Abstract
Although in situ hybridization (ISH) and polymerase chain reaction (PCR) have extensively been used on cytology specimens, there have been limited reports of the usefulness of these techniques in relation to confirmed histologic findings. In this study, we used PCR and ISH to detect human papillomavirus (HPV) in cytologic and histologic specimens, respectively. By using positive and negative likelihood ratios, we attempted to identify any predictive role of ISH testing alone or in combination with PCR for the development of high-grade histologic lesions (cervical intraepithelial neoplasia [CIN] 2+). In our study, ISH was a useful method for detection of HPV, even in a large fraction of samples with normal cytologic or biopsy findings. We suggest that when used together and evaluated in conjunction with histologic sections, ISH is a useful tool for ancillary molecular testing of HPV infection in cervical lesions, especially in CIN 2+ histological lesions where its analytic sensitivities and specificities were as good as those of PCR testing.
Keywords: Human papillomavirus, HPV, In situ hybridization, Polymerase chain reaction, PCR, Cervical intraepithelial neoplasia, Cancer
Infection with human papillomavirus (HPV) is a major risk factor for the development of precancerous and cancerous cervical lesions.1 HPV DNA is found in more than 90% of cervical cancers,1 but it can also be detected in low-grade lesions. Integration of HPV DNA into host-cell DNA is a major event in development of HPV-related cancer.2 The strong correlation between viral integration and invasive carcinoma has led to the increasing use of HPV DNA testing as an additional diagnostic tool in cervical cancer screening.3–5 In situ hybridization (ISH), a direct signal detection assay, has the advantage of preserving the morphologic context of HPV DNA signals.6 A historic issue concerning the use of ISH for viral detection has been its low sensitivity,7 but improved signal-detecting methods have shown higher sensitivity.8,9
Although ISH and polymerase chain reaction (PCR) have been extensively used on liquid-based cytology (LBC) specimens,10–12 there have been limited reports of the usefulness of these techniques in relation to histologic findings. Immunohistochemical detection of p16INK4a,13–19 Ki-67,13–19 and HPV L120 is a valuable adjunctive aid in the diagnosis of difficult-to-interpret cervical biopsy specimens and has been used for immunohistochemical detection of HPV in many studies.13–20 However, there are limited data comparing rates of HPV detection by ISH in cervical tissues with similar rates obtained by HPV-specific PCR performed in cytologic samples. In addition, a study using ISH to detect HPV in cervical tissues found poor overall sensitivity of L1-based testing for HPV detection and concluded that broad-spectrum HPV ISH could possibly provide a better “gold standard” for immunohistochemical diagnosis of HPV as the probes kits improve.20
In this study, we examined the association between HPV infection detected by PCR and broad-spectrum HPV ISH with cytologic and histologic findings. Moreover, we attempted to identify any predictive role of ISH testing on histologic sections alone or in combination with PCR testing on liquid-based cytologic specimens for the development of high-grade squamous intraepithelial lesion (HSIL).
Materials and Methods
Cervical Tissue Specimen Selection
Archived, formalin-fixed, paraffin-embedded cervical biopsy specimens obtained from 2005 to 2007 were retrieved from the Department of Pathology at St Elizabeth’s Medical Center, Boston, MA. The study was approved by the Human Research Subjects Committee of the institution. In consecutive order, 210 cervical tissue specimens from biopsies, loop electrosurgical excision procedures, cone biopsies, and hysterectomies were selected. Four pathologists independently reviewed the H&E-stained slides. If there was diagnostic agreement between the first 2 reviewers, consensus diagnosis was achieved, and no additional reviews were conducted. Cases with diagnostic disagreement between the 2 reviewers were rereviewed together, masked to the earlier diagnoses, by 4 reviewing pathologists at a multiheaded microscope until a consensus diagnosis was reached. This consensus was reached by reviewing H&E stains only; no immunohistochemical results were used.
The presence of koilocytotic atypia, the morphologic hallmark of HPV cytopathic effect (CPE) and the earliest cytologic manifestation of cervical intraepithelial neoplasia (CIN) that may suggest presence of HPV in the tissue,21 was also determined by 4 experienced cytopathologists. Specimens were eliminated from the study for any of the following reasons: (1) no consensus in diagnosis; (2) no available LBC sample from the same patient; and (3) samples from patients having one of the following conditions that may be associated with equivocal cytologic findings: pregnancy, HIV infection, and immunosuppressant therapy.22 Thus, from 210 samples that were initially screened, 176 tissue samples were included in the final study.
Liquid-Based Cytology
Only data for patients with available LBC specimens were included in the study. Specimens were collected and slides prepared using the SurePath Liquid-Based Pap Test (BD, Franklin Lakes, NJ) Slides were examined by 2 independent cytopathologists (L.A. and I.S.A.) and were classified according to the 2001 Bethesda System.23
In Situ Hybridization
Thin-section microtome sections were prepared from biopsy tissue. Serial sections adjacent to those used for histologic diagnosis were tested for HPV DNA. ISH was performed using the GenPoint Catalyzed Signal Amplification System (DAKO, Carpinteria, CA)24 for high-risk (HR)-HPV (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68, code Y1443) and low-risk (LR)-HPV (types 6 and 11) according to manufacturer protocols. This method allowed us to detect as few as 1 or 2 copies of HPV DNA as previously described.24 Two CIN 3 cases were used as positive control cases that were positive in previous reactions.
