Abstract
Background
To evaluate the effects of vitamin D (vitD) supplement on uterine fibroid growth.
Materials and Methods
A randomized blinded clinical trial was conducted at a tertiary university-based hospital from August 2017 to September 2018. Totally, 204 women were enrolled into the study. They had at least one uterine fibroid >10 mm on transvaginal ultrasound and their vitD level was insufficient (i.e. 20-30 ng/ml). The intervention group was treated with vitD 50000 U supplements for two months. After 2 months, ultrasound screening and vitD level measurement was done in both groups.
Results
At first, the mean serum vitD levels in intervention and control group were 23.62 and 23.20 ng/ml, respec- tively. After 8 weeks, the mean serum vitD levels in the control and intervention group were 22.72 and 28.56 ng/ml respectively (P<0.05). Also, mean fibroma diameter in the intervention group before and after 8 weeks of vitD supple- mentation was 43 ± 4.68 and 42.6 ± 1.31 mm, respectively. Mean uterine fibroid diameter in the control group which did not receive vitD supplements, before and after 8 weeks was 41.98 ± 5.25 and 47.81 ± 3.42 mm, respectively. The variation in the mean size of the uterine fibroid between the control and intervention group which was respectively about 5.83 mm increase and 0.48 mm decrease, was significant (P<0.001).
Conclusion
Our results showed that vitD supplementation prevents fibroid growth. It seems that vitD supple- ment is a simple, safe and inexpensive modality for leiomyoma growth prevention (Registration number: IRCT201703122576N15).
Keywords: Cell Proliferation, Dietary Supplements, Leiomyoma, Premenopausal Women, Vitamin D
Introduction
In women of the reproductive age, uterine fibroids (leiomyoma) are monoclonal is a benign and most common gynecological tumor that depends on hormonal changes (1-5). These tumors affect 25-80% of reproductive age women depending on their demographic characteristic [race, age, and body mass index (BMI)], past medical history [hypertension (HTN), infertility, and premenopausal period], nutritional habits (use of food additive or soy bean milk) and family history (6-9).
It has been reported that some intrinsic abnormalities during the menstrual period including abnormal myometrial receptors for estrogen and hormonal changes or altered responses to ischemic injury, may be accountable for the start of (epi) genetic changes found in uterine myomas (10, 11).
Most uterine fibroids are asymptomatic, but they can cause symptoms, according to their size and location, like abnormal or heavy menstrual bleeding, iron deficiency anemia, bloating, constipation, urinary symptoms, pelvic pain, problems in intercourse, or pregnancy complications like recurrent miscarriage, premature labor and infertility (5, 12-16).
Symptomatic tumors are mainly treated by surgery. In addition to high direct costs of fibroma surgery treatments, these treatments are not suitable for women who wish to maintain fertility. However, nonsurgical treatments are approved just for short-term treatment and have their own disadvantages (5, 17). On the other hand, some therapeutic agents like gonadotropin hormone-releasing hormone (GnRH) analogue, oral GnRH antagonist (Elagolix), selective progesterone receptor modulators (SPRM), vitamin D (vitD) and green tea extracts are candidates to blunt the fibroid growth (18, 19).
Of medical therapeutic agents, vitD is a safe, inexpensive and available treatment without major side effects in therapeutic dose, which is assumed theoretically as an antitumor agent (20). It modulates gene (like cell growth and division genes and encoding estrogen and progesterone receptors genes) expression via vitD receptor and can result in regulation of cellular proliferation and cellular differentiation, stimulation of apoptosis that finally results in inhibition of malignant transformation and prevention of tumor cells proliferation (21, 22). Exposure to 1α, 1,25(OH)2 D3 inhibits the growth of melanoma, and lung, colon, prostate and breast cancer (23-27). So, vitD can be assumed as a preventive tool for high-risk group of patients that are susceptible to uterine fibroma (28).
Since, there is a high prevalence of vitD deficiency in Iran, there are interesting articles about the theoretical role of vitD in growth inhibition of uterine fibroid and the present data are inadequate to show the role of vitD as a medical therapy for the treatment of uterine fibroids, there is a need for a clinical trial that can assess inhibitory effects of vitD on the uterine fibroid size. Therefore, we conducted a randomized blinded clinical trial for evaluating the effects of vitD on the leiomyoma mean diameter in women with at least one uterine fibroid>10 mm referred to gynecology clinic in Tehran University of Medical Sciences’ Hospital.
