Abstract
Objectives
Vitamin K is hypothesised to play a role in osteoarthritis (OA) pathogenesis through effects on vitamin K-dependent bone and cartilage proteins, and therefore may represent a modifiable risk factor. A genetic variant in a vitamin K-dependent protein that is an essential inhibitor for cartilage calcification, matrix Gla protein (MGP), was associated with an increased risk for OA. Vitamin K antagonist anticoagulants (VKAs), such as warfarin and acenocoumarol, act as anticoagulants through inhibition of vitamin K-dependent blood coagulation proteins. VKAs likely also affect the functioning of other vitamin K-dependent proteins such as MGP.
Methods
We investigated the effect of acenocoumarol usage on progression and incidence of radiographic OA in 3494 participants of the Rotterdam Study cohort. We also examined the effect of MGP and VKORC1 single nucleotide variants on this association.
Results
Acenocoumarol usage was associated with an increased risk of OA incidence and progression (OR=2.50, 95% CI=1.94–3.20), both for knee (OR=2.34, 95% CI=1.67–3.22) and hip OA (OR=2.74, 95% CI=1.82–4.11). Among acenocoumarol users, carriers of the high VKORC1(BB) expression haplotype together with the MGP OA risk allele (rs1800801-T) had an increased risk of OA incidence and progression (OR=4.18, 95% CI=2.69–6.50), while this relationship was not present in non-users of that group (OR=1.01, 95% CI=0.78–1.33).
Conclusions
These findings support the importance of vitamin K and vitamin K-dependent proteins, as MGP, in the pathogenesis of OA. Additionally, these results may have direct implications for the clinical prevention of OA, supporting the consideration of direct oral anticoagulants in favour of VKAs.
Keywords: osteoarthritis, epidemiology, pharmacogenetics
Key messages.
What is already known about this subject?
Osteoarthritis (OA) is the most common form of arthritis worldwide, affecting 320 million people, and is a leading cause of disability. To date, there are no disease-modifying therapies available, and treatment development has been hampered by existence of only few recognised modifiable risk factors.
Vitamin K and vitamin K-dependent proteins, such as matrix Gla protein (MGP), have been implicated in OA by epidemiological studies, genetic studies and subsequent in functional genomics studies, indicating vitamin K as possible modifiable risk factor for OA.
What does this study add?
This study shows that the use of vitamin K antagonist anticoagulants (VKAs) significantly increases the risk of progression of hip and knee OA, by inhibiting the vitamin K pathway.
This study also demonstrates that known OA genetic risk variants in MGP and pharmacogenetic variants known to affect vitamin K metabolism increase the risk of OA progression when using VKAs.
How might this impact on clinical practice or future developments?
The findings suggest the consideration of novel (or direct) oral anticoagulants in favour of VKAs, such as acenocoumarol and warfarin, in people with OA.
Introduction
Osteoarthritis (OA) is a chronic disabling joint disease that also increases in prevalence with age. It is the most common form of arthritis, one of the fastest growing chronic diseases worldwide,1 and is the fourth leading cause of years lived with disability globally.2 To date, there are no known therapies that can alter its progression or prevent its occurrence. Apart from obesity and knee injury, very few other modifiable risk factors have been identified. Vitamin K has been hypothesised to play a role in OA pathogenesis through its effects on several vitamin K-dependent bone and cartilage proteins,3 and therefore may represent a modifiable risk factor. A number of observational studies reported an association between vitamin K status and prevalence and incidence of OA.4–6 There has been one modestly sized clinical trial studying the effect of vitamin K supplementation on OA progression. This ancillary study, originally designed to study vascular calcification, reported no overall beneficial effects of vitamin K supplementation. However, in individuals with insufficient vitamin K levels at baseline, a beneficial effect was observed.7 No studies to date have evaluated the relation between vitamin K antagonist anticoagulants (VKAs) and OA, which can be expected to result in low vitamin K functioning, which may lead to increased OA incidence or progression.4–6
Vitamin K is an essential cofactor in the post-translational γ‐carboxylation of glutamic acid to form γ‐carboxyglutamic acid (Gla) residues, which confer functionality to Gla proteins. VKAs deplete the active form of vitamin K by inhibiting the enzyme vitamin K epoxide reductase complex 1 (VKORC1). Genetic variants of VKORC1 account for approximately 25% of the variance in VKA dose.8 Matrix Gla protein (MGP) is a vitamin K-dependent Gla protein that is an essential inhibitor of cartilage and vascular mineralisation.9 10 Recently, genome-wide association study (GWAS) and functional studies identified MGP to be causally involved in OA.11 12
VKAs such as warfarin and acenocoumarol are primarily prescribed for the prevention of thromboembolic events in patients with atrial fibrillation (AF).13 With ageing-related increases in prevalence of AF, the projected number of individuals with AF needing anticoagulation is predicted to rise to 17.9 million by 2060 in the European Union.14 While a new class of anticoagulants are available, the non-vitamin K oral anticoagulants (NOACs), VKAs are still widely prescribed, particularly to older adults.15 Whether long-term VKA use with resultant impairment of vitamin K-dependent proteins such as MGP increases risk of OA incidence or progression is not known. Given the high prevalence of VKA users in addition to the high prevalence of OA globally, clarifying this relationship would have substantial public health impact by identifying a potentially modifiable risk factor for OA.
