Abstract
Background
In vitro fertilization (IVF) is a useful assisted reproductive technology to achieve pregnancy in infertile couples. However, it is very important to optimize the success rate after IVF by controlling for its influencing factors. This study aims to classify successful deliveries after IVF according to couples’ characteristics and available data on oocytes, sperm, and embryos using several classification methods.
Materials and Methods
This historical cohort study was conducted in a referral infertility centre located in Tehran, Iran. The patients’ demographic and clinical variables for 6071 cycles during March 21, 2011 to March 20, 2014 were collected. We used six different machine learning approaches including support vector machine (SVM), extreme gradient boosting (XGBoost), logistic regression (LR), random forest (RF), naïve Bayes (NB), and linear discriminant analysis (LDA) to predict successful delivery. The results of the performed methods were compared using accuracy tools.
Results
The rate of successful delivery was 81.2% among 4930 cycles. The total accuracy of the results exposed RF had the best performance among the six approaches (ACC=0.81). Regarding the importance of variables, total number of embryos, number of injected oocytes, cause of infertility, female age, and polycystic ovary syndrome (PCOS) were the most important factors predicting successful delivery.
Conclusion
A successful delivery following IVF in infertile individuals is considerably affected by the number of embryos, number of injected oocytes, cause of infertility, female age, and PCOS.
Keywords: Assisted Reproductive Technology, Classification, Infertility, In Vitro Fertilization, Live Birth
Introduction
In vitro fertilization (IVF) is considered a popular technique used in assisted reproductive technology (ART) to promote the achievement of childbirth in the population of infertile individuals. Numerous aspects of IVF treatments have changed over time. Substantial research has been conducted to improve IVF results by taking into consideration its influencing factors; however, there is still a lack of knowledge about the predictors of IVF outcomes while the overall pregnancy rates have only reached approximately 30% (1, 2).
Many factors have been known to affect IVF outcomes including age, sperm quality, fertilization rate, embryo quality, frequency of transferred embryos, and endometrial thickness (3, 4). Determining influencing factors, could potentially influence the likelihood for a successful IVF treatment; this would enable clinicians and physicians to make better decisions in order to apply IVF based on patients’ characteristics (5). Patients who failed treatments might experience adverse psychological problems such as depression and anxiety (6). Therefore, it is essential to assess factors associated with the outcome after IVF and determine the influencing factors. In order to reduce psychological and other negative outcomes after IVF, patients could evaluate the likelihood of successful IVF based on their characteristics.
Thus, machine learning approaches have been designed to assess the relationship of an outcome and its effective variables; The use of a hybrid intelligence method for knowledge exploring of a clinical database on IVF (7), an ordered mechanism in comparison with naïve Bayes (NB) classifier to estimate the odds of success after IVF (8), random forest (RF) and adaptive boosting in classifying the state of ART (9), and logistic regression (LR) to predict implantation after blastocyst transfer (10) are some examples of application of this approach on IVF data.
Here, we used a clinical database that included each couple’s characteristics and available data on oocytes, sperm and embryos, as well as the cycle outcomes to classify the IVF outcome (successful/unsuccessful delivery) by NB, RF, support vector machine (SVM), extreme gradient boosting (XGBoost), linear discriminant analysis (LDA), and LR.
Materials and Methods
Participants and study design
We conducted this historical cohort study in a referral infertility centre located in Tehran, Iran. Data from 6071 cycles performed during March 21, 2011 to March 20, 2014 were analysed. We included only those women for whom clinical pregnancy was confirmed observing an intrauterine gestational sac. The collected demographic and clinical variables comprised women’s ages, source of infertility (female factor, male factor, combined male-female factor infertility, unexplained), infertility type (primary, secondary), body mass index (BMI), infertility duration (years), number of previous abortions, polycystic ovary syndrome (PCOS), number of previous IVF attempts, total number of retrieved oocyte, number of injected oocytes, number of embryos, number of transferred embryos, spermogram, fertilization rate after intracytoplasmic sperm injection (ICSI), number of two-pronuclear embryos to number of metaphase II (MII) oocytes (2PN/MII ratio), and data on embryo quality (number of compact, blastocysts, grade A, grade AB, early blastocysts, A compact, and AB compact), as well as the day of the embryo transfer (ET).
Statistical analysis
The descriptive characteristics of the data are shown using mean (standard error) and frequency (percentage) for continuous and categorical variables, respectively. We used the independent samples t test after checking the normality of data distribution to compare the mean of the variables across the categories of the response. The chi-squaretest was used to assess the independence of categorical variables with the outcome.
