Abstract
Background and aim
COVID-19 is a global public health concern. As no standard treatment has been found for it yet, several minerals and vitamins with antioxidants, immunomodulators, and antimicrobials roles can be sufficient for the immune response against the disease. The present study evaluates the serum vitamin D, calcium, and Zinc levels in patients with COVID-19.
Materials & methods
This research is a case–control study performed in May 2020 on 93 patients with COVID-19 hospitalized in a Shoushtar city hospital and on 186 healthy subjects with no symptoms of COVID-19. The serum vitamin D, calcium, and zinc levels were collected and analyzed using correlation coefficient and independent t-test via SPSS 18.
Results
Vitamin D levels had a significant difference between the case and control groups (p = 0.008). Serum calcium and serum zinc levels also had statistically significant differences between the two groups (p < 0.001).
Conclusion
The research results showed that serum zinc, calcium, and vitamin D levels in COVID-19 patients are lower than in the control group. The supplementation with such nutrients is a safe and low-cost measure that can help cope with the increased demand for these nutrients in risk of acquiring the COVID-19 virus.
Keywords: COVID-19, Vitamin D, Calcium, Zinc
1. Introduction
COVID-19 is a global public health concern [1]. The outbreak of pneumonia from an unknown cause in Wuhan, Hubei province of China, in December 2019 prompted the World Health Organization (WHO) to announce the prevalence of the novel coronavirus as the sixth cause of public health emergency worldwide on 11 January 2020 as a threat not only for China but also for all countries [[2], [3], [4]]. The WHO has presented this previously unknown virus as a mutated form of coronavirus unknown previously and called 2019-nCOV [5]. Some minerals and vitamins have antioxidant, immunomodulatory, and antimicrobial roles. They can help the immune response against the SARS-CoV-2 virus considering the absence of any standard treatment for the novel coronavirus [6]. Supplementation of micronutrients emerges as an essential measure to improve the immune system and prevent the development of severe symptoms. Some of these micronutrients are vitamins, D, and minerals such as calcium and zinc [[7], [8], [9]]. Vitamin D deficiency is regarded as a significant public health problem. Recent studies have shown that depletion of these supplements has emerged worldwide [6,10,11]. In this regard, studies show that hypocalcemia and vitamin D deficiency increases the risk of respiratory infections. The available data regarding vitamin D effects show that vitamin D deficient individuals are more likely to experience diseases including pediatric tuberculosis, acute media otitis, and acute bronchitis [12].
The role of calcium and vitamin D has attracted attention as immunomodulators [13]. The active form of vitamin D is a potent immunomodulator. Vitamin D receptor exists on many cells of the body's immune system, including T lymphocytes, macrophages, and dendritic cells. There is extensive evidence suggesting that 1–25 dihydroxy vitamin D3 has various effects on the immune system. Hence, it enhances immunity and reduces autoimmunity [14]. Vitamin D directly interacts with the cells responsible for fighting infection. Accordingly, researchers have concluded that vitamin D deficiency can increase the risk viral infections. The molecular functional mechanism is based on the interference between the signaling pathway of 1-alpha-25-dihydroxy vitamin D3 with other growth hormone factors, contributing to proliferation, differentiation, and viability of cells [15]. Studies in this regard, such as Cristiano et al. (2020), indicated that vitamin D3 has various useful effects, including the body's human system to coat the glycoprotein envelope of the viral protein confirmed its role in COVID-19 [16]. The results of William et al. (2020) suggested the significance of vitamin D for these patients [17]. Calcium is an essential mineral element involved in normal respiratory functioning, energy generation, immunity strength, nerve conduction, blood coagulation, regulating heart rate, secretion of hormones, enzymes, and contraction of muscles [18]. Shai et al. (2020) reported a high prevalence of hypocalcemia in patients with coronavirus. Thus, they propounded this hypothesis of serum calcium level is associated with the severity and prognosis of the disease in patients with COVID-19 [19].
Zinc also plays an essential catalytic role in a wide range of enzymes - considering the developed structure - and is a critical component in the immune system's antioxidant activity. It also plays a substantial role in controlling and preventing infection [20]. By inhibiting the protease enzyme of rhinovirus, Zinc prevents its replication [21]. Zinc supplement in children significantly reduces the prevalence of pneumonia [22,23]. It is still unknown how zinc exerts its antiviral effects; Zinc can inhibit viral attachment while suppressing inflammatory events [24,25].
Meanwhile, the most critical role of Zinc in the human system body has been shown. Briefly, Zinc regulates the proliferation, differentiation, maturity, and function of leukocytes and lymphocytes [26]. Mahmudian et al. (2017) showed that Zinc causes a reduced incidence of upper respiratory infections [27]. The studies performed on the factors affecting this newly emerging disease are minimal, and currently, no research has been conducted in our country. Concerning the epidemics and high mortality of these viral diseases, and for paying more attention to the role of vitamin D, calcium, and Zinc in boosting the immune system to lower the incidence of disease, the present case–control study aims at investigating the serum vitamin D, calcium, and Zinc levels in patients with COVID-19 in Shoushtar city.