We therefore examined the relation of VKA use to progression and incidence of hip and knee OA in two subcohorts of the large prospective population-based cohort of the Rotterdam study (RS). We additionally examined how the impact of VKA use varies by the presence of the MGP risk allele that influences MGP expression and single nucleotide variations (SNVs) affecting VKORC1 gene expression, which impact VKA dosage.
Methods
Study population and clinical data
The Rotterdam Study (RS) is a large prospective population-based cohort study ongoing since 1990 to study determinants of chronic disabling diseases in the elderly.16 It consists of separate subcohorts (RS-I, RS-II, RS-III). All RS cohort participants live in the Ommoord district of the city of Rotterdam, the Netherlands. Residents of 55 years and older were first recruited in 1990. In 2000, a second cohort, RS-II, was started with individuals who had become 55 years of age or moved into the study district since the start of the study. Follow-up data were collected at follow-up visits every ~5–6 years. Details of the design and rationale of the RS have been published elsewhere.16 Participant measurements at baseline and follow-up were obtained during visits to the research centre for physical examinations, computerised pharmacy records and from home interviews. Our study included participants of RS-I and RS-II for whom radiographs of knee and hip joints at baseline and follow-up visit were present, obtained and scored (online supplemental figure S1). Additional information included sex, age at baseline visit, body mass index (BMI, kg/m2), physical activity (metabolic equivalent of task/hours per week), smoking (never, former and current smoker), locomotor disability, education level (UNESCO education classification), diabetes mellitus, hypertension, femoral neck bone mineral density (FN-BMD), HDL/total cholesterol ratio and the Stanford Health Assessment Questionnaire (see online supplemental text for details).
annrheumdis-2020-219483supp001.pdf (118.4KB, pdf)
annrheumdis-2020-219483supp002.pdf (132.8KB, pdf)
The RS has been approved by the institutional review board (Medical Ethics Committee) of the Erasmus Medical Center and by the review board of The Netherlands Ministry of Health, Welfare and Sports. The approval has been renewed every 5 years (MEC 02.1015). All participants provided written informed consent for participation in the RS.
Incidence and progression of OA
Our study included participants of RS-I and RS-II for whom radiographs of knee and hip joints at baseline and follow-up visit were obtained and scored by trained medical professionals for OA severity using Kellgren and Lawrence Grade (KLG)17 18 (online supplemental figure S1). Individuals who had at baseline locomotor disability were excluded from our study population19 (online supplemental figure S1). We analysed OA incidence and progression together, as both definitions cannot be accurately defined based on radiographic examination alone, and by combining both into one definition reduces this bias.20 We evaluated any OA progression, defined as an increase of KLG between baseline and follow-up of ≥1 and/or joint replacement; if baseline KLG was 0, progression was defined as an increase of KLG ≥2 (incidence).21 Joints with a baseline KLG of 4 or baseline joint replacement were excluded from analysis (online supplemental figure S1). OA progression was defined in a joint-specific and side-specific manner (knee, hip; left and right). Joints with no progression of OA comprised the referent group. Joints with missing data were excluded (online supplemental figure S1), with the exception of joints with missing baseline data and a KLG of ≤1 at follow-up, which were included in the referent group (online supplemental figure S1).
Vitamin K antagonist anticoagulants
For each participant, we extracted the usage of VKAs (acenocoumarol) for the period between baseline visit (RS-I-1, RS-II-1) and follow-up visits (RS-I-3, RS-II-2), from computerised pharmacy data (online supplemental text). Acenocoumarol is the main prescribed VKA in the Netherlands as warfarin is not registered for use as a drug. All participants taking VKAs attended an anticoagulation clinic, which is standard practice in the Netherlands.22 We excluded participants who were taking VKA (n=148) during the baseline visit to avoid prevalent user bias. We defined VKA usage as any acenocoumarol usage during the period between the baseline (RS-I, RS-II) and follow-up visit (RS-I-3, RS-II-2), regardless of duration or dosage. To examine the effects of increasing duration of use, we defined duration of use by tertiles: ≤180 days, between >180 days and ≤556 days of use, and >556 days of use.
Genetic data and haplotype analysis
Methods for DNA isolation, genotyping, quality control and data processing have been described elsewhere.11 Data from 11 SNVs were extracted from the genotyped and imputed genetic dataset: the MGP SNV previously found associated with OA11 and 10 SNVs needed for the VKORC1 H-haplotypes as described on the PharmGKB database23 (online supplemental table S1). Haplotypes were inferred from all available genotypes (N=8448), using imputed genotype dosage data (HRC panel v.1.124) and the R-package haplo.stats.25 Haplotypes were grouped based on VKA maintenance dose/VKORC1 expression association: (A) low-dose VKA requirement/low VKORC1 expression and (B) high-dose VKA requirement/high VKORC1 expression.26 Study participants were further stratified into: low expression/dose (AA), intermediate expression/dose (AB) and high expression/dose (BB) groups (table 1 and online supplemental table S1).
Table 1.
