Abstract
Background
Noninvasive methods to assess treatment response in eosinophilic esophagitis are needed.
Aims
Our aim was to determine whether a blood-based biomarker panel centered on immune parameters could identify histologic response to treatment in eosinophilic esophagitis patients.
Methods
A pilot study involving adult patients with active eosinophilic esophagitis recruited at two Ear, Nose, Throat clinics in Sweden was designed. The patients (n = 20) donated blood and esophageal biopsies and filled in three questionnaires before and after a 2-month course of topical corticosteroids. Blood samples were analyzed for absolute levels of granulocytes and T cells and the fractions of eosinophils expressing 10 different surface markers by flow cytometry. All data were analyzed by multivariate methods of pattern recognition.
Results
Multivariate modeling revealed that a combination of 13 immune parameters and 10 patient-reported outcome scores were required to create a model capable of separating responders (n = 15) from non-responders (n = 5). Questions regarding symptoms of esophageal dysfunction and capacity to eat certain foods from two of the questionnaires were discriminatory in the multivariate model, as were absolute counts of T cells, eosinophils, and eosinophil expression of activation markers and cell adhesion molecules.
Conclusions
A combination of blood-based immune parameters and directed questions may prove helpful to monitor response to treatment, perhaps reducing the need for repeat endoscopies in eosinophilic esophagitis patients in the future.
Keywords: Eosinophilic esophagitis, Biomarker, Eosinophils, Patient-reported outcome, T cells, CD16
Introduction
Eosinophilic esophagitis is a relatively new inflammatory disease of the esophagus that is considered to be a variant of food allergy [1]. This chronic Th2 inflammation [2, 3] is driven by dietary allergens and/or other antigens [1] and leads to fibrosis and poor esophageal function if left untreated for a long period of time [4–6]. Although the inflammation in the esophagus is dominated by eosinophils, the leukocyte that defines the disease, there are also elevated levels of T cells, basophils, mast cells and B cells [2, 3, 7, 8]. Elimination of food allergens from the diet, topical corticosteroids, and proton pump inhibitors are the three main therapeutic options at present [9].
The unresolved question is to know when and how often it is necessary to treat eosinophilic esophagitis patients to curb the inflammatory process in the esophagus. Today, response to treatment is generally defined histologically, as a reduction of peak eosinophil counts in the esophagus to < 15 eosinophils/high-power field (HPF), although 75–90% reduction of peak eosinophil counts in esophageal biopsies have also been used as response measures [10]. An additional important therapeutic goal is improved capacity to ingest foodstuffs and reduced burden of symptoms, which can be difficult to estimate since most patients have developed coping mechanisms to compensate for their difficulties in eating and swallowing, such as avoidance of certain foods, helping the swallowing process by drinking copious amounts of water and eating slowly [10].
Monitoring of eosinophil counts in the esophagus requires repeated invasive procedures involving endoscopy with collection of at least six biopsies at different levels of the esophageal mucosa. The overall aim of this study was to evaluate the possibility of assessing the response to treatment in adult patients by analysis of a panel of immune parameters in the blood focusing on T cells and eosinophils, including a subpopulation of eosinophils with T cell suppressive capacity. These “suppressive eosinophils” can be identified by analysis of surface molecules including CD16. In addition, the suppressive eosinophils express a higher level of CD4, CD40, CD44, CD54 (ICAM-1), CD66c, CD183 (CXCR3), CD194 (CCR4), CD199 (CCR9), CD274 (PD-L1), TSLPR, FPR1 and galectin-10 and a lower level of CD9, CD11a, CD45, CD49d, CD66b, CD71, CD294 (CRTH2) and Siglec-8 compared with conventional eosinophils [11]. Most striking is the higher expression of CD54 which is necessary for the formation of immune synapses with T cells [12] and galectin-10 which is necessary for the suppression of T cells for both eosinophils [11] and regulatory T cells [13]. The rationale for our choice to include CD16+ suppressive eosinophils in the panel of immune parameters was that we have previously found that eosinophils isolated from the blood of adult eosinophilic esophagitis patients had reduced T cell suppressive capacity compared with eosinophils from healthy donors [14]. However, we could not determine whether this apparent diminished suppressive capacity was because the patients had too few suppressive eosinophils or if it was their suppressive function that was impaired. One possibility raised by the previous study was that the levels of suppressive eosinophils in the blood might be relatively reduced in untreated patients and return back to normal after treatment, and hence a potential biomarker to monitor response to therapy. A second aim of the study was to evaluate how histologic response to therapy related to patient-reported outcomes as symptomatic improvement and enhanced quality of life are the main goals of treatment in eosinophilic esophagitis.