2. Materials and methods
2.1. Methods and type of study
This research is a case–control study performed in 2020 to investigate the serum vitamin D, calcium, and Zinc levels in patients with COVID-19 and healthy individuals after acquiring the necessary permissions from Shoushtar faculty of medical sciences. After receiving the required approval for data collection, the researcher referred to the centers of interest. After explaining the research objectives and gaining patients' consent and a written informed consent form from the subjects, he performed the sampling. This study was conducted from 13 May to 30 May on 93 patients with COVID-19 disease hospitalized in Khatam-al- Anbya Hospital of Shoushtar city (A city in southwestern Iran), and 186 healthy subjects had no symptom of COVID-19.
2.2. Patients and data collection
Considering the research objectives, previous studies [19], and based on the parameters of respiratory distress in patients with COVID-19, in the two groups, normal serum calcium level and low serum calcium level, the ratio of individuals with respiratory problems in the two groups P1 = .06 and P2 = .016, and considering α = 0.9, β = .05, and d = .08, and using the formula of the sample size ratio for the case group, the sample size was estimated as 93 for the case group. Then, we considered 1.5 times as large (n = 140) for the control group. The nasal swab sample or throat swab was used for nucleic acid detection of SARS-CoV-2 using real-time reverse transcriptase-polymerase chain reaction (RT-PCR). The healthy individuals were added to the plan to know the serum vitamin D, calcium, and Zinc levels in the research's general population and compare with the target group. The sampling method for the case group was purposeful out of patients with COVID-19 referring to Khatam-al- Anbya Hospital in Shoushtar city. Meanwhile, the control group's sampling method was also random from among healthy individuals referring to healthcare centers of Shoushtar city. The control group was matched against the treatment group regarding gender and age (±2 years).
Blood samples were taken by a nurse (a colleague in the research plan) and were sent to a private laboratory. The individuals were selected through simple randomized sampling from the list of names To investigate healthy individuals upon referral to healthcare centers. They were then contacted, after which they received an explanation about the plan and then signed a written informed consent form. They were then invited to take blood samples for laboratory results (serum vitamin D, Zinc, and calcium levels).
The inclusion criteria for the healthy group are as follows:
-
1.
Having Iranian nationality and residence in Shoushtar city;
-
2.
Having no COVID-19 symptom;
-
3.
the person should have been quarantined and had no contact with patients over the past week;
-
4.
having no record of affliction with COVID-19;
-
5.
Being consent to participate in the study;
-
6.
Being above 15 years old; and
-
7.
fasting at the time of sampling.
The exclusion criteria for the patient and healthy groups are as follows:
-
1.
Being resident outside Shoushtar city;
-
2.
Having no consent;
-
3.
consuming vitamin D, calcium, and Zinc supplements over the past 24 h;
-
4.
consuming corticosteroids, cholesterol-reducing drugs such as cholestyramine, barbiturates, and phenytoin (which can cause reduced vitamin D, calcium, and Zinc levels in the body);
-
5.
being with parathyroid disease, bone disease, chronic liver disease, kidney disease, and cancer; and
-
6.
Undergoing treatment with vitamin D, calcium, and Zinc.
2.3. Clinical and laboratory results
In this study, we measured serum levels of vitamin D, calcium, and zinc. Serum vitamin D levels were measured by ELISA (Enzyme-Linked Immunosorbent Assay). Vitamin D levels were divided into 30ng/ml-100 ng/ml as sufficient, 10 ng/ml −30 ng/ml as insufficient, and <10 ng/ml as deficient. Calcium Arsenazo measured serum calcium levels with a reference range of 8.6–10.3 mg/dl. Serum zinc levels were measured by (AAS) Atomic Absorption Spectrophotometry with a reference range of 70–127 μg/dl.
Data such as age; gender; place of residence; consuming calcium, zinc, and vitamin D supplements; a record of the underlying diseases such as diabetes, hypertension, cardiovascular disease, pulmonary disease, hyperlipidemia; possible symptoms and complications of the disease such as cough, dyspnea, fever, muscle weakness and pain, nausea and vomiting, headache, business, abdominal pain, convulsion, chest pain, tachycardia, blurred vision, diarrhea, restlessness, agitation, diminished sense of smell, etc.; and laboratory results of serum vitamin D, calcium, zinc, Blood Urea Nitrogen (Bun), Creatinine (Cr), Sodium (Na), Potassium (K), White Blood Cell (Wbc), Red Blood Cell (Rbc), Hemoglobin [28], Hematocrit (Hema), Platelet (Plt), Lymphocyte (Lym), Neutrophils (Neut), Arterial Blood Gas Test (Ph, Po2, Pco2, Hco3), Erythrocyte Sedimentation Rate (Esr), Blood Sugar (Bs) levels were recorded.
2.4. Statistical analysis
The results of blood tests and demographic and clinical information of the subjects were introduced into SPSS v 18. Quantitative variables have been reported as mean (median), standard deviation (quartile median range), minimum and maximum, while qualitative variables were reported as a percentage. The Kolmogorov–Smirnov test checked the normality of quantitative variables. The Pearson correlation coefficient test investigated the relationship between quantitative variables (in non-normality, Spearman correlation coefficient test was used). An independent t-test compared the means (in case of nonnormality of variables, Mann–Whitney test was employed). ANOVA compared the level of variables (in the nonnormality of variables, the Kruskal–Wallis test was used). Finally, a ROC diagram was applied to test the test's effectiveness in differentiating between healthy and diseased subjects. The significance level of the above tests was considered as less than .05. The significance level of the above tests was viewed as less than .05.
