Abstract
Background
Viral infections can cause significant morbidity in cystic fibrosis (CF). The current Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic could therefore have a serious impact on the health of people with CF (pwCF).
Methods
We used the 38-country European Cystic Fibrosis Society Patient Registry (ECFSPR) to collect case data about pwCF and SARS-CoV-2 infection.
Results
Up to 30 June 2020, 16 countries reported 130 SARS-CoV-2 cases in people with CF, yielding an incidence of 2.70/1000 pwCF. Incidence was higher in lung-transplanted patients (n=23) versus non-transplanted patients (n=107) (8.43 versus 2.36 cases/1000). Incidence was higher in pwCF versus the age-matched general population in the age groups <15, 15-24, and 25-49 years (p<0.001), with similar trends for pwCF with and without lung transplant. Compared to the general population, pwCF (regardless of transplantation status) had significantly higher rates of admission to hospital for all age groups with available data, and higher rates of intensive care, although not statistically significant.
Most pwCF recovered (96.2%), however 5 died, of whom 3 were lung transplant recipients. The case fatality rate for pwCF (3.85%, 95% CI: 1.26-8.75) was non-significantly lower than that of the general population (7.46%; p=0.133).
Conclusions
SARS-CoV-2 infection can result in severe illness and death for pwCF, even for younger patients and especially for lung transplant recipients. PwCF should continue to shield from infection and should be prioritized for vaccination.
Keywords: Cystic fibrosis, Covid-19, SARS-CoV-2, Europe, Incidence, Epidemiology
1. Background
SARS-CoV-2, the novel Severe Acute Respiratory Syndrome Coronavirus 2 causing Covid-19, was declared a pandemic in March 2020 by the World Health Organization (WHO). Western Europe was heavily affected in the first half of 2020. Covid-19 morbidity and mortality are highest in the elderly and in people with underlying chronic illnesses [1].
Cystic fibrosis (CF) arises from gene mutations in cystic fibrosis transmembrane conductance regulator (CFTR), which lead to chronic CF lung disease and compromised function of multiple other organ systems. Repeated cycles of respiratory infection and chronic inflammation cause progressive lung function decline. Pulmonary exacerbations can be triggered by infection with viruses such as influenza [2]. The 2009-2010 H1N1 pandemic caused significant morbidity in people with CF (pwCF) [3]. Therefore, the SARS-CoV-2 pandemic caused considerable anxiety in the CF community [4]. Early case series of pwCF in various regions who tested positive for SARS-CoV-2 suggest that pwCF have better outcomes than expected [5], [6], [7], [8].
We aimed to assess the incidence, clinical course and outcome of SARS-CoV-2 infection in pwCF versus the general population from February to June 2020 in countries that report data to the European Cystic Fibrosis Society Patient Registry (ECFSPR).
2. Methods
2.1. Study design
This observational study was nested within the ECFSPR. We collected case reports for pwCF diagnosed with polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection between 01 February 2020 and 30 June 2020, with data follow-up until 07 January 2021. Case definitions were based on WHO guidance [9], [10], [11].
ECFSPR's 38 member countries provided data under existing ethical approvals and data governance structures. Most countries contributed data directly to ECFSPR. Belgium, France, Germany and the UK contributed via their national registries. Italy contributed via their national registry and the Italian CF society. Turkey contributed via their national registry and directly to ECFSPR. No cases were doubly reported from Italy or Turkey. ECFSPR covers 35-99% of the CF population per country [12]; 9 countries have coverage <80% (Armenia, Belarus, Bulgaria, Lithuania, Poland, Romania, Spain, Turkey and Ukraine) [12] in 2018.
The ECFSPR structure and operations have been previously described [13] (www.ecfs.eu/ecfspr). Participating pwCF provide written informed consent, including consent to use their data for future research. This provided the framework for collecting data regarding SARS-CoV-2 infection, as confirmed in writing by the ECFSPR data protection officer.
2.2. Data collected
A case report form, developed collaboratively within ECFS, was used to collect anonymized data about demographics, pre-infection CF characteristics (medical history data taken pre-infection or from 2019 registry data) and SARS-CoV-2 infection (symptoms, treatment, complications, and outcomes). Most national registries collected data in a different format, then shared a core dataset of patient-level data. One national registry shared aggregated data.
The CF population seen in the calendar year per country was from the most recent ECFSPR report (2018) [12] (and the 2017 report for France). Country-specific SARS-CoV-2 aggregate data up to 30 June 2020 for the general population (cases, deaths, hospitalizations and intensive care admissions) were from the European Centre for Disease Prevention and Control (ECDC) [14], [15], [16]. General population data were only available by different age groups for incidence (<15, 15-24, 25-49, 50-64, 65-79, >80 years) and hospitalization/ICU (0-9, 10-19, 20-29, 30-39, 40-49, 50-59, 60-69, 70-79, >80 years). Incidence by age group was available only for a subset of 24 countries: Austria, Belgium, Croatia, Czech Republic, Cyprus, Denmark, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden. Hospitalization/ICU data were available for a different subset of 30 countries (the 24 countries above plus Bulgaria, Estonia, Finland, Iceland, Malta, United Kingdom) [14]. Of note, pwCF are included in these data reported for the general European population.
2.3. Statistics
Standard ECFSPR definitions applied to all variables (www.ecfs.eu/projects/ecfs-patient-registry/Variables-Definitions). Percent predicted forced expiratory volume was calculated using Global Lung Initiative reference values [17]. Patient-level data were summarized, then aggregate data were added where available. Continuous data were categorized and presented as number and percentage (computed excluding missing data).
Clinical and demographic characteristics of pwCF with SARS-CoV-2 infection (and the subgroups of non-lung-transplanted and lung-transplanted pwCF) are reported using descriptive statistics. SARS-CoV-2 incidence was estimated in pwCF, and by country and age group. 95% confidence intervals (CIs) were computed using binomial exact distribution. Age-specific SARS-CoV-2 incidence rates were compared in pwCF versus the general population in corresponding countries, using the exact binomial test and p-values are provided [18]. P-values below 0.05 were considered statistically significant.
ECFSPR statisticians analyzed data using SAS (v9.4, SAS Institute Inc., Cary, NC, USA) and R v4.0. . Maps were produced using www.datawrapper.de.
3. Results
3.1. Participation and patient characteristics
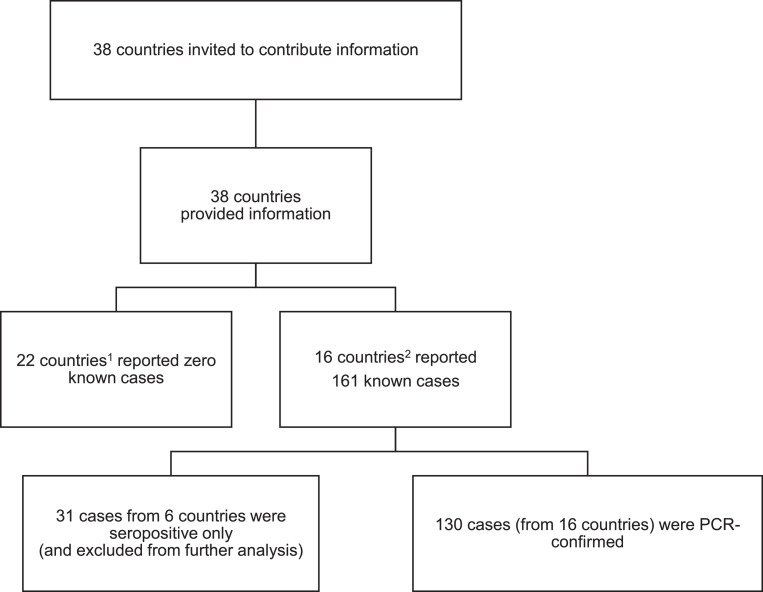
Of the 38 ECFSPR member countries, 22 reported zero known cases and 16 reported 130 PCR-confirmed cases up to 30 June 2020 (Fig. 1 ). We also received notification of 31 patients seropositive for SARS-CoV-2 but without confirmatory PCR. We excluded these patients from analyses.
