Abstract
MAP/ERK kinase 1 and 2 (MEK 1/2) inhibitors (MEKi) are investigated in several trials to treat lesions that arise from pathogenic variants of the Neurofibromatosis type 1 and type 2 genes (NF1, NF2). These trials showed that MEKi are capable to shrink volume of low grade gliomas and plexiform neurofibromas in NF1. Targeting other lesions being associated with a high morbidity in NF1 seems to be promising. Due to involvement of multiple pathways in NF2 associated lesions as well as in malignant tumors, MEKi are also used in combination therapies. This review outlines the current state of MEKi application in neurofibromatosis and associated benign and malignant lesions.
Keywords: MEK inhibitor, Neurofibromatosis, NF1, NF2, Schwannomatosis, LGG, Neurofibroma, MPNST, Glioblastoma, RASopathy
Background
A targeted therapy of Neurofibromatosis (NF) ideally would start early to inhibit tumor development and, at best, would cure the disease. Soon restoration of the mutational effect would raise the amount of functional protein and compensate impaired functions. Currently, substantial improvement has been made regarding targeted therapies by using MAP/ERK kinase 1 and 2 (MEK 1/2) inhibitors (MEKi) to block RAS-MAPK overactivation and to minimize the mutational effect on the somatic level. Here, the published status of MEKi therapies in NF with special respect to Neurofibromatosis type 1 associated lesions is reviewed.
RAS-MAPK signaling cascade and selective MEK1/2 inhibition
The mitogen-activated protein kinase kinase kinase (MAP 4 K) hierarchical pathway (RAS-RAF-MEK-ERK) is important for proliferation, differentiation and survival of cells and is overactive in many cancers [1]. Cell surface tyrosine kinase receptors, Ca2+, protein kinase C or G protein-coupled receptor activate nucleotide guanosine triphosphate (GTP)ase bound Kirsten rat sarcoma virus (RAS/MAP 4 K) which transduces the extracellular signal to many profound substrates through phosphorylation of the following intracellular kinases: rapidly accelerated fibrosarcoma (RAF/MAP 3 K or MAPKK), MAP/ERK kinase 1 and 2 (MEK 1/2 / MAP 2 K), and extracellular signal-related kinase (ERK/MAPK). Activated ERK finally transfers the signals into the cellular transcription network [1]. Phosphorylation of protein kinases and substrates is a highly significant regulatory mechanism in cells. Therefore, inhibition of phosphorylation for therapy of disease can be expected to lead to multiple side effects.
Enzymes MEK1 and MEK2 are conserved, important dual specificity serine/tyrosine protein kinases of 44 and 45 kDa molecular weight. They can be specifically targeted by inhibitors (MEKi) which arrest MEK1/2-dependent signaling in a highly selective way. MEK proteins of the MEK family structurally share an amino-terminal domain, a conserved kinase domain, and a carboxyl-terminal domain [2]. MEK1/2 are encoded by MAP 2 K1 (15q22.31) and MAP 2 K2 (19p13.3). Many inhibitors block components of the RAS-MAPK signaling cascade but MEKi inhibitors were the first selective ones that effectively approached patients. Thus, trametinib was the first clinically successful MEKi used for melanoma with BRAF pathogenic variants [3]. Targetable catalytic processes occur within ATP binding site of the kinase domain. MEKi act non-competitive or competitive with ATP, but only those that bind allosteric to the ATP binding site are very specific [2].
Currently, MEKi therapy is limited by two major problems: drug resistance and toxicity.
Resistance due to reactivation of RAS-MAPK signaling can arise from alterations of RAS, RAF, NF1, or MEK, from reactivation of upstream receptor tyrosine kinases (e. g. hepatocyte growth factor (HGF) / HGF receptor (MET) signaling) due to adaptation, from activated parallel pathways (PI3K, STAT, Hippo and signal transducer and activator of transcription (STAT) signaling, loss of PTEN or PP2A) as well as from activated transcription factors to control phenotypes and metabolism [4, 5]. Thus, pathogenic variants in MAP 2 K1 or MAP 2 K2 influence sensitivity to MEKi [6]. Hence, acquired pathogenic variants such as MEK1V211D can induce resistance to allosteric MEKi, which may be overcome by a new class of adenosine triphosphate (ATP)-competitive MEKi [7]. In principle, allosteric ATP-non-competent compounds bind MEK adjacent to the conserved ATP binding pocket. Non-competitive binding induces enzyme inactivation due to a change of protein conformation: the activation loop that needs to be phosphorylated at serine residues S127 and S221 to enable a complete biological activity, remains incompletely phosphorylated. MEKi trametinib also blocks S218. Subsequently, the catalytically inactive state of protein kinase MEK is locked and activated RAF kinases are not able to pass activity. An overactive (e.g. BRAF mutated) RAF kinase that is not able to phosphorylate MEK leads to suppression of downstream signaling (via phosphorylation of ERK) and blocks pathway overactivation. Similarly, upstream overactivated (e. g. RAS or NF1 mutated) RAS cannot transduce oncogenic signals when MEK is blocked (Fig. 1a). However, upstream kinases can stimulate other effectors and induce drug escape: For example, KRAS can stimulate the phosphatidylinositol 3-kinase (PI3K). Therefore, in KRAS mutant cancers additional PI3K mutations lead to reduced sensitivity to MEKi. Those cancers require combination therapy, and identification of biomarkers is important [8]. Trametinib is one of those MEKi that is currently tested in combination with others inhibitors [9]. During therapy drug resistance can also occur due to negative feedback signaling downstream of RAS [4]. To overcome those adaptive mechanism novel drugs such as trametiglue that enhance binding are investigated [9].
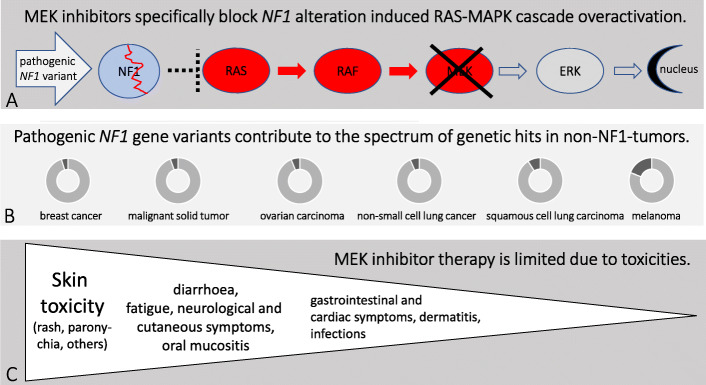
Fig. 1.