Materials and Methods
This randomized blinded clinical trial was conducted between August 2017 and September 2018 in a tertiary university-based hospital.
All reproductive age women who referred to gynecologic clinic and had at least one uterine fibroid>10 mm in transvaginal ultrasound, were evaluated using a blood sample for vitD levels. According to the Endocrine Society Practice Guideline on vitD status, deficiency was defined as vitD<20 ng/ml, insufficiency as 21-29 ng/ml, and sufficiency as at least 30 ng/ml (29).
All the patients with uterine fibroid and insufficient levels of vitD (21-29 ng/ml) were included in the present study. Due to ethical concerns, patients with deficient levels of vitD (<20 ng/ml) were excluded from the study and received vitD as treatment. Other exclusion criteria were refusing follow-up visit or being candidate for hysterectomy or myomectomy due to related symptoms of uterine fibroid like bleeding, pain, pregnancy, menopause, use of oral contraceptive during the last 3 months, consuming vitD supplements during the last 3 months, having BMI of <18 or >30 kg/m2 (30) and malignancy
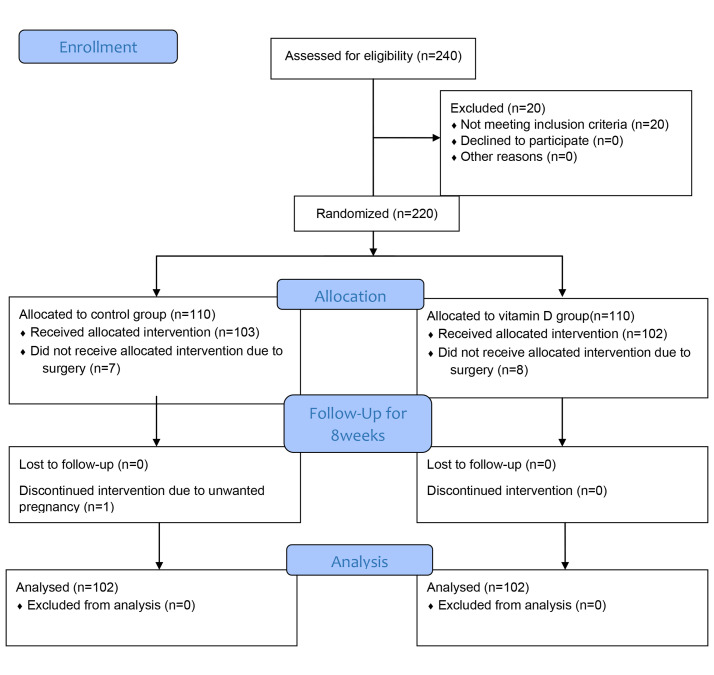
Two-hundred and forty women with uterine fibroid were referred to the clinic. Of them, 20 patients had sufficient levels of vitD, and seven patients underwent surgery in the control group and eight patients in the intervention group and one woman with unwanted pregnancy was excluded from the control group during the study. To tally 204 women, 102 in each group, completed the study protocol and data was analyzed (according to intention to treat analysis) (Fig.1).
Fig.1.
Consort flow diagram of study.
The vitD level was measured by a Kit (Narvanteb Company Kit, Iran) based on a chemiluminescence technology. Each patient had two blood samples, one in the initiation of the study and one after 8 weeks of observation or treatment with vitD in the same laboratory following the same method.
According to the sample size formula (α=0.05, β=90%, r=-0.31), we needed 212 patients for this study (106 patients for each groups) (30). Randomization of the patients was done based on block randomization by a computer program (Random Allocation Software) that was performed by a secretory that was blinded to the study group.
The uterine fibroid size was measured by a radiologist who was experienced in gynecologic ultrasound in the initiation of study and after 8 weeks of treatment with vitD supplement for both study groups. The radiologist was blinded to the grouping. Uterine fibroid was defined as well-defined, hypoechoic, heterogenous mass. Regarding the anatomical site in the uterus, patients divided as subserosal if the development was in the outer portion of uterus, intramural if the leiomyoma localized in the myometrium, and submucosal if the development was in to uterine cavity. The leiomyoma was measured by three perpendicular diameter and the mean of them was calculated. The scans were performed by a 5.5-MHZ probe of Voluson 730 GE Healthcare, Milwaukee, WI.
