Abstract
Breast cancer is the second common cancer and the leading cause of malignancy among females overall. Breast cancer stem cells (BCSCs) are a small population of breast cancer cells that play a critical role in the metastasis of breast cancer to other organs in the body. BCSCs have both self-renewal and differentiation capacities, which are thought to contribute to the aggressiveness of metastatic lesions. Therefore, targeting BCSCs can be a suitable approach for the treatment and metastasis of breast cancer. Growing evidence has indicated that the Wnt, NFκB, Notch, BMP2, STAT3, and hedgehog (Hh) signaling pathways govern epithelial-to-mesenchymal transition (EMT) activation, growth, and tumorigenesis of BCSCs in the primary regions. miRNAs as the central regulatory molecules also play critical roles in BCSC self-renewal, metastasis, and drug resistance. Hence, targeting these pathways might be a novel therapeutic approach for breast cancer diagnosis and therapy. This review discusses known signaling mechanisms involved in the stimulation or prevention of BCSC self-renewal, metastasis, and tumorigenesis.
Keywords: Breast cancer, Breast cancer stem cells, Signaling pathways, miRNAs, Metastasis, Tumorigenesis
Introduction
Breast cancer (BC) is the most invasive cancer and the second common cause of malignancy among females overall [1–3]. BC involves different areas of the breast (lobules, ducts, and connective tissue) and shows various physiological properties and different clinical outcomes [4, 5]. Based on the cancer response, BC can be divided into estrogen receptor (ER)-positive (response to estrogen signaling), progesterone receptor (PR)-positive (response to progesterone), ER/PR-positive, and human epidermal growth factor receptor-2 (HER2)-positive tumors [6–8]. Triple-negative breast cancer (TNBC) as a subtype of basal-like breast cancer (BLBC) is defined with negative expression of the ER, PR, and HER2 [9]. The main treatments for BC include chemotherapy [10, 11], radiation therapy [12, 13], hormone-blocking therapy [14, 15], surgery [16], and biological treatment [17]. Despite available interventions, these strategies may not always be the best treatment options for targeting BC metastasis [18]. Therefore, a better understanding of the molecular mechanisms involved in tumorigenesis of BC is required to develop more effective therapeutic strategies [19, 20]. Breast cancer stem cells (BCSCs) are a small population of BC cells that play a critical role in the metastasis of BC to other organs in the body [21]. BCSCs have the ability to self-renew and to differentiate into specialized cells that are found in malignancy [22, 23]. Accumulating evidence shows that BCSCs are the leading cause of tumor progression and resistance against conventional therapy [24–26]. Therefore, targeting BCSCs may be an appropriate approach for the treatment of BC [27–30]. This review discusses known signaling mechanisms involved in the stimulation or prevention of BCSC self-renewal, metastasis, and tumorigenesis.
Cellular and molecular characteristics of BCSCs
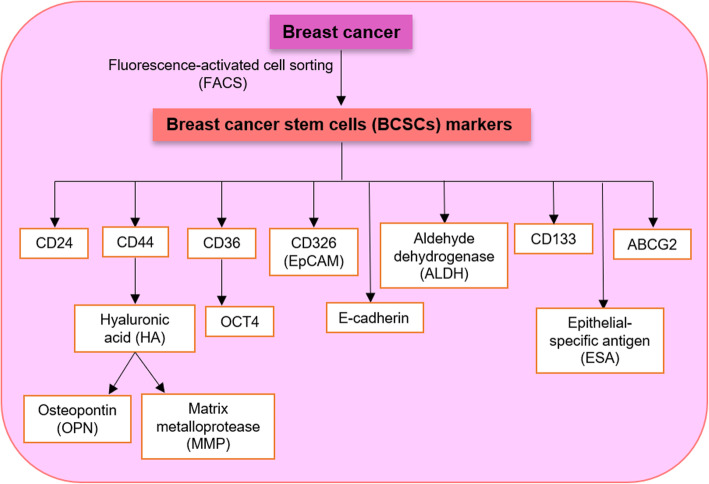
In recent years, the existence of BCSCs or breast cancer-initiating cells (BCICs) in BC has been confirmed [31–33]. BCSCs as a subset of cancer cells exhibit similar properties with normal stem cells [34]. These cells have a slow cell cycle and the potential to divide asymmetrically and to seed tumors when transplanted into a host [35, 36]. BCSCs have antioxidative, tumorsphere formation, tumorigenicity, and chemoresistance properties [37]. Based on cell surface marker expression (by fluorescence-activated cell sorting (FACS)), BCSCs are CD44(+)/CD24(−/low) tumorigenic cells that initiate tumors in xenografts [34, 38]. CD44 is a cell surface glycoprotein and stemness marker in BCSCs [39]. CD44 binds to hyaluronic acid (HA) and mediates the interactions between cell/cell and cell/matrix proteins such as matrix metalloprotease (MMP) and osteopontin (OPN) [37, 40]. Therefore, the HA hydrogel might be an efficient strategy that targets BCSCs [37]. CD24 is a glycosylated cell surface protein that negatively controls the function of CXCR4 (chemokine receptor) and regulates BCSC metastasis and proliferation [18, 41]. Gene expression of embryonic stem cell factors, including Oct4, Nanog, SOX2, and DNA (cytosine-5)-methyltransferase 1 (DNMT1), is observed in BCSCs [36]. It was validated that BCSCs express CD326 (EpCAM), aldehyde dehydrogenase (ALDH), epithelial-specific antigen (ESA), and E-cadherin [42, 43]. The ALDH1 enzyme is a useful therapeutic target that regulates BCSC functions and malignancies [44, 45]. EpCAM by Wnt signaling can stimulate cell adhesion, proliferation, and invasion of BCSCs [46]. CD36 through uptaking fatty acids and induction of STAT3 and nuclear factor kappa B (NFkB) can promote expression of the stem cell marker OCT4, metastasis, and migration of BCSCs [47, 48]. Histone deacetylases (HDACs) such as HDAC1 and HDAC7 play essential roles in BCSC maintenance [49]. CD47, CD133, CD166, CD61, ABCG2, and Lgr5 are the other markers used for the isolation of BCSCs [50] (Fig. 1).
Fig. 1.
Cellular and molecular characteristics of breast cancer stem cells (BCSCs)
There is a high degree of intertumor and intratumor heterogeneity in breast cancer [51, 52]. Thus, a single tumor may contain BCSCs with distinct molecular profiles [53, 54]. Based on immunohistochemical analyses, cells with the CD44+CD24−/low phenotype are not enough to characterize BCSC. Several candidate markers such as the ESA antigen, ALDH1 expression, Prominin-1 (CD133), and CD131 and the capacity to form spheroid can be independent factors for the characterization of BCSCs [49, 55, 56].
Critical signaling pathways involved in the stimulation or prevention of BCSC propagation and metastasis
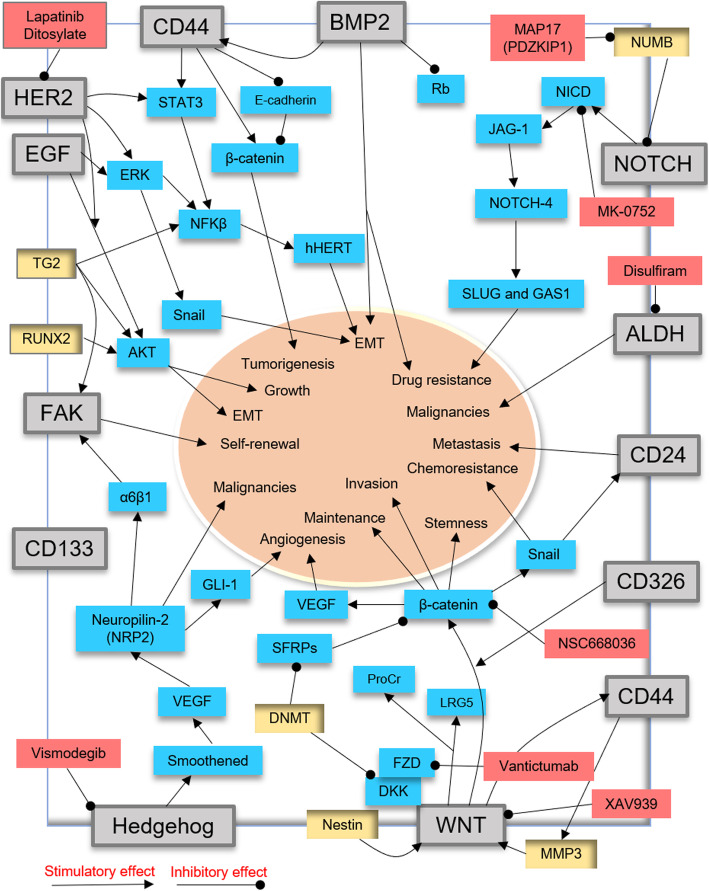
Tumor microenvironment and signaling pathways have critical roles in the propagation and differentiation of BCSCs [57, 58] (Fig. 2). Growing evidences have indicated that the Wnt/β-catenin, NFκB, BMP2, Notch, STAT3, and hedgehog (Hh) signaling pathways govern epithelial-to-mesenchymal transition (EMT) activation, growth, and tumorigenesis of BCSCs in the primary regions [38, 59–62]. However, many of these crucial signaling pathways play important functional roles in normal stem cells [63, 64]. Several specific molecules, including NFkB, BCL6, SOX2, FOXC2, and hypoxia-inducible factor-1 (HIF1), have been contributed to BCSC EMT and malignancies [39]. Recent studies showed that the Wnt-, STAT3-, HDAC-, and estrogen receptor alpha (ESR1)-related pathways can cause TNBC-associated BCSCs (TNBCSC) to undergo unexpected differentiation, EMT, and metastasis [49, 65].
