Abstract
Patients with worsening heart failure with reduced ejection fraction (HFrEF) spend a large proportion of time in the hospital and other health care facilities. The benefits of guideline-directed medical therapy (GDMT) in the outpatient setting have been shown in large randomized controlled trials. However, the decision to initiate, continue, switch, or withdraw HFrEF medications in the inpatient setting is often based on multiple factors and subject to significant variability across providers. Based on available data, in well-selected, treatment-naïve patients who are hemodynamically stable and clinically euvolemic after stabilization during hospitalization for HF, elements of GDMT can be safely initiated. Inpatient continuation of GDMT for HFrEF appears safe and well-tolerated in most hemodynamically stable patients. Hospitalization is also a potential time for switching from an angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker to sacubitril/valsartan therapy in eligible patients, and is the subject of ongoing study. Therapy withdrawal or need for dose reduction is rarely required, but if needed identifies a particularly at-risk group of patients with progressive HF. If recurrent intolerance to neurohormonal blockers is observed, these patients should be evaluated for advanced HF therapies. There is an enduring need for using the teachable moment of HFrEF hospitalization for optimal initiation, continuation, and switching of GDMT to improve post-discharge patient outcomes and the quality of chronic HFrEF care.
Keywords: heart failure, medical decision making, medication adherence, medication discontinuation
For patients with heart failure with reduced ejection fraction (HFrEF), a series of landmark randomized clinical trials conducted in stable outpatients identified multiple therapies to improve morbidity and mortality (1). Nonetheless, substantial gaps in provision of guideline-directed medical therapy (GDMT) remain. Given the persistently high rates of morbidity and mortality seen in the general HFrEF population, the hospital setting provides a key opportunity to readdress medical therapies.
The decision whether to initiate, continue, switch, withdraw, or withhold initiation of HF medications in the hospital-based setting is at the discretion of the treating physician and may be driven by factors such as patient symptoms at presentation, blood pressure, heart rate, and renal function. Despite the central importance of these clinical decisions in the routine care of hospitalized HF patients, data surrounding in-hospital management of chronic HFrEF medications are modest compared to that for the medical management in the stable outpatient setting. In this review, we discuss the data regarding safety and logistics surrounding new initiation, continuation, switching, and withdrawal of HFrEF medical therapy during HF hospitalization. We focus on beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin II receptor blockers (ARBs), sacubitril/valsartan, and mineralocorticoid receptor antagonists (MRAs) because these medications form the foundation of most GDMT regimens and are applicable to the large majority of patients with HFrEF (Table 1).
TABLE 1.
Select Studies of In-Hospital Use of Beta-Blocker, ACEI/ARB/ARNI, and MRA Therapy Among Patients Hospitalized for HF With Reduced Ejection Fraction
| Therapy First Author (Ref. #) Trial | Study Design | N | Key Results |
|---|---|---|---|
| Beta-blocker Initiation | |||
| Gattis et al. (3) (IMPACT-HF) | Randomized (open-label) clinical trial: Carvedilol initiation pre-hospital discharge vs. initiation >2 weeks postdischarge at physician discretion | 363 | At 60 days post-randomization, 91% randomized to pre-discharge carvedilol initiation were treated with a beta-blocker, compared with 73% randomized to post-discharge initiation (p < 0.001). No difference in rates of serious adverse events or index hospitalization length of stay between groups. |
| Hernandez et al. (5) (OPTIMIZE-HF registry linked to Medicare claims) | Observational: Among patients eligible for beta-blockers, in-hospital beta-blocker initiation vs. no initiation | 3,001 (subset with reduced ejection fraction) | At 1 year post-discharge, beta-blocker initiation associated with lower adjusted risk for all-cause mortality (HR: 0.77; 95% CI: 0.68–0.87), all-cause rehospitalization (HR: 0.89; 95% CI: 0.80–0.99), and mortality or rehospitalization (HR: 0.87; 95% CI: 0.79–0.96). |
| Continuation or withdrawal | |||
| Fonarow et al. (7) (OPTIMIZE-HF Registry) |
Observational: Among patients eligible for beta-blockers, in-hospital beta-blocker continuation vs. no beta-blocker; beta-blocker withdrawal vs. continuation | 2,373 | At 60–90 days post-discharge, beta-blocker continuation associated with a lower propensity adjusted risk for mortality (HR: 0.60; 95% CI: 0.37–0.99; p - 0.044) and mortality or rehospitalization (odds ratio: 0.69; 95% CI: 0.52–0.92; p = 0.012), compared with no beta-blocker. 92% of patients newly initiated on beta-blocker therapy remained on therapy. Beta-blocker withdrawal associated with higher adjusted mortality risk compared with continuation (HR: 2.3; 95% CI: 1.2–4.6; p = 0.013). 57% of patients with in-hospital beta-blocker discontinuation were restarted on therapy within 60–90 days. |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker Initiation | |||
