Abstract
Objectives
To generate and validate state-of-the-art radiomics models for prediction of radiation-induced lung injury and oncologic outcome in non-small cell lung cancer (NSCLC) patients treated with robotic stereotactic body radiation therapy (SBRT).
Methods
Radiomics models were generated from the planning CT images of 110 patients with primary, inoperable stage I/IIa NSCLC who were treated with robotic SBRT using a risk-adapted fractionation scheme at the University Hospital Cologne (training cohort). In total, 199 uncorrelated radiomic features fulfilling the standards of the Image Biomarker Standardization Initiative (IBSI) were extracted from the outlined gross tumor volume (GTV). Regularized models (Coxnet and Gradient Boost) for the development of local lung fibrosis (LF), local tumor control (LC), disease-free survival (DFS) and overall survival (OS) were built from either clinical/ dosimetric variables, radiomics features or a combination thereof and validated in a comparable cohort of 71 patients treated by robotic SBRT at the Radiosurgery Center in Northern Germany (test cohort).
Results
Oncologic outcome did not differ significantly between the two cohorts (OS at 36 months 56% vs. 43%, p = 0.065; median DFS 25 months vs. 23 months, p = 0.43; LC at 36 months 90% vs. 93%, p = 0.197). Local lung fibrosis developed in 33% vs. 35% of the patients (p = 0.75), all events were observed within 36 months. In the training cohort, radiomics models were able to predict OS, DFS and LC (concordance index 0.77–0.99, p < 0.005), but failed to generalize to the test cohort. In opposite, models for the development of lung fibrosis could be generated from both clinical/dosimetric factors and radiomic features or combinations thereof, which were both predictive in the training set (concordance index 0.71– 0.79, p < 0.005) and in the test set (concordance index 0.59–0.66, p < 0.05). The best performing model included 4 clinical/dosimetric variables (GTV-Dmean, PTV-D95%, Lung-D1ml, age) and 7 radiomic features (concordance index 0.66, p < 0.03).
Conclusion
Despite the obvious difficulties in generalizing predictive models for oncologic outcome and toxicity, this analysis shows that carefully designed radiomics models for prediction of local lung fibrosis after SBRT of early stage lung cancer perform well across different institutions.
Introduction
Stereotactic body radiation therapy (SBRT) is an effective therapy for early-stage, node-negative, medically inoperable non-small cell lung cancer (NSCLC). Dose-fractionation schemes usually depend on tumor size and location and have been largely standardized by current guidelines [1–4]. However, after irradiation, about 10–15% of the tumors will recur locally, up to 50% of the patients will experience systemic disease progression despite PET-based staging before SBRT [5], and 25–30% of the patients will develop radiation-induced lung injury (RILI) on follow-up chest imaging. Apart from an established dose–response relationship for local control [6], dosimetric and clinical factors have only shown limited capability in predicting these events [7–14].
Radiomics aims at extraction of biomarkers from high-dimensional analysis of digital images and has been extensively studied in lung cancer by using computed tomography (CT) or Fluor-Deoxyglucose Positron Emission Tomography (FDG-PET) of the chest [15–20]. Several studies have applied radiomic analysis in SBRT of NSCLC [21–34], but so far, the clinical impact of the developed algorithms has been low due to low reproducibility of the results [35], lack of standardization of the extracted radiomic features and lack of external validation on data from other institutions.
The availability of open-source software solutions allows the extraction of standardized radiomic features and generation of complex, non-linear models which are able to account for complex interactions between features and have the potential to achieve high performance. The primary objective of the present study was to build a model for the development of radiation-induced lung injury by use of state-of-the-art feature extraction and machine-learning algorithms in order to determine the extra value of imaging tumor biomarkers when used in addition to dosimetric and clinical factors in a cohort of patients with NSCLC treated by robotic SBRT. Secondary objectives of the study were the development of models for local control, disease free survival and overall survival. The models were trained on data from one institution and tested on a cohort from a separate institution that treated patients based on similar inclusion criteria and fractionation schemes. This work extends an earlier single institution report [36].
Patients and methods
Patients, treatment and follow-up
Two cohorts of patients with stage I/IIa NSCLC (according to staging classification of the Union for International Cancer Control [UICC], 8th edition) who underwent definitive robotic SBRT were retrospectively analyzed. The first cohort comprised 110 patients treated at the University Hospital of Cologne, Germany and was used for identification of clinical, dosimetric and image-derived parameters to predict local control (LC), overall survival (OS), disease free survival (DFS) and occurrence of local lung fibrosis (LF) as a manifestation of radiation-induced lung injury after SBRT. This cohort had already been analyzed in a previous study using a simpler radiomics approach that only included first-level features and a small set of 5 texture features from the Gray-Level Co-occurrence Matrix (GLCM) without wavelet-filtering [36] and served as the training data set. A second cohort of 71 patients was treated at the Radiosurgery Center Northern Germany, Guestrow, and was used as test set (in machine learning terminology) for the predictive power of the models developed in the training set.
In both cohorts, patients suffering from a peripheral T1/2 (UICC 8) NSCLC without lymph node metastases who were either medically inoperable or refused resection were treated solely by means of the CyberknifeR system (Accuray, Sunnyvale, USA) without concomitant therapy using a risk-adapted fractionation scheme (peripheral T1 tumors 3 × 13–18 Gy, T1 tumors with broad contact to the chest wall and T2 tumors 5 × 10–11 Gy, near-central or true central tumors 8 × 6–7.5 Gy). The dose was calculated using a Monte Carlo dose calculation algorithm (Multiplan 4.5, Accuray, Sunnyvale, USA) and the prescribed dose was referred to the 65–70% isodose in most cases. The GTV was manually outlined for clinical use on the planning CT, and the PTV was generated by adding a margin of 3-4 mm (Table 1). A set of volumetric and dosimetric parameters was extracted from the planning system including GTV (gross tumor volume), PTV (planning target volume), GTV-Dmax (maximal dose in GTV), GTV-Dmean (mean dose in GTV), GTV-D95% (dose achieved in 95% of the GTV), PTV-D95% (dose achieved in 95% of the PTV) and lung doses Lung-D1ml, Lung-D10ml, Lung-D50ml, Lung-D100ml [13, 37–40], see Table 1. According to the different dose/ fractionation schemes, the doses covered a wide range in both datasets. The GTV-Dmax which is reported as a point dose from the Cyberknife system might have been biased upwards due to noise induced by the Monte Carlo algorithm, but showed a close correlation with the GTV-Dmean in both sets (Pearson correlation coefficient 0.98 and 0.89), indicating that this bias was small. The cohorts also contained 12(8) patients with local stage T1/2 tumors who had been successfully treated for oligo-metastatic disease, and who were free from tumor activity besides the primary tumor. Patient characteristics and treatment parameters are shown in Table 1. All patients had the (3D) planning CT performed under breath-hold conditions which was used for both treatment planning and radiomics image analysis (Table 2).
Table 1.
