Abstract
Flaviviruses, including Zika, dengue and West Nile virus, are important human pathogens. The highly conserved NS2B-NS3 protease of Flavivirus is essential for viral replication and therefore a promising drug target. Through compound screen followed by medicinal chemistry studies, a novel series of 2,5,6-trisubstituted pyrazine compounds are found to be potent, allosteric inhibitors of Zika virus protease (ZVpro) with IC50 values as low as 130 nM. Their structure-activity relationships are discussed. The ZVpro inhibitors also inhibit homologous proteases of dengue and West Nile virus and their inhibitory activities are correlated. The most potent compounds 47 and 103 potently inhibited Zika virus replication in cells with EC68 values of 300–600 nM and in a mouse model of Zika infection. These compounds represent novel pharmacological leads for drug development against Flavivirus infections.
Keywords: Zika virus, NS2B-NS3 protease, allosteric inhibitor, structure-activity relationship
Graphical Abstract
INTRODUCTION
Zika virus (ZIKV) is a species of the arthropod-borne Flavivirus in the Flaviviridae family of RNA viruses, which includes other important human pathogens such as yellow fever, dengue and West Nile virus. ZIKV was first discovered and isolated from a sentinel Rhesus monkey in the Zika Forest of Uganda in 19471. The virus is transmitted among humans through Aedes mosquitoes. About 20% people infected with ZIKV show flu-like symptoms and can recover naturally. However, ZIKV infections has been found to cause a 20-fold increased incidence of severe neurological disorders, including birth defects of central nervous systems (mostly microcephaly)2, 3 and Guillain-Barré syndrome4, 5. An outbreak of ZIKV in Brazil and the other 48 American countries in 2015–16 afflicted more than 2 million people6 . During mid-2015 through Jan-2016, >4,000 cases of microcephaly with suspected ZIKV involvement were reported, as compared to <200 cases/year previously in Brazil7, 8. WHO announced that ZIKV is a “Public Health Emergency of International Concern”. Except for mosquito control, there have been no antiviral drugs or vaccines for the prevention and treatment of ZIKV infection9, 10.
ZIKV contains a ~10.8 kb RNA genome encoding a polyprotein11, which is cleaved by the viral NS2B-NS3 protease (ZVpro) as well as host proteases into three structural (C, prM/M, and E) proteins and seven non-structural proteins, including NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5. NS3 contains a N-terminal serine protease domain (1–170), but complexation with NS2B is required for the catalytic activity12. In the active (or known as “closed”) conformation of ZVpro, NS2B is fully wrapped around NS3 and constitutes part of the active site of the enzyme11, 13–16. In the “open” conformation, NS2B is partially associated with NS3 and the enzyme is inactive. Due to its pivotal role in viral replication, ZVpro is a promising drug target for ZIKV infection9, 12, 17.
Peptide-based covalent inhibitors of ZVpro and other Flavivirus proteases have been reported12, 18–21, but they did not show significant antiviral activities in cell or animal models, presumably due to low cell permeability or metabolic stability. Non-peptidic inhibitors have also been disclosed, whose activities are relatively weak and interactions with the protease are less characterized12–14, 22. In a recent communication15, we reported that several 2,5,6-trisubstituted pyrazine compounds are novel potent inhibitors of the NS2B-NS3 proteases of Zika and related dengue and West Nile viruses. These compounds inhibited in vitro and in vivo replication of ZIKV. X-ray crystallographic studies show these inhibitors bind to an allosteric pocket of dengue NS2B-NS3 protease. In this full article, we report the synthesis, comprehensive structure-activity relationships (SAR), and biological activities of 104 pyrazine and related compounds targeting ZVpro, which have led to the discovery of compound 47 with potent anti-ZIKV activity. Moreover, selected ZVpro inhibitors were found to also inhibit NS2B-NS3 proteases of dengue and West Nile virus, showing these compounds are broad-spectrum inhibitors of Flaviviruses.
RESULTS AND DISCUSSION
Chemical synthesis
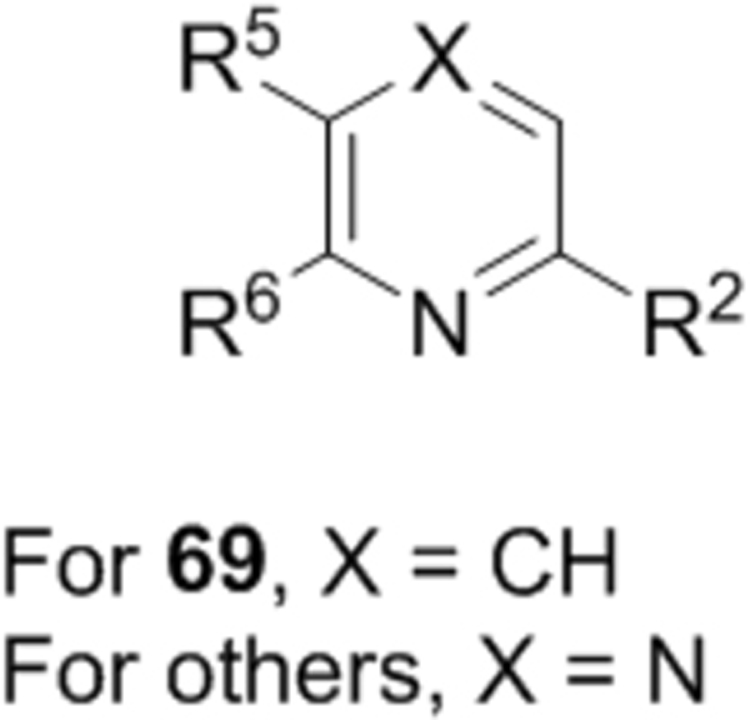
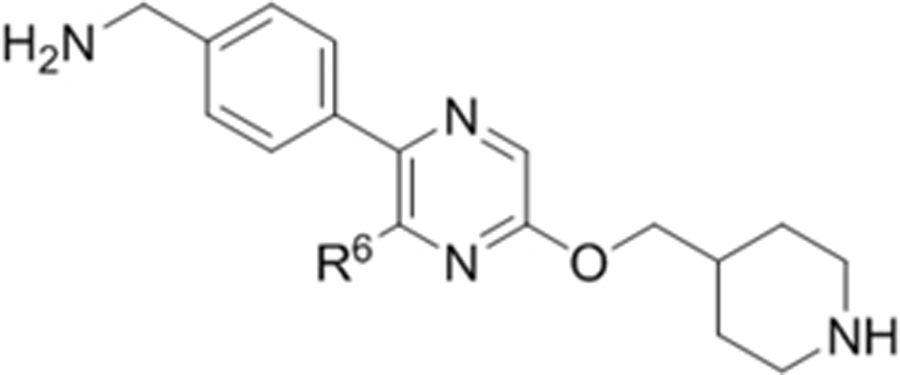
Compounds 1–18 with the same R5 and R6 substituents were synthesized using general methods shown in Schemes 1. Synthesis of compounds 1–4 was started with a cyclization reaction of 4,4′-dibromo- or diiodo-benzil (105) with glycine amide (106) in the presence of sodium hydroxide to give 2-hydroxypyrazine 107. The hydroxy group was alkylated with tert-butyloxycarbonyl (Boc)-protected 4-piperidinemethanol using a Mitsunobu reaction and the product was deprotected to give compound 1–4.
Scheme 1.
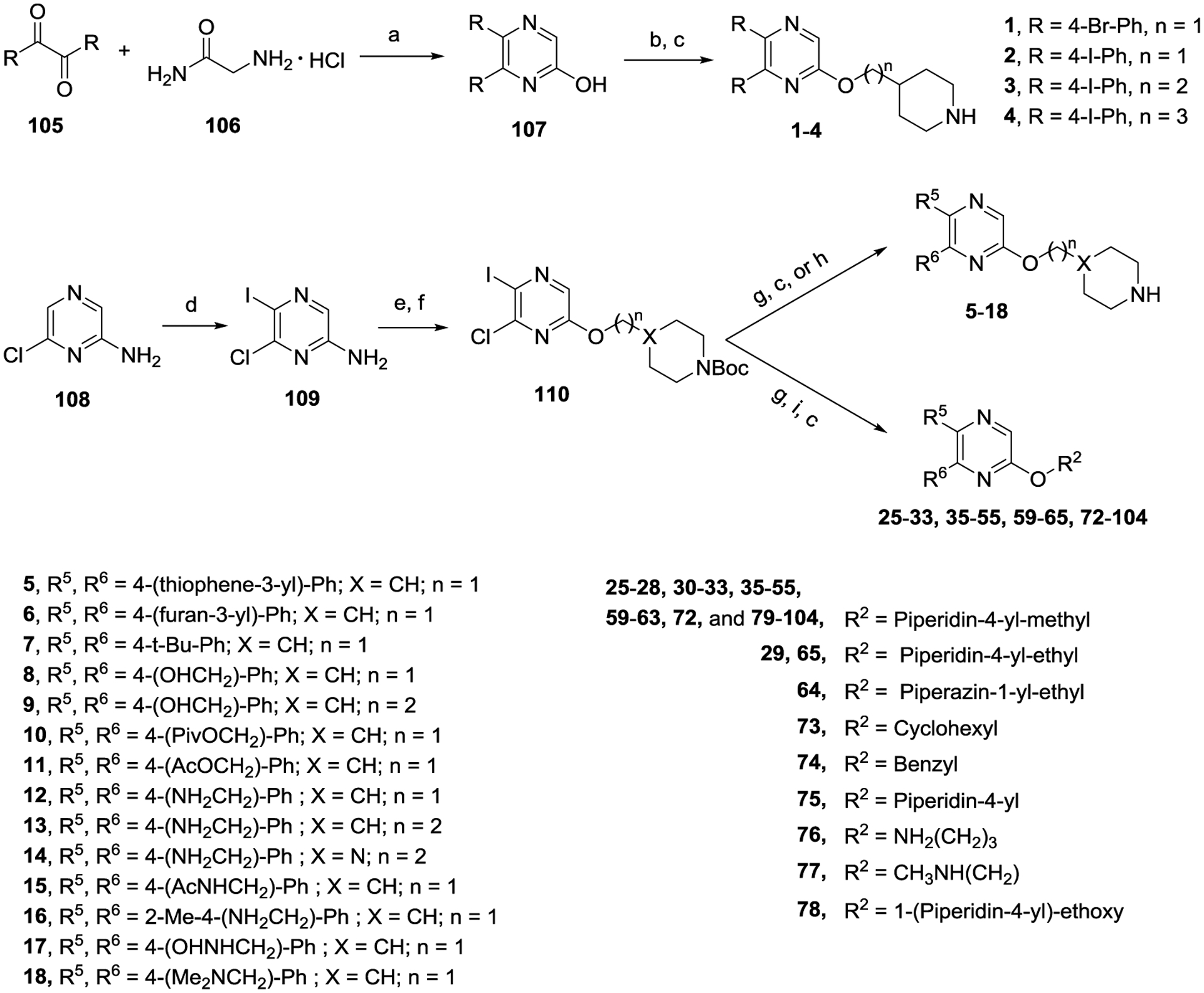
Synthesis of pyrazine compounds 1−18, 25−33, 35−55, 59−65, 72−104.a
aReagents and conditions: (a) NaOH, MeOH, reflux; (b) N-Boc-4-piperidinemethanol, PPh3, diisopropyl azodicarboxylate, THF; (c) HCl (4 M in 1,4-dioxane), CH2Cl2, 0 °C; (d) N-iodosuccinimide, DMSO, 72 h; (e) NaNO2, H2SO4 (conc.), 1 h; (f) Alcohols, PPh3, diisopropyl azodicarboxylate, THF; (g) Aryl boronic acid or Aryl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, Pd(PPh3)4, Na2CO3, 1,4-dioxane-H2O, 110 °C; (h) For synthesis of 10 and 11, diisopropylethylamine, CH2Cl2, 0 °C, acetyl chloride and pivaloyl chloride; (i) For synthesis of 33, 35, 36 and 37, K2CO3, DMSO, 110 °C, overnight; For other compounds: R6-boronic acid or R6-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, Pd(PPh3)4, Na2CO3, 1,4-dioxane-H2O, 110 °C.
For synthesis of compounds 5–18, 6-chloro-2-aminopyrazine (108) was selectively iodized with N-iodosuccinimide to give 6-chloro-5-iodo-2-aminopyrazine (109), whose amino group was converted to a hydroxy by treatment with NaNO2 in H2SO4. A Mitsunobu reaction between the hydroxy group and a Boc-protected piperidine- or piperazine-containing alcohol gave compound 110. Palladium-catalyzed Suzuki reactions on the 5-iodo and 6-chloro groups followed by deprotection produced compounds 5–18. Synthesis of compounds with different R5 and R6 substituents was also started from compound 108. Two selective Suzuki reactions were performed to introduce the R5 and R6 substituents, followed by deprotection to produce compound 47 and its analogs. For compounds 33, 35, 36 and 37, a nucleophilic aromatic substitution reaction was used to replace the 6-Cl of the pyrazine ring to generate the corresponding intermediates.
For synthesis of compounds with a 2-amino substituent (Scheme 2), mono-substitution of 1,6-dibromo-pyridine or -pyrazine (111) with (N-Boc-piperidin-4-yl)methylamine produced compound 112, which was iodized to give 5-iodo product 113. The target compounds can then be obtained following the reactions described above.
Scheme 2.
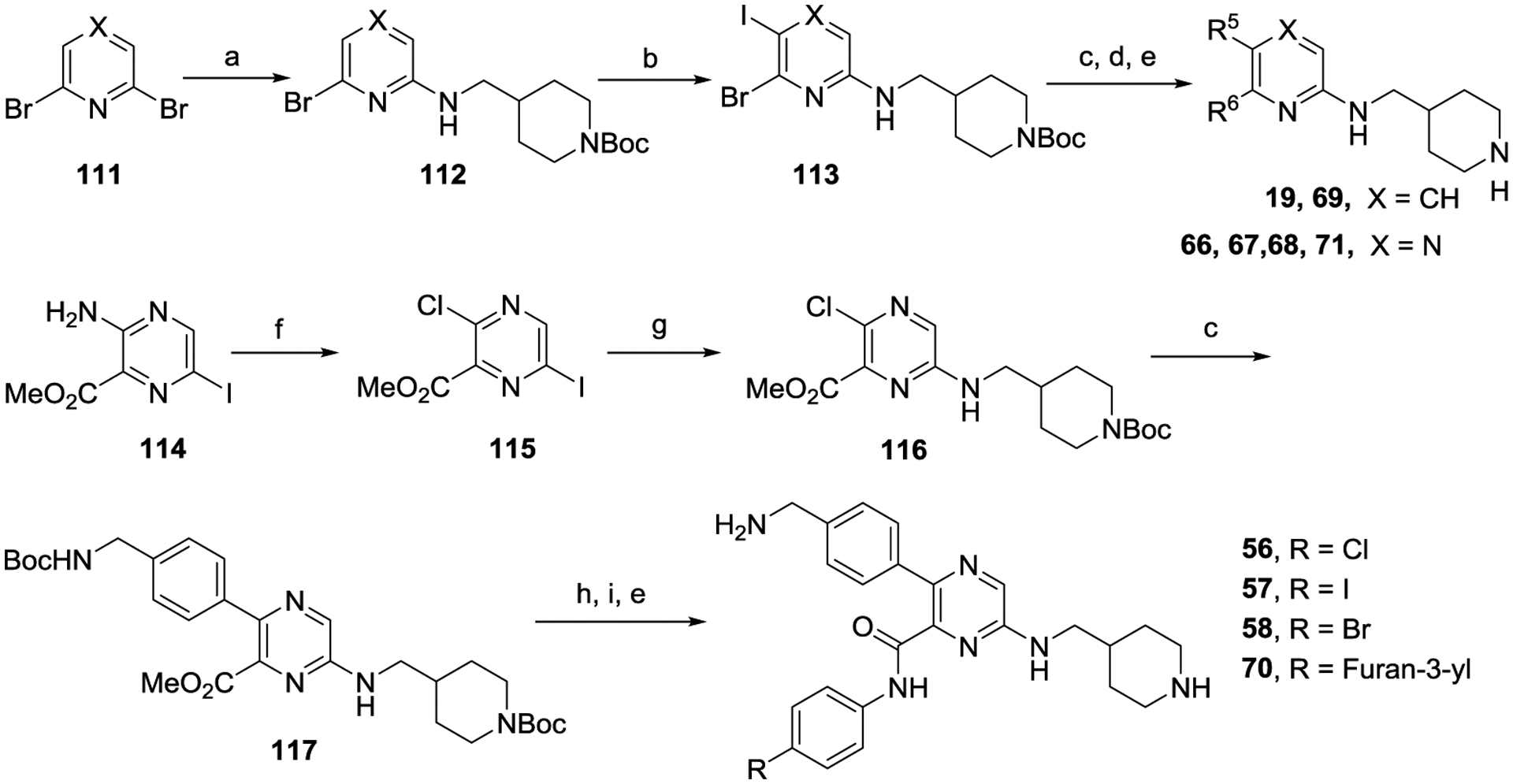
Synthesis of compounds 19, 56−58, and 66−71.a
aReagents and conditions: (a) (N-Boc-piperidin-4-yl)methylamine, K2CO3, DMF, 100 °C, 12 h; (b) N-iodosuccinimide, CH3CN-DMSO, 24 h; (c) Aryl boronic acid or aryl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, Pd(PPh3)4, Na2CO3, 1,4-dioxane-H2O, 80 °C; (d) 2-(4-(furan-3-yl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, Pd(PPh3)4, Na2CO3, 1,4-dioxane-H2O, 100 °C; (e) HCl (4 M in 1,4-dioxane), CH2Cl2, 0 °C; (f) NaNO2, CuCl, HCl (12 M), −10 °C; (g) (N-Boc-piperidin-4-yl)methylamine, K2CO3, DMSO, 50 °C; (h) LiOH, H2O-MeOH, rt; (i) An aniline, HATU (1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate), diisopropylethylamine, DMF.
Scheme 2 also shows the synthesis of compounds 56, 57, 58 and 70. Methyl 3-amino-6-iodopyrazine-2-carboxylate (114) was successively subjected to a Sandmeyer reaction to convert its 3-amino group to a -Cl, a nucleophilic substitution reaction on 6-I, and a Suzuki reaction on 3-Cl to give compound 117. Upon hydrolysis of the methyl ester, the resulting acid was converted to an amide, followed by deprotection to give the target compounds.
The general methods for synthesis of compounds 20-24 and 34 are depicted in Scheme 3. The aldehyde group of 4,5-dibromothiophene-2-carbaldehyde (127) was reduced and converted to a -Cl to give 128, which was reacted with (N-Boc-piperidin-4-yl)methylamine to produce compound 129. Compound 130 was obtained using Suzuki coupling reactions, which was then deprotected to give tri-substituted thiophene 20. Starting from 2-amino-6-chloropyrazine (108), compound 21 was synthesized through bromination, nucleophilic substitution, Suzuki coupling, and deprotection. The reaction between 3,5-dibromo-6-chloropyrazin-2-amine (121) and chloroacetaldehyde produced the imidazo[1,2-a]pyrazine core in compound 124, from which compounds 22-24 were obtained using similar methods as described for compound 21. The reaction between pyrazine compound 118 with a 6-Cl and potassium vinyltrifluoroborate gave compound 119 with a 6-vinyl group, which was subjected to an amination reaction to give, after removing the Boc groups, compound 34.
Scheme 3.
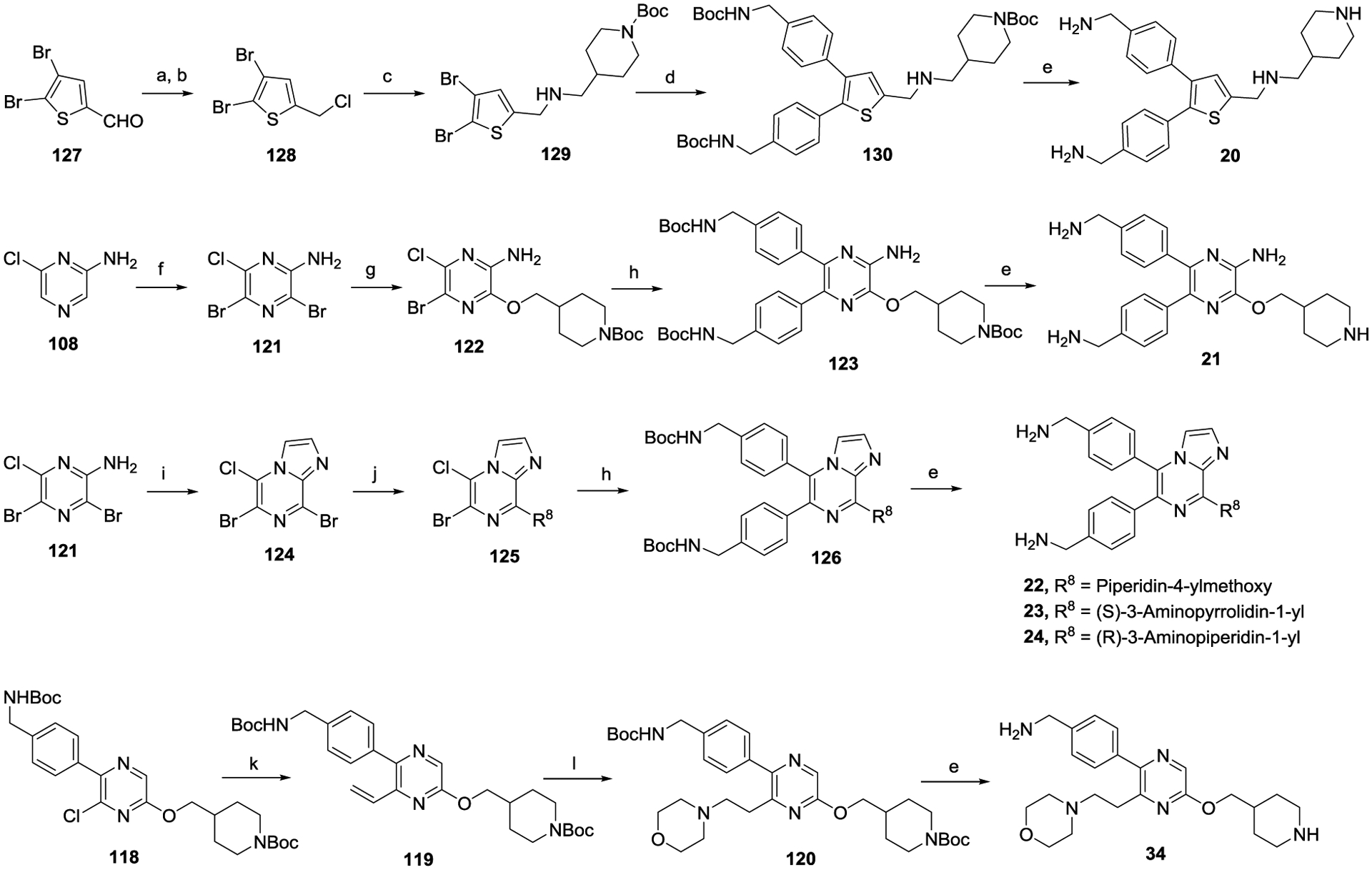
Synthesis of compounds 20−24 and 34.a
aReagents and conditions: (a) NaBH4, MeOH, 0 °C; (b) Cyanuric chloride, DMF; (c) 4-(aminomethyl)-1-Boc-piperidine, K2CO3, DMF; (d) Aryl-boronic acid, Pd(PPh3)4, Na2CO3, 100 °C; (e) HCl (4 M in 1,4-dioxane); (f) N-bromosuccinimide, CH3CN, 0 °C to rt, 18 h; (g) N-Boc-4-piperidinemethanol, NaOH, 1,4-dioxane, 75 °C, 4 h; (h) Aryl-boronic acid, (A-taPhos)2PdCl2, Na2CO3, 1,4-dioxane-H2O, 100 °C, 16 h; (i) Chloroacetaldehyde, isopropyl alcohol, 105 °C, 18 h; (j) an alcohol, NaOH, 1,4-dioxane, 4 h, 75 °C; or an amine, diisopropylethylamine, CH3CN, 4 h, 75 °C; (k) Potassium vinyltrifluoroborate, Cs2CO3, THF-H2O, 100 °C; (l) Morpholine, DBU, 120 °C.
Biochemical assay and initial inhibitor discovery
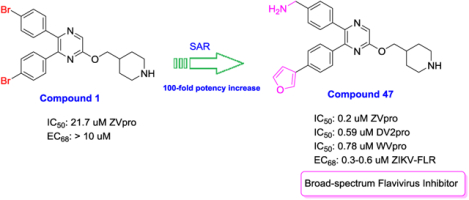
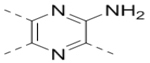
We applied a biochemical assay for recombinant ZVpro, containing NS2B (47–95) and NS3 (1–170) covalently connected with a flexible Gly4-Ser-Gly4 linker. This strategy has been commonly used for Flavivirus protease assay12, 18, 19. Benzoyl-norleucine-lysine-lysine-arginine-(7-amino-4-methylcoumarine) was used as the substrate. Upon ZVpro-mediated hydrolysis, there is a significant increase of fluorescence (Ex/Em: 360/460 nm). We screened our proprietary library of ~1,200 compounds synthesized targeting histone modifying enzymes such as lysine specific demethylase 1 (LSD1)23. 5,6-Di(4-bromophenyl)-2-(piperidin-4-ylmethoxy)pyrazine (Compound 1, Table 1) was found to be an inhibitor of ZVpro with an IC50 value of 21.7 μM. Structure-activity relationship studies based on compound 1 were performed to find more potent inhibitors.
Table 1.
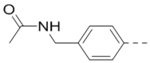
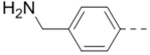
Structures and inhibitory activities of compounds 1–18.a
 | |||||
|---|---|---|---|---|---|
| Compd. | R5=R6 | IC50 (μM) | Compd. | R5=R6 | IC50 (μM) |
| 1b | 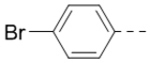 |
21.7 | 10 | 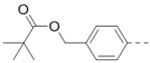 |
9.8 |
| 2 |  |
0.52 | 11 | 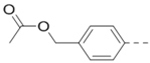 |
>10 |
| 3 (n = 2) |  |
4.7 | 12 |  |
0.62 |
| 4 (n = 3) |  |
>10 | 13 (n = 2) | 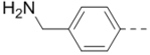 |
>10 |
| 5 | 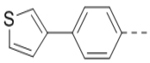 |
1.4 | 14 (X = N, n = 2) | 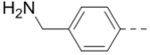 |
>10 |
| 6 | 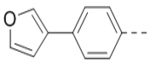 |
1.0 | 15 | 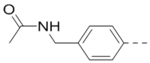 |
>10 |
| 7 | 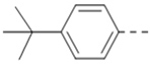 |
3.1 | 16 | 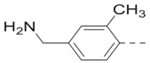 |
2.1 |
| 8 |  |
0.51 | 17 (n = 2) | 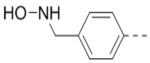 |
1.6 |
| 9 (n = 2) | 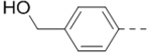 |
2.1 | 18 | 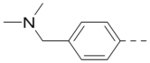 |
0.39 |
Standard errors of all IC50 values are less than 30%.