A principle component approach was utilized to reduce the dimension of multiple independent variables into smaller components. To do so, the variables that included the numbers of compact, blastocyst, grade A, grade AB, early blastocyst, A compact and AB compact, and the day of the ET were entered in the principle component analysis. The best number of components is decided according to the highest determined variance of the variables so that the majority of variability in the independent variables is available in the result antcomponents.
For the classification approaches, we randomly divided the data into two sets of train (70%) and test (30%). The train set was used to fit the model and the validation of the results was checked by the test set. In order to classify the status of delivery (successful/unsuccessful), we compared the results from the following six techniques: LR, SVM, XGBoost, RF, NB, and LDA. Sensitivity (SE), specificity (SP), positive predictive value (PPV), negative predictive value (NPV), accuracy (ACC), area under the curve (AUC) and 95% confidence interval were used to assess the performance of the models. In order to find more reliable results, we repeated each technique 500 times. The mean ACC measures are presented.
The statistical programing R software version 3.2.3 (http://www.R-project.org) packages that included RF, NB, e1071, XGBoost, and MASS were used for data analysis. The type one error was assumed as 0.05.
Ethical consideration
The Ethics Committee of Royan Institute (approval number: IR.ACECR.ROYAN.REC.1395.62), Tehran, Iran approved this study. The information used in this study was obtained from the data routinely registered in the patients’ medical records.
Results
Among the assessed cycles, 4930 (81.2%) cycles resulted in successful deliveries. In the analysis, 23 variables were assessed and eight variables were summarized into four components using the principle component analysis. Finally, the association of IVF outcome and the 19 variables were evaluated. Table 1 lists the mean or frequency of the variables for both successful and unsuccessful deliveries. The unadjusted results are shown using the t test and chi-square test for continuous and categorical variables, respectively. The duration of infertility for those who delivered successfully was 0.40 years less than those with unsuccessful deliveries (t-score: 2.75, P=0.006). The mean number of previous IVF cycles was higher for cases without successful deliveries (t-score: 2.46, P=0.014). The number of injected oocytes among cases with successful deliveries was higher than those with unsuccessful deliveries (t-score:-1.99, P=0.046). Cases with successful deliveries were significantly 1.35 years younger (t-score: 8.78, P<0.001) and had 0.60 kg/m2 lower BMI (tscore: 4.67, P<0.001). We noted that patients with PCOS had more successful deliveries (chi-square: 6.83, degree of freedom [df]: 1, P=0.009). Male factor (chi-square: 18.25, df: 5, P=0.003), frequency of previous abortions(chi-square: 19.62, df: 2, P<0.001), and primary type of infertility (chisquare: 5.02, df: 1, P=0.025) were associated with a higher probability of successful delivery. Table 1 provides additional details of the patients’ characteristics.
Table 1.
Patients’ characteristics in the successful and unsuccessful delivery groups
| Variables | Successful deliveryn (%) | t-score or chi-square (df) | P value | |||
|---|---|---|---|---|---|---|
| No1141 (18.8%) | Yes4930 (81.2%) | |||||
| Mean or frequency | SD or percentage | Mean or frequency | SD or percentage | |||