HPV DNA Testing by PCR
Genomic HPV DNA was extracted at Esoterix Molecular Genetics, Eden Prairie, MN, from the submitted SurePath AutoCyte liquid-based sample media and amplified by PCR using consensus oligonucleotide primers specific for the L1 region of the HPV genome, as previously described.25 Concurrently, the integrity of the extracted DNA was evaluated by amplification of β-globin, a common housekeeping gene. Amplified products were subjected to digestion by restriction endonuclease(s) HaeIII, PstI, and RSAI. Digested DNA fragments were separated on a 5% polyacrylamide gel and visualized by ethidium bromide intercalation. A digital image of the gel was captured, and the specific HPV type was determined by matching the restriction fragment patterns of the respective specimens to that of known HPV controls. HPV DNA testing was carried out blindly without knowledge of the pathologic diagnosis.
Statistical Analysis
Categorical data are presented as rates. Comparisons between rates of positive HPV DNA or ISH testing between groups with and without high-grade lesions were made using Mann-Whitney testing or 1-way analysis of variance for continuous variables and the χ2 or Fisher exact test for categorical data. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) are presented for HPV DNA detection, ISH, and cytologic findings (HSIL+) with regard to CIN 2+ lesions. Likelihood ratios were used to calculate the odds of posttest probabilities when multiplied by the odds of the prevalence of the disease. Statistical analyses were performed using SPSS, version 16.0 (SPSS, Chicago, IL) statistical software. All statistical tests were 2-tailed. A P value of .05 or less was considered statistically significant.
Results
Cytologic Findings
Specimens for 176 patients were examined (median age, 29 years; interquartile range, 24–37 years). Two cases of adenocarcinoma were noted in patients older than 45 years and 1 in a 30-year-old subject. In all 3 cases, adenocarcinoma was present in the distal portion of the endocervical canal and at the squamocolumnar junction with small foci of adjacent HSIL. No cases of invasive squamous cell carcinoma were identified.
HPV Detection and Cytologic Results
The association between HPV detection methods and cytologic results is shown in ▮Table 1▮ and ▮Table 2▮. The results are stratified according to the oncogenic potential of the virus. From 176 LBC samples, 102 (58.0%) were positive for HPV by PCR; 77 (76.2%) samples were positive for 1 HPV genotype and 24 (23.8%) were positive for multiple genotypes. We identified 30 HPV genotypes, ie, 6, 11, 16, 18, 31, 33, 35, 39, 45, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 69, 72, 82, 83, 84, MM4, CP8304, CP141, LVX160, and CP6108, and 7 were unknown types (X). Of 101 samples, 78 (77.2%), 9 (8.9%), and 14 (13.9%) were positive for HR, LR, and unknown-risk HPV genotypes, respectively. The most common HPV genotypes that were detected were HPV-16 (16/101 [15.8%]), HPV-53 (10/101 [9.9%]), HPV-66 (8/101 [7.9%]), HPV-31 (7/101 [6.9%]), HPV-84 (6/101 [5.9%]), and HPV-18 (5/101 [5.0%]). HPV-45 was detected in 3 cases (3.0%). The most common genotype identified in mixed infections was genotype 16 (12/24 with multiple infections [50%]). HPV genotypes were identified in all 17 patients with HSIL+ cytology.
Table 1.
Associations of Liquid-Based Cytology, CPE, and HPV+ Testing by PCR*
| Liquid-Based Cytology Results | ||||||
|---|---|---|---|---|---|---|
| Negative | ASCUS | LSIL | HSIL | Adenocarcinoma | Total | |
| CPE | ||||||
| Absent | 28 (54) | 10 (19) | 8 (15) | 3 (6) | 3 (6) | 52 (100) |
| Present | 19 (15.3) | 34 (27.4) | 60 (48.4) | 11 (8.9) | 0 (0) | 124 (100.0) |
| PCR for HPV | ||||||
| Negative | 44 (59) | 13 (18) | 16 (22) | 0 (0) | 1 (1) | 74 (100) |
| Low-risk HPV | 0 (0) | 3 (33) | 5 (56) | 0 (0) | 1 (11) | 9 (100) |
| High-risk HPV | 2 (3) | 23 (29) | 40 (51) | 12 (15) | 1 (1) | 78 (100) |
| Unknown-risk HPV | 1 (7) | 5 (36) | 6 (43) | 2 (14) | 0 (0) | 14 (100) |
ASCUS, atypical squamous cells of undetermined significance; CPE, cytopathic effect; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; PCR, polymerase chain reaction.
Data are given as number (percentage) within the individual CPE and PCR categories.
Table 2.
Associations Between Liquid-Based Cytology Results and HPV+ Testing by ISH*
| Liquid-Based Cytology Results | ||||||
|---|---|---|---|---|---|---|
| ISH | Negative | ASCUS | LSIL | HSIL | Adenocarcinoma | Total |
| Negative | 44 (71) | 8 (13) | 9 (15) | 0 (0) | 1 (2) | 62 (100) |
| Low-risk HPV | 1 (25) | 1 (25) | 2 (50) | 0 (0) | 0 (0) | 4 (100) |
| High-risk HPV | 1 (1.0) | 33 (32.4) | 52 (51.0) | 14 (13.7) | 2 (2.0) | 102 (100) |
| High-and low-risk HPV | 1 (13) | 2 (25) | 5 (63) | 0 (0) | 0 (0) | 8 (100) |
| Total | 47 (26.7) | 44 (25.0) | 68 (38.6) | 14 (8.0) | 3 (1.7) | 176 (100.0) |
ASCUS, atypical squamous cells of undetermined significance; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; ISH, in situ hybridization; LSIL, low-grade squamous intraepithelial lesion.