Unless indicated, X = CH and n = 1.
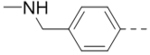
Structure-activity relationship studies
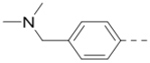
First, a series of pyrazine compounds with identical R5 and R6 substituents were synthesized and their inhibitory activities are shown in Table 1. Replacing the bromo groups at the para-position of the phenyl ring in compound 1 with tert-butyl groups in compound 7 (IC50: 3.14 μM), thiophene-3-yl groups in 5 (IC50: 1.42 μM), furan-3-yl groups in 6 (IC50: 1.0 μM), and iodo groups in 2 (IC50: 0.52 μM) increases the activity against ZVpro by ~7, 15, 20 and 40-fold, respectively. This suggests increased bulk and/or hydrophobicity are favorable. Interestingly, introduction of more polar hydroxymethyl groups at these positions as found in compound 8 (IC50: 0.52 μM) also resulted in a 40-fold increase of the activity, while masking the hydroxy with an acetyl in compound 11 (IC50 >10 μM) or a pivaloyl group in 10 (IC50: 9.8 μM) significantly reduced activity. Similarly, compound 12 (IC50: 0.62 μM) with aminomethyl groups exhibits a comparable activity (to compounds 2 and 8). An additional ortho-methyl group as found in compound 16 (IC50: 2.14 μM) leads to a 3-fold activity reduction, suggesting the substitution is disfavored. While acetylation of the primary amine groups in 12 yielded inactive compound 15 (IC50 >10 μM), masking the amine with N,N-dimethyl groups as found in compound 18 (IC50: 0.39 μM) increased the inhibitory activity.
Next, the length of R2 sidechain was evaluated. Generally, a longer R2 linker leads to significant reduction in activity. Compounds 3 (IC50: 4.65 μM), 17 (IC50: 1.62 μM), 4 (IC50 >10 μM), 9 (IC50: 2.1 μM), 13 (IC50 >10 μM) and 14 (IC50 >10 μM) are less active than their analogs with a piperidin-4-ylmethoxy substituent.
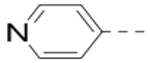
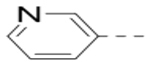
Several analogs of compound 12 with different central aromatic rings were synthesized to evaluate their SARs (Table 2). Switching to a pyridine in compound 19, thiophene in 20, 3-aminopyrazine in 21, and imidazo[1,2-a]pyrazine in compounds 22-24 resulted in considerable (3–30×) activity reduction.
Table 2.
Structures and inhibitory activities of compounds 19–24.a
 | |||
|---|---|---|---|
| Compd. | R2 | Heterocycle | IC50 (μM) |
| 19 | 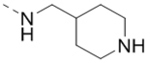 |
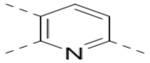 |
3.7 |
| 20 | 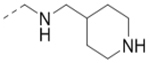 |
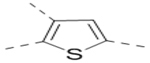 |
10.7 |
| 21 | 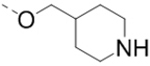 |
 |
2.1 |
| 22 | 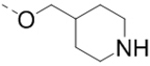 |
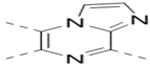 |
9.3 |
| 23 | 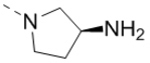 |
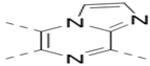 |
19.3 |
| 24 | 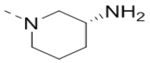 |
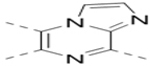 |
3.5 |
Standard errors of all IC50 values are less than 30%.
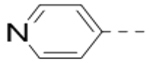
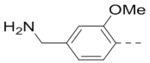
Based on the SARs described above, the pyrazine core with a 2-piperidin-4-yl-methoxy group is favorable for ZVpro inhibition. We next synthesized compounds to optimize the R5 substituent of compounds 12 and 18. Compound 25 (IC50: 8.1 μM, Table 3) containing a 4-hydroxyphenyl group at the R5 position exhibits more than 10-fold activity reduction, as compared to 12 (IC50: 0.62 μM). Dimethyl analog 26 is also a weak inhibitor (IC50: 20.5 μM). Acetylation of the 5-substituent (of 12) is disfavored in 27 (IC50: 30 μM). A pyrindin-4-yl group in compound 28 (IC50: 0.32 μM) increases the inhibitory potency, while its analog 29 (IC50: 1.7 μM) with a longer R2 side chain is less potent. Similar to compound 16, adding an ortho-methoxy group to the 5-substituent of 28 resulted in a significant activity reduction in compound 30 (IC50: 3.5 μM). Compound 31 with a pyrin-3-yl group at the R5 position is a weak inhibitor (IC50: 9.9 μM).
Table 3.
Structures and inhibitory activities of compounds 25–31.a
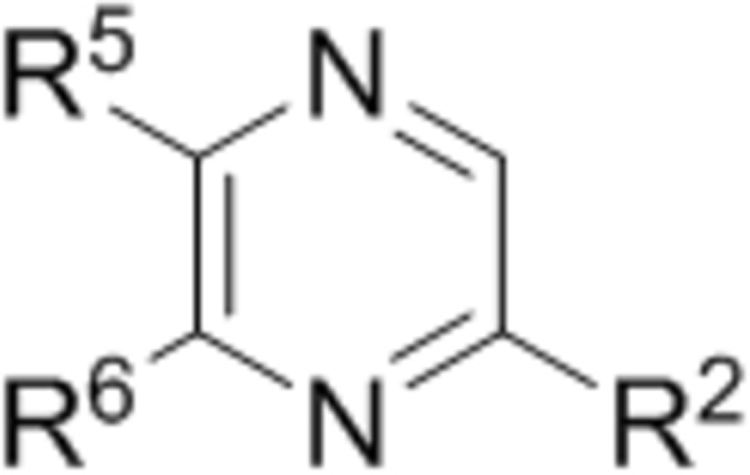 | ||||
|---|---|---|---|---|
| Compd. | R2 | R5 | R6 | IC50 (μM) |
| 25 | 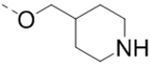 |
 |
 |
8.1 |
| 26 | 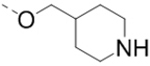 |
 |
 |
20.5 |
| 27 | 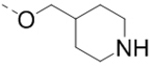 |
 |
 |
30.0 |
| 28 | 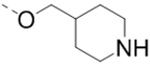 |
 |
 |
0.32 |
| 29 | 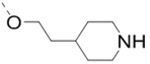 |
 |
 |
1.7 |
| 30 | 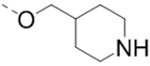 |
 |
 |
3.5 |
| 31 | 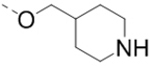 |
 |
 |
9.9 |
Standard errors of all IC50 values are less than 30%.
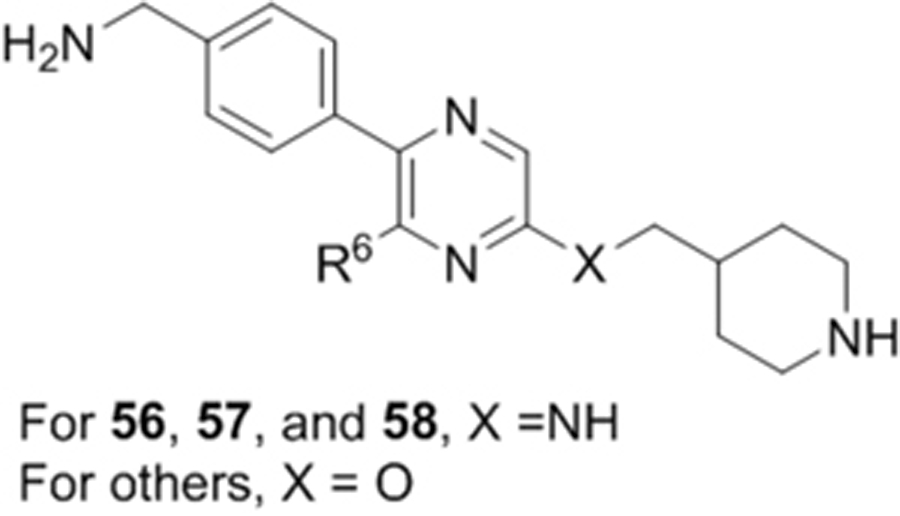
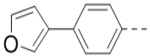
We next wanted to optimize the R6 substituent in potent compounds 8 and 12 and the results are summarized in Table 4. Surprisingly, adding a meta-F group to the phenyl ring for compound 32 (IC50 >50 μM) significantly reduced the activity, which might indicate a strict electronic and/or steric tolerance for the 6-phenyl ring. A series of smaller 6-substituents were introduced. Compounds 33 (IC50: 7.3 μM) with a 2-aminoethylamino and 34 (IC50 >50 μM) with a 2-morpholinyl-ethyl group are weak inhibitors. Similarly, compounds 35 (IC50 >50 μM), 36 (IC50 >10 μM), 37 (IC50: 6.6 μM), 38 (IC50 : 24.6 μM), 39 (IC50: 5.8 μM), and 40 (IC50: 11.1 μM) containing 5-membered heterocycles are significantly less active than 12. Compounds 41-47 with a terminal aromatic group were evaluated. While compounds 41-45 show reduced activities, compound 46 (IC50: 0.71μM) with a pyrazol-4-yl group retains the activity and compound 47 with a furan-3-yl group has a significantly enhanced activity with an IC50 value of 200 nM. More variety of the 4-substituent at the 6-phenyl ring were evaluated. As compared to 12, an acetyl group in compound 48 (IC50: 3.16 μM) and isopropyl in 49 (IC50: 2.02 μM) reduce the inhibitory activity by 3- and 5-fold, while compound 50 with a hydroxy group (IC50: 0.76 μM) shows a comparable activity. Compounds 51-55 with a fused bicyclic aromatic ring at the R6 position were found to give reduced activities with IC50 values of 1.1–21 μM. Three aromatic amide 6-substituents in compounds 56-58 were found to be disfavored (IC50: 12–17 μM).
Table 4.
Structures and inhibitory activities of compounds 32–58.a
 | |||||
|---|---|---|---|---|---|
| Compd. | R6 | IC50 (μM) | Compd. | R6 | IC50 (μM) |
| 32 | 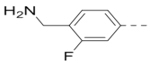 |
>50 | 46 | 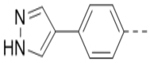 |
0.71 |
| 33 | 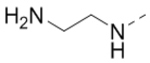 |
7.3 | 47 | 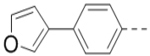 |
0.20 |
| 34 | 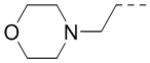 |
>50 | 48 | 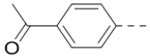 |
3.2 |
| 35 | 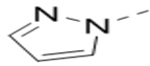 |
>50 | 49 | 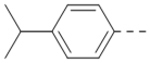 |
2.0 |
| 36 | 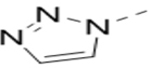 |
>10 | 50 | 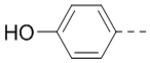 |
0.76 |
| 37 | 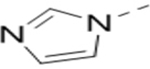 |
6.6 | 51 | 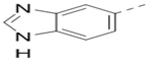 |
2.22 |
| 38 | 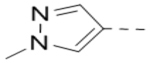 |
24.6 | 52 |  |
1.6 |
| 39 |  |
5.8 | 53 |  |
21.1 |
| 40 |  |
11.1 | 54 | 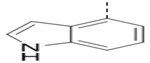 |
7.1 |
| 41 |  |
>10 | 55 |  |
1.1 |
| 42 |  |
>10 | 56 | 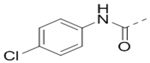 |
17 |
| 43 |  |
>10 | 57 | 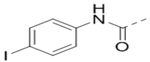 |
12.6 |
| 44 | 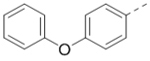 |
32.6 | 58 |  |
12.6 |
| 45 |  |
26.5 | |||
Standard errors of all IC50 values are less than 30%.
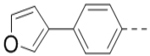
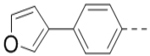
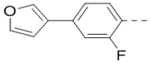
With the discovery of compound 47 showing an IC50 of 200 nM against ZVpro (as well as potent cellular anti-ZIKV activity described below), it became the focus of the SAR studies. Switching the positions of the R5 and R6 substituents produced compound 59 (Table 5) with a reduced inhibitory activity (IC50: 0.68 μM). Masking the primary amino group of 59 with one and two methyl groups for compounds 60 (IC50: 3.3 μM) and 61 (IC50: 1.8 μM) further reduces the potency. Similarly, mono- and di-methylation of 47 led to activity reduction for compounds 62 (IC50: 0.53 μM) and 63 (IC50: 6.1 μM). Compound 64 and 65 with a longer R2 sidechain are considerably less active than their corresponding analogs 59 and 47. In addition, analogous compounds 66-71 with an -NH-containing R2 substituent were synthesized. A simple change from -O- in 47 to -NH- in 66 (IC50: 0.40 μM) resulted in a 2-fold activity decrease. Adding a F- to either the 6- (in 67) or 5-subsituent (in 68) led to a significant activity reduction. Changing the central pyrazine ring in 66 to a pyridine in 69 (IC50: 0.79 μM) also reduced the potency. Insertion of an amide linker into the R6 group for compound 70 is disfavored (IC50: 1.1 μM). Compounds 71 and 72 with a thiophene-3-yl terminal group for the R6 were found to have modest activities, suggesting a thiophene is less favored than a furan-3-yl group.
Table 5.
Structures and inhibitory activities of compounds 59–72.a
 | ||||
|---|---|---|---|---|
| Compd. | R2 | R5 | R6 | IC50 (μM) |
| 59 | 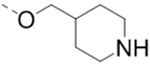 |
 |
 |
0.68 |
| 60 | 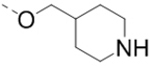 |
 |
 |
3.3 |
| 61 | 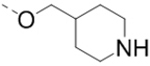 |
 |
 |
1.8 |
| 62 | 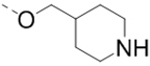 |
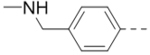 |
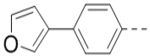 |
0.53 |
| 63 | 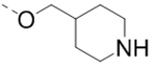 |
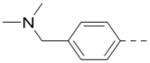 |
 |
6.1 |
| 64 | 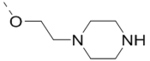 |
 |
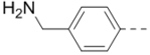 |
1.8 |
| 65 | 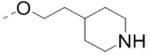 |
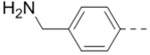 |
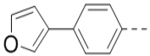 |
1.3 |
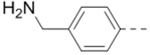
| 66 | 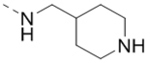 |
 |
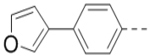 |
0.40 |
| 67 | 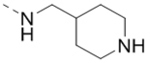 |
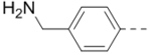 |
 |
2.3 |
| 68 |  |
 |
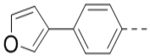 |
5.3 |
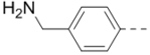
| 69 | 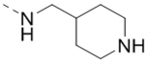 |
 |
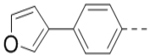 |
0.79 |
| 70 | 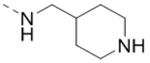 |
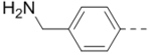 |
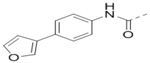 |
1.1 |
| 71 | 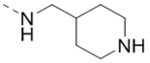 |
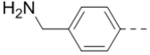 |
 |
18.5 |
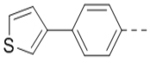
| 72 |  |
 |
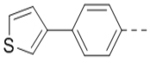 |
4.7 |
Standard errors of all IC50 values are less than 30%.
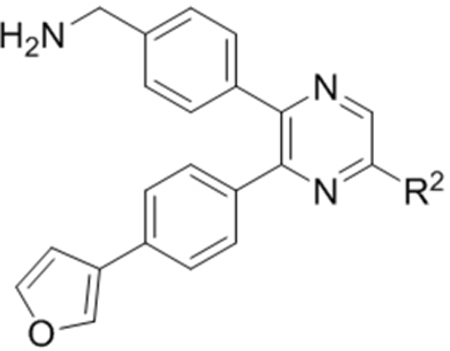
Compounds 73-78 (Table 6) were synthesized to find how modifications of the R2 substituent affects ZVpro inhibition. Changing the piperidine group to a cyclohexyl ring in 73 (IC50: 3.2 μM) or a phenyl ring in 74 (IC50: 3.2 μM) caused >15-fold activity loss. Shortening the R2 length resulted in a 7-fold reduction of potency in 75 (IC50: 1.6 μM). Changing to linear amines in 76 (IC50: 1.3 μM) and 77 (IC50: 2.1 μM) were also disfavored. Compound 78 (IC50: 240 nM) bearing an additional (racemic) methyl group exhibits a comparable activity to that of compound 47.
Table 6.
Structures and inhibitory activities of compounds 73–78.a
 | ||
|---|---|---|
| Compd. | R8 | IC50 (μM) |
| 73 | 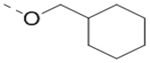 |
3.2 |
| 74 | 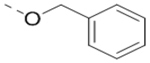 |
3.2 |
| 75 | 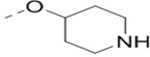 |
1.6 |
| 76 | 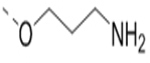 |
1.3 |
| 77 | 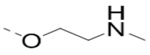 |
2.1 |
| 78 | 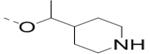 |
0.24 |
Standard errors of all IC50 values are less than 30%.
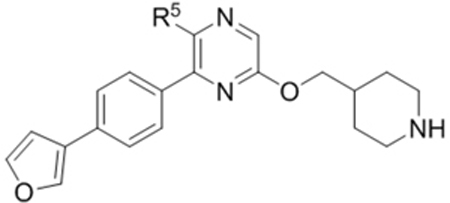
Compounds 79-83 (Table 7) were synthesized to fine-tune the terminal amino group of the R5 substituent of compound 47. Changing it to an amide group in 79 (IC50: 1.1 μM) considerably reduced the activity by ~5×, while further activity reduction was observed upon addition of an ortho-F group for compound 80 (IC50: 2.3 μM). A hydroxyamino group in 81 (IC50: 1.2 μM) is less favored than a primary amine. Adding a methyl group to the R5 phenyl ring in 82 (IC50: 2.6 μM), or moving the aminomethyl group to the meta-position in compound 83 (IC50: 2.9 μM) considerably reduced the activity.
Table 7.
Structures and inhibitory activities of compounds 79–83.a
 | ||
|---|---|---|
| Compd. | R5 | IC50 (μM) |
| 79 | 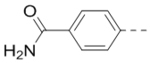 |
1.1 |
| 80 | 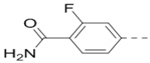 |
2.3 |
| 81 | 1.2 | |
| 82 | 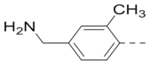 |
2.6 |
| 83 | 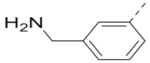 |
2.9 |
Standard errors of all IC50 values are less than 30%.
Compounds 84-103 (Table 8) were synthesized to optimize the R6 substituent of compound 47. The substituent at the ortho-position for compounds 85, 86, 87, and 88, or at the meta-position for 89, 90 and 91 of the phenyl ring dramatically decreased the inhibitory activity, showing a strict steric and/or electronic requirement for the pocket. Next, the furan-3-yl group is optimized. Changing it to an amide in compounds 91-93 (IC50: 3.5–13 μM) was disfavored. Switching to saturated tetrahydrofuran-3-yl substituent for compound 94 (IC50: 0.59 μM) resulted in 3-fold activity drop, while moving the tetrahydrofuran-3-yl group to the meta-position for 95 further reduced the potency. Moreover, a variety of analogous cyclic groups with different steric, electronic, or hydrogen bond forming properties were synthesized and evaluated, including a pyrrolin-1-yl group in compound 96 (IC50: 2.5 μM), piperidin-1-yl group in 97 (IC50 >10 μM), cyclohexyl group in 98 (IC50: 16.2 μM), piperdin-4-yl group in 99 (IC50: 3.1 μM) and 100 (IC50: 2.9 μM), peperazin-1-yl group in 101 (IC50: 0.65 μM), and O-containing groups in compounds 102 (IC50: 1.0 μM), 103 (IC50: 130 nM) and 104 (IC50 >10 μM). It is of interest that compound 103 with a flexible tetrahydropyran-3-yl ring exhibited improved inhibitory activity as compared to 47, while others are less active.
Table 8.
Structures and inhibitory activities of compounds 84–104.a
 | |||||
|---|---|---|---|---|---|
| Compd. | R6 | IC50 (μM) | Compd. | R6 | IC50 (μM) |
| 84 | 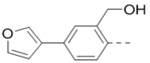 |
>10 | 95 | 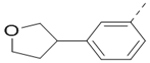 |
>10 |
| 85 | 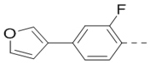 |
8.4 | 96 | 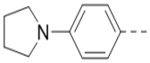 |
2.5 |
| 86 | 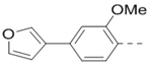 |
1.9 | 97 | 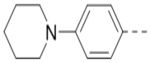 |
>10 |
| 87 | 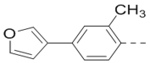 |
17.1 | 98 | 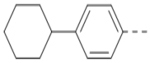 |
16.2 |
| 88 | 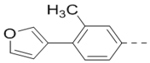 |
10.6 | 99 | 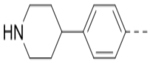 |
3.1 |
| 89 | 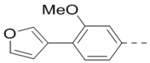 |
8.0 | 100 | 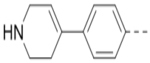 |
2.9 |
| 90 | 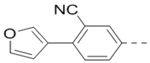 |
1.8 | 101 | 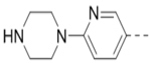 |
0.65 |
| 91 | 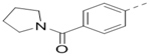 |
13.0 | 102 | 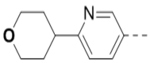 |
1.0 |
| 92 | 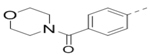 |
>10 | 103 | 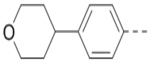 |
0.13 |
| 93 | 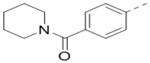 |
3.5 | 104 | 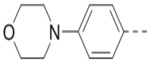 |
>10 |
| 94 | 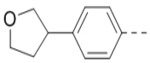 |
0.59 | |||
Standard errors of all IC50 values are less than 30%.
Activity against proteases of dengue and West Nile virus
Dengue and West Nile virus also belong to the Flavivirus family with a high homology to ZIKV, particularly for their NS2B-NS3 proteases. The proteases of the four serotypes of dengue viruses show 49–54% sequence identity and 71–73% similarity to ZVpro, while that of West Nile virus has 66% sequence identity and 78% similarity. 18 compounds with a wide range of inhibitory activities against ZVpro were tested for their activity against the protease of dengue serotype-2 and West Nile virus (DV2pro and WVpro). As shown in Table 9 and Figure 1, these ZVpro inhibitors also inhibit the activity of DV2pro and WVpro, and their activities are correlated with those against ZVpro, showing R2 values of 0.76 and 0.77, respectively. These results suggest these three Flavivirus proteases exhibit similar susceptibility to this series of compounds. Moreover, compound 47 did not inhibit 5 selected human proteases at 10 μM15, showing a high selectivity.
Table 9.
Inhibitory activity IC50 (μM) against Flavivirus proteases ZVpro, DV2pro and WVpro.a
| ZVpro | DV2pro | WVpro | |
|---|---|---|---|
| 44 | 32.6 | >50 | >50 |
| 45 | 26.5 | >50 | >50 |
| 1 | 21.7 | 35 | >50 |
| 20 | 10.7 | >50 | >50 |
| 10 | 9.8 | 13.4 | 20.0 |
| 3 | 4.6 | 10.2 | 1.8 |
| 7 | 3.1 | 8.4 | 9.5 |
| 16 | 2.1 | 6.3 | 3.6 |
| 17 | 1.6 | 3.8 | 3.2 |
| 5 | 1.4 | 3.6 | 6.6 |
| 55 | 1.1 | 0.64 | 0.93 |
| 79 | 1.1 | 0.98 | 1.34 |
| 69 | 0.79 | 0.86 | 1.3 |
| 46 | 0.71 | 0.21 | 0.12 |
| 62 | 0.53 | 0.73 | 0.87 |
| 66 | 0.40 | 0.29 | 0.51 |
| 47 | 0.20 | 0.59 | 0.78 |
| 103 | 0.13 | 2.4 | 0.82 |
Standard errors of all IC50 values are less than 30%.
Figure 1.
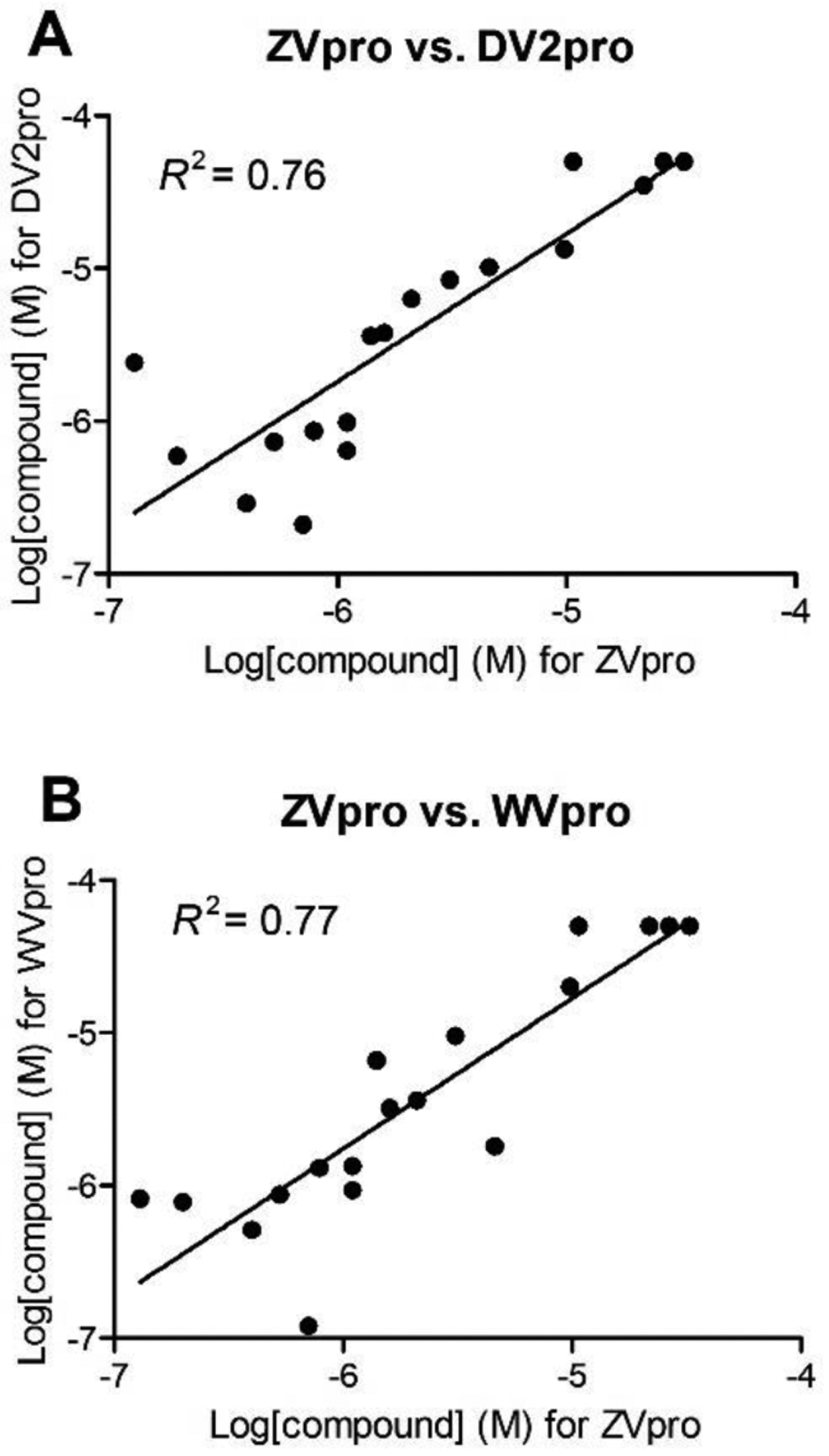
Activities of ZVpro inhibitors are correlated with those of (A) DV2pro and (B) WVpro with R2 values of 0.76 and 0.77, respectively.