Fig. 2.
Critical signaling pathways involved in breast cancer stem cell (BCSC) propagation and metastasis
Signaling pathways induced by CD44 in BCSCs
CD44 is known to cooperate with the receptor tyrosine kinase (RTK) and regulate BCSC proliferation and migration [39]. Blockade of CD44 impairs the properties of BCSCs, including cell adhesion, malignancy, progression, metastasis, EMT, and therapy resistance [39, 66]. Several signaling pathways such as Wnt/β-catenin, PI3K/Akt, Ras-MAPK, and Rho GTPases are stimulated by CD44 [67, 68]. Thus, CD44 may be a predictor biomarker for BCSC isolation and therapy resistance [69]. However, CD44 is not a suitable marker for the detection of luminal BCSCs (CD44−/CD24+ or CD44−/CD24−) [39, 70]. CD44 can suppress the formation of the E-cadherin/β-catenin complex and enhance nuclear β-catenin and genes related to cell invasion and tumorigenesis in BCSCs [71]. CD44 also interacts with STAT3 and NF-kB to activate the catalytic subunit of telomerase (hTERT), enhance metastasis, and trigger the EMT process in BCSCs [69, 72, 73].
WNT signaling
The Wnt pathway plays a pivotal role in BCSC phenotype shaping, proliferation, migration, chemoresistance, and radioresistance [62, 74]. Canonical and noncanonical Wnt signaling by targeting CD44 promotes the “stemness” of BCSCs [75, 76]. The Wnt/β-catenin/TCF4 axis through the Snail protein promotes the expression of miR-125b and chemoresistance in BCSCs [77]. Snail enhances the expression of CD44 marker and ALDH activity in BCSCs [78]. Protein C receptor (ProCr) and LRG5 are novel Wnt targets and potent biomarkers of BCSCs [79, 80]. MMP3 has been proven that targets Wnt signaling and contributes to the maintenance of BCSCs [81]. Nestin is a type VI intermediate filament protein that positively targets the Wnt/β-catenin pathway and enhances the metastatic ability of BCSCs [82]. DNMT has been proposed to inactivate the cytoplasmic β-catenin antagonists such as secreted frizzled-related proteins (SFRPs) and DICKKOPF protein (DKK) and to promote the expression of Wnt/β-catenin signaling in BCSCs [83, 84]. Therefore, targeting Wnt/β-catenin signaling may be a potent marker for removing BCSCs [18, 71]. Recent studies showed that NSC668036 targeted the PDZ domain and suppressed Disheveled (Dvl) and FZD interactions in Wnt/β-catenin signaling (pre-clinical trial) [85]. OMP-18R5 (Vantictumab) is a monoclonal antibody against FZD receptors that targets FZDs and blocks BCSC growth (phase I) [86]. XAV939 has been demonstrated that interacts with the type 1 and 2 tankyrase-binding domain (TBD) of the Axin molecule and blocks Wnt/β-catenin signaling in BCSCs (phase I) [85]. PKF118-310 (PKF) is a small molecule inhibitor of Wnt/β-catenin signaling that targets BCSCs in a HER2-overexpressing mouse model [87]. Pyrvinium pamoate (PP) is an anti-helminthic drug and a WNT pathway suppressor that inhibits the expression of the NANOG, SOX2, and OCT4 genes, and the growth of BCSCs [88].
BMP2 signaling
In breast cancer xenograft models, BMP-2 can promote EMT transition and bone metastasis [89]. BMP2 via targeting CD44 expression and suppressing the Rb signaling pathway induces EMT, stemness, and chemoresistance in BCSCs [89]. Activation of the PI3K/Akt pathway as well as Rb interaction with CD44 has been shown to play essential roles in BMP-2-dependent EMT in BCSCs [89]. However, BMPs are able to cause G1 arrest, increase apoptosis, and suppress BCSC proliferation [90]. Therefore, the BMP family may have dual behaviors in stimulation or suppression of BCSCs [91]. Thus, employing BMP family inhibitors may be useful for targeting BCSCs [49, 92, 93].
Hedgehog signaling
Hedgehog signaling by interaction with the Smoothened (SMO) protein can influence BCSC stemness and malignancies [49, 94]. Neuropilin-2 (NRP2) is a VEGF receptor that stimulates the expression of glioma-associated oncogene-1 (GLI-1) and α6β1 integrins and contributes to BCSC initiation [95]. GLI-1 by promoting angiogenesis accelerates BCSC progression [96]. Studies suggest that α6β1 integrins can trigger focal adhesion kinase (FAK) signaling and mediate BCSC self-renewal ability [97]. Therefore, targeting the VEGF/NRP2, α6β1, GLI1, and FAK signaling pathways can provide an attractive strategy for BC treatment [98]. Genistein is an isoflavone component present in soy products that has been shown to suppress hedgehog downstream signaling and block BCSC growth and survival [99]. Besides, aqueous extract of Trametes robiniophila Murr (Huaier) by blocking hedgehog downstream signaling can decrease BCSC growth, self-renewal, and proliferation [100, 101].
Notch signaling
The Notch pathway through JAG-1 and NOTCH-4 can stimulate and maintain the invasion, mesenchymal-like properties, and drug resistance of BCSCs [102, 103]. NOTCH4 by targeting SLUG and GAS1 is involved in BC development [104]. In normal cells, the NUMB protein blocks the Notch intracellular domain (NICD) in the cytoplasm and inhibits the Notch pathway. miR-146a has been reported to suppress the function of NUMB, activate the Notch pathway, and trigger the formation of BCSCs [105]. Thus, downregulation of miR-146a and miR-146b expression may weaken the capacity for self-renewal in BCSCs [106]. MAP 17 (PDZKIP1) is a small cargo protein that negatively regulates the NUMB activity, activates the Notch pathway, and promotes the maintenance of BCSCs [107]. 6-Shogaol as a ginger-derived compound by targeting the expression of the Notch-Hes1-Cyclin D1 (CYLD) axis can suppress autophagy and apoptosis and then blocks the growth of BCSCs [108]. MK-0752 is a gamma-secretase inhibitor that inhibits the NICD domain and targets the BCSC population (phases I and II) [109]. Vismodegib (GDC-0449) is a Notch/hedgehog inhibitor drug that inhibits BCSC growth in tamoxifen-resistant breast cancer (phase I) [110] (http://clinicaltrials.gov).
PI3K-AKT signaling
Epidermal growth factor receptor (EGFR/HER)-related signaling have been implicated in the pathogenesis of BCSCs and resistance to chemotherapeutic drugs [111, 112]. This signaling activates molecules such as STAT3, protein kinase B (PKB or AKT), and tyrosine kinase Src and stimulates the MAPK (Ras/Raf/Mek/Ek), PI3K/Akt, and STATs pathways [35]. PI3K-AKT signaling is required for BCSC phenotype, EMT, and drug resistance [113]. The role of PI3K/Akt in BCSCs may be mediated by HER2 [114]. HER1- and HER2-positive BCSCs are able to self-renew [115, 116]. HER2 dysregulation leads to a rise in the phosphorylation of Akt in the ALDH+ population of BCSCs [35]. Therefore, the effects of HER2 signaling in BCSCs can be increased through the PI3K/Akt pathway [35]. Transglutaminase (TG2) has been shown that stimulates NFkβ, Akt, and FAK signaling and initiates BCSC growth and survival [117]. Tbox transcription factor 3 (Tbx3) through FGF signaling is associated with BCSC phenotypes and oncogenesis [118, 119]. In addition, the ZFHX3 transcription factor by enhancing TBX3 transcription increases the proliferation and tumor growth of BCSCs [120]. A recent study indicated that Runt-related transcription factor 2 (RUNX2) by activating the PI3K/AKT pathway contributes to tumorigenicity, metastasis, and EMT in BCSCs [121]. It has been reported that Disulfiram (DS) as an anti-alcoholism drug can inhibit NFκB activation, inhibit ALDH enzymatic activity, and reduce BCSC stemness and chemoresistance [122]. Everolimus (RAD001) is a PI3K/Akt/mTOR pathway inhibitor that blocks BCSC growth [123, 124]. Lapatinib ditosylate (NCT01868503) by targeting EGFR/HER2 impacts on the subpopulation of BCSCs (phase II) [125] (http://clinicaltrials.gov/).