Fig. 1.
Flow chart on SARS-CoV-2 data collection.
1 Albania, Armenia, Austria, Belarus, Bulgaria, Croatia, Cyprus, Czech Republic, Georgia, Hungary, Israel, Latvia, Lithuania, Luxembourg, Republic of Moldova, North Macedonia, Portugal, Romania, Serbia, Slovak Republic, Slovenia, Ukraine
2 Belgium, Denmark, France, Germany, Greece, Ireland, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, Turkey, United Kingdom
Of the 130 pwCF with SARS-CoV-2 infection, 72 (55.4%) were male and 39 (30.0%)were aged <18 years. Demographics and pre-infection disease characteristics are presented by pwCF with lung transplant (n=23) and without (n=107, including 1 patient with a liver transplant) (Table 1 ). Compared to those without transplant, lung-transplanted pwCF were older, had higher pre-infection BMI, higher lung function, more frequent CF-related diabetes (CFRD) and pancreatic insufficiency and lower use of CFTR modulators. Lung-transplanted pwCF more frequently used azithromycin maintenance therapy (although data were often missing for this variable).
Table 1.
Demographics and pre-infection characteristics of people with cystic fibrosis with SARS-CoV-2.
| Total N=130 | Non-lung transplant1 N=107 | Lung-transplant N=23 | |
|---|---|---|---|
| Age, years, n (%) | |||
| 0-11 | 15 (11.5%) | 15 (14%) | 0 (0%) |
| 12-17 | 24 (18.5%) | 24 (22.4%) | 0 (0%) |
| 18-29 | 37 (28.5%) | 33 (30.8%) | 4 (17.4%) |
| 30-49 | 49 (37.7%) | 30 (28%) | 19 (82.6%) |
| 50+ | 5 (3.8%) | 5 (4.7%) | 0 (0%) |
| Sex, n (%) | |||
| Female | 58 (44.6%) | 50 (46.7%) | 8 (34.8%) |
| Male | 72 (55.4%) | 57 (53.3%) | 15 (65.2%) |
| Genotype, n (%) | |||
| F508del homozygous | 57 (43.8%) | 45 (42.1%) | 2 (8.7%) |
| F508del heterozygous | 46 (35.4%) | 37 (34.6%) | 9 (39.1%) |
| Other | 27 (20.8%) | 25 (23.4%) | 12 (52.2%) |
| Unknown | 0 | 0 | 0 |
| BMI2, kg/m2, n (%) | |||
| <18.5 | 31 (26.3%) | 27 (28.1%) | 4 (18.2%) |
| 18.5-30 | 82 (69.5%) | 64 (66.7%) | 18 (81.8%) |
| >30 | 5 (4.2%) | 5 (5.2%) | 0 (0%) |
| Unknown | 8 | 7 | 1 |
| Azithromycin (maintenance therapy pre-infection), n (%) | |||
| Yes | 30 (39.0%) | 23 (35.4%) | 7 (58.3%) |
| No | 47 (61.0%) | 42 (64.6%) | 5 (41.7%) |
| Unknown | 53 | 42 | 11 |
| ppFEV13, n (%) | |||
| ≤40% | 31 (26.5%) | 27 (28.4%) | 4 (18.2%) |
| 41-70% | 31 (26.5%) | 28 (29.5%) | 3 (13.6%) |
| >70% | 55 (47.0%) | 40 (42.1%) | 15 (68.2%) |
| Unknown | 5 | 4 | 1 |
| Pseudomonas aeruginosa4, n (%) | |||
| Yes | 65 (51.2%) | 53 (50.5%) | 12 (54.5%) |
| No | 62 (48.8%) | 52 (49.5%) | 10 (45.5%) |
| Unknown | 3 | 2 | 1 |
| CF related diabetes, n (%) | |||
| Yes | 40 (32.0%) | 26 (25.2%) | 14 (63.6%) |
| No | 85 (68.0%) | 77 (74.8%) | 8 (36.4%) |
| Unknown | 5 | 4 | 1 |
| Pancreatic insufficiency, n (%) | |||
| Yes | 82 (79.6%) | 62 (75.6%) | 20 (95.2%) |
| No | 21 (20.4%) | 20 (24.4%) | 1 (4.8%) |
| Unknown | 27 | 25 | 2 |
| CFTR modulator therapy, n (%) | |||
| Yes | 31 (24.6%) | 30 (29.1%) | 1 (4.3%) |
| No | 95 (75.4%) | 73 (70.9%) | 22 (95.7%) |
| Unknown | 4 | 4 | 0 |
Abbreviations: BMI=body mass index, CFTR=cystic fibrosis transmembrane conductance regulator, ppFEV1=percent predicted forced expiratory volume in one second
Percentages are calculated based on non-missing data.
1This group included 1 patient with a liver transplant
2 BMI was only calculated for patients aged 2 years and older. Data were not available to calculate z-scores.
3 Percent predicted FEV1 was only calculated for patients not lung transplanted and aged 6 years and older.
4 In the last 12 months.
3.2. Symptoms of SARS-CoV-2 infection in pwCF
We had data about at least one symptom for 128 pwCF, but data collection was not uniform across all countries. Of the 128 pwCF with partial data available, 101 pwCF (78.9%) had symptoms of SARS-CoV-2 infection, while 27 (21.1%) were asymptomatic. Lung-transplanted pwCF had a higher rate of symptomatic SARS-CoV-2 than non-lung transplanted pwCF (91.3% versus 76.2%). PwCF most commonly reported general symptoms (≥1 event of fever, fatigue, headache, arthralgia/myalgia) (84/122 [68.9%] with information about these symptoms), followed by pulmonary symptoms (66/112; 58.9%) (≥1 event of increased cough, dyspnea, chest tightness, wheezing, sputum production, hemoptysis).
3.3. Incidence of SARS-CoV-2 infection in pwCF
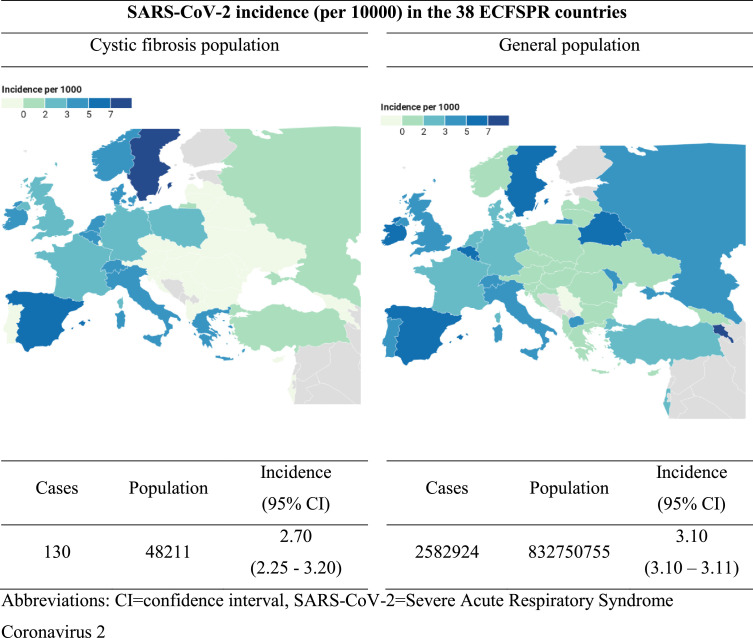
The incidence of PCR-confirmed SARS-CoV-2 in pwCF (2.70 cases per 1000; 95% CI: 2.25-3.20) was not statistically different to that of the general population (3.10/1000; 95% CI: 3.10-3.11) of the entire 38-country ECFSPR area in the same period. SARS-CoV-2 infections were concentrated in Western Europe both in pwCF and the general population (Fig. 2 ), although this could be due to differing rates of testing between countries. Incidence per 1000 pwCF by country varied from 1.11 to 7.26, with large confidence intervals for all countries (Supplementary Table 1).
Fig. 2.