| Infertility duration (Y) | 6.02 | 4.59 | 5.62 | 4.29 | 2.75 | 0.006 |
| Number of previous IVF | 0.94 | 1.27 | 0.85 | 1.15 | 2.46 | 0.014 |
| Number of retrieved oocyte | 8.36 | 4.16 | 8.56 | 4.19 | -1.08 | 0.280 |
| Number of injected oocytes | 7.16 | 3.84 | 7.47 | 3.58 | -1.99 | 0.046 |
| Total number of embryos | 4.77 | 3.00 | 4.94 | 2.87 | -1.63 | 0.101 |
| Number of transferred embryos | 2.38 | 0.97 | 2.38 | 1.02 | -0.19 | 0.848 |
| Spermogram | 3.30 | 3.02 | 3.37 | 3.70 | -0.51 | 0.612 |
| Fertilization rate | 0.68 | 0.44 | 0.70 | 0.26 | -1.28 | 0.199 |
| C1 | 0.01 | 0.88 | 0.00 | 1.03 | 0.48 | 0.626 |
| C2 | 0.00 | 0.98 | 0.00 | 1.00 | 0.05 | 0.959 |
| C3 | 0.05 | 1.35 | -0.01 | 0.90 | 1.82 | 0.068 |
| C4 | 0.03 | 1.23 | -0.01 | 0.94 | 1.08 | 0.277 |
| Age of women (Y) | 84.1(3) | <0.001 | ||||
| <35 | 882 | 16.90 | 4341 | 83.10 | ||
| 35-37 | 106 | 20.40 | 414 | 79.60 | ||
| 37-40 | 127 | 26.70 | 348 | 73.30 | ||
| >40 | 91 | 36.40 | 159 | 63.60 | ||
| Age (continuous form) | 32.25 | 5.38 | 30.90 | 4.87 | 8.78 | <0.001 |
| BMI (kg/m2) | 24.02(3) | <0.001 | ||||
| Underweight | 14 | 12.30 | 100 | 87.70 | ||
| Normal | 396 | 16.60 | 1990 | 83.40 | ||
| Overweight | 446 | 18.60 | 1958 | 81.40 | ||
| Obese | 350 | 22.40 | 1214 | 77.60 | ||
| BMI (continuous form) | 26.53 | 4.26 | 25.93 | 4.14 | 4.67 | <0.001 |
| PCOS | 6.83(1) | 0.009 | ||||
| Yes | 915 | 17.90 | 4190 | 82.10 | ||
| No | 254 | 21.20 | 945 | 78.80 | ||
| Cause of infertility | 18.25(5) | 0.003 | ||||
| Female | 297 | 19.80 | 1204 | 80.20 | ||
| Male | 529 | 17.10 | 2571 | 82.90 | ||
| Both | 155 | 21.30 | 573 | 78.70 | ||
| Unknown | 189 | 19.52 | 779 | 80.48 | ||
| History of abortion | 19.62(2) | <0.001 | ||||
| None | 908 | 17.70 | 4231 | 82.30 | ||
| One | 176 | 20.80 | 669 | 79.20 | ||
| ≥Two | 1222 | 25.20 | 362 | 74.80 | ||
| Infertility type | 5.02(1) | 0.025 | ||||
| Primary | 813 | 17.80 | 3742 | 82.20 | ||
| Secondary | 320 | 20.40 | 1249 | 79.60 | ||
| Type of cycle | 0.401 | |||||
| ET | 441 | 18.30 | 1972 | 81.70 | ||
| ICSI | 700 | 19.10 | 2958 | 80.90 | ||
C1; Number of compact and blastocysts, C2; Number of grade A and grade AB, C3; Number of early blastocysts, A compact and the day of ET, and C4; Number of AB compact, SD; Standard deviation, df; Degree of freedom, IVF; In vitro fertilisation, BMI; Body mass index, PCOS; Polycystic ovary syndrome, ET; Embryo transfer, and ICSI; Intracytoplasmic sperm injection.
The principle component analysis reduced eight embryo factors (number of compact, blastocyst, grade A, grade AB, early blastocyst, A compact, AB compact, and day of ET) to four components. The components were: C1 (number of compact and blastocysts); C2 (number of grade A and grade AB); C3 (number of early blastocysts, A compact and the day of ET), and C4 (number of AB compact).
The six classification methods, including NB, RF, LDA and LR, were applied. Table 2 shows a comparison of their ACC measures. Except for LR, other classification methods resulted in almost the same and high SE and PPV (SE>0.80, PPV>0.99). In contrast, the SP and NPV of LR was higher than the other approaches (SP=0.50, NPV=0.27). The total accuracy of the results showed that LR (ACC=0.64) had the worst performance where as RF (ACC=0.81) had the best performance among the six applied approaches. Moreover, the AUC for RF (AUC=60; 0.55–0.64), LDA (AUC=0.57; 0.51–0.63), LR (AUC=0.55; 0.49–0.61), and NB (AUC=0.53; 0.47–0.58) confirmed as lightly higher accuracy for RF compared to the other methods.
Table 2.