Signaling pathways induced by miRNAs in BCSCs
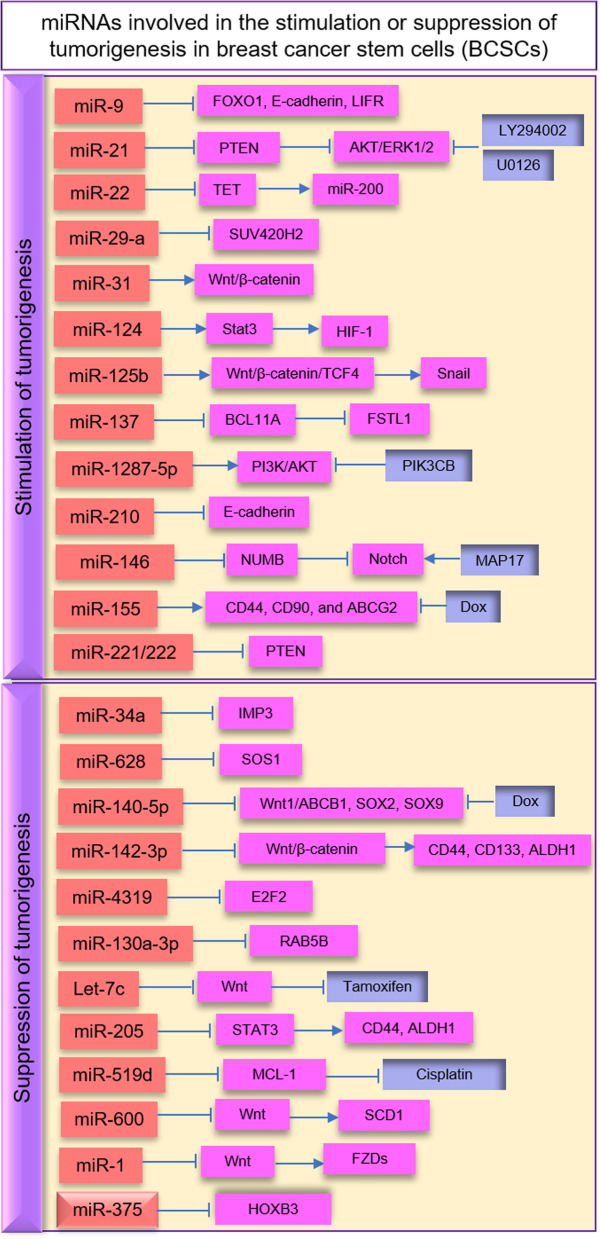
Recently, studies have been suggested that epigenetic changes such as DNA methylation and histone modifications enhance the events of BCSC metastasis [126–128]. miRNAs are epigenetic modulators that target mRNAs without modifying the gene sequences [129, 130]. In human cancer, miRNA expression can be controlled by epigenetic modifications [131]. miRNAs also play an important role in the proliferation, migration, and invasion of BCSCs [132–135]. The expression of microRNAs can be deregulated in BCSCs [130]. Several miRNAs including mir-21, mir-22, mir-29a, and mir-221/222 increase tumorigenesis, and miR-34a, miR-628, miRNA-140-5p, and miRNA-4319 decrease metastasis in BCSCs [39, 136, 137].
Stimulation of tumorigenesis
miRNAs as the central regulatory molecules serve critical roles in BCSC self-renewal, metastasis, and drug resistance [138–140] (Fig. 3). miR-21 by targeting the phosphatase and tensin homolog (PTEN) protein stimulates AKT/ERK1/2 signaling and contributes to the BCSC progression, EMT, and metastasis [113]. LY294002 and U0126 as the inhibitors of the PI3K-AKT and ERK1/2 pathways suppress EMT and BCSC phenotype [113]. miR-22 has been shown to target the TET (ten eleven translocation) family of methylcytosine dioxygenases and inhibit demethylation of the miR-200 promoter, promote EMT, BCSC stemness, and metastasis [141]. miR-31 targets Wnt/β-catenin signaling and increases BCSC stemness and tumorigenesis [142]. It has been evident that miR-29a represses SUV420H2 (a histone methyltransferase) and promotes EMT progress, migration, and metastasis in BCSCs [143]. miR-124 through targeting STAT3 regulates the HIF-1 pathway and enhances doxorubicin (DOX) resistance of BCSCs [144]. miR-125b has been suggested that targets the Snail protein and increases the CD44+ and chemoresistance BCSCs [77]. miR-1287-5p through PI3K/AKT signaling plays critical roles in the prognosis and survival of BCSCs [145]. PIK3CB is a PI3Kinase pathway chemical inhibitor that interacts with miR-1287-5p and suppresses breast carcinogenesis [145]. It has been validated that miR-137 via targeting BCL11A (a zinc-finger transcription factor) and Wnt signaling enhances FSTL1 levels and chemoresistance in BCSCs [146]. The hypoxic microenvironment around BCSCs can induce the expression of miR-210. Hypoxia-mediated miR-210 by targeting E-cadherin improves BCSC invasion and proliferation [147]. miR-155 enhances BCSC stemness markers, including CD44, CD90, and ABCG2. Thus, downregulation of miR-155 promotes DOX sensitivity in BCSC [148]. It has been shown that miR-9 and miR-221 via targeting multiple genes involved with carcinogenesis are able to promote BCSC stemness and the capacity for tumor cell renewal [149]. miR-9 by targeting forkhead box O1 (FOXO1), E-cadherin, and leukemia inhibitory factor receptor (LIFR) promotes the BCSC recurrence and invasiveness [150]. miR-221/222 has been reported to regulate the expression of PTEN and enhance the growth and maintenance of BCSC [151]. Some findings suggest that miR-146a and miR-146b by targeting the Notch pathway are involved in the development of BCSCs [105].
Fig. 3.
miRNAs involved in the stimulation or suppression of tumorigenesis in breast cancer stem cells (BCSCs)
Suppression of tumorigenesis
Some miRNAs may act as tumor suppressors and overcome tumorigenesis and drug resistance in BSCS [138, 152]. miR-34a is an important miRNA that targets the insulin-like growth factor II (IGFII), mRNA-binding protein (IMP3)-induced stemness, and Wnt/β-catenin signaling and decreases BCSC self-renewal [153]. miR-628 by targeting SOS Ras/Rac guanine nucleotide exchange factor 1 (SOS1) inhibits BCSC migration and invasion [154]. miR-140-5p as a critical tumor suppressor blocks the Wnt/β-catenin, SOX2, and SOX9 pathways and inhibits the growth, tumorsphere formation, and progression of BCSCs [155, 156]. This miRNA through the Wnt1/ABCB1 pathway promotes the sensitivity of BCSCs to Dox [156]. miR-142-3p by targeting β-catenin pathway can reduce CD44, CD133, ALDH1, and radioresistance in BCSCs [157]. miR-4319 can suppress the expression of the E2F2 transcription factor and decrease the tumorigenicity of TNBCSC [158]. Another investigation shows that miR-130a-3p by targeting the expression of RAB5B (member of RAS oncogene family) inhibits the carcinogenic features of BCSCs [159, 160]. miRNA Let-7 has been shown to block the Wnt pathway, inhibit the growth and stemness of BCSCs, and promote the anti-cancer effect of tamoxifen (a chemotherapeutic drug) [161]. Recent work has also identified Let-7c through estrogen-activated Wnt signaling can suppress the self-renewal abilities of BCSCs [162]. miR-205 via modulating STAT3 signaling reduces the expression of CD44 and ALDH1 stem-cell markers and inhibits BCSC migration and invasion [163]. miR-519d by targeting MCL-1 (a member of the proapoptotic Bcl-2 family) increases the sensitivity of BCSC to cisplatin (a chemotherapeutic drug) [164]. miR-600 through the Wnt pathway targets stearoyl desaturase 1 (SCD1) and reduces BCSC self-renewal and tumorigenicity [165]. Also, miRNA-1 has been identified that targets frizzled receptors (FZDs) in the Wnt pathway and decreases BCSC proliferation and metastasis [166]. miR-375 by degrading the HOXB3 gene reduces BCSC phenotypes, EMT, and tamoxifen resistance [167]. Therefore, tumor-suppressing miRNAs with their functional pathways could be introduced as an effective strategy for targeting BCSCs [168, 169].
Conclusion
Several signal transduction pathways, including Wnt/β-catenin, hedgehog, Notch, BMPs, and PI3K/Akt/NFkB, are deregulated in BCSCs. These signaling pathways stimulate proliferation, migration, invasion, EMT, chemotherapy, and radiotherapy resistance in BCSCs. miRNAs also through several signaling pathways can regulate the stemness features and tumorigenesis of BCSCs. Inhibition of key signaling pathways with small molecule inhibitors, nanoparticles, herbal medicine, and genetic modifications might be effective therapeutic approaches for targeting BCSCs [31, 85, 170, 171].
Acknowledgements
Xuzhou Key Research and Development Plan, KC19078.
