Abstract
Main conclusion
Modulation of the gaseous environment using oxygen absorbers and/or silica gel shows potential for enhancing seed longevity through trapping toxic volatiles emitted by seeds during artificial ageing.
Abstract
Volatile profiling using non-invasive gas chromatography–mass spectrometry provides insight into the specific processes occurring during seed ageing. Production of alcohols, aldehydes and ketones, derived from processes such as alcoholic fermentation, lipid peroxidation and Maillard reactions, are known to be dependent on storage temperature and relative humidity, but little is known about the potential modulating role of the gaseous environment, which also affects seed lifespan, on volatile production. Seeds of Lolium perenne (Poaceae), Agrostemma githago (Caryophyllaceae) and Pisum sativum (Fabaceae) were aged under normal atmospheric oxygen conditions and in sealed vials containing either oxygen absorbers, oxygen absorbers and silica gel (equilibrated at 60% RH), or silica gel alone. Seeds of A. githago that were aged in the absence of oxygen maintained higher viability and produced fewer volatiles than seeds aged in air. In addition, seeds of A. githago and L. perenne aged in the presence of silica gel were longer lived than those aged without silica, with no effect on seed moisture content or oxygen concentration in the storage containers, but with silica gel acting as a volatile trap. These results indicate that the use of inexpensive oxygen absorbers and silica gel could improve seed longevity in storage for some species and suggests a potential, and previously unidentified, role for silica gel in ultra-dry storage.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00425-021-03620-5.
Keywords: Anoxia, Longevity, Oxygen, Seed storage, Silica gel
Introduction
The effects of temperature and moisture on seed longevity in storage have been well characterised, and in general, the life span of orthodox, desiccation tolerant, seeds increases as temperature and moisture content decrease, within certain limits (Dickie et al. 1990; Ellis et al. 1990). Although, this does not hold true for all species, particularly under ultra-dry conditions (Ballesteros and Walters 2011). The international standards for long-term ex situ seed storage (FAO 2014) recommend that seeds are stored at low temperature (ca. -18 °C) after drying to low relative humidity (ca. 15% RH). Besides temperature and moisture content, gaseous environment may affect seed life span (Roberts and Abdalla 1968; Ibrahim and Roberts 1983; Ibrahim et al. 1983; Tompsett 1983). Research into the process of seed deterioration during storage has indicated the involvement of reactive oxygen species and accumulation of oxidative damage to cellular macromolecules e.g. lipids, proteins and nucleic acids, which eventually leads to loss of cell function and seed death (Bailly 2004; Rajjou et al. 2008; Kranner et al. 2011). For this reason, much of the focus of research into the effect of the gaseous environment on seed storage has been on oxygen. Comparisons of hermetic storage and open storage at a range of seed moisture contents showed that longevity was higher in hermetic storage for seeds with low moisture content, and the difference is likely due to the limited availability of oxygen in hermetic storage (Ellis and Hong 2007).
The life span of seeds in storage varies greatly between species (Walters et al. 2005; Probert et al. 2009) and also between seed lots of the same species (Nagel et al. 2009), but the characteristics underpinning seed lifespan have yet to be determined. A recent analysis of seed life span under a range of conditions has shown that most species may produce seeds that are comparatively short-lived i.e. more than half of species have seed life spans < 20% of the longest lived under the same conditions (Colville and Pritchard 2019). Predicting how long seeds will survive in ex situ storage is difficult. Viability equations for the estimation of storage life at a range of moisture and temperature conditions have been defined but require determination of species-specific temperature and moisture constants (Ellis and Roberts 1980). Therefore, monitoring of seed viability during storage is a critical component of the management of ex situ seed collections and requires optimisation of testing intervals to minimise wastage of seeds by testing too frequently or conversely reduce the risk of not detecting deterioration of seed collections by testing too infrequently. The standard approach for monitoring seed viability is germination testing, which is time-consuming and destructive (Hay and Whitehouse 2017). Techniques for the rapid, and ideally non-destructive screening of seed viability have been the focus of research (e.g. Costanzo et al. 2008; Kranner et al. 2010; Xin et al. 2013). However, there is not yet a single approach that can be universally applied for monitoring seed viability of diverse species. One technique that has received attention is the analysis of volatile metabolites evolved by seeds during storage. These metabolites accumulate in sealed storage containers and can be detected using gas chromatography–mass spectrometry (GC–MS). A wide range of volatile compounds have been detected during seed storage, among them ethanol, methanol, acetaldehyde and acetone are frequently reported to accumulate during storage of seeds of a number of species including Pisum sativum (Zhang et al. 1995; Colville et al. 2012); Lathyrus pratensis and Cytisus scoparius (Colville et al. 2012); Glycine max (Zhang et al. 1994, 1995; Lee et al. 2001); Phaseolus vulgaris (Lee et al. 2001); Lactuca sativa (Zhang et al. 1994, 1995; Mira et al. 2010, 2016); Carum carvi and Eruca vescaria (Mira et al. 2016); Oryza sativa (Zhang et al. 1994, 1995); Xanthium pennsylvanicum (Zhang et al. 1995); Brassica napus (Buckley and Buckley 2009); Helianthus annuus and Daucus carota (Zhang et al. 1994).
In addition to its potential as a non-invasive technique for monitoring seed viability, seed volatile profiling also provides an insight into the processes taking place within seeds during storage (Mira et al. 2016). The volatile compounds released by seeds of three legume species (C. scoparius, L. pratensis and P. sativum) during storage are reported to derive from a number of processes including respiration, lipid peroxidation and Maillard reactions (Colville et al. 2012). The occurrence of these reactions is highly dependent on the moisture content of the seeds and temperature (Wettlaufer and Leopold 1991), which will determine viscosity of the cytoplasm and molecular mobility therein, i.e., whether the seed cytoplasm is in a glassy state (Sun 1997). The high viscosity of intracellular glasses minimises molecular mobility and therefore limits the occurrence of chemical reactions that contribute to seed deterioration during storage (Buitink et al. 2000). Mitochondrial respiration does not occur in seeds stored at < 75% RH, but glycolysis has been shown to occur at a slow rate leading to production of ethanol and acetaldehyde, which have been detected from G. max and P. vulgaris seeds stored between 12 and 75% RH, via alcoholic fermentation (Lee et al. 2001). Mira et al. (2010, 2016) reported that at > 30% RH volatile organic compounds (VOCs) emitted by seeds of C. carvi, E. vescaria and L. sativa derived from fermentation-type reactions, and at < 30% RH, there was a switch towards peroxidation-type reactions. However, viability was more strongly correlated with emission of fermentation products and there was no correlation between ageing and emission of lipid peroxidation products.
The presence of oxygen may also influence the types of reactions that take place during seed storage, particularly those related to respiration and oxidative processes, e.g., lipid peroxidation. In metabolically active plant tissues, a reduction in oxygen concentration leads to a rapid inhibition of respiration and downregulation of the Krebs cycle and glycolysis to reduce oxygen consumption and avoid internal anoxia (Geigenberger 2003). Developing seeds of Vicia faba and P. sativum have been shown to have very low levels of oxygen of around 2–3%, which limits the rate of metabolism, but anoxia and induction of fermentation were not detected (Rolletschek et al. 2002). The internal oxygen concentration of mature, dry pea seeds was reported to be in equilibrium with the atmosphere, but rapidly falls during imbibition due to the onset of respiration and low oxygen diffusion through the seed coat, leading to fermentation (Rolletschek et al. 2009). Despite the potential benefits to seed life span of storage under hypoxia or anoxia (Groot et al. 2015), there have been few studies of the biochemical effects of reduced oxygen levels during seed storage (Rutzke et al. 2008).
In this study, the influence of oxygen in the gaseous storage environment on the life span of seeds of three species, Agrostemma githago, Lolium perenne and P. sativum, during artificial ageing at 60% RH and 50 °C was investigated. The species were chosen to represent a diverse range of phylogeny, seed size and structure, particularly embryo: seed ratio, to determine the variation in response to ageing in modified gaseous environments. Oxygen absorbing sachets were used to generate an anoxic storage environment (< 0.1% oxygen), and comparison was made with seeds aged in ambient atmospheric conditions, and with seeds aged in the presence of sachets of silica gel both alone and in combination with O2 absorbers. The silica gel had been equilibrated at 60% RH and was included to control for a potential moisturising effect of the oxygen absorbers. Seed deterioration during artificial ageing was monitored by germination testing and assessment of seedling growth. In addition, volatile profiling of the headspace of the storage containers using GC–MS was conducted to gain an insight into the processes taking place within seeds during ageing in the presence and absence of oxygen. To assess the potential toxicity of the most abundant volatile metabolites which accumulate in the headspace of storage containers during ageing, seeds of P. sativum were subjected to 10 d of artificial ageing in the presence of exogenously applied pure volatile compounds. The adsorption of these volatile compounds by O2 absorbers, silica gel and the seeds themselves was determined by solid phase microextraction (SPME)-GC–MS analysis.
Materials and methods
Seed material
Seeds of L. perenne L. and A. githago L. were obtained from Emorsgate Seeds (King's Lynn, Norfolk, UK) and P. sativum ‘Meteor’ seeds were purchased from CN Seeds Ltd (Pymoor, UK). All seeds were stored at 15% RH and 15 °C prior to use.