| Sanam et al. (11) (Medicare beneficiaries) | Observational: Among patients without prior ACEI/ARB use and without known contraindications, discharge ACEI/ARB prescription vs. no prescription | 954 (propensity matched cohort) | At 30 days post-discharge, ACEI/ARB prescription associated with significantly lower propensity adjusted all-cause readmission (HR: 0.74; 95% CI: 0.56–0.97; p = 0.030) and 30-day all-cause mortality (HR: 0.56; 95% CI: 0.33–0.98; p = 0.041). All associations remained significant at 1 year post-discharge. |
| Continuation or withdrawal | |||
| Gilstrap et al. (10) (GWTG-HF linked to Medicare claims) | Observational: Among eligible patients, ACEI/ARB withdrawal vs. continuation | 16,052 | At 1-year post-discharge, in-hospital ACEI/ARB withdrawal was associated higher adjusted mortality risk compared with continuation (HR: 1.35; 95% CI: 1.13–1.61; p < 0.001). |
| Angiotensin receptor-neprilysin inhibitor Initiation | |||
| Velazquez et al. (32) (PIONEER-HF) | Randomized clinical trial: Eligible patients randomized to in-hospital initiation of sacubitril/valsartan vs. enalapril with 12-week follow-up. | 882 | Trial ongoing and results not yet available. |
| Mineralocorticoid receptor antagonist Initiation | |||
| Ferreira et al. (15) | Nonrandomized clinical trial, single-blind (patients): Patients not receiving background MRA therapy and meeting other study criteria assigned short in-hospital course of spironolactone 50–100 mg/d plus standard care vs. standard care alone. | 100 | Spironolactone not associated with excess in-hospital worsening
renal function or hyperkalemia. Greater proportions of patients receiving spironolactone were free of congestion at day 3: less edema, rales, jugular venous pressure and orthopnea (all p < 0.05). |
| Butler J et al. (17) (ATHENA-HF) | Randomized clinical trial: High-dose spironolactone 100 mg/d for 4 days plus standard care vs. standard care alone. Overall, 11% of patients on spironolactone at baseline. | 360 | Spironolactone not associated with excess in-hospital worsening renal function or hyperkalemia. Spironolactone therapy did decrease NT-proBNP level or improve clinical markers of congestion compared with standard care. |
| Lam et al. (20) (Medicare beneficiaries) | Observational: Among patients without MRA use at admission and without known contraindications, discharge MRA prescription vs. no prescription | 648 (propensity matched cohort) | At 30 days post-discharge, MRA therapy not associated with propensity adjusted risk of all-cause readmission (HR: 0.92; 95% CI: 0.64-1.32; p = 0.650), all-cause mortality (HR: 0.84; 95% CI: 0.38–1.88; p = 0.678), or HF readmission (HR: 0.74; 95% CI: 0.41-1.31; p = 0.301). Associations remained consistent at 1-year follow-up. |
| Prescription at discharge | |||
| Hamaguchi et al. (19) (JCARE-CARD registry) | Observational: Use of spironolactone at discharge vs. no use at discharge | 946 | Over mean post-discharge follow-up of 2.2 years, discharge use of spironolactone associated with lower adjusted risk of all-cause (HR: 0.62; 95% CI: 0.41–0.93; p = 0.020) and cardiovascular death (HR: 0.52; 95% CI: 0.32–0.87; p = 0.013). Spironolactone not associated with adjusted risk of all-cause hospitalization (HR: 0.79; 95% CI: 0.59–1.05; p = 0.101). |
| Hernandez et al. (21) (GWTG-HF linked to Medicare claims) | Observational: Among patients eligible for therapy, discharge MRA prescription vs. no prescription | 5,887 | At 3 years post-discharge, MRA therapy not associated with
adjusted risk of mortality (HR: 1.04; 95% CI: 0.96–1.14; p = 0.32) or cardiovascular rehospitalization (HR: 1.00; 95% CI: 0.91–1.09; p = 0.94). At 3 years, MRA therapy associated with lower adjusted risk of HF rehospitalization (HR: 0.87; 95% CI: 0.77-0.98; p = 0.02). MRA therapy associated with higher adjusted risk of hospitalization for hyperkalemia at 30 days (HR: 2.54; 95% CI: 1.51–4.29; p < 0.001) and 1 year (HR: 1.50; 95% CI: 1.23–1.84; p < 0.001). |
| Curtis et al. (22) (GWTG-HF linked to Medicare claims) | Observational: Among patients eligible for therapy, discharge MRA prescription vs. no prescription | 2,086 | Within 90 days post-discharge, 79% of patients with a discharge prescription filled a prescription for therapy, compared with 13% without a discharge prescription (p < 0.001). 8% of patients with a discharge prescription discontinued therapy within 1 year. |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; ATHENA-HF = Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure trial; CI = confidence interval; GWTG-HF = Get With the Guidelines-Heart Failure; HF = heart failure; HR = hazard ratio; IMPACT-HF = Initiation Management Pre-discharge: Process for Assessment of Carvedilol Therapy in Heart Failure trial; MRA = mineralocorticoid receptor antagonist; NT-proBNP = N-terminal pro-B-type natriuretic peptide; OPTIMIZE-HF = Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure registry; PIONEER-HF = Comparison of Sacubitril/valsartan Versus Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode trial.
INITIATION OF HF MEDICATIONS
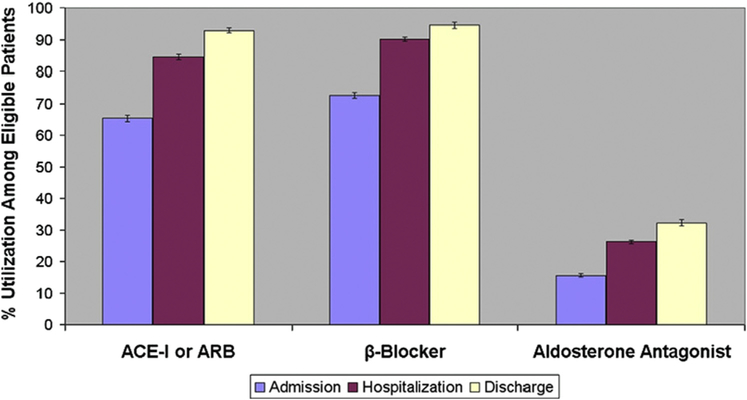
In treatment-naïve patients included in the GWTG-HF (Get With the Guidelines-Heart Failure) registry, 90% were initiated on beta-blockers during hospitalization or at discharge, 87% were initiated on ACEIs/ARBs, and 25% were initiated on MRAs (Figure 1) (2).