The crystal structures of compounds 46, 47 and 66 in complex with DV2pro have been published in our previous communication15 Given the high homology between DV2pro and ZVpro, particularly in the active site, the binding of these molecules to Zika protease is expected to be similar, which has been confirmed with our enzyme kinetics studies. Other analogs described above should adopt a similar binding pose in the protein. In addition, it could explain many of the SARs described above. For example, the crystal structures show there are strong hydrogen bond and electrostatic interactions between their -NH2 group and Asp75, which explains, as compared to 47 (IC50 = 0.20 μM), compounds 62 (IC50 = 0.53 μM) and 63 (IC50 = 6.1 μM) with -NHMe and -N(Me)2 exhibited significantly reduced activities. Moreover, the steric and hydrophobic nature of the R6 binding pocket may explain many of the observed SARs for compounds in Table 8.
Anti-ZIKV activity
Antiviral activities of selected ZVpro inhibitors were evaluated in human U87 glioma cells and monkey Vero cells15, in which ZIKV replicates rapidly. ZIKV does not cause cytopathic effects (CPE) in U87 cells24, but it causes significant CPE and, eventually, cell death and lysis in ~5–7 days in Vero cells lacking interferon-mediated defense25. This feature can be conveniently used to detect ZIKV in Vero cells. The passage-3 stock of the FLR strain of ZIKV, which was isolated from the serum of a patient infected in Colombia in 201526, was used for the antiviral experiments. 0.01 multiplicity of infection (MOI, the number of infectious viral particles per cell) of ZIKV was added to a monolayer of cells to initiate viral infection. After 1h for virus attachment, cells were washed and incubated with fresh media containing increasing concentrations of a compound for 48h. The viral titer of the supernatant containing newly generated ZIKV was determined using an end-point dilution assay and the anti-ZIKV activity of the compound can be evaluated15.
First, selected ZVpro inhibitors were tested for their cytotoxicity against U87 and Vero cells, using MTT assay. Compounds in Table 8 did not inhibit proliferation of these cells at 10 μM. These compounds were next evaluated for their anti-ZIKV activity using the method described above. As shown in Table 8, except for compounds 1 and 50, other compounds inhibited ZIKV replication with EC68 (concentration at which the number of infectious ZIKV in the supernatant is reduced by 68% (half-log)) values of 0.3–5 μM. In addition, their anti-ZIKV activities are generally correlated with the inhibitory activities against ZVpro (Table 10), suggesting ZVpro is the cellular target. Compound 50, a potent inhibitor of ZVpro, did not inhibit ZIKV replication at 10 μM. In addition to its 5-aminomethylphenyl substituent, compound 50 contains a polar 4-hydroxyphenyl (phenol) group at the 6-position, which might significantly reduce its cell permeability.
Table 10.
Antiviral EC68 (μM) against ZIKV-FLR in U87 cells.
| ZVpro IC50 (μM) | ZIKV-FLR EC68 (μM) | |
|---|---|---|
| 1 | 21.7 | >10 |
| 50 | 0.76 | >10 |
| 7 | 3.1 | 5.0 |
| 102 | 1.0 | 3 |
| 79 | 1.1 | 2.5 |
| 55 | 1.1 | 2.5 |
| 46 | 0.71 | 2.5 |
| 69 | 0.79 | 1.2 |
| 59 | 0.68 | 1.2 |
| 62 | 0.53 | 1.2 |
| 66 | 0.40 | 1.2 |
| 78 | 0.24 | 1.2 |
| 103 | 0.13 | 0.6 |
| 47 | 0.20 | 0.3–0.6 |
The most active compound 47 was found to potently inhibit replication of ZIKV strain HN16 and dengue virus (serotype-2, strain K0049) with comparable activities in our previous communication15. Compound 47 exhibited strong in vivo antiviral activity in a mouse model of ZIKV infection. Treatment with compound 47 (30 mg/kg) for 3 days inhibited ZIKV viral loads in plasma and brain by 96% and 98% (at 24h) and significantly prolonged survivals of the experimental mice15.
CONCLUSION
Compound screening found 2,5,6-trisubstituted pyrazine compound 1 is a novel inhibitor of ZVpro. Iterative SAR and medicinal chemistry studies were performed find more potent inhibitors. The initial series of compounds with identical R5 and R6 groups led to the discovery of compound 12 with an IC50 of 620 nM. Subsequent scaffold hopping did not yield a better core, suggesting the importance of the central pyrazine ring. Next, optimization of the R6 group of compound 12 gave rise to a potent inhibitor 47 having a furanylphenyl substituent showing an IC50 of 200 nM. Further modification based on 47 produced compound 103 with an IC50 of 130 nM against ZVpro. Activity optimization for the R5 or R2 group did not yield more potent compounds. ZVpro inhibitors also inhibit activity of homologous proteases of dengue and West Nile virus and their inhibitory activities are well correlated with a R2 of 0.77, showing these compounds are broad-spectrum inhibitors of Flavivirus proteases. Cell-based assays showed compounds 47 and 103 exhibited the most potent antiviral activity against ZIKV with EC68 values of 300–600 nM. Compound 47 also showed strong in vivo antiviral activity in a mouse model of ZIKV infection. Compound 47 and related compounds are potent, broadly active inhibitors of Flavivirus proteases and therefore, represent novel pharmacological leads for developing antiviral drugs against Zika, dengue or West Nile virus infections.
Experimental Section
All chemicals for synthesis were purchased from Alfa Aesar (Ward Hill, MA) or Aldrich (Milwaukee, WI). Unless otherwise stated, all solvents and reagents used as received. All reactions were performed using a Teflon-coated magnetic stir bar at the indicated temperature and were conducted under an inert atmosphere when stated. The identity of the synthesized compounds was characterized by 1H and 13C NMR on a Varian (Palo Alto, CA) 400-MR spectrometer and mass spectrometer (Shimadzu LCMS-2020). Chemical shifts were reported in parts per million (ppm, δ) downfield from tetramethylsilane. Proton coupling patterns are described as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), and broad (br). The identity of the potent inhibitors was confirmed with high resolution mass spectra (HRMS) using an Agilent 6550 iFunnel quadrupole-time-of-flight (Q-TOF) mass spectrometer with electrospray ionization (ESI). The purities of the final compounds were determined to be >95% with a Shimadzu Prominence HPLC using a Zorbax C18 (or C8) column (4.6 × 250 mm) monitored by UV at 254 nm.
General Synthetic Procedure for compounds 1–4:
Sodium hydroxide solution (12.5 M, 3.2 mL, 40 mmol) was added over 30 min to a refluxing mixture of 4,4’-dibromobenzil (105, 7.36 g, 20 mmol), glycine amide hydrogen chloride (106, 2.21 g, 20 mmol), and 50 mL methanol. After refluxing for another 30 min, the mixture was treated with HCl (12 N, 2.5 mL), followed by KHCO3 (2 g). The following yellow solid formed was filtered off, washed well with water and recrystallized from tBuOH. Yellow needles of 5,6-bis(4-bromophenyl)pyrazin-2-ol (107, 5.94 g) was obtained after filtration. 1H NMR (400 MHz, CDCl3) δ 8.12 (s, 1H), 7.54 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 7.26 (d, J = 8.4 Hz, 2H), and 7.15 (d, J = 8.4 Hz, 2H).
To a solution of 2-hydroxypyrazine 107 (1.12 g, 2.76 mmol) and N-Boc-4-piperidinemethanol (594 mg, 2.76 mmol) in anhydrous THF (27 mL) was added triphenylphosphine (1.16 g, 4.42 mmol) and DIAD (894 mg, 4.42 mmol). The reaction mixture was stirred at room temperture for 17 h. The solution was then concentrated and purified to give the product tert-butyl 4-(((5,6-bis(4-bromophenyl)pyrazin-2-yl)oxy)methyl)piperidine-1-carboxylate 1 precursor (1.5 g, 90%). 1H NMR (400 MHz, CDCl3) δ 8.22 (s, 1H), 7.47–7.41 (m, 4H), 7.30–7.24 (m, 4H), 4.26 (d, J = 6.8 Hz, 2H), 4.15 (br, 2H), 2.75 (t, J = 12.8 Hz, 2H), 2.03 (br, 1H), 1.81 (d, J = 13.2 Hz, 2H), 1.46 (s, 9H), and 1.35 – 1.25 (m, 2H).
To a solution of the above 1 precursor (1.5 g, 2.49 mmol) in CH2Cl2 (25 mL) was added dropwise hydrogen chloride (4 N in p-dioxane, 12.5 mL) at 0 °C. The solution was stirred overnight and precipitated solid was filtered off to give 1.1 g compound 1 as pale yellow powder. 2,3-bis(4-Bromophenyl)-5-(piperidin-4-ylmethoxy)pyrazine hydrochloride (1). 1H NMR (400 MHz, DMSO-d6) δ 8.92 (br, 2H), 8.33 (s, 1H), 7.53 (d, J = 8.0 Hz, 2H), 7.49 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 8.4 Hz, 2H), 7.24 (d, J = 8.4 Hz, 2H), 4.26 (d, J = 5.6 Hz, 2H), 3.26 (d, J = 12.0 Hz, 2H), 2.88 (t, J = 12.4 Hz, 2H), 2.11 (br, 1H), 1.90 (d, J = 12.8 Hz, 2H), 1.57–1.47 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.2, 147.1, 143.4, 137.8, 137.4, 133.3, 132. 1, 131.9, 131.79, 131.70, 122.9, 122.0, 70.1, 43.1, 33.3, 25.5; MS (ESI) [M+H]+ 504.2.
2,3-bis(4-Iodophenyl)-5-(piperidin-4-ylmethoxy)pyrazine hydrochloride (2) was prepared from 4,4′-Iodobenzil, following the same procedure as compound 1, as a hydrochloric acid salt (pale yellow powder). 1H NMR (400 MHz, DMSO-d6) δ 8.93 (s, 1H), 8.59 (s, 1H), 8.33 (s, 1H), 7.72–7.60 (m, 4H), 7.16–7.08 (m, 4H), 4.5 (s, 2H), 3.26 (s, 2H), 2.85 (brs, 2H), 2.10 (s, 1H), 1.87 (d, J = 10.4 Hz, 2H), 1.46 (s, 1H); MS (ESI) [M+H]+ 598.0.
2,3-bis(4-iodophenyl)-5-(2-(piperidin-4-yl)ethoxy)pyrazine hydrochloride (3) was prepared from 4,4’-Iodobenzil, following the same procedure as compound 1, as a hydrochloric acid salt (pale yellow powder). 1H NMR (400 MHz, DMSO-d6) δ 8.93 (s, 1H), 8.70 (s, 1H), 8.35 (s, 1H), 7.72 (dd, J = 15.6, 7.2 Hz, 4H), 7.16 (dd, J = 28.0, 7.2 Hz, 4H), 4.44 (s, 2H), 3.22 (d, J = 12.0 Hz, 2H), 2.82 (dd, J = 22.8, 11.6 Hz, 2H), 1.86 (d, J = 13.2 Hz, 2H), 1.75 (s, 3H), 1.40 (dd, J = 19.2, 10.4 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.8, 146.8, 142.9, 137.8, 137.7, 137.4, 137.2, 137.1, 132.9, 131.6, 131.5, 130.8, 63.8, 43.0, 34.3, 30.2, 28.2; MS (ESI) [M+H]+ 612.0
2,3-bis(4-Iodophenyl)-5-(3-(piperidin-4-yl)propoxy)pyrazine hydrochloride (4) was prepared from 4,4’-Iodobenzil, following the same procedure as compound 1, as a hydrochloric acid salt (pale yellow powder).1H NMR (400 MHz, DMSO-d6) δ 8.90 (s, 1H), 8.67 (s, 1H), 8.36 (s, 1H), 7.72 (dd, J = 15.3, 8.2 Hz, 4H), 7.16 (dd, J = 26.0, 8.1 Hz, 4H), 4.38 (s, 2H), 3.21 (d, J = 10.8 Hz, 2H), 2.80 (d, J = 9.9 Hz, 2H), 1.79 (s, 4H), 1.56 (s, 1H), 1.41 – 1.27 (m, 4H); 13C NMR (100 MHz, DMSO-d6) δ 157.9, 146.8, 142.9, 137.7, 137.4, 137.2, 137.1, 133.0, 131.6, 131.5, 95.8, 94.8, 66.4, 43.1, 32.6, 31.8, 28.3, 25.2; MS (ESI) [M+H]+ 626.0.
General Synthetic Procedure for compounds (5)-(18):
To a solution of 2-amino-6-chloropyrazine (108, 8 g, 62 mmol) in DMSO (50 mL) was added N-iodosuccinimide (NIS, 15.3 g, 68 mmol) in portions. After stirring at room temperature for 72 h, the reaction was quenched with sodium thiosulfate aqueous solution (50 mL). The mixture was extracted with ethyl acetate (3 × 100 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 5:1 to 2:1) to afford 6-chloro-5-iodopyrazin-2-amine (109, 12.7 g, 80%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ 7.72 (s, 1H), and 4.73 (br, 2H).
To a suspension of 109 (3.78 g, 14.9 mmol) in sulfuric acid (18 mL) at 0 °C was added sodium nitrite (1.09 g, 15.8 mmol) in 3 portions. The resulting reaction mixture was stirred at 0 °C for 1 h. The mixture was then poured into a beaker with ice while stirring. The resulting precipitate was collected by filtration, washed with water and dried under vacuum to afford 6-chloro-5-iodopyrazin-2-ol (3.6 g) as a yellowish solid, which is used directly for the next step. 1H NMR (400 MHz, DMSO-d6) δ 7.98 (s, 1H). Crude product 6-chloro-5-iodopyrazin-2-ol (3.6 g, 14 mmol), N-Boc-4-piperidinemethanol (3.1 g, 14.5 mmol), and triphenylphosphine (5.9 g, 22.5 mmol) were dissolved in THF (40 mL) and cooled to 0 °C. Diisopropyl azodicarboxylate (4.55 g, 22.5 mmol) was added dropwise under nitrogen atmosphere. The mixture was warmed to room temperature and stirred for 12 h. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 10:1 to 5:1) to afford compound 110 (5.2 g, 77% for 2 steps) as an off-white solid. 1H NMR (400 MHz, CDCl3) δ 7.96 (s, 1H), 4.16 (d, J = 6.4 Hz, 4H), 2.73 (t, J = 12.0 Hz, 2H), 1.96 (s, 1H), 1.77 (d, J = 12.8 Hz, 2H), 1.46 (s, 9H), and 1.33 – 1.19 (m, 2H).
Compound 110 (1.94 mmol), arylboronic acid or aryl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (2.51 mmol), tetrakis(triphenylphosphine)palladium (110 mg, 0.095 mmol), and sodium carbonate (610 mg, 5.75 mmol) in p-dioxane/H2O (15/3 mL) were placed in a sealed tube. The mixture was degassed and heated to 110 °C for 24 h. The reaction was then cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 5:1 to 1:2) to afford the R5 and R6 identically substituted product. To a solution of this intermediate (1.5 mmol) in DCM (5 mL) was added dropwise HCl (1.2 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 12 h. The volatiles were removed in vacuo to afford an oil, which was triturated in diether ether and solidified to give the final product hydrochloric salt.
For the synthesis precursors of 10 and 11 only: To a solution of the above R5 and R6 identically substituted product (R = 4-(OHCH2) -Ph, 0.2 mmol) and N-diisopropylethylamine (87 μL, 0.5 mmol) in anhydrous dichloromethane (3 mL) was added Acetyl chloride (18 μL, 0.25 mmol) or Pivaloyl chloride (31 μL, 0.25 mmol) at °C, respectively. The mixture was stirred for 2 h before it was quenched with saturated NaHCO3. The mixture was extracted with ethyl acetate (3 × 80 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 1.5:1 to 1:1) to afford precursors of 10 (112 mg, 95%) or 11 (124 mg, 92%) as colorless oil. For precursor of 10: 1H NMR (400 MHz, CDCl3) δ 8.22 (s, 1H), 7.41 (dd, J = 25.6, 7.4 Hz, 4H), 7.29 – 7.18 (m, 4H), 5.09 (s, 4H), 4.27 (d, J = 6.0 Hz, 2H), 4.15 (s, 2H), 2.82 – 2.65 (m, 2H), 2.15 – 1.91 (m, 7H), 1.82 (d, J = 11.7 Hz, 2H), 1.51 – 1.34 (m, 9H), 1.29 (dd, J = 25.3, 13.6 Hz, 2H); For precursor of 11: 1H NMR (400 MHz, CDCl3) δ 8.24 (s, 1H), 7.44 (d, J = 6.9 Hz, 2H), 7.37 (d, J = 8.3 Hz, 2H), 7.26 (s, 4H), 5.10 (s, 4H), 4.29 (d, J = 6.0 Hz, 2H), 4.16 (s, 2H), 2.76 (t, J = 14.2 Hz, 2H), 2.01 (s, 1H), 1.83 (d, J = 13.0 Hz, 2H), 1.55 (s, 2H), 1.47 (s, 9H), 1.28–1.18 (m, 18H).
5-(Piperidin-4-ylmethoxy)-2,3-bis(4-(thiophen-3-yl)phenyl)pyrazine hydrochloride (5) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.13 (s, 1H), 8.79 (s, 1H), 8.34 (s, 1H), 7.94 – 7.85 (m, 2H), 7.68 (t, J = 7.7 Hz, 4H), 7.60 (dd, J = 5.5, 2.4 Hz, 2H), 7.55 (dd, J = 3.0, 1.4 Hz, 2H), 7.46 (d, J = 8.4 Hz, 2H), 7.38 (d, J = 8.4 Hz, 2H), 4.29 (d, J = 6.3 Hz, 2H), 3.27 (d, J = 11.5 Hz, 2H), 2.88 (dd, J = 22.6, 11.5 Hz, 2H), 2.13 (s, 1H), 1.91 (d, J = 13.2 Hz, 2H), 1.54 (dd, J = 22.8, 11.1 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.6, 147.3, 143.8, 140.7, 140.6, 137.1, 136.7, 135.2, 134.5, 132.3, 130.2, 129.9, 127.3, 127.2, 126.1, 126.1, 125.8, 125.8, 121.7, 121.4, 69.5, 42.7, 33.0, 25.2; MS (ESI) [M+H]+ 510.2.
2,3-bis(4-(Furan-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazine hydrochloride (6) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.93 (s, 1H), 8.57 (s, 1H), 8.33 (s, 1H), 8.19 (d, J = 6.0 Hz, 2H), 7.75 – 7.67 (m, 2H), 7.61 – 7.49 (m, 4H), 7.44 (d, J = 8.0 Hz, 2H), 7.36 (d, J = 8.0 Hz, 2H), 6.95 (s, 2H), 4.30 (d, J = 6.0 Hz, 2H), 3.30 (d, J = 11.6 Hz, 2H), 2.91 (t, J = 12.4 Hz, 2H), 2.14 (s, 1H), 1.94 (d, J = 13.6 Hz, 2H), 1.51 (dd, J = 24.4, 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) 158.0, 147.7, 144.9, 144.8, 144.2, 140.3, 140.1, 137.3, 136.9, 132.6, 131.8, 130.5, 130.2, 125.7, 109.0; MS (ESI) [M+H]+ 429.2.
2,3-bis(4-(tert-Butyl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazine hydrochloride (7) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.00 – 8.27 (br, 4H), 8.30 (s, 1H), 7.38 – 7.26 (m, 8H), 4.27 (d, J = 6.2 Hz, 2H), 3.33 – 3.24 (m, 2H), 2.90 (t, J = 11.8 Hz, 2H), 2.13 (br, 1H), 1.93 (d, J = 13.0 Hz, 2H), 1.58 – 1.48 (m, 2H), 1.26 (s, 18H); 13C NMR (100 MHz, DMSO-d6) 157.4, 151.2, 150.3, 147.4, 144.0, 135.7, 135.4, 131.9, 129.2, 129.0, 125.0, 124.9, 69.4, 42.7, 34.4, 34.3, 33.0, 31.1, 31.0, 25.2; HRMS (ESI+) calcd for C30H40N3O [M+H]+ 458.3166, found 458.3179.
((5-(Piperidin-4-ylmethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))dimethanol hydrochloride (8) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.00 (s, 1H), 8.65 (s, 1H), 8.33 (s, 1H), 7.58 (d, J = 19.6 Hz, 2H), 7.36 (s, 2H), 7.26 (br, 6H), 4.49 (br, 4H), 3.56 (s, 2H), 3.28 (s, 2H), 2.89 (s, 2H), 2.14 (s, 1H), 1.92 (s, 2H), 1.52 (s, 2H); δ 158.0, 148.0, 144.6, 143.5, 142.6, 137.3, 137.0, 132.4, 132.0, 129.8, 129.5, 126.6, 69.8, 63.0, 62.9, 43.2, 33.4, 25.6; MS (ESI) [M+H]+ 406.2
((5-(2-(Piperidin-4-yl)ethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))dimethanol hydrochloride (9) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.90 (s, 1H), 8.67 (s, 1H), 8.36 (s, 1H), 7.72 (dd, J = 15.3, 8.2 Hz, 4H), 7.16 (dd, J = 26.0, 8.1 Hz, 4H), 4.38 (s, 2H), 3.21 (d, J = 10.8 Hz, 2H), 2.80 (d, J = 9.9 Hz, 2H), 1.79 (s, 4H), 1.56 (s, 1H), 1.41 – 1.27 (m, 4H); 1H NMR (400 MHz, DMSO-d6) δ 8.98 (s, 1H), 8.74 (s, 1H), 8.31 (s, 1H), 7.36 (d, J = 7.7 Hz, 2H), 7.31 – 7.21 (m, 6H), 4.49 (s, 4H), 4.45 (s, 2H), 3.21 (d, J = 11.4 Hz, 2H), 2.82 (dd, J = 22.4, 11.0 Hz, 2H), 1.87 (d, J = 13.8 Hz, 2H), 1.76 (s, 3H), 1.41 (dd, J = 22.8, 12.5 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.5, 147.6, 144.0, 143.0, 142.2, 137.1, 136.9, 136.6, 132.1, 129.3, 129.1, 126.1, 63.6, 62.5, 43.0, 34.4, 30.2, 28.2; MS (ESI) [M+H]+ 420.2
((5-(Piperidin-4-ylmethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))bis(methylene) bis(2,2-dimethylpropanoate) hydrochloride (10) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.04 (s, 1H), 8.69 (s, 1H), 8.36 (s, 1H), 7.41 (d, J = 7.6 Hz, 2H), 7.36 – 7.19 (m, 6H), 5.08 (s, 4H), 4.30 (d, J = 6.0 Hz, 2H), 3.27 (s, 2H), 2.90 (d, J = 11.2 Hz, 2H), 2.14 (s, 1H), 1.93 (d, J = 14.4 Hz, 2H), 1.61 – 1.45 (m, 2H), 1.16 (s, 18H); 13C NMR (100 MHz, DMSO-d6) δ 177.6, 158.1, 147.8, 144.3, 138.3, 138.0, 137.4, 136.6, 132.9, 130.1, 129.9, 127.6, 127.6, 69.9, 65.4, 43.1, 38.7, 33.4, 27.3, 25.6; MS (ESI) [M+H]+ 574.3.
N,N’-(((5-(Piperidin-4-ylmethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))bis(methylene))diacetamide hydrochloride (11) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.13 (d, J = 8.4 Hz, 1H), 8.78 (d, J = 9.6 Hz, 1H), 8.36 (s, 1H), 7.42 (d, J = 7.6 Hz, 2H), 7.37 – 7.27 (m, 6H), 5.07 (d, J = 4.4 Hz, 4H), 4.30 (d, J = 6.0 Hz, 2H), 3.29 (d, J = 12.4 Hz, 2H), 2.90 (q, J = 11.6 Hz, 2H), 2.15 (s, 1H), 2.07 (s, 6H), 1.93 (d, J = 14.0 Hz, 2H), 1.54 (q, J = 11.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 170.2, 157.6, 147.3, 143.8, 138.0, 137.7, 136.7, 135.8, 132.5, 129.6, 129.4, 127.7, 69.5, 65.02, 64.97, 42.6, 32.9, 25.1, 20.7; MS (ESI) [M+H]+ 488.3.
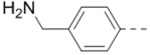
((5-(Piperidin-4-ylmethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))dimethanamine hydrochloride (12) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.02 (s, 1H), 7.20 (d, J = 7.6 Hz, 2H), 7.11 (s, 6H), 4.10 (s, 2H), 3.89 (s, 4H), 3.22 (d, J = 10.0 Hz, 2H), 2.79 (t, J = 10.4 Hz, 2H), 1.99 (s, 1H), 1.84 (d, J = 11.6 Hz, 2H), 1.41 – 1.32 (m, 2H); MS (ESI) [M+H]+ 404.2.