A comparison of the six applied classification techniques using the accuracy measures
| Tools | Set | Methods Tool (95% confidence interval) | |||||
|---|---|---|---|---|---|---|---|
| XGBoost | SVM | NB | RF | LDA | LR | ||
| SE | Train | 0.75 (0.71–0.79) | 0.78 (0.75–0.81) | 0.81 (0.80–0.82) | 0.99 (0.98–1.00) | 0.81 (0.80–0.82) | 0.68 (0.67–0.69) |
| Test | 0.75 (0.72–0.78) | 0.76 (0.73–0.79) | 0.82 (0.81–0.83) | 0.81 (0.80–0.82) | 0.80 (0.79–0.81) | 0.67 (0.66–0.68) | |
| SP | Train | 0.65 (0.62–0.68) | 0.35 (0.32–0.38) | 0.32 (0.31–0.33) | 0.99 (0.98–1.00) | 0.64 (0.61–0.67) | 0.48 (0.47–0.49) |
| Test | 0.62 (0.56–0.70) | 0.34 (0.32–0.36) | 0.25 (0.22–0.28) | 0.39 (0.34–0.44) | 0.41 (0.35–0.47) | 0.50 (0.49–0.51) | |
| PPV | Train | 0.90 (0.86–0.94) | 0.60 (0.56–0.64) | 0.97 (0.96–0.98) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.85 (0.84–0.86) |
| Test | 0.89 (0.85–0.93) | 0.58 (0.52–0.64) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.84 (0.83–0.85) | |
| NPV | Train | 0.38 (0.33–0.43) | 0.56 (0.53–0.59) | 0.05 (0.04–0.06) | 0.99 (0.98–1.00) | 0.01 (0.01–0.02) | 0.26 (0.25–0.27) |
| Test | 0.37 (0.32–0.42) | 0.55 (0.53–0.57) | 0.09 (0.06–0.12) | 0.01 (0.01–0.02) | 0.01 (0.01–0.02) | 0.27 (0.26–0.28) | |
| ACC | Train | 0.74 (0.70–0.78) | 0.59 (0.56–0.62) | 0.79 (0.78–0.80) | 0.99 (0.98–1.00) | 0.81 (0.80–0.82) | 0.65 (0.64–0.66) |
| Test | 0.73 (0.68–0.78) | 0.58 (0.55–0.61) | 0.77 (0.76–0.78) | 0.81 (0.80–0.82) | 0.80 (0.79–0.81) | 0.64 (0.63–0.65) | |
| AUC | Train | 0.62 (0.57–0.67) | 0.58 (0.55–0.61) | 0.60 (0.56–0.64) | 0.68 (0.64–0.72) | 0.63 (0.58–0.68) | 0.62 (0.56–0.68) |
| Test | 0.60 (0.57–0.63) | 0.57 (0.53–0.61) | 0.53 (0.47–0.58) | 0.60 (0.55–0.64) | 0.57 (0.51–0.63) | 0.55 (0.49–0.61) | |
SVM; Support vector machine, XGBoost; Extreme gradient boosting, LDA; Linear discriminant analysis, LR; Logistic regression, RF; Random forest, NB; Naïve Bayes, SE; Sensitivity, SP; Specificity, PPV; Positive predictive value, NPV; Negative predictive value, ACC; Accuracy, and AUC; area under the curve.
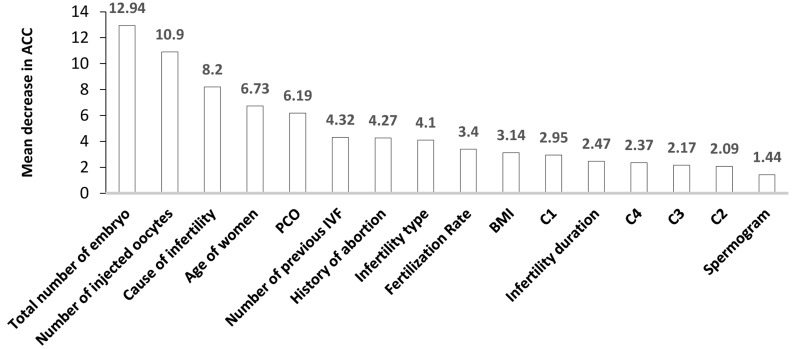
Fig.1.
The importance of variables that affect successful delivery according to the RF approach, which had the best performance among the classification approaches. C1; Number of compact and blastocysts, C2; Number of grade A and grade AB, C3; Number of early blastocysts, A compact and the day of ET, and C4; Number of AB compact, RF; Random forest, PCOS; Polycystic ovary syndrome, IVF; In vitro fertilisation, BMI; Body mass index, and ACC; Accuracy.
Figure 1shows the importance of variables that affected successful delivery using the RF method. The total number of embryos, number of injected oocytes, cause of infertility, women’s age, and PCOS were the affecting predictors for a successful delivery that had a higher amount of importance in comparison to the other variables.
Discussion
The aim of this study was to compare classical regression based methods with machine learning methods. We compared these techniques in an attempt to gain a better understanding and prediction of IVF outcomes. The application of these methods in IVF data is supposed to improve efficiency by estimating the chance of success. Generalizable and reliable prediction methods can help fulfil this purpose.