Principles of MEK inhibition in NF1 associated lesions. Legend: a NF1 pathogenic variants induce overactivation of the MAPK signaling cascade. Specific MEK inhibition blocks phosphorylation of ERK and subsequent signal transduction to the transcription network of the nucleus. b Apart from Neurofibromatosis type 1, somatic pathogenic NF1 gene variants occur in non-NF1 associated tumors and can be targeted by MEK inhibitors. c MEK inhibition is associated with side effects which occur at different percentages
Inhibitors of MEK1/2 are currently used for therapy of BRAF mutated as well as Neurofibromatosis type 1 (NF1) mutated, KRAS and NRAS mutated tumors including treatment of RASopathies (Fig. 1). Of those, trametinib, cobimetinib, selumetinib, binemetinib and mirdametinib are in use to treat patients. As reported by the database of the National Cancer Institute (“ClinicalTrials.gov”) four MEKi studies have been completed for neurofibromatoses: NCT02096471, NCT02124772, NCT01885195, and NCT03649165. They involved PD-0325901 (mirdametinib), trametinib, dabrafenib, and MEK162 (binimetinb) in phase 1 and 2 studies and included individuals with NF1-associated plexiform neurofibromas and other cancers harboring V600 mutations or RAS/RAF/MEK activated tumors. Inhibition of MEK1/2 by selumetinib affects MAP 2 K dependent pathways in NF1 mutated inoperable plexiform tumors and was recently approved by the U. S. Food and Drug Administration (FDA). Other MEKi therapies target patients with ganglioglioma, non-small cell lung carcinoma, pilocytic astrocytoma, pleomorphic xanthoastrocytoma, malignant solid tumor, hematologic disorders, and colorectal cancer [10].
RAS-MAPK signaling cascade in Neurofibromatosis
Neurofibromatosis (NF) type 1 (NF1), type 2 (NF2) and type 3 (Schwannomatosis) are inherited neurocutaneous tumor syndromes that affect multiple organs and share development of multiple benign peripheral nerve sheath tumors eponymous for the disease. They are caused by germline pathogenic loss-of-function variants of tumor suppressor genes on 17q11.2 (Neurofibromatosis type 1 gene, NF1), on 22q11.2 (Neurofibromatosis type 2 gene, NF2), and on 22q.23 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily b, member 1 gene, SMARCB1 and on 22q11.21 (Leucine zipper like transcription regulator gene, LZTR1). Besides germline events in these syndromes, somatic pathogenic variants of the involved genes can also arise in several sporadic, non-NF associated cancers (Fig. 1b) [11, 12]. NF belong to RASopathies. RASopathy-associated tumors are treated in trials using MEKi such as cobimetinib (NCT02639546). In most RASopathies germline pathogenic variants in genes encoding RAS pathway proteins affect functions upstream of MEK1/2. Thus, drug escape can be anticipated by upstream genetic alterations [13]. A new NCI initiative (“Advancing RAS/RASopathy (ART)”) aims to develop therapeutic strategies for RASopathy associated lesions [14].
In NF2, therapy of brain tumors precedes therapy of peripheral tumors since associated vestibular schwannomas, ependymomas and meningiomas lead to more severe complications. Therefore, endpoints of trials for NF2 associated tumors differ compared to other cancer studies [15]. NF2 pathogenic germline variants of moesin-ezrin-radixin-like protein called merlin or schwannomin effect not only RAS-MAPK signaling but also tyrosine receptor kinases and many other downstream pathways underlining a complex multi-suppressor function of merlin [2, 16]. Nevertheless, pre-clinical studies extensively studied MEKi. A large study analyzed MEKi selumetinib, trametinib, PD0325901, MEK162, cobimetinib and refametinib in NF2-associated merlin-deficient schwannoma cells and mouse models and identified trametinib, PD0325901 and cobimetinib to be the most effective as well as uncovered resistance mechanisms [3]. In a NF2-mutation associated tumor model application of MEKi trametinib alone as well as in combination with vistusertib was effective [6]. Somatic pathogenic variants can occur additionally to NF2 gene variants such as in AKT1 (e.g. AKT1E17K variant) which highlights the importance of tumor genome analysis prior to a targeted therapy [7]. Consequently, merlin deficiency can be rescued not only by MEKi but several other drugs and multiple alternative ways give rise to drug resistance in patients. Therapeutics that targeted single tyrosine kinases in trials were not successful so far [15, 17]. NF2-associated tumors, although benign, need a specific multi-target approach explaining why FDA-approved systemic MEKi therapies are not established and why (radio) surgery in NF2 is still a successful first line approach [18]. Currently, an open trial (SEL-TH-1601, NCT03095248) investigates response rate of NF2-associated tumors by selumitinib. Ongoing trials investigate MEKi selumitinib and cobimetinib for NF2-tumor-associated hearing loss and MEKi trametinib in combination therapy for aggressive and recurrent meningiomas.
Currently, no trial exists that investigates any kind of therapy of Schwannomatosis. Associated schwannomas show a combination of “first and second hits” of SMARCB1, LZTR1, NF2 and others. A complex inactivation of different tumor suppressor genes leads to involvement of several pathways in development of benign tumors. As in NF2, more than RAS-MAPK signaling would need to be targeted.
In contrast, the RAS-MAPK signaling cascade seems to be a very promising target in NF1, at least in benign nerve sheath tumors, low grade gliomas and non-tumor lesions since RAS-MAPK activation is the main pathomechanism. As there is rapidly growing knowledge, MEKi therapy in NF1 is reviewed in the following sections.
RAS-MAPK signalling cascade in Neurofibromatosis type 1 (NF1)
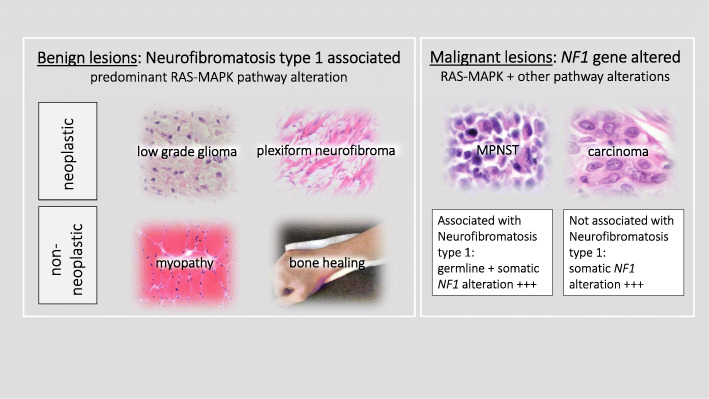
Among neurofibromatoses, NF1 is most frequent and results from germline pathogenic variants of the NF1 gene in about 50% of cases. NF1 is associated with a broad spectrum of symptoms including benign peripheral nerve sheath tumours (neurofibromas), café-au-lait macules (CALM), skinfold freckling, iris Lisch nodules, low grade gliomas, bone malformation and others [19, 20]. Clinical diagnosis of NF1 is defined by NIH criteria that are revised in 2021 to support differentiation from related syndromes [21]. NF1 patients are at increased risk for malignant transformation of neurofibromas. Benign lesions such as CALM, pseudarthrosis, and benign tumors arise from a “second (somatic) NF1 hit” followed by loss of function of the gene product neurofibromin. The central, highly conserved guanosine triphosphatase (GTPase) activating protein (GAP)- related domain (GRD) of neurofibromin is crucial to downregulate RAS in many cells. NF1 pathogenic variants that involve important binding sites of GRD dramatically reduce GAP activity of RAS [22, 23]. Essential regulation of RAS via GRD has prompted therapeutic targeting of the RAS-MAPK signaling cascade in NF1 long ago. In malignant, sporadic non-NF1-associated tumors such as pheochromocytoma, lung adenocarcinoma, breast cancer, ovarian cancer, glioblastoma and many others, somatic pathogenic variants of NF1 are also important targets. MEKi have been investigated in several human and animal NF1 studies and to date trametinib and selumitinib are used in nearly 20 ongoing studies in NF1 patients. Although to date binimetinib, cobimetinib, trametinib, selumitinib, and mirdametinib (PD0325901) are investigated in trials, only selumitinib is FDA approved for plexiform neurofibromas. To summarize, MEKi can be used to neutralize a pathogenic NF1 gene variant in following modes (Fig. 1a, b; Fig. 2):
In NF1 patients, MEKi are effective in blocking RAS-MAPK overactivation in benign tumors (low grade gliomas, neurofibromas) that display a “first” and a “second (somatic) NF1 hit” (Knudson’s hypothesis).