Data are given as number (percentage).
Table 2 depicts the association between ISH results and cytologic findings. Overall, 112 (86.8%) of 129 patients with abnormal cytologic smears had ISH+ cervical tissues. Positive ISH signals were observed in 59 (87%) of 68 samples with low-grade squamous intraepithelial lesion (LSIL), 14 (100%) of 14 with HSIL, and 3 (100%) of 3 with adenocarcinoma for a combined 76 (89%) of 85 LSIL+ (LSIL or higher lesions) cytologic lesions. Moreover, CPE was noted in 71 (84%) of 85 LSIL+ lesions, and mild dysplasia (CIN 1) was present in 49 (58%) of 85 LSIL+ lesions ▮Table 3▮.
Table 3.
Associations in Histologic Diagnosis, LBC, CPE, and HPV+ Testing by ISH vs PCR*
| Cervical Biopsy Result | ||||||
|---|---|---|---|---|---|---|
| Results | Negative | CIN 1 | CIN 2 | CIN 3 | Adenocarcinoma | Total |
| LBC | ||||||
| Negative | 44 (9) | 2 (4) | 1 (2) | 0 (0) | 0 (0) | 47 (100) |
| ASCUS | 13 (30) | 17 (39) | 10 (23) | 2 (5) | 2 (5) | 44 (100) |
| LSIL | 13 (19) | 45 (66) | 9 (13) | 1 (1) | 0 (0) | 68 (100) |
| HSIL | 0 (0) | 4 (29) | 6 (43) | 3 (21) | 1 (7) | 14 (100) |
| Adenocarcinoma | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 2 (67) | 3 (100) |
| Total | 70 (39.8) | 68 (38.6) | 26 (14.8) | 7 (4.0) | 5 (2.8) | 176 (100.0) |
| CPE | ||||||
| Absent | 34 (65) | 6 (12) | 4 (8) | 4 (8) | 4 (8) | 52 (100) |
| Present | 36 (29.0) | 62 (50.0) | 22 (17.7) | 3 (2.4) | 1 (0.8) | 124 (100.0) |
| Total | 70 (39.8) | 68 (38.6) | 26 (14.8) | 7 (4.0) | 5 (2.8) | 176 (100.0) |
| ISH | ||||||
| Positive | 22 (18.8) | 61 (52.1) | 23 (19.7) | 7 (6.0) | 4 (3.4) | 117 (100.0) |
| Negative | 48 (81) | 7 (12) | 3 (5) | 0 (0) | 1 (2) | 59 (100) |
| Total | 70 (39.8) | 68 (38.6) | 26 (14.8) | 7 (4.0) | 5 (2.8) | 176 (100.0) |
| PCR for HPV in LBC samples | ||||||
| Negative | 51 (69) | 16 (22) | 6 (8) | 0 (0) | 1 (1) | 74 (100) |
| Low-risk HPV | 4 (44) | 4 (44) | 0 (0) | 1 (11) | 0 (0) | 9 (100) |
| High-risk HPV | 14 (18) | 36 (46) | 19 (24) | 5 (6) | 4 (5) | 78 (100) |
| Unknown-risk HPV | 1 (7) | 11 (79) | 1 (7) | 1 (7) | 0 (0) | 14 (100) |
| Total | 70 (40.0) | 67 (38.3) | 26 (14.9) | 7 (4.0) | 5 (2.9) | 175 (100.0) |
ASCUS, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; CPE, cytopathic effect; HSIL, high-grade squamous intraepithelial lesion; HPV, human papillomavirus; ISH, in situ hybridization; LBC, liquid-based cytology; LSIL, low-grade squamous intraepithelial lesion; PCR, polymerase chain reaction.
Data are given as number (percentage).
HPV was detected by PCR and ISH in 89 cases (50.6%) and by PCR alone in 53 (30.1%) cases. The presence of HPV by ISH was found in 34 (19.3%) of 176 cases with negative PCR for HPV in cytologic samples. More specifically, HPV was detected by ISH in 5 cases with negative cytology, 13 cases with atypical squamous cells of undetermined significance (ASCUS), 15 cases with LSIL, and 1 case with HSIL cytology.
Histologic Results
Of the samples, 38 (21.6%) were identified as CIN 2+ and 12 (6.8%) as CIN 3 or worse. The association between cytologic findings and histologic diagnosis is shown in Table 3. Abnormal cytology (ASCUS or worse) was more common in CIN 2+ cases (odds ratio [OR], 18.5; 95% confidence interval [CI], 2.46–139.2; P < .0001). A cytologic diagnosis of HSIL+ correlated with a histologic diagnosis of CIN 2+ (OR, 17.42; 95% CI, 5.25–57.8; P < .0001). The sensitivity of HSIL+ cytology for the diagnosis of CIN 2+ was 34% (13/38), and the specificity, PPV, and NPV were 97.1% (134/138), 76% (13/17), and 84.3% (134/159), respectively ▮Table 4▮.
Table 4.