Patient and treatment characteristics
| Training set | Test set | |||
|---|---|---|---|---|
| (n = 110) | (n = 71) | |||
| Age (median/range) | 73y (50–94 year) | 75y (48–88 year) | ||
| Gender (male/female) | 58/52 (53%/47%) | 47/24 (66%/34%) | ||
| Tumor diameter (median/range) | 2.2 cm (0.8–6.6 cm)* | 2.6 cm (1.1–6.0 cm)# | ||
| Tumor stage (UICC8), T1/T2 | 89/21 (81%/19%) | 45/26 (63%/37%) | ||
| Pathological confirmation (Yes/No) | 91/19 (83%/17%) | 55/16 (77%/23%) | ||
| Mediastinal staging | ||||
| CT only | 18 (16%) | 5 (7%) | ||
| CT + PET | 52 (47%) | 33 (47%) | ||
| CT + EBUS | 18 (16%) | 16 (23%) | ||
| CT + EBUS + PET | 18 (16%) | 17 (24%) | ||
| CT + mediastinoscopy | 3 (3%) | – | ||
| CT + PET + mediastinoscopy | 1 (1%) | – | ||
| Histology | ||||
| Adenocarcinoma | 37 (34%) | 23 (32%) | ||
| Squamous cell | 42 (38%) | 28 (39%) | ||
| Other | 12 (11%) | 4 ( 6%) | ||
| Unknown | 19 (17%) | 16 (23%) | ||
| Fractionation scheme | ||||
| Number of fractions | Dose per fraction | n Pat | Dose per fraction | n Pat |
| 1 | 25 Gy | 5 (5%) | 26–27 Gy | 2 (3%) |
| 3 | 17 Gy | 45 (41%) | 13–18 Gy | 65 (90%)§ |
| 5 | 11 Gy | 43 (39%) | 10–11 Gy | 3 (6%) |
| 8 | 7.5 Gy | 17 (16%) | 6.0 Gy | 1 (1%) |
| Doses to GTV, PTV and lung (median/ range) | ||||
| GTV Dmax | 84.6 (28.2–95.2) Gy | 70.9 (41.5–84.6) Gy | ||
| GTV Dmean | 71.6 (26.2–84.0) Gy | 62.7 (37.9–72.5) Gy | ||
| GTV D95% | 61.9 (21.8–75.9) Gy | 53.8 (33.0–64.6) Gy | ||
| PTV D95% | 54.0 (19.0–67.1) Gy | 45.3 (25.2–55.2) Gy | ||
| Lung D1ml | 65.6 (23.6–81.0) Gy | 55.5 (37.7–71.6) Gy | ||
| Lung D10ml | 52.1 (15.8–78.9) Gy | 47.5 (25.8–66.9) Gy | ||
| Lung D50ml | 31.3 (6.9–77.7) Gy | 31.6 (11.2–51.9) Gy | ||
| Lung D100ml | 20.5 (4.5–77.0) Gy | 20.5 (6.6–43.0) Gy | ||
| GTV-PTV margin | 3–4 mm | 3–5 mm | ||
| Tracking Mode (Fiducials/XSightLung) | 15/95 (14%/86%) | 6/65 (9%/91%) |
*1 pt. > 5 cm
#3 pts. > 5 cm
§1 pt. 4 × 10 Gy
Table 2.
Imaging parameters
| Training set | Test set | |
|---|---|---|
| CT scanner | Aquilion LB-CT, Toshiba | Brilliance 16, Philips |
| Slice thickness | 1.0 mm | 1.5 mm |
| Transversal resolution | 0.93–1.37 mm | 0.93–0.97 mm |
| Voltage | 120KV | 120KV |
| Current–time product | 400mAs | 400-450mAs |
| Image matrix | 512 × 512 | 512 × 512 |
| Reconstruction kernel | FC17 | B |
| Contrast agent | None (84%), AccupaqueR 300 (16%)* | None (100%) |
*No significant impact on GTV radiodensity
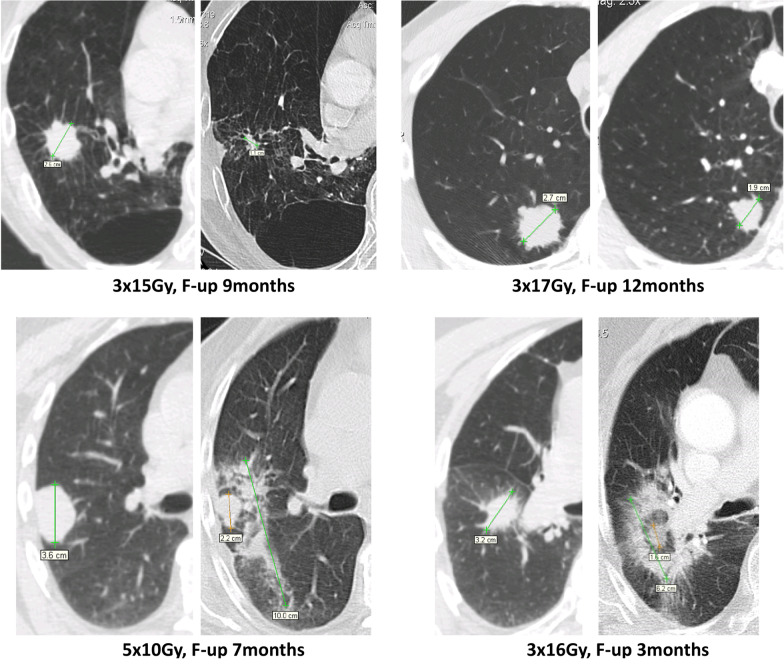
Clinical and radiological follow-up including chest CT scans was scheduled at 3 and 6 months after radiotherapy and every 6 months thereafter. A local recurrence was assumed if the irradiated lesion showed a solid core that increased by at least 25% compared to the last follow-up and exhibited further growth. The first occurrence of diffuse or patchy consolidation, diffuse or patchy ground glass opacity or modified or mass like consolidation in the lung tissue adjacent to the tumor was regarded as radiation induced lung injury (termed local fibrosis, LF, see Fig. 1) and recorded as an event with regard to the time interval to the date of first irradiation [41, 42]. Lung tissue changes smaller than the original tumor, scar-like patterns distant to the tumor and lung toxicities without clear spatial or temporal relation to radiotherapy (early acute pneumonia, late acute pneumonia, pneumonitis spatially not correlated to the PTV) were not considered. In cases where a growing lesion could not be differentiated from local fibrosis, an FDG-PET-CT scan or a biopsy was performed in order to confirm or reject the diagnosis of a local recurrence.
Fig. 1.
Representative chest CT images of patients who did not (upper row) or did (lower row) develop local lung injury induced by robotic stereotactic body radiation therapy of early-stage non-small cell lung cancer
Image processing and feature extraction
Image processing was performed using Python 3.6.7 (Python Software Foundation, Beaverton, Oregon, USA). The original DICOM data containing manual delineations of the gross tumor volume (GTV) and anatomical image data were restored from the CyberknifeR archive and subsequently used to extract the target volumes for radiomic analysis. For all further image processing, the software package pyradiomics 2.0.1 [43] was used that allows the extraction of standardized features which were defined by the IBSI (Image Biomarker Standardization Initiative) [44]. Preprocessing included resampling of the CT images and masks to isotropic voxels of 1 mm3 by the standard procedures of pyradiomics (B-spline interpolation for the CT images and nearest-neighbour interpolation for the binary masks) and removal of all voxels with Hounsfield units (HU) below (− 400) HU and above 1000 HU from the volume which were assumed to represent normal lung and bony tissue unintentionally included in the GTV by manual segmentation. Radiomic features were calculated based on the original image and after wavelet filtering, yielding eight additional image types based on the application of wavelet-based high-pass or low-pass filters to each of the three dimensions. In addition to 14 features descriptive of the target's shape, 93 features were calculated for each of the nine image types, resulting in a total of 851 radiomic features.