3. Results
In this study, 93 patients with COVID-19 were confirmed as a case group, and 186 healthy individuals with no COVID-19 symptoms were included as the control group.
The average age of the patients was 51 years (40–61, IQR), 52 (55.9%) of them were women. The average duration of hospitalization was 4 days (3–5 days, IQR). Almost 40% (n = 37) patients presented a severe form of COVID-19 disease with acute lung injury. Clinical comorbidities in the patients were as follows: 16.1% patients (n = 15) had diabetes, 10.8% (n = 10) had hypertension, 8.6% (n = 8) had pulmonary, and 21.5% (n = 20) had cardiovascular diseases. Disease symptoms in patients are as follows: 29.0% (n = 27) had fevers, 60.2% (n = 56) had a cough, 40.9% (n = 38) had dyspnea, 19.4% (n = 18) had muscle pain, 11.8% (n = 11) had headaches, 19.4%ones (n = 18) had anorexia, 12.9% (n = 12) had fatigue, 6.5% (n = 6) had a sore throat, 8.6% (n = 8) had digestive symptoms, and 2.2% (n = 2) suffered from a diminished smelling capacity (Table 1 ).
Table 1.
Demographic characteristics, underlying diseases, symptoms.
| Characteristics | Total (n = 93) | |
|---|---|---|
| Age, median (IQR), years | 51 (40–61) | |
| Gender | Male | 41 (44.1%) |
| Female | 52 (55.9%) | |
| Underlying diseases | Diabetes | 15 (16.1%) |
| Hypertension | 10 (10.8%) | |
| Pulmonary diseases | 8 (8.6%) | |
| Cardiovascular diseases | 20 (21.5%) | |
| Disease symptoms | Fever | 27 (29.0%) |
| Cough | 56 (60.2%) | |
| Dyspnea | 38 (40.9%) | |
| Muscle pain | 18 (19.4%) | |
| Headache | 11 (11.8%) | |
| Anorexia | 18 (19.4%) | |
| Fatigue | 12 (12.9%) | |
| Sore throat | 6 (6.5%) | |
| Digestive symptoms | 8 (8.6%) | |
| A diminished sense of smell | 2 (2.2%) | |
There was no significant difference between the patient and control groups in terms of age (p = 0.12). The number of men and women had no significant difference either between the two groups (p = 0.33).
In the blood serum of patients, 73% (n = 69), 42% (n = 39), and 52% (n = 49) had vitamin D, calcium, and Zinc deficiency respectively.
Vitamin D levels had a significant difference between the case and controls group (p = 0.008) it is interesting that both (case and controls) have lower vitamin D levels. The calcium serum level had a statistically significant difference between the two groups (p < 0.001) but the mean value it is between the normal range. ultimately, the serum Zinc level had a significant difference between the two groups (p < 0.001) its value in covid patients is pathological and it is normal in controls group (Table 2 ).
Table 2.
Serum vitamin D, calcium, and Zinc levels in the case and control groups.
| Serum levels (reference range) | Case | control | p-value t-test |
|---|---|---|---|
| Vitamin D (30–100 ng/ml) | 22.83 ± 12.97 | 27.50 ± 15.35 | .008 |
| Calcium (8.6–10.3 mg/dl) | 9.14 ± .39 | 9.50 ± .52 | <.001 |
| Zinc (70–127 μg/dl) | 67.61 ± 15.10 | 86.66 ± 11.76 | <.001 |
The serum vitamin D level had no significant difference between the patient and case groups (p = 0.625). However, this difference was significant across women (p < 0.001). The serum calcium level significantly differed between patient and healthy groups among men and women (p < 0.001). The serum Zinc level had a significant difference between patient and control groups among men and women (p < 0.001) (Table 3 ).
Table 3.
The serum vitamin D, calcium, and Zinc levels indicate and control groups for each gender.
| Case | control | p-value t-test |
||
|---|---|---|---|---|
| Vitamin D (30–100 ng/ml) | Male | 25.37 ± 14.04 | 25.92 ± 13.62 | .625 |
| Female | 20.82 ± 11.82 | 27.82 ± 15.41 | .001 | |
| p-value | .093 | .366 | ||
| Calcium (8.6–10.3 mg/dl) | Male | 9.05 ± .35 | 9.46 ± .58 | <.001 |
| Female | 9.21 ± .41 | 9.53 ± .49 | <.001 | |
| p-value | .059 | .483 | ||
| Zinc (70–127 μg/dl) | Male | 66.65 ± 16.15 | 86.62 ± 14.04 | <.001 |
| Female | 68.36 ± 14.33 | 79.37 ± 10.89 | <.001 | |
| p-value | .591 | .025 | ||
There was a significant and direct relationship between serum vitamin D level and BUN (P = 0.004). There was also a significant and direct relationship between serum vitamin D level and CR (P = 0.023).
