Abstract
Objectives
Anti-centromere antibodies (ACAs) are detected in patients with various autoimmune diseases such as Sjögren’s syndrome (SS), systemic sclerosis (SSc) and primary biliary cholangitis (PBC). However, the targeted antigens of ACAs are not fully elucidated despite the accumulating understanding of the molecular structure of the centromere. The aim of this study was to comprehensively reveal the autoantigenicity of centromere proteins.
Methods
A centromere antigen library including 16 principal subcomplexes composed of 41 centromere proteins was constructed. Centromere protein/complex binding beads were used to detect serum ACAs in patients with SS, SSc and PBC. ACA-secreting cells in salivary glands obtained from patients with SS were detected with green fluorescent protein-fusion centromere antigens and semiquantified with confocal microscopy.
Results
A total of 241 individuals with SS, SSc or PBC and healthy controls were recruited for serum ACA profiling. A broad spectrum of serum autoantibodies was observed, and some of them had comparative frequency as anti-CENP-B antibody, which is the known major ACA. The prevalence of each antibody was shared across the three diseases. Immunostaining of SS salivary glands showed the accumulation of antibody-secreting cells (ASCs) specific for kinetochore, which is a part of the centromere, whereas little reactivity against CENP-B was seen.
Conclusions
We demonstrated that serum autoantibodies target the centromere–kinetochore macrocomplex in patients with SS, SSc and PBC. The specificity of ASCs in SS salivary glands suggests kinetochore complex-driven autoantibody selection, providing insight into the underlying mechanism of ACA acquisition.
Keywords: autoimmunity, Sjogren's syndrome, scleroderma, systemic, autoantibodies
Key messages.
What is already known about this subject?
Anti-centromere antibodies (ACAs) are detected in various autoimmune diseases such as Sjögren’s syndrome (SS), systemic sclerosis (SSc) and primary biliary cholangitis (PBC) and correlate with characteristic symptoms such as Raynaud’s phenomenon and sclerodactyly.
What does this study add?
Comprehensive serum ACA profiling revealed broad specificity for the centromere–kinetochore macrocomplex, and the specificity of autoantibodies was not different in patients with SS, SSc and PBC.
Antibody-secreting cells in the salivary glands of ACA-positive SS patients were specific to the part of the centromeric structure, termed the ‘kinetochore’ rather than CENP-B, which is known as the major autoantigen corresponding to ACA.
How might this impact on clinical practice or future developments?
This study conducted a detailed analysis of the specificity of ACAs, providing further insights into pathognomonic autoantibodies common to multiple autoimmune diseases.
The combination of multiple conformational centromere antigens could detect serum ACAs with higher sensitivity than conventional ACA detection methods.
Introduction
Anti-centromere antibodies (ACAs) are well-known autoantibodies detected in various autoimmune diseases. Although serum ACAs are frequently detected in patients with systemic sclerosis (SSc), they are also detected in other autoimmune diseases such as Sjögren’s syndrome (SS) and primary biliary cholangitis (PBC), and the presence of ACAs is associated with the overlap of these three diseases.1–3
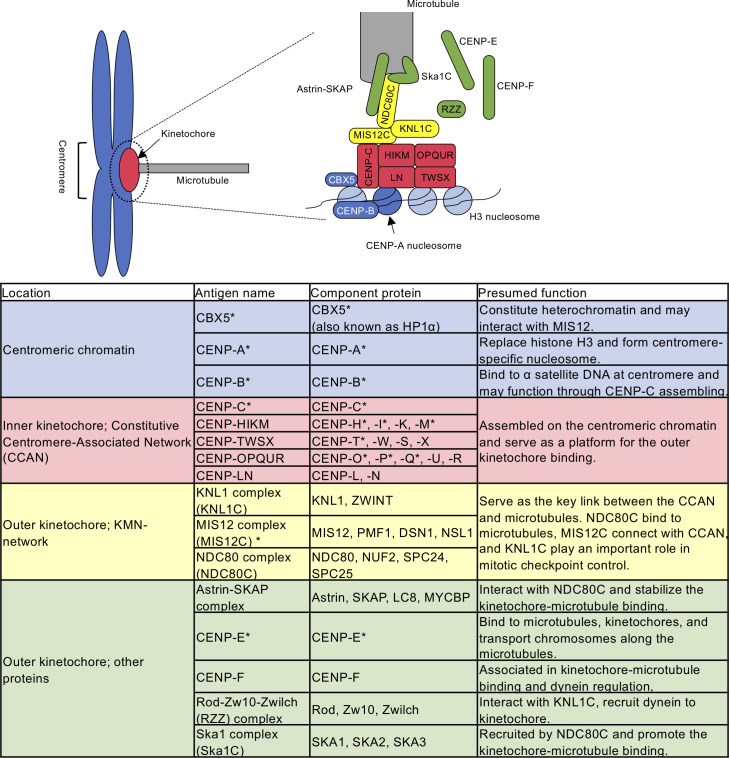
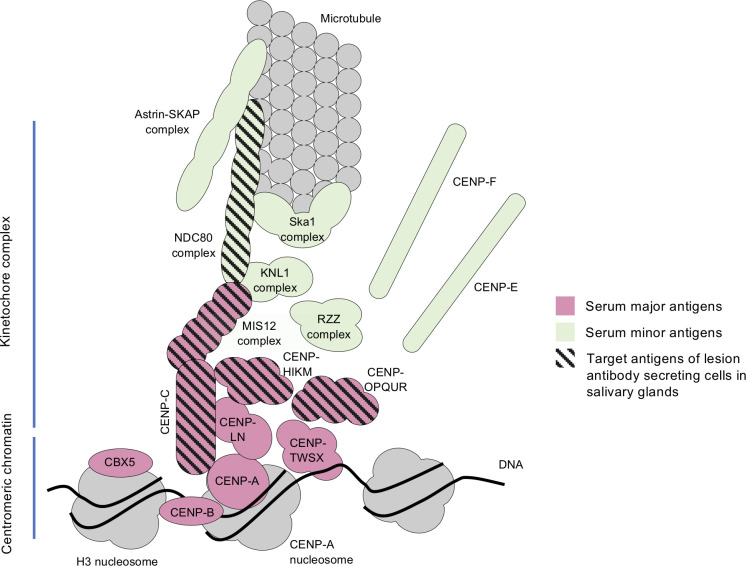
In the anti-nuclear antibody (ANA) test, ACAs show a characteristic staining pattern called the discrete-speckled pattern, which reflects the localisation of the centromere.4 Recently, the molecular structure of the centromere has been rapidly clarified. Its framework structure is understood as a combination of specific centromeric chromatin, characterised by the replacement of histone H3 by CENP-A and the macromolecular complex ‘kinetochore’, which is assembled on the centromere-specific nucleosome.5 The centromere binds to microtubules via inner and outer kinetochore structure during cell division. Schematic illustration of centromere–kinetochore–microtubule interface is described in figure 1 based on the cited references.6–11
Figure 1.
