-Spike vaccines are likely to offer reduced protection against some of these variants [87], so the interest in CCP is likely to remain high for months. It should be mandatory to test pretransfusion nAb titres (employing fast viral neutralization test variants or surrogate high-throughput serology) in candidate CCP recipients. Although there is still rationale for using high nAb titre CCP units in advanced disease stages of COVID-19, we feel that best practices (summarized in Table 1 ) should be implemented in randomized controlled trials. Those practices will largely facilitate the creation of mathematical models aimed at predicting the corrected nAb increase for a given therapeutic dose, and finally develop customized therapeutic doses.
Table 1.
Best practices for running CCP trials
| Transfuse CCP in the framework of randomized controlled trial |
| Measure pre-transfusion nAb titre employing fast methods (pseudotype viral neutralization tests or anti-Spike IgG high-throughput serology having high correlation coefficients) |
| Exclude from enrolment patients with high pretransfusion nAb titre |
| Use equal volume of fresh frozen non-convalescent plasma in the control arm to assess the relative contribution of non-nAb factors in plasma |
| Adjust dose among CCP-treated patients according to body weight, nAb titre and eventually affinity |
| Use a CCP loading dose (ideally from different donors or pooled donations) rather than multiple low-volume CCP doses at different time points |
| Repeat nAb titre in recipient serum at short-range time points to assess kinetics |
| Perform genomic studies in refractory/relapsing patients treated with CCP to assess emergence of mutated SARS-CoV-2 strains |
Abbreviations: CCP, COVID-19 convalescent plasma; COVID-19, coronavirus disease 2019; nAb, neutralizing antibody; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Transparency declaration
We declare that we have no conflict of interest related to this manuscript. The authors received no funding related to this manuscript.
Author contributions
DF designed the paper, analysed the data and wrote the first draft. FM and ML designed the figure; and MF, AA, AM and FM revised the final version.
Editor: J. Rodriguez-Baño
References
- 1.Schwarzkopf S., Krawczyk A., Knop D., Klump H., Heinold A., Heinemann F.M., et al. Cellular immunity in COVID-19 convalescents with PCR-confirmed infection but with undetectable SARS-CoV-2-specific IgG. Emerg Infect Dis. 2020;27 doi: 10.3201/2701.203772. [DOI] [PubMed] [Google Scholar]
- 2.Focosi D., Farrugia A. The art of the possible in approaching efficacy trials for COVID-19 convalescent plasma. Int J Infect Dis. 2020;102:244–246. doi: 10.1016/j.ijid.2020.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyner M.J., Carter R.E., Senefeld J.W., Klassen S.A., Mills J.R., Johnson P.W., et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2031893. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2020 doi: 10.1056/NEJMoa2033700. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2031304. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO R & D blueprint – novel coronavirus COVID-19 therapeutic trial synopsis. 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf?ua=1 Available at:
- 7.Focosi D., Mazzetti P., Pistello M.M. Viral infection neutralization tests: a focus on SARS-CoV-2 with implications for convalescent plasma therapy. Rev Med Virol. 2020 doi: 10.1002/rmv.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Focosi D., Farrugia A. Urgent need to regulate convalescent plasma differently from thawed plasma. Transf Med Hemother. 2020 doi: 10.1159/000513035. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585:273–276. doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munster V.J., Feldmann F., Williamson B.N., van Doremalen N., Pérez-Pérez L., Schulz J., et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585:268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., Oude Munnink B.B., et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snyder W., Cook M., Nasset E., Karhausen L., Parry Howells G., Tipton I. Report of the task group on reference man. Oxford: Pergamon Press; 1975;3:288–298. [Google Scholar]