Seed morphology
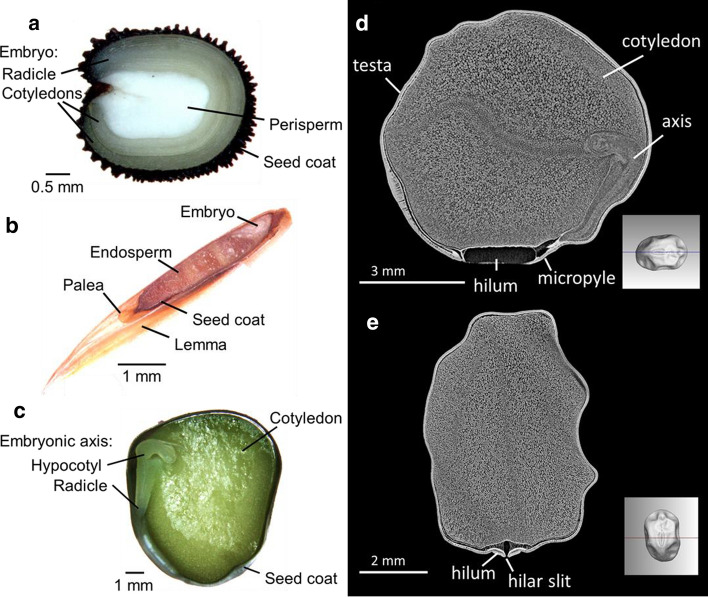
Seeds were imbibed for 24 h, frozen in Optimal Cutting Temperature compound and cut using a CM3050 cryostat (Leica Microsystems, Wetzlar, Germany) at − 20 °C. The seeds were then imaged using a digital microscopy camera (Zeiss Axiocam) mounted on a stereomicroscope (Zeiss Stemi SV11). The images were taken using Axiovision version 3.1 (Carl Zeiss, Jena, Germany), see Fig. 1.
Fig. 1.
Longitudinal sections of imbibed seeds of Agrostemma githago (a), Lolium perenne (b) and Pisum sativum (c) showing the differences in size and structure between the three species. X-ray micro-computed tomography images of simulated thin sections of a dry Pisum sativum seed showing longitudinal (d) and transverse (e) sections through the hilum
X-ray micro-computed tomography
A dry (15% RH) seed of P. sativum was characterised with a Zeiss Xradia 520 Versa CT system (Carl Zeiss Microscopy) using X-ray micro-computed tomography (XCT). The instrument was set up to obtain an appropriate transmission of X-rays through the sample. The experimental conditions consisted of voltage of 60 kV and current of 83.3 µA (power of 5 W); no filter. Data were recorded with an indirect detector (scintillator screen, 0.39 × optical lens, Andor iKon-L Charge-coupled device camera with 2048 × 2048 active pixels of 13.5 × 13.5 µm). The acquisition consisted of 2401 projections of 6 s each. The combined magnification from the X-ray conical beam geometry (Source-Object distance: 15.02 mm; Object-Detector distance: 110.00 mm) and the specifications of the indirect detector generated data with an isotropic voxel size of 4.14 µm. The tomographic acquisition and reconstruction were performed using Zeiss Scout-and-Scan software (Carl Zeiss Microscopy). The reconstruction first consisted of a 32-bit volume and was later converted to a 16-bit stack of tiff files using Zeiss XRController software, see Fig. 1.
Seed oil content
Seed oil content was quantified by time domain-nuclear magnetic resonance (TD-NMR) using a Bruker mq20 minispec, with a 0.47 T magnet (20 MHz proton resonance frequency) operating at 40 °C and a 10 mm probe assembly. Samples were analysed using a calibration with seeds of H. annuus of known oil content, where the method consisted of the acquisition of 16 scans with a recycle delay of 2 s. The method was modified from Borisjuk et al. (2011). Seed samples for each species were divided into five replicates of 100 mg and placed in 10 mm diameter NMR vials. For P. sativum, seeds were first cut into small, irregular fragments with a scalpel. 100 mg of the resulting seed fragments were then transferred to the NMR vials at random.
Seed ageing treatments
Seeds were aged under the conditions used for P. sativum in previous experiments (Kranner et al. 2006; Colville et al. 2012; Chen et al. 2013) Seeds were equilibrated over non-saturated lithium chloride solutions at 60% RH and 20 °C. The RH of the lithium chloride solution and the equilibrium RH of the seeds was determined using a hygrometer (HygroPalm, Rotronic Instruments Ltd, Crawley, UK). Following equilibration, 1 g of seeds were placed into 20 mL glass vials containing an oxygen indicating tablet (RP System Indicating eye, Conservation by Design, Milton Keynes, UK), which turn pink when oxygen concentrations are below 1%, and either a 20 cc oxygen absorbing sachet (O-Buster FT-type, Hsiao Sung Non-Oxygen Chemicals, Taichung City, Taiwan), a sachet of silica gel which had been pre-equilibrated at 60% RH, or an oxygen absorbing sachet and silica gel. The O-Buster FT-type oxygen absorbers are a self-reacting type, containing moistened zeolites to provide the water required for the oxidation of iron powder. The oxygen absorbers are effective at low RH and do not require additional water (from the sample) to be activated. Control vials contained only seeds and an oxygen indicating tablet (Fig. 2). The vials were hermetically sealed and placed in an oven at 50 °C. Vials were removed from the oven at intervals during artificial ageing (after 0, 5, 10, 15 and 20 days for P. sativum and L. perenne; after 0, 1, 2, 3 and 4 days for A. githago). The length of ageing was determined in trial experiments (unpublished). Three replicate vials were used for each treatment and ageing time point.
Fig. 2.

Photograph showing the experimental set up. 20 mL glass headspace vials were filled with 1 g of seeds (seeds pictured are Lolium perenne) which had been equilibrated to 60% RH. Control vials (left) contained just seeds and an oxygen indicating tablet, which was blue/purple in the presence of oxygen (> 0.5%) and turned to pink when oxygen levels were < 0.1%. Other vials (second from left to right) contained seeds, an oxygen indicating tablet and either a 20 cc oxygen absorber (O2 absorber), a 20 cc oxygen absorber and a silica gel sachet (O2 absorber + silica), or a silica gel sachet which had been pre-equilibrated to 60% RH (silica). All vials were sealed with PTFE-lined crimp seals
Volatile profiling
Volatiles accumulated in the headspace of the vials were sampled using solid phase microextraction (SPME) and analysed using gas chromatography–mass spectrometry (GC–MS; Colville et al. 2012; Paulsen et al. 2013). Prior to headspace sampling, vials were incubated at room temperature for 24 h to allow the headspace to equilibrate following the transfer from 50 °C. Volatile analysis was performed using automated SPME with a 75 μm Carboxen polydimethylsiloxane (PDMS) fibre (Supelco, Bellefonte, PA, USA) and an extraction time of 30 min at 30 °C followed by 5 min of desorption in the GC injector port at 240 °C. The volatiles were separated using GC (Thermo Finnigan Trace GC Ultra; Thermo Fisher Scientific, Waltham, MA, USA) on a FAMEWAX column (30 m length, 0.25 mm internal diameter, 0.25 μm film thickness; Restek, Bellefonte, PA, USA) running a temperature program (3 min hold at 35 °C, 3 °C min−1 to 60 °C, 10 °C min−1 to 220 °C and 1 min hold; helium carrier gas at constant flow rate of 1 mL min−1). The volatiles were detected using mass spectrometry (MS; Thermo Finnigan Trace DSQ; ionisation energy 70 eV and scan frequency range m/z 10–350 per 0.5 s). The volatile compounds were identified by spectral library matching with the NIST (National Institute of Standards and Technology, Gaithersburg, MD, USA) mass spectral database. The identities of the volatile compounds were confirmed and quantified through comparison with analytical standards (Sigma Aldrich). Standards were prepared as a mixture containing 24 compounds and diluted with dimethyl sulfoxide to produce nine different concentrations, which ranged from 2.5 to 100 μM for ethyl acetate (lowest concentration standard) to 0.9–35 mM for acetic acid (highest concentration standard). Aliquots of 10 μL were placed into 20 mL headspace vials, which were sealed and analysed using SPME–GC–MS as described above.
Moisture content, germination and seedling growth evaluation
Subsequently, seed moisture content was determined gravimetrically following oven drying at 103 °C for 17 h to assess the effect of the treatments, and particularly the presence of oxygen absorbers and/or silica gel on seed moisture content. In addition, the equilibrium relative humidity (eRH) of silica gel sachets was measured using a hygrometer probe (Rotronic). Silica gel sachets were removed from the sample vials containing seeds following ageing, and two sachets were immediately sealed in a 10 mL vial and RH was recorded after 30 min at 20 °C. Seed viability was assessed by germination testing. For each replicate (n = 3), 10 (P. sativum) or 20 (L. perenne and A. githago) seeds were sown in 10 cm diameter Petri dishes on 1% (w/v) agar. Only 10 seeds were used per replicate for P. sativum due to the size of the seeds (1 g of seeds was used per replicate for the ageing experiments, which is ca. 13–14 seeds for P. sativum). L. perenne and P. sativum seeds were incubated at a constant temperature of 20 °C with a 12 h photoperiod, and A. githago seeds were incubated at an alternating temperature of 20/10 °C with a 12 h photoperiod, as per the germination conditions for these species recorded in Royal Botanic Gardens, Kew’s Seed Bank Database. Germination was scored daily as the emergence of a radicle > 2 mm in length (hereafter referred to as total germination). After 14 d (P. sativum), 21 d (L. perenne) and 28 d (A. githago), normal seedling development was assessed to determine that seedlings showed vigorous and balanced growth of roots and shoots, and had potential to develop into healthy plants. Seedlings were photographed and root and shoot length was measured using Image J (Schneider et al. 2012).
Volatile treatments
To assess the effects of individual volatile compounds on seed viability during ageing, P. sativum seeds were aged for 10 d at 60% RH and 50 °C in the presence of either 1.25 μmol ethanol, 3 μmol methanol, 0.6 μmol acetic acid, 0.85 μmol 2-propanol, 90 nmol acetone or 40 nmol acetaldehyde. The concentrations of the compounds were in the range of those measured in the headspace of control P. sativum seeds following artificial ageing for 15 d. The pure compounds were pipetted individually into 2 mL glass vials. Each 2 mL vial was immediately placed inside a 20 mL glass vial containing 1 g of P. sativum seeds. Three replicates were used for each treatment. Following ageing, the volatile accumulation in the headspace was assessed by SPME–GC–MS and germination and root and shoot length determined as described above. For comparison with the previous experiment, control vials containing only seeds, vials containing seeds and an oxygen absorbing sachet, and vials containing seeds and silica gel were set up and volatile accumulation, germination and seedling growth were determined in unaged (0 d) and aged (10 d) seeds.