Abbreviations
- ALDH
Aldehyde dehydrogenase
- BC
Breast cancer
- BCICs
Breast cancer-initiating cells
- BCSCs
Breast cancer stem cells
- BLBC
Basal-like breast cancer
- DKK
DICKKOPF protein
- Dox
Doxorubicin
- DNMT
DNA methyltransferase
- ESA
Epithelial-specific antigen
- EMT
Epithelial-to-mesenchymal transition
- EGFRs
Epidermal growth factor receptors
- ER
Estrogen receptor
- FAK
Focal adhesion kinase
- FACS
Fluorescence-activated cell sorting
- GLI-1
Glioma-associated oncogene-1
- HA
Hyaluronic acid
- HER2
Human epidermal growth factor receptor-2
- Hh
Hedgehog
- hTERT
Human telomerase
- IGFII
Insulin-like growth factor II
- IMP3
IGFII mRNA-binding protein
- MMP3
Matrix metalloproteinase 3
- NFkB
Nuclear factor kappa B
- NRP2
Neuropilin-2
- OCT4
Transcription factor 4
- OPN
Osteopontin
- PKB
Protein kinase B
- PR
Progesterone receptor
- ProCr
Protein C receptor
- PTEN
Phosphatase and tensin homolog
- RUNX2
Runt-related transcription factor 2
- RTK
Receptor tyrosine kinase
- SCD1
Stearoyl desaturase 1
- SFRPs
Secreted frizzled-related proteins
- SOS1
SOS Ras/Rac guanine nucleotide exchange factor 1
- SMO
Smoothened
- Tbx3
Tbox transcription factor 3
- TG2
Transglutaminase
- TET
Ten eleven translocations
- TNBCSC
TNBC-associated BCSCs
Authors’ contributions
Dr. Kai Song and Dr. Maryam Farzaneh have made contributions to the writing of the manuscript, the design of the figures, and the revision of the manuscript. All authors have approved the submitted version of the article and have agreed to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kai Song, Email: songkai2534@163.com.
Maryam Farzaneh, Email: maryamfarzaneh2013@yahoo.com.
References
- 1.Sopik V, Sun P, Narod SA. Impact of microinvasion on breast cancer mortality in women with ductal carcinoma in situ. Breast Cancer Res Treat. 2018;167:787–795. doi: 10.1007/s10549-017-4572-2. [DOI] [PubMed] [Google Scholar]
- 2.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15:408–414. doi: 10.1016/j.jacr.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, Ji X, Liu W, Huang B, Luo W, Liu B, Lei Y, Du S, Vuppalapati A, Luu HH, Haydon RC, He T-C, Ren G. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106. doi: 10.1016/j.gendis.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali SMNM. Sudan University of science and technology. 2019. Characterization of female breast tumors using ultrasonography. [Google Scholar]
- 5.Galea M. Benign breast disorders. Surgery (Oxford). 2019;37:151–6.
- 6.Rueda OM, Sammut S-J, Seoane JA, Chin S-F, Caswell-Jin JL, Callari M, Batra R, Pereira B, Bruna A, Ali HR. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature. 2019;567:399–404. doi: 10.1038/s41586-019-1007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia Y, Shi L, Yun F, Liu X, Chen Y, Wang M, Chen C, Ren Y, Bao Y, Wang L. Transcriptome sequencing profiles reveal lncRNAs may involve in breast cancer (ER/PR positive type) by interaction with RAS associated genes. Pathol Res Pract. 2019;215:152405. doi: 10.1016/j.prp.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Gao X, Dong Q-Z. Advance in metabolism and target therapy in breast cancer stem cells. World J Stem Cells. 2020;12:1295. doi: 10.4252/wjsc.v12.i11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin L, Duan J-J, Bian X-W, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:61. doi: 10.1186/s13058-020-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons J, Maaskant-Braat A, Luiten E, Leidenius M, van Nijnatten T, Boelens P, Koppert L, van der Pol C, van de Velde C, Audisio R. Reply to: sentinel node biopsy after neoadjuvant chemotherapy for breast cancer in patients with pre-treatment node-positive: recommendation to optimize the performance. Eur J Surg Oncol. 2020;46:218–219. doi: 10.1016/j.ejso.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Thakur V, Kutty RV. Recent advances in nanotheranostics for triple negative breast cancer treatment. J Exp Clin Cancer Res. 2019;38(1):430. doi: 10.1186/s13046-019-1443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demissei BG, Freedman G, Feigenberg SJ, Plastaras JP, Maity A, Smith AM, McDonald C, Sheline K, Simone CB, II, Lin LL. Early changes in cardiovascular biomarkers with contemporary thoracic radiation therapy for breast cancer, lung cancer, and lymphoma. Int J Radiat Oncol Biol Phys. 2019;103:851–860. doi: 10.1016/j.ijrobp.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster B, Sindhu K, Hepel J, Wazer D, Graves T, Taneja C, Wiggins D, Leonard K. Three-dimensional bioabsorbable tissue marker placement is associated with decreased tumor bed volume among patients receiving radiation therapy for breast cancer. Pract Radiat Oncol. 2019;9:e134–e141. doi: 10.1016/j.prro.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 14.J-w L, G-y L, Y-j J, Yan X, Pang D, Z-f J, D-d C, Zhang B, Xu B-h, Z-m S. Switching to anastrozole plus goserelin vs continued tamoxifen for adjuvant therapy of premenopausal early-stage breast cancer: preliminary results from a randomized trial. Cancer Manag Res. 2019;11:299. doi: 10.2147/CMAR.S183672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakatsukasa K, Koyama H, Ouchi Y, Sakaguchi K, Fujita Y, Matsuda T, Kato M, Konishi E, Taguchi T. Effects of denosumab on bone mineral density in Japanese women with osteoporosis treated with aromatase inhibitors for breast cancer. J Bone Miner Metab. 2019;37:301–306. doi: 10.1007/s00774-018-0917-0. [DOI] [PubMed] [Google Scholar]
- 16.Landercasper J, Ramirez LD, Borgert AJ, Ahmad HF, Parsons BM, Dietrich LL, Linebarger JH. A reappraisal of the comparative effectiveness of lumpectomy versus mastectomy on breast cancer survival: a propensity score–matched update from the National Cancer Data Base (NCDB) Clin Breast Cancer. 2019;19:e481–e493. doi: 10.1016/j.clbc.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Tang J-Y, Ho Y, Chang C-Y, Liu H-L. Discovery of novel irreversible HER2 inhibitors for breast cancer treatment. J Biomed Sci Eng. 2019;12:225. doi: 10.4236/jbise.2019.124016. [DOI] [Google Scholar]
- 18.Sin WC, Lim CL. Breast cancer stem cells—from origins to targeted therapy. Stem Cell Investig. 2017;4;96. [DOI] [PMC free article] [PubMed]
- 19.Chiotaki R, Polioudaki H, Theodoropoulos PA. Stem cell technology in breast cancer: current status and potential applications. Stem Cells Cloning. 2016;9:17. doi: 10.2147/SCCAA.S72836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suter R, Marcum JA. The molecular genetics of breast cancer and targeted therapy. Biologics. 2007;1:241–258. [PMC free article] [PubMed] [Google Scholar]
- 21.Luo M, Brooks M, Wicha MS. Epithelial-mesenchymal plasticity of breast cancer stem cells: implications for metastasis and therapeutic resistance. Curr Pharm Des. 2015;21(10):1301–1310. doi: 10.2174/1381612821666141211120604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23(43):7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 23.Gangopadhyay S, Nandy A, Hor P, Mukhopadhyay A. Breast cancer stem cells: a novel therapeutic target. Clin Breast Cancer. 2013;13:7–15. doi: 10.1016/j.clbc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Olivares-Urbano MA, Griñán-Lisón C, Marchal JA, Núñez MI. CSC radioresistance: a therapeutic challenge to improve radiotherapy effectiveness in cancer. Cells. 2020;9(7):1651. doi: 10.3390/cells9071651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Angelis ML, Francescangeli F, Zeuner A. Breast cancer stem cells as drivers of tumor chemoresistance, dormancy and relapse: new challenges and therapeutic opportunities. Cancers (Basel) 2019;11(10):1569. doi: 10.3390/cancers11101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Zhang Y, Huang Q, Li C. Integrated bioinformatics analysis reveals key candidate genes and pathways in breast cancer. Mol Med Rep. 2018;17:8091–8100. doi: 10.3892/mmr.2018.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dey P, Rathod M, De A. Targeting stem cells in the realm of drug-resistant breast cancer. Breast Cancer. 2019;11:115. doi: 10.2147/BCTT.S189224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palomeras S, Ruiz-Martínez S, Puig T. Targeting breast cancer stem cells to overcome treatment resistance. Molecules. 2018;23(9):2193. doi: 10.3390/molecules23092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pindiprolu S K S, Krishnamurthy P T, Chintamaneni P K, Karri V V S R, Nanocarrier based approaches for targeting breast cancer stem cells. Artif Cells Nanomed Biotechnol 2018; 46: 885–898. [DOI] [PubMed]
