Abstract
Background and Aims
Much of our understanding of the ecology and evolution of seed dispersal in the Neotropics is founded on studies involving the animal-dispersed, hyperdiverse plant clade Miconia (Melastomataceae). Nonetheless, no formal attempt has been made to establish its relevance as a model system or indeed provide evidence of the role of frugivores as Miconia seed dispersers.
Methods
We built three Miconia databases (fruit phenology/diaspore traits, fruit–frugivore interactions and effects on seed germination after gut passage) to determine how Miconia fruiting phenology and fruit traits for >350 species interact with and shape patterns of frugivore selection. In addition, we conducted a meta-analysis evaluating the effects of animal gut passage/seed handling on Miconia germination.
Key Results
Miconia produce numerous small berries that enclose numerous tiny seeds within water- and sugar-rich pulps. In addition, coexisting species provide sequential, year long availability of fruits within communities, with many species producing fruits in periods of resource scarcity. From 2396 pairwise interactions, we identified 646 animal frugivore species in five classes, 22 orders and 60 families, including birds, mammals, reptiles, fish and ants that consume Miconia fruits. Endozoochory is the main dispersal mechanism, but gut passage effects on germination were specific to animal clades; birds, monkeys and ants reduced seed germination percentages, while opossums increased it.
Conclusions
The sequential fruiting phenologies and wide taxonomic and functional diversity of animal vectors associated with Miconia fruits underscore the likely keystone role that this plant clade plays in the Neotropics. By producing fruits morphologically and chemically accessible to a variety of animals, Miconia species ensure short- and long-distance seed dispersal and constitute reliable resources that sustain entire frugivore assemblages.
Keywords: Fruiting phenology, germination, Melastomataceae, Miconia, mutualism, seed ecology
‘… I made systematic observations for two years on the various species of melastomaceous trees and shrubs of the genus Miconia, which are a conspicuous element in the secondary vegetation and whose fruits bulk large in the diet of the smaller frugivorous birds such as manakins and tanagers [...] as well as some that are not normally considered to be fruit-eaters’.
D. W. Snow, 1965
INTRODUCTION
Frugivore-mediated seed dispersal constitutes ancient interactions driving plant evolution and diversification worldwide (Lomáscolo et al., 2010; Eriksson, 2016; Brodie, 2017; Onstein et al., 2017; Valenta and Nevo, 2020). These mutualistic interactions directly affect the dynamics of plant communities and are a vital ecosystem function for biodiversity maintenance (García and Martínez, 2012; Valiente-Banuet et al., 2015; Morán-López et al., 2018). The consequences of seed dispersal range from seedling recruitment to carbon storage–release balance in tropical forests (Bello et al., 2015; Peres et al., 2016; Culot et al., 2017). Moreover, fruit–frugivore interactions have direct applications in ecosystem management, and support more effective restoration practices (Cole et al., 2010; Zahawi et al., 2013; Peters et al., 2016; González-Castro et al., 2018). Frugivory and seed dispersal are recognized as important issues spanning ecology, evolution and conservation (Jordano et al., 2011). Nevertheless, the seminal studies that have shaped the theoretical development of fruit–frugivore interactions date back to five decades ago, and were mostly catalysed by the groundbreaking studies of David W. Snow (e.g. Snow, 1965, 1971).
Snow’s landmark studies paved the way for the novel research field of ecology and evolution of fruit–frugivore interactions. His observations on sympatric Miconia species in the tropical forests of Trinidad showed little overlap in fruiting phenology, leading him to propose that the staggered fruiting pattern could have evolved as a strategy to reduce competition for avian seed dispersers (Snow, 1965). Staggered fruiting not only provides a competitive advantage for plants, but is also beneficial for frugivores, as the year-round fruit availability would allow maintenance of their populations (Snow, 1971). The validity of Snow’s proposition regarding staggered phenologies was subject to later debate by some who disputed his ideas because of failure to detect a non-random uniform fruiting sequence in other systems (e.g. Rathcke and Lacey, 1985; Wheelwright, 1985a; van Schaik et al., 1993). However, his pioneering and influential studies highlighted the coevolutionary processes shaping the traits of both fruiting plants and their seed dispersers.
Snow’s ideas also stimulated the publication of seminal papers (e.g. McKey, 1975; Howe and Estabrook, 1977) which set the theoretical background for exploration of this topic in the next decades (Howe, 1993). McKey (1975) proposed that the evolution of plant strategies related to attraction and reward of seed dispersers would fall along an ecological gradient of seed dispersal specialization, with specialist and generalist species at the opposite ends of an adaptive continuum. Specialized plants such as Virola (Myristicaceae), Casearia (Salicaceae) and mistletoes, which produce large, lipid-rich rewards and one or a few large seeds, and have low fecundity and an extended fruiting season, are expected to rely on a small number of more specialized frugivore species acting as highly effective seed dispersers. At the other end of the continuum, Ficus (Moraceae), Cecropia (Urticaceae) and Miconia (Melastomataceae) species are examples of generalized seed dispersal systems, characterized by the production of fruit which are small in size with water- and sugar-rich fruit pulp enclosing many small seeds. These species produce much larger crops, albeit over a shorter fruiting season, and attract large assemblages of less specialized frugivores, with consequential lower seed dispersal effectiveness. The early postulates of the ‘specialized vs. generalized paradigm’ were targets of much criticism due to conceptual problems and limited capacity to explain the observed patterns (Wheelwright and Orians, 1982; Jordano, 1987; Fleming et al., 1993). However, the ‘paradigm’ was important for recognizing that fruit traits and plant phenology may determine the identity of the interacting partners. Why do some plants have a small set of frugivores dispersing their seeds, whereas fruits of other species are consumed by much broader animal assemblages within the same community? This question would lead to the subsequent quest for keystone mutualists in tropical forests (Howe, 1993).
Terborgh (1986) was the first to suggest that a relatively small number of plant species have a disproportionally large role in the maintenance of frugivore communities in tropical forests. Species providing fruit resources during periods of scarcity, especially at the wet and dry season transitions, may sustain a higher diversity of seed dispersers and structure species-rich communities. Therefore, the notion of keystone plant species is linked to widespread resource use by fauna and their significance for the stability of trophic interactions within communities. Since Terborgh’s proposal, the concept of keystone plant resources (KPRs) has been widely discussed, especially regarding their underlying ecological attributes (Lambert and Marshall, 1991; Peres, 2000; Escribano-Avila et al., 2018). Traits supporting identification of KPRs include (1) copious fruit production to sustain a large assemblage of frugivorous animals; (2) fruiting phenology extended through periods of relative food scarcity; (3) low redundancy with other food sources; and (4) small to medium fruit size enabling consumption by a wide diversity of frugivorous animals. The KPR concept is currently largely accepted, and many taxa have been recognized as keystones based on their widespread consumption by fauna; examples include palms (340 frugivore species associated with 126 palm species; Terborgh, 1986; Zona and Henderson, 1989; Muñoz et al., 2019), Ficus trees (1274 bird and mammal frugivores associated with 260 Ficus species worldwide; Lambert and Marshall, 1991; Shanahan et al., 2001), Parkia pods (Peres, 2000), mistletoes (associated frugivores include 66 families within 12 orders of birds and 30 families within ten orders of mammals worldwide; Watson, 2001), some Myrtaceae species (Staggemeier et al., 2017; Escribano-Avila et al., 2018) and melastome berries (Galetti and Stotz, 1996; Maruyama et al., 2013; Escribano-Avila et al., 2018; Messeder et al., 2020).
Decades before the proposal of the KPR concept, Land (1963) reported his pioneer observations on frugivory in the tropics and recognized the importance of Miconia trinervia fruits as a resource for birds in a Guatemalan rain forest. However, Galetti and Stotz (1996) were the first to suggest a Miconia species as a KPR, mostly based on observations of copious fruit production and widespread consumption by a primate and avian frugivores during periods of fruit scarcity in the Atlantic Forest. Later, Maruyama et al. (2013) also highlighted M. chamissois as a KPR in a savannah–swamp–forest habitat mosaic in Central Brazil, as it produced fruits consistently across years during periods of scarcity when most avian frugivores consumed its fruits. More recently, Escribano-Avila et al. (2018) emphasized the role of Miconia species as KPRs in Neotropical fruit–frugivore networks. Although experimental removal of plant species to test their role as keystones are scarce (e.g. Watson and Herring, 2012), Messeder et al. (2020) recently developed an interaction network-based approach to identify KPRs over a broader ecological scale. By recognizing the most central plant species in 38 Neotropical fruit–frugivore interaction networks and by simulating their removal, they were able to compare distinct plant taxa for the effects of their removal on community stability descriptors derived from networks. They concluded that Miconia species were – by far – the top candidates as KPRs for Neotropical avian frugivores. However, no formal attempt has yet been made to address the importance of these plants as keystone resources for frugivores across animal clades.
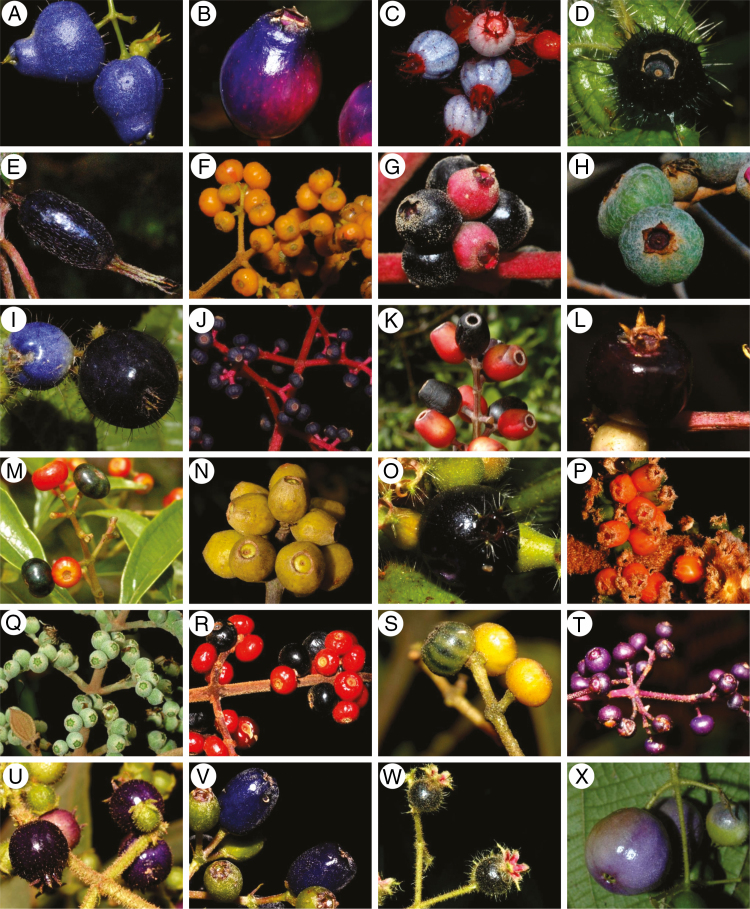
Miconia belongs to the Melastomataceae, a pantropical family with >5000 species in nearly 150 genera (www.melastomataceae.net), with the highest diversity in the Neotropics (approx. 3000 species; Renner et al., 2001; Michelangeli et al., 2013). More than a third of its diversity is within the tribe Miconieae, the largest clade with >1900 species (Michelangeli et al., 2004; Goldenberg et al., 2008). Due to paraphyly in many lineages within the Miconieae (Michelangeli et al., 2019), recent taxonomic and nomenclatural changes recognized Miconia as the only genus within the tribe, making this the most diverse flowering plant genus exclusively distributed in the Neotropics (Michelangeli et al., 2019). Miconia is not only a taxonomically hyperdiverse clade, but it is also a functionally diverse group with a variety of life forms, including lianas, herbs, epiphytes, shrubs, treelets and trees. Miconia is also recognized for producing berries with a variety of shapes, sizes and colours (Fig. 1). The geographic distribution of Miconia spans the entire Neotropical realm, with species ranging from microendemics to those with widespread distribution. Miconia species are usually pioneers and can be abundant in several plant physiognomies, and occur from sea level to highlands mostly in the mainland and even on distant islands such as the Galapagos (Michelangeli et al., 2013; Silveira et al., 2013a). Despite the historical importance of Miconia to our current understanding of the ecology of fruit–frugivore mutualisms (Land, 1963; Snow, 1965), no study to date has summarized the major findings on the seed dispersal ecology of these plants.
Fig. 1.
Diversity in shapes, sizes and colours of Miconia fruits. (A) M. capilliflora; (B) M. subciliata; (C) M. blepharodes; (D) M. macrosperma; (E) M. loligomorpha; (F) Miconia sp.; (G) M. calvescens; (H) M. albicans; (I) M. leacordifolia; (J) M. affinis; (K) M. gratissima; (L) M. dolichostachya; (M) M. sellowiana; (N) M. ampla; (O) Miconia sp.; (P) M. candelabriforme; (Q) M. heliotropoides; (R) M. impetiolaris; (S) M. rimalis; (T) M. minutiflora; (U) M. dasytricha; (V) M. oxymeris; (W) M. neourceolata; (X) Miconia sp. (All pictures were kindly provided by Renato Goldenberg.)
In this study, we synthesize the published information on frugivory and seed dispersal of Miconia species in order to produce an integrative review that highlights the role of its fruits as KPRs for Neotropical fauna. Our study had three major goals. First, we aimed at compiling data on fruit traits and fruiting phenology of Miconia species relevant to their keystone role. Second, we integrated the scattered published information on frugivory of Miconia species to quantitatively assess the number of animal taxa consuming their fruits. The effectiveness of animals as seed dispersers is determined by their handling behaviour, ingestion and deposition patterns which directly affect seedling establishment (Schupp et al., 2010). Because the quality of seed dispersal services is intimately associated with the capacity of handled or ingested seeds to germinate (Traveset et al., 2007), our third goal was to quantitatively evaluate the effects of consumption by different animal clades on the germination of Miconia species. Through a meta-analytical approach, we compared the consequences for Miconia seed germination of fruit manipulation by ants and vertebrate gut passage of seeds across a broad range of frugivore groups.