FIGURE 1. Medication Utilization at Admission, During Hospitalization, and at Discharge.
Analysis from the Get With the Guidelines Heart Failure Registry: Proportion of patients on guideline-directed medical therapies at admission (purple bars), during hospitalization (dark red bars), and at discharge (light yellow bars) with associated upper and lower boundaries of 95% confidence intervals in patients hospitalized for heart failure. ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker. Adapted with permission from Krantz et al. (2).
BETA-BLOCKER.
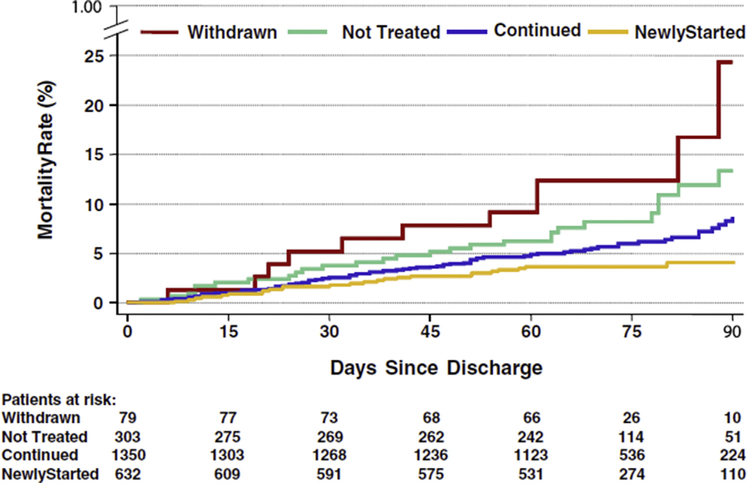
There are no randomized data for in-hospital initiation of metoprolol succinate or bisoprolol. In contrast, the IMPACT-HF (Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure) trial found that carvedilol initiation pre-discharge was feasible without untoward side effects or prolonged length of stay, and appeared to improve post-discharge beta-blocker use (3). Another small randomized clinical trial found the strategy of pre-discharge initiation of low-dose carvedilol coupled with biweekly nursing care significantly reduced the need for hospitalization and improved functional status at 6 months (4). Likewise, several observational studies from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) registry support the relative benefits of in-hospital beta-blocker initiation on post-discharge clinical outcomes (5,6). For example, an analysis from OPTIMIZE-HF suggested lower mortality in patients started on beta-blockers compared with those who were not at 60 to 90 days after discharge (7). Initiation of beta-blockers in the hospital was generally well-tolerated and translated to high rates of post-discharge use, as 92% of patients continued therapy at 60 to 90 days post-discharge (Figure 2) (7).
FIGURE 2. Post-Discharge Survival by Beta-Blocker Treatment Groups.
Kaplan-Meier survival curve of patients newly started, continued, withdrawn, and not treated with beta-blocker therapy. Reproduced with permission from Fonarow et al. (7).
Acknowledging potential differences in patient characteristics and clinical stability, data from landmark randomized controlled trials of beta-blocker therapy among stable outpatients with chronic HFrEF may help contextualize in-hospital initiation of therapy. MERIT-HF (Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure) randomized patients to either initiation/titration of metoprolol controlled release/extended release or placebo and showed that beta-blocker therapy could be safely tolerated in most patients with low risk of deterioration. In addition, the trial found risk of deterioration greatest between 4 and 8 weeks of initiation and by week 8 the mortality and hospitalization rates trended in favor of beta-blockade (8). Likewise, the COPERNICUS (Carvedilol Prospective Randomized Cumulative Survival) trial randomized 2,289 clinically euvolemic patients with severe HF (i.e., symptoms at rest or with minimal exertion) to carvedilol or placebo and found clinical benefits on mortality and hospitalization with beta-blocker therapy over the first 8 weeks of the trial to be consistent with the long-term trial results (9).
ACEI/ARB.
To our knowledge, there are no robust randomized clinical outcome data regarding in-hospital ACEI/ARB initiation. Observational data from the GWTG-HF registry found that among 16,052 patients, those who were newly started on ACEI/ARB before discharge had lower mortality and readmission rates up to 1 year (10). A matched-cohort analysis of Medicare beneficiaries hospitalized for HF between 1998 and 2001 found patients initiated on an ACEI/ARB had lower 30-day all-cause readmissions (18% vs. 24%) and all-cause mortality (7% vs. 14%), both of which remained significant 1 year after discharge (11). In addition, there was a significantly lower risk of 30-day HF readmission (7% vs. 15%) (11). Another relevant analysis from the Medicare population specifically assessed those who also developed acute kidney injury during hospitalization. Overall, 54% of this subgroup received a discharge prescription for an ACEI/ARB and the benefits of ACEI/ARB therapy remained consistent, with significantly lower 30-day all-cause readmission, 30-day HF readmission, and 30-day all-cause mortality, which persisted to 12-months post-discharge (12).
MRA.