Study population characteristics
| Study population (RSI and RSII) | ||
| Non-users (N=3255) | Acenocoumarol users (N=239) | |
| General characteristics | ||
| RSI | 2394 (73.5%) | 207 (86.6%) |
| RSII | 861 (26.5%) | 33 (13.4%) |
| Age, years | 64.2 (6.3) | 66.6 (6.8) |
| Females (%) | 1778 (54.6%) | 117 (49.0%) |
| Follow-up period, months, median (IQR) | 76.1 (55.2, 78.5) | 76.7 (75.1, 79.4) |
| Smoking status | ||
| Never | 1028 (31.6%) | 65 (27.2%) |
| Former smoker | 1538 (47.3%) | 119 (49.8%) |
| Current smoker | 689 (21.2%) | 55 (23.0%) |
| Education | ||
| Primary education | 385 (11.7%) | 34 (14.2%) |
| Intermediate general education | 1433 (44.0%) | 103 (43.1%) |
| Higher general education | 1026 (31.5%)) | 69 (28.9%) |
| Higher vocational education/University | 411 (12.6%) | 33 (13.8%) |
| Physical activity (MET/week) | 89.9 (44.69) | 78.27 (41.5) |
| Body mass index (BMI), kg/m2 | 26.3 (3.5) | 26.9 (3.5) |
| Total cholesterol/HDL ratio | 5.0 (1.6) | 5.3 (1.5) |
| Hypertension (%) | 1709 (52.3%) | 143 (59.8%) |
| Diabetes mellitus (%) | 386 (11.9%) | 41 (17.2%) |
| Femoral neck BMD (g/cm2) | 0.88 (0.13) | 0.88 (0.12) |
| Osteoarthritis status* | ||
| Hip OA (%) | 65 (2.0%) | 10 (4.6%) |
| Knee OA (%) | 262 (8.5%) | 26 (12.0%) |
| MGP risk allele (rs1800801) | ||
| Non-risk allele (A/A) | 1168 (38.6%) | 79 (36.3%) |
| Risk allele carrier (T/*) | 1856 (61.4%) | 137 (63.4%) |
| VKORC1 Haplotype groups† | ||
| Low—AA | 447 (14.8%) | 42 (19.5%) |
| Intermediate—AB | 1425 (47.3%) | 92 (42.8%) |
| High—BB | 1142 (37.9%) | 81 (37.7%) |
| MGP risk allele carrier and VKORC1 BB-haplotype | 684 (22.7%) | 51 (23.7%) |
*Osteoarthritis at baseline was defined as radiographic OA, Kellgren-Lawrence score ≥2 in either the left or right or both investigated joints.
†VKORC1 groups based on VKORC1 H-Haplotypes and their association with VKORC1 expression/VKAs maintenance dosage; low: low VKORC1 expression and associated with lower required dosage; high: high VKORC1 expression and associated with higher required dosage, also see online supplemental table S1.
BMD, bone mineral density; HDL, high-density lipoprotein; MET, metabolic equivalent of task hours; OA, osteoarthritis; RS, Rotterdam Study.
annrheumdis-2020-219483supp003.pdf (206.4KB, pdf)
Statistical analysis
We evaluated the relation of VKA to the risk of overall progression of OA of either the knee or hip using logistic regression with generalised estimating equations (GEE) to account for correlations between joints within an individual.21 We repeated the analyses stratified by VKORC1 and MGP genotype/haplotype. The following covariates were included in all analyses: sex, age, BMI, physical activity, smoking, education level, diabetes mellitus, hypertension, FN-BMD, HDL/total cholesterol ratio, baseline OA severity, time between follow-up visits, joint modelled and RS cohort (online supplemental text).
Results
Relation of acenocoumarol use to OA progression
A total 3494 of participants of two large prospective older-age population-based cohorts, the Rotterdam Study (RS), were included in this study, with RS-I contributing 2601 individuals, while RSII contributed 894 participants.16 See table 1 for the general characteristics of the study population. At baseline, there were 363 individuals with OA (KLG ≥2), 75 with hip OA and 288 with knee OA. We identified 239 new users of acenocoumarol (VKA) (RS-I n=207, RS-II n=33) in our study population in our follow-up period.
When we examined the incidence/progression of OA in acenocoumarol users and non-users, there was a >2-fold higher risk for overall OA incidence/progression (ie, OA of the knee or hip) in acenocoumarol users compared with non-users (OR=2.50, 95% CI=1.94–3.20; table 2, online supplemental table S2). This association was also observed in each subcohort (RS-I and RS-II) separately (online supplemental table S3) and for OA incidence and OA progression separately (online supplemental table S4). Overall OA incidence/progression risk estimates remained similar for longer duration of acenocoumarol use (tertiles): ≤180 days (OR=2.82, 95% CI=1.90–4.20), >180 days and ≤556 days (OR=2.94, 95% CI=2.00–4.32), with only a reduction of risk with long-term use, >556 days of acenocoumarol use (OR=1.74, 95% CI=1.10–2.76) (table 2).
Table 2.