Schematic illustration of the centromere-kinetochore-microtubule interface. CENP-A replaces histone H3 and forms centromere-specific nucleosome. CBX5 and CENP-B bind to H3 nucleosome and centromeric DNA, respectively. The kinetochore complex is constructed on the CENP-A nucleosome and interacts with microtubules. The key kinetochore subcomplexes are the constitutive centromere-associated network (CCAN; divided into CENP-C, CENP-HIKM, CENP-TWSX, CENP-LN, and CENP-OPQUR) and the KMN-network (divided into the KNL1 complex, the MIS12 complex, and the NDC80 complex). The Astrin-SKAP complex and the Ska1 complex stabilise the kinetochore-microtubule binding. CENP-E, CENP-F, and the RZZ complex associate in kinetochore-microtubule binding and chromosome transportation. *Known autoantigens in autoimmune diseases.12 15 17
Among a number of component molecules, CENP-A, CENP-B, CENP-C and CBX5 are known targets of ACAs.12 13 In particular, CENP-B is thought to be the main autoantigen because the presence of anti-CENP-B antibody is highly consistent with the ACAs detected by the ANA test.14 Some other centromere proteins were also identified as autoantigens in the sera of ACA-positive patients: CENP-D, -E, -G, -H, -I, -J, -M, -T, -O, -P and -Q12 15; however, the spatial relationship of these antigens has not been taken into account, and the autoantigenicity of newly identified centromere proteins remains unclear. In addition, although several studies have focused on the distinct epitope specificity of major antigens (ie, CENP-A, -B and -C), comparing patients with SS and SSc,13 16 few studies have been performed on the prevalence of antibodies against other centromere proteins.
Our recent study of autoantibodies produced in salivary glands demonstrated that many autoantibodies recognise native conformational epitopes. We developed an antigen-binding bead assay by using mammalian cell line-derived proteins as antigens, which provided higher sensitivity for the detection of autoantibodies than conventional ELISA.17
In this study, we constructed a centromere antigen library including 41 centromere proteins, which were selected based on the latest information about the centromere structure. To clarify the true target of ACAs and to identify differences by disease, we examined serum autoantibodies against this library using an antigen-binding bead assay and investigated the spatial relationship of antibody-secreting cells (ASCs) in salivary gland tissue.
Methods
Clinical samples
Serum samples were obtained from patients with SS, SSc or PBC, and salivary gland samples were collected from patients with clinically suspected SS who underwent a lip biopsy at Keio University Hospital. The diagnosis was made according to the 2016 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for primary SS,18 the 2013 ACR/EULAR classification criteria for SSc19 and the clinical practice guidelines for PBC established in 2017.20 Sera of healthy controls (HCs) were used as controls.
Preparation of the centromere antigen library
A total of 41 centromere proteins were cloned into pEFs vector or pcDNA3.4 vector as a centromere protein library, combined with the streptavidin-binding peptide tag and green fluorescent protein (GFP) at the N-terminus and expressed by 293T cells. Most of them were cotransfected to construct subcomplexes according to the molecular structure of the centromere.8 9 21–23 Antigens were purified by streptavidin beads and electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by silver staining (Aproscience, Tokushima, Japan) and western blotting with anti-human GFP antibody (unconjugated, 1GFP63, BioLegend, California, USA) and horseradish peroxidase-conjugated sheep anti-mouse IgG antibody (GE Healthcare, Buckinghamshire, UK) (see online supplemental figure S1). Nucleic acid of CENP-E was purchased from Kazusa DNA Research Institute (Chiba, Japan). Other detailed methods were described previously.17
annrheumdis-2020-218881supp001.pdf (24MB, pdf)
Serum autoantibody detection by antigen-binding bead assay
The protocols for bead coupling and measurements of serum antibody titres were described previously.17 In short, antigens expressed by 293T cells were attached to Dynabeads M-280 Streptavidin (Thermo Fisher Scientific, Massachusetts, USA). Antigen-binding beads were incubated with sera of subjects, washed and then stained with anti-human IgG-Fc antibody (APC, goat-F(ab’)2 fragment, Jackson ImmunoResearch, Pennsylvania, USA) and anti-human IgA-Fc antibody (DL405, goat-F(ab’)2 fragment, Jackson ImmunoResearch). The titres of antibodies were analysed by FACSVerse and FlowJo software (BD Biosciences, California, USA). Anti-CENP-B antibody was measured by anti-CENP-B ELISA (ORGENTEC, Mainz, Germany) according to the manufacturer’s instructions.
Monoclonal antibodies against newly identified centromere autoantigens
We previously examined lesion antibody specificity by cloning antibodies from human salivary glands and analysed their reactivity to recombinant centromere antigens.17 In this study, these cloned antibodies were comprehensively analysed with a newly developed centromere antigen library. The detailed method is described in online supplemental information.
Direct detection of antibody-producing cells in salivary glands
Fresh-frozen sections of labial salivary gland samples were incubated with GFP fusion antigens and anti-CD138 antibody and anti-mouse IgG1 antibody. ASCs were semiquantified using confocal microscopy. The detailed method is provided in online supplemental information.
Statistics
The cut-off value of a serum antibody against a specific antigen was determined by the median plus 5IQR of the mean fluorescent intensity in 68 HCs. A doubling of the difference between the third quartile and the median (Q3−Q2) was substituted for IQR when the skewness of a distribution in HCs was over 1.24 Exceptionally, the cut-off value of anti-CENP-B IgG antibody was determined by receiver operating characteristic analysis, in which patients with positive anti-CENP-B antibody by ELISA were defined as positive. The Wilcoxon rank sum test was applied to compare continuous variables, and two-sided Fisher’s exact test was applied to compare categorical variables. P values <0.05 were considered statistically significant. Unsupervised hierarchical clustering by Ward’s method and principal component analysis (PCA) were performed to analyse the serum autoantibody profile. GraphPad Prism 8 (GraphPad Software, California, USA) and JMP V.13 (SAS Institute, North Carolina, USA) were used to perform the analyses.