To determine the adsorption of volatile compounds by silica gel, oxygen absorbing sachets, A. githago seeds, and L. perenne seeds the same amounts of pure compounds as used above were placed into 2 mL glass vials and added to 20 mL glass vials either alone (controls) or along with sachets of silica gel (equilibrated to 60% RH), oxygen absorbing sachets or 1 g of A. githago or L. perenne seeds. The vials were sealed and placed at 50 °C for 10 d following which volatile analysis was performed using SPME–GC–MS. Three replicates were performed for each treatment.
Statistical analysis
One-way ANOVA followed by Dunnett’s post hoc test was performed using Genstat (Version 12, VSN International, 2011) to test for significant (P ≤ 0.05) differences between treatments and the control for each species. Principal component analysis (PCA) was performed on 26 variables (total germination, normal germination, emission rate of individual VOCs and total VOC emission rate) for all species combined, using XLSTAT. Data were automatically standardised, and Pearson correlation coefficients were computed by the software (Suppl. Table S1).
Results
Seed morphology
The seeds of the three species differed in size and morphology. L. perenne are the smallest, with an average 1000 seed weight of 2.0 g, compared to 12.0 g for A. githago and 139.9 g for P. sativum (Royal Botanic Gardens Kew 2020). A. githago has a peripheral embryo, which occupies around half of the seed volume, and surrounds the perisperm tissue. The L. perenne caryopsis consists of a small lateral embryo and abundant endosperm enclosed in a thin seed coat with lemma and palea attached. P. sativum has a bent embryo, which occupies the total seed volume with the cotyledons forming the storage reserves for the seed instead of endosperm tissue (Fig. 1; Martin 1946). Micro-computed tomography of a dry P. sativum seed showed more detail of the dry seed structure, particularly the hilum, which was open. Intercellular spaces could be seen in the cotyledons, but were not visible in the axis, in which cell density appeared to be higher (Fig. 1d).
Effect of storage environment on germination and seedling growth during artificial ageing
The moisture content of the unaged control seeds was 12.7 ± 0.38%, 10.8 ± 0.33% and 11.9 ± 0.07% for A. githago, L. perenne and P. sativum, respectively. Moisture content did not change during ageing and was also unaffected by the presence of O2 absorbers or silica gel (Table 1). Likewise, the eRH of silica gel sachets was unaffected by the ageing treatments, and there was no difference in the eRH of silica gel which had been incubated with seeds or with seeds and O2 absorbers (Table 1). The oxygen indicators sealed within each vial remained pink throughout the duration of the ageing time courses in all vials containing O2 absorbers, showing that the oxygen levels were below 0.1%. In vials without O2 absorbers, the oxygen indicators were blue, with no change in colour to indicate any changes in oxygen levels (Fig. 2).
Table 1.
Moisture content of seeds of Agrostemma githago, Lolium perenne and Pisum sativum, and equilibrium relative humidity (eRH) of silica gel sachets following equilibration at 60% RH (at 20 °C) and incubation at 50 °C
| Aged (d) | Seed moisture content (% FWB) | Silica eRH (%) | ||||
|---|---|---|---|---|---|---|
| Control | O2 ab | Si | O2 ab. + Si | Si | O2 ab. + Si | |
| A. githago | ||||||
| 0 | 12.69 (0.384) | 10.98 (0.944) | 12.11 (0.123) | 12.36 (0.258) | 59.4 | 58.3 |
| 1 | 12.21 (0.081) | 11.83 (0.036) | 12.08 (0.184) | 11.63 (0.153) | 58.1 | 57.4 |
| 2 | 11.77 (0.088) | 11.48 (0.203) | 11.31 (0.128) | 11.28 (0.096) | 57.6 | 57.3 |
| 3 | 11.75 (0.582) | 11.51 (0.228) | 11.47 (0.199) | 11.73 (0.403) | 58.5 | 59 |
| 4 | 11.19 (0.089) | 11.42 (0.324) | 11.82 (0.190) | 12.31 (0.670) | 59.3 | 58.6 |
| L. perenne | ||||||
| 0 | 10.84 (0.331) | 9.90 (1.053) | 11.43 (0.178) | 10.91 (0.708) | 58.7 | 58.4 |
| 5 | 10.74 (0.350) | 10.70 (0.451) | 10.55 (0.087) | 10.83 (0.561) | 59.1 | 59 |
| 10 | 10.88 (0.136) | 10.41 (0.179) | 9.91 (0.110) | 10.19 (0.056) | ||
| 15 | 10.59 (0.223) | 10.14 (0.311) | 10.40 (0.291) | 10.94 (0.672) | 59 | 57.8 |
| 20 | 11.04 (0.041) | 10.59 (0.026) | 10.64 (0.025) | 10.33 (0.057) | 60.2 | 59.3 |
| P. sativum | ||||||
| 0 | 11.92 (0.074) | 12.13 (0.160) | 12.02 (0.052) | 11.87 (0.120) | 58.7 | 58.6 |
| 5 | 11.00 (0.024) | 11.02 (0.069) | 11.06 (0.079) | 11.05 (0.011) | 59.8 | 59.5 |
| 10 | 11.62 (0.037) | 11.60 (0.091) | 11.67 (0.106) | 11.60 (0.058) | ||
| 15 | 11.45 (0.030) | 11.36 (0.021) | 11.40 (0.059) | 11.43 (0.130) | 60.8 | 58.9 |
| 20 | 11.35 (0.043) | 11.21 (0.116) | 11.32 (0.041) | 11.18 (0.179) | 61.5 | 59.6 |
Moisture content is expressed on a fresh weight basis. Values represent mean (n = 3) with SE shown in parentheses. There were no significant differences in seed moisture content between storage treatments. Silica eRH was measured using a hygrometer. A single measurement was performed per treatment using silica gel sachets from two of the three replicates
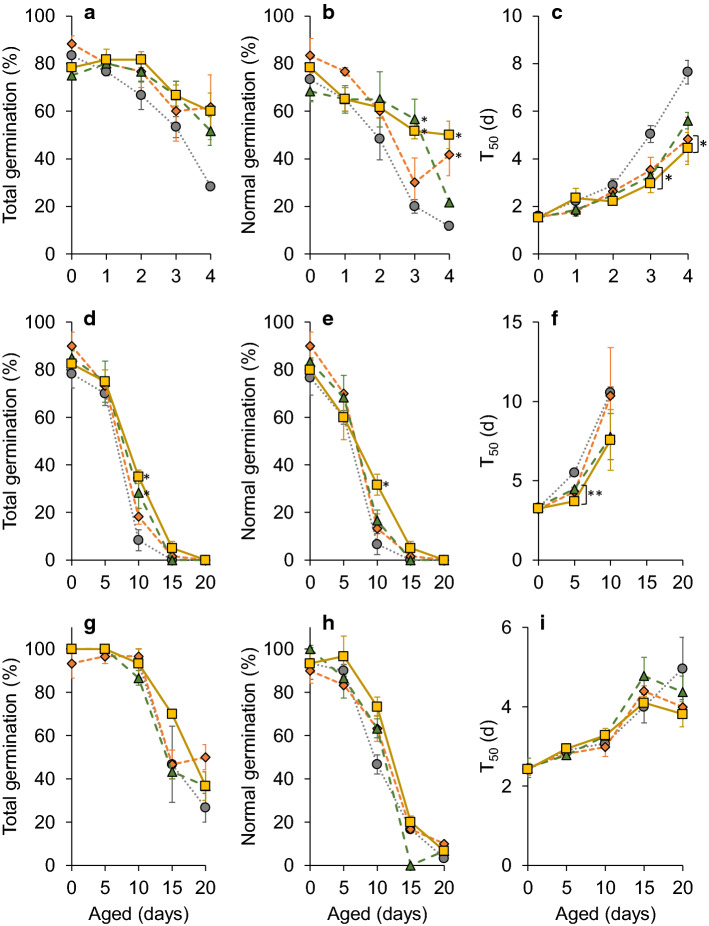
Total germination, assessed as radicle emergence, of all three species declined during artificial ageing. Control A. githago seeds had initial total germination of 83 ± 4.41%, but after 4 d of ageing total germination decreased to 28 ± 1.67%. Seeds aged in the presence of O2 absorbers did not show such a large decline in germination, and after 4 d of ageing, total germination was 62 ± 13.64%. Silica also reduced loss of germinability, with total germination of 52 ± 6.01% after 4 d of ageing. However, the differences between the treatments were not statistically significant (Fig. 3a). Similar patterns were observed for L. perenne, where total germination was significantly (P < 0.05) higher in seeds aged for 10 d in the presence of silica and O2 absorbers + silica compared to control seeds (Fig. 3d). Likewise, O2 absorbers and silica treatments reduced the decline in germination during artificial ageing of P. sativum seeds, albeit not significantly (Fig. 3g).
Fig. 3.
Total germination, normal germination and time taken for germination to reach 50% (T50) for A. githago (a–c), L. perenne (d–f) and P. sativum (g–i) seeds following ageing at 50 °C and 60% RH. The coloured symbols/lines represent different treatments: control (grey circles and dotted line), seeds aged in the presence of O2 absorbers (orange diamonds and short dashed line), silica gel (green triangles and long dashed line) or O2 absorbers and silica gel (yellow squares and solid line). Values are means of 3 replicates ± SE. Asterisks denote significant differences (*, P < 0.05; **, P < 0.01) between treated and control seeds at a particular ageing time point determined using one-way ANOVA
Normal germination followed a similar pattern to total germination and was significantly (P < 0.05) higher in A. githago seeds aged in the presence of silica and O2 absorbers + silica for 3 d, and O2 absorbers and O2 absorbers + silica for 4 d compared to control seeds (Fig. 3b). L. perenne also showed significantly (P < 0.05) higher normal germination of seeds aged with O2 absorbers + silica for 10 d compared to control seeds (Fig. 3e).