Association between acenocoumarol use and risk of OA incidence and progression in RSI and RSII
| Overall osteoarthritis progression | Overall progression of knee osteoarthritis | Overall progression of hip osteoarthritis | ||||||||||||||
| Joints N* |
Incidence/Progression N (%) |
OR adj.† | 95% CI | P value | Joints N* |
Incidence/Progression N (%) | OR adj.† | 95% CI | P value | Joints N* |
Incidence/Progression N (%) |
OR adj.† | 95% CI | P value | ||
| Non-users | 12 594 | 506 (4.0%) | 1 | – | – | 6162 | 329 (5.3%) | 1 | 6432 | 177 (2.8%) | 1 | – | – | |||
| Users | 863 | 94 (10.9%) | 2.5 | 1.94 to 3.20 | >0.001 | 426 | 55 (12.9%) | 2.34 | 1.69 to 3.22 | >0.001 | 437 | 39 (8.9%) | 2.74 | 1.82 to 4.11 | >0.001 | |
| Duration of acenocoumarol usage | ||||||||||||||||
| Non users | 12 594 | 506 (4.0%) | 1 | – | – | 6162 | 329 (5.3%) | 1 | – | – | 6432 | 177 (2.8%) | 1 | – | – | |
| ≤180 days | 279 | 35 (12.5%) | 2.82 | 1.90 to 4.20 | >0.001 | 144 | 15 (10.4%) | 1.78 | 1.01 to 3.18 | 4.8×10–02 | 135 | 20 (14.8%) | 4.84 | 2.73 to 8.56 | >0.001 | |
| >180 days and ≤556 days | 285 | 36 (12.6%) | 2.94 | 2.00 to 4.32 | >0.001 | 135 | 20 (14.8%) | 2.69 | 1.62 to 4.46 | >0.001 | 150 | 16 (10.7%) | 3.33 | 1.79 to 6.18 | >0.001 | |
| >556 days | 299 | 23 (7.7%) | 1.74 | 1.10 to 2.76 | 1.9×10–02 | 147 | 20 (13.6%) | 2.65 | 1.57 to 4.46 | >0.001 | 152 | 3 (2.0%) | 0.27 | 0.18 to 1.85 | 0.35 | |
Incidence and progression of osteoarthritis (OA) in RS-I and RS-II within the follow-up time associated with acenocoumarol use. Model used is a GEE (generalised estimated equations) multivariate logistic regression model including acenocoumarol use and adjusted for age, sex, BMI, smoking, time between baseline and follow-up visit, baseline OA severity in Kellgren-Lawrence score, joint modelled, femoral neck BMD, HDL/total cholesterol ratio, physical activity, education level, hypertension, diabetes mellitus and Rotterdam Study cohort.
Progression: number of joints showing overall progression of either hip or knee joints or both. Acenocoumarol usage examined by tertiles: first: ≤180 days, second: >180 and ≤556 days, third: >556 days of acenocoumarol use.
*Number of individual knee and/or hip joints studied from RSI and RSII (online supplemental figure S1 for exclusions).
†Unadjusted (raw) ORs are reported in online supplemental table S2.
BMI, body mass index; HDL, high-density lipoprotein; RS, Rotterdam Study.
Increased risk of overall OA incidence/progression in acenocoumarol users was also observed in the knee and hip joints separately (table 2) (knee OA (OR=2.34, 95% CI=1.69–3.22) and hip OA (OR=2.74, 95% CI=1.82–4.11)), as well as in each subcohort separately (online supplemental table S3). Interestingly, longer duration of acenocoumarol use does seem to have slight different effects on overall knee OA incidence/progression than on hip OA. Knee OA risk seems to increase for longer duration of acenocoumarol use, whereas risk for hip OA seems to decrease for longer durations of acenocoumarol use. These differences may represent true biological differences between the joints. However, this is more likely the effect of low statistical power (tertiles analysis), the statistical power difference between the joints as there were more cases of overall knee OA incidence/progression (n=385 joints) in our study cohorts than for hip OA (n=216 joints). In addition, the absence of a stronger effect in long-term acenocoumarol users could be explained by depletion of susceptible bias.27 This bias suggests that the cohort is depleted of all its susceptible subjects who had the event, OA incidence/progression, early on. Seemingly decreasing the risk with longer acenocoumarol (exposure) use. Thus, the remaining acenocoumarol users will include fewer OA incidence/progression predisposed subjects over the longer follow‐up, causing the risk to decrease over time.
MGP, VKORC1 genetics and acenocoumarol affect OA progression
Maintenance dosages of VKAs are dependent on genetic variants (SNVs) affecting VKORC1 expression activity.22 28 This altered VKORC1 expression can also affect MGP γ-carboxylation, as VKORC1 is needed for γ-carboxylation by vitamin K, a process that is needed to activate MGP. We therefore examined the extent to which acenocoumarol users with a genetic predisposition for decreased MGP and/or altered VKORC1 expression had an altered risk for overall incidence/progression of OA. The study population was stratified in MGP risk allele carriers (T/*) and non-carriers (A/A) (table 1).
Acenocoumarol users among both MGP genotype groups had a significantly higher risk of overall OA incidence/progression than non-users (table 3). Using the VKORC1-Haplotypes, the study population could be also stratified into low (AA), intermediate (AB) and high (BB) VKORC1 expression/VKA dose groups26 (table 1 and online supplemental table S1). Similar to the MGP genotypes, we observed no effect of the VCORC1 genotypes on the risk for overall OA incidence/progression (table 3), although the VKORC1-BB group had the highest risk of overall OA incidence/progression (OR=3.35, 95% CI=2.22–5.05, table 3).
Table 3.