Incidence of SARS-CoV-2 infection up to 30 June 2020 in people with cystic fibrosis and in the general population by country.
Abbreviations: CI=confidence interval, SARS-CoV-2=Severe Acute Respiratory Syndrome Coronavirus 2
Notes: All cases of SARS-CoV-2 in pwCF and the general population were PCR-confirmed. Incidence was calculated as (SARS-CoV-2 cases/number of people in the population)*1000.
CF population size was from the 2018 ECFSPR report.
SARS-CoV-2 incidence was significantly higher in lung-transplanted pwCF (8.43/1000, 95% CI: 5.35-12.62) versus non-lung transplanted pwCF (2.36/1000, 95% CI: 1.94-2.86).
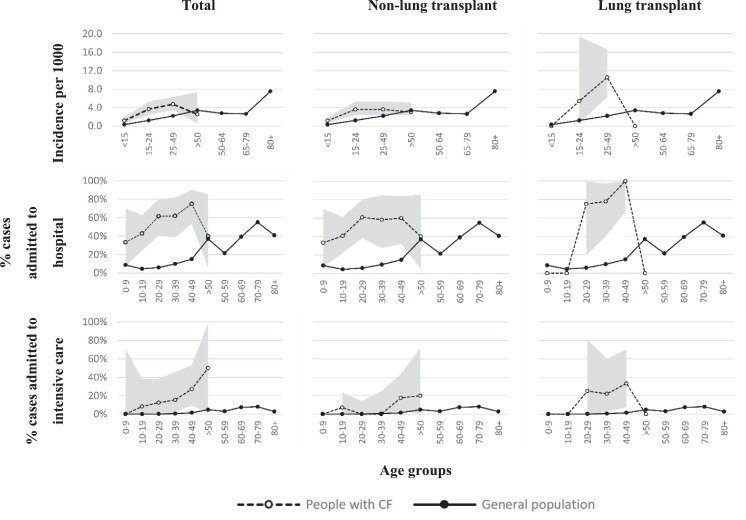
When incidence was considered by age group for the 24 countries with data for both populations, SARS-CoV-2 incidence was significantly higher in pwCF versus the general population for those aged <15, 15-24, and 25-49 years (p<0.001). This pattern was maintained when incidence by age group was considered for pwCF with and without lung transplant (Fig. 3 , Supplementary Table 2).
Fig. 3.
Incidence and rates of hospitalization and intensive care admission by age group and transplant status in people with cystic fibrosis compared to the general population.
Notes: Confidence intervals shown as grey shaded area. People with CF aged ≥50 years were grouped together due to low numbers.
The 24 countries included in age-banded analysis of incidence in people with CF and the general population were: Austria, Belgium, Croatia, Czech Republic, Cyprus, Denmark, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden.
The 30 countries included in age-banded analysis of hospital and intensive care admission in people with CF and the general population were the 24 countries above plus Bulgaria, Estonia, Finland, Iceland, Malta, United Kingdom.
3.4. Management and outcomes
Overall, 75 pwCF (58.1%) were admitted to hospital and 12 (9.2%) to intensive care (Table 2 ). Lung-transplanted pwCF had higher rates than non-lung transplanted pwCF of hospital (82.6% versus 52.8%) and intensive care (26.1% versus 5.6%) admission (Fig. 3, Supplementary Tables 4 and 5).
Table 2.
Outcome of SARS-CoV-2 infection in people with cystic fibrosis, by lung transplant status.
| Total (N=130) | Non-lung transplant N=107 | Lung-transplant N=23 | |
|---|---|---|---|
| Hospital admission | |||
| Yes | 75 (58.1%) | 56 (52.8%) | 19 (82.6%) |
| No | 54 (41.9%) | 50 (47.2%) | 4 (17.4%) |
| Missing | 1 | 1 | 0 |
| Intensive care unit | |||
| Yes | 12 (9.2%) | 6 (5.6%) | 6 (26.1%) |
| No | 118 (90.8%) | 101 (94.4%) | 17 (73.9%) |
| Missing | 0 | 0 | 0 |
| Death | |||
| Yes | 5 (3.8%) | 2 (1.9%) | 3 (13%) |
| No | 125 (96.2%) | 105 (98.1%) | 20 (87%) |
| Missing | 0 | 0 | 0 |
Abbreviations: SARS-CoV-2=Severe Acute Respiratory Syndrome Coronavirus 2
Percentages are calculated based on non-missing data
Compared to the general population, pwCF (regardless of transplantation status) had significantly higher rates of admission to hospital for all age groups with available data. Importantly, this pattern of higher hospitalization rates in pwCF was maintained even when we excluded pwCF lung transplant from the analysis (Fig. 3, Supplementary Table 4).
The rate of intensive care admissions was notably higher in pwCF versus the general population. Some differences were observed by age group when pwCF with and without lung transplant were compared versus the general population (Fig. 3, Supplementary Table 5), but low numbers in some subgroups prevent robust comparisons.
PwCF with SARS-CoV-2 infection were most commonly treated with additional intravenous antibiotics (49.5%) and additional oral antibiotics (36.8%) (Supplementary Table 3).
Oxygen was administered to 24/86 (27.9%) pwCF and 10/80 (12.5%) received some form of respiratory support. Five pwCF received non-invasive ventilation, 5 required invasive ventilation and 2 had an additional extracorporeal membrane oxygenation (ECMO). Most pwCF recovered (96.2%), however 5 (3.8%) died.
Of the 5 pwCF who died, 3 had received a lung transplant and 2 did not. Complete case information was available for 3 of the 5 patients who died. All 3 patients had general and pulmonary symptoms of SARS-CoV-2 infection. They were all F508del homozygous lung transplant recipients with pancreatic insufficiency and aged over 20 years at the time of SARS-CoV-2 infection. Two had CFRD. All 3 had invasive ventilation in intensive care (2 of whom required ECMO) and 2 received treatment with steroids.
Case fatality rates were calculated for pwCF and the general population of the 16 countries with SARS-CoV-2 cases in both populations. The case fatality rate for pwCF (3.85%, 95% CI: 1.26-8.75) was non-significantly lower than that of the general population (7.46%; 95% CI: 7.43-7.49; p=0.133).
4. Discussion
We used the 38-country ECFSPR framework to collect case data about PCR-confirmed SARS-CoV-2 infection in pwCF. Up to 30 June 2020 we report that incidence was significantly higher in pwCF versus the age-matched general population, where comparisons could be made. PwCF had significantly higher rates of hospitalization, even when lung-transplanted pwCF were excluded and notably higher rates of intensive care admissions.
In the ECFSPR geographic area, the overall incidence of SARS-CoV-2 in pwCF was not significantly different to the incidence of that of the corresponding general population (2.70 versus 3.10/1000). SARS-CoV-2 was lower in pwCF versus the general population in studies from a global group (0.7/1000 versus 1.5/1000 up to mid-April 2020) [7], Spain (3.2/1000 versus 4.9/1000 up to 16 May 20) [19], France (4.1/1000 pwCF up to 30 June 20) [5], Italy (15.8/1000 versus 29.1/1000 up to November 2020) [20] and Verona, Italy (1.9/1000 versus 4/1000 up to 23 July 20) [21].
However, age represents a major confounding factor to these comparisons of incidence, due to different age distributions in pwCF versus the general population. PwCF have a shorter lifespan than the general population, with a median age of survival in the range of 40-55 years and the median age at death of 31.2 years [22]. We addressed this confounder with subgroup analysis of incidence by age group and found that SARS-Co-V2 incidence was significantly higher in pwCF versus the general population for all age groups up to 49 years of age (p<0.001). According to the 2018 ECFSPR report, 96% of pwCF are aged below 50 years [12]. Therefore, for the vast majority of the European CF population, incidence of SARS-CoV-2 is significantly higher than in the age-matched general population.
