Abstract
Background and Aims
An arbuscular mycorrhiza is a mutualistic symbiosis with plants as carbon providers for fungi. However, achlorophyllous arbuscular mycorrhizal species are known to obtain carbon from fungi, i.e. they are mycoheterotrophic. These species all have the Paris type of arbuscular mycorrhiza. Recently, two chlorophyllous Paris-type species proved to be partially mycoheterotrophic. In this study, we explore the frequency of this condition and its association with Paris-type arbuscular mycorrhiza.
Methods
We searched for evidence of mycoheterotrophy in all currently published 13C, 2H and 15N stable isotope abundance patterns suited for calculations of enrichment factors, i.e. isotopic differences between neighbouring Paris- and Arum-type species. We found suitable data for 135 plant species classified into the two arbuscular mycorrhizal morphotypes.
Key Results
About half of the chlorophyllous Paris-type species tested were significantly enriched in 13C and often also enriched in 2H and 15N, compared with co-occurring Arum-type species. Based on a two-source linear mixing model, the carbon gain from the fungal source ranged between 7 and 93 % with ferns > horsetails > seed plants. The seed plants represented 13 families, many without a previous record of mycoheterotrophy. The 13C-enriched chlorophyllous Paris-type species were exclusively herbaceous perennials, with a majority of them thriving on shady forest ground.
Conclusions
Significant carbon acquisition from fungi appears quite common and widespread among Paris-type species, this arbuscular mycorrhizal morphotype probably being a pre-condition for developing varying degrees of mycoheterotrophy.
Keywords: Arbuscular mycorrhiza, Arum-type, Ellenberg values, ferns, horsetails, mycoheterotrophy, mycorrhizal networks, Paris-type, seed plants, stable isotopes, 13C, 2H
INTRODUCTION
A number of plant species are known to cover their energy consumption entirely or partly by parasitism. Some parasitic species transfer photosynthates from another plant through haustoria (Těšitel et al., 2010; Westwood et al., 2010), but many parasitize endophytic fungi (‘mycoheterotrophy’; Leake, 1994; Merckx, 2013; Waterman et al., 2013). Ultimately, mycoheterotrophy must be based on hyphal connection to photo-assimilating plants or to photosynthetic products in organic debris. The physiology of parasitic plants holds a considerable fascination. Access to photosynthates either directly from another plant or through hyphae can be associated with a dramatic reduction of chlorophyll and thus of photosynthetic capacity (Westwood et al., 2010; Merckx, 2013), but often some photosynthetic activity is retained (‘hemiparasites’ or ‘partial mycoheterotrophs’) (Těšitel et al., 2010; Hynson et al., 2013).
The role that parasitic plants play in the natural habitats where they occur is generally understudied and probably underestimated (Quested et al., 2003; Quested, 2008). The vast majority of parasitic plants are herbaceous, often small in stature, and the condition is thought to be confined to certain specialized plant families (e.g. Burmanniaceae, Ericaceae, Gentianaceae, Orchidaceae, Orobanchaceae, Polygalaceae, Santalaceae, Thismiaceae and Triuridaceae; Westwood et al., 2010; Merckx et al., 2013). In a recent study, however, we demonstrated partial mycoheterotrophy in two plant species with Paris-type arbuscular mycorrhiza (AM): Paris quadrifolia (Melanthiaceae) and Anemone nemorosa (Ranunculaceae) (Giesemann et al., 2020b). Nevertheless, both plant species have the outer appearance of typical photo-assimilating plants and no close relationship with previously known mycoheterotrophic species (Giesemann et al., 2020b). This supports the suggestion that a capacity towards mycoheterotrophy may coincide with the Paris type of AM, as suggested by Imhof (1999), and be more widespread than previously recognized. The impact on plant communities could thus be quite considerable, if it amounts to regular and substantial transfers of photosynthates between common plant species.
In this study, we explore the prevalence of partial mycoheterotrophy and its suspected link to the Paris type of AM by surveying published information. Evidence of carbon (C) gain from fungi may be found in the stable isotope composition in leaves of the species in question, where species with mycoheterotrophy deviate characteristically in composition from the purely photo-assimilating species (Gebauer and Meyer, 2003). Thus, we extracted information on carbon (13C), nitrogen (15N) and hydrogen (2H) stable isotope composition in plant species with AM and sorted them into three groups: chlorophyllous Arum-type species, chlorophyllous Paris-type species and achlorophyllous Paris-type species. The distinction into morphotypes (Arum vs. Paris) was developed by Gallaud (1905) and is based on the infection pattern of hyphae within the roots. Since then the morphotyping has been applied to a wide range of plant species. About 80 % of higher plant species are estimated to form AM, and published records suggest that the morphotypes occur with about equal frequency, with few intermediate or variable forms. Furthermore, the morphotype is mostly consistent across the species of the same genus (Dickson et al., 2007). AM is based on ubiquitous fungi belonging to Glomeromycotina.
We intended to test the following hypotheses: (i) that chlorophyllous Paris-type AM plant species would be enriched in 13C, 15N and 2H stable isotopes compared with chlorophyllous Arum-type species, but less so than the achlorophyllous Paris-type AM species (cf. Gomes et al., 2020; Giesemann et al., 2020b); (ii) that the proportion of C gained by photosynthesis and through fungi, respectively, would vary within the group of chlorophyllous Paris-type species, reflecting different degrees of reliance on mycoheterotrophy, in the same way as was found in partial mycoheterotrophs with other mycorrhizal relationships (associated with either ectomycorrhizal or saprotrophic basidio- or ascomycetes; cf. Gebauer and Meyer, 2003; Zimmer et al., 2007; Hynson et al., 2009; Gebauer et al., 2016; Schiebold et al., 2018); and (iii) that shade-adapted Paris-type AM plant species would show a greater reliance on fungi as their C source, and thus a higher 13C and 2H enrichment, than Paris-morphotype AM plant species adapted to full sunlight. A similar light dependence is known in partial mycoheterotrophs on ectomycorrhizal fungi (Preiss et al., 2010) and on saprotrophic rhizoctonia fungi (Schweiger et al., 2019).
MATERIALS AND METHODS
Distinction of species to AM morphotype
The morphotype of each chlorophyllous plant genus/species was obtained from Dickson et al. (2007). This information was supplemented with data from Diallo et al. (2001), Becerra et al. (2007), Menoyo et al. (2007), Turnau et al. (2008), Zubek et al. (2008, 2011a, b), Shah et al. (2009), Zubek and Błaszkowski (2009), Druva-Lusite and Ievinsh (2010), Velázquez et al. (2010), Burni and Hussain (2011), Kołaczek et al. (2013), Shi et al. (2013) and Nobis et al. (2015) (see details in Supplementary data Table S1). All achlorophyllous plant species on AM fungi were assumed to possess Paris-type AM, as no contradictory evidence is present so far (Dickson et al., 2007; Imhof et al., 2013).