Model development and statistical analysis
All model development was performed on the training cohort and the model parameters were optimized using cross-validation schemes. First, the primary set of radiomics features was reduced by identifying and removing linearly correlated features with a Pearson correlation coefficient > 0.90. Out of the 851 extracted features, 652 were found to be highly linearly correlated and removed from the analysis. The remaining 199 features were z-normalized and used to develop predictive models for each of the four endpoints: LC, OS, DFS and occurrence of local lung fibrosis (LF) after SBRT. Two different models implemented in the scikit-survival package for Python [45] were applied. The (linear) Cox Proportional Hazard model was used in conjunction with elastic net regularization of the feature coefficients (Coxnet, [46]) by means of a grid search for the optimal penalty parameter (alpha) with 10-times repeated fivefold random cross validation within the training set. Thus, the algorithm tries to reduce as many feature coefficients as possible to zero, and to achieve good predictions from the resulting smaller selection of features in the 10 × 5 validation data sets each comprising 20% of the training data. The model with the best cross-validated average training performance was then re-evaluated both in the complete training and the test set, where the feature values of the test set were subjected to the z-transformation parameterized from the training set. In order to allow for complex non-linear relationships between feature values and treatment outcome, a gradient-boosted ensemble of 100 regression trees (Gradient Boost, [47]) with the partial likelihood loss of the Cox’s proportional hazards model used as the loss function was also chosen as a model. In the training set, the main parameters of the model (learning rate, dropout rate and subsampling rate) were optimized by a 10-times repeated fivefold grid search with cross-validation. Thus, an intermediate model that returns a list of features and their importance was generated. The list was then used to build models with increasing number of features, by starting with the one feature with the largest importance and successively adding the next important ones. At each step, the model was retrained and cross-validated (10-times repeated fivefold cross validation) in the training set. Typically, the cross-validated performance increased initially, but decreased by including more and more features due to overfitting. As before, the model with the optimal cross-validated performance was applied to the feature-normalized training and test sets.
In addition to the radiomics features, the following continuous clinical and dosimetric variables were analyzed in univariate Cox regression models with respect to their potential impact on any of the endpoints in order to select them as predictive features: GTV, PTV, GTV-Dmax, GTV-Dmean, GTV-D95%, PTV-D95%, Lung-D1ml, Lung-D10ml, Lung-D50ml, Lung-D100ml, tumor diameter, age and Charlson Comorbidity Score. Categorical clinical and treatment related factors were investigated using the Kaplan–Meier method and survival estimates were compared using two-sided log rank tests. These included: gender, T-Stage (T1 vs. T2), histology (squamous cell/ adeno /other/ unknown) and fiducial tracking (no/ yes).
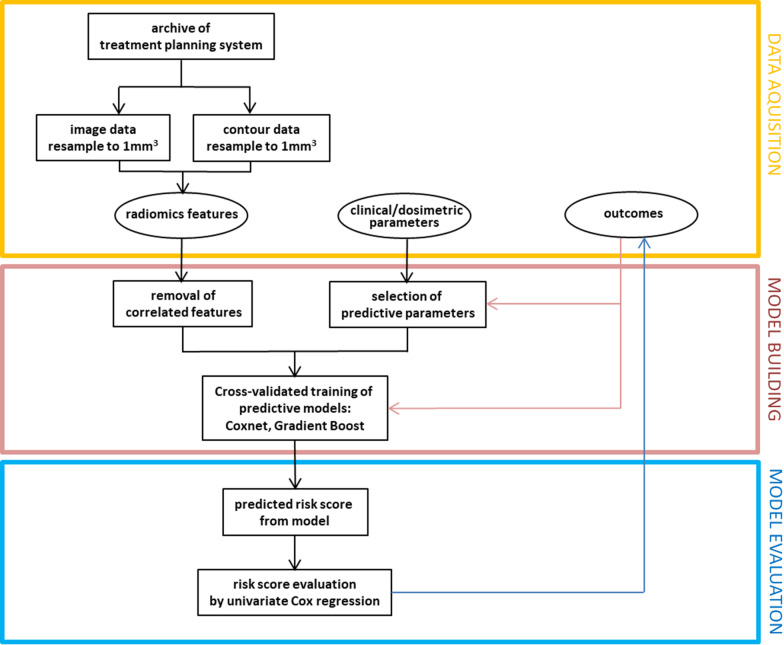
For prediction of radiation-induced lung injury, Coxnet and Gradient Boost models were computed using only clinical/dosimetric features, only imaging features or both types of features. In case of the default settings for the Coxnet model used here, a baseline survival function is not calculated and the predictions are risk scores of arbitrary scale, while the scores of the Gradient Boost models built with the partial likelihood loss of the Cox’s proportional hazards model used as the loss function can be interpreted as log hazard ratios. The performance of any model was evaluated in the test set by means of the concordance index and the significance level of the predicted risk score when used as a continuous variable in a univariate Cox regression (pCox). For purposes of illustration, the risk scores were dichotomized by their median and the Kaplan–Meier curves for the resulting low- and high risk groups were depicted. All statistical analyses were performed with the Lifelines python package (version 0.25.10, https://doi.org/10.5281/zenodo.4579431) and cross-checked by SPSS (vs. 24, Armonk, NY, USA). A p-value of < 0.05 was considered significant. The complete workflow is depicted in Fig. 2.
Fig. 2.
Workflow for generating and validating the developed models
Results
Clinical outcome
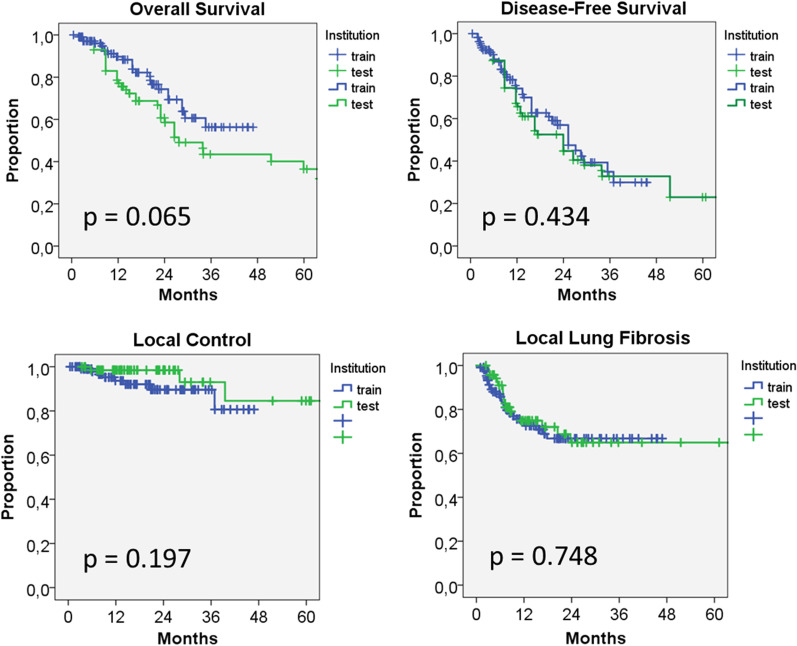
The outcome in terms of the analyzed clinical endpoints did not differ significantly between the two cohorts (Fig. 3). Overall survival at 36 months amounted to 56% versus 43%, p = 0.065), median DFS was 25 months versus 23 months, p = 0.43 and local control rates at 36 months were 90% vs. 93%, p = 0.197). In the training set, none of the clinical and dosimetric factors had a significant influence on these endpoints. Local lung fibrosis developed in 33% vs. 35% of the patients (p = 0.75), all events were observed within 36 months after irradiation. Three dosimetric factors (GTVmean, PTV-D95%, Lung-D1ml) and the patient's age had a significant impact (p < 0.05) on the development of local lung fibrosis with an increase in hazard of approximately 6% per Gy and per year of age.