Sensitivity, Specificity, PPV, and LRs of the Various Methods*
| CIN 2 or Worse | ||||||
|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | PLR (%) | NLR (%) | |
| LBC | ||||||
| ASCUS or worse | 97.4 (87.5–99.5) | 33.3 (30.6–33.9) | 28.7 (25.8–29.3) | 97.9 (89.9–99.6) | 1.5 (1.3–1.5) | 0.079 (0.01–0.41) |
| LSIL or worse | 57.9 (43.9–70.9) | 55.1 (51.2–58.7) | 26.2 (19.9–32.1) | 82.6 (76.8–88) | 1.3 (0.9–1.7) | 0.77 (0.5–1.1) |
| HSIL or worse | 34.2 (24.5–40.3) | 97.1 (94.4–98.8) | 76.5 (54.7–90.1) | 84.3 (81.9–85.7) | 11.8 (4.4–33.2) | 0.68 (0.6–0.8) |
| ISH + | ||||||
| All | 89.5 (77.4–95.7) | 39.9 (36.5–41.6) | 29.1 (25.1–31.1) | 93.2 (85.4–97.3) | 1.5 (1.2–1.6) | 0.26 (0.1–0.62) |
| High-risk HPV only | 89.5 (77.3–95.7) | 45.7 (42.3–47.4) | 31.2 (27–33.4) | 94 (87.1–97.6) | 1.6 (1.3–1.8) | 0.23 (0.09–0.54) |
| HPV PCR + | ||||||
| All | 94.7 (83.8–98.5) | 52.2 (49.2–53.2) | 35.3 (31.2–36.7) | 97.3 (91.7–99.2) | 1.9 (1.6–2.1) | 0.1 (0.03–0.33) |
| Types 16, 18, 31, and 33 | 73.7 (63.7–78.7) | 97.8 (95.1–99.2) | 90.3 (78.1–96.4) | 93.1 (90.5–94.4) | 33.9 (12.9–98.3) | 0.27 (0.22–0.38) |
ASCUS, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; ISH, in situ hybridization; LSIL, low-grade squamous intraepithelial lesion; NLR, negative likelihood ratio; NPV, negative predictive value; PCR, polymerase chain reaction; PLR, positive likelihood ratio; PPV, positive predictive value.
Values in parentheses are 95% confidence intervals.
HPV Detection and Correlations With Histologic Findings
Results of the distribution of HPV genotypes according to histologic diagnosis are shown in Table 3. ▮Image 1▮ shows representative cases of CIN 1 and 2 and adenocarcinoma. HR-HPV genotypes were detected in 28 (78%) of 36 cases of CIN 2+ compared with 51 (40.5%) of 126 cases of LSIL (OR, 4.8; 95% CI, 1.8–14.10; P < .0001). Rates of LR-HPV genotypes by PCR were higher in cases with lower grade histologic features (OR, 5.1; 95% CI, 2.2–12.2; P < .0001). Rates of infection with multiple HPV genotypes by PCR were higher in cases with CIN 2+ compared with cases with lower grade histologic features (P = .016).
Image 1.
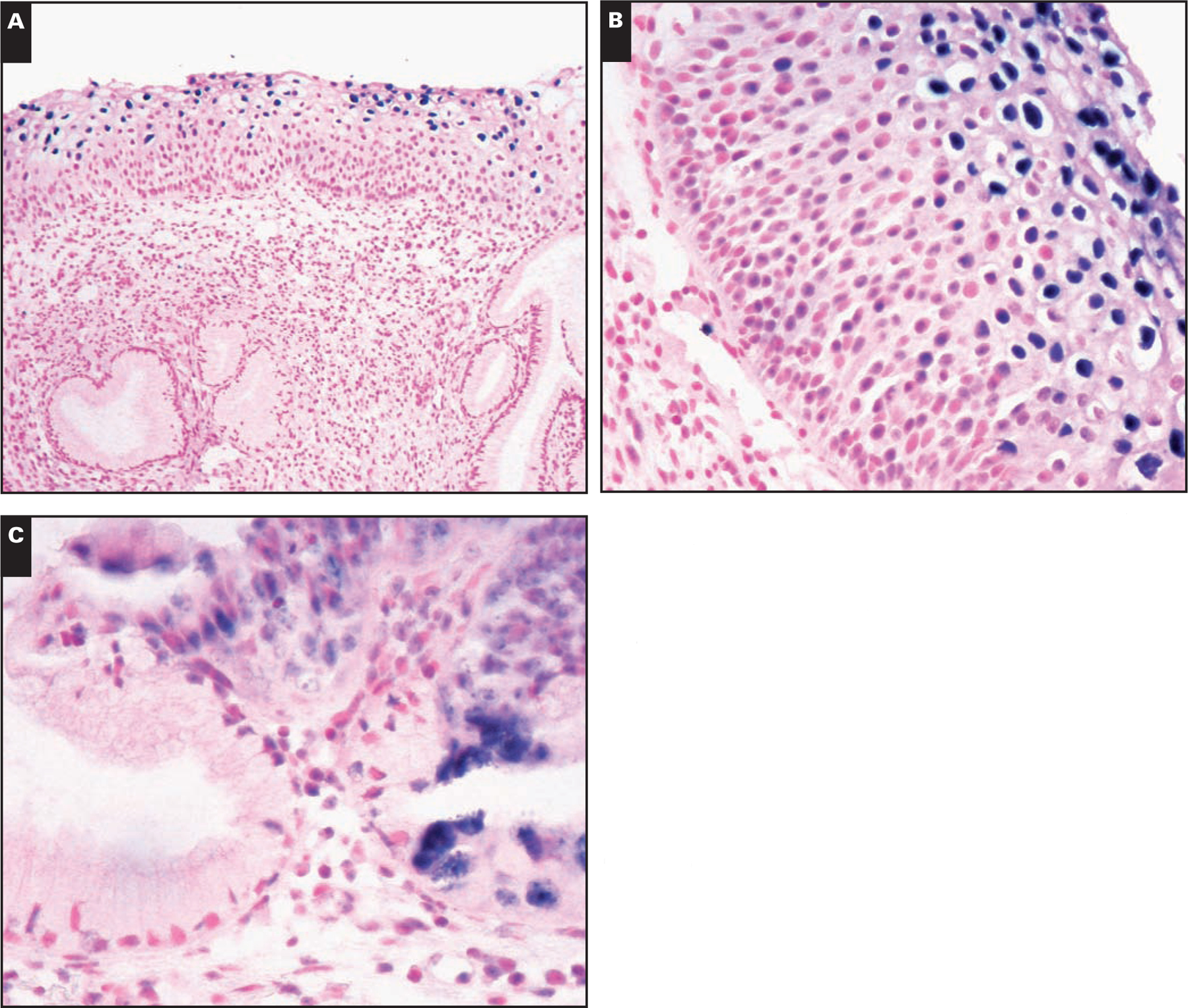
A, Cervical intraepithelial neoplasia (CIN) 1 with mild dysplasia and human papillomavirus (HPV) cytopathic effect with diffuse signal pattern of HPV staining (NBT/BCIP substrate with nuclear fast red counterstain, ×100). B, CIN 2 with moderate dysplasia and HPV cytopathic effect with diffuse signal pattern of HPV staining (NBT/BCIP substrate with nuclear fast red counterstain, ×400). C, Endocervical adenocarcinoma. In situ hybridization is positive for high-risk HPV types in rare adenocarcinoma cells and negative in benign endocervical glands. There is moderate dysplasia and HPV cytopathic effect with diffuse signal pattern of HPV staining (NBT/BCIP substrate with nuclear fast red counterstain, ×400).
The association between HPV ISH and histologic findings is shown in Table 3. From 176 cervical tissue samples, 59 (33.5%) were negative for HPV by ISH and 106 (60.2%), 4 (2.3%), and 7 (4.0%) were positive for HR-, LR-, and both HR- and LR-HPV, respectively. The incidence of detection of HPV by ISH was higher in tissue samples with CIN 2+ histologic features compared with tissue samples with low-grade histologic features (OR, 5.6; 95% CI, 1.9–16.8; P < .0001). HPV CPE and its association with histologic classification are shown in Table 3. We found that approximately 40% of cases with negative or ASCUS cytologic features and 70% of cases with normal or CIN 1 histologic features had CPE in cervical tissues.