MATERIALS AND METHODS
Phenology and fruit and seed traits
To produce a comprehensive database of dispersal-relevant fruit and seed traits and phenological patterns of Miconia species, we performed a systematic literature review of papers published from 1945 to 2017 in the Web of Science, Scopus and SciELO databases, using the following terms and their combinations in the title, abstract and keywords: ‘frugivory’, ‘fruit chemical content’, ‘fruit dispersal syndromes’, ‘fruit trait’, ‘fruiting phenology’, ‘Neotropics’ and ‘seed dispersal’. In addition, we checked for fruit/seed trait information on the Kew Seed Information Database (http://data.kew.org/sid/), TRY Plant Trait Database (Kattge et al., 2011; https://www.try-db.org/TryWeb/Home.php), FRUBASE data set (Jordano, 1995) and the ATLANTIC-FRUGIVORY data set (Bello et al., 2017) to complement our database.
To be included in our database, studies must have reported at least one of the following traits – life form, duration of fruiting season (months with availability of ripe fruit), ripe fruit colour, fruit size (diameter in centimetres) and weight (fresh fruit mass in milligrams), fruit crop size (average number of fruit estimated for the individual), seed size (largest dimension in centimetres) and weight (in milligrams), number of seeds per fruit and fruit nutritional content (percentage of water, sugar, lipids and proteins on a centesimal composition basis) – for any species currently recognized as Miconia (sensuMichelangeli et al., 2019). This included species previously distributed in 17 genera within the Miconieae (i.e. Anaectocalyx, Calycogonium, Catocoryne, Charianthus, Clidemia, Conostegia, Killipia, Leandra, Maieta, Mecranium, Necramium, Ossaea, Pachyanthus, Pleiochiton, Sagraea, Tetrazygia and Tococa; all species names were checked and updated using the MEL names database at http://www.melastomataceae.net/ and Michelangeli et al., 2019). We also gathered information on IUCN conservation status, if available (the target year for IUCN listing was 2017; http://www.iucnredlist.org).
To envisage fruiting phenology patterns, we constructed phenological line plots for each sampled community where interactions among at least three sympatric fruiting Miconia species and frugivores were recorded. Production of berries is costly, requiring allocation of high amounts of nutrients and water for their development and maintenance (Coombe, 1976; Fenner, 1998; Jordano, 2014). Consequently, water availability is regarded as one of the main factors constraining the number of fruiting species and the length of the fruiting period in a community (van Schaik et al., 1993; Fenner, 1998; Mendonza et al., 2017). For these reasons, the dry season is a period of food scarcity for frugivores (Terborgh, 1986; Peres, 2000; Jordano, 2014), when a lower proportion of fleshy fruit is available to sustain frugivores across different ecosystems (Hilty, 1980; Peres, 2000; Batalha and Martins, 2004; Jordano, 2014; Brito et al., 2017a; Staggemeier et al., 2017; Maruyama et al., 2019). To investigate whether Miconia species have phenological patterns related to seasonality, for each community we also retrieved its geographical location and average monthly historical precipitation (mm) from 1901 to 2018 (data obtained from Harris et al., 2020), which was plotted within the community phenology plots to evaluate the relationship between precipitation and phenology. For each community, we also calculated the seasonality index (SI), as the sum of the absolute deviations of mean monthly rainfall from the overall monthly mean divided by the mean annual rainfall (for detailed information on the calculation, see Walsh and Lawler, 1981). In theory, the SI can vary from 0 (precipitation equally distributed throughout the year) to 1.83 (precipitation restricted to a single month; Walsh and Lawler, 1981). We classified each community as seasonal or non-seasonal, and evaluated the number of fruiting species and their fruiting patterns.
In addition, to evaluate fruiting phenological patterns in Miconia at a large spatial scale, we performed circular analysis with the phenological data using the R package ‘Circular’ (Lund et al., 2017). We then calculated the circular standard deviation, the circular mean (µ) and the length of the mean vector (r) which informs how the data are clustered around the mean (with 0 meaning uniformly distributed and 1 meaning perfectly clustered). We then performed Rayleigh’s test of uniformity (at α = 0.05), which indicates unimodal distribution and significant seasonality in fruiting patterns (Morellato et al., 2010).
Fruit consumption by fauna
To produce a comprehensive database of frugivore interactions with Miconia fruits, we performed a systematic literature review using ‘ecological networks’, ‘fruit diet’, ‘fruit–frugivore’, ‘frugivore community’, ‘frugivory’, ‘Neotropics’, ‘plant seed disperser interaction’ and ‘seed dispersal’ as keywords in electronic databases. We also checked the Interaction Web Database (www.nceas.ucsb.edu/interactionweb/), the Web of Life Ecological Networks Database (http://www.web-of-life.es/) and the ATLANTIC-FRUGIVORY data set (Bello et al., 2017) to complement our database. Finally, we searched the references listed in the studies surveyed to ensure that we did not miss any interactions between frugivores and Miconia fruits.
To be included in our database, studies must have reported at least one event of frugivory of any Miconia species. Our database consists of data generated by different methods to record the consumption of Miconia fruits, including focal plant observations, diet studies reporting seed presence in faecal samples, regurgitation samples and stomach contents, and direct foraging observations. We recorded taxonomy and IUCN status for all animal species interacting with Miconia. For each interaction reported, we recorded geographical co-ordinates and elevation, and classified the ecoregion according to Olson et al. (2001). To estimate the richness of frugivores consuming Miconia fruits, we constructed rarefaction curves for animal species belonging to distinct taxonomic groups using Miconia species as samples in the R package ‘vegan’ (Oksanen et al., 2019). To determine the average number of seed dispersers of Miconia species, we considered only focal plant studies (in our database this method was used only by studies dealing with avian seed dispersers). The same approach was used to determine the average number of ant species attracted to Miconia fruits in studies of fruit removal by ants. We also investigated whether different animal taxonomic groups have fruit colour preferences by partitioning their consumption records according to different fruit colours.
Meta-analysis assessing effects of gut passage on Miconia seed germination
Investigation of how fruit handling (i.e. manipulation by invertebrates and vertebrate gut passage) by a wide range of taxa affects seed germination patterns is especially relevant for comparing the effect of services provided by different groups on the fate of dispersed seeds. We used a meta-analytical approach (Hillebrand and Gurevitch, 2014) to determine the overall magnitude of the effect of animal manipulation/gut passage on the percentage and speed of seed germination. We then partitioned the magnitude of these effects by animal taxonomic group.
To build a germination database, we searched for the following terms and combinations: ‘ant seed germination’, ‘frugivore gut effect’, ‘gut passage effect’, ‘Neotropics’, ‘vertebrate gut passage’, ‘seed fate’ and ‘seed germination’. We also checked the reference list of the studies surveyed to supplement our literature survey. To be included in our database, a study must have met the following criteria: (1) reported data as germination proportion (or percentage) of manipulated/gut-passed and control treatments (manually extracted seeds from fruits); and (2) reported a measure of germination kinetics (speed of or time to germination, which we converted to days whenever needed) for both treatment and control seeds. Whenever the data were unclear, or not clearly reported in the results or supplementary materials, we contacted the corresponding author to ask for permission to use the original data. In some cases, we were not able to determine the exact sample sizes (the number of replicates and seeds set to germinate in each replicate), so we instead used the values given for total seed sample size. When data were available only in figures, we digitized them and extracted the data using ImageJ software (Abramoff et al., 2004).
First, we provided information on the overall percentage of seed germinated in different experimental groups (Samuels and Levey, 2005). We plotted the germination percentage of each plant species reported in each study according to control I (seeds germinating within intact fruits), control II (manually extracted seeds from fruits) and treatment, according to handling by each animal group. For the four vertebrate orders (Aves, Primates, Didelphimorphia and Rodentia) included in our review, we considered gut passage as the treatment, while for ants (Formicidae) we considered seed cleaning (depulping) as the treatment. We then conducted separate meta-analyses for different aspects of germination performance: germination percentage, days to germination of the first seed and mean germination time for total germination (Traveset et al., 2007). As most of the studies did not consider seed germination within fruits (control I) as an additional control treatment, we only included manually extracted seeds from fruits (control II) as the control group (see Samuels and Levey, 2005). We investigated the total effect of animal fruit handling/gut passage on germination performance and moderated further analyses by taxonomic categories (class, order and family) to explore specific germination outcomes.
We used the unweighted log response ratio (Hedges et al., 1999) to summarize gut passage effects of vertebrate and ant manipulation on seed germination of Miconia species. We chose this metric because most data from the literature lacked the information needed to calculate standard deviations of individual effect size estimates. The 95 % confidence intervals (CIs) around the effect size were calculated, and estimates of the effect sizes were considered significant only when the CIs did not overlap zero (Hedges et al., 1999).
The response ratio is the ratio of some measured quantity in experimental vs. control treatments and is commonly used as a measure of experimental effects because it quantifies the proportional change that results from experimental manipulation (Hedges et al., 1999). We calculated the natural log of the response ratio for each effect studied (Hedges et al., 1999) as
Effects were reported as the proportional change from control groups (seeds manually extracted from fruits). Negative percentage changes indicate decreases in seed germination percentage and/or germination time of ingested/manipulated seeds compared with control groups, and positive values indicate an increase in the effect measured due to passage through vertebrate gut or ant manipulation (Rosenberg et al., 2000). To estimate the cumulative effect size (E++) for a sample of studies addressing the same effect, effect sizes were combined across studies using an unweighted randomization test (Rosenberg et al., 2000). All analyses were conducted using Open MEE (Wallace et al., 2017).
By using meta-analysis, it is possible to split the variance within groups and to evaluate whether or not categorical groups (in our study taxonomic groups) are homogeneous by using heterogeneity analysis (Q; Gurevitch and Hedges, 1999). We calculated total heterogeneity (QT) and heterogeneity within (QW) and between groups (QB). We used a χ 2 distribution to assess whether or not the value of Q was significant. Fail-safe numbers were calculated for each effect tested, indicating how many non-significant, unpublished or missing studies would need to be added to the sample to change the results from significant to non-significant (α = 0.05; Rosenberg et al., 2000). Studies that show large and significant effects are more likely to be published than studies that show weak or no effects (Rosenthal, 1979). As a rule of thumb, results are considered robust when the fail-safe number exceeds 5n + 10, where n is the number of comparisons (Møller and Jennions, 2001). To evaluate publication bias, we used funnel plots as a graphical method, where a symmetrical funnel shape is obtained in the absence of bias when the effect size of each study is plotted against sample size.
RESULTS
Traits of Miconia diaspores
Our trait database included at least one trait datum for 357 species derived from 126 papers (Supplementary data Appendix 1). Data on life form were available for 68.3 % of the species, followed by fruit diameter (60.7 %), ripe fruit colour (51.8 %), fruiting season (46.7 %), seed size (35 %), seeds per fruit (24.3 %) and seed weight (20.7 %). Other traits were less commonly reported (<12 %). The IUCN conservation status was available only for five species: M. robinsoniana and M. quadrangularis (both endangered) and M. amoena, M. paucidens and M. rimalis (least concern).
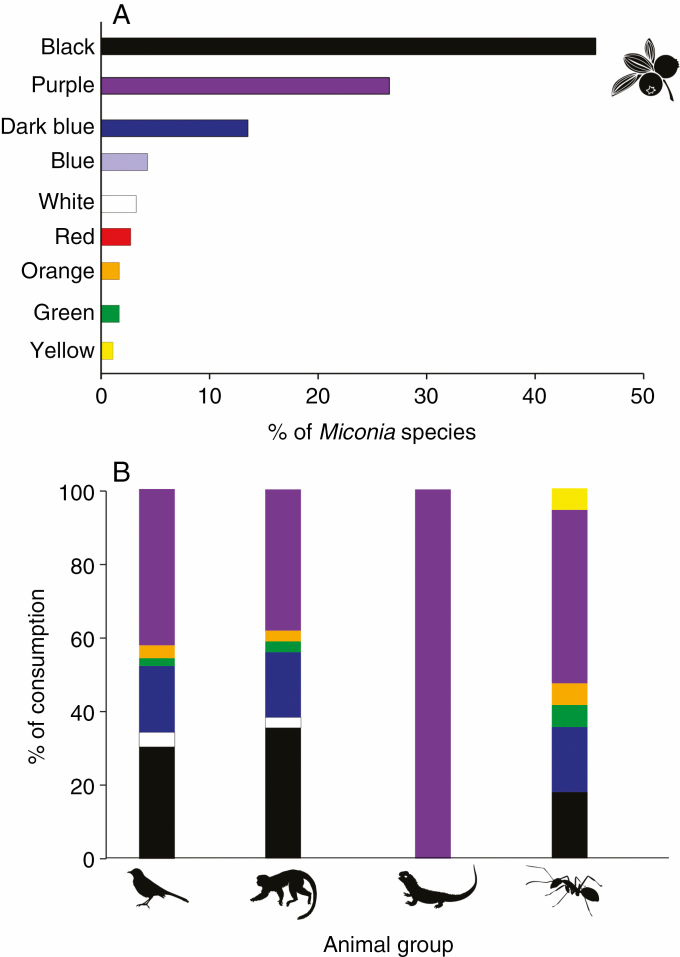
Miconia species showed a variety of life forms, with shrubs (160 species) dominating, followed by trees (49), shrubs/trees (16; dual life form depending on the habitat), treelets (16), lianas (4) and herbs (4). Most fruits were small berries [diameter 0.57 ± 0.01 cm (mean ± s.e.), range 0.2–1.76 cm] with many tiny seeds (seed size: 0.23 ± 0.02 cm, range 0.002–1.3 cm; seeds/fruit: 106.92 ± 19.01, range 1.44–1005.2; Table 1; Supplementary data Appendix 5, Fig. S1A–C). Water was the predominant component in fruit pulp, with sugars (mainly glucose and fructose) figuring as the most important reward, followed by smaller amounts of lipids and proteins (Table 1). Ripe fruit colour in Miconia varied, but 72.3 % of the species exhibited black and purple berries (Fig. 2A). Other colours, in decreasing order of frequency, were dark blue, blue, white, red, orange, green and yellow (Fig. 2A).
Table 1.