- 13.ICRP Basic anatomical and physiological data for use in radiological protection: reference values. Ann ICRP. 2002;32:1–277. [PubMed] [Google Scholar]
- 14.Gallichotte E.N., Quicke K.M., Sexton N.R., Fitzmeyer E., Young M.C., Janich A.J., et al. Longitudinal surveillance for SARS-CoV-2 among staff in six Colorado long-term care facilities: epidemiologic, virologic and sequence analysis. medRxiv. 2020 doi: 10.1101/2020.06.08.20125989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sender R., Bar-On Y.M., Flamholz A., Gleizer S., Bernsthein B., Phillips R., et al. The total number and mass of SARS-CoV-2 virions in an infected person. medRxiv. 2020 doi: 10.1101/2020.11.16.20232009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fajnzylber J., Regan J., Coxen K., Corry H., Wong C., Rosenthal A., et al. The Massachusetts Consortium for Pathogen, SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1101/2020.07.18.20155374. [DOI] [Google Scholar]
- 18.Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.T., et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369:956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suthar M.S., Zimmerman M.G., Kauffman R.C., Mantus G., Linderman S.L., Hudson W.H., et al. Rapid Generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. 2020;1:100040. doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klingler J., Weiss S., Itri V., Liu X., Oguntuyo K.Y., Stevens C., et al. Role of IgM and IgA antibodies in the neutralization of SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.08.18.20177303. [DOI] [Google Scholar]
- 21.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., clear L., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Viant C., Gaebler C., et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.abf1555. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao H., Song Y., Chen Y., Wu N., Xu J., Sun C., et al. Molecular architecture of the SARS-CoV-2 virus. Cell. 2020;183:730–738. doi: 10.1016/j.cell.2020.09.018. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turoňová B., Sikora M., Schürmann C., Hagen W.J.H., Welsch S., Blanc F.E.C., et al. In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges. Science. 2020;370:203–208. doi: 10.1126/science.abd5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor H.P., Armstrong S.J., Dimmock N.J. Quantitative relationships between an influenza virus and neutralizing antibody. Virology. 1987;159:288–298. doi: 10.1016/0042-6822(87)90466-1. [DOI] [PubMed] [Google Scholar]
- 26.Pierson T.C., Diamond M.S. A game of numbers: the stoichiometry of antibody-mediated neutralization of flavivirus infection. Prog Mol Biol Transl Sci. 2015;129:141–166. doi: 10.1016/bs.pmbts.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Shennawy L., Hoffmann A.D., Dashzeveg N.K., Mehl P.J., Yu Z., Tokars V.L., et al. Circulating ACE2-expressing exosomes block SARS-CoV-2 virus infection as an innate antiviral mechanism. biorXiv. 2020 doi: 10.1101/2020.12.03.407031. [DOI] [Google Scholar]
- 28.Lindemann M., Krawczyk A., Dolff S., Konik M., Rohn H., Platte M., et al. SARS-CoV-2-specific humoral and cellular immunity in two renal transplants and two hemodialysis patients treated with convalescent plasma. J Med Viro. 2021;93:3047–3054. doi: 10.1002/jmv.26840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dugas M., Grote-Westrick T., Merle U., Fontenay M., Kremer A.E., Vollenberg R., et al. J Clin Virol; 2020. Lack of antibodies against seasonal coronavirus OC43 nucleocapsid protein identifies patients at risk of critical COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deleers M., Breiman A., Daubie V., Maggetto C., Barreau I., Besse T., et al. Covid-19 and blood groups: ABO antibody levels may also matter. Int J Infect Dis. 2021;104:242–249. doi: 10.1016/j.ijid.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schön M.P., Berking C., Biedermann T., Buhl T., Erpenbeck L., Eyerich K., et al. COVID-19 and immunological regulations - from basic and translational aspects to clinical implications. J D Dermatol Gesellsch. 