The rate of germination, measured as the time taken for 50% of seeds to germinate (T50), increased as ageing progressed e.g. T50 increased from 1.6 ± 0.15 d in unaged control A. githago seeds to 7.6 ± 0.50 d in seeds aged for 4 d. The increase in T50 was significantly (P < 0.05) lower for seeds aged for 3 or 4 d in the presence of O2 absorbers and/or silica (Fig. 3c). L. perenne seeds aged for 5 d with O2 absorbers and/or silica also had significantly (P < 0.01) lower T50 compared to control seeds (Fig. 3f). There were no significant differences in T50 between treatments for P. sativum seeds (Fig. 3i).
The longevity of the three species during artificial ageing was calculated using Probit analysis to determine the time taken for half of the seeds to lose viability (P50). The P50 of control seeds ranged from 2.8 d for A. githago to 16 d for P. sativum. Treatment with O2 absorbers, silica and O2 absorbers + silica significantly increased P50 for A. githago to 4.8, 5.2 and 5.5 d, respectively. Treatment with O2 absorbers + silica also increased P50 of L. perenne seeds from 5.1 to 10.7 d. In contrast, the P50 of P. sativum was unaffected by the treatments (Table 2).
Table 2.
Time taken for total germination to fall to 50% of initial germination (P50) determined using Probit analysis
| P50 (d) | ||||
|---|---|---|---|---|
| Control | O2 absorbers | Silica | O2 ab. + Silica | |
| A. githago | 2.843 (2.481–3.303) | 4.815 (3.444–12.59) | 5.159 (3.799–10.38) | 5.545 (4.094–10.89) |
| L. perenne | 5.106 (3.563–6.517) | 6.563 (5.529–7.572) | 6.714 (5.312–8.051) | 10.65 (8.835–12.66) |
| P. sativum | 16.08 (14.32–18.25) | 18.75 (15.09–27.89) | 16.35 (14.95–18.08) | 18.01 (16.81–19.59) |
Values in parentheses represent lower and upper 95% confidence intervals. Values in bold are significantly different compared to the control seeds based on non-overlapping confidence intervals
Effect of storage environment on volatile profiles
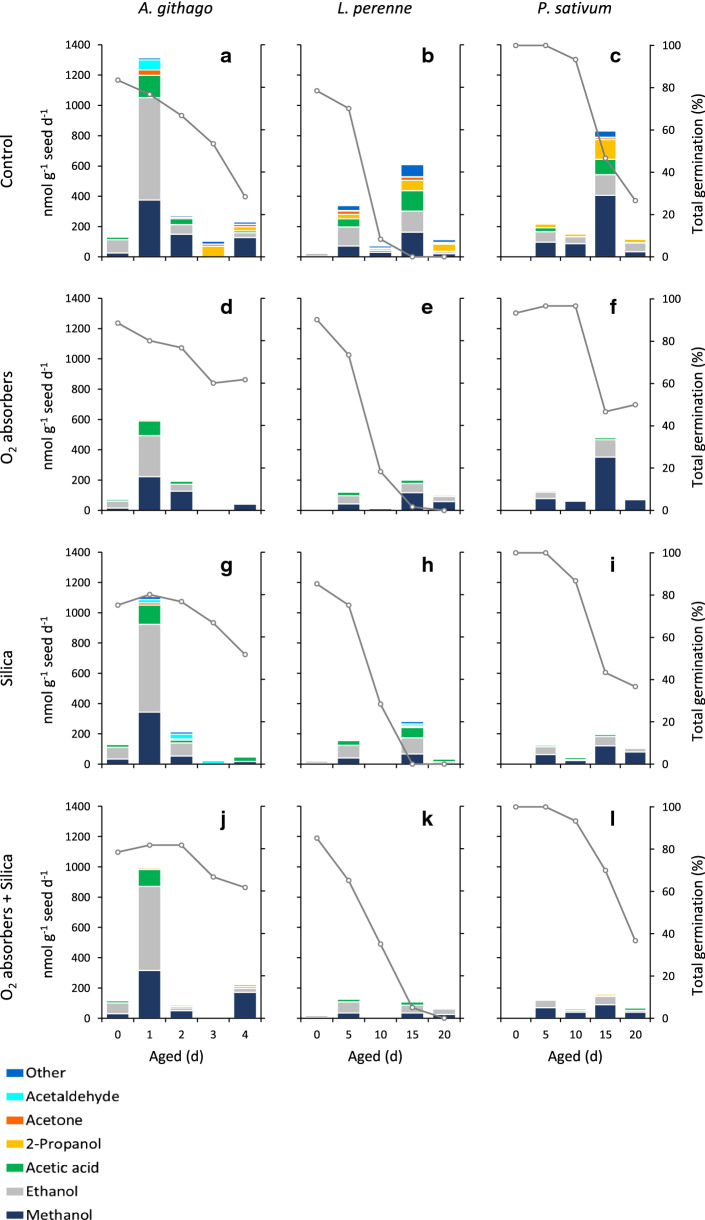
Total volatile emission, calculated as the sum of the amount of identified and quantified volatiles, from unaged control seeds ranged from 368 nmol g−1 FW for P. sativum up to 658 nmol g−1 FW for L. perenne. In all three species, volatile emission was higher from aged seeds but did not follow a consistent increase as ageing progressed. Volatile emission from A. githago peaked at 1344 nmol g−1 FW after 2 d of ageing, representing around a 2.5-fold increase compared to unaged seeds. In contrast, volatile emission increased over eightfold between 0 and 20 d aged L. perenne seeds, and over 16-fold between 0 and 15 d aged P. sativum seeds (Table 3). However, when the rate of total volatile emission per day of ageing was calculated after subtraction of the total volatiles detected at the previous time point, the highest rate of volatile emission was similar for A. githago and P. sativum seeds at around 800 nmol g−1 seed day−1. For unaged controls, seeds were incubated at 20 °C for the duration of the ageing time course (4 d for A. githago and 20 d for L. perenne and P. sativum), and the total volatile emission rate was calculated by dividing the total volatile emission by the number of days of incubation at 20 °C. Ageing seeds in the presence of O2 absorbers generally decreased volatile emission by around twofold compared to control seeds. Silica and O2 absorbers + silica had little effect on volatile emission from A. githago seeds but did significantly reduce volatile emission from L. perenne and P. sativum seeds, by around two- to fourfold at the later ageing time points (Fig. 4).
Table 3.
Total volatile emission of Agrostemma githago, Lolium perenne and Pisum sativum seeds during ageing at 50 °C and 60% RH determined using SPME–GC–MS
| Aged (d) | Total volatile emission (nmol g−1 FW) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | O2 absorbers | Silica | O2 ab. + Silica | |||||
| A. githago | ||||||||
| 0 | 545.4 | ab | 299.3 | a | 547.6 | ab | 475.4 | ab |
| 1 | 1313.4 | f | 597.9 | abcd | 1106.2 | ef | 993.6 | cdef |
| 2 | 1343.5 | f | 793.1 | bcde | 1303.7 | f | 985.7 | cdef |
| 3 | 1000.8 | def | 499.4 | ab | 776.9 | bcde | 560.5 | abc |
| 4 | 1190.6 | ef | 519.0 | ab | 621.8 | abcd | 783.2 | bcde |
| L. perenne | ||||||||
| 0 | 658.0 | ab | 310.8 | a | 511.5 | a | 416.7 | a |
| 5 | 1689.4 | cde | 623.7 | ab | 880.1 | abc | 642.9 | ab |
| 10 | 1962.0 | de | 681.2 | ab | 613.2 | ab | 611.5 | ab |
| 15 | 5005.0 | f | 1721.4 | cde | 2018.7 | e | 1153.7 | abcd |
| 20 | 5368.6 | f | 2242.4 | e | 1932.8 | de | 1456.6 | bcde |
| P. sativum | ||||||||
| 0 | 367.9 | ab | 243.6 | a | 280.5 | ab | 268.8 | ab |
| 5 | 1167.3 | abc | 646.6 | abc | 642.7 | abc | 621.1 | ab |
| 10 | 1918.2 | abcde | 979.1 | abc | 863.1 | abc | 933.1 | abc |
| 15 | 6062.4 | g | 3394.8 | de | 1928.3 | abcde | 1744.8 | abcd |
| 20 | 5275.8 | fg | 3605.7 | ef | 2396.0 | cde | 2039.7 | bcde |
Values are mean ± SE (n = 3). Letters indicate significant differences (P < 0.05) between treatments and ageing time points determined using two-way ANOVA and Tukey’s post hoc test
Fig. 4.
Stacked bars showing the rate of volatile emission during ageing of seeds of Agrostemma githago, Lolium perenne, and Pisum sativum under control conditions (a–c), in the presence of O2 absorbers (d–f), in the presence of silica gel (g–i), and in the presence of O2 absorbers and silica gel (j–l). The overall height of the bars corresponds to the mean total volatile emission (n = 3), whilst the coloured bands represent individual volatile compounds. Volatile emission was calculated as nmol g−1 seed d−1 on a rolling basis throughout the ageing time course, in that at each ageing time point, the amounts of each volatile accumulated at the previous time point were subtracted, and the remaining amount was divided by the number of days of ageing between sampling intervals. The line plots represent mean total germination (n = 3) under each of the conditions
Twenty-four different volatile compounds were detected across the 3 species, including a range of alcohols, aldehydes, ketones and furans (Supplementary Fig. S1). However, the volatile profiles of all three species were dominated by ethanol, methanol, acetic acid and 2-propanol (Fig. 4). Ageing in the presence of O2 absorbers and/or silica tended to reduce the emission of individual volatiles. O2 absorbers were more effective than silica at reducing the emission of ethanol, acetic acid and acetone, whilst silica was more effective at reducing emission of methanol, particularly in L. perenne and P. sativum seeds (Fig. 4).