Acenocoumarol use interacts with MGP OA risk variants and VKORC1 haplotype groups, leading to increased risk of overall incidence/progression of osteoarthritis
| Acenocoumarol use | Joints* | Incidence/Progression | OR | 95% CI | OR | 95% CI | P value |
| N | N (%) | Adj. | Adj. | Adj. | |||
| MGP rs1800801 alleles | |||||||
| Non-users MGP non-risk allele carriers (A/A) | 4332 | 205 (4.7%) | 1 | – | 1 | – | – |
| Non-users MGP risk allele carriers (T/*) | 7211 | 274 (3.8%) | 0.83 | 0.69 to 1.00 | 0.86 | 0.71 to 1.04 | 0.11 |
| Users MGP non-risk allele carriers (A/A) | 286 | 26 (9.1%) | 2.11 | 1.37 to 3.24 | 2.01 | 1.29 to 3.13 | 2.0×10–03 |
| Users MGP risk allele carriers (T/*) | 492 | 58 (11.8%) | 2.82 | 2.08 to 3.84 | 2.57 | 1.87 to 3.54 | 1.1×10–08 |
| VKORC1 haplotype groups | |||||||
| Non-users low VKORC1 group (AA) | 1735 | 75 (4.3%) | 1 | 1 | – | – | |
| Non-users intermediate VKORC1 gGroup (AB) | 5525 | 203 (3.7%) | 0.84 | 0.64 to 1.11 | 0.84 | 0.64 to 1.10 | 0.2 |
| Non-users high VKORC1 group (BB) | 4448 | 201 (4.5%) | 1.05 | 0.80 to 1.37 | 1.04 | 0.79 to 1.36 | 0.8 |
| Users low VKORC1 group (AA) | 152 | 16 (10.1%) | 2.6 | 1.48 to 4.59 | 2.31 | 1.28 to 4.17 | 5.3×10–03 |
| Users intermediate VKORC1 group (AB) | 317 | 24 (7.6%) | 1.81 | 1.13 to 2.92 | 1.53 | 0.34 to 2.51 | 9.1×10–02 |
| Users high VKORC1 group (BB) | 305 | 42 (13.8%) | 3.53 | 2.37 to 5.27 | 3.35 | 2.22 to 5.05 | 7.2×10–09 |
Overall progression of osteoarthritis (OA) in RSI and RSII within the follow-up time associated with acenocoumarol use effect MGP rs1800801 OA risk variant and VKORC1 expression/VKA dosage haplotypes. Model used is a GEE (generalised estimated equations) multivariate logistic regression model including acenocoumarol use adjusted (Adj.) for age, sex, BMI, smoking, time between baseline and follow-up visit, baseline OA severity in Kellgren-Lawrence score, joint modelled, femoral neck BMD, HDL/total cholesterol ratio, physical activity, education level, hypertension, diabetes mellitus and Rotterdam Study cohort. VKORC1 haplotype groups are based on the H haplotypes, see online supplemental table S1. For MGP risk variants and carriers, see Table 1. Progression: number of joints showing overall OA progression; T/*: MGP osteoarthritis risk variant carrier (T/A) or (T/T).
*Number of individual knee and hip joints included in the analysis.
BMD, bone mineral density; BMI, body mass index.
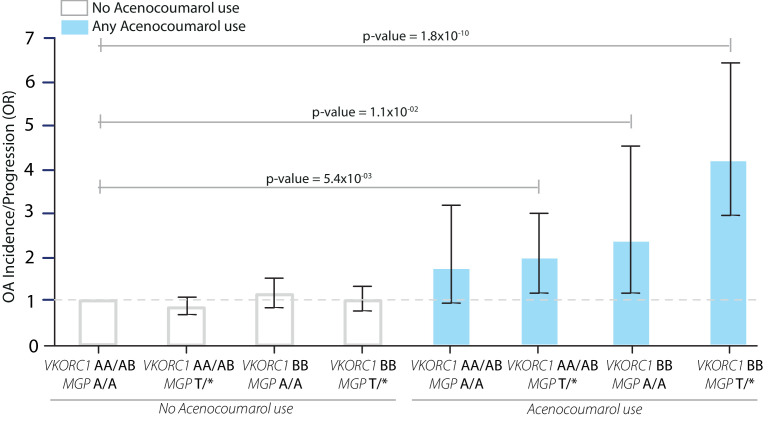
As individuals can be both carriers of MGP risk alleles and VKROC1 haplotypes, we examined the combined effects of the VKORC1-BB haplotype and MGP risk allele carriers (T/*). We stratified our study population into VKORC1 BB-haplotype or AA/AB carriers, which was then further stratified into carriers (T/*) and non-carriers (A/A) of the MGP risk allele, rs1800801 (figure 1 and online supplemental table S5). Acenocoumarol users whom either were carriers of the MGP risk alleles (T/*) or carriers of the VKORC1 BB-haplotype had a significant increased risk of overall OA incidence/progression. Individuals whom were carriers of both the VKORC1 BB-haplotype and the MGP risk allele had a fourfold increased risk of OA incidence/progression (OR=4.18, 95% CI=2.69–6.50, figure 1). Interestingly, VKORC1 AA/AB-haplotype carriers who were not carriers of the MGP risk allele (A/A) did not have a significant increased risk of overall OA progression/incidence when using acenocoumarol (OR=1.72, 95% CI=0.93–3.19, figure 1).
Figure 1.
Acenocoumarol use interacts with MGP osteoarthritis (OA) risk single nucleotide variants (SNVs) and VKORC1 haplotype groups, leading to increased risk of overall progression of OA. VKORC1 haplotype groups are based on the VKORC1 H-haplotypes, which can be divided into three groups based on their VKA dosage/VKORC1 expression association: AA=low VKA dose/VKORC1 expression; AB=intermediate VKA dose/VKORC1 expression; and BB=high VKA dose/VKORC1 expression. Acenocoumarol use in VKORC1 BB haplotype carriers and MGP risk allele carriers and OA risk. OR, CI and p value based on GEE multivariate logistic regression adjusted for age, sex, BMI, smoking, time between baseline and follow-up visit, baseline OA severity in Kellgren-Lawrence score, joint modelled, femoral neck BMD, HDL/total cholesterol ratio, physical activity, education level, hypertension and diabetes mellitus. A/A: non-carrier of MGP risk allele; T/*: carrier of MGP risk allele; VKORC1 AA: homozygous VKORC1 A haplotype carriers; VKORC1 BB: homozygous VKORC1 BB haplotype carriers. error bars indicate 95% CI for the OR. P values belong to depicted OR. See online supplemental table S3 for the exact values depicted in this graph.