Stable isotope data for plant species with known AM morphotype, nitrogen concentration and light requirement
The literature was surveyed for C, N, H and oxygen (O) stable isotope natural abundance data suitable for the calculation of the stable isotope enrichment factors, ε (see below) (Supplementary data Table S1). These factors average the effect of habitat conditions, such as microclimate, soil respiration and soil conditions, on the stable isotope composition in leaves of autotrophic C3 plants growing together in the respective habitats. Those drivers for variations in the stable isotope composition of plants collected from different habitats may thus be disregarded (Farquhar et al., 1982, 1989; Sternberg et al., 1984; Ziegler, 1988; Dawson et al., 2002; Cernusak et al., 2004; Gebauer et al., 2016). We obtained raw data of 1300 stable isotope records on ε 13C/15N and 225 on ε 2H/18O from the following publications: Gebauer and Meyer (2003), Bidartondo et al. (2004), Zimmer et al. (2007, 2008), Hynson et al. (2009, 2015), Cameron and Bolin (2010), Liebel et al. (2010, 2015), Preiss et al. (2010), Girlanda et al. (2011), Ercole et al. (2015), Lee et al. (2015), Hynson (2016), Shutoh et al. (2016), Schiebold et al. (2017, 2018), Suetsugu et al. (2017), Ogura-Tsujita et al. (2018), Giesemann et al. (2020a, b) and Gomes et al. (2020). Additionally, unpublished data from the BayCEER Laboratory of Isotope Biogeochemistry Bayreuth were kindly provided. From this data set, 155 plant individuals (22 species) forming either intermediate morphotypes or establishing both Arum and Paris type were excluded (Dickson, 2004). Raw data from Courty et al. (2011) not made available are the only currently published data with AM reference plants missing in our survey. Thus, 1145 stable isotope records on ε 13C/15N and 218 on ε 2H/18O were analysed (135 plant species).
The isotope data were sorted into three functional groups: 13 species of achlorophyllous, full mycoheterotrophs on Paris-type AM (Merckx et al., 2010; Gomes et al., 2020), 63 species of chlorophyllous Paris- and 59 species of chlorophyllous Arum-type AM. From this data pool, leaf N concentrations were also available for 107 plant species (n = 890, 12 full mycoheterotrophs, 50 chlorophyllous Paris-type and 45 chlorophyllous Arum-type). Most belonged to the temperate climate zone in Europe (e.g. Austria, Germany and Italy) but there were a few records from other continents (e.g. Australia, Japan and the USA) (Supplementary data Table S1).
We compared the Paris-type enrichment factor ε (target plant, TP) with neighbouring Arum-type species (reference plant, RP) as εx = δxTP – mean(δxRP) (x = 13C, 15N, 18O, 2H) (Preiss and Gebauer, 2008; Hynson et al., 2013) [see hypotheses (i) and (ii)].
We classified the species with respect to light and temperature preferences by means of Ellenberg indicator values (Ellenberg et al., 2001) which is a system of ordinally/quasi-cardinally scaled classification of plants in Central Europe, finding information on 96 Paris- and 149 Arum-type species. For a sub-set of plant species from Central Europe (81 species, n = 787), we had both Ellenberg indicator values and ε 13C enrichment factors. The 41 Paris-type species of this sub-set (n = 315) were analysed for a correlation between light availability and ε 13C enrichment factors, reflecting the degree of mycoheterotrophy [see hypothesis (iii)].
Statistical analysis
The degree of mycoheterotrophy was approximated based on the two-source linear mixing model on 13C (Gebauer and Meyer, 2003; Hynson et al., 2013). Plant species considered fully autotrophic (chlorophyllous Arum-type plant species) and plant species considered fully mycoheterotrophic (achlorophyllous Paris-type plant species; Merckx et al., 2010; Gomes et al., 2020) represent the two end members between which the partially mycoheterotrophic Paris-type species are expected to fall (Giesemann et al., 2020b).
RStudio 1.1 (R Core Team, 2019) and SigmaPlot 11.0 (Systat Software, San Jose, CA, USA) were applied for data analyses. The platform from Lenhard and Lenhard (2016) was used for estimations of effect sizes (Cohen’s d). An effect size of d > 0.8 is usually counted as relevant (Cohen, 1992). Shapiro–Wilk tests for normal distribution (‘stats’ R package by R Core Team, 2019) and Levene tests for variance homogeneity (‘car’ R package by Fox and Weisberg, 2019) recommended a conservative, non-parametric test procedure for the comparison between Arum- and Paris-morphotype species. The one-tailed Mann–Whitney U-test using the ‘stats’ R package was performed to evaluate differences in Ellenberg indicator values between Arum- and Paris-morphotype species. One-tailed Kruskal–Wallis H-tests using the ‘stats’ R package were applied for a comparison of full mycoheterotrophs, chlorophyllous Paris- and Arum-morphotype species as well as for chlorophyllous Arum- and Paris-morphotype species separated into ferns, horsetails and seed plants, respectively. Dunn’s post-hoc (Z) test for multiple comparison was performed after a significant H-test using the ‘dunn.test’ R package (Dinno, 2017). P-values were corrected according to the sequential Holm–Bonferroni method. A linear regression (Pearson correlation, r2adj) was performed for light availability and ε 13C enrichment of Paris-morphotype plant species. The critical level of significance was set to α ≤ 0.05. The data are expressed as their mean value with their s.d. (x̄ ± s.d.).
RESULTS
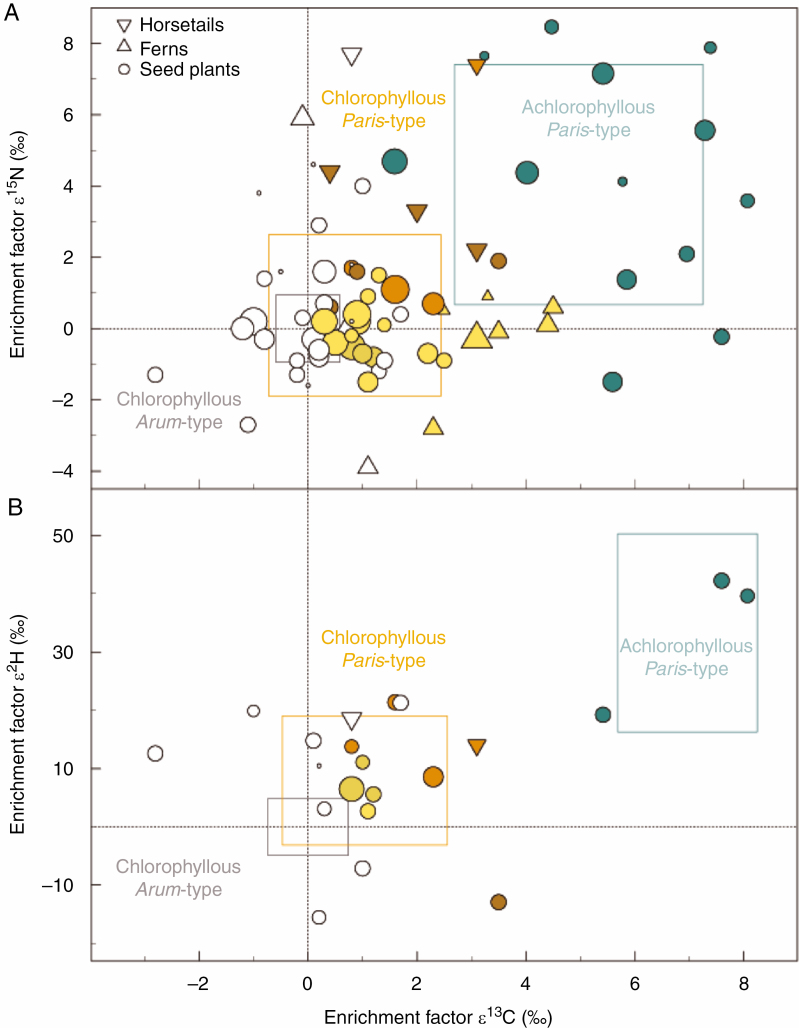
For Arum-morphotype AM species, as reference plants, the mean enrichment factor ε was zero by definition. Thus with standard deviation it was for 13C = 0.0 ± 0.6 ‰, 15N = 0.0 ± 0.9 ‰ and 2H = 0.0 ± 5.0 ‰, while enrichments in achlorophyllous Paris-morphotype species were pronounced: 13C = 5.0 ± 2.3 ‰, 15N = 4.0 ± 3.4 ‰ and 2H = 33.3 ± 17.0 ‰ (Fig. 1). Chlorophyllous Paris-type species were located between chlorophyllous Arum-type and achlorophyllous fully mycoheterotrophic plants, with intermediate enrichment values: 13C = 0.9 ± 1.6 ‰, 15N = 0.4 ± 2.3 ‰ and 2H = 8.0 ± 11.1 ‰. (Fig. 1). These average figures cover a large variation among chlorophyllous Paris-type species, ranging from –2.7 ± 0.8 ‰ for Asarum europaeum to 4.5 ± 1.4 ‰ for Athyrium filix-femina in 13C, from –3.9 ± 1.1 ‰ for Asplenium ruta-muraria to 7.7 ± 3.2 ‰ for Equisetum palustre in 15N and from –15.5 ± 7.8 ‰ for Gentiana bavarica to 21.4 ± 6.4 ‰ for Mercurialis perennis in 2H (Supplementary data Table S1).