Fig. 3.
Kaplan-Meier curves for overall survival (OS), local control (LC), disease free survival (DFS) and occurrence of local lung fibrosis after SBRT for the training and testing cohort. No significant difference between the cohorts was measured for any endpoint
Radiomics models for OS, DFS and local tumor control
As shown in Table 3, the Coxnet model failed to substantially reduce the optimal feature number for these endpoints. Although highly predictive models (CCI 0.77–0.94, pCox < 0.005) were such found in the training set, these models showed poor cross-validation (CCI 0.52–0.54) and test set (CCI 0.36–0.49) performance. By application of the Gradient Boost model, a substantial feature set reduction to 5–22 features was achieved. However, despite reasonable cross-validation scores of 0.68–0.89, these models also failed to generalize to the test set. Due to the absence of any predictive models from clinical/radiological and radiomics features, combined models were not evaluated for these endpoints.
Table 3.
Results of radiomics machine learning models for predicting overall survival, disease-free survival and local tumor control
| Endpoint | Coxnet | Gradient boost | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of features | CCI train-set | CCI cross-valid | CCI test-set | Number of features | CCI train-set | CCI cross-valid | CCI test-set | |
| Overall survival | 191 |
0.80 p < 0.005 |
0.52 ± 0.15 |
0.46 n.s |
22 |
0.99 p < 0.005 |
0.68 ± 0.13 |
0.45 n.s |
| Disease free SV | 197 |
0.94 p < 0.005 |
0.54 ± 0.11 |
0.49 n.s |
10 |
0.97 p < 0.005 |
0.76 ± 0.09 |
0.52 n.s |
| Local control | 199 |
0.77 p < 0.005 |
0.54 ± 0.24 |
0.36 n.s |
5 |
0.98 p < 0.005 |
0.89 ± 0.11 |
0.17 n.s |
CCI concordance index, means ± standard deviation are shown, p values: significance level of the model risk score in univariate Cox regression analysis.
Clinical/dosimetric, radiomics and combined models for development of lung fibrosis
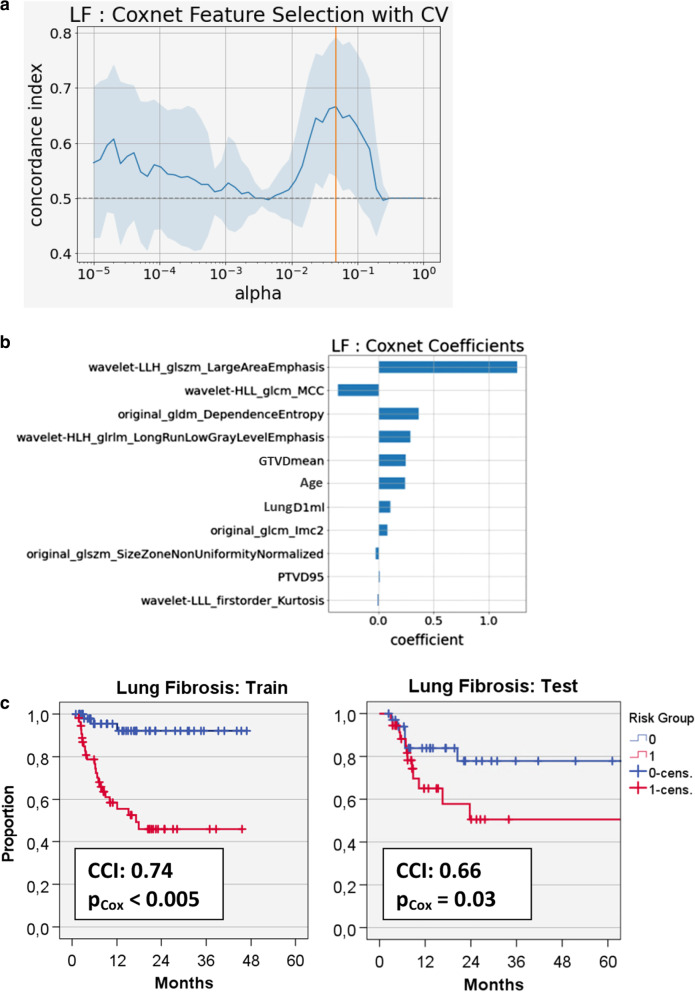
Using the 4 identified clinical/dosimetric variables, both the Coxnet and Gradient Boost algorithms selected 3 of them (age, GTVmean and Lung-D1ml) for building predictive models from the training set (CCI 0.71/0.73) which in case of the Coxnet also achieved a CCI of 0.65 (pCox = 0.04) in the test set (Table 4). Feature selection was also successful in both radiomics models where the Gradient Boost Model had a CCI of 0.59 (pCox = 0.02) using two features (Maximal Correlation Coefficient of the GLCM extracted from images filtered by two different wavelet kernels); this model could not be further improved by including any clinical/dosimetric variables. The best combined model resulted from the Coxnet that selected the 4 clinical/dosimetric variables and another 7 radiomics features and had a CCI of 0.66 (pCox = 0.03) in the test set (Fig. 4). The two radiomics features with the largest coefficients in this model were the Large Area Emphasis from the wavelet-filtered gray-level size zone matrix (GLSZM) and, as in the radiomics Gradient Boost model, the GLCM Maximal Correlation Coefficient from the wavelet-filtered images.
Table 4.
Results of machine learning models for predicting local lung fibrosis
| Coxnet | Gradient boost | |||||||
|---|---|---|---|---|---|---|---|---|
| Features | number of features | CCI train-set | CCI cross-valid | CCI test-set | Number of features | CCI train-set | CCI cross-valid | CCI test-set |
| Clinical/dosimetric | 3§ |
0.71 p < 0.005 |
0.68 ± 0.11 |
0.65 p = 0.04* |
3§ |
0.73 p < 0.005 |
0.64 ± 0.12 |
0.62 n.s |
| Radiomics | 10 |
0.79 p < 0.005 |
0.64 ± 0.13 |
0.58 n.s |
2† |
0.75 p < 0.005 |
0.72 ± 0.11 |
0.59 p = 0.02* |
| Combined | 4 + 7 |
0.74 p < 0.005 |
0.67 ± 0.12 |
0.66 p = 0.03* |
0 + 2† |
0.72 p < 0.005 |
0.72 ± 0.11 |
0.59 p = 0.02* |
CCI concordance index, means ± standard deviation are shown, p-values: significance level of the model risk score in univariate Cox regression analysis
§Age/ GTVMeanDose/LungD1ml
†wavelet_HLH_glcm_MCC/wavelet_HLL_glcm_MCC (= GrayLevelCo-occurrence matrix maximal correlation coefficient)
Fig. 4.
a Regularization and feature selection by repeated cross validation (CV) in a combined Coxnet model for development of lung fibrosis (LF) in the training set. The optimal model arose at an alpha-value of 0.5 × 10–2 where a mean concordance index (CCI) of 0.67 ± 0.12 was achieved. b Coefficients for the optimal Coxnet model that comprised 4 clinical/dosimetric and 7 radiomics features. c Kaplan–Meier curves displaying performance of the radiomics model in the training and test cohorts when stratifying patients into low and high risk groups by the respective medians of the model risk scores (train: 40.2, range 31.4–46.0; test: 42.4, range 25.0–60.4); pCox: Significance level for the model risk score used as a continuous variable in a univariate Cox regression analysis
Discussion
Summary of findings
In the present analysis, two cohorts of early-stage lung cancer patients treated with robotic stereotactic body radiotherapy at two different institutions were investigated. Although slightly different fractionation schedules were applied, oncologic outcome in terms of local tumor control, disease-free survival and overall survival were well comparable. Importantly, the frequency and time course of development of radiation-induced local lung injury was also similar in the two cohorts. Radiomics analysis based on a selected set of standardized features and state-of-the-art modelling in the training cohort resulted in models for prediction of radiation-induced local lung injury that performed well also in the test cohort. The predictive ability of the radiomics models resembled that of a model from a selection of clinical/dosimetric variables, but the marginally best performance was achieved in a model that combined a small number of clinical/dosimetric and radiomics features. However, the models for the endpoints of oncologic outcome (OS, DFS, local control) failed to generalize to the test cohort.