Discrepancies Between Cytologic and Histologic Findings: Likelihood Ratios
Overall, in 40 (22.7%) of 176 cases, there were discrepancies between cytologic and histologic diagnoses. Sensitivities, specificities, PPVs, NPVs, positive likelihood ratios (PLRs), and negative likelihood ratios (NLRs) for cytologic and molecular HPV testing with regard to the histologic diagnosis of CIN 2+ are provided in Table 4. PLRs of 33.9 and 11.8 were noted for positive HPV testing for types 16, 18, 31, and 33 and cytologic findings of HSIL+, respectively (Table 4). For CIN 2+ histologic lesions, the highest PLRs were observed with cytologic testing and with detection of HR-HPV types by PCR (Table 4). NLRs of 0.68 and 0.27 were noted for cytologic findings of HSIL+ and positive HPV testing for types 16, 18, 31, and 33, respectively (Table 4).
Concordance for HPV Detection Between ISH and PCR
The overall rate of concordance for HPV detection between ISH and PCR was 78.2%, and the percentage of concordance of these 2 methods for different histologic and cytologic categories is shown in ▮Table 5▮. The percentage of concordance for HPV detection between ISH and PCR for histologic specimens was lower for nondysplastic tissues (79% [54/68]) and CIN 1 (74% [39/53]) and was higher for CIN 2+ (83% [29/35]) and CIN 3+ (91% [10/11]) (Table 5). The percentage of concordance for HPV detection between ISH and PCR for cytologic specimens was 96% (44/46) for negative LBC specimens, 63% (24/38) for ASCUS, 67% (38/57) for LSIL, and 93% (14/15) for HSIL+ (Table 5).
Table 5.
Concordance of PCR Data and Results of ISH in Histologic and Cytologic Diagnoses*
| Histologic Result | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nondysplastic Tissues | CIN 1 ISH Data | CIN 2 | CIN 3 | Carcinoma | |||||||||
| Cytologic/PCR Data | HR-HPV | LR-HPV | – | HR-HPV | LR-HPV | – | HR-HPV | LR-HPV | – | HR-HPV | HR-HPV | – | Total |
| Negative LBC | |||||||||||||
| Negative | 0 | 0 | 42 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 44 |
| HR-HPV | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| LR-HPV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ASCUS | |||||||||||||
| Negative | 4 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 11 |
| HR-HPV | 3 | 0 | 2 | 8 | 0 | 0 | 5 | 0 | 1 | 2 | 2 | 0 | 23 |
| LR-HPV | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| LSIL | |||||||||||||
| Negative | 1 | 0 | 2 | 8 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 14 |
| HR-HPV | 5 | 0 | 2 | 24 | 0 | 0 | 6 | 0 | 0 | 1 | 0 | 0 | 38 |
| LR-HPV | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| HSIL | |||||||||||||
| Negative | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HR-HPV | 0 | 0 | 0 | 3 | 0 | 0 | 6 | 0 | 0 | 2 | 1 | 0 | 12 |
| LR-HPV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Carcinoma | |||||||||||||
| Negative | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| HR-HPV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| LR-HPV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Total | 15 | 1 | 52 | 48 | 2 | 3 | 21 | 0 | 3 | 6 | 4 | 1 | 156 |
| Total concordance | 8/15 (53) | 1/1 (100) | 45/52 (87) | 35/48 (73) | 1/2 (50) | 3/3 (100) | 17/21 (81) | 0/0 (0) | 2/3 (67) | 5/6 (83) | 4/4 (100) | 1/1 (100) | 122/156 (78.2) |
ASCUS, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HR, high-risk; HSIL, high-grade squamous intraepithelial lesion; ISH, in situ hybridization; LBC, liquid-based cytology; LR, low-risk; LSIL, low-grade squamous intraepithelial lesion; PCR, polymerase chain reaction.