Volatile emission increased as seed germination decreased, and for acetic acid and methanol emission was significantly higher from samples with total germination less than 85% for A. githago (47/60 samples) and P. sativum (28/60 samples) and less than 70% for L. perenne (40/60 samples). There was also significantly higher emission of ethanol from seeds of L. perenne and P. sativum with total germination less than 70% and 85%, respectively. Whilst acetone showed significantly higher emission only from seeds of L. perenne with total germination less than 70% (Fig. 5).
Fig. 5.
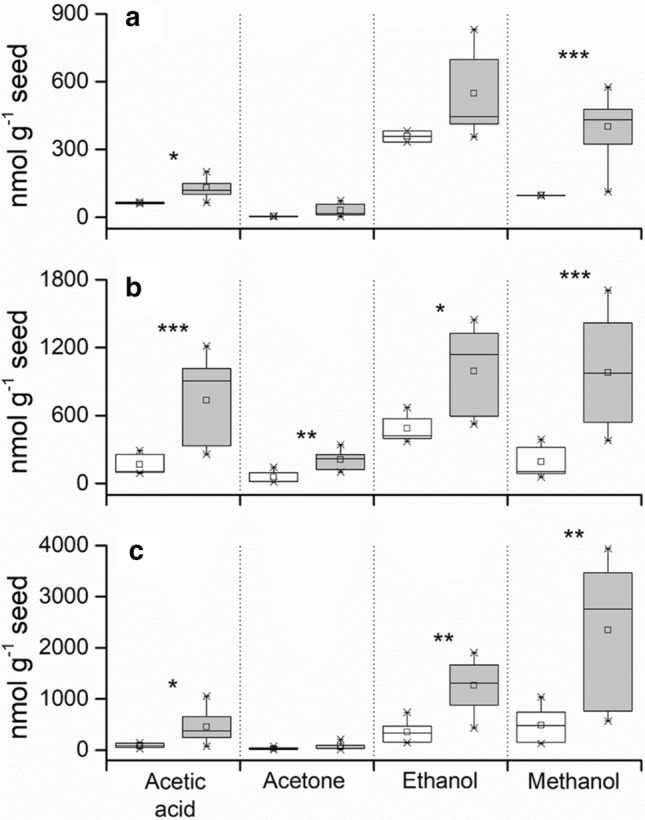
Box plots showing emission of acetic acid, acetone, ethanol and methanol during ageing of control seeds of Agrostemma githago (a), Lolium perenne (b), Pisum sativum (c). White boxes represent seeds with > 85% total germination (> 70% for Lolium perenne), and grey boxes represent seeds with < 85% total germination (< 70% for Lolium perenne). The boxes span the 25th to 75th percentiles, with the median and mean represented by a line and square, respectively. Whiskers show the minimum and maximum values. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) in the emission of individual volatiles from seeds with high (> 85% or 70%) and low (< 85% or 70%) germination determined using a two-sample, two-tailed t-test
PCA based on 26 VOC and germination variables separated the three species and the four storage treatments. The first four factors explained 70% of the observed variation (Suppl. Table S2). The first factor (F1) accounted for 36% of the variation. The variables with the highest loading in F1 were methyl acetate, 2-methyl-1-propanol, 2-methyl-1-butanol, 1-butanol, 2-butanol, 2-pentanone, 2-ethylfuran and 2-methylfuran. The second factor (F2) accounted for 17% of the variation, and total VOC, ethanol, methanol, and acetic acid emission were the variables with the highest loading in F2 (see Supplementary Fig. S2). F3 and F4 explained 10% and 7% of the observed variation, respectively. Total germination and normal germination showed the greatest correlation with F4 (Table 4). Species and treatments were projected as supplementary variables onto the PCA space defined by F1 and F2. F1 separated the control treatment from the other three treatments, with the control treatment centred towards greater VOC emission. A. githago showed the greatest separation from L. perenne in F2 (Supplementary Fig. S2). This corresponded to greater emission of acetaldehyde and ethanol from A. githago seeds aged for 1 d in the control and Silica treatments, whilst aged, control L. perenne seeds with declining or no germinability were characterised by higher emission of furans, branched alcohols, 2-butanone and 2-pentanone.
Table 4.
Correlations between 26 variables and the first 6 factors (PCA axes) for germination and VOC emission for Agrostemma githago, Lolium perenne and Pisum sativum across all 4 storage treatments
| Variables | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Total germination | − 0.336 | 0.276 | − 0.199 | 0.813 | 0.040 | − 0.041 |
| Normal germination | − 0.315 | 0.182 | − 0.288 | 0.798 | 0.018 | − 0.097 |
| Acetaldehyde | 0.112 | 0.447 | − 0.251 | − 0.135 | − 0.121 | − 0.342 |
| Dimethylsulfide | 0.060 | − 0.152 | 0.027 | − 0.537 | − 0.276 | − 0.168 |
| Propanal | 0.561 | 0.331 | 0.587 | 0.155 | − 0.003 | 0.178 |
| Acetone | 0.520 | 0.327 | − 0.264 | − 0.045 | 0.503 | − 0.163 |
| Methyl acetate | 0.853 | − 0.194 | 0.114 | − 0.082 | 0.221 | 0.123 |
| 2-Methylfuran | 0.744 | − 0.447 | − 0.216 | − 0.030 | 0.121 | − 0.145 |
| Ethyl acetate | 0.212 | − 0.081 | − 0.087 | − 0.217 | 0.775 | − 0.192 |
| Methanol | 0.439 | 0.746 | − 0.084 | − 0.094 | − 0.051 | 0.014 |
| 2-Butanone | 0.692 | − 0.362 | − 0.283 | 0.063 | 0.290 | − 0.119 |
| 2-Methylbutanal | 0.532 | 0.343 | 0.571 | 0.099 | − 0.035 | − 0.339 |
| 3-Methylbutanal | 0.584 | 0.352 | 0.600 | 0.118 | − 0.054 | − 0.276 |
| 2-Propanol | 0.708 | 0.142 | 0.555 | 0.075 | 0.043 | 0.057 |
| Ethanol | 0.308 | 0.763 | − 0.462 | − 0.086 | − 0.126 | − 0.132 |
| 2-Ethylfuran | 0.770 | − 0.366 | − 0.039 | 0.069 | − 0.360 | − 0.168 |
| 2-Pentanone | 0.783 | − 0.397 | − 0.282 | 0.161 | − 0.112 | − 0.065 |
| Acetonitrile | 0.130 | 0.436 | − 0.439 | − 0.217 | − 0.226 | 0.397 |
| 2-Butanol | 0.772 | 0.114 | 0.369 | 0.177 | − 0.019 | 0.299 |
| 1-Propanol | 0.578 | 0.343 | − 0.027 | − 0.118 | 0.117 | 0.302 |
| 2-Methyl-1-propanol | 0.844 | − 0.318 | − 0.118 | 0.100 | − 0.055 | 0.120 |
| 1-Butanol | 0.804 | − 0.352 | − 0.279 | 0.162 | 0.037 | 0.146 |
| 2-Methyl-1-butanol | 0.821 | − 0.376 | − 0.204 | 0.111 | − 0.111 | 0.167 |
| 2-Pentylfuran | 0.570 | − 0.414 | − 0.123 | 0.018 | − 0.445 | − 0.306 |
| Acetic acid | 0.647 | 0.622 | − 0.267 | 0.002 | − 0.052 | 0.040 |
| Total | 0.528 | 0.779 | − 0.262 | − 0.078 | − 0.072 | -0.064 |
| Eigenvalue | 9.241 | 4.468 | 2.662 | 1.913 | 1.548 | 1.047 |
| Variability (%) | 35.542 | 17.183 | 10.238 | 7.358 | 5.956 | 4.029 |
| Cumulative % | 35.542 | 52.725 | 62.963 | 70.321 | 76.277 | 80.306 |
Values in bold indicate variables with a high contribution to each factor. The eigenvalues, variability and cumulative variability for each factor are shown in the lower part of the table
Influence of volatile accumulation in the headspace on seed storage longevity
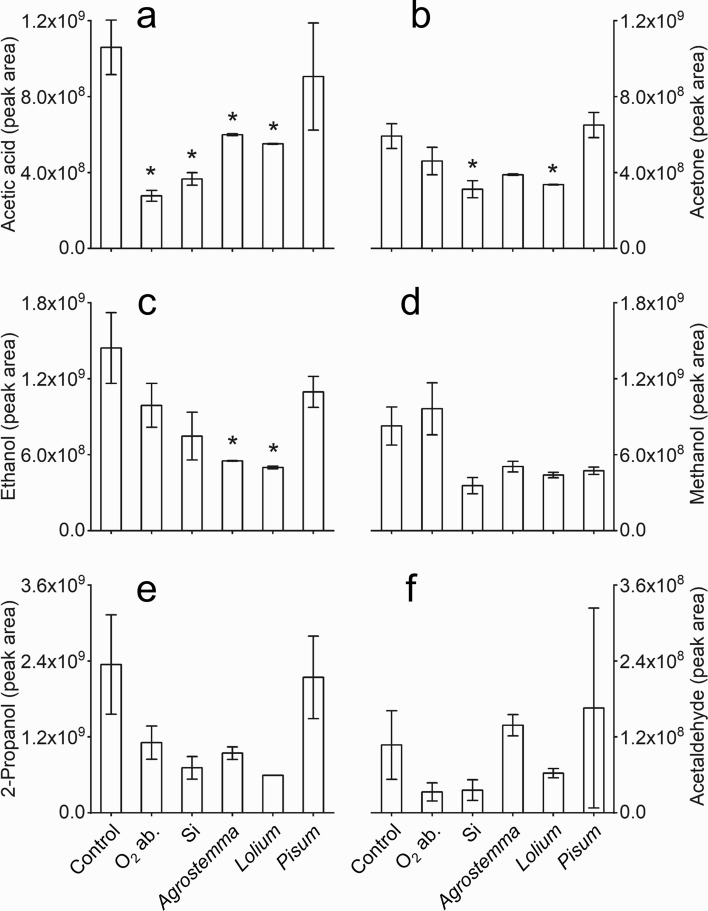
The adsorption of volatiles by O2 absorbers, silica, A. githago, L. perenne and P. sativum seeds was tested by comparing the abundance of acetic acid, acetone, ethanol, methanol, 2-propanol and acetaldehyde after 10 d incubation at 50 °C. Significantly lower levels of acetic acid were detected in vials containing O2 absorbers, silica, A. githago and L. perenne seeds (P < 0.05; Fig. 6a) whilst silica and L. perenne seeds significantly reduced the abundance of acetone (P < 0.05; Fig. 6b), and levels of ethanol were significantly reduced by A. githago and L. perenne seeds (P < 0.05; Fig. 6c). There were no significant effects on the abundance of the other volatile compounds, although methanol abundance appeared to be reduced by silica and seeds of all three species; and O2 absorbers, silica, A. githago and L. perenne seeds appeared to reduce levels of 2-propanol (Fig. 6).