Prediction of local radiation-induced lung injury
To the best of our knowledge, this is the first report that aimed at generating machine-learning models for the development of local lung injury from the GTV after lung SBRT [36]. Radiation-induced local lung injury that finally develops into local lung fibrosis is a typical event after lung SBRT, although it remains asymptomatic in most cases. It is probably triggered by the release of inflammatory cytokines such as TGF-ß from the tumor which subsequently initiate an immunological response [48, 49]. At first sight, it seems far from obvious how a texture pattern detectable by radiomics could predict for this event. However, an association between a pre-therapeutic radiomics feature (LoG standard deviation) with the TGF-ß signaling pathway has recently been observed, and in the same report, a radiomics score was correlated with the amount of tumor infiltration by T-lymphocytes [50]. The view that image features correlate with the presence of immune-competent cells in lung tumor tissue is also supported by the observation that lung tumors characterized by low CT intensity and high CT heterogeneity exhibited a high CD3 (T-lymphocyte) infiltration, suggestive of an activated immune state [51]. Interestingly, the most predictive features found in the present analysis were related to the heterogeneity of the lung tumor, as the GLSZM Large Area Emphasis is a measure of the distribution of large area size zones where a greater value is indicative of more larger size zones and more coarse textures, and the Maximal Correlation Coefficient of the GLCM is a measure of complexity of the texture resulting in values approaching unity for flat, homogenous regions.
In the present report, one of the radiomics models slightly improved the pure clinical/ dosimetric model (Coxnet) for the development of local lung fibrosis while the other radiomics model (Gradient Boost) did not benefit from the potential inclusion of the clinical/dosimetric features. In a comparable approach that has been applied for prediction of radiation pneumonitis from features of the total lung tissue in lung cancer patients treated with intensity-modulated radiotherapy (IMRT), the radiomics features also only slightly improved the predictive value of the model when added to clinical and dosimetric factors [47]. Interestingly, the inhomogeneous dose distribution usually generated by robotic radiosurgery and volumetric arc therapy has itself been analyzed with respect to dose distribution patterns (“dosiomics”) which in turn have been found to predict the incidence of radiation pneumonitis [52]. Thus, a more comprehensive model of radiation-induced lung injury could probably be built from incorporating texture analysis of the tumor, a shell [53, 54] comprising the adjacent lung tissue and the dose distribution.
Prediction of local control, disease-free survival and overall survival
Although the two cohorts resembled each other in terms of oncologic outcome, the radiomics models did not generalize from the training to the test cohort with respect to these endpoints. In case of the Coxnet model that utilizes a simple, linear combination of features values, a substantial reduction of the feature space was not possible even during cross-validation within the training set itself. This is probably due to the fact that in the present cohort of inoperable patients with small lung tumors, local control could be achieved in most such that only a small number of events was available for training, and DFS and OS were largely independent from single radiomic properties of the tumors and confounded by other factors. Such, the predictive models for these endpoints were only found by chance and due to overfitting by using almost all features available. The Coxnet model used an ensemble of regression trees that can account for complicated, non-linear relationships between features and time to survival or other endpoints. Much higher cross-validation scores were found from a lower number of features, but the principle obstacles of relating confounded or low event-populated endpoints to a small set of radiomic features in an independent test set remained. As discussed above, the relation between selected radiomic tumor features, dose distribution and development of local lung fibrosis seemed be much stronger such that the type of the applied model became less important.
A compilation of recent studies on the impact of radiomics features on oncologic outcome for lung cancer patients after SBRT is presented in Table 5. Most of the studies applied single institution cross-validation or validation by test sets from the same institution and were able to predict local tumor recurrence, regional/nodal recurrence, distant failures and overall survival with a moderate accuracy. Of note, one report failed to observe features predictive of local recurrence [24]. Only in a minority of series were the results validated in test sets from independent institutions. In a large study from the Cleveland Clinic (Ohio, USA), a convolutional neural network (CNN) was trained to predict local recurrence in a group of > 900 lung cancer patients treated by SBRT. The stratification resulted in two groups with highly significant different risk for recurrence in both the training and test set [55]. Also in another study where a CNN was applied to both CT and PET images, a highly accurate classification of survival probability was achieved in an independent data set [21]. These results suggest that the application of CNN's that learn the relevant features for time-dependent oncologic predictions may be more effective than training models on predefined features [56, 57].
Table 5.
Reports on outcome prediction of SBRT in lung cancer from analysis of radiomic features
| Author | N | Modality/features (software applied) | # features selected | Model type | Outcome measures | Validation | Result/comment |
|---|---|---|---|---|---|---|---|
| Huynh [24] | 113 | CT:1605 (in-house software) | 12 + clinical | Survival analysis, cc-index | Recurrence, Distant mets., OS | Single institution cross validation | Risk for recurrence: no significant features Risk for dist. metastases: 1 sign. Feature OS: 4 significant features, cc = 0.67 |
| Li [28, 29] | 92 | CT: 219 (Definiens Developer) | 8–68 + clinical + semantic | ROC-analysis | Recurrence, RFS, OS | Single institution cross-validation | Risk stratification: AUC = 0.69–0.75 |
| Zhang [58] | 112 | CT: 30 (ProCanVAS) | dependent on model | 8 models: Random forest GLM, SVM etc | Recurrence, Distal failure, OS | Single institution cross validation | Risk stratification: AUC = 0.60–0.77 |
| Yu [34] | 442 | CT: 12 (IBEX) | 2 | Random survival forests | Regional recurrence, OS | Single institution test set: 67% | OS risk stratification: p = 0.017 Recurrence risk stratification: p < 0.05 2 sign. features: kurtosis, homogeneity |
| Li [30] | 110 | CT + FDG-PET (learned by model) | from model | Kernelled support tensor machine | Distant failure | Single institution test set: 30% | Risk stratification: AUC = 0.80 |
| Oikonomou [31] | 150 | CT + FDG-PET 2 × 21 (ProCanVAS) | 6–8, 4 from PCA | PCA, logistic regression | Local control, Distant control, DSS, OS | Single institution cross validation | Risk stratification: p = 0.004–0.02 features: heterogeneity and morphology |
| Starkov [32] | 116 | CT: 2D-textures from solid core and GGO | 2–30 | Cox regression lasso | PFS, distant failure | Single institution cross validation | Risk stratification: p = 0.03 dependent on wavelet filtering |
| Lafata [26] | 70 | CT: 43 | 2 | Logistic regression regularized | Local recurrence | none | Risk stratification: p = 0.048 features: density |
| Franceschini [23] | 102 | CT: 41 (LifeX) | 4–6 | Cox regression elastic net, back selection | Nodal relapse, PFS, DSS | Single institution Test set: 32% | Nodal Relapse: accuracy = 85% PFS: 53 vs.45 months features: heterogeneity |
| Lou [59] | 944* | CT | learned by model | CNN, Multivariate competing risk | Local recurrence | Multi institution test set: 10% | Risk stratification: p < 0.002 |
| Baek [21] | 122 |
CT + FDG-PET 2 × 55,296 |
Features from k-medoids pool | CNN (U-Net) logistic regression | OS | Independent institution test set: 21% | Risk stratification: AUC = 0.87 |
ProCanVAS prostate cancer visualization and analysis system, PCA principal component analysis, cc-index: concordance index, RFS Recurrence-free survival, ROC receiver-operator-characteristics; validation by independent test sets shown in bold
*Includes recurrent lung cancers and pulmonary metastases
#Features from PET and CT
Limitations of the present study
The present study, although based on the results of two independent cohorts, probably still lacks a sufficient number of patients and events needed for an informative analysis of the interaction between dosimetric parameters and radiologic tissue characteristics for prediction of local events (recurrence, local lung fibrosis) after SBRT of NSCLC. Also, the classification of local lung injury and tumor control is purely image-based and remains somewhat ambiguous, as tissue specimens are rarely available following SBRT. Differences in therapeutic strategies for detecting and treating metastases may have prevented the creation of a general radiomics-based model for prediction of DFS and OS.