Randomized controlled trial data regarding in-hospital initiation of MRA therapy among HFrEF patients are limited. The EPHESUS (Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival) study randomized patients after an acute myocardial infraction (MI) with left ventricular dysfunction (ejection fraction < 40%) and found initiation of eplerenone 3–14 days after MI reduced the risk of cardiovascular events (13). Although this trial focused on use after acute MI, early MRA use in acute HF exacerbations may play a role in reducing sudden cardiac death and arrhythmia (14). In a prospective single-blinded trial of patients hospitalized for HF (15), spironolactone was associated with greater congestion relief and reductions in natriuretic peptides at day 3 (15). Another randomized controlled trial conducted among 116 patients hospitalized for HF found that those who were initiated on spironolactone versus placebo had a reduced rate of arrhythmias (16). Most recently, the ATHENA-HF (Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure) trial compared initiation of high-dose spironolactone 100 mg daily plus usual care versus usual care alone among patients hospitalized for HF and found no significant difference between 30-day all-cause mortality/HF hospitalization rates. Moreover, there was no significant difference in the natriuretic peptide trajectory at 96 h between treatment arms (17).
Regarding associations with clinical outcomes, observational data have shown mixed results regarding comparative effectiveness of in-hospital MRA initiation (18–21). For example, a study of 946 patients found the 46% of patients discharged on spironolactone to have lower rates of all-cause and cardiovascular death (19). In contrast, an analysis of Medicare patients found that when compared with those not receiving therapy, prescription of MRA therapy at discharge was not associated with a difference in mortality and cardiovascular readmissions, but was associated with a lower rate of HF readmissions (21). Speculating as to why the reliable benefits of MRA therapy in randomized trials differ from the inconsistent results in observational studies, residual confounding and the potential for MRA therapy to be selectively prescribed to higher-risk patients may be likely explanations.
Despite limited randomized data regarding clinical effects of in-hospital MRA initiation, observational data support the hospitalization as a tool to increase downstream long-term MRA use. For instance, GWTG-HF data found discharge prescription for MRA therapy to be strongly correlated with continued post-discharge adherence with 79% of patients with a discharge prescription filling a prescription within 90 days (22). Among patients who filled a prescription within 90 days, most remained on therapy at 1 year with only an 8% discontinuation rate. In contrast, only 13% of eligible patients without a discharge prescription initiated therapy as outpatients (22). Although these relationships may be seen with other HFrEF therapies, the particularly low rates of MRA use among eligible patients in routine practice highlight in-hospital initiation as a particularly valuable strategy for consideration (23).
CONTINUATION VERSUS WITHDRAWAL OF HF MEDICATIONS
The GWTG-HF registry showed that continuation rates of medications during hospitalization for HF were 92% for beta-blockers, 89% for ACEI/ARB, and 72% for MRA (2).
BETA-BLOCKER.
Continuation of beta-blockers during an acute HF exacerbation in the inpatient setting has been consistently associated with improved clinical outcomes (6,24). Data from OPTIMIZE-HF showed that among 2,373 patients eligible for beta-blockers at discharge, 1,350 were previously on therapy and continued and 79 patients were withdrawn from previous therapy (7). Those who continued on therapy had a significantly lower risk of post-discharge death and death/rehospitalization compared with patients on no beta-blocker. In contrast, withdrawal of beta-blocker was associated with substantially higher risk-adjusted mortality compared with those who continued on beta-blockers. OPTIMIZE-HF also found a statistically significant benefit of beta-blocker use (i.e., initiation or continuation) at discharge, showing that death from any cause at 60 to 90 days was significantly lower among those discharged with a beta-blocker as compared with those discharged without such therapy (6% vs. 11%).
ACEI/ARB.
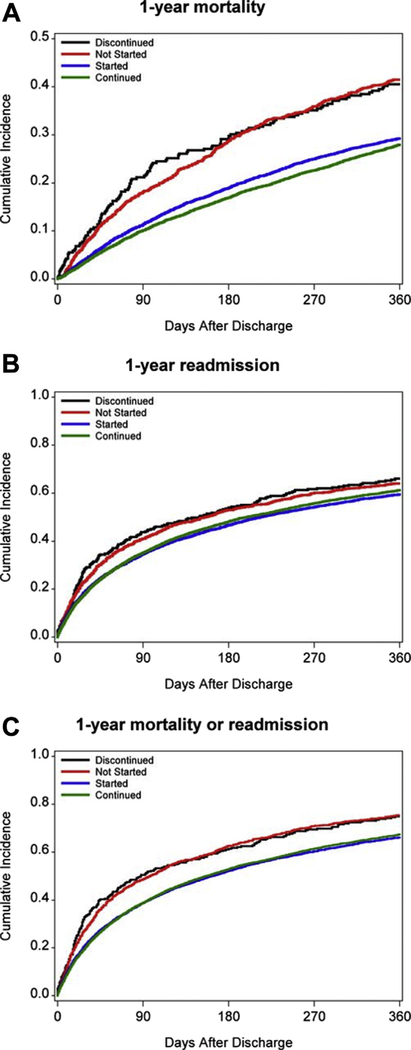
The OPTIMIZE-HF registry found the in-hospital discontinuation rate for ACEI/ARB to be 28%, as compared to 7% for beta-blockers (25). In an analysis from the GWTG-HF registry, continuation of ACEI/ARB among patients hospitalized for HFrEF was associated with significantly lower 30-day, 90-day, and 1-year mortality and 30-day readmission, as compared to those who were discontinued (Figure 3) (10). In African American patients with HFrEF, ACEI/ARB dose reduction or discontinuation occurred more frequently than for beta-blockers (17% vs. 7%). Patients who were discontinued versus continued on ACEI/ARB had an associated greater median length of stay at 5.5 days versus 3.0 days and a shorter time to HF readmission; however, this trend did not meet statistical significance (26).
FIGURE 3. Post-Discharge Outcomes by ACEI Treatment Groups.