((5-(2-(Piperidin-4-yl)ethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))dimethanamine hydrochloride (13) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.28 (s, 1H), 7.51 – 7.43 (m, 2H), 7.44 – 7.30 (m, 6H), 4.57 – 4.44 (m, 2H), 4.20 (s, 4H), 3.44 (d, J = 12.8 Hz, 2H), 3.00 (t, J = 12.6 Hz, 2H), 2.05 (d, J = 14.3 Hz, 2H), 1.93 – 1.78 (m, 3H), 1.50 (dd, J = 24.7, 13.4 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.8, 149.2, 143.4, 138.0, 137.8, 133.4, 132.8, 131.7, 130.4, 130.3, 128.7, 128.6, 64.9, 43.9, 42.6, 34.0, 30.2, 28.2; MS (ESI) [M+H]+ 418.3.
((5-(2-(Piperazin-1-yl)ethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))dimethanamine hydrochloride (14) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.39 (s, 1H), 7.54 (d, J = 8.2 Hz, 2H), 7.43 (dd, J = 7.8, 5.0 Hz, 6H), 4.90 (d, J = 4.7 Hz, 2H), 4.21 (s, 4H), 3.88 – 3.80 (m, 6H), 3.67 (d, J = 4.6 Hz, 4H); 13C NMR (100 MHz, D2O) δ 157.5, 148.8, 144.8, 138.2, 138.1, 133.4, 132.8, 132.5, 130.4, 130.3, 128.7, 128.7, 60.1, 56.0, 48.8, 42.6, 42.6, 40.6; MS (ESI) [M+H]+ 419.3.
N,N’-(((5-(Piperidin-4-ylmethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))bis(methylene))diacetamide hydrochloride (15) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.17 (d, J = 10.1 Hz, 1H), 8.82 (d, J = 10.0 Hz, 1H), 8.45 (dt, J = 11.2, 5.7 Hz, 2H), 8.32 (d, J = 1.4 Hz, 1H), 7.36 (d, J = 7.3 Hz, 2H), 7.28 (d, J = 7.2 Hz, 2H), 7.19 (t, J = 9.0 Hz, 4H), 4.28 (d, J = 6.3 Hz, 2H), 4.25 (d, J = 5.7 Hz, 4H), 3.28 (d, J = 12.1 Hz, 2H), 2.89 (q, J = 11.7 Hz, 2H), 2.14 (s, 1H), 1.92 (d, J = 12.7 Hz, 2H), 1.88 (s, 6H), 1.54 (dd, J = 23.1, 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 169.3, 169.3, 157.5, 147.4, 143.9, 140.2, 139.3, 137.0, 136.7, 132.2, 129.5, 129.3, 126.9, 126.9, 69.4, 54.9, 42.6, 41.7, 41.7, 33.0, 25.2, 22.6; MS (ESI) [M+H]+ 488.3
((5-(Piperidin-4-ylmethoxy)pyrazine-2,3-diyl)bis(3-methyl-4,1-phenylene))dimethanamine hydrochloride (16) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.26 (s, 1H), 7.17 (d, J = 9.6 Hz, 2H), 7.02 (s, 4H), 4.20 (d, J = 5.6 Hz, 2H), 3.99 (s, 2H), 3.39 (d, J = 12.4 Hz, 2H), 2.96 (t, J = 11.2 Hz, 2H), 2.14 (br, 1H), 2.09 (br, 3H), 2.00 (br, 3H), 1.52 (dd, J = 23.6, 11.6 Hz, 2H); MS (ESI) [M+H]+ 432.3.
N,N’-(((5-(2-(Piperidin-4-yl)ethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))bis(methylene))bis(Hydroxylamine) hydrochloride (17) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.16 (s, 1H), 7.37 (d, J = 7.7 Hz, 2H), 7.30 (d, J = 7.7 Hz, 6H), 4.39 (s, 2H), 4.31 (s, 4H), 3.29 (d, J = 12.3 Hz, 2H), 2.85 (t, J = 12.2 Hz, 2H), 1.91 (d, J = 13.5 Hz, 2H), 1.82 – 1.71 (m, 3H), 1.41 – 1.31 (m, 2H); 13C NMR (100 MHz, D2O) δ 158.8, 148.8, 143.8, 139.0, 138.9, 132.3, 130.52, 130.48, 130.3, 130.2, 129.2, 128.5, 64.8, 54.4, 43.9, 34.0, 30.2, 28.2; MS (ESI) [M+H]+ 450.2.
1,1’-((5-(Piperidin-4-ylmethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))bis(N,N-dimethylmethanamine) hydrochloride (18) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.20 (s, 1H), 7.38 (d, J = 7.2 Hz, 2H), 7.30 (s, 6H), 4.26 (d, J = 4.0 Hz, 2H), 4.18 (s, 4H), 3.37 (d, J = 11.6 Hz, 2H), 2.94 (t, J = 12.4 Hz, 2H), 2.71 (s, 12H), 2.15 (s, 1H), 2.00 (d, J = 12.8 Hz, 2H), 1.51 (dd, J = 26.4, 12.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.9, 147.4, 143.7, 139.2, 138.8, 132.9, 131.0, 130.8, 130.8, 130.2, 129.9, 129.8, 69.6, 58.9, 58.8, 42.6, 41.5, 41.4, 32.9, 25.1; MS (ESI) [M+H]+ 406.3.
General Synthetic Procedure for compounds 25–32, 38–55 and 72–104:
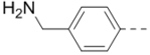
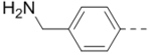
Compound 110 (1.2 g, 2.66 mmol), 4-[(tert-Butoxycarbonylamino)methyl]phenylboronic acid pinacol ester (2.66 mmol), tetrakis(triphenylphosphine)palladium (154 mg, 0.13 mmol), and sodium carbonate (564 mg, 5.32 mmol) in p-dioxane/H2O (15/3 mL) were placed in a sealed tube. The mixture was degassed and heated to 80 °C for 12 h. The reaction was cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 5:1 to 1:1) to afford the corresponding R5 substituted product. For intermediate of 47: 1H NMR (400 MHz, CDCl3) δ 8.19 (s, 1H), 7.67 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 5.04 (s, 1H), 4.34 (d, J = 5.2 Hz, 2H), 4.21 (d, J = 6.4 Hz, 2H), 4.15 (s, 2H), 2.73 (t, J = 11.2 Hz, 2H), 1.99 – 1.92 (m, 1H), 1.79 (d, J = 12.4 Hz, 2H), 1.44 (s, 18H), and 1.30 – 1.20 (m, 2H).
The above R5 substituted compound (1.94 mmol), arylboronic acid or aryl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (2.51 mmol), tetrakis(triphenylphosphine)palladium (110 mg, 0.095 mmol), and sodium carbonate (610 mg, 5.75 mmol) in p-dioxane/H2O (15/3 mL) were placed in a sealed tube. The mixture was degassed and heated to 110 °C for 24 h. The reaction was then cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 5:1 to 1:2) to afford the corresponding R6 substituted product. For precursor of 47: 1H NMR (400 MHz, CDCl3) δ 8.22 (s, 1H), 7.75 (s, 1H), 7.50 – 7.34 (m, 7H), 7.20 (d, J = 7.8 Hz, 2H), 6.70 (s, 1H), 4.85 (s, 1H), 4.36 – 4.24 (m, 4H), 4.20 – 4.07 (m, 2H), 2.76 (t, J = 12.4 Hz, 2H), 2.03 – 1.97 (m, 1H), 1.84 (d, J = 12.4 Hz, 3H), 1.54 – 1.40 (m, 18H), and 1.37 – 1.27 (m, 2H).
To a solution of R6 substituted compound (1.5 mmol) in DCM (5 mL) was added dropwise HCl (1.2 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 12 h. The volatiles were removed in vacuo to afford an oil, which was triturated in diether ether and solidified to give the final product hydrochloric salt.
4-(3-(4-(Aminomethyl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenol hydrochloride (25) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.03 (bs, 1H), 8.76 (bs, 1H), 8.43 (bs, 3H), 8.28 (s, 1H), 7.42 (s, 4H), 7.10 (d, J = 8.4 Hz, 2H), 6.67 (d, J = 8.8 Hz, 2H), 4.25 (d, J = 6 Hz, 2H), 3.99 (d, J = 6 Hz, 2H), 3.26 (d, J = 13.2 Hz, 2H), 2.87 (dd, J = 12.4 Hz, 24 Hz, 2H), 2.11 (bs, 1H), 1.89 (d, J = 12.4 Hz, 2H), 1.51 (dd, J = 12 Hz, 22.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.8, 158.2, 147.7, 144.1, 139.3, 138.9, 133.0, 132.2, 130.2, 130.2, 130.10, 130.06, 70.0, 51.1, 43.0, 33.4, 25.6; MS (ESI) [M+H]+ 391.1.
4-(3-(4-((Dimethylamino)methyl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenol hydrochloride (26) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.23 (s, 1H), 7.46 (d, J = 7.9 Hz, 2H), 7.41 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 8.4 Hz, 2H), 6.79 (d, J = 8.3 Hz, 2H), 4.31 (s, 4H), 3.51 (d, J = 12.6 Hz, 2H), 3.06 (t, J = 12.1 Hz, 2H), 2.23 (s, 1H), 2.10 (d, J = 13.8 Hz, 2H), 1.63 (dd, J = 23.6, 11.5 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.3, 155.9, 148.3, 144.3, 139.3, 131.5, 131.2, 130.6, 130.4, 129.7, 129.1, 115.0, 70.2, 60.5, 43.6, 42.0, 32.9, 25.0; MS (ESI) [M+H]+ 419.2.
N-(4-(3-(4-(Aminomethyl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)benzyl)acetamide hydrochloride (27) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.15 (s, 1H), 7.33 (d, J = 8.1 Hz, 2H), 7.25 (d, J = 8.1 Hz, 2H), 7.16 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 8.1 Hz, 2H), 4.23 (s, 4H), 4.06 (s, 2H), 3.38 (d, J = 12.8 Hz, 2H), 2.94 (t, J = 12.8 Hz, 2H), 2.13 (s, 1H), 1.99 (d, J = 13.6 Hz, 2H), 1.92 (s, 3H), 1.51 (dd, J = 24.0, 11.5 Hz, 2H); 13C NMR (100 MHz, D2O) δ 174.0, 158.6, 148.7, 144.4, 138.1, 136.4, 133.2, 131.9, 130.3, 129.8, 128.6, 126.9, 110.0, 70.2, 43.6, 42.65, 42.63, 32.9, 25.0, 21.8; MS (ESI) [M+H]+ 446.2.
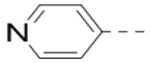
(4-(6-(Piperidin-4-ylmethoxy)-3-(pyridin-4-yl)pyrazin-2-yl)phenyl)methanamine hydrochloride (28) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.59 (d, J = 5.6 Hz, 2H), 8.40 (s, 1H), 7.81 (d, J = 6.0 Hz, 2H), 7.51 (dd, J = 21.2, 8.0 Hz, 4H), 4.42 (d, J = 6.0 Hz, 2H), 4.24 (s, 2H), 3.53 (d, J = 12.4 Hz, 2H), 3.09 (t, J = 12.4 Hz, 2H), 2.29 (s, 1H), 2.14 (d, J = 13.2 Hz, 2H), 1.66 (dd, J = 24.0, 11.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.3, 152.7, 149.3, 142.3, 138.7, 136.1, 135.1, 133.3, 131.7, 129.6, 128.8, 126.2, 69.6, 42.2, 41.4, 32.4, 24.6; MS (ESI) [M+H]+ 476.2.
(4-(6-(2-(Piperidin-4-yl)ethoxy)-3-(pyridin-4-yl)pyrazin-2-yl)phenyl)methanamine hydrochloride (29) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.66 (s, 2H), 8.42 (s, 1H), 8.01 (s, 2H), 7.57 (br, 2H), 7.52 (br, 2H), 4.61 (s, 2H), 4.26 (s, 2H), 3.45 (d, J = 12.4 Hz, 2H), 3.01 (t, J = 12.4 Hz, 2H), 2.07 (d, J = 13.6 Hz, 2H), 1.92 (br, 3H), 1.51 (dd, J = 23.2, 11.2 Hz, 2H); 13C NMR (100 MHz, D2O) δ 159.9, 155.7, 151.0, 140.9, 138.3, 136.8, 134.5, 134.2, 130.6, 129.2, 127.3, 65.2, 43.9, 42.6, 33.9, 30.2, 28.2 MS (ESI) [M+H]+ 390.2.
(3-Methoxy-4-(6-(piperidin-4-ylmethoxy)-3-(pyridin-4-yl)pyrazin-2-yl)phenyl)methanamine hydrochloride (30) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.18 (bs, 1H), 8.88 (bs, 1H), 8.73 (bs, 2H), 8.65 (bs, 3H), 8.46 (s, 1H), 7.70 (bs, 2H), 7.26 (d, J = 7.2 Hz, 1H), 7.21 (s, 1H), 4.28 (d, J = 5.6 Hz, 2H), 4.04 (d, J = 4.4 Hz, 2H), 3.26 (s, 3H), 2.87 (d, J = 11.2 Hz, 2H), 2.12 (bs, 1H), 1.89 (d, J = 13.2 Hz, 2H), 1.52 (d, J = 12.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 159.7, 155.6, 147.5, 143.6, 141.6, 138.2, 133.8, 133.6, 126.1, 124.8, 122.2, 113.0, 70.4, 55.2, 43.0, 33.3, 25.5; MS (ESI) [M+H]+ 406.2.
N,N-Dimethyl-1-(4-(6-(piperidin-4-ylmethoxy)-3-(pyridin-3-yl)pyrazin-2-yl)phenyl)methanamine hydrochloride (31) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.70 (s, 1H), 8.61 (d, J = 5.4 Hz, 1H), 8.38 (d, J = 7.5 Hz, 1H), 8.31 (s, 1H), 7.85 (t, J = 6.8 Hz, 1H), 7.44 (dd, J = 19.5, 7.6 Hz, 4H), 4.31 (d, J = 6.0 Hz, 2H), 4.25 (s, 2H), 3.39 (d, J = 11.1 Hz, 2H), 2.96 (t, J = 12.8 Hz, 2H), 2.17 (s, 1H), 2.01 (d, J = 13.9 Hz, 2H), 1.53 (q, J = 12.7 Hz, 2H); 13C NMR (100 MHz, D2O) δ 159.7, 149.7, 147.1, 141.4, 140.2, 137.8, 137.7, 137.4, 134.2, 131.3, 130.8, 130.7, 126.9, 70.4, 60.5, 43.6, 42.2, 42.0, 32.8, 24.9; MS (ESI) [M+H]+ 404.2.
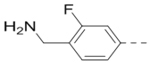
(4-(3-(4-(Aminomethyl)-3-fluorophenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (32) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.32 (s, 1H), 7.40 (d, J = 14.0 Hz, 5H), 7.28 (t, J = 8.4 Hz, 2H), 4.38 (d, J = 6.0 Hz, 2H), 4.25 (s, 2H), 4.19 (s, 2H), 3.51 (d, J = 12.4 Hz, 2H), 3.07 (t, J = 12.8 Hz, 2H), 2.27 (s, 1H), 2.12 (d, J = 14.0 Hz, 2H), 1.64 (dd, J = 23.6, 11.6 Hz, 2H); 13C NMR (100 MHz, D2O) δ 161.7, 159.2, 158.8, 147.6, 143.7, 140.9, 140.8, 137.7, 132.9, 132.6, 131.1, 131.0, 130.2, 128.8, 126.2, 126.2, 120.4, 120.2, 117.1, 116.8, 110.0, 70.3, 43.6, 42.6, 36.9, 36.8, 32.8, 25.0; MS (ESI) [M+H]+ 422.2.
(4-(3-(1-Methyl-1H-pyrazol-4-yl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (38) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.28 (s, 1H), 9.06 (d, J = 9.6 Hz, 1H), 8.67 (s, 3H), 8.13 (s, 1H), 7.74 (s, 1H), 7.59 (d, J = 7.6 Hz, 2H), 7.43 (d, J = 7.6 Hz, 2H), 7.06 (s, 1H), 4.29 (d, J = 6.0 Hz, 2H), 4.09 (d, J = 5.6 Hz, 2H), 3.79 (s, 3H), 3.27 (d, J = 11.6 Hz, 2H), 2.89 (dd, J = 22.4, 11.2 Hz, 2H), 2.13 (s, 1H), 1.92 (d, J = 13.2 Hz, 2H), 1.58 (dd, J = 23.6, 11.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.2, 142.9, 141.7, 139.4, 138.8, 134.7, 131.9, 130.8, 129.65, 129.60, 120.0, 69.7, 43.0, 42.3, 33.4, 25.6; MS (ESI) [M+H]+ 379.2.
(4-(5-(Piperidin-4-ylmethoxy)-3-(1H-pyrazol-4-yl)pyrazin-2-yl)phenyl)methanamine hydrochloride (39) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.06 (s, 1H), 7.54 (d, J = 5.3 Hz, 4H), 7.43 (d, J = 7.6 Hz, 2H), 4.29 (d, J = 11.6 Hz, 4H), 3.51 (d, J = 12.4 Hz, 2H), 3.07 (t, J = 12.6 Hz, 2H), 2.21 (s, 1H), 2.10 (d, J = 14.0 Hz, 2H), 1.62 (q, J = 11.6 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.6, 142.3, 141.9, 137.6, 134.4, 133.6, 129.7, 129.7, 129.3, 119.2, 70.0, 43.6, 42.7, 32.8, 25.0; MS (ESI) [M+H]+ 365.2.
(4-(3-(3,5-Dimethylisoxazol-4-yl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (40) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.37 (s, 1H), 7.48 (s, 4H), 4.34 (d, J = 5.6 Hz, 2H), 4.21 (s, 2H), 3.52 (d, J = 12.4 Hz, 2H), 3.08 (t, J = 12.8 Hz, 2H), 2.27 (s, 1H), 2.13 (d, J = 14.0 Hz, 2H), 2.03 (s, 3H), 1.99 (s, 3H), 1.69 – 1.59 (m, 2H); 13C NMR (100 MHz, D2O) δ 169.2, 160.4, 158.8, 145.1, 139.6, 137.5, 133.9, 132.9, 129.6, 129.0, 113.9, 70.3, 43.6, 42.6, 32.8, 24.9, 10.6, 9.5; MS (ESI) [M+H]+ 394.2
(4-(3-(4-(3,5-Dimethylisoxazol-4-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (41) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.12 (s, 1H), 8.81 (s, 1H), 8.47 (s, 3H), 8.39 (s, 1H), 7.51 (d, J = 8.0 Hz, 2H), 7.47 – 7.32 (m, 6H), 4.31 (d, J = 6.0 Hz, 2H), 4.02 (d, J = 5.2 Hz, 2H), 3.29 (d, J = 11.2 Hz, 2H), 2.91 (dd, J = 22.8, 12.4 Hz, 2H), 2.41 (s, 3H), 2.23 (s, 3H), 2.16 (s, 1H), 1.94 (d, J = 13.6 Hz, 2H), 1.56 (dd, J = 24.4, 11.2 Hz, 2H); 13C NMR (100 MHz, D2O) δ 165.4, 158.0, 157.7, 147.3, 143.6, 138.4, 137.0, 133.8, 132.6, 130.3, 130.0, 129.5, 128.7, 128.6, 115.3, 69.6, 42.6, 41.8, 32.9, 25.2, 11.6, 10.6; MS (ESI) [M+H]+ 470.2.
(4-(3-(4-(1H-1,2,3-Triazol-1-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (42) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.38 (s, 1H), 8.19 (s, 1H), 7.93 (s, 1H), 7.59 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 7.34 (dd, J = 21.2, 8.0 Hz, 4H), 4.24 (d, J = 6.0 Hz, 2H), 4.16 (s, 2H), 3.50 (d, J = 12.4 Hz, 2H), 3.05 (t, J = 12.4 Hz, 2H), 2.16 (s, 1H), 2.06 (d, J = 13.6 Hz, 2H), 1.60 (dd, J = 24.2, 11.2 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.5, 147.7, 143.5, 137.9, 136.3, 134.5, 132.8, 132.0, 131.2, 130.1, 128.8, 123.8, 120.3, 116.0, 70.1, 43.6, 42.6, 32.9, 25.0; MS (ESI) [M+H]+ 442.2.
(1-(4-(3-(4-(Aminomethyl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)-1H-1,2,3-triazol-4-yl)methanol hydrochloride (43) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.18 (s, 1H), 8.87 (s, 1H), 8.73 (s, 1H), 8.49 (br, 3H), 8.42 (s, 1H), 7.90 (d, J = 8.0 Hz, 2H), 7.61 (d, J = 8.0 Hz, 2H), 7.43 (dd, J = 20.0, 8.0 Hz, 4H), 4.61 (s, 2H), 4.32 (d, J = 5.6 Hz, 2H), 4.04 (s, 2H), 3.29 (d, J = 10.4 Hz, 2H), 2.90 (dd, J = 22.4, 10.8 Hz, 2H), 1.94 (d, J = 13.2 Hz, 2H), 1.63 – 1.49 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.8, 149.3, 146.7, 143.8, 138.2, 138.0, 136.6, 133.8, 132.9, 131.1, 129.6, 128.8, 120.9, 119.6, 69.7, 54.9, 42.6, 41.8, 32.9, 25.2; MS (ESI) [M+H]+ 472.2.
(4-(3-(4-Phenoxyphenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (44) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.11 (s, 1H), 7.25 (d, J = 7.7 Hz, 2H), 7.13 (dd, J = 15.2, 7.6 Hz, 4H), 7.03 – 6.93 (m, 3H), 6.64 (d, J = 7.6 Hz, 2H), 6.46 (d, J = 7.6 Hz, 2H), 4.01 (s, 4H), 3.42 (d, J = 12.0 Hz, 2H), 2.93 (t, J = 12.6 Hz, 2H), 1.98 (s, 1H), 1.87 (d, J = 13.6 Hz, 2H), 1.49 (dd, J = 24.8, 12.8 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.2, 157.3, 155.5, 148.1, 142.9, 138.3, 132.5, 131.9, 131.3, 129.9, 129.4, 128.9, 127.0, 124.1, 118.9, 117.4, 69.7, 43.4, 42.5, 32.8, 24.9; MS (ESI) [M+H]+ 46.2.7
(4-(5-(Piperidin-4-ylmethoxy)-3-(4-(pyridin-4-yl)phenyl)pyrazin-2-yl)phenyl)methanamine hydrochloride (45) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.77 (d, J = 6.0 Hz, 2H), 8.29 (d, J = 7.2 Hz, 3H), 7.85 (d, J = 7.6 Hz, 2H), 7.63 (d, J = 7.6 Hz, 2H), 7.41 (dd, J = 14.8, 7.6 Hz, 4H), 4.38 (d, J = 5.6 Hz, 2H), 4.18 (s, 2H), 3.51 (d, J = 12.0 Hz, 2H), 3.07 (t, J = 12.0 Hz, 2H), 2.27 (s, 1H), 2.13 (d, J = 13.2 Hz, 2H), 1.65 (dd, J = 23.2, 10.0 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.7, 157.1, 148.1, 144.0, 141.0, 140.7, 138.1, 134.6, 132.8, 132.5, 130.8, 130.2, 128.7, 127.8, 124.3, 70.2, 43.5, 42.6, 32.8, 24.9; MS (ESI) [M+H]+ 452.2.
(4-(3-(4-(1H-Pyrazol-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methan-amine hydrochloride (46) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.18 (s, 2H), 8.15 (s, 1H), 7.41 (s, 2H), 7.34 – 7.27 (m, 6H), 4.20 (s, 2H), 4.14 (s, 2H), 3.49 (d, J = 12.9 Hz, 2H), 3.02 (t, J = 13.2 Hz, 2H), 2.12 (br, 1H), 2.03 (d, J = 15.3 Hz, 2H), 1.64 – 1.54 (m, 2H); 13C NMR (100 MHz, D2O) 158.5, 148.8, 143.2, 138.1, 135.9, 132.7, 131.11, 131.04, 131.02, 131.00, 130.95, 130.4, 130.1, 128.8, 125.1, 70.1, 43.5, 42.6, 32.9, 25.0; MS (ESI) [M+H]+ 441.2.
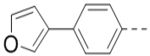
(4-(3-(4-(Furan-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (47) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.24 (s, 1H), 7.94 (s, 1H), 7.61 (s, 1H), 7.51 (dd, J = 8.4, 1.8 Hz, 2H), 7.41 (br, 6H), 6.85 (s, 1H), 4.35 (br, 2H), 4.17 (s, 2H), 3.50 (d, J = 13.3 Hz, 2H), 3.06 (t, J = 13.3 Hz, 2H), 2.12 (br, 1H), 2.11 (d, J = 14.6 Hz, 2H), 1.68 – 1.58 (m, 2H); 13C NMR (100 MHz, D2O) 158.4, 148.8, 144.5, 143.3, 139.4 (2), 138.3, 135.6, 132.6, 132.4, 131.1, 130.3, 130.0, 128.7, 125.0, 108.1, 70.0, 43.5, 42.6, 32.9, 24.9; HRMS (ESI+) calcd for C27H29N4O2 [M+H]+ 441.2291, found 441.2285.
1-(4-(3-(4-(Aminomethyl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)ethan-1-one hydrochloride (48) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.08 (br, 1H), 8.78 (br, 1H), 8.41 (br, 4H), 7.89 (d, J = 8.0 Hz, 2H), 7.53 (d, J = 8.4 Hz, 2H), 7.42 (d, J = 8.4 Hz, 2H), 7.35 (d, J = 8.4 Hz, 2H), 4.29 (d, J = 6 Hz, 2H), 3.99 (d, J = 5.2 Hz, 2H), 3.27 (d, J = 12.0 Hz, 2H), 2.88 (d, J = 10.4 Hz, 2H), 2.57 (s, 3H), 2.15 (br, 1H), 1.91 (d, J = 11.6 Hz, 2H), 1.53 (d, J = 12 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 198.0, 158.2, 147.2, 144.4, 143.0, 138.5, 136.9, 134.3, 133.6, 130.4, 130.0, 129.2, 128.6, 70.1, 55.4, 43.0, 42.2, 33.4, 25.6; MS (ESI) [M+H]+ 417.2.
(4-(3-(4-Isopropylphenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (49) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.65 (s, 4H), 8.34 (s, 1H), 7.44 (d, J = 7.6 Hz, 2H), 7.41 – 7.29 (m, 4H), 7.21 (d, J = 7.2 Hz, 2H), 4.29 (d, J = 6.0 Hz, 2H), 4.01 (s, 2H), 3.28 (d, J = 12.4 Hz, 2H), 2.90 (t, J = 10.8 Hz, 3H), 2.14 (s, 1H), 1.93 (d, J = 13.2 Hz, 2H), 1.55 (dd, J = 24.2, 11.8 Hz, 2H), 1.20 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 157.6, 149.0, 147.8, 143.4, 138.6, 135.6, 133.6, 132.1, 129.5, 129.4, 128.6, 126.2, 69.5, 42.6, 41.8, 33.1, 33.0, 25.2, 23.7; MS (ESI) [M+H]+ 417.3.