Results
Serum anti-centromere antibody analysis
We recruited a total of 241 individuals with SS (n=86), SSc (n=35), PBC (n=10) or two or more diseases above (overlap; n=42) and HC (n=68) for serum antibody analysis. The clinical characteristics of the subjects are shown in table 1.
Table 1.
Clinical characteristics of the patients who underwent serum analysis
| Disease type | HC n=68 | SS n=86 | SSc n=35 | PBC n=10 | Overlap n=42 |
| Female % | 82 | 97 | 86 | 100 | 100 |
| Age (y), median (IQR) | 44 (31–50) | 61 (47–72) | 59 (50–71) | 60 (56–72) | 64 (55–74) |
| ANA discrete-speckled % | NA | 10 | 34 | 30 | 69 |
| Anti-CENP-B antibody positive % | 1.5 | 10 | 37 | 30 | 71 |
| Disease specific antibody positive % | Anti-SSA 83 Anti-SSB 42 |
Anti-Topo 1 31 Anti-RNAPIII 6 |
AMA 80 | ||
| Disease type/ complicated disease % | Primary SS 85 Secondary SS 15 |
lcSSc 71 dcSSc 29 |
SS+SSc 27 SS+PBC 17 SSc+PBC 29 SS +SSc+ PBC 29 |
ACA, anti-centromere antibody; AMA, anti-mitochondrial antibody; dcSSc, diffuse cutaneous systemic sclerosis; HC, healthy controls; lcSSc, limited cutaneous systemic sclerosis; NA, not assessed; PBC, primary biliary cholangitis; RNAPIII, RNA polymerase III; SS, Sjögren’s syndrome; SSc, systemic sclerosis; Topo 1, topoisomerase 1.
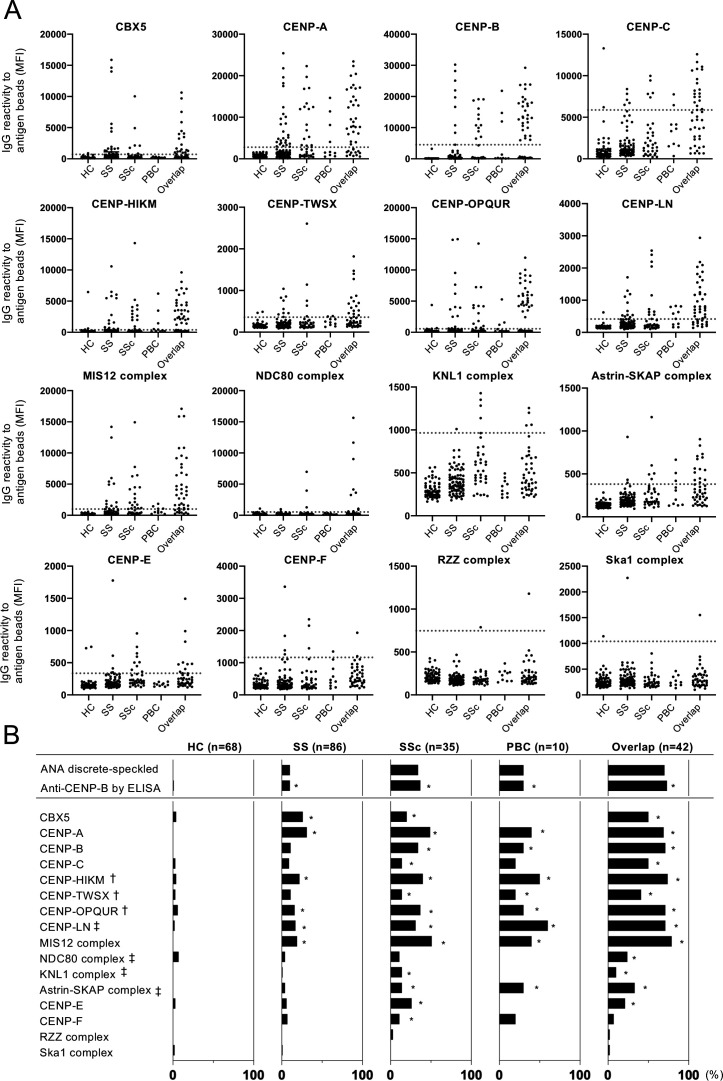
The IgG reactivity of each individual sera against centromere antigens is shown in figure 2A. The reactivity against CENP-HIKM, CENP-TWSX, CENP-OPQUR and CENP-LN was observed with a similar tendency to those against previously known autoantigens: CBX5, CENP-A, CENP-B, CENP-C and the MIS12 complex (MIS12C). Reactivity against the NDC80 complex (NDC80C), the KNL1 complex (KNL1C), the Astrin–SKAP complex, CENP-E and CENP-F was also seen with relatively low frequency. The Rod-Zw10-Zwilch (RZZ) complex and the Ska1 complex showed negligibly low reactivity in the sera. Although we also performed the same analysis with IgA antibodies, the titres and antibody positivity were low, and the differences between the HC group and each disease group were less apparent than those of IgG antibodies (online supplemental figure S2).
Figure 2.
Profiling of serum IgG ACAs. The serum IgG autoantibody titres against each centromere antigen were analysed by the antigen-binding bead assay with the sera of patients with SS (n=86), SSc (n=35), PBC (n=10), overlap disease (n=42), and healthy controls (HC; n=68). (A) Each symbol represents the antibody level in an individual’s serum and the dotted line indicates the cut-off value, which was determined by the median plus 5IQR of MFI in HC. (B) Bar graphs show the prevalence of autoantibodies against centromere antigens measured as MFI in each disease group. The prevalence of the discrete-speckled pattern by the ANA test and anti-CENP-B antibody by ELISA are shown in the top. *p<0.05 between each disease group and the HC group; †novel autoantigen as a form of complex, at least one component molecule is known as an autoantigen; ‡novel autoantigen identified in this assay. The data of CBX5, CENP-A, CENP-B, CENP-C, and the MIS12 complex were obtained from our previous study.17 ANA, anti-nuclear antibody; ELISA, enzyme-linked immunosorbent assay; MFI, mean fluorescence intensity.
As shown in figure 2B, the positive rates of antibodies against CENP-HIKM, CENP-TWSX, CENP-OPQUR, CENP-LN, NDC80C, KNL1C and the Astrin–SKAP complex were significantly higher in at least one disease group than in the HC group. CENP-LN, NDC80C, KNL1C, and the Astrin-SKAP complex were newly identified as centromere autoantigens. In CENP-HIKM, CENP-TWSX, and CENP-OPQUR, at least one component molecule was previously reported as an autoantigen; however, this is the first study to examine in complex form.