Fig. 6.
The effect of oxygen absorbers, silica gel and P. sativum seeds on the detection of acetic acid (a), acetone (b), ethanol (c), methanol (d), 2-propanol (e) and acetaldehyde (f) in the headspace of 20 mL vials containing 0.6 μmol acetic acid, 90 nmol acetone, 1.25 μmol ethanol, 3 μmol methanol, 0.85 μmol 2-propanol, or 40 nmol acetaldehyde. Vials either contained only the volatile compound (control), or the volatile compound in addition to an O2 absorbing sachet (O2 ab.), a silica gel sachet (Si; equilibrated to 60% RH) or 1 g of A. githago, L. perenne or P. sativum seeds that had been equilibrated to 60% RH. All vials were incubated at 50 °C for 10 d. Bars represent the mean volatile emission ± SE. (n = 3). Asterisks denote significant differences (P < 0.05) in volatile abundance between the control vials and those containing O2 absorbers, silica or P. sativum seeds determined using one-way ANOVA and a post hoc Dunnett’s test
To test the effects of individual volatile compounds on seed germinability, P. sativum seeds were aged for 10 d in the presence of acetaldehyde, acetic acid, acetone, ethanol, methanol or 2-propanol. Only acetone and methanol had a significant effect, reducing total germination from 77% in 10 d aged controls to 43% and 20%, respectively (P < 0.05; Table 5).
Table 5.
Total germination of Pisum sativum seeds after ageing for 0 or 10 days at 50 °C and 60% RH in the presence of either a 20 cc oxygen absorber (O2 absorber), silica gel sachet, acetaldehyde, acetic acid, acetone, ethanol, methanol or 2-propanol
| Aged (d) | Total germination (%) | ||
|---|---|---|---|
| Control | 0 | 100.0 ± 3.33 | |
| O2 absorbers | 0 | 100.0 ± 4.08 | |
| Silica | 0 | 93.3 ± 2.33 | |
| Control | 10 | 76.7 ± 0.00 | c |
| O2 absorbers | 10 | 76.7 ± 14.53 | c |
| Silica | 10 | 73.3 ± 12.02 | bc |
| Acetaldehyde | 10 | 80.0 ± 18.56 | c |
| Acetic acid | 10 | 66.7 ± 13.33 | bc |
| Acetone | 10 | 43.3 ± 1.11 | ab |
| Ethanol | 10 | 60.0 ± 8.16 | bc |
| Methanol | 10 | 20.0 ± 5.88 | a |
| 2-Propanol | 10 | 66.7 ± 13.47 | bc |
Values are means ± SE of 3 replicates. Different letters indicate significant differences (P < 0.05) between treatments for seeds aged for 10 d determined using ANOVA and a post hoc Tukey’s test
Discussion
The three species are phylogenetically distant, L. perenne belongs to the Poaceae family within the monocotyledons, P. sativum belongs to Leguminosae within the Rosid clade and A. githago belongs to the Caryophyllaceae family within the order Caryophyllales, which is considered to be sister to the Asterids. They also differ in seed structure; the seeds of both L. perenne and A. githago are endospermic, whilst those of P. sativum are non-endospermic (Fig. 1). Endospermic seeds have been reported to be shorter lived than non-endospermic seeds (Probert et al. 2009) and the results obtained in this study follow this pattern with seeds of P. sativum being the longest lived with a P50 of 16 d compared to 2.8 d and 5 d for A. githago and L. perenne, respectively (Table 2). Similarly, P. sativum was classified as long-lived under ambient (P50 = 15.9 y) and cold (P50 = 97 y) storage conditions, whilst L. perenne was classified as medium-lived with P50 values of 7.2 y and 41 y under ambient and cold storage conditions, respectively (Colville and Pritchard 2019).
Despite the differences in phylogeny and seed structure, the volatile profiles of the three species were very similar, with the major volatiles being methanol, ethanol, acetic acid, 2-propanol and acetone. Methanol, ethanol and acetone have also been widely reported in other studies of seed volatile production during storage (Buckley and Buckley 2009; Mira et al. 2010, 2016; Colville et al. 2012). This indicates that the major volatile compounds are derived from core processes occurring in seeds during artificial ageing, and probably not greatly influenced by the chemical composition or structure of seeds. However, the oil content of the seeds of all three species was fairly low at 0.7% for P. sativum, 1.5% for L. perenne, and 4.4% for A. githago, as determined by time domain-nuclear magnetic resonance (TD-NMR), so the seeds are dominated by starchy reserves. Although lower in abundance, VOCs likely to derive from lipid peroxidation, such as alcohols (2-methyl-1-propanol, 2-methyl-1-butanol, and 2-propanol, etc.), ketones (2-butanone and 2-pentanone), and 2-methylfuran and 2-pentylfuran showed a significant, albeit weak negative correlation with germination (Supplementary Table S1). For oily seeds, it might be expected that a greater proportion of volatiles deriving from lipid peroxidation e.g. aldehydes would be emitted, although fermentation-type reactions have been shown to dominate in seeds with 10—32% oil content stored under humid conditions (75% RH) compared to drier (< 33% RH) conditions (Mira et al. 2016). Based on the mean seed moisture content and oil content of the seeds used in this study, the eRH at the elevated temperature of ageing (50 °C) was estimated using Royal Botanic Gardens, Kew’s Seed Information Database (2020). The eRH was estimated to be 76, 61 and 69% for A. githago, L. perenne, P. sativum, respectively, which means that during ageing at 50 °C the seeds are close to or just over the eRH threshold at which mitochondrial respiration can occur.
Storage of seeds under anoxia has been shown to increase longevity. Celery and celeriac seeds stored in sealed jars at 35 °C for 17 d with an oxygen absorber and zeolite Drying Beads™ showed 97% and 85% normal germination, respectively, compared to 0% and 23% when stored without oxygen absorbers or Drying Beads™ (Groot et al. 2015). In that experiment, storage with either an oxygen absorber or Drying Beads™ alone did not improve longevity, which was likely due to the moisturisation effect of the oxygen absorbers, and the ultra-drying effect of the Drying Beads™, respectively. In this study, neither the oxygen absorbers nor silica gel had any significant effect on the moisture content of the seeds (Table 1). This suggests that the moisturising agent in the O-Buster O2 absorbers did not release sufficient excess water to affect the moisture content of the seeds, which had been equilibrated at 60% RH prior to ageing. Perhaps this could be an issue for seeds at lower moisture content, and in this case, alternative oxygen absorbers, which function at low RH without requiring water for absorption of oxygen should be used. The silica gel had no drying effect on the seeds because it had been equilibrated at 60% RH along with the seeds prior to the ageing experiments. The incubation of silica gel with O2 absorbers had no effect on the eRH of the silica gel, confirming that the O2 absorbers did not cause measurable changes in the moisture status within the sealed vials. The life span (P50) of A. githago seeds was significantly increased by the presence of O2 absorbers (69% increase), silica (81% increase) and O2 absorbers + silica (95% increase), whilst that of L. perenne was increased by O2 absorbers + silica (108% increase; Table 2). This indicates that the positive effects of the treatments on life span are not solely due to reduction of oxygen levels. Volatile emission during ageing was monitored, and it was shown that ageing in the presence of O2 absorbers and/or silica tended to reduce total volatile emission by around twofold, particularly at the later ageing time points (Table 3). Since none of the treatments had any effect on seed moisture content, and silica treatment did not influence oxygen levels in the storage containers it is likely that the reduction in the abundance of volatiles detected is at least partly due to adsorption of seed-derived volatiles by the silica gel and possibly also the O2 absorbers. This was tested by incubating the silica gel sachets and O2 absorbers in sealed vials containing either methanol, ethanol, acetic acid, acetone, 2-propanol, or acetaldehyde at 50 °C for 10 d. There was a fairly high degree of variability in the data, probably because of the volatility of compounds and therefore the difficulty of minimising losses during preparation of the samples. However, significant reduction of acetic acid by O2 absorbers and silica, and acetone by silica, was observed. Although not significant, 2-propanol levels appeared to be reduced by O2 absorbers, and 2-propanol and methanol were detected at lower levels in the presence of silica (Fig. 6). This indicates that silica, and O2 absorbers to a lesser degree, could adsorb volatiles released by seeds during artificial ageing. The O2 absorbers contain zeolites, which along with silica gel have been found to adsorb VOCs. A comparison of the adsorption of a range of VOCs by silica gel, zeolites and activated charcoal showed that silica gel was the most adsorbent overall, and acetic acid was the VOC that was most strongly adsorbed by silica gel and zeolites (McGath et al. 2014).