Fig. 1.
(A) Carbon and nitrogen enrichment factors (ε 13C and ε 15N) and (B) carbon and hydrogen enrichment factors (ε 13C and ε 2H) for chlorophyllous Arum-type arbuscular mycorrhizal (AM) plant species (grey frame, s.d.), chlorophyllous Paris-type AM plant species (brownish tones, brown frame, s.d.) and achlorophyllous, full mycoheterotrophs on AM fungi (blue, blue frame, s.d.). AM morphotype assignment was obtained from the literature (see the Materials and Methods). Each species is represented by mean values and the s.d. is omitted for clarity. Symbol size reflects the sample size of the Paris-type species (n = 1–31, see Supplementary data Table S3). Each chlorophyllous Paris-type AM plant species was tested for significance of differences in ε 13C, ε 15N and ε 2H from co-occurring chlorophyllous Arum-type AM plant species (see Supplementary data Table S3). Chlorophyllous Paris-type AM plant species shown in coloured symbols are significant in at least one trait (13C enrichment, light gold; 13C + 2H enrichment, light brown; 13C + 15N enrichment, dark brown, 13C + 2H + 15N enrichment, dark gold; no significant enrichment, white). Achlorophyllous plant species were not included in the test procedure (see Gomes et al., 2020). The data comprise for 13C/15N: 13 achlorophyllous Paris-type species (n = 99), 63 chlorophyllous Paris-type species (n = 520) and 59 chlorophyllous Arum-type species (n = 530). The data comprise for 2H: three achlorophyllous species (n = 14), 18 chlorophyllous Paris-type species (n = 100) and 15 chlorophyllous Arum-type species (n = 104).
Kruskal–Wallis tests indicated significant effects for the comparisons of Arum-type AM, achlorophyllous Paris-type AM and chlorophyllous Paris-type AM plants in ε 13C [H(2) = 330.523, P < 0.001, Cohen’s d = 1.276], ε 15N [H(2) = 139.422, P < 0.001, Cohen’s d = 0.742] and ε 2H [H(2) = 71.985, P < 0.001, Cohen’s d = 1.389]. Pairwise comparisons by Dunn’s post-hoc test showed significant differences in ε 13C, ε 15N and ε 2H between each of the three functional groups (Table 1). Although Kruskal–Wallis test detected a significant effect between groups in 18O enrichment [H(2) = 6.001, P = 0.05, Cohen’s d = 0.275], the low Cohen’s d value suggested that the effect was not relevant. Furthermore, no significant effect between groups was detected for leaf total N concentrations [H(2) = 4.856, P = 0.09, Cohen’s d = 0.113]. The chlorophyllous Paris-type species were also consistently 13C and 2H enriched when Paris- and Arum-type species were separated into groups of horsetails (Equisetum), ferns and seed plants (Supplementary data Table S2). The 13C enrichment follows the sequence ferns > horsetails > seed plants, while the limited data on 2H enrichment suggest horsetails > seed plants (see below).
Table 1.
Pairwise Dunn’s post-hoc test (Z) for significance of differences between the three functional groups of achlorophyllous species on Paris-type AM (full mycoheterotrophs), chlorophyllous potentially partial mycoheterotrophs on Paris-type AM and chlorophyllous Arum-type AM plant species as references in enrichment factors ε 13C, ε 15N and ε 2H
| ε 13C | ε 15N | ε 2H | ||||
|---|---|---|---|---|---|---|
| Test statistics | P | Test statistics | P | Test statistics | P | |
| Achlorophyllous Paris-type vs. chlorophyllous Paris-type | Z = 11.193 | <0.001 | Z = 10.384 | <0.001 | Z = 3.885 | <0.001 |
| Achlorophyllous Paris-type vs. chlorophyllous Arum-type | Z = 17.176 | <0.001 | Z = 11.772 | <0.001 | Z = 7.009 | <0.001 |
| Chlorophyllous Paris-type vs. chlorophyllous Arum-type | Z = 10.570 | <0.001 | Z = 2.451 | 0.007 | Z = 6.331 | <0.001 |
Significances are highlighted in bold.
For 13C/15N, the data comprise 13 achlorophyllous Paris-type species (n = 99), 63 chlorophyllous Paris-type species (n = 520) and 59 chlorophyllous Arum-type species (n = 530). For 2H, the data comprise three achlorophyllous species (n = 14), 18 chlorophyllous Paris-type species (n = 100) and 15 chlorophyllous Arum-type species (n = 104).
A total of 63 chlorophyllous Paris-type AM species were compared with their respective chlorophyllous Arum-type AM reference plants in stable isotope enrichment factors ε 13C, ε 15N and ε 2H (Supplementary data Table S3). Of these, 31 were found to be significantly 13C enriched (Fig. 1, brownish symbols) while 32 remained inconspicuously enriched or depleted in their 13C patterns (Fig. 1, white). For the group that was significantly 13C enriched, a mean proportional C gain from the fungal source was calculated based on the linear two-source mixing model (Table 2). This mean proportional C gain from the fungal source ranged from 7 to 93 % (38±19 %) and follows the sequence ferns > horsetails > seed plants (Table 2).
Table 2.