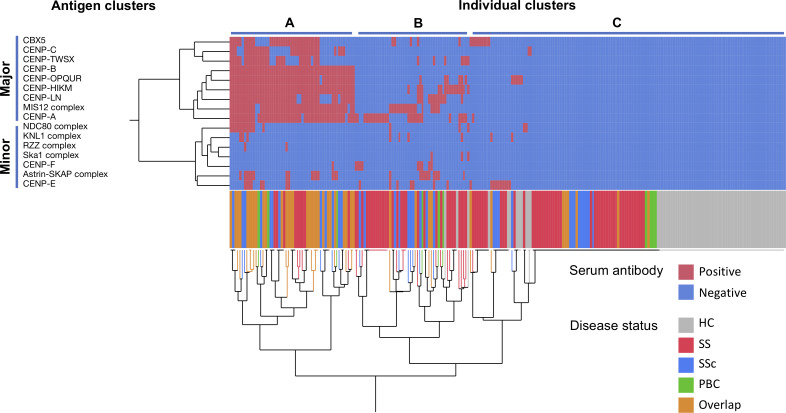
Clustering of autoantigens and individuals
Next, we performed clustering analysis to visualise the serum autoantibody profile of each individual and analysed the correlations among antigens. Unsupervised hierarchical clustering identified two antigen clusters (figure 3). We refer to the first cluster, including CBX5, CENP-A, CENP-B, CENP-C, CENP-HIKM, CENP-TWSX, CENP-OPQUR, CENP-LN, and MIS12C, as the ‘major antigen’ cluster and the second cluster, including the others, as the ‘minor antigen’ cluster. In the major autoantigen cluster, the titre of each antibody was mutually correlated in addition to the prevalence (online supplemental figure S3A).
Figure 3.
Clustering analysis of the centromere antigens and individuals. The sera of patients with SS (n=86), SSc (n=35), PBC (n=10), or overlap disease (n=42), and HC (n=68) were analysed by the antigen-binding bead assay. The serum antibody positivity for each target antigen (rows) in each individual (columns) is indicated by the appropriate colour. Hierarchical clustering produced a dendrogram among target antigens (left) and individuals (bottom). Coloured lines below the matrix indicate the disease status of the individuals. HC, healthy controls; PBC, primary biliary cholangitis; SS, Sjögren’s syndrome;SSc, systemic sclerosis.
When we focused on the disease status, the participants seemed to be classified into three groups. A total of 54 individuals in cluster A showed a broad spectrum of autoantibodies against the major antigens. For the remaining participants, cluster B, with 50 individuals, had 1–7 antibodies against major or minor antigens, whereas cluster C, with 137 individuals, had few or none. Although patients with overlapping diseases tended to be classified as cluster A and most HCs were in cluster C, the result of clustering based on the antibody profile was not consistent with disease specificity. We further performed PCA of serum IgG antibody reactivity and revealed overlapping 95% confidence ellipses across patients in the SS, SSc, PBC and overlap groups, suggesting similar patterns of antibody specificity in these diseases (online supplemental figure S3B). These results indicated that the specificity of ACAs is generally shared across disease phenotypes.
Antigen bead assay identified potential ACA-positive patients
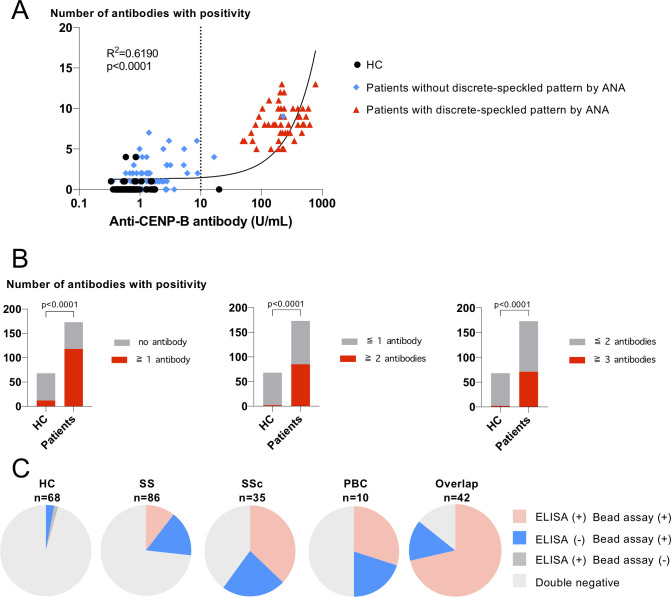
Next, we analysed the clinical significance of comprehensive bead-based autoantibody detection compared with standard ACA detection methods, ANA tests and anti-CENP-B antibodies by ELISA. As shown in figure 4A, the numbers of autoantibodies against centromere proteins were well correlated with their titres of anti-CENP-B antibody by ELISA (r2=0.6190, p<0.0001). The presence of a discrete-speckled pattern by the ANA test was remarkably consistent with the positivity of the anti-CENP-B antibody by ELISA.
Figure 4.
Comparison between the antigen-binding bead assay and conventional methods; ANA test and anti-CENP-B antibody by ELISA. (A) The numbers of antibodies with positivity in sera were plotted against the titres of anti-CENP-B antibody. Linear regression lines, the correlation coefficient, and p value of the regression line are shown. The dotted line indicates cut-off value by ELISA. (B) Number of individuals with and without bead assay positivity depending on various cut-off values. (C) Pie charts show positive rates for anti-CENP-B antibody by ELISA and the centromere antigen-binding bead assay. Having two or more antibodies against centromere antigens was considered as positive. Individuals who were additionally identified as ACA positive by the bead assay are shown by blue. ANA, anti-nuclear antibody; ELISA, enzyme-linked immunosorbent assay; HC, healthy controls.
Although most HCs have no more than one antibody, some patients without ACA by standard ACA detection methods have two or more antibodies. Defining the bead assay positivity as having two or more antibodies, the bead assay clearly distinguished patients with SS, SSc or PBC with high specificity (figure 4B, online supplemental table S1). As shown in figure 4C, the bead assay identified 14%–23% of additional patients as ACA positive compared with standard methods in each disease group (15% in SS, 23% in SSc, 20% in PBC and 14% in overlap).