Several caveats exist for incidence estimates. First, testing in early 2020 was restricted to symptomatic cases with usual respiratory symptoms, therefore many cases of asymptomatic or mild infections probably went undetected, both in pwCF and the general population. ECDC data show that the rate of testing for SARS-CoV-2 increased steadily throughout the reported period and varied widely between countries [23]. Second, cases could have been under-reported by overwhelmed hospital teams. Third, pwCF may have been tested more frequently than the general population due to increased vigilance and established care routines, particularly for younger age groups. Testing rates by age group are not available from the ECDC. Conversely, we also know that many CF teams switched to conducting care and clinical trial visits remotely as much as possible to minimize the risk of exposure [19, 24] which could have led to missed opportunities to test asymptomatic and mildly ill pwCF for SARS-CoV-2 infection. Fourth, comparing incidence by country (either in our study or in other published reports) is difficult due to differing impacts of the pandemic, case definitions and public health measures between countries. Fifth, pwCF and their families are expert in infection control. Anecdotal evidence suggests that they took early action to avoid infection and effectively started lockdown or shielding earlier than it was imposed in countries across Europe to proactively avoid infection [25]. Sixth, we do not know details about the type of PCR tests used to diagnosis SARS-CoV-2 infection in pwCF across the different countries. PCR tests vary in brand, sensitivity and the platform used [26]. Given these caveats, the true incidence in pwCF will only become apparent with large scale, comprehensive and timely serological studies.
Nonetheless, our data show the importance that pwCF continue to protect themselves from infection by maintaining good lung health (via exercise, physiotherapy and medical treatment) and by shielding, reducing social contacts, wearing a mask and maintaining high standards of hand hygiene.
4.1. Disease course and outcomes
SARS-CoV-2 caused symptomatic illness in 101/128 (78.9%) of our cohort, where data about symptoms were available, lower than the 82-100% reported in other studies in CF [5, 7, 19] but comparable to the reported rate of symptomatic illness in the general population in the first half of 2020 (80%) [27]. In our cohort of pwCF, the most common symptoms were general or pulmonary, in line with symptoms commonly experienced by the general population. Just over half of pwCF with SARS-CoV-2 were admitted to hospital and 9.2% were admitted to intensive care. PwCF were more frequently admitted to hospital and intensive care than the general population in all age groups up to 49 years (after which age too few cases in pwCF are available for robust comparison). The use of hospitalization as a proxy for severe Covid-19 should be interpreted with caution, as hospitalization could have been precautionary, or for reasons other than Covid-19. Of note, a meta-analysis has identified male sex as a risk factor for death and intensive care admission in the general population [28]. It is therefore possible that the over-representation of male pwCF in our cohort (55.4%) could have partly driven rates of intensive care.
The case fatality rate in pwCF was lower (but not significantly) than the general population (3.85% vs 7.46%, p=0.133). More data is required to confirm this, and to untangle whether this could be due to the younger age and/or the absence of other risk factors for more severe course of the infection in our cohort of pwCF with SARS-CoV-2, or the heterogeneous testing and reporting between countries.
People with solid organ transplants have an increased risk of severe disease from SARS-CoV-2 infection [29]. ECFSPR 2018 data report that 1816 pwCF (4.4% of the ECFSPR population) were living with a lung transplant [12], representing a large group at risk for severe complications if infected with SARS-CoV-2. Our cohort included 23 pwCF with lung transplant. Upon SARS-CoV-2 infection, lung-transplanted pwCF were more frequently symptomatic, were admitted to hospital 1.7-fold more frequently and required intensive care 8-fold more frequently than pwCF without lung transplant. Previous studies also found that solid organ transplant recipients require more treatment and medical care upon SARS-CoV-2 infection, whether transplant was for CF [5, 7] or other reasons [30, 31].
4.2. Limitations and future directions
Our study had several limitations, as well as the caveats related to testing and incidence discussed above. Throughout the study period, countries had differing impact of the pandemic and heterogeneous access to testing. Case definitions were also heterogeneous between countries, as reflected in the different case definitions in published studies in CF [5, 7, 19, 21]. Reporting to ECFSPR was voluntary, therefore cases may be under-reported with possible selection bias for more severe cases. In addition, ECFSPR has low coverage in some countries, especially in Eastern Europe [12]. Since these countries reported a low incidence of SARS-CoV-2 in the general population during our study period, we do not believe this to be problematic. However, it could however skew future updates of incidence, as SARS-CoV-2 cases have risen significantly in these countries since summer 2020, whether through increased testing or increased impact of the pandemic. The denominator for calculating SARS-CoV-2 incidence in pwCF was from the 2018 ECFSPR report. It is possible that the CF population has increased since then. In some countries and regions, lung transplanted pwCF are treated by another care team or at another hospital. Therefore, it is possible that some lung-transplanted pwCF no longer participate in ECFSPR and the number of infections in pwCF living with a transplant could be under-reported here. Of note, pwCF from 11 countries in our study were also included in the global study [7] and in national or center-based studies [5, 19, 21, 32] in pwCF.
We sourced comparator data for the general population from the ECDC and included only pwCF with PCR-confirmed SARS-CoV-2 to facilitate comparisons. However, ECDC datasets were limited to certain subsets of countries and age groups, forcing us to exclude some cases in pwCF from comparisons.
Finally, some comparisons reported here should be interpreted with caution due to low sample sizes, particularly in subgroup analyses. We are currently enlarging our dataset by collecting cases up to the end of 2020. This will increase the robustness of our analyses and will facilitate multivariate analyses to identify risk factors associated with severe disease and worse outcomes. We also plan to assess the long-term effect of SARS-CoV-2 infection on lung function and pulmonary exacerbations in pwCF, as well any effects of concomitant CFTR modulator therapy. This is important since pulmonary exacerbation and lung function decline have been previously associated with viral infection by H1N1 [3], RSV [2] and influenza B [33].
5. Conclusion
Since the case cutoff of 30 June 2020 in this study, the number of cases has risen significantly, with expansion into Eastern Europe (1126 cases reported up to 08 March 2021, www.ecfs.eu). At the time of writing, several vaccines have been approved by the EMA and vaccination campaigns have started. Here we show that, versus the age-matched general population, pwCF have a higher incidence of SARS-CoV-2 infection and hospitalization, especially lung-transplanted pwCF. Although more data are required to confirm these findings, it is clear that SARS-CoV-2 infection can result in severe illness and death for pwCF, especially for those with lung transplants. PwCF should continue to protect their health by shielding and maintaining good adherence to treatments and exercise. Governments should prioritize pwCF of all ages for vaccination, by listing CF as an underlying condition with an increased risk of more severe disease.
Contributors
Belgium
Hedwige Boboli, Department of Pediatrics, Pediatric Pulmonology, University Hospital Liège, Liège, Belgium.
Elke De Wachter, Department of Paediatric Pulmonology, Universitair ziekenhuis Brussel, Brussels, Belgium
Lieven Dupont, Department of Pneumology, University Hospitals Leuven, Leuven, Belgium
Sophie Gohy, Department of Pulmonology Cliniques Universitaires Saint-Luc UCL, Brussels, Belgium & Cystic Fibrosis Reference Center Cliniques Universitaires Saint-Luc UCL, Brussels, Belgium
Laurence Hanssens, CF centre, Hôpital Universitaire des Enfants Reine Fabiola (HUDERF), Brussels, Belgium & Institut de mucoviscidose de l'université libre de Bruxelles (ULB), hôpital universitaire des enfants Reine Fabiola - ULB, Brussels, Belgium
Christiane Knoop, Institut de mucoviscidose de l'université libre de Bruxelles (ULB), hôpital universitaire Erasme – ULB, Brussels, Belgium
Vicky Nowé, Department of Pulmonology, GZA Sint-Vincentius Hospital, Antwerp, Belgium
Jessica Pirson, Service de Pneumologie, CHR Citadelle, Liège, Belgium.