Discussion
We demonstrated that use of the acenocoumarol was associated with a higher risk of overall OA incidence/progression in non-users. We observed that the increased OA risk in acenocoumarol users varied based on genetic variants affecting the vitamin K cycle with VKA use and MGP risk allele status. Acenocoumarol users with the MGP risk allele and VKORC1 BB-haplotype had a fourfold higher risk for overall OA incidence/progression.
The VKORC1 BB-haplotype is associated with a higher expression of VKORC1, associated with greater vitamin K activity; however, individuals who are VKORC1 BB-haplotype carriers also require and receive higher dosages of VKA for the desired anticoagulation effect.26 Intuitively, the anticoagulation, amount of VKORC1 inhibition, should be similar in all users regardless of VKORC1 haplotype since the amount of anticoagulation is the dosing measurement, not VKORC1 expression. Previous research in animal models has indicated that vitamin K availability levels differ significantly between tissues29 30 and warfarin affects vitamin K inhibition differently in liver compared with bone.31–33 In liver, another enzyme in addition to VKORC1 is available for the recycling of vitamin K into its active form, NQO1 (NAD(P)H quinone oxidoreductase 1). This enzyme is not present in bone tissue, causing the bone tissue to be potentially more susceptible to VKA dosages than liver tissue.32–34 Thus, we propose the following hypothesis: that the higher VKA dosages in VKORC1 BB-haplotype carriers, needed for desired inhibition of vitamin K-dependent blood coagulation proteins in the liver, might be too high of a dosage for VKORC1 functioning in the joint. This hypothesis, however, needs to be further examined in functional studies, particularly in human functional studies.
Oral VKAs, which include acenocoumarol and warfarin, were the only oral anticoagulants available for decades.35 New oral anticoagulant drugs developed over the past decade target thrombin (IIa) or factor X (Xa) instead of vitamin K. These are known as non-vitamin K inhibiting anticoagulants (NOACs) or direct oral anticoagulants (DOACs). Recent years have seen a rise in the use of NOACs,15 which have an improved efficacy-to-safety ratio over VKAs. Additionally, they do not need routine coagulation monitoring and have fewer food and drug interactions compared with VKAs; however, NOACs are more costly and difficult to reverse.36–38 Nonetheless, VKAs continue to be commonly prescribed and are the only indicated anticoagulant class for certain indications (eg, antiphospholipid antibody syndrome, mechanical heart valves). With ageing of the population, the number of people with OA and requiring anticoagulation medication will continue to rise. Given our findings of increased risk of OA incidence/progression with VKA use and the lack of effective treatment options for OA, it may be reasonable to consider NOACs over VKAs for medical indications in which NOACs can be used. This may be particularly the case for those who are carriers of the MGP risk allele and the VKORC1 BB-haplotype, which is an estimated 21% of individuals of European ancestry.
The strengths of our study include the robust underlying biological hypothesis, large sample size and high-quality prospectively collected data. However, some limitations of our study should be acknowledged. First, while we found similar results in RS-I and RS-II, analyses in other independent and even larger cohorts are warranted, as in our large sample size, the numbers of acenocoumarol users is still relatively low. Specifically in RSII, which has a much smaller sample size and number of acenocoumarol users compared with RSI. Also replication in non-Central European ancestry-based cohorts is warranted. Second, the association we noted in this study may be due to a shared disease pathology between OA and VKA indications.39 40 We addressed this issue by adjusting for multiple cardiovascular disease risk factors in our analysis (hypertension, HDL/total cholesterol ratio, diabetes mellitus, BMI, physical activity, smoking and age). However, we cannot rule out possible confounding by indication. This potential bias needs to be addressed more directly. This could be done by examining direct (new) oral anticoagulants (DOAC/NOAC) users as a comparator group, as these oral anticoagulants do not inhibit the vitamin K cycle. Unfortunately, our study population contains too few DOAC/NOAC users for such an analysis (n=9). Third, as we only have radiographs available of participants whom were able, healthy and survived long enough to come to our research centre at baseline and follow-up visits, our study may contain health and survivor bias. However, this could also possibly indicate that our found effect may even be larger.20 Last, as with all observational studies, we cannot rule out residual confounding.
In summary, we found an increased risk of overall OA incidence/progression in users of the VKA acenocoumarol, which was further increased in VKORC1 BB-haplotype and MGP risk allele carriers. These findings are consistent with the known biology of MGP and vitamin K, and are in keeping with prior studies of vitamin K in OA. Taken together, these studies, including the current one, highlight the importance of vitamin K and vitamin K-dependent Gla proteins such as MGP in the pathogenesis of OA. Importantly, our results may also indicate a role for other vitamin K-dependent proteins which occur and function in cartilage and bone tissues, such as osteocalcin and Gla-rich protein.3 Given that there are as yet no treatment options for preventing OA onset or progression, vitamin K may represent a modifiable risk factor. These data provide strong rationale for a properly powered randomised clinical trial of vitamin K in an appropriate patient population, such as those with insufficient vitamin K levels and/or MGP risk allele carriers. Additionally, these data lend support to the consideration of DOAC/NOACs in favour of VKAs when appropriate for a supported indication, and highlight the future possibility of genetic screening to identify individuals at high risk of OA incidence/progression.