Fruit and seed traits and fleshy pulp nutritional contents of Miconia species.
| Mean | Median | s.e. | Range | n | |
|---|---|---|---|---|---|
| Morphological | |||||
| Fruit diameter (cm) | 0.57 | 0.54 | 0.01 | 0.2–1.76 | 217 |
| Fruit weight (mg) | 166.15 | 100 | 35.36 | 10–1300 | 39 |
| Seed size (cm) | 0.23 | 0.1 | 0.02 | 0.002–1.3 | 125 |
| Seed weight (mg) | 4.89 | 0.093 | 2.37 | 0.002–150 | 74 |
| Seeds per fruit | 106.92 | 33.4 | 19.01 | 1.44–1005.2 | 87 |
| Fruit crop size | 11 817.09 | 1358 | 5099.24 | 74.3–168 696 | 41 |
| Fleshy pulp chemical composition (%) | |||||
| Water | 80.93 | 79.86 | 2.01 | 70.05–93.7 | 12 |
| Lipids | 1.43 | 0.65 | 0.46 | 0.04–5.98 | 14 |
| Protein | 2.34 | 1.55 | 0.59 | 0.045–8 | 14 |
| Total non-structural carbohydrates | 13.04 | 8.74 | 2.58 | 0.67–59.22 | 28 |
| Sugar composition | |||||
| Fructose (%) | 44.25 | 46 | 4.43 | 1–62 | 12 |
| Glucose (%) | 48.54 | 48 | 1.97 | 37–56 | 11 |
| Sucrose (%) | 7.25 | 3 | 4.01 | 1–51 | 12 |
Values for chemical composition were obtained in a centesimal composition basis (s.e. = standard error, n = sample size).
Fig. 2.
Percentage of Miconia species with corresponding ripe fruit colour (n = 185 species) (A) and partitioned consumption by different animal groups according to Miconia ripe fruit colours; animal icons corresponds to the animal clades Aves (n = 90 Miconia species), Mammalia (n = 34), Reptilia (n = 3) and Insecta (Formicidae; n = 17) (B).
Fruit production and phenology
Average crop size ranged from 74.3 fruits per plant in M. auricoma to 168 696 fruits per plant in M. rubiginosa. However, 56 % of the 41 species with estimated average crop size produced fruit very copiously, usually exceeding 1000 fruits per plant (Table 1; Supplementary data Appendix 5, Fig. S1D). Data on fruiting phenology were available for 167 Miconia species, and showed a large diversity of fruiting patterns. Most species fruited once a year, 11 species twice a year and M. rufescens three times a year (Supplementary data Appendix 4, Table S1). Average duration of the fruiting period was 6.5 ± 0.27 months, ranging from a single month to year-round fruit production (29 species) (Supplementary data, Appendix 4, Table S1).
At the community level, sympatric Miconia species provided food for frugivores during virtually the whole year across several ecoregions, regardless of the rainfall regime, and exhibited a highly staggered fruiting pattern (Supplementary data Appendix 5, Fig. S2). According to the SI, 54 % of the communities (n = 35) were classified as seasonal (SI ≥0.4) (Supplementary data Appendix 4, Table S2). The seasonal communities had on average 4.5 ± 0.9 fruiting species, with around 50 % of these (2.33 ± 0.28 species) fruiting during or comprising the dry season, and 14 communities had on average 1.5 ± 0.13 species with long fruiting periods (≥7 months). Only three communities had one species each with year-round fruit production (Supplementary data Appendix 4, Table S2). Non-seasonal communities, on the other hand, had on average 6.6 ± 1.1 fruiting species, and all communities, except one, had on average 2.86 ± 0.57 species with long fruiting periods, and around half of them (1.45 ± 0.28 species) with year-round fruit production (Supplementary data Appendix 4, Table S2). Overall, the large-scale pattern of the Miconia fruiting season indicated no phenological peak during the year, with Miconia species fruiting every month in many ecoregions across the Neotropics (Supplementarydata Appendix 5, Fig. S3).
Fruit consumption by fauna
Our literature survey found 182 studies reporting Miconia fruit consumption and handling by animals, including both vertebrates and ants (Supplementary data Appendix 2), that yielded a total of 2396 pairwise interactions. A third of the pairwise interaction records came from community-wide studies, 29 % from species-focused studies and 28 % from dietary studies (other type of studies accounted for <7 % of the recorded interactions).We recorded frugivory events for 243 Miconia species, 78 (32 %) of which were not fully identified to the species level. Fruit–frugivore interactions were found in 47 ecoregions across latitudinal and elevational gradients, with elevation ranging from zero to 4200 m a.s.l., and extending from 19°31′N to 26°15′S (Supplementary data Appendix 5, Fig. S4; Appendix 4, Table S3).
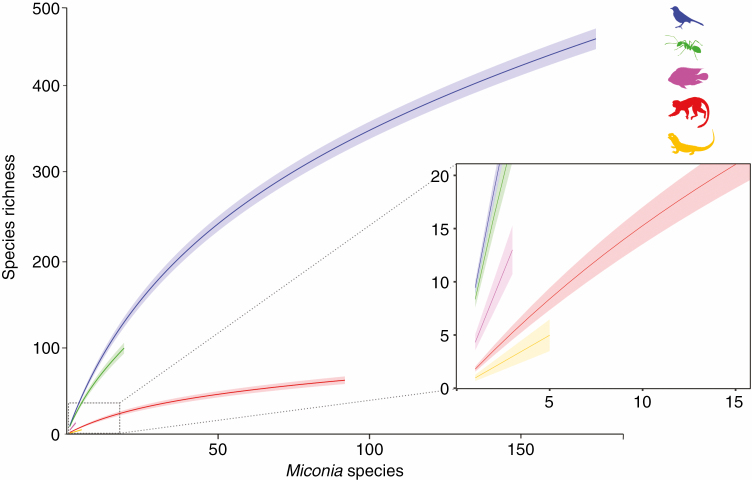
Pairwise interactions were recorded between 243 Miconia species and 646 animal species distributed across five taxonomic classes: Aves, Mammalia, Actinopterygii, Reptilia and Insecta (Fig. 3; Supplementary data Appendix 4, Table S4). The shape and steepness of the rarefaction curves indicated that the diversity of frugivores associated with Miconia fruits was underestimated (Fig. 3). Birds were the most diverse group consuming Miconia fruits, representing 73 % of total animal interactions. Within Aves, frugivore diversity was distributed across ten orders and 37 families, with the Passeriformes standing out as the most diverse order (384 species representing >80 % of the within-Aves diversity) and the Thraupidae as the most diverse family (117 species) (Supplementary data Appendix 4, Table S4). Ants were ranked second for species interacting with Miconia fruits, with 99 species unevenly distributed amongst six subfamilies, with the Myrmicinae representing 75 % of total diversity (Supplementary dataAppendix 4, Table S4). Sixty-one species of mammals consumed Miconia fruits; these were distributed in seven orders and 13 families, including Primates (41.3 %), Chiroptera (17.5 %), Didelphimorphia (17.5 %), Rodentia (14.3 %), Carnivora (4.8 %), Perissodactyla (3.17 %) and Cetartiodactyla (1.6 %) (Supplementary data Appendix 4, Table S4).
Fig. 3.
Species rarefaction curves describing the number of animal species of different clades increasing in function of Miconia species with reports of fruit consumption. Lines represent means and shaded areas the 95 % confidence interval. Blue = Aves; green = Formicidae; pink = Actinopterygii; red = Mammalia; yellow = Reptilia.
We also found reports of 13 fish species in five families consuming Miconia fruits (Supplementary data Appendix 4, Table S4). Reptiles were represented by only five species from the families Iguanidae and Teiidae (Squamata) and Geoemydidae and Testudinidae (Testudines) (Supplementary data Appendix 4, Table S4). IUCN conservation status information was available for nearly 78 % of animal species consuming Miconia fruits, with 36 species (distributed within three classes, 11 orders and 20 families) listed as threatened with extinction to some extent (Supplementary data Appendix 4, Table S5).
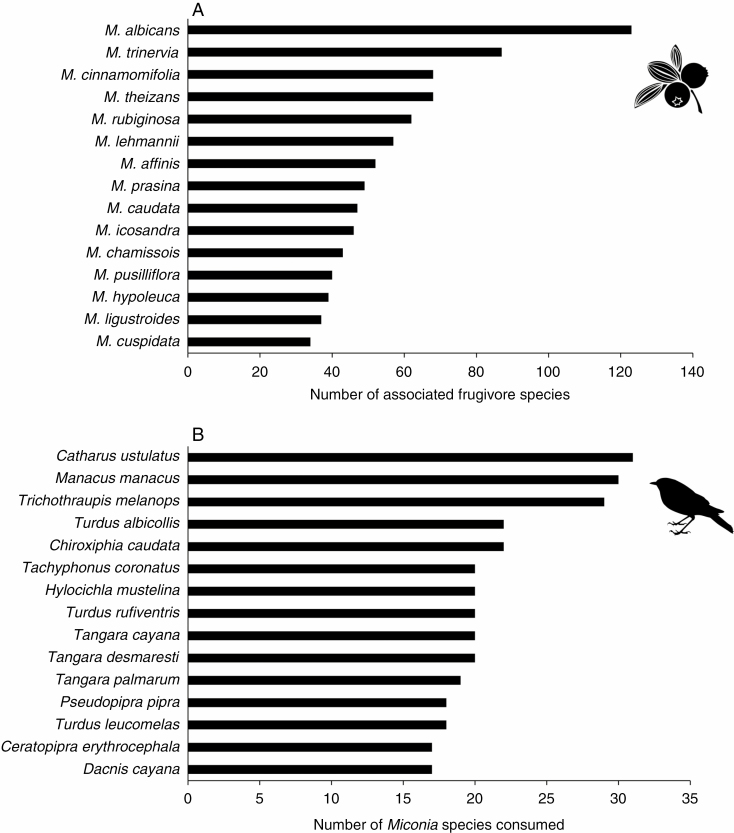
According to 21 plant-focal studies assessing frugivory, each Miconia species had an average of 16.5 ± 1.4 avian seed dispersers (n = 37). According to ant fruit removal studies (14 studies), Miconia fruits attracted on average 6 ± 1 ant species (n = 28). Miconia albicans attracted the most diverse assemblage of frugivores, with records of consumption by at least 122 animal species (Fig. 4A). The passerine bird Catharus ustulatus (Turdidae) consumed the highest number of Miconia species (32 species; Fig. 4B). In the case of plant life form, shrubs were associated with the highest number of frugivore species (401 species), followed by trees (328).
Fig. 4.
Top 15 Miconia species consumed by Neotropical frugivores (A) and top 15 animal species consuming Miconia fruits (B).
Birds and mammals consumed Miconia fruits regardless of their colour, except for yellow fruits, which were consumed exclusively by ants (Fig. 2B). Reptiles only consumed purple fruits, although this group accounted for a small proportion of total fruit consumption (Fig. 2B).
Effects of vertebrate gut passage and ant manipulation on seed germination
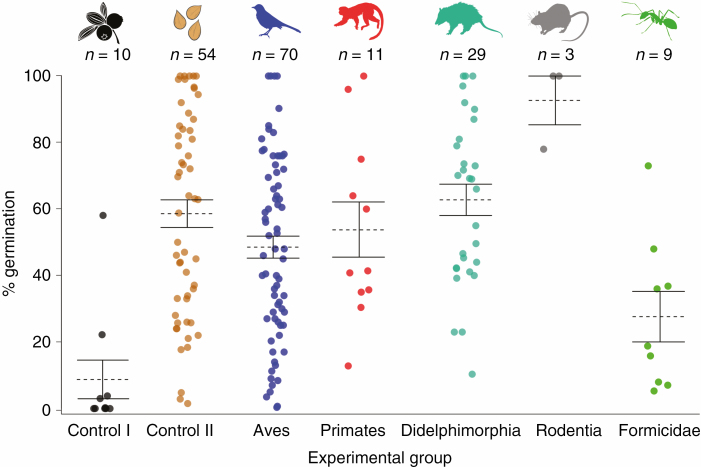
Our literature survey found 25 published studies addressing the effect of vertebrate gut passage or ant manipulation on seed germination that met our inclusion criteria (Supplementary data Appendix 3). Germination data were reported for 35 Miconia species and generated 207 outcomes: 121 for germination percentage, 78 for germination time and 8 for days until first germination. With the exception of ants, most animal groups seem to substantially increase germination percentage relative to seeds germinating within fruits (control I), with no apparent differences compared with manually extracted seeds (control II; Fig. 5).
Fig. 5.
Percentage of seed germination in Miconia species across different experimental treatments. Control I, seeds within fruits; control II, manually extracted seeds; Aves; Primates; Didelphimorphia; Rodentia; Formicidae. Dots are the independent germination percentage of each plant species reported in each study according to the experimental group. Dashed lines represent means, and whiskers the standard error. n is the number of observations for each experimental group.
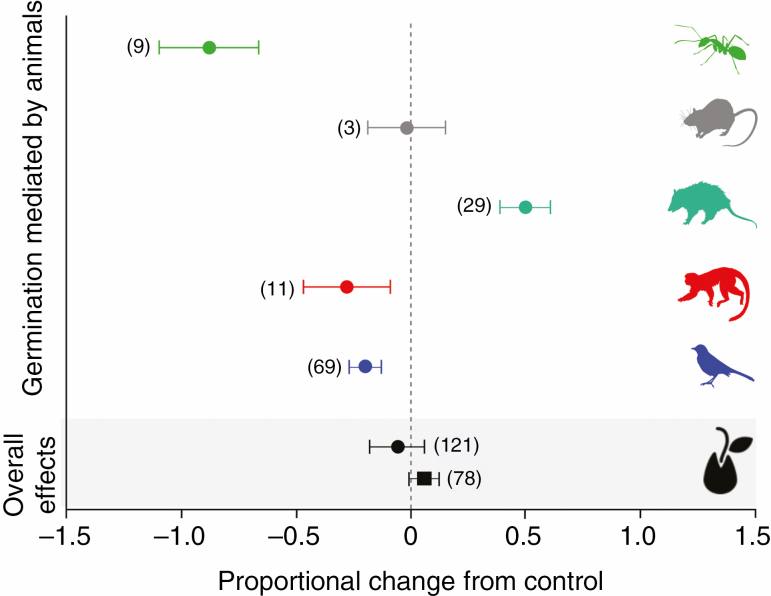
Overall, we found a non-significant effect of gut passage on germination percentage (E++ = –0.06, 95 % CI = –0.1435 to 0.0217), days to first seed germination (E++ = 0.10, 95 % CI = –0.2270 to 0.4198) and mean germination time (E++ = 0.05, 95 % CI = –0.0071 to 0.1169; Fig. 6). However, when effects were moderated by taxonomic class (QB = 60.63, P ≤ 0.001), gut passage had significant effects on Miconia seed germination.
Fig. 6.