Data in parentheses are number/total [percentage], except for total concordance data, which are number/total (percentage). Twenty cases positive for high- and low-risk HPV types by one method and/or unknown HPV types (by PCR) were excluded from this analysis, and only cases negative for HPV or positive for HR-HPV or LR-HPV (by PCR or ISH) were included in the analysis.
Discussion
Although widely used in research studies for detection of HPV, adjudicated histologic diagnosis is not the standard of care. A recent study addressed interobserver variation in the histologic diagnosis of HPV and limitations of this method.20 Immunohistochemical detection of HPV is a valuable adjunctive aid in the diagnosis of difficult cervical biopsy specimens and has been used in many studies.13–20 However, there are limited data on the correlation of immunohistochemical detection of HPV detection in cervical tissues with genotyping data in cytologic samples. In addition, recent studies suggest that a broad-spectrum HPV ISH test could possibly provide a better gold standard for the immunohistochemical diagnosis of HPV as the probes and kits used for this purpose continue to improve.20
In this study, we found significant association of abnormal cytology (ASCUS or worse) with high-grade cervical dysplasia (moderate and severe dysplasia, CIN 2+). Approximately 9 of 10 patients with abnormal cytologic smears had detection of HPV in cervical tissues by ISH. The presence of HR-HPV genotypes in cytologic samples was significantly associated with the presence of high-grade cervical dysplasia (CIN 2+). Overall, positive HR-HPV testing by PCR in cytologic samples and high-grade abnormal cytology (HSIL+) had high predictive value for the presence of high-grade cervical dysplasia (CIN 2+). The presence of HPV by ISH in 1 of 5 cases with negative PCR for HPV in cytologic samples may suggest inadequate sampling for these PCR samples. Although the presence of CPE may suggest the presence of HPV in tissue samples, this morphologic method has not been used widely for the detection of HPV and has not been evaluated extensively in correlation with confirmed cytologic or histologic findings.12 We found that CPE was present in approximately 4 of 10 cases with negative or ASCUS cytologic features and 7 of 10 cases with normal or low-grade dysplasia (CIN 1). The presence of CPE may be used in combination with other morphologic tests or newer diagnostic methods to further assist in the screening for cervical disease.
Our results confirm the higher prevalence of HPV infection in women with abnormal cytologic findings, in concordance with most studies so far published that have observed that the increase in HPV prevalence is related to the increasing grade of squamous intraepithelial lesions.26,27
Integration of HPV DNA into host-cell DNA is a major event in the development of HPV-related cancer.2 There are 3 types of nucleic acid hybridization method formats used to detect HPV: (1) the direct nucleic acid probe methods (ISH); (2) hybridization signal amplification (Hybrid Capture 2 system); and (3) target amplification methods (PCR). Although there are studies comparing ISH and PCR in LBC samples and in cervical samples, there are limited data regarding correlation of PCR testing of HPV DNA in LBC samples with ISH detection of HPV in cervical tissues.
The overall concordance between ISH and PCR testing in our study was approximately 75%. However, the presence of HPV was found by ISH in approximately 20% of cases with negative PCR for HPV, suggesting that inadequate sampling may increase the rate of false-negative results with PCR. The most prevalent genotypes found in our study were HPV-16, HPV-53, HPV-66, and HPV-31. Although detection of the episomal form of HPV-16 has been reported in cervical cancers,29 our results show that the episomal form of HPV infection can be present in cervical tissues of patients with negative PCR in cytologic samples. This finding suggests that integration of HPV in cervical tissues is an early event in pathogenesis of HPV infection and can be detected with the use of ISH.
A possible explanation for the partially discordant results between ISH and PCR in our study is the fact that L1 primers may actually miss some cases deleted in the L1 region of the virus.30 An increased concentration of human DNA or blood in the samples may also reduce the sensitivity or inhibit the PCR.30 In addition, some of the samples exhibiting discordance may have infection with HPV types not amplified by the used primers but still detectable by the ISH cocktails. Finally, different demographic parameters between studies may affect the prevalence of latent infection and the sensitivity and specificity of the 2 tests.
This study was not designed to present the usefulness of these techniques as screening methods because it includes a selected population and would, therefore, be largely biased. Rather it is an analytic study examining 2 techniques and their yield with regard to HPV detection.
With regard to sensitivity and specificity, PCR exhibited high sensitivity (94.7%) for the detection of CIN 2+ lesions, but it lacked specificity and PPV in our population. Detection of HPV by ISH had good sensitivity (89.5%) but poor specificity (39.9%) for detection of CIN 2+. Thus, ISH remains a valuable tool for the detection of HPV in cervical tissues.