There was a significant inverse relationship between serum Zinc level and BUN (P = 0.021). There was a significant inverse relationship between serum Zinc level and CR (P = 0.033). There was a significant correlation between calcium serum level and PLT (P = 0.008). There was a significant inverse correlation between serum Zinc level and WBC (P = 0.005). There was a significant inverse correlation between serum Zinc level and Neut (P = 0.012). Finally, there was a significant direct correlation between serum Zinc level and PCO2 (P = 0.019) (Table 4, Table 5 ).
Table 4.
Comparing the clinical parameters in patients with normal and abnormal serum vitamin D, calcium, and zinc levels.
| Blood test results | Vitamin D N (%) Median (IQR) |
P-Value | Calcium N (%) Median (IQR) |
P-Value | Zinc N (%) Median (IQR) |
P-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| insufficient (10–30 ng/ml) N = 68 |
sufficient (30–100 ng/ml) N = 25 |
insufficient (<8.6 mg/dl) N = 39 |
sufficient (8.6–10.3 mg/dl) N = 54 |
insufficient (<70 mg/dl) N = 49 |
sufficient (70–127 mg/dl) N = 44 |
|||||
| Vit-D | 19.20 (13–30.8) | 15.15 (11.4–21.8) | 34.2 (31.5–50) | <.001 | 16.7 (10.8–26.4) | 22.95 (14.5–31.7) | .046 | 19.2 (13–31) | 20.2 (13.5–29) | .969 |
| Calcium | 9.1 (8.9–9.4) | 9.1 (8.8–9.4) | 9.1 (8.9–9.5) | .891 | 8.8 (8.6–8.9) | 9.3 (9.2–9.5) | <.001 | 9 (8.8–9.3) | 9.2 (9–9.5) | .009 |
| Zinc | 68 (56–78) | 69 (57–78) | 65 (52–76) | .445 | 63 (52–72) | 73 (59–79) | .004 | 57 (50–63) | 78 (73–82) | <.001 |
| Duration of hospitalization | 4 (3–5) | 4 (3–5) | 4 (3–6) | .346 | 4 (3–5) | 4 (3–5) | .808 | 4 (3–5) | 4 (3–5) | .657 |
| BUN | 14 (12–19) | 14 (11–18.5) | 16 (13–25) | .004 | 13 (10–18) | 15 (13–19) | .06 | 14 (11.7–19.2) | 14 (12–19) | .966 |
| CR | 1 (1–1.1) | .95 (.80–1.1) | 1 (.9–1.18) | .244 | .9 (.8–1.1) | 1 (.9–1.1) | .166 | 1 (.8–1.1) | 1 (.8–1.1) | .756 |
| NA | 140 (138–141.2) | 140 (138–141.2) | 140 (139–141.75) | .583 | 139.5 (138–141) | 140 (138–143) | .144 | 140 (137.75–141) | 140 (138.2–142) | .394 |
| K | 4.2 (4–4.62) | 4.2 (4–4.52) | 4.35 (4–4.78) | .664 | 4.3 (3.9–4.68) | 4.2 (4.07–4.62) | .554 | 4.3 (3.9–4.7) | 4.2 (4.02–4.57) | .901 |
| WBC | 6.4 (4.7–8.1) | 6.3 (4.6–8.25) | 6.85 (5.28–7.7) | .740 | 6.44 (4.7–9.2) | 6.35 (4.85–7.48) | .908 | 7.05 (4.7–9.9) | 5.7 (4.7–7.2) | .079 |
| RBC | 4.4 (4.1–4.95) | 4.4 (4.22–5.1) | 4.35 (4–4.78) | .272 | 4.4 (4.03–5.1) | 4.4 (4.12–4.7) | .556 | 4.46 (4.05–5.1) | 4.4 (4.2–4.7) | .399 |
| HB | 12.6 (11.4–13.5) | 12.4 (11.3–13.5) | 12.75 (11.72–13.52) | .803 | 12.5 (11.12–13.58) | 12.7 (11.6–13.5) | .87 | 12.5 (11.2–13.5) | 12.7 (11.6–13.6) | .593 |
| HEMA | 37.7 (35.15–41.32) | 37.7 (35.5–41.32) | 37.5 (34.2–41.35) | .605 | 37.6 (35–41.1) | 37.9 (35.5–41.5) | .82 | 37.85 (34.5–41.2) | 37.7 (35.4–42) | .882 |
| PLT | 211 (164–256) | 206.5 (161.7–248.2) | 224.5 (166–286.2) | .152 | 196 (154–233) | 224 (168–279) | .298 | 195.5 (164–261.5) | 221 (164.7–259.5) | .685 |
| LYM | 22.3 (16.1–33.4) | 22.4 (16.5–30.4) | 22.2 (14.65–38.1) | .938 | 20.1 (14.98–31.22) | 23.3 (18.52–34.18) | .272 | 21.2 (15.4–30.4) | 24.3 (16.9–35.9) | .185 |
| Neut | 69.25 (61.08–76.28) | 69.1 (58.4–75.6) | 70.3 (61.4–81.45) | .252 | 69 (60.72–76.75) | 69.45 (60.55–76.08) | .471 | 69.5 (59.2–77.5) | 68.9 (61.15–75.15) | .279 |