Matthieu Thimmesch, Service de Pédiatrie, CHC Clinique du MontLégia, Liège, Belgium
Eva Van Braeckel, Cystic Fibrosis Reference Centre, Department of Respiratory Medicine, Ghent University Hospital, Ghent, Belgium & Department of Internal Medicine and Pediatrics, Ghent University, Ghent, Belgium
Kim Van Hoorenbeeck, Department of Pediatrics, Antwerp University Hospital, Edegem, Belgium
Eef Vanderhelst, Respiratory Division, University Hospital UZ Brussel, Brussels, Belgium
Denmark
Tania Pressler,Rigshospitalet, Copenhagen Cystic Fibrosis Centre, Copenhagen, Denmark
France
French Cystic Fibrosis Reference Network study group:
Michel Abely, Centre Hospitalier Universitaire de Reims, Reims, France
Carole Bailly Piccini, Centre Hospitalier Universitaire de Nice, Nice, France
Chantal Belleguic, Centre Hospitalier Universitaire de Rennes - Hôpital Pontchaillou, Rennes, France
Tiphaine Bihouee, Centre Hospitalier Universitaire de Nantes, Nantes, France
Yves Billon, Centre Hospitalier Régional Universitaire de Nancy, Nancy, France
Stéphanie Bui, Centre Hospitalier Universitaire Bordeaux, Bordeaux, France
Boubou Camara, Centre Hospitalier Universitaire de Grenoble, La Tronche, France
Marie-Christine Cheraud, Centre Hospitalier Universitaire de Clermont-Ferrand, Clermont-Ferrand France
Raphael Chiron, Centre Hospitalier Universitaire de Montpellier, Montpellier, France
Emmanuelle Coirier Duet, Centre Hospitalier de Versailles, Le Chesnay-Rocquencourt, France
Laure Cosson, Centre Hospitalier Régional Universitaire de Tours - Clocheville Hospital, Tours, France
Marie-Laure Dalphin, Centre hospitalier universitaire de Besançon, Besançon, France
Isabelle Danner Boucher, Centre Hospitalier Universitaire de Nantes, Nantes, France
Sandra De Miranda, Hôpital Foch, Suresnes, France
Eric Deneuville, Centre Hospitalier Universitaire de Rennes - Hôpital Sud, Rennes, France
Jean-Christophe Dubus, Hôpitaux Universitaires de Marseille, Marseille, France
Isabelle Durieu, Centre Hospitalier Lyon-Sud, Pierre-Bénite, France
Ralph Epaud, Hôpital Intercommunal de Créteil, Créteil, France
Michèle Gerardin, AP-HP - Hôpital Robert-Debré, Paris, France
Dominique Grenet, Hôpital Foch, Suresnes, France
Véronique Houdouin, AP-HP - Hôpital Robert-Debré, Paris, France
Frédéric Huet, Centre Hospitalier Universitaire de Dijon Bourgogne, Dijon, France
Kanaan Reem, AP-HP Hôpital Cochin, Paris, France
Romain Kessler, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
Jeanne Languepin, Centre Hospitalier Universitaire de Limoges, Limoges, France
Muriel Laurans, Centre Hospitalier Universitaire de Caen, Caen, France
Sylvie Leroy, Centre Hospitalier Universitaire de Nice, Nice, France
Cathie Llerena, Centre Hospitalier Universitaire de Grenoble, La Tronche, France
Julie Macey, Centre Hospitalier Universitaire de Bordeaux - Hôpital Haut-Lévêque, Pessac, France
Julie Mankikian, Centre Hospitalier Régional Universitaire de Tours – Bretonneau, Tours, France
Christophe Marguet, Centre Hospitalier Universitaire de Rouen, Rouen, France
Clémence Martin, AP-HP Hôpital Cochin, Paris, France
Laurent Mely, Hospices Civils de Lyon - Hôpital Renée Sabran, Giens-Hyères, France
Marie Mittaine, Centre Hospitalier Universitaire de Toulouse - Hôpital des Enfants, Toulouse, France
Marlène Murris-Espin, Centre Hospitalier Universitaire de Toulouse - Hôpital Larrey, Toulouse, France
Caroline Perisson, Centre Hospitalier Universitaire de La Réunion - sites Sud, Saint-Pierre, France
Anne Prevotat, Centre Hospitalier Universitaire de Lille, Lille, France
Sophie Ramel, Fondation ILYDS, Roscoff, France
Cinthia Rames, Centre Hospitalier Universitaire Amiens-Picardie, Amiens, France
Philippe Reix, Hospices Civils de Lyon, Hôpital Femme Mère Enfant, Bron, France
Marine Revillon, Centre Hospitalier Universitaire de Lille, Lille, France
Martine Reynaud-Gaubert, Hôpitaux Universitaires de Marseille, Marseille, France
Bénédicte Richaud-Thiriez, Centre hospitalier universitaire de Besançon, Besançon, France
Jean-Luc Rittie, Centre Hospitalier Universitaire de La Réunion - site Felix Guyon, Saint-Denis, France
Manuëla Scalbert-Dujardin, Centre Hospitalier Dunkerque, Dunkerque, France
Isabelle Sermet-Gaudelus, AP-HP - Hôpital Necker Enfants malades, Paris, France
Véronique Storni, Centre hospitalier Bretagne-Atlantique, Vannes, France
Aurélie Tatopoulos, Centre Hospitalier Régional Universitaire de Nancy, Nancy, France
Guillaume Thouvenin, AP-HP Hôpital Armand-Trousseau, Paris, France
Françoise Troussier, Centre hospitalier universitaire d'Angers, Angers, France
Laurence Weiss, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
Nathalie Wizla, Centre Hospitalier Universitaire de Lille, Lille, France
Germany
Eva-Susanne Behl, Klinikum Westbrandenburg, Klinik für Kinder- und Jugendmedizin, Potsdam, Germany
Martin Claßen, Klinikverbund Bremen gGmbH, Klinikum Links der Weser, Christiane Herzog-Ambulanz für Mukoviszidose, Bremen, Germany
Ute Graepler-Mainka, Department of General Pediatrics, Hematology and Oncology, Children's Hospital, Eberhard-Karls-University, Tübingen, Germany
Birte Kinder, Dietrich Bonhoeffer Klinikum Neubrandenbrurg, Klinik für Kinder- und Jugendmedizin, Neubrandenburg, Germany
Holger Köster, Klinikum Oldenburg, Oldenburg, Germany
Jochen Mainz, Brandenburg Medical School (MHB), University, Klinikum Westbrandenburg, Brandenburg an der Havel, Germany
Angelika Mayer, Robert-Bosch-Krankenhaus, Klinik Schillerhöhe, Pneumologie, Gerlingen, Germany
Susanne Naehrig, Medizinische Klinik V (Pneumology), LMU University of Munich, Pneumology, Medizinische Klinik Innenstadt, University of Munich, Munich, Germany
Thomas Nüßlein, Gemeinschaftsklinikum Mittelrhein - Klinik für Kinder- und Jugendmedizin, Koblenz, Koblenz, Germany
Markus Rose, Klinikum Stuttgart, Olgahospital- Pediatric Pulmonology, Stuttgart, Germany
Josef Rosenecker, Fachkliniken Wangen, Wangen, Germany
Anette Scharschinger, Paediatric and Adolescent Medicine, University Medical Center Augsburg, Augsburg, Germany
Christian Schropp, Children's Hospital Dritter Orden, Passau, Germany
Carsten Schwarz, Department of Pediatric Pneumology, Immunology and Intensive Care Medicine, Cystic Fibrosis Center, Charité - Universitätsmedizin Berlin, Berlin, Germany
Simone Stolz, Klinik für Kinder- und Jugendmedizin, Carl-Thiem-Klinikum gGmbH, Cottbus, Germany
Wolfgang Thomas, Klinikum Mutterhaus der Borromäerinnen, Kinder- und Jugendmedizin, Trier, Germany
Sabine Wege, Department of Pneumology and Critical Care Medicine, Thoraxklinik at the University Hospital Heidelberg, Heidelberg, Germany
Britta Welzenbach, Josefinum hospital for children and adolescents, Augsburg, Germany
Bettina Wollschläger, Martin-Luther-University Halle, Clinic for Internal Medicine, Halle, Germany
Greece
Filia Diamantea, Sismanoglio General Hospital of Attica, Adult Cystic Fibrosis Unit, Athens, Greece
Ireland
Barry J. Plant, University College Cork, Cork, Ireland
Cedric Gunaratnam, Beaumont Hospital, Dublin, Ireland
Italy
Annalisa Amato and Gianluca Ferrari, technical board of ICFR, Italian Cystic Fibrosis Ligue, Rome, Italy
Carlo Castellani and Rosaria Casciaro, CF Referral Center Liguria Region, IRCCS Istituto Giannina Gaslini Genova, Italy