In NF1 patients, MEKi might also be very useful to block RAS-MAPK overactivation in non-tumor lesions. Promising pre-clinical approaches demonstrated positive effects on bone lesions and fracture healing (see below). Even NF1 associated myopathic features or intimal proliferation (neointima) are successfully targeted or rescued with MEKi such as PD0325901 in murine models [24, 25].
In tumors being unassociated with NF1, pathogenic variants of the NF1 gene occur additionally to other genetic events on the somatic level. Here, MEKi need to be applied within combination therapy. Even in NF1-associated malignant tumors such as in MPNST, multi-step mutational processes afford combination therapy.
Fig. 2.
MEK inhibitors neutralize pathogenic NF1 mutations. Legend: Main applications MEK inhibitors: MEKi can be principally applied for NF1 associated benign lesions or malignant tumors that harbor NF1 gene pathogenic variants
MEKi therapy remodels the kinome activity and gene expression in NF1 mutant tumor cells [21]. Therefore, it is not surprising that MEKi therapy leads to multiple toxicities among which skin toxicities are most common and require dose adjustments (Fig. 1c). Detailed side effects have been published and recommendations for management have been compiled by the Clinical Care Advisory board of the Children’s tumor Foundation [26]. However, long term experience onof continuous therapies is missing.
MEKi treatment of neurofibromas in Neurofibromatosis type 1
NF1 associated childhood plexiform neurofibromas occur in up to 50% of NF1 patients. They are congenital, can undergo malignant change and cause severe complications by invading neighboring structures. Inhibition of NF1 associated nerve sheath tumor growth by MEKi PD0325901 has been shown in extensive human and murine, in-vitro and pre-clinical studies [27]. For inoperable plexiform neurofibromas, MEKi treatment is now a valuable option, and response is standardized by volumetric MRI measurements [28]. Meanwhile several trials and single case studies using selumitinib, trametinib or PD-0325901 reported size reduction of plexiform and/or spinal neurofibromas in children with NF1 [29–37]. For instance, following up promising data from a phase 1 study, Gross and co-workers recently described durable tumor shrinkage in NF1 patients with plexiform neurofibromas with selumitinib (NCT01362803). They reported partial response in 70% of cases, durable responses over ≥1 year in 28 cases, clinical benefits, but also disease progression in 6 cases [36]. Selumetinib was also successfully applied in NF1 patients with spinal neurofibromas and was shown to reduce tumor burden, effect on the spinal canal, cerebrospinal fluid distribution, and spinal cord shape in 18 of 24 patients [38]. In one pilot study selumetinib is applied to reduce the size and number of neurofibromas in adult NF1 patients (NCT012839720). In summary, MEK inhibitors effectively decrease volume of plexiform neurofibromas and may be also be beneficial in cases with a high cutaneous neurofibroma burden or complicated spinal neurofibromas.
MEKi treatment of low grade glioma in Neurofibromatosis type 1
MEKi selumetinib and trametinib are currently employed in trials for low grade gliomas (LGG) including NF1 patients (NCT03363217, PNOC021, NCT03871257, NCT04166409, NCT04576117, NCT033263388, NCT01089101). LGG are common brain tumors in children. About 20% of NF1 patients develop brain tumors of which pilocytic astrocytoma of the optic pathway is the most common [39]. Only 2–3% of NF1 patients with optic gliomas need standard chemotherapy [21]. Clinical trials with MEKi are implemented only for recurrent or refractive disease. Selective MEKi are clearly superior to multikinase inhibitors [40]. A multi-center study of 18 LGG cases including 8 NF1-related tumors demonstrated disease control in all patients with progressive BRAF, fibroblast growth factor receptor 1 (FGFR1) or NF1 mutated tumors using trametinib [41]. NF1-associated tumors comprised posterior fossa and midline pilocytic astrocytoma as well as other tumors which showed typical DNA methylation profiles. Most NF1-associated LGGs displayed a partial response. One co-occuring plexiform neurofibroma showed a volume reduction of 26% under treatment whereas other non-LGG tumors did not. As already demonstrated for selumetinib, some individuals showed tumor progression after treatment, nevertheless re-challenge seemed to be an option. In two other studies, selumetinib led to a partial response in up to 40% of recurrent LGGs and to a high percentage of progression free survivals. Only one patient had a progressive disease [42, 43]. TRAM-01 (NCT03363217) is a current prospective phase 1 study based on significant responses to trametinib in patients with refractory pediatric LGG [44]. Trametinib was also successfully applied in 5 pediatric cases with NF1 or NF1 mutated LGGs [45–47] as well as in single small cohorts [48].
MEKi treatment of high grade NF1 associated tumors
NF1 patients are predisposed to malignant tumors such as malignant peripheral nerve sheath tumors, glioblastomas, breast cancers, juvenile myelomonocytic leukemia, lymphoblastic leukemia, pheochromocytomas, and rhabdomyosarcomas.
Although predisposition to LGG is more common, NF1 patients are also at risk for high grade gliomas [49]. In contrast to LGG, high grade gliomas harbor a distinct molecular landscape and are enriched in tumor protein 53 (TP53), cyclin dependent kinase inhibitor 2A (CDKN2A), alpha-thalassemia/mental retardation, X-linked (ATRX) and telomerase reverse rranscriptase (TERT) alterations as well as in the chromatin regulation and PI3K pathway alterations [39, 50–52]. In a mouse model, proliferation of malignant glial tumor cells has been shown to depend on MEK as well as PI3K signalling pathways and manifestation of tumors does not depend on a particular germline pathogenic NF1 variant [39, 53]. DNA methylation profiles indicate that NF1 associated gliomas belong to a poorly defined Isocitrate dehydrogenase 1 wild-type subgroup (LGm6, mesenchymal subtype) of sporadic gliomas [39]. MEKi trametinib therapy of NF1 associated high grade gliomas is reported only in single NF1 adult cases: in a 24-year-old male with NF1 and treatment-refractory glioblastoma and in a 19-year-old male with NF1 and a recurrent mesencephalic glioblastoma [54, 55]. In-vitro studies using cell lines, glioblastoma 3D oncospheres or precursor cells demonstrate sensitivity of tumor cells or of mesenchymal transition due to MEKi [52, 56–59].