Methods
Study Design
Eosinophilic esophagitis patients were studied before and after completion of a 2-month course of topical corticosteroids regarding levels of eosinophils in the blood, their molecular patterns and the subpopulation of suppressive eosinophils, as well as the absolute levels of granulocytes, and of CD4+ and CD8+ T cells. The study patients provided self-assessment of response to treatment by filling in three written questionnaires before and after treatment: the Short Form Health Survey (SF-36) [15], the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Oesophageal Module 18 (EORTC QLQ-OES18) [16] and the Watson dysphagia scale (WDS) form [17]; these forms have been used previously for evaluation of eosinophilic esophagitis patients although they have not been validated for this disease [18, 19]. The immune parameters and questionnaire scores were analyzed by multivariate pattern recognition methods to determine whether any combinations of parameters could be used to assess histological response to treatment.
Study Patients
Thirty adult patients with active eosinophilic esophagitis were recruited at NÄL Medical Hospital, Trollhättan, Sweden and Skaraborg Hospital, Skövde, Sweden. Ten patients were excluded because they either did not complete treatment, declined repeated endoscopic examination, or because blood samples were not taken at the same time point as the biopsies. The diagnostic criteria for eosinophilic esophagitis in use at the time of patient recruitment were employed [9]. Inclusion requirements were ≥ 15 peak eosinophil counts/HPF (HPF = 0.229 mm2) in at least one of six biopsies collected from the proximal and distal parts of the esophagus, together with symptoms of esophageal dysfunction. Fourteen of the patients were newly diagnosed, and six were known eosinophilic esophagitis patients who had not been treated for at least three months. The patients completed a 2-month course of topical corticosteroids (200 µg mometasone furoate aerosol swallowed q.i.d.) and donated 10 mL of EDTA blood before and after treatment for flow cytometry analyses. A second endoscopic examination with collection of biopsies was performed after treatment. Table 1 summarizes the patient characteristics. The study was approved by the Regional Ethical Review Board of Gothenburg, Sweden (137-09, March 30, 2009, and T664-11, July 13, 2011). Written informed consent was acquired from all study participants. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Table 1.
Clinical characteristics of study patients
| Clinical data | Responders | Non-responders | P value | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Patients | 15 | 75 | 5 | 25 | |
| Age | 43 (20–79)a | 34 (18–66)a | NS | ||
| Male | 9 | 60 | 5 | 100 | NS |
| Allergy | |||||
| Inhalant allergy | 11 | 73 | 2 | 40 | NS |
| Hay fever | 7 | 47 | 1 | 20 | NS |
| Food allergy | 4 | 27 | 2 | 40 | NS |
| No allergy | 3 | 20 | 2 | 40 | NS |
| Food and inhalant allergy | 3 | 20 | 1 | 20 | NS |
| Eczema | 0 | 0 | 1 | 20 | NS |
| Eosinophilic esophagitis | |||||
| Previous bolus obstructionb | 8 | 53 | 4 | 80 | NS |
| Previous esophageal dilation | 2 | 13 | 0 | 0 | NS |
| Current symptoms | |||||
| Dysphagia | 15 | 100 | 5 | 100 | NS |
| Chest pain | 13 | 87 | 1 | 20 | 0.014 |
| Food impaction | 10 | 67 | 4 | 80 | NS |
| Cough | 8 | 53 | 1 | 20 | NS |
| Nausea/vomiting | 6 | 40 | 3 | 60 | NS |
| Current esophageal findings | |||||
| Linear furrows | 10 | 67 | 3 | 60 | NS |
| Plaques | 7 | 47 | 4 | 80 | NS |
| Trachealization | 11 | 73 | 4 | 80 | NS |
| Strictures | 8 | 53 | 1 | 20 | NS |
| Peak eosinophil counts/HPF before treatment | 30 (15–80)a | 25 (17–70)a | NS | ||
| Peak eosinophil counts/HPF after treatment | 0 (0–13)a | 30 (20–38)a | < 0.001 | ||
| Histologic response to treatment | |||||
| < 15 peak eosinophil counts/HPF | 15 | 75 | 0 | 0 | < 0.001 |
| > 50% reduction of peak eosinophil counts/HPF | 14 | 70 | 0 | 0 | < 0.001 |
| > 75% reduction of peak eosinophil counts/HPF | 13 | 65 | 0 | 0 | 0.0014 |
| Blood eosinophil countsc | NS | ||||
| before treatment | 0.36 (0.054–1.08)a,d | 0.46 (0.25–1.97)a,e | |||
| after treatment | 0.20 (0.077–0.47)a,d | 0.36 (0.29–0.60)a,e | 0.0037 | ||
| Watson dysphagia scale summary score | |||||
| before treatment | 18 (4–31)a | 17 (0–23)a | NS | ||