Effect sizes of vertebrate gut passage and fruit handling by ants on germination performance. Overall effects are for germination percentage (circle) and mean germination time (square). Animal groups mediating germination are Formicidae, Rodentia, Didelphimorphia, Primates and Aves (from top to bottom). The cumulative effect size is reported with its 95 % confidence interval. Numbers in parentheses indicate the number of outcomes for each effect. Effects are significant if the confidence intervals do not overlap zero.
Unexpectedly, passage through the gut of bird species significantly reduced germination percentage by 15 % (E++ = –0.15, 95 % CI = –0.2618 to –0.0504) and diaspore handling by ants reduced seed germination by 90 % (E++ = –0.90, 95 % CI = –1.2369 to –0.5669; Fig. 6). Conversely, gut passage in mammals significantly increased germination percentage by 26 % (E++ = 0.26, 95 % CI = 0.1288 to 0.3956; Fig. 6). Birds (E++ = 0.07, 95 % CI = –0.0017 to 0.1542), mammals (E++ = 0.07, 95 % CI = –0.0450 to 0.1917) and ants (E++ = –0.34, 95 % CI = –0.7899 to 0.1084) had no significant effect on germination time. We could not compare the effects between different animal classes on days to first seed germination due to small sample sizes.
Among birds, gut passage in the Passeriformes resulted in a significant reduction in germination percentage by 20 % (E++ = –0.20, 95 % CI = –0.2761 to –0.1279), whereas it was non-significant for the Galliformes (E++ = 1.32, 95 % CI = –1.6024 to 4.2404). To evaluate the gut passage effect of passerine birds on germination percentage, we partitioned the variance among bird families (Supplementary data Appendix 4, Table S5). The overall trend was a reduction in germination percentage. Species within the Turdidae and Thraupidae caused a significant reduction of 27 % (E++ = –0.27, 95 % CI = –0.4209 to –0.1173) and 17 % (E++ = –0.17, 95 % CI = –0.2895 to –0.0494), respectively (Supplementary data Appendix 4, Table S6).
For mammals, we found contrasting within-class effects of gut passage on seed germination. Primates significantly reduced germination percentage by 28 % (E++ = –0.28, 95 % CI = –0.4879 to –0.0799), whereas other non-flying mammals (i.e. Didelphimorphia and Rodentia grouped together) significantly increased it by 45 % (E++ = 0.45, 95 % CI = 0.3406 to 0.5595). Both primates (E++ = 0.19, 95 % CI = –0.0321 to 0.4103) and non-flying mammals (E++ = –0.00, 95 % CI = –0.1659 to 0.1648) had no significant effect on mean germination time. Gut passage in didelphimorphs increased germination percentage by 50 % (E++ = 0.50, 95 % CI = 0.3889 to 0.6102), but the effects were not significant for rodents (E++ = –0.02, 95 % CI = –0.7512 to 0.6939; Fig. 6).
Fail-safe numbers for the effects of vertebrate gut passage and ant fruit manipulation on seed germination percentage and mean germination time were small relative to the number of outcomes included in the meta-analysis, indicating that our results should be interpreted with caution and we should avoid generalizations. The scatter plots of effect size against sample sizes of pooled data showed a classical funnel shape (Supplementary data Appendix 5, Fig. S5), indicating that studies with small sample sizes had a larger dispersion of effect size around the true effect, whereas studies with large sample sizes had an effect size closer to the true value.
DISCUSSION
Our results support the idea of Miconia species as KPRs (Escribano-Avila et al., 2018; Messeder et al., 2020) for most fruit-eating vertebrates and ant lineages. We showed that Miconia frugivory and seed dispersal involve a functionally diverse range of animals, including mostly terrestrial but also aquatic species, varying in morphology, body size, behaviour and habitat use. As we reported, Miconia produces large fruit crops year round, especially at times of resource scarcity, and sustain frugivore assemblages, including threatened species, in both forest and non-forest ecosystems.
Diaspore traits and fruiting phenology in Miconia
Most Miconia fruits are blackish/purplish small-sized berries, enclosing numerous small seeds within water- and sugar-rich fleshy pulp – traits typical of ornithochoric species (Snow, 1981; Wheelwright et al., 1984; Stiles and Rosselli, 1993). However, we showed that a much broader spectrum of vertebrates acts as primary seed dispersers of Miconia. The suite of traits of Miconia fruits may largely explain the widespread consumption by Neotropical fauna. Small fruits and seeds are key traits that drive patterns of interaction in fruit–frugivore networks (Burns, 2013; González-Castro et al., 2015; Sebastián-González et al., 2017). Large fruits containing large seeds constrain interactions with small frugivores, whereas plants producing small-sized fruits and seeds allow consumption by animal species with a much wider range of gape sizes, encompassing frugivores across different sizes and taxonomic groups (Wheelwright, 1985b; Levey, 1987; Jordano, 2014; Fuzessy et al., 2018).
Likewise, the high sugar content of the fruit pulp of Miconia berries also contributes to consumption by fauna (Wheelwright et al., 1984; Jordano, 1995; Baker et al., 1998; Galetti et al., 2011). Although lipid-rich fruits are supposedly more rewarding in energetic terms, their digestion is costly, with longer retention times, because lipids need to be emulsified, hydrolysed, absorbed and then metabolized (Witmer and van Soest, 1998; Levey and Martinez-del-Rio, 2001). In contrast, digestion of soluble monosaccharides (Wheelwright et al., 1984; Baker et al., 1998) is much simpler and entails faster absorption favouring greater net energy gains (Karasov and Diamond, 1988; Worthington, 1989; Witmer and van Soest, 1998; Lepczyk et al., 2000). Therefore, it is reasonable to expect that most frugivores are able to digest fruits, such as Miconia, primarily containing non-structural carbohydrates (Baker et al., 1998; Maruyama et al., 2019).
Fruit production and phenology also help to explain the widespread consumption of Miconia fruits. First, we found that even small shrubs or treelets usually produce copious fruits, with crop sizes often exceeding thousands of fruits per plant (Levey, 1990; Blendinger et al., 2008; Christianini and Oliveira, 2009; Kessler-Rios and Kattan, 2012; Guerra et al., 2017; Santos et al., 2017), but large trees produce up to 160 000 fruits (Christianini and Oliveira, 2009). Second, Miconia species are abundant in plant communities, leading to high fruit density in habitat patches where they occur (Levey, 1990; Blendinger et al., 2008; Christianini and Oliveira, 2009; Maruyama et al., 2013; Guerra et al., 2017). Third, as we have shown, the fruiting phenology of most Miconia species extends on average over 6 months, with year-round fruiting patterns common in species in non-seasonal habitats (Levey, 1988; Poulin et al., 1999) where water is less limited (Morellato et al., 2000).
Our investigation shows that Miconia species commonly produce fruits during the dry season in several seasonal habitats, making them a reliable food source during periods of food scarcity (Rathcke and Lacey, 1985; Terborgh, 1986; Peres, 2000; Jordano, 2014; Mendonza et al., 2017; Maruyama et al., 2019). Year-round fruit availability at the community level entails a higher probability of interaction (Jordano et al., 2003; Olesen et al., 2011), for both resident and migratory species, ensures seed dispersal services and helps to maintain biodiversity (Messeder et al., 2020).
Current evidence also indicates that Miconia species produce fruits consistently between years (Christianini and Oliveira, 2009; Kessler-Rios and Kattan, 2012; Maruyama et al., 2013; Brito et al., 2017a). With the phenological patterns identified in our study, we emphasize the role of Miconia in providing consistent and reliable food resources to frugivores over the years, as well as being one of the plant taxa most likely to sustain many animal species during periods of food scarcity in seasonal ecosystems throughout the Neotropics. The combination of fruit traits and fruiting phenology is similar to those identified for other KPRs such as Ficus and Cecropia (Terborgh, 1986; Lambert and Marshall, 1991; Shanahan et al., 2001), and largely explains the relevance of Miconia species to Neotropical frugivores (Messeder et al., 2020).
Frugivores associated with Miconia fruits
Miconia fruits are consumed by taxonomically and functionally highly diverse vertebrates across all lineages, except amphibians (Table 2; Supplementary data Appendix 4, Table S4). Remarkably, bird species accounted for >70 % of recorded interactions, with >460 species, encompassing a wide range of lineages, body sizes and functional groups. Although our study represents <10 % of Miconia diversity, we have shown that Miconia fruits are indeed a significant food source for 13 % of all Neotropical bird species and for >70 % of the species classified as primarily frugivorous (Kissling et al., 2009, 2012).
Table 2.
A summary of the main seed dispersal agent clades of Miconia species (based on the number of interaction records and species diversity) of each animal class and their preferential fruit traits
| Frugivore clade | Species | Interactions | Fruit colour (%) | Crop size | Fruit size | Seeds/fruit | %Water | %Carbohydrates | %Lipids | %Protein | Key references |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aves Passeriformes Thraupidae |
117 | 744 | Purple (44 %); black (25.23 %); dark blue (13.88 %); green (7.09 %); blue (6.15 %); orange (1.57 %); red (1.26 %); white (0.78 %) | 16 450.4 (26) | 0.62 (45) | 105.97 (35) | 80.06 (8) | 16.04 (16) | 1.27 (11) | 2.88 (11) | Silva et al. (2002); Parrini and Pacheco (2011); Vidal et al. (2014); Palacio et al. (2016) |
| Aves Passeriformes Tyrannidae |
76 | 235 | Purple (50.47 %); green (17.61 %); black (16.66 %); dark blue (12.38 %); orange (1.90 %); red (0.47 %); blue (0.47 %) | 24 486.75 (16) | 0.64 (28) | 36.46 (22) | 80.06 (8) | 12.90 (15) | 1.04 (9) | 2.21 (8) | Luck and Daily (2003); Maruyama et al. (2013); Camargo et al. (2015) |
| Aves Passeriformes Pipridae |
26 | 237 | Purple (38.70 %); black (27.95 %); dark blue (43.11 %); blue (3.76 %); orange (3.22 %); green (1.61 %); white (1.61 %) | 24 061.86 (18) | 0.58 (39) | 132.57 (31) | 78.64 (6) | 15.89 (17) | 1.24 (8) | 2.66 (8) | Snow (1965); Loiselle and Blake (1990); Gorchov et al. (1995); |
| Aves Passeriformes Turdidae |
26 | 228 | Purple (44.14 %); black (23.40 %); dark blue (17.55 %); blue (6.91 %); green (3.19 %); orange (2.65 %); white (1.59 %); red (0.53 %) | 14 789.39 (24) | 0.63 (41) | 100.15 (28) | 80.06 (8) | 11.99 (19) | 1.23 (10) | 3.11 (10) | Blake and Loiselle (1992); Kessler-Rios and Kattan (2012) |
| Aves Passeriformes Fringilidae |
16 | 61 | Purple (38.18 %); black (30.90 %); dark blue (12.72 %); blue (7.27 %); orange (7.27 %); green (3.63 %) | 12 825.17 (13) | 0.67 (24) | 139.23 (13) | 80.94 (4) | 11.58 (10) | 1.37 (6) | 3.18 (6) | Silva et al. (2002); Vidal et al. (2014) |
| Mammalia Primates Callitrichidae |
12 | 31 | Purple (54.16 %); black (20.83 %); orange (12.5 %); dark blue (8.33 %); green (4.16 %) | 31 921.94 (6) | 0.42 (10) | 74.45 (14) | 77.46 (3) | 30.44 (4) | 1.63 (4) | 2.46 (4) | Dietz et al. (1997); Miranda and Faria (2000); Lapenta et al. (2008) |
| Mammalia Didelphimorphia Didelphidae |
11 | 54 | Black (64.58 %); purple (29.16 %); green (6.25 %) | 2239.66 (5) | 0.60 (12) | 133.32 (11) | 77.19 (2) | 12.73 (2) | 1.32 (2) | 2.88 (2) | Lessa and Costa (2010); de Camargo et al. (2011); Lessa et al. (2013) |
| Mammalia Chiroptera Phyllostomidae |
11 | 18 | Purple (36.36 %); black (27.27 %); dark blue (27.27 %); white (9.09 %) | 3656.66 | 0.7 (6) | 181 (3) | 74.76 | 12.08 | 2.56 | 3.6 | Hernández-Conrique et al. (1997); Hernández-Montero et al. (2015); Lima et al. (2016) |
| Mammalia Perissodactyla Tapiridae |
2 | 21 | Dark blue (66.66 %); black (33.34 %) | NA | 0.60 (3) | 360 | NA | NA | NA | NA | Lizcano and Cavelier (2004); Tobler et al. (2010); Barcelos et al. (2013) |
| Mammalia Primates Cebidae |
4 | 15 | Purple (54.54 %); black (18.18 %); orange (18.18 %); blue (9.09 %) | 12 135.62 (6) | 0.47 (8) | 84.2 (6) | 78 | 15.52 (3) | 2.60 (2) | 4.48 (2) | Wehncke and Dalling (2005); Canale et al. (2016) |
| Insecta Hymenoptera Formicidae Myrmicinae |
74 | 126 | Purple (52.41 %); green (23.38 %); dark blue (12.09 %); black (9.67 %); yellow (2.61 %); orange (0.8 %) | 25 790.45 (8) | 0.58 (14) | 38.59 (15) | 76.27 (4) | 14.16 (8) | 0.99 (5) | 2.47 (5) | Kaspari (1993); Leal and Oliveira (1998); Christianini et al. (2012) |
| Insecta Hymenoptera Formicidae Formicinae |
10 | 21 | Green (38.09 %); purple (33.33 %); black (28.57 %) | 2609.5 (2) | 0.65 (5) | 33.56 (6) | 79.63 | 25.24 (2) | 1.14 (2) | 2.83 (2) | Leite et al. (2013); Lima et al. (2013) |
| Insecta Hymenoptera Formicidae Ponerinae |
7 | 10 | Purple (66.66 %); green (33.34 %) | 85 895.5 (2) | 0.61 (4) | 44.53 (3) | 74.84 (2) | 22.95 (3) | 0.79 (3) | 2.43 (3) | Christianini et al. (2012); Santana et al. (2013) |
| Actinopterygii Characiformes Serrasalmidae |
6 | 6 | NA | NA | NA | NA | NA | NA | NA | NA | Hawes and Peres (2014a) |
| Actinopterygii Characiformes Bryconidae |
3 | 3 | NA | NA | NA | NA | NA | NA | NA | NA | Correa et al. (2016) |
| Reptilia Testudines Geoemydidae |
2 | 2 | Purple (100 %) | NA | 0.55 | 40 | NA | 7.65 | NA | NA | Moll and Jansen (1995) |
Morphological and chemical fruit traits are reported by the mean value and sample size in parentheses.