- 30.Gao Y, Tang M, Leung E, Svirskis D, Shelling A, Wu Z. Dual or multiple drug loaded nanoparticles to target breast cancer stem cells. RSC Adv. 2020;10:19089–19105. doi: 10.1039/D0RA02801K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du F-Y, Zhou Q-F, Sun W-J, Chen G-L. Targeting cancer stem cells in drug discovery: current state and future perspectives. World J Stem Cells. 2019;11:398–420. doi: 10.4252/wjsc.v11.i7.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scioli MG, Storti G, D’Amico F, Gentile P, Fabbri G, Cervelli V, Orlandi A. The role of breast cancer stem cells as a prognostic marker and a target to improve the efficacy of breast cancer therapy. Cancers (Basel) 2019;11:1021. doi: 10.3390/cancers11071021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badve S, Nakshatri H. Breast-cancer stem cells—beyond semantics. Lancet Oncol. 2012;13:e43–e48. doi: 10.1016/S1470-2045(11)70191-7. [DOI] [PubMed] [Google Scholar]
- 35.Alanazi IO, Khan Z, Understanding EGFR. Signaling in breast cancer and breast cancer stem cells: overexpression and therapeutic implications. Asian Pac J Cancer Prev. 2016;17:445–453. doi: 10.7314/APJCP.2016.17.2.445. [DOI] [PubMed] [Google Scholar]
- 36.Ko CCH, Chia WK, Selvarajah GT, Cheah YK, Wong YP, Tan GC. The role of breast cancer stem cell-related biomarkers as prognostic factors. Diagnostics. 2020;10:721. doi: 10.3390/diagnostics10090721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan S, Yamashita A, Gao SJ, Kurisawa M. Hyaluronic acid hydrogels with defined crosslink density for the efficient enrichment of breast cancer stem cells. Acta Biomater. 2019;94:320–329. doi: 10.1016/j.actbio.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 38.Yang F, Xu J, Tang L, Guan X. Breast cancer stem cell: the roles and therapeutic implications. Cell Mol Life Sci. 2017;74:951–966. doi: 10.1007/s00018-016-2334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yousefnia S, Seyed Forootan F, Seyed Forootan S, Nasr Esfahani MH, Gure AO, Ghaedi K. Mechanistic pathways of malignancy in breast cancer stem cells. Front Oncol. 2020;10:452. doi: 10.3389/fonc.2020.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S J, Owen S C, Hyaluronic acid binding to CD44S is indiscriminate of molecular weight. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2020;1862(9):183348. [DOI] [PubMed]
- 41.Schabath H, Runz S, Joumaa S, Altevogt P. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci. 2006;119:314–325. doi: 10.1242/jcs.02741. [DOI] [PubMed] [Google Scholar]
- 42.Zhou J, Chen Q, Zou Y, Chen H, Qi L, Chen Y. Stem cells and cellular origins of breast cancer: updates in the rationale, controversies, and therapeutic implications. Front Oncol. 2019;9:820. doi: 10.3389/fonc.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark DW, Palle K. Aldehyde dehydrogenases in cancer stem cells: potential as therapeutic targets. Ann Transl Med. 2016;4(24):518. doi: 10.21037/atm.2016.11.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomita H, Tanaka K, Tanaka T, Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 2016;7:11018–11032. doi: 10.18632/oncotarget.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vassalli G. Aldehyde dehydrogenases: not just markers, but functional regulators of stem cells. Stem Cells Int. 2019;2019:3904645. doi: 10.1155/2019/3904645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Gao J, Sun Y, Zhang D, Liu T, Yan Q, Yang X. Mutation of N-linked glycosylation in EpCAM affected cell adhesion in breast cancer cells. Biol Chem. 2017;398:1119–1126. doi: 10.1515/hsz-2016-0232. [DOI] [PubMed] [Google Scholar]
- 47.Enciu A-M, Radu E, Popescu ID, Hinescu ME, Ceafalan LC. Targeting CD36 as biomarker for metastasis prognostic: how far from translation into clinical practice? Biomed Res Int. 2018;2018:7801202. doi: 10.1155/2018/7801202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS-O, Berenguer A, Prats N, Toll A, Hueto JA. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541(7635):41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 49.Nilendu P, Kumar A, Kumar A, Pal JK, Sharma NK. Breast cancer stem cells as last soldiers eluding therapeutic burn: a hard nut to crack. Int J Cancer. 2018;142:7–17. doi: 10.1002/ijc.30898. [DOI] [PubMed] [Google Scholar]
- 50.Collina F, Di Bonito M, Li Bergolis V, De Laurentiis M, Vitagliano C, Cerrone M, Nuzzo F, Cantile M, Botti G. Prognostic value of cancer stem cells markers in triple-negative breast cancer. Biomed Res Int. 2015;2015:158682. [DOI] [PMC free article] [PubMed]
- 51.Turashvili G, Brogi E. Tumor heterogeneity in breast cancer. Front Med. 2017;4:227. doi: 10.3389/fmed.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fumagalli C, Ranghiero A, Gandini S, Corso F, Taormina S, De Camilli E, Rappa A, Vacirca D, Viale G, Guerini-Rocco E. Inter-tumor genomic heterogeneity of breast cancers: comprehensive genomic profile of primary early breast cancers and relapses. Breast Cancer Res. 2020;22:1–11. doi: 10.1186/s13058-020-01345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiaobin Z, Chengxiao L, Jie Y, Haoqiang W, Guoqing W, Yingpeng L, Chen N. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol Res. 2021;163:105320. [DOI] [PubMed]
- 54.Strietz J, Stepputtis SS, Follo M, Bronsert P, Stickeler E, Maurer J. Human primary breast cancer stem cells are characterized by epithelial-mesenchymal plasticity. Int J Mol Sci. 2021;22:1808. doi: 10.3390/ijms22041808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorico A, Rappa G. Phenotypic heterogeneity of breast cancer stem cells. J Oncol. 2011;2011:135039. [DOI] [PMC free article] [PubMed]
- 56.Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22(3):457–472. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng S-Q, Alexandrou AT, Li JJ. Breast cancer stem cells: multiple capacities in tumor metastasis. Cancer Lett. 2014;349:1–7. doi: 10.1016/j.canlet.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barriga V, Kuol N, Nurgali K, Apostolopoulos V. The complex interaction between the tumor micro-environment and immune checkpoints in breast cancer. Cancers (Basel) 2019;11(8):1205. doi: 10.3390/cancers11081205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koury J, Zhong L, Hao J. Targeting signaling pathways in cancer stem cells for cancer treatment. Stem Cells Int. 2017;2017:2925869. doi: 10.1155/2017/2925869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, Li X, Wang T, Guo Q, Xi T, Zheng L. Emerging agents that target signaling pathways in cancer stem cells. J Hematol Oncol. 2020;13:60. doi: 10.1186/s13045-020-00901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu X, Zhang M, Xu F, Jiang S. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020;19:1–35. doi: 10.1186/s12943-019-1085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clarke MF. Clinical and therapeutic implications of cancer stem cells. N Engl J Med. 2019;380:2237–2245. doi: 10.1056/NEJMra1804280. [DOI] [PubMed] [Google Scholar]
- 64.Park S-Y, Choi J-H, Nam J-S. Targeting cancer stem cells in triple-negative breast cancer. Cancers. 2019;11(7):965. doi: 10.3390/cancers11070965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim Y-J, Kim JY, Lee N, Oh E, Sung D, Cho T-M, Seo JH. Disulfiram suppresses cancer stem-like properties and STAT3 signaling in triple-negative breast cancer cells. Biochem Biophys Res Commun. 2017;486:1069–1076. doi: 10.1016/j.bbrc.2017.03.164. [DOI] [PubMed] [Google Scholar]
- 66.Al-Othman N, Alhendi A, Ihbaisha M, Barahmeh M, Alqaraleh M, Al-Momany BZ. Role of CD44 in breast cancer. Breast Dis. 2020;39(1):1–13. doi: 10.3233/BD-190409. [DOI] [PubMed] [Google Scholar]