Acknowledgments
The authors are grateful to the study participants, the staff from the Rotterdam Study and the participating general practitioners and pharmacists. The generation and management of GWAS genotype data for the Rotterdam Study (RS-I, RS-II, RS-III) was executed by the Human Genotyping Facility of the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, Rotterdam, The Netherlands. We thank Pascal Arp, Mila Jhamai, Marijn Verkerk, Lizbeth Herrera, Marjolein Peters, MSc, and Carolina Medina-Gomez, MSc, for their help in creating the GWAS database, and Linda Broer, PhD, for the creation of the imputed genotype data.
Footnotes
Handling editor: Josef S Smolen
Twitter: @CurlyGeneticist
Contributors: CGB designed the study, performed the analyses, made the figures and tables, and wrote the manuscript. IS contributed to study design. NLN performed analysis. TN and IM contributed to study design. AGU and MAI provided access to the Rotterdam study dataset. SB-Z contributed to study design. BS provided pharmacological data, contributed to study design and analysis. JBvM designed the study and supervised this work. All authors critically assessed the manuscript.
Funding: This research was funded by the Dutch Arthritis Society (ReumaNederland). The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII) and the Municipality of Rotterdam. The Rotterdam Study GWAS datasets are supported by the Netherlands Organisation of Scientific Research NWO Investments (numbers 175.010.2005.011, 911-03-012), the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, the Research Institute for Diseases in the Elderly (014-93-015; RIDE2), the Netherlands Genomics Initiative (NGI)/Netherlands Organisation for Scientific Research (NWO) Netherlands Consortium for Healthy Aging (NCHA), project number 050-060-810. TN was supported by NIH K24 AR070892.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All relevant data supporting the key findings of this study are available within the article and its supplementary data. Due to ethical and legal restrictions, individual-level data of the Rotterdam Study (RS) cannot be made publicly available. Data are available upon request to the data manager of the Rotterdam Study Frank van Rooij (f.vanrooij@erasmusmc.nl) and subject to local rules and regulations. This includes submitting a proposal to the management team of RS, where upon approval, analysis needs to be done on a local server with protected access, complying with GDPR regulations.
References
- 1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 3. Azuma K, Inoue S. Multiple modes of vitamin K actions in aging-related musculoskeletal disorders. Int J Mol Sci 2019;20. 10.3390/ijms20112844. [Epub ahead of print: 11 Jun 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neogi T, Booth SL, Zhang YQ, et al. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum 2006;54:1255–61. 10.1002/art.21735 [DOI] [PubMed] [Google Scholar]
- 5. Misra D, Booth SL, Tolstykh I, et al. Vitamin K deficiency is associated with incident knee osteoarthritis. Am J Med 2013;126:243–8. 10.1016/j.amjmed.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shea MK, Kritchevsky SB, Hsu F-C, et al. The association between vitamin K status and knee osteoarthritis features in older adults: the health, aging and body composition study. Osteoarthritis Cartilage 2015;23:370–8. 10.1016/j.joca.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neogi T, Felson DT, Sarno R, et al. Vitamin K in hand osteoarthritis: results from a randomised clinical trial. Ann Rheum Dis 2008;67:1570–3. 10.1136/ard.2008.094771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yin T, Miyata T. Warfarin dose and the pharmacogenomics of CYP2C9 and VKORC1 - rationale and perspectives. Thromb Res 2007;120:1–10. 10.1016/j.thromres.2006.10.021 [DOI] [PubMed] [Google Scholar]
- 9. Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix Gla protein. Nature 1997;386:78–81. 10.1038/386078a0 [DOI] [PubMed] [Google Scholar]
- 10. Newman B, Gigout LI, Sudre L, et al. Coordinated expression of matrix Gla protein is required during endochondral ossification for chondrocyte survival. J Cell Biol 2001;154:659–66. 10.1083/jcb.200106040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. den Hollander W, Boer CG, Hart DJ, et al. Genome-Wide association and functional studies identify a role for matrix Gla protein in osteoarthritis of the hand. Ann Rheum Dis 2017;76:2046–53. 10.1136/annrheumdis-2017-211214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shepherd C, Skelton AJ, Rushton MD, et al. Expression analysis of the osteoarthritis genetic susceptibility locus mapping to an intron of the MCF2L gene and marked by the polymorphism rs11842874. BMC Med Genet 2015;16:108. 10.1186/s12881-015-0254-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holbrook A, Schulman S, Witt DM, et al. Evidence-Based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ED: American College of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e152S–84. 10.1378/chest.11-2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–51. 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu J, Alexander GC, Nazarian S, et al. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010-2017. Pharmacotherapy 2018;38:907–20. 10.1002/phar.2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ikram MA, Brusselle G, Ghanbari M, et al. Objectives, design and main findings until 2020 from the Rotterdam study. Eur J Epidemiol 2020;35:483–517. 10.1007/s10654-020-00640-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kerkhof HJM, Meulenbelt I, Akune T, et al. Recommendations for standardization and phenotype definitions in genetic studies of osteoarthritis: the TREAT-OA Consortium. Osteoarthritis Cartilage 2011;19:254–64. 10.1016/j.joca.2010.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Odding E, Valkenburg HA, Algra D, et al. Association of locomotor complaints and disability in the Rotterdam study. Ann Rheum Dis 1995;54:721–5. 10.1136/ard.54.9.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Niu J, Felson DT, et al. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res 2010;62:1527–32. 10.1002/acr.20287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clockaerts S, Van Osch GJVM, Bastiaansen-Jenniskens YM, et al. Statin use is associated with reduced incidence and progression of knee osteoarthritis in the Rotterdam study. Ann Rheum Dis 2012;71:642–7. 10.1136/annrheumdis-2011-200092 [DOI] [PubMed] [Google Scholar]
- 22. Teichert M, Eijgelsheim M, Rivadeneira F, et al. A genome-wide association study of acenocoumarol maintenance dosage. Hum Mol Genet 2009;18:3758–68. 10.1093/hmg/ddp309 [DOI] [PubMed] [Google Scholar]
- 23. Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 2012;92:414–7. 10.1038/clpt.2012.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCarthy S, Das S, Kretzschmar W, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 2016;48:1279–83. 10.1038/ng.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. haplo.stats: Statistical Analysis of Haplotypes with Traits and Covariates when linkag pahs is Ambigous [program]. 1.7.9 version 2018.