The intervention group with insufficient levels of vitD, received vitD 50000 IU (D-Vigel 50000, DaanaPharma Company, Iran) orally once per a week for 8 weeks and then a blood sample for vitD levels measurement was collected and the second transvaginal ultrasound was performed (1 to 2 weeks after the last dose of vitD). The control group with insufficient levels of vitD was observed for 8 weeks without vitD supplementation and 1 to 2 weeks after that the second blood sample was collected and transvaginal ultrasound was performed, then they received vitD supplement to reach sufficient vitD concentration.
Finally, variations in the mean diameter of uterine fibroid and vitD levels were measured and compared between thetwogroup as the primary outcomes.
Statistical analysis
All the statistical analyses were performed using SPSS version 24.0 (IBM, New York, USA). Quantitative data are expressed as mean ± standard deviation and categorical data are expressed as frequency and percentage. The Kolmogorov-Smirnov test was used to evaluate the distribution of the data. Independent samples, one-sample t test and Crosstabs were used in assessing the variable relationship. P<0.05 was considered significant.
Ethical considerations
The study was approved by the local Ethical Committee (IR.TUMS.VCR.REC.1396.2701) and the trial was registered as IRCT201703122576N15. All the patients signed the informed consent before being enrolled in to the study groups.
Results
Totally, 204 women, 102 in each group, completed the study protocol, and data were analyzed. The mean age and BMI in the control and intervention group were 37.21 and 34.89 years and 26.71 and 25.63 kg/m2 respectively. About 6.86% of women had a history of infertility in the intervention group versus 5.88% in the control group. The group were not statistically different in terms of age, BMI and history of infertility (Table 1).
Table 1.
Demographic data of women in the two groups
| Variable | Control group | Interventiongroup | P value |
|---|---|---|---|
| Age (Y) | 37.2 ± 91.13 | 34.89 ± 10.10 | 0.080 |
| BMI (kg/m2) | 26.7 ± 2.51 | 25.63 ± 3.31 | 0.090 |
| History of infertility | 6 | 7 | 0.770 |
| Vitamin D level before (ng/ml) | 23.62 ± 4.80 | 23.20 ± 4.64 | 0.520 |
| Vitamin D level after (ng/ml) | 22.72 ± 5.21 | 28.56 ± 5.04 | 0.001 |
| Mean leiomyoma diameter before (mm) | 41.98 ± 11.31 | 43.09 ± 14.36 | 0.180 |
| Mean leiomyoma diameter after (mm) | 47.81 ± 14.13 | 42.61 ± 15.53 | 0.018 |
| Subserosal leiomyoma | 44 (43.14) | 50 (49.02) | 0.561 |
| Intramural leiomyoma | 45 (44.12) | 43 (42.16) | |
| Submucosal leiomyoma | 13 (12.75) | 9 (8.82) | |
Data are presented as mean ± SD or n (%). BMI; Body mass index.
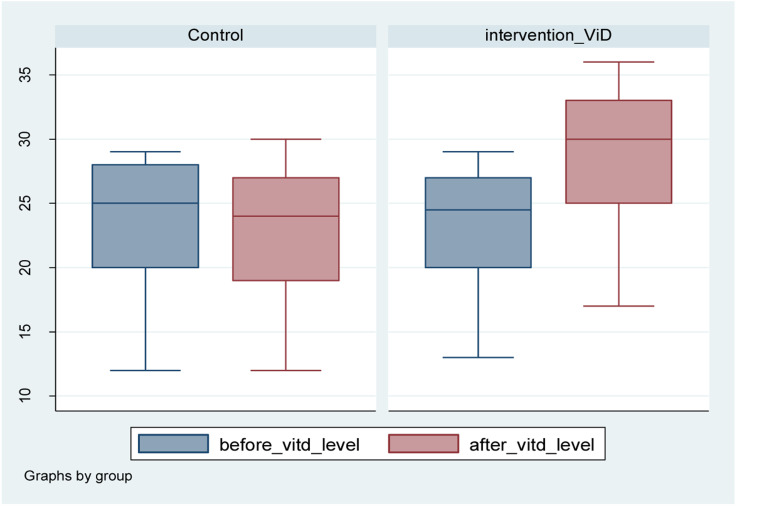
The mean vitD levels in the control and intervention group was 23.62 and 23.20 ng/ml, respectively which was not statistically different. At the end of the study, the mean vitD levels in the control and intervention group was 22.72 and 28.56 ng/ml, respectively.