NA = information not available.
Frugivory has emerged independently several times in the evolutionary history of birds (Fleming and Kress, 2011), with the Passeriformes standing out as the most species-rich order with the highest proportion of frugivorous species (Kissling et al., 2009). Accordingly, we have demonstrated the relevance of Miconia fruits to small-sized passerines, especially tanagers (Thraupidae), flycatchers (Tyrannidae), thrushes (Turdidae) and manakins (Pipridae; Table 2; Supplementary data Appendix 4, Table S4). Thus, coevolution with highly frugivorous passerine bird clades may be a major force driving changes in Miconia fruit traits towards smaller size and purplish pulp rich in water and sugar (Table 2). However, studies on coevolution between fruiting plants and frugivores remain scarce and inconclusive, and it is a topic worthy of future investigation (Valenta and Nevo, 2020).
Although mostly overlooked, the reports gathered here also emphasize the importance of Miconia fruits for a functionally diverse group of mammals, ranging from large terrestrial to small volant and non-volant species. Frugivory is well distributed along mammalian lineages, having evolved independently numerous times (Fleming and Kress, 2011). The major families of Neotropical frugivorous mammals are the Atelidae, Cebidae, Pitheciidae and Aotidae (Primates), Phyllostomidae (Chiroptera), and Echimyidae and Dasyproctidae (Rodentia; Fleming and Kress, 2011). However, we document a much broader diversity of mammals relying on Miconia fruits as a food resource, including some threatened species (Table 2; Supplementary data Appendix 4, Tables S4 and S5).
Primates are by far the most frugivorous clade of mammals, and accordingly are the most diverse group of mammals consuming Miconia. Mutualistic interactions with fruits have played an important role in promoting primate diversification, especially in the Neotropics (Gómez and Verdú, 2012), where most species depend greatly on fruit as part of their overall diet (Hawes and Peres, 2014b). These large-bodied vertebrates are considered generalist frugivores, due to their capacity for consuming a wide variety of fruit, ranging from very small to very large seeded (Fuzessy et al., 2018). We emphasize the importance of the small-seeded Miconia fruits as food resources for many primate species, which rely heavily on fruits of several different Miconia species, especially during periods of resource scarcity (Pavelka and Knopff, 2004; Lapenta et al., 2008; Canale et al., 2016).
Although less common, Miconia fruit consumption by fishes and reptiles is underestimated. We found at least 13 species of fish that consume Miconia fruits (Kubitzki and Ziburski, 1994; Hawes and Peres, 2014a; Correa et al., 2016; Weiss et al., 2016) in the Amazon forest (Supplementary data 4, Table S4) where, once a year, seasonal flooding allows aquatic fauna to access forest interiors and consume diaspores that fall into the water (Kubitzki and Ziburski, 1994). Miconia fruits are also consumed by reptiles, including tortoises (Moll and Jansen, 1995; Guzmán and Stevenson, 2008) and lizards (Swanson, 1950; Magnusson and Sanaiotti, 1987; Guerra et al., 2018). Although documented infrequently, consumption of Miconia fruits by fishes and reptiles is not necessarily rare, simply because diet studies sampling stomach contents rarely provide precise taxonomic identification of plant items.
Despite the scarcity of studies (17 studies) providing data on interactions with Miconia (19 species), ants were ranked as the second most diverse group of consumers of the genus (Supplementary data Appendix 4, Table S4). In fact, the shape of the species accumulation curve indicates that the diversity of ants interacting with Miconia fruits could match that of birds. Miconia species are primarily vertebrate dispersed and lack specialized structures, such as elaiosomes, to mediate ant interactions. Ants are, therefore, important secondary seed dispersers that use both fruit pulp and seeds as food resources (Kaspari, 1993; Christianini et al., 2007; Christianini and Oliveira, 2009; Lima et al., 2013; Guerra et al., 2018). Miconia fruits are consumed by fungi-growing ants (Leal and Oliveira, 1998; Dalling and Wirth, 1998), opportunistic exploiters of fruit pulp (Christianini et al., 2007; Christianini and Oliveira, 2009; Lima et al., 2013) and granivores (Kaspari, 1993; Levey and Byrne, 1993; Guerra et al., 2018). Therefore, our results highlight the overlooked relevance of Miconia fruits and seeds as food resources for Neotropical ants.
Effects of gut passage on germination of Miconia seeds
Endozoochory is the main mechanism of Miconia seed dispersal. Germination of Miconia seeds is light dependent and rarely occurs within fleshy pulp (Silveira et al., 2013a; Santos et al., 2017). Miconia fruits have germination inhibitors; therefore, release of seeds from fleshy pulp is a key service provided by dispersers (Silveira et al., 2013a, b; Ribeiro et al., 2016). Although our results showed that some animal groups caused an overall decrease in seed germination when compared with hand extraction, fruit consumption and seed handling allow a much higher likelihood of germination than situations when seed cleaning does not take place.
Our meta-analysis highlighted that vertebrates and ants may affect Miconia seed germination, but also revealed that the outcomes of gut passage are clade specific. Fruit-eating birds are recognized to enhance seed germination, as passage through their guts generally increases germination by up to 40 % and reduces germination time (Traveset et al., 2001; Traveset and Verdú, 2002; Lehouck et al., 2011). Contrary to this expectation, we found that birds negatively affected seed germination. Passage through the avian gut may reduce resistance to breakage (Traveset et al., 2008) in some Miconia species (Ribeiro et al., 2016). The reduced germination of gut-passed seeds may be related to their small size and thinner coats, which could be damaged during passage through muscular gizzards, or because some species produce physiologically dormant seeds (Silveira et al., 2012a).
Seed germination outcomes were far from general, showing very specific results strongly dependent on frugivore identity. Studies with specialized frugivores have shown positive or neutral influences on Miconia seed germination (Ellison et al., 1993; Gomes et al., 2008; Acosta-Rojas et al., 2012; Silveira et al., 2012b). However, most of the studies in our database evaluated omnivorous birds that included both fruit and insects in their diets (Alves et al., 2008; Gomes et al., 2008; Silveira et al., 2012b). Generalist birds often possess more muscular gizzards, leading to longer retention times and mechanical and chemical abrasion of seeds, consequently decreasing the likelihood of germination (Traveset et al., 2008; Jordano, 2014). However, the frequency of Miconia seeds in faecal samples of some passerines may reach >90 % (Stiles and Rosselli, 1993), whereas the average number of seeds in bird droppings can reach hundreds in some cases (Loiselle and Blake, 1999). Therefore, even with <50 % of seeds germinating after gut passage, birds could still effectively disperse many viable seeds, successfully contributing to Miconia seedling establishment (Ellison et al., 1993; Krijger et al., 1997; Blendinger et al., 2011).
Our meta-analysis showed that diaspore handling by ants considerably reduced germination of Miconia seeds. However, it is important to acknowledge that seeds handled by ants are those that have fallen under mother plants, after being mashed or defecated by birds (Kaspari, 1993; Christianini and Oliveira, 2009; Lima et al., 2013; Guerra et al., 2018). Therefore, most seeds collected and transported by ants have already suffered – to some degree – from pre-dispersal predation and mandibulation, and/or ingestion by primary dispersers. Despite their apparent negative effects on seed germination, several ant species present behaviours that allow their recognition as legitimate dispersers of Miconia seeds, including depulping and transporting intact seeds to sites favourable to establishment (Dalling and Wirth, 1998; Christianini and Oliveira, 2009; Lima et al., 2013). Some ants seem to be attracted mostly to fruit pulp rewards, and transport seeds unintentionally while carrying fruit pulp (Christianini and Oliveira, 2009; Lima et al., 2013; Guerra et al., 2018). Although ants may harm Miconia seeds during handling and reduce their germination potential, their contribution to short-distance seed dispersal must be acknowledged (Christianini and Oliveira, 2009; Lima et al., 2013; Guerra et al., 2018).
Previous meta-analytical studies investigating the gut passage effect on seed germination across a wide variety of animals and plant taxa (Traveset, 1998; Traveset and Verdú, 2002; Traveset et al., 2008; Torres et al., 2020) showed that non-flying small mammals caused the lowest positive effect on seeds (approx. 5 % increase in germination percentage). However, there is emerging evidence that rodents and opossums can play important roles as seed dispersers, with seeds showing positive germination responses after fruit ingestion, especially in small-seeded species (Grenha et al., 2010; Lessa et al., 2013, 2019; Lessa and Geise, 2014; Sahley et al., 2015; Genrich et al., 2017). Our results support the view that small mammals, especially opossums, are important dispersers of Miconia seeds, considerably increasing germination percentages.
The germination percentage of seeds of Miconia fruit consumed by primates decreased by 28 % when compared with that of hand-extracted seeds. Despite this reduction, germination of primate gut-passed seeds was greater than that of seeds enclosed in fruits, suggesting that seed depulping is an important service also provided by primates. Although primates seem to have a restricted direct role in Miconia seed dispersal, they may complement the mostly short-distance services provided by birds and ants (Christianini and Oliveira, 2009; Guerra et al., 2018). The longer transit times and wider home ranges of primarily frugivorous primates could lead to long-distance dispersal of some Miconia seeds (Fuzessy et al., 2017). Although the results of our meta-analysis were highly informative, they must be interpreted with caution. Future experimental germination studies with Miconia species should investigate gut passage effects across a broader range of frugivores.
CONCLUSIONS AND PERSPECTIVES FOR FUTURE STUDIES
Although studies of Miconia species and their dispersers catalysed the early debate on coevolution between frugivores and their plants (Snow, 1971; Stiles and Rosselli, 1993), the role of fruit–frugivore interactions as drivers of diversification and geographic range in this hyperdiverse genus stands as a major knowledge gap (Reginato et al., 2020). Indeed, the evolution and diversification of Miconia fruits remain an enigma, as fleshy fruits have not been found to be a key innovation within the Melastomataceae (Reginato et al., 2020). Therefore, several aspects of the evolutionary ecology of Miconia fruits remain unexplored. Data gathered in our integrative review strongly support previous studies (Escribano-Avila et al., 2018; Messeder et al., 2020) emphasizing the relevance of Miconia species as keystone resources for other animals in addition to birds. By producing large crops of small fruits year round, especially at times of resource scarcity, Miconia species are able to entirely sustain a wide diversity of frugivore assemblages.
Current evidence indicates that selection by frugivores may drive evolution of fruit and seed traits, including size, colour and nutrient content (Janson, 1983; Jordano, 1995; Galetti et al., 2013; Brodie, 2017; Nevo et al., 2018). Nevertheless, studies addressing phenotypic selection of Miconia fruits and seeds by dispersers remain scarce (Camargo et al., 2015), with available information indicating that avian frugivores may select for individual plants with larger crop sizes (Blendinger et al., 2008; Christianini and Oliveira, 2009; Guerra et al., 2017). We argue that the diverse suite of traits exhibited by Miconia fruits and their exposure to different selective pressures exerted by different frugivore functional groups can be a suitable model system for testing coevolutionary hypotheses (Table 2). Although consumption of Miconia fruits by frugivores seems diffuse with no clearly apparent coevolutionary patterns at this time (Table 2), future studies with proper experimental designs may be valuable. For instance, studies combining observational, experimental and phylogenetic comparative approaches should investigate the role of frugivore clades in shaping the evolution and diversification of Miconia species. Can species dispersed by different frugivore clades evolve specific traits? We hypothesize that, given the germination outcomes specific to animal clades, fruit and seed traits could be positively shaped according to the disperser functional group.
While many frugivory and seed dispersal studies have highlighted the importance of Miconia species for sustaining and structuring frugivore assemblages, their broader role at higher organizational levels (e.g. community, ecosystem and landscape) remains overlooked. Miconia species can be highly diverse and abundant across many communities throughout the Neotropics; thus, their contribution to the structure and function of forest and non-forest ecosystems probably extends far beyond the maintenance of fruit–frugivore interactions. Of note, Miconia flowers are an important resource for pollen-collecting bees, wasps, beetles, flies, moths and hummingbirds (Kriebel and Zumbado, 2014; Brito et al., 2016, 2017b). Moreover, Miconia species are known as hosts of many specialized herbivores, including leaf-chewing lepidopteran larvae (Badenes-Pérez et al., 2010; Scherrer et al., 2010), twig girdler beetles (Paulino-Neto et al., 2005; Paro et al., 2014), seed-feeding carabid beetles (Paarmann et al., 2002), sap-sucking treehoppers (Lopes, 1996; Chacón-Madrigal et al., 2012; Swing, 2012; Alfaro-Alpízar et al., 2020), fruit-galling insects (Centrella and Shaw, 2010, and leaf-galling nematodes (Santos et al., 2012; Viana et al., 2013), among others. Therefore, we highlight the need for further studies addressing their importance for primary consumers other than frugivores, as well as their role in maintaining biodiversity and ecosystem functioning, while facilitating habitat connectivity and providing ecosystem services across Neotropical ecoregions.
Finally, the recognition of Miconia as a keystone plant taxon for Neotropical frugivores is the first step towards applying this knowledge to better conservation and management practices (Peters et al., 2016). Birds are the most common frugivores attending Miconia plants, and also the main group delivering diaspores into sites targeted for restoration (Wunderle, 1997; Cole et al., 2010; Zahawi et al., 2013, Bechara et al., 2016; González-Castro et al., 2018). As a result, planting of Miconia species could intensify the attraction of avian frugivorous species to areas targeted for restoration and increase both the amount and diversity of seed rain. Since we have shown their broad use and dispersal by fauna, we hypothesize that the return of frugivore assemblages and their ecosystem functions can be facilitated by Miconia species. Furthermore, Miconia species are recognized as pioneers present in early successional stages, forest borders and gaps (Levey, 1990; Ellison et al., 1993; Silveira et al., 2013a). Therefore, we suggest that prioritizing the planting of Miconia species selected from regional pools could maximize natural regeneration in increasingly fragmented landscapes.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Appendix 1: Miconia fruit phenology and trait database reference papers. Appendix 2: Miconia–frugivore interaction database reference papers. Appendix 3: Miconia gut passage seed germination database reference papers. Appendix 4: Table S1: list of Miconia species and their respective fruiting seasons; Table S2: seasonality index, precipitation regime and seasonal/non-seasonal simple classification for each community sampled with at least three sympatric Miconia species with phenological data; Table S3: Neotropical ecoregions encompassing fruit–frugivore interactions with Miconia; Table S4: diversity of frugivores consuming Miconia; Table S5: list of species classified as some level of degree of threat according to the IUCN; Table S6: gut passage effect size on seed germination percentage according to bird families. Appendix 5: Figure S1: data distribution of dispersal-related traits in species of Miconia; Figure S2: fruiting phenology line plots and precipitation data for sampled communities; Figure S3: rose diagram showing the number of fruiting Miconia across the Neotropics; Figure S4: geographical map of the Neotropical realm and its terrestrial ecoregions showing each sampled community; Figure S5: scatter plot of effect size against sample size.