NF1 associated MPNST are aggressive and infiltrative tumors characterized by high recurrence rates and early metastases. They are responsible for decreased life expectancy in NF1. They derive from benign plexiform neurofibromas and some NF1 patients are at increased risk [21]. So far, sufficient therapies do not exist, and surgical complete resection with adequate margins followed by adjuvant therapies is still the most important protective measure [60]. Loss of CDKN2A/CDKN2B genes in tumor Schwann cells first leads to development of premalignant, atypical neurofibromatosis neoplasms of uncertain biological potential (ANNUBP) [61]. MPNST arise when further somatic alterations occur in Polycomb repressive complex 2 component (PRC2) genes (that cause loss of histone H3 lysine 27 trimethylation) as well as in TP53. Many pre-clinical and clinical approaches to target MPNST have been followed and cannot be fully reviewed here [62]. In murine models, sarcomas generated by loss of NF1 were sensitive to MEKi [63]. Among those, combination of MEKi with other drugs to catch multiple signaling pathways seems most promising. Experimental combination therapies include treatment of human MPNST cells with MEKi PD0235901 and all-trans retinoic acid, with MEKi and bone morphogenic protein 2 type I receptor inhibitor, with MEKi and Src homology region 2 domain-containing phosphatase-2 inhibitor and others [64–66]. Presently, only one trial recruits NF1 patients with MPNST for therapy with MEKi selumitinib in combination with mTOR inhibitor (NCT03433183). Recently it was demonstated that activation of receptor tyrosine kinases HGF/MET mediated resistance to MEKi in MPNST, and points towards a useful combination of MEK and MET inhibition NF1 patients with MPNST [5].
Although rare, NF1 associated leukemias are currently investigated in single trials using trametinib (NCT04439318, NCT03190915). Study reports have not been published so far.
Specific knowledge of the individual genetic landscape in any high grade NF1 associated tumor by comprehensive molecular characterization will drive selection of targeted drugs beyond current approaches and will influence choice of personalized combination therapy.
MEKi treatment of bone abnormalities in Neurofibromatosis type 1
NF1 associated bone dysplasias comprises idiopathic scoliosis, osteopenia, tibial dysplasia, short stature, pseudarthrosis, sphenoid wing dysplasia, and chest wall deformaties [67, 68]. Therapies are still challenging and patients often need repetitive surgery. Alike in other benign NF1 associated lesions, inactivation of both NF1 gene copies is present in skeletal abnormalities due to a “second hit” leading to overactivation of the RAS-MAPK signaling cascade [69–72]. This affects early bone osteoblasts and impairs bone formation and fracture healing [73–75]. Additionally, ERK was shown to be important for osteoclast functions [76]. Deletion of NF1 in osteoprogenitor cells in mice led to upregulation of inorganic pyrophosphate pathway related genes which finally inhibited hydroxyapatite formation and bone mineralization dependent of MEK [77]. Limited pre-clinical studies demonstrated induction of osteoblast differentiation and bone healing with combined MEKi PD98059 or trametinib and bone morphogenetic protein 2 treatment [73, 75, 77]. Recently, it was hypothesized that treatment of neurofbromas with MEKi may also improve skeletal lesions since selumitinib positively affected dysregulation of pyrophosphate homeostasis in adjacent NF tumors and partially rescued reduced tumor-associated bone mineral density in a patient [78]. In a murine fracture model of NF1 pseudarthrosis MEKi therapy with PD0325901 in combination with bone morphogenetic protein 2 led to significantly increased bone volume [79]. Recently, combination of MEKi PD0325901 with bisphosphonate zoledronic acid improved bone morphogenetic protein 2 induced spine fusion in a modified murine NF1 model [80]. In these experiments, MEKi increased bone volume and bisphosphonate zoledronic acid increased bone density versus bone morphogenetic protein 2 alone indicating a complex interaction of bone formation, deposition of fibrous tissue and repair. Beside the role of RAS-MAPK cascade in bone abnormalities in NF1 other signalling pathways such as aberrant jun N-terminate kinase (JNK) activity seem to be important for osteogenesis in NF1 [81]. These preclinical studies indicate that MEKI may be a powerful tool for therapy of severe orthopaedic problems in NF1 which would substantially improve life quality.
Conclusions
MEKi therapy has become an important and highly selective tool to neutralize mutational events of the RAS-MAPK signaling cascade affected in Neurofibromatosis.
They seem especially promising for therapy of Neurofibromatosis type 1 patients with refractive low grade gliomas, inoperable plexiform and other neurofibromas as well as other non-tumor lesions that mainly depend on RAS-MAPK overactivation. Malignant tumors that harbor pathogenic NF1 variants additionally to other genetic events show increased response when MEK inhibition is combined with other therapeutics.
Acknowledgements
None.
Abbreviations
- ANNUBP
Atypical neurofibromatosis neoplasms of uncertain biological potential
- ATRX
Alpha-thalassemia/mental retardation, X-linked
- AKT1
Serine/threonine protein kinase 1
- ATP
Adenosine tri-phophate
- BRAF
B-RAF
- CALM
Café-au-lait macules
- CDKN2A/B
Cyclin dependent kinase inhibitor 2A/B
- FDA
Food and drug administration
- FGFR1
Fibroblast growth factor receptor 1
- ERK
Extracellular signal-related kinase
- GAP
GTPase activating protein
- GTP
Guanosine tri-phosphate
- GRD
GAP related domain
- HGF
Hepatocyte growth factor
- JNK
Jun N-terminate kinase
- KRAS
Kirsten rat sarcoma virus
- LGG
Low grade glioma
- LZTR1
Leucine zipper like transcription regulator 1
- MAP
Mitogen activated protein kinase
- MAPK
Mitogen activated protein kinase kinase
- MAPKK
Mitogen activated protein kinase kinase (MAP 3 K)
- MEK
MAP/ERK kinase
- MEKi
MEK inhibitor
- MET
HGF receptor
- MPNST
Malignant peripheral nerve sheath tumor
- NF1
Neurofibromatosis type 1
- NF2
Neurofibromatosis type 2
- PI3K
Phosphatidylinositol 3-kinase
- NRAS
Neuroblastoma RAS
- PRC2
Polycomb repressive complex 2
- PTEN
Phosphatase and tensin homolog
- PP2A
Protein phosphatase 2
- RAF
Rapidly accelerated fibrosarcoma
- RAS
Rat sarcoma
- SMARCB1
SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1
- STAT
Signal transducer and activator of transcription
- TP53
Tumor protein 53
- TERT
Telomerase reverse transcriptase
Author’s contributions
The author (s) read and approved the final manuscript.