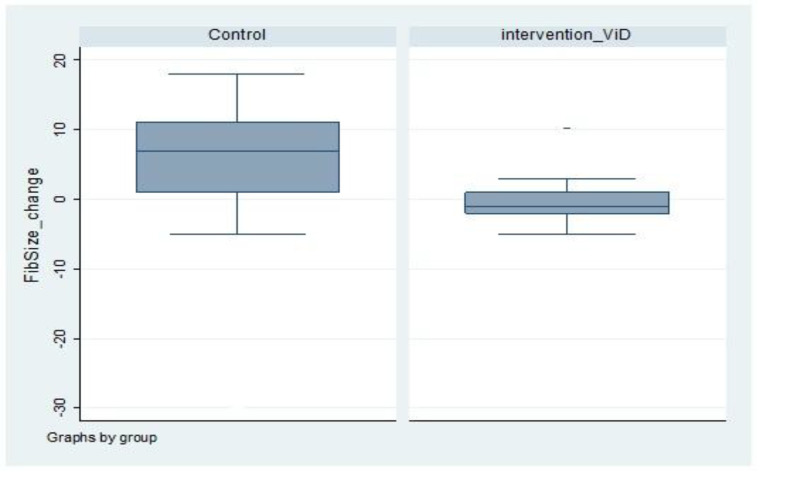
The mean uterine fibroids diameter in the control group before and after the study was 41.98 ± 5.25 and 47.813 ± 3.42 mm, respectively. The mean uterine fibroid size increased for 14.5% in control group. Mean vitD levels before and after the study were 23.6 and 22.7 ng/ml, respectively in the control group. It showed 0.9 unit or 4% decrement; the levels were assumed stable, if the laboratory error was considered (Fig.2).
Fig.2.
Diagram of variations in the mean diameter of uterine fibroid in the two groups.
Regarding the site of uterine fibroids in the control group, 43.14% of them were subserosal, 44.12% intramural and 12.75% submucosal. In the intervention group, 49.02% were subserosal, 42.16% intramural, and 8.82% submucosal uterine fibroid.
The most common type of uterine fibroids in the control group was intramural followed by subserosal, versus the intervention group in which, the subserosal was the most common type followed by intramural. The submucosal leiomyoma had the lowest incidence in both groups.
The mean uterine fibroid size in the intervention group before and after the study was 43.09 ± 14.36 and 42.61 ±15.53 mm, respectively. The size decreased to 0.48 mm. Mean vitD levels before and after intervention qwew23.2 and 28.5 ng/ml, respectively. It showed 5.36 unit or 24% increase in vitD levels (Fig.3).
Fig.3.
Diagram shows vitamin D levels in both group, before and after the study.
The change in the size of the uterine fibroid between the intervention and control group had a significant difference, the mean changes in size was 5.83 mm in the control group compare to - 0.48 mm in the intervention group (P=0.001). The control group had increases in size versus a slight decrease in the intervention group (Table 2).
Table 2.
Comparison of uterine fibroid size and vitD level changes in the two groups after the intervention
| Groups | Uterine fibroid size (mm) | % Size change (ng/ml) | Vitamin D | % Size change |
|---|---|---|---|---|
| Control n=102 | 5.833 ± 0.642 | 14.5 | -0.902 ± 1.29 | -4 |
| Intervention n=102 | -0.480 ± 0.246 | -2.5 | 5.362 ± 2.567 | 24 |
| P value | <0.001 | <0.001 | ||
Data are presented as mean ± SD or %.