ACEI Initiation, Continuation, Discontinuation, and Never Initiated rates for 1-year mortality (A), 1-year readmission (B), and 1-year mortality/readmission (C). Adapted with permission from Gilstrap et al. (10). ACE-I = angiotensin-converting enzyme inhibitor.
MRA.
Continuation of spironolactone as an inpatient has not been well elucidated in clinical trials or observational studies. Perhaps the most informative work in this area comes from an analysis of the COACH (Co-ordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure) biomarker study, which found patients who were initiated or continued on spironolactone had a lower 30-day mortality (18). However, regardless of whether MRA therapy is newly initiated or continued during the hospitalization, data strongly support the association between MRA use at discharge and higher rates of post-discharge use (22).
SWITCHING
BETA-BLOCKER.
Specific beta-blockers (carvedilol, metoprolol succinate, and bisoprolol) have been well-studied and proven to improve clinical outcomes in patients with chronic HFrEF. Currently, there are no published studies in hospitalized patients for HFrEF regarding transition from non–evidence-based beta-blockers, such as atenolol, to evidence-based beta-blockers. However, clinical experience suggests that transitioning from non–evidence-based beta-blockers to evidence-based beta-blockers is generally well tolerated in clinically stable hospitalized patients.
ACEI/ARB TO ANGIOTENSIN RECEPTOR-NEPRILYSIN INHIBITOR.
Although the PARADIGM-HF (Prospective Comparison of ARNI With an ACEInhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial showed that sacubitril/valsartan was superior to enalapril in reducing cardiovascular events in stable patients with HFrEF, limited data are available regarding the use and safety among patients hospitalized for HF (27). Subsequent analyses of PARADIGM-HF have suggested that patients recently hospitalized for HFrEF are just as likely to benefit from sacubitril/valsartan as more stable HFrEF patients with remote or no prior hospitalizations (28). Consistent safety and efficacy of angiotensin receptor-neprilysin inhibitor (ARNI) even in patients with recent HF hospitalization suggests clinical benefit with in-hospital switching among stable, clinically euvolemic patients. Similarly, the benefits of ARNI therapy appear consistent across a spectrum of risk in the PARADIGM-HF trials; higher-risk patients identified in this study may be analogous to stable patients hospitalized for HF (29). Furthermore, there appears to be important clinical benefits of ARNI early after initiation and after HF hospitalization. In patients who experienced hospitalization for HF in PARADIGM-HF, sacubitril/valsartan appears to reduce 30-day all-cause readmissions compared with enalapril (28).
Unfortunately, the uptake and use of sacubitril/valsartan has been sluggish; an analysis of the GWTG-HF registry found that only 2.3% of hospitalized patients were discharged with ARNI therapy (30). As evidenced by numerous trials for beta-blockers, ACEI/ARBs, and MRAs, postponing the initiation of optimal medical therapy in the hospital-based setting often leads to failure to initiate medication in the outpatient setting. Failure of ARNI prescription at discharge and therefore potential lack of optimal use in the outpatient setting may be directly attributed to 28,484 deaths per year in the United States (31). As with all other GDMT for HFrEF, data from chronic HFrEF trials have been readily extrapolated as best practice for treatment and optimization of medical therapy during hospitalization for HF. Nonetheless, to provide more specific data, the PIONEER-HF (Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode) study is a multicenter randomized double-blind controlled trial currently underway to evaluate the effect of in-hospital initiation of sacubitril/valsartan on changes in N-terminal pro-B-type natriuretic peptide (NT-proBNP) and safety and tolerability (32). Likewise, the LIFE (Entresto in Advanced Heart Failure) (NCT02816736) trial aims to evaluate the safety and efficacy of sacubitril/valsartan in New York Heart Association functional class IV HFrEF patients and will be inclusive of patients hospitalized for HF.
SAFETY
A significant number of patients hospitalized for HFrEF have worsening hemodynamics and/or worsening renal function. These characteristics and others may lead to clinical reluctance of initiating or continuing hemodynamically active therapies (33,34).
BETA-BLOCKER.
The IMPACT-HF trial found low rates of worsening HF (0.5%), hypotension (1.6%), and bradycardia (1.6%) requiring discontinuation in patients started on beta-blockers during hospitalization. Rates of discontinuation did not appear different between treatment and control groups. Further, length of stay in the hospital was the same between both groups (~5 days) (3). The ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization) trial found that beta-blockers were commonly discontinued for respiratory rate >24 breaths/min, heart rate >100 beats/min, lower EF, diabetes mellitus, and systolic blood pressure <100 mm Hg during hospitalization (24). OPTIMIZE-HF found beta-blocker therapy was not prescribed at discharge due to symptomatic bradycardia (1%), reactive airway disease (3%), symptomatic hypotension (3%), second- or third-degree heart block (0.4%), allergy (0.2%), and other reasons (4%) (35). The COPERNICUS trial showed that patients randomized to carvedilol therapy were more likely to report dizziness, hypotension, edema, and bradycardia and required medication withdrawal, but this was limited to a small number of cases (9). However, safety data on risk of worsening HF was reassuring, with rates of worsening HF early after therapy initiation numerically higher among patients receiving placebo (9).
ACEI/ARB.
In the inpatient setting, reasons for discontinuation of ACEI/ARBs in the GWTG-HF registry were primarily renal dysfunction and hyperkalemia (10). Another analysis among African American patients found that ACEI/ARB medications were reduced or discontinued because of acute kidney injury (57%), hypotension (23%), and hyperkalemia (10%); serum creatinine and systolic blood pressure at admission were significant independent predictors of in-hospital dose reduction or discontinuation (26). However, this study also found that despite renal dysfunction cited as the most frequent reason for de-escalation of ACEI/ARB therapy, 24% of patients had no significant in-hospital rise in creatinine level (i.e., did not meet criteria for an acute kidney injury) and medication changes were made over concern for worsening renal function rather than its actual occurrence (26).