Funding
Writing the manuscript was not funded. Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. Aall information in this review can be found in the reference list.
Declarations
Ethics approval and consent to participate
No ethics approval was required for this review that did not involve patients or patient data.
Consent for publication
Not applicable.
Competing interests
The author declares that she has no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou L, Hanemann CO. Merlin, a multi-suppressor from cell membrane to the nucleus. FEBS Lett. 2012;586(10):1403–1408. doi: 10.1016/j.febslet.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Fuse MA, Dinh CT, Vitte J, Kirkpatrick J, Mindos T, Plati SK, Young JI, Huang J, Carlstedt A, Franco MC, Brnjos K, Nagamoto J, Petrilli AM, Copik AJ, Soulakova JN, Bracho O, Yan D, Mittal R, Shen R, Telischi FF, Morrison H, Giovannini M, Liu XZ, Chang LS, Fernandez-Valle C. Preclinical assessment of MEK1/2 inhibitors for neurofibromatosis type 2-associated schwannomas reveals differences in efficacy and drug resistance development. Neuro-Oncology. 2019;21(4):486–497. doi: 10.1093/neuonc/noz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kun E, Tsang YTM, Ng CW, Gershenson DM, Wong KK. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat Rev. 2021;92:102137. doi: 10.1016/j.ctrv.2020.102137. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Pollard K, Calizo A, Pratilas CA. Activation of receptor tyrosine kinases mediates acquired resistance to MEK inhibition in malignant peripheral nerve sheath tumors. Cancer Res. 2021;81(3):747–762. doi: 10.1158/0008-5472.CAN-20-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Lu XY, Shi WY, Mao FJ, Yang XY, Luo YB, Li W. Combined mTOR/MEK inhibition prevents proliferation and induces apoptosis in NF2-mutant tumors. Eur Rev Med Pharmacol Sci. 2019;23(13):5874–5883. doi: 10.26355/eurrev_201907_18331. [DOI] [PubMed] [Google Scholar]
- 7.Yesiloz U, Kirches E, Hartmann C, Scholz J, Kropf S, Sahm F, et al. Frequent AKT1E17K mutations in skull base meningiomas are associated with mTOR and ERK1/2 activation and reduced time to tumor recurrence. Neuro-Oncology. 2017;19(8):1088–1096. doi: 10.1093/neuonc/nox018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon JH, Hong SW, Kim JE, Shin JS, Kim JS, Jung SA, Ha SH, Lee S, Kim J, Lee DH, Park YS, Kim DM, Park SS, Hong JK, Kim DY, Kim EH, Jung J, Kim MJ, Kim SM, Deming DA, Kim K, Kim TW, Jin DH. Targeting beta-catenin overcomes MEK inhibition resistance in colon cancer with KRAS and PIK3CA mutations. Br J Cancer. 2019;120(9):941–951. doi: 10.1038/s41416-019-0434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan ZM, Real AM, Marsiglia WM, Chow A, Duffy ME, Yerabolu JR, et al. Structural basis for the action of the drug trametinib at KSR-bound MEK. Nature. 2020;588(7838):509-14. 10.1038/s41568-020-2760-4. [DOI] [PMC free article] [PubMed]
- 10.Han J, Liu Y, Yang S, Wu X, Li H, Wang Q. MEK inhibitors for the treatment of non-small cell lung cancer. J Hematol Oncol. 2021;14(1):1. doi: 10.1186/s13045-020-01025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrilli AM, Fernandez-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene. 2016;35(5):537–548. doi: 10.1038/onc.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamenkovic I, Yu Q. Merlin, a "magic" linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci. 2010;11(6):471–484. doi: 10.2174/138920310791824011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jindal GA, Goyal Y, Burdine RD, Rauen KA, Shvartsman SY. RASopathies: unraveling mechanisms with animal models. Dis Model Mech. 2015;8(8):769–782. doi: 10.1242/dmm.020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross AM, Frone M, Gripp KW, Gelb BD, Schoyer L, Schill L, Stronach B, Biesecker LG, Esposito D, Hernandez ER, Legius E, Loh ML, Martin S, Morrison DK, Rauen KA, Wolters PL, Zand D, McCormick F, Savage SA, Stewart DR, Widemann BC, Yohe ME. Advancing RAS/RASopathy therapies: an NCI-sponsored intramural and extramural collaboration for the study of RASopathies. Am J Med Genet A. 2020;182(4):866–876. doi: 10.1002/ajmg.a.61485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blakeley JO, Evans DG, Adler J, Brackmann D, Chen R, Ferner RE, Hanemann CO, Harris G, Huson SM, Jacob A, Kalamarides M, Karajannis MA, Korf BR, Mautner VF, McClatchey AI, Miao H, Plotkin SR, Slattery W, III, Stemmer-Rachamimov AO, Welling DB, Wen PY, Widemann B, Hunter-Schaedle K, Giovannini M. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am J Med Genet A. 2012;158A(1):24–41. doi: 10.1002/ajmg.a.34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White SM, Avantaggiati ML, Nemazanyy I, Di Poto C, Yang Y, Pende M, et al. YAP/TAZ inhibition induces metabolic and signaling rewiring resulting in targetable vulnerabilities in NF2-deficient tumor cells. Dev Cell. 2019;49(3):425–443. doi: 10.1016/j.devcel.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goutagny S, Raymond E, Esposito-Farese M, Trunet S, Mawrin C, Bernardeschi D, Larroque B, Sterkers O, Giovannini M, Kalamarides M. Phase II study of mTORC1 inhibition by everolimus in neurofibromatosis type 2 patients with growing vestibular schwannomas. J Neuro-Oncol. 2015;122(2):313–320. doi: 10.1007/s11060-014-1710-0. [DOI] [PubMed] [Google Scholar]
- 18.Coy S, Rashid R, Stemmer-Rachamimov A, Santagata S. An update on the CNS manifestations of neurofibromatosis type 2. Acta Neuropathol. 2020;139(4):643–665. doi: 10.1007/s00401-019-02029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, Lalloo F. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327–332. doi: 10.1002/ajmg.a.33139. [DOI] [PubMed] [Google Scholar]
- 20.Blakeley JO, Plotkin SR. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro-Oncology. 2016;18(5):624–638. doi: 10.1093/neuonc/nov200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legius E, Brems H. Genetic basis of neurofibromatosis type 1 and related conditions, including mosaicism. Childs Nerv Syst. 2020;36(10):2285–2295. doi: 10.1007/s00381-020-04771-8. [DOI] [PubMed] [Google Scholar]