The mean uterine fibroid size changes in the control group regarding the type of uterine fibroid was 5.15,7.33, and 2.92 mm for subserosal, intramural, and submucosal, respectively. Maximum change in mean leiomyoma diameter was found for intramural followed by subserosal.
The mean uterine fibroid size changes in the intervention group was 0.3 mm decrease for intramural and subserosal but for submucosal 2.33 mm decrease in size occurred after the intervention.
For evaluating the error of measurement of uterine fibroid diameter, another analysis was done and it showed that the mean change was 4.54 and -0.17 mm for the control and intervention group, respectively (P=0.001).
Discussion
This trial showed that vitD given to at a therapeutic dose vitD insufficient women, can inhibit the leiomyoma growth which was detectable by ultrasound; however, women in the control group (who did not receive vitD treatment) had some growth in leiomyoma size.
There are some articles about the role of vitD as antitumoral agent (7, 9, 17, 19, 28). The receptor of vitD is a nuclear receptor which is activated by 1, 25(OH)2 D3 , resulting in modulation of the transcription rate of target gene (like cell growth and division genes and estrogen and progesterone receptors encoding genes) (31). These functions are the orgin of anti-tumor effects of 1,25(OH)2 D3 on uterine fibroid.
Accumulating evidence suggests that the metabolic pathways of vitD may play a key role in the developing of several gynecological diseases. Indeed, the vitD receptor (VDR)-mediated signaling pathways and vitD levels seem to (deeply) affect the risk of polycystic ovary syndrome (PCOS), endometriosis, infertility, ovarian and even breast cancer, and affect a woman’s response to menopausal status (32-35).
Also another study evaluated vitD concentration and uterine fibroid in premenopausal women by ultrasound (36). About 90% of black women and 50% of white women had insufficient vitD levels. These women had 62% chance of uterine fibroid in comparison with women who had sufficient vitD concentrations.The study was showed that sufficient levels of vitD are associated with decreased risk of liomyoma. VitD concentration was checked from stored plasma of patients and 620 blacks and 416 whites were evaluated. Their samples were randomly chosen from National Institue of Environmental Health Sciences Uterine Fibroid Study during 1996- 1999. Women were asked regarding sun exposure by a questionaire that evaluted sun exposure>1 hour/day
Another cross-sectional study (37) showed that 52 women with uterine fibroid diagnosed by magnetic resonance imaging (MRI) or ultrasound had vitD level<30 ng/ml. The study showed that 85% of women with documented uterine fibroid were vitD deficient and that confirmed our study results.
Another prospective cross-sectional study in Turkish premenopausal women showed that traditional costume, being a house wife and low eduction are risk factor for vitD deficiency. Also, the study showed that vitD defficiency in women with leiomyoma was more prevalent versus in women without leiomyoma (38).
Moreover, a study by Mitro and Zota (39) in 3590 women with uterine leiomyomata, in the National Health and Nutrition Examination Survey indicated that there was no relationship between vitD and odds of uterine fibroids. Although, subgroup analysis performed on the same data showed that insufficient serum vitD was associated with significantly higher odds of uterine fibroids in white women but not in black patients.
In vivo vitD production is affected by sun exposure, geographical locations, latitude, season, weather condition, clothing and use of sunscreens have an important effect on vitD level. Indoor activity and dark skin are risk factor for black house-wife women for having fibroma (28, 40).
The strengths of our study were the design as a randomized clinical trial and accurate inclusion and exclusion criteria. The limitation of the present study is the short follow-up period (i.e.8 weeks). We recommend a clinical trial with longer period of follow-up (i.e. 12 months) for further measurement of leiomyoma size. Another limitation was that due to ethical considerations, we could not include women with vitD<20 ng/ml in the study and compare the effect of treatment on these women with the control group, and the reluctance of patients to participate in the study were another limitations of this study
Conclusion
Our results showed that vitD supplementation prevents fibroid growth. It seems that vitD supplement is a simple, safe and inexpensive modality for leiomyoma growth prevention.
Acknowledgements
The study was financially supported by Vice-Chancellor for Research and Technology of Tehran University of Medical Sciences, Tehran, Iran. The authors declare that they have no conflict of interest.