In the statistical analysis of our paper, six data mining procedures were fitted and compared to investigate successful delivery. Based on the ACC tools, the RF best fitted the data. There are several possible explanations for the better performance of the RF method in this study. First, it might be explained by the fact that modern modelling methods such as RF tend to be “data hungry” and are believed to perform better with a higher eventsper-variable ratio than classical methods (11). The larger number of continuous variables than categorical variables in this study could be another possible explanation for the better RF performance. It may also be due to the fact that tree based methods like RF account for variable interactions, while regression based methods like LR do not (12). The goodness of fit for determining a machine learning approach is a function of rates in levels of the outcome. Therefore, it does not seem to be quite rational to focus only on the total accuracy (13). A few other research indicate inconsistent performance of various classification algorithms with respect to the small/ high prevalence of the outcome (14, 15). The manner under which the predictor variables influence the result is essential for deciding the correct form of method. Therefore, discrepancies could be reported in performing classification techniques in various data areas.
Several studies have assessed machine learningbased prediction models in different outcomes during ART (e.g., embryo implantation, ongoing pregnancy, clinical pregnancy, pregnancy) (16). Uyar et al. (17) found that higher accuracy rates might be obtained by using morphological variables of individual embryos utilizing NB method for implantation prediction. Hafiz et al. (9) demonstrated that RF performed better than SVM, recursive partitioning (RPART), adaptive boosting (Adaboost), and nearest neighbour in predicting implantation outcomes of IVF and ICSI. The dataset in their study was highly unbalanced as the number of those with negative implantation was more than the positive. They explained the poor performance of SVM with the unbalanced nature of medical datasets, in particular, the one used in their study. In another study, Hassan et al. (18) compared a series of classifiers (SVM, RF, multilayer perceptron neural network [MLP], decision tree, classification and regression trees [CART] and artificial neural networks [ANN]) to predict pregnancy outcomes for IVF treatment. They reported that SVM and RF performed almost the same and both were better than the other classifiers in terms of prediction ACC and AUC. The results demonstrated that selection of a set of features for each method significantly improved the prediction ACC of pregnancy success.
The result of this study showed that the total number of embryos obtained in each cycle was associated with successful live birth. This finding supported those reported by Bartmann et al. (19), who used an artificial intelligence system to calculate pregnancy chance by taking into consideration the patients’ clinical and laboratory information. They showed that the number of embryos obtained was the best discriminant variable for pregnancy prediction; according to the artificial intelligent system developed in this study, women with more embryos tended to have greater chances for pregnancy. It was reported that the total number of embryos might be a surrogate marker for hormonal factors that act via uterine receptivity (20).
In the current study, the number of injected oocytes was another important variable that predicted IVF outcome. In a historical cohort study on 996 infertile women, modified Poisson regression analysis demonstrated that females who attained clinical pregnancy had a significantly greater number of injected oocytes compared with those who failed to achieve pregnancy (21). In another study, the number of injected oocytes was positively associated with the number of grade A embryos and could be a determinant of a successful ART (22). Zorn et al. (23) conducted a study of influencing gender characteristics of ICSI outcome in azoospermic and aspermic patients. They observed a positive association between the frequency of injected oocytes with reaching the blastocyst stage and live birth.
Cause of infertility is another important variable that affects IVF outcome, which has been confirmed by the results of numerous similar studies (24). Nelson and Lawlor (25) predicted live birth and weight at birth among infants born from IVF. They observed that male cause of infertility was linked to lower chances of successful pregnancy in patients who did not receive ICSI. Factors associated with failed treatment were evaluated by Bhattacharya et al. (26); the results showed that the risk of poor fertilization was more common among patients with tubal disease, male factor, and endometriosis. Moreover, they noted that the risk of non-live births among those with tubal disease and male factor was higher than those with unexplained infertility. It has been demonstrated that cause of infertility plays a role in determining poor intermediate outcomes. Elizur et al. (27) investigated the predictive factors for IVF treatment pregnancy results; they observed that delivery rate among those with male factor was significantly higher than other aetiologies.
A woman’s age was another significant factor for achieving a successful pregnancy. It has been widely debated that with increasing of female age, the IVF outcomes become increasingly worse. Among infertile cases, the Society for ART (SART) stated that 47% of ETs among women younger than 35 years of age resulted in successful delivery. The proportion was 38% for ages 35–37, 28% for ages 38–40, 16% for ages 41–42 and 6% for older than 42 years of age (28). Nazemian et al. (29) investigated the impact of age on IVF outcome. They reported that cases younger than 25 years of age have lower fertilization rates as well as a decreased frequency of high quality embryos. In their study, clinical pregnancy and implantation rates were similar to those who were 30-35years of age. In another research, Yan et al. (30) evaluated the mechanism by which maternal age affects the outcomes of IVF cases. Patients older than 40 years had a disadvantaged IVF outcome and increased numbers of miscarriages.