| PH | 7.4 (7.32–7.41) | 7.39 (7.32–7.41) | 7.4 (7.33–7.42) | .665 | 7.4 (7.32–7.41) | 7.39 (7.32–7.42) | .873 | 7.4 (7.38–7.41) | 7.36 (7.32–7.4) | .158 |
| PCO2 | 37 (33.07–41.1) | 37.75 (34.75–41.72) | 35.65 (30.65–40.05) | .445 | 37.75 (36–41.05) | 36.25 (32–43.2) | .708 | 36.3 (29.6–38) | 39.5 (35.8–44.2) | .085 |
| PO2 | 31 (25.72–42.72) | 31 (25.8–43.58) | 32.5 (25.58–40.82) | .995 | 29.95 (21.68–42.72) | 34.55 (26.68–42.52) | .54 | 30.9 (27.1–54) | 31.1 (25.55–37.85) | .144 |
| HCO3 | 22.05 (20.82–23.88) | 22.15 (20.98–23.65) | 21.2 (17.72–24.18) | .263 | 22.25 (21.38–24.7) | 21.65 (20.65–23.75) | .66 | 21.6 (20–24.1) | 22.2 (20.95–23.55) | .445 |
| ESR | 28 (19.5–55.5) | 27.5 (15.75–48) | 40 (22–87) | .170 | 32.5 (15–63.25) | 28 (21–48) | .871 | 32 (22–48) | 27 (17.25–64.75) | .704 |
| BS | 118 (89–192) | 115.5 (87.5–192) | 138 (104–190) | .436 | 116 (82–238.25) | 121 (98.5–191) | .522 | 118.5 (86.25–220.5) | 118 (97–192) | .880 |
Note: IQR, interquartile range; Blood Urea Nitrogen(BUN), creatinine(cr), Sodium(Na), Potassium(K), WHITE BLOOD CELL(WBC), RED BLOOD CELL(RBC), HEMOGLOBIN (HB), HEMATOCRIT(HEMA), PLATELET(PLT), LYMPHOCYTE(Lym), neutrophils(Neut), Arterial Blood Gas Test(PH,PO2,PCO2, HCO3), Erythrocyte sedimentation rate(ESR), Blood Sugar(BS).).
Table 5.
The relationship between serum vitamin D, calcium, and Zinc levels and clinical parameters in patients.
| Vitamin D |
Calcium |
Zinc |
||||
|---|---|---|---|---|---|---|
| Pearson Correlation | Sig. (2−tailed) | Pearson Correlation | Sig. (2−tailed) | Pearson Correlation | Sig. (2−tailed) | |
| Vitamin D | 1 | .041 | .693 | −.094 | .368 | |
| Calcium | .041 | .693 | 1 | .342∗∗ | .001 | |
| Zinc | −.094 | .368 | .342∗∗ | .001 | 1 | |
| Age | .300∗∗ | .003 | −.061 | .564 | −.169 | .105 |
| Duration of hospitalization | .175 | .093 | −.048 | .647 | −.035 | .743 |
| BUN | .338∗∗ | .004 | .029 | .812 | −.278∗ | .021 |
| CR | .276 | .023 | .054 | .664 | −.259∗ | .033 |
| NA | .078 | .520 | .021 | .865 | .138 | .255 |
| K | .030 | .803 | −.148 | .220 | −.026 | .830 |
| WBC | .098 | .416 | −.030 | .803 | −.333∗∗ | .005 |
| RBC | −.087 | .468 | −.124 | .303 | .082 | .499 |
| HB | .003 | .979 | .021 | .864 | .089 | .461 |
| HEMA | −.065 | .593 | −.047 | .698 | .122 | .316 |
| PLT | .161 | .182 | .312∗∗ | .008 | .014 | .907 |
| LYM | −.010 | .935 | .231 | .058 | .210 | .085 |
| Neut | .218 | .075 | −.210 | .086 | −.303∗ | .012 |
| PH | .061 | .756 | .009 | .962 | −.223 | .253 |
| PCO2 | −.117 | .553 | .033 | .866 | .439 | .019 |
| PO2 | .132 | .503 | .079 | .690 | −.319 | .098 |
| HCO3 | −.184 | .348 | −.087 | .661 | .316 | .101 |
| ESR | .318 | .071 | −.135 | .454 | .013 | .944 |
| BS | .130 | .345 | −.027 | .845 | −.048 | .730 |
∗∗ : Correlation is significant at the 0.01 level (2-tailed).
∗ : Correlation is significant at the 0.05 level (2-tailed).
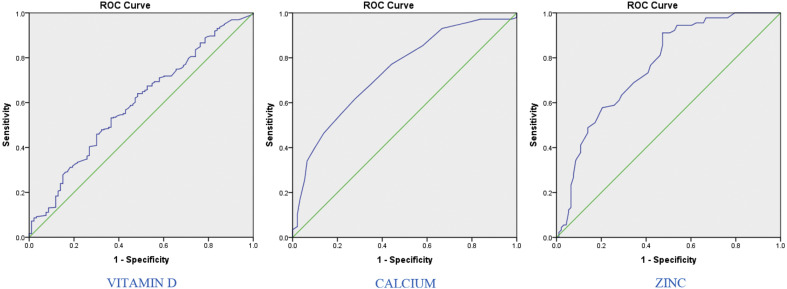
Based on the ROC curve and its area under the curve, the level of serum vitamin D, calcium, and zinc have been useful in predicting the status of healthy and patient individuals. The area under the curve for the serum zinc level has been larger for zinc than calcium and vitamin D, which means that it is more effective in differentiating patients from healthy individuals (Table 6 and Fig. 1 ).