ISH techniques are becoming increasingly sensitive and are currently more often used in routine diagnostics to detect or exclude malignancy.31 New ISH techniques can be used for the detection of very low copy number of HPV DNA sequences in paraffin-embedded tissue sections. Detection of HPV DNA with the more commonly used HPV DNA tests, such as Hybrid Capture or PCR assays, does not exclude contamination with viral DNA from other sites and lacks the morphologic details of nuclear HPV DNA integration provided by ISH. It is interesting that we found a significant rate of HPV detection in patients with negative biopsy results and CIN 1 using ISH. The rate was higher than in other studies in which a more sensitive technique (PCR) was used. Although the more sensitive methods such as PCR can be performed in formalin-fixed and paraffin-embedded tissues, DNA damage and DNA extraction in these tissues can reduce the sensitivity of PCR. Thus, ISH can detect HPV in cases that may not be identified by PCR.9 However, a potential limitation of this technique is the failure to detect HPV subtypes other than the genotypes tested with the commercial probes.
The high NPVs of both molecular tests underlie their importance in detecting HPV in high-grade lesions. In high-grade histologic lesions, HPV detection rates seemed to be a little higher with PCR than with ISH testing. However, PLRs and NLRs were similar for positive ISH (any result) or PCR HPV+ testing (any result) for CIN 2+ and for CIN 3+ histologic findings. Actually, PCR had a better PLR, whereas ISH had a better NLR in both histologic categories. This means that with a positive PCR, there was a greater likelihood of disease (CIN 2+) than with ISH, whereas with a negative ISH, there was a lesser likelihood of disease (CIN 2+) than with PCR. Nevertheless, in essence, both tests gave similar results. However, both molecular HPV tests did not outperform cytologic studies that had comparable results. Finally, positive PCR testing for HPV genotypes that are included in the HPV vaccine such as HPV-16 and HPV-18 had a very high PLR for CIN 2+ and for CIN 3+ histologic findings. The current observations further confirm the association of HR-HPV types with cytologic detection of HSIL or invasive carcinoma and also with histologically confirmed premalignant or malignant lesions.1 All cases of CIN 2, CIN 3, or carcinomas harbored single or multiple HR-HPV oncogenic-type infections. In our study, single infections with HPV type 16 or 18 in cases of invasive carcinoma and the high PLR of detection of HPV genotypes that are included in the HPV vaccine such as HPV-16 and HPV-18 for CIN 2+ and CIN 3+ support the strong rationale for preventive HPV vaccination in our population.
In our study, we found comparable results in terms of HPV detection by cytologic studies, ISH, and PCR (Tables 1–5). The concordance rate for HPV detection between ISH and PCR was higher for CIN 3+ and HSIL lesions (>90%). This finding may be explained by higher false-negative results for HPV detection using PCR owing to inadequate sampling or by improved sensitivity or increased false-positive results for HPV detection using the newer ISH probes. Further studies are needed to compare the sensitivity of these methods to detect HPV alone or in combination in cervical tissues and cytologic samples. Although other molecular techniques (such as detection of p16) may be technically easier to use compared with ISH, the newer ISH methods also allow synchronous detection of different HPV types (HR or LR) rather than just detection of HPV antigens, and this increases diagnostic information.20 In the era of HPV vaccine, there is increasing interest in molecular methods that detect multiple types of HPV simultaneously and newer ISH methods could provide this information in addition to morphologic details about the physical status of the HPV. Thus, our study may form the basis for further studies on the usefulness of ISH in comparison with other well-established diagnostic methods.
Conclusion
The present study focused on HPV detection by PCR in correlation with histologic findings and ISH testing. HPV rates were high in high-grade lesions in accordance with published literature; however, HPV was also identified in a large fraction of samples with normal cytologic findings and in samples with normal biopsy results. Newer methods assessing the integration of the virus may be more appropriate in the workup of such cases. In our study, LBC and molecular HPV tests performed similarly as tests for detection of high-grade lesions and sometimes were complementary to each other. We conclude that when used together and evaluated in conjunction with histologic sections, ISH is a useful tool for ancillary molecular testing of HPV infection in cervical lesions. Both PCR and ISH could be used in the evaluation of CIN 2+ lesions. This approach may lead to more appropriate screening strategies after the implementation of HPV vaccination and may be needed to fully evaluate the prevalence of HPV in cervical tissues and further understand attribution of individual HPV types to the development of cervical cancer.