- 22.Klose A, Ahmadian MR, Schuelke M, Scheffzek K, Hoffmeyer S, Gewies A, Schmitz F, Kaufmann D, Peters H, Wittinghofer A, Nürnberg P. Selective disactivation of neurofibromin GAP activity in neurofibromatosis type 1. Hum Mol Genet. 1998;7(8):1261–1268. doi: 10.1093/hmg/7.8.1261. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadian MR, Kiel C, Stege P, Scheffzek K. Structural fingerprints of the Ras-GTPase activating proteins neurofibromin and p120GAP. J Mol Biol. 2003;329(4):699–710. doi: 10.1016/S0022-2836(03)00514-X. [DOI] [PubMed] [Google Scholar]
- 24.Summers MA, Vasiljevski ER, Mikulec K, Peacock L, Little DG, Schindeler A. Developmental dosing with a MEK inhibitor (PD0325901) rescues myopathic features of the muscle-specific but not limb-specific Nf1 knockout mouse. Mol Genet Metab. 2018;123(4):518–525. doi: 10.1016/j.ymgme.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Stansfield BK, Bessler WK, Mali R, Mund JA, Downing BD, Kapur R, Ingram DA., Jr Ras-Mek-Erk signaling regulates Nf1 heterozygous neointima formation. Am J Pathol. 2014;184(1):79–85. doi: 10.1016/j.ajpath.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klesse LJ, Jordan JT, Radtke HB, Rosser T, Schorry E, Ullrich N, Viskochil D, Knight P, Plotkin SR, Yohay K. The use of MEK inhibitors in Neurofibromatosis type 1-associated tumors and management of toxicities. Oncologist. 2020;25(7):e1109–e1e16. doi: 10.1634/theoncologist.2020-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jessen WJ, Miller SJ, Jousma E, Wu J, Rizvi TA, Brundage ME, Eaves D, Widemann B, Kim MO, Dombi E, Sabo J, Hardiman Dudley A, Niwa-Kawakita M, Page GP, Giovannini M, Aronow BJ, Cripe TP, Ratner N. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123(1):340–347. doi: 10.1172/JCI60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, Barker FG, Connor S, Evans DG, Fisher MJ, Goutagny S, Harris GJ, Jaramillo D, Karajannis MA, Korf BR, Mautner V, Plotkin SR, Poussaint TY, Robertson K, Shih CS, Widemann BC, For the REiNS International Collaboration Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21 Suppl 1):S33–S40. doi: 10.1212/01.wnl.0000435744.57038.af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, Whitcomb P, Martin S, Aschbacher-Smith LE, Rizvi TA, Wu J, Ershler R, Wolters P, Therrien J, Glod J, Belasco JB, Schorry E, Brofferio A, Starosta AJ, Gillespie A, Doyle AL, Ratner N, Widemann BC. Activity of Selumetinib in Neurofibromatosis type 1-related Plexiform Neurofibromas. N Engl J Med. 2016;375(26):2550–2560. doi: 10.1056/NEJMoa1605943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss BPS, Widemann B, et al. NF106: Phase 2 trial of the MEK inhibitor PD-0325901 in adolescents and adults with NF1-related plexiform neurofibromas: an NF clinical trials consortium study. Neuro Oncol. 2018;20(Suppl 2):i143. 10.1093/neuonc/noy059.514.
- 31.Vaassen P, Durr N, Rohrig A, Willing R, Rosenbaum T. Trametinib induces Neurofibroma shrinkage and enables surgery. Neuropediatrics. 2019;50(5):300–303. doi: 10.1055/s-0039-1691830. [DOI] [PubMed] [Google Scholar]
- 32.Caruana M, Hatami A, Marcoux D, Perreault S, McCuaig CC. Isotretinoin for the treatment of severe acneiform eruptions associated with the MEK inhibitor trametinib. JAAD Case Rep. 2020;6(10):1056–1058. doi: 10.1016/j.jdcr.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papalia H, Audic F, Riviere GR, Verschuur A, Andre N. Quick and sustained clinical response to MEK inhibitor I in a NF1 patient with neurofibromas. Ecancermedicalscience. 2018;12:862. doi: 10.3332/ecancer.2018.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burki TK. Selumetinib for children with plexiform neurofibromas. Lancet Oncol. 2017;18(2):e69. doi: 10.1016/S1470-2045(17)30009-8. [DOI] [PubMed] [Google Scholar]
- 35.Coyne GGA, Dombi E, et al. Phase II trial of the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886 Hydrogen Sulfate in adults with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas) J Clin Oncol. 2020;38(15_suppl):3612. doi: 10.1200/JCO.2020.38.15_suppl.3612. [DOI] [Google Scholar]
- 36.Gross AM, Wolters PL, Dombi E, Baldwin A, Whitcomb P, Fisher MJ, Weiss B, Kim AR, Bornhorst M, Shah AC, Martin S, Roderick MC, Pichard DC, Carbonell A, Paul SM, Therrien J, Kapustina O, Heisey K, Clapp DW, Zhang C, Peer CJ, Figg WD, Smith M, Glod J, Blakeley JO, Steinberg SM, Venzon DJ, Doyle LA, Widemann BC. Selumetinib in children with inoperable Plexiform Neurofibromas. N Engl J Med. 2020;382(15):1430–1442. doi: 10.1056/NEJMoa1912735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross AWP, Baldwin A, et al. SPRINT: phase II study of the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886) in children with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PN) J Clin Oncol. 2018;36(15Suppl):10503. doi: 10.1200/JCO.2018.36.15_suppl.10503. [DOI] [Google Scholar]
- 38.Jackson S, Baker EH, Gross AM, Whitcomb P, Baldwin A, Derdak J, et al. The MEK inhibitor selumetinib reduces spinal neurofibroma burden in patients with NF1 and plexiform neurofibromas. Neurooncol Adv. 2020;2(1):vdaa095. doi: 10.1093/noajnl/vdaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Angelo F, Ceccarelli M, Garofano L, Zhang J, Frattini V, et al. The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med. 2019;25(1):176–187. doi: 10.1038/s41591-018-0263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karajannis MA, Legault G, Fisher MJ, Milla SS, Cohen KJ, Wisoff JH, Harter DH, Goldberg JD, Hochman T, Merkelson A, Bloom MC, Sievert AJ, Resnick AC, Dhall G, Jones DTW, Korshunov A, Pfister SM, Eberhart CG, Zagzag D, Allen JC. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro-Oncology. 2014;16(10):1408–1416. doi: 10.1093/neuonc/nou059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selt F, van Tilburg CM, Bison B, Sievers P, Harting I, Ecker J, Pajtler KW, Sahm F, Bahr A, Simon M, Jones DTW, Well L, Mautner VF, Capper D, Hernáiz Driever P, Gnekow A, Pfister SM, Witt O, Milde T. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neuro-Oncol. 