Conclusion
The present analysis provides evidence that radiomics analysis can, in principle, be used for prediction of local lung injury after SBRT of NSCLC in independent data sets and as such complements existing results on the successful prediction of other oncologic endpoints in this setting.
Acknowledgements
None.
Abbreviations
- NSCLC
non-small cell lung cancer
- SBRT
stereotactic body radiation therapy
- RILI
radiation-induced lung injury
- UICC
Union for International Cancer Control
- LC
local control
- DFS
disease-free survival
- OS
overall survival
- LF
lung fibrosis
- GTV
gross tumor volume
- PTV
planning target volume
- GTV-Dmean
mean dose in GTV
- GTV-Dmax
maximal dose in GTV
- PTV-D95%
dose covering 95% of the PTV
- GTV-D95%
dose covering 95% of the GTV
- Lung-D1ml
dose achieved in 1 ml of the lung volume
- Lung-D10ml
dose achieved in 10 ml of the lung volume
- CT
computed tomography
- PET
positron emission tomography
- FDG-PET
fluor-deoxyglucose-positron emission tomograpy
- DICOM
digital imaging and communications in medicine
- HU
hounsfield unit
- IBSI
image biomarker standardization initiative
- CNN
convolutional neural network
- LoG
Laplacian-of Gaussian
- TGF-ß
transforming growth factor beta
- CD3
cluster of differentiation (protein) 3
- GLCM
gray-level co-occurrence matrix
- GLSZM
gray-level size zone matrix
- Coxnet
Cox proportional hazard model with elastic net regularization
- Gradient Boost
gradient-boosted ensemble of regression trees
Authors' contributions
KB: design, data acquisition, data analysis, data interpretation, manuscript draft. OB: conception, design, data acquisition, data analysis, data interpretation, manuscript draft. ST: data acquisition, data interpretation, manuscript draft. MLW: data acquisition, data interpretation, manuscript draft. MH: data analysis, data interpretation, manuscript draft. WWB: data aquisition, data interpretation, manuscript draft. DR: data interpretation, manuscript draft. VVV: data interpretation, manuscript draft. MIR: data interpretation, manuscript draft. HT: conception, design, data interpretation, manuscript draft. MK: conception, design, data acquisition, data analysis, data interpretation, manuscript draft. all: approved the submitted version. all: agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of supporting data
The datasets generated and/or analyzed during the current study are not publicly available due but are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
This study was approved by the Ethics Committee of the Medical Faculty, University of Cologne, Protocol No. 17-009 and by the Ethics Committee of the Medical Faculty, University of Kiel, Protocol No. D421/18. All data were processed in anonymized form. Individual informed consent was waived as this was a retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NCCN: National Comprehensive Cancer Network. Non Small-Cell Lung Cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2019:Version 7.2019.
- 2.Guckenberger M, Aerts JG, Van Schil P, Weder W. The American Society of Clinical Oncology-endorsed American Society for Radiation Oncology Evidence-Based Guideline of stereotactic body radiotherapy for early-stage non-small cell lung cancer: an expert opinion. J Thorac Cardiovasc Surg. 2019;157(1):358–361. doi: 10.1016/j.jtcvs.2018.09.107. [DOI] [PubMed] [Google Scholar]
- 3.Guckenberger M, Andratschke N, Alheit H, Holy R, Moustakis C, Nestle U, Sauer O. Deutschen Gesellschaft fur R: definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol. 2014;190(1):26–33. doi: 10.1007/s00066-013-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guckenberger M, Andratschke N, Dieckmann K, Hoogeman MS, Hoyer M, Hurkmans C, Tanadini-Lang S, Lartigau E, Mendez Romero A, Senan S, et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol. 2017;124(1):11–17. doi: 10.1016/j.radonc.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Febbo JA, Gaddikeri RS, Shah PN. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: a primer for radiologists. Radiographics. 2018;38(5):1312–1336. doi: 10.1148/rg.2018170155. [DOI] [PubMed] [Google Scholar]
- 6.Guckenberger M, Klement RJ, Allgauer M, Andratschke N, Blanck O, Boda-Heggemann J, Dieckmann K, Duma M, Ernst I, Ganswindt U, et al. Local tumor control probability modeling of primary and secondary lung tumors in stereotactic body radiotherapy. Radiother Oncol. 2016;118(3):485–491. doi: 10.1016/j.radonc.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Guckenberger M, Klement RJ, Kestin LL, Hope AJ, Belderbos J, Werner-Wasik M, Yan D, Sonke JJ, Bissonnette JP, Xiao Y, et al. Lack of a dose-effect relationship for pulmonary function changes after stereotactic body radiation therapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85(4):1074–1081. doi: 10.1016/j.ijrobp.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Okubo M, Itonaga T, Saito T, Shiraishi S, Mikami R, Nakayama H, Sakurada A, Sugahara S, Koizumi K, Tokuuye K. Predicting risk factors for radiation pneumonitis after stereotactic body radiation therapy for primary or metastatic lung tumours. Br J Radiol. 2017;90(1073):20160508. doi: 10.1259/bjr.20160508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Yorke ED, Li L, Kavanagh BD, Li XA, Das S, Miften M, Rimner A, Campbell J, Xue J, et al. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: a pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys. 2016;95(5):1357–1366. doi: 10.1016/j.ijrobp.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoll MA, Salvatore M, Sheu RD, Knoll AD, Kerns SL, Lo YC, Rosenzweig KE. The use of isodose levels to interpret radiation induced lung injury: a quantitative analysis of computed tomography changes. Quant Imaging Med Surg. 2016;6(1):35–41. doi: 10.3978/j.issn.2223-4292.2016.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue T, Shiomi H, Oh RJ. Stereotactic body radiotherapy for Stage I lung cancer with chronic obstructive pulmonary disease: special reference to survival and radiation-induced pneumonitis. J Radiat Res. 2015;56(4):727–734. doi: 10.1093/jrr/rrv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Mantovani C, Fiandra C, Anglesio S, Ragona R. Dosimetric predictors of radiation-induced lung injury in stereotactic body radiation therapy. Acta Oncol. 2009;48(4):571–577. doi: 10.1080/02841860802520821. [DOI] [PubMed] [Google Scholar]