| after treatment | 12 (0–27)a | 21 (0–24)a | NS | ||
HPF high-power field = 0.229 mm2; NS Nonsignificant
aMedian (min–max)
bRequiring hospital care for removal of bolus
cEosinophil number × 109 cells/L blood, ref 0.04–0.4 × 109/L
dP = 0.015 when comparing responders before and after treatment. Wilcoxon matched-pairs test
eP = NS when comparing non-responders before and after treatment. Wilcoxon matched-pairs test
Patient-Reported Outcomes
The “Short Form Health Survey” (SF-36) is a validated multi-purpose questionnaire on general health consisting of 36 questions that cover eight domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health. The scores from each domain are summarized in two categories: Physical Component Summary and Mental Component Summary, which were included in the multivariate analysis.
The “Watson Dysphagia Scale” (WDS) is used for patients with dysphagia but has not yet been validated for eosinophilic esophagitis patients [17]. The patients answer whether they never (= 0), sometimes (= 0.5) or always (= 1) experience trouble swallowing food of different textures, ranging from liquids to solid foodstuffs. The score is multiplied by a factor for each foodstuff, giving a final score ranging from 0 (no dysphagia) to 45 (severe dysphagia). The scores for each foodstuff were included in the multivariate analysis.
The EORTC QLQ-OES18 was designed for patients with esophageal cancer [16], but because of its focus on swallowing difficulties has also been deemed to be suitable for eosinophilic esophagitis patients. The form consists of 18 questions divided into 10 domains: dysphagia scale, saliva, choking, eating scale, dryness, taste, cough, speaking, reflux scale and local pain scale. Scores for each domain, as well as a summary score, were included in the multivariate analysis.
Flow Cytometric Analyses
EDTA blood was analyzed by 5-color flow cytometry within 24 h of collection as previously described [20], using the mAbs listed in Table 2. Granulocytes were gated based on high side- and forward-scatter and the eosinophils were separated from the CD16+ neutrophils based on the eosinophils’ higher side-scatter and high levels of CCR3, as previously described [11]. CD4+ and CD8+ T cells were identified based on low side-scatter, high CD3 expression, and either high expression of CD4 or of CD8. The data are expressed as median fluorescence intensity (median-FI) or percent cells expressing a particular marker.
Table 2.
Monoclonal antibodies used in the flow cytometry analyses
| Antigen | Clone | Isotype | Cell target | Fluorochrome |
|---|---|---|---|---|
| CD3 | SK7 | IgG1, κ | T cell | FITC |
| CD4 | SK3 | IgG1, κ | T cell | APC-H7 |
| CD8 | SK1 | IgG1, κ | T cell | PE |
| CD16 | 3G8 | IgG1, κ | Eosinophil | FITC |
| CD25 | 2A3 | IgG1, κ | Eosinophil | APC |
| CD44 | G44-26 | IgG2b, κ | Eosinophil | PE |
| CD49d | 9F10 | IgG1, κ | Eosinophil | PE |
| CD54 | HA58 | IgG1, κ | Eosinophil | APC |
| CD66c | KOR-SA3544 | IgG1, κ | Eosinophil | PE |
| CD193 (CCR3) | 5E8 | IgG2b, κ | Eosinophil | BV421 |
| CD193 (CCR3) | 5E8 | IgG2b, κ | Eosinophil | AF647 |
| CD199 (CCR9) | L053E8 | IgG2a, κ | Eosinophil | PE |
| CD274 (PDL1) | 29E.2A3 | IgG2b, κ | Eosinophil | APC |
| CD294 (CRTH2) | BM16 | rat IgG2a, κ | Eosinophil | APC |
All antibodies were from BD Biosciences, Franklin Lakes, NJ, USA
Statistical Methods
The paired Wilcoxon test and Fisher’s exact test were used for comparisons of two groups and the Spearman test to determine correlations between data sets. GraphPad Prism 7.0 software (GraphPad, San Diego, CA, USA) was employed. P < 0.05 was considered statistically significant. Multivariate analyses of pattern recognition “Orthogonal-Projection to Latent Structures” (OPLS) were performed using the SIMCA-P statistical package version 13.03 (MKS Data Analytics Solutions, Malmö, Sweden). Multivariate models with outcome variable (Y) “histologic response to therapy” were constructed with input X-variables, i.e., immune parameters, clinical data, and questionnaire scores. The quality of the obtained models was assessed by their explanatory power (R2Y) and robustness (Q2Y), respectively. The Variable Importance Parameter (VIP) module was employed to evaluate the contribution of the X-variables to the tested models and enable the selection of the parameters with the highest impact on the test models.