MRA.
Although the precise reasons for poor use of MRA therapy by time of hospital discharge among eligible patients are unclear, likely barriers include perceived risks of hyperkalemia and worsening renal function. An analysis from the EPHESUS trial found that estimated glomerular filtration rate was decreased at 24-month follow-up in patients taking eplerenone as opposed to placebo (36). Whereas MRA-associated adverse effects of hyperkalemia and worsening renal function are well recognized, more contemporary evidence supports a relatively sound safety profile for in-hospital use of MRA therapy. Most notably, despite use of high doses of 100 mg daily, the ATHENA-HF trial found spironolactone to be well-tolerated among patients hospitalized with HF with no significant change in potassium level or renal function, as compared with usual care (17).
OPTIMIZATION OF GDMT DURING HOSPITALIZATION FOR HF
Decisions to initiate, continue, switch, or withdraw HFrEF medications in the inpatient setting are complex, often based on multiple factors, and subject to significant variability across providers. However, although randomized clinical trial data and safety data regarding in-hospital use of GDMT are modest, clinical treatment guidelines, hospital performance measures, and ongoing quality improvement initiatives all strongly emphasize prescription of these medications by time of hospital discharge (1,2,37).
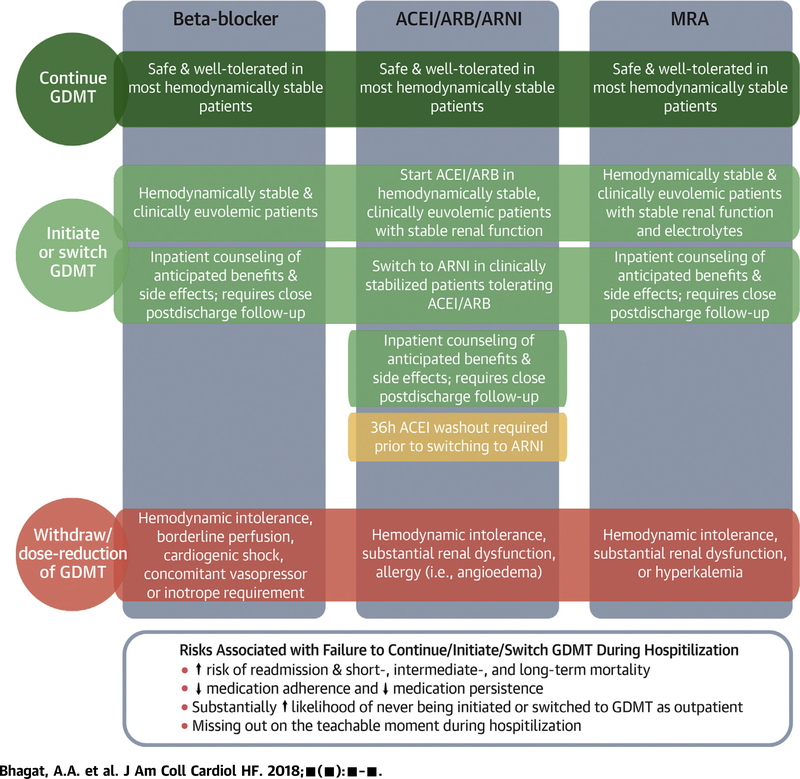
We provide a conceptual framework regarding inpatient decision-making to optimize GDMT in HFrEF patients (Central Illustration). After stabilization during hospitalization for HFrEF, in well-selected treatment-naïve patients who are hemodynamically stable (e.g., not requiring inotropes or vasopressors) and clinically euvolemic (e.g., being transitioned to oral diuretic therapy), guideline-directed medications can be safely initiated (38). Moreover, in the presence of close monitoring, multiple guideline medications can generally be safely initiated or up-titrated during a single hospitalization. For example, in the EPHESUS trial, low rates of prior MI (27%) and HF (15%) in the context of high rates of ACEI/ARB (87%) and beta-blocker (75%) at study baseline (3 to 14 days after MI) suggest many patients were initiated on 1 or both therapies during the index hospitalization (before randomization), with additional benefits to those further randomized to eplerenone (39). Nonetheless, some important considerations should be noted during inpatient management of these therapies. Patients expected to be unable or unwilling to comply with appropriate post-discharge clinical and laboratory monitoring are not candidates for inpatient initiation or escalation of MRA therapy. In addition, caution should be used when initiating ACEI/ARB therapy in hypovolemic patients (such as patients who are “over-diuresed”) because renin-angiotensin-aldosterone system activation is high and ACEI/ARB may cause excessive blood pressure lowering. Likewise, careful attention is required when initiating or up-titrating beta-blocker therapy among patients with relative bradycardia or compensatory tachycardia, as presence of the former may increase risk for symptomatic bradycardia (at rest or with exertion) while the latter may be compensatory in the setting of severely reduced stroke volume.
CENTRAL ILLUSTRATION. Key Elements Related to Initiation, Switching, Continuation, and Withdrawal of GDMT During Hospitalization for HF.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blockers; ARNI = angiotensin receptor neprilysin inhibitor; GDMT = guideline-directed medical therapies; HF = heart failure; MRA = mineralocorticoid receptor antagonist.