ACKNOWLEDGEMENTS
We would like to thank M. A. Pizo and P. K. Maruyama for valuable comments and suggestions on the manuscript, M. L. Bueno for the map, R. Goldenberg for providing fruit pictures of Miconia species and suggestions on the manuscript, and especially all the researchers that kindly provided data for our study (A. Shiels, A. A. B. Darosci, J. C. Motta-Junior, J F. Saracco, D. C. Acosta-Rojas, J. Hawes, R. Ruggera, P. Blendinger, M. M. Vidal and M. Passamani). We are grateful to Mick Hanley and three anonymous revieweres for providing substantial contributions to our manuscript. We thank GSG fellows for logistic and intellectual support.
FUNDING
J.V.S.M. was supported with a master student scholarship and T.J.G. with a post-doctoral researcher scholarship funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). J.V.S.M. acknowledges his current PhD scholarship funded by the Fulbright Commission and CAPES. F.A.O.S. and T.G.C. acknowledge the financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
LITERATURE CITED
- Abramoff MD, Magalhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics International 11: 36–42. [Google Scholar]
- Acosta-Rojas DC, Muñoz MC, Torres GAM, Corredor G. 2012. Dieta y dispersión de semillas: ¿afecta la guacharaca colombiana (Ortalis columbiana) la germinación de las semillas consumidas? Ornitologia Neotropical 23: 439–453. [Google Scholar]
- Alfaro-Alpízar MA, Koster SJC, Johnson MT, Badenes-Pérez FR. 2020. Description, biology, and impact of the fruit-feeding moth, Mompha luteofascia sp. n. (Lepidoptera: Momphidae), on Miconia calvescens (Melastomataceae) in Costa Rica. Annals of the Entomological Society of America 113: 30–39. [Google Scholar]
- Alves MAS, Ritter PD, Antonini RD, Almeida EM. 2008. Two thrush species as dispersers of Miconia prasina (Sw.) DC. (Melastomataceae): an experimental approach. Brazilian Journal of Biology 62: 397–401. [DOI] [PubMed] [Google Scholar]
- Badenes-Pérez FR, Alfaro-Alpízar MA, Johnson MT. 2010. Diversity, ecology and herbivory of hairstreak butterflies (Theclinae) associated with the velvet tree, Miconia calvescens in Costa Rica. Journal of Insect Science 10: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker HG, Baker I, Hodges SA. 1998. Sugar composition of nectars and fruits consumed by birds and bats in the tropics and subtropics. Biotropica 30: 559–586. [Google Scholar]
- Barcelos AR, Bobrowiec PED, Sanaiotti TM, Gribel R. 2013. Seed germination from lowland tapir (Tapirus terrestris) fecal samples collected during the dry season in the northern Brazilian Amazon. Integrative Zoology 8: 63–73. [DOI] [PubMed] [Google Scholar]
- Batalha MA, Martins FR. 2004. Reproductive phenology of the cerrado plant community in Emas National Park (central Brazil). Australian Journal of Botany 52: 149–161. [Google Scholar]
- Bechara FC, Dickens SJ, Farrer EC, et al. 2016. Neotropical rainforest restoration: comparing passive, plantation and nucleation approaches. Biodiversity and Conservation 25: 2021–2034. [Google Scholar]
- Bello C, Galetti M, Montan D, et al. 2017. Atlantic frugivory: a plant–frugivore interaction data set for the Atlantic Forest. Ecology 98: 1729. [DOI] [PubMed] [Google Scholar]
- Bello C, Galetti M, Pizo MA, et al. 2015. Defaunation affects carbon storage in tropical forests. Science Advances 1: e1501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake JG, Loiselle BA. 1992. Fruits in the diets of Neotropical migrant birds in Costa Rica. Biotropica 24: 200–210. [Google Scholar]
- Blendinger PG, Blake JG, Loiselle BA. 2011. Connecting fruit production to seedling establishment in two co-occurring Miconia species: consequences of seed dispersal by birds in upper Amazonia. Oecologia 167: 61–73. [DOI] [PubMed] [Google Scholar]
- Blendinger PG, Loiselle BA, Blake JG. 2008. Crop size, plant aggregation, and microhabitat type affect fruit removal by birds from individual melastome plants in the Upper Amazon. Oecologia 158: 273–283. [DOI] [PubMed] [Google Scholar]
- Brito VLG, Fendrich TG, Smidt EC, Varassin IG, Goldenberg R. 2016. Shifts from specialised to generalised pollination systems in Miconieae (Melastomataceae) and their relation with anther morphology and seed number. Plant Biology 18: 585–593. [DOI] [PubMed] [Google Scholar]
- Brito VLG, Maia FR, Silveira FAO, et al. 2017. a. Reproductive phenology of Melastomataceae species with contrasting reproductive systems: contemporary and historical drivers. Plant Biology 19: 806–817. [DOI] [PubMed] [Google Scholar]
- Brito VLG, Rech AR, Ollerton J, Sazima M. 2017b. Nectar production, reproductive success and the evolution of generalised pollination within a specialised pollen-rewarding plant family: a case study using Miconia theizans. Plant Systematics and Evolution 303: 709–718. [Google Scholar]
- Brodie JF. 2017. Evolutionary cascades induced by large frugivores. Proceedings of the National Academy of Sciences, USA 114: 11998–12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno RS, Guevara R, Ribeiro MC, Culot L, Bufalo FS, Galetti M, 2013. Functional redundancy and complementarities of seed dispersal by the last Neotropical megafrugivores. PLoS One 8: e56252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KC. 2013. What causes size coupling in fruit–frugivore interaction webs? Ecology 94: 295–300. [DOI] [PubMed] [Google Scholar]
- Canale GR, Suscke P, Rocha-Santos L, São Bernardo CS, Kierulff MCM, Chivers DJ. 2016. Seed dispersal of threatened tree species by a critically endangered primate in a Brazilian hotspot. Folia Primatologica 87:123–140. [DOI] [PubMed] [Google Scholar]
- Camargo MGG, Schaefer HM, Habermann G, Cazetta E, Soares NC, Morellato LPC. 2015. Bicolored display of Miconia albicans fruits: evaluating visual and physiological functions of fruit colors. American Journal of Botany 102: 1453–1461. [DOI] [PubMed] [Google Scholar]
- de Camargo NF, Cruz RMS, Ribeiro JF, Vieira EM. 2011. Frugivoria e potencial dispersão de sementes pelo marsupial Gracilinanus agilis (Didelphidae: Didelphimorphia) em áreas de Cerrado no Brasil central. Acta Botanica Brasilica 25: 646–656. [Google Scholar]
- Centrella ML, Shaw SR. 2010. A new species of phytophagous braconid Allorhogas minimus (Hymenoptera: Braconidae: Doryctinae) reared from fruit galls on Miconia longifolia (Melastomataceae) in Costa Rica. International Journal of Tropical Insect Science 30: 101–107. [Google Scholar]
- Chacón-Madrigal E, Johnson MT, Hanson P. 2012. The life history and immature stages of the weevil Anthonomus monostigma Champion (Coleoptera: Curculionidae) on Miconia calvescens DC (Melastomataceae). Proceedins of the Entomological Society of Washington 114: 173–185. [Google Scholar]
- Christianini AV, Oliveira PS. 2009. The relevance of ants as seed rescuers of a primarily bird-dispersed tree in the Neotropical cerrado savanna. Oecologia 160: 735–745. [DOI] [PubMed] [Google Scholar]
- Christianini AV, Mayhé-Nunes AJ, Oliveira PS. 2007. The role of ants in the removal of non-myrmecochorous diaspores and seed germination in a Neotropical savanna. Journal of Tropical Ecology 23: 343–351. [Google Scholar]
- Christianini AV, Mayhé-Nunes AJ, Oliveira PS. 2012. Exploitation of fallen diaspores by ants: are there ant–plant partner choices? Biotropica 44: 360–367. [Google Scholar]
- Cole RJ, Holl KD, Zahawi RA. 2010. Seed rain under tree islands planted to restore degraded lands in a tropical agricultural landscape. Ecological Application 20: 1255–1269. [DOI] [PubMed] [Google Scholar]
- Correa SB, Arujo JK, Penha J, Nunes da Cunha C, Bobier KE, Anderson JT. 2016. Stability and generalization in seed dispersal networks: a case study of frugivorous fish in Neotropical wetlands. Proceedings of the Royal Society B: Biological Sciences 283: 20161267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe BG. 1976. The development of fleshy fruits. Annual Review of Plant Physiology 27: 507–528. [Google Scholar]
- Culot L, Bello C, Batista JLF, Couto HTZ, Galetti M. 2017. Synergistic effects of seed disperser and predator loss on recruitment success and long-term consequences for carbon stocks in tropical rainforests. Scientific Reports 7: 7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalling JW, Wirth R. 1998. Dispersal of Miconia argentea seeds by the leaf-cutting ant Atta colombica. Journal of Tropical Ecology 14: 705–710. [Google Scholar]
- Dietz JM, Peres CA, Pinder L. 1997. Foraging ecology and use of space in wild golden lion tamarins (Leontopithecus rosalia). American Journal of Primatology 41: 289–305. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Denslow JS, Loiselle BA. 1993. Seed and seedling ecology of Neotropical Melastomataceae. Ecology 74: 1733–1749. [Google Scholar]
- Eriksson O. 2016. Evolution of angiosperm seed disperser mutualisms: the timing of origins and their consequences for coevolutionary interactions between angiosperms and frugivores. Biological Reviews 91: 168–186. [DOI] [PubMed] [Google Scholar]
- Escribano-Avila G, Lara-Romeno C, Heleno R, Traveset A. 2018. Tropical seed dispersal networks: emerging patterns, biases, and keystone species traits. In: Dáttilo W, Rico-Gray V, eds. Ecological networks in the tropics. Springer International Publishing, 93–110. [Google Scholar]
- Fenner M. 1998. The phenology of growth and reproduction in plants. Perspectives in Plant Ecology, Evolution and Systematics 1: 78–91. [Google Scholar]
- Fleming TH, Kress WJ. 2011. A brief history of fruits and frugivores. Acta Oecologica 37: 521–530. [Google Scholar]
- Fleming TH, Venable DL, Herrera MLG. 1993. Opportunism vs. specialization: the evolution of dispersal strategies in fleshy-fruited plants. Vegetatio 107/108: 106–120. [Google Scholar]
- Fuzessy LF, Janson CH, Silveira FAO. 2017. How far do Neotropical primates disperse seeds? American Journal of Primatology 79: e22659. [DOI] [PubMed] [Google Scholar]
- Fuzessy LF, Janson CH, Silveira FAO. 2018. Effects of seed size and frugivory degree on dispersal by Neotropical frugivores. Acta Oecologica 93: 41–47. [Google Scholar]
- Galetti M, Stotz D. 1996. Miconia hypoleuca (Melastomataceae) como espécie-chave para aves frugívoras no sudeste do Brasil. Revista Brasileira de Biologia 56: 435–439. [Google Scholar]
- Galetti M, Pizo MA, Morellato LPC. 2011. Diversity of functional traits of fleshy fruits in a species-rich Atlantic rain forest. Biota Neotropica 11: 181–193. [Google Scholar]
- Galetti M, Guevara R, Côrtes MC, et al. 2013. Functional extinction of birds drives rapid evolutionary changes in seed size. Science 340: 1086–1090. [DOI] [PubMed] [Google Scholar]
- García D, Martínez D. 2012. Species richness matters for the quality of ecosystem services: a test using seed dispersal by frugivorous birds. Proceedings of the Royal Society B: Biological Sciences 279: 3106–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genrich CM, Mello MAR, Silveira FAO, Bronstein JL, Paglia AP. 2017. Duality of interaction outcomes in a plant–frugivore multilayer network. Oikos 126: 361–368. [Google Scholar]
- Goldenberg R, Penneys DS, Almeda F, Judd WS, Michelangeli FA. 2008. Phylogeny of Miconia (Melastomataceae): patterns of stamen diversification in a megadiverse Neotropical genus. International Journal of Plant Sciences 169: 963–979. [Google Scholar]
- Gomes VSM, Correia MCR, Lima HA, Alves MAS. 2008. Potential role of frugivorous birds (Passeriformes) on seed dispersal of six plant species in a restinga habitat, southeastern Brazil. Revista de Biologia Tropical 56: 205–216. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Verdú M. 2012. Mutualism with plants drives primate diversification. Systematic Biology 61: 567–577. [DOI] [PubMed] [Google Scholar]
- González-Castro A, Yang S, Nogales M, Carlo TA. 2015. Relative importance of phenotypic trait matching and species abundances in determining plant–avian seed dispersal interactions in a small insular community. AoB Plants 7: plv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Castro A, Yang S, Carlo TA. 2018. How does avian seed dispersal shape the structure of early successional tropical forests? Functional Ecology 33: 229–238. [Google Scholar]
- Gorchov DL, Cornejo F, Ascorra CF, Jaramillo M. 1995. Dietary overlap between frugivorous birds and bats in the Peruvian Amazon. Oikos 74: 235–250. [Google Scholar]
- Grenha V, Macedo MV, Pires AS, Monteiro RF. 2010. The role of Cerradomys subflavus (Rodentia, Cricetidae) as seed predator and disperser of the palm Allagoptera arenaria. Mastozoologia Neotropical 17: 61–68. [Google Scholar]
- Guerra TJ, Dayrell RLC, Arruda AJ, et al. 2017. Intraspecific variation in fruit–frugivore interactions: effects of fruiting neighborhood and consequences for seed dispersal. Oecologia 185: 233–243. [DOI] [PubMed] [Google Scholar]
- Guerra TJ, Messeder JVS, Arruda AJ, et al. 2018. Handling by avian frugivores affects diaspore secondary removal. PLoS One 13: e0202435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitch J, Hedges LV. 1999. Statistical issues in ecological meta-analyses. Ecology 80: 1142–1149. [Google Scholar]