Results
Combination of Immune Parameters and Self-Assessment Data Required for Construction of Multivariate Models
Three-quarters of the patients (15/20) responded to topical corticosteroids and attained < 15 peak eosinophils/HPF, whereas one quarter of the patients (5/20) did not and were defined as non-responders. We tried to construct multivariate models to segregate the responders from the non-responders using clinical data, immune parameters (absolute blood counts of eosinophils, granulocytes, CD4+ T cells and CD8+ T cells, the expression of ten molecules by blood eosinophils indicated as median-FI and % expression, and peak eosinophil counts/HPF in esophageal biopsies), and domain and global self-assessment scores obtained from three questionnaires (SF-36, WDS, EORTC QLQ-OES18). All immune parameters were analyzed before and after treatment. Similarly, scores were retrieved from the three questionnaires that the patients filled in before and after treatment. The change in the levels of the immune parameters and questionnaire scores were also used as input variables. The outcome variable for all multivariate models was histological response to treatment, which was defined as < 15 peak eosinophils/HPF in the esophagus.
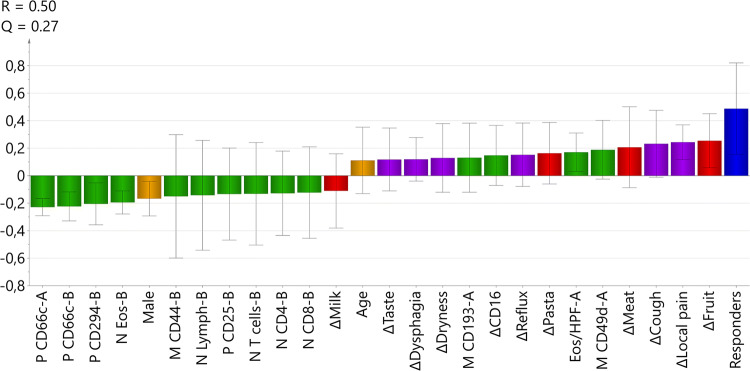
The modeling revealed that it was necessary to include 13 immune parameters (11 derived from flow cytometry assay and 2 from blood differential counts) and 10 questionnaire scores to obtain a stable model. Models solely based on either immune parameters or on questionnaire scores were incapable of segregating the responders from the non-responders. It was also necessary to include immune parameters measured before and after treatment to create a good model. Moreover, inclusion of sex and age of the patient contributed to the generation of a stable model. The best model is shown in Fig. 1. Parameters above the line (> 0) are positively associated with response to therapy, whereas parameters below the line are associated with lack of response.
Fig. 1.
Multivariate OPLS model of histological response to topical corticosteroids. The outcome Y-variable (blue) was set as a histological response of < 15 peak eosinophil counts/HPF in the esophagus after treatment. Input X-variables with discriminatory power are shown as bars and consisted of clinical data, immune parameters and questionnaire scores, collected before (A) and after treatment (B). Immune parameters (green) are presented as percent (P) blood eosinophils expressing a particular molecule and its median fluorescence intensity (M), and absolute numbers (N) of leukocyte subpopulations in the blood. Scores from the EORTC QLQ-OES18 (purple) and Watson dysphagia scale (red) questionnaires and clinical data (yellow) are shown. Δ is the difference between values before and after treatment for each parameter. The X-variables indicated by bars in the same direction as the output Y-variable were positively associated, and the variables indicated by bars in the opposite direction were negatively associated with being a responder. The model had an explanatory power (R2Y) of 50% and a stability (Q2) of 27%
EORTC and Watson Dysphagia Scale Were the Best Questionnaires
Several questionnaire scores reflecting symptomatic relief and improved capacity to eat certain foods turned out to be important parameters in the multivariate model. Self-assessed improvements regarding local pain, coughing, reflux, dryness, dysphagia and taste alterations retrieved from the EORTC questionnaire were positively associated with being a responder (Fig. 1). Moreover, an increased ability to eat fruit was the strongest parameter, followed in order of decreasing strength by the capacity to eat meat, and pasta; these queries were from the WDS (Fig. 1). None of the questions from the SF-36 questionnaire contributed to the model.