- 13.Baumann R, Chan MKH, Pyschny F, Stera S, Malzkuhn B, Wurster S, Huttenlocher S, Szucs M, Imhoff D, Keller C, et al. Clinical results of mean GTV dose optimized robotic-guided stereotactic body radiation therapy for lung tumors. Front Oncol. 2018;8:171. doi: 10.3389/fonc.2018.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura M, Nishikawa R, Mayahara H, Uezono H, Harada A, Hashimoto N, Nishimura H. Pattern of recurrence after CyberKnife stereotactic body radiotherapy for peripheral early non-small cell lung cancer. J Thorac Dis. 2019;11(1):214–221. doi: 10.21037/jtd.2018.12.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coroller TP, Agrawal V, Huynh E, Narayan V, Lee SW, Mak RH, Aerts H. Radiomic-based pathological response prediction from primary tumors and lymph nodes in NSCLC. J Thorac Oncol. 2017;12(3):467–476. doi: 10.1016/j.jtho.2016.11.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coroller TP, Agrawal V, Narayan V, Hou Y, Grossmann P, Lee SW, Mak RH, Aerts HJ. Radiomic phenotype features predict pathological response in non-small cell lung cancer. Radiother Oncol. 2016;119(3):480–486. doi: 10.1016/j.radonc.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coroller TP, Grossmann P, Hou Y, Rios Velazquez E, Leijenaar RT, Hermann G, Lambin P, Haibe-Kains B, Mak RH, Aerts HJ. CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother Oncol. 2015;114(3):345–350. doi: 10.1016/j.radonc.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grove O, Berglund AE, Schabath MB, Aerts HJ, Dekker A, Wang H, Velazquez ER, Lambin P, Gu Y, Balagurunathan Y, et al. Quantitative computed tomographic descriptors associate tumor shape complexity and intratumor heterogeneity with prognosis in lung adenocarcinoma. PLoS ONE. 2015;10(3):e0118261. doi: 10.1371/journal.pone.0118261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee G, Lee HY, Park H, Schiebler ML, van Beek EJR, Ohno Y, Seo JB, Leung A. Radiomics and its emerging role in lung cancer research, imaging biomarkers and clinical management: State of the art. Eur J Radiol. 2017;86:297–307. doi: 10.1016/j.ejrad.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Baek S, He Y, Allen BG, Buatti JM, Smith BJ, Tong L, Sun Z, Wu J, Diehn M, Loo BW, et al. Deep segmentation networks predict survival of non-small cell lung cancer. Sci Rep. 2019;9(1):17286. doi: 10.1038/s41598-019-53461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dissaux G, Visvikis D, Do-Ano R, Pradier O, Chajon E, Barillot I, Duverge L, Masson I, Abgral R, Santiago Ribeiro MJ et al. Pre-treatment (18)F-FDG PET/CT Radiomics predict local recurrence in patients treated with stereotactic radiotherapy for early-stage non-small cell lung cancer: a multicentric study. J Nucl Med 2019. [DOI] [PubMed]
- 23.Franceschini D, Cozzi L, De Rose F, Navarria P, Fogliata A, Franzese C, Pezzulla D, Tomatis S, Reggiori G, Scorsetti M. A radiomic approach to predicting nodal relapse and disease-specific survival in patients treated with stereotactic body radiation therapy for early-stage non-small cell lung cancer. Strahlenther Onkol 2019. [DOI] [PubMed]
- 24.Huynh E, Coroller TP, Narayan V, Agrawal V, Hou Y, Romano J, Franco I, Mak RH, Aerts HJ. CT-based radiomic analysis of stereotactic body radiation therapy patients with lung cancer. Radiother Oncol. 2016;120(2):258–266. doi: 10.1016/j.radonc.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Huynh E, Coroller TP, Narayan V, Agrawal V, Romano J, Franco I, Parmar C, Hou Y, Mak RH, Aerts HJ. Associations of radiomic data extracted from static and respiratory-gated CT scans with disease recurrence in lung cancer patients treated with SBRT. PLoS ONE. 2017;12(1):e0169172. doi: 10.1371/journal.pone.0169172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafata KJ, Hong JC, Geng R, Ackerson BG, Liu JG, Zhou Z, Torok J, Kelsey CR, Yin FF. Association of pre-treatment radiomic features with lung cancer recurrence following stereotactic body radiation therapy. Phys Med Biol. 2019;64(2):025007. doi: 10.1088/1361-6560/aaf5a5. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Galperin-Aizenberg M, Pryma D, Simone CB, 2nd, Fan Y. Unsupervised machine learning of radiomic features for predicting treatment response and overall survival of early stage non-small cell lung cancer patients treated with stereotactic body radiation therapy. Radiother Oncol. 2018;129(2):218–226. doi: 10.1016/j.radonc.2018.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Kim J, Balagurunathan Y, Liu Y, Latifi K, Stringfield O, Garcia A, Moros EG, Dilling TJ, Schabath MB, et al. Imaging features from pretreatment CT scans are associated with clinical outcomes in nonsmall-cell lung cancer patients treated with stereotactic body radiotherapy. Med Phys. 2017;44(8):4341–4349. doi: 10.1002/mp.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Kim J, Balagurunathan Y, Qi J, Liu Y, Latifi K, Moros EG, Schabath MB, Ye Z, Gillies RJ, et al. CT imaging features associated with recurrence in non-small cell lung cancer patients after stereotactic body radiotherapy. Radiat Oncol. 2017;12(1):158. doi: 10.1186/s13014-017-0892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Yang N, Li B, Zhou Z, Hao H, Folkert MR, Iyengar P, Westover K, Choy H, Timmerman R, et al. A pilot study using kernelled support tensor machine for distant failure prediction in lung SBRT. Med Image Anal. 2018;50:106–116. doi: 10.1016/j.media.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oikonomou A, Khalvati F, Tyrrell PN, Haider MA, Tarique U, Jimenez-Juan L, Tjong MC, Poon I, Eilaghi A, Ehrlich L, et al. Radiomics analysis at PET/CT contributes to prognosis of recurrence and survival in lung cancer treated with stereotactic body radiotherapy. Sci Rep. 2018;8(1):4003. doi: 10.1038/s41598-018-22357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starkov P, Aguilera TA, Golden DI, Shultz DB, Trakul N, Maxim PG, Le QT, Loo BW, Diehn M, Depeursinge A, et al. The use of texture-based radiomics CT analysis to predict outcomes in early-stage non-small cell lung cancer treated with stereotactic ablative radiotherapy. Br J Radiol. 2019;92(1094):20180228. doi: 10.1259/bjr.20180228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda K, Takanami K, Shirata Y, Yamamoto T, Takahashi N, Ito K, Takase K, Jingu K. Clinical utility of texture analysis of 18F-FDG PET/CT in patients with Stage I lung cancer treated with stereotactic body radiotherapy. J Radiat Res 2017:1–8. [DOI] [PMC free article] [PubMed]
- 34.Yu W, Tang C, Hobbs BP, Li X, Koay EJ, Wistuba, II, Sepesi B, Behrens C, Rodriguez Canales J, Parra Cuentas ER et al. Development and validation of a predictive radiomics model for clinical outcomes in stage i non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017. [DOI] [PubMed]