The current work shows that PCOS is a potential influencing factor for live birth. Beydoun et al. (31) have reported that PCOS has a distinct effect on the early stages of pregnancy among women who undergo IVF/ICSI, but not on the later stages. Ryan et al. (32), in a study of a large number of infertile women, showed that women with PCOS had increased odds for childbirth. Moreover, PCOS significantly confounded the relationship between the duration of ovarian stimulation and treatment success. Earlier findings also showed a greater number of oocytes were retrieved in PCOS women compared to women without PCOS (31), and greater number of follicles>16 mm and MII oocytes in PCOS women compared to women with subfertile male partners and those with unexplained infertility (33). These results imply a higher amount of ovarian capacity in PCOS women and the compensatory impact of this capacity (34, 35). However, the results from a large number of studies mentioned that the role of PCOS in ART success mainly depended on obesity, insulin resistance, and other metabolic syndrome features (36, 37).
This study had several limitations. First, this research was carried out in one infertility clinic and this limits the generaliz ability of our findings. Second, other predictors such as basal FSH and somegenetic features are potential factors that were not recorded by the Centre (38, 39). Third, the distribution of IVF outcome was not balanced (unbalanced dataset). Fourth, the AUCs were relatively small and the performance of the models was compared using accuracy tools in conjunction with the AUC.
RF performance has less dependence on parameter values than other machine learning methods. However, in future investigations it might be possible to achieve more improvements in this method by using optimization procedures to simultaneously tune the RF parameters or use RF based on conditional inference trees to address the problem of variable selection (40).
Results obtained from machine learning could help to determine the risk factors and their impact in real world settings. It could also help to predict the personalized chance of an ART outcome before the treatment procedure. This would assist clinicians decide whether it is worth to startan ART procedure and would also provide infertile couples information about the chances for success.
Conclusion
This study sought to classify IVF successful delivery based on six machine learning approaches: SVM, XGBoost, LDA, LR, RF and NB by using couples’ characteristics and available data on oocytes, sperm, and embryos. This study indicated that successful delivery after ART is strongly dependent on various characteristics of the patients, which included total number of embryos, number of injected oocytes, cause of infertility, age of women, and PCOS. Our results indicated that the RF approach could be a better choice to classify ART outcome among other classification methods. These results could assist clinicians to have a better prediction and management of ART treatment and advise patients accordingly.
Acknowledgements
The authors would like to express their appreciation to Royan Institute for financial and scientific support. The authors declare that they have no conflicts of interest.
Authors’ Contributions
A.G., P.A., M.P.-K.; Study conception and design, data extraction, data analysis and interpretation, and drafting of the manuscript. F.R., S.M., R.O.-S.; Study conception and design, data interpretation, and drafting of the manuscript. All authors approved the final version of this manuscript for publication.
References
- 1.Somigliana E, Vigano P, Busnelli A, Paffoni A, Vegetti W, Vercellini P. Repeated implantation failure at the crossroad between statistics, clinics and over-diagnosis. Reprod Biomed Online. 2018;36(1):32–38. doi: 10.1016/j.rbmo.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Wade JJ, MacLachlan V, Kovacs G. The success rate of IVF has significantly improved over the last decade. Aust N Z J Obstet Gynaecol. 2015;55(5):473–476. doi: 10.1111/ajo.12356. [DOI] [PubMed] [Google Scholar]
- 3.Vaegter KK, Lakic TG, Olovsson M, Berglund L, Brodin T, Holte J. Which factors are most predictive for live birth after in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments?. Analysis of 100 prospectively recorded variables in 8,400 IVF/ICSI single-embryo transfers. Fertil Steril. 2017;107(3):641–648. doi: 10.1016/j.fertnstert.2016.12.005. e642. [DOI] [PubMed] [Google Scholar]