Table 6.
The values of area under the curve, best cutoff point, sensitivity, and specificity for serum levels of vitamin D, calcium, and zinc in healthy and diseased individuals.
| Vitamin D | Calcium | Zinc | |
|---|---|---|---|
| AUC | .594(.529–.659) | .739(.675–.803) | .761(.692–.830) |
| Asymptotic Sig | .005 | <.0001 | <.0001 |
| Cut of point | 20.05 | 9.35 | 68.5 |
| Sensitivity | .621 | .618 | .911 |
| Specificity | .527 | .720 | .527 |
AUC: The areas under the receiver operating characteristic curves.
Fig. 1.
ROC diagram for the serum levels of vitamin D, calcium, and zinc for differentiating healthy and diseased individuals.
4. Discussion
The present study aimed to investigate the serum vitamin D, calcium, and Zinc levels in patients with COVID-19. The studies demonstrate that Zinc and vitamins D, and Calcium are integral parts of the immune system and show synergistic functions at various stages of the host defenses, such as maintaining the integrity of biological barriers and the functionality of cells that make up the innate and adaptive systems. Therefore, the deficiency or insufficiency of these critical nutrients, acting in synergy in tight and adherens junction proteins, can lead to impairment of mucosal epithelial cells, possibly making them more susceptible to pathogen entry, such as SARS-CoV-2.
In this scenario, we explained that vitamin D, calcium, and Zinc were lower in coronavirus positive patients than healthy individuals (Table 1). Serum vitamin D levels in the two groups were lower than normal, but this slight difference between the two groups was statistically significant. It is interesting that both (case and controls) have lower vitamin D levels, the important data is that mean value of vitamin D is pathological in both cases. Akriti et al. (2020) investigated the role of vitamin D supplementation in preventing and treating COVID-19 and found that vitamin D has antiviral and anti-inflammatory effects. Also, vitamin D, by increasing the production of antimicrobial peptides, including Cathelicidin (LL-37) in respiratory epithelial cells, boosts innate immunity, which can cause impairment in the bacterial membranes through electrostatic interactions [29]. In their study, Viktor Christiano et al. (2020) showed that vitamin D has various effects on the body, including influence on the immune system against coronavirus [16].
A study by William et al. (2020) emphasized the effect of vitamin D in these patients [17]. Kent Vier et al. (2020) also showed that low vitamin D levels are associated with elevated inflammatory cytokines, which can be involved in affliction with COVID-19 [30], consistent with the present study results. Therefore, preparing preventive supplements and adjuvant therapy of vitamin D to boost the immune system and prevent and mitigate the severity of COVID-19 infection seems logical [29].
In our study, there was a significant difference between the two groups in terms of serum calcium levels, which was lower in the case group compared to the control, but the mean value it is between the normal range, so it is not pathological. According to our observations, people with higher calcium levels may be more resistant to the virus than people with lower calcium levels. Shai et al. (2020) reported a high prevalence of hypocalcemia in patients with coronavirus. Therefore, they hypothesized that serum calcium level is associated with patients' severity and prognosis with Covid-19 [19]. Bara El Cardi et al. (2020) also showed that maintaining normal serum calcium levels (such as oral feeding through calcium anti-carbonate) in the early stages of COVID-19 infection may prevent disease progression [31], which is in line with our study. Based on this research's findings, Zinc's serum level had a significant difference between the two groups, which was lower in the case group than the control. Responding to the question of whether zinc can maintain balance and optimal resistance to COVID-19 in the host, Rozak (2020) in the US stated that consuming at most 50 mg/day of Zinc may create another protection against the virus. COVID-19 pandemic disease may minimize the disease burden by increasing the host resistance to the viral infection. As mentioned earlier, the useful potential role of Zinc in COVID-19 infection requires greater clinical validity.
Nevertheless, Zinc's consumption for reducing disease burden can be a good test [32]. Shakoor et al. (2020) discussed Zinc's role in immunity and its particular effect on patients with COVID-19. They also discussed consuming this nutrient as a potential therapeutic method for reducing the complications and mortality rate of patients with COVID-19 [1]. Foster et al. showed that Zinc supplements positively affected reducing fever duration in patients with respiratory infections. Still, it had no significant effect on respiratory rate, coughs duration, and hospitalization time [33]. This study suggested that Zinc deficiency could be a predictor for a critical illness of COVID-19. It may be helpful to use zinc supplementation for non-patients for prevention and zinc supplementation for patients who may have lower than normal serum zinc levels. However, trials with an increased number of patients should be evaluated.
Our study showed that the serum vitamin D level in men had no significant difference between the case and control groups. Still, this difference was significant in women, where the level was higher in control than in diseased women. In Hosseininezhad et al., the prevalence of vitamin D deficiency did not differ significantly between the two genders [34]. In the study by Salk et al. (2007), no significant difference was observed between the two genders [35]. However, in Banajeh et al. in Yemen, the male gender was strongly associated with more incidence and more severe acute lower respiratory infection [36]. The serum calcium level had a significant difference in the men and women between the patient and healthy groups. The serum calcium level was higher in both males and females of the control group than the patient group.