2020;149(3):499–510. doi: 10.1007/s11060-020-03640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolaides T, Young Poussaint T, Fangusaro J, Phillips J, Perry A, Turner D, Prados M, Packer RJ, Qaddoumi I, Gururangan S, Pollack IF, Goldman S, Doyle LA, Stewart CF, Boyett JM, Kun LE, Fouladi M. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a pediatric brain tumor consortium (PBTC) study. Neuro-Oncology. 2017;19(8):1135–1144. doi: 10.1093/neuonc/now282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, Banerjee A, Packer RJ, Kilburn LB, Goldman S, Pollack IF, Qaddoumi I, Jakacki RI, Fisher PG, Dhall G, Baxter P, Kreissman SG, Stewart CF, Jones DTW, Pfister SM, Vezina G, Stern JS, Panigrahy A, Patay Z, Tamrazi B, Jones JY, Haque SS, Enterline DS, Cha S, Fisher MJ, Doyle LA, Smith M, Dunkel IJ, Fouladi M. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–1022. doi: 10.1016/S1470-2045(19)30277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perreault S, Larouche V, Tabori U, Hawkin C, Lippe S, Ellezam B, et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer. 2019;19(1):1250. doi: 10.1186/s12885-019-6442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondyli M, Larouche V, Saint-Martin C, Ellezam B, Pouliot L, Sinnett D, Legault G, Crevier L, Weil A, Farmer JP, Jabado N, Perreault S. Trametinib for progressive pediatric low-grade gliomas. J Neuro-Oncol. 2018;140(2):435–444. doi: 10.1007/s11060-018-2971-9. [DOI] [PubMed] [Google Scholar]
- 46.Manoharan N, Choi J, Chordas C, Zimmerman MA, Scully J, Clymer J, Filbin M, Ullrich NJ, Bandopadhayay P, Chi SN, Yeo KK. Trametinib for the treatment of recurrent/progressive pediatric low-grade glioma. J Neuro-Oncol. 2020;149(2):253–262. doi: 10.1007/s11060-020-03592-8. [DOI] [PubMed] [Google Scholar]
- 47.Knight T, Shatara M, Carvalho L, Altinok D, Poulik J, Wang ZJ. Dramatic response to trametinib in a male child with neurofibromatosis type 1 and refractory astrocytoma. Pediatr Blood Cancer. 2019;66(1):e27474. doi: 10.1002/pbc.27474. [DOI] [PubMed] [Google Scholar]
- 48.Paul MR, Pehlivan KC, Milburn M, Yeh-Nayre L, Elster J, Crawford JR. Trametinib-based treatment of pediatric CNS tumors: a single institutional experience. J Pediatr Hematol Oncol. 2020;42(8):e730–e7e7. doi: 10.1097/MPH.0000000000001819. [DOI] [PubMed] [Google Scholar]
- 49.Sellmer L, Farschtschi S, Marangoni M, Heran MK, Birch P, Wenzel R, et al. Non-optic glioma in adults and children with neurofibromatosis 1. Orphanet J Rare Dis. 2017;12(1):34. doi: 10.1186/s13023-017-0588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8(2):119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez PP, Kim J, Galvao RP, Cruickshanks N, Abounader R, Zong H. p53 and NF 1 loss plays distinct but complementary roles in glioma initiation and progression. Glia. 2018;66(5):999–1015. doi: 10.1002/glia.23297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gursel DB, Connell-Albert YS, Tuskan RG, Anastassiadis T, Walrath JC, Hawes JJ, Amlin-van Schaick JC, Reilly KM. Control of proliferation in astrocytoma cells by the receptor tyrosine kinase/PI3K/AKT signaling axis and the use of PI-103 and TCN as potential anti-astrocytoma therapies. Neuro-Oncology. 2011;13(6):610–621. doi: 10.1093/neuonc/nor035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ameratunga M, McArthur G, Gan H, Cher L. Prolonged disease control with MEK inhibitor in neurofibromatosis type I-associated glioblastoma. J Clin Pharm Ther. 2016;41(3):357–359. doi: 10.1111/jcpt.12378. [DOI] [PubMed] [Google Scholar]
- 55.Awada G, Serruys D, Schwarze JK, Van De Voorde L, Duerinck J, Neyns B. Durable complete response of a recurrent Mesencephalic Glioblastoma treated with Trametinib and low-dose Dabrafenib in a patient with Neurofibromatosis type 1. Case Rep Oncol. 2020;13(2):1031–1036. doi: 10.1159/000509773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.See WL, Tan IL, Mukherjee J, Nicolaides T, Pieper RO. Sensitivity of glioblastomas to clinically available MEK inhibitors is defined by neurofibromin 1 deficiency. Cancer Res. 2012;72(13):3350–3359. doi: 10.1158/0008-5472.CAN-12-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson KM, Mathews-Griner LA, Williamson T, Guha R, Chen L, Shinn P, McKnight C, Michael S, Klumpp-Thomas C, Binder ZA, Ferrer M, Gallia GL, Thomas CJ, Riggins GJ. Mutation profiles in Glioblastoma 3D Oncospheres modulate drug efficacy. SLAS Technol. 2019;24(1):28–40. doi: 10.1177/2472630318803749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho HJ, Zhao J, Jung SW, Ladewig E, Kong DS, Suh YL, Lee Y, Kim D, Ahn SH, Bordyuh M, Kang HJ, Sa JK, Seo YJ, Kim ST, Lim DH, Dho YS, Lee JI, Seol HJ, Choi JW, Park WY, Park CK, Rabadan R, Nam DH. Distinct genomic profile and specific targeted drug responses in adult cerebellar glioblastoma. Neuro-Oncology. 2019;21(1):47–58. doi: 10.1093/neuonc/noy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho KH, Chen PH, Shih CM, Lee YT, Cheng CH, Liu AJ, et al. MiR-4286 is involved in connections between IGF-1 and TGF-beta signaling for the mesenchymal transition and invasion by glioblastomas. Cell Mol Neurobiol. 2020. 10.1007/s10571-020-00977-1. [DOI] [PMC free article] [PubMed]
- 60.Cai Z, Tang X, Liang H, Yang R, Yan T, Guo W. Prognosis and risk factors for malignant peripheral nerve sheath tumor: a systematic review and meta-analysis. World J Surg Oncol. 2020;18(1):257. doi: 10.1186/s12957-020-02036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miettinen MM, Antonescu CR, Fletcher CDM, Kim A, Lazar AJ, Quezado MM, Reilly KM, Stemmer-Rachamimov A, Stewart DR, Viskochil D, Widemann B, Perry A. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1–10. doi: 10.1016/j.humpath.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams KB, Largaespada DA. New Model systems and the development of targeted therapies for the treatment of neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Genes (Basel). 2020;11(5):477. 10.3390/genes/11050477. [DOI] [PMC free article] [PubMed]