2020 doi: 10.1111/ddg.14169. Epub haead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klasse P.J. How to assess the binding strength of antibodies elicited by vaccination against HIV and other viruses. Expert Rev Vaccines. 2016;15:295–311. doi: 10.1586/14760584.2016.1128831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider M.M., Scheidt T., Priddey A.J., Xu C.K., Hu M., Devenish S.R.A., et al. Microfluidic antibody affinity profiling for in-solution characterisation of alloantibody–HLA interactions in human serum. bioRxiv. 2020 doi: 10.1101/2020.09.14.296442. [DOI] [PubMed] [Google Scholar]
- 34.Chi X., Liu X., Wang C., Zhang X., Li X., Hou J., et al. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting the spike receptor binding domain. Nat Commun. 2020;11:4528. doi: 10.1038/s41467-020-18387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seydoux E., Homad L.J., MacCamy A.J., Parks K.R., Hurlburt N.K., Jennewein M.F., et al. Analysis of a SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation. Immunity. 2020;53:98–105. doi: 10.1016/j.immuni.2020.06.001. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider M.M., Emmenegger M., Xu C.K., Condado Morales I., Turelli P., Zimmermann M.R., et al. Microfluidic affinity profiling reveals a broad range of target affinities for anti-SARS-CoV-2 antibodies in plasma of covid survivors. 2020. https://www.medrxiv.org/content/medrxiv/early/2020/09/23/2020.09.20.20196907.full.pdf Avaiable at:
- 38.Kirchdoerfer R.N., Wang N., Pallesen J., Wrapp D., Turner H.L., Cottrell C.A., et al. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci Rep. 2018;8:15701. doi: 10.1038/s41598-018-34171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer G., Struck F., Schreiner P., Staschik E., Soutschek E., Motz M. Research Square; 2020. The serological response to SARS corona virus-2 is characterized by frequent incomplete maturation of functional affinity (avidity) [Google Scholar]
- 40.Castro A., Ozturk K., Zanetti M., Carter H. biorXiv; 2020. MHC-II constrains the natural neutralizing antibody response to the SARS-CoV-2 spike RBM in humans. [Google Scholar]
- 41.Benner S.E., Patel E.U., Laeyendecker O., Pekosz A., Littlefield K., Eby Y., et al. SARS-CoV-2 antibody avidity responses in COVID-19 patients and convalescent plasma donors. J Infect Dis. 2020;222:1974–1984. doi: 10.1093/infdis/jiaa581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winkler E.S., Gilchuk P., Yu J., Bailey A.L., Chen R.E., Zost S.J., et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions and monocytes for optimal therapeutic protection. Cell. 2021;184(7):1804–1820. doi: 10.1016/j.cell.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Havervall S., Jernbom Falk A., Klingstrom J., Ng H., Greilert Norin N., Gabrielsson L., et al. medrXiv; 2021. SARS-CoV-2 induces a durable and antigen specific humoral immunity after asymptomatic to mild COVID-19 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haddad N.S., Nguyen D.C., Kuruvilla M.E., Morrison-Porter A., Anam F., Cashman K.S., et al. bioRxiv; 2020. Elevated SARS-CoV-2 antibodies distinguish severe disease in early COVID-19 infection. [Google Scholar]
- 45.Focosi D., Franchini M. Clinical predictors of SARS-CoV-2 neutralizing antibody titers in COVID-19 convalescents: implications for convalescent plasma donor recruitment. Eur J Haematol. 2021 doi: 10.1111/ejh.13630. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matzinger P. The evolution of the danger theory. Interview by Lauren constable, commissioning editor. Exp Rev Clin Immunol. 2012;8:311–317. doi: 10.1586/eci.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karthik K., Senthilkumar T.M.A., Udhayavel S., Raj G.D. Role of antibody-dependent enhancement (ADE) in the virulence of SARS-CoV-2 and its mitigation strategies for the development of vaccines and immunotherapies to counter COVID-19. Hum Vaccines Immunother. 