Limited data from observational studies and small randomized clinical trials suggest that inpatient continuation of foundational therapies (beta-blockers, ACEI/ARBs, and MRAs) is safe and well-tolerated in most hemodynamically stable patients. It is important to recognize that observational studies assessing changes in medical therapy are subject to significant patient selection and confounding. Nonetheless, although unable to definitively prove causality, such data clearly identify tolerance of GDMT to be (at minimum) a very strong marker of a more favorable post-discharge clinical course. Patients should be counseled and educated with respect to the importance, treatment benefits, and anticipated side effect profiles of these therapies during hospitalization. Early post-discharge follow-up with close monitoring of hemodynamics, renal function, electrolytes, and symptoms in the weeks after initiation of these therapies is required, especially in treatment-naïve patients. Therapy withdrawal or need for dose reduction identifies a particularly at-risk group of patients with progressive HF. If recurrent intolerance to neurohormonal antagonists is observed, these patients should be evaluated for advanced HF therapies.
Pre-discharge switching to sacubitril/valsartan may be a mechanism to improve overall use of ARNI. Most patients hospitalized for worsening chronic HFrEF are admitted on either ACEI or ARBs. In appropriately selected patients who were tolerating ACEI/ARB, pre-discharge transition to sacubitril/valsartan should be considered. ACEI must be stopped at least 36 h before the first dose of sacubitril/valsartan and ARNI should not be considered in patients who have had prior angioedema or hypersensitivity to ACEI/ARB. Depending on the degree of clinical stability, dose of prior ACEI/ARB, and systemic blood pressures, sacubitril/valsartan can be initiated at 24 mg/26 mg twice daily or 49 mg/51 mg twice daily. Close outpatient follow-up is required for serial monitoring of symptoms, side effects, electrolytes, renal function, and hemodynamics. The dose of sacubitril/valsartan can be doubled every 2 to 4 weeks depending on clinical tolerance, but more gradual up-titration strategies in the early post-discharge period may optimize dosing (40).
In the stable outpatient setting, the robust clinical benefits of GDMT are well established. Hospitalization for HF identifies patients at high risk for progressive HF and thus represents an important opportunity to revisit and optimize GDMT. Significant time spent in the inpatient and post-acute care settings allows frequent provider engagement and patient education regarding these therapies. As such, the inpatient setting affords an important opportunity to initiate, switch, or continue GDMT that may improve long-term post-discharge prognosis in this high-risk cohort. There is an enduring need for data to guide these inpatient decisions regarding new initiation of novel and established therapies.
Acknowledgments
Dr. Greene has received the NHLBI T32 postdoctoral training grant (T32HL069749–14), a Heart Failure Society of America/Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis; research support from Novartis and Amgen. Dr. Vaduganathan has received the NHLBI T32 postdoctoral training grant (T32HL007604). Dr. Fonarow has received personal fees from Novartis, Amgen, Janssen, Medtronic, and St. Jude Medical. Dr. Butler is a principal investigator of the EMPEROR program (Boehringer Ingelheim); has received research support from the NIH and the European Union; and has received personal fees from Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Novartis, Relypsa, ZS Pharma, Medtronic, Merck, CVRx, G3 Pharmaceuticals, Lutipold, Stealth Peptide, SC Pharma, and Vifor. All other authors have reported that they have no relationships relevant to the contents of this paper to report.
ABBREVIATIONS AND ACRONYMS
- ACEI
angiotensin-converting enzyme inhibitor
- ARB
angiotensin II receptor blocker
- ARNI
angiotensin receptor-neprilysin inhibitor
- GDMT
guided-directed medical therapy
- HF
heart failure
- HFrEF
heart failure with reduce ejection fraction
- MI
myocardial infarction
- MRA
mineralocorticoid receptor antagonist
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2013;62:1495–539. [DOI] [PubMed] [Google Scholar]
- 2.Krantz MJ, Ambardekar AV, Kaltenbach L, et al. Patterns and predictors of evidence-based medication continuation among hospitalized heart failure patients (from Get with the Guidelines-Heart Failure). Am J Cardiol 2011;107:1818–23. [DOI] [PubMed] [Google Scholar]
- 3.Gattis WA, O’Connor CM, Gallup DS, et al. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol 2004; 43:1534–41. [DOI] [PubMed] [Google Scholar]
- 4.Krantz MJ, Havranek EP, Haynes DK, Smith I, Bucher-Bartelson B, Long CS. Inpatient initiation of β-blockade plus nurse management in vulnerable heart failure patients: a randomized study. J Card Fail 2008;14:303–9. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol 2009;53:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonarow GC, Abraham WT, Albert NM, et al. Carvedilol use at discharge in patients hospitalized for heart failure is associated with improved survival: an analysis from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 2007;153:82.e1–11. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Abraham WT, Albert NM, et al. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE-HF program. J Am Coll Cardiol 2008;52:190–9. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb SS, Fisher ML, Kjekshus J, et al. Tolerability of beta-blocker initiation and titration in the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Circulation 2002;105:1182–8. [DOI] [PubMed] [Google Scholar]
- 9.Krum H, Roecker EB, Mohacsi P, et al. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA 2003;289:712–8. [DOI] [PubMed] [Google Scholar]