To confirm the clinical significance of additionally identified ACAs, we compared the characteristics of patients with or without anti-CENP-B antibody. The clinical characteristics of bead assay-positive SS patients with or without anti-CENP-B antibody by ELISA were comparable between the two groups (online supplemental table S2). For patients with SSc, the clinical characteristics differed in the two groups (online supplemental table S3). Although all anti-CENP-B antibody-positive patients presented limited cutaneous SSc, half of the bead assay-positive and ELISA-negative patients showed diffuse cutaneous SSc with anti-topoisomerase 1 antibody (ATA). The number of patients with PBC was too small to compare clinical characteristics (online supplemental table S4).
Monoclonal antibodies from SS salivary glands recognised the newly identified centromere antigens
In a previous study, we produced 256 antibodies from the ASCs of SS salivary glands, some of which were reacted against known centromere autoantigens.17 In this study, we searched for antibodies that recognise new centromere autoantigens. The results are shown in online supplemental table S5. We identified two antibodies against CENP-HIKM, one against CENP-OPQUR and one against NDC80C in addition to the previously identified ACAs.
Antigen specificity of ASCs in salivary glands
Next, we comprehensively analysed the specificity of ASCs in target organs using a centromere antigen library. Due to limitations in sample collection, the study was limited to salivary glands in patients with SS. Fresh-frozen sections of labial salivary glands were stained with GFP-fusion centromere antigens and CD138 as a cell surface marker of ASCs. Representative images are shown in online supplemental figure S4.
The results are summarised in table 2. Among ACA-positive patients (n=8), 6 patients had lesion ASCs targeting centromere antigens. The most frequent target antigens were CENP-C (n=6) and MIS12C (n=6), followed by CENP-HIKM (n=4), NDC80C (n=3) and CENP-OPQUR (n=2). ASCs targeting other major antigens were scarce or not identified in salivary glands. ASCs in patients with ACA-negative SS (n=5) and patients with sicca symptoms without fulfilling SS diagnosis (n=2) showed negative results. These results were consistent with the results of the specificity of monoclonal antibodies from ACA-positive SS salivary glands.
Table 2.
Clinical characteristics and antibody specificity of lesion antibody-secreting cells in salivary glands
| Patient ID | S3 | S10 | LB32 | LB73 | LB90 | LB117 | LB101 | LB93 | LB17 | LB25 | LB46 | LB19 | LB23 | LB47 | LB48 |
| Age, sex | 51, F | 71, F | 31, F | 29, F | 70, F | 70, F | 65, F | 65, F | 47, F | 86, F | 50, F | 54, F | 60, F | 38, F | 47, F |
| Disease | sSS, PBC, MCTD |
pSS | pSS | sSS, SSc | pSS | pSS | sSS, PMR | pSS | pSS | pSS | pSS | pSS | pSS | nonSS | nonSS |
| Anti-CENP-B antibody | + | + | + | + | + | + | + | + | NA | NA | NA | NA | – | – | NA |
| ANA discrete-speckled | - * | + | + | + | + | + | + | + | – | – | – | – | – | – | – |
| ANA | >2560 Sp+C | 640 H+D | 640 H+D+N | 2560 D | 320 H+D | 160 D | 320 D | 160 D | 40 Sp | 80 H+Sp | 80 Sp | <40 | <40 | 40 H+Sp | 80 H+Sp |
| Anti-SSA antibody | + | + | – | – | – | + | – | – | + | + | + | – | – | – | – |
| Anti-SSB antibody | + | – | – | – | – | – | – | – | + | + | – | – | – | – | – |
| Rheumatoid factor | + | + | – | + | NA | + | – | – | – | – | + | + | – | – | – |
| Greenspan grade | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 1 | 3 | 4 | 4 | 3 | 4 | 2 | 1 |
| Medication | + † | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Extraglandular symptom | PH | – | Erythema | – | – | – | – | – | – | – | – | – | – | – | – |
| Antibody-secreting cell | |||||||||||||||
| CBX5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| CENP-A | ± | ± | – | – | ± | ± | – | – | – | – | – | – | – | – | – |
| CENP-B | – | ± | – | ± | – | ± | – | – | – | – | – | – | – | – | – |
| CENP-C | +++ | +++ | ++ | +++ | ++ | + | – | – | – | – | – | – | – | – | – |
| CENP-HIKM | +++ | – | ++ | ± | +++ | +++ | – | – | – | – | – | – | – | – | – |
| CENP-TWSX | – | – | – | – | – | ± | – | – | ± | ± | – | – | – | – | – |
| CENP-OPQUR | ++ | – | – | – | – | + | – | – | – | – | – | – | – | – | – |
| CENP-LN | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| MIS12 complex | +++ | ++ | +++ | +++ | +++ | +++ | – | – | – | – | – | – | – | – | – |
| NDC80 complex | + | + | – | ++ | – | – | – | – | – | – | – | – | – | – | – |
| KNL1 complex | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Astrin-SKAP complex | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| CENP-E | – | – | – | – | – | ± | – | – | – | – | – | – | – | – | – |
| CENP-F | – | – | – | ± | – | – | – | – | – | – | – | – | – | – | – |
| RZZ complex | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Ska1 complex | – | – | – | ± | – | – | – | – | – | – | – | – | – | – | – |
Slides were examined at a magnification of ×200. –, undetectable; ±, one cell in multiple fields; +, 1–3 cells in one field; ++, 4–8 cells in one field; +++,>8 cells in one field
The patient IDs correspond to those in our previous study.17
*ACA converted to positive after 1 year of immunosuppressive therapy.
†Treated with prednisolone 10 mg/day.
C, cytosol; D, discrete-speckled; F, female; H, homogeneous; LB, lip biopsy; MCTD, mixed connective tissue disease; N, nucleolar; NA, not assessed; PH, pulmonary hypertension; PMR, polymyalgia rheumatica; pSS, primary Sjögren’s syndrome; RZZ, Rod-Zw10-Zwilch; S, salivary gland; Sp, speckled; SS, Sjögren’s syndrome; sSS, secondary Sjögren’s syndrome.