2020;16(12):3055–3060. doi: 10.1080/21645515.2020.1796425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D., Miller T.E., Feldman J., et al. COVID-19 neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2):476–488. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X., Pan Z., Yue S., Yu F., Zhang J., Yang Y., et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transd Targ Ther. 2020;5:180. doi: 10.1038/s41392-020-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cervia C., Nilsson J., Zurbuchen Y., Valaperti A., Schreiner J., Wolfensberger A., et al. Systemic and mucosal antibody secretion specific to SARS-CoV-2 during mild versus severe COVID-19. Allergy Clin Immunol. 1987;147(2):545–557. doi: 10.1016/j.jaci.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.London J., Boutboul D., Lacombe K., Pirenne F., Heym B., Zeller V., et al. Severe COVID-19 in patients with B cell alymphocytosis and response to convalescent plasma therapy. J Clin Immunol. 2020;41(2):356–361. doi: 10.1007/s10875-020-00904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Damme K.F.A., Tavernier S., Van Roy N., De Leeuw E., Declercq J., Bosteels C., et al. Case report: convalescent plasma, a targeted therapy for patients with CVID and severe COVID-19. Front Immunol. 2020;11:596761. doi: 10.3389/fimmu.2020.596761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milošević I., Jovanović J., Stevanovic O. Atypical course of COVID-19 in patient with Bruton agammaglobulinemia. J Infect Dev Ctries. 2020;14:1248–1251. doi: 10.3855/jidc.13840. [DOI] [PubMed] [Google Scholar]
- 55.Jin H., Reed J.C., Liu S.T.H., Ho H.E., Lopes J.P., Ramsey N.B., et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allerg Clin Immunol Pract. 2020;8:3594–3596. doi: 10.1016/j.jaip.2020.08.059. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mira E., Yarce O.A., Ortega C., Fernández S., Pascual N.M., Gómez C., et al. Rapid recovery of a SARS-CoV-2-infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma. J Allerg Clin Immunol Pract. 2020;8:2793–2795. doi: 10.1016/j.jaip.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yokoyama A.P.H., Wendel S., Bonet-Bub C., Fachini R.M., Dametto A.P.F., Blumm F., et al. medrXiv; 2020. Impact of convalescent plasma transfusion (CCP) in patients with previous circulating neutralizing antibodies (nAb) to COVID-19. [Google Scholar]
- 58.Klein M.N., Wang E.W., Zimand P., Beauchamp H., Donis C., Ward M.D., et al. J Clin Pathol; 2021. Kinetics of SARS-CoV-2 antibody responses pre- and post- COVID-19 convalescent plasma transfusion in patients with severe respiratory failure: an observational case-control study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2020;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ducloyer M., Gaborit B., Toquet C., Castain L., Bal A., Arrigoni P.P., et al. Complete post-mortem data in a fatal case of COVID-19: clinical, radiological and pathological correlations. Int J Leg Med. 2020;134:2209–2214. doi: 10.1007/s00414-020-02390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindner D., Fitzek A., Bräuninger H., Aleshcheva G., Edler C., Meissner K., et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deinhardt-Emmer S., Wittschieber D., Sanft J., Kleemann S., Elschner S., Haupt K.F., et al. Early postmortem mapping of SARS-CoV-2 RNA in patients with COVID-19 and correlation to tissue damage. Elife. 2020:e60361. doi: 10.7554/eLife.60361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skok K., Stelzl E., Trauner M., Kessler H.H., Lax S.F. Post-mortem viral dynamics and tropism in COVID-19 patients in correlation with organ damage. Virchows Archiv. 2020 doi: 10.1007/s00428-020-02903-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casciola-Rosen L., Thiemann D.R., Andrade F., Trejo Zambrano M.I., Hooper J.E., Leonard E.K., et al. medrXiv; 2020. IgM autoantibodies recognizing ACE2 are associated with severe COVID-19. [Google Scholar]
- 66.Gupta S., Nakabo S., Chu J., Hasni S., Kaplan M.J. medrXiv; 2020. Association between anti-interferon-alpha autoantibodies and COVID-19 in systemic lupus erythematosus. [Google Scholar]