Marco Cipolli and Francesca Lucca, CF Referral Center Veneto Region, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy
Valeria Daccò, Vanessa Gagliano and Giovanna Pizzamiglio, CF Referral Center Lombardia Region, Fondazione IRCCS Cà Granda - Ospedale Maggiore Policlinico, Milan, Italy
Elisabetta Bignamini and Anna Folino, CF Referral Center Piemonte and Valle D'Aosta Regions, Ospedale Infantile Regina Margherita – Sant’ Anna, Torino, Italy
Massimo Maschio, CF Referral Center Friuli-Venezia Giulia Region, IRCCS Materno Infantile Burlo Garofolo, Trieste, Italy
Mirco Ros, Veneto Region CF Support Center of Treviso, Ospedale Ca' Foncello, Treviso Italy
Barbara Messore, Adult CF Centre Torino, Pulmonolgy Dept, Azienda Ospedaliero Universitaria San Luigi Gonzaga, Orbassano, Italy
Piercarlo Poli, Department of Pediatrics, Regional support Centre for Cystic Fibrosis, Children's Hospital – ASST Spedali Civili Pz.le Spedali Civili, University of Brescia, Brescia, Italy
Stefano Pantano, CF Referral Center Abruzzi and Molise Region, Teramo, Italy
Fiorella Battistini and Valentina Donati, CF Referral Center Emilia-Romagna Region, Cesena, Italy
Valeria Mencarini and Nicola Palladino, CF Referral Center Umbria Region, Gubbio, Italy
Giuseppe Cimino, CF Referral Center Lazio Region, Rome, Italy
Valeria Raia and Caterina Laezza, CF Pediatric Referral Center Campania Region, Naples, Italy
Pamela Vitullo, CF Support Center, Puglia Region, Cerignola, Italy
Salvatore Leonardi and Novella Rotolo, CF Support Center, Sicily Region, Catania, Italy
Giovanna Pisi, Cinzia Spaggiari, CF Referral Center, Emilia-Romagna Region, Parma, Italy
Vincenzo Carnovale, Adult CF Referral Center, Campania Region, Naples, Italy
Maria Cristina Lucanto and Ester Quattromano, CF Referral Center, Sicily Region, Messina, Italy
Antonio Manca and Giuseppina Leonetti, CF Referral Center, Puglia Region, Bari, Italy
Mirella Collura and Francesca Ficili, CF Referral Center, Sicily Region, Palermo, Italy
Vincenzina Lucidi, Federico Alghisi, Fabiana Ciciriello and Fabio Majo, CF UOC, Paediatric Hospital “Bambino Gesù”, Rome, Italy
Luxembourg
Hélène De la Barrière, Department of Pulmonology, Hôpitaux Robert Schuman, Luxembourg, Luxembourg
Russia
Evgeniya Boitsova, Department of propaedeutics of children's diseases. Federal state budgetary scientific institution of higher education "Saint Petersburg state pediatric medical University" of the Russian Federation Ministry of Health, Saint Petersburg, Russia
Yuliya Gorinova , National Medical Research Center for Children's Health, Moscow, Russia
Stanislav Krasovskiy, Laboratory of cystic fibrosis, Scientific Research Institute of Pulmonology of the Federal Medical and Biological Agency of Russia, Moscow, Russia
Maria Mukhina, Medical and genetic Department, cystic fibrosis office of the State budgetary healthcare institution "Morozovskaya Children's Municipal Clinical Hospital" Moscow, Russia
Victoria Sherman, The scientific and clinical Department of cystic fibrosis, "Research Centre for Medical Genetics", Moscow, Russia
Spain
Antonio Alvarez Fernàndez, Hospital Universitario Vall d'Hebron, Adult Cystic Fibrosis unit, Barcelona, Spain
Isodoro Cortell-Aznar, Hospital Universitario y Politécnico La Fe, Unidad de Trasplante Pulmonar y Fibrosis Quística, Valencia, Spain
Layla Diab Cáceres, Hospital 12 de Octubre, Unidad de Fibrosis Quística, Madrid, Spain
Silvia Gartner, Hospital Vall d'Hebron, Unidad Fibrosis Quística y Neumología Pediátrica, Barcelona, Spain
Rosa Maria Girón-Moreno, Hospital Universitario La Princesa, Neumología Adultos, Madrid, Spain
Carlos Peñalver Mellado, Hospital Clinico Universitario Virgen de la Arrixaca, Murcia, Spain
Rosa Nieto-Royo, Hospital Universitario de Ramón y Cajal, Unidad de Fibrosis Quística, Madrid, Spain
Concepción Prados-Sanchez, Hospital Universitario La Paz, Unidad de Fibrosis Quìstica Adultos, Servicio de Neumología, Madrid, Spain
Isabel Ramos Cancelo, Hospital Clinico Universitario de Valladolid, Vallalodid, Spain
Marta Ruiz de Valbuena, Hospital Infantil La Paz, Sección de Neumología Pediátrica, Unidad de Fibrosis Quística Pediátrica, Madrid, Spain
Sweden
Stefanie Diemer, Lunds university hospital, Sweden
Marita Gilljam, Gothenburg CF center, Sahlgrenska University Hospital, Sweden
Christina Krantz, Department of Women's and Children's Health, Research group; Paediatric Inflammation, Metabolism and Child Health Research, Uppsala University, Uppsala, Sweden
Ulrika Lindberg, Department of Respiratory Medicine and Allergology, Lund CF center, Skane University Hospital, Lund, Sweden
Switzerland
Christian Clarenbach, Universitätsspital Zürich, Klinik für Pneumologie, Adultes CF Zentrum, Zürich, Switzerland
Reta Fischer, Lindenhofspital Quartier Bleu, Bern, Switzerland
Isabelle Rochat, Centre Hospitalier Universitaire Vaudois (CHUV), Département femme-mère-enfant, Service de pédiatrie, Unité de pneumologie et mucoviscidose pédiatrique, Lausanne, Switzerland
Ukraine
Lyudmyla Bober, Western Ukrainian Specialised Children's Medical Centre, Lviv, Ukraine
Author Statement
Naehrlich Lutz: Conceptualization, Methodology, Resources, Writing - Review & Editing, Project administration, Supervision, Funding acquisition. Orenti Annalisa:Conceptualization, Methodology, Formal analysis, Data Curation, Writing - Review & Editing, Project administration. Dunlevy Fiona: Conceptualization, Writing - Original Draft, Writing - Review & Editing, Project administration. Kasmi Irena: Resources, Writing - Review & Editing.Harutyunyan Satenik: Resources, Writing - Review & Editing. Pfleger Andreas: Resources, Writing - Review & Editing. Bobrovnichy Vladimir: Resources, Writing - Review & Editing. Keegan Svetlana: Resources, Writing - Review & Editing. Daneau Géraldine: Resources, Writing - Review & Editing. Petrova Guergana: Resources, Writing - Review & Editing. Bambir Ivan: Resources, Writing - Review & Editing. Dugac Vukic Andrea: Resources, Writing - Review & Editing. Tješić-DrinkovićDuška: Resources, Writing - Review & Editing. Yiallouros Panayiotis: Resources, Writing - Review & Editing.Drevinek Pavel: Resources, Writing - Review & Editing.Macek Milan Jr.: Resources, Writing - Review & Editing. Bilkova Alena: Resources, Writing - Review & Editing. Olesen Hanne Vebert: Resources, Writing - Review & Editing. Burgel Pierre-Régis: Resources, Writing - Review & Editing. Corvol Harriet: Resources, Writing - Review & Editing.Lemonnier-Videau Lydie: Resources, Writing - Review & Editing. Parulava Tsitsino: Resources, Writing - Review & Editing. Hatziagorou Elpis: Resources, Writing - Review & Editing. Diamantea Filia: Resources, Writing - Review & Editing. Párniczky Andrea: Resources, Writing - Review & Editing. Fletcher Godfrey:Resources, Writing - Review & Editing.McKone Edward F: Resources, Writing - Review & Editing. Mei-Zahav Meir: Resources, Writing - Review & Editing. Padoan Rita: Resources, Writing - Review & Editing. Salvatore Marco: Resources, Writing - Review & Editing. Colombo Carla: Resources, Writing - Review & Editing. Aleksejeva Elina: Resources, Writing - Review & Editing. Malakauskas Kestutis: Resources, Writing - Review & Editing. Schlesser Marc: Resources, Writing - Review & Editing. Fustik Stojka: Resources, Writing - Review & Editing. Turcu Oxana: Resources, Writing - Review & Editing. Gulmans Vincent: Resources, Writing - Review & Editing. Zomer-van Ommen Domenique: Resources, Writing - Review & Editing. Senstad Wathne Anita: Resources, Writing - Review & Editing. Bakkeheim Egil: Resources, Writing - Review & Editing. Woźniacki Łukasz: Resources, Writing - Review & Editing. Pereira Luísa: Resources, Writing - Review & Editing, Pop Liviu: Resources, Writing - Review & Editing. Kondratyeva Elena: Resources, Writing - Review & Editing. Amelina Elena: Resources, Writing - Review & Editing. Zhekaite Elena: Resources, Writing - Review & Editing. Simonova Olga: Resources, Writing - Review & Editing. Kashirskaya Nataliya: Resources, Writing - Review & Editing. Rodić Milan: Resources, Writing - Review & Editing. Kayserova Hana: Resources, Writing - Review & Editing. Krivec Uros: Resources, Writing - Review & Editing. Mondejar-Lopez Pedro: Resources, Writing - Review & Editing. Pastor-Vivero Maria Dolores: Resources, Writing - Review & Editing.de Monestrol Isabelle: Resources, Writing - Review & Editing. Lindblad Anders: Resources, Writing - Review & Editing. Dogru Deniz: Resources, Writing - Review & Editing. Yasemin Gökdemir: Resources, Writing - Review & Editing. Pekcan Sevgi: Resources, Writing - Review & Editing. Makukh Halyna: Resources, Writing - Review & Editing. Brownlee Keith: Resources, Writing - Review & Editing. Cosgriff Rebecca: Resources, Writing - Review & Editing. McClenaghan Eliott: Resources, Writing - Review & Editing. Carr Siobhán: Resources, Writing - Review & Editing. Lammertyn Elise: Writing - Review & Editing. Zolin Anna: Writing - Review & Editing. Fox Alice: Writing - Review & Editing, Project administration. Krasnyk Marco: Writing - Review & Editing, Project administration. Van Rens Jacqui: Writing - Review & Editing, Project administration. van Koningsbruggen-Rietschel Silke: Conceptualization, Methodology, Resources, Writing - Review & Editing. Jung Andreas: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Funding acquisition.
All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
Dr. Naehrlich reports that he has received institutional fees for site participation in clinical trials from Vertex Pharmaceuticals and Boehringer Ingelheim; Dr. Orenti has nothing to disclose; Dr. Dunlevy reports institutional grants from Chiesi, during the conduct of the study; Dr. Kasmi has nothing to disclose; Dr. Harutyunyan has nothing to disclose; Dr. Pfleger has nothing to disclose; Dr. Bobrovnichy has nothing to disclose;Dr. Keegan has nothing to disclose; Dr. Daneau has nothing to disclose; Dr. Petrova has nothing to disclose; Dr. Bambir has nothing to disclose; Dr. Vukić Dugac has nothing to disclose; Dr. Tješić-Drinković has nothing to disclose; Dr. Yiallouros has nothing to disclose; Dr. Drevinek reports personal fees from Vertex Pharmaceuticals, outside the submitted work; Prof. Milan Macek reports grants from Vertex Pharmaceuticals, outside the submitted workr r; Mrs. Bilkova has nothing to disclose; Dr. Olesen has nothing to disclose; Dr. Burgel reports personal fees from Astra-Zeneca, personal fees from Boehringer Ingelheim, personal fees from Chiesi, personal fees from GSK, personal fees from Insmed, personal fees from Novartis, personal fees from Pfizer, grants and personal fees from Vertex, personal fees from Zambon, outside the submitted work; Dr. Corvol has nothing to disclose; Ms. Lemmonier has nothing to disclose; Dr. Parulava has nothing to disclose; Dr. Hatziagorou has nothing to disclose; Dr. Diamantea has nothing to disclose; Dr. Párniczky has nothing to disclose; G. Fletcher has nothing to disclose; Prof. McKone reports travel support from A Menarini, speaker fees from Roche Pharmaceuticals, consultancy fees from Insmed, consultancy fees from Janssen Pharmaceuticals, grants to institution and consultancy fees from Vertex, outside the submitted work; Dr. Mei-Zahav has nothing to disclose; Dr. Padoan has nothing to disclose; Dr. Salvatore has nothing to disclose; Dr. Colombo has nothing to disclose; Dr. Aleksejeva has nothing to disclose; Dr. Malakauskas has nothing to disclose; Dr. Schlesser has nothing to disclose; Dr. Fustik has nothing to disclose; Dr. Turcu has nothing to disclose; V. Gulmans has nothing to disclose; D. Zomer-van Ommen has nothing to disclose; Dr. Wathne has nothing to disclose; Dr. Bakkeheim has nothing to disclose; Dr. Wozniacki has nothing to disclose; Dr. Pereira has nothing to disclose; Dr. Pop has nothing to disclose; Dr. Kondratyeva has nothing to disclose; Dr. Amelina has nothing to disclose; Dr. Zhekaite has nothing to disclose; Dr. O. Simonova has nothing to disclose; Dr. Kashirskaya has nothing to disclose; Dr. Rodic has nothing to disclose; Dr. Kayserova has nothing to disclose; Dr. Krivec has nothing to disclose; Dr. Mondejar-Lopez has nothing to disclose; Dr. Pastor-Vivero has nothing to disclose; Dr. de Monestrol reports grants from Vertex, outside the submitted work; Dr. Lindblad has nothing to disclose; Dr. Dogru has nothing to disclose; Dr. Gokdemir has nothing to disclose; Dr. Pekcan has nothing to disclose; Dr. Makukh has nothing to disclose; Dr. Brownlee has nothing to disclose; Ms. Cosgriff has nothing to disclose; Mr. McClenaghan has nothing to disclose; Dr. Carr reports personal fees from Chiesi Pharmaceuticals, personal fees and non-financial support from Vertex, personal fees from Zambon, personal fees from Insmed, outside the submitted work; Dr. Lammertyn has nothing to disclose; Dr. Zolin has nothing to disclose; Ms.. Fox reports grants from ECFS, during the conduct of the study; Mr Krasnyk has nothing to disclose; Mrs. Van Rens has nothing to disclose; Dr. van Koningsbruggen-Rietschel reports grants and personal fees from Algipharma (HORIZON2020), personal fees from Deutsches Zentrum für Infektionsforschung, personal fees from Antabio, personal fees from Proteostasis, personal fees from Roche, personal fees from Vertex, outside the submitted work; Dr. Jung reports grants from Chiesi Pharmaceuticals, during the conduct of the study.
Acknowledgements
We thank the people with CF, and their families, for consenting to their data being included in the ECFSPR. We thank the centers and individual country representatives for allowing the use of the anonymized patient data.
This work was supported by an unrestricted grant from Chiesi Farmaceutici SpA, Parma, Italy. The funder had no role in the design, conduct or reporting of this study.
Datasets for the general population were provided by the European Centre for Disease Prevention and Control (ECDC) based on data provided by WHO and Ministries of Health from the affected countries. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the ECDC. The accuracy of the authors' statistical analysis and the findings they report are not the responsibility of ECDC. ECDC is not responsible for conclusions or opinions drawn from the data provided. ECDC is not responsible for the correctness of the data and for data management, data merging and data collation after provision of the data. ECDC shall not be held liable for improper or incorrect use of the data.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcf.2021.03.017.