Further, the serum Zinc level significantly differed between the healthy and patient groups in men and women. The serum Zinc level was lower in the patient group's men and women than the men and women of the control group. However, in the study by Hirashkur et al., no significant difference was found between the genders. They stated that the elderly, most probably regarding nutrient deficiency and immunity through immunosenescence, significantly increase the risk of poor prognosis for COVID-19, thus considerably highlighting adequate nutrition [30]. Our results showed no significant difference between serum Zinc level and BS, while Kasmer et al. showed Zinc's critical role in the synthesis, storage, and secretion of insulin [37]. There was a significant and direct relationship between serum vitamin D level and CR and BUN in the present research.
On the other hand, Saeidi et al. reported that vitamin D analogs significantly reduced proteinuria in kidney disease patients. It also caused a significant reduction in BUN and creatinine, thereby improving kidney function [38]. There was also a significant inverse relationship between Zinc and CR, and BUN. It can be concluded that Zinc consumption helps in improving kidney function and thus mitigate kidney problems in patients with coronavirus. In the study by SOMI et al., improvement in CR and BUN test results was observed in liver disease patients who consumed Zinc supplements [39]. Shahni et al. indicated Zinc's effect on reducing kidney problems in patients with chronic liver failure [40].
5. Conclusion
The results showed that serum of Zinc, calcium, and vitamin D levels in COVID-19 patients are lower than in healthy individuals. So, such nutrients are characterized to be widely available, safe, and low-cost measure that helps cope with the increased demand for these nutrients in case of contact with the virus and onset of the immune responses. They also lower the risk of severe progression and prognosis of this viral infection. More clinical trials are needed to say that it can control the disease.
6. Suggestions
Since COVID-19 is a pandemic and there is no definite treatment for it, we should seek methods that promote prevention. It is suggested that other studies with larger sample sizes be conducted across different parts of the world in this regard so that a definitive conclusion could be obtained about consuming vitamin D, calcium, and Zinc supplements to reduce affliction with coronavirus disease.
Financial support and sponsorship
Nil.
Ethical statement
We herewith confirm that the trial protocol has been approved by the Central Ethics Commission of the Ministry of Healthcare of IRAN.This article is from a research project entitled Serum levels of vitamin D, calcium and zinc in people with coronavirus 19 in Shoushtar city with ethics code IR.SHOUSHTAR.REC.1399.017.
Declaration of competing interest
The authors declare that there is no conflict of interest concerning this paper.
Acknowledgments
We would like to thank all the participants in this study, as well as the material and spiritual efforts of the Vice Chancellor for Research of Shoushtar Faculty of Medical Sciences.
References
- 1.Shakoor H., Feehan J., Al Dhaheri A.S., Ali H.I., Platat C., Ismail L.C. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2020 doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavakoli A., Vahdat K., Keshavarz M. Novel coronavirus disease 2019 (COVID-19): an emerging infectious disease in the 21st century. ISMJ. 2020;22(6):432–450. [Google Scholar]
- 5.Constantin S. 2020. Dose-response effects of viral exposure in COVID-19. [Google Scholar]
- 6.Wang X., Chen L., Wang L., Fan Q., Pan D., Li J. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci China Chem. 2019;62(8):933–967. [Google Scholar]
- 7.Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune System–Working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jovic T.H., Ali S.R., Ibrahim N., Jessop Z.M., Tarassoli S.P., Dobbs T.D. Could vitamins help in the fight against COVID-19? Nutrients. 2020;12(9):2550. doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souza A.C.R., Vasconcelos A.R., Prado P.S., Pereira C.P.M. Zinc, vitamin D and vitamin C: perspectives for COVID-19 with a focus on physical tissue barrier integrity. Front Nutr. 2020;7:295. doi: 10.3389/fnut.2020.606398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bikle D.D. What is new in vitamin D: 2006–2007. Curr Opin Rheumatol. 2007;19(4):383–388. doi: 10.1097/BOR.0b013e32818e9d58. [DOI] [PubMed] [Google Scholar]
- 11.Gray T.K., Lowe W., Lester G.E. Vitamin D and pregnancy: the maternal-fetal metabolism of vitamin D. Endocr Rev. 1981;2(3):264–274. doi: 10.1210/edrv-2-3-264. [DOI] [PubMed] [Google Scholar]
- 12.Zisi D., Challa A., Makis A. The association between vitamin D status and infectious diseases of the respiratory system in infancy and childhood. Hormones. 2019;18(4):353–363. doi: 10.1007/s42000-019-00155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Etten E., Mathieu C. Immunoregulation by 1, 25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97(1–2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Griffin M.D., Xing N., Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23(1):117–145. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 15.Moukayed M., Grant W.B. Molecular link between vitamin D and cancer prevention. Nutrients. 2013;5(10):3993–4021. doi: 10.3390/nu5103993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christianto V., Smarandache F., Umniyati Y. A review of major role of vitamin D3 in human immune system and its possible use for novel corona virus treatment. Jurnal Penelitian Fisika dan Aplikasinya (JPFA) 2020;10(1):1–6. [Google Scholar]