- Guzmán A, Stevenson PR. 2008. Seed dispersal, habitat selection and movement patterns in the Amazonian tortoise, Geochelone denticulata. Amphibia-Reptilia 29: 463–472. [Google Scholar]
- Harris I, Osborn TJ, Jones P, Lister D. 2020. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Scientific Data 7: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes JE, Peres CA. 2014a. Fruit–frugivore interactions in Amazonian seasonally flooded and unflooded forests. Journal of Tropical Ecology 30: 381–399. [Google Scholar]
- Hawes JE, Peres CA. 2014b. Ecological correlates of trophic status and frugivory in Neotropical primates. Oikos 123: 365–377. [Google Scholar]
- Hedges LV, Gurevitch J, Curtis PS. 1999. The meta-analysis of response ratios in experimental ecology. Ecology 80: 1150–1156. [Google Scholar]
- Hernández-Conrique D, Iñiguez-Dávalos LI, Storz JF. 1997. Selective feeding by phyllostomid fruit bats in a subtropical montane cloud forest. Biotropica 29: 376–379. [Google Scholar]
- Hernández-Montero JR, Saldaña-Vázquez RA, Galindo-González J, Sosa VJ. 2015. Bat–fruit interactions are more specialized in shaded-coffee plantations than in tropical mountain cloud forest fragments. PLoS One 10: e0126084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand H, Gurevitch J. 2014. Meta-analysis results are unlikely to be biased by differences in variance and replication between ecological lab and field studies. Oikos 123: 794–799. [Google Scholar]
- Hilty SL. 1980. Flowering and fruiting periodicity in a premontane rain forest in Pacific Colombia. Biotropica 12: 292–306. [Google Scholar]
- Howe HF. 1993. Specialized and generalized dispersal systems: where does ‘the paradigm’stand? Vegetatio 107: 3–13. [Google Scholar]
- Howe HF, Estabrook GF. 1977. On intraspecific competition for avian dispersers in tropical trees. The American Naturalist 111: 817–832. [Google Scholar]
- Janson CH. 1983. Adaptation of fruit morphology to dispersal agents in a Neotropical forest. Science 219: 187–189. [DOI] [PubMed] [Google Scholar]
- Jordano P. 1987. Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution. The American Naturalist 129: 657–677. [Google Scholar]
- Jordano P. 1995. Angiosperm fleshy fruits and seed dispersers: a comparative analysis of adaptation and constraints in plant-animal interactions. The American Naturalist 145: 163–191. [Google Scholar]
- Jordano P. 2014. Fruits and frugivory. In: Gallagher RS, ed. Seeds: the ecology of regeneration in plant communities. Wallingford, UK: CABI Publishing, 18–61. [Google Scholar]
- Jordano P, Bascompte J, Olesen JM. 2003. Invariant properties in coevolutionary networks of plant–animal interactions. Ecology Letters 6: 69–81. [Google Scholar]
- Jordano P, Forget P, Lambert JE, Böhning-Gaese K, Traveset A, Wright SJ. 2011. Frugivores and seed dispersal: mechanisms and consequences for biodiversity of a key ecological interaction. Biology Letters 7: 321–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov WH, Diamond JM. 1988. Interplay between physiology and ecology in digestion. BioScience 38: 602–611. [Google Scholar]
- Kaspari M. 1993. Removal of seeds from Neotropical frugivore droppings. Oecologia 95: 81–88. [DOI] [PubMed] [Google Scholar]
- Kattge J, Díaz S, Lavorel S, et al. 2011. TRY – a global database of plant traits. Global Change Biology 17: 2905–2935. [Google Scholar]
- Kessler-Rios MM, Kattan GH. 2012. Fruits of Melastomataceae: phenology in Andean forest and role as a food resource for birds. Journal of Tropical Ecology 28: 11–21. [Google Scholar]
- Kissling WD, Böhning-Gaese K, Jetz W. 2009. The global distribution of frugivory in birds. Global Ecology and Biogeography 18: 150–162. [Google Scholar]
- Kissling WD, Sekercioglu CH, Jetz W. 2012. Bird dietary guild richness across latitudes, environments and biogeographic regions. Global Ecology and Biogeography 21: 328–340. [Google Scholar]
- Kriebel R, Zumbado MA. 2014. New reports of generalist insect visitation to flowers of species of Miconia (Miconieae: Melastomataceae) and their evolutionary implications. Brittonia 66: 396–404. [Google Scholar]
- Krijger CL, Opdam M, Théry M, Bongers F. 1997. Courtship behaviour of manakins and seed bank composition in a French Guianan rain forest. Journal of Tropical Ecology 13: 631–635. [Google Scholar]
- Kubitzki K, Ziburski A. 1994. Seed dispersal in flood plain forests of Amazonia. Biotropica 26: 30–43. [Google Scholar]
- Land HC. 1963. A tropical feeding tree. The Wilson Bulletin 75: 199–200. [Google Scholar]
- Lambert FR, Marshall AG. 1991. Keystone characteristics of bird-dispersed Ficus in a Malaysian lowland rain forest. Journal of Ecology 79: 793–809. [Google Scholar]
- Lapenta MJ, Procópio-de-Oliveira P, Kierulff MCM, Motta-Junior JC. 2008. Frugivory and seed dispersal of golden lion tamarin (Leontopithecus rosalia (Linnaeus, 1766)) in a forest fragment in the Atlantic Forest, Brazil. Brazilian Journal of Biology 68: 241–249. [DOI] [PubMed] [Google Scholar]
- Leal IR, Oliveira PS. 1998. Interactions between fungus-growing ants (Attini), fruits and seeds in Cerrado vegetation in southeast Brazil. Biotropica 30: 170–178. [Google Scholar]
- Leite RR, Araujo SSC, Oliveira EG. 2013. Remoção dos frutos de Miconia albicans (Sw.) Triana (Melastomataceae) por formigas na borda e no interior de um fragmento de Cerrado, Curvelo, MG. Revista Árvore 37: 469–478. [Google Scholar]
- Lehouck V, Spanhove T, Lens L. 2011. Avian fruit ingestion differentially facilitates seed germination of four fleshy-fruited plant species of an Afrotropical forest. Plant Ecology and Evolution 144: 96–100. [Google Scholar]
- Lepczyk CA, Murray KG, Winnett-Murray K, Bartell P, Geyer E, Work T. 2000. Seasonal fruit preferences for lipids and sugars by American robins. The Auk 117: 709–717. [Google Scholar]
- Lessa LG, Costa FN. 2010. Diet and seed dispersal by five marsupials (Didelphimorphia:Didelphidae) in a Brazilian cerrado reserve. Mammalian Biology 75: 10–16. [Google Scholar]
- Lessa LG, Geise L. 2014. Food habits of Metachirus nudicaudatus (Didelphimorphia, Didelphidae) in a Brazilian Cerrado: diet composition and dietary seasonality. Studies of the Neotropical Fauna and Environment 49: 75–78. [Google Scholar]
- Lessa LG, Geise L, Costa FN. 2013. Effects of gut passage on the germination of seeds ingested by didelphid marsupials in a Neotropical savanna. Acta Botanica Brasilica 27: 519–525. [Google Scholar]
- Lessa LG, Paula CS, Pessoa RS. 2019. Food habits and endozoochorous seed dispersal by small rodents (Cricetidae and Echimyidae) in a riparian forest in Southeastern Brazil. Neotropical Biology and Conservation 14: 349–359. [Google Scholar]
- Levey DJ. 1987. Seed size and fruit-handling techniques of avian frugivores. The American Naturalist 129: 471–485. [Google Scholar]
- Levey DJ. 1988. Spatial and temporal variation in Costa Rican fruit and fruit-eating bird abundance. Ecological Monographs 58: 251–269. [Google Scholar]
- Levey DJ. 1990. Habitat-dependent fruiting behaviour of an understorey tree, Miconia centrodesma, and tropical treefall gaps as keystone habitats for frugivores in Costa Rica. Journal of Tropical Ecology 6: 409–420. [Google Scholar]
- Levey DJ, Byrne MM. 1993. Complex ant–plant interactions: rain-forest ants as secondary dispersers and post-dispersal seed predators. Ecology 74: 1802–1812. [Google Scholar]
- Levey DJ, Martínez del Rio C. 2001. It takes guts (and more) to eat fruit: lessons from avian nutritional ecology. The Auk 118: 819–831. [Google Scholar]
- Lima IP, Nogueira MR, Monteiro LR, Peracchi AL. 2016. Floresta atlântica de tabuleiro: diversidade e endemismos na reserva natural vale. In: Rolim SG, de Menezes LFT, Srbek-Araujo AC, eds. Floresta Atlântica de Tabuleiro: diversidade e endemismos na Reserva Natural Vale. Belo Horizonte, Brazil: Rona Editora, 433–452. [Google Scholar]
- Lima MH, Oliveira EG, Silveira FAO. 2013. Interactions between ants and non-myrmecochorous fruits in Miconia (Melastomataceae) in a Neotropical savanna. Biotropica 45: 217–223. [Google Scholar]
- Lizcano DJ, Cavelier J. 2004. Características químicas de salados y hábitos alimenticios de la danta de montaña (Tapirus pinchaque Roulin, 1829) en los andes centrales de Colombia. Mastozoologia Neotropical 11:193–201. [Google Scholar]
- Loiselle BA, Blake JG. 1990. Diets of understory fruit-eating birds in Costa Rica: seasonality and resource abundance. Studies in Avian Biology 13: 91–103. [Google Scholar]
- Loiselle BA, Blake JG. 1999. Dispersal of melastome seeds by fruit-eating birds of tropical forest understory. Ecology 80: 330–336. [Google Scholar]
- Lomáscolo SB, Levey DJ, Kimball RT, Bolker BM, Alborn HT. 2010. Dispersers shape fruit diversity in Ficus (Moraceae). Proceedings of the National Academy of Sciences, USA 107: 14668–14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes BC. 1996. Treehoppers (Homoptera: Membracidae) in southeastern Brazil: use of host plants. Revista Brasileira de Zoologia 12: 595–608. [Google Scholar]
- Luck GW, Daily GC. 2003. Tropical countryside bird assemblages: richness, composition, and foraging differ by landscape context. Ecological Applications 13: 235–247. [Google Scholar]
- Lund U, Agostinelli C, Arai H, et al. 2017. circular: Circular statistics. R Package Version: 0.4-93. https://CRAN.R-project.org.
- Magnusson WE, Sanaiotti TM. 1987. Dispersal of Miconia seeds by the rat Bolomys lasiurus. Journal of Tropical Ecology 3: 277–278. [Google Scholar]
- Maruyama PK, Borges MR, Silva PA, Burns KC, Melo C. 2013. Avian frugivory in Miconia (Melastomataceae): contrasting fruiting times promote habitat complementarity between savanna and palm swamp. Journal of Tropical Ecology 29: 99–109. [Google Scholar]
- Maruyama PK, Melo C, Pascoal C, et al. 2019. What is on the menu for frugivorous birds in the Cerrado? Fruiting phenology and nutritional traits highlight the importance of habitat complementarity. Acta Botanica Brasilica 33: 572–583. [Google Scholar]
- McKey D. 1975. The ecology of coevolved seed dispersal systems. In: Gilbert LE, Raven PH, eds. Coevolution of animals and plants. Austin, TX: University of Texas Press, 159–191. [Google Scholar]
- Mendonza I, Peres CA, Morellato LPC. 2017. Continental-scale patterns and climatic drivers of fruiting phenology: a quantitative Neotropical review. Global and Planetery Change 148: 227–241. [Google Scholar]
- Messeder JVS, Guerra TJ, Dáttilo W, Silveira FAO. 2020. Searching for keystone plant resources in fruit–frugivore interaction networks across the Neotropics. Biotropica 52: 857–870. [Google Scholar]
- Michelangeli FA, Penneys DS, Giza J, Soltis D, Hils MH, Skean JD. 2004. A preliminary phylogeny of the tribe Miconieae (Melastomataceae) based on nrITS sequence data and its implications on inflorescence position. Taxon 53: 279–290. [Google Scholar]
- Michelangeli FA, Guimarães PJF, Penneys DS, Almeda F, Kriebel R. 2013. Phylogenetic relationships and distribution of new world Melastomeae. Botanical Journal of the Linnean Society 171: 38–60. [Google Scholar]
- Michelangeli FA, Goldenberg R, Almeda F, et al. 2019. Nomenclatural novelties in Miconia (Melastomataceae: Miconieae). Brittonia 71: 82–121. [Google Scholar]
- Miranda GHB, Faria DS. 2000. Ecological aspects of black-pincelled marmoset (Callithrix penicillata) in the cerradão and dense cerrado of the brazilian central plateau. Brazilian Journal of Biology 61: 397–404. [DOI] [PubMed] [Google Scholar]
- Moll D, Jansen KP. 1995. Evidence for a role in seed dispersal by two tropical herbivorous turtles. Biotropica 27: 121–127. [Google Scholar]
- Møller AP, Jennions MD. 2001. Testing and adjusting for publication bias. Trends in Ecology and Evolution 16: 580–586. [Google Scholar]
- Morán-López T, Carlo TA, Morales JM. 2018. The role of frugivory in diversity maintenance – a simulation approach. Ecography 41: 24–31. [Google Scholar]
- Morellato LPC, Alberti LF, Hudson IL. 2010. Applications of circular statistics in plant phenology: a case studies approach. In: Hudson IL, Keatley MR, eds. Phenological research. Dordrecht: Springer, 339–359. [Google Scholar]
- Morellato LPC, Talora DC, Takahashi A, Bencke CC, Romera EC, Ziparro VB. 2000. Phenology of Atlantic rainforest trees: a comparative study. Biotropica 32: 811–823. [Google Scholar]
- Muñoz G, Trøjelsgaard K, Kissling WD. 2019. A synthesis of animal-mediated seed dispersal of palms reveals distinct biogeographical differences in species interactions. Journal of Biogeography 46: 466–484. [Google Scholar]
- Nevo O, Valenta K, Razafimandimby D, Melin AD, Ayasse M, Chapman CA. 2018. Frugivores and the evolution of fruit colour. Biology Letters 14: 20180377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, et al. 2019. ‘vegan’ Community Ecology Package. R package version: 2.5-6. https://CRAN.R-project.org.