- 35.van Timmeren JE, Carvalho S, Leijenaar RTH, Troost EGC, van Elmpt W, de Ruysscher D, Muratet JP, Denis F, Schimek-Jasch T, Nestle U, et al. Challenges and caveats of a multi-center retrospective radiomics study: an example of early treatment response assessment for NSCLC patients using FDG-PET/CT radiomics. PLoS ONE. 2019;14(6):e0217536. doi: 10.1371/journal.pone.0217536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bousabarah K, Temming S, Hoevels M, Borggrefe J, Baus WW, Ruess D, Visser-Vandewalle V, Ruge M, Kocher M, Treuer H. Radiomic analysis of planning computed tomograms for predicting radiation-induced lung injury and outcome in lung cancer patients treated with robotic stereotactic body radiation therapy. Strahlenther Onkol. 2019;195(9):830–842. doi: 10.1007/s00066-019-01452-7. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt D, Blanck O, Gauer T, Fix MK, Brunner TB, Fleckenstein J, Loutfi-Krauss B, Manser P, Werner R, Wilhelm ML, et al. Technological quality requirements for stereotactic radiotherapy: expert review group consensus from the DGMP Working Group for Physics and Technology in Stereotactic Radiotherapy. Strahlenther Onkol. 2020;196(5):421–443. doi: 10.1007/s00066-020-01583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stera S, Balermpas P, Chan MKH, Huttenlocher S, Wurster S, Keller C, Imhoff D, Rades D, Dunst J, Rodel C, et al. Breathing-motion-compensated robotic guided stereotactic body radiation therapy: patterns of failure analysis. Strahlenther Onkol. 2018;194(2):143–155. doi: 10.1007/s00066-017-1204-z. [DOI] [PubMed] [Google Scholar]
- 39.Temming S, Kocher M, Stoelben E, Hagmeyer L, Chang DH, Frank K, Hekmat K, Wolf J, Baus WW, Semrau R, et al. Risk-adapted robotic stereotactic body radiation therapy for inoperable early-stage non-small-cell lung cancer. Strahlenther Onkol. 2018;194(2):91–97. doi: 10.1007/s00066-017-1194-x. [DOI] [PubMed] [Google Scholar]
- 40.Wilke L, Andratschke N, Blanck O, Brunner TB, Combs SE, Grosu AL, Moustakis C, Schmitt D, Baus WW, Guckenberger M. ICRU report 91 on prescribing, recording, and reporting of stereotactic treatments with small photon beams: statement from the DEGRO/DGMP working group stereotactic radiotherapy and radiosurgery. Strahlenther Onkol. 2019;195(3):193–198. doi: 10.1007/s00066-018-1416-x. [DOI] [PubMed] [Google Scholar]
- 41.Dahele M, Palma D, Lagerwaard F, Slotman B, Senan S. Radiological changes after stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol. 2011;6(7):1221–1228. doi: 10.1097/JTO.0b013e318219aac5. [DOI] [PubMed] [Google Scholar]
- 42.Trovo M, Linda A, El Naqa I, Javidan-Nejad C, Bradley J. Early and late lung radiographic injury following stereotactic body radiation therapy (SBRT) Lung Cancer. 2010;69(1):77–85. doi: 10.1016/j.lungcan.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Van Griethuysen JJ, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RG, Fillion-Robin J-C, Pieper S, Aerts HJ. Computational radiomics system to decode the radiographic phenotype. Can Res. 2017;77(21):e104–e107. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zwanenburg A, Leger S, Löck S: Image biomarker standardisation initiative. 161207003 2016:Accessed January 2, 2020.
- 45.Pölsterl S. Scikit-survival: a library for time-to-event analysis built on top of scikit-learn. J Mach Learn Res. 2020;21(212):1–6. [Google Scholar]
- 46.Benner A, Zucknick M, Hielscher T, Ittrich C, Mansmann U. High-dimensional Cox models: the choice of penalty as part of the model building process. Biom J. 2010;52(1):50–69. doi: 10.1002/bimj.200900064. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Jia Z, Mercola D, Xie X. A gradient boosting algorithm for survival analysis via direct optimization of concordance index. Comput Math Methods Med. 2013;2013:873595. doi: 10.1155/2013/873595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66(5):1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Campbell J, Stenmark MH, Zhao J, Stanton P, Matuszak MM, Ten Haken RK, Kong FS. Plasma levels of IL-8 and TGF-beta1 predict radiation-induced lung toxicity in non-small cell lung cancer: a validation study. Int J Radiat Oncol Biol Phys. 2017;98(3):615–621. doi: 10.1016/j.ijrobp.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grossmann P, Stringfield O, El-Hachem N, Bui MM, Rios Velazquez E, Parmar C, Leijenaar RT, Haibe-Kains B, Lambin P, Gillies RJ, et al. Defining the biological basis of radiomic phenotypes in lung cancer. Elife. 2017;6:e23421. doi: 10.7554/eLife.23421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang C, Hobbs B, Amer A, Li X, Behrens C, Canales JR, Cuentas EP, Villalobos P, Fried D, Chang JY, et al. Development of an immune-pathology informed radiomics model for non-small cell lung cancer. Sci Rep. 2018;8(1):1922. doi: 10.1038/s41598-018-20471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang B, Yan H, Tian Y, Chen X, Yan L, Zhang T, Zhou Z, Wang L, Dai J. Dosiomics: extracting 3D spatial features from dose distribution to predict incidence of radiation pneumonitis. Front Oncol. 2019;9:269. doi: 10.3389/fonc.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diamant A, Chatterjee A, Faria S, Naqa IE, Bahig H, Filion E, Robinson C, Al-Halabi H, Seuntjens J: Can dose outside the PTV influence the risk of distant metastases in stage I lung cancer patients treated with stereotactic body radiotherapy (SBRT)? Radiother Oncol 2018. [DOI] [PubMed]
- 54.Hao H, Zhou Z, Li S, Maquilan G, Folkert MR, Iyengar P, Westover KD, Albuquerque K, Liu F, Choy H, et al. Shell feature: a new radiomics descriptor for predicting distant failure after radiotherapy in non-small cell lung cancer and cervix cancer. Phys Med Biol. 2018;63(9):095007. doi: 10.1088/1361-6560/aabb5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lou B, Doken S, Zhuang T, Wingerter D, Gidwani M, Mistry N, Ladic L, Kamen A, Abazeed ME. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit Health. 2019;1(3):e136–e147. doi: 10.1016/S2589-7500(19)30058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lombardo E, Kurz C, Marschner S, Avanzo M, Gagliardi V, Fanetti G, Franchin G, Stancanello J, Corradini S, Niyazi M, et al. Distant metastasis time to event analysis with CNNs in independent head and neck cancer cohorts. Sci Rep. 2021;11(1):6418. doi: 10.1038/s41598-021-85671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Starke S, Leger S, Zwanenburg A, Leger K, Lohaus F, Linge A, Schreiber A, Kalinauskaite G, Tinhofer I, Guberina N, et al. 2D and 3D convolutional neural networks for outcome modelling of locally advanced head and neck squamous cell carcinoma. Sci Rep. 2020;10(1):15625. doi: 10.1038/s41598-020-70542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Oikonomou A, Wong A, Haider MA, Khalvati F. Radiomics-based prognosis analysis for non-small cell lung cancer. Sci Rep. 2017;7:46349. doi: 10.1038/srep46349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo Y, McShan DL, Matuszak MM, Ray D, Lawrence TS, Jolly S, Kong FM, Ten Haken RK, El Naqa I. A multiobjective Bayesian networks approach for joint prediction of tumor local control and radiation pneumonitis in nonsmall-cell lung cancer (NSCLC) for response-adapted radiotherapy. Med Phys 2018. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due but are available from the corresponding author on reasonable request.