- 17.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey R.L., Gahche J.J., Lentino C.V., Dwyer J.T., Engel J.S., Thomas P.R. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141(2):261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J.-K., Zhang W.-H., Zou L., Liu Y., Li J.-J., Kan X.-H. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019: a retrospective cross-sectional study. 2020 Jun 30;12(12):11287–11295. doi: 10.18632/aging.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster M., Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4(7):676–694. doi: 10.3390/nu4070676. Epub 2012/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habibian R., Khoshdel A., Kheiri S., Torabi A. The effect of zinc sulphate syrup on children's respiratory tract infections. J Babol Univ Med Sci. 2013;15(4):22–29. [Google Scholar]
- 22.Bhutta Z., Black R.E., Brown K., Gardner J.M., Gore S., Hidayat A. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr. 1999;135(6):689–697. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 23.Prasad A.S., Fitzgerald J.T., Bao B., Beck F.W., Chandrasekar P.H. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000;133(4):245–252. doi: 10.7326/0003-4819-133-4-200008150-00006. [DOI] [PubMed] [Google Scholar]
- 24.Cakman I., Kirchner H., Rink L. Zinc supplementation reconstitutes the production of interferon-α by leukocytes from elderly persons. J Interferon Cytokine Res. 1997;17(8):469–472. doi: 10.1089/jir.1997.17.469. [DOI] [PubMed] [Google Scholar]
- 25.Mocchegiani E., Muzzioli M. Therapeutic application of zinc in human immunodeficiency virus against opportunistic infections. J Nutr. 2000;130(5) doi: 10.1093/jn/130.5.1424S. 1424S-31S. [DOI] [PubMed] [Google Scholar]
- 26.Wessels I., Rolles B., Rink L. The potential impact of Zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020:11. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmoudian S.A., Poya A. Effects of zinc and. Tehran Univ Med J. 2007;65(6):29–35. [Google Scholar]
- 28.Camargo C.A., Ganmaa D., Frazier A.L., Kirchberg F.F., Stuart J.J., Kleinman K. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130(3):e561–e567. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 29.Khemka A., Suri A., Singh N.K., Bansal S.K. Role of vitamin D supplementation in prevention and treatment of COVID-19. Indian J Clin Biochem. 2020:1–2. doi: 10.1007/s12291-020-00908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weir E.K., Thenappan T., Bhargava M., Chen Y. Does vitamin D deficiency increase the severity of COVID-19? Clin Med. 2020 Jul;20(4):e107–e108. doi: 10.7861/clinmed.2020-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Kurdi B., Khatua B., Rood C., Snozek C., Cartin-Ceba R., Singh V.P. Mortality from COVID-19 increases with unsaturated fat, and may be reduced by early calcium and albumin supplementation. Gastroenterology. 2020 Sep;159(3):1015–1018. doi: 10.1053/j.gastro.2020.05.057. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razzaque M. COVID-19 pandemic: can maintaining optimal zinc balance enhance host resistance? Tohoku J Exp Med. 2020 Jul;251(3):175–181. doi: 10.1620/tjem.251.175. [DOI] [PubMed] [Google Scholar]
- 33.Foster M., Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4(7):676–694. doi: 10.3390/nu4070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosseininejad N., Kalbasi Z., Afshar J. Vitamin D and childhood pneumonia. Razi J Med Sci. 2016;22(140):109–116. [Google Scholar]
- 35.Salek M., Rafati H., Hashemipour M., Memar A.P., Nezhadnik H., Amini M. Is vitamin D deficiency prevalent in healthy 6-yearold children in Isfahan City? 2007;25(85):95–103. [Google Scholar]
- 36.Banajeh S.M. Nutritional rickets and vitamin D deficiency—association with the outcomes of childhood very severe pneumonia: a prospective cohort study. Pediatr Pulmonol. 2009;44(12):1207–1215. doi: 10.1002/ppul.21121. [DOI] [PubMed] [Google Scholar]
- 37.Chausmer A.B. Zinc, insulin and diabetes. J Am Coll Nutr. 1998;17(2):109–115. doi: 10.1080/07315724.1998.10718735. [DOI] [PubMed] [Google Scholar]
- 38.Saeedi N., Rezvanfar M., Hadidi M., Mahani F.A., Ahmadlou M. The effect of active VitaminD on treatment of proteinuria in patients with diabetic nephropathy without vitamin D deficiency. J Arak Univ Med Sci. 2016;19(6):57–67. [Google Scholar]
- 39.Somi M.H., Rezaeifar P., Rahimi A.O., Moshrefi B. Effects of low dose zinc supplementation on biochemical markers in non-alcoholic cirrhosis: a randomized clinical trial. Arch Iran Med. 2012;15(8) [PubMed] [Google Scholar]
- 40.Sahni N., Gupta K., Rana S., Prasad R., Bhalla A. Intake of antioxidants and their status in chronic kidney disease patients. J Ren Nutr. 2012;22(4):389–399. doi: 10.1053/j.jrn.2011.09.002. [DOI] [PubMed] [Google Scholar]