Proportional C gain (%) of all 31 chlorophyllous Paris-type species that were identified as significantly different in stable isotope enrichment factors ε 13C from neighbouring Arum-type species
| Paris-type species | Family | n | Proportional C gain (%) | ||
|---|---|---|---|---|---|
| Range | Mean s.d. | ||||
| H,o | Equisetum arvense | Equisetaceae | 5 | 53–81 | 63 ± 10 |
| H,f | Equisetum fluviatile | Equisetaceae | 5 | 6–12 | 9 ± 3 |
| H,f | Equisetum sylvaticum | Equisetaceae | 4 | 48–81 | 63 ± 14 |
| H,f | Equisetum telmateia | Equisetaceae | 5 | 12–73 | 41 ± 24 |
| F,f | Pteridium sp. | Dennstaedtiaceae | 2 | 64–72 | 68 |
| F,f | Blechnum sp. | Blechnaceae | 2 | 25–79 | 52 |
| F,f | Athyrium filix-femina | Athyriaceae | 5 | 69–139 | 93 ± 28 |
| F,f | Diplazium sandwichianum | Athyriaceae | 6 | 50–122 | 91 ± 30 |
| F,f | Dryopteris filix-mas | Dryopteridaceae | 5 | 33–99 | 73 ± 25 |
| F,f | Polystichum sp. | Dryopteridaceae | 16 | 3–113 | 64 ± 31 |
| F,f | Polypodium vulgare | Polypodiaceae | 5 | 30–70 | 48 ± 16 |
| S,f | Tamus communis | Dioscoreaceae | 5 | –13 to 41 | 19 ± 22 |
| S,f | Paris quadrifolia | Melanthiaceae | 13 | 24–79 | 47 ± 16 |
| S,f | Smilax aspera | Smilacaceae | 5 | 5–48 | 26 ± 19 |
| S,o | Asphodelus aestivus | Asphodelaceae | 25 | –16 to 91 | 18 ± 26 |
| S,o | Bromus erectus | Poaceae | 29 | –7 to 54 | 18 ± 15 |
| S,o | Bromus sp. | Poaceae | 21 | –42 to 50 | 11 ± 22 |
| S,f | Melica nutans | Poaceae | 4 | 5–42 | 16 ± 17 |
| S,f | Anemone nemorosa | Ranunculaceae | 10 | –5 to 53 | 25 ± 17 |
| S,o | Ranunculus sp. | Ranunculaceae | 23 | –41 to 38 | 7 ± 20 |
| S,o | Alchemilla vulgaris | Rosaceae | 4 | 18–36 | 29 ± 8 |
| S,o | Alchemilla sp. | Rosaceae | 6 | –81 to 64 | 8 ± 49 |
| S,f | Oxalis acetosella | Oxalidaceae | 9 | –9 to 59 | 20 ± 21 |
| S,f | Mercurialis perennis | Euphorbiaceae | 31 | –3 to 85 | 33 ± 22 |
| S,f | Geranium sylvaticum | Geraniaceae | 5 | 42–66 | 52 ± 10 |
| S,f | Lysimachia nummularia | Primulaceae | 5 | 5–32 | 23 ± 11 |
| S,o | Gentiana lutea | Gentianaceae | 5 | 55–108 | 73 ± 21 |
| S,o | Astrantia major | Apiaceae | 25 | –31 to 50 | 17 ± 19 |
| S,o | Ligusticum mutellina | Apiaceae | 5 | 13–25 | 16 ± 5 |
| S,o | Meum athamanticum | Apiaceae | 10 | 23–76 | 46 ± 17 |
| S,o | Scandix pecten-veneris | Apiaceae | 10 | –4 to 44 | 22 ± 17 |
In total, 63 chlorophyllous Paris-type plant species were tested, thus 32 species were insignificantly enriched in 13C (see Supplementary data Table S3 for the complete list). Nomenclature follows the sources APG IV (2016) and PPG I (2016). Family sequences are according to PPG I (2016) (pteridophytes), Haston et al. (2009) and APG IV (2016) (angiosperms).
F, fern; H, horsetail; S, seed plant; f, forest; o, open-land.
Among the 13C-enriched chlorophyllous Paris-type species were seven out of ten forest ferns (ε 13C mean: 3.4 ± 1.1 ‰), four out of five forest horsetails (ε 13C mean: 2.2 ± 1.3 ‰) and nine out of 24 forest-floor seed plants (ε 13C mean: 1.4 ± 0.8 ‰). These species were all herbaceous perennials. In addition to 13C enrichment, two species were also 2H enriched (8.4 ± 3.9 ‰; Anemone nemorosa and Oxalis acetosella; Fig. 1, light brown) and four were 15N enriched (2.8 ± 1.2 ‰; Equisetum arvense, E. fluviatile, E. telmateia and Tamus communis; Fig. 1, dark brown). The herbaceous forest species Paris quadrifolia, Mercurialis perennis and Equisetum sylvaticum turned out to be simultaneously 13C (mean: 2.3 ± 0.8 ‰), 15N (mean: 3.1 ± 3.8 ‰) and 2H enriched (mean: 14.7 ± 6.4 ‰) (Fig. 1, dark gold).
Among the chlorophyllous Paris-type species that did not show significant 13C enrichment, the forest perennial Asarum europaeum was nevertheless significantly 15N and 2H enriched (Supplementary data Table S3). Tree saplings of Acer campestre tended towards both 13C and 15N enrichment, and saplings of Cornus controversa tended towards a 13C enrichment that was uncertain because of the small sample size. Saplings of Acer pseudoplatanus were not conspicuously 13C enriched while they were significantly 2H enriched.
In addition to the frequently 13C-enriched chlorophyllous Paris-type species from forest sites, 13C enrichments were also observed for 11 out of 23 herbaceous open-land species (ε 13C mean: 1.2 ± 1.0 ‰), such as Astrantia major, Gentiana lutea and Ligusticum mutellina. Their conspicuousness was supported by either an 15N enrichment (1.3 ± 0.9 ‰, Gentiana lutea and Alchemilla sp.; Fig. 1, dark brown), an 2H enrichment (6.5 ± 6.0 ‰, Astrantia major; Fig. 1, light brown) or both (1.7 ± 1.1 ‰ and 13.8 ± 4.4 ‰, Ligusticum mutellina, Fig. 1, dark gold; Table 2). The open-land species Aquilegia atrata and Trollius europaeus were found to be 13C inconspicuous (0.9 ± 0.9 ‰) while a significant 2H enrichment was disclosed (14.8 ± 6.3 ‰ and 21.3 ± 5.6 ‰, respectively; Supplementary data Table S3). Species that were 13C enriched but where data on 2H are not available could be suspected of partial mycoheterotrophic nutrition and should be further investigated (2.0 ± 1.3 ‰, e.g. Asphodelus aestivus, Geranium sylvaticum, Meum athamanticum, Scandix pecten-veneris and ferns). Botrychium lunaria, Brachypodium sylvaticum, Molinia caerulea and Pimpinella saxifraga were found to be only 15N enriched, while data on 2H enrichment are still missing for these species.
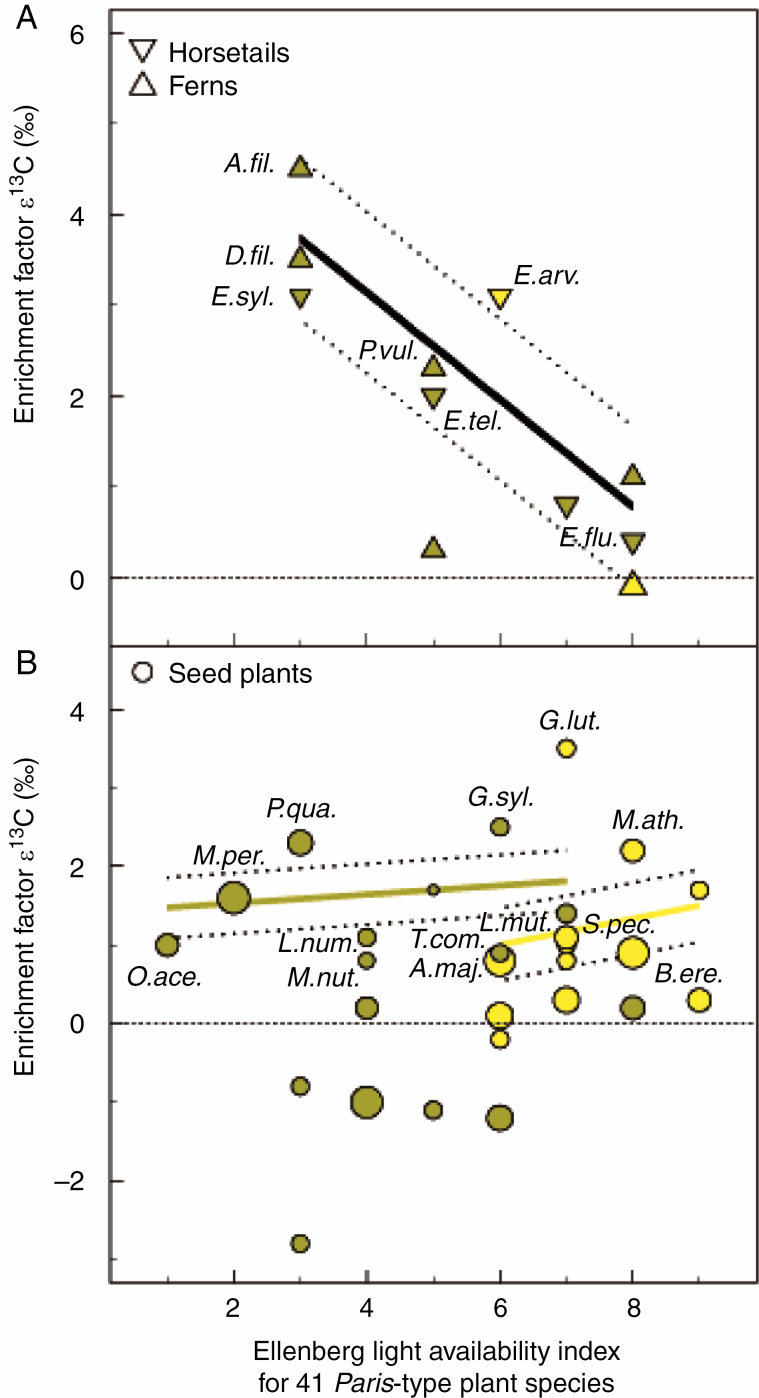
Ellenberg indicator values found chlorophyllous Paris-type species to be significantly distinguished from chlorophyllous Arum-type species in their preferences for light availability [U(94,148) = 5416.5, P = 0.003, Cohen’s d = 0.4] and temperature [H(66,111) = 2605.5, P < 0.001, Cohen’s d = 0.5]. Although the Cohen’s d index probably penalizes the imbalance in sample size distribution, these differences tendentially indicate that Paris-type species occur preferentially in habitats of lower light availability and lower temperature (Supplementary data Fig. S1). Among the chlorophyllous Paris-type ferns and horsetails for which both Ellenberg light availability values and ε 13C enrichment factors of Paris-type species were available, significant relationships were found to 13C enrichments. This means proportionally higher C gains from the fungal source under conditions of lower light availability (Fig. 2). There was no significant correlation between Ellenberg light availability values and ε 13C in seed plants, which could be due to the existence of a few highly 13C-enriched open-land plant species (Gentiana lutea and Meum athamanticum). Chlorophyllous Paris-type ferns, horsetails and seed plants from forests together were more 13C enriched than open-land Paris-type plants [U(148,163) = 6272.6, P < 0.001, Cohen’s d = 0.9]. This relationship also holds when only comparing seed plants that were significantly 13C enriched from forests and open lands [U(73,84) = 2368, P = 0.014, Cohen’s d = 0.4; Fig. 2].