Authors’ Contributions
F.D.T.; Project development and patients examinations. E.F.; Data analysis and manuscript editing and submitting. M.V.F., H.A.; Data collection and patients examinations. B.M.; Vaginal ultrasound performing. S.S.S.; Data management and manuscript writing. All authors read and approved the final manuscript.
References
- 1.De Vivo A, Mancuso A, Giacobbe A, Maggio Savasta L, De Dominici R, Duga N, et al. Uterine myomas during pregnancy: a longitudinal sonographic study. Ultrasound Obstet Gynecol. 2011;37(3):361–365. doi: 10.1002/uog.8826. [DOI] [PubMed] [Google Scholar]
- 2.Benaglia L, Cardellicchio L, Filippi F, Paffoni A, Vercellini P, Somigliana E, et al. The rapid growth of fibroids during early pregnancy. PLoS One. 2014;9(1):e85933–e85933. doi: 10.1371/journal.pone.0085933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol. 2014;210(3):194–199. doi: 10.1016/j.ajog.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metwally M, Farquhar CM, Li TC. Is another meta-analysis on the effects of intramural fibroids on reproductive outcomes needed? Reprod Biomed Online. 2011;23(1):2–14. doi: 10.1016/j.rbmo.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Ali M, Al-Hendy A, Yang Q. Vit D, a promising natural compound with anti-uterine fibroid characteristics. Fertil Steril. 2019;111(2):268–269. doi: 10.1016/j.fertnstert.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Eldar-Geva T, Healy DL. Other medical management of uterine fibroids. Bailliere's Clinical Obstetrics and Gynaecology. 1998;12(2):269–288. doi: 10.1016/s0950-3552(98)80064-3. [DOI] [PubMed] [Google Scholar]
- 7.Guo XC, Segars J. The impact and management of fibroids for fertility an evidence-based approach. Obstet Gynecol Clin North Am. 2012;39(4):521–533. doi: 10.1016/j.ogc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matyjaszek-Matuszek B, Lenart-Lipińska M, Woźniakowska E. Clinical implications of vitamin D deficiency. Menopause Rev. 2015;14:75–81. doi: 10.5114/pm.2015.52149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holick MF. Vit D: a millenium perspective. J Cell Biochem. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 10.Laganà AS, Vergara D, Favilli A, La Rosa VL, Tinelli A, Gerli S, et al. Epigenetic and genetic landscape of uterine leiomyomas: a current view over a common gynecological disease. Arch Gynecol Obstet. 2017;296(5):855–867. doi: 10.1007/s00404-017-4515-5. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Mas A, Diamond MP, Al-Hendy A. The mechanism and function of epigenetics in uterine leiomyoma development. Reprod Sci. 2016;23(2):163–175. doi: 10.1177/1933719115584449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 13.Al-Hendy A, Myers ER, Stewart EA. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35(6):473–480. doi: 10.1055/s-0037-1607264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87(4):725–736. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 15.Scalia P, Durand MA, Forcino RC, Schubbe D, Barr PJ, O'Brien N, et al. Implementation of the uterine fibroids option grid patient decision aids across five organizational settings: a randomized stepped-wedge study protocol. Implementation Sci. 2019;14(1) doi: 10.1186/s13012-019-0933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciebiera M, Łukaszuk K, Męczekalski B, Ciebiera M, Wojtyła C, Słabuszewska-Jóźwiak A, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18(12) doi: 10.3390/ijms18122586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise LA, Palmer JR, Stewart EA, Rosenberg L. Age-specific incidence rates for self-reported uterine leiomyomata in the black women’s health study. Obstet Gynecol. 2005;105(3):563–568. doi: 10.1097/01.AOG.0000154161.03418.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair R, Maseeh A. Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3(2):118–126. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bikle DD. Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep. 2009;7(2) doi: 10.1007/s11914-009-0011-6. [DOI] [PubMed] [Google Scholar]
- 20.Parazzini F, Di Martino M, Candiani M, Vigano P. Dietary components and uterine leiomyomas: a review of published data. Nutr Cancer. 2015;67(4):569–579. doi: 10.1080/01635581.2015.1015746. [DOI] [PubMed] [Google Scholar]