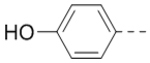
4-(3-(4-(Aminomethyl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenol hydrochloride (50) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.79 (s, 1H), 8.25–9.82 (m, 5H), 7.34–7.40 (m, 4H), 7.20 (d, J = 8.8 Hz, 2H), 6.68 (d, J = 8.4 Hz, 2H), 4.27 (d, J = 6.4 Hz, 2H), 4.00 (d, J = 5.2 Hz, 2H), 3.27 (br, 2H), 2.89 (d, J = 11.2 Hz, 2H), 2.11 (br, 1H), 1.91 (d, J = 13.2 Hz, 2H), 1.48–1.51 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.5, 158.0, 148.4, 143.5, 139.5, 133.8, 131.8, 131.4, 129.8, 129.0, 128.8, 115.5, 69.8, 43.2, 42.3, 33.4, 25.6; MS (ESI) [M+H]+ 391.2.
(4-(3-(1H-Benzo[d]imidazol-6-yl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (51) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6): δ = 9.59 (s, 1H), 8.82 (s, 1H), 9.07 (s, 1H), 8.43 (bs, 4H), 7.95 (s, 1H), 7.74 (d, J = 8.8 Hz, 1H), 7.46 (d, J = 8.4 Hz, 1H), 7.34–7.40 (m, 4H), 4.29 (d, J = 6.4 Hz, 2H), 3.98 (d, J = 6.4 Hz, 2H), 3.28 (d, J = 12 Hz, 2H), 2.90 (d, J = 11.6 Hz, 2H), 2.15 (bs, 1H), 1.92 (d, J = 12.8 Hz, 2H), 1.52–1.60 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ = 158.19, 147.32, 144.34, 141.85, 138.63, 136.45, 134.25, 131.12, 130.05, 129.21, 128.01, 116.12, 114.61, 65.35, 43.07, 42.20, 31.75, 15.61; MS (ESI) [M+H]+ 414.2.
(4-(3-(1H-Benzo[d]imidazol-4-yl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (52) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.43 (s, 1H), 9.06 (bs, 1H), 8.78 (bs, 1H), 8.50 (s, 1H), 8.39 (bs, 3H), 7.83 (d, J = 7.6 Hz, 2H), 7.38–7.40 (m, 2H), 7.23 (d, J = 7.2 Hz, 2H), 4.25 (d, J = 6.4 Hz, 2H), 3.93 (d, J = 4.8 Hz, 2H), 2.87 (d, J = 11.6 Hz, 4H), 2.11 (bs, 1H), 1.89 (d, J = 12.4 Hz, 2H), 1.51 (d, J = 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 170.8, 158.2, 144.9, 144.2, 142.0, 137.9, 134.6, 134.3, 129.8, 129.0, 127.0, 126.5, 125.8, 115.6, 114.9, 70.3, 65.4, 60.2, 34.6, 21.2; MS (ESI) [M+H]+ 415.2.
(4-(3-(1H-Indol-2-yl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (53) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 11.51 (s, 1H), 9.11 (s, 1H), 8.88 (s, 1H), 8.53 (s, 3H), 8.24 (s, 1H), 7.64 – 7.47 (m, 5H), 7.32 (d, J = 7.5 Hz, 1H), 7.19 – 7.05 (m, 1H), 7.00 – 6.89 (m, 1H), 4.51 (d, J = 4.6 Hz, 2H), 4.13 (d, J = 4.4 Hz, 2H), 3.31 (d, J = 8.9 Hz, 2H), 2.92 (dd, J = 21.7, 11.0 Hz, 2H), 2.17 (s, 1H), 1.99 (d, J = 11.7 Hz, 2H), 1.61 (dd, J = 22.3, 10.1 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.6, 143.7, 140.1, 139.2, 136.6, 134.3, 133.7, 131.2, 129.3, 129.1, 127.8, 122.9, 120.8, 119.6, 112.0, 104.2, 69.7, 42.6, 42.0, 33.1, 25.2; MS (ESI) [M+H]+ 414.2.
(4-(3-(1H-Indol-4-yl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (54) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.27 (s, 1H), 7.55 (d, J = 8.4 Hz, 1H), 7.34 (d, J = 9.2 Hz, 3H), 7.24 (d, J = 7.2 Hz, 2H), 7.12 (t, J = 7.2 Hz, 1H), 6.97 (d, J = 7.2 Hz, 1H), 4.26 (d, J = 4.8 Hz, 2H), 4.09 (s, 2H), 3.49 (d, J = 12.4 Hz, 2H), 3.03 (t, J = 12.4 Hz, 2H), 2.18 (s, 1H), 2.05 (d, J = 14.0 Hz, 2H), 1.59 (dd, J = 25.6, 13.2 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.5, 149.8, 143.9, 138.4, 135.9, 132.3, 131.5, 129.6, 129.0, 128.4, 126.3, 126.1, 121.5, 121.2, 112.4, 70.2, 43.6, 42.6, 32.8, 24.9; MS (ESI) [M+H]+ 414.2.
(4-(3-(1H-Indol-5-yl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (55) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.27 (s, 1H), 7.71 (s, 1H), 7.47 – 7.35 (m, 6H), 7.32 (d, J = 7.4 Hz, 1H), 7.21 (d, J = 7.3 Hz, 1H), 4.36 (s, 2H), 4.17 (s, 2H), 3.51 (d, J = 11.5 Hz, 2H), 3.06 (t, J = 13.1 Hz, 2H), 2.22 (s, 1H), 2.10 (d, J = 13.5 Hz, 2H), 1.65 (dd, J = 24.7, 12.0 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.6, 151.1, 143.4, 139.3, 136.1, 132.8, 130.6, 130.3, 129.1, 128.8, 127.6, 126.6, 123.6, 122.5, 111.6, 70.2, 43.7, 42.7, 33.2, 25.3; MS (ESI) [M+H]+ 414.2.
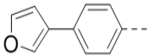
(4-(3-(4-(Furan-3-yl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (59) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.01 (s, 1H), 7.69 (s, 1H), 7.41 (s, 1H), 7.21 (dd, J = 20.0, 7.6 Hz, 6H), 7.04 (d, J = 8.1 Hz, 2H), 6.61 (s, 1H), 4.09 (d, J = 6.0 Hz, 2H), 3.98 (s, 2H), 3.34 (d, J = 12.4 Hz, 3H), 2.88 (t, J = 12.0 Hz, 2H), 2.00 (s, 1H), 1.91 (d, J = 14.0 Hz, 2H), 1.44 (dd, J = 23.6, 10.8 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.3, 148.5, 144.4, 143.8, 139.3, 138.2, 135.6, 133.3, 131.8, 131.6, 130.7, 130.2, 130.1, 128.7, 125.2, 108.2, 70.2, 43.5, 42.6, 32.9, 24.9; MS (ESI) [M+H]+ 441.2
1-(4-(3-(4-(Furan-3-yl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)-N-methylmethanamine hydrochloride (60) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.17 (s, 1H), 7.85 (s, 1H), 7.57 (s, 1H), 7.48 – 7.24 (m, 6H), 7.18 (d, J = 7.4 Hz, 2H), 6.76 (s, 1H), 4.24 (d, J = 4.8 Hz, 2H), 4.16 (s, 2H), 3.49 (d, J = 10.4 Hz, 2H), 3.03 (t, J = 11.6 Hz, 2H), 2.68 (s, 3H), 2.17 (s, 1H), 2.06 (d, J = 12.4 Hz, 2H), 1.60 (dd, J = 24.4, 12.8 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.3, 148.2, 144.4, 144.0, 139.3, 138.7, 135.8, 131.9, 131.8, 131.3, 130.3, 130.1, 129.6, 125.2, 125.1, 108.2, 70.2, 51.7, 43.6, 32.9, 32.0, 25.0; MS (ESI) [M+H]+ 455.2.
1-(4-(3-(4-(Furan-3-yl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)-N,N-dimethylmethanamine hydrochloride (61) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.07 (s, 1H), 7.73 (s, 1H), 7.44 (s, 1H), 7.28 (t, J = 7.3 Hz, 4H), 7.23 (d, J = 7.7 Hz, 2H), 7.07 (d, J = 7.8 Hz, 2H), 6.64 (s, 1H), 4.16 – 4.11 (m, 4H), 3.36 (d, J = 12.6 Hz, 2H), 2.89 (d, J = 12.8 Hz, 2H), 2.67 (s, 6H), 2.05 (s, 1H), 1.94 (d, J = 13.4 Hz, 2H), 1.47 (dd, J = 24.5, 11.9 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.4, 148.2, 144.4, 144.1, 139.3, 135.8, 132.0, 131.8, 130.6, 130.4, 130.4, 130.1, 129.8, 125.2, 125.1, 108.2, 70.2, 60.5, 43.6, 42.0, 32.9, 24.9; MS (ESI) [M+H]+ 469.2
1-(4-(3-(4-(Furan-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)-N-methylmethanamine hydrochloride (62) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.07 (s, 1H), 7.76 (s, 1H), 7.54 (s, 1H), 7.28 (d, J = 7.9 Hz, 2H), 7.24 – 7.16 (m, 4H), 7.12 (d, J = 7.8 Hz, 2H), 6.67 (s, 1H), 4.1 – 4.00 (m, 4H), 3.43 (d, J = 6.1 Hz, 2H), 2.97 (t, J = 12.6 Hz, 2H), 2.64 (s, 3H), 2.06 (s, 2H), 1.95 (d, J = 13.2 Hz, 2H), 1.59 – 1.48 (m, 2H) ; 13C NMR (100 MHz, D2O) δ 158.5, 148.8, 144.7, 143.2, 139.5, 138.9, 135.6, 132.6, 131.3, 130.8, 130.4, 130.2, 129.9, 125.1, 116.0, 108.3, 70.1, 52.3, 43.7, 33.07, 32.11, 25.0; MS (ESI) [M+H]+ 455.2.
1-(4-(3-(4-(Furan-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)-N,N-dimethylmethanamine hydrochloride (63) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.25 (s, 1H), 7.94 (s, 1H), 7.61 (s, 1H), 7.50 (d, J = 7.2 Hz, 2H), 7.45 – 7.35 (m, 6H), 6.84 (s, 1H), 4.34 (d, J = 6.0 Hz, 2H), 4.29 (s, 2H), 3.50 (d, J = 11.6 Hz, 2H), 3.06 (t, J = 13.2 Hz, 2H), 2.24 (s, 1H), 2.11 (d, J = 14.4 Hz, 2H), 1.63 (dd, J = 24.0, 11.6 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.7, 149.1, 144.5, 143.6, 139.5, 135.8, 132.6, 131.6, 130.6, 130.4, 130.3, 129.2, 125.2, 125.1, 108.2, 70.1, 60.6, 43.5, 42.0, 32.9, 24.9; MS (ESI) [M+H]+ 429.2.
(4-(3-(4-(Furan-3-yl)phenyl)-6-(2-(piperazin-1-yl)ethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (64) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.36 (s, 1H), 7.93 (s, 1H), 7.60 (s, 1H), 7.53 (d, J = 8.0 Hz, 4H), 7.41 – 7.34 (m, 4H), 6.85 (s, 1H), 4.18 (s, 2H), 3.85 (s, 7H), 3.66 (s, 6H); 13C NMR (100 MHz, D2O) δ 157.3, 148.6, 145.1, 144.5, 139.4, 138.2, 135.8, 133.4, 132.3, 132.1, 130.3, 130.2, 128.7, 125.4, 125.2, 108.3, 60.0, 56.0, 48.8, 42.6, 40.5; MS (ESI) [M+H]+ 456.2.
(4-(3-(4-(Furan-3-yl)phenyl)-5-(2-(piperidin-4-yl)ethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (65) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.00 (s, 1H), 7.71 (s, 1H), 7.44 (s, 1H), 7.28 – 7.04 (m, 6H), 6.61 (s, 1H), 4.19 (s, 1H), 3.99 (s, 2H), 3.25 (d, J = 11.2 Hz, 2H), 2.77 (t, J = 12.4 Hz, 1H), 1.80 (d, J = 13.6 Hz, 2H), 1.62 (s, 2H), 1.29 (dd, J = 21.2, 10.4 Hz, 1H); 13C NMR (100 MHz, D2O) δ 158.5, 149.0, 144.5, 143.2, 139.4, 138.3, 135.8, 132.6, 132.5, 131.2, 130.3, 130.1, 128.8, 125.1, 108.2, 64.7, 43.8, 42.6, 34.0, 30.2, 28.2; MS (ESI) [M+H]+ 455.2.
2-Methoxy-4-(5-(piperidin-4-ylmethoxy)-3-(4-(thiophen-3-yl)phenyl)pyrazin-2-yl)benzamide hydrochloride (72) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.91 (br, 1H), 8.57 (br, 1H), 8.39 (s, 1H), 7.96 (br, 1H), 7.74 (d, J = 7.9 Hz, 2H), 7.72 (d, J = 8.0 Hz, 1H), 7.65–7.59 (m, 2H), 7.55 (br, 1H), 7.47 (d, J = 7.9 Hz, 2H), 7.11 (s, 1H), 7.00 (d, J = 8.0 Hz, 1H), 4.33 (d, J = 6.2 Hz, 2H), 3.68 (s, 3H), 3.31 (d, J = 12.5 Hz, 2H), 2.96–2.88 (m, 2H), 2.16 (br, 1H), 1.94 (d, J = 13.6 Hz, 2H), 1.58–1.49 (m, 2H); 13C NMR (100 MHz, DMSO-d6) 165.9, 157.9, 147.9, 143.2, 142.4, 140.6, 136.5, 135.4, 132.4, 132.1, 131.5, 130.7, 130.2, 128.8, 127.4, 126.2, 125.9, 121.9, 113.2, 69.6, 66.4, 42.8, 32.9, 26.3; MS (ESI) [M+H]+ 501.2.
(4-(5-(Cyclohexylmethoxy)-3-(4-(furan-3-yl)phenyl)pyrazin-2-yl)phenyl)methanamine hydrochloride (73) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.45 (bs, 3H), 8.33 (s, 1H), 8.21 (s, 1H), 7.73 (s, 1H), 7.57 (d, J = 7.6 Hz, 2H), 7.38–7.42 (m, 6H), 6.96 (s, 1H), 4.20 (d, J = 5.2 Hz, 2H), 3.99 (d, J = 4.8 Hz, 2H), 1.80 (d, J = 10.8 Hz, 2H), 1.70 (d, J = 11.2 Hz, 2H), 1.64 (d, J = 10.8 Hz, 1H), 1.21 (dd, J = 12.8 Hz, 25.6 Hz, 4H), 1.04–1.07 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.4, 147.9, 144.9, 143.7, 140.4, 139.1, 137.0, 134.0, 132.8, 132.6, 130.5, 130.0, 129.1, 125.7, 125.6, 109.0, 71.6, 42.2, 37.3, 29.7, 26.4, 25.7. MS (ESI) [M+H]+ 440.2.
(4-(5-(Benzyloxy)-3-(4-(furan-3-yl)phenyl)pyrazin-2-yl)phenyl)methanamine hydrochloride (74) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ = 8.40–8.42 (m, 3H), 8.23 (s, 1H), 7.74 (s, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.52–7.57 (m, 3H), 7.38–7.41 (m, 8H), 7.34–7.36 (m, 2H), 6.70 (s, 1H), 5.48 (s, 2H), 3.99 (d, J = 5.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.9, 147.9, 145.0, 144.1, 140.4, 139.1, 137.0, 136.8, 134.0, 133.0, 132.7, 130.5, 130.0, 129.1, 128.9, 128.8, 128.6, 125.7, 125.6, 109.0, 68.1, 42.3; MS (ESI) [M+H]+ 434.2.
(4-(3-(4-(Furan-3-yl)phenyl)-5-(piperidin-4-yloxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (75) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.29 (s, 1H), 8.20 (s, 1H), 7.73 (s, 1H), 7.56 (d, J = 8 Hz, 2H), 7.38–7.40 (m, 4H), 7.29 (d, J = 8 Hz, 2H), 7.15 (d, J = 8 Hz, 2H), 6.96 (s, 1H), 5.27 (bs, 1H), 4.10 (d, J = 5.6 Hz, 2H), 3.65 (bs, 2H), 3.23 (bs, 2H), 2.00 (bs, 2H), 1.64–1.66 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.2, 156.3, 154.4, 147.6, 144.9, 144.3, 140.3, 137.4, 137.0, 133.1, 132.6, 130.4, 129.7, 127.0, 125.7, 109.0, 79.2, 78.3, 71.6, 43.5; MS (ESI) [M+H]+ 427.2.
3-((5-(4-(Aminomethyl)phenyl)-6-(4-(furan-3-yl)phenyl)pyrazin-2-yl)oxy)propan-1-amine hydrochloride (76) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.44 (bs, 3H), 8.36 (s, 1H), 8.22 (s, 1H), 8.09 (bs, 3H), 7.74 (s, 1H), 7.57 (d, J = 8 Hz, 2H), 7.37–7.42 (m, 6H), 6.97 (s, 1H), 4.48 (t, J = 6 Hz, 2H), 3.99 (d, J = 5.6 Hz, 2H), 2.96 (d, J = 6 Hz, 2H), 2.09–2.13 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.0, 147.9, 145.0, 144.0, 140.4, 139.0, 136.8, 134.1, 132.9, 132.7, 130.5, 129.9, 129.1, 125.7, 125.6, 109.0, 64.0, 42.2, 36.6, 34.6; MS (ESI) [M+H]+ 401.2.
2-((5-(4-(Aminomethyl)phenyl)-6-(4-(furan-3-yl)phenyl)pyrazin-2-yl)oxy)-N-methylethan-1-amine hydrochloride (77) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.32 (bs, 1H), 8.20 (s, 1H), 7.73 (s, 1H), 7.57 (d, J = 7.2 Hz, 2H), 7.38–7.40 (m, 3H), 7.26–7.28 (m, 2H), 7.15 (d, J = 8 Hz, 2H), 6.96 (s, 1H), 4.53 (t, J = 5.2 Hz, 2H), 4.10 (d, J = 5.2 Hz, 2H), 2.85 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 157.8, 156.3, 144.9, 144.4, 140.4, 140.3, 137.4, 137.0, 132.8, 132.6, 130.4, 129.7, 127.0, 125.7, 125.6, 109.0, 78.9, 78.3, 63.8, 43.5; MS (ESI) [M+H]+ 401.2.
(4-(3-(4-(Furan-3-yl)phenyl)-5-(1-(piperidin-4-yl)ethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (78) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.09 (bs, 1H), 8.78 (bs, 1H), 8.45 (bs, 3H), 8.30 (s, 1H), 7.75 (s, 1H), 7.57 (d, J = 8 Hz, 2H), 7.39–7.41 (m, 6H), 7.26 (d, J = 8 Hz, 1H), 6.77 (d, J = 8 Hz, 1H), 5.14–5.16 (m, 1H), 3.99 (bs, 2H), 3.27 (d, J = 13.6 Hz, 2H), 2.83 (bs, 2H), 1.93 (bs, 2H), 1.81–1.84 (m, 1H), 1.52–1.56 (m, 2H), 1.32 (d, J = 6 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 157.9, 154.8, 147.6, 143.9, 143.8, 138.8, 137.0, 132.9, 132.6, 130.2, 129.8, 127.3, 125.9, 125.5, 115.4, 108.6, 79.4, 75.1, 41.5, 28.4, 27.8, 16.9; MS (ESI) [M+H]+ 455.2.
4-(3-(4-(Furan-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)benzamide hydrochloride (79) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.77 (br, 2H), 8.40 (s, 1H), 8.37 (s, 1H), 8.22 (s, 1H), 7.99 (s, 1H), 7.81 (d, J = 7.7 Hz, 2H), 7.74 (s, 1H), 7.59 (d, J = 7.7 Hz, 2H), 7.43–7.40 (m, 4H), 6.98 (s, 1H), 4.33 (d, J = 5.6 Hz, 2H), 3.09–3.05 (m, 2H), 2.92 (br, 2H), 2.16 (br, 1H), 1.94 (d, J = 14.6 Hz, 2H), 1.56–1.47 (m, 2H); 13C NMR (100 MHz, DMSO-d6) 167.6, 158.0, 147.8, 144.6, 143.4, 141.4, 140.1, 136.3, 133.4, 132.5, 130.2, 129.4, 127.53, 127.46, 125.4, 125.3, 108.7, 69.7, 42.7, 33.0, 25.3; MS (ESI) [M+H]+ 455.5.
2-Fluoro-4-(3-(4-(furan-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)benzamide hydrochloride (80) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.80 (br, 1H), 8.48 (br, 1H), 8.39 (s, 1H), 7.77–7.75 (m, 1H), 7.74 (br, 1H), 7.66 (br, 1H), 7.63 (d, J = 8.3 Hz, 2H), 7.59 (t, J = 7.8 Hz, 2H), 7.45 (d, J = 8.3 Hz, 2H), 7.26–7.19 (m, 2H), 7.00 (s, 1H), 4.33 (d, J = 6.3 Hz, 2H), 3.33–3.30 (m, 2H), 2.96–2.87 (m, 2H), 2.16 (br, 1H), 1.94 (d, J = 14.6 Hz, 2H), 1.56–1.48 (m, 2H); 13C NMR (100 MHz, DMSO-d6) 164.8, 159.0, 158.0, 147.9, 144.6, 142.9, 142.0, 140.1, 135.9, 132.6, 131.5, 130.2, 128.9, 128.7, 125.4, 125.3, 108.6, 70.0, 43.2, 35.3, 25.6; MS (ESI) [M+H]+ 473.2.
N-(4-(3-(4-(Furan-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)benzyl)Hydroxylamine hydrochloride (81) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.20 (s, 1H), 7.87 (s, 1H), 7.58 (s, 1H), 7.49 – 7.21 (m, 8H), 6.78 (s, 1H), 4.39 (d, J = 7.6 Hz, 2H), 4.26 (d, J = 6.4 Hz, 2H), 3.52 – 3.42 (m, 2H), 3.03 (s, 2H), 2.17 (s, 1H), 2.06 (d, J = 12.4 Hz, 2H), 1.59 (dd, J = 23.6, 10.4 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.6, 148.9, 144.4, 143.6, 139.4, 139.2, 135.7, 132.4, 131.5, 130.5, 130.3, 130.0, 128.4, 125.2, 115.8, 108.2, 70.1, 54.3, 43.5, 32.9, 24.9; MS (ESI) [M+H]+ 457.2.
(4-(3-(4-(Furan-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)-3-methylphenyl)methanamine hydrochloride (82) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.10 (s, 1H), 7.77 (s, 1H), 7.47 (s, 1H), 7.28 (d, J = 8.0 Hz, 2H), 7.20 (d, J = 8.0 Hz, 1H), 7.13 (s, 3H), 6.67 (s, 1H), 4.18 (d, J = 6.0 Hz, 2H), 4.02 (s, 2H), 3.37 (d, J = 12.4 Hz, 2H), 2.92 (q, J = 11.2 Hz, 2H), 2.09 (br, 1H), 1.97 (d, J = 12.4 Hz, 2H), 1.8 (br, 3H), 1.49 (dd, J = 23.6, 11.6 Hz, 2H); MS (ESI) [M+H]+ 455.2
(3-(3-(4-(Furan-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (83) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.14 (br, 1H), 8.84 (br, 1H), 8.48 (br, 3H), 8.37 (s, 1H), 8.24 (s, 1H), 7.76 (s, 1H), 7.72 (s, 1H), 7.58 (d, J = 8.0 Hz, 2H), 7.46 (d, J = 8.0 Hz, 1H), 7.43 (d, J = 8.0 Hz, 2H), 7.28 (t, J = 8.0 Hz, 1H), 7.14 (d, J = 8.0 Hz, 1H), 6.98 (s, 1H), 4.33 (d, J = 6.2 Hz, 2H), 4.02 (q, J = 5.1 Hz, 2H), 3.29 (d, J = 12.3 Hz, 2H), 2.91 (q, J = 12.1 Hz, 2H), 2.16 (br, 1H), 1.94 (d, J = 14.6 Hz, 2H), 1.60 – 1.52 (m, 2H); 13C NMR (100 MHz, DMSO-d6) 157.8, 147.4, 144.6, 143.6, 140.0, 138.9, 136.2, 134.4, 132.4, 132.3, 130.1, 129.9, 129.6, 128.5, 128.3, 125.3, 125.2, 108.6, 69.6, 42.7, 42.2, 33.0, 25.2; MS (ESI) [M+H]+ 441.5.
(2-(3-(4-(Aminomethyl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)-5-(furan-3-yl)phenyl)methanol hydrochloride (84) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.34 (s, 1H), 7.97 (s, 1H), 7.70 (s, 1H), 7.64 (s, 1H), 7.43 – 7.29 (m, 5H), 7.10 (s, 1H), 6.88 (s, 1H), 4.54 (s, 2H), 4.27 (s, 2H), 4.14 (s, 2H), 3.51 (d, J = 12.0 Hz, 2H), 3.07 (t, J = 12.0 Hz, 2H), 2.22 (s, 1H), 2.10 (d, J = 13.2 Hz, 2H), 1.63 (dd, J = 25.6, 13.6 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.3, 148.8, 144.5, 144.1, 139.5, 139.3, 137.8, 134.5, 132.9, 132.8, 132.5, 131.4, 129.9, 128.5, 125.2, 125.1, 124.5, 108.2, 70.2, 61.4, 43.6, 42.6, 32.8, 24.9; MS (ESI) [M+H]+ 471.2.
(4-(3-(2-Fluoro-4-(furan-3-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (85) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.40 (d, J = 8.8 Hz, 1H), 9.04 (d, J = 8.8 Hz, 1H), 8.59 (br, 3H), 8.44 (s, 1H), 8.34 (s, 1H), 7.77 (s, 1H), 7.63 – 7.54 (m, 2H), 7.40 (dd, J = 23.6, 8.4 Hz, 5H), 7.05 (s, 1H), 4.26 (d, J = 6.0 Hz, 2H), 3.97 (d, J = 5.2 Hz, 2H), 3.26 (d, J = 11.6 Hz, 2H), 2.88 (dd, J = 22.0, 11.2 Hz, 2H), 2.14 (s, 1H), 1.91 (d, J = 12.6 Hz, 2H), 1.56 (dd, J = 23.2, 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 160.3, 157.9, 157.8, 144.9, 144.7, 142.9, 140.8, 138.1, 135.2, 135.1, 133.8, 133.5, 132.4, 132.4, 128.6, 128.5, 124.6, 124.49, 124.47, 124.4, 121.8, 112.6, 112.4, 108.6, 69.8, 42.5, 41.7, 32.9, 25.1; MS (ESI) [M+H]+ 459.2.