Contributor Information
European Cystic Fibrosis COVID project group:
Vladimir Bobrovnichy, Ivan Bambir, Andrea Dugac Vukic, Pavel Drevinek, Milan Macek Jr, Harriet Corvol, Lydie Lemonnier-Videau, Elpis Hatziagorou, Godfrey Fletcher, Rita Padoan, Vincent Gulmans, Egil Bakkeheim, Elena Kondratyeva, Elena Amelina, Elena Zhekaite, Olga Simonova, Maria Dolores Pastor-Vivero, Anders Lindblad, Yasemin Gökdemir, Sevgi Pekcan, Keith Brownlee, Elliott McClenaghan, Siobhán Carr, Elise Lammertyn, Anna Zolin, Alice Fox, Marko Krasnyk, and Jacqui Van Rens
Appendix. Supplementary materials
References
- 1.Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiedrowski MR, Bomberger JM. Viral-Bacterial Co-infections in the Cystic Fibrosis Respiratory Tract. Front Immunol. 2018;9:3067. doi: 10.3389/fimmu.2018.03067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viviani L, Assael BM, Kerem E, group EHNs. Impact of the A (H1N1) pandemic influenza (season 2009-2010) on patients with cystic fibrosis. J Cyst Fibros. 2011;10:370–376. doi: 10.1016/j.jcf.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Pinar Senkalfa B, Sismanlar Eyuboglu T, Aslan AT, Ramasli Gursoy T, Soysal AS, Yapar D. Effect of the COVID-19 pandemic on anxiety among children with cystic fibrosis and their mothers. Pediatr Pulmonol. 2020;55:2128–2134. doi: 10.1002/ppul.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corvol H, de Miranda S, Lemonnier L, Kemgang A, Reynaud Gaubert M, Chiron R. First Wave of COVID-19 in French Patients with Cystic Fibrosis. J Clin Med. 2020;9:3624. doi: 10.3390/jcm9113624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosgriff R, Ahern S, Bell SC, Brownlee K, Burgel PR, Byrnes C. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cyst Fibros. 2020;19:355–358. doi: 10.1016/j.jcf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClenaghan E, Cosgriff R, Brownlee K, Ahern S, Burgel PR, Byrnes CA. The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J Cyst Fibros. 2020;19:868–871. doi: 10.1016/j.jcf.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bain R, Cosgriff R, Zampoli M, Elbert A, Burgel PR, Carr SB. Clinical characteristics of SARS-CoV-2 infection in children with cystic fibrosis: An international observational study. J Cyst Fibros. 2021;20:25–30. doi: 10.1016/j.jcf.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Public health surveillance for COVID-19.2020. https://www.who.int/publications/i/item/who-2019-nCoV-surveillanceguidance-2020. 7 Date accessed: 23 November 2020.
- 10.World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases.2020. https://www.who.int/publications/i/item/10665-331501. Date accessed: 23 November 2020.
- 11.World Health Organization. International guidelines for certification and classification (coding) of Covid-19 as cause of death.2020. https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19.pdf?ua=1. Date accessed: 23 November 2020.
- 12.ZolinA, OrentiA, NaehrlichL, JungA, van RensJ. ECFS Patient Registry Annual Report 2018. 2020. https://www.ecfs.eu/projects/ecfs-patient-registry/annual-reports
- 13.Viviani L, Zolin A, Mehta A, Olesen HV. The European Cystic Fibrosis Society Patient Registry: valuable lessons learned on how to sustain a disease registry. Orphanet J Rare Dis. 2014;9:81. doi: 10.1186/1750-1172-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dataset. European Centre for Disease Control.Daily number of new reported cases of COVID-19 by country worldwide.2020. https://www.ecdc.europa.eu/en/publications-data/download-todays-data-geographic-distribution-covid-19-cases-worldwide. Date accessed: 06 Dec 2020.
- 15.Dataset. European Centre for Disease Control.Data on hospital and ICU admission rates and current occupancy for COVID-19.2020. https://www.ecdc.europa.eu/en/publications-data/download-data-hospital-and-icu-admission-rates-and-current-occupancy-covid-19. Date accessed: 06 Dec 2020.
- 16.European Centre for Disease Control. How ECDC collects and processes Covid-19 data. 2021. https://www.ecdc.europa.eu/en/covid-19/data-collection. Date accessed: 11 Jan 2021.
- 17.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EngelsB. XNomial: Exact Goodness-of-Fit Test for Multinomial Data with Fixed Probabilities. R package version 1.0.4 2015. https://cran.r-project.org/package=XNomial
- 19.Mondejar-Lopez P, Quintana-Gallego E, Giron-Moreno RM, Cortell-Aznar I, Ruiz de Valbuena-Maiz M, Diab-Caceres L. Impact of SARS-CoV-2 infection in patients with cystic fibrosis in Spain: Incidence and results of the national CF-COVID19-Spain survey. Respir Med. 2020;170 doi: 10.1016/j.rmed.2020.106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padoan R, Carnovale V, Salvatore D, Quattrucci S, Taruscio D, Floridia G. First and second wave of SARS-CoV2 in Italian Cystic Fibrosis patients: Data from Italian Cystic Fibrosis Registry. J Cyst Fibros. 2021;26:S1569–S1993. doi: 10.1016/j.jcf.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bezzerri V, Lucca F, Volpi S, Cipolli M. Does cystic fibrosis constitute an advantage in COVID-19 infection? Ital J Pediatr. 2020;46:143. doi: 10.1186/s13052-020-00909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephenson AL, Stanojevic S, Sykes J, Burgel PR. The changing epidemiology and demography of cystic fibrosis. Presse Med. 2017;46:e87–e95. doi: 10.1016/j.lpm.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Dataset. European Centre for Disease Control.Data on testing for COVID-19 by week and country.2020. https://www.ecdc.europa.eu/en/publications-data/covid-19-testing.
- 24.van Koningsbruggen-Rietschel S, Dunlevy F, Bulteel V, Downey DG, Dupont L. SARS-CoV-2 disrupts clinical research: the role of a rare disease-specific trial network. Eur Respir J. 2020;56 doi: 10.1183/13993003.02114-2020. 2002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombo C, Burgel PR, Gartner S, van Koningsbruggen-Rietschel S, Naehrlich L, Sermet-Gaudelus I. Impact of COVID-19 on people with cystic fibrosis. Lancet Respir Med. 2020;8:e35–ee6. doi: 10.1016/S2213-2600(20)30177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Kasteren PB, van der Veer B, van den Brink S, Wijsman L, de Jonge J, van den Brandt A. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for disease control and prevention (CDC). Evidence used to update the list of underlying medical conditions that increase a person's risk of severe illness from COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html. Date accessed: 22 December 2020. [PubMed]
- 30.Raja MA, Mendoza MA, Villavicencio A, Anjan S, Reynolds JM, Kittipibul V. COVID-19 in solid organ transplant recipients: A systematic review and meta-analysis of current literature. Transplant Rev (Orlando) 2021;35 doi: 10.1016/j.trre.2020.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saez-Gimenez B, Berastegui C, Barrecheguren M, Revilla-Lopez E, Los Arcos I, Alonso R. COVID-19 in lung transplant recipients: A multicenter study. Am J Transplant. 2020 doi: 10.1111/ajt.16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondratyeva EI, Krasovsky SA, Kashirskaya NY, Amelina EL, Zhekaite EK, Sherman VD. COVID-19 in cystic fibrosis patients. Pulmonologiya. 2020;30:544–552. doi: 10.18093/0869-0189-2020-30-5-544-552. [DOI] [Google Scholar]
- 33.Dennis JB, Jones AM, Davies EA, Welfare W, Barry PJ, Collier L. Influenza B outbreak at an adult cystic fibrosis centre - Clinical impact and factors influencing spread. J Cyst Fibros. 2020;19:808–814. doi: 10.1016/j.jcf.2020.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.