- 67.Franke C., Ferse C., Kreye J., Reincke M., Sanchez-Sendin E., Rocco A., et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun; 2021;93:115–119. doi: 10.1016/j.bbi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woodruff M.C., Ramonell R.P., Lee F.E.H., Sanz I. medrXiv; 2020. Clinically identifiable autoreactivity is common in severe SARS-CoV-2 infection. [Google Scholar]
- 69.Gomes C., Zuniga M., Crotty K.A., Qian K., Hsu Lin L., Argyropoulos K., et al. medrXiv; 2021. Autoimmune anti-DNA antibodies predict disease severity in COVID-19 patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zuniga M., Gomes C., Carsons S.E., Bender M.T., Cotzia P., Miao Q.R., et al. medrXiv; 2021. Autoimmunity to the lung protective phospholipid-binding protein annexin A2 predicts mortality among hospitalized COVID-19 patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu C., Wang Q., Wang Y., Wang G., Wang L., Chen H., et al. medrXiv; 2020. Analysis of the correlation between anti-MDA5 antibody and the severity of COVID-19: a retrospective study. [Google Scholar]
- 73.Althaus K., Marini I., Zlamal J., Pelzl L., Haeberle H., Mehrlaender M., et al. Severe COVID-19 infection is associated with increased antibody-mediated platelet apoptosis. Blood. 2020;137(8):1061–1071. doi: 10.1182/blood.2020008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hasan Ali O., Bomze D., Risch L., Brugger S.D., Paprotny M., Weber M., et al. Severe COVID-19 is associated with elevated serum IgA and antiphospholipid IgA-antibodies. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1496. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12(570):eabd3876. doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borghi M.O., Beltagy A., Garrafa E., Curreli D., Cecchini G., Bodio C., et al. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front Immunol. 2020;11(2692):584241. doi: 10.3389/fimmu.2020.584241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Liu F., et al. medrXiv; 2020. Diverse functional autoantibodies in patients with COVID-19. [DOI] [PubMed] [Google Scholar]
- 78.Tzilas V., Manali E., Papiris S., Bouros D. Intravenous immunoglobulin for the treatment of COVID-19: a promising tool. Resp Int Rev Thorac Dis. 2020;99(12):1087–1089. doi: 10.1159/000512727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L., et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020 doi: 10.1016/j.cell.2020.10.049. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi B., Choudhary M.C., Regan J., Sparks J.A., Padera R.F., Qiu X., et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kemp S.A., Collier D.A., Datir R., Gayed S., Jahun A., Hosmillo M., et al. Neutralising antibodies in Spike mediated SARS-CoV-2 adaptation. Nature. 2020 doi: 10.1101/2020.12.05.20241927. [DOI] [Google Scholar]
- 82.Rambaut A., Loman N., Pybus O., Barclay W., Barrett J., Carabelli A., et al. On behalf of COVID-19 Genomics Consortium UK. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. 2020. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 Available at:
- 83.Aydillo T., Gonzalez-Reiche A.S., Aslam S., van de Guchte A., Khan Z., Obla A., et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naveca F., da Costa C., Nascimento V., Souza V., Corado A., Nascimento F., et al. SARS-CoV-2 reinfection by the new variant of concern (VOC) P.1 in Amazonas, Brazil. 2021. https://virological.org/t/sars-cov-2-reinfection-by-the-new-variant-of-concern-voc-p-1-in-amazonas-brazil/596 Available at:
- 85.Kosakovsky Pond S., Wilkison E., Weaver S., James S., Tegally H., de Oliveira T., et al. 2020. A preliminary selection analysis of the South African V501.V2 SARS-CoV-2 clade.https://virological.org/t/a-preliminary-selection-analysis-of-the-south-african-v501-v2-sars-cov-2-clade/573 Available at: [Google Scholar]
- 86.Rees-Spear C., Muir L., Griffith S.A., Heaney J., Aldon Y., Snitselaar J., et al. The impact of Spike mutations on SARS-CoV-2 neutralization. biorXiv. 2021 doi: 10.1101/2021.01.15.426849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. 2021. https://www.biorxiv.org/content/biorxiv/early/2021/01/19/2021.01.15.426911.full.pdf Available at: [DOI] [PMC free article] [PubMed]