- 4.Azmoudeh A, Shahraki Z, Hoseini F, Akbari-Asbagh F, DavariTanha F, Mortazavi F. In vitro fertilization success and associated factors: a prospective cohort study. Int J Women's Health Reprod Sci. 2018;6(3):350–355. [Google Scholar]
- 5.Gunderson S, Jungheim ES, Kallen CB, Omurtag K. Public reporting of IVF outcomes influences medical decision-making and physician training. Fertil Res Pract. 2020;6(1):1–6. doi: 10.1186/s40738-020-00070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Abbudi S. Major depressive disorder association with unsuccessful in-vitro fertilization (IVF) of primary infertile women. International Journal of Contemporary Research and Review. 2019;10(4):20717–20725. [Google Scholar]
- 7.Guh R, Wu T, Weng SP. Integrating genetic algorithm and decision tree learning for assistance in predicting in vitro fertilization. Medi Biol Eng Comput. 2015;53(9):911–920. [Google Scholar]
- 8.Güvenir HA, Misirli G, Dilbaz S, Ozdegirmenci O, Demir B, Dilbaz B. Estimating the chance of success in IVF treatment using a ranking algorithm. Medi Biol Eng Comput. 2015;53(9):911–920. doi: 10.1007/s11517-015-1299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hafiz P, Nematollahi M, Boostani R, Namvar Jahromi B. Predicting implantation outcome of in vitro fertilization and intracytoplasmic sperm injection using data mining techniques. Int J Fertil Steril. 2017;11(3):184–190. doi: 10.22074/ijfs.2017.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blank C WR, Wildeboer RR, DeCroo I, Tilleman K, Weyers B, de Sutter P, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111(2):318–326. doi: 10.1016/j.fertnstert.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 11.van der Ploeg T, Austin PC, Steyerberg EW. Modern modelling techniques are data hungry: a simulation study for predicting dichotomous endpoints. BMC Med Res Methodol. 2014;14(1):137–137. doi: 10.1186/1471-2288-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Pinto LN, Venable LR, Fahrenbach J, Churpek MM. Comparison of variable selection methods for clinical predictive modeling. Int J Med Inform. 2018;116:10–17. doi: 10.1016/j.ijmedinf.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapak L, Mahjub H, Hamidi O, Poorolajal J. Real-data comparison of data mining methods in prediction of diabetes in Iran. Healthcare Inform Res. 2013;19(3):177–185. doi: 10.4258/hir.2013.19.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer D, Leisch F, Hornik K. The support vector machine under test. Neurocomputing. 2003;55(1-2):169–186. [Google Scholar]
- 15.Amini P, Ahmadinia H, Poorolajal J, Amiri MM. Evaluating the high risk groups for suicide: a comparison of logistic regression, support vector machine, decision tree and artificial neural network. Iran J Public Health. 2016;45(9):1179–1187. [PMC free article] [PubMed] [Google Scholar]
- 16.Raef B, Ferdousi R. A review of machine learning approaches in assisted reproductive technologies. Acta Inform Med. 2019;27(3):205–205. doi: 10.5455/aim.2019.27.205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uyar A, Bener A, Ciray HN. Predictive modeling of implantation outcome in an in vitro fertilization setting: an application of machine learning methods. Med Decis Making. 2015;35(6):714–725. doi: 10.1177/0272989X14535984. [DOI] [PubMed] [Google Scholar]
- 18.Hassan MR, Al-Insaif S, Hossain MI, Kamruzzaman J. A machine learning approach for prediction of pregnancy outcome following IVF treatment. Neural Comput Appl. 2020;32(7):2283–2297. [Google Scholar]
- 19.Bartmann AK, Junior MF, de Barros GP, de Paula LS, Bettini NR. The number of embryos obtained can offset the age factor in IVF resultsaccording to an artificial intelligence system. Womans Health Gynecol. 2016;2(5):1–5. [Google Scholar]
- 20.Roberts S, Hirst WM, Brison DR, Vail A. Embryo and uterine influences on IVF outcomes: an analysis of a UK multi-centre cohort. Hum Reprod. 2010;25(11):2792–2802. doi: 10.1093/humrep/deq213. [DOI] [PubMed] [Google Scholar]
- 21.Almasi-Hashiani A, Mansournia MA, Sepidarkish M, Vesali S, Ghaheri A, Esmailzadeh A, et al. Comparison of in vitro fertilization/ intracytoplasmic sperm injection cycle outcome in patients with and without polycystic ovary syndrome: a modified poisson regression model. Int J Fertil Steril. 2018;11(4):309–313. doi: 10.22074/ijfs.2018.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almasi-Hashiani A, Ghaheri A, Omani Samani R. Determinants of the grade a embryos in infertile women; zero-inflated regression model. Cell J. 2017;19(3):506–511. doi: 10.22074/cellj.2017.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zorn B, Virant-Klun I, Drobni S, Šinkovec J, Meden-Vrtovec H. Male and female factors that influence ICSI outcome in azoospermia or aspermia. Reprod Biomed Online. 2009;18(2):168–176. doi: 10.1016/s1472-6483(10)60252-0. [DOI] [PubMed] [Google Scholar]