- 67.Kwong LN, Dove WF. APC and its modifiers in colon cancer. Adv Exp Med Biol. 2009;656:85–106. doi: 10.1007/978-1-4419-1145-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmitt M, Metzger M, Gradl D, Davidson G, Orian-Rousseau V. CD44 functions in Wnt signaling by regulating LRP6 localization and activation. Cell Death Differ. 2015;22:677–689. doi: 10.1038/cdd.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu H, Niu M, Yuan X, Wu K, Liu A. CD44 as a tumor biomarker and therapeutic target. Exp Hematol Oncol. 2020;9:36. doi: 10.1186/s40164-020-00192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vikram R, Chou WC, Hung S-C, Shen C-Y. Tumorigenic and metastatic role of CD44-/low/CD24-/low cells in luminal breast cancer. Cancers (Basel) 2020;12:1239. doi: 10.3390/cancers12051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jang G-B, Kim J-Y, Cho S-D, Park K-S, Jung J-Y, Lee H-Y, Hong I-S, Nam J-S. Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci Rep. 2015;5:12465. doi: 10.1038/srep12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell RG, Wu K. The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Ther. 2015;8:3783–3792. doi: 10.2147/OTT.S95470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung SS, Aroh C, Vadgama JV. Constitutive activation of STAT3 signaling regulates hTERT and promotes stem cell-like traits in human breast cancer cells. PLoS One. 2013;8:e83971. doi: 10.1371/journal.pone.0083971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu X, Zhang M, Xu F, Jiang S. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020;19:165. doi: 10.1186/s12943-020-01276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, Lin Y, Li J, Kang T, Tao M. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell. 2014;25:210–225. doi: 10.1016/j.ccr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang S, Zhang M, Zhang Y, Zhou W, Zhu T, Ruan Q, Chen H, Fang J, Zhou F, Sun J. WNT5B governs the phenotype of basal-like breast cancer by activating WNT signaling. Cell Commun Signal. 2019;17:1–19. doi: 10.1186/s12964-019-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Z, Liu H, Desai S, Schmitt DC, Zhou M, Khong HT, Klos KS, McClellan S, Fodstad O, Tan M. miR-125b functions as a key mediator for snail-induced stem cell propagation and chemoresistance. J Biol Chem. 2013;288:4334–4345. doi: 10.1074/jbc.M112.419168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma SY, Park J-H, Jung H, Ha S-M, Kim Y, Park DH, Lee DH, Lee S, Chu I-H, Jung SY. Snail maintains metastatic potential, cancer stem-like properties, and chemoresistance in mesenchymal mouse breast cancer TUBO-P2J cells. Oncol Rep. 2017;38:1867–1876. doi: 10.3892/or.2017.5834. [DOI] [PubMed] [Google Scholar]
- 79.Wang D, Cai C, Dong X, Yu QC, Zhang XO, Yang L, Zeng YA. Identification of multipotent mammary stem cells by protein C receptor expression. Nature. 2015;517:81–84. doi: 10.1038/nature13851. [DOI] [PubMed] [Google Scholar]
- 80.de Visser KE, Ciampricotti M, Michalak EM, Tan DWM, Speksnijder EN, Hau CS, Clevers H, Barker N, Jonkers J. Developmental stage-specific contribution of LGR5+ cells to basal and luminal epithelial lineages in the postnatal mammary gland. J Pathol. 2012;228:300–309. doi: 10.1002/path.4096. [DOI] [PubMed] [Google Scholar]
- 81.Pindiprolu SKSS, Krishnamurthy PT, Chintamaneni PK. Pharmacological targets of breast cancer stem cells: a review. Naunyn Schmiedeberg’s Arch Pharmacol. 2018;391(5):463–479. doi: 10.1007/s00210-018-1479-3. [DOI] [PubMed] [Google Scholar]
- 82.Zhao Z, Lu P, Zhang H, Xu H, Gao N, Li M, Liu C. Nestin positively regulates the Wnt/β-catenin pathway and the proliferation, survival and invasiveness of breast cancer stem cells. Breast Cancer Res. 2014;16(4):408. doi: 10.1186/s13058-014-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klarmann GJ, Decker A, Farrar WL. Epigenetic gene silencing in the Wnt pathway in breast cancer. Epigenetics. 2008;3:59–63. doi: 10.4161/epi.3.2.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pathania R, Ramachandran S, Elangovan S, Padia R, Yang P, Cinghu S, Veeranan-Karmegam R, Arjunan P, Gnana-Prakasam JP, Sadanand F, Pei L, Chang CS, Choi JH, Shi H, Manicassamy S, Prasad PD, Sharma S, Ganapathy V, Jothi R, Thangaraju M. DNMT1 is essential for mammary and cancer stem cell maintenance and tumorigenesis. Nat Commun. 2015;6:6910. doi: 10.1038/ncomms7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abetov D, Mustapova Z, Saliev T, Bulanin D, Batyrbekov K, Gilman CP. Novel small molecule inhibitors of cancer stem cell signaling pathways. Stem Cell Rev Rep. 2015;11:909–918. doi: 10.1007/s12015-015-9612-x. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:1–16. doi: 10.1186/s13045-019-0838-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hallett RM, Kondratyev MK, Giacomelli AO, Nixon AM, Girgis-Gabardo A, Ilieva D, Hassell JA. Small molecule antagonists of the Wnt/beta-catenin signaling pathway target breast tumor-initiating cells in a Her2/Neu mouse model of breast cancer. PLoS One. 2012;7:e33976. doi: 10.1371/journal.pone.0033976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu L, Zhang L, Hu C, Liang S, Fei X, Yan N, Zhang Y, Zhang F. WNT pathway inhibitor pyrvinium pamoate inhibits the self-renewal and metastasis of breast cancer stem cells. Int J Oncol. 2016;48:1175–1186. doi: 10.3892/ijo.2016.3337. [DOI] [PubMed] [Google Scholar]
- 89.Huang P, Chen A, He W, Li Z, Zhang G, Liu Z, Liu G, Liu X, He S, Xiao G, Huang F, Stenvang J, Brünner N, Hong A, Wang J. BMP-2 induces EMT and breast cancer stemness through Rb and CD44. Cell Death Discov. 2017;3:17039. doi: 10.1038/cddiscovery.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen A, Wang D, Liu X, He S, Yu Z, Wang J. Inhibitory effect of BMP-2 on the proliferation of breast cancer cells. Mol Med Rep. 2012;6:615–620. doi: 10.3892/mmr.2012.962. [DOI] [PubMed] [Google Scholar]
- 91.Bach D-H, Park HJ, Lee SK. The dual role of bone morphogenetic proteins in cancer. Mol Ther Oncolytics. 2018;8:1–13. doi: 10.1016/j.omto.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bach D-H, Park HJ, Lee SK. The dual role of bone morphogenetic proteins in cancer. Mol Ther Oncolytics. 2017;8:1–13. doi: 10.1016/j.omto.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdullah MF, Muhamad M, Ab-Rahim S. The role of bone morphogenetic protein 2 in the reprogramming of cancer stem cells. 2019. [Google Scholar]
- 94.Di Mauro C, Rosa R, D’Amato V, Ciciola P, Servetto A, Marciano R, Orsini RC, Formisano L, De Falco S, Cicatiello V. Hedgehog signalling pathway orchestrates angiogenesis in triple-negative breast cancers. Br J Cancer. 2017;116:1425–1435. doi: 10.1038/bjc.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goel HL, Pursell B, Chang C, Shaw LM, Mao J, Simin K, Kumar P, Vander Kooi CW, Shultz LD, Greiner DL, Norum JH, Toftgard R, Kuperwasser C, Mercurio AM. GLI1 regulates a novel neuropilin-2/α6β1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol Med. 2013;5(4):488–508. doi: 10.1002/emmm.201202078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ge X, Lyu P, Gu Y, Li L, Li J, Wang Y, Zhang L, Fu C, Cao Z. Sonic hedgehog stimulates glycolysis and proliferation of breast cancer cells: modulation of PFKFB3 activation. Biochem Biophys Res Commun. 2015;464:862–868. doi: 10.1016/j.bbrc.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 97.Habib JG, O’Shaughnessy JA. The hedgehog pathway in triple-negative breast cancer. Cancer Med. 2016;5:2989–3006. doi: 10.1002/cam4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mercurio AM. VEGF/neuropilin signaling in cancer stem cells. Int J Mol Sci. 2019;20:490. doi: 10.3390/ijms20030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan P, Fan S, Wang H, Mao J, Shi Y, Ibrahim MM, Ma W, Yu X, Hou Z, Wang B. Genistein decreases the breast cancer stem-like cell population through Hedgehog pathway. Stem Cell Res Ther. 2013;4:1–10. doi: 10.1186/scrt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang L, Duan W, Kang L, Mao J, Yu X, Fan S, Li L, Tao Y. Smoothened activates breast cancer stem-like cell and promotes tumorigenesis and metastasis of breast cancer. Biomed Pharmacother. 2014;68:1099–1104. doi: 10.1016/j.biopha.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Zhang N, Kong X, Yan S, Yuan C, Yang Q. Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci. 2010;101:2375–2383. doi: 10.1111/j.1349-7006.2010.01680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simões BM, O’Brien CS, Eyre R, Silva A, Yu L, Sarmiento-Castro A, Alférez DG, Spence K, Santiago-Gómez A, Chemi F. Anti-estrogen resistance in human breast tumors is driven by JAG1-NOTCH4-dependent cancer stem cell activity. Cell Rep. 2015;12:1968–1977. doi: 10.1016/j.celrep.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou L, Wang D, Sheng D, Xu J, Chen W, Qin Y, Du R, Yang X, He X, Xie N. NOTCH4 maintains quiescent mesenchymal-like breast cancer stem cells via transcriptionally activating SLUG and GAS1 in triple-negative breast cancer. Theranostics. 2020;10:2405. doi: 10.7150/thno.38875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bai J-W, Wei M, Li J-W, Zhang G-J. Notch signaling pathway and endocrine resistance in breast cancer. Front Pharmacol. 2020;11. 10.3389/fphar.2020.00924. [DOI] [PMC free article] [PubMed]
- 105.Zhang Y, Xu B, Zhang X-P. Effects of miRNAs on functions of breast cancer stem cells and treatment of breast cancer. Onco Targets Ther. 2018;11:4263–4270. doi: 10.2147/OTT.S165156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shimono Y, Mukohyama J, Nakamura S, Minami H. MicroRNA regulation of human breast cancer stem cells. J Clin Med. 2015;5(1). 10.3390/jcm5010002. [DOI] [PMC free article] [PubMed]