References
- 1.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. [DOI] [PubMed] [Google Scholar]
- 2.zur Hausen H. Viruses in human cancers. Science. 1991;254:1167–1173. [DOI] [PubMed] [Google Scholar]
- 3.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. [DOI] [PubMed] [Google Scholar]
- 4.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–257. [DOI] [PubMed] [Google Scholar]
- 5.Wright TC Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304–309. [DOI] [PubMed] [Google Scholar]
- 6.Bryan JT, Taddeo F, Skulsky D, et al. Detection of specific human papillomavirus types in paraffin-embedded sections of cervical carcinomas. J Med Virol. 2006;78:117–124. [DOI] [PubMed] [Google Scholar]
- 7.Zehbe I, Hacker GW, Su H, et al. Sensitive in situ hybridization with catalyzed reporter deposition, streptavidin-Nanogold, and silver acetate autometallography: detection of single-copy human papillomavirus. Am J Pathol. 1997;150:1553–1561. [PMC free article] [PubMed] [Google Scholar]
- 8.Evans MF, Aliesky HA, Cooper K. Optimization of biotinyl-tyramide-based in situ hybridization for sensitive background-free applications on formalin-fixed, paraffin-embedded tissue specimens. BMC Clin Pathol. 2003;3:2. doi: 10.1186/1472-6890-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo M, Gong Y, Deavers M, et al. Evaluation of a commercialized in situ hybridization assay for detecting human papillomavirus DNA in tissue specimens from patients with cervical intraepithelial neoplasia and cervical carcinoma. J Clin Microbiol. 2008;46:274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lizard G, Demares-Poulet MJ, Roignot P, et al. In situ hybridization detection of single-copy human papillomavirus on isolated cells, using a catalyzed signal amplification system: GenPoint. Diagn Cytopathol. 2001;24:112–116. [DOI] [PubMed] [Google Scholar]
- 11.Guo M, Patel SJ, Chovanec M, et al. A human papillomavirus testing system in women with abnormal Pap results: a comparison study with follow-up biopsies. Acta Cytol. 2007;51:749–754. [DOI] [PubMed] [Google Scholar]
- 12.Zaravinos A, Mammas IN, Sourvinos G, et al. Molecular detection methods of human papillomavirus (HPV). Int J Biol Markers. 2009;24:215–222. [DOI] [PubMed] [Google Scholar]
- 13.Conesa-Zamora P, Domenech-Peris A, Orantes-Casado FJ, et al. Effect of human papillomavirus on cell cycle–related proteins p16, Ki-67, cyclin D1, p53, and ProEx C in precursor lesions of cervical carcinoma: a tissue microarray study. Am J Clin Pathol. 2009;132:378–390. [DOI] [PubMed] [Google Scholar]
- 14.Griesser H, Sander H, Walczak C, et al. HPV vaccine protein L1 predicts disease outcome of high-risk HPV+ early squamous dysplastic lesions. Am J Clin Pathol. 2009;132:840–845. [DOI] [PubMed] [Google Scholar]
- 15.Huang MZ, Li HB, Nie XM, et al. An analysis on the combination expression of HPV L1 capsid protein and p16INK4a in cervical lesions. Diagn Cytopathol. 2010;38:573–578. [DOI] [PubMed] [Google Scholar]
- 16.Klaes R, Benner A, Friedrich T, et al. p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2002;26:1389–1399. [DOI] [PubMed] [Google Scholar]
- 17.Liang J, Mittal KR, Wei JJ, et al. Utility of p16INK4a, CEA, Ki67, p53 and ER/PR in the differential diagnosis of benign, premalignant, and malignant glandular lesions of the uterine cervix and their relationship with Silverberg scoring system for endocervical glandular lesions. Int J Gynecol Pathol. 2007;26:71–75. [DOI] [PubMed] [Google Scholar]
- 18.Sarian LO, Derchain SF, Yoshida A, et al. Expression of cyclooxygenase-2 (COX-2) and Ki67 as related to disease severity and HPV detection in squamous lesions of the cervix. Gynecol Oncol. 2006;102:537–541. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Kuhn L, Denny LA, et al. Impact of utilizing p16INK4A immunohistochemistry on estimated performance of three cervical cancer screening tests. Int J Cancer. 2007;120:351–356. [DOI] [PubMed] [Google Scholar]
- 20.Galgano MT, Castle PE, Atkins KA, et al. Using biomarkers as objective standards in the diagnosis of cervical biopsies. Am J Surg Pathol. 2010;34:1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoler MH. Human papillomavirus biology and cervical neoplasia: implications for diagnostic criteria and testing. Arch Pathol Lab Med. 2003;127:935–939. [DOI] [PubMed] [Google Scholar]
- 22.Maucort-Boulch D, Plummer M, Castle PE, et al. Predictors of human papillomavirus persistence among women with equivocal or mildly abnormal cytology. Int J Cancer. 2010;126:684–691. [DOI] [PubMed] [Google Scholar]
- 23.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. [DOI] [PubMed] [Google Scholar]
- 24.De Marchi TR, Metze K, Zeferino LC, et al. HPV in situ hybridization signal patterns as a marker for cervical intraepithelial neoplasia progression. Gynecol Oncol. 2009;112:114–118. [DOI] [PubMed] [Google Scholar]
- 25.Tsiodras S, Georgoulakis J, Chranioti A, et al. Hybrid Capture vs PCR screening of cervical human papilloma virus infections: cytological and histological associations in 1270 women. BMC Cancer. 2010;10:53. doi: 10.1186/1471-2407-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nobbenhuis MA, Walboomers JM, Helmerhorst TJ, et al. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20–25. [DOI] [PubMed] [Google Scholar]
- 27.Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605–1610. [DOI] [PubMed] [Google Scholar]
- 28.Cooper K, Herrington CS, Stickland JE, et al. Episomal and integrated human papillomavirus in cervical neoplasia shown by non-isotopic in situ hybridisation. J Clin Pathol. 1991;44:990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujii T, Masumoto N, Saito M, et al. Comparison between in situ hybridization and real-time PCR technique as a means of detecting the integrated form of human papillomavirus 16 in cervical neoplasia. Diagn Mol Pathol. 2005;14:103–108. [DOI] [PubMed] [Google Scholar]
- 30.Flores-Munguia R, Siegel E, Klimecki WT, et al. Performance assessment of eight high-throughput PCR assays for viral load quantitation of oncogenic HPV types. J Mol Diagn. 2004;6:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiegl M, Haun M, Massoner A, et al. Combination of cytology, fluorescence in situ hybridization for aneuploidy, and reverse-transcriptase polymerase chain reaction for human mammaglobin/mammaglobin B expression improves diagnosis of malignant effusions. J Clin Oncol. 2004;22:474–483. [DOI] [PubMed] [Google Scholar]