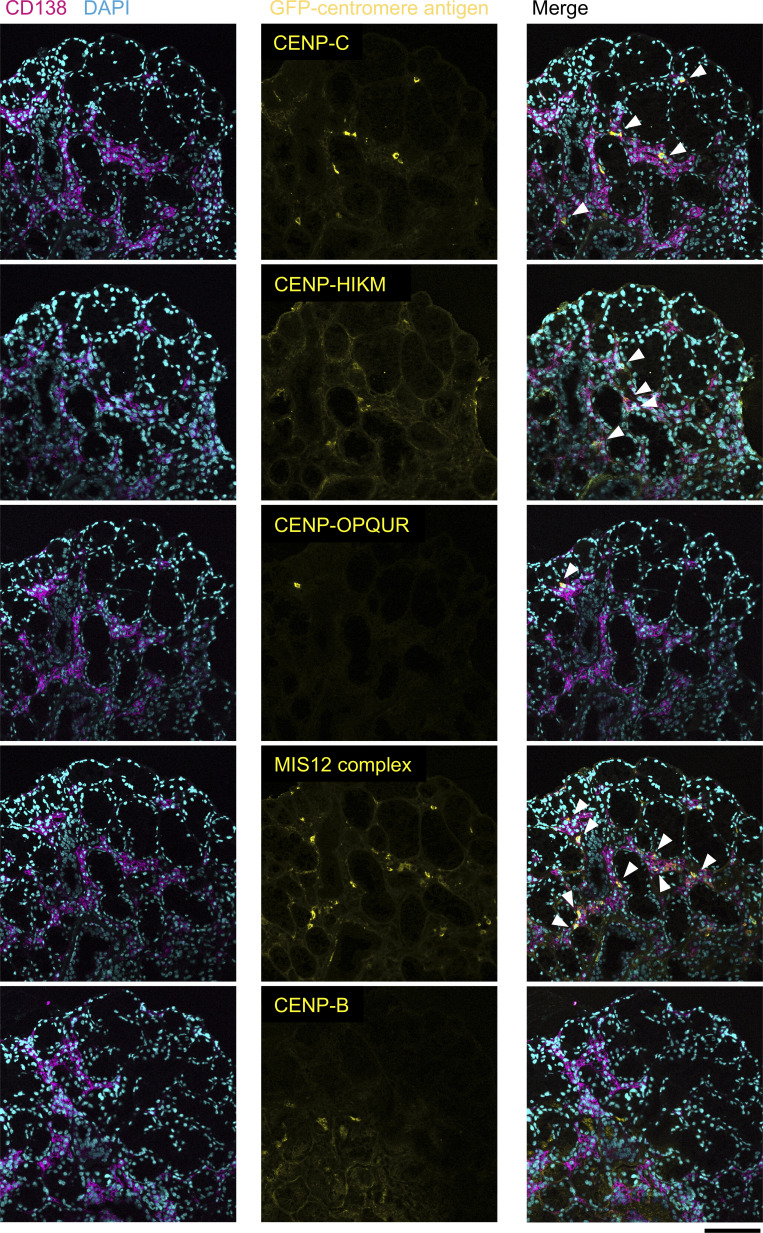
In addition, an analysis of serial sections demonstrated the distribution of ASCs against various antigens (CENP-C, CENP-HIKM, CENP-OPQUR and MIS12C) in the same area (figure 5). This result suggested that the centromere complex, particularly the kinetochore protein complex, was processed and presented to ASCs in SS salivary glands.
Figure 5.
The distribution of ASCs against various kinetochore antigens observed in serial salivary gland sections despite a lack of ASCs against CENP-B (magnification, ×200). Serial sections of salivary gland samples obtained from a serum ACA-positive patient (LB117) were stained with GFP-centromere antigens (yellow), CD138 (magenta), and DAPI (cyan). Arrowheads indicate ASCs against centromere antigens. Antibodies against CENP-C, CENP-HIKM, CENP-OPQUR, and the MIS12 complex were secreted in the same area of salivary glands from distinct ASCs. Scale bar: 100 µm. ASCs, antibody-secreting cells; DAPI, 4',6-diamidino-2-phenylindole; GFP, green fluorescent protein.
Finally, we illustrated a schematic figure of the centromere–kinetochore complex and its autoantigenicity in the sera of patients with various autoimmune diseases and in SS salivary glands (figure 6). Serum antibodies target centromeric chromatin (CENP-A, CENP-B and CBX5), the constitutive centromere-associated network (CCAN; consisting of CENP-C, CENP-HIKM, CENP-TWSX, CENP-LN and CENP-OPQUR) and MIS12C, whereas target antigens of lesion ASCs were dominant in the kinetochore complex.
Figure 6.
Schematic summary of autoantigenicity of centromere-kinetochore macrocomplex. Centromeric antigens, CCAN (CENP-C, CENP-HIKM, CENP-TWSX, CENP-LN, and CENP-OPQUR), and the MIS12 complex were the main targets of serum antibodies in patients with SS, SSc, and PBC. By contrast, antibody-secreting cells in salivary glands in patients with SS were specific to kinetochore antigens. CCAN, constitutive centromere-associated network; PBC, primary biliary cholangitis; SS, Sjögren’s syndrome; SSc, systemic sclerosis.
Discussion
We provided the comprehensive mapping of ACAs targets within autoimmune diseases. We demonstrated that the CCAN was the major target of serum autoantibodies as well as the previously known autoantigens CBX5, CENP-A, CENP-B, CENP-C and MIS12C in patients with SS, SSc and PBC. These results indicated that the centromere–kinetochore macrocomplex is the main target of serum ACAs. In addition, the autoantigenicity of centromere antigens was shared among patients with SS, SSc and PBC. With regards to the ASCs in SS salivary glands, kinetochore antigens, rather than centromeric proteins such as CENP-B, were the dominant targets of ASCs as opposed to serum ACAs.
Several studies indicated that ACA may cross-react with some non-centromere proteins.25 26 Although we could not rule out cross-reactivity with non-centromere proteins, however, we found that when an autoantibody is acquired against at least one major centromere antigen, it is likely to be accompanied by multiple ACAs as shown in figure 3. These results could not be explained by molecular mimicry alone and suggest that ACAs would recognise the structure of the centromere complex, rather than a single epitope.
Although a previous study showed that serum ACAs had relatively low or no reactivity against centromere proteins other than CENP-A, CENP-B and CENP-C,12 their reactivity might be underestimated due to the usage of non-mammalian cell-derived individual proteins regardless of their intermolecular association. We previously reported that using human cell-derived antigens and coexpression of the complexed proteins enables highly sensitive detection of autoantibody. Accumulating evidence about the molecular structure of the centromere6 21 enabled us to build a centromere antigen library in which the intermolecular conformation was taken into account. In this study, we identified multiple novel targets of ACAs, such as CENP-HIKM, CENP-TWSX, CENP-OPQUR, CENP-LN, NDC80C, KNL1C and the Astrin–SKAP complex. We believe that this concept, using conformational antigens for antibody detection, could be applicable to identify novel autoantibodies in other autoimmune conditions.