Fig. 2.
Relationship between the Ellenberg light availability index and stable isotope enrichment factors ε 13C for 41 Paris-morphotype arbuscular mycorrhizal (AM) plant species. Significance of 13C enrichment tested towards co-occurring Arum-type AM species is highlighted by species labels (see Supplementary data Table S3). (A) Pteridophytes (horsetails and ferns). A negative correlation between light and fungal carbon gain [black solid line, t(34) = 12.8, r2adj = 0.7, P = 0.016, dotted line: 95 % confidence intervals]. (B) Seed plant species. No clear correlation. The coloured solid lines illustrate a regression among seed plant species significantly 13C enriched separated by forest habitats [dark khaki-coloured solid line and symbols; t(73) = 0.1, r2adj = 0.0, P = 0.741] and open lands [light khaki-coloured solid line and symbols; t(84) = 0.1, r2adj = 0.0, P = 0.729] (dotted 95 % confidence intervals). Ellenberg light availability index and AM morphotype were obtained from the literature (see the Materials and Methods). The single Paris-type AM plant species are represented by mean values. Standard deviations are omitted for clarity. Symbol size reflects the sample size of the Paris-type species (n = 1–31, see Supplementary data Table S3). The figure counts only Paris-type AM species with data on ε 13C and Ellenberg light availability values. Paris-type plant species that were significantly 13C enriched: (A) A.fil. Athyrium filix-femina, D.fil. Dryopteris filix-mas, E. syl. Equisetum sylvaticum, P.vul. Polypodium vulgare, E.tel. Equisetum telmateia, E.arv. Equisetum arvense, E.flu. Equisetum fluviatile; (B) O.ace. Oxalis acetosella, M.per. Mercurialis perennis, P.qua. Paris quadrifolia, L.num. Lysimachia nummularia, M.nut. Melica nutans, G.syl. Geranium sylvaticum, T.com. Tamus communis, A.maj. Astrantia major, G.lut. Gentiana lutea, S.pec. Scandix pecten-veneris, L.mut. Ligusticum mutellina, M.ath. Meum athamanticum, B.ere. Bromus erectus.
DISCUSSION
We found that partial mycoheterotrophy is common in plants with AM. In plants with Paris-type AM, we found significant 13C enrichment in about half of the species under study (31 out of 63 Paris-type species). Two of these, Paris quadrifolia and Anemone nemorosa, coincide with our previous report (Giesemann et al., 2020b). Among some of the remaining species with Paris-type AM, the enrichment in 15N, 2H or both may suggest that some mycoheterotrophy did occur. Plant species with Paris-type AM might thus present a continuous range between full autotrophy and full mycoheterotrophy. Our results are in support of Imhof (1999) who suggested that hyphal growth within root cells, such as the intracellular hyphal coils of the Paris morphotype, is an important prerequisite for the evolution of mycoheterotrophy.
Paris-type partial mycoheterotrophy seemed to be common in the group of pteridophytes, and among seed plants we found it in 13 families, both mono- and dicotyledons. This suggests that the capacity to parasitize Glomeromycotina fungi in a Paris-type AM relationship is widespread in the plant kingdom and, important to note, exists among plants with fully developed leaves which up to now have been thought to be fully photo-assimilating.
Our 31 species with partial mycoheterotrophy on Paris-type AM add to the steadily increasing list of plant species with this kind of nutrition; currently 124 plant species are known. Most of these belong in Orchidaceae (orchid mycorrhiza) and Ericales (ericoid mycorrhiza) (Hynson et al., 2013, 2016; Gebauer et al., 2016; Schiebold et al., 2018).
Previous discoveries of partial mycoheterotrophy on Paris-type AM amount to a few species with apparent physiological and evolutionary prerequisites. Ophioglossum kawamurae, O. parvum and O. thermale (sporophyte, Ophioglossaceae), found by Suetsugu et al. (2020b) to be partially mycoheterotrophic, belong to a family where the gametophyte generation is known to be achlorophyllous and mycoheterotrophic. Other examples belong to families known to contain achlorophyllous members: Bartonia virginica, Obolaria virginica and Pterygocalyx volubilis in Gentianaceae (Cameron and Bolin, 2010; Suetsugu et al., 2020a) and Burmannia coelestis in Burmanniaceae (Bolin et al., 2017). In view of our new records, these first few examples appear to just graze the surface of something much bigger. In surveying plants that have served as references in other studies, we were able to make an assessment without any pre-conceived expectations regarding certain species or groups. Thus, we could demonstrate significant mycoheterotrophy not only in most pteridophytes tested, but also in ten families of seed plants where it has not been recorded, or even suspected, previously (Apiaceae, Asphodelaceae, Dioscoreaceae, Euphorbiaceae, Geraniaceae, Oxalidaceae, Poaceae, Primulaceae, Rosaceae and Smilacaceae). To this list we may add two more families: Melanthiaceae and Ranunculaceae for Paris quadrifolia and Anemone nemorosa which we identified as partially mycoheterotrophic quite recently (Giesemann et al., 2020b).
Since organic C within the fungi ultimately comes from photo-assimilating plants (either alive or as plant debris), partial mycoheterotrophy does not interact only with the fungi in question, but also with potential donor plants in the ecosystem. Since fungi belonging to the Glomeromycotina are considered to be obligate biotrophs, we should be expecting live donor plants. We found partial mycoheterotrophy only in herbaceous species with Paris-type AM. The woody species under study were either Arum-type plants, or Paris-type without any clear indication of mycoheterotrophy. Transfer of photosynthates from the roots of woody species could be a way for understorey AM species to compensate for a light-limited environment. The negative relationship in pteridophyte species between light and 13C enrichment suggests such a mechanism and is consistent with our third hypothesis. Previous studies indicated a limited photosynthetic capacity of many ferns and horsetails (e.g. Ludlow and Wolf, 1975; Gago et al., 2013; Nadal et al., 2018), and photosynthetic rates lower than in corresponding Arum-type species were found in several Paris-type AM deciduous forest trees (Wright et al., 2004) and Paris-type AM forest ground herbaceous species (Dalke et al., 2018).