Adsorption of exogenously applied volatile compounds by seeds was tested by incubating A. githago, L. perenne and P. sativum seeds with methanol, ethanol, acetic acid, acetone, 2-propanol or acetaldehyde at 50 °C for 10 d. A. githago and L. perenne seeds significantly reduced the levels of acetic acid and ethanol, and L. perenne also reduced the levels of acetone. There were no significant reductions in the abundance of volatiles detected in the presence of P. sativum seeds (Fig. 6). This implies that for P. sativum seeds under the conditions used in this study, adsorption of volatiles by the seeds is not a major factor contributing to viability loss. Although, it is possible that seeds are re-adsorbing some of the volatiles released during ageing and reaching saturation, preventing further adsorption of exogenously applied volatile compounds. The adsorption of acetic acid, ethanol and acetone by A. githago and/or L. perenne seeds, but not P. sativum seeds, could explain why the O2 absorber and silica gel treatments only had a beneficial effect on the lifespan of A. githago and L. perenne seeds. Mira et al. (2016) showed that the levels of volatiles released by mixtures of seeds were not equal to what would be expected from the sum of volatile profiles of the individual species, and proposed that this indicated that the seeds were adsorbing volatiles from the headspace of the storage container. However, they found no detrimental effect on longevity as a result of volatile adsorption, so concluded that volatile accumulation during seed storage did not contribute to seed deterioration.
In this study, the total germination of P. sativum seeds artificially aged for 10 d in the presence of exogenously applied acetone or methanol was significantly decreased compared to control seeds, but ethanol, acetic acid, 2-propanol and acetaldehyde had no effect. This suggests that some seed-derived compounds including acetone and methanol may be toxic and accumulation within seed storage containers has the potential to shorten seed longevity. Acetone was also shown to be toxic when pea seeds were soaked in acetone and then subjected to accelerated ageing (100% RH and 40 °C for 4 d) or controlled deterioration (20% MC, 40 °C for 4 d). Susceptibility was dependent on MC and was more apparent after ageing, particularly in seeds subjected to controlled deterioration (Coolbear et al. 1991). However, the toxicity of volatile compounds may be species-dependent and influenced by storage conditions. Zhang et al. (1994) also reported that some exogenously applied volatile compounds were toxic to seeds, but in contrast to our results, they found that methanol had no deleterious effects, whilst acetone reduced germination of rice seeds and soybean seeds stored at 23 °C, but had no effect on lettuce or sunflower seeds. In their study, acetaldehyde and acetic acid were the most toxic across all storage conditions and all four species, ethanol was toxic only at higher RH conditions, and 2-propanol only affected rice and soybean seeds. They applied the volatile treatments at a concentration of 4 mM for all except acetaldehyde which was 1 mM. In contrast, the concentrations used in this study ranged from 2 μM for acetaldehyde to 150 μM for methanol. We attempted to use physiologically relevant concentrations of volatile compounds, i.e., treatments were applied at levels similar to those released by P. sativum seeds with moisture content of 11.9% during ageing at 50 °C, so the ‘naturally’ low concentrations used could explain why we did not observe any toxic effects of acetaldehyde, acetic acid, ethanol and 2-propanol.
The rate of volatile release from seeds depends upon the rate of volatile formation and the rate of diffusion from the site of formation to the exterior of the seed. The latter is determined by the properties of the seed matrix and the volatile compound and the diffusion distance. Oxygen diffusion can occur in cellular glasses, such as those found in dry seeds (El-Maarouf-Bouteau and Bailly 2008). Intercellular spaces are the main path for oxygen diffusion within seeds (Cloetens et al. 2006). Micro-CT images of a P. sativum seed (Fig. 1d and e) showed intercellular spaces, particularly in the cotyledons, indicating that gas diffusion via intercellular spaces may vary between different seed tissues.
Cuticular layers are a barrier to oxygen uptake/release (Borisjuk and Rolletschek 2009). Cuticles are a hydrophobic layer comprising cutin and cuticular waxes. Seeds may have multiple cuticles, which can be associated with the seed coat, and also surround the embryo and endosperm. They play a role in controlling water uptake/loss as well as gas diffusion (Ingram and Nawrath 2017). In developing seeds, the internal oxygen concentration is low (Borisjuk and Rolletschek 2009), and cuticles have been proposed to limit oxygen diffusion and thereby reactive oxygen species production to protect against oxidation and viability loss (De Giorgi et al. 2015). The cuticle also provides the greatest resistance to volatile emission (Widhalm et al. 2015). The composition and permeability of cuticles varies between species and organs and this may explain the different responses of the three species to ageing under hypoxia. The life span of A. githago seeds showed the greatest increase when aged with O2 absorbers, silica or O2 absorbers + silica. Perhaps the cuticle of A. githago is more permeable to oxygen and volatile compounds than that of L. perenne or P. sativum. This could explain the higher volatile emission rate from unaged A. githago seeds compared to unaged L. perenne and P. sativum seeds (Fig. 4a–c). Volatile emission from A. githago followed a different pattern to that of L. perenne and P. sativum seeds, and volatile emission rate was highest after 1 d of ageing, it then fell to its lowest level after 3 d of ageing before rising slightly after 4 d ageing. In contrast, L. perenne and P. sativum both showed a biphasic pattern of volatile emission, with an initial small increase in volatile emission rate after 5 d of ageing and then a larger increase after 15 d of ageing (Fig. 4). The peak in volatile emission rate after 15 d of ageing coincided with the onset of rapid viability loss in P. sativum and almost complete loss of viability in L. perenne (Fig. 3d and g). This could indicate that barriers to gas diffusion, e.g., cuticles were degraded during ageing leading to the large increase in volatile emission. The seeds were aged in sealed headspace vials and volatile analysis measured the accumulation of volatiles over the ageing time course. Therefore, decreases in volatile abundance indicate that the volatile compounds were being re-adsorbed by the seeds. In P. sativum seeds gas exchange is restricted mainly to the micropylar region (Wager 1974), although in dry P. sativum seeds the hilar slit is open (Fig. 1e) and may also be a path for gas diffusion. The seeds are also larger (Fig. 1) so diffusion of oxygen and volatile compounds into and out of the seeds will likely take longer than for A. githago and L. perenne seeds.
These experiments reveal that seed ageing can be slowed for some species by storage of seeds in an oxygen-free atmosphere. Furthermore, the volatile compounds released by seeds during storage may be re-adsorbed by seeds leading to toxic effects and loss of viability. Therefore, volatile traps such as silica gel may also be beneficial for extending seed life span in storage. There is variation between species in terms of their response to oxygen and in their temporal patterns of volatile emission, which may relate to differences in gas permeability due to seed size, structure and the presence of diffusion barriers such as cuticles. Future work will assess the influence of these seed traits on the potential for storage under anoxia to extend seed life span. Cuticles are considered to be the main barrier to oxygen diffusion, but the permeability of cuticles increases markedly at temperatures above 35 °C (Schreiber et al. 2001; Riederer 2006), so under the artificial ageing conditions used in this study the effect of any inter-specific differences in cuticle composition and permeability may be less marked than under lower temperature storage conditions. Therefore, the extent to which anoxia may extend seed life span may be even greater at lower temperatures such as those used for commercial storage (c. 12 °C) or gene bank storage (-20 °C).
Author contribution statement
Conceptualization: LC; Data curation: LC; Formal analysis: LC; Investigation: BH, VF, LC; Visualisation: LC; Writing–original draft: LC; Writing–review and editing: BH, VF, HWP, LC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Jack Rhodes for assistance with TD-NMR measurements, seed images and preliminary data, and Stefan Grossfurthner for assistance with setting up storage experiments. The Royal Botanic Gardens, Kew receives grant-in-aid from Defra.
Abbreviations
- eRH
Equilibrium relative humidity
- VOC
Volatile organic compound
Data availability statement
The data that support the findings of this study are available from the corresponding author upon request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Biao Han, Email: hanbiaook831228@163.com.
Vincent Fernandez, Email: v.fernandez@nhm.ac.uk.
Hugh W. Pritchard, Email: h.pritchard@kew.org
Louise Colville, Email: l.colville@kew.org.