- 10.Gilstrap LG, Fonarow GC, Desai AS, et al. Initiation, continuation, or withdrawal of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J Am Heart Assoc 2017;6:pii: e004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanam K, Bhatia V, Bajaj NS, et al. Renin-angiotensin system inhibition and lower 30-day all-cause readmission in Medicare beneficiaries with heart failure. Am J Med 2016;129:1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui M, Sanders PW, Arora G, et al. Use of ACE inhibitors or angiotensin receptor blockers is associated with a significantly lower risk of 30-day all-cause and heart failure readmissions and all-cause mortality in older Medicare beneficiaries hospitalized for heart failure developing acute kidney injury (abstr.). Circulation 2015;132:A19094. [Google Scholar]
- 13.Pitt B, White H, Nicolau J, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol 2005;46:425–31. [DOI] [PubMed] [Google Scholar]
- 14.Brown K, Chee J, Kyung S, Vettichira B, Papadimitriou L, Butler J. Mineralocorticoid receptor antagonism in acute heart failure. Curr Treat Options Cardiovasc Med 2015;17:402. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P, Carvalho H. Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med 2014;25: 67–72. [DOI] [PubMed] [Google Scholar]
- 16.Gao X, Peng L, Adhikari CM, Lin J, Zuo Z. Spironolactone reduced arrhythmia and maintained magnesium homeostasis in patients with congestive heart failure. J Card Fail 2007;13:170–7. [DOI] [PubMed] [Google Scholar]
- 17.Butler J, Anstrom KJ, Felker GM, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol 2017;2:950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maisel A, Xue Y, van Veldhuisen DJ, et al. Effect of spironolactone on 30-day death and heart failure rehospitalization (from the COACH Study). Am J Cardiol 2014;114:737–42. [DOI] [PubMed] [Google Scholar]
- 19.Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, et al. Spironolactone use at discharge was associated with improved survival in hospitalized patients with systolic heart failure. Am Heart J 2010;160:1156–62. [DOI] [PubMed] [Google Scholar]
- 20.Lam PH, Dooley DJ, Inampudi C, et al. Lack of evidence of lower 30-day all-cause readmission in Medicare beneficiaries with heart failure and reduced ejection fraction discharged on spironolactone. Int J Cardiol 2017;227:462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez AF, Mi X, Hammill BG, et al. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA 2012;308:2097–107. [DOI] [PubMed] [Google Scholar]
- 22.Curtis LH, Mi X, Qualls LG, et al. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J 2013;165:979–86.e1. [DOI] [PubMed] [Google Scholar]
- 23.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation 2010;122: 585–96. [DOI] [PubMed] [Google Scholar]
- 24.Butler J, Young JB, Abraham WT, et al. Beta-blocker use and outcomes among hospitalized heart failure patients. J Am Coll Cardiol 2006;47:2462–9. [DOI] [PubMed] [Google Scholar]
- 25.Fonarow GC, Abraham WT, Albert NM, et al. Prospective evaluation of beta-blocker use at the time of hospital discharge as a heart failure performance measure: results from OPTIMIZE-HF. J Card Fail 2007;13:722–31. [DOI] [PubMed] [Google Scholar]
- 26.Kane JA, Kim JK, Haidry SA, Salciccioli L, Lazar J. Discontinuation/dose reduction of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers during acute decompensated heart failure in African-American patients with reduced left-ventricular ejection fraction. Cardiology 2017; 137:121–5. [DOI] [PubMed] [Google Scholar]
- 27.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 28.Desai AS, Claggett BL, Packer M, et al. Influence of sacubitril/valsartan (LCZ696) on 30-Day readmission after heart failure hospitalization. J Am Coll Cardiol 2016;68:241–8. [DOI] [PubMed] [Google Scholar]
- 29.Simpson J, Jhund PS, Silva Cardoso J, et al. Comparing LCZ696 with enalapril according to baseline risk using the MAGGIC and EMPHASIS-HF risk scores: an analysis of mortality and morbidity in PARADIGM-HF. J Am Coll Cardiol 2015;66: 2059–71. [DOI] [PubMed] [Google Scholar]
- 30.Luo N, Fonarow GC, Lippmann SJ, et al. Early adoption of sacubitril/valsartan for patients with heart failure with reduced ejection fraction: insights from Get With the Guidelines–Heart Failure (GWTG-HF). J Am Coll Cardiol HF 2017;5:305–9. [DOI] [PubMed] [Google Scholar]
- 31.Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiol 2016;1:714–7. [DOI] [PubMed] [Google Scholar]
- 32.Velazquez EJ, Morrow DA, DeVore AD, et al. Rationale and design of the comParIson Of sacubitril/valsartaN versus Enalapril on Effect on nt-pRo-bnp in patients stabilized from an acute Heart Failure episode (PIONEER-HF) trial. Am Heart J 2018;198:145–51. [DOI] [PubMed] [Google Scholar]
- 33.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009;53:557–73. [DOI] [PubMed] [Google Scholar]
- 34.Vaduganathan M, Butler J, Pitt B, Gheorghiade M. Contemporary drug development in heart failure: call for hemodynamically neutral therapies. Circ Heart Fail 2015;8:826–31. [DOI] [PubMed] [Google Scholar]
- 35.Fonarow GC, Abraham WT, Albert NM, et al. Dosing of beta-blocker therapy before, during, and after hospitalization for heart failure (from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure). Am J Cardiol 2008;102:1524–9. [DOI] [PubMed] [Google Scholar]
- 36.Rossignol P, Cleland JG, Bhandari S, et al. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients post myocardial infarction: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 2012;125:271–9. [DOI] [PubMed] [Google Scholar]
- 37.Yancy CW, Januzzi JL Jr., Allen LA, et al. 2017 ACC Expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;71: 201–30. [DOI] [PubMed] [Google Scholar]
- 38.Ambrosy AP, Butler J, Gheorghiade M. Clinical trials in acute heart failure: beginning of the end or end of the beginning? Eur J Heart Fail 2017;19:1358–60. [DOI] [PubMed] [Google Scholar]
- 39.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–21. [DOI] [PubMed] [Google Scholar]
- 40.Senni M, McMurray JJ, Wachter R, et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur Heart J Fail 2016;18:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]