Some Paris-type AM tree saplings (Acer campestre, A. pseudoplatanus and Cornus controversa) did show indications towards partial mycoheterotrophy. Whether this condition only characterizes the juvenile trees or persists throughout life is a matter for future investigations.
Between herbaceous plant species, however, partial mycoheterotrophy could also be a competitive advantage in relation to species with Arum-type mycorrhizal connections. Partial mycoheterotrophy in some species might increase biodiversity by restricting development in other, more vigorously growing members, in analogy with the effect that hemiparasitic plant species may have on the surrounding vegetation and ecosystem (Quested et al., 2003; Quested, 2008; Hartley et al., 2015). Partial mycoheterotrophy may thus have profound effects with respect not only to C cycling but also to the cycling of nutrients such as N.
Interspecific and interfamilial variations in 15N enrichments are known from other groups of mycoheterotrophic plants (Orchidaceae and Pyroloideae; Hynson et al., 2016) and also from AM full mycoheterotrophs (Merckx et al., 2010; Courty et al., 2011; Gomes et al., 2020). The various 15N enrichments were attributed to different fungal partners that access different N nutrient sources (Schiebold et al., 2017). The variations in this study of 15N enrichments in the partially mycoheterotrophic species might indicate different forms of N utilized by Glomeromycotina fungi. Significant 15N enrichments without any 13C and 2H enrichments might, however, also be attributed to a functional role in N acquisition by entirely different fungal endophytes (Hoysted et al., 2019; Giesemann et al., 2020a). In addition to stable isotope natural abundance, the total leaf N concentration has been used to differentiate between mycoheterotrophy on orchid mycorrhiza (high leaf N concentrations) and ericoid mycorrhiza (low leaf N concentrations) (Hynson et al., 2016). This difference is thought to be due to different fungal matter uptake mechanisms by the various types of mycoheterotrophic plants: digestion of fungal ‘pelotons’ in addition to active membrane transport in orchid mycorrhiza and exclusively active membrane transport in ericoid mycorrhiza (Hynson et al., 2016). Since partially mycoheterotrophic Paris-type AM species do not differ markedly in leaf total N concentration from fully autotrophic Arum-type AM species, a similar active membrane transport mechanism for the uptake of N is suggested for both. However, digestion of Paris-morphotype coils has also been documented (Imhof et al., 2013) and life history strategies rather than fungal substrate and trophic strategies could contribute to leaf total N concentration patterns as well (cf. Hynson et al., 2016).
The high diversity in partial mycoheterotrophs as found here is in agreement with the recent finding of low phylogenetic constraints for developing mycoheterotrophy (Perez-Lamarque et al., 2020). A significant proportion of the AM plants, and thus also of all plant species globally, possess the required infection pattern (Paris-type AM) to obtain C from a fungal source. Based on these findings, partial mycoheterotrophy, indeed, is more widespread than recognized so far (Giesemann et al., 2020b). Nonetheless, there still remain a lot of open questions to be resolved. Our current survey on the occurrence of Paris-type AM is mostly based on sometimes old lists from the literature that might require careful re-evaluation. A matter of further research has to be also the consideration of intermediate AM types, plant species that are able to form either Arum- or Paris-type AM and the influences of environmental conditions and AM fungal taxa on the formation of either Arum- or Paris-type AM.
We conclude that the fungal Paris-coiling type appears to be a necessary prerequisite for partial mycoheterotrophy in chlorophyllous AM plant species. However, not all chlorophyllous Paris-morphotype AM plant species turned out to be partially mycoheterotrophic.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: Ellenberg indicator value for light availability and temperature between Arum- and Paris-type arbuscular mycorrhizal plant species. Table S1: stable isotope enrichment factors ε with s.d., leaf total N concentrations with s.d. and the arbuscular mycorrhizal type. Table S2: pairwise Dunn’s post-hoc test for significance of differences between chlorophyllous Arum- and Paris-type arbuscular mycorrhizal plant species separated by groups of horsetails, ferns and seed plants in enrichment factors ε 13C, ε 15N and ε 2H. Table S3: Mann–Whitney U-test for significance of differences between chlorophyllous Paris-type species and their respective chlorophyllous Arum-type reference plant species in stable isotope enrichment factors ε 13C, ε 15N and ε 2H.
ACKNOWLEDGEMENTS
Some species were sampled during students’ field courses in 2007, 2008, 2016, and 2018 and we are grateful for the support of student field assistants. The authors thank Milena Opgenoorth for providing data on fern species, and Finn N. Rasmussen for enabling a taxonomic ordering of Table 2 and Supplementary data Table S3. The authors thank Ian Wright and Peter Reich for access to the ‘GLOPNET’ leaf ecomonics data set. P.G. surveyed the data and made a synthesis, analysed and treated the results, and wrote the first manuscript draft. H.N.R. essentially initiated the basic idea for this research originating from an unpublished literature review. G.G. coordinated the project, supervised the isotope abundance survey, supported data treatment and provided access to unpublished data. All authors contributed to interpretation and presentation of results in the manuscript.
FUNDING
P.G. and this project were funded by the Elite Network of Bavaria.
LITERATURE CITED
- APG IV . 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Becerra A, Cabello M, Chiarini F. 2007. Arbuscular mycorrhizal colonization of vascular plants from the Yungas forests, Argentina. Annals of Forest Science 64: 765–772. [Google Scholar]
- Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ. 2004. Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proceedingsof the Royal Society B: Biological Sciences 271: 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin JF, Tennakoon KU, Majid MBA, Cameron DD. 2017. Isotopic evidence of partial mycoheterotrophy in Burmannia coelestis (Burmanniaceae). Plant Species Biology 32: 74–80. [Google Scholar]
- Burni T, Hussain F. 2011. Diversity in arbuscular mycorrhizal morphology in some medicinal plants of family Lamiaceae. Pakistan Journal of Botany 43: 1789–1792. [Google Scholar]
- Cameron DD, Bolin JF. 2010. Isotopic evidence of partial mycoheterotrophy in the Gentianaceae: Bartonia virginica and Obolaria virginica as case studies. American Journal of Botany 97: 1272–1277. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Pate JS, Farquhar GD. 2004. Oxygen and carbon isotope composition of parasitic plants and their hosts in southwestern Australia. Oecologia 139: 199–213. [DOI] [PubMed] [Google Scholar]
- Cohen J. 1992. A power primer. Psychological Bulletin 112: 155–159. [DOI] [PubMed] [Google Scholar]
- Courty PE, Walder F, Boller T, et al. . 2011. Carbon and nitrogen metabolism in mycorrhizal networks and mycoheterotrophic plants of tropical forests: a stable isotope analysis. Plant Physiology 156: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalke IV, Novakovskiy AB, Maslova SP, Dubrovskiy YA. 2018. Morphological and functional traits of herbaceous plants with different functional types in the European Northeast. Plant Ecology 219: 1295–1305. [Google Scholar]
- Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. 2002. Stable isotopes in plant ecology. Annual Review of Ecology and Systematics 33: 507–559. [Google Scholar]
- Diallo A, Weber H, Imhof S. 2001. Sonderstellung der Mykorrhiza von Mercurialis perennis L. im Vergleich mit anderen Euphorbiaceae. Beiträge zur Biologie der Pflanzen 72: 315–323. [Google Scholar]
- Dickson S. 2004. The Arum–Paris continuum of mycorrhizal symbioses. New Phytologist 163: 187–200. [DOI] [PubMed] [Google Scholar]
- Dickson S, Smith FA, Smith SE. 2007. Structural differences in arbuscular mycorrhizal symbioses: more than 100 years after Gallaud, where next? Mycorrhiza 17: 375–393. [DOI] [PubMed] [Google Scholar]
- Dinno A. 2017. dunn.test: Dunn’s test of multiple comparisons using rank sums. R package version 1.3.5.https://cran.r-project.org/package=dunn.test.