References
- Bailly C. Active oxygen species and antioxidants in seed biology. Seed Sci Res. 2004;14:93–107. doi: 10.1079/SSR2004159. [DOI] [Google Scholar]
- Ballesteros D, Walters C. Detailed characterization of mechanical properties and molecular mobility within dry seed glasses: relevance to the physiology of dry biological systems. Plant J. 2011;68:607–619. doi: 10.1111/j.1365-313X.2011.04711.x. [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H. The oxygen status of the developing seed. New Phytol. 2009;182:17–30. doi: 10.1111/j.1469-8137.2008.02752.x. [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H, Fuchs J, Melkus G, Neuberger T. Low and hugh field magnetic resonance for in vivo analysis of seeds. Materials. 2011;4:1426–1439. doi: 10.3390/ma4081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley W, Buckley K. Low-molecular-weight volatile indicators of canola seed deterioration. Seed Sci Technol. 2009;37:676–690. doi: 10.15258/sst.2009.37.3.15. [DOI] [Google Scholar]
- Buitink J, Leprince O, Hemminga MA, Hoekstra FA. Molecular mobility in the cytoplasm: an approach to describe and predict lifespan of dry germplasm. Proc Natl Acad Sci USA. 2000;97:2385–2390. doi: 10.1073/pnas.040554797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Osuna D, Colville L, Lorenzo O, Graeber K, Kuester H, Leubner-Metzger G, Kranner I. Transcriptome-wide mapping of pea seed ageing reveals a pivotal role for genes related to oxidative stress and programmed cell death. PLoS One. 2013;8:e78471. doi: 10.1371/journal.pone.0078471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloetens P, Mache R, Schlenker M, Lerbs-Mache S. Quantitative phase tomography of Arabidopsis seeds reveals intercellular void network. Proc Natl Acad Sci USA. 2006;103:14626–14630. doi: 10.1073/pnas.0603490103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville L, Pritchard HW. Seed life span and food security. New Phytol. 2019;224:557–562. doi: 10.1111/nph.16006. [DOI] [PubMed] [Google Scholar]
- Colville L, Bradley EL, Lloyd AS, Pritchard HW, Castle L, Kranner I. Volatile fingerprints of seeds of four species indicate the involvement of alcoholic fermentation: lipid peroxidation: and Maillard reactions in seed deterioration during ageing and desiccation stress. J Exp Bot. 2012;63:6519–6530. doi: 10.1093/jxb/ers307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolbear P, McGill CR, Sakunnarak N. Susceptibility of pea seeds to acetone toxicity: interactions with seed moisture content and ageing treatments. Seed Sci Technol. 1991;19:519–526. [Google Scholar]
- Costanzo E, Gulino M, Lanzanò L, Musumeci F, Scordino A, Tudisco S, Sui L. Single seed viability checked by delayed luminescence. Eur Biophys J. 2008;37:235–238. doi: 10.1007/s00249-007-0221-8. [DOI] [PubMed] [Google Scholar]
- De Giorgi J, Piskurewicz U, Loubery S, Utz-Pugin A, Bailly C, Mène-Saffrané L, Lopez-Molina L. An endosperm-associated cuticle is required for Arabidopsis seed viability: dormancy and early control of germination. PLoS Genet. 2015;11:e1005708. doi: 10.1371/journal.pgen.1005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie JB, Ellis RH, Kraak HL, Ryder K, Tompsett PB. Temperature and seed storage longevity. Ann Bot. 1990;65:197–204. doi: 10.1093/oxfordjournals.aob.a087924. [DOI] [Google Scholar]
- Ellis RH, Hong TD. Seed longevity. Moisture content relationships in hermetic and open storage. Seed Sci Technol. 2007;35:423–431. doi: 10.15258/sst.2007.35.2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RH, Roberts EH. Improved equations for the prediction of seed longevity. Ann Bot. 1980;45:13–30. doi: 10.1093/oxfordjournals.aob.a085797. [DOI] [Google Scholar]
- Ellis RH, Hong TD, Roberts EH, Tao KL. Low moisture content limits to relations between seed longevity and moisture. Ann Bot. 1990;65:493–504. doi: 10.1093/oxfordjournals.aob.a087961. [DOI] [Google Scholar]
- El-Maarouf-Bouteau H, Bailly C. Oxidative signaling in seed germination and dormancy. Plant Signal Behav. 2008;3:175–182. doi: 10.4161/psb.3.3.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . Genebank standards for plant genetic resources for food and agriculture. Rome: FAO; 2014. [Google Scholar]
- Geigenberger P. Response of plant metabolism to too little oxygen. Curr Opin Plant Biol. 2003;6:247–256. doi: 10.1016/S1369-5266(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Groot SPC, De Groot L, Kodde J, Van Treuren R. Prolonging the longevity of ex situ conserved seeds by storage under anoxia. Plant Genet Resour. 2015;13:18–26. doi: 10.1017/S1479262114000586. [DOI] [Google Scholar]
- Hay FR, Whitehouse KJ. Rethinking the approach to viability monitoring in seed genebanks. Conserv Physiol. 2017;5:cox009. doi: 10.1093/conphys/cox009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AE, Roberts EH. Viability of lettuce seeds: I. Survival in hermetic storage. J Exp Bot. 1983;34:620–630. doi: 10.1093/jxb/34.5.620. [DOI] [Google Scholar]
- Ibrahim AE, Roberts EH, Murdoch AJ. Viability of lettuce seeds: II. Survival and oxygen uptake in osmotically controlled storage. J Exp Bot. 1983;34:631–640. doi: 10.1093/jxb/34.5.631. [DOI] [Google Scholar]
- Ingram G, Nawrath C. The roles of the cuticle in plant development: organ adhesions and beyond. J Exp Bot. 2017;68:5307–5321. doi: 10.1093/jxb/erx313. [DOI] [PubMed] [Google Scholar]
- Kranner I, Birtić S, Anderson KM, Pritchard HW. Glutathione half-cell reduction potential: a universal stress marker and modulator ofprogrammed cell death? Free Radic Biol Med. 2006;40:2155–2165. doi: 10.1016/j.freeradbiomed.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Kranner I, Kastberger G, Hartbauer M, Pritchard HW. Noninvasive diagnosis of seed viability using infrared thermography. Proc Natl Acad Sci USA. 2010;107:3912–3917. doi: 10.1073/pnas.0914197107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranner I, Chen H, Pritchard HW, Pearce SR, Birtić S. Inter-nucleosomal DNA fragmentation and loss of RNA integrity during seed ageing. Plant Growth Regul. 2011;63:63–72. doi: 10.1007/s10725-010-9512-7. [DOI] [Google Scholar]
- Lee PC, Taylor AG, Zhang M, Esashi Y. Volatile compounds and accumulation of acetaldehyde-protein adducts in relation to seed quality and storage conditions. J New Seed. 2001;2:59–76. doi: 10.1300/J153v02n01_04. [DOI] [Google Scholar]
- Martin AC. The comparative internal morphology of seeds. Am Midl Nat. 1946;36:513–660. doi: 10.2307/2421457. [DOI] [Google Scholar]
- McGath M, McCarthy B, Bosworth J. Environmental monitoring of volatile organic compounds using silica gel: zeolite and activated charcoal. MRS Proc. 2014;1656:51–72. doi: 10.1557/opl.2014.812. [DOI] [Google Scholar]
- Mira S, González-Benito ME, Hill LM, Walters C. Characterization of volatile production during storage of lettuce (Lactuca sativa) seed. J Exp Bot. 2010;61:3915–3924. doi: 10.1093/jxb/erq202. [DOI] [PubMed] [Google Scholar]
- Mira S, Hill LM, González-Benito ME, Ibáñez MA, Walters C. Volatile emission in dry seeds as a way to probe chemical reactions during initial asymptomatic deterioration. J Exp Bot. 2016;67:1783–1793. doi: 10.1093/jxb/erv568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Vogel H, Landjeva S, Buck-Sorlin G, Lohwasser U, Scholz U, Börner A. Seed conservation in ex situ genebanks-genetic studies on longevity in barley. Euphytica. 2009;170:5–14. doi: 10.1007/s10681-009-9975-7. [DOI] [Google Scholar]
- Paulsen TR, Colville L, Kranner I, Daws MI, Högstedt G, Vandvik V, Thompson K. Physical dormancy in seeds: a game of hide and seek? New Phytol. 2013;198:496–503. doi: 10.1111/nph.12191. [DOI] [PubMed] [Google Scholar]
- Probert RJ, Daws MI, Hay FR. Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Ann Bot. 2009;104:57–69. doi: 10.1093/aob/mcp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajjou L, Lovigny Y, Groot SPC, Belghazi M, Job C, Job D. Proteome-wide characterization of seed aging in Arabidopsis: a comparison between artificial and natural aging protocols. Plant Physiol. 2008;148:620–641. doi: 10.1104/pp.108.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer M. Thermodynamics of the water permeability of plant cuticles: characterization of the polar pathway. J Exp Bot. 2006;57:2937–2942. doi: 10.1093/jxb/erl053. [DOI] [PubMed] [Google Scholar]
- Roberts EH, Abdalla FH. The influence of temperature, moisture, and oxygen on period of seed viability in barley, broad beans, and peas. Ann Bot. 1968;32:97–117. doi: 10.1093/oxfordjournals.aob.a084202. [DOI] [Google Scholar]
- Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H. Legume embryos develop in a hypoxic environment. J Exp Bot. 2002;53:1099–1107. doi: 10.1093/jexbot/53.371.1099. [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Stangelmayer A, Borisjuk L. Methodology and significance of microsensor-based oxygen mapping in plant seeds: an overview. Sensors. 2009;9:3218–3227. doi: 10.3390/s90503218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal Botanic Gardens Kew (2020) Seed information database (SID). Version 7.1. http://data.kew.org/sid/. Accessed 19 Aug 2020
- Rutzke CFJ, Taylor AG, Obendorf RL. Influence of aging: oxygen: and moisture on ethanol production from cabbage seeds. J Am Soc Hortic Sci. 2008;133:158. doi: 10.21273/JASHS.133.1.158. [DOI] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L, Skrabs M, Hartmann KD, Diamantopoulos P, Simanova E, Santrucek J. Effect of humidity on cuticular water permeability of isolated cuticular membranes and leaf disks. Planta. 2001;214:274–282. doi: 10.1007/s004250100615. [DOI] [PubMed] [Google Scholar]
- Sun WQ. Glassy state and seed storage stability: the WLF kinetics of seed viability loss at T> T g and the plasticization effect of water on storage stability. Ann Bot. 1997;79:291–297. doi: 10.1006/anbo.1996.0346. [DOI] [Google Scholar]
- Tompsett PB. The influence of gaseous environment on the storage life of Araucaria hunsteinii seed. Ann Bot. 1983;52:229–237. doi: 10.1093/oxfordjournals.aob.a086568. [DOI] [Google Scholar]
- Wager HG. The effect of subjecting peas to air enriched with carbon dioxide I. The path of gaseous diffusion, the content of CO2 and the buffering of the tissue. J Exp Bot. 1974;25:330–337. doi: 10.1093/jxb/25.2.330. [DOI] [Google Scholar]
- Walters C, Wheeler LM, Grotenhuis JM. Longevity of seeds stored in a genebank: species characteristics. Seed Sci Res. 2005;15:1–20. doi: 10.1079/SSR2004195. [DOI] [Google Scholar]
- Wettlaufer SH, Leopold AC. Relevance of Amadori and Maillard products to seed deterioration. Plant Physiol. 1991;97:165–169. doi: 10.1104/pp.97.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widhalm JR, Jaini R, Morgan JA, Dudareva N. Rethinking how volatiles are released from plant cells. Trends Plant Sci. 2015;20:545–550. doi: 10.1016/j.tplants.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Xin X, Wan Y, Wang W, Yin G, McLamore ES, Lu X. A real-time: non-invasive: micro-optrode technique for detecting seed viability by using oxygen influx. Sci Rep. 2013;3:3057. doi: 10.1038/srep03057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Maeda Y, Furihata Y, Nakamaru Y, Esashi Y. A mechanism of seed deterioration in relation to the volatile compounds evolved by dry seeds themselves. Seed Sci Res. 1994;4:49–56. doi: 10.1017/S0960258500001999. [DOI] [Google Scholar]
- Zhang M, Nakamaru Y, Tsuda S, Nagashima T, Esashi Y. Enzymatic conversion of volatile metabolites in dry seeds during storage. Plant Cell Physiol. 1995;36:157–164. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.