- Olesen JM, Bascompte J, Dupont YL, Elberling H, Rasmussen C, Jordano P. 2011. Missing and forbidden links in mutualistic networks. Proceedings of the Royal Society B: Biological Sciences 278: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, et al. 2001. Terrestrial ecoregions of the world: a new map of life on earth: a new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 51: 933–938. [Google Scholar]
- Onstein RE, Baker WJ, Couvreur TLP, Faurby S, Svenning JC, Kissling WD. 2017. Frugivory-related traits promote speciation of tropical palms. Nature Ecology and Evolution 1: 1903–1911. [DOI] [PubMed] [Google Scholar]
- Paarmann W, Gutzmann B, Stumpe P, et al. 2002. The structure of ground beetle assemblages (Coleoptera: Carabidae) at fruit falls of Melastomataceae trees in a Brazilian Terra Firme Rainforest. Biotropica 34: 368–375. [Google Scholar]
- Palacio RD, Valderrama-Ardila C, Kattan GH. 2016. Generalist species have a central role in a highly diverse plant–frugivore network. Biotropica 48: 349–355. [Google Scholar]
- Paulino-Neto HF, Romero GQ, Vasconcellos-Neto J. 2005. Interactions between Oncideres humeralis Thorms (Coleoptera: Cerambycidae) and Melastomataceae: host plant selection and patterns of host use in south-east Brazil. Neotropical Entomology 34: 7–14. [Google Scholar]
- Pavelka MS, Knopff KH. 2004. Diet and activity in black howler monkeys (Alouatta pigra) in southern Belize: does degree of frugivory influence activity level? Primates 45: 105–111. [DOI] [PubMed] [Google Scholar]
- Paro CM, Arab A, Vasconcellos-Neto J. 2014. Specialization of Atlantic rain forest twig-girdler beetles (Cerambycidae: Lamiinae: Onciderini): variation in host–plant use by microhabitat specialists. Arthropod-Plant Interactions 8: 557–569. [Google Scholar]
- Parrini R, Pacheco JF. 2011. Frugivoria por aves em seis espécies arbóreas do gênero Miconia (Melastomataceae) na Mata Atlântica do Parque Nacional da Serra dos Órgãos, Região Sudeste do Brasil. Atualidades Ornitologicas 159: 51–58. [Google Scholar]
- Peres CA. 2000. Identifying keystone plant resources in tropical forests: the case of gums from Parkia pods. Journal ofTropical Ecology 16: 287–317. [Google Scholar]
- Peres CA, Emilio T, Schietti J, Desmoulière SJM, Levi T. 2016. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proceedings of the National Academy of Sciences, USA 113: 892–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters V, Carlo TA, Mello MAR, et al. 2016. Using plant–animal interactions to inform tree selection in tree-based agroecosystems for enhanced biodiversity. Bioscience 66:1046–1056. [Google Scholar]
- Poulin B, Wright SJ, Lefebvre G, Calderon O. 1999. Interspecific synchrony and asynchrony in the fruiting phenologies of congeneric bird-dispersed plants in Panama. Journal of Tropical Ecology 15: 213–227. [Google Scholar]
- Rathcke B, Lacey EP. 1985. Phenological patterns of terrestrial plants. Annual Review of Ecology and Systematics 16: 179–214. [Google Scholar]
- Reginato M, Vasconcelos TN, Kriebel R, Simões AO. 2020. Is dispersal mode a driver of diversification and geographical distribution in the tropical plant family Melastomataceae? Molecular Phylogenetics and Evolution 148: 106815. [DOI] [PubMed] [Google Scholar]
- Renner SS, Clausing G, Meyer K. 2001. Historical biogeography of Melastomataceae: the roles of tertiary migration and long-distance dispersal. American Journal of Botany 88: 1290–1300. [PubMed] [Google Scholar]
- Ribeiro RC, Figueiredo MLN, Picorelli A, Oliveira DMT, Silveira FAO. 2016. Does seed coat structure modulate gut-passage effects on seed germination? Examples from Miconieae DC. (Melastomataceae). Seed Science Research 26: 139–147. [Google Scholar]
- Rosenberg MS, Adams DC, Gurevitch J. 2000. MetaWin: statistical software for meta-analysis, ver. 2.0. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Rosenthal R. 1979. The ‘file drawer’ problem and tolerance for null results. Psychological Bulletin 86: 638–641. [Google Scholar]
- Sahley CT, Cervantes K, Pacheco V, Salas E, Paredes D, Alonso A. 2015. Diet of a sigmodontine rodent assemblage in a Peruvian montane forest. Journal of Mammalogy 96: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IA, Levey DJ. 2005. Effects of gut passage on seed germination: do experiments answer the questions they ask? Functional Ecology 19: 365–368. [Google Scholar]
- Santana FD, Cazetta E, Delabie JHC. 2013. Interactions between ants and non-myrmecochorous diaspores in a tropical wet forest in southern Bahia, Brazil. Journal of Tropical Ecology 29:71–80. [Google Scholar]
- Santos AMO, Jacobi CM, Silveira FAO. 2017. Frugivory and seed dispersal effectiveness in two Miconia (Melastomataceae) species from ferruginous Campo Rupestre. Seed Science Research 27: 65–73. [Google Scholar]
- Santos JC, Santos CIR, Cares JE, Almeida-Cortez JS. 2012. Impact of nematode-induced galls on Miconia prasina (Sw.) DC (Melastomataceae) traits in the Atlantic forest of northeastern Brazil. Journal of Plant Interactions 7: 197–203. [Google Scholar]
- van Schaik CP, Terborgh JW, Wright SJ. 1993. The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics 24: 353–377. [Google Scholar]
- Scherrer S, Diniz IR, Morais HC. 2010. Climate and host plant characteristics effects on lepidopteran caterpillar abundance on Miconia ferruginata DC. and Miconia pohliana Cogn (Melastomataceae). Brazilian Journal of Biology 70: 103–109. [DOI] [PubMed] [Google Scholar]
- Schupp EW, Jordano P, Gómez JM. 2010. Seed dispersal effectiveness revisited: a conceptual review. New Phytologist 188: 333–353. [DOI] [PubMed] [Google Scholar]
- Sebastián-González E, Pires MM, Donatti CI, Guimarães PR, Dirzo R. 2017. Species traits and interaction rules shape a species-rich seed-dispersal interaction network. Ecology and Evolution 7: 4496–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M, Compton SG, So S, Corlett R. 2001. Fig-eating by vertebrate frugivores: a global review. Biological Reviews 76: 529–572. [DOI] [PubMed] [Google Scholar]
- Silva WR, De Marco P, Hasui E, Gomes VSM. 2002. Patterns of fruit–frugivores interactions in two Atlantic Forest bird communities of South-eastern Brazil: implications for conservation. In: Levey DJ, Silva WR, Galetti M, eds. Seed dispersal and frugivory: ecology, evolution and conservation. Wallinford, UK: CAB Publishing, 423–435. [Google Scholar]
- Silveira FAO, Ribeiro RC, Oliveira DM, Fernandes GW, Lemos-Filho JP. 2012a. Evolution of physiological dormancy multiple times in Melastomataceae from Neotropical montane vegetation. Seed Science Research 22: 37–44. [Google Scholar]
- Silveira FAO, Mafia PO, Lemos-Filho JP, Fernandes GW. 2012b. Species-specific outcomes of avian gut passage on germination of Melastomataceae seeds. Plant Ecology and Evolution 145: 350–355. [Google Scholar]
- Silveira FAO, Fernandes GW, Lemos-Filho JP. 2013a. Seed and seedling ecophysiology of Neotropical Melastomataceae: implications for conservation and restoration of savannas and rainforests. Annals of the Missouri Botanical Garden 99: 82–99. [Google Scholar]
- Silveira FAO, Ribeiro RC, Soares S, Rocha D, Oliveira C. 2013b. Physiological dormancy and seed germination inhibitors in Miconia (Melastomataceae). Plant Ecology and Evolution 146: 290–294. [Google Scholar]
- Snow DW. 1965. A possible selective factor in the evolution of fruiting seasons in tropical forest. Oikos 15: 274–281. [Google Scholar]
- Snow DW. 1971. Evolutionary aspects of fruit-eating by birds. Ibis 113: 194–202. [Google Scholar]
- Snow DW. 1981. Tropical frugivorous birds and their food plants: a world survey. Biotropica 13: 1–14. [Google Scholar]
- Staggemeier VG, Cazetta E, Morellato LPC. 2017. Hyperdominance in fruit production in the Brazilian Atlantic rain forest: the functional role of plants in sustaining frugivores. Biotropica 49: 71–82. [Google Scholar]
- Stiles FG, Rosselli L. 1993. Consumption of fruits of the Melastomataceae by birds: how diffuse is coevolution? Vegetatio 107: 57–73. [Google Scholar]
- Swanson PL. 1950. The iguana Iguana iguana iguana (L). Herpetologica 6: 187–193. [Google Scholar]
- Swing K. 2012. Preliminary observations on the natural history of representative treehoppers (Hemiptera, Auchenorrhyncha, Cicadomorpha: Membracidae and Aetalionidae) in the Yasuní Biosphere Reserve, including first reports of 13 genera for Ecuador and the province of Orellana. Avances en Ciencias e Ingenierias 4: 17–30. [Google Scholar]
- Terborgh J. 1986. Keystone plant resources in the tropical forest. In: Soulé ME, ed. Conservation biology: the science of scarcity and diversity. Sunderland, MA: Sinauer Associates, 330–344. [Google Scholar]
- Tobler MW, Janovec JP, Cornejo F. 2010. Frugivory and seed dispersal by the lowland tapir Tapirus terrestris in the Peruvian Amazon. Biotropica 42: 215–222. [Google Scholar]
- Torres DA, Castaño JH, Carranza-Quiceno JA. 2020. Global patterns in seed germination after ingestion by mammals. Mammal Review 50: 278–290. [Google Scholar]
- Traveset A. 1998. Effect of seed passage through vertebrate frugivores’ guts on germination: a review. Perspectives in Plant Ecology Evolution and Systematics 1: 151–190. [Google Scholar]
- Traveset A, Verdú M. 2002. A meta-analysis of gut treatment on seed germination. In: Levey DJ, Silva WR, Galetti M, eds. Frugivores and seed dispersal: ecological, evolutionary and conservation issues. Wallingford, UK: CABI Publishing, 339–350. [Google Scholar]
- Traveset A, Riera N, Mas RE. 2001. Passage through bird guts causes interspecific differences in seed germination characteristics. Functional Ecology 15: 669–675. [Google Scholar]
- Traveset A, Robertson AW, Rodríguez-Pérez J. 2007. A review of the role of endozoochory on seed germination. In: Dennis AJ, Schupp EW, Green RJ, Westcott DA, eds. Seed dispersal: theory and its application in a changing world. Wallingford, UK: CABI Publishing, 79–103. [Google Scholar]
- Traveset A, Rodríguez-Pérez J, Pías B. 2008. Seed trait changes in dispersers’ guts and consequences for germination and seedling growth. Ecology 89: 95–106. [DOI] [PubMed] [Google Scholar]
- Valenta K, Nevo O. 2020. The dispersal syndrome hypothesis: how animals shaped fruit traits, and how they did not. Functional Ecology 34: 1158–1169. [Google Scholar]
- Valiente-Banuet A, Aizen MA, Alcántara JM, et al. 2015. Beyond species loss: the extinction of ecological interactions in a changing world. Functional Ecology 29: 299–307. [Google Scholar]
- Viana LR, Silveira FAO, Santos JC, et al. 2013. Nematode-induced galls in Miconia albicans: effect of host plant density and correlations with performance. Plant Species Biology 28: 63–69. [Google Scholar]
- Vidal MM, Hasui E, Pizo MA, Tamashiro JY, Silva WR, Guimarães PR. 2014. Frugivores at higher risk of extinction are the key elements of a mutualistic network. Ecology 95: 3440–3447. [Google Scholar]
- Wallace BC, Lajeunesse MJ, Dietz G, et al. 2017. Open MEE: intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods in Ecology and Evolution 8: 941–947. [Google Scholar]
- Walsh RPD, Lawler DM. 1981. Rainfall seasonality: description, spatial patterns and change through time. Weather 36: 201–208. [Google Scholar]
- Watson DM. 2001. Mistletoe – a keystone resource in forests and woodlands worldwide. Annual Reviews in Ecology and Systematics 32: 219–249. [Google Scholar]
- Watson DM, Herring M. 2012. Mistletoe as a keystone resource: an experimental test. Proceedings of the Royal Society B: Biological Sciences 279: 3853–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehncke EV, Dalling JW. 2005. Post-dispersal seed removal and germination selected tree species dispersed by Cebus capucinus on Barro Colorado Island, Panama. Biotropica 37: 73–80. [Google Scholar]
- Weiss B, Zuanon JA, Piedade MT. 2016. Viability of seeds consumed by fishes in a lowland forest in the Brazilian Central Amazon. Tropical Conservation Science 9: 1940082916676129. [Google Scholar]
- Wheelwright NT. 1985a. Competition for dispersers, and the timing of flowering and fruiting in a guild of tropical trees. Oikos 44: 465–477. [Google Scholar]
- Wheelwright NT. 1985b. Fruit-size, gape width, and the diets of fruit-eating birds. Ecology 66: 808–818. [Google Scholar]
- Wheelwright NT, Orians GH. 1982. Seed dispersal by animals: contrasts with pollen dispersal, problems with terminology, and constraints on coevolution. The American Naturalist 119: 402–413. [Google Scholar]
- Wheelwright NT, Haber WA, Murray KG, Guindon C. 1984. Tropical fruit-eating birds and their food plants: a survey of a Costa Rican lower montane forest. Biotropica 16: 173–192. [Google Scholar]
- Witmer MC, van Soest PJ. 1998. Contrasting digestive strategies of fruit-eating birds. Functional Ecology 12: 728–741. [Google Scholar]
- Worthington AH. 1989. Adaptations for avian frugivory: assimilation efficiency and gut transit time of Manacus vitellinus and Pipra mentalis. Oecologia 80: 381–389. [DOI] [PubMed] [Google Scholar]
- Wunderle JM. 1997. The role of animal seed dispersal in accelerating native forest regeneration on degraded tropical lands. Forest Ecology and Management 99: 223–235. [Google Scholar]
- Zahawi RA, Holl KH, Cole RJ, Reid JL. 2013. Testing applied nucleation as a strategy to facilitate tropical forest recovery. Journal of Applied Ecology 50: 88–96. [Google Scholar]
- Zona S, Henderson A. 1989. A review of animal-mediated seed dispersal of palms. Selbyana 11: 6–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.