(4-(3-(4-(Furan-3-yl)-2-methoxyphenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (86) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.26 (s, 1H), 7.95 (s, 1H), 7.62 (s, 1H), 7.42 – 7.23 (m, 6H), 7.02 (s, 1H), 6.85 (s, 1H), 4.22 (s, 2H), 4.13 (s, 2H), 3.49 (d, J = 14.0 Hz, 2H), 3.31 (s, 3H), 3.03 (t, J = 13.2 Hz, 2H), 2.15 (s, 1H), 2.05 (d, J = 12.8 Hz, 2H), 1.59 (dd, J = 24.4, 13.2 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.8, 156.0, 146.8, 145.1, 144.5, 139.6, 138.6, 134.9, 132.4, 131.9, 131.8, 128.9, 128.4, 125.3, 125.2, 118.5, 108.8, 108.3, 70.2, 55.1, 43.6, 42.6, 32.9, 24.9; MS (ESI) [M+H]+ 471.2.
(4-(3-(4-(Furan-3-yl)-2-methylphenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (87) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.15 (s, 1H), 8.83 (s, 1H), 8.49 (s, 3H), 8.36 (s, 1H), 7.94 (s, 1H), 7.77 (d, J = 1.6 Hz, 1H), 7.45 – 7.38 (m, 5H), 7.31 (d, J = 7.8 Hz, 1H), 7.14 (d, J = 7.6 Hz, 1H), 6.82 (s, 1H), 4.31 (d, J = 5.6 Hz, 2H), 4.01 (d, J = 4.8 Hz, 2H), 3.29 (d, J = 12.0 Hz, 2H), 2.90 (d, J = 10.8 Hz, 2H), 2.34 (s, 3H), 2.14 (s, 1H), 1.93 (d, J = 13.2 Hz, 2H), 1.56 (d, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.7, 147.6, 143.6, 143.4, 140.9, 138.6, 136.6, 135.2, 133.6, 132.3, 132.1, 131.9, 129.5, 128.7, 128.4, 127.3, 124.4, 111.1, 69.6, 42.6, 41.8, 33.0, 25.2, 21.4; MS (ESI) [M+H]+ 455.2
(4-(3-(4-(Furan-3-yl)-3-methylphenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (88) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.36 (d, J = 11.0 Hz, 1H), 8.99 (d, J = 8.8 Hz, 1H), 8.55 (s, 3H), 8.42 (s, 1H), 8.23 (s, 1H), 7.75 (s, 1H), 7.51 (s, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.36 (dd, J = 23.4, 8.0 Hz, 4H), 7.17 (d, J = 8.0 Hz, 1H), 6.98 (s, 1H), 4.22 (d, J = 6.0 Hz, 2H), 3.95 (d, J = 5.2 Hz, 2H), 3.26 (d, J = 11.6 Hz, 2H), 2.87 (q, J = 11.2 Hz, 2H), 2.11 (s, 1H), 2.06 (br, 3H), 1.91 (d, J = 13.2 Hz, 2H), 1.55 (dd, J = 22.8, 10.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.5, 148.4, 144.4, 144.0, 139.8, 138.1, 136.5, 136.1, 133.6, 133.0, 132.0, 130.6, 128.9, 128.5, 127.3, 125.3, 123.0, 108.6, 69.6, 42.5, 41.7, 33.0, 25.1, 19.5; MS (ESI) [M+H]+ 455.2.
(4-(3-(4-(Furan-3-yl)-3-methoxyphenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (89) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.17 (s, 1H), 8.85 (s, 1H), 8.48 (br, 3H), 8.37 (s, 1H), 8.16 (s, 1H), 7.73 (s, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.50 – 7.36 (m, 4H), 7.04 (d, J = 21.6 Hz, 3H), 4.33 (d, J = 5.6 Hz, 2H), 4.01 (s, 2H), 3.67 (s, 3H), 3.29 (d, J = 10.0 Hz, 2H), 2.90 (dd, J = 21.6, 10.8 Hz, 2H), 2.16 (s, 1H), 1.94 (d, J = 12.4 Hz, 2H), 1.56 (dd, J = 22.4, 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.7, 155.5, 147.5, 143.8, 143.1, 141.9, 138.8, 137.1, 133.6, 132.4, 129.5, 128.7, 127.3, 121.9, 120.9, 120.8, 112.9, 109.3, 69.6, 55.3, 42.6, 41.8, 33.0, 25.2; MS (ESI) [M+H]+ 471.2.
5-(3-(4-(Aminomethyl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)-2-(furan-3-yl)benzonitrile hydrochloride (90) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.25 (s, 1H), 8.92 (s, 1H), 8.54 (s, 3H), 8.41 (d, J = 2.1 Hz, 1H), 8.29 (s, 1H), 7.97 (s, 1H), 7.86 (s, 1H), 7.63 (dd, J = 27.6, 8.0 Hz, 2H), 7.46 (d, J = 7.2 Hz, 2H), 7.40 (d, J = 6.4 Hz, 2H), 7.02 (s, 1H), 4.30 (s, 2H), 4.00 (s, 2H), 3.26 (d, J = 11.2 Hz, 2H), 2.88 (dd, J = 19.6, 8.8 Hz, 2H), 2.13 (s, 1H), 1.91 (d, J = 13.2 Hz, 2H), 1.55 (dd, J = 22.0, 10.4 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.8, 145.4, 144.6, 143.9, 141.9, 137.9, 137.5, 135.2, 134.9, 134.5, 134.0, 133.4, 129.6, 128.9, 128.6, 122.4, 118.6, 109.8, 108.7, 69.8, 42.6, 41.7, 32.9, 25.1; MS (ESI) [M+H]+ 466.2
(4-(3-(4-(Aminomethyl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)(pyrrolidin-1-yl)methanone hydrochloride (91) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.22 (s, 1H), 8.92 (d, J = 9.2 Hz, 1H), 8.51 (br, 3H), 8.40 (s, 1H), 7.50 – 7.40 (m, 6H), 7.37 (d, J = 8.0 Hz, 2H), 4.30 (d, J = 6.0 Hz, 2H), 4.00 (s, 2H), 3.45 (t, J = 6.0 Hz, 2H), 3.36 (t, J = 6.0 Hz, 2H), 3.28 (d, J = 12.0 Hz, 2H), 2.90 (dd, J = 22.4, 10.4 Hz, 2H), 2.15 (s, 1H), 1.93 (d, J = 13.6 Hz, 2H), 1.83 (dt, J = 23.6, 11.6 Hz, 4H), 1.56 (dd, J = 23.6, 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 168.1, 158.2, 147.6, 144.2, 139.6, 138.7, 137.6, 134.2, 133.3, 130.0, 129.9, 129.2, 127.4, 70.1, 49.3, 46.4, 43.0, 42.2, 33.4, 26.4, 25.6, 24.3; MS (ESI) [M+H]+ 472.3.
(4-(3-(4-(Aminomethyl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)(morpholino)methanone hydrochloride (92) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.27 (d, J = 10.4 Hz, 1H), 8.98 (d, J = 9.6 Hz, 1H), 8.55 (s, 3H), 8.40 (s, 1H), 7.46 (dd, J = 9.6, 8.4 Hz, 4H), 7.41 – 7.32 (m, 4H), 4.29 (d, J = 6.0 Hz, 2H), 4.01 (d, J = 5.6 Hz, 2H), 3.60 (s, 11H), 3.28 (d, J = 11.6 Hz, 2H), 2.90 (dd, J = 22.4, 10.8 Hz, 2H), 2.15 (s, 1H), 1.93 (d, J = 12.8 Hz, 2H), 1.57 (q, J = 12.4 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 169.0, 158.1, 147.5, 144.0, 139.5, 138.6, 135.8, 133.7, 133.2, 129.97, 129.95, 129.0, 127.3, 69.9, 66.6, 66.3, 42.9, 41.9, 33.1, 25.3; MS (ESI) [M+H]+ 488.3
(4-(3-(4-(Aminomethyl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)(piperidin-1-yl)methanone hydrochloride (93) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.17 (s, 1H, NH), 8.86 (s, 1H, NH), 8.47 (s, 3H, NH), 8.37 (s, 1H), 7.46 – 7.20 (m, 8H), 4.26 (s, 2H), 3.97 (s, 2H), 3.53 (s, 2H), 3.24 (s, 4H), 2.86 (s, 2H), 2.11 (s, 1H), 1.90 (d, J = 13.2 Hz, 2H), 1.60 – 1.32 (m, 8H); 13C NMR (100 MHz, DMSO-d6) δ 168.3, 157.7, 147.2, 143.7, 138.8, 138.3, 136.5, 133.8, 132.8, 129.6, 129.5, 128.7, 126.6, 69.6, 42.6, 41.8, 32.9, 25.1, 24.0; MS (ESI) [M+H]+ 486.3.
(4-(5-(Piperidin-4-ylmethoxy)-3-(4-(tetraHydrofuran-3-yl)phenyl)pyrazin-2-yl)phenyl)methanamine hydrochloride (94) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.16 (bs, 1H), 8.86 (bs, 1H), 8.48 (bs, 3H), 8.31 (s, 1H), 7.41 (d, J = 8.4 Hz, 2H), 7.32–7.40 (m, 4H), 7.21 (d, J = 8.4 Hz, 2H), 4.26 (d, J = 5.6 Hz, 2H), 3.99 (d, J = 7.6 Hz, 3H), 3.88–3.93 (m, 1H), 3.76 (dd, J = 8 Hz, 15.6 Hz, 1H), 3.35 (t, J = 8.4 Hz, 1H), 3.25 (d, J = 13.2 Hz, 2H), 2.87 (d, J = 11.2 Hz, 2H), 2.26–2.29 (m, 1H), 2.12 (bs, 1H), 1.88–1.92 (m, 3H), 1.52–1.56 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.1, 148.0, 143.9, 143.8, 139.0, 136.5, 134.1, 132.7, 130.1, 129.9, 129.1, 127.6, 74.1, 69.9, 68.1, 55.4, 44.3, 43.0, 34.2, 33.4, 25.6; MS (ESI) [M+H]+ 445.3.
(4-(5-(Piperidin-4-ylmethoxy)-3-(3-(tetraHydrofuran-3-yl)phenyl)pyrazin-2-yl)phenyl)methanamine hydrochloride (95) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.30 (s, 1H), 7.14–7.33 (m, 8H), 4.23 (d, J = 5.6 Hz, 2H), 3.85 (t, J = 6.8 Hz, 1H), 3.65–3.67 (m, 4H), 3.21–3.27 (m, 4H), 3.08 (d, J = 12 Hz, 2H), 2.63–2.66 (m, 2H), 2.12 (bs, 1H), 1.98 (bs, 1H), 1.77 (d, J = 14 Hz, 2H), 1.60 (bs, 1H), 1.28–1.36 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.2, 148.0, 144.6, 143.0, 138.6, 137.3, 132.7, 129.6, 128.9, 128.8, 128.2, 127.9, 127.6, 127.1, 74.0, 70.6, 67.9, 55.3, 44.6, 44.2, 34.7, 34.2, 27.8; MS (ESI) [M+H]+ 444.6.
(4-(5-(Piperidin-4-ylmethoxy)-3-(4-(pyrrolidin-1-yl)phenyl)pyrazin-2-yl)phenyl)methanamine hydrochloride (96) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.07 (s, 1H), 8.75 (s, 1H), 8.45 (s, 3H), 8.19 (s, 1H), 7.43 (q, J = 8.4 Hz, 4H), 7.26 (d, J = 8.8 Hz, 2H), 6.45 (d, J = 8.8 Hz, 2H), 4.29 (d, J = 6.4 Hz, 2H), 4.02 (d, J = 6.0 Hz, 2H), 3.29 (d, J = 12.0 Hz, 2H), 3.23 (s, 4H), 2.90 (dd, J = 23.6, 12.4 Hz, 2H), 2.14 (s, 1H), 1.95 (s, 7H), 1.54 (dd, J = 24.2, 10.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.5, 148.2, 147.7, 142.6, 139.6, 133.3, 130.7, 130.3, 129.3, 128.6, 123.90, 111.1, 69.2, 47.2, 42.7, 41.8, 33.0, 25.2, 25.0; MS (ESI) [M+H]+ 444.3.
(4-(3-(4-(Piperidin-1-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (97) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.32 (s, 1H), 7.59 (dd, J = 22.2, 7.9 Hz, 4H), 7.41 (br, 4H), 4.38 (s, 2H), 4.21 (s, 2H), 3.64 (br, 4H), 3.52 (d, J = 12.4 Hz, 2H), 3.09 (t, J = 12.0 Hz, 2H), 2.27 (s, 1H), 2.13 (d, J = 12.8 Hz, 2H), 2.03 (br, 4H), 1.77 (s, 2H), 1.66 (dd, J = 25.2, 12.8 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.8, 147.6, 143.8, 141.9, 139.3, 137.9, 132.8, 132.5, 131.8, 130.2, 128.8, 120.9, 70.2, 57.1, 43.6, 42.6, 32.9, 25.0, 23.3, 20.5; MS (ESI) [M+H]+ 458.3.
(4-(3-(4-Cyclohexylphenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (98) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.97 (bs, 1H), 8.68 (bs, 1H), 8.37 (bs, 3H), 8.30 (s, 1H), 7.29–7.39 (m, 6H), 7.15 (d, J = 8 Hz, 2H), 4.27 (s, 2H), 3.99 (s, 2H), 3.27 (d, J = 11.6 Hz, 2H), 2.88 (d, J = 11.2 Hz, 2H), 2.11 (bs, 1H), 1.90 (d, J = 13.6 Hz, 3H), 1.65–1.76 (m, 5H), 1.51 (d, J = 12 Hz, 2H), 1.35 (bs, 4H), 1.12–1.20 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.1, 148.7, 148.2, 143.8, 139.1, 136.0, 134.0, 132.6, 130.0, 129.9, 129.0, 127.0, 110.0, 69.9, 43.9, 43.1, 42.2, 34.2, 33.3, 26.7, 26.0, 25.6; MS (ESI) [M+H]+ 457.3.
(4-(3-(4-(Piperidin-4-yl)phenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (99) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.24 (s, 1H), 7.38 (s, 6H), 7.25 (d, J = 7.6 Hz, 2H), 4.36 (d, J = 5.2 Hz, 2H), 4.18 (s, 2H), 3.51 (t, J = 12.0 Hz, 4H), 3.18 – 3.01 (m, 4H), 2.97 – 2.88 (m, 1H), 2.25 (s, 1H), 2.16 – 2.02 (m, 4H), 1.87 (dd, J = 26.4, 12.8 Hz, 2H), 1.63 (dd, J = 25.6, 12.0 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.7, 149.2, 145.21, 143.6, 138.4, 135.7, 132.5, 131.3, 130.1, 130.0, 128.7, 126.5, 70.1, 44.2, 43.5, 42.6, 38.8, 32.8, 29.1, 24.9; MS (ESI) [M+H]+ 458.3.
(4-(5-(Piperidin-4-ylmethoxy)-3-(4-(1,2,3,6-tetraHydropyridin-4-yl)phenyl)pyrazin-2-yl)phenyl)methanamine hydrochloride (100) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.52 (s, 2H, NH), 9.15 (s, 1H, NH), 8.86 (s, 1H, NH), 8.49 (s, 4H), 8.34 (s, 1H), 7.44 – 7.32 (m, 8H), 6.24 (s, 6H), 4.26 (d, J = 5.2 Hz, 2H), 3.97 (d, J = 4.0 Hz, 2H), 3.69.18 (s, 2H), 3.60 – 3.24 (m, 4H), 2.90 – 2.85 (m, 2H), 2.65 (s, 2H), 2.11 (s, 1H), 1.88 (d, J = 12.0 Hz, 2H), 1.54 (dd, J = 25.6, 12.0 Hz, 2H); MS (ESI) [M+H]+ 456.3.
(4-(3-(6-(Piperazin-1-yl)pyridin-3-yl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (101) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.20 (s, 1H), 7.97 (s, 1H), 7.79 (d, J = 12.0 Hz, 1H), 7.35 (s, 4H), 7.04 (d, J = 12.0 Hz, 1H), 4.27 (s, 2H), 4.09 (s, 1H), 3.83 (s, 4H), 3.35 (s, 6H), 2.94 (t, J = 12.0 Hz, 4H), 2.15 (s, 2H), 1.99 (d, J = 12.0 Hz, 2H), 1.50 (d, J = 12.0 Hz, 2H); 13C NMR (100 MHz, D2O) δ 158.9, 152.1, 144.6, 143.7, 143.3, 138.5, 137.4, 133.4, 133.2, 130.2, 129.2, 124.5, 111.8, 70.2, 43.6, 42.9, 42.6, 42.2, 32.8, 25.0; MS (ESI) [M+H]+ 460.3.
(4-(5-(Piperidin-4-ylmethoxy)-3-(6-(tetraHydro-2H-pyran-4-yl)pyridin-3-yl)pyrazin-2-yl)phenyl)methanamine hydrochloride (102) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.11 (bs, 1H), 8.82–8.85 (m, 1H), 8.60 (s, 1H), 8.49 (bs, 3H), 8.43 (s, 1H), 8.01 (d, J = 7.6 Hz, 1H), 7.56 (d, J = 8 Hz, 1H), 7.46 (d, J = 8 Hz, 2H), 7.40 (d, J = 8.4 Hz, 2H), 4.28 (d, J = 6 Hz, 2H), 4.00 (d, J = 5.2 Hz, 2H), 3.93 (d, J = 10.8, 2H), 3.32–3.62 (m, 4H), 3.26 (d, J = 11.6 Hz, 2H), 3.14 (bs, 1H), 2.88 (d, J = 12 Hz, 2H), 2.14 (bs, 1H), 1.90 (d, J = 12.8 Hz, 2H), 1.59–1.74 (m, 4H), 1.51–1.59 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.3, 146.2, 144.6, 144.0, 138.0, 134.6, 134.2, 133.6, 130.1, 129.4, 129.3, 129.2, 122.8, 70.3, 67.2, 65.4, 43.0, 42.2, 41.1, 34.6, 33.3, 31.8, 31.7, 25.5, 15.6; MS (ESI) [M+H]+ 460.3.
(4-(5-(Piperidin-4-ylmethoxy)-3-(4-(tetraHydro-2H-pyran-4-yl)phenyl)pyrazin-2-yl)phenyl)methanamine hydrochloride (103) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.02 (bs, 1H), 8.70–8.72 (m, 1H), 8.40 (bs, 3H), 8.32 (s, 1H), 7.32–7.41 (m, 6H), 7.20 (d, J = 8 Hz, 2H), 4.26 (d, J = 6 Hz, 2H), 3.99 (d, J = 5.2 Hz, 2H), 3.91 (d, J = 10.8 Hz, 2H), 2.88 (dd, J = 11.2 Hz, 22.8 Hz, 2H), 2.73–2.76 (m, 1H), 2.11 (bs, 1H), 1.90 (d, J = 13.2 Hz, 2H), 1.64 (bs, 4H), 1.51 (d, J = 12 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 158.1, 148.1, 147.0, 143.8, 139.1, 136.3, 134.0, 132.6, 130.1, 129.9, 129.0, 127.0, 69.9, 67.7, 43.0, 42.2, 40.5, 33.8, 33.3, 25.6; MS (ESI) [M+H]+ 459.3.
(4-(3-(4-Morpholinophenyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (104) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 9.24 (d, J = 10.4 Hz, 1H), 8.91 (dd, J = 20.8, 10.8 Hz, 1H), 8.54 (s, 3H), 8.27 (s, 1H), 7.45 (d, J = 8.0 Hz, 2H), 7.39 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.4 Hz, 2H), 4.29 (d, J = 6.0 Hz, 2H), 4.01 (d, J = 5.2 Hz, 2H), 3.75 (br, 4H), 3.27 (d, J = 12.4 Hz, 2H), 3.18 (br, 4H), 2.89 (q, J = 11.6 Hz, 2H), 2.14 (s, 1H), 1.92 (d, J = 12.6 Hz, 2H), 1.55 (q, J = 11.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 157.6, 150.3, 147.6, 143.1, 139.0, 133.5, 131.3, 130.6, 129.4, 128.7, 114.6, 69.4, 65.8, 48.1, 42.6, 41.8, 40.2, 39.9, 39.7, 39.5, 39.3, 39.1, 38.9, 33.0, 25.2; MS (ESI) [M+H]+ 460.3.
General Synthetic Procedure for compounds 33, 35, 36, and 37.
To a solution of R5 substituted compound (88 mg, 0.16 mmol) in DMSO (3 mL) was added pyrazole (17 mg, 0.25 mmol) and potassium carbonate (45 mg, 0.32 mmol). The resulting mixture was heated at 120 °C for 24 h before it was cooled to room temperature. The mixture was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 2:1 to 1.5:1) to give corresponding R6 substituted product (35 mg, 38%). For precursor of 35: 1H NMR (400 MHz, CDCl3) δ 8.32 (s, 1H), 7.69 (s, 1H), 7.65 (s, 1H), 7.21 (s, 4H), 4.86 (s, 1H), 4.29 (br, 1H), 4.25 (d, J = 6.8 Hz, 2H), 4.18 (br, 2H), 2.74 (t, J = 12.8 Hz, 2H), 2.03 (s, 1H), 1.81 (d, J = 12.8 Hz, 2H), 1.45 (br, 18H), 1.28–1.21 (m, 2)
To a solution of R6 substituted compound (0.08 mmol) in DCM (5 mL) was added dropwise HCl (0.1 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 5 h. The volatiles were removed in vacuo to afford an oil, which was triturated in diether ether and solidified to give the final product hydrochloric salt.
N1-(3-(4-(Aminomethyl)phenyl)-6-(piperidin-4-ylmethoxy)pyrazin-2-yl)ethane-1,2-diamine hydrochloride (33) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 7.62 – 7.43 (m, 4H), 7.41 (s, 1H), 4.24 (d, J = 6.0 Hz, 2H), 4.16 (s, 2H), 3.62 (d, J = 5.6 Hz, 2H), 3.38 (d, J = 11.2 Hz, 2H), 3.15 (s, 2H), 2.94 (t, J = 12.0 Hz, 2H), 2.10 (s, 1H), 1.98 (d, J = 13.6 Hz, 2H), 1.56 – 1.42 (m, 2H); 13C NMR (100 MHz, D2O) δ 160.3, 152.8, 134.8, 131.4, 129.8, 129.4, 128.0, 112.4, 70.6, 43.6, 42.6, 38.5, 32.9, 25.0; MS (ESI) [M+H]+ 357.2.
(4-(5-(Piperidin-4-ylmethoxy)-3-(1H-pyrazol-1-yl)pyrazin-2-yl)phenyl)methanamine hydrochloride (35). 1H NMR (400 MHz, D2O) δ 8.26 (s, 1H), 7.76 (s, 1H), 7.57 (s, 1H), 7.30 (d, J = 7.2 Hz, 2H), 7.16 (d, J = 7.2 Hz, 2H), 4.22 (d, J = 6.0 Hz, 2H), 4.07 (s, 2H), 3.37 (d, J = 12.4 Hz, 2H), 2.94 (t, J = 12.4 Hz, 2H), 2.13 (s, 1H), 1.98 (d, J = 13.2 Hz, 2H), 1.57 – 1.46 (m, 2H); 13C NMR (100 MHz, D2O) δ 158.2, 142.2, 141.9, 138.6, 136.0, 134.2, 133.1, 132.1, 128.9, 128.8, 108.1, 70.5, 43.5, 42.6, 33.0, 25.0; 365.2; MS (ESI) [M+H]+ 365.2
(4-(5-(Piperidin-4-ylmethoxy)-3-(1H-1,2,3-triazol-1-yl)pyrazin-2-yl)phenyl)methanamine hydrochloride (36) was prepared following the same procedure as a hydrochloric acid salt.. 1H NMR (400 MHz, D2O) δ 8.43 (s, 1H), 7.87 (s, 2H), 7.30 (d, J = 7.6 Hz, 2H), 7.11 (d, J = 7.6 Hz, 2H), 4.26 (d, J = 5.6 Hz, 2H), 4.07 (s, 2H), 3.38 (d, J = 12.4 Hz, 2H), 2.95 (t, J = 12.8 Hz, 2H), 2.16 (s, 1H), 1.99 (d, J = 13.6 Hz, 2H), 1.57 – 1.46 (m, 2H); 13C NMR (100 MHz, D2O) δ 158.4, 141.1, 139.5, 137.1, 136.6, 135.4, 133.4, 129.0, 128.7, 70.7, 43.5, 42.6, 32.6, 24.8; MS (ESI) [M+H]+ 366.2.
(4-(3-(1H-Imidazol-1-yl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (37) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 9.46 (s, 1H), 8.57 (s, 1H), 8.13 (s, 1H), 7.86 (d, J = 7.6 Hz, 2H), 7.59 (s, 1H), 7.49 (d, J = 8.0 Hz, 2H), 4.36 (d, J = 6.0 Hz, 2H), 4.16 (s, 2H), 3.34 (d, J = 12.8 Hz, 2H), 2.90 (t, J = 12.8 Hz, 2H), 2.15 (s, 1H), 1.94 (d, J = 14.4 Hz, 2H), 1.46 (dd, J = 24.8, 11.6 Hz, 2H); 13C NMR (100 MHz, D2O) δ 156.6, 143.9, 139.6, 134.7, 134.4, 133.6, 129.8, 128.8, 125.1, 121.0, 119.0, 70.9, 43.5, 42.7, 32.6, 25.1; MS (ESI) [M+H]+ 365.2.
General Synthetic Procedure for compounds 19, 66–69, and 71.