- 107.Garcia-Heredia JM, Lucena-Cacace A, Verdugo-Sivianes EM, Pérez M, Carnero A. The cargo protein MAP 17 (PDZK1IP1) regulates the cancer stem cell pool activating the Notch pathway by abducting NUMB. Clin Cancer Res. 2017;23:3871–3883. doi: 10.1158/1078-0432.CCR-16-2358. [DOI] [PubMed] [Google Scholar]
- 108.Bawadood AS, Al-Abbasi FA, Anwar F, El-Halawany AM, Al-Abd AM. 6-Shogaol suppresses the growth of breast cancer cells by inducing apoptosis and suppressing autophagy via targeting notch signaling pathway. Biomed Pharmacother. 2020;128:110302. doi: 10.1016/j.biopha.2020.110302. [DOI] [PubMed] [Google Scholar]
- 109.Schott AF, Landis MD, Dontu G, Griffith KA, Layman RM, Krop I, Paskett LA, Wong H, Dobrolecki LE, Lewis MT, Froehlich AM, Paranilam J, Hayes DF, Wicha MS, Chang JC. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res. 2013;19:1512–1524. doi: 10.1158/1078-0432.CCR-11-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramaswamy B, Lu Y, Teng KY, Nuovo G, Li X, Shapiro CL, Majumder S. Hedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathway. Cancer Res. 2012;72(19):5048–5059. doi: 10.1158/0008-5472.CAN-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ji X, Lu Y, Tian H, Meng X, Wei M, Cho WC. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed Pharmacother. 2019;114:108800. doi: 10.1016/j.biopha.2019.108800. [DOI] [PubMed] [Google Scholar]
- 112.Zhao R, Kaakati RN, Hollenbeck ST, Li CY. Epidermal growth factor receptor target in breast cancer treatment. J Am Coll Surg. 2018;227:e80. doi: 10.1016/j.jamcollsurg.2018.08.216. [DOI] [Google Scholar]
- 113.Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y, Zhao L, Qu H, Fan Y, Wu C. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. Plos One. 2012;7:e39520. doi: 10.1371/journal.pone.0039520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nami B, Wang Z. HER2 in breast cancer stemness: a negative feedback loop towards trastuzumab resistance. Cancers. 2017;9:40. doi: 10.3390/cancers9050040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duru N, Candas D, Jiang G, Li JJ. Breast cancer adaptive resistance: HER2 and cancer stem cell repopulation in a heterogeneous tumor society. J Cancer Res Clin Oncol. 2014;140:1–14. doi: 10.1007/s00432-013-1494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tabolacci C, De Martino A, Mischiati C, Feriotto G, Beninati S. The role of tissue transglutaminase in cancer cell initiation, survival and progression. Med Sci. 2019;7:19. doi: 10.3390/medsci7020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Willmer T, Cooper A, Peres J, Omar R, Prince S. The T-Box transcription factor 3 in development and cancer. Biosci Trends. 2017;11:254–266. doi: 10.5582/bst.2017.01043. [DOI] [PubMed] [Google Scholar]
- 119.Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, Kuperwasser C. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci. 2010;107:21737–21742. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dong G, Ma G, Wu R, Liu J, Liu M, Gao A, Li X, Liu X, Zhang Z, Zhang B. ZFHX3 promotes the proliferation and tumor growth of ER-positive breast cancer cells likely by enhancing stem-like features and MYC and TBX3 transcription. Cancers. 2020;12(11):3415. doi: 10.3390/cancers12113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fritz AJ, Hong D, Boyd J, Kost J, Finstaad KH, Fitzgerald MP, Hanna S, Abuarqoub AH, Malik M, Bushweller J. RUNX transcription factor mediated control of breast cancer stem cells. J Cell Physiol. 2020;235(10):7261–7272. doi: 10.1002/jcp.29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu P, Wang Z, Brown S, Kannappan V, Tawari PE, Jiang W, Irache JM, Tang JZ, Armesilla AL, Darling JL, Tang X, Wang W. Liposome encapsulated Disulfiram inhibits NFκB pathway and targets breast cancer stem cells in vitro and in vivo. Oncotarget. 2014;5:7471–7485. doi: 10.18632/oncotarget.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee JJ, Loh K, Yap Y-S. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med. 2015;12:342–354. doi: 10.7497/j.issn.2095-3941.2015.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ortega MA, Fraile-Martínez O, Asúnsolo Á, Buján J, García-Honduvilla N, Coca S. Signal transduction pathways in breast cancer: the important role of PI3K/Akt/mTOR. J Oncol. 2020;2020:9258396. doi: 10.1155/2020/9258396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holmes FA, Espina V, Liotta LA, Nagarwala YM, Danso M, McIntyre KJ, Osborne CR, Anderson T, Krekow L, Blum JL. Pathologic complete response after preoperative anti-HER2 therapy correlates with alterations in PTEN, FOXO, phosphorylated Stat5, and autophagy protein signaling. BMC Res Notes. 2013;6:1–10. doi: 10.1186/1756-0500-6-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhuang J, Huo Q, Yang F, Xie N. Perspectives on the role of histone modification in breast cancer progression and the advanced technological tools to study epigenetic determinants of metastasis. Front Genet. 2020;11:603552. doi: 10.3389/fgene.2020.603552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Völker-Albert M, Bronkhorst A, Holdenrieder S, Imhof A. Histone modifications in stem cell development and their clinical implications. Stem Cell Rep. 2020;15:1196–1205. doi: 10.1016/j.stemcr.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pei J, Wang Y, Li Y, Identification of key genes controlling breast cancer stem cell characteristics via stemness indices analysis. J Transl Med. 2020;18:1–15. [DOI] [PMC free article] [PubMed]
- 129.Yao Q, Chen Y, Zhou X. The roles of microRNAs in epigenetic regulation. Curr Opin Chem Biol. 2019;51:11–17. doi: 10.1016/j.cbpa.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 130.Ali Syeda Z, Langden SSS, Munkhzul C, Lee M, Song SJ. Regulatory mechanism of microRNA expression in cancer. Int J Mol Sci. 2020;21:1723. doi: 10.3390/ijms21051723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bao-Caamano A, Rodriguez-Casanova A, Diaz-Lagares A. Epigenetics of circulating tumor cells in breast cancer. Adv Exp Med Biol. 2020;1220:117-34. [DOI] [PubMed]
- 132.Chi Y, Wang D, Wang J, Yu W, Yang J. Long non-coding RNA in the pathogenesis of cancers. Cells. 2019;8(9):1015. doi: 10.3390/cells8091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yousefi H, Maheronnaghsh M, Molaei F, Mashouri L, Reza Aref A, Momeny M, Alahari SK. Long noncoding RNAs and exosomal lncRNAs: classification, and mechanisms in breast cancer metastasis and drug resistance. Oncogene. 2020;39:953–974. doi: 10.1038/s41388-019-1040-y. [DOI] [PubMed] [Google Scholar]
- 134.Wu Y, Shao A, Wang L, Hu K, Yu C, Pan C, Zhang S. The Role of lncRNAs in the Distant Metastasis of Breast Cancer. Front Oncol. 2019;9:407. [DOI] [PMC free article] [PubMed]
- 135.Li L, Meng D, Wang R. Long non-coding RNA SOX21-AS1 enhances the stemness of breast cancer cells via the Hippo pathway. FEBS Open Bio. 2020;11(1):251–64. [DOI] [PMC free article] [PubMed]
- 136.Singh R, Mo Y-Y. Role of microRNAs in breast cancer. Cancer Biol Ther. 2013;14:201–212. doi: 10.4161/cbt.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ding L, Gu H, Xiong X, Ao H, Cao J, Lin W, Yu M, Lin J, Cui Q. MicroRNAs involved in carcinogenesis, prognosis, therapeutic resistance and applications in human triple-negative breast cancer. Cells. 2019;8:1492. doi: 10.3390/cells8121492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khan AQ, Ahmed EI, Elareer NR, Junejo K, Steinhoff M, Uddin S. Role of miRNA-regulated cancer stem cells in the pathogenesis of human malignancies. Cells. 2019;8:840. doi: 10.3390/cells8080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Si W, Shen J, Zheng H, Fan W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenetics. 2019;11:25. doi: 10.1186/s13148-018-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Das PK, Siddika M, Asha SY, Aktar S, Rakib M, Khanam JA, Pillai S, Islam F. MicroRNAs, a promising target for breast cancer stem cells. Mol Diagn Ther. 2019;24:69–83. doi: 10.1007/s40291-019-00439-5. [DOI] [PubMed] [Google Scholar]