Although several studies have focused on the distinct epitope specificity comparing patients with SS and SSc, demonstrating that antibodies against CBX5 and CENP-C are frequently seen in SS compared with SSc,13 16 our present study clarified the similarity in serum autoantibodies against nine major autoantigens. Several reports demonstrated the frequent concurrence of SS/SSc, SS/PBC and SSc/PBC in the presence of ACAs.27 28 Moreover, the presence of ACAs is associated with characteristic symptoms such as Raynaud’s phenomenon, sclerodactyly and sicca syndrome regardless of whether classification criteria are fulfilled.29 30 Taken together, these results indicate that patients with ACA-positive SS, SSc and PBC have common clinical and immunological characteristics, strengthening our idea that novel disease classification, ‘ACA-related disease’, could be added to the disease category.
We further demonstrated the potential for the clinical application of the assay with multiple centromere antigens. Previous studies showed that the ELISA results of the anti-CENP-B antibody highly corresponded with the discrete-speckled pattern by the ANA test31; hence, these principal 2 methods could only detect the common population. Our data demonstrated that 14%–23% of the patients with SS, SSc or PBC had autoantibodies against multiple centromere antigens but not CENP-B. Furthermore, these antibodies were highly specific to autoimmune diseases. Additionally identified ACA positivity might have comparable clinical significance to anti-CENP-B antibody in patients with SS because the clinical characteristics were similar regardless of the presence of anti-CENP-B antibody. In SSc patients, although the clinical characteristics of ACA-positive patients with or without anti-CENP-B antibody were different in accordance with the prevalence of ATA, this result was consistent with that of previous reports that ATA and ACA were not mutually exclusive, and the clinical manifestations of ATA and ACA double-positive patients were similar to those of ATA single-positive patients.32 33
ASCs in the salivary glands of ACA-positive patients with SS showed reactivity to various centromere antigens and characterised by specificity to kinetochore antigens. There is accumulating evidence of local antibody production in target organs of systemic autoimmune diseases.34 In SS, several studies described the antigen-driven immune response and autoantibody production from B cells within salivary glands.35 36 In our study, the observed diversity of ASCs against kinetochore proteins corroborated the presence of serum various ACAs in autoimmune diseases. Although serum autoantibodies showed similar reactivity against major autoantigens, including both centromeric chromatin and kinetochore proteins, ASCs in the salivary glands showed specificity to kinetochore proteins. In addition, immunostaining of serial sections with various kinetochore antigens showed the local accumulation of distinct anti-kinetochore ASCs. These results suggested that the kinetochore complex is presented to B cells and causes kinetochore-driven antibody selection within sialadenitis in SS.
We note that this study has several limitations. The diagnostic potential of our method should be verified in a large cohort of seronegative autoimmune disease patients because many of the patients in our cohort were already diagnosed with conventional disease-specific autoantibodies. In addition, our result might not reflect the serology in early stage of each disease because the patients, especially in SSc group, had relatively long disease duration. Moreover, the result from ASCs of SS salivary glands could show the indirect evidence of kinetochore-driven antibody selection, however, further research is needed to clarify the precise mechanism of ACA acquisition. The specificity of ASCs in affected organs other than SS salivary glands remains a challenge due to the limitation of sample collection.
In conclusion, our study presented the precise mapping of ACA targets, indicating that the centromere–kinetochore macrocomplex is the main target of serum ACAs and that patients with ACA-positive SS, SSc and PBC form distinct subgroups in terms of the similarity of antibody specificity. The acquisition of ACAs might be the result of kinetochore complex-driven antibody selection in affected organs.
Acknowledgments
We thank Ms. Harumi Kondo and Ms. Mayumi Ota for collecting clinical samples. This study was supported by the Collaborative Research Resources, Keio University School of Medicine, which provided technical assistance. The pEFs vector was kindly gifted from Dr. A. Yamashita, Yokohama City University School of Medicine, Japan.
Footnotes
Handling editor: Josef S Smolen
Contributors: Study design: NK, MT, KS, KT and TT. Data acquisition: NK, MT, YK, HY, KI, HS, SK, HS, and KT. Data analysis and interpretation: NK, MT and KS. Manuscript drafting: NK, MT, KS and TT. All authors approved the final version of the manuscript.
Funding: This work was supported by JSPS KAKENHI, Grant numbers JP 16K19609 and JP 17H04216.
Competing interests: MT, KS, and TT have applied for a patent of anti-MIS12C antibody as diagnostic marker.
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was approved by the Ethics Committee of Keio University School of Medicine and was conducted in accordance with the principles of the Declaration of Helsinki.
References
- 1. Kayser C, Fritzler MJ. Autoantibodies in systemic sclerosis: unanswered questions. Front Immunol 2015;6:2–7. 10.3389/fimmu.2015.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fayyaz A, Kurien BT, Scofield RH. Autoantibodies in Sjögren's syndrome. Rheum Dis Clin North Am 2016;42:419–34. 10.1016/j.rdc.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liberal R, Grant CR, Sakkas L, et al. Diagnostic and clinical significance of anti-centromere antibodies in primary biliary cirrhosis. Clin Res Hepatol Gastroenterol 2013;37:572–85. 10.1016/j.clinre.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 4. Stochmal A, Czuwara J, Trojanowska M, et al. Antinuclear antibodies in systemic sclerosis: an update. Clin Rev Allergy Immunol 2020;58:40–51. 10.1007/s12016-018-8718-8 [DOI] [PubMed] [Google Scholar]
- 5. Fukagawa T, Earnshaw WC. The centromere: chromatin foundation for the kinetochore machinery. Dev Cell 2014;30:496–508. 10.1016/j.devcel.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Monda JK, Cheeseman IM. The kinetochore-microtubule interface at a glance. J Cell Sci 2018;131:jcs214577. 10.1242/jcs.214577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol 2008;9:33–46. 10.1038/nrm2310 [DOI] [PubMed] [Google Scholar]