- 63.Dodd RD, Mito JK, Eward WC, Chitalia R, Sachdeva M, Ma Y, Barretina J, Dodd L, Kirsch DG. NF1 deletion generates multiple subtypes of soft-tissue sarcoma that respond to MEK inhibition. Mol Cancer Ther. 2013;12(9):1906–1917. doi: 10.1158/1535-7163.MCT-13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischer-Huchzermeyer S, Dombrowski A, Wilke G, Stahn V, Streubel A, Mautner VF, Harder A. MEK inhibitors enhance therapeutic response towards ATRA in NF1 associated malignant peripheral nerve sheath tumors (MPNST) in-vitro. PLoS One. 2017;12(11):e0187700. doi: 10.1371/journal.pone.0187700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahsan S, Ge Y, Tainsky MA. Combinatorial therapeutic targeting of BMP2 and MEK-ERK pathways in NF1-associated malignant peripheral nerve sheath tumors. Oncotarget. 2016;7(35):57171–57185. doi: 10.18632/oncotarget.11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Pollard K, Allen AN, Tomar T, Pijnenburg D, Yao Z, Rodriguez FJ, Pratilas CA. Combined inhibition of SHP2 and MEK is effective in models of NF1-deficient malignant peripheral nerve sheath tumors. Cancer Res. 2020;80(23):5367–5379. doi: 10.1158/0008-5472.CAN-20-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elefteriou F, Kolanczyk M, Schindeler A, Viskochil DH, Hock JM, Schorry EK, Crawford AH, Friedman JM, Little D, Peltonen J, Carey JC, Feldman D, Yu X, Armstrong L, Birch P, Kendler DL, Mundlos S, Yang FC, Agiostratidou G, Hunter-Schaedle K, Stevenson DA. Skeletal abnormalities in neurofibromatosis type 1: approaches to therapeutic options. Am J Med Genet A. 2009;149A(10):2327–2338. doi: 10.1002/ajmg.a.33045. [DOI] [PubMed] [Google Scholar]
- 68.Stevenson DA, Little D, Armstrong L, Crawford AH, Eastwood D, Friedman JM, Greggi T, Gutierrez G, Hunter-Schaedle K, Kendler DL, Kolanczyk M, Monsell F, Oetgen M, Richards BS, Schindeler A, Schorry EK, Wilkes D, Viskochil DH, Yang FC, Elefteriou F. Approaches to treating NF1 tibial pseudarthrosis: consensus from the Children's Tumor Foundation NF1 bone abnormalities consortium. J Pediatr Orthop. 2013;33(3):269–275. doi: 10.1097/BPO.0b013e31828121b8. [DOI] [PubMed] [Google Scholar]
- 69.Stevenson DA, Zhou H, Ashrafi S, Messiaen LM, Carey JC, D'Astous JL, et al. Double inactivation of NF1 in tibial pseudarthrosis. Am J Hum Genet. 2006;79(1):143–148. doi: 10.1086/504441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paria N, Cho TJ, Choi IH, Kamiya N, Kayembe K, Mao R, Margraf RL, Obermosser G, Oxendine I, Sant DW, Song MH, Stevenson DA, Viskochil DH, Wise CA, Kim HKW, Rios JJ. Neurofibromin deficiency-associated transcriptional dysregulation suggests a novel therapy for tibial pseudoarthrosis in NF1. J Bone Miner Res. 2014;29(12):2636–2642. doi: 10.1002/jbmr.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sant DW, Margraf RL, Stevenson DA, Grossmann AH, Viskochil DH, Hanson H, Everitt MD, Rios JJ, Elefteriou F, Hennessey T, Mao R. Evaluation of somatic mutations in tibial pseudarthrosis samples in neurofibromatosis type 1. J Med Genet. 2015;52(4):256–261. doi: 10.1136/jmedgenet-2014-102815. [DOI] [PubMed] [Google Scholar]
- 72.Margraf RL, VanSant-Webb C, Mao R, Viskochil DH, Carey J, Hanson H, D’Astous J, Grossmann A, Stevenson DA. NF1 somatic mutation in dystrophic scoliosis. J Mol Neurosci. 2019;68(1):11–18. doi: 10.1007/s12031-019-01277-0. [DOI] [PubMed] [Google Scholar]
- 73.Sharma R, Wu X, Rhodes SD, Chen S, He Y, Yuan J, Li J, Yang X, Li X, Jiang L, Kim ET, Stevenson DA, Viskochil D, Xu M, Yang FC. Hyperactive Ras/MAPK signaling is critical for tibial nonunion fracture in neurofibromin-deficient mice. Hum Mol Genet. 2013;22(23):4818–4828. doi: 10.1093/hmg/ddt333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Hoss J, Sullivan K, Cheng T, Yu NY, Bobyn JD, Peacock L, et al. A murine model of neurofibromatosis type 1 tibial pseudarthrosis featuring proliferative fibrous tissue and osteoclast-like cells. J Bone Miner Res. 2012;27(1):68–78. doi: 10.1002/jbmr.528. [DOI] [PubMed] [Google Scholar]
- 75.de la Croix NJ, Stevens DM, Vignaux G, Uppuganti S, Perrien DS, Yang X, et al. Combined MEK inhibition and BMP2 treatment promotes osteoblast differentiation and bone healing in Nf1Osx −/− mice. J Bone Miner Res. 2015;30(1):55–63. doi: 10.1002/jbmr.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He Y, Staser K, Rhodes SD, Liu Y, Wu X, Park SJ, Yuan J, Yang X, Li X, Jiang L, Chen S, Yang FC. Erk1 positively regulates osteoclast differentiation and bone resorptive activity. PLoS One. 2011;6(9):e24780. doi: 10.1371/journal.pone.0024780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de la Croix NJ, Makowski AJ, Uppuganti S, Vignaux G, Ono K, Perrien DS, et al. Asfotase-alpha improves bone growth, mineralization and strength in mouse models of neurofibromatosis type-1. Nat Med. 2014;20(8):904–910. doi: 10.1038/nm.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma Y, Gross AM, Dombi E, Pemov A, Choi K, Chaney K, Rhodes SD, Angus SP, Sciaky N, Clapp DW, Ratner N, Widemann BC, Rios JJ, Elefteriou F. A molecular basis for neurofibroma-associated skeletal manifestations in NF1. Genet Med. 2020;22(11):1786–1793. doi: 10.1038/s41436-020-0885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El-Hoss J, Cheng T, Carpenter EC, Sullivan K, Deo N, Mikulec K, et al. A combination of rhBMP-2 (recombinant human bone morphogenetic Protein-2) and MEK (MAP kinase/ERK kinase) inhibitor PD0325901 increases bone formation in a murine model of Neurofibromatosis type I Pseudarthrosis. J Bone Joint Surg Am. 2014;96(14):e117. doi: 10.2106/JBJS.M.00862. [DOI] [PubMed] [Google Scholar]
- 80.Bobyn JD, Deo N, Little DG, Schindeler A. Modulation of spine fusion with BMP-2, MEK inhibitor (PD0325901), and zoledronic acid in a murine model of NF1 double inactivation. J Orthop Sci. 2020. 10.1016/j.jos.2020.05.016. [DOI] [PubMed]
- 81.Sullivan K, El-Hoss J, Little DG, Schindeler A. JNK inhibitors increase osteogenesis in Nf1-deficient cells. Bone. 2011;49(6):1311–1316. doi: 10.1016/j.bone.2011.09.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. Aall information in this review can be found in the reference list.