- 141.Song SJ, Poliseno L, Song MS, Ala U, Webster K, Ng C, Beringer G, Brikbak NJ, Yuan X, Cantley LC, Richardson AL, Pandolfi PP. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell. 2013;154:311–324. doi: 10.1016/j.cell.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lv C, Li F, Li X, Tian Y, Zhang Y, Sheng X, Song Y, Meng Q, Yuan S, Luan L, Andl T, Feng X, Jiao B, Xu M, Plikus MV, Dai X, Lengner C, Cui W, Ren F, Shuai J, Millar SE, Yu Z. MiR-31 promotes mammary stem cell expansion and breast tumorigenesis by suppressing Wnt signaling antagonists. Nat Commun. 2017;8:1036. doi: 10.1038/s41467-017-01059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu Y, Shi W, Tang T, Wang Y, Yin X, Chen Y, Zhang Y, Xing Y, Shen Y, Xia T, Guo C, Pan Y, Jin L. miR-29a contributes to breast cancer cells epithelial-mesenchymal transition, migration, and invasion via down-regulating histone H4K20 trimethylation through directly targeting SUV420H2. Cell Death Dis. 2019;10:176. doi: 10.1038/s41419-019-1437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu C, Xing H, Guo C, Yang Z, Wang Y, Wang Y. MiR-124 reversed the doxorubicin resistance of breast cancer stem cells through STAT3/HIF-1 signaling pathways. Cell Cycle. 2019;18:2215–2227. doi: 10.1080/15384101.2019.1638182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schwarzenbacher D, Klec C, Pasculli B, Cerk S, Rinner B, Karbiener M, Ivan C, Barbano R, Ling H, Wulf-Goldenberg A, Stanzer S, Rinnerthaler G, Stoeger H, Bauernhofer T, Haybaeck J, Hoefler G, Jahn SW, Parrella P, Calin GA, Pichler M. MiR-1287-5p inhibits triple negative breast cancer growth by interaction with phosphoinositide 3-kinase CB, thereby sensitizing cells for PI3Kinase inhibitors. Breast Cancer Res. 2019;21:20. doi: 10.1186/s13058-019-1104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cheng S, Huang Y, Lou C, He Y, Zhang Y, Zhang Q. FSTL1 enhances chemoresistance and maintains stemness in breast cancer cells via integrin β3/Wnt signaling under miR-137 regulation. Cancer Biol Ther. 2019;20(3):328–337. doi: 10.1080/15384047.2018.1529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tang T, Yang Z, Zhu Q, Wu Y, Sun K, Alahdal M, Zhang Y, Xing Y, Shen Y, Xia T, Xi T, Pan Y, Jin L. Up-regulation of miR-210 induced by a hypoxic microenvironment promotes breast cancer stem cells metastasis, proliferation, and self-renewal by targeting E-cadherin. Faseb j. 2018;32(12):6965-81. [DOI] [PubMed]
- 148.Zuo J, Yu Y, Zhu M, Jing W, Yu M, Chai H, Liang C, Tu J. Inhibition of miR-155, a therapeutic target for breast cancer, prevented in cancer stem cell formation. Cancer Biomark. 2018;21:383–392. doi: 10.3233/CBM-170642. [DOI] [PubMed] [Google Scholar]
- 149.Cheng CW, Yu JC, Hsieh YH, Liao WL, Shieh JC, Yao CC, Lee HJ, Chen PM, Wu PE, Shen CY. Increased cellular levels of microRNA-9 and microRNA-221 correlate with cancer stemness and predict poor outcome in human breast cancer. Cell Physiol Biochem. 2018;48:2205–2218. doi: 10.1159/000492561. [DOI] [PubMed] [Google Scholar]
- 150.Li X, Zeng Z, Wang J, Wu Y, Chen W, Zheng L, Xi T, Wang A, Lu Y. MicroRNA-9 and breast cancer. Biomed Pharmacother. 2020;122:109687. doi: 10.1016/j.biopha.2019.109687. [DOI] [PubMed] [Google Scholar]
- 151.Li B, Lu Y, Yu L, Han X, Wang H, Mao J, Shen J, Wang B, Tang J, Li C, Song B. miR-221/222 promote cancer stem-like cell properties and tumor growth of breast cancer via targeting PTEN and sustained Akt/NF-κB/COX-2 activation. Chem Biol Interact. 2017;277:33–42. doi: 10.1016/j.cbi.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 152.Zhou K, Liu M, Cao Y. New insight into microRNA functions in cancer: oncogene-microRNA-tumor suppressor gene network. Front Mol Biosci. 2017;4:46. doi: 10.3389/fmolb.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bonetti P, Climent M, Panebianco F, Tordonato C, Santoro A, Marzi MJ, Pelicci PG, Ventura A, Nicassio F. Dual role for miR-34a in the control of early progenitor proliferation and commitment in the mammary gland and in breast cancer. Oncogene. 2019;38:360–374. doi: 10.1038/s41388-018-0445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lin C, Gao B, Yan X, Lei Z, Chen K, Li Y, Zeng Q, Chen Z, Li H. MicroRNA 628 suppresses migration and invasion of breast cancer stem cells through targeting SOS1. Onco Targets Ther. 2018;11:5419–5428. doi: 10.2147/OTT.S164575. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Wolfson B, Eades G, Zhou Q. Roles of microRNA-140 in stem cell-associated early stage breast cancer. World J Stem Cells. 2014;6:591. doi: 10.4252/wjsc.v6.i5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu D, Zhang J, Lu Y, Bo S, Li L, Wang L, Zhang Q, Mao J. miR-140-5p inhibits the proliferation and enhances the efficacy of doxorubicin to breast cancer stem cells by targeting Wnt1. Cancer Gene Ther. 2019;26:74–82. doi: 10.1038/s41417-018-0035-0. [DOI] [PubMed] [Google Scholar]
- 157.Troschel FM, Böhly N, Borrmann K, Braun T, Schwickert A, Kiesel L, Eich HT, Götte M, Greve B. miR-142-3p attenuates breast cancer stem cell characteristics and decreases radioresistance in vitro. Tumour Biol. 2018;40:1010428318791887. doi: 10.1177/1010428318791887. [DOI] [PubMed] [Google Scholar]
- 158.Chu J, Li Y, Fan X, Ma J, Li J, Lu G, Zhang Y, Huang Y, Li W, Huang X, Fu Z, Yin Y, Yuan H. MiR-4319 suppress the malignancy of triple-negative breast cancer by regulating self-renewal and tumorigenesis of stem cells. Cell Physiol Biochem. 2018;48(2):593–604. doi: 10.1159/000491888. [DOI] [PubMed] [Google Scholar]
- 159.Kong X, Zhang J, Li J, Shao J, Fang L. MiR-130a-3p inhibits migration and invasion by regulating RAB5B in human breast cancer stem cell-like cells. Biochem Biophys Res Commun. 2018;501:486–493. doi: 10.1016/j.bbrc.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 160.Pan Y, Wang R, Zhang F, Chen Y, Lv Q, Long G, Yang K. MicroRNA-130a inhibits cell proliferation, invasion and migration in human breast cancer by targeting the RAB5A. Int J Clin Exp Pathol. 2015;8:384–393. [PMC free article] [PubMed] [Google Scholar]
- 161.Sun X, Xu C, Xiao G, Meng J, Wang J, Tang SC, Qin S, Du N, Li G, Ren H, Liu D. Breast cancer stem-like cells are sensitized to tamoxifen induction of self-renewal inhibition with enforced Let-7c dependent on Wnt blocking. Int J Mol Med. 2018;41(4):1967–1975. doi: 10.3892/ijmm.2018.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sun X, Xu C, Tang SC, Wang J, Wang H, Wang P, Du N, Qin S, Li G, Xu S, Tao Z, Liu D, Ren H. Let-7c blocks estrogen-activated Wnt signaling in induction of self-renewal of breast cancer stem cells. Cancer Gene Ther. 2016;23(4):83–89. doi: 10.1038/cgt.2016.3. [DOI] [PubMed] [Google Scholar]
- 163.Mayoral-Varo V, Calcabrini A, Sánchez-Bailón M P, Martín-Pérez J, miR205 inhibits stem cell renewal in SUM159PT breast cancer cells. Plos One 2017; 12: e0188637. [DOI] [PMC free article] [PubMed]
- 164.Xie Q, Wang S, Zhao Y, Zhang Z, Qin C, Yang X. MiR-519d impedes cisplatin-resistance in breast cancer stem cells by down-regulating the expression of MCL-1. Oncotarget. 2017;8:22003–22013. doi: 10.18632/oncotarget.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.El Helou R, Pinna G, Cabaud O, Wicinski J, Bhajun R, Guyon L, Rioualen C, Finetti P, Gros A, Mari B, Barbry P, Bertucci F, Bidaut G, Harel-Bellan A, Birnbaum D, Charafe-Jauffret E, Ginestier C. miR-600 acts as a bimodal switch that regulates breast cancer stem cell fate through WNT signaling. Cell Rep. 2017;18:2256–2268. doi: 10.1016/j.celrep.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 166.Liu T, Hu K, Zhao Z, Chen G, Ou X, Zhang H, Zhang X, Wei X, Wang D, Cui M, Liu C. MicroRNA-1 down-regulates proliferation and migration of breast cancer stem cells by inhibiting the Wnt/β-catenin pathway. Oncotarget. 2015;6:41638–41649. doi: 10.18632/oncotarget.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fu H, Fu L, Xie C, Zuo WS, Liu YS, Zheng MZ, Yu JM. miR-375 inhibits cancer stem cell phenotype and tamoxifen resistance by degrading HOXB3 in human ER-positive breast cancer. Oncol Rep. 2017;37:1093–1099. doi: 10.3892/or.2017.5360. [DOI] [PubMed] [Google Scholar]
- 168.Bozorgi A, Khazaei S, Khademi A, Khazaei M. Natural and herbal compounds targeting breast cancer, a review based on cancer stem cells. Iran J Basic Med Sci. 2020;23(8):970–983. doi: 10.22038/ijbms.2020.43745.10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Scioli MG, Storti G, D’Amico F, Gentile P, Fabbri G, Cervelli V, Orlandi A. The role of breast cancer stem cells as a prognostic marker and a target to improve the efficacy of breast cancer therapy. Cancers. 2019;11:1021. doi: 10.3390/cancers11071021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li W, Yang H, Li X, Han L, Xu N, Shi A. Signaling pathway inhibitors target breast cancer stem cells in triple-negative breast cancer. Oncol Rep. 2019;41(1):437–446. doi: 10.3892/or.2018.6805. [DOI] [PubMed] [Google Scholar]
- 171.Zhu Q, Shen Y, Chen X, He J, Liu J, Zu X. Self-renewal signalling pathway inhibitors: perspectives on therapeutic approaches for cancer stem cells. Onco Targets Ther. 2020;13:525–540. doi: 10.2147/OTT.S224465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.