- 8. Nagpal H, Fukagawa T. Kinetochore assembly and function through the cell cycle. Chromosoma 2016;125:645–59. 10.1007/s00412-016-0608-3 [DOI] [PubMed] [Google Scholar]
- 9. Kern DM, Monda JK, Su K-C, et al. Astrin-SKAP complex reconstitution reveals its kinetochore interaction with microtubule-bound Ndc80. Elife 2017;6:e26866. 10.7554/eLife.26866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Obuse C, Iwasaki O, Kiyomitsu T, et al. A conserved Mis12 centromere complex is linked to heterochromatic HP1 and outer kinetochore protein Zwint-1. Nat Cell Biol 2004;6:1135–41. 10.1038/ncb1187 [DOI] [PubMed] [Google Scholar]
- 11. Auckland P, Roscioli E, Coker HLE, et al. Cenp-F stabilizes kinetochore-microtubule attachments and limits dynein stripping of corona cargoes. J Cell Biol 2020;219:e201905018. 10.1083/jcb.201905018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song G, Hu C, Zhu H, et al. New centromere autoantigens identified in systemic sclerosis using centromere protein microarrays. J Rheumatol 2013;40:461–8. 10.3899/jrheum.120264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanaka N, Muro Y, Suzuki Y, et al. Anticentromere antibody-positive primary Sjögren's syndrome: epitope analysis of a subset of anticentromere antibody-positive patients. Mod Rheumatol 2017;27:115–21. 10.1080/14397595.2016.1176327 [DOI] [PubMed] [Google Scholar]
- 14. Hudson M, Mahler M, Pope J, et al. Clinical correlates of CENP-A and CENP-B antibodies in a large cohort of patients with systemic sclerosis. J Rheumatol 2012;39:787–94. 10.3899/rheum.111133 [DOI] [PubMed] [Google Scholar]
- 15. Fritzler MJ, Rattner JB, Luft LM, et al. Historical perspectives on the discovery and elucidation of autoantibodies to centromere proteins (CENP) and the emerging importance of antibodies to CENP-F. Autoimmun Rev 2011;10:194–200. 10.1016/j.autrev.2010.09.025 [DOI] [PubMed] [Google Scholar]
- 16. Gelber AC, Pillemer SR, Baum BJ, et al. Distinct recognition of antibodies to centromere proteins in primary Sjogren's syndrome compared with limited scleroderma. Ann Rheum Dis 2006;65:1028–32. 10.1136/ard.2005.046003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takeshita M, Suzuki K, Kaneda Y, et al. Antigen-driven selection of antibodies against SSA, SSB and the centromere 'complex', including a novel antigen, MIS12 complex, in human salivary glands. Ann Rheum Dis 2020;79:150–8. 10.1136/annrheumdis-2019-215862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League against rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. 10.1136/annrheumdis-2016-210571 [DOI] [PubMed] [Google Scholar]
- 19. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. 10.1136/annrheumdis-2013-204424 [DOI] [PubMed] [Google Scholar]
- 20. European Association for the Study of the Liver . EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145–72. 10.1016/j.jhep.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 21. Weir JR, Faesen AC, Klare K, et al. Insights from biochemical reconstitution into the architecture of human kinetochores. Nature 2016;537:249–53. 10.1038/nature19333 [DOI] [PubMed] [Google Scholar]
- 22. Kops GJPL, Kim Y, Weaver BAA, et al. Zw10 links mitotic checkpoint signaling to the structural kinetochore. J Cell Biol 2005;169:49–60. 10.1083/jcb.200411118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt JC, Arthanari H, Boeszoermenyi A, et al. The kinetochore-bound Ska1 complex tracks depolymerizing microtubules and binds to curved protofilaments. Dev Cell 2012;23:968–80. 10.1016/j.devcel.2012.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwertman NC, Owens MA, Adnan R. A simple more General boxplot method for identifying outliers. Comput Stat Data Anal 2004;47:165–74. 10.1016/j.csda.2003.10.012 [DOI] [Google Scholar]
- 25. Gkoutzourelas A, Barmakoudi M, Bogdanos DP. A bioinformatics analysis reveals novel pathogens as molecular mimicry triggers of systemic sclerosis. Mediterr J Rheumatol 2019;31:50. 10.31138/mjr.31.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fritzler MJ, Hudson M, Choi MY, et al. Bicaudal D2 is a novel autoantibody target in systemic sclerosis that shares a key epitope with CENP-A but has a distinct clinical phenotype. Autoimmun Rev 2018;17:267–75. 10.1016/j.autrev.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 27. Salliot C, Gottenberg J-E, Bengoufa D, et al. Anticentromere antibodies identify patients with Sjögren's syndrome and autoimmune overlap syndrome. J Rheumatol 2007;34:2253–8. [PubMed] [Google Scholar]
- 28. Miyawaki S, Asanuma H, Nishiyama S, et al. Clinical and serological heterogeneity in patients with anticentromere antibodies. J Rheumatol 2005;32:1488–94. [PubMed] [Google Scholar]
- 29. Caramaschi P, Biasi D, Manzo T, et al. Anticentromere antibody--clinical associations. A study of 44 patients. Rheumatol Int 1995;14:253–5. 10.1007/BF00262092 [DOI] [PubMed] [Google Scholar]
- 30. Tsukamoto M, Suzuki K, Takeuchi T. Clinical and immunological features of anti-centromere antibody-positive primary Sjögren's syndrome. Rheumatol Ther 2018;5:499–505. 10.1007/s40744-018-0126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rothfield N, Whitaker D, Bordwell B, et al. Detection of anticentromere antibodies using cloned autoantigen CENP-B. Arthritis Rheum 1987;30:1416–9. 10.1002/art.1780301214 [DOI] [PubMed] [Google Scholar]
- 32. Jarzabek-Chorzelska M, Błaszczyk M, Kołacińska-Strasz Z, et al. Are ACA and SCL 70 antibodies mutually exclusive? Br J Dermatol 1990;122:201–8. 10.1111/j.1365-2133.1990.tb08266.x [DOI] [PubMed] [Google Scholar]
- 33. Heijnen IAFM, Foocharoen C, Bannert B, et al. Clinical significance of coexisting antitopoisomerase I and anticentromere antibodies in patients with systemic sclerosis: a EUSTAR group-based study. Clin Exp Rheumatol 2013;31:96–102. [PubMed] [Google Scholar]
- 34. Reparon-Schuijt CC, van Esch WJ, van Kooten C, et al. Functional analysis of rheumatoid factor-producing B cells from the synovial fluid of rheumatoid arthritis patients. Arthritis Rheum 1998;41:2211–20. [DOI] [PubMed] [Google Scholar]
- 35. Stott DI, Hiepe F, Hummel M, et al. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. the salivary glands of patients with Sjögren's syndrome. J Clin Invest 1998;102:938–46. 10.1172/JCI3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maier-Moore JS, Koelsch KA, Smith K, et al. Antibody-secreting cell specificity in labial salivary glands reflects the clinical presentation and serology in patients with Sjögren's syndrome. Arthritis Rheumatol 2014;66:3445–56. 10.1002/art.38872 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-218881supp001.pdf (24MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.