- 24.Women's NCCf Health Cs. Fertility: assessment and treatment for people with fertility problems.London.RCOG Press. RCOG Press; 2004. [PubMed] [Google Scholar]
- 25.Nelson SM, Lawlor DA. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: a prospective study of 144,018 treatment cycles. PLoS Med. 2011;8(1):e1000386–e1000386. doi: 10.1371/journal.pmed.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharya S, Maheshwari A, Mollison J. Factors associated with failed treatment: an analysis of 121,744 women embarking on their first IVF cycles. PLoS One. 2013;8(12):e82249–e82249. doi: 10.1371/journal.pone.0082249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elizur SE, Lerner-Geva L, Levron J, Shulman A, Bider D, Dor J. Factors predicting IVF treatment outcome: a multivariate analysis of 5310 cycles. Reprod Biomed Online. 2005;10(5):645–649. doi: 10.1016/s1472-6483(10)61673-2. [DOI] [PubMed] [Google Scholar]
- 28.Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Criteria for number of embryos to transfer: a committee opinion. Fertil Steril. 2012;99(1):44–46. doi: 10.1016/j.fertnstert.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 29.Nazemian Z, Esfandiari N, Javed M, Casper RF. T The effect of age on in vitro fertilization outcome: Is too young possible? J Assist Reprod Genet. 2011;28(2):101–106. doi: 10.1007/s10815-010-9499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan J, Wu K, Tang R, Ding L, Chen ZJ. Effect of maternal age on the outcomes of in vitro fertilization and embryo transfer (IVF-ET) Sci China Life Sci. 2012;55(8):694–698. doi: 10.1007/s11427-012-4357-0. [DOI] [PubMed] [Google Scholar]
- 31.Beydoun HA, Stadtmauer L, Beydoun MA, Russell H, Zhao Y, Oehninger S. Polycystic ovary syndrome, body mass index and outcomes of assisted reproductive technologies. Reprod Biomed Online. 2009;18(6):856–863. doi: 10.1016/s1472-6483(10)60037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan A, Wang S, Alvero R, Polotsky AJ. Prolonged gonadotropin stimulation for assisted reproductive technology cycles is associated with decreased pregnancy rates for all women except for women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31(7):837–842. doi: 10.1007/s10815-014-0253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nafiye Y, Sevtap K, Muammer D, Emre O, Senol K, Leyla M. The effect of serum and intrafollicular insulin resistance parameters and homocysteine levels of nonobese, nonhyperandrogenemic polycystic ovary syndrome patients on in vitro fertilization outcome. Fertil Steril. 2010;93(6):1864–1869. doi: 10.1016/j.fertnstert.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Okohue JE, Onuh SO, Ikimalo JI. Comparison of IVF/ICSI outcome in patients with polycystic ovarian syndrome or tubal factor infertility. Niger J Clin Pract. 2013;16(2):207–210. doi: 10.4103/1119-3077.110164. [DOI] [PubMed] [Google Scholar]
- 35.Urman B, Tiras B, Yakin K. Assisted reproduction in the treatment of polycystic ovarian syndrome. Reprod Biomed Online. 2004;8(4):419–430. doi: 10.1016/s1472-6483(10)60926-1. [DOI] [PubMed] [Google Scholar]
- 36.Sheng Y, Lu G, Liu J, Liang X, Ma Y, Zhang X, et al. Effect of body mass index on the outcomes of controlled ovarian hyperstimulation in Chinese women with polycystic ovary syndrome: a multicenter, prospective, observational study. J Assist Reprod Genet. 2017;34(1):61–70. doi: 10.1007/s10815-016-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cakiroglu Y, Doger E, Vural F, Kopuk SY, Vural B. Impact of insulin resistance and obesity on intracytoplasmic sperm injection outcomes in young women with polycystıc ovary syndrome. North Clin Istanb. 2017;4(3):218–224. doi: 10.14744/nci.2017.79663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Liu D, Wang J, Deng H, Luo X, Shen X, et al. Meta-analysis identifies candidate key genes in endometrium as predictive biomarkers for clinical pregnancy in IVF. Oncotarget. 2015;8(60):102428–102428. doi: 10.18632/oncotarget.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Loendersloot LL, van Wely M, Limpens J, Bossuyt PMM, Repping S, van Der Veen F. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16(6):577–589. doi: 10.1093/humupd/dmq015. [DOI] [PubMed] [Google Scholar]
- 40.Strobl C, Boulesteix AL, Zeileis A, Hothorn T. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8(1):25–25. doi: 10.1186/1471-2105-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]