- 21.Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22(6):665–686. doi: 10.1093/humupd/dmw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciebiera M, Włodarczyk M, Ciebiera M, Zaręba K, Łukaszuk K, Jakiel G. Vitamin D and Uterine fibroids—review of the literature and novel concepts. Int J Mol Sci. 2018;19(7) doi: 10.3390/ijms19072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 24.Halder SK, Osteen KG, Al-Hendy A. 1,25-dihydroxyvitamin D3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol Reprod. 2013;89(6) doi: 10.1095/biolreprod.113.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Mateo GT, Fernandez-Millara V, Bellon T, Liappas G, Ruiz-Ortega M, Lopez-Cabrera M, et al. Paricalcitol reduces peritoneal fibrosis in mice through the activation of regulatory T cells and reduction in IL-17 production. PLoS One. 2014;9(10):e108477–e108477. doi: 10.1371/journal.pone.0108477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Protic O, Toti P, Islam MS, Occhini R, Giannubilo SR, Catherino WH, et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016;364(2):415–427. doi: 10.1007/s00441-015-2324-3. [DOI] [PubMed] [Google Scholar]
- 27.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211–211. doi: 10.1016/j.ajog.2011.12.002. e1-211e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourdet AT, Luton D, Koskas M. Clinical utility of ulipristal acetate for the treatment of uterine fibroids: current evidence. Int J Womens Health. 2015;7:321–330. doi: 10.2147/IJWH.S50016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 30.Abedi S, Taebi M, Nasr Esfahani MH. Effect of vitamin D Supplementation on intracytoplasmic sperm injection outcomes: a randomized double-blind placebo-controlled trial. Int J Fertil Steril. 2019;13(1):18–23. doi: 10.22074/ijfs.2019.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Hendy A, Adam S. Can vitamin D reduce the risk of uterine fibroids? Womens Health (Lond Engl) 2014;10(4):353–358. doi: 10.2217/whe.14.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laganà AS, Vitale SG, Ban Frangež H, Vrtačnik Bokal E, D'Anna R. Vitamin D in human reproduction: the more, the better?. An evidence-based critical appraisal. Eur Rev Med Pharmacol Sci. 2017;21(18):4243–4251. [PubMed] [Google Scholar]
- 33.Colonese F, Laganà AS, Colonese E, Sofo V, Salmeri FM, Granese R, et al. The pleiotropic effects of vitamin D in gynaecological and obstetric diseases: an overview on a hot topic. Biomed Res Int. 2015;2015:986281–986281. doi: 10.1155/2015/986281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colonese F, La Rosa VL, Laganà AS, Vitale SG, Cortinovis D, Bidoli P. Comment on: Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Investig. 2016;29(1):37–38. doi: 10.1515/hmbci-2016-0040. [DOI] [PubMed] [Google Scholar]
- 35.Fichera M, Török P, Tesarik J, Corte LD, Rizzo G, Garzon S, et al. Vitamin D, reproductive disorders and assisted reproduction: evidences and perspectives. Int J Food Sci Nutr. 2020;71(3):276–285. doi: 10.1080/09637486.2019.1661978. [DOI] [PubMed] [Google Scholar]
- 36.Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin D and the risk of uterine fibroids. Epidemiology. 2013;24(3):447–53. doi: 10.1097/EDE.0b013e31828acca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen LD, Fenske SK, Isola HM, Ascher-Walsh CJ. Vitamin D deficiency in women with uterine fibroids versus Vitamin D deficiency in the general population. Clin Obstet Gynecol Reprod Med. 2018;4(6):1–3. [Google Scholar]
- 38.Oskovi Kaplan ZA, Taşçi Y, Topçu HO, Erkaya S. 25-Hydroxy vitamin D levels in premenopausal Turkish women with uterine leiomyoma. Gynecol Endocrinol. 2018;34(3):261–264. doi: 10.1080/09513590.2017.1391774. [DOI] [PubMed] [Google Scholar]
- 39.Mitro SD, Zota AR. Vitamin D and uterine leiomyoma among a sample of US women: findings from NHANES, 2001-2006. Reprod Toxicol. 2015;57:81–86. doi: 10.1016/j.reprotox.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brakta S, Diamond JS, Al-Hendy A, Diamond MP, Halder SK. Role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104(3):698–706. doi: 10.1016/j.fertnstert.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]