Leukocyte Counts and Eosinophil Molecules for Identification of Responders
The non-responders tended to have a higher fraction of eosinophils expressing CD66c both before (A) and after treatment (B), and higher absolute numbers (N) of eosinophils, lymphocytes, T cells, CD4+ T cells, and CD8+ T cells after completion of the steroid course (Fig. 1). Moreover, a higher fraction of the non-responders’ eosinophils expressed the markers CD294 and CD25, and had a higher median expression of CD44 after treatment (Fig. 1). Fewer immune parameters were associated with response to treatment: an increased median expression of CD49d and of CD193 (CCR3) on eosinophils before treatment, and higher pre-treatment peak counts of esophageal eosinophils were characteristics of the responders (Fig. 1). Reduced levels of CD16-expressing eosinophils in the blood after treatment was also associated with being a responder (Fig. 1).
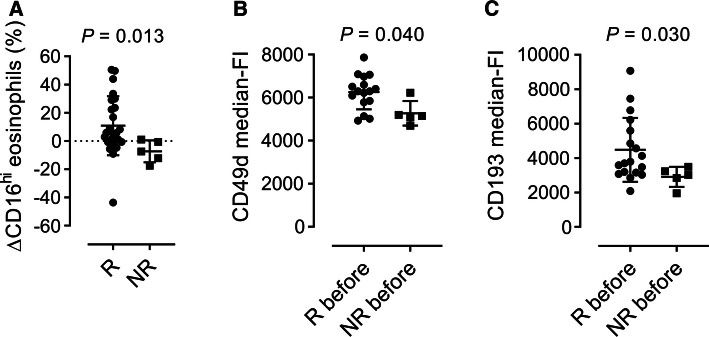
Only a few of the immune parameters from the multivariate model were found to be statistically significant when analyzed singly: The responder group had a statistically significant reduction in the fraction of CD16-positive eosinophils (ΔCD16) post-therapy compared with the non-responders (Fig. 2a). In addition, eosinophils from responders had statistically significant higher levels of CD49d and CCR3 before treatment compared with non-responders (Fig. 2b, c). Importantly, none of these immune parameters could separate responders from non-responders on their own, as shown by the considerable overlap in the levels of each parameter between responders and non-responders (Fig. 2a–c).
Fig. 2.
Univariate analysis of a, the difference in % of CD16-expressing eosinophils in the blood before and after treatment (ΔCD16), and median intensity expression by blood eosinophils of b, CD49d, and c, CD193 before treatment between responders (R, n = 15) and non-responders (NR, n = 5). Wilcoxon matched-pairs signed-rank test was used
Reduction of CD16-Expressing “Suppressive” Eosinophils in the Blood After Successful Corticosteroid Treatment
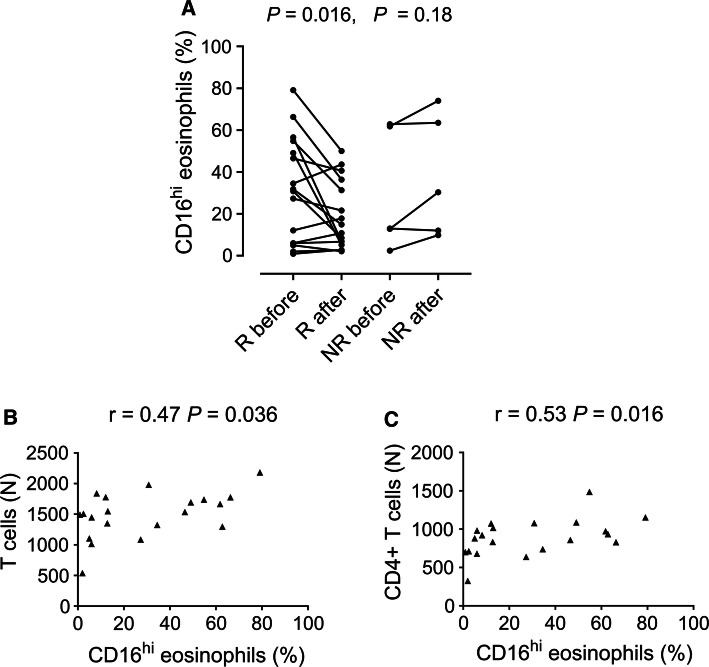
We found that whereas responders had lower levels of CD16-expressing “suppressive” eosinophils in the circulation after treatment (Fig. 3a), this was not seen among the non-responders. In fact, there was a trend for increased levels of CD16+ eosinophils post-therapy in patients who did not respond to treatment (Fig. 3a). Moreover, the percentage of CD16+ eosinophils in the blood correlated with both the total numbers of CD3+ T cells and of CD4+ T cells in the blood, respectively (Fig. 3b, c), indicative of a relationship between these cell types.