2,6-Dibromopyrazine (111a, X =N, 7.14 g, 30 mmol) or 2,6-dibromopyridine (111b, X = CH, 7.14 g, 30 mmol), tert-butyl 4-(aminomethyl)piperidine-1-carboxylate (7.07 g, 33 mmol) and potassium carbonate (8.28 g, 60 mmol) in DMF (30 mL) were placed in a sealed tube. The mixture was stirred at 100 °C for 12 h. The reaction was cooled and quenched with saturated brine (50 mL). The mixture was extracted with ethyl acetate (3 × 100 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford 112a or 112b as a white solid, which was used in the next step without further purification. For compound 112a: 1H NMR (400 MHz, CDCl3): δ 7.85 (s, 1H), 7.76 (s, 1H), 5.00 (s, 1H), 4.14 – 4.11 (m, 2H), 3.25 (t, J = 5.9 Hz, 2H), 2.68 (t, J = 11.8 Hz, 2H), 1.73 – 1.70 (m, 3H), 1.44 (s, 9H), and 1.21 – 1.09 (m, 2H). For compound 112b: 1H NMR (400 MHz, CDCl3): δ 7.23 (t, J = 7.8 Hz, 1H), 6.70 (d, J = 7.8 Hz, 1H), 6.26 (d, J = 7.8 Hz, 1H), 4.76 (t, J = 5.4 Hz, 1H), 4.10 (br, 2H), 3.14 (t, J = 6.1 Hz, 2H), 2.67 (t, J = 12.8 Hz, 2H), 1.77 – 1.67 (m, 3H), 1.44 (s, 9H), and 1.19 – 1.09 (m, 2H).
To a solution of compound 112a or 112b (~30 mmol) in CH3CN/DMSO (20/10 mL) was added N-Iodosuccinimide (8.1 g, 36 mmol). After stirring at room temperature for 24 h, the reaction was quenched with sodium thiosulfate aqueous solution (50 mL). The mixture was extracted with ethyl acetate (3 × 100 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 10:1 to 3:1) to give 113a or 113b as a white solid (8.95 g, 60% over two steps). For compound 113a: 1H NMR (400 MHz, CDCl3): δ 7.64 (s, 1H), 4.86 (t, J = 5.6 Hz, 1H), 4.14 – 4.11 (m, 2H), 3.24 (t, J = 5.9 Hz, 2H), 2.69 (t, J = 12.2 Hz, 2H), 1.73 – 1.69 (m, 3H), 1.45 (s, 9H), and 1.21 – 1.13 (m, 2H). For compound 113b: 1H NMR (400 MHz, CDCl3): δ 7.64 (d, J = 8.5 Hz, 1H), 6.10 (d, J = 8.5 Hz, 1H), 4.74 (t, J = 5.4 Hz, 1H), 4.14 – 4.09 (m, 2H), 3.14 (t, J = 5.9 Hz, 2H), 2.69 (t, J = 12.2 Hz, 2H), 1.73 – 1.69 (m, 3H), 1.45 (s, 9H), and 1.20 – 1.11 (m, 2H).
Compound 113a or 113b (994 mg, 2 mmol), 4-((N-Boc-amino)methyl)phenylboronic acid (527 mg, 2.1 mmol), tetrakis(triphenylphosphine)palladium (69 mg, 3 mol%), and sodium carbonate (424 mg, 4 mmol) in p-dioxane/H2O (15/3 mL) were placed in a sealed tube. The mixture was degassed and heated to 80 °C for 24 h. The reaction was then cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine, dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 5:1 to 1:1) to give the corresponding R5 substituted product a and b (842 mg, 73% yield for a). For compound a (Intermediate of 66): 1H NMR (400 MHz, CDCl3): δ 7.88 (s, 1H), 7.63 (d, J = 7.8 Hz, 2H), 7.34 (d, J = 7.8 Hz, 2H), 4.94 (t, J = 5.9 Hz, 1H), 4.88 (br, 1H), 4.37 (d, J = 5.9 Hz, 2H), 4.16 – 4.11 (m, 2H), 3.31 (t, J = 5.9 Hz, 2H), 2.71 (t, J = 12.2 Hz, 2H), 1.77 – 1.74 (m, 3H), 1.46 (s, 9H), 1.45 (s, 9H), and 1.25 – 1.15 (m, 2H). compound b (Intermediate of 69): 1H NMR (400 MHz, CDCl3): δ 7.38 (d, J = 8.4 Hz, 1H), 7.34 (d, J = 8.4 Hz, 2H), 7.30 (d, J = 8.4 Hz, 2H), 6.36 (d, J = 8.4 Hz, 1H), 4.88 (br, 2H), 4.36 (br, 2H), 4.16 – 4.11 (m, 2H), 3.18 (t, J = 5.9 Hz, 2H), 2.68 (t, J = 12.2 Hz, 2H), 2.10 (br, 1H), 1.77 – 1.74 (m, 2H), 1.46 (s, 9H), 1.45 (s, 9H), and 1.22 – 1.15 (m, 2H).
The above R5 substituted compound a or b (1 mmol), 2-(4-(furan-3-yl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1.1 mmol), tetrakis(triphenylphosphine)palladium (57.7 mg, 5 mol%), and sodium carbonate (216 mg, 2 mmol) in p-dioxane/H2O (8/2 mL) were placed in a sealed tube. The mixture was degassed and heated to 100 °C for 24 h. The reaction was then cooled and quenched with brine (10 mL). The mixture was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 5:1 to 1:2) to afford the corresponding R6 substituted a or b (78% yield for a). For Compound a (precursor of 66): 1H NMR (400 MHz, CDCl3): δ 7.90 (s, 1H), 7.73 (s, 1H), 7.46 (s, 1H), 7.42 (d, J = 8.2 Hz, 2H), 7.39 (d, J = 8.2 Hz, 2H), 7.33 (d, J = 7.9 Hz, 2H), 7.16 (d, J = 7.9 Hz, 2H), 6.69 (s, 1H), 4.89 (t, J = 5.9 Hz, 1H), 4.85 (br, 1H), 4.28 (d, J = 5.9 Hz, 2H), 4.16 – 4.11 (m, 2H), 3.36 (t, J = 6.1 Hz, 2H), 2.71 (t, J = 11.8 Hz, 2H), 1.79 – 1.76 (m, 3H), 1.45 (s, 9H), 1.44 (s, 9H), and 1.26 – 1.16 (m, 2H). For Compound b(precursor of 69): 1H NMR (400 MHz, CDCl3): δ 7.70 (s, 1H), 7.47 (d, J = 8.4 Hz, 1H), 7.45 (br, 1H), 7.34 (br, 4H), 7.13 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 8.0 Hz, 2H), 6.67 (s, 1H), 6.42 (d, J = 8.4 Hz, 1H), 4.83 (t, J = 5.5 Hz, 2H), 4.28 (d, J = 5.3 Hz, 2H), 4.14 – 4.09 (m, 2H), 3.25 (t, J = 5.8 Hz, 2H), 2.71 (t, J = 13.4 Hz, 2H), 1.81 – 1.78 (m, 3H), 1.46 (s, 9H), 1.45 (s, 9H), and 1.27 – 1.20 (m, 2H).
The above R6 substituted (0.2 mmol) was dissolved in dichloromethane (2 mL). HCl (0.2 mL, 4 N in p-dioxane) was slowly added to the reaction mixture at 0 °C. After 0.5 h, the reaction was warmed to room temperature and stirred for 12 h. The volatiles were removed in vacuo to afford an oil, which was triturated in diether ether and solidified to give 66 and 69 hydrochloric salt as a white and pale-yellow powder, respectively.
5-(4-(Aminomethyl)phenyl)-6-(4-(furan-3-yl)phenyl)-N-(piperidin-4-ylmethyl)pyrazin-2-amine hydrochloride (66). 1H NMR (400 MHz, DMSO-d6) δ 9.00 (br, 2H), 8.67 (br, 2H), 8.38 (br, 4H), 8.21 (s, 1H), 8.02 (s, 1H), 7.75 (s, 1H), 7.54 (d, J = 7.3 Hz, 2H), 7.37–7.30 (m, 6H), 6.96 (s, 1H), 3.98 (br, 2H), 3.28–3.24 (m, 4H), 2.83 (q, J = 10.8 Hz, 2H), 1.89–1.85 (m, 3H), 1.46–1.38 (m, 2H); 13C NMR (100 MHz, DMSO-d6) 153.0, 148.1, 144.5, 143.8, 139.8, 139.6, 137.9, 137.6, 132.5, 131.7, 130.0, 129.3, 128.5, 125.3, 125.1, 108.6, 55.0, 42.9, 41.9, 33.5, 26.4; MS (ESI) [M+H]+ 440.5.
5-(4-(Aminomethyl)phenyl)-6-(4-(furan-3-yl)phenyl)-N-(piperidin-4-ylmethyl)pyridin-2-amine hydrochloride (69). 1H NMR (400 MHz, DMSO-d6) δ 9.05 (br, 1H), 8.83 (br, 2H), 8.44 (br, 3H), 8.28 (s, 1H), 7.91 (br, 2H), 7.77 (s, 1H), 7.62 (d, J = 8.1 Hz, 2H), 7.41 (d, J = 8.1 Hz, 2H), 7.36 (d, J = 8.1 Hz, 2H), 7.19 (d, J = 8.1 Hz, 2H), 7.01 (s, 1H), 3.97 (d, J = 6.3 Hz, 2H), 3.38 (br, 2H), 3.28 (d, J = 13.5 Hz, 2H), 2.85 (q, J = 12.3 Hz, 2H), 1.95–1.91 (m, 3H), 1.48–1.40 (m, 2H); 13C NMR (100 MHz, DMSO-d6) 153.9, 144.6, 140.4, 136.6, 133.2, 130.4, 129.5, 129.0, 125.3, 124.9, 123.1, 108.5, 46.3, 42.5, 41.6, 33.1, 25.9; MS (ESI) [M+H]+ 439.6.
((6-((Piperidin-4-ylmethyl)amino)pyridine-2,3-diyl)bis(4,1-phenylene))dimethanamine hydrochloride (19) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.09 (d, J = 9.3 Hz, 2H), 7.44 (s, 2H), 7.36 (d, J = 8.1 Hz, 2H), 7.26 (d, J = 8.1 Hz, 2H), 7.22 (d, J = 9.3 Hz, 2H), 4.21 (s, 2H), 4.15 (s, 2H), 3.52–3.46 (m, 4H), 3.05 (t, J = 13.2 Hz, 2H), 2.13–2.08 (m, 3H), 1.59–1.49 (m, 2H); 13C NMR (100 MHz, D2O) 152.8, 146.4, 144.5, 136.5, 134.8, 132.4, 132.2, 130.4, 130.2, 128.9, 128.8, 124.8, 110.3, 46.6, 43.6, 42.55, 42.48, 32.8, 25.9; MS (ESI) [M+H]+ 402.3.
5-(4-(Aminomethyl)phenyl)-6-(2-fluoro-4-(furan-3-yl)phenyl)-N-(piperidin-4-ylmethyl)pyrazin-2-amine hydrochloride (67) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O): δ 8.13 (s, 1H), 7.84 (br, 2H), 7.60 (d, J = 7.6 Hz, 2H), 7.50 (d, J = 7.6 Hz, 2H), 7.33–7.26 (m, 3H), 6.72 (s, 1H), 4.21 (s, 2H), 3.72 (s, 2H), 3.44 (d, J = 13.1 Hz, 2H), 2.99–2.93 (m, 2H), 2.06–1.96 (m, 3H), 1.51–1.42 (m, 2H); 13C NMR (100 MHz, D2O): 159.4, 153.7, 152.2, 144.3, 140.1, 136.5, 136.1, 135.6, 132.9, 132.7, 130.4, 129.7, 129.3, 128.8, 128.6, 121.5, 112.5, 108.1, 45.3, 43.6, 42.6, 32.7, 26.0; MS (ESI) [M+H]+ 458.2.
5-(4-(Aminomethyl)-3-fluorophenyl)-6-(4-(furan-3-yl)phenyl)-N-(piperidin-4-ylmethyl)pyrazin-2-amine hydrochloride (68) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, D2O) δ 8.11 (s, 1H), 7.81 (br, 1H), 7.47 (br, 1H), 7.39 (d, J = 8.0 Hz, 2H), 7.28 (d, J = 8.0 Hz, 2H), 7.25 (br, 1H), 7.08–7.05 (m, 2H), 6.72 (s, 1H), 4.22 (d, J = 6.0 Hz, 2H), 4.10 (s, 2H), 3.36 (d, J = 13.6 Hz, 2H), 2.92 (t, J = 12.9 Hz, 2H), 2.11 (br, 1H), 1.98 (d, J = 14.8 Hz, 2H), 1.54–1.44 (m, 2H); 13C NMR (100 MHz, D2O): 160.5, 158.6, 148.8, 144.5, 141.2, 139.4, 138.5, 135.3, 132.6, 131.5, 131.1, 130.3, 125.9, 125.1, 125.0, 119.6, 116.6, 108.2, 70.6, 43.5, 36.7, 32.9, 25.0; MS (ESI) [M+H]+ 458.6.
5-(4-(Aminomethyl)phenyl)-N-(piperidin-4-ylmethyl)-6-(4-(thiophen-3-yl)phenyl)pyridin-2-amine hydrochloride (71) was prepared following the same procedure as a hydrochloric acid salt. 1H NMR (400 MHz, DMSO-d6) δ 8.84 (br, 2H), 8.61 (br, 2H), 8.31 (br, 4H), 7.95 (s, 1H), 7.71–7.58 (m, 3H), 7.38–7.35 (m, 4H), 7.18 (d, J = 8.1 Hz, 2H), 3.97 (q, J = 5.4 Hz, 2H), 3.35 (br, 2H), 3.28 (d, J = 13.5 Hz, 2H), 2.85 (q, J = 11.6 Hz, 2H), 1.95–1.91 (m, 3H), 1.46–1.36 (m, 2H); MS (ESI) [M+H]+ 455.2.
General Synthetic Procedure for compounds 56, 57, 58, and 70.
To a solution of 3-amino-6-iodopyrazine-2-carboxylate (114, 1.8 g, 6.6 mmol) in 12N HCl-H2O (v/v = 5/1, 54 mL) at - 10 °C was added sodium nitrite solution (0.7 M solution, 18 mL) in 3 portions. The resulting reaction mixture was stirred at - 10 °C for 1 h before Copper(I) chloride (0.78 g, 7.88 mmol) in 12 N HCl solution (1.8 mL) was added slowly and stirred for additional 2 h. The mixture was extracted with ethyl acetate (3 × 50 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a crude residue, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 20:1 to 10:1) to afford 3-Chrolo-6-iodopyrazine-2-carboxylate 115 (770 mg, 39%). 1H NMR (400 MHz, CDCl3) δ 8.75 (s, 1H), 4.02 (s, 3H).
3-Chrolo-6-iodopyrazine-2-carboxylate (115, 298 mg, 1 mmol), tert-butyl 4-(aminomethyl)piperidine-1-carboxylate (206 mg, 0.98 mmol) and potassium carbonate (300 mg, 2.2 mmol) in DMSO (6 mL) were placed in a sealed tube. The mixture was stirred at 50 °C for 12 h. The reaction was cooled and quenched with saturated brine (30 mL). The mixture was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a residue, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 2:1 to 1.5:1) to afford 116 (270 mg, 70%). 1H NMR (400 MHz, CDCl3) δ 8.35 (s, 1H), 8.06 (t, J = 5.3 Hz, 1H), 4.07 (dd, J = 13.9, 6.9 Hz, 2H), 3.91 (s, 3H), 3.36 (t, J = 6.1 Hz, 2H), 2.65 (t, J = 11.5 Hz, 2H), 1.78 – 1.63 (m, 3H), 1.41 (s, 9H), 1.19 – 1.08 (m, 2H).
Compound 116 (270 mg, 0.70 mmol), 4-[(tert-Butoxycarbonylamino)methyl]phenylboronic acid pinacol ester (300 mg, 0.9 mmol), tetrakis(triphenylphosphine)palladium (40 mg, 0.035 mmol), and sodium carbonate (220 mg, 2.07 mmol) in p-dioxane/H2O (10/2 mL) were placed in a sealed tube. The mixture was degassed and heated to 105 °C for 24 h. The reaction was then cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 30 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 1.5:1 to 1:1) to afford the product 117 (244 mg, 63%), and a small portion of crude product (38 mg). 1H NMR (400 MHz, CDCl3) δ 8.34 (s, 1H), 8.02 (t, J = 5.3 Hz, 1H), 7.49 (d, J = 7.6 Hz, 2H), 7.32 (d, J = 7.6 Hz, 2H) 4.02 (dd, J = 13.9, 6.9 Hz, 2H), 3.88 (s, 3H), 3.36 (t, J = 6.1 Hz, 2H), 2.65 (t, J = 11.5 Hz, 2H), 1.78 – 1.63 (m, 3H), 1.41 (s, 9H), 1.19 – 1.08 (m, 2H).
To a solution of compounds 117 (244 mg, 0.44 mmol) in MeOH-H2O (v/v = 4/1, 5 mL) was added Lithium hydroxide (105 mg, 4.4 mmol) at room temperature. It was allowed to stir for 24 h before removing the solvents to give a residue, which was acidified by 1N HCl and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a crude acid, which was used in the next step without further purification.
To a solution of the crude acid (70 mg) and 4-aminomethyl-1-Boc-piperidine (42 mg, 0.2 mmol) in DMF (3 mL) was added N, N-diisopropylethylamine (87 μL, 0.5 mmol) and HATU (114 mg, 0.3 mmol). The mixture was stirred for 12 h before it was quenched with H2O. The mixture was extracted with ethyl acetate (3 × 80 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 1.5:1 to 1:1) to afford corresponding amides (62 mg) as colorless oil. For 56 precursor: 1H NMR (400 MHz, CDCl3) δ 9.92 (s, 1H), 8.74 (br, 1H), 8.66 (s, 1H), 7.80 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 8.4 Hz, 2H), 7.39 (d, J = 8.0 Hz, 2H), 7.33 (d, J = 8.0 Hz, 2H), 4.98 (br, 1H), 4.36 (d, J = 5.2 Hz, 2H), 4.16 (br, 1H), 3.47 (t, J = 5.6 Hz, 2H), 2.70 (br, 1H), 1.90–1.78 (m, 3H), 1.91 (s, 1H), 1.47 (s, 9H), 1.45 (s, 9H); For 57 precursor: 1H NMR (400 MHz, CDCl3) δ 9.93 (s, 1H), 8.75 (t, J = 5.6 Hz, 1H), 8.68 (s, 1H), 7.82 (d, J = 8.0 Hz, 2H), 7.69 (d, J = 8.5 Hz, 2H), 7.48 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 7.9 Hz, 2H), 4.93 (br, 1H), 4.38 (d, J = 5.2 Hz, 2H), 4.12 (br, 2H), 3.48 (t, J = 6.0 Hz, 2H), 2.71 (t, J = 13.3 Hz, 2H), 1.90–1.78 (m, 3H), 1.48 (s, 9H), 1.45 (s, 9H), 1.25 (s, 2H); For 58 precursor: 1H NMR (400 MHz, CDCl3) δ 9.94 (s, 1H), 8.75 (t, J = 5.4 Hz, 1H), 8.68 (s, 1H), 7.82 (d, J = 8.1 Hz, 2H), 7.60 (d, J = 8.8 Hz, 2H), 7.49 (d, J = 8.8 Hz, 2H), 7.40 (d, J = 7.9 Hz, 2H), 4.96 (s, 1H), 4.37 (d, J = 4.8 Hz, 2H), 4.22 – 4.06 (m, 2H), 3.48 (t, J = 6.0 Hz, 2H), 2.71 (t, J = 12.3 Hz, 2H), 1.49 – 1.42 (m, 3H), 1.49 – 1.42 (m, 18H), 1.25 – 1.20 (m, 2H).
To a solution of the above amides (1.5 mmol) in DCM (5 mL) was added dropwise HCl (1.2 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 5 h. The volatiles were removed in vacuo to afford an oil, which was triturated in diether ether and solidified to give the final product hydrochloric salt.
For the synthesis of 70 precursor: Compound 57 precursor (X = I, 65 mg, 0.088 mmol), furan-3-yl boronic acid (17 mg, 0.15 mmol), tetrakis(triphenylphosphine)palladium (10 mg, 0.009 mmol), and sodium carbonate (43 mg, 0.4 mmol) in p-dioxane/H2O (4/1 mL) were placed in a sealed tube. The mixture was degassed and heated to 80 °C for 12 h. The reaction was then cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 30 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 1.5:1 to 1:1) to afford the product 70 precursor (244 mg, 63%).
3-(4-(Aminomethyl)phenyl)-N-(4-chlorophenyl)-6-((piperidin-4-ylmethyl)amino)pyrazine-2-carboxamide hydrochloride (56). 1H NMR (400 MHz, DMSO-d6) δ 10.55 (s, 1H), 9.23 – 9.21 (m, 2H), 8.98 (s, 1H), 8.92 (d, J = 10.0 Hz, 2H), 8.74 (s, 1H), 8.65 (br, 3H), 8.25 (d, J = 8.4 Hz, 2H), 7.84 (d, J = 8.8 Hz, 2H), 7.60 (d, J = 8.0 Hz, 2H), 7.43 (d, J = 8.8 Hz, 2H), 4.04 (d, J = 5.6 Hz, 2H), 3.45 (s, 2H), 3.21 (d, J = 11.2 Hz, 2H), 2.79 (dd, J = 23.2, 11.2 Hz, 2H), 1.91 (s, 1H), 1.81 (d, J = 12.8 Hz, 2H), 1.43 (dd, J = 25.2, 13.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.9, 153.4, 144.2, 136.9, 136.8, 135.7, 134.0, 129.4, 128.5, 128.1, 125.7, 124.5, 123.1, 44.8, 42.8, 41.9, 33.4, 26.3; MS (ESI) [M+H]+ 451.2.
3-(4-(Aminomethyl)phenyl)-N-(4-iodophenyl)-6-((piperidin-4-ylmethyl)amino)pyrazine-2-carboxamide hydrochloride (57). 1H NMR (400 MHz, DMSO-d6) δ 10.51 (s, 1H), 9.03 – 8.87 (m, 2H), 8.69 (ddd, J = 31.6, 19.2, 8.4 Hz, 2H), 8.49 (br, 3H), 8.26 (d, J = 8.0 Hz, 2H), 7.73 (d, J = 8.8 Hz, 2H), 7.63 (dd, J = 29.2, 8.4 Hz, 4H), 4.06 (d, J = 5.2 Hz, 2H), 3.55 (s, 2H), 3.24 (d, J = 11.2 Hz, 2H), 2.81 (dd, J = 23.2, 11.2 Hz, 2H), 1.91 (s, 1H), 1.82 (d, J = 13.2 Hz, 2H), 1.42 (dd, J = 25.2, 13.2 Hz, 2H); 13C NMR (400 MHz, DMSO-d6) δ 165.3, 153.8, 144.6, 138.0, 137.7, 137.3, 136.1, 134.3, 129.8, 126.1, 124.9, 123.9, 88.8, 45.2, 43.2, 42.3, 33.8, 26.7; MS (ESI) [M+H]+ 543.2.
3-(4-(Aminomethyl)phenyl)-N-(4-bromophenyl)-6-((piperidin-4-ylmethyl)amino)pyrazine-2-carboxamide hydrochloride (58). 1H NMR (400 MHz, DMSO-d6) δ 10.54 (s, 1H), 8.99 (s, 2H), 8.79 – 8.60 (m, 2H), 8.50 (s, 3H), 8.26 (d, J = 8.4 Hz, 2H), 7.79 (d, J = 8.8 Hz, 2H), 7.63 – 7.48 (m, 4H), 4.05 (dd, J = 11.2, 5.2 Hz, 2H), 3.45 (t, J = 6.0 Hz, 2H), 3.23 (d, J = 11.6 Hz, 2H), 2.86 – 2.72 (m, 2H), 1.91 (s, 1H), 1.81 (d, J = 13.2 Hz, 2H), 1.41 (dd, J = 23.6, 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.9, 153.4, 144.2, 137.2, 136.9, 135.7, 133.9, 131.4, 129.3, 125.7, 124.5, 123.4, 116.2, 61.9, 44.8, 42.8, 41.9, 33.3, 31.5, 26.3; MS (ESI) [M+H]+ 495.1.
3-(4-(Aminomethyl)phenyl)-N-(4-(furan-3-yl)phenyl)-6-((piperidin-4-ylmethyl)amino)pyrazine-2-carboxamide hydrochloride (70) was prepared by cross coupling with 49 precursor followed by deprotection. 1H NMR (400 MHz, DMSO-d6) δ 10.51 (s, 1H), 9.01 (s, 1H), 8.90 (d, J = 8.0 Hz, 1H), 8.83 (t, J = 6.0 Hz, 1H), 8.66 – 8.56 (m, 1H), 8.46 (s, 3H), 8.29 (d, J = 8.0 Hz, 2H), 8.19 (s, 1H), 7.84 (d, J = 8.4 Hz, 2H), 7.75 (d, J = 1.6 Hz, 1H), 7.63 (dd, J = 18.4, 8.4 Hz, 4H), 6.98 (s, 1H), 4.09 (q, J = 6.0 Hz, 2H), 3.44 (s, 2H), 3.26 (d, J = 12.0 Hz, 2H), 2.83 (dd, J = 24.4, 12.4 Hz, 2H), 1.95 (s, 1H), 1.85 (d, J = 12.8 Hz, 2H), 1.50 – 1.37 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.7, 153.5, 144.3, 144.1, 139.1, 136.8, 136.5, 135.8, 133.9, 129.3, 128.1, 125.7, 125.4, 124.7, 121.7, 108.6, 44.8, 42.9, 41.9, 33.4, 26.3; MS (ESI) [M+H]+ 483.2.
General Synthetic Procedure for compound 20
To a solution of 4,5-dibromothiophene-2-carboxaldehyde 127 (540 mg, 2 mmol) in MeOH (4 mL), NaBH4 (79 mg, 2.1 mmol) was added slowly 0 °C. The reaction mixture was stirred at room temperature for 1 h and quenched with water (5 mL). The product was extracted with diethyl ether (3 × 20 mL) and the combined organic layers were washed with water and brine, dried over Na2SO4. Upon removal of the solvent carefully, the residual oil was dried and used in the next step without purification. It was dissolved in DMF (10 mL) and the solution was cooled to 0 °C. Cyanuric chloride (369 mg, 2 mmol) was added slowly at 0 °C and stirred at room temperature for 10 h. The reaction was quenched with saturated NaHCO3 (10 mL). The product was extracted with diethyl ether (3 × 20 mL) and the combined organic layers were washed with water and brine, dried over Na2SO4. Removal of the solvents in vacuo afforded compound 128 as a colorless oil, which is used without purification.
To a solution of 4-(aminomethyl)-1-BOC-piperidine (214 mg, 1 mmol) and potassium carbonate (166 mg, 1.2 mmol) in DMF (5 mL), then a solution of 128 (145 mg, 0.5 mmol) in DMF (3 mL) was added slowly at 0 °C and the mixture was stirred at room temperature for 10 h. Upon quenching with saturated NaHCO3 (10 mL), the product was extracted with diethyl ether (3 × 50 mL) and the combined organic layers were washed with water and brine, dried over Na2SO4. A column chromatography (silica gel, hexanes: ethyl acetate from 40:1 to 2:1) for the residue oil gave compound 129 as a pale-yellow oil (69% yield for the three steps).