- 26. Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 2005;352:2285–93. 10.1056/NEJMoa044503 [DOI] [PubMed] [Google Scholar]
- 27. Renoux C, Dell'Aniello S, Brenner B, et al. Bias from depletion of susceptibles: the example of hormone replacement therapy and the risk of venous thromboembolism. Pharmacoepidemiol Drug Saf 2017;26:554–60. 10.1002/pds.4197 [DOI] [PubMed] [Google Scholar]
- 28. Teichert M, Eijgelsheim M, Uitterlinden AG, et al. Dependency of phenprocoumon dosage on polymorphisms in the VKORC1, CYP2C9, and CYP4F2 genes. Pharmacogenet Genomics 2011;21:26–34. 10.1097/FPC.0b013e32834154fb [DOI] [PubMed] [Google Scholar]
- 29. Roncaglioni MC, Soute BA, de Boer-vd Berg MA, et al. Warfarin-Induced accumulation of vitamin K-dependent proteins. Comparison between hepatic and non-hepatic tissues. Biochem Biophys Res Commun 1983;114:991–7. 10.1016/0006-291X(83)90658-7 [DOI] [PubMed] [Google Scholar]
- 30. Thijssen HH, Drittij-Reijnders MJ. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr 1996;75:121–7. 10.1079/BJN19960115 [DOI] [PubMed] [Google Scholar]
- 31. Price PA, Kaneda Y. Vitamin K counteracts the effect of warfarin in liver but not in bone. Thromb Res 1987;46:121–31. 10.1016/0049-3848(87)90212-X [DOI] [PubMed] [Google Scholar]
- 32. Sato T, Ohtani Y, Yamada Y, et al. Difference in the metabolism of vitamin K between liver and bone in vitamin K-deficient rats. Br J Nutr 2002;87:307–14. 10.1079/BJN2001519 [DOI] [PubMed] [Google Scholar]
- 33. Hara K, Kobayashi M, Akiyama Y. Comparison of inhibitory effects of warfarin on gamma-carboxylation between bone and liver in rats. J Bone Miner Metab 2005;23:366–72. 10.1007/s00774-005-0614-7 [DOI] [PubMed] [Google Scholar]
- 34. Ulrich MM, Knapen MH, Herrmann-Erlee MP, et al. Vitamin K is no antagonist for the action of warfarin in rat osteosarcoma UMR 106. Thromb Res 1988;50:27–32. 10.1016/0049-3848(88)90171-5 [DOI] [PubMed] [Google Scholar]
- 35. Gómez-Outes A, Suárez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol 2012;9:83–104. 10.2174/1570163811209020083 [DOI] [PubMed] [Google Scholar]
- 36. Mekaj YH, Mekaj AY, Duci SB, et al. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag 2015;11:967–77. 10.2147/TCRM.S84210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim I-S, Kim H-J, Kim T-H, et al. Non-Vitamin K antagonist oral anticoagulants have better efficacy and equivalent safety compared to warfarin in elderly patients with atrial fibrillation: a systematic review and meta-analysis. J Cardiol 2018;72:105–12. 10.1016/j.jjcc.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 38. Haas S, Camm AJ, Bassand J-P, et al. Predictors of NOAC versus VKA use for stroke prevention in patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Am Heart J 2019;213:35–46. 10.1016/j.ahj.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 39. Veronese N, Trevisan C, De Rui M, et al. Association of osteoarthritis with increased risk of cardiovascular diseases in the elderly: findings from the Progetto Veneto Anziano study cohort. Arthritis Rheumatol 2016;68:1136–44. 10.1002/art.39564 [DOI] [PubMed] [Google Scholar]
- 40. Wang H, Bai J, He B, et al. Osteoarthritis and the risk of cardiovascular disease: a meta-analysis of observational studies. Sci Rep 2016;6:39672. 10.1038/srep39672 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-219483supp001.pdf (118.4KB, pdf)
annrheumdis-2020-219483supp002.pdf (132.8KB, pdf)
annrheumdis-2020-219483supp003.pdf (206.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All relevant data supporting the key findings of this study are available within the article and its supplementary data. Due to ethical and legal restrictions, individual-level data of the Rotterdam Study (RS) cannot be made publicly available. Data are available upon request to the data manager of the Rotterdam Study Frank van Rooij (f.vanrooij@erasmusmc.nl) and subject to local rules and regulations. This includes submitting a proposal to the management team of RS, where upon approval, analysis needs to be done on a local server with protected access, complying with GDPR regulations.