Fig. 3.
Univariate analysis of the levels of CD16-expressing eosinophils in the blood before and after treatment in a, responders (R) and non-responders (NR). Wilcoxon matched-pairs signed-rank test was used. Correlation between percent CD16-expressing eosinophils in the blood and b, absolute counts (N) of T cells and c, absolute counts of CD4+ T cells in the blood before treatment. The Spearman test was used
Discussion
The main objective of this study was to test the hypothesis that a combination of blood-based immune parameters could segregate responders from non-responders to topical corticosteroid therapy in a study cohort of 20 adult eosinophilic esophagitis patients. Blood-based diagnostic and therapeutic biomarkers have been much sought after within the field of eosinophilic esophagitis research since biomarkers might reduce the need for repeated endoscopy to monitor disease activity [21–24]. How to measure disease activity in eosinophilic esophagitis is a complex issue but should include objective clinician-reported measures and patient-reported measures [25]. We chose to use histologic response to therapy as the clinician-reported outcome and three different self-assessment questionnaires covering quality of life, as well as symptoms and behavioral adaptations related to esophageal dysfunction as patient-reported outcomes. When we attempted to construct models to differentiate histologic responders from non-responders to therapy we found that it was necessary to include blood-based immune parameters collected before and after treatment, as well as patient-reported questionnaire scores. This was unexpected against the background that several studies have reported poor congruence between histologic response and patient-reported response to treatment in eosinophilic esophagitis [25, 26].
An advantage with multivariate modeling is the possibility to remove parameters that contribute noise to the model by using the unbiased “variable of importance” module, which may in part explain why several patient-reported outcome scores were shown to be important for separating responders from non-responders. Furthermore, we found that several questions derived from both the Watson dysphagia scale and EORTC questionnaires, which cover dysphagia-related symptoms and corresponding behavioral adaptations, were of value for creating a stable model, whereas the more general questions relating to quality of life from the SF-36 form did not. Unexpectedly, local pain and cough were the symptoms that most responders reported to have become improved after treatment. In fact, the majority of patients in this study suffered from chest pain (75%) and cough (65%), respectively. In line with this, a retrospective study of adult eosinophilic esophagitis patients identified baseline abdominal pain to be predictive of response to topical corticosteroids [27]. Furthermore, eosinophilic esophagitis is increasingly being recognized to be a differential diagnosis for chronic cough in children [28, 29]. In contrast, although dysphagia, the defining symptom of eosinophilic esophagitis, was also a patient-reported parameter that lent stability to the model, its contribution was much lower than the aforementioned symptoms of cough and local pain. On the other hand, enhanced ability to eat fruit and meat were the biggest improvements in terms of altered eating habits, both of which are alternate measures of dysphagia. This is in agreement with earlier studies reinforcing the notion that general questions regarding dysphagia can be misleading in this group of patients who in general have modified their eating patterns to minimize dysphagia [10, 25]. It might be valuable for clinicians assessing response to therapy in adult eosinophilic esophagitis patients to ask more precise questions regarding particular symptoms and specific foods rather than more general questions concerning dysphagia.