A mixture of 129 (186 mg, 0.4 mmol), 4-[(tert-Butoxycarbonylamino)methyl]phenylboronic acid pinacol ester (300 mg, 0.9 mmol), tetrakis(tri-phenylphosphine)palladium (46 mg, 0.04 mmol) and sodium carbonate (212 mg, 2 mmol) in 1,4-dioxane/H2O (5/1 mL) were heated to 100 °C for 12 h. The reaction was then quenched with brine (10 mL). The product was extracted with diethyl ether (3 × 20 mL) and the combined organic layers were washed with water and brine, dried over Na2SO4. Upon removal of the solvent, the product was purified by column chromatography (silica gel, n-hexanes: ethyl acetate from 5:1 to 1:2) to give 130 as a white solid (260 mg, 90%).
To a solution of 130 (0.1 mmol) in dichloromethane (2 mL) at 0 °C, HCl (0.2 mL, 4 N in 1,4-dioxane) was added slowly and then stirred at room temperature for 12 h. Upon removal of the solvent carefully, the residual oil was treated with anhydrous diethyl ether and vacuum dried to give 20 (white powder) as a hydrochloric salt (92% yield).
((5-(((Piperidin-4-ylmethyl)amino)methyl)thiophene-2,3-diyl)bis(4,1-phenylene))dimethanamine Hydrochloride (20–1226). 1H NMR (400 MHz, D2O) δ 7.43 (s, 1H), 7.40 (br, 8H), 4.57 (s, 2H), 4.19 (s, 4H), 3.50 (d, J = 12.8 Hz, 2H), 3.15 (d, J = 6.8 Hz, 2H), 3.05 (t, J = 12.0 Hz, 2H), 2.18 (s, 1H), 2.08 (d, J = 14.0 Hz, 2H), 1.59–1.50 (m, 2H); 13C NMR (100 MHz, D2O) 140.6, 137.9, 136.1, 133.9, 133.8, 132.6, 131.8, 130.5, 129.9, 129.6, 129.1, 129.0, 50.8, 45.4, 43.1, 42.64, 42.57, 30.7, 25.8; MS (ESI) [M+H]+ 421.6.
Synthesis of compound 21.
To a solution of 2-amino-6-chloropyrazine 108 (2.0 g, 15.5 mmol) in acetonitrile (20 mL) was added N-bromosuccinimide (6.9 g, 39 mmol) at 0 °C. The resulting mixture was allowed to warm to room temperature, and stirred for 18 h, and to this was added water. The mixture was extracted with ethyl acetate (3 × 50 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude residue, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 5:1 to 2:1) to give 121 as a yellow solid (3.93 g, 89%). 1H NMR (400 MHz, CDCl3) δ 5.23 (s, 2H, NH).
To a solution of 121 (990 mg, 3.44 mmol) in 1,4-dioxane (10 mL) was added N-Boc-4-piperidinemethanol (832 mg, 3.85 mmol) and sodium hydroxide (320 mg, 8.0 mmol). The resulting mixture was heated at 75 for 4 h °C. After cooled to room temperature, it was extracted with ethyl acetate (3 × 30 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude residue, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 2:1 to 1:1) to give 122 as a yellow solid (885 mg, 61%). 1H NMR (400 MHz, CDCl3) δ 4.87 (s, 1H), 4.84 (s, 1H), 4.22 (d, J = 6.4 Hz, 2H), 4.12 (s, 2H), 2.74 (t, J = 12.4 Hz, 2H), 1.98 (s, 1H), 1.76 (d, J = 13.0 Hz, 2H), 1.46 (s, 9H), 1.27 (d, J = 12.2 Hz, 2H).
Compound 122 (90 mg, 0.214 mmol), 4-[(tert-Boc-methyl]phenylboronic acid pinacol ester (166 mg, 0.5 mmol), Bis[di-tert-butyl(4-dimethylaminophenyl)phosphine] dichloropalladium(II) (15 mg, 0.021 mmol), and potassium phosphate (212 mg, 1 mmol) in p-doxane-H2O (6/1.5 mL) were placed in a sealed tube. The mixture was degassed and heated to 110 °C for 16 h. The reaction was then cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 30 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 2:1 to 1:2) to afford 123 (66 mg, 43%). 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 8.0 Hz, 2H), 7.73 (d, J = 7.8 Hz, 2H), 7.39 (d, J = 7.8 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 5.12 (s, 1H), 5.01 (s, 1H), 4.35 (s, 4H), 4.23 (s, 2H), 4.16 – 4.10 (m, 2H), 2.81 – 2.70 (m, 2H), 2.02 (s, 1H), 1.82 (d, J = 12.6 Hz, 2H), 1.49 (s, 27H), 1.28 (d, J = 6.8 Hz, 2H).
To a solution of compound 123 (66 mg, 0.092 mmol) in DCM (3 mL) was added dropwise HCl (0.3 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 5 h. The volatiles were removed in vacuo to afford an oil, which was triturated in diether ether and solidified to give 21 (44 mg, 91%) as a hydrochloric salt.
((5-Amino-6-(piperidin-4-ylmethoxy)pyrazine-2,3-diyl)bis(4,1-phenylene))dimethanamine hydrochloride (21). 1H NMR (400 MHz, D2O) δ 7.85 (d, J = 6.4 Hz, 2H), 7.75 (d, J = 7.2 Hz, 2H), 7.61 (dd, J = 25.6, 7.2 Hz, 4H), 4.36 (d, J = 5.2 Hz, 2H), 4.29 (s, 2H), 4.27 (s, 2H), 3.48 (d, J = 12.8 Hz, 2H), 3.03 (t, J = 12.0 Hz, 2H), 2.23 (s, 1H), 2.05 (d, J = 13.6 Hz, 2H), 1.57 (dd, J = 24.0, 11.8 Hz, 2H); 13C NMR (100 MHz, D2O) δ 156.6, 151.7, 134.3, 133.9, 133.7, 133.0, 129.5, 129.42, 129.36, 128.8, 128.4, 127.6, 70.1, 43.5, 42.7, 32.7, 25.1; MS (ESI) [M+H]+ 419.2.
General Synthetic Procedure for compounds 22–24.
To a solution of 121 (600 mg, 2.09 mmol) in isopropyl alcohol (10 mL) was added chloroacetaldehyde (50% wt in water, 1.7 mL, 14 mmol). The resulting mixture was refluxed at 105 °C for 18 h then cooled to room temperature and concentrated. The residue was dissolved in dichloromethane (30 mL) and washed sequentially with Sat. NaHCO3 (10 mL), H2O (10 mL), and Brine (10 mL). The organic layer was dried over Na2SO4, filtered, and removed in vacuo to give a residue, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 5:1 to 2:1) to afford 124 (260 mg, 40%). 1H NMR (400 MHz, CDCl3) δ 7.94 (s, 1H), 7.90 (s, 1H).
A solution of 124 (122 mg, 0.39 mmol), alcohol nucleophiles (0.38 mmol), and sodium hydroxide (20 mg, 0.5 mmol) in 1,4-dioxane (3 mL), or A solution of amine nucleophiles (0.38 mmol), and N, N-diisopropylethylamine (87 μL, 0.5 mmol) in acetonitrile (3 mL) was heated at 75 for 4 h °C. After cooled to room temperature, it was concentrated, and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude residue, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 2:1 to 1:2) to give 125 as a solid (885 mg, 58%). For intermediate of compound 24: 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 1.1 Hz, 1H), 7.56 (s, 1H), 4.96 (s, 1H), 4.29 (s, 1H), 4.22 (s, 2H), 4.15 – 4.07 (m, 1H), 3.82 (s, 1H), 1.97 – 1.66 (m, 4H), 1.43 (s, 9H).
Compound 125 (0.16 mmol), 4-[(tert-Butoxycarbonylamino)methyl]phenylboronic acid pinacol ester (133 mg, 0.4 mmol), Bis[di-tert-butyl(4-dimethylaminophenyl)phosphine] dichloropalladium(II) (15 mg, 0.021 mmol), and potassium phosphate (127 mg, 0.6 mmol) in p-dioxane-H2O (5/1 mL) were placed in a sealed tube. The mixture was degassed and heated to 110 °C for 16 h. The reaction was then cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 30 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 2:1 to 1:2) to afford 126. For precursor of compound 24: 1H NMR (400 MHz, CDCl3)) δ 7.48 (s, 1H), 7.32 – 7.25 (m, 7H), 7.08 (d, J = 7.6 Hz, 2H), 5.60 (s, 1H), 4.97 (s, 1H), 4.79 (s, 1H), 4.42 – 4.35 (m, 4H), 4.24 (d, J = 3.6 Hz, 2H), 4.02 – 3.87 (m, 3H), 1.89 (br, 2H), 1.71 (br, 2H), 1.47 – 1.40 (m, 27H).
To a solution of compound 126 (0.08 mmol) in DCM (3 mL) was added dropwise HCl (0.1 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 5 h. The volatiles were removed in vacuo to afford an oil, which was triturated in diether ether and solidified to give final products as hydrochloric salts.
((8-(Piperidin-4-ylmethoxy)imidazo[1,2-a]pyrazine-5,6-diyl)bis(4,1-phenylene)) dimethanamine hydrochloride (22) was prepared following the general procedure. 1H NMR (400 MHz, D2O) δ 7.97 (s, 1H), 7.78 (s, 1H), 7.58 – 7.48 (m, 4H), 7.46 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 4.63 (d, J = 6.0 Hz, 2H), 4.23 (s, 2H), 4.13 (s, 2H), 3.50 (d, J = 12.4 Hz, 2H), 3.08 (t, J = 12.0 Hz, 2H), 2.37 (s, 1H), 2.16 (d, J = 13.6 Hz, 2H), 1.74 – 1.58 (m, 2H); 13C NMR (100 MHz, D2O) δ 149.6, 138.7, 136.6, 134.9, 133.0, 131.4, 130.5, 130.0, 129.7, 128.5, 128.0, 124.9, 124.4, 116.2, 71.1, 43.5, 42.5, 32.7, 24.9; MS (ESI) [M+H]+ 443.2
(S)-((8-(3-Aminopyrrolidin-1-yl)imidazo[1,2-a]pyrazine-5,6-diyl)bis(4,1-phenylene))dimethanamine hydrochloride (23) was prepared following the general procedure. 1H NMR (400 MHz, DMSO-d6) δ 8.61 (br, 3H), 8.52 (br, 3H), 8.36 (br, 3H), 7.65 – 7.59 (m, 3H), 7.46 (d, J = 8.0 Hz, 2H), 7.41 – 7.33 (m, 4H), 7.21 (s, 1H), 5.16 (t, J = 13.2 Hz, 2H), 4.09 (s, 2H), 3.95 (s, 2H), 3.29 (s, 1H), 2.12 (s, 1H), 1.88 (s, 1H), 1.77 – 1.62 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 145.6, 137.7, 135.1, 134.6, 133.4, 132.9, 132.5, 132.3, 131.6, 130.4, 130.2, 128.8, 119.7, 115.2, 110.0, 52.8, 49.5, 47.3, 42.1, 42.0, 29.1; MS (ESI) [M+H]+ 414.2.
(R)-((8-(3-Aminopiperidin-1-yl)imidazo[1,2-a]pyrazine-5,6-diyl)bis(4,1-phenylene))dimethanamine hydrochloride (24) was prepared following the general procedure. 1H NMR (400 MHz, D2O) δ 8.61 (br, 3H), 8.51 (br, 3H), 8.36 (br, 3H), 7.62 – 7.59 (m, 3H), 7.44 (d, J = 7.2 Hz, 2H), 7.35 (dd, J = 13.6, 4.4 Hz, 2H), 7.19 (s, 1H), 5.14 (t, J = 9.8 Hz, 2H), 4.07 (d, J = 5.6 Hz, 2H), 3.93 (d, J = 5.6 Hz, 2H), 3.42 (s, 2H), 3.27 (s, 2H), 2.09 (s, 1H), 1.87 (d, J = 9.2 Hz, 2H), 1.71 – 1.56 (m, 2H); MS (ESI) [M+H]+ 428.2.
Synthesis of compound 34.
Compound 118 (120 mg, 0.23 mmol), potassium vinyltrifluoroborate (39 mg, 0.29 mmol), tetrakis(triphenylphosphine)palladium (14 mg, 0.012 mmol), and cesium carbonate (147 mg, 0.45 mmol) in THF-H2O (5/1 mL) were placed in a sealed tube. The mixture was degassed and heated to 110 °C for 12 h. The reaction was then cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 4:1 to 2:1) to afford 119 (108 mg, 90%). 1H NMR (400 MHz, CDCl3) δ 8.14 (s, 1H), 7.47 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 7.9 Hz, 2H), 6.83 (dd, J = 16.9, 10.6 Hz, 1H), 6.43 (dd, J = 16.9, 2.1 Hz, 1H), 5.46 (dd, J = 10.6, 2.1 Hz, 1H), 5.06 (s, 1H), 4.34 (d, J = 5.0 Hz, 2H), 4.26 (d, J = 6.4 Hz, 2H), 4.18 – 4.06 (m, 2H), 2.73 (t, J = 10.8 Hz, 2H), 2.00 – 1.94 (m, 1H), 1.81 (d, J = 12.9 Hz, 2H), 1.45 (s, 18H), 1.35 – 1.25 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 158.1, 156.0, 154.9, 144.4, 143.6, 139.2, 136.8, 133.9, 132.9, 130.0, 127.4, 120.8, 79.5, 70.3, 44.4, 35.9, 28.5, 28.5, 22.0.
To a solution of compound 119 (80 mg, 0.15 mmol) in DBU (2 mL) was added morpholine (0.1 mL). The resulting mixture was heated at 120 °C for 24 h before it was cooled to room temperature. The mixture was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 1:1 to 1:2) to give 120 (52 mg, 57%).1H NMR (400 MHz, CDCl3) δ 8.08 (s, 1H), 7.42 (d, J = 7.8 Hz, 2H), 7.32 (d, J = 7.8 Hz, 2H), 5.00 (s, 1H), 4.33 (d, J = 5.3 Hz, 2H), 4.18 (d, J = 6.4 Hz, 2H), 4.14 – 4.04 (m, 2H), 3.67 – 3.57 (m, 4H), 2.96 – 2.89 (m, 2H), 2.78 – 2.67 (m, 4H), 2.36 (s, 4H), 1.96 (s, 1H), 1.79 (d, J = 12.6 Hz, 2H), 1.44 (s, 18H), 1.31 – 1.24 (m, 2H).
To a solution of compound 120 (52 mg, 0.085 mmol) in DCM (3 mL) was added dropwise HCl (0.3 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 5 h. The volatiles were removed in vacuo to afford an oil, which was triturated in diether ether and solidified to give 34 (41 mg, 92%) as a hydrochloric salt.
(4-(3-(2-Morpholinoethyl)-5-(piperidin-4-ylmethoxy)pyrazin-2-yl)phenyl)methanamine hydrochloride (34). 1H NMR (400 MHz, D2O) δ 8.22 (s, 1H), 7.69 – 7.53 (m, 4H), 4.36 (d, J = 5.6 Hz, 2H), 4.28 (s, 2H), 4.08 (d, J = 13.2 Hz, 2H), 3.81 – 3.72 (m, 2H), 3.60 (t, J = 7.2 Hz, 2H), 3.56 – 3.41 (m, 4H), 3.30 (t, J = 7.2 Hz, 2H), 3.17 (t, J = 12.4 Hz, 2H), 3.08 (t, J = 12.8 Hz, 2H), 2.26 (s, 1H), 2.13 (d, J = 14.0 Hz, 2H), 1.65 (dd, J = 25.6, 13.2 Hz, 2H); 13C NMR (100 MHz, D2O) δ 159.0, 146.9, 144.2, 136.9, 133.4, 131.6, 129.7, 129.2, 70.2, 63.6, 55.2, 51.7, 43.6, 42.7, 32.8, 27.6, 25.0; MS (ESI) [M+H]+ 412.3.
Activity and inhibition assays for Flavivirus proteases.
Expression and purification of Flavivirus proteases were performed as described previously15. Activity and inhibition assay for ZVpro was performed using the enzyme (1 nM) and benzoyl-norleucine-lysine-lysine-arginine 7-amino-4-methylcoumarine (Bz-Nle-Lys-Lys-Arg-AMC, 20 μM) as the substrate in a HEPES buffer (20mM, pH 7.3) containing 0.05% Triton X-100. To determine IC50, triplicate samples of a compound with concentrations ranging from 1 nM to 10 μM were incubated with the enzyme for 10 min before adding the substrate to initiate the reaction in 96-well plate (100 μL final volume). The fluorescence signal (Ex: 360 nm, Em: 460 nm) of each well was monitored every 30s, using a Tecan microplate reader. The initial velocity data were imported into Prism (version 5.0), and IC50 values from 3 independent experiments with standard deviation were obtained by using a standard dose-response curve fitting. Enzyme inhibition assays for DV2pro and WVpro were performed similarly.
Cellular antiviral activity testing.
Anti-ZIKV activity was evaluated in human U87 glioma following our previous methods15. 2×104 U87 cells/well were seeded in 96-well plates and cultured in DMEM media with 2% FBS to form a monolayer of cells. 0.01 MOI (multiplicity of infection) of ZIKV was added. After incubation for 1h, the supernatant was removed and cells were washed with PBS. Fresh medium (150 μL/well) containing various concentrations of a compound in triplicate were added. Upon incubation at 37 °C for 48h, aliquots of the supernatant from each well were used to determine ZIKV titers. Half-log serial dilution of the viral supernatant (50 μL) was added to a monolayer of Vero cells in quadruplicate in 96-well plates and cultured for 7 days. CPE/cell lysis was determined with microscope followed by MTT assay. TCID50 was calculated based on the highest dilution in which ≥50% (i.e., ≥2 out of the 4 quadruplicate wells) of Vero cells were infected with ZIKV. Compared to controls, the ability for a compound to reduce TCID50 can be determined. The results were from at least 2 independent experiments.
Supplementary Material
Acknowledgement
This work was supported by a grant (W81XWH-18-1-0368) from USAMRAA of the US Department of Defense and grants (RP180177) from Cancer Prevention and Research Institute of Texas to Y.S., NIH grants R01AI099483 and R01AI098715 to R.R.-H.
ABBREVATIONS:
- Ac-CoA
acetyl coenzyme A
- AR
androgen receptor
- SAR
structure-activity relationships
- DMF
N,N-Dimethylformamide
- LSD1
lysine specific demethylase 1
- ZVpro
Zika virus protease
- DV2pro
Dengue (serotype 2) virus protease
- WVpro
West Nile virus protease
- CPE
cytopathic effects
- MOI
multiplicity of infection
- NIS
N-iodosuccinimide
Footnotes
Supporting Information Available. Molecular Formula Strings for all compounds are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Dick GW; Kitchen SF; Haddow AJ Zika Virus. I. Isolations and Serological Specificity. Trans R Soc Trop Med Hyg 1952, 46, 509–520. [DOI] [PubMed] [Google Scholar]
- 2.Cauchemez S; Besnard M; Bompard P; Dub T; Guillemette-Artur P; Eyrolle-Guignot D; Salje H; Van Kerkhove MD; Abadie V; Garel C; Fontanet A; Mallet HP Association between Zika Virus and Microcephaly in French Polynesia, 2013–15: a Retrospective Study. Lancet 2016, 387, 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jouannic JM; Friszer S; Leparc-Goffart I; Garel C; Eyrolle-Guignot D Zika Virus Infection in French Polynesia. Lancet 2016, 387, 1051–1052. [DOI] [PubMed] [Google Scholar]
- 4.Oehler E; Watrin L; Larre P; Leparc-Goffart I; Lastere S; Valour F; Baudouin L; Mallet H; Musso D; Ghawche F Zika Virus Infection Complicated by Guillain-Barre Syndrome--Case Report, French Polynesia, December 2013. Euro Surveill 2014, 19. [DOI] [PubMed] [Google Scholar]
- 5.Cao-Lormeau VM; Blake A; Mons S; Lastere S; Roche C; Vanhomwegen J; Dub T; Baudouin L; Teissier A; Larre P; Vial AL; Decam C; Choumet V; Halstead SK; Willison HJ; Musset L; Manuguerra JC; Despres P; Fournier E; Mallet HP; Musso D; Fontanet A; Neil J; Ghawche F Guillain-Barre Syndrome Outbreak Associated with Zika Virus Infection in French Polynesia: a Case-Control Study. Lancet 2016, 387, 1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Zika Virus Outbreaks in the Americas. Wkly. Epidemiol. Rec 2015, 90, 609–610. [PubMed] [Google Scholar]
- 7.Schuler-Faccini L; Ribeiro EM; Feitosa IM; Horovitz DD; Cavalcanti DP; Pessoa A; Doriqui MJ; Neri JI; Neto JM; Wanderley HY; Cernach M; El-Husny AS; Pone MV; Serao CL; Sanseverino MT Possible Association Between Zika Virus Infection and Microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016, 65, 59–62. [DOI] [PubMed] [Google Scholar]
- 8.Victora CG; Schuler-Faccini L; Matijasevich A; Ribeiro E; Pessoa A; Barros FC Microcephaly in Brazil: How to Interpret Reported Numbers? Lancet 2016, 387, 621–624. [DOI] [PubMed] [Google Scholar]
- 9.Yun SI; Lee YM Zika Virus: An Emerging Flavivirus. J Microbiol 2017, 55, 204–219. [DOI] [PubMed] [Google Scholar]
- 10.Medin CL; Rothman AL Zika Virus: The Agent and Its Biology, With Relevance to Pathology. Arch Pathol Lab Med 2017, 141, 33–42. [DOI] [PubMed] [Google Scholar]
- 11.Yun SI; Song BH; Frank JC; Julander JG; Polejaeva IA; Davies CJ; White KL; Lee YM Complete Genome Sequences of Three Historically Important, Spatiotemporally Distinct, and Genetically Divergent Strains of Zika Virus: MR-766, P6–740, and PRVABC-59. Genome Announc 2016, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitsche C; Holloway S; Schirmeister T; Klein CD Biochemistry and Medicinal Chemistry of the Dengue Virus Protease. Chem Rev 2014, 114, 11348–11381. [DOI] [PubMed] [Google Scholar]
- 13.Li Z; Brecher M; Deng YQ; Zhang J; Sakamuru S; Liu B; Huang R; Koetzner CA; Allen CA; Jones SA; Chen H; Zhang NN; Tian M; Gao F; Lin Q; Banavali N; Zhou J; Boles N; Xia M; Kramer LD; Qin CF; Li H Existing Drugs as Broad-Spectrum and Potent Inhibitors for Zika Virus by Targeting NS2B-NS3 interaction. Cell Res 2017, 27, 1046–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brecher M; Li Z; Liu B; Zhang J; Koetzner CA; Alifarag A; Jones SA; Lin Q; Kramer LD; Li H A Conformational Switch High-throughput Screening Assay and Allosteric Inhibition of the Flavivirus NS2B-NS3 Protease. PLoS Pathog 2017, 13, e1006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y; Huo T; Lin YL; Nie S; Wu F; Hua Y; Wu J; Kneubehl AR; Vogt MB; Rico-Hesse R; Song Y Discovery, X-ray Crystallography and Antiviral Activity of Allosteric Inhibitors of Flavivirus NS2B-NS3 Protease. J Am Chem Soc 2019, 141, 6832–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z; Li Y; Loh YR; Phoo WW; Hung AW; Kang C; Luo D Crystal Structure of Unlinked NS2B-NS3 Protease from Zika Virus. Science 2016, 354, 1597–1600. [DOI] [PubMed] [Google Scholar]
- 17.Kang C; Keller TH; Luo D Zika Virus Protease: An Antiviral Drug Target. Trends Microbiol 2017, 25, 797–808. [DOI] [PubMed] [Google Scholar]
- 18.Lei J; Hansen G; Nitsche C; Klein CD; Zhang L; Hilgenfeld R Crystal Structure of Zika Virus NS2B-NS3 Protease in Complex with a Boronate Inhibitor. Science 2016, 353, 503–505. [DOI] [PubMed] [Google Scholar]
- 19.Nitsche C; Zhang L; Weigel LF; Schilz J; Graf D; Bartenschlager R; Hilgenfeld R; Klein CD Peptide-Boronic Acid Inhibitors of Flaviviral Proteases: Medicinal Chemistry and Structural Biology. J Med Chem 2017, 60, 511–516. [DOI] [PubMed] [Google Scholar]
- 20.Phoo WW; Li Y; Zhang Z; Lee MY; Loh YR; Tan YB; Ng EY; Lescar J; Kang C; Luo D Structure of the NS2B-NS3 Protease from Zika Virus after Self-cleavage. Nat Commun 2016, 7, 13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun NJ; Quek JP; Huber S; Kouretova J; Rogge D; Lang-Henkel H; Cheong EZK; Chew BLA; Heine A; Luo D; Steinmetzer T Structure-Based Macrocyclization of Substrate Analogue NS2B-NS3 Protease Inhibitors of Zika, West Nile and Dengue viruses. ChemMedChem 2020, 15, 1439–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y; Zhang Z; Phoo WW; Loh YR; Li R; Yang HY; Jansson AE; Hill J; Keller TH; Nacro K; Luo D; Kang C Structural Insights into the Inhibition of Zika Virus NS2B-NS3 Protease by a Small-Molecule Inhibitor. Structure 2018, 26, 555–564 e553. [DOI] [PubMed] [Google Scholar]
- 23.Wu F; Zhou C; Yao Y; Wei L; Feng Z; Deng L; Song Y 3-(Piperidin-4-ylmethoxy)pyridine Containing Compounds Are Potent Inhibitors of Lysine Specific Demethylase 1. J Med Chem 2016, 59, 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tricarico PM; Caracciolo I; Crovella S; D’Agaro P Zika Virus Induces Inflammasome Activation in the Glial Cell Line U87-MG. Biochem Biophys Res Commun 2017, 492, 597–602. [DOI] [PubMed] [Google Scholar]
- 25.Chan JF; Yip CC; Tsang JO; Tee KM; Cai JP; Chik KK; Zhu Z; Chan CC; Choi GK; Sridhar S; Zhang AJ; Lu G; Chiu K; Lo AC; Tsao SW; Kok KH; Jin DY; Chan KH; Yuen KY Differential Cell Line Susceptibility to the Emerging Zika Virus: Implications for Disease Pathogenesis, Non-vector-borne Human Transmission and Animal Reservoirs. Emerg Microbes Infect 2016, 5, e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahon A; Arya RP; Kneubehl AR; Vogt MB; Dailey Garnes NJ; Rico-Hesse R Characterization of a Zika Virus Isolate from Colombia. PLoS Negl Trop Dis 2016, 10, e0005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