- Druva-Lusite I, Ievinsh G. 2010. Diversity of arbuscular mycorrhizal symbiosis in plants from coastal habitats. Environmental and Experimental Biology 8: 17–34. [Google Scholar]
- Ellenberg H, Weber HE, Düll R, Wirth V, Werner W. 2001. Zeigerwerte von Pflanzen in Mitteleuropa, 3., durchgesehene Auflage. Göttingen: Verlag Erich Goltze GmbH & Co KG. [Google Scholar]
- Ercole E, Adamo M, Rodda M, Gebauer G, Girlanda M, Perotto S. 2015. Temporal variation in mycorrhizal diversity and carbon and nitrogen stable isotope abundance in the wintergreen meadow orchid Anacamptis morio. New Phytologist 205: 1308–1319. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, O’Leary MH, Berry JA. 1982. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Functional Plant Biology 9: 121. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Biology 40: 503–537. [Google Scholar]
- Fox J, Weisberg S. 2019. An R companion to applied regression, 3rd edn. Thousand Oaks, CA: Sage. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. [Google Scholar]
- Gago J, Coopman RE, Cabrera HM, et al. . 2013. Photosynthesis limitations in three fern species. Physiologia Plantarum 149: 599–611. [DOI] [PubMed] [Google Scholar]
- Gallaud I. 1905. Études sur les mycorrhizes endotrophes. Revue générale de botanique 17: 5–48; 66–83, 123–135; 223–239; 313–325; 425–433; 479–500. [Google Scholar]
- Gebauer G, Meyer M. 2003. 15N and 13C natural abundance of autotrophic and myco-heterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytologist 160: 209–223. [DOI] [PubMed] [Google Scholar]
- Gebauer G, Preiss K, Gebauer AC. 2016. Partial mycoheterotrophy is more widespread among orchids than previously assumed. New Phytologist 211: 11–15. [DOI] [PubMed] [Google Scholar]
- Giesemann P, Eichenberg D, Stöckel M, et al. . 2020. a. Dark septate endophytes and arbuscular mycorrhizal fungi (Paris-morphotype) affect the stable isotope composition of ‘classically’ non-mycorrhizal plants. Functional Ecology 34: 2453–2466. [Google Scholar]
- Giesemann P, Rasmussen HN, Liebel HT, Gebauer G. 2020b. Discreet heterotrophs: green plants that receive fungal carbon through Paris-type arbuscular mycorrhiza. New Phytologist 226: 960–966. [DOI] [PubMed] [Google Scholar]
- Girlanda M, Segreto R, Cafasso D, et al. . 2011. Photosynthetic Mediterranean meadow orchids feature partial mycoheterotrophy and specific mycorrhizal associations. American Journal of Botany 98: 1148–1163. [DOI] [PubMed] [Google Scholar]
- Gomes S, Merckx V, Kehl J, Gebauer G. 2020. Mycoheterotrophic plants living on arbuscular mycorrhizal fungi are generally enriched in 13C, 15N, and 2H isotopes. Journal of Ecology 108: 1250–1261. [Google Scholar]
- Hartley SE, Green P, Massey FP, Press MC, Stewart JA, John EA. 2015. Hemiparasitic plant impacts animal and plant communities across four trophic levels. Ecology 96: 2408–2416. [DOI] [PubMed] [Google Scholar]
- Haston E, Richardson JE, Stevens PF, Chase MW, Harris DJ. 2009. The Linear Angiosperm Phylogeny Group (LAPG) III: a linear sequence of the families in APG III. Botanical Journal of the Linnean Society 161: 128–131. [Google Scholar]
- Hoysted GA, Jacob AS, Kowal J, et al. . 2019. Mucoromycotina fine root endophyte fungi form nutritional mutualisms with vascular plants. Plant Physiology 181: 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynson NA. 2016. The carbon and nitrogen ecophysiologies of two endemic tropical orchids mirrors those of their temperate relatives and the local environment. Royal Society Open Science 3: 160427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynson NA, Preiss K, Gebauer G, Bruns TD. 2009. Isotopic evidence of full and partial myco-heterotrophy in the plant tribe Pyroleae (Ericaceae). New Phytologist 182: 719–726. [DOI] [PubMed] [Google Scholar]
- Hynson NA, Madsen TP, Selosse M-A, et al. . 2013. The physiological ecology of mycoheterotrophy. In: Merckx VSFT, ed. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer, 297–342. [Google Scholar]
- Hynson NA, Bidartondo MI, Read DJ. 2015. Are there geographic mosaics of mycorrhizal specificity and partial mycoheterotrophy? A case study in Moneses uniflora (Ericaceae). New Phytologist 208: 1003–1007. [DOI] [PubMed] [Google Scholar]
- Hynson NA, Schiebold JM, Gebauer G. 2016. Plant family identity distinguishes patterns of carbon and nitrogen stable isotope abundance and nitrogen concentration in mycoheterotrophic plants associated with ectomycorrhizal fungi. Annals of Botany 118: 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof S. 1999. Root morphology, anatomy and mycotrophy of the achlorophyllous Voyria aphylla (Jacq.) Pers. (Gentianaceae). Mycorrhiza 9: 33–39. [Google Scholar]
- Imhof S, Massicotte HB, Melville LH, Peterson RL. 2013. Subterranean morphology and mycorrhizal structures. In: Merckx VSFT, ed. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer, 157–214. [Google Scholar]
- Kołaczek P, Zubek S, Błaszkowski J, Mleczko P, Margielewski W. 2013. Erosion or plant succession – How to interpret the presence of arbuscular mycorrhizal fungi (Glomeromycota) spores in pollen profiles collected from mires. Review of Palaeobotany and Palynology 189: 29–37. [Google Scholar]
- Leake JR. 1994. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytologist 127: 171–216. [DOI] [PubMed] [Google Scholar]
- Lee YI, Yang CK, Gebauer G. 2015. The importance of associations with saprotrophic non-Rhizoctonia fungi among fully mycoheterotrophic orchids is currently under-estimated: novel evidence from sub-tropical Asia. Annals of Botany 116: 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard W, Lenhard A. 2016. Berechnung von Effektstärken. https://www.psychometrica.de/effektstaerke.html. [Google Scholar]
- Liebel HT, Bidartondo MI, Preiss K, et al. . 2010. C and N stable isotope signatures reveal constraints to nutritional modes in orchids from the Mediterranean and Macaronesia. American Journal of Botany 97: 903–912. [DOI] [PubMed] [Google Scholar]
- Liebel HT, Bidartondo MI, Gebauer G. 2015. Are carbon and nitrogen exchange between fungi and the orchid Goodyera repens affected by irradiance? Annals of Botany 115: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CJ, Wolf FT. 1975. Photosynthesis and respiration rates of ferns. American Fern Journal 65: 43. [Google Scholar]
- Menoyo E, Beccera A, Rension D. 2007. Mycorrhizal assiciations in Polylepsis woodlands of Central Argentina. Canadian Journal of Botany 85: 526–531. [Google Scholar]
- Merckx VSFT. 2013. Mycoheterotrophy: an introduction. In: Merckx VSFT, ed. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer, 1–17. [Google Scholar]
- Merckx V, Stöckel M, Fleischmann A, Bruns TD, Gebauer G. 2010. 15N and 13C natural abundance of two mycoheterotrophic and a putative partially mycoheterotrophic species associated with arbuscular mycorrhizal fungi. New Phytologist 188: 590–596. [DOI] [PubMed] [Google Scholar]
- Merckx VS, Freudenstein JV, Kissling J, et al. . 2013. Taxonomy and classification. In: Merckx VSFT, ed. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer, 19–101. [Google Scholar]