Another hypothesis we wanted to test was if levels of CD16-expressing “suppressive eosinophils” could be used to monitor treatment response. Indeed, a decreased fraction of CD16-expressing eosinophils in the blood after treatment was one of the immune parameters associated with being a responder to therapy. This was contrary to our original hypothesis, that untreated patients would have depressed levels of CD16-expressing eosinophils in the blood, which would return to normal after successful treatment. Instead, it appears that there is increased release of CD16-expressing eosinophils from the bone marrow to the blood in patients with symptomatic disease, which decreases in successfully treated patients. We have previously shown that CD16-expressing eosinophils are more potent T cell suppressors than conventional eosinophils in vitro [11]. Our finding that the levels of CD16-expressing, potentially suppressive eosinophils, correlated with the numbers of CD3+ T cells and CD4+ T cells alike, at least hints at an association between these two types of leukocytes. Since eosinophilic esophagitis is claimed to be a Th2-driven disorder [2, 3], it is tempting to speculate that the activated CD16-expressing eosinophils in the blood of eosinophilic esophagitis patients might have a T cell suppressive function in the esophagus.
One limitation of this study is the lack of validated questionnaires in Swedish for patients with eosinophilic esophagitis. Nevertheless, the WDS, EORTC QLQ-OES18 and SF-36 questionnaires available in the Swedish language have been used previously to assess patient-reported outcomes in adult Swedish eosinophilic esophagitis patients [18, 19]. This pilot study was designed to assess if it would be possible to identify a panel of immune parameters in the blood to monitor response to treatment in eosinophilic esophagitis. Intriguingly, we found by multivariate modeling that patient-reported outcomes were required in addition to the immune parameters, suggesting that it may be difficult to rely solely on blood-based biomarkers to evaluate eosinophilic esophagitis patients. The combination of blood-based immune parameters with a select number of patient-reported outcome queries may prove to be a good strategy for noninvasive monitoring of response to therapy in eosinophilic esophagitis patients in the future. However, our findings are based on a relatively small cohort of patients and need to be reproduced using a larger number of patients, preferably from different study centers.
Acknowledgements
Open access funding provided by University of Gothenburg. We thank Mikaela Engelin and Kerstin Andersson for expert logistical and technical assistance.
Author contributions
C.L. and S.A. did the analysis of flow cytometry data, multivariate modeling, compilation of results, and participated in the writing of the manuscript. L.J. and H.L. recruited and evaluated all study patients, performed all endoscopies, and participated in the design of the study. H.L participated in the writing of the MS. C.W. designed the study, interpreted the results, was chiefly responsible for writing the manuscript and supervised the entire study.
Funding
This work was funded by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (71580), the Cancer and Allergy Foundation (18-228), Health & Medical Care Committee of the Regional Executive Board of Region Västra Götaland (94920), IngaBritt and Arne Lundberg Research Foundation, and the University of Gothenburg.
Compliance with Ethical Standards
Conflict of interest
Helen Larsson has been part of an advisory board for EsoCap AG, June 2019, but has no other conflicts of interest. Christine Lingblom, Sofie Albinsson, Leif Johansson and Christine Wennerås have no conflicts of interest to declare.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Regional Ethical Review Board of Gothenburg.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christine Lingblom, Email: christine.lingblom@microbio.gu.se.
Sofie Albinsson, Email: sofie.albinsson@gu.se.
Leif Johansson, Email: leif.e.johansson@vgregion.se.
Helen Larsson, Email: helen.m.larsson@vgregion.se.
Christine Wennerås, Email: christine.wenneras@microbio.gu.se.
References
- 1.Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med. 2015;373:1640–1648. doi: 10.1056/NEJMra1502863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–961. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 3.Wen T, Aronow BJ, Rochman Y, et al. Single-cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J Clin Invest. 2019;129:2014–2028. doi: 10.1172/jci125917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aceves SS. Remodeling and fibrosis in chronic eosinophil inflammation. Dig Dis. 2014;32:15–21. doi: 10.1159/000357004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230–1236. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577–585. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwakura N, Fujiwara Y, Tanaka F, et al. Basophil infiltration in eosinophilic oesophagitis and proton pump inhibitor-responsive oesophageal eosinophilia. Aliment Pharmacol Ther. 2015;41:776–784. doi: 10.1111/apt.13141. [DOI] [PubMed] [Google Scholar]
- 8.Vicario M, Blanchard C, Stringer KF, et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucendo AJ, Molina-Infante J, Arias A, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5:335–358. doi: 10.1177/2050640616689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2019;17:2149–2160. doi: 10.1016/j.cgh.2019.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingblom C, Andersson J, Andersson K, Wenneras C. Regulatory eosinophils suppress T cells partly through galectin-10. J Immunol. 2017;198:4672–4681. doi: 10.4049/jimmunol.1601005. [DOI] [PubMed] [Google Scholar]
- 12.Fooksman DR, Vardhana S, Vasiliver-Shamis G, et al. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubach J, Lutter P, Bopp T, et al. Human CD4 + CD25 + regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110:1550–1558. doi: 10.1182/blood-2007-01-069229. [DOI] [PubMed] [Google Scholar]
- 14.Lingblom C, Wallander J, Ingelsten M, et al. Eosinophils from eosinophilic oesophagitis patients have T cell suppressive capacity and express FOXP3. Clin Exp Immunol. 2017;187:455–465. doi: 10.1111/cei.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan M, Karlsson J, Ware JE. The Swedish SF-36 Health Survey–I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med. 1995;41:1349–1358. doi: 10.1016/0277-9536(95)00125-q. [DOI] [PubMed] [Google Scholar]
- 16.Blazeby JM, Conroy T, Hammerlid E, et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384–1394. doi: 10.1016/S0959-8049(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 17.Dakkak M, Bennett JR. A new dysphagia score with objective validation. J Clin Gastroenterol. 1992;14:99–100. doi: 10.1097/00004836-199203000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Larsson H, Bergman K, Finizia C, Johansson L, Bove M, Bergquist H. Dysphagia and health-related quality of life in patients with eosinophilic esophagitis: a long-term follow-up. Eur Arch Otorhinolaryngol. 2015;272:3833–3839. doi: 10.1007/s00405-015-3696-4. [DOI] [PubMed] [Google Scholar]
- 19.Larsson H, Norder Grusell E, Tegtmeyer B, Ruth M, Bergquist H, Bove M. Grade of eosinophilia versus symptoms in patients with dysphagia and esophageal eosinophilia. Dis Esophagus. 2016;29:971–976. doi: 10.1111/dote.12417. [DOI] [PubMed] [Google Scholar]
- 20.Lingblom C, Kappi T, Bergquist H, et al. Differences in eosinophil molecular profiles between children and adults with eosinophilic esophagitis. Allergy. 2017;72:1406–1414. doi: 10.1111/all.13140. [DOI] [PubMed] [Google Scholar]
- 21.Bhardwaj N, Ghaffari G. Biomarkers for eosinophilic esophagitis: a review. Ann Allergy Asthma Immunol. 2012;109:155–159. doi: 10.1016/j.anai.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Dellon ES, Rusin S, Gebhart JH, et al. Utility of a noninvasive serum biomarker panel for diagnosis and monitoring of eosinophilic esophagitis: a prospective study. Am J Gastroenterol. 2015;110:821–827. doi: 10.1038/ajg.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlag C, Miehlke S, Heiseke A, et al. Peripheral blood eosinophils and other non-invasive biomarkers can monitor treatment response in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;42:1122–1130. doi: 10.1111/apt.13386. [DOI] [PubMed] [Google Scholar]
- 24.Subbarao G, Rosenman MB, Ohnuki L, et al. Exploring potential noninvasive biomarkers in eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr. 2011;53:651–658. doi: 10.1097/MPG.0b013e318228cee6. [DOI] [PubMed] [Google Scholar]
- 25.Schoepfer A, Safroneeva E, Straumann A. How to measure disease activity in eosinophilic esophagitis. Dis Esophagus. 2016;29:959–966. doi: 10.1111/dote.12391. [DOI] [PubMed] [Google Scholar]
- 26.Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology. 2016;150:581–590. doi: 10.1053/j.gastro.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf WA, Cotton CC, Green DJ, et al. Predictors of response to steroid therapy for eosinophilic esophagitis and treatment of steroid-refractory patients. Clin Gastroenterol Hepatol. 2015;13:452–458. doi: 10.1016/j.cgh.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fracchia MS, Diercks G, Cook A, et al. The diagnostic role of triple endoscopy in pediatric patients with chronic cough. Int J Pediatr Otorhinolaryngol. 2019;116:58–61. doi: 10.1016/j.ijporl.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Orizio P, Cinquini M, Minetti S, et al. Chronic cough and eosinophilic esophagitis: an uncommon association. Case Rep Gastroenterol. 2011;5:497–501. doi: 10.1159/000331510. [DOI] [PMC free article] [PubMed] [Google Scholar]