- Nadal M, Flexas J, Gulías J. 2018. Possible link between photosynthesis and leaf modulus of elasticity among vascular plants: a new player in leaf traits relationships? Ecology Letters 21: 1372–1379. [DOI] [PubMed] [Google Scholar]
- Nobis A, Błaszkowski J, Zubek S. 2015. Arbuscular mycorrhizal fungi associations of vascular plants confined to river valleys: towards understanding the river corridor plant distribution. Journal of Plant Research 128: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura-Tsujita Y, Gebauer G, Xu H, et al. . 2018. The giant mycoheterotrophic orchid Erythrorchis altissima is associated mainly with a divergent set of wood-decaying fungi. Molecular Ecology 27: 1324–1337. [DOI] [PubMed] [Google Scholar]
- Perez-Lamarque B, Selosse MA, Öpik M, Morlon H, Martos F. 2020. Cheating in arbuscular mycorrhizal mutualism: a network and phylogenetic analysis of mycoheterotrophy. New Phytologist 226: 1822–1835. [DOI] [PubMed] [Google Scholar]
- PPG I . 2016. A community-derived classification for extant lycophytes and ferns. Journal of Systematics and Evolution 54: 563–603. [Google Scholar]
- Preiss K, Adam IK, Gebauer G. 2010. Irradiance governs exploitation of fungi: fine-tuning of carbon gain by two partially myco-heterotrophic orchids. Proceedings of the Royal Society B: Biological Sciences 277: 1333–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss K, Gebauer G. 2008. A methodological approach to improve estimates of nutrient gains by partially myco-heterotrophic plants. Isotopes in Environmental and Health Studies 44: 393–401. [DOI] [PubMed] [Google Scholar]
- Quested HM. 2008. Parasitic plants – impacts on nutrient cycling. Plant and Soil 311: 269–272. [Google Scholar]
- Quested HM, Cornelissen JHC, Press MC, et al. . 2003. Decomposition of sub-arctic plants with differing nitrogen economies: a functional role for hemiparasites. Ecology 84: 3209–3221. [Google Scholar]
- R Core Team . 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org. [Google Scholar]
- Schiebold JM, Bidartondo MI, Karasch P, Gravendeel B, Gebauer G. 2017. You are what you get from your fungi: nitrogen stable isotope patterns in Epipactis species. Annals of Botany 119: 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebold JM-I, Bidartondo MI, Lenhard F, Makiola A, Gebauer G. 2018. Exploiting mycorrhizas in broad daylight: partial mycoheterotrophy is a common nutritional strategy in meadow orchids. Journal of Ecology 106: 168–178. [Google Scholar]
- Schweiger JM, Kemnade C, Bidartondo MI, Gebauer G. 2019. Light limitation and partial mycoheterotrophy in rhizoctonia-associated orchids. Oecologia 189: 375–383. [DOI] [PubMed] [Google Scholar]
- Shah MA, Reshi ZA, Khasa D. 2009. Arbuscular mycorrhizal status of some Kashmir Himalayan alien invasive plants. Mycorrhiza 20: 67–72. [DOI] [PubMed] [Google Scholar]
- Shi Z, Hou X, Chen Y, Wang F, Miao Y. 2013. Foliar stoichiometry under different mycorrhizal types in relation to temperature and precipitation in grassland. Journal of Plant Ecology 6: 270–276. [Google Scholar]
- Shutoh K, Kaneko S, Suetsugu K, Naito YI, Kurosawa T. 2016. Variation in vegetative morphology tracks the complex genetic diversification of the mycoheterotrophic species Pyrola japonica sensu lato. American Journal of Botany 103: 1618–1629. [DOI] [PubMed] [Google Scholar]
- Sternberg LO, DeNiro MJ, Ting IP. 1984. Carbon, hydrogen, and oxygen isotope ratios of cellulose from plants having intermediary photosynthetic modes. Plant Physiology 74: 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu K, Yamato M, Miura C, et al. . 2017. Comparison of green and albino individuals of the partially mycoheterotrophic orchid Epipactis helleborine on molecular identities of mycorrhizal fungi, nutritional modes and gene expression in mycorrhizal roots. Molecular Ecology 26: 1652–1669. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Matsubayashi J, Ogawa NO, Murata S, Sato R, Tomimatsu H. 2020a. Isotopic evidence of arbuscular mycorrhizal cheating in a grassland gentian species. Oecologia 192: 929–937. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Taketomi S, Tanabe AS, Haraguchi TF, Tayasu I, Toju H. 2020b. Isotopic and molecular data support mixotrophy in Ophioglossum at the sporophytic stage. New Phytologist 228: 415–419. [DOI] [PubMed] [Google Scholar]
- Těšitel J, Plavcová L, Cameron DD. 2010. Interactions between hemiparasitic plants and their hosts: the importance of organic carbon transfer. Plant Signaling & Behavior 5: 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnau K, Anielska T, Ryszka P, Gawroński S, Ostachowicz B, Jurkiewicz A. 2008. Establishment of arbuscular mycorrhizal plants originating from xerothermic grasslands on heavy metal rich industrial wastes – new solution for waste revegetation. Plant and Soil 305: 267–280. [Google Scholar]
- Velázquez S, Biganzoli F, Cabello M. 2010. Arbuscular mycorrhizal fungi in El Palmar National Park (Entre Rios Province, Argentina) – a protected reserve. Sydowia 62: 149–163. [Google Scholar]
- Waterman RJ, Klooster MR, Hentrich H, Bidartondo MI. 2013. Species interactions of mycoheterotrophic plants: specialization and its potential consequences. In: Merckx VSFT, ed. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer, 267–296. [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, dePamphilis CW. 2010. The evolution of parasitism in plants. Trends in Plant Science 15: 227–235. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. . 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Ziegler H. 1988. Hydrogen isotope fractionation in plant tissues. In: Rundel PW, Ehleringer JR, Nagy KA, eds. Stable isotopes in ecological research. Berlin: Springer, 105–123. [Google Scholar]
- Zimmer K, Hynson NA, Gebauer G, Allen EB, Allen MF, Read DJ. 2007. Wide geographical and ecological distribution of nitrogen and carbon gains from fungi in pyroloids and monotropoids (Ericaceae) and in orchids. New Phytologist 175: 166–175. [DOI] [PubMed] [Google Scholar]
- Zimmer K, Meyer C, Gebauer G. 2008. The ectomycorrhizal specialist orchid Corallorhiza trifida is a partial myco-heterotroph. New Phytologist 178: 395–400. [DOI] [PubMed] [Google Scholar]
- Zubek S, Błaszkowski J. 2009. Medicinal plants as hosts of arbuscular mycorrhizal fungi and dark septate endophytes. Phytochemistry Reviews 8: 571–580. [Google Scholar]
- Zubek S, Turnau K, Błaszkowski J. 2008. Arbuscular mycorrhiza of endemic and endangered plants from the Tatra Mts. Acta Societatis Botanicorum Poloniae 77: 149–156. [Google Scholar]
- Zubek S, Blaszkowski J, Mleczko P. 2011a. Arbuscular mycorrhizal and dark septate endophyte associations of medicinal plants. Acta Societatis Botanicorum Poloniae 80: 285–292. [Google Scholar]
- Zubek S, Nobis M, Błaszkowski J, Mleczko P, Nowak A. 2011b. Fungal root endophyte associations of plants endemic to the Pamir Alay Mountains of Central Asia. Symbiosis (